Introduction
Cisplatin is one of the most widely used
chemotherapeutics and has been applied for treating ovarian cancer.
However, resistance to cisplatin can develop, which results in poor
prognosis and high patient mortality (1,2).
Over the last 40 years, many biological analyses of the mechanism
of cisplatin resistance have been performed, and it is now
recognized to be more complicated than originally thought.
Mechanisms of cisplatin resistance in many types of cancer were
thought to be associated with enhanced drug efflux, reduced drug
uptake, repair of DNA adducts, and evasion of apoptosis (3,4). In
addition, previous studies by the authors demonstrated that
cisplatin resistance in ovarian cancer cells was associated with
increased autophagic degradation of ubiquitinated proteins
(5), enhanced lysosomal function
in cisplatin-induced autophagic processes (6), and close communication between the
endoplasmic reticulum (ER) and mitochondria that was induced by the
overexpression of Bcl-2 (7).
Members of the B-cell lymphoma 2 (Bcl-2) protein
family are the major regulators of the apoptotic process, and the
pro-survival members of this family (Bcl-2, Bcl-w and Bcl-xL) that
inhibit apoptosis are overexpressed in many types of cancer. The
altered expression of the pro-survival members of the Bcl-2 has
been reported to be associated with resistance to cytotoxic
antineoplastic drugs (8-11). The mechanisms by which Bcl-2 family
proteins regulate apoptosis were thought to depend primarily on
their ability to modulate the release of apoptosis-associated
proteins (12). Consistent with
mitochondria being the major sites of activity of members of the
Bcl-2 protein family, studies have suggested that the
overexpression of Bcl-2 and Bcl-xL are also able to stimulate
mitochondrial respiration, with increases in the activity of
complex I, oxygen consumption, adenosine triphosphate (ATP) levels,
and mitochondrial transmembrane potential (∆Ψm) in osteosarcoma and
lung cancer cells (13,14). Therefore, apart from their role in
the apoptotic process, the anti-apoptotic members of the Bcl-2
family may also have additional roles associated with mitochondrial
metabolism to regulate cell fate.
It was previously hypothesized that cancer cells are
highly dependent on aerobic glycolysis to survive, which is also
termed the 'Warburg effect' (15,16).
However, recent studies have demonstrated that the sources of ATP
from mitochondria are also of great importance to cancer cells
(17). Recently, evidence in
support of the hypothesis that resistance to cytotoxic
antineoplastic drugs induces a metabolic shift from aerobic
glycolysis towards oxidative phosphorylation (OXPHOS) has been
presented (18-20), demonstrating that metabolic
plasticity has an important role in tumor recurrence. However,
research on the metabolic changes in cisplatin-resistant ovarian
cancer cells (C13) has indicated increased dependence on glucose
and reduced oxygen consumption compared with those in
cisplatin-sensitive (2008) cells (21). Therefore, the metabolic phenotypes
of drug-resistant cancer cells may differ for different drugs or
cancer cell lines.
ABT-737, a powerful inhibitor of anti-apoptotic
proteins, Bcl-2, Bcl-xL, and Bcl-w, displays synergistic
cytotoxicity (9) and induces
considerable apoptosis in assorted cancer types, including ovarian
cancer, cholangiocarcinoma and lung cancer (7,22).
Moreover, the authors of the present study previously found that
ABT737 was able to enhance cisplatin-induced apoptosis by
regulating ER-mitochondrial Ca2+ signal transduction or
modulating mitochondrial dynamics in ovarian cancer cells (7,22).
Based on the roles of pro-survival members of Bcl-2 family in
mitochondrial metabolism, it was proposed that ABT737 might affect
resistance to cisplatin by modulating glucose metabolism in human
ovarian cancer cells.
The data reported in the present study indicated
that glucose demand in human ovarian cancer SKOV3/DDP
(cisplatin-resistant) cells increased, and metabolism shifted in
these cells towards OXPHOS via the stable overexpression of Bcl-2.
Furthermore, inhibiting Bcl-2 reduced OXPHOS and selectively
sensitized SKOV3/DDP cells to cisplatin. The combination of
inhibiting Bcl-2 and glycolysis dramatically decreased the survival
of SKOV3/DDP cells. Therefore, targeting Bcl-2 family members may
be a promising approach for treating ovarian cancer. Furthermore,
in the modulation of cancer glucose metabolism, pro-survival
members of Bcl-2 family appear to be closely related to
hypoxia-inducible factor 1α (HIF-1α).
Materials and methods
Reagents and antibodies
The stock solutions of ABT-737 were prepared in DMSO
(Selleck Chemicals, Houston, TX, USA). Cisplatin,
2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]-D-glucose
(2-NBDG), Rotenone and 6-aminonicotinamide (6-AN) were from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). MitoSOX Red
(mitochondrial super-oxide indicator) and MitoTracker Green were
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP)
and antimycin A were purchased from Abcam (Cambridge, MA, USA).
2-deoxy-D-glucose (2-DG), rotenone and dehydroepiandrosterone
(DHEA) were from Aladdin industrial Corporation (Shanghai, China).
2′,7′-Dichlorofluorescin diacetate (DCFH-DA) was purchased from
Beyotime Institute of Biotechnology (Shanghai, China). Anti-HK2
(cat. no. 22029-1-AP), anti-Bcl-2 (cat. no. 12789-1-AP), anti-G6PD
(cat. no. 25413-1-AP), anti-β-actin (cat. no. 60008-1-Ig) and
anti-PDHB (cat. no. 14744-1-AP) antibodies were purchased from
ProteinTech Group, Inc., (Chicago, IL, USA) (1:1,000). Anti-HIF-1α
(1:200; cat. no. sc-10790) and anti-Glut1 (1:200; cat. no. sc-7903)
were purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX,
USA). Peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L;
cat. no. SA00001-2) and peroxidase-conjugated AffiniPure goat
anti-mouse IgG (H+L; cat. no. SA00001-1) from ProteinTech Group,
Inc.
Cell culture
Human ovarian carcinoma cell lines SKOV3
(cisplatin-sensitive) and SKOV3/DDP (cisplatin-resistant) cells
were obtained from the Chinese Academy of Medical Sciences and
Peking Union Medical College (Beijing, China). The two cell lines
were maintained in Roswell Park Memorial Institute-1640 (RPMI-1640)
culture medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS, Invitrogen; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 mg/ml
streptomycin (complete medium) at 37°C and 5% CO2 with
high humidity. To maintain resistance to cisplatin, SKOV3/DDP cells
were cultured in complete medium with 1 μg/ml cisplatin
(Sigma-Aldrich, Merck KGaA) (6).
Cell viability assays
Cell viability was determined by MTT assay. The
cells were seeded in 96-well plates with 100 μl complete
RPMI-1640 medium at 8×103 per cells/well. Following
overnight incubation at 37°C, increasing concentrations of
cisplatin were applied followed by culture at 37°C for 24 h. For
each group, replicate experiments were performed in five wells. A
total of 20 μl MTT was added to each well and incubated for
4 h. Then, 150 μl DMSO was added to each well to dissolve
the formazan crystals. The absorbance at 570 nm was determined
using a microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Glucose metabolism RT2 profiler
polymerase chain reaction (PCR) array
The expression levels of 84 key genes in glucose
metabolism were determined by Human Glucose Metabolism
RT2 Profiler™ PCR Array (SABiosciences; Qiagen GmbH,
Hilden, Germany). Total RNA was isolated from cultured cells, and 1
μg of total RNA was reverse-transcribed to single-stranded
cDNA using the RT2 First Strand kit (SABiosciences;
Qiagen GmbH). The expression levels of genes of interest were
determined by quantitative PCR using the Applied Biosystems 7300
Fast Real-Time PCR system (Thermo Fisher Scientific, Inc.) with
SYBR Green fluorophore using the RT2 SYBR Green Master Mix
(SABiosciences; Qiagen GmbH). The reaction program involved 40
cycles of 95°C for 10 min, 95°C for 15 sec and 60°C for 1 min. The
results were analyzed using the manufacturer's software and
relative gene expression was quantified using the 2−ΔΔCq
method (23). The altered
expression of the 84 genes was displayed using heat imaging with
normalization to β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In accordance with the manufacturer's protocol,
total RNA of cultured cells was extracted using TRIzol (Invitrogen,
Thermo Fisher Scientific Inc.). A total of 1 μg RNA was
reverse-transcribed to cDNA using SuperScript™ IV First-Strand
Synthesis System (Invitrogen, Thermo Fisher Scientific Inc.). The
relative expression of genes of interest was determined with
StepOne™ Real-Time PCR system and Power SYBR® Green PCR
Master Mix (Applied Biosystems; Thermo Fisher Scientific Inc.) on
an ABI 7300 instrument. The RT reaction was initially run at 50°C
for 10 min, followed by a denaturation step at 94°C for 2 min.
