Introduction
Breast cancer is one of the most frequently
diagnosed types of cancer and is the leading cause of mortality in
Western women (1). However, the
specific developmental mechanisms of this cancer remain to be fully
elucidated (2). Chemotherapy is a
basic strategy for treating breast cancer (3,4),
however, this approach has several limitations, including limited
drug delivery due to the blood-brain barrier and drug resistance
(5,6). Paclitaxel, as one of the most
effective chemotherapeutic drugs, is used to treat breast cancer
(7,8), however, its failure in treatment and
poor prognosis are mainly due to the development of drug resistance
(9).
In previous years, small non-coding RNAs, known as
microRNAs (miRNAs), have appeared as a pivotal regulators in human
tumorigenesis, including breast cancer (10,11).
miRNAs bind to the 3′-untranslated regions (3′-UTRs) of the target
gene, thus inhibiting expression of the target gene at the
transcriptional or posttranscriptional level. Dysregulated miRNAs
have significant roles in tumor occurrence (12–14).
Additionally, miRNA (miR)-3p and miR-5p, which are processed from
the 5′ and 3′ precursors of pre-miRNA, have been identified to be
involved in different regulatory loops and exert the same or
different effects on tumors (15,16).
For example, miR-409-3p/-5p have an oncogenic effect on prostate
cancer bone metastasis and can serve as a therapeutic target
(17). Deep sequencing revealed
that the opposite strands of miR-144-5p/-3p, miR-145-5p/-3p, and
miR-139-5p/-3p can function as dual-strand tumor-suppressor miRNAs
(18–20). Sakaguchi et al also found
that the miR-199 family (miR-199a-3p/5p and miR-199b-3p/5p) may
function as tumor suppressors by regulating common the target gene
integrin α3 (21). Despite this,
miR-3p and miR-5p can have opposite effects on carcinogenesis. For
example, a previous study showed that mature miR-96-5p was
significantly upregulated in cirrhosis and dysplastic nodules in
hepatocellular carcinoma, whereas the expression of passenger
strand miR-96-3p was detectable in cirrhosis and dysplastic nodules
(22). Based on the previous
studies, the miR-155 family was found to be involved in the
regulation of corresponding biological activity in breast cancer.
miR-155-3p was found to be downregulated whereas miR-155-5p acted
as an oncogenic gene in breast cancer cell lines. However, the
mechanisms involving 21-residue N-terminal of viral macrophage
inflammatory protein II (vMIP-II), termed NT21MP, and the miR-155
family remains to be fully elucidated.
Previous studies have demonstrated that NT21MP,
derived from vMIP-II, efficiently inhibits proliferation, invasion,
cell cycle, and apoptosis in breast cancer cells by inhibiting CXC
chemokine receptor 4 (CXCR4) and its ligand stromal cell-derived
factor-1α (SDF-1α; also known as CXCL12) in vitro and in
vivo (23–25). Although NT21MP has been shown to
reverse breast cancer, the underlying specific molecular mechanism
requires further investigation.
The present study aimed to determine whether the
miR-155 family can be regulated using NT21MP in breast cancer cells
and whether the overexpression of miR-155-3p or downregulation of
miR-155-5p combined with NT21MP can reverse paclitaxel-resistant
(PR) breast cancer cells more than the single treatment group. In
addition, by analyzing the respective target genes of miR-155-3p
and miR-155-5p, the present study aimed to verify whether NT21MP
combined with the downregulation of myeloid differentiation primary
response gene 88 (MYD88), the target gene of miR-155-3p, or
upregulation of tumor protein 53-induced nuclear protein 1
(TP53INP1), the target gene of miR-155-5p, can significantly
inhibit carcinogenesis in vitro. The findings provided novel
insight into the potential efficacy of NT21MP as an adjuvant
chemotherapy for breast cancer through regulating the miRNA
family.
Materials and methods
Cell culture
The MCF-7 human breast cancer cell line was obtained
from the Shanghai Cell Institute of Chinese Academy of Science
(Shanghai, China). The corresponding PR cells (MCF-7/PR) were
treated with 25 µg/ml paclitaxel. All cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) maintained at 37°C in a saturated humidity atmosphere
containing 5% CO2.
Transfection
Cell transfections were performed using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocol. Final concentrations
of 50 nM miRNA mimics and 0.75 µg/ml plasmids were added
into a 6-well plate with 2 ml of culture medium. MYD88 small
interfering (si)RNA (si-MYD88) and pcDNA-TP53INP1 (GenePharma,
Shanghai, China) were used for stable transfection. The
pcDNA-TP53INP1 was constructed using G418 (200 µg/ml). The
sequences for the siRNAs and RNA oligoribonucleotides were as
follows: si-MYD88-1, 5′-CCCAUCAGAAGCGACUGAUTTAUCAGUCGCUUCU GAUGG
GTT-3′; si-MYD88-2, 5′-GGCAACUGGAACAGAC
AAATTUUUGUCUGUUCCAGUUGCCTT-3′; si-MYD88-3,
5′-GCCUGUCUCUGUUCUUGAATTUUCAAGAACAGAG ACAGGCTT-3′.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was isolated from breast cancer cells
using TRIzol reagent according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized
using the First-Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). The qPCR analysis was performed using the SYBR
premix Ex TaqII kit (Takara Biotechnology, Co., Ltd., Dalian,
China) through an ABI 7500 fast real-time PCR system (Applied
Systems, Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Additionally, glyceraldehyde 3-phosphate
dehydrogenase was used as an internal control. The PCR procedure
[SYBR Premix (2X), 10 µl; forward and reverse primer (10
µM), 0.8 µl; ROX reference dye II (50X), 0.4
µl; cDNA, 2 µl; sterile purified water, 6 µl;
total volume, 20 µl) was performed under the following
conditions: 95.0°C for 30 sec, followed by 40 cycles at 95.0°C for
15 sec, 57°C for 30 sec and 72°C for 34 sec, and a final extension
step at 72°C for 5 min. Data were processed using the
2−∆∆Cq method (26).
The corresponding primers are listed in Table I.
 | Table ISequences of primers. |
Table I
Sequences of primers.
| Name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| MYD88 |
CTGCCTCCTCCTTTCGTTGTAG |
GCTCTGCTGGTCCTTCTTAGTC |
| TP53INP1 |
GACTTCATAGATACTTGCAC |
ATTGGACATGACTCAAACTG |
| Bax |
GGGGACGAACTGGACAGTAA |
CAGTTGAAGTTGCCGTCAGA |
| Caspase-3 |
ACAAATGGACCTGTTGACCTGA |
ACACCACTGTCTGTCTCAATGC |
| Bcl-2 |
ATGTGTGTGGAGAGCGTCAA |
ACAGTTCCACAAAGGCATCC |
| GAPDH |
CAGCCTCAAGATCATCAGCA |
TGTGGTCATGAGTCCTTCCA |
Western blot analysis
Cell lysates were prepared in lysis buffer
containing 150 mM NaCl, 50 mM Tris, 1% Triton X-100, 0.1% sodium
dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 0.02% sodium
azide, 1 mM sodium vanadate, and protease inhibitors (10
µg/ml leupeptin, 10 µg/ml aprotinin, and 1 mM
phenylmethylsulfonyl fluoride). The protein concentration was
measured using a Bio-Rad protein assay. Equal quantities of
proteins were subjected to 10% SDS-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene difluoride
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membranes were blocked using phosphate-buffered saline (PBS) with
5% non-fat milk and incubated overnight at 4°C with primary
antibodies. Following incubation with peroxidase-conjugated
AffiniPure goat anti-mouse immunoglobulin G (IgG) or
peroxidase-conjugated affinipure goat anti-rabbit IgG for 2 h at
37°C. The immune complexes were detected using an Enhanced
Chemiluminescence Western Blotting kit (EMD Millipore, Billerica,
MA, USA) and Bio-Rad Image-Lab software 5.2.1 (Bio-Rad
Laboratories, Inc.). The relative protein expression was determined
using ImageJ V1.8.0 (National Institutes of Health, Bethesda, MD,
USA), with GAPDH used as the internal reference. The following
antibodies and dilutions were used: MYD88 (1:2,000; cat. no.
ab2068, Abcam, Cambridge, MA, USA), TP53INP1 (1:2,000; cat.
no. ab154877, Abcam), B-cell lymphoma 2 (Bcl-2; 1:1,500; cat. no.
ab196495, Abcam), caspase-3 (1:5,000; cat. no. ab13586, Abcam),
Bcl-2-associated X protein (Bax; 1:1,000; cat. no. 23931-1-AP,
ProteinTech Group, Inc., Chicago, IL, USA), β-actin (1:3,000; cat.
no. sc-130065, Santa Cruz Biotechnology Co., Ltd., Dallas, TX,
USA), goat anti- rabbit IgG-horseradish peroxidase (1:5,000; cat.
no. sc-2004, Santa Cruz Biotechnology, Inc.), and goat anti-mouse
IgG-horseradish peroxidase (1:5,000; cat. no. sc-2005, Santa Cruz
Biotechnology, Inc.).
