Introduction
Colon cancer continues to be a major health concern,
due to high rates of incidence and mortality (1,2).
Current treatments for advanced-stage colon cancer are limited in
their capacity to target tumor cells employing adaptive mechanisms
necessary for survival, often resulting in the recurrence of
disease and a poor prognosis. In addition, the adverse side effects
from toxicity associated with conventional treatment approaches
continue to hinder the quality of life for vulnerable patients
(3–6). Therefore, it is critical to develop
novel therapeutic approaches targeting pro-survival mechanisms in
colon cancer that are also less toxic.
Conventional chemotherapeutic drugs are commonly
used in combination as a neoadjuvant and/or adjuvant treatment for
advanced-stage colon cancer, or when surgical resection and
radiation are no longer deemed effective treatment options. Several
combination chemotherapy regimens may be considered, with each of
these regimens commonly consisting of two or three drugs with
different mechanisms of action. In the United States,
advanced-stage colon cancer patients are generally offered
conventional chemotherapies, such as 5-flurouracil (5-FU),
oxaliplatin, or a combination of both as a first-line regimen,
which has shown success in prolonging patient survival, but still
leaves the patient vulnerable to recurrence of the disease
(3). In addition, the conventional
regimens are also associated with high costs and a multitude of
debilitating side effects which can hinder the quality of life of
patients (7). Of note, recent
studies have demonstrated the use of natural plant products, such
as flavonoids, in combination with conventional chemotherapy, as a
therapeutic strategy to mitigate toxicity and enhance efficacy
(8–11). These findings show the potential
for natural plant products to help increase survival and improve
the quality of life of colon cancer patients, and provide the basis
for further investigation into combinations of other natural agents
and conventional chemotherapy in colon cancer.
Proanthocyanidins are naturally occurring
polyphenolic bioflavonoids, which are diverse in biological
function (12,13). Both in vitro and in
vivo experimental data support the anticancer capacity of
proanthocyanidins, as they have been shown to reduce survival of
tumor cells by inducing cell cycle arrest and apoptosis (14–18).
Multiple studies have also revealed the various molecular targets
of proanthocyanidins, which could prove to be useful in the
prevention or treatment of different cancers (19–22).
Although numerous proanthocyanidins have been identified, grape
seed proanthocyanidins have been more extensively studied for their
anti-cancer effects, as compared with many which have yet to be
holistically evaluated in different types of cancer.
Cinnamtannin B-1 (CTB-1) is a naturally occurring
trimeric proanthocyanidin, present in a limited number of plants,
including Cinnamomum zeylanicum and Laurus nobilis
(23,24). CTB-1 has been mostly studied for
its ability to inhibit platelet aggregation and potentiate the
action of insulin, likely due to its antioxidant properties
(25–28). Researchers have also investigated
the anti-cancer properties of CTB-1, revealing its cytotoxicity in
melanoma cells, and its capacity to induce cell cycle arrest and
apoptosis in hepatocellular carcinoma and cervical cancer cells
(29,30). Given the observed efficacy of CTB-1
in a select number of cancers, further studies are warranted to
determine its efficacy and mechanism of action in other cancers,
particularly colon cancer. The current study investigated the
anti-survival and pro-apoptotic effects of CTB-1 in colon cancer,
while also elucidating cellular and molecular mechanisms underlying
CTB-1 function and evaluating the potential for CTB-1 to enhance
the potency of conventional chemotherapy. Collectively, these
findings, for the first time, at least to the best of our
knowledge, implicate CTB-1 as a potential therapeutic alternative
to improve colon cancer outcomes.
Materials and methods
Materials
CTB-1, isolated from the L. nobilis plant, was
purchased from Enzo Life Sciences (Farmingdale, NY, USA), and was
dissolved in DMSO (Corning Life Sciences, Corning, NY, USA).
5-Fluorouracil (5-FU) was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and was also dissolved in DMSO. For western blot analysis,
p53 rabbit antibody (Ab; cat. no. 2527P), phospho-p53 (Ser6) rabbit
Ab (cat. no. 9285P), phospho-p53 (Ser9) rabbit Ab (cat. no. 9288P),
Bak (D4E4) rabbit monoclonal antibody (mAb; cat. no. 12105P),
cytochrome c rabbit Ab (cat. no. 11940S), GAPDH (D16H11)
XP® rabbit mAb (cat. no. 5174S), anti-rabbit IgG
HRP-linked Ab (cat. no. 7074P2), and anti-mouse IgG HRP-linked Ab
(cat. no. 7076P2) were purchased from Cell Signaling Technology
(Danvers, MA, USA). Anti-mouse Bcl-2 mAb (cat. no. 05–826) was
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Primary antibodies were diluted 1:1,000 and secondary antibodies
were diluted 1:2,000 in 5% non-fat milk dissolved in TBS with 0.1%
Tween-20. For immunofluorescence, PE-Annexin V Ab (cat. no. 640908)
was purchased from Biolegend (San Diego, CA, USA) and used at a
concentration of 5 µg/ml. The ProLong™ Gold Antifade
Mountant with DAPI for nuclear stain was purchased from Thermo
Fisher Scientific, Inc.
Cell lines and cell culture
Normal colon epithelial cells (CCD 841 CoN) and
colon cancer cells (DLD-1 from node-positive adenocarcinoma
patients; and COLO 201 cells from adenocarcinoma patients with
distant metastases) were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA). These cell lines were
cultured in RPMI-1640 medium (Corning Cellgro, Manassas, VA, USA)
supplemented with 10% fetal bovine serum (FBS), 100 µg/ml of
streptomycin and 100 U/ml of penicillin (both from HyClone,
Pittsburgh, PA, USA). Wild-type p53 and p53-null HCT-116 colorectal
carcinoma cells were generously donated by Dr Upender Manne
(University of Alabama at Birmingham, Birmingham, AL, USA) and
cultured in McCoy's 5A medium (ATCC) supplemented with glutamine,
penicillin/streptomycin and 10% FBS. All cells were maintained at
37°C with 5% CO2, and cultured in either RPMI-1640 or
McCoy's 5A media with 1% FBS, 24 h prior to each experiment.
Cell viability
The effect of CTB-1, either alone or in combination
with 5-FU, on colon cancer cell survival, was assessed by cell
viability assay using the tetrazolium dye, MTT. Briefly, normal
colon epithelial cells or colon cancer cells (5×104)
were cultured in triplicate in a 96-well plate for 24 h at 37°C
with 5% CO2. Cells were then treated with increasing
concentrations of CTB-1 (10, 20, 50 and 100 µM) or an
equivalent dilution of the DMSO vehicle control for 24, 48 and 72
h. For drug combination experiments, cells were treated with
increasing concentrations of 5-FU (10, 20 and 40 µM) alone,
5-FU in combination (1:1) with CTB-1 (10, 20 and 40 µM), or
an equivalent dilution of the DMSO vehicle control for 72 h. To
each well, 20 µl of MTT reagent (0.5 mg/ml in 1X PBS) was
added following treatment, followed by incubation for 2 h to allow
the formation of insoluble formazan crystals. The media was then
discarded, and DMSO was added to dissolve formazan crystals. The
optical density was measured at 570 nm using a SpectraMax M5
spectrophotometer (Molecular Devices, San Jose, CA, USA). All
concentrations were tested in quadruplicate and experiments were
repeated 3 times.
Flow cytometric analysis
To ascertain the effects of CTB-1 on cell cycle
progression and apoptosis, DLD-1 and COLO 201 colon cancer cells
were treated with CTB-1 (20 or 40 µM) or the vehicle control
for 24 h (cell cycle) or 48 h (apoptosis). The range of CTB-1
concentrations was selected based on suboptimal (20 µM) and
above-optimal (40 µM) concentrations relative to the half
maximal effective concentration (EC50) values determined
from the cell viability assay, and the time points based on the
inferred sequence and timing of intracellular biological events.
For cell cycle analysis, 1×106 cells were harvested and
washed 3 times with fluorescence-activated cell-sorting (FACS)
buffer (PBS supplemented with 2% FBS). Cells were then stained with
propidium iodide containing RNase (Cell Signaling Technology,
Danvers, MA, USA) according to the manufacturer's instructions. To
assess the populations of apoptotic cells following CTB-1
treatment, cells were harvested and washed in ice-cold PBS.
Subsequently, 1×105 cells were stained with PE-Annexin V
and 7-aminoactinomycin D (7-AAD) using the PE-Annexin V Apoptosis
Detection kit I (BD Biosciences, San Jose, CA, USA), according to
the manufacturer's instructions. The fluorescent intensity of the
stained cells was acquired (20,000 events/sample) using a Guava
flow cytometer (Millipore, Billerica, MA, USA) and analyzed using
FlowJo 10.0.06 software (Treestar Inc., Ashland, OR, USA).
Apoptotic cells could be grouped into early-stage apoptosis
(Annexin V+ and 7-AAD-) and late-stage
apoptosis (Annexin V+ and 7-AAD+) shown in
the lower right and upper right quadrants of the FACS dot plots,
respectively. The experiment was repeated 3 times for both cell
cycle and apoptotic analyses.