Also, the amplification reaction was continued for 35 cycles of
denaturing (94°C for 15 sec) and annealing (55°C for 30 sec),
followed by final extending step at 68°C for 1 min. The primer
sequences are listed in Table
I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer name | Primer sequence
(5′-3′) |
|---|
| HK2 | |
| Forward |
GAGCCACCACTCACCCTACT |
| Reverse |
CCAGGCATTCGGCAATGTG |
| GPI | |
| Forward |
GCTTTGCTGCGTACTTCCA |
| Reverse |
GTCCACACGGGTTCCAGA |
| PFKL | |
| Forward |
GGCTTCGACACCCGTGTAA |
| Reverse |
CGTCAAACCTCTTGTCATCCA |
| G6PD | |
| Forward |
ATGGCAGAGCAGGTGGCCCT |
| Reverse |
TCATGCAGGACTCGTGAATG |
| PDHB | |
| Forward |
GTAGAGGACACGGGCAAGAT |
| Reverse |
TTCACGAACTGTCAACTGCAC |
| LDHA | |
| Forward |
TTGACCTACGTGGCTTGGAAG |
| Reverse |
GGTAACGGAATCGGGCTGAAT |
| CS | |
| Forward |
TCCGACCCTTACCTGTCCTT |
| Reverse |
ACTTCCTGATTTGCCAGTCC |
| ACO2 | |
| Forward |
AGATTGTGTATGGACACCTGGA |
| Reverse |
TACGACTTGCCTCGCTCAAT |
| IDH2 | |
| Forward |
CCATCATCTGCAAAAACATCC |
| Reverse |
CCAATGGTGATGGGCTTG |
| MDH2 | |
| Forward |
CAGGACCAGCTGACAGCAC |
| Reverse |
AGCCTGCTCCGGCTTTAG |
| GYS | |
| Forward |
GCCTTTCCAGAGCACTTCAC |
| Reverse |
CTCCTCGTCCTCATCGTAGC |
| HIF-1α | |
| Forward |
TGGATGGCTTTGTTATGGTG |
| Reverse |
TGGTCACATGGATGGGTAAA |
| β-actin | |
| Forward |
TGTATGCCTCTGGTCGTACC |
| Reverse |
CAGGTCCAGACGCAGGATG |
Oxygen consumption and extracellular
acidification rates
The rates of oxygen consumption (OCR) and
extracellular acidification were determined using the fluorescent
oxygen-sensitive and pH-sensitive probes Mito-Xpress and pH-Xtra
(Luxcel Bioscience, Cork, Ireland). Briefly, SKOV3 or SKOV3/DDP
cells were seeded in 96-well plates at 8×104 cells/well.
Following overnight incubation at 37°C, different drug treatments
were applied followed by culture for 6 h. For each group, replicate
experiments were performed in three wells (24).
Glucose, lactate concentrations and
glucose uptake
The cells were seeded in 6-well plates at
5×105 cells per well. Following overnight incubation at
37°C, the medium was changed to fresh complete medium. After 24 h,
the medium of the cells was collected after which the proteins were
extracted by sonication and quantified using the Bradford Protein
Assay kit (Beyotime Institute of Biotechnology). Then, the glucose
and lactate concentrations were determined using glucose assay
(RsBio, Shanghai, China) and lactate assay kits (Jiancheng Bio,
Nanjing, China), respectively. The untreated groups were collected
and measured using 2-NBDG (50 μM; Sigma-Aldrich; Merck KGaA)
in Dulbecco's modified Eagle's medium (Gibco Life Technologies;
without serum and glucose) for 30 min to determine the capacity for
glucose uptake using a flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA).
ATP assay of cell viability and glycogen
concentration
A total of 1×106 cells were plated in a
25 cm2-cell culture flask. Following overnight
incubation at 37°C, the medium was changed to fresh medium. After
24 h, in accordance with the manufacturer's instructions, cells
were washed with PBS and then their ATP levels were determined by
CellTiter-Glo® Luminescent Cell Viability Assay
(Promega, Madison, MI, USA). The glycogen concentrations were
determined from the cell lysate using a glycogen assay kit
(Jiancheng Bio) in accordance with the manufacturer's protocol.
Western blot analysis
Western blot determination of cell extracts was
measured as described previously (25). After different treatments, the
SKOV3 or SKOV3/DDP cells were harvested and washed with cold
phosphate-buffered saline (PBS), and then incubated in ice-cold
radioimmunoprecipitation assay buffer. The cell lysates were
sonicated and centrifuged at 5,000 × g for 10 min. The
concentration of the protein was determined using the Bradford
Protein Assay kit (Beyotime Institute of Biotechnology). The
proteins samples (30-50 μg) were separated by 12% w/v
SDS-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes. Then, the membranes were
blocked with 5% (w/v) skim milk in PBST buffer [100 mM NaCl, 10 mM
Tris-HCl (pH 7.6) and 0.1% (v/v) Tween-20] for 1 h at room
temperature, and incubated overnight at 4°C with the primary
antibody (anti-HK2, 1:1,000 dilution; anti-Bcl-2, 1:1,000 dilution;
anti-G6PD, 1:1,000 dilution; anti-β-actin, 1:1,000 dilution;
anti-PDHB, 1:1,000 dilution; anti-HIF-1α, 1:200 dilution and
anti-Glut1, 1:200 dilution). The following day, the membranes were
washed with PBST and incubated with secondary antibodies (1:2,000
dilution). The membranes were detected using the ECL reagents and
captured using the Syngene Bio Imager (Synoptics Ltd., Cambridge,
UK).
Biochemical measurements
A total of 1×106 SKOV3 or SKOV3/DDP cells
were seeded in a 25 cm2-cell culture flask. Following
overnight incubation at 37°C, the complete medium was changed to
fresh medium. After 24 h, 5×106 cells were collected
followed by protein extraction by sonication and quantification
using the Bradford Protein Assay kit. Then, the cells were
subjected to analysis with the NADP+/NADPH
Quantification kit (BioVision, Inc., Milpitas, CA, USA) to
determine NADPH levels and NADPH/NADP+ ratios. The
cellular glutathione (GSH) and glutathione disulfide (GSSG) levels
and GSH/GSSG ratio were determined using commercial colorimetric
kits (Jiancheng Bio) (26). The
glucose 6-phosphate dehydrogenase (G6PD) activity was determined
using commercial colorimetric kits (BioVision Inc.). The absorbance
was determined using a microplate reader (BioTek Instruments,
Inc.).
Flow cytometric analysis of
apoptosis
A total of 5×105 cells were plated in
6-well plates and incubated overnight at 37°C. Following exposure
to ABT737 and/or cisplatin at 37°C for 24 h, the cells were
collected. The induction of apoptosis in these cells was determined
using Annexin V-FITC (Annexin V Apoptosis Detection Kit II, BD
Biosciences, San Diego, CA, USA). The cells were analyzed using a
flow cytometer (BD Biosciences).
Assessment of intracellular ROS level and
intramitochondrial superoxide anion
The intracellular reactive oxygen species (ROS) and
intramitochondrial superoxide anion (O2−)
were also determined using DCFH-DA and MitoSOX Red, respectively. A
total of 5×105 cells were plated in 6-well plates and
incubated overnight at 37°C. Following treatment as indicated in
the figure legends, the cells were washed with PBS. DCFH-DA (10
μM) or MitoSOX Red (5 μM) was added to the cells and
cultured at 37°C and 5% CO2 for 20 min. Then, the cells
were collected. The fluorescence of the stained cells was detected
using a flow cytometer (BD Biosciences).
Live/dead cell viability assay
Cell death was detected using Calcein-AM/PI Double
Stain Kit (Shanghai Yeasen Biotechnology Co., Ltd, Shanghai,
China). Briefly, following treatment, the SKOV3 or SKOV3/DDP cells
were cultured with 2 μM propidium iodide and 4 μM
calcein acetoxymethyl ester in an incubator at 37°C and 5%
CO2 for 30 min. After rinsing with PBS, the viability of
the cells was then determined using an IX71 fluorescence
microscope. The dead and alive cells represented by bright red or
green fluorescence were identified upon excitation at 544 and 485
nm, respectively.
Statistical analyses
The data were analyzed by one-way analysis of
variance using SPSS (version 21.0; IBM Corp., Armonk, NY, USA).