Wound-healing assay
The transfected breast cancer cells were seeded into
6-well plates and then wounded by scratching with a sterile
10-µl pipette tip. The detached cells were removed by
washing twice with PBS, and fresh culture medium without serum was
added. The wound closure was monitored at 0 and 24 h using a
fluorescence microscope (×40 magnification; IX71; Olympus
Corporation, Tokyo, Japan). The wound surface area was quantified
by image analysis (Image J V1.8.0; National Institutes of
Health).
Cell cycle and apoptosis assays
The cells were seeded into 6-well plates. Following
transfection for 24 h, the cells were collected by trypsinization
and then analyzed with a flow cytometer (Muse Cell Analyzer, Merck
Millipore, Darmstadt, Germany) using an Annexin V and Dead Cell kit
and a Cell Cycle Detection kit (Merck Millipore). All experiments
were repeated three times.
Cell counting kit-8 (CCK-8) assay
The MCF-7 and MCF-7/PR cells were trypsinized and
seeded into 96-well plates at 1×105 cells/ml. After 24
h, various concentrations of paclitaxel (0, 20, 40, 60, 80, and 100
µmol/l) were added, and 50 nmol/l miR-155-3p
mimics/miR-155-5p inhibitor/NT21MP was transfected into each well
and incubated at 37°C for 72 h. Subsequently, 10 µl of CCK-8
(Beyotime Institute of Biotechnology, Shanghai, China) was added to
100 µl of DMEM medium containing 10% FBS. The values of
absorbance were measured at 450 nm.
Statistical analysis
All analyses were performed in triplicate.
Statistical analysis was performed using one-way analysis of
variance and the post hoc Least Significant Difference test with
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. The
data are reported as the mean ± standard deviation.
Results
NT21MP reverses the SDF-1α-induced
decrease of miR-155-3p and increase of miR-155-5p in MCF-7 and
MCF-7/PR cells
Previous studies have confirmed that NT21MP can
inhibit SDF-1α-induced proliferation, migration and invasion, and
promote apoptosis by downregulating the expression of CXCR4 in
breast cancer cells. The results of the RT-qPCR analysis showed
that NT21MP (1 µg/ml) inhibited the SDF-1α-induced decrease
of miR-155-3p in the parental and PR cells compared with the
control group (Fig. 1A). By
contrast, SDF-1α (0.1 µg/ml) promoted the expression level
of miR-155-5p, whereas NT21MP inhibited its effect (Fig. 1B). These results suggested that
miR-155-3p/5p was involved in the regulation of NT21MP, not only in
breast cancer parental cells, but also in drug-resistant cells.
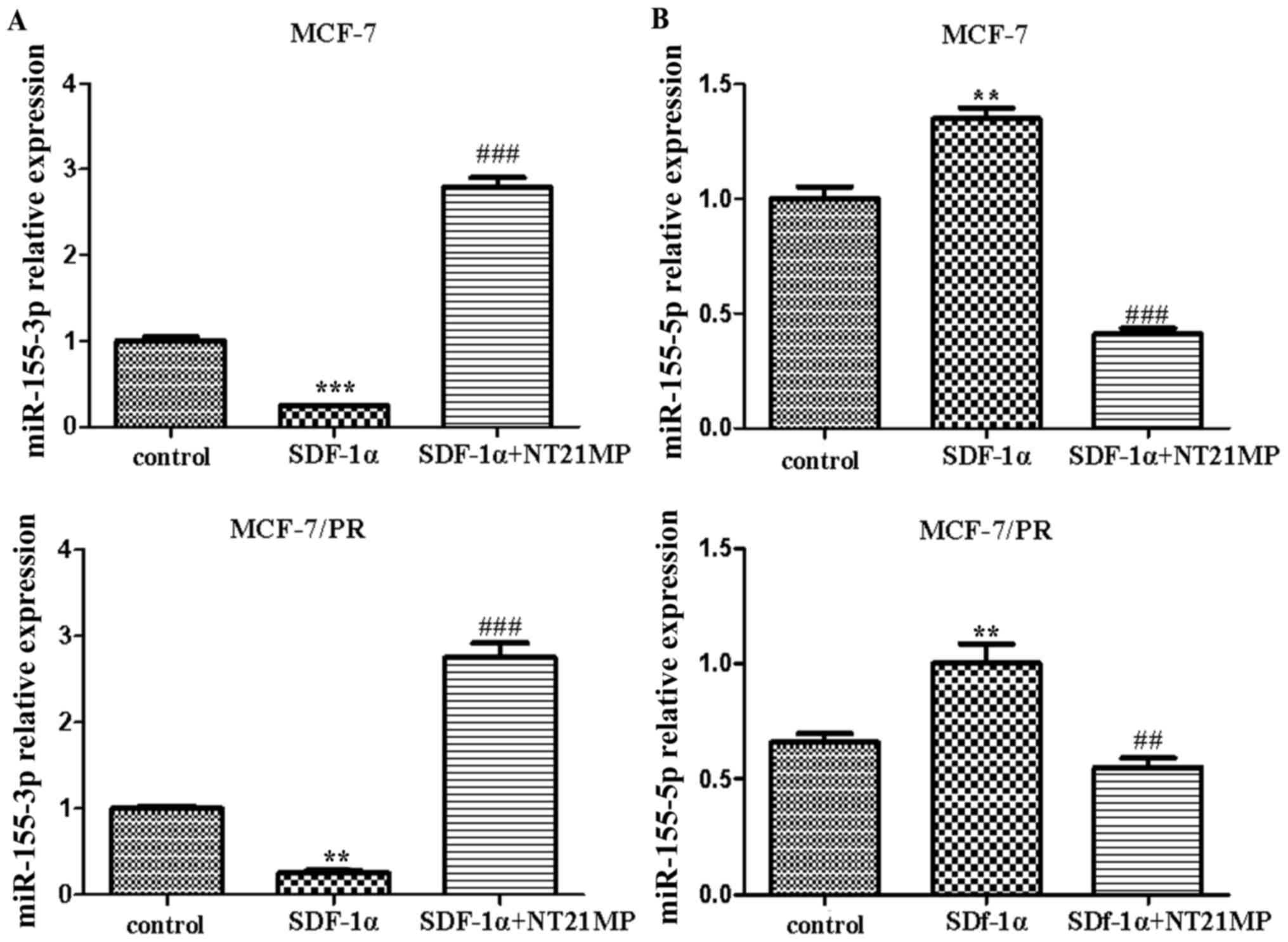 | Figure 1Effects of NT21MP on the expression
level of miR-155-3p/5p in MCF-7 and MCF-7/PR cell lines. (A)
Effects of NT21MP on the expression level of miR-155-3p in MCF-7
and MCF-7/PR cells, detected using RT-qPCR analysis, compared with
control groups. (B) Effects of NT21MP on the expression level of
miR-155-5p in MCF-7 and MCF-7/PR cells, detected using RT-qPCR
analysis, compared with control groups. **P<0.01,
***P<0.001, ##P<0.01 and
###P<0.001, compared with SDF-1α treatment. NT21MP;
21-residue peptide derived from viral macrophage inflammatory
protein II; miR, microRNA; SDF-1α, stromal cell-derived factor-1α;
PR, paclitaxel-resistant; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
NT21MP inhibits the SDF-1α-induced
increase of MYD88 and decrease of TP53INP1 in MCF-7 and MCF-7/PR
cells
Previous studies have demonstrated that MYD88
functions as the target gene of miR-155-3p and TP53INP1
functions as the target gene of miR-155-5p using TargetScan v7.1,
miRanda, and miRTarbase (27). The
same experiments for miR-155-3p/5p were performed in the present
study to further elucidate whether the targets of miR-155-3p/5p
were also involved in the regulatory effect of NT21MP in drug
resistance in breast cancer. The results showed that SDF-1α
promoted the expression level of MYD88 whereas NT21MP
suppressed this effect in the MCF-7 and MCF-7/PR cells (Fig. 2A). Additionally, NT21MP inhibited
the SDF-1α-induced decrease of TP53INP1 (Fig. 2B). The corresponding protein levels
are shown in Fig. 2C.
