Introduction
Liver cancer is one of the most common types of
malignant tumor worldwide, which is associated with relatively poor
prognosis and rapid progression (1,2).
Liver cancer consists of two subtypes: Hepatocellular carcinoma and
hepatoblastoma. It has previously been reported that 20% of
patients with unresectable liver cancer exhibit reduced sensitivity
to chemotherapy, and no survival benefit is observed. Although
chemotherapy prolongs patient survival, a large number of patients
with cancer benefit less due to a poor response to chemotherapeutic
drugs (3,4). Multidrug resistance (MDR) serves
crucial roles in drug-resistant cancer cells (5). Multidrug resistance protein 1 (MDR1)
serves as an ATP-dependent drug efflux pump that reduces the
intracellular accumulation of chemotherapeutic drugs, such as
cisplatin (DDP), 5-fluoracil (5-FU) and doxorubicin, and thereby
limits their anticancer efficacy (6,7). Due
to the important role of MDR1 in drug resistance, blockade of MDR1
may help to avert drug resistance.
Twist family bHLH transcription factor 1 (TWIST) is
a highly conserved basic helix-loop-helix transcription factor,
which has an important role in epithelial-mesenchymal transition
(EMT). EMT is a transient phase initially observed in embryonic
development (8,9). Downregulation of TWIST by small
interfering RNA leads to decreased metastatic potential and
invasion of prostate carcinoma cells (10,11).
Recent studies have reported that EMT is associated
with chemoresistance in cancer (12,13).
Consistent with a previous report (14), it was revealed that TWIST is
involved in the development of acquired drug resistance in human
cancer cells. TWIST overexpression is also correlated with
chemotherapy resistance in various types of cancer and leads to a
poorer prognosis (15,16). Therefore, TWIST may be considered a
novel therapeutic target in overcoming MDR in liver cancer. The
present study aimed to investigate the relationship between TWIST
and MDR1 in liver cancer cell-associated drug resistance.
The present study provided evidence to suggest that
TWIST was highly expressed in liver cancer tissues and was
positively correlated with MDR1 expression. Furthermore, the
results confirmed that MDR1 was negatively correlated with
E-cadherin expression in cancer samples. Knockdown of TWIST
enhanced the cytotoxicity of chemotherapeutic drugs in R-HepG2
cells by suppressing MDR1 and reversing EMT.
Materials and methods
Tissue specimens
A total of 49 paraffin-embedded samples were
obtained from patients who were diagnosed at the Affiliated
Hospital of the Guangdong Medical University (Zhanjiang, China)
between April and September 2015. Patient characteristics are
presented in Table I. A total of
22 corresponding non-cancerous liver tissues were also obtained
immediately following surgical resection; these 22 non-cancerous
tissues were paired with cancer samples. However, not all
corresponding non-cancerous samples were collected for all cancer
samples as some non-cancerous samples were missing due to surgical
reasons. All patients signed a consent form, which disclosed that
the samples were to be used for scientific research. None of the
patients received preoperative therapy, such as with transarterial
chemoem-bolization or percutaneous ethanol injection. The present
study was approved by the Institutional Ethics Committee of
Guangdong Medical University. All patients provided written
informed consent.
 | Table IPatient clinical parameters. |
Table I
Patient clinical parameters.
| Variable | n |
|---|
| Age (years) | |
| ≤50 | 19 |
| >50 | 30 |
| Sex | |
| Male | 45 |
| Female | 4 |
| Liver cirrosis | |
| Yes | 13 |
| No | 36 |
| Histological
differentiation | |
| High | 5 |
| Moderate | 15 |
| Poor | 29 |
| T stage | |
| I + II | 19 |
| III + IV | 30 |
| Metastasis | |
| Yes | 16 |
| No | 33 |
Immunohistochemistry (IHC)
Paraffin-embedded sections (4 μm) were
prepared for IHC. Briefly, sections were blocked with 2% bovine
serum albumin (cat. no. 05470; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature for 1 h, followed by the
clearance of endogenous peroxidases with 0.3%
H2O2 for 15 min. Sections were then incubated
with primary antibodies against TWIST (Abcam, Cambridge, MA, USA,
cat. no. ab49254), MDR1 (cat. no. ab170904; Abcam), E-cadherin
(cat. no. sc-7870; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
or vimentin (cat. no. 3932; Cell Signaling Technology Inc.,
Danvers, MA, USA) at 4°C overnight, whereas non-immune
immunoglobulin G (IgG, cat. no. A7016; Beyotime Institute of
Biotechnology, Haimen, China) was used as a negative control.
Antigenic sites were localized using a SP9000 kit and DAB kit (cat.
nos. SP9000 and C02-100; OriGene Technologies, Inc., Beijing,
China). Horseradish peroxidase-labeled secondary antibodies were
subsequently added to the sections at room temperature for 1 h
according to the manufacturer's protocol (supplied with the DAB
kit, cat. no. C02-100; Origene Technologies, Inc.). IHC staining
results were interpreted independently by two pathologists (DM300
LED; Leica Microsystems GmbH, Wetzlar, Germany). Briefly, the
immunoreactive scores (IRS) of TWIST, MDR1, E-cadherin and vimentin
were calculated as follows: 0, negative; 1, weak; 2, moderate and
3, strong. The percentage of positive cells was scored as 1, 0–9%;
2, 10-50%; 3, 51–75% and 4, >76%. The two scores were multiplied
together and samples with a total IRS of 0–1, 2–3, 4–5 and ≥6 were
considered (−), (+), (++) and (+++), respectively. According to the
final score, the expression levels of TWIST, MDR1, E-cadherin and
Vimentin were categorized as low (<6) or high (≥6).
Cell cultures and establishment of a
TWIST-silenced cell line
HepG2 cells (cat. no. HB-8065; American Type Culture
Collection, Manassas, VA, USA) were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin G and 100
μg/ml streptomycin. HepG2 cells were initially misidentified
as a hepatocellular carcinoma cell line; however, they have now
been identified as hepatoblastoma cells (17). Cells were incubated at 37°C in a
humidified atmosphere containing 5% CO2. To maintain
drug resistance, doxorubicin-resistant HepG2 (R-HepG2) cells were
cultured for 2 weeks and continuously maintained in medium
containing 1.2 μM doxorubicin (cat. no. D1515;
Sigma-Aldrich; Merck KGaA). R-HepG2 cells were provided by Dr Ye
Caiguo (Chinese University of Hong Kong, Hong Kong, China).
Doxorubicin resistance was induced by continued incubation of the
parental HepG2 cells with 1.2 μM doxorubicin, until a single
stable clone was obtained, which was named R-HepG2.
TWIST-silenced HepG2 and R-HepG2, and negative
control (NC) HepG2 and R-HepG2 cell lines were established as
previously described (18).
Packaged lentiviruses containing TWIST-specific short hairpin RNA
(shRNA), which were labeled with green fluorescent protein as a
transfection marker were provided by Shanghai GenePharma Co., Ltd.
(Shanghai, China). The shRNA sequences were as follows: TWIST
shRNA, 5′-CCTGA GCAACAGCGAGGAA-3′; and NC shRNA, 5′-TTCTCCGA
ACGTGTCACGT-3′. Cells were infected with lentiviruses (multiplicity
of infection=25) for 24 h at 37°C. Subsequently, cells were
cultured with fresh complete RPMI-1640 medium containing 1
μg/ml puromycin for 14 days. The efficiency of TWIST
silencing was determined by western blotting. Stable TWIST-silenced
HepG2 or R-HepG2 cells (si-TWIST group), and NC HepG2 or R-HepG2
cells (NC group) were maintained in similar medium to HepG2 or
R-HepG2 cells supplemented with 0.5 μg/ml (90 μg/ml)
puromycin.