Tukey's post hoc test was used to determine the significance for
all pairwise comparisons of interest. The data were obtained from
three experiments and presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Glucose metabolism is altered in
cisplatin-resistant cells
A pair of isogenic ovarian cancer cell lines that
were cisplatin-sensitive (SKOV3) or cisplatin-resistant (SKOV3/DDP)
was used. SKOV3/DDP, a subline of SKOV3, acquired resistance to
cisplatin in vitro (5). As
expected, SKOV3/DDP cells exhibited considerable resistance to
cisplatin, while SKOV3 cells also exhibited resistance to cisplatin
as determined by the MTT assay following exposure to increasing
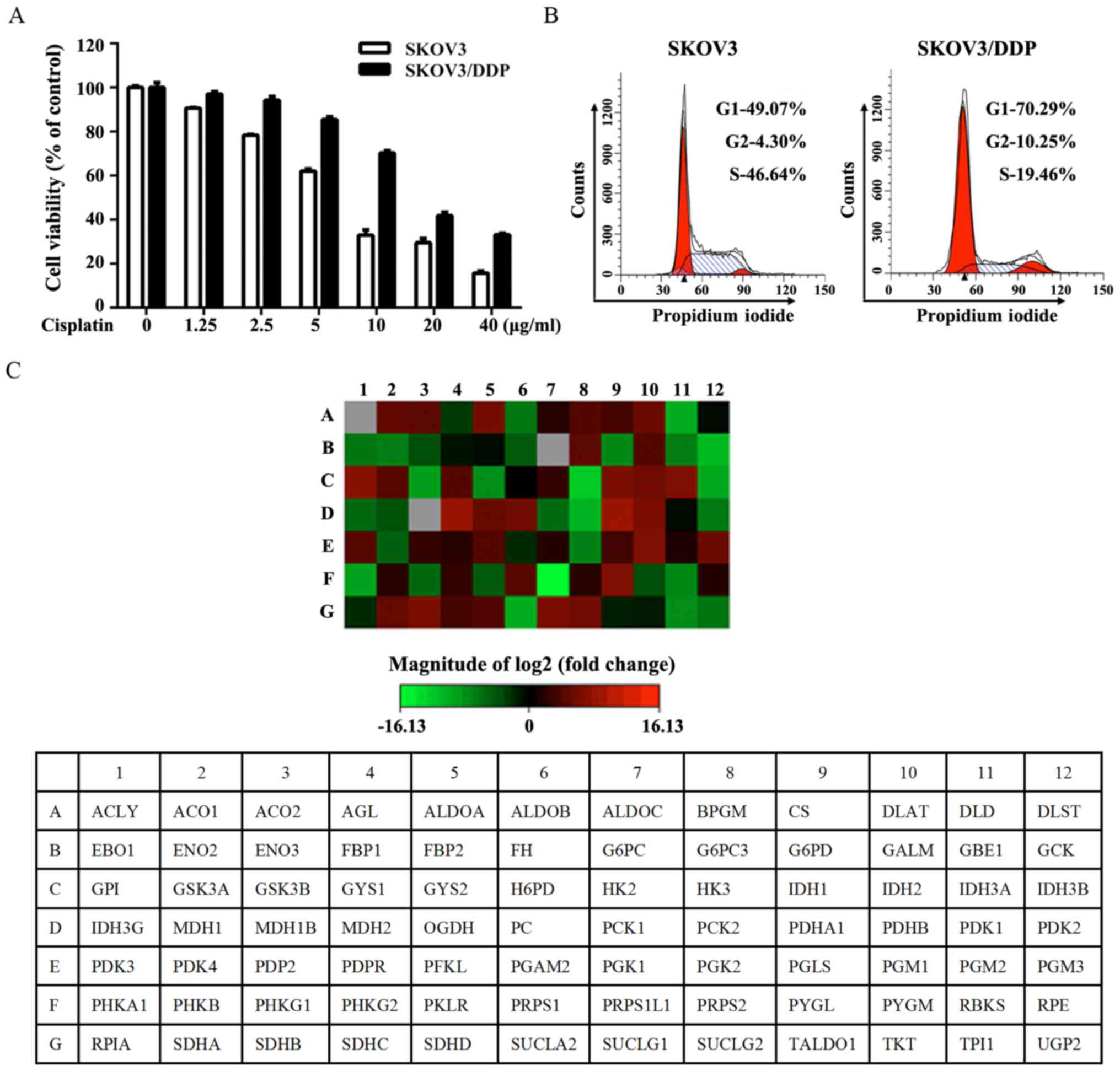
concentrations of cisplatin for 24 h (Fig. 1A). As shown in Fig. 1B, SKOV3/DDP cells were
preferentially enriched for G0/G1 quiescent cells and had a lower
proliferation rate. The expression of genes associated with glucose
metabolism was assessed by RT2 Human Glucose Metabolism Profiler
PCR array. The obtained results indicated the upregulation of
glycolysis, the tricarboxylic acid cycle (TCA) cycle and
gluconeogenesis in SKOV3/DDP cells (Fig. 1C and Table II).
 | Table IIFunctional grouping of gene
expression. |
Table II
Functional grouping of gene
expression.
| Functional gene
grouping | Upregulated | Downregulated |
|---|
| Glucose
metabolism | | |
| Glycolysis | ALDOA, BPGM, GALM,
GPI, HK2, PFKL, PGM1, PGM3 | ALDOB, ENO1, ENO2,
ENO3, GCK, HK3, PGK2, PKLR, TPI1 |
|
Gluconeogenesis | G6PC3, PC, | G6PC, PCK1,
PCK2 |
| Regulation | PDK3, PDP2 | PDK2, PDK4 |
| TCA cycle | ACO1, ACO2, CS,
DLAT, IDH1, IDH2, IDH3A, MDH2, OGDH, PDHA1, PDHB, SDHA, SDHB, SDHC,
SDHD, SUCLG1, SUCLG2 | ACLY, DLD, FH,
IDH3B, IDH3G, MDH1, MDH1B, SUCLA2 |
| PPP | PGLS | PRPS1L1, RBKS,
RPIA |
| Glycogen
metabolism | | |
| Synthesis | GYS1 | UGP2, GYS2,
GBE1 |
| Degradation | PYGL | AGL, PYGM |
| Regulation | PHKG2, GSK3A | PHKA1, PHKG1,
GSK3B |
Cisplatin-resistant cells exhibit a
higher glucose demand
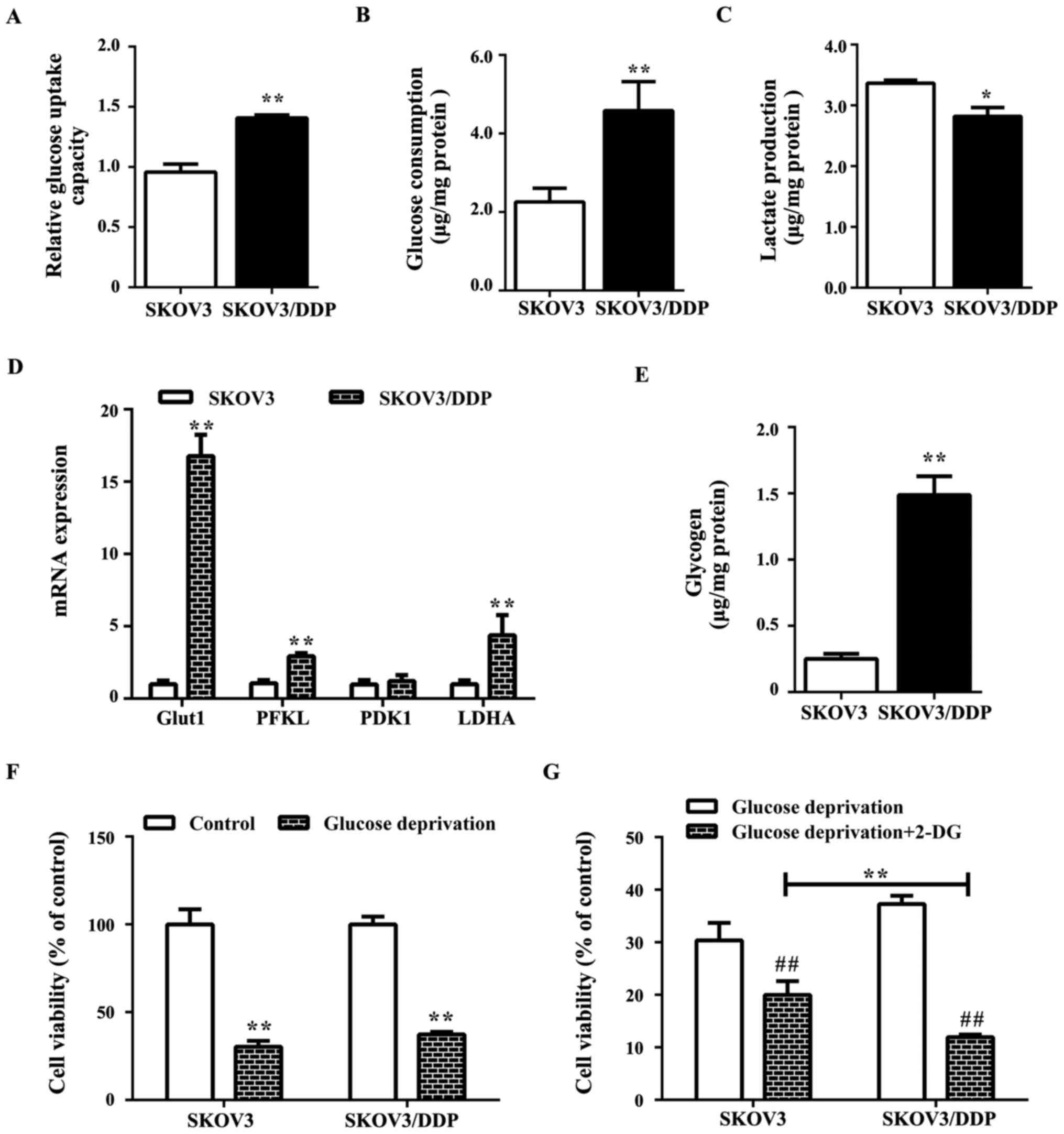
Further study suggested that glucose metabolism in
SKOV3/DDP cells may be altered compared with SKOV3 cells with
increased glucose uptake and consumption (Fig. 2A and B), decreased lactate
production (Fig. 2C), and
overexpression of the glucose metabolism-associated genes,
PFKL and LDHA, and the glucose transporter
Glut1 as well as elevated glycogen levels (Fig. 2D). As glycogen is a branched
polymer of glucose that acts as an intracellular glucose store,
high glycogen levels may render the cells less sensitive to glucose
deprivation (Fig. 2E). Notably,
SKOV3/DDP cells exhibited reduced sensitivity to glucose
deprivation compared with SKOV3 cells (Fig. 2F), while the combined treatment
with 2-DG (glycolysis inhibitor) induced significant cell death
compared with the glucose deprivation alone group (Fig. 2G).