Immunofluorescence
To determine the effect of CTB-1 on apoptosis, colon
cancer cells (DLD-1 and COLO 201) were cultured on Nunc™ Lab-Tek™
II Chamber Slides™ (Thermo Fisher Scientific, Inc.) and then
treated with CTB-1 (20 or 40 µM) or the vehicle control for
48 h. Following treatment, cells were washed twice with ice-cold
PBS and once with 1X Annexin V Binding Buffer (BD Biosciences).
Cells were then incubated with the PE-conjugated Annexin V primary
antibody for 20 min. Samples were then mounted on a slide with
ProLong™ Gold Antifade Mountant with the nuclear stain DAPI. Images
were acquired using Observer.Z1 microscope (Carl Zeiss Microscopy
GmbH, Königsallee, Germany).
Antibody array
Antibody microarrays (cat. no. APP069 and PCC076;
Full Moon Biosystems, Inc., Sunnyvale, CA, USA) were used to
determine the changes in the expression and phosphorylation profile
of molecules involved in survival and apoptosis in colon cancer
cells (DLD-1 and COLO 201) following CTB-1 treatment. The array
layout consisted of highly specific and well characterized
antibodies against apoptosis and cell cycle markers, each
replicated 6 times, with β-actin and GAPDH as controls. Briefly,
colon cancer cells were treated with CTB-1 (40 µM) or DMSO
vehicle control for 48 h, and their total protein extracted and
biotinylated (75 µg protein/reaction) using an antibody
array assay kit (Full Moon Biosystems, Inc.) as per the
manufacturer's instructions. Biotinylated samples were coupled with
the array slides, which were then labeled with 0.5 mg/ml
Cy3-Streptavadin and processed following the manufacturer's
instructions. The arrays were scanned and signal quantified by an
Axon GenePix 4000B microarray scanner (Molecular Devices,
Sunnyvale, CA, USA). For each antibody, the average signal
intensity of replicate spots is normalized first to the median
signal of the slide, then to the normalized average signal
intensity of the GAPDH internal control. Fold changes in protein
expression and/or phosphorylation were calculated by dividing
normalized average signal intensities for CTB-1 treated samples by
untreated controls. Array data was used to generate a heat map
using the CIMminer platform (https://discover.nci.nih.gov/cimminer/oneMatrix.do)
developed by the Genomics and Bioinformatics Group at the National
Cancer Institute.
Isolation of cytoplasmic protein
fraction
To determine the effects of CTB-1 treatment on the
level of cytosolic cytochrome c, cytoplasmic protein was
extracted from DLD-1 and COLO 201 colon cancer cells treated with
CTB-1 (20 or 40 µM) or the DMSO vehicle control for 48 h
using the NE-PER™ Nuclear and Cytoplasmic Extraction Reagents
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Briefly, 5×106 cells were harvested, washed
and centrifuged at 500 × g for 3 min. Cytoplasmic Extraction
Reagent 1 (CER I) was added, followed by vortexing and incubation
on ice for 10 min. CER II was then added, followed by vortexing and
incubation on ice for 1 min. The cytoplasmic extract was collected
by centrifugation at 16,000 × g and stored at -80°C for further
analysis.
Western blot analysis
The expression and/or phosphorylation profile of
apoptosis related proteins was assessed by western blot analysis.
Total protein from colon cancer cells (DLD-1, COLO 201 and HCT-116
WT p53) treated with CTB-1 (20 or 40 µM) or DMSO vehicle
control for 48 h were isolated using RIPA lysis buffer containing
protease and phosphatase inhibitor cocktail, and quantified by the
Pierce bicinconic acid (BCA) method (both from Thermo Fisher
Scientific, Inc.). Isolated proteins (30 µg) were resolved
on 10% SDS-PAGE and transferred to PVDF membranes using a semidry
transfer apparatus (both from Bio-Rad, Hercules, CA, USA). The
membranes were incubated in blocking buffer (5% non-fat milk
dissolved in Tris-buffered saline with 0.1% Tween-20) for 1 h at
room temperature, followed by overnight incubation with primary
antibodies at 4°C. Subsequently, the membranes were incubated with
secondary antibody for 2 h after washing (3X) with TBST. GAPDH was
used as the loading control. Protein bands were developed using
Super Signal West Pico Chemiluminescent substrate kit (Thermo
Fisher Scientific, Inc.) and images were captured using the
ImageQuant LAS 4000 (GE Healthcare Life Sciences, Logan, UT, USA).
ImageJ software (http://www.rsbweb.nih.gov/ij) was used to quantify the
optical density for treated samples, which were normalized to the
GAPDH internal controls.
Evaluating drug interactions
Calcusyn software was used to evaluate drug
interactions between CTB-1 and 5-FU, which utilizes the combination
index method derived from the median-effect principle established
by Chou and Talalay (31–33). The basis of the mathematical model
is the median-effect equation fa/fu =
[D/Dm]m, where 'fa' is the
fraction of cells affected, fu is the fraction
unaffected (1-fa), 'D' equals the concentration of the
drug, 'Dm' the drug dose required for 50% inhibition,
and 'm' is a measurement of the sigmoidicity of the dose-effect
curve yielded by Calcusyn. The combination index (CI) is a
quantitative representation of the pharmacological interactivity
between CTB-1 and 5-FU, which factors in both the potency
(Dm) and the shape of the dose-effect curve. CI is
derived from the formula CI =
(D)1/(Dx)1 +
(D)2(Dx)2 +
(D)1(D)2/(Dx)1(Dx)2,
where (Dx)1 and (Dx)2
are the concentrations for Drug 1 (CTB-1) and Drug 2 (5-FU)
separately, resulting in X% inhibition, and (D)1 and
(D)2 the concentrations of the respective drugs in
combination, resulting in the same percentage inhibition. The CI
quantitatively defines synergism as CI<1, additive effect as
CI=1 and antagonism as CI>1. The CalcuSyn software generated CI
values over a range of Fa levels at different growth
inhibition percentages (EC50, EC75 and
EC90). The Fa-CI plot, also generated by
Calcusyn, is a graphical representation of the pharmacological
interaction, which connects the Fa points against the
fixed ratio combinations of Drug 1 (CTB-1) and Drug 2 (5-FU) on the
X and Y axes. The dose-reduction index (DRI) is a measure of how
much the dose of each drug in a synergistic combination may be
reduced at a given effect level compared with the doses for each
drug alone, and is calculated by the equation (DRI)Drug
A = (Dx)Drug A/(D)Drug A.
Statistical analysis
Statistically significant differences between
control and treatment groups were determined by one-way ANOVA
followed by Dunnett's multiple comparisons test using GraphPad
Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). A two-way
ANOVA followed by Tukey's multiple comparisons test was done to
compare statistically significant differences in cell viability
between different time points. An unpaired t-test with Welch's
correction was used to compare CTB-1 EC50 values in
HCT-116 WT p53 and HCT-116 p53 null colon cancer cells. Statistical
significance was established at p<0.05 and results are shown as
mean ± SEM of 3 independent experiments.
Results
CTB-1 decreases the survival of colon
cancer cells
The effect of CTB-1 on the survival of colon cancer
cells was assessed using an MTT assay. A dose- and time-dependent
reduction in cell viability was observed following treatment with
increasing concentrations of CTB-1 in DLD-1 and COLO 201 cells.
Compared with the vehicle-treated controls (presented as VT in the
figures), a significant reduction was observed after 72 h (DLD-1
cells, 76%, p<0.0001; COLO 201 cells, 74%, p<0.0001) and to a
lesser degree at 24 h (DLD-1 cells, 41%, p<0.0001; COLO 201
cells, 39%, p<0.0001) following treatment with CTB-1 (50
µM). Similar time-dependent trends occurred at all
concentrations of CTB-1. The EC50 value of CTB-1 was
32.4±3.3 µM in the DLD-1 cells and 35.0±1.3 µM in the
COLO 201 cells, calculated from the equation for the
line-of-best-fit for the 72-h treatment (Fig. 1A and B). However, minimal effects
on the viability of the normal colon cells (CCD 841 CoN) treated
within the effective concentration range of CTB-1 (40 µM)
were observed (72 h with 40 µM CTB-1 treatment, 16%
reduction, p=0.0037), as compared with the vehicle-treated controls
(Fig. 1C). These findings
highlight the potential of CTB-1 to selectively target colon cancer
cells, while possibly decreasing the propensity for adverse side
effects from treatment.