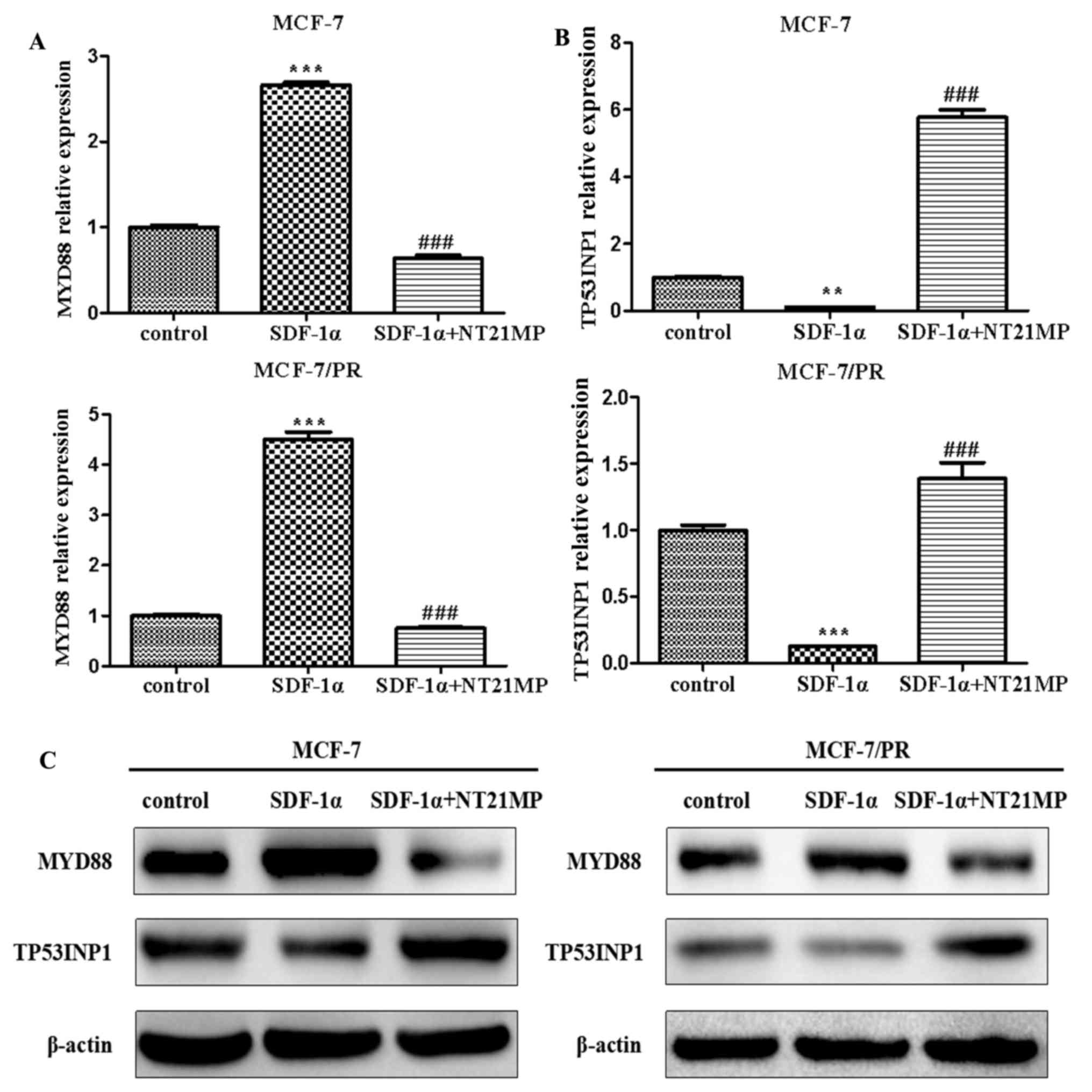 | Figure 2Effects of NT21MP on the expression
of MYD88 or TP53INP1 in MCF-7 and MCF-7/PR cells. (A)
Effects of NT21MP on the expression of MYD88 using RT-qPCR
analysis, compared with the control groups. (B) Effects of NT21MP
on the expression of TP53INP1 using RT-PCR analysis,
compared with the control groups. (C) Western blot analysis was
performed to identify the effects of NT21MP on the expression of
MYD88 or TP53INP1 in MCF-7 and MCF-7/PR cells,
compared with control groups. The results are representative of
three independent experiments. **P<0.01,
***P<0.001 and ###P<0.001, compared
with SDF-1α treatment. NT21MP; 21-residue peptide derived from
viral macrophage inflammatory protein II; SDF-1α, stromal
cell-derived factor-1α; PR, paclitaxel-resistant; MYD88, myeloid
differentiation primary response gene 88; TP53INP1, tumor protein
53-induced nuclear protein 1; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
NT21MP, combined with the overexpression
of miR-155-3p, inhibits target gene MYD88 and biological activities
in MCF-7/PR cells
A wound-healing assay was performed to assess the
ability of SDF-1α to promote cell migration and the ability of
miR-155-3p or NT21MP to weaken this effect, particularly in the
combined groups, in order to examine the molecular effect of NT21MP
and the overexpression of miR-155-3p in breast cancer-resistant
cells (Fig. 3A). Subsequent cell
cycle and apoptotic analyses were performed using flow cytometry to
detect the combined effect of NT21MP and miR-155-3p. The percentage
of cells in the G0/G1 phase decreased from 70.2 to 68.4%, whereas
the number of cells in the S phase increased from 23.6 to 24.6%
following SDF-1α stimulation, suggesting that SDF-1α promoted cell
transformation from the G0/G1 phase to the S phase. When the cells
were treated with NT21MP or miR-155-3p mimics, an increased
percentage of cells were arrested in the G0/G1 phase, from 68.4 to
78.9% in the NT21MP group, and from 68.4 to 79.9% in the miR-155-3p
mimics group, particularly in the combined group (Fig. 3B). Additionally, the changes in
apoptotic cells were in line with the cell cycle results (Fig. 3C). RT-qPCR and western blot
analyses were used to analyze the mRNA and protein expression
levels of cell cycle-related factors. As shown in Fig. 3D and E, SDF-1α upregulated the
expression of target gene MYD88 and Bcl-2, and
downregu-lated the expression of apoptotic genes Bax and caspase-3.
NT21MP inhibited the anti-apoptotic effects of SDF-1α, indicating
that NT21MP induced breast cancer-resistant cell apoptosis.
Furthermore, MYD88 was significantly attenuated in the
NT21MP and overexpression of miR-155-3p treatment group compared
with the univariate treatment group. Bcl-2 was also downregulated
under the same treatment. By contrast, Bax and caspase-3 exhibited
opposite expression trends. These results indicated that NT21MP and
miR-155-3p inhibited the stimulatory effect of SDF-1α on the
biological activity in PR cells.