Cisplatin-resistant cells exhibit an
increase in oxygen consumption
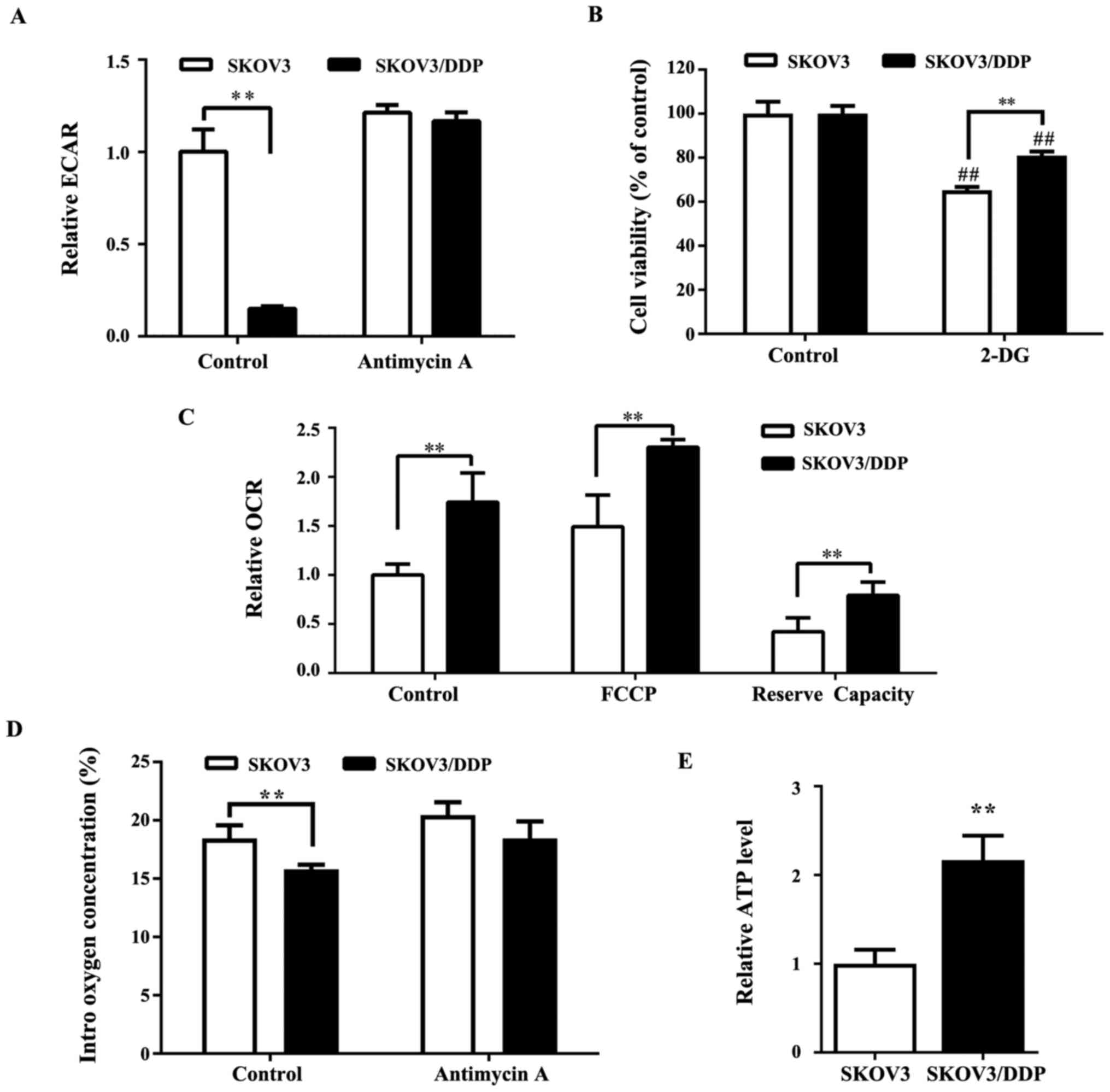
Numerous studies have previously demonstrated that
the Warburg effect is extremely important to ovarian tumor growth
(27,28). An analysis of the extracellular
acidification rate (ECAR) indicated that ECAR was significantly
lower (Fig. 3A) in SKOV3/DDP cells
compared with SKOV3 cells, indicating a reduction of the Warburg
effect in cisplatin-resistant cancer cells. 2-DG, a glycolytic
inhibitor, blocks glycolysis by inhibiting hexokinase, which is the
key rate-limiting enzyme of glycolysis. The results of the present
study suggested that SKOV3/DDP cells were less sensitive to 2-DG
compared with SKOV3 cells (Fig.
3B). As the basal rate of glycolysis in SKOV3/DDP cells was
lower compared with SKOV3 cells, the metabolic status of SKOV3/DDP
cells might involve downregulation of glycolysis and a shift toward
OXPHOS. To investigate this hypothesis, the OCR (which is
indicative of OXPHOS) in SKOV3 and SKOV3/DDP cells was measured. As
shown in Fig. 3C, SKOV3/DDP cells
demonstrated significantly higher OCR, which represented higher
oxidative metabolism compared with SKOV3 cells. Consistent with
this finding, the intracellular oxygen concentration was lower in
SKOV3/DDP cells compared with SKOV3 cells (Fig. 3D). Furthermore, the ATP level was
higher in SKOV3/DDP compared with SKOV3 cells (Fig. 3E).
Cisplatin-resistant cells have elevated
levels of intramitochondrial superoxide anion
(O2−) and intracellular ROS
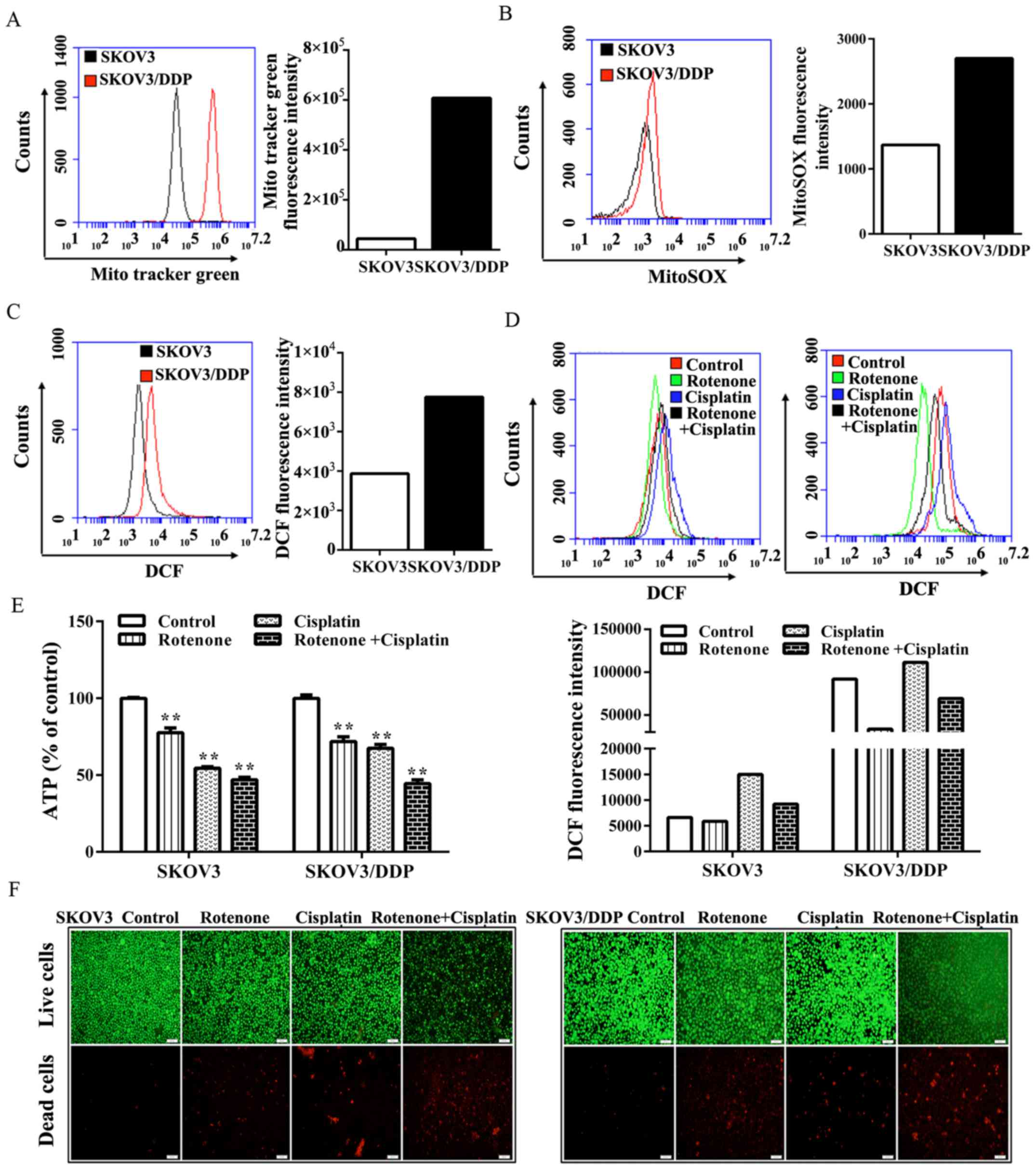
As oxidative processes mainly take place in the
mitochondria, whether the increased oxygen consumption demonstrated
in SKOV3/DDP cells was a result of increased mitochondrial mass was
investigated.
Notably, compared with the level in SKOV3 cells,
SKOV3/DDP cells exhibited a marked increase in staining with MTG
(MitoTracker green) (Fig. 4A).