Cell viability assay
Cell viability and half maximal inhibitory
concentration (IC50) were analyzed using the Cell
Counting kit-8 (CCK-8, cat. no. C0037; Beyotime Institute of
Biotechnology) assay. Cells were seeded at 2.5×103
cells/well in 96-well plates for 24 h prior to experimentation,
after which various concentrations of DDP (0–1 μg), 5-FU
(0–500 μg) or doxorubicin (0–24 μM) were added to
each well; the CCK-8 assay was performed after 48 h. Briefly, 10
μl CCK-8 reagent was added to each well and incubated for 2
h at 37°C. Absorbance was subsequently measured at 450 nm using a
Synergy 2 Multi-Mode microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). The assay was conducted in quadruplicate for
each sample and three parallel experiments were performed. The
IC50 values of 5-FU, DDP and doxorubicin were calculated
using GraphPad Prism (Version 5.01; GraphPad Software, Inc., La
Jolla, CA, USA).
Dual luciferase reporter assay
The dual luciferase reporter assay was conducted
according to the manufacturer's protocol
(Dual-Luciferase® Reporter Assay system, cat. no. E1910;
Promega Corporation, Madison, WI, USA). The luciferase detection
kit was used to analyze cells, which were transfected with MDR1
promoter reporter plasmids or blank plasmids. Briefly,
2×105 cells were seeded in a 6-well plate 24 h prior to
experimentation. The MDR1 promoter ranging from −1,040 kbp to +288
bp was cloned by PCR reaction using HepG2 genomic DNA as a
template. The PCR products were sequenced and subjected to BLAST
analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The
pRL-SV40-blank vector (cat. no. 27163; Addgene Inc., Cambridge, MA,
USA) was digested with KpnI (cat. no. R0142S; New England
Biolabs, Inc., Ipswich, MA, USA) and HindIII (cat. no.
R0104S; New England Biolabs, Inc.). PCR products were mixed with
digested blank vectors for ligation. The ligation products were
then transformed into competent DH5α bacteria (cat. no. 9057,
Takara Biotechnology Co., Ltd., Dalian, China) for cloning and
plasmid amplification. Subsequently, 2 μg constructed
pRL-SV40-MDR1 reporter vector or pRL-SV40-blank vector were mixed
with Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 30 min, after which the
mixture was added to the cells and incubated at 37°C for 24 h.
Cells were then lysed with lysis buffer after washing with PBS
twice, and luciferin substrate was mixed with the lysate for 5 min.
The firefly luciferase reporter signal was detected by a microplate
spectrophotometer in luminescence format. Renilla luciferase
activity was also measured for normalization. Three replicate wells
were used for each condition and the experiment was conducted three
times for validation.
Intracellular accumulation of
doxorubicin
Once the cells reached 70–80% confluence in 6-well
plates, a final concentration of 10 μM doxorubicin was added
to each well and incubated at 37°C for 3 h. Subsequently, the cells
were washed and resuspended at 5–10×105 cells/ml in PBS.
After washing with PBS, the cells were observed by fluorescence
microscopy and analyzed by flow cytometry: Excitation wavelength,
488 nm; emission wavelength, 575 nm. For intracellular doxorubicin
analysis, red mean fluorescence intensity (MFI) of the gated cells
was measured to determine drug accumulation by flow cytometry
(EPICS XL-MCL; Beckman Coulter, Inc., Brea, CA, USA) using with
ModFit LT software version 2.0 (Beckman Coulter, Inc.). Briefy,
1×104 cells were collected under the following settings:
forward scatter (FSC), 200 mV; side scatter (SSC), 250 mV and
fluorescein isothiocyanate (FITC) channel, 400 mV. Single cells in
the FSC-SSC window were gated for 575 nm channel signal
detection.
Confocal microscopy
Cells were detected by indirect immunofluorescence.
Cells were fixed with 1:1 acetone-methanol at room temperature for
30 min, and then incubated with the following primary antibodies
overnight at 4°C, TWIST (1:100), vimentin (1:100) and E-cadherin
(1:100). Antigenic sites were subsequently localized using
FITC-labeled secondary antibody (E-cadherin; 1:100, cat. no.
111095045; Jackson ImmunoResearch Europe Ltd., Ely, UK) or
TRITC-conjugated goat anti-rabbit secondary antibody (Vimentin;
1:100, cat. no. 111025045; Jackson ImmunoResearch Europe Ltd.).
DAPI was used to stain the nuclei. Images were captured under a
Leica laser scanning confocal microscope (TCS SP5; Leica
Microsystems GmbH).
Western blot analysis
Western blotting was performed as previously
described (18). Cells were
collected and lysed with radioimmunoprecipitation assay lysis
buffer [50 mM Tris-HCl (pH 8.0), 150 mM sodium chloride, 1.0%
Igepal CA-630 (NP-40), 0.5% sodium deoxycholate). Protein
quantification was measured using NanoDrop 3000 (NanoDrop; Thermo
Fisher Scientific, Inc., Wilmington, DE, USA) at A280. Total
proteins (60–80 μg) were separated by 10% SDS-PAGE and were
transferred to polyvinylidene difluoride membranes (pore size, 0.22
μM). After washing twice with Tris-buffered saline
containing 0.1% Tween-20 (TBST), the membranes were incubated with
5% not-fat dried milk in TBST at room temperature for 1 h, and the
membranes were incubated with primary antibodies against TWIST
(1:250, cat. no. ab49254; Abcam), vimentin (1:1,000, cat. no. 3932;
Cell Signaling Technology Inc.), E-cadherin (1:500, cat. no. 3195;
Cell Signaling Technology Inc.), MDR1 (1:1,000, cat. no. ab170904),
multidrug resistance associated protein 1 (MRP1, 1:1,000, cat. no.
14685; Cell Signaling Technology Inc.), N-cadherin (1:500, cat. no.
13116; Cell Signaling Technology Inc.) and Snail (1:1,000, cat. no.
9782; Cell Signaling Technology Inc.) and β-actin (1:2,000, cat.
no. 4970; Cell Signaling Technology Inc.) at 4°C overnight. After a
further two washes in TBST, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (1:2,000,
cat. no. 111035003; Jackson ImmunoResearch Europe Ltd.) for 1 h at
room temperature. Signals were detected on X-ray film using an
enhanced chemiluminescence detection system (Pierce; Thermo Fisher
Scientific, Inc.).
Cell migration and wound-healing
assays
The migratory ability of cells was evaluated using
Transwell inserts with 8 mm pores (Corning Incorporated, Corning,
NY, USA), as previously described (19). Briefly, 2×105 cells in
100 μl serum-free medium and 600 μl RPMI-1640
containing 10% FBS were added to the upper and lower chambers,
respectively. After 48-h incubation at room temperature,
non-migrated cells were removed with a cotton swab, and the
membrane inserts were fixed with 1:1 methanol and acetone for 15
min, and stained with 0.1% crystal violet for 45 min. Migrated
cells were counted within five representative fields in triplicate
under a microscope (TS100-F serial; Nikon Corporation, Tokyo,
Japan).
For the wound-healing assay, normal cells or cells
infected with si-TWIST or NC (5×104) for 24 h were
harvested and seeded into 6-well plates in RPMI-1640 supplemented
with 0.5% FBS; the cells were then cultured for 24 h, according to
a previously published study (20). Once the cells reached 80%
confluence, three parallel scratches were made using a 10-μl
pipette tip; 1X PBS was used to remove the free-floating cells and
serum-free medium was added to the 6-well plate. After 24, 48 and
72 h, a microscope in bright light mode (BX51; Olympus Corporation,
Tokyo, Japan) was used to observe the distance migrated by the
cells. Wound-healing area was calculated according to the following
equation: Area = length × width. The wound-healing rate at 24, 48
and 72 h was calculated through normalization to the area at 0 h.
Triplicate wells were used for each treatment.