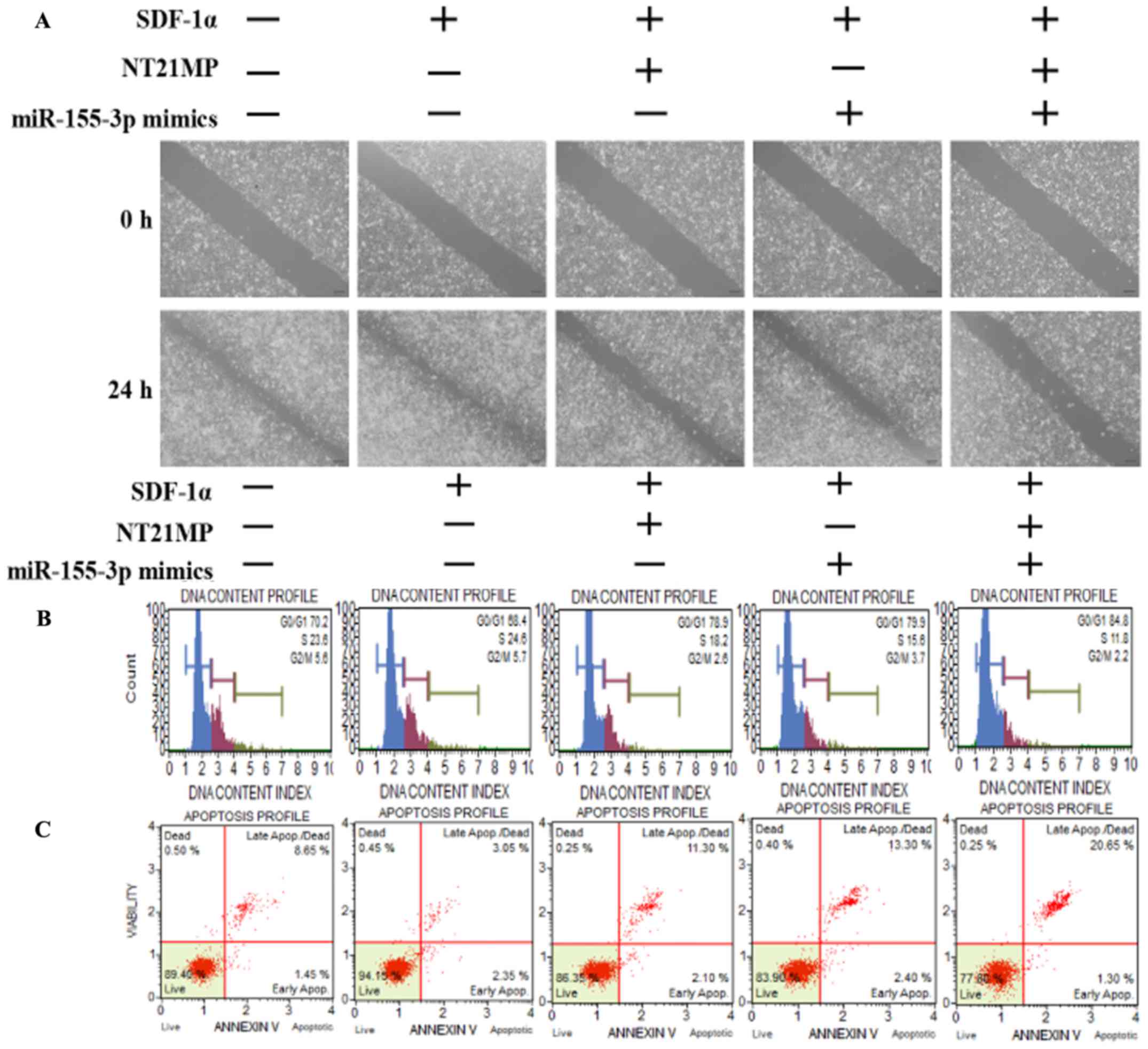 | Figure 3Biological effects of NT21MP and
miR-155-3p mimics on PR breast cancer cells. (A) Effects of NT21MP
and miR-155-3p mimics on cell migration and invasion were measured
using a wound-healing assay. (B) Effects of NT21MP and miR-155-3p
mimics on cell cycle were analyzed using PI staining and flow
cytometry. (C) Effects of NT21MP and miR-155-3p mimics on cell
apoptosis were evaluated using the Annexin V/PI staining and flow
cytometry. (D) Reverse transcription-quantitative polymerase chain
reaction analysis was used to detect the effects of NT21MP and
miR-155-3p mimics on target gene mRNA level, cell cycle, and
apoptosis-related factors. (E) Results of western blot analysis of
the protein levels of target genes, cell cycle, and
apoptosis-related factors in PR cells were consistent with the mRNA
results. Data are presented as the mean ± standard deviation of
three independent experiments. @P<0.05 and
@@@P<0.001, compared with the control group;
*P<0.05, **P<0.01 and
***P<0.001, compared with SDF-1α treatment;
##P<0.01 and ###P<0.001, compared with
S + NT21MP treatment; ∆P<0.05 and
∆∆∆P<0.001, compared with S + 3p mimics treatment.
NT21MP; 21-residue peptide derived from viral macrophage
inflammatory protein II; miR, microRNA; S/SDF-1α, stromal
cell-derived factor-1α; NT, NT21MP; PR, paclitaxel-resistant;
MYD88, myeloid differentiation primary response gene 88; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein; PI, propidium
iodide. |
NT21MP, combined with the downregulation
of MYD88, inhibited its biological effects in MCF-7/PR cells
The present study further examined the biological
effect of MYD88, the target gene of miR-155-3p, when
presented in NT21MP. The wound-healing assay also showed that the
scratch wounds underwent slower closing following the knockdown of
MYD88, particularly when combined with NT21MP (Fig. 4A). The cell cycle and apoptosis
assays showed an increased percentage of cells in the G0/G1 phase
but a decreased percentage of cells in the S phase. The number of
apoptotic cells increased significantly in the si-MYD88 and NT21MP
combined group (Fig. 4B and C). In
addition, the results of the RT-q PCR and western blot analyses
showed that the expression levels of MYD88 and Bcl-2
increased in the SDF-1α group but decreased in the NT21MP and/or
downregulation of MYD88 groups. The expression levels of Bax
and caspase-3 were decreased in the SDF-1α group but increased in
the NT21MP and/or downregulation of MYD88 groups (Fig. 4D and E).
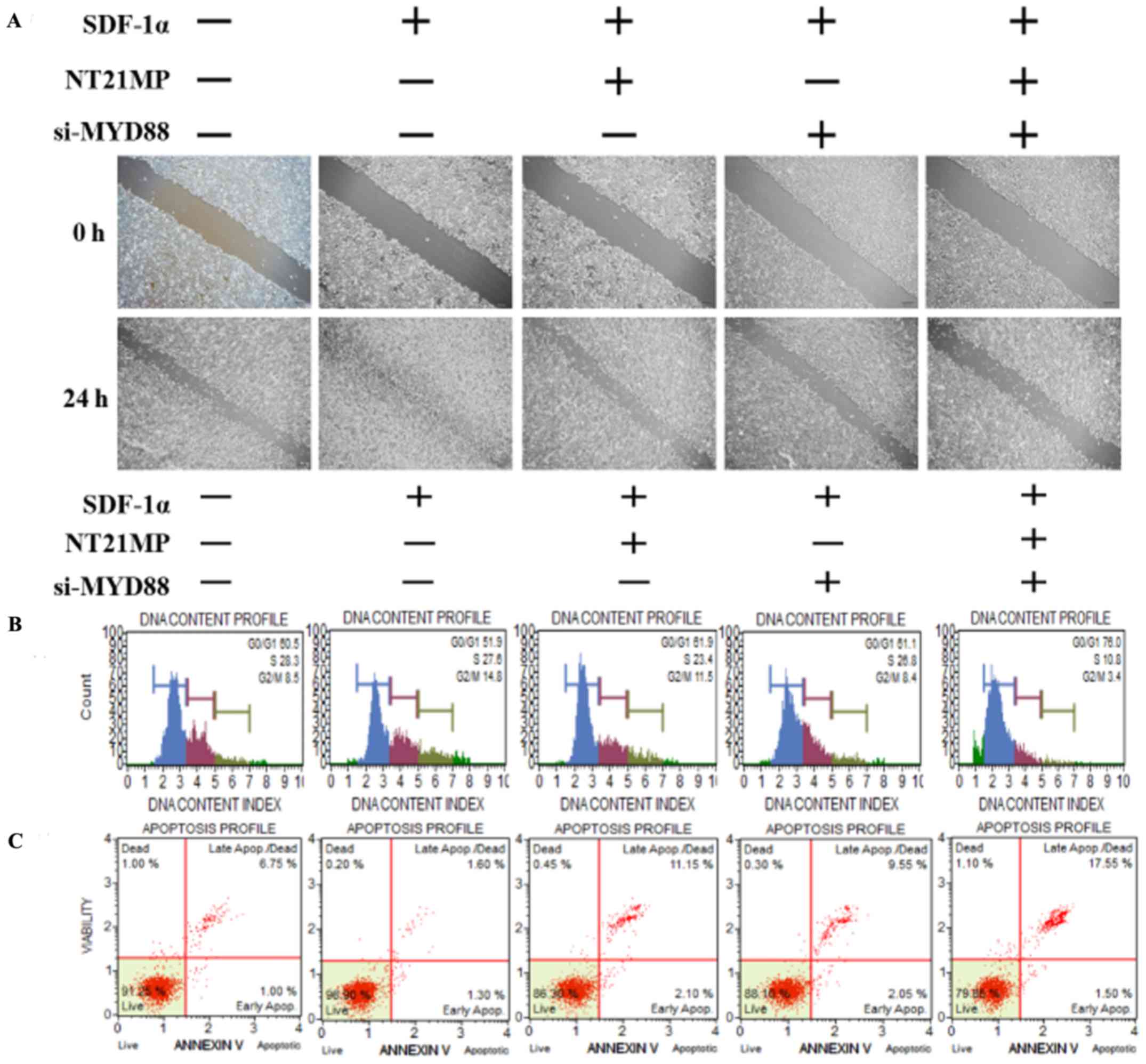 | Figure 4Biological effects of NT21MP and
si-MYD88 on PR cells. (A) Effects of NT21MP and si-MYD88 on cell
migration and invasion were measured using a wound-healing assay.
(B) Effects of NT21MP and si-MYD88 on cell cycle were analyzed
using PI staining and flow cytometry. (C) Effects of NT21MP and
si-MYD88 on cell apoptosis were evaluated using Annexin V/PI
staining and flow cytometry. (D) Reverse transcription-quantitative
polymerase chain reaction analysis was used to detect the effect of
NT21MP and si-MYD88 on the mRNA levels of target genes, cell cycle,
and apoptosis-related factors. (E) Western blot analysis results of
the protein level of target gene, cell cycle, and apoptosis-related
factors in PR cells were consistent with the mRNA results. The data
are presented as the mean ± standard deviation of three independent
experiments. @@@P<0.001, compared with the control
group; ***P<0.001, compared with SDF-1α treatment;
#P<0.05, ##P<0.01 and ###P<0.001,
compared with S + NT21MP treatment; ∆∆P<0.01 and
∆∆∆P<0.001, compared with S + si-MYD88 treatment.