Furthermore, SKOV3/DDP cells exhibited a marked increase in
labeling with the mitochondrial-specific redox probe, MitoSox-Red,
suggesting that increased ROS content originated from the
mitochondria and that mitochondria were the main source of
oxidative metabolism in SKOV3/DDP cells (Fig. 4B). Moreover, the level of
intracellular ROS in SKOV3/DDP cells was markedly increased in
comparison to SKOV3 cells (Fig.
4C). Given their increased oxygen consumption, SKOV3/DDP cells
exhibit a more pro-oxidant state.
To investigate whether the inhibition of
mitochondrial respiration was able to lead to oxidative stress and
induce the death of SKOV3/DDP cells, the complex I inhibitor,
rotenone, was used. It was found that the intracellular levels of
ROS that were detected by fluorescence of the redox dye DCFH-DA,
decreased (Fig. 4D). By contrast,
the number of dead cells, as detected by bright red fluorescence in
the live/dead cell viability assay, was markedly increased upon
treatment with cisplatin plus rotenone compared with treatment with
cisplatin alone (Fig. 4F).
Therefore, it was hypothesized that the marked cell death induced
by rotenone may be associated with energy depletion. It was found
that the treatment with rotenone and/or cisplatin caused a marked
reduction in the ATP level in SKOV3/DDP cells (Fig. 4E). Notably, the present study
demonstrated that compared with SKOV3 cells, SKOV3/DDP cells might
rely more on the respiration of mitochondria rather than the
Warburg effect to meet their energy demands.
Redox homeostasis in SKOV3/DDP cells is
maintained intrinsically by pairing OXPHOS with pentose phosphate
pathway (PPP)
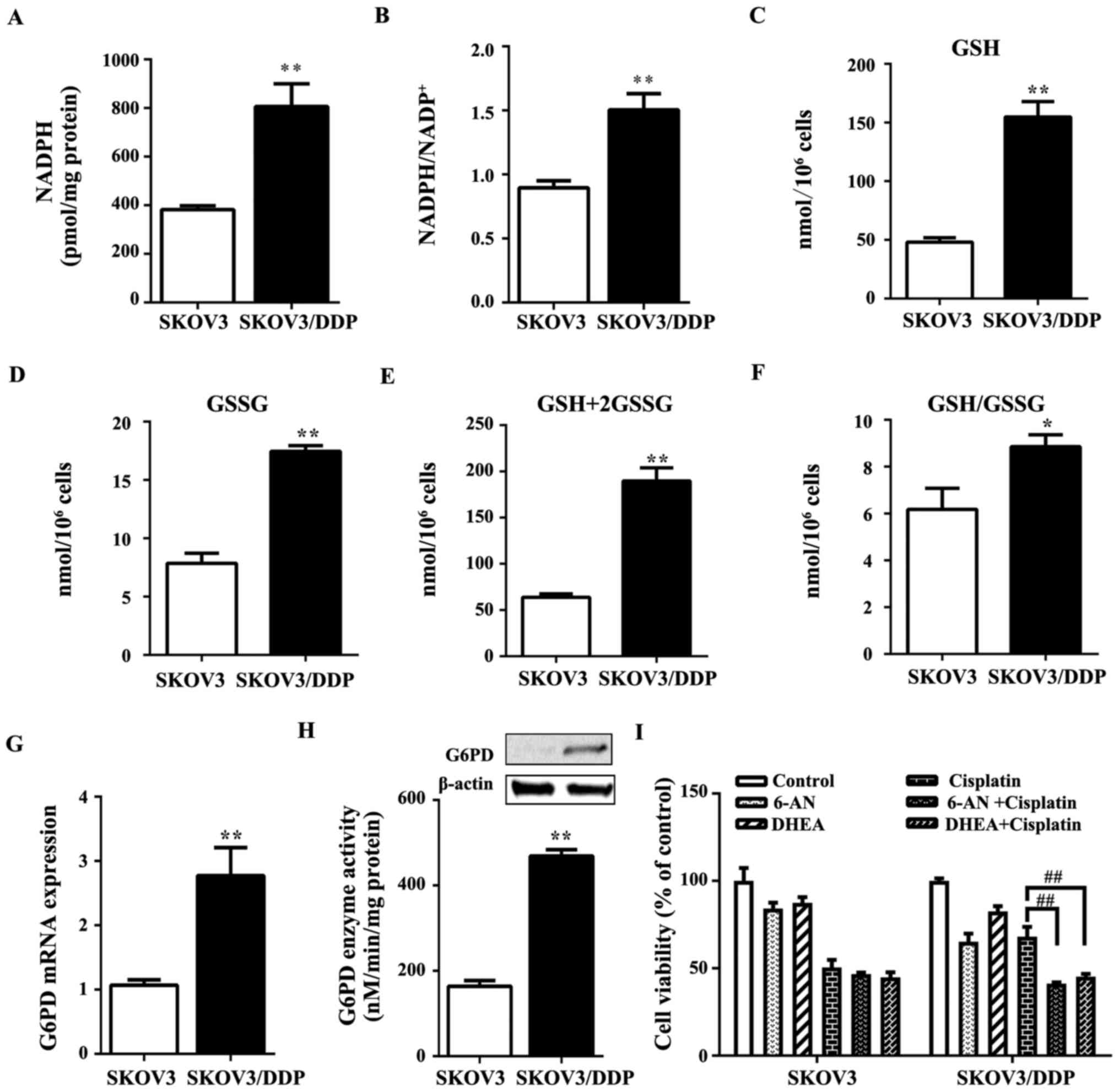
The level of the antioxidant molecule, NADPH, which
can be used to scavenge ROS (29),
increased in SKOV3/DDP cells compared with SKOV3 cells (Fig. 5A). The ratio of NADPH to
NADP+ in SKOV3/DDP cells also increased relative to the
ratio in SKOV3 cells (Fig. 5B).
GSH, an important redox buffer in cancer cells, has been considered
to participate in sustaining cisplatin resistance (30). In SKOV3/DDP cells, GSH and GSSG
contents, total GSH (GSH plus GSSG), and the GSH to GSSG ratio were
significantly higher than those in SKOV3 cells (Fig. 5C–F). Moreover, the oxidative PPP
branch is a major source of NADPH for cells, and substantial
evidence has demonstrated that cancer cells mainly rely on the
oxidative PPP branch to maintain redox homeostasis (31). Therefore, the authors hypothesized
that SKOV3/DDP cells might exploit the PPP pathway to increase GSH
biosynthesis to compensate for the increased ROS generated by
OXPHOS. G6PD is a major rate-limiting enzyme for the activity of
PPP. Notably, the gene and protein expression of G6PD (Fig. 5G) as well as its activity (Fig. 5H) were increased in SKOV3/DDP cells
compared with the levels in SKOV3 cells.
To better understand the role of PPP in cisplatin
resistance, the cells were treated with the competitive G6PD
inhibitor 6-AN (6-aminonicotinamide) (32) or the uncompetitive G6PD inhibitor
DHEA (dehydroepiandrosterone) (33) with or without 6 μg/ml
cisplatin. In SKOV3 cells, the treatment of 6-AN or DHEA together
with cisplatin for 24 h induced no significant effect on cell
viability compared with the cisplatin alone group (Fig. 5I). By contrast, in SKOV3/DDP cells,
treatment with 6-AN or DHEA together with cisplatin significantly
reduced cell viability compared with the cisplatin alone group
(Fig. 5I). These results suggested
that SKOV3/DDP cells exploit oxidative PPP as a resistance
mechanism. These results also demonstrated that SKOV3/DDP cells
utilize the oxidative PPP branch as a mechanism of resistance to
cisplatin by contributing to redox buffering.