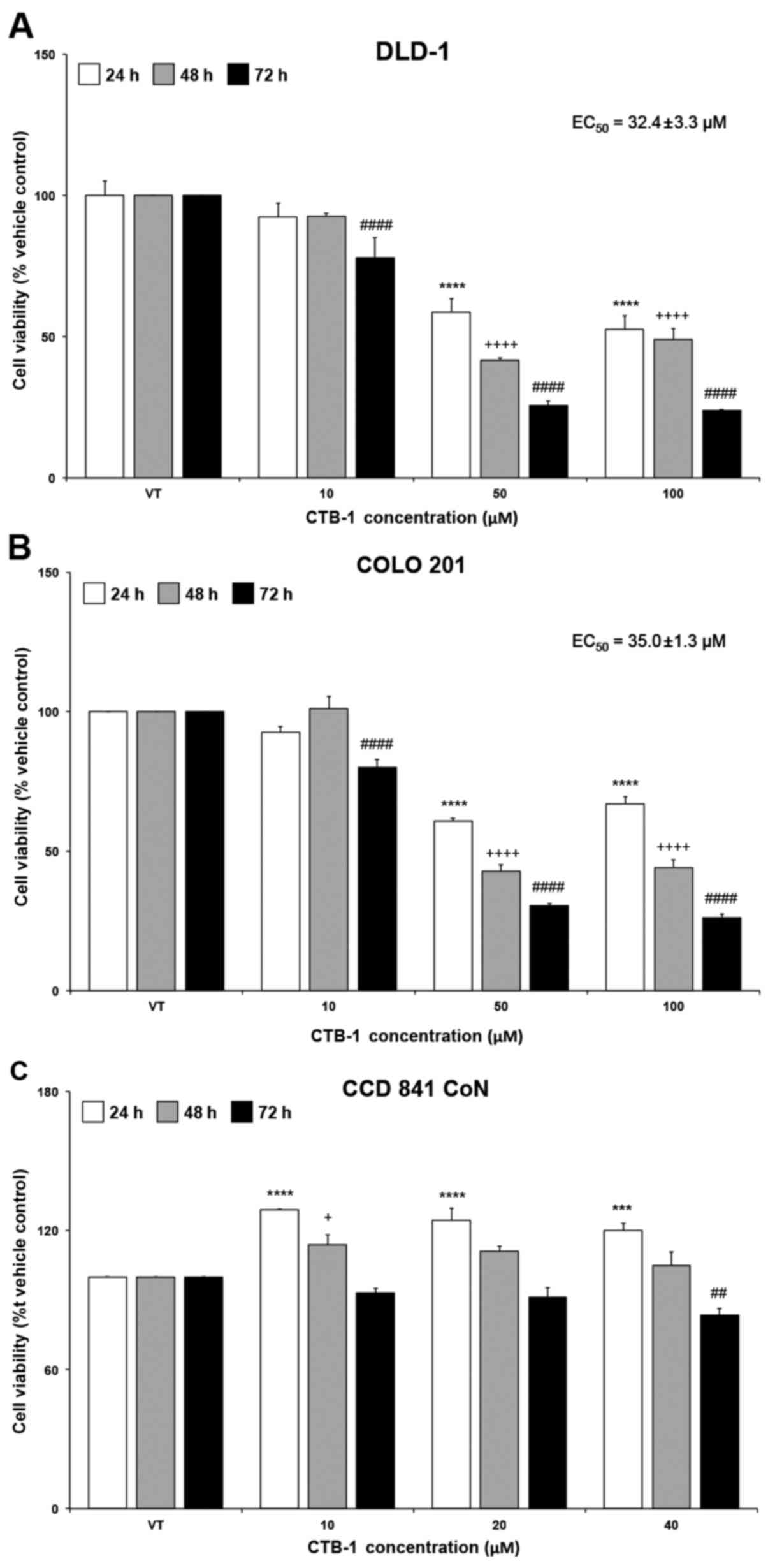 | Figure 1Cinnamtannin B-1 (CTB-1) decreases
the viability of colon cancer cells. (A) DLD-1 and (B) COLO 201
colon cancer cell lines were treated with increasing concentrations
of CTB-1 or the DMSO vehicle control for 24, 48 and 72 h, then
assessed for cell viability via MTT assay. The half maximal
effective concentration (EC50) of CTB-1 against colon
cancer cell lines following treatment for 72 h is displayed in the
graph, calculated from the equation of the line-of-best-fit. (C)
CCD 841 CoN normal colon epithelial cells were treated with CTB-1
within the effective concentration range to assess cytotoxicity via
MTT assay. For all graphs, the mean value (% vehicle control-VT) ±
SEM from 3 replicates is shown. Significant differences between
time-matched CTB-1-treated and vehicle-treated samples are denoted
by (*) for 24 h, (+) for 48 h, and (#) for 72 h
(+p<0.05, ##p<0.01, ***p<0.001,
****,++++,####p<0.0001, Dunnett's multiple
comparisons test). |
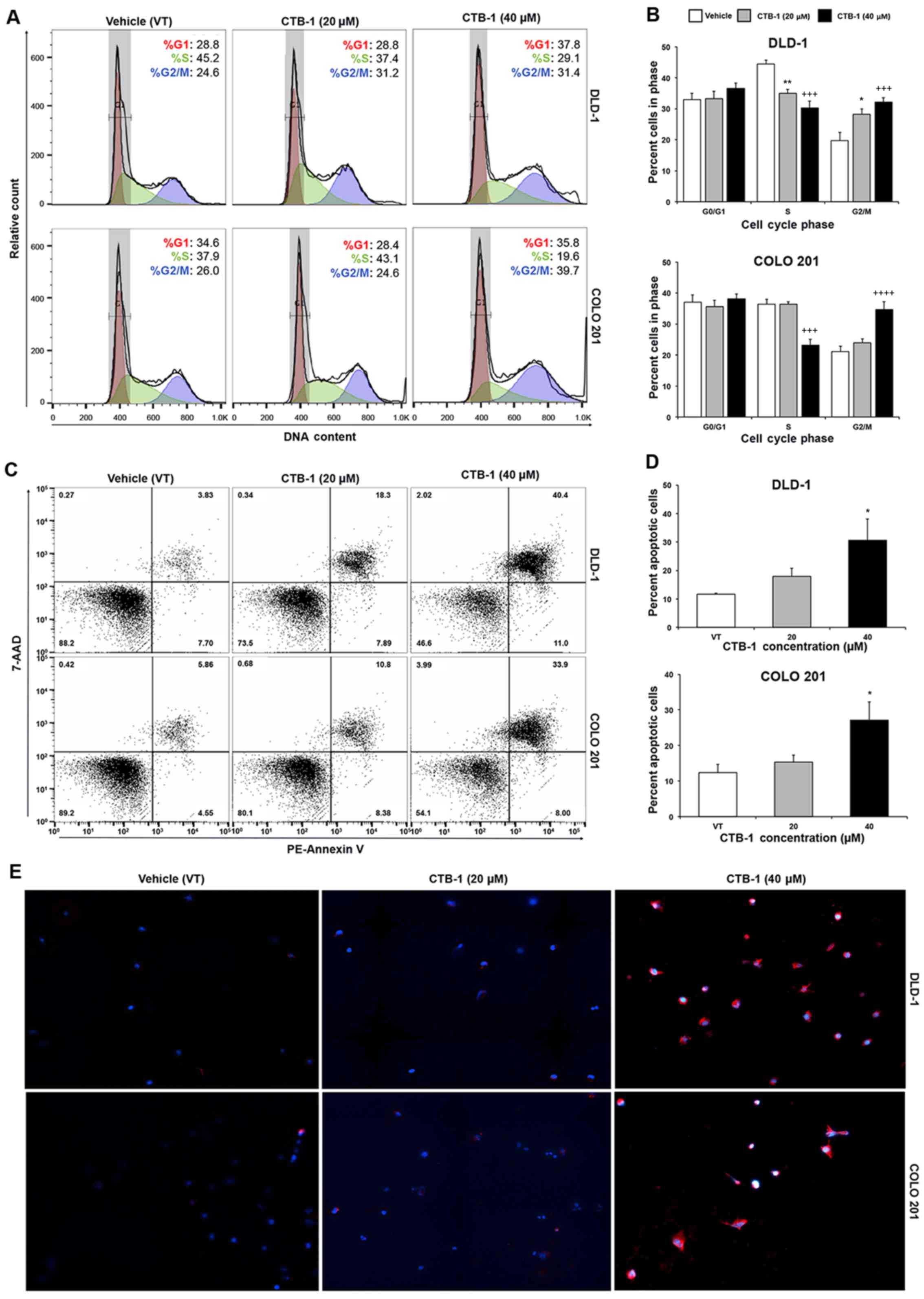
CTB-1 induces cell cycle arrest in colon
cancer cells
Flow cytometric analysis was performed to ascertain
the effects of CTB-1 on the cell cycle phase distribution in colon
cancer cells. The DLD-1 (%G2/M: 19.8 to 32.1, p=0.0009) and COLO
201 (%G2/M: 19.8 to 34.7, p=0.0001) colon cancer cells were both
significantly arrested in the G2/M phase of the cell cycle
following CTB-1 (40 µM) treatment. Our data also revealed
that CTB-1 (40 µM) treatment resulted in a significant
reduction in the number of DLD-1 (%S: 44.4 to 30.2, p=0.0003) and
COLO 201 (%S: 36.4 to 23.2, p=0.0003) cells in the S phase,
compared to the vehicle-treated controls (Fig. 2A and B). The ability of CTB-1 to
arrest the cell cycle progression of colon cancer cells indeed
contributes to the reduction in viable cells observed following
treatment, and demonstrates its potential to slow down the growth
of tumors.
CTB-1 induces the apoptosis of colon
cancer cells
Flow cyto-metric analysis of apoptosis was performed
to ascertain whether the reduction in the viability of the colon
cancer cells following CTB-1 treatment was due to the induction of
apoptosis. Our data revealed an almost 3-fold increase (p<0.05)
in the percentage of total apoptotic DLD-1 cells treated with 40
µM CTB-1 (30.6%), as compared with the vehicle-treated
controls (11.7%). Similarly, there was >2-fold increase
(p<0.05) in the percentage of total apoptotic COLO 201 cells
treated with 40 µM CTB-1 (27.1%), as compared with the
vehicle-treated controls (12.4%) (Fig.