Statistical analysis
SPSS version 16.0 software package (SPSS, Inc.,
Chicago, IL, USA) and GraphPad Prism were used for statistical
analysis and data plotting. The experiments were repeated in
triplicate and the data are expressed as the means ± sttandard
deviation. For comparisons between two groups, a Mann-Whitney U
test was performed. When multiple comparisons were made, the
Bonferroni correction was applied. Pearson's correlation
coefficient was used for association analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
TWIST is highly expressed in cancer
specimens and is correlated with MDR1
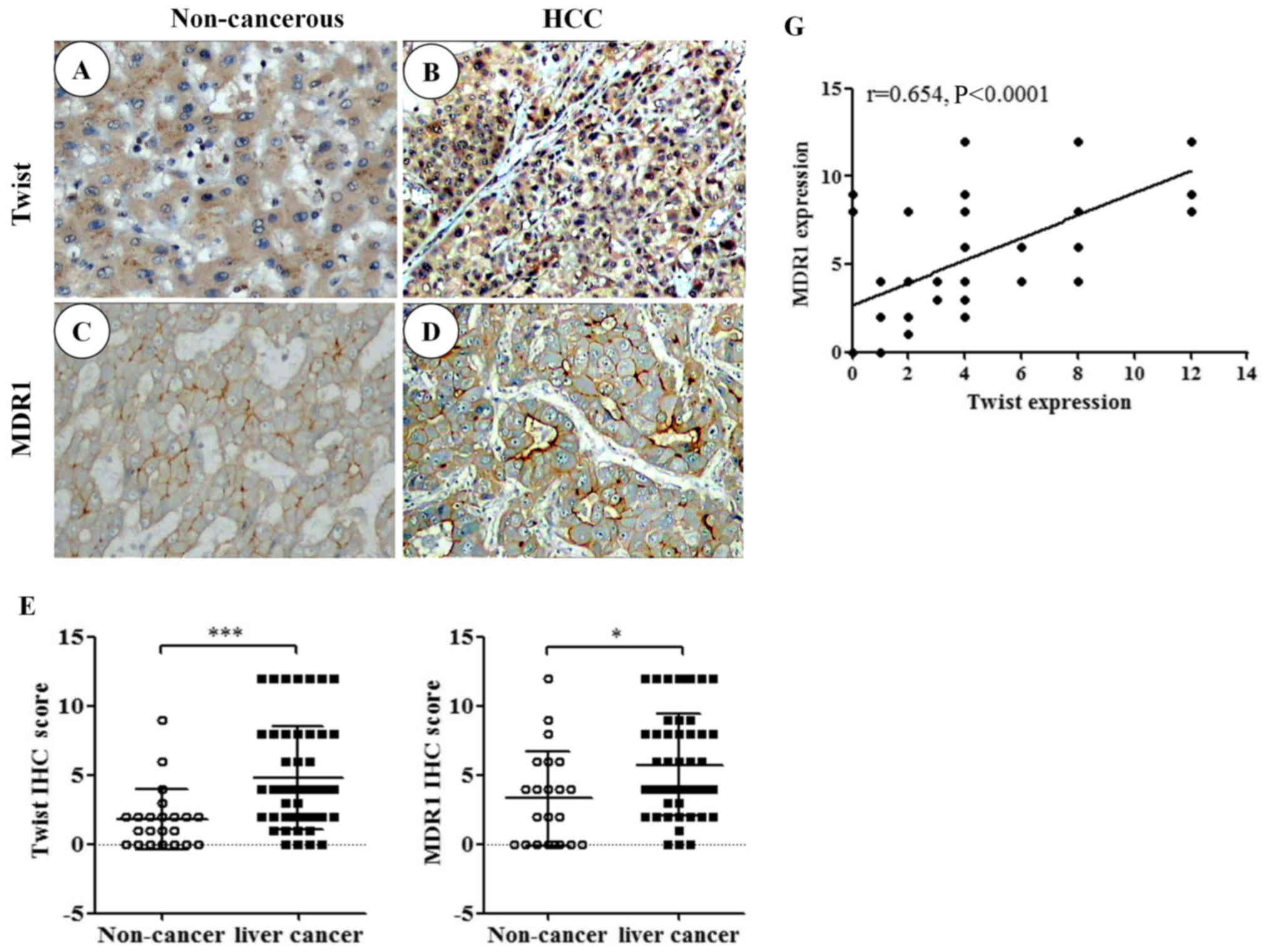
To evaluate the expression of TWIST in liver cancer,
IHC was used to examine TWIST expression in 49 specimens and 22
corresponding non-cancerous specimens. TWIST was predominantly
expressed in the nuclei, and mixed nuclear and cytoplasmic
expression was also observed in some tumor cells (Fig. 1A and B). In addition, MDR1 was
predominantly expressed in epithelial liver tumor cell membranes,
and cytoplasmic expression was also observed in some tumor cells
(Fig. 1C and D). High expression
of TWIST was detected in 17/49 cancer samples and 2/22
non-cancerous tissues (P=0.0004; Fig.
1E). High expression of MDR1 was detected in 23/49 cancer
samples and 6/22 non-cancerous tissues (P=0.01; Fig. 1F). The present study also
demonstrated that there was a positive correlation between TWIST
and MDR1 expression (Fig. 1G;
Pearson's correlation coefficient, r=0.654, P<0.0001).
Association between MDR1 and EMT markers
in cancer samples
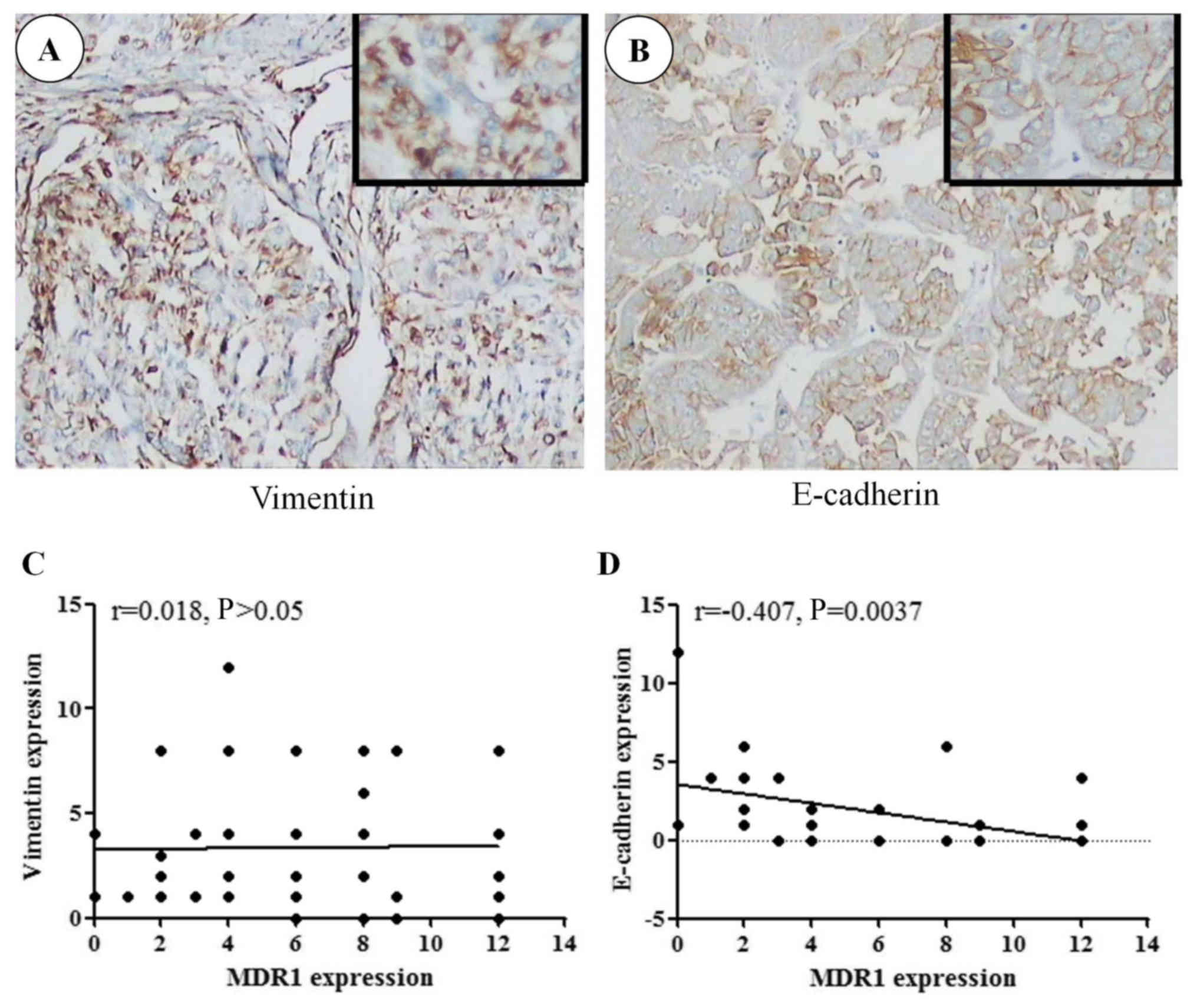
The present study aimed to analyze the effects of
MDR1 on liver cancer cell EMT transformation (Fig. 2). The association between MDR1, and
membrane E-cadherin and vimentin expression in cancer samples was
analyzed using Pearson's correlation coefficient. A negative
correlation was determined between MDR1 and membrane E-cadherin
expression (r =−0.407, P=0.004; Fig.