ABT737 sensitizes ovarian cancer cells to
cisplatin treatment
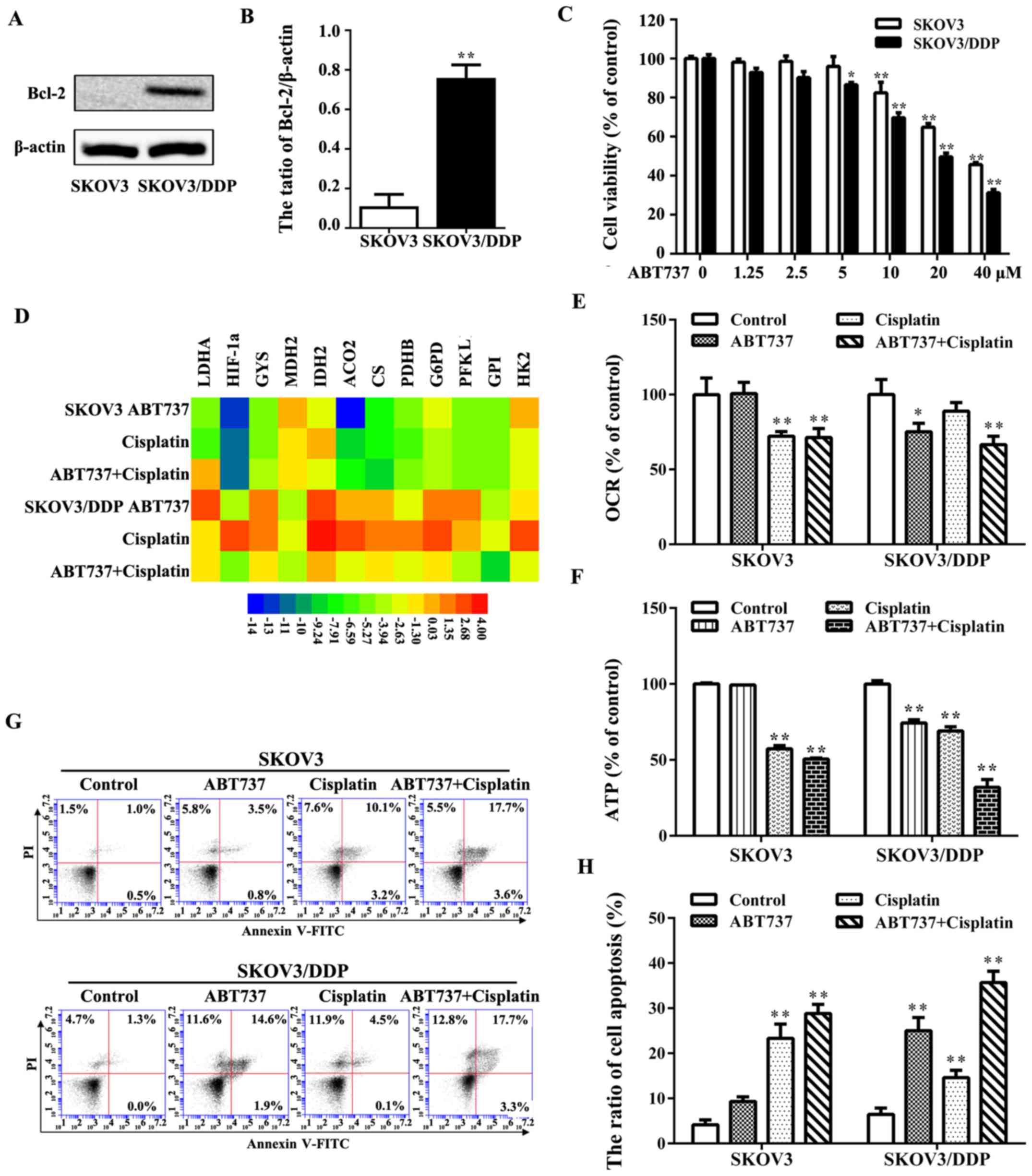
SKOV3/DDP cells exhibit an overexpression of the
Bcl-2 protein, which might render them more resistant to cisplatin
(Fig. 6A and B). The increased
expression of Bcl-2 in SKOV3/DDP cells is potentially important
because Bcl-2 not only participates in the apoptotic pathway but
also is involved in mitochondrial metabolism (34). To examine the mechanisms of Bcl-2
in cisplatin resistance, the cell survival rate was examined via
MTT assays following exposure to various doses of the Bcl-2
inhibitor, ABT737, for 24 h. SKOV3/DDP cells were more sensitive to
ABT737 than SKOV3 cells. Based on these MTT results, we treated
both cell lines with 10 μM ABT737 (Fig. 6C). The effects of ABT737 on glucose
metabolism-associated genes and OCR were analyzed in the two cell
lines to clarify whether ABT737 affects glucose metabolism before
exhibiting notable cytotoxicity, such as by inducing apoptosis.
Treatment of the cells ABT737 with or without cisplatin was able to
induce a significant decrease in the expression of glucose
metabolism-associated genes and a decrease in OCR in SKOV3/DDP
cells compared with the control group (Fig. 6D and E), but it had no marked
effect on either the expression of glucose metabolism-associated
genes or OCR in SKOV3 cells. The ATP level was significantly
decreased in SKOV3/DDP cells upon treatment with cisplatin plus
ABT737 compared with single treatment of cisplatin or ABT737
(Fig. 6F). Annexin V-FITC staining
indicated that treatment with cisplatin plus ABT737 induced
significant apoptosis in SKOV3/DDP cells compared with other
treatment groups (Fig. 6G and H).
These findings indicated that ABT737 induced significant
cytotoxicity mainly by inhibiting the respiration of mitochondria
in SKOV3/DDP cells and sensitizing the cells to cisplatin.
Combination of ABT737 and 2-DG
significantly induces cell death by disrupting glucose
metabolism
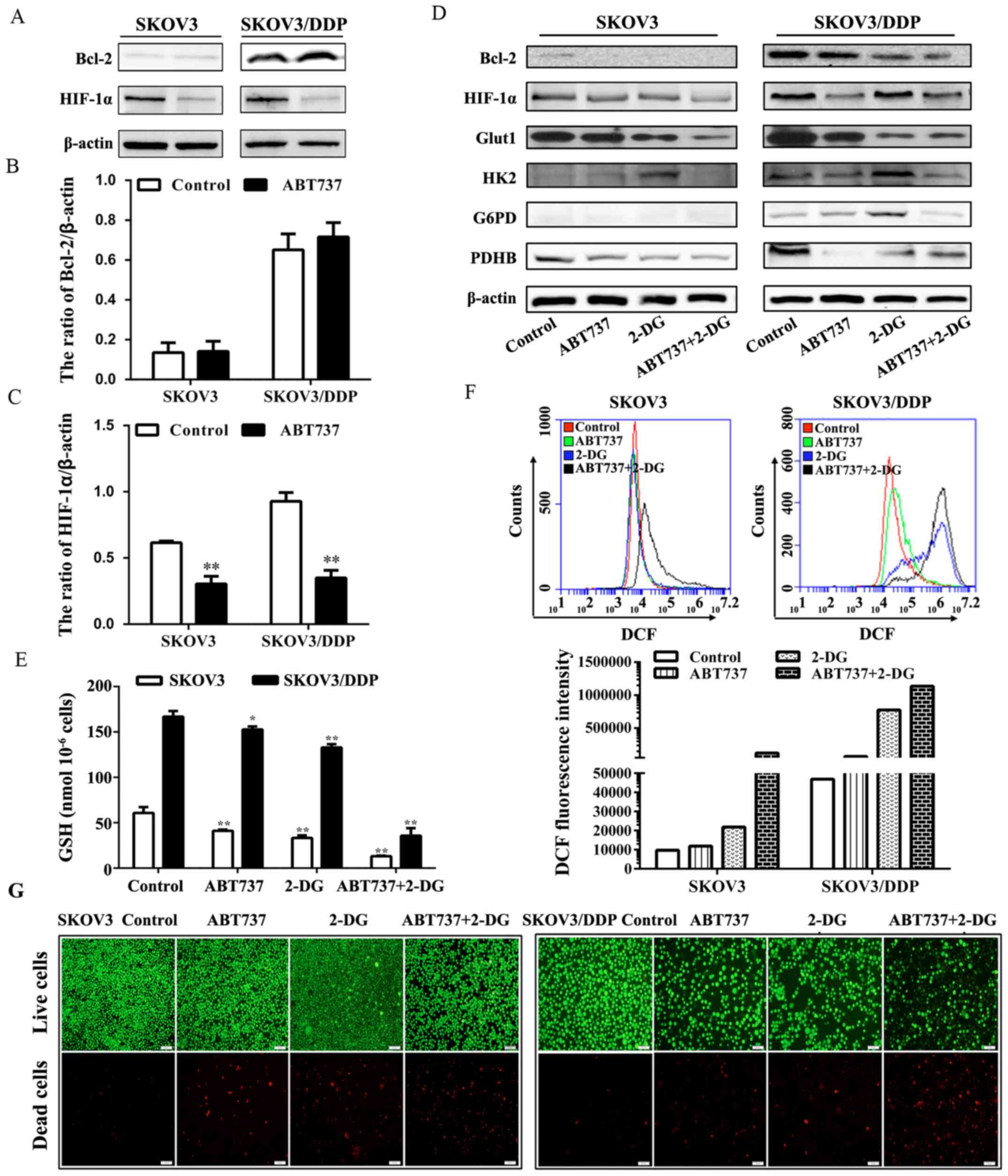
Notably, the expression of HIF-1α was inhibited by
ABT737 (Fig. 7A–C), suggesting
that ABT737 had a direct effect on HIF-1α that in turn may affect
glycolysis. Since ABT737 could reduce the respiration of
mitochondria in SKOV3/DDP cells, we proposed that the combined
treatment of ABT737 and glycolysis inhibitor 2-DG would play an
important therapeutic role in SKOV3/DDP cells. To estimate the
effect of ABT737 and 2-DG in combination, we examined changes in
the expression of enzymes associated with glucose metabolism
(Fig. 7D). In SKOV3/DDP cells,
among several proteins related to glucose metabolism, Bcl-2,
HIF-1α, Glut1, HK2, G6PD, IDH1 and PDHB exhibited marked decreases
in expression following this combination treatment compared with
those in the other groups. Compared with that in SKOV3 cells, the
GSH level in SKOV3/DDP cells was markedly decreased following
treatment with ABT737 plus 2-DG compared with that following
treatment with either ABT737 alone or 2-DG alone (Fig. 7E), while the intracellular ROS
level in SKOV3/DDP cells was increased accordingly (Fig. 7F). Furthermore, we observed a
remarkable increase of the death (Fig.
7G) of SKOV3/DDP cells treated with ABT737 and 2-DG compared
with that in the other groups. These results indicate that the
glycolysis inhibitor 2-DG enhanced the activation of apoptosis
induced by ABT737.
Discussion
Warburg reported that despite the exposure to
sufficient oxygen cancer cells mainly depend on increased
glycolysis rather than oxidative respiration to meet their energy
demands (15). He suggested that
this is due to their defective mitochondria. However, it has become
increasingly clear that the mitochondria of cancer cells are
generally normal and may participate in tumor growth (35,36).