2C and D). To qualitatively observe the effects of CTB-1 on the
apoptosis of colon cancer cells, the DLD-1 and COLO 201 colon
cancer cells were treated with CTB-1 (20 or 40 µM) or the
vehicle control for 48 h, and then analyzed by immunofluorescence
following the PE-Annexin V/DAPI double staining of apoptotic cells.
As shown in Fig. 2E, the colon
cancer cells treated with CTB-1 exhibited increased PE-Annexin V
(red) fluorescent intensity as compared with the vehicle-treated
controls. These results indicate that CTB-1 is capable of inducing
apoptosis in colon cancer cells.
CTB-1 regulates cell-signaling molecules
impacting cell survival and apoptosis
Given the effects of CTB-1 on cell viability and the
induction of apoptosis, it was of interest to broadly evaluate
proteins that may be regulated and/or activated by CTB-1 using an
antibody array. Treatment with CTB-1 (40 µM) was shown to
modulate the expression of anti-apoptotic (Bcl-10, Bcl-2a, Bcl-6,
Bcl-x and Bcl-xL) and pro-apoptotic (Bak and Bax) Bcl-2 family
proteins located on the mitochondrial membrane. Treatment with
CTB-1 also modulated the expression of initiator (caspase-2 and
-8), effector (caspase-3, -6 and -7) and inflammation-related
caspases (caspase-1 and -5), as well as downstream proteins
involved in DNA fragmentation (PARP and DFF40/CAD). In addition,
CTB-1 modulated the expression of anti-apoptotic proteins involved
in the inhibition of extrinsic (SODD) and intrinsic (survivin)
apoptotic signaling cascades (Fig.
3A). Most notably, an increase in the phosphorylation of Ser9
(72% increase) and Ser6 (83% increase) p53 residues in the DLD-1
cells was observed following treatment with CTB-1. However,
antibody microarray data did not reveal any change in the
phosphorylation status of p53 serine residues in CTB-1-treated COLO
201 cells. In addition, there was an increase in the expression of
Bak (114% increase) in COLO 201 cells, while no variation was
observed in DLD-1 cells. Taken together, these data suggest that
CTB-1 may be regulating p53, thereby leading to a modulation of
downstream molecules involved in apoptosis.
 | Figure 3Cinnamtannin B-1 (CTB-1) modulates
the expression and phosphorylation of apoptotic proteins. DLD-1 and
COLO 201 colon cancer cell lines treated with increasing
concentrations of CTB-1 or the DMSO vehicle control for 48 h were
assessed for fold changes in i) total protein or ii) phosphorylated
protein to normal protein. (A) Heatmap representing modulated
apoptosis-related proteins included in apoptosis and
phospho-specific cell cycle protein antibody arrays. Each row
represents one phosphorylation site. Cells shaded red indicate
increased expression or phosphorylation and cells shaded green
indicate a decrease. (B) Protein expression levels of total p53 and
phosphorylation of Ser6 and Ser9 p53 residues were assessed by
western blot analysis. (C) Graphs representing the expression of
total p53, p53 (Ser6) and p53 (Ser9) based on the optical density
(OD) of each protein normalized to the GAPDH control. (D and E)
Western blot images of the apoptotic proteins Bcl-2, Bak and
cytosolic cytochrome c are shown, as well as (F) graphs
representing the expression of each protein based on the optical
density of each protein normalized to the GAPDH control. The mean
OD value ± SEM from 3 replicates is shown. Significant differences
between CTB-1-treated and vehicle-treated samples are denoted by
(*) for DLD-1 cells, and (+) for COLO 201 cells
(*,+p<0.05, **,++p<0.01,
***,+++p<0.001, ****,++++p<0.0001,
Dunnett's multiple comparisons test). |
CTB-1 increases the expression and
phosphorylation of p53
The results from the antibody arrays led us to
validate the expression of p53, as well as the phosphorylation of
Ser6 and Ser9 p53 residues by western blot analysis. There was a
significant increase (p=0.005) in the expression of total p53
following treatment with 20 µM CTB-1 as compared with the
vehicle-treated controls in the DLD-1 cells, yet a decrease was
observed at 40 µM CTB-1. There was also a significant
increase in the phosphorylation of Ser6 (40 µM CTB-1,
p=0.0036) and Ser9 (20 µM CTB-1, p=0.0026; 40 µM
CTB-1, p=0.0319) p53 residues following CTB-1 treatment; however,
the increase in Ser9 phosphorylation was more pronounced at 20
µM CTB-1 than at 40 µM CTB-1. In the COLO 201 cells,
there was a significant dose-dependent increase (40 µM
CTB-1, p=0.0002) in total p53, as well as in the phosphorylation of
Ser6 (40 µM CTB-1, p=0.0001) and Ser9 (40 µM CTB-1,
p=0.02) p53 residues, contrasting from the array data (Fig. 3B and C). The discrepancy in the
results of the arrays and western blot analysis were likely due to
the maintenance of the tertiary structure for the protein targets
in our sample for the array, limiting the number of exposed
epitopes to bind to antibody. Our data further revealed that CTB-1
might contribute to the stabilization of p53, and the
phosphorylation of activating residues.
CTB-1 regulates the expression of Bcl-2,
Bak and the release of cytochrome c into the cytosol
To better understand the downstream molecular
consequences following p53 regulation by CTB-1, the expression of
key proteins related to apoptosis, namely Bcl-2, Bak and cytochrome
c, was evaluated by western blot analysis. In the DLD-1
cells, a significant dose-dependent decrease (40 µM CTB-1,
p=0.0049) in the expression of the anti-apoptotic Bcl-2 protein was
observed, coupled with a significant increase (p=0.0032) in the
expression of the pro-apoptotic Bak protein. Additionally, there
was a significant dose-dependent increase (20 µM CTB-1,
p=0.0033; 40 µM CTB-1, p=0.0001) in the amount of cytosolic
cytochrome c in the CTB-1-treated samples. Of note, in the
COLO 201 cells, there was a significant dose-dependent increase (40
µM CTB-1, p=0.02) in the expression of Bcl-2, coupled with
no notable change in Bak expression, in contrast to the antibody
array results. However, a significant increase (20 µM CTB-1,
p=0.0036; 40 µM CTB-1, p=0.0017) in the levels of cytosolic
cytochrome c was observed response to CTB-1 (Fig. 3D–F). These findings shed light on
the propensity for CTB-1 to induce the initiation of apoptosis by
regulating the expression and localization of mitochondrial
proteins, possibly through a p53-dependent mechanism.
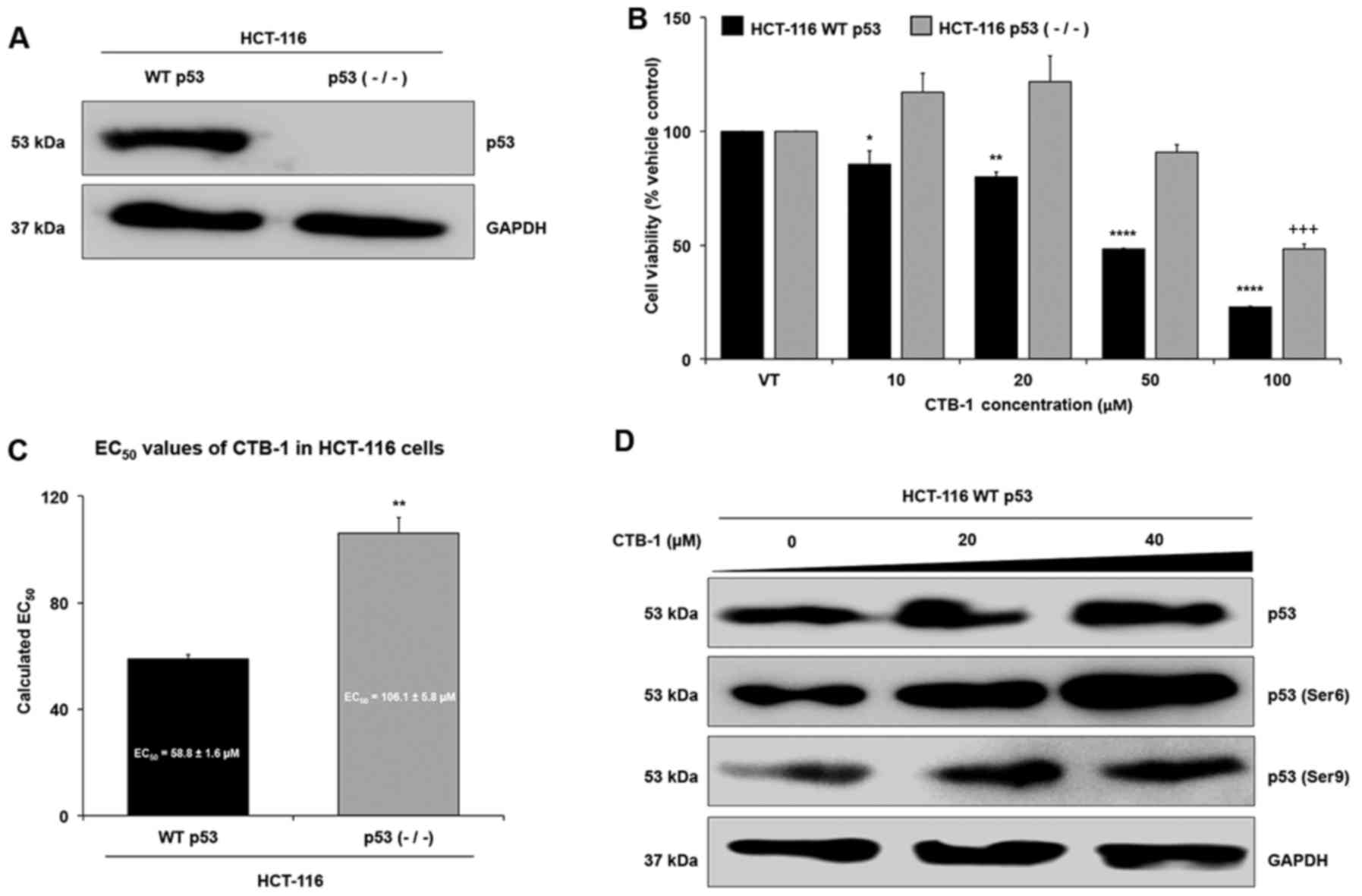
Loss of p53 mitigates the effect of CTB-1
on colon cancer cells
In order to further validate whether CTB-1 exerts
its effects on survival via p53, we wished to determine whether
CTB-1 similarly decreased the survival and regulated p53 in a colon
cancer model with wild-type (WT) p53 rather than the mutated form
of p53 in DLD-1 and COLO 201 cells. To do so, HCT-116 colon cancer
cells with WT p53, or HCT-116 cells with knockdown of p53 [p53
(−/−)] were compared for the expression of p53 (Fig. 4A) and for the extent to which CTB-1
affected cell viability (Fig. 4B).