NT21MP; 21-residue peptide derived from viral macrophage
inflammatory protein II; S/SDF-1α, stromal cell-derived factor-1α;
NT, NT21MP; PR, paclitaxel-resistant; MYD88, myeloid
differentiation primary response gene 88; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; PI, propidium iodide; si, small
interfering RNA. |
NT21MP, combined with the downregulation
of miR-155-5p, induces the expression of its target gene TP53INP1
and biological effects in PR cells
The same functional experiments as in miR-155-3p
were performed to examine the association between miR-155-5p and
NT21MP in breast cancer. The SDF-1α group underwent faster closing
of scratch wounds compared with the negative control group. The
NT21MP and miR-155-5p inhibitor groups showed slower closing of
scratch wounds compared with the SDF-1α group, particularly in the
combination groups (Fig. 5A). The
results of cell cycle analysis revealed a decreased number of cells
in the G0/G1 phase but an increased number of cells in the S phase
when transfected with NT21MP or downregulation of miR-155-5p,
compared with the SDF-1α treatment group, and this was more marked
in the combination group (Fig.
5B). The ratio of apoptotic cells decreased with SDF-1α
treatment but increased with NT21MP or miR-155-5p inhibitor; it
also decreased significantly in the combined group (Fig. 5C). The RT-qPCR and western blot
analyses verified that the expression level of target gene
TP53INP1 decreased with SDF-1α treatment, but increased
significantly with NT21MP or downregulation of miR-155-5p (Fig. 5D and E). The expression level of
Bcl-2 increased following SDF-1α treatment but decreased following
NT21MP treatment or the downregulation of miR-155-5p, whereas Bax
and caspase-3 exhibited the opposite results. These results
suggested that NT21MP had a more marked inhibitory effect in
reversing PR cells when combined with the expression of
miR-155-5p.
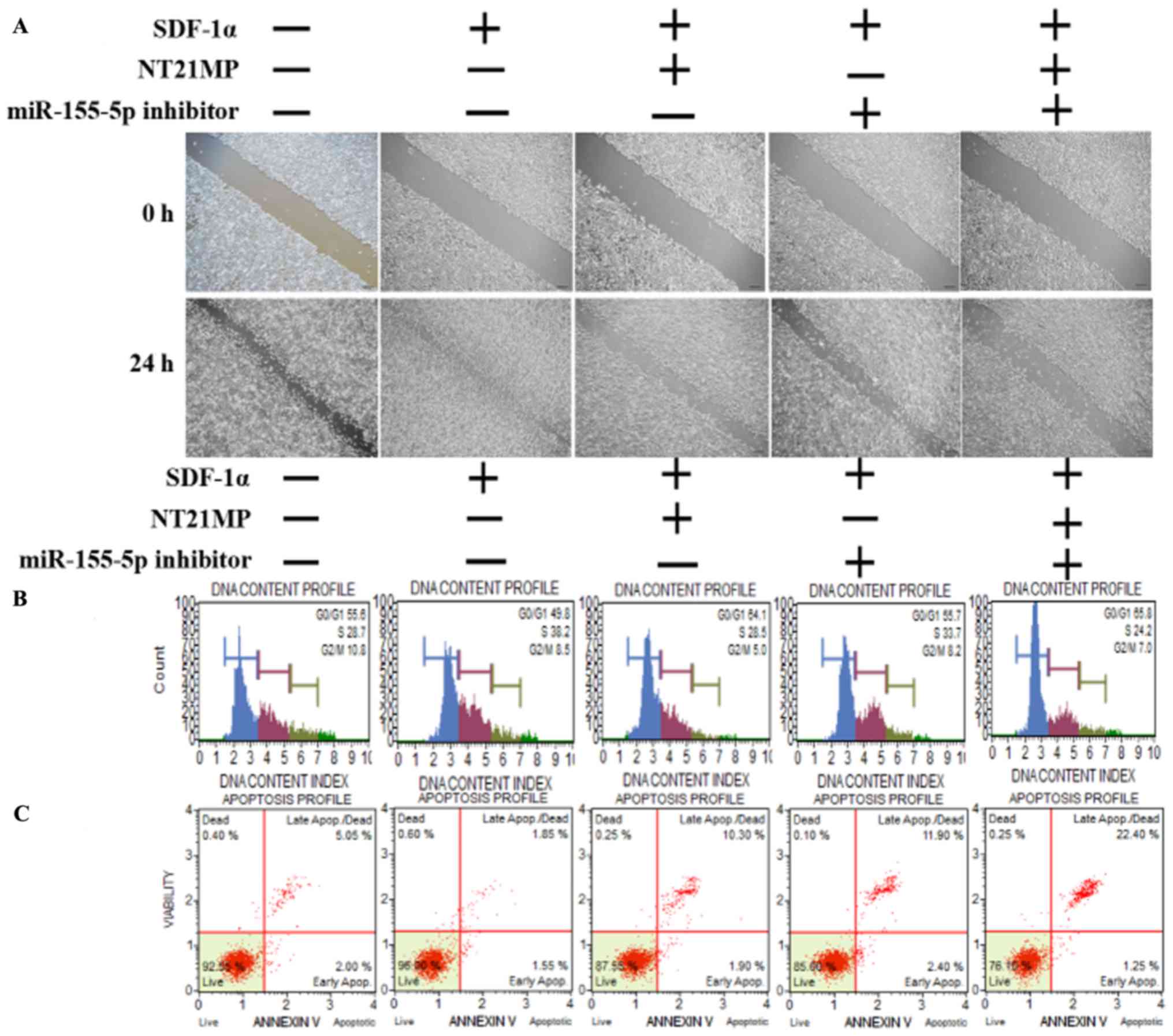 | Figure 5Biological effects of NT21MP and
miR-155-5p inhibitor on PR cells. (A) Effects of NT21MP and
miR-155-5p inhibitor on cell migration and invasion were measured
using the wound-healing assay. (B) Effects of NT21MP and miR-155-5p
inhibitor on cell cycle were analyzed using PI staining and flow
cytom-etry. (C) Effects of NT21MP and miR-155-5p inhibitor on cell
apoptosis were evaluated using the Annexin V/PI staining and flow
cytometry. (D) Reverse transcription-quantitative polymerase chain
reaction analysis was used to detect the effect of NT21MP and
miR-155-5p inhibitor on the mRNA levels of target gene, cell cycle,
and apoptosis-related factors. (E) Western blot analysis results of
the protein levels of target genes, cell cycle, and
apoptosis-related factors in PR cells were consistent with the mRNA
results. Data are presented as the mean ± standard deviation of
three independent experiments. @@P<0.01, compared
with the control group; *P<0.05,
**P<0.01 and ***P<0.001, compared with
SDF-1α treatment; ###P<0.001, compared with S +
NT21MP treatment; ∆P<0.05 and
∆∆∆P<0.001, compared with S + 5p inhibitor treatment.
NT21MP; 21-residue peptide derived from viral macrophage
inflammatory protein II; miR, microRNA; S/SDF-1α, stromal
cell-derived factor-1α; NT, NT21MP; PR, paclitaxel-resistant;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein;
TP53INP1, tumor protein 53-induced nuclear protein 1; PI, propidium
iodide. |
NT21MP, combined with the upregulation of
TP53INP1, inhibits its biological effects in MCF-7/PR cells
As shown in Fig.
6A, transfection with NT21MP and/or pC-TP53INP1 led to slower
closure of wounds. Changes in cell cycle and cell apoptosis showed
that the number of cells in the G0/G1 phase increased but the
number of cells in the S phase decreased. In addition, the
percentage of apoptotic cells increased with NT21MP and/or
pC-TP53INP1 treatment (Fig. 6B and
C). The RT-qPCR and western blot analyses showed that the
expression levels of TP53INP1, Bax, and caspase-3 increased
with NT21MP treatment and/or the overexpression of TP53INP1,
whereas the expression level of Bcl-2 showed the opposite effect
(Fig. 6D and E). Therefore, these
results indicated that miR-155-3p/5p was important in reversing PR,
and its target gene had a regulatory effect when combined with
NT21MP.