Whether cancer cells utilize glycolysis or oxidative
phosphorylation to meet their energy demands depends upon various
factors, including the type and stage of cancer cells, the
proliferation rate of cells and the sequence of activated oncogenes
that is directly associated with mitochondria (37-39).
Recently, a study has suggested that the main role of aerobic
glycolysis is to maintain glycolytic intermediates at high levels
to sustain anabolic reactions, which is selected by highly
proliferating cancer cells (37).
However, slow-cycling cells depend more on OXPHOS (40). In the present study, SKOV3/DDP
cells had increased mitochondrial mass, oxygen consumption, ATP
level and lower intracellular oxygen concentration compared with
SKOV3 cells, suggesting a shift in glucose metabolism in
cisplatin-resistant SKOV3/DDP cells from aerobic glycolysis to
OXPHOS.
Glucose is predominantly used in ovarian cancer
cells to generate ATP and maintain the energy and redox balance
(41-43). Many studies have identified that
drug-resistant cells are great exploiters of glucose (19,44).
For example, Catanzaro et al (45) demonstrated that cisplatin-resistant
ovarian cancer cells have an increased demand for glucose and
higher sensitivity to glucose deprivation. In line with this, the
present study indicated that SKOV3/DDP cells had an increased
demand for glucose and exhibited increased glucose uptake and
consumption and upregulated expression of the glucose transporter
Glut1. However, SKOV3/DDP cells were less sensitive to glucose
deprivation due to their larger stores of glycogen. Furthermore,
SKOV3/DDP cells exhibited remarkable decreases in extracellular
lactate and ECAR (indicative of glycolysis), therefore it was
suggested that the metabolism of cisplatin-resistant ovarian cancer
cells may vary among different cell lines.
In highly proliferating cancer cells, the shift in
metabolism to aerobic glycolysis could avoid damage resulting from
oxidative stress, whereas this shift may not be so important in
slowly proliferating/quiescent drug-resistant cells (37,46).
As the endogenous ROS are mainly derived from mitochondrial OXPHOS,
oxidative stress is induced by the accumulation of ROS, which is
caused by the imbalance between ROS production and elimination
(47). The drug-resistant cells
may develop sufficient antioxidant mechanisms. The oxidative branch
of PPP is the strongest supporter of cellular ROS defense
mechanisms among the antioxidant mechanisms (48). G6PD, the rate-limiting enzyme of
PPP, catalyzes the generation of the first molecule of NADPH and is
relatively overexpressed in primary breast carcinoma and gastric
cancer cells (49,50). Furthermore, the acquisition of
cisplatin resistance is associated with the upregulation of G6PD,
whose increased expression was previously linked to a
cisplatin-resistant phenotype (45). In line with this, the data in the
present study demonstrated that in SKOV3/DDP cells despite
exhibiting a pro-oxidizing state with higher levels of
intramitochondrial superoxide anion (O2−) and
intracellular ROS in comparison to SKOV3 cells, the oxidative
branch of PPP was elevated with higher NADPH content, and increased
G6PD protein expression and enzymatic activity compared with the
levels in SKOV3 cells. Furthermore, the combined treatment with the
G6PD inhibitors 6-AN or DHEA and cisplatin was more effective
compared with treatment with cisplatin alone in SKOV3/DDP cells.
This suggested that the redox homeostasis of SKOV3/DDP cells is
maintained by pairing ROS generated from OXPHOS and cisplatin
toxicity with reductive equivalent NADPH produced by the oxidative
branch of PPP.
Apart from their role in the regulation of
mitochondrial apoptosis, Bcl-2 proteins are also able to stimulate
mitochondrial respiration (51-53).
Moreover, it was found that Bcl-2 protein was upregulated in
SKOV3/DDP cells compared with the level in SKOV3 cells. To enhance
the cisplatin sensitivity of SKOV3/DDP cells, ABT-737 was utilized
to inhibit Bcl-2, Bcl-w and Bcl-xL (54,55).
In the present study, it was indicated that ABT737 significantly
inhibited mitochondrial OXPHOS and the expression of glucose
metabolism-associated genes, causing a reduction in ATP content and
impairing the survival of SKOV3/DDP cells. Accordingly, the vital
function of OXPHOS in metabolic reprogramming and the induction of
cisplatin resistance led the authors to examine the effect of
rotenone (inhibitor of mitochondrial complex I) on cisplatin
resistance. It was also found that rotenone markedly improved the
sensitivity of SKOV3/DDP cells to cisplatin. While ABT737 had no
significant effect on OXPHOS in SKOV3 cells, it exhibited a weaker
effect on cell survival compared with SKOV3/DDP cells. Therefore,
it was hypothesized that ABT737 may inhibit viability less
effectively in cancer cells with a lower expression of Bcl-2
protein.
As heterogeneous systems, tumors are composed of
both highly proliferative cells and slowly proliferating or
quiescent cells, including tumor-initiating cells or cancer stem
cells (8). Therefore, to obtain a
comprehensive understanding of them, their different metabolic
phenotypes need to be considered. HIF-1α initiates the
transcription of genes encoding glucose transporters and glycolytic
enzymes (57,58) and has been shown to be associated
with chemoresistance in many preclinical and clinical studies
(58,59). Notably, HIF-1α may be more stable
in SKOV3/DDP cells with a lower intracellular oxygen concentration
and a higher level of ROS. In turn, glycogen synthesis is induced
through HIF-mediated induction of GYS, which is consistent with the
elevated glycogen level in SKOV3/DDP cells. Interestingly, ABT737
caused a remarkable decrease in HIF-1α expression in SKOV3/DDP
cells and SKOV3 cells. Owing to HIF-1α also initiating the
transcription of glucose metabolism-associated genes (59,60), it
was suggested that Bcl-2 proteins might also be modulators of
HIF-1α and might thereby contribute to many other parts of glucose
metabolism apart from OXPHOS. The previous study by the authors
demonstrated that the glycolysis inhibitor, 2-DG, was able to
enhance apoptosis that was induced by S1 (Bcl-2 inhibitor) via the
upregulation of SIRT3 in SKOV3 cells (25). Furthermore, the use of 2-DG also
enhanced the sensitivity of SKOV3/DDP cells to ABT737. The
combination of ABT737 and 2-DG significantly decreased GSH content,
the expression of HIF-1α and glucose metabolism-associated
proteins, while also inducing marked cell death in SKOV3/DDP cells.
Therefore, it was hypothesized that the combination of 2-DG with
ABT737 may be applied to exploit the metabolic weakness of
cisplatin-resistant cells, circumventing treatment resistance and
enhancing treatment efficacy in the heterogeneous systems that are
tumors.
Abbreviations:
|
Bcl-2
|
B-cell lymphoma 2
|
|
Bcl-w
|
BCL-2 like protein 2
|
|
Bcl-xL
|
B-cell lymphoma extra large
|
|
OXPHOS
|
oxidative phosphorylation
|
|
ATP
|
adenosine triphosphate
|
|
ECAR
|
extracellular acidification rate
|
|
OCR
|
oxygen consumption rate
|
|
ROS
|
reactive oxygen species
|
|
TCA
|
tricarboxylic acid cycle
|
Acknowledgments
The present authors would like to thank Xiao Song
Wang, Kuo Wang and Yan Liu for technical contributions and also
Liwen Bianji, Edanz Group China, for editing the English text of a
draft of this manuscript.
References
|
1
|
Tew WP and Fleming GF: Treatment of
ovarian cancer in the older woman. Gynecol Oncol. 136:136–142.
2015. View Article : Google Scholar
|
|
2
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H, Su J, Xu Y, Kang J, Li H, Zhang L,
Yi H, Xiang X, Liu F and Sun L: p62/SQSTM1 involved in cisplatin
resistance in human ovarian cancer cells by clearing ubiquitinated
proteins. Eur J Cancer. 47:1585–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma L, Xu Y, Su J, Yu H, Kang J, Li H, Li
X, Xie Q, Yu C, Sun L, et al: Autophagic flux promotes cisplatin
resistance in human ovarian carcinoma cells through ATP-mediated
lysosomal function. Int J Oncol. 47:1890–1900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie Q, Su J, Jiao B, Shen L, Ma L, Qu X,
Yu C, Jiang X, Xu Y and Sun L: ABT737 reverses cisplatin resistance
by regulating ER-mitochondria Ca2+ signal transduction
in human ovarian cancer cells. Int J Oncol. 49:2507–2519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lagadinou ED, Sach A, Callahan K, Rossi
RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O'Dwyer
KM, et al: BCL-2 inhibition targets oxidative phosphorylation and
selectively eradicates quiescent human leukemia stem cells. Cell
Stem Cell. 12:329–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weiler M, Bähr O, Hohlweg U, Naumann U,
Rieger J, Huang H, Tabatabai G, Krell HW, Ohgaki H, Weller M, et
al: BCL-xL: Time-dependent dissociation between modulation of
apoptosis and invasiveness in human malignant glioma cells. Cell
Death Differ. 13:1156–1169. 2006. View Article : Google Scholar
|
|
11
|
Bae IH, Yoon SH, Lee SB, Park JK, Ho JN
and Um HD: Signaling components involved in Bcl-w-induced migration
of gastric cancer cells. Cancer Lett. 277:22–28. 2009. View Article : Google Scholar
|
|
12
|
Kelekar A and Thompson CB: Bcl-2-family
proteins: The role of the BH3 domain in apoptosis. Trends Cell
Biol. 8:324–330. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manfredi G, Kwong JQ, Oca-Cossio JA,
Woischnik M, Gajewski CD, Martushova K, D'Aurelio M, Friedlich AL
and Moraes CT: BCL-2 improves oxidative phosphorylation and
modulates adenine nucleotide translocation in mitochondria of cells
harboring mutant mtDNA. J Biol Chem. 278:5639–5645. 2003.