As shown in Fig. 4C, the
EC50 value of CTB-1 (calculated from the equation for
the line-of-best-fit for the 72-h treatment) in the HCT-116 WT p53
cells (58.8±1.6 µM) was significantly lower (p=0.003) than
the EC50 value of CTB-1 in the HCT-116 p53 null cells
(106.1±5.8 µM). In the HCT-116 WT p53 cells, CTB-1 treatment
also increased the expression of total p53, and the phosphorylation
of Ser6 and Ser9 p53 residues (Fig.
4D), as was observed in the DLD-1 and COLO 201 cells. These
data strengthen the notion that CTB-1 may be mediating its effects,
in part, via p53.
CTB-1 synergizes with 5-FU in exerting
its effects on colon cancer cells
To determine whether CTB-1 can function in concert
with conventional chemotherapeutic agents, such as 5-FU, to achieve
an enhanced potency against colon cancer cells, MTT assay was
carried out. Following treatment of the DLD-1 and COLO 201 colon
cancer cells with increasing concentrations of 5-FU alone, or in
combination with either 10 µM CTB-1, 20 µM CTB-1 or
40 µM CTB-1, viability was significantly lower in cells
treated with the combination of 5-FU and CTB-1, as compared with
that of cells treated with 5-FU alone (40 µM 5-FU/CTB-1;
p=0.0001). In contrast to the DLD-1 cells, in which an enhanced
potency was observed with 5-FU treatment in combination with all
concentrations of CTB-1, the enhanced potency of the combination
treatment in the COLO 201 cells was more prominent in the cells
treated with 5-FU and either 20 µM CTB-1 or 40 µM
CTB-1 (p=0.0001), as compared with the cells treated with 5-FU and
10 µM CTB-1 (Fig. 5A). To
elucidate whether the enhanced effect was due to additive or
synergistic drug interactions between 5-FU and CTB-1, Calcusyn
software was used to generate dose-effect curves and
Fa-CI plots (Fig. 5B).
In the dose-effect curve for the DLD-1 and COLO 201 cells, it is
shown that the effect of the combination of 5-FU and CTB-1 (1:1
ratio) is greater than the effect of either 5-FU or CTB-1 given
alone. The Fa-CI plots for the DLD-1 and COLO 201 cells
revealed that the combination index (CI) for 5-FU and CTB-1 (1:1
ratio) at multiple concentrations of both drugs (10, 20 and 40
µM) was below CI=1, indicating a synergistic, rather than
additive (CI=1) or antagonistic (CI>1), association between the
two drugs. The quantification of the CI at the EC50
(DLD-1, 0.54; COLO 201, 0.62), EC75 (DLD-1, 0.55; COLO
201, 0.39) and EC90 (DLD-1, 0.61; COLO 201, 0.33) of the
drug combination is shown in Table
I. Additionally in Table I,
the Dm, another metric of drug potency, of both 5-FU and CTB-1 is
shown to be significantly reduced when given in combination. This
increase in drug potency for both 5-FU and CTB-1 was quantified by
the drug reduction index (DRI), revealing that in the DLD-1 cells,
5-FU had a DRI=3.85 and CTB-1 had a DRI=3.53 at Fa=0.5.
In the COLO 201 cells, 5-FU had a DRI=2.72 and CTB-1 had a DRI=3.99
at Fa=0.5. Overall, these findings indicate there is a
true synergistic association between 5-FU and CTB-1 (Table II), indicating CTB-1 may be able
to reduce the dosage of 5-FU needed to maintain effectiveness in
colon cancer.
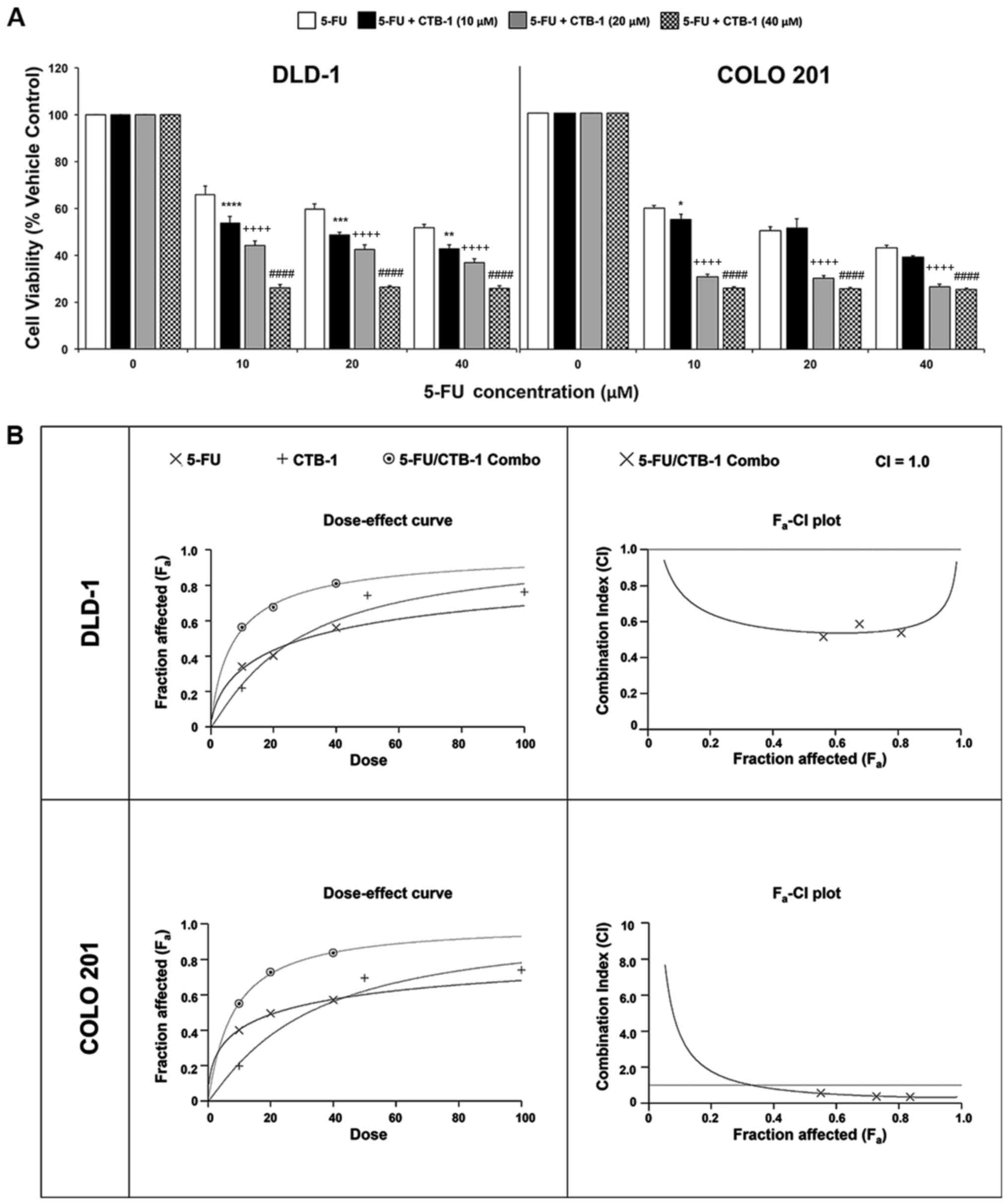 | Figure 5Cinnamtannin B-1 (CTB-1) synergizes
with 5-fluorouracil (5-FU) in colon cancer cells. (A) DLD-1 and
COLO 201 colon cancer cells were treated with increasing
concentrations of 5-FU either alone or in combination with CTB-1
(10, 20 and 40 µM) for 72 h, then assessed for cell
viability via MTT assay. The mean value (% vehicle control) ± SEM
from 3 replicates is shown. Significant differences between
5-FU/CTB-1-treated samples and samples treated with 5-FU alone are
denoted by (*) for 5-FU + CTB-1 (10 µM), (+) for 5-FU +
CTB-1 (20 µM), and (#) for 5-FU + CTB-1 (40 µM)
(*p<0.05, **p<0.01,
***p<0.001, ****,++++,####p<0.0001,
Dunnett's multiple comparisons test). (B) Dose-effect curve and
Fa-CI plot generated by Calcusyn software for DLD-1
cells and COLO 201 cells treated with 5-FU alone, CTB-1 alone, or a
combination of 5-FU and CTB-1 (1:1 ratio). On the Fa-CI
plot, combination index (CI) values below 1 indicate synergism
between two drugs. |
 | Table ICombination index values for colon
cancer cells treated with combination of 5-fluorouracil and
cinnamtannin B-1. |
Table I
Combination index values for colon
cancer cells treated with combination of 5-fluorouracil and
cinnamtannin B-1.