2B and D); however, there was no correlation between MDR1 and
vimentin (r=0.018, P>0.05; Fig. 2A
and C). These results indicated that MDR1 expression may be
associated with EMT.
R-HepG2 cells exhibit EMT
characteristics
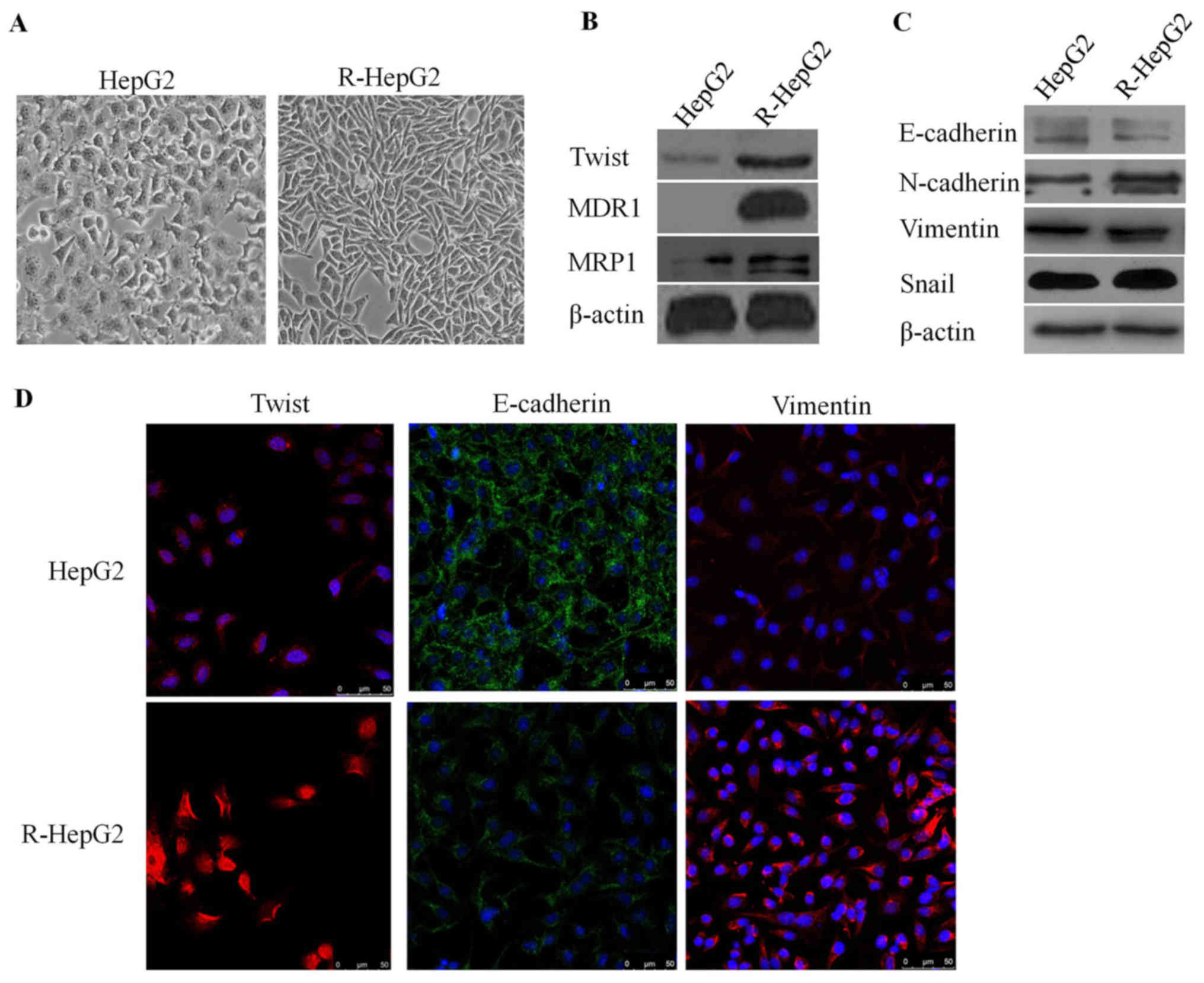
To determine whether R-HepG2 cells could obtain EMT
characteristics, R-HepG2 cells were cultured and analyzed. As
expected, R-HepG2 cells exhibited phenotypic alterations consistent
with EMT. R-HepG2 cells possessed elongated, irregular fibroblastic
morphology compared with HepG2 parental cells, which exhibited an
irregular flat morphology and few pseudopodia (Fig. 3A). Notably, the expression levels
of the epithelial molecule E-cadherin were decreased and the
expression levels of mesenchymal markers, including N-cadherin,
vimentin, TWIST and Snail were increased in R-HepG2 cells, as
determined by immunofluorescence staining and western blotting
(Fig. 3B–D). These findings
suggested that R-HepG2 cells obtained specific EMT molecular
markers.
Knockdown of TWIST decreases MDR1
expression in R-HepG2 cells
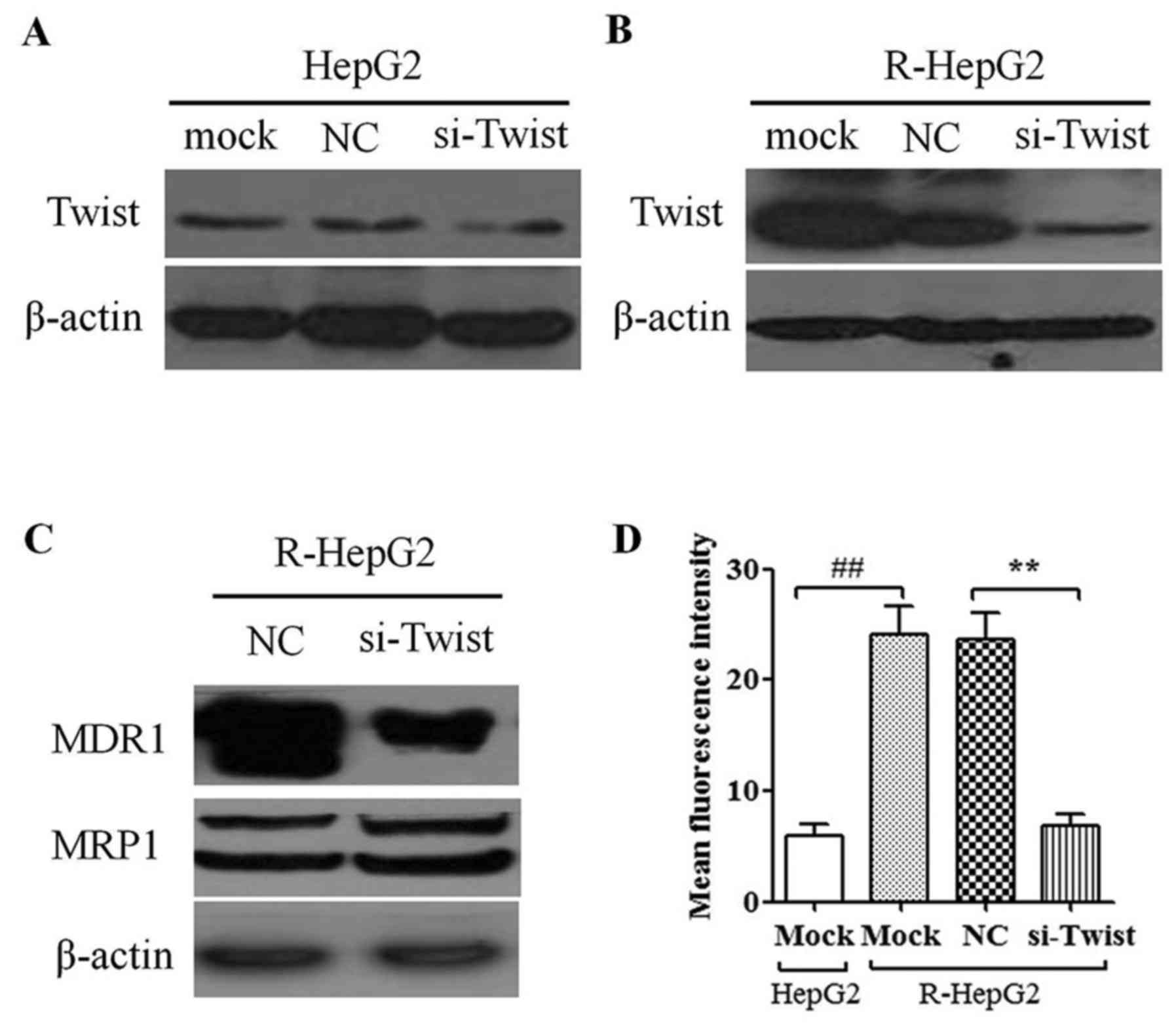
To determine the knockdown efficiency of
TWIST-specific shRNA-containing lentiviruses, si-TWIST and NC cells
were harvested (Fig. 4A and B).