View Article : Google Scholar
|
|
14
|
Dey R and Moraes CT: Lack of oxidative
phosphorylation and low mitochondrial membrane potential decrease
susceptibility to apoptosis and do not modulate the protective
effect of Bcl-x(L) in osteosarcoma cells. J Biol Chem.
275:7087–7094. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Warburg O: Iron, the oxygen-carrier of
respiration-ferment. Science. 61:575–582. 1925. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandel NS: Mitochondria and cancer.
Cancer Metab. 2:82014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matassa DS, Amoroso MR, Lu H, Avolio R,
Arzeni D, Procaccini C, Faicchia D, Maddalena F, Simeon V,
Agliarulo I, et al: Oxidative metabolism drives
inflammation-induced platinum resistance in human ovarian cancer.
Cell Death Differ. 23:1542–1554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ippolito L, Marini A, Cavallini L, Morandi
A, Pietrovito L, Pintus G, Giannoni E, Schrader T, Puhr M, Chiarugi
P, et al: Metabolic shift toward oxidative phosphorylation in
docetaxel resistant prostate cancer cells. Oncotarget.
7:61890–61904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Denise C, Paoli P, Calvani M, Taddei ML,
Giannoni E, Kopetz S, Kazmi SM, Pia MM, Pettazzoni P, Sacco E, et
al: 5-fluorouracil resistant colon cancer cells are addicted to
OXPHOS to survive and enhance stem-like traits. Oncotarget.
6:41706–41721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montopoli M, Bellanda M, Lonardoni F,
Ragazzi E, Dorigo P, Froldi G, Mammi S and Caparrotta L: 'Metabolic
reprogramming' in ovarian cancer cells resistant to cisplatin. Curr
Cancer Drug Targets. 11:226–235. 2011. View Article : Google Scholar
|
|
22
|
Fan Z, Yu H, Cui N, Kong X, Liu X, Chang
Y, Wu Y, Sun L and Wang G: ABT737 enhances cholangiocarcinoma
sensitivity to cisplatin through regulation of mitochondrial
dynamics. Exp Cell Res. 335:68–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Bol V, Bol A, Bouzin C, Labar D, Lee JA,
Janssens G, Porporato PE, Sonveaux P, Feron O and Grégoire V:
Reprogramming of tumor metabolism by targeting mitochondria
improves tumor response to irradiation. Acta Oncol. 54:266–274.
2015. View Article : Google Scholar
|
|
25
|
Xiang XY, Kang JS, Yang XC, Su J, Wu Y,
Yan XY, Xue YN, Xu Y, Liu YH, Yu CY, et al: SIRT3 participates in
glucose metabolism interruption and apoptosis induced by BH3
mimetic S1 in ovarian cancer cells. Int J Oncol. 49:773–784. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Floreani M, Petrone M, Debetto P and
Palatini P: A comparison between different methods for the
determination of reduced and oxidized glutathione in mammalian
tissues. Free Radic Res. 26:449–455. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deberardinis RJ, Sayed N, Ditsworth D and
Thompson CB: Brick by brick: Metabolism and tumor cell growth. Curr
Opin Genet Dev. 18:54–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caneba CA, Yang L, Baddour J, Curtis R,
Win J, Hartig S, Marini J and Nagrath D: Nitric oxide is a positive
regulator of the Warburg effect in ovarian cancer cells. Cell Death
Dis. 5:e13022014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stanton RC: Glucose-6-phosphate
dehydrogenase, NADPH, and cell survival. IUBMB Life. 64:362–369.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Borst P, Evers R, Kool M and Wijnholds J:
A family of drug transporters: The multidrug resistance-associated
proteins. J Natl Cancer Inst. 92:1295–1302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patra KC and Hay N: The pentose phosphate
pathway and cancer. Trends Biochem Sci. 39:347–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Köhler E, Barrach H and Neubert D:
Inhibition of NADP dependent oxidoreductases by the
6-aminonicotinamide analogue of NADP. FEBS Lett. 6:225–228. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raineri R and Levy HR: On the specificity
of steroid interaction with mammary glucose 6-phosphate
dehydrogenase. Biochemistry. 9:2233–2243. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gross A: BCL-2 family proteins as
regulators of mitochondria metabolism. Biochim Biophys Acta.
1857.1243–1246. 2016.
|
|
35
|
Caro P, Kishan AU, Norberg E, Stanley IA,
Chapuy B, Ficarro SB, Polak K, Tondera D, Gounarides J, Yin H, et
al: Metabolic signatures uncover distinct targets in molecular
subsets of diffuse large B cell lymphoma. Cancer Cell. 22:547–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weinberg SE and Chandel NS: Targeting
mitochondria metabolism for cancer therapy. Nat Chem Biol. 11:9–15.
2015. View Article : Google Scholar :
|
|
37
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berridge MV, Herst PM and Tan AS:
Metabolic flexibility and cell hierarchy in metastatic cancer.
Mitochondrion. 10:584–588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jose C, Hébert-Chatelain E, Bellance N,
Larendra A, Su M, Nouette-Gaulain K and Rossignol R: AICAR inhibits
cancer cell growth and triggers cell-type distinct effects on
OXPHOS biogenesis, oxidative stress and Akt activation. Biochim
Biophys Acta. 1807.707–718. 2011.
|
|
40
|
Roesch A, Vultur A, Bogeski I, Wang H,
Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA,
Philipp SE, et al: Overcoming intrinsic multidrug resistance in
melanoma by blocking the mitochondrial respiratory chain of
slow-cycling JARID1B(high) cells. Cancer Cell. 23:811–825. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vander Heiden MG, Locasale JW, Swanson KD,
Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G,
Rabinowitz JD, Asara JM, et al: Evidence for an alternative
glycolytic pathway in rapidly proliferating cells. Science.
329:1492–1499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahn CS and Metallo CM: Mitochondria as
biosynthetic factories for cancer proliferation. Cancer Metab.
3:12015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vander Heiden MG: Targeting cancer
metabolism: A therapeutic window opens. Nat Rev Drug Discov.
10:671–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee YJ, Galoforo SS, Berns CM, Tong WP,
Kim HR and Corry PM: Glucose deprivation-induced cytotoxicity in
drug resistant human breast carcinoma MCF-7/ADR cells: Role of
c-myc and bcl-2 in apoptotic cell death. J Cell Sci. 110:681–686.
1997.PubMed/NCBI
|
|
45
|
Catanzaro D, Gaude E, Orso G, Giordano C,
Guzzo G, Rasola A, Ragazzi E, Caparrotta L, Frezza C and Montopoli
M: Inhibition of glucose-6-phosphate dehydrogenase sensitizes
cisplatin-resistant cells to death. Oncotarget. 6:30102–30114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Z, Fukushima H, Gao D, Inuzuka H, Wan
L, Lau AW, Liu P and Wei W: The two faces of FBW7 in cancer drug
resistance. BioEssays. 33:851–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sengupta N, Rose ST and Morgan JA:
Metabolic flux analysis of CHO cell metabolism in the late
non-growth phase. Biotechnol Bioeng. 108:82–92. 2011. View Article : Google Scholar
|
|
49
|
Polimeni M, Voena C, Kopecka J, Riganti C,
Pescarmona G, Bosia A and Ghigo D: Modulation of doxorubicin
resistance by the glucose-6-phosphate dehydrogenase activity.
Biochem J. 439:141–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang J, Yuan W and Chen Z, Wu S, Chen J,
Ge J, Hou F and Chen Z: Overexpression of G6PD is associated with
poor clinical outcome in gastric cancer. Tumour Biol. 33:95–101.
2012. View Article : Google Scholar
|
|
51
|
Clément MV, Hirpara JL and Pervaiz S:
Decrease in intracellular superoxide sensitizes
Bcl-2-overexpressing tumor cells to receptor and drug-induced
apoptosis independent of the mitochondria. Cell Death Differ.
10:1273–1285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen ZX and Pervaiz S: Bcl-2 induces
pro-oxidant state by engaging mitochondrial respiration in tumor
cells. Cell Death Differ. 14:1617–1627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen ZX and Pervaiz S: Involvement of
cytochrome c oxidase subunits Va and Vb in the regulation of cancer
cell metabolism by Bcl-2. Cell Death Differ. 17:408–420. 2010.
View Article : Google Scholar
|
|
54
|
Chen L, Willis SN, Wei A, Smith BJ,
Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM and Huang DC:
Differential targeting of prosurvival Bcl-2 proteins by their
BH3-only ligands allows complementary apoptotic function. Mol Cell.
17:393–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dai Y, Jin S, Li X and Wang D: The
involvement of Bcl-2 family proteins in AKT-regulated cell survival
in cisplatin resistant epithelial ovarian cancer. Oncotarget.
8:1354–1368. 2017.
|
|
56
|
Semenza GL: HIF-1: Upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar :
|
|
57
|
Wang GL and Semenza GL: General
involvement of hypoxia-inducible factor 1 in transcriptional
response to hypoxia. Proc Natl Acad Sci USA. 90:4304–4308. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar :
|
|
59
|
Ai Z, Lu Y, Qiu S and Fan Z: Overcoming
cisplatin resistance of ovarian cancer cells by targeting
HIF-1-regulated cancer metabolism. Cancer Lett. 373:36–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|