| Cell line | Drug | Combination index
(CI) values at
| Dm | DRI
(Fa=0.5) |
|---|
|
ED50 |
ED75 |
ED90 |
|---|
| DLD-1 | 5-FU | – | – | – | 30.20
µM | 3.85 |
| CTB-1 | – | – | – | 27.68
µM | 3.53 |
| 5-FU + CTB-1
(1:1) | 0.54 | 0.55 | 0.61 | 7.85 µM | – |
| COLO 201 | 5-FU | – | – | – | 21.80
µM | 2.72 |
| CTB-1 | – | – | – | 31.99
µM | 3.99 |
| 5-FU + CTB-1
(1:1) | 0.62 | 0.39 | 0.33 | 8.03 µM | – |
 | Table IIInterpretation of combination index
values as it relates to additive, synergistic, or antagonistic
association between two or more drugs [as described by Chou
(33) and analyzed using Calcusyn
software]. |
Table II
Interpretation of combination index
values as it relates to additive, synergistic, or antagonistic
association between two or more drugs [as described by Chou
(33) and analyzed using Calcusyn
software].
| Range of CI | Symbol | Description |
|---|
| <0.1 | +++++ | Very strong
synergism |
| 0.1–0.3 | ++++ | Strong
synergism |
| 0.3–0.7 | +++ | Synergism |
| 0.7–0.85 | ++ | Moderate
synergism |
| 0.85–0.90 | + | Slight
synergism |
| 0.90–1.10 | ± | Nearly
additive |
| 1.10–1.20 | - | Slight
antagonism |
| 1.20–1.45 | -- | Moderate
antagonism |
| 1.45–3.3 | --- | Antagonism |
| 3.3–10 | ---- | Strong
antagonism |
| >10 | ----- | Very strong
antagonism |
Discussion
The majority of colon cancer patients are diagnosed
at an advanced stage, at which point chemotherapy often becomes the
best, if not only, therapeutic option (34–36).
While there have been effective advancements in treatment
approaches, from combination therapy to targeted therapy, the fact
remains that the 5-year survival rate for advanced stage (stage
IIIB-stage IV) patients ranges from 30 to 80%, lower than that of
early-stage patients (1).
Additionally, the adverse side effects associated with chemotherapy
prove to be burdensome. These realities demonstrate room for
improvement in therapy, and warrant investigation into alternative
treatment approaches, which may simultaneously address issues of
efficacy and toxicity.
In the initiation and progression of colon cancer,
pro-survival cellular and molecular mechanisms are elevated,
allowing cells to divide uncontrollably and avoid apoptosis,
normally designed to eliminate abnormal cells (37). As the evasion of apoptosis in
advanced-stage colon cancer has been shown to be an important
factor in poor response to chemotherapy and radiation, therapies
designed to induce apoptosis in target cells would play a critical
role in controlling tumor growth (38).
A key cell cycle regulator and apoptosis-related
transcription factor is p53, which functions in response to stress
and DNA damage in cells. Regarded as 'the guardian of the genome',
active p53 causes growth arrest, which provides a window for DNA
repair or elimination of cells with severely damaged DNA (39–41).
The phosphorylation of serine residues, including Ser6 and Ser9,
are essential for the stabilization and activation of p53 (42,43).
Once activated, p53 can regulate anti-apoptotic and pro-apoptotic
mitochondrial membrane proteins such as Bcl-2 and Bak,
respectively, which play a role in the permeabilization of the
mitochondrial membrane by opening of voltage-gated anion channels.
This consequently leads to the release of cytochrome c from
the mitochondria into the cytosol coinciding with the initiation of
apoptosis (44). Inactivating
mutations in p53 are found in approximately 50% of human cancers,
inhibiting its function of transcriptional transactivation of
downstream target genes that regulate apoptosis (45).
Our study demonstrates CTB-1, a proanthocyanidin
with multiple observed biological functions, can selectively reduce
the survival of colon cancer cells (both representative of
advanced-stage colon cancer) by approximately 75%, due, to a
certain extent, to the induction of apoptosis resulting from
regulation of pro- and anti-apoptotic proteins. Of note, the
CTB-1-induced increase in the apoptosis of colon cancer cells did
not fully correlate with the observed reduction in cell viability,
indicating that CTB-1 may be influencing the survival of DLD-1 and
COLO 201 cells by alternate cell death mechanisms, such as
autophagy. Given the effect of CTB-1 on cell cycle progression, the
proliferative capacity of the colon cancer cells may also be
reduced in response to treatment, causing the cells to enter
cellular senescence. In both p53-mutated DLD-1 and COLO 201 colon
cancer cells, CTB-1 treatment resulted in an increased expression
of total p53, suggesting that CTB-1 may contribute to the
stabilization of p53 within the cells. Furthermore, in response to
CTB-1, both cell lines exhibited an increase in the phosphorylation
of Ser6 and Ser9, demonstrating that CTB-1 may selectively cause
stress in colon cancer cells, and can subsequently restore the
activation and function of p53. Although it is possible that the
p53 mutations in DLD-1 and COLO 201 cells may have a
dominant-negative effect, the fact that CTB-1 treatment
demonstrated higher potency against HCT-116 WT p53-expressing cells
than HCT-116 p53 null cells, while also increasing the expression
of p53 and the phosphorylation of Ser6 and Ser9 residues in HCT-116
WT p53-expressing cells, further demonstrates that CTB-1 may exert
its effects, at least in part, through p53-dependent
mechanisms.
In this study, while CTB-1 appeared to induce
apoptosis by regulating p53 and inducing the release of cytochrome
c into the cytosol in both DLD-1 and COLO 201 cells, there
were differences observed in the downstream regulation of the
mitochondrial proteins, Bcl-2 and Bak, suggesting that p53 may be
uniquely interacting with Bcl-2 family proteins in either cell
line. One possible reason for the contrasting mechanisms of
p53-induced apoptosis may be related to the p53 mutation status, as
DLD-1 cells have a missense mutation in p53 (E7 codon 241, Ser to
Phe), while COLO 201 cells have a frameshift mutation in p53 (E4
codon 103, T insertion and 25 bp deletion) (46). Since p53 interacts with many
pro-apoptotic transcriptional targets, mutations can alter p53
DNA-binding domains via different mechanisms, depending on the
cellular context, and can ultimately hinder the ability of p53 to
bind to p53-responsive elements on Bcl-2 and Bak genes, or
transactivate the transcription of protein inhibitors to Bcl-2,
such as PUMA and NOXA (47). As a
result, p53 in COLO 201 cells may not have the same capacity as
DLD-1 cells to regulate Bcl-2 or Bak expression, and may instead
interact with other Bcl-2 family proteins to induce apoptosis. It
is also possible that the 48 h time point we selected to evaluate
the expression of apoptotic proteins did not coincide with the
timing of molecular changes underlying the apop-tosis of COLO 201
cells as it did in the DLD-1 cells. It is also noteworthy that
although CTB-1 caused an increase in cytosolic cytochrome c
levels in the DLD-1 and COLO 201 cells, the quantification of the
mitochondrial membrane potential (MMP) measured via JC-1 assay
(data not shown) revealed that CTB-1 treatment did not
significantly alter MMP in the colon cancer cell line. The lack of
any change in MMP following treatment does not particularly
correspond with the regulation of Bcl-2 and Bak mitochondrial
proteins and their role in membrane channel assembly. Overall, our
findings warrant additional investigation to substantiate the
observed reduction in survival.
The idea of using anti-cancer agents together is
hardly novel, as the use of combination chemotherapy has yielded
increased survival in multiple cancer types and is often standard
of care, particularly for advanced-stage cancer patients (3). However, the prospect of finding
alternative drug combinations, including ones employing the use of
natural plant products, to enhance the potency of conventional
chemotherapy is still of great interest, due to the possibility to
reduce the required dosage of chemotherapy and the associated
adverse side effects from toxicity. Therefore, the ability of CTB-1
to significantly enhance the potency of 5-FU by almost 3–4-fold
through synergistic drug interactions highlights the
translational/clinical implications that CTB-1 may have by
simultaneously combating the high costs and adverse side effects
associated with colon cancer treatment, likely leading to improved
patient-centered outcomes.