The data demonstrated that the protein expression levels of TWIST
were reduced in R-HepG2 si-TWIST cells compared with in mock or NC
cells (Fig. 4B); however, no
marked effect was detected in HepG2 cells (Fig. 4A). Furthermore, knockdown of TWIST
decreased MDR1 protein expression in R-HepG2 cells; however, it had
no effect on MRP1 protein expression (Fig. 4C). In addition, luciferase assays
indicated that knockdown of TWIST significantly suppressed MDR1
promoter activity in R-HepG2 cells (Fig. 4D). These results supported the
possibility that knockdown of TWIST may suppress MDR1 expression in
cancer cells.
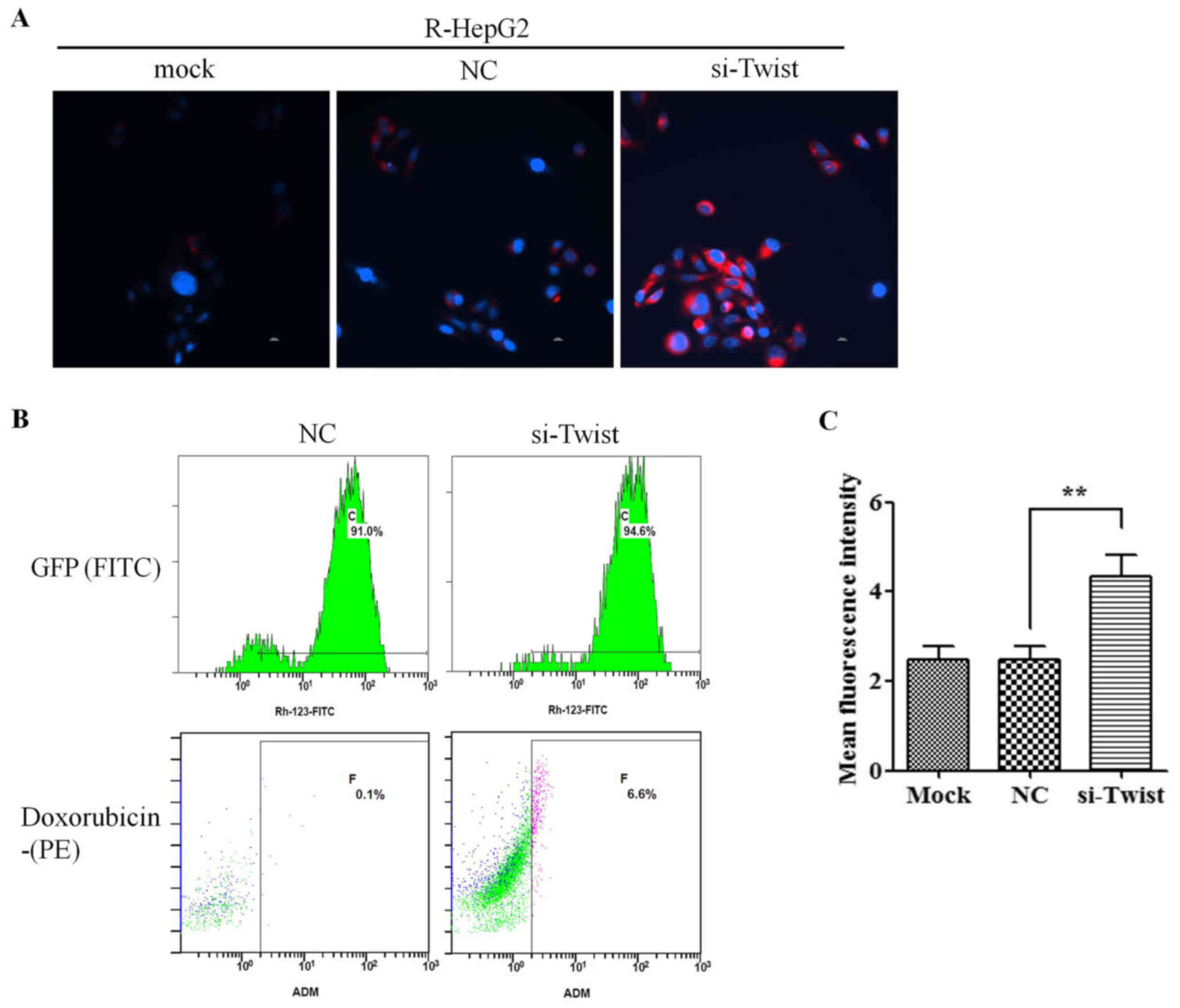
Knockdown of TWIST reduces drug efflux
from R-HepG2 cells
To evaluate the effects of TWIST on regulating MDR1
transporter activity, R-HepG2 cells were harvested and treated with
doxorubicin, which emits a natural red fluorescence. Intracellular
doxorubicin fluorescence intensity was measured by fluorescence
microscopy. Red fluorescence was stronger in si-TWIST cells
compared with in NC cells following treatment with doxorubicin,
indicating increased doxorubicin accumulation in si-TWIST cells
(Fig. 5A). Flow cytometry also
revealed that the intracellular concentration of doxorubicin was
augmented in si-TWIST cells (Fig. 5B
and C). The red MFI was significantly increased in si-TWIST
cells compared with in NC cells treated with doxorubicin for 3 h.
Taken together, these data suggested that knockdown of TWIST may
decrease drug efflux, leading to higher intracellular accumulation
of doxorubicin in R-HepG2 cells.