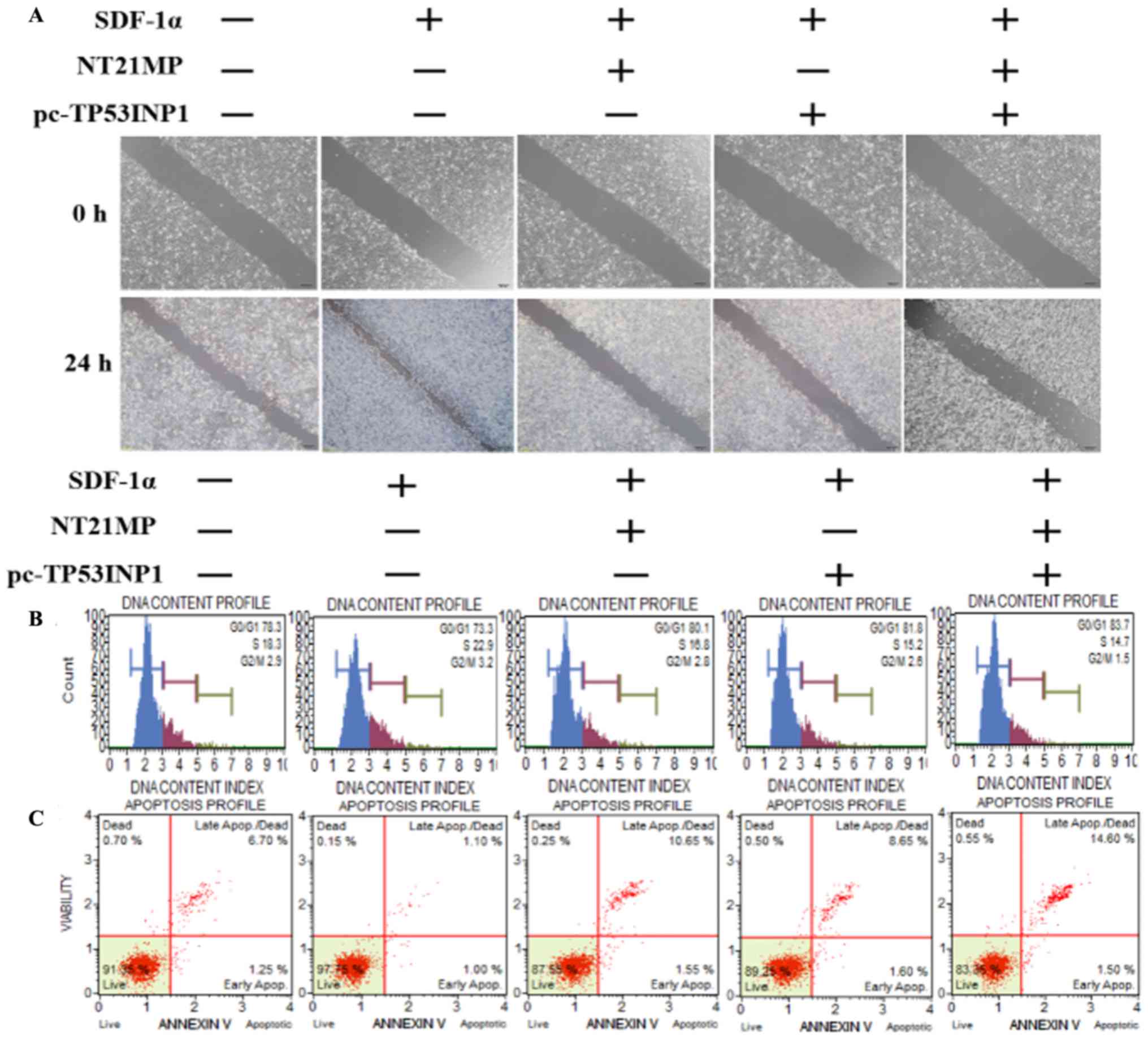 | Figure 6Biological effects of NT21MP and
pc-TP53INP1 on PR cells. (A) Effects of NT21MP and pc-TP53INP1 on
cell migration and invasion were measured using the wound-healing
assay. (B) Effects of NT21MP and pc-TP53INP1 on cell cycle were
analyzed using propidium iodide (PI) staining and flow cytometry.
(C) Effects of NT21MP and pc-TP53INP1 on cell apoptosis were
evaluated using Annexin V/PI staining and flow cytometry. (D)
Reverse transcription-quantitative polymerase chain reaction
analysis was used to detect the effect of NT21MP and pc-TP53INP1 on
the mRNA levels of target genes, cell cycle, and apoptosis-related
factors. (E) Western blot analysis results of the protein levels of
target genes, cell cycle, and apoptosis-related factors in PR cells
were consistent with the mRNA results. Data are presented as the
mean ± standard deviation of three independent experiments.
@P<0.05, @@P<0.01 and
@@@P<0.001, compared with the control group;
***P<0.001, compared with SDF-1α treatment;
##P<0.01 and ###P<0.001, compared with
S + NT21MP treatment; ∆P<0.05 and
∆∆∆P<0.001, compared with S + pc-TP53INP1 treatment.
NT21MP; 21-residue peptide derived from viral macrophage
inflammatory protein II; S/SDF-1α, stromal cell-derived factor-1α;
NT, NT21MP; PR, paclitaxel-resistant; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; TP53INP1, tumor protein 53-induced
nuclear protein 1; PI, propidium iodide. |
NT21MP, combined with miR-155-3p/5p,
promotes PR cell sensitivity in breast cancer
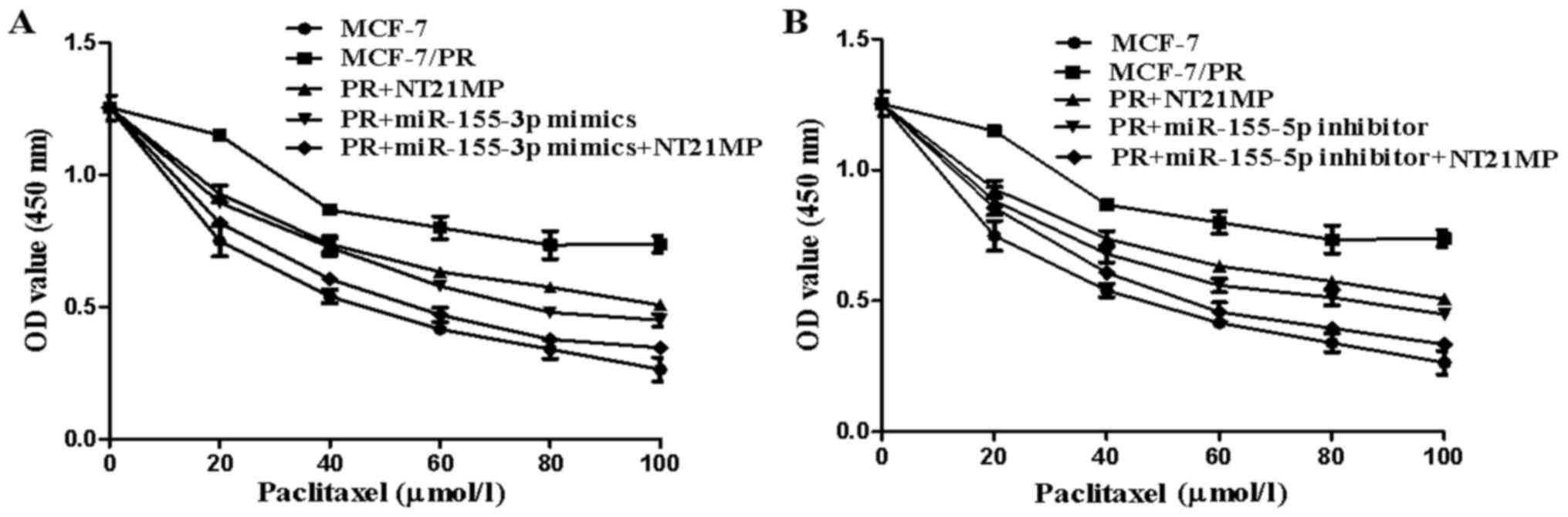
The CCK-8 assay was used to measure the
proliferation of cells incubated with paclitaxel (0, 20, 40, 60,
80, and 100 µmol/l) for 72 h to clarify the efficiency of
NT21MP in reversing drug resistance. The results showed that
NT21MP/miR-155-3p mimics/miR-155-5p inhibitor inhibited the
proliferation of PR cells (Fig. 7A and
B, P<0.05). The efficiency of NT21MP in reversing drug
resistance was found to be more marked when combined with the
miR-155-3p mimics or miR-155-5p inhibitor.
Discussion
Chemotherapy is currently widely used in breast
cancer treatment (28). Despite
its impressive therapeutic effect, drug resistance has become a
hurdle in several clinical cases (29,30).
miRNAs have emerged as pivotal regulators of tumorigen-esis,
particularly in drug resistance of breast cancer (31–33).
However, the molecular mechanism of action of miRNAs in regulating
drug resistance remains to be fully elucidated. The present study
focused on the mature miR-155 family (miR-155-3p and miR-155-5p),
which are expressed abnormally in PR breast cancer cells. A
previous study showed that the overexpression of miR-155-3p
enhanced cell proliferation and tumorigenesis by inhibiting the
expression of F-Box and WD repeat domain containing 7 in
hepatocellular carcinoma (34).