Although our findings establish the anti-survival,
pro-apoptotic and synergistic effects of CTB-1 on colon cancer
cells in vitro, preclinical studies using a mouse model are
required to further strengthen clinical utility of CTB-1 and
synergism between CTB-1 and 5-FU.
The data reported in this study add to a growing
body of evidence demonstrating the ability of polyphenols,
specifically proanthocyanidins, to selectively target cancer cells
by inducing apoptosis (14,16–18,20)
and to be used in combination with conventional chemotherapy to
enhance potency (8–11). Grape seed proanthocyanidins and
oligomeric proanthocyanidins have been more extensively studied for
their anti-survival properties in multiple cancer types, including
colon cancer, and have also been shown to mediate their
pro-apoptotic effects via p53 activation (18,21,48,49).
By contrast, CTB-1 has been shown to be cytotoxic to melanoma cells
(30), and to induce cell cycle
arrest and the apoptosis of hepatocellular carcinoma and cervical
cancer cells (29); however, it
has yet to be holistically evaluated for its anti-cancer properties
in colon cancer. The present study, for the first time, at least to
the best of our knowledge, revealed CTB-1 increased the expression
and phosphorylation of activating p53 residues in both a
p53-mutated and WT p53 colon cancer model, which likely precedes
the induction of apoptosis and significant reduction in colon
cancer cell survival. Given the high frequency of p53 mutations in
colon cancer, it is markedly consequential that CTB-1 may restore
the p53 function in p53-mutated colon cancer cell lines (50).
Reactive oxygen species (ROS) formation and
oxidative stress have been shown to induce neoplastic
transformation, as they are involved in several key events of
tumorigenesis, including self-sufficiency in growth signals
(51,52), and resistance to apoptosis
(53,54). The anti-cancer effects of
polyphenolic compounds, such as CTB-1, are often attributed to
their capacity to scavenge ROS as potent antioxidant molecules.
This theory also underlies the ability of polyphenols to target
cancer cells over normal cells, as cancer cells may be more
sensitive to changes in the redox state, given their increased
oxidative stress (55,56). More recently, however, it has been
established that polyphenols are also able to induce ROS (mainly
superoxide anions or hydrogen peroxide) formation in cancer cells,
activating the DNA-damage response pathway (57–61).
Since there is evidence to indicate that ROS act as both an
upstream signal that triggers p53 activation and as a downstream
factor that mediates apoptosis (62–64),
it is possible that CTB-1 may influence p53 stabilization,
activation and function by affecting ROS levels in colon cancer
cells. However, further investigations are warranted to conclude
whether this is true, and if so, to determine whether CTB-1
functions as a scavenger or inducer of ROS in colon cancer
cells.
In conclusion, in this study, CTB-1 exhibited the
capacity to significantly reduce colon cancer survival and induce
apoptosis of colon cancer cells, seemingly by modulating the
expression of survival-related proteins, specifically those with
pro-apoptotic and anti-apoptotic functions, such as p53 and
downstream molecular targets. In addition to its anti-cancer
efficacy, CTB-1 also exhibited minimal cytotoxicity in normal colon
epithelial cells, suggesting a decreased likelihood for adverse
side effects. Lastly, CTB-1 displayed a synergistic association
with 5-FU, strengthening its clinical implications. Collectively,
the data presented in this study taps into the potential of CTB-1
as a novel low-toxicity therapeutic agent for advanced-stage colon
cancer.
Acknowledgments
The content of this manuscript benefited from many
fruitful conversations with members of the Morehouse School of
Medicine, Atlanta, GA, USA. We are also grateful to Dr. Upender
Manne from the University of Alabama at Birmingham for generously
donating the HCT-116 WT p53 and HCT-116 p53 null colon cancer cell
lines for use in our experiments.
Funding
This study was supported in part by the funds
(CA169716, CA180212, CA118638 and CA179701) from NCI and Morehouse
School of Medicine flow cytometry core. The content is solely the
responsibility of the authors and does not necessarily represent
the official views of the National Institutes of Health.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PPC developed experimental design, conducted
experiments, analyzed data and drafted the manuscript. HM assisted
in flow cytometric analysis and manuscript preparation. NK assisted
in microscope imaging and manuscript preparation. ABW assisted in
western blot analysis and manuscript preparation. SS conceptualized
the study, developed study design, supervised the authors
throughout the study, and provided his expertise in manuscript
preparation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
CTB-1
|
cinnamtannin B-1
|
|
5-FU
|
5-fluorouracil
|
|
VT
|
vehicle-treated
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
PE
|
phycoerythrin
|
|
7-AAD
|
7-aminoactinomycin D
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
ROS
|
reactive oxygen species
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
Ab
|
antibody
|
|
mAB
|
monoclonal antibody
|
|
HRP
|
horseradish peroxidase
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et
al: SEER Cancer Statistics Review, 1975–2013. National Cancer
Institute; Bethesda, MD: 2016
|
|
3
|
Braun MS and Seymour MT: Balancing the
efficacy and toxicity of chemotherapy in colorectal cancer. Ther
Adv Med Oncol. 3:43–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu CY, Chan W, Delclos GP and Du XL:
Adjuvant chemotherapy and risk of gastrointestinal, hematologic,
and cardiac toxicities in elderly patients with stage III colon
cancer. Am J Clin Oncol. 35:228–236. 2012. View Article : Google Scholar
|
|
5
|
Nadeem H, Jayakrishnan TT, Gamblin TC and
Turaga K: Cost differential among systemic therapies for colon
cancer. Ann Surg Oncol. 21:S832014.
|
|
6
|
Liu CJ, Lin JK, Chen W-S, Lin TC, Yang SH,
Jiang JK, Chang SC, Lan YT, Yen CC, Tzeng CH, et al: The efficacy
of chemotherapy in patients with high-grade metastatic colon
cancer. Hepatogastroenterology. 58:1495–1501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Engstrom PF, Arnoletti JP, Benson AB III,
Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS,
Enzinger PC, et al National Comprehensive Cancer Network: NCCN
Clinical Practice Guidelines in Oncology: Colon cancer. J Natl
Compr Canc Netw. 7:778–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel BB, Sengupta R, Qazi S, Vachhani H,
Yu Y, Rishi AK and Majumdar AP: Curcumin enhances the effects of
5-fluorouracil and oxaliplatin in mediating growth inhibition of
colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer.
122:267–273. 2008. View Article : Google Scholar
|
|
9
|
Patel BB, Gupta D, Elliott AA, Sengupta V,
Yu Y and Majumdar AP: Curcumin targets FOLFOX-surviving colon
cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res.
30:319–325. 2010.PubMed/NCBI
|
|
10
|
Chen WT-L, Yang T-S, Chen H-C, Chen HH,
Chiang HC, Lin TC, Yeh CH, Ke TW, Chen JS, Hsiao KH, et al:
Effectiveness of a novel herbal agent MB-6 as a potential adjunct
to 5-fluoracil-based chemotherapy in colorectal cancer. Nutr Res.
34:585–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amin ARMR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Plumb GW, De Pascual-Teresa S,
Santos-Buelga C, Cheynier V and Williamson G: Antioxidant
properties of catechins and proanthocyanidins: Effect of
polymerisation, galloylation and glycosylation. Free Radic Res.
29:351–358. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santos-Buelga C and Scalbert A:
Proanthocyanidins and tannin-like compounds - nature, occurrence,
dietary intake and effects on nutrition and health. J Sci Food
Agric. 80:1094–1117. 2000. View Article : Google Scholar
|
|
14
|
Prasad R, Vaid M and Katiyar SK: Grape
proanthocyanidin inhibit pancreatic cancer cell growth in vitro and
in vivo through induction of apoptosis and by targeting the
PI3K/Akt pathway. PLoS One. 7:e430642012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma SD, Meeran SM and Katiyar SK:
Proanthocyanidins inhibit in vitro and in vivo growth of human
non-small cell lung cancer cells by inhibiting the prostaglandin
E(2) and prostaglandin E(2) receptors. Mol Cancer Ther. 9:569–580.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh T, Sharma SD and Katiyar SK: Grape
proanthocyanidins induce apoptosis by loss of mitochondrial
membrane potential of human non-small cell lung cancer cells in
vitro and in vivo. PLoS One. 6:e274442011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nomoto H, Iigo M, Hamada H, Kojima S and
Tsuda H: Chemoprevention of colorectal cancer by grape seed
proanthocyanidin is accompanied by a decrease in proliferation and
increase in apoptosis. Nutr Cancer. 49:81–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Q, Liu X-FF and Zheng P-SS: Grape
seed proanthocyanidins (GSPs) inhibit the growth of cervical cancer
by inducing apoptosis mediated by the mitochondrial pathway. PLoS
One. 9:e1070452014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vaid M, Singh T and Katiyar SK: Grape seed
proanthocyanidins inhibit melanoma cell invasiveness by reduction
of PGE2 synthesis and reversal of epithelial-to-mesenchymal
transition. PLoS One. 6:e215392011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Engelbrecht AM, Mattheyse M, Ellis B, Loos
B, Thomas M, Smith R, Peters S, Smith C and Myburgh K:
Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB
pathway and induces apoptosis in a colon cancer cell line. Cancer
Lett. 258:144–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nandakumar V, Singh T and Katiyar SK:
Multi-targeted prevention and therapy of cancer by
proanthocyanidins. Cancer Lett. 269:378–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ting-Ting L, Tong L, Yu-Cong Z and Ke-Yuan
Z: Inhibitive effect of proanthocyanidins on cyclooxygenase-2
expression in A549 cells induced by cytokine interleukin-1 beta. J
Shanghai Jiaotong Univ. 17:500–504. 2012. View Article : Google Scholar
|
|
23
|
Jayaprakasha GK, Ohnishi-Kameyama M, Ono
H, Yoshida M and Jaganmohan Rao L: Phenolic constituents in the
fruits of Cinnamomum zeylanicum and their antioxidant activity. J
Agric Food Chem. 54:1672–1679. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dall'Acqua S, Cervellati R, Speroni E,
Costa S, Guerra MC, Stella L, Greco E and Innocenti G:
Phytochemical composition and antioxidant activity of Laurus
nobilis L. leaf infusion J Med Food. 12:869–876. 2009. View Article : Google Scholar
|
|
25
|
Taher M: Majid F adibah abdul and Sarmidi
MRS: A proanthocyanidin from cinnamomum zeylanicum stimulates
phosphorylation of insulin receptor in 3t3-l1 adipocytes. J Teknol.