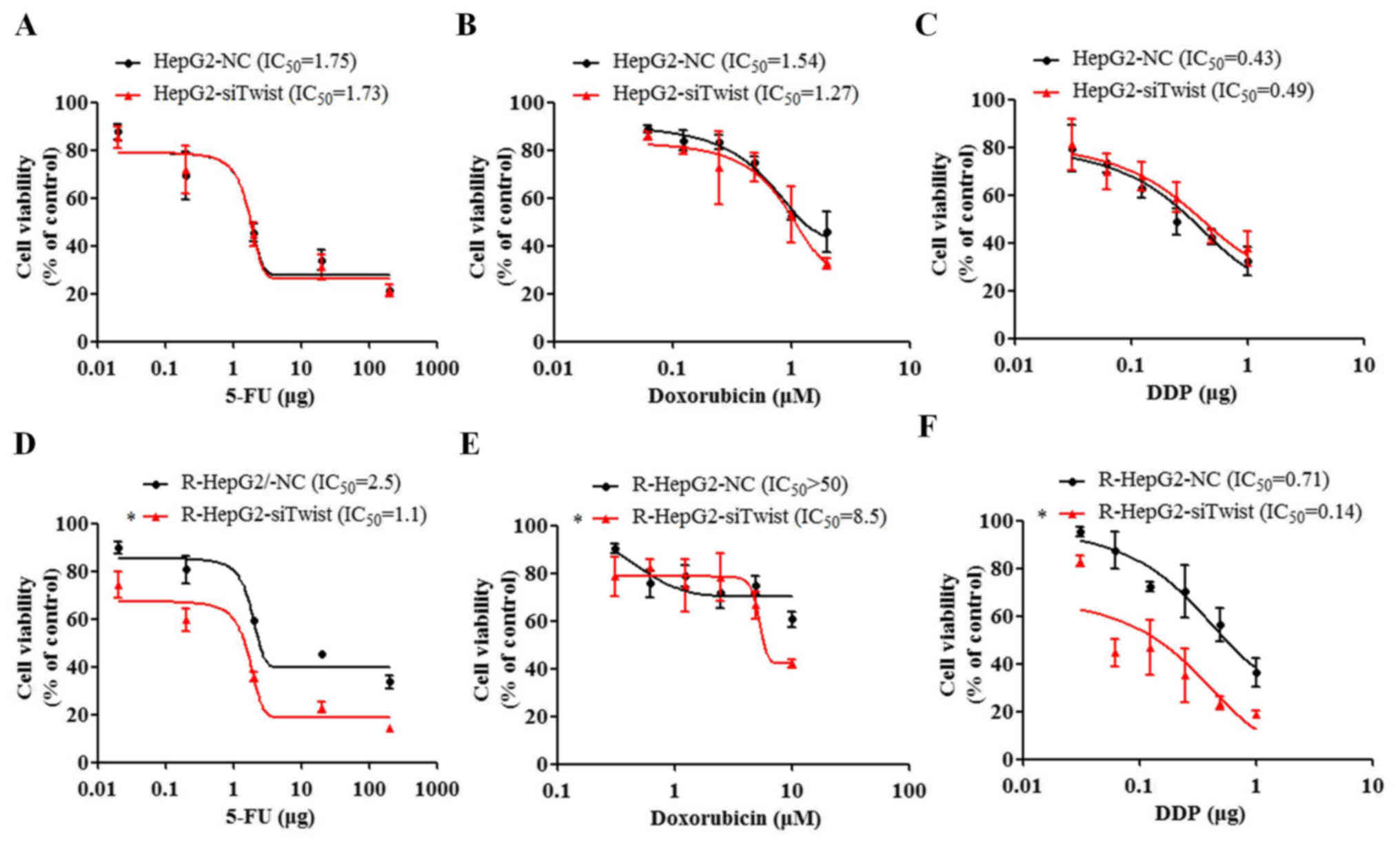
Knockdown of TWIST increases the
cytotoxicity of chemotherapeutic drugs
Knockdown of TWIST suppressed MDR1, thus indicating
that altering TWIST may increase drug sensitivity in cancer cells.
To assess this hypothesis, the cytotoxicity index of 5-FU, DDP and
doxorubicin was determined in si-TWIST cells (Fig. 6). The results demonstrated that
TWIST knockdown in R-HepG2 cells resulted in 2–10-fold increased
cytotoxicity of the chemotherapeutic drugs (5-FU, DDP and
doxorubicin) compared with in NC cells, as determined by CCK-8
assays (P<0.05, U test; Fig.
6D–F). The P-value provided refers to the comparison of the
end-point dosage between the two groups. The findings were further
confirmed by the IC50 assay; si-TWIST resulted in
decreasing 5-FU, DDP and doxorubicin IC50 values. These
data indicated that knockdown of TWIST may lead to enhanced
sensitivity of R-HepG2 cells to anticancer drugs.
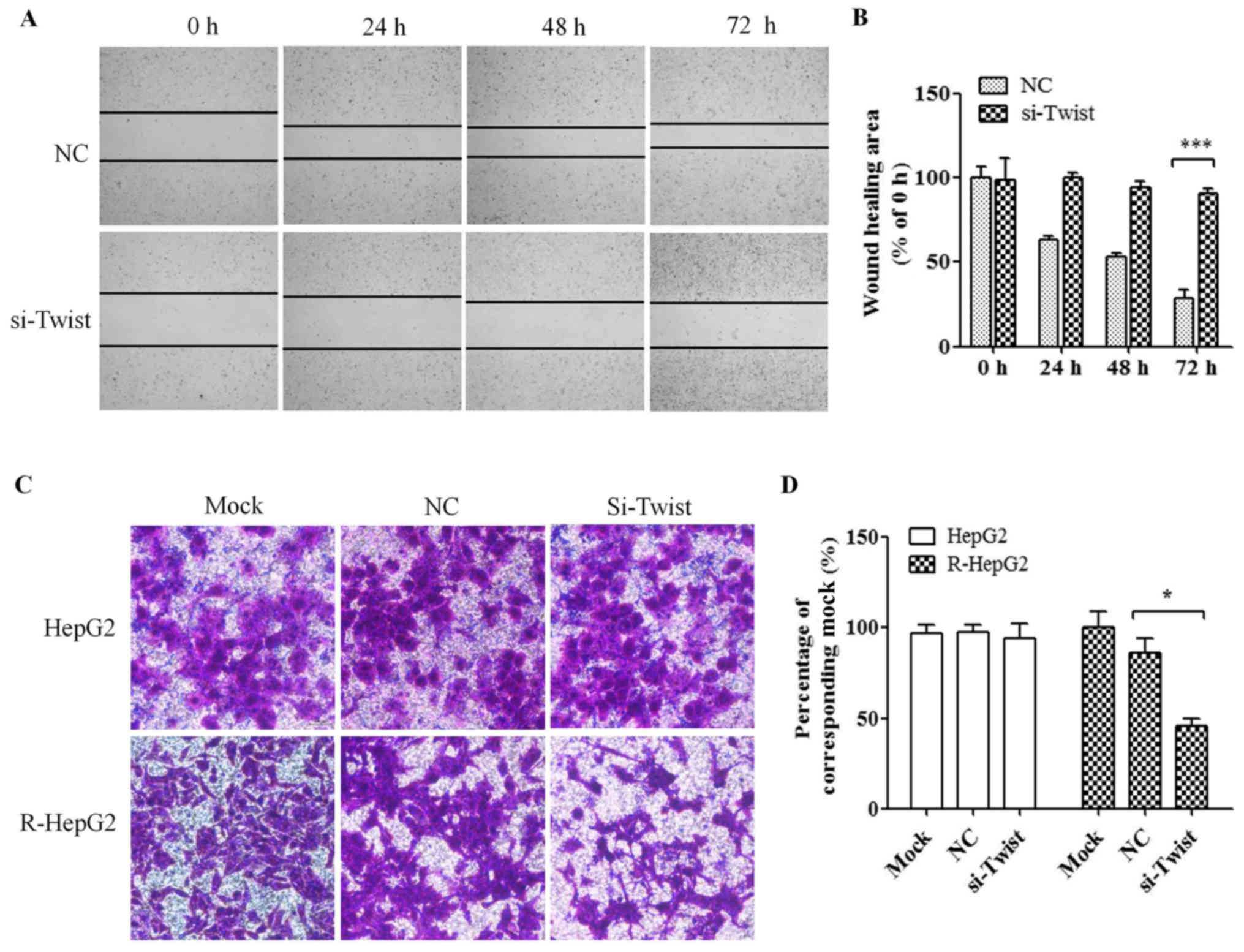
Knockdown of TWIST expression inhibits
the migratory ability of R-HepG2 cells
The wound-healing assay revealed that knockdown of
TWIST suppressed cell migration in R-HepG2 cells (U test; Fig. 7A and B). Transwell assay was used
to detect the effects of TWIST silencing on R-HepG2 cell motility.
The migration of si-TWIST cells was significantly decreased
compared with mock and NC cells (U test; Fig. 7C and D); these findings were
similar to the findings of the wound-healing assay.