Yim et al showed that the upregulation of miR-155-3p led to
an increased number of sub-G1 apoptotic cells and reduced cellular
viability, suggesting its tumor-suppressive effects (35). However, investigations on
miR-155-3p in terms of the development of breast cancer or drug
resistance have not been performed. miR-155-5p has also been shown
to be upregulated in triple-negative breast cancer (36) and involved in cell progression and
the epithelial-mesenchymal transition process sponged by myocardial
infarction-associated transcript (37). The present study demonstrated that
not only miR-155-3p, a tumor-suppressor gene, but also miR-155-5p,
an oncogenic gene, were involved in the biological activities of PR
breast cancer, including cell invasion, cell cycle and cell
apoptosis.
Previous studies have shown that these two miRNAs
have opposite effects in breast cancer cells and predicted that
their target genes were MYD88 and TP53INP1, using
bioinformatics software and dual-luciferase assays, respectively.
MYD88 was previously reported to be pivotal in anticancer
treatment via activation of the Toll-like receptor-mediated MYD88
signaling pathway in tumor biology, providing a novel potential
target for cancer immunotherapy (38–42).
Studies have reported that MYD88 is involved in the
regulation of drug resistance in breast cancer cells (43,44).
For example, using antibody microarrays, MYD88 was found to
be differentially expressed as a predictive biomarker of
neoadjuvant chemotherapy resistance in breast cancer (45). Xiang et al suggested that
the downregulation of MYD88 reduced the proliferation,
migration and invasion of breast cancer cells, and increased tumor
cell sensitivity to paclitaxel treatment through inhibiting the
activation of nuclear factor-κB via the phosphoinositide
3-kinase/Akt pathway (46).
TP53INP1, which is located on the chromosome 8q22, acts as a
tumor suppressor involved in breast cancer cell proliferation and
apoptotic activity, negatively regulated by miR-155 (47,48).
Dysregulation on the 3′-UTR of this gene regulates competitive
endogenous messenger RNA-mediated migration and invasion and is
correlated with drug resistance (49,50).
In line with these findings, the present study suggested that
MYD88, the target gene of miR-155-3p, and TP53INP1,
the target gene of miR-155-5p, were also crucial in promoting and
suppressing the biological effects in PR breast tumor cells.
Interference of MYD88 or restoration of TP53INP1
enhanced the effects more markedly compared with the single
treatment group, which was mediated by NT21MP.
NT21MP, a synthetic 21-mer peptide antagonist of
CXCR4, derived from vMIP-II, was previously identified to inhibit
cancer growth and metastasis by competitively binding SDF-1α to
CXCR4 (23–25,51).
The present study verified that SDF-1α inhibited the expression of
miR-155-3p and enhanced the level of miR-155-5p, whereas these
effects were reversed with NT21MP in MCF-7 parental and PR cells.
This indicated that NT21MP acted as a potential antagonist
attenuating the effect of the SDF-1α/CXCR4 axis. The combined use
of NT21MP with miR-155-3p mimics, miR-155-5p inhibitor, si-MYD88,
and pc-TP53INP1 contributed to decreases of cell proliferation,
migration, invasion, cell cycle, and apoptosis, compared with that
in MCF-7 PR cells. These changes suggested that NT21MP may reverse
breast cancer drug resistance and improve the efficacy of
paclitaxel through regulating miR-155-3p/5p or
MYD88/TP53INP1.
In conclusion, the present study elucidated that,
not only miR-155-3p/5p, but also their target genes
MYD88/TP53INP1 are involved in the molecular regulation of
PR breast cancer cells. Their combination with NT21MP significantly
improved the sensitivity to paclitaxel, thus providing a novel
clinical therapeutic strategy for the majority of women with breast
cancer. However, the specific mechanism underlying how NT21MP
regulates the potential miRNAs or the corresponding targets to
reverse drug resistance clinically requires further
investigation.
Acknowledgments
Not applicable.
References
|
1
|
Weigelt B, Peterse JL and van 't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Jin K, van Pelt GW, van Dam H, Yu X,
Mesker WE, Ten Dijke P, Zhou F and Zhang L: c-Myb enhances breast
cancer invasion and metastasis through the Wnt/β-catenin/Axin2
pathway. Cancer Res. 76:3364–3375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hackshaw A, Roughton M, Forsyth S, Monson
K, Reczko K, Sainsbury R and Baum M: Long-term benefits of 5 years
of tamoxifen: 10-year follow-up of a large randomized trial in
women at least 50 years of age with early breast cancer. J Clin
Oncol. 29:1657–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trail PA, Dubowchik GM and Lowinger TB:
Antibody drug conjugates for treatment of breast cancer: Novel
targets and diverse approaches in ADC design. Pharmacol Ther.
181:126–142. 2018. View Article : Google Scholar
|
|
5
|
Phuong NT, Lim SC, Kim YM and Kang KW:
Aromatase induction in tamoxifen-resistant breast cancer: Role of
phosphoinositide 3-kinase-dependent CREB activation. Cancer Lett.
351:91–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim YJ, Sung D, Oh E, Cho Y, Cho TM,
Farrand L, Seo JH and Kim JY: Flubendazole overcomes trastuzumab
resistance by targeting cancer stem-like properties and HER2
signaling in HER2-positive breast cancer. Cancer Lett. 412:118–130.
2018. View Article : Google Scholar
|
|
7
|
Vuylsteke P, Huizing M, Petrakova K,
Roylance R, Laing R, Chan S, Abell F, Gendreau S, Rooney I, Apt D,
et al: Pictilisib PI3Kinase inhibitor (a phosphatidylinositol
3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of
hormone receptor-positive, HER2-negative, locally recurrent, or
metastatic breast cancer: Interim analysis of the multicentre,
placebo-controlled, phase II randomised PEGGY study. Ann Oncol.
27:2059–2066. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pellegrino B, Boggiani D, Tommasi C, Palli
D and Musolino A: Nab-paclitaxel after docetaxel hypersensitivity
reaction: Case report and literature review. Acta Biomed.
88:329–333. 2017.PubMed/NCBI
|
|
9
|
Liu T, Sun H, Liu S, Yang Z, Li L, Yao N,
Cheng S, Dong X, Liang X, Chen C, et al: The suppression of DUSP5
expression correlates with paclitaxel resistance and poor prognosis
in basal-like breast cancer. Int J Med Sci. 15:738–747. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang F, Wang B, Long H, Yu J, Li F, Hou H
and Yang Q: Decreased miR-124-3p expression prompted breast cancer
cell progression mainly by targeting Beclin-1. Clin Lab.
62:1139–1145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han L, Liu B, Jiang L, Liu J and Han S:
MicroRNA-497 downregulation contributes to cell proliferation,
migration, and invasion of estrogen receptor alpha negative breast
cancer by targeting estrogen-related receptor alpha. Tumour Biol.
37:13205–13214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samaeekia R, Adorno-Cruz V, Bockhorn J,
Chang YF, Huang S, Prat A, Ha N, Kibria G, Huo D, Zheng H, et al:
miR-206 inhibits stemness and metastasis of breast cancer by
targeting MKL1/IL11 pathway. Clin Cancer Res. 23:1091–1103. 2017.
View Article : Google Scholar
|
|
15
|
Yu T, Li J, Yan M, Liu L, Lin H, Zhao F,
Sun L, Zhang Y, Cui Y, Zhang F, et al: MicroRNA-193a-3p and -5p
suppress the metastasis of human non-small-cell lung cancer by
down-regulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway.
Oncogene. 34:413–423. 2015. View Article : Google Scholar
|
|
16
|
Zhou H, Huang X, Cui H, Luo X, Tang Y,
Chen S, Wu L and Shen N: miR-155 and its star-form partner miR-155*
cooperatively regulate type I interferon production by human
plasmacytoid dendritic cells. Blood. 116:5885–5894. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsushita R, Seki N, Chiyomaru T,
Inoguchi S, Ishihara T, Goto Y, Nishikawa R, Mataki H, Tatarano S,
Itesako T, et al: Tumour-suppressive microRNA-144-5p directly
targets CCNE1/2 as potential prognostic markers in bladder cancer.