44:53–68. 2006.
|
|
26
|
Bouaziz A, Salido S, Linares-Palomino PJ,
Sanchez A, Altarejos J, Bartegi A, Salido GM and Rosado JA:
Cinnamtannin B-1 from bay wood reduces abnormal intracellular
Ca2+ homeostasis and platelet hyperaggregability in type
2 diabetes mellitus patients. Arch Biochem Biophys. 457:235–242.
2007. View Article : Google Scholar
|
|
27
|
Ben Amor N, Bouaziz A, Romera-Castillo C,
Salido S, Linares-Palomino PJ, Bartegi A, Salido GM and Rosado JA:
Characterization of the intracellular mechanisms involved in the
antiaggregant properties of cinnamtannin B-1 from bay wood in human
platelets. J Med Chem. 50:3937–3944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bouaziz A, Romera-Castillo C, Salido S,
Linares-Palomino PJ, Altarejos J, Bartegi A, Rosado JA and Salido
GM: Cinnamtannin B-1 from bay wood exhibits antiapoptotic effects
in human platelets. Apoptosis. 12:489–498. 2007. View Article : Google Scholar
|
|
29
|
Wen L, You L, Yang X, Yang J, Chen F,
Jiang Y and Yang B: Identification of phenolics in litchi and
evaluation of anticancer cell proliferation activity and
intracellular antioxidant activity. Free Radic Biol Med.
84:171–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kashiwada Y, Nonaka G, Nishioka I, Chang
JJ and Lee KH: Antitumor agents, 129. Tannins and related compounds
as selective cytotoxic agents. J Nat Prod. 55:1033–1043. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chou TC and Talalay P: Analysis of
combined drug effects: A new look at a very old problem. Trends
Pharmacol Sci. 4:450–454. 1983. View Article : Google Scholar
|
|
32
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kelly C and Cassidy J: Chemotherapy in
metastatic colorectal cancer. Surg Oncol. 16:65–70. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chibaudel B, Tournigand C, André T and de
Gramont A: Therapeutic strategy in unresectable metastatic
colorectal cancer. Ther Adv Med Oncol. 4:75–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goodwin RA and Asmis TR: Overview of
systemic therapy for colorectal cancer. Clin Colon Rectal Surg.
22:251–256. 2009. View Article : Google Scholar :
|
|
37
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abraha AM and Ketema EB: Apoptotic
pathways as a therapeutic target for colorectal cancer treatment.
World J Gastrointest Oncol. 8:583–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fridman JS and Lowe SW: Control of
apoptosis by p53. Oncogene. 22:9030–9040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Haupt S, Berger M, Goldberg Z and Haupt Y:
Apoptosis - the p53 network. J Cell Sci. 116:4077–4085. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lane DP: Cancer. p53, guardian of the
genome. Nature. 358:15–16. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Higashimoto Y, Saito S, Tong XH, Hong A,
Sakaguchi K, Appella E and Anderson CW: Human p53 is phosphorylated
on serines 6 and 9 in response to DNA damage-inducing agents. J
Biol Chem. 275:23199–23203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaeser MD, Pebernard S and Iggo RD:
Regulation of p53 stability and function in HCT116 colon cancer
cells. J Biol Chem. 279:7598–7605. 2004. View Article : Google Scholar
|
|
44
|
Schuler M, Bossy-Wetzel E, Goldstein JC,
Fitzgerald P and Green DR: p53 induces apoptosis by caspase
activation through mitochondrial cytochrome c release. J Biol Chem.
275:7337–7342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Soussi T, Ishioka C, Claustres M and
Béroud C: Locus-specific mutation databases: Pitfalls and good
practice based on the p53 experience. Nat Rev Cancer. 6:83–90.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
Stat. 2014:9–29. 2014.
|
|
47
|
Hemann MT and Lowe SW: The p53-Bcl-2
connection. Cell Death Differ. 13:1256–1259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Al-Suhaibani ES: Antiproliferation and
antiactivity of proanthocyanidins against colorectal cancer cells
(Caco-2) line through mitochondrial pathway. Int J Adv Sci Tech
Res. 4:152–161. 2015.
|
|
49
|
Roy AM, Baliga MS, Elmets CA and Katiyar
SK: Grape seed proanthocyanidins induce apoptosis through p53, Bax,
and caspase 3 pathways. Neoplasia. 7:24–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Y and Bodmer WF: Analysis of P53
mutations and their expression in 56 colorectal cancer cell lines.
Proc Natl Acad Sci USA. 103:976–981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yoo MH, Xu XM, Carlson BA, Patterson AD,
Gladyshev VN and Hatfield DL: Targeting thioredoxin reductase 1
reduction in cancer cells inhibits self-sufficient growth and DNA
replication. PLoS One. 2:e11122007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun G and Kemble DJ: To C or not to C:
Direct and indirect redox regulation of Src protein tyrosine
kinase. Cell Cycle. 8:2353–2355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vaughn AE and Deshmukh M: Glucose
metabolism inhibits apoptosis in neurons and cancer cells by redox
inactivation of cytochrom c. Nat Cell Biol. 10:1477–1483. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Salvioli S, Storci G, Pinti M, Quaglino D,
Moretti L, Merlo-Pich M, Lenaz G, Filosa S, Fico A, Bonafè M, et
al: Apoptosis-resistant phenotype in HL-60-derived cells HCW-2 is
related to changes in expression of stress-induced proteins that
impact on redox status and mitochondrial metabolism. Cell Death
Differ. 10:163–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mileo AM and Miccadei S: Polyphenols as
modulator of oxidative stress in cancer disease: New therapeutic
strategies. Oxid Med Cell Longev. 2016:64756242016. View Article : Google Scholar
|
|
56
|
Cos P, De Bruyne T, Hermans N, Apers S,
Berghe DV and Vlietinck AJ: Proanthocyanidins in health care:
Current and new trends. Curr Med Chem. 11:1345–1359. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lee DH, Lee TH, Jung CH and Kim YH:
Wogonin induces apoptosis by activating the AMPK and p53 signaling
pathways in human glioblastoma cells. Cell Signal. 24:2216–2225.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sharif T, Auger C, Alhosin M, Ebel C,
Achour M, Etienne-Selloum N, Fuhrmann G, Bronner C and Schini-Kerth
VB: Red wine polyphenols cause growth inhibition and apoptosis in
acute lymphoblastic leukaemia cells by inducing a redox-sensitive
up-regulation of p73 and down-regulation of UHRF1. Eur J Cancer.
46:983–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shankar S and Srivastava RK: Involvement
of Bcl-2 family members, phosphatidylinositol 3'-kinase/AKT and
mitochondrial p53 in curcumin (diferulolylmethane)-induced
apoptosis in prostate cancer. Int J Oncol. 30:905–918.
2007.PubMed/NCBI
|
|
60
|
Ye R, Goodarzi AA, Kurz EU, Saito S,
Higashimoto Y, Lavin MF, Appella E, Anderson CW and Lees-Miller SP:
The isoflavonoids genistein and quercetin activate different stress
signaling pathways as shown by analysis of site-specific
phosphorylation of ATM, p53 and histone H2AX. DNA Repair (Amst).
3:235–244. 2004. View Article : Google Scholar
|
|
61
|
Su CC, Lin JG, Li TM, Chung JG, Yang JS,
Ip SW, Lin WC and Chen GW: Curcumin-induced apoptosis of human
colon cancer colo 205 cells through the production of ROS,
Ca2+ and the activation of caspase-3. Anticancer Res.
26:4379–4389. 2006.
|
|
62
|
Méplan C, Richard MJ and Hainaut P: Redox
signalling and transition metals in the control of the p53 pathway.
Biochem Pharmacol. 59:25–33. 2000. View Article : Google Scholar
|
|
63
|
Maillet A and Pervaiz S: Redox regulation
of p53, redox effectors regulated by p53: A subtle balance.
Antioxid Redox Signal. 16:1285–1294. 2012. View Article : Google Scholar
|
|
64
|
Liu B, Chen Y and St Clair DK: ROS and
p53: A versatile partnership. Free Radic Biol Med. 44:1529–1535.
2008. View Article : Google Scholar : PubMed/NCBI
|