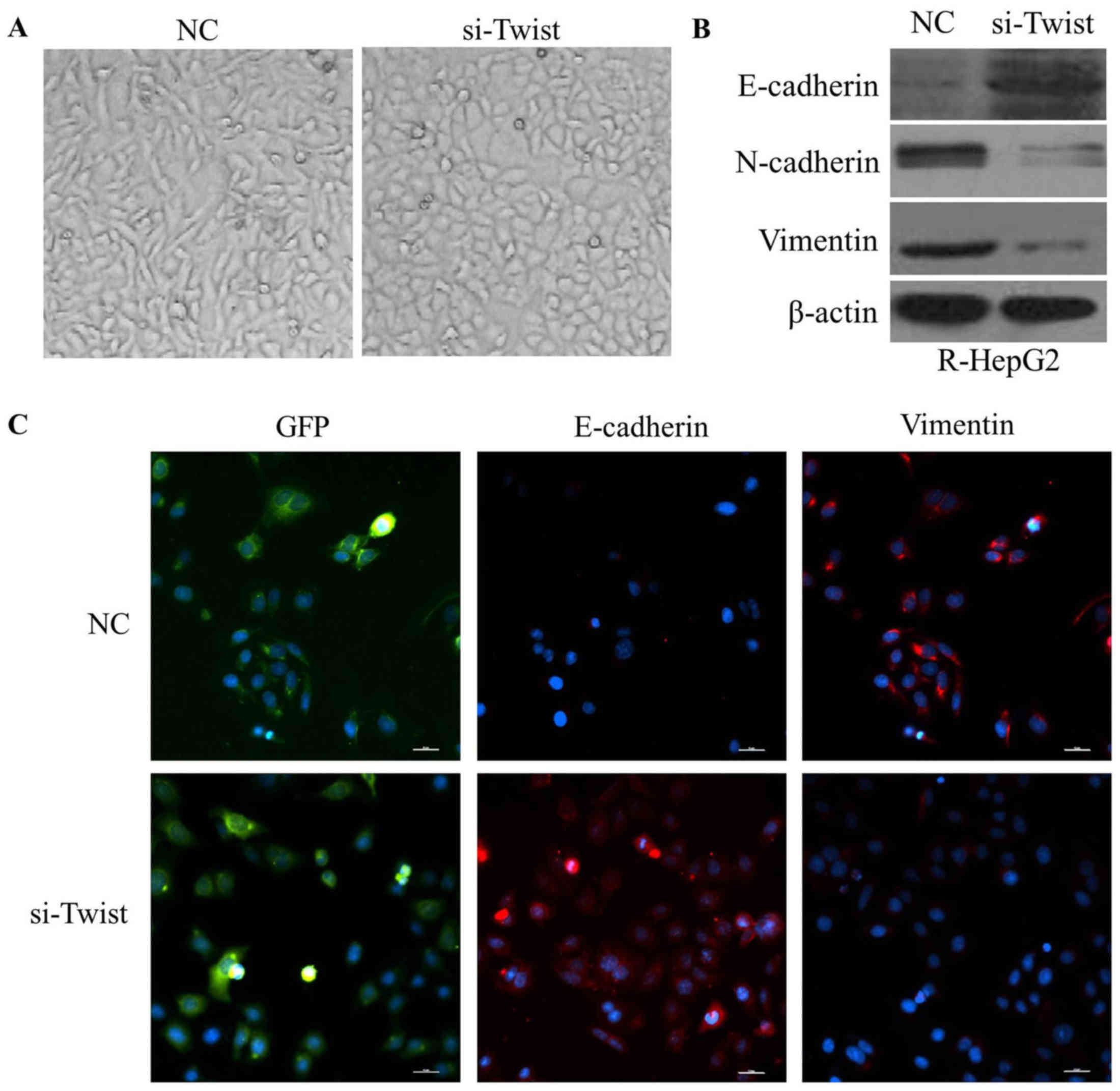
Knockdown of TWIST reverses EMT to
mesenchymal-epithelial transition (MET) in R-HepG2 cells
R-HepG2-si-TWIST cells exhibited round cell-like
phenotype (Fig. 8A). Furthermore,
the expression levels of E-cadherin were elevated, whereas vimentin
and N-cadherin were reduced in R-HepG2-si TWIST cells (Fig. 8B and C). These findings indicated
that downregulation of TWIST may reverse the EMT phenotype to a MET
phenotype.
Discussion
TWIST has been reported to promote EMT resulting in
the promotion of tumor invasion (21,22).
TWIST expression is elevated in hepatocellular carcinoma, breast
cancer, nasopharyngeal carcinoma and oral carcinoma (21,23–25).
Previous studies have revealed a novel function of TWIST, and it
has been reported to be involved in the development of acquired
chemoresistance in human cancer cells (15,16,26).
In the present study, TWIST and MDR1 were expressed to a
significantly higher level in liver tissues compared with in
non-cancerous tissues. In addition, the present study demonstrated
that there was a positive correlation between TWIST and MDR1
expression in liver cancer tissues.
Accumulating evidence has demonstrated that MDR
cancer cells are associated with the EMT process. For example,
tamoxifen-resistant MCF7/TR breast cancer cells acquire EMT
features (27). Similarly,
cisplatin-resistant cervical cancer cells possess more EMT
characteristics, and exhibit increased migratory abilities and
invasiveness (28). Furthermore,
gemcitabine-resistant pancreatic cancer cells possess EMT
characteristics (29). The present
study revealed that increased MDR1 and MRP1 expression was detected
in R-HepG2 cells alongside acquired EMT phenotypic characteristics,
reduced E-cadherin, and elevated vimentin, Snail and TWIST
expression. Due to the lack of a suitable hepatocellular
carcinoma-derived drug-resistant cell line, HepG2 cells were
selected for use in this study. Although HepG2 cells have been
identified as a hepatoblastoma cell line (17), the evaluation of its role in
drug-induced resistance is still meaningful, as drug resistance is
a prevalent phenomenon in various types of cancer. Therefore,
parental HepG2 and drug-resistant R-HepG2 cells were used in the
present study to evaluate liver cancer drug resistance. HepG2 and
drug-resistant R-HepG2 cells have been studied in numerous papers
on drug resistance (30–32). In addition, hepatoblastoma samples
are difficult to collect, as they are rare in our hospital. For the
present study, both hepatocellular carcinoma and hepatoblastoma
cases were therefore collected. The results provided evidence to
suggest that there was a negative correlation between MDR1 and
E-cadherin expression. The results indicated that a link may exist
between MDR and EMT in cancer development. Whether the expression
of MDR1 is regulated by TWIST remains to be elucidated.
Overexpression of MDR1 causes the efflux of various
hydrophobic compounds and xenobiotics, resulting in drug
resistance. The transcriptional levels of MDR1 are regulated by
numerous pathways, such as those mediated by activator protein 1,
nuclear transcription factor Y subunit α (NF-Y), GC rich-box and
p53, and even methylation and acetylation (33). In addition, MDR1 is regulated by
microRNAs (miRs), such as miR-495 and miR-127 (34,35).
Ma et al (36) revealed
that zinc fingers and homeoboxes 2 decreases NF-Y-mediated
activation of MDR1 transcription and improves the effects of
chemotherapy in hepatocellular carcinoma cells. A potential
transcriptional regulatory role of TWIST1 has been confirmed in
MDR. Zhu et al (26)
reported that knockdown of TWIST decreases MDR1 expression in Hela
cells, inhibits cell proliferation, suppresses Rhodamine 123 efflux
activity of cells and enhances cytotoxicity following treatment
with DDP. In the present study, knockdown of TWIST suppressed MDR1
expression and significantly decreased MDR1 promoter activity.
Furthermore, knockdown of TWIST reduced drug efflux, leading to
increased intracellular doxorubicin levels in R-HepG2 cells and
increased sensitivity of R-HepG2 cells to these chemotherapeutic
drugs. These findings indicated that knockdown of TWIST may enhance
the cytotoxicity of chemotherapeutic drugs in R-HepG2 cells by
inhibiting MDR1.
It has previously been demonstrated that EMT
inducers can increase migratory, invasive potential and promote MDR
by upregulating ABC transporters, which efflux chemotherapeutic
drugs (37). EMT regulators, such
as TWIST, Snail and Zeb1 transcription factors, and some signal
pathways, including transforming growth factor β, Wnt and Notch,
are known to induce EMT (38). In
agreement with those previous studies, the present results
demonstrated that knockdown of TWIST inhibited the migratory
ability of R-HepG2 cells and reversed EMT to MET in R-HepG2 cells.