Br J Cancer. 113:282–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsushita R, Yoshino H, Enokida H, Goto
Y, Miyamoto K, Yonemori M, Inoguchi S, Nakagawa M and Seki N:
Regulation of UHRF1 by dual-strand tumor-suppressor microRNA-145
(miR-145-5p and miR-145-3p): Inhibition of bladder cancer cell
aggressiveness. Oncotarget. 7:28460–28487. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yonemori M, Seki N, Yoshino H, Matsushita
R, Miyamoto K, Nakagawa M and Enokida H: Dual tumor-suppressors
miR-139-5p and miR-139-3p targeting matrix metalloprotease 11 in
bladder cancer. Cancer Sci. 107:1233–1242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakaguchi T, Yoshino H, Yonemori M,
Miyamoto K, Sugita S, Matsushita R, Itesako T, Tatarano S, Nakagawa
M and Enokida H: Regulation of ITGA3 by the dual-stranded
microRNA-199 family as a potential prognostic marker in bladder
cancer. Br J Cancer. 116:1077–1087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Xing AY, Ma RR, Wang YW, Liu YH
and Gao P: Diagnostic value of miRNA-96-5p/3p in dysplastic nodules
and well-differentiated small hepatocellular carcinoma. Hepatol
Res. 46:784–793. 2016. View Article : Google Scholar
|
|
23
|
Yang Q, Zhang F, Ding Y, Huang J, Chen S,
Wu Q, Wang Z, Wang Z and Chen C: Antitumour activity of the
recombination polypeptide GST-NT21MP is mediated by inhibition of
CXCR4 pathway in breast cancer. Br J Cancer. 110:1288–1297. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Q, Wu H, Wang H, Li Y, Zhang L, Zhu
L, Wang W, Zhou J, Fu Y, Chen S, et al: N-terminal polypeptide
derived from vMIP-II exerts its antitumor activity by inhibiting
the CXCR4 pathway in human glioma. Int J Oncol. 50:1160–1174. 2017.
View Article : Google Scholar :
|
|
25
|
Wang Y, Wang H, Ding Y, Li Y, Chen S,
Zhang L, Wu H, Zhou J, Duan K, Wang W, et al: N-peptide of vMIP-II
reverses paclitaxel- resistance by regulating miRNA-335 in breast
cancer. Int J Oncol. 51:918–930. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Tang B, Xiao B, Liu Z, Li N, Zhu ED, Li
BS, Xie QH, Zhuang Y, Zou QM and Mao XH: Identification of MyD88 as
a novel target of miR-155, involved in negative regulation of
Helicobacter pylori-induced inflammation. FEBS Lett. 584:1481–1486.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rabanal C, Ruiz R, Neciosup S and Gomez H:
Metronomic chemotherapy for non-metastatic triple negative breast
cancer: Selection is the key. World J Clin Oncol. 8:437–446. 2017.
View Article : Google Scholar
|
|
29
|
Jones SK and Merkel OM: Tackling breast
cancer chemore-sistance with nano-formulated siRNA. Gene Ther.
23:821–828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Monteiro IP, Madureira P, de Vasconscelos
A, Pozza DH and de Mello RA: Targeting HER family in HER2-positive
metastatic breast cancer: Potential biomarkers and novel targeted
therapies. Pharmacogenomics. 16:257–271. 2015. View Article : Google Scholar
|
|
31
|
Mi Y, Zhang D, Jiang W, Weng J, Zhou C,
Huang K, Tang H, Yu Y, Liu X, Cui W, et al: miR-181a-5p promotes
the progression of gastric cancer via RASSF6-mediated MAPK
signalling activation. Cancer Lett. 389:11–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Emmrich S, Engeland F, El-Khatib M, Henke
K, Obulkasim A, Schöning J, Katsman-Kuipers JE, Michel Zwaan C,
Pich A, Stary J, et al: miR-139-5p controls translation in myeloid
leukemia through EIF4G2. Oncogene. 35:1822–1831. 2016. View Article : Google Scholar
|
|
33
|
Bahrami A, Aledavood A, Anvari K,
Hassanian SM, Maftouh M, Yaghobzade A, Salarzaee O, ShahidSales S
and Avan A: The prognostic and therapeutic application of microRNAs
in breast cancer: Tissue and circulating microRNAs. J Cell Physiol.
233:774–786. 2018. View Article : Google Scholar
|
|
34
|
Tang B, Lei B, Qi G, Liang X, Tang F, Yuan
S, Wang Z, Yu S and He S: MicroRNA-155-3p promotes hepatocellular
carcinoma formation by suppressing FBXW7 expression. J Exp Clin
Cancer Res. 35:932016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yim RL, Wong KY, Kwong YL, Loong F, Leung
CY, Chu R, Lam WW, Hui PK, Lai R and Chim CS: Methylation of
miR-155-3p in mantle cell lymphoma and other non-Hodgkin's
lymphomas. Oncotarget. 5:9770–9782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W,
Chang G, Li X, Li Q, Wang S, et al: MicroRNA profiling implies new
markers of chemoresistance of triple-negative breast cancer. PLoS
One. 9:e962282014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luan T, Zhang X, Wang S, Song Y, Zhou S,
Lin J, An W, Yuan W, Yang Y, Cai H, et al: Long non-coding RNA MIAT
promotes breast cancer progression and functions as ceRNA to
regulate DUSP7 expression by sponging miR-155-5p. Oncotarget.
8:76153–76164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hassan F, Islam S, Tumurkhuu G, Naiki Y,
Koide N, Mori I, Yoshida T and Yokochi T: Intracellular expression
of toll-like receptor 4 in neuroblastoma cells and their
unresponsiveness to lipopolysaccharide. BMC Cancer. 6:2812006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma FJ, Liu ZB, Hu X, Ling H, Li S, Wu J
and Shao ZM: Prognostic value of myeloid differentiation primary
response 88 and Toll-like receptor 4 in breast cancer patients.
PLoS One. 9:e1116392014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chow A, Zhou W, Liu L, Fong MY, Champer J,
Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, et al: Macrophage
immunomodulation by breast cancer-derived exosomes requires
Toll-like receptor 2-mediated activation of NF-κB. Sci Rep.
4:57502014. View Article : Google Scholar
|
|
41
|
Shi M, Yao Y, Han F and Li Y and Li Y:
MAP1S controls breast cancer cell TLR5 signaling pathway and
promotes TLR5 signaling-based tumor suppression. PLoS One.
9:e868392014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou L, Liu Z, Wang Z, Yu S, Long T, Zhou
X and Bao Y: Astragalus polysaccharides exerts immunomodulatory
effects via TLR4-mediated MyD88-dependent signaling pathway in
vitro and in vivo. Sci Rep. 7:448222017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ge X, Cao Z, Gu Y, Wang F, Li J, Han M,
Xia W, Yu Z and Lyu P: PFKFB3 potentially contributes to paclitaxel
resistance in breast cancer cells through TLR4 activation by
stimulating lactate production. Cell Mol Biol (Noisy-le-grand).
62:119–125. 2016.
|
|
44
|
Edwardson DW, Boudreau J, Mapletoft J,
Lanner C, Kovala AT and Parissenti AM: Inflammatory cytokine
production in tumor cells upon chemotherapy drug exposure or upon
selection for drug resistance. PLoS One. 12:e01836622017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hodgkinson VC, ELFadl D, Agarwal V,
Garimella V, Russell C, Long ED, Fox JN, McManus PL, Mahapatra TK,
Kneeshaw PJ, et al: Proteomic identification of predictive
biomarkers of resistance to neoadjuvant chemotherapy in luminal
breast cancer: A possible role for 14-3-3 theta/tau and tBID? J
Proteomics. 75:1276–1283. 2012. View Article : Google Scholar
|
|
46
|
Xiang F, Ni Z, Zhan Y, Kong Q, Xu J, Jiang
J, Wu R and Kang X: Increased expression of MyD88 and association
with paclitaxel resistance in breast cancer. Tumour Biol.
37:6017–6025. 2016. View Article : Google Scholar
|
|
47
|
Zhang C, Zhao J and Deng H: 17β-estradiol
up-regulates miR-155 expression and reduces TP53INP1 expression in
MCF-7 breast cancer cells. Mol Cell Biochem. 379:201–211. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang CM, Zhao J and Deng HY: miR-155
promotes proliferation of human breast cancer MCF-7 cells through
targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci.
20:792013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng L, Li X, Chou J, Xiang C, Guo Q,
Zhang Z, Guo X, Gao L, Xing Y and Xi T: StarD13 3′-untranslated
region functions as a ceRNA for TP53INP1 in prohibiting migration
and invasion of breast cancer cells by regulating miR-125b
activity. Eur J Cell. 97:23–31. 2018. View Article : Google Scholar
|
|
50
|
Yamamoto Y, Yoshioka Y, Minoura K,
Takahashi RU, Takeshita F, Taya T, Horii R, Fukuoka Y, Kato T,
Kosaka N, et al: An integrative genomic analysis revealed the
relevance of microRNA and gene expression for drug-resistance in
human breast cancer cells. Mol Cancer. 10:1352011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang QL, Zhang LY, Wang HF, Li Y, Wang YY,
Chen TT, Dai MF, Wu HH, Chen SL, Wang WR, et al: The N-terminal
polypeptide derived from viral macrophage inflammatory protein II
reverses breast cancer epithelial-to-mesenchymal transition via a
PDGFRα-dependent mechanism. Oncotarget. 8:37448–37463.
2017.PubMed/NCBI
|