In addition, the involvement of other EMT inducers in
TWIST/MDR1-mediated R-HepG2 cells will be investigated in future
studies.
Acknowledgments
The authors would like to thank Dr Kangrong Cai
(Department of Analytic Center, Guangdong Medical University) for
setting up the flow cytometer and providing software
instruction.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81441118 and
81572782), the Project of Constructing Strong Province of
Traditional Chinese Medicine by the Traditional Chinese Medicine
Bureau of Guangdong Province, China (grant no. 20152150), the
Sci-Tech Project Foundation of Zhanjiang City, China (grant nos.
2013C301015 and 2015A01040), and the Key Cultivation Project of
Guangdong Medical University, China (grant no. Z2015001).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RL and CW designed the study, conducted experiments
and performed data analysis; they also wrote the manuscript. HL
performed immunohistochemistry staining, and wrote this part of the
Materials and methods and Results. YZ carried out western blotting,
and wrote the Results section of the manuscript. CL performed the
cell cytotoxicity assay experiment. XZ and CY designed the MDR1
gene promoter reporter assay vector, performed the luciferase
reporter experiment, revised the manuscript and examined the
validity of all experiments. CY also gave final approval of the
version to be submitted and published.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of Guangdong Medical University (Zhanjiang,
China). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wada Y, Takami Y, Tateishi M, Ryu T,
Mikagi K and Saitsu H: Impact of more detailed categorization of
shrinkage or progression ratio at initial imaging response after
sorafenib treatment in advanced hepatocellular carcinoma patients.
OncoTargets Ther. 8:3193–3202. 2015. View Article : Google Scholar
|
|
2
|
Zender L, Villanueva A, Tovar V, Sia D,
Chiang DY and Llovet JM: Cancer gene discovery in hepatocellular
carcinoma. J Hepatol. 52:921–929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhangoo MS, Karnani DR, Hein PN, Giap H,
Knowles H, Issa C, Steuterman S, Pockros P and Frenette C:
Radioembolization with Yttrium-90 microspheres for patients with
unresectable hepatocellular carcinoma. J Gastrointest Oncol.
6:469–478. 2015.PubMed/NCBI
|
|
4
|
Song MJ and Bae SH: Newer treatments for
advanced hepatocellular carcinoma. Korean J Intern Med (Korean
Assoc Intern Med). 29:149–155. 2014.
|
|
5
|
Follit CA, Brewer FK, Wise JG and Vogel
PD: In silico identified targeted inhibitors of P-glycoprotein
overcome multidrug resistance in human cancer cells in culture.
Pharmacol Res Perspect. 3:e001702015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCormick JW, Vogel PD and Wise JG:
Multiple drug transport pathways through human P-glycoprotein.
Biochemistry. 54:4374–4390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Janigro D, Perju C, Fazio V, Hallene K,
Dini G, Agarwal MK and Cucullo L: Alternating current electrical
stimulation enhanced chemotherapy: A novel strategy to bypass
multidrug resistance in tumor cells. BMC Cancer. 6:722006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vesuna F, Bergman Y and Raman V: Genomic
pathways modulated by Twist in breast cancer. BMC Cancer.
17:522017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang H, Massi D, Hemmings BA, Mandalà M,
Hu Z, Wicki A and Xue G: AKT-ions with a TWIST between EMT and MET.
Oncotarget. 7:62767–62777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Gong J, Kong D and Liu HY:
Anti-proliferation effects of Twist gene silencing in gastric
cancer SGC7901 cells. World J Gastroenterol. 21:2926–2936. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nantajit D, Lin D and Li JJ: The network
of epithelial-mesen-chymal transition: Potential new targets for
tumor resistance. J Cancer Res Clin Oncol. 141:1697–1713. 2015.
View Article : Google Scholar
|
|
13
|
Jiang ZS, Sun YZ, Wang SM and Ruan JS:
Epithelial-mesenchymal transition: Potential regulator of ABC
transporters in tumor progression. J Cancer. 8:2319–2327. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng JJ, Zhang W, Xu XM, Zhang F, Tao WP,
Ye JJ and Ge W: Twist mediates an aggressive phenotype in human
colorectal cancer cells. Int J Oncol. 48:1117–1124. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YR, Liang L, Zhao JM, Zhang Y, Zhang
M, Zhong WL, Zhang Q, Wei JJ, Li M, Yuan J, et al: Twist1 confers
multidrug resistance in colon cancer through upregulation of
ATP-binding cassette transporters. Oncotarget. 8:52901–52912.
2017.PubMed/NCBI
|
|
16
|
Lu S, Yu L, Mu Y, Ma J, Tian J, Xu W and
Wang H: Role and mechanism of Twist1 in modulating the
chemosensitivity of FaDu cells. Mol Med Rep. 10:53–60. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Wu C, Xiong W, Chen C, Li R and
Zhou G: Knockdown of p54nrb inhibits migration, invasion and TNF-α
release of human acute monocytic leukemia THP1 cells. Oncol Rep.
35:3742–3748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li R, Zhao Y, Chen J, Shao S and Zhang X:
Fisetin inhibits migration, invasion and epithelial-mesenchymal
transition of LMP1-positive nasopharyngeal carcinoma cells. Mol Med
Rep. 9:413–418. 2014. View Article : Google Scholar
|
|
20
|
de Jong E, Winkel P, Poelstra K and
Prakash J: Anticancer effects of 15d-prostaglandin-J2 in wild-type
and doxorubicin-resistant ovarian cancer cells: Novel actions on
SIRT1 and HDAC. PLoS One. 6:e251922011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhuo X, Chang A, Huang C, Yang L, Xiang Z
and Zhou Y: Expression of TWIST, an inducer of
epithelial-mesenchymal transition, in nasopharyngeal carcinoma and
its clinical significance. Int J Clin Exp Pathol. 7:8862–8868.
2014.
|
|
22
|
Zhang YQ, Wei XL, Liang YK, Chen WL, Zhang
F, Bai JW, Qiu SQ, Du CW, Huang WH and Zhang GJ: Over-expressed
Twist associates with markers of epithelial mesenchymal transition
and predicts poor prognosis in breast cancers via ERK and Akt
activation. PLoS One. 10:e01358512015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chun HW and Hong R: Significance of the
hedgehog pathway-associated proteins Gli-1 and Gli-2 and the
epithelial-mesenchymal transition-associated proteins Twist and
E-cadherin in hepatocellular carcinoma. Oncol Lett. 12:1753–1762.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang L, Hou Y, Yuan J, Tang S, Zhang H,
Zhu Q, Du YE, Zhou M, Wen S, Xu L, et al: Twist promotes
reprogramming of glucose metabolism in breast cancer cells through
PI3K/AKT and p53 signaling pathways. Oncotarget. 6:25755–25769.
2015.PubMed/NCBI
|
|
25
|
Zhou Y, Zhang H, Zhuo X, Liu Y, Zhang G
and Tan Y: Over-expression of TWIST, an epithelial-mesenchymal
transition inducer, predicts poor survival in patients with oral
carcinoma. Int J Clin Exp Med. 8:9239–9247. 2015.PubMed/NCBI
|
|
26
|
Zhu K, Chen L, Han X and Wang J and Wang
J: Short hairpin RNA targeting Twist1 suppresses cell proliferation
and improves chemosensitivity to cisplatin in HeLa human cervical
cancer cells. Oncol Rep. 27:1027–1034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi XP, Miao S, Wu Y, Zhang W, Zhang XF,
Ma HZ, Xin HL, Feng J, Wen AD and Li Y: Resveratrol sensitizes
tamoxifen in antiestrogen-resistant breast cancer cells with
epithelial-mesenchymal transition features. Int J Mol Sci.
14:15655–15668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song J and Li Y: miR-25-3p reverses
epithelial-mesenchymal transition via targeting Sema4C in
cisplatin-resistance cervical cancer cells. Cancer Sci. 108:23–31.
2017. View Article : Google Scholar
|
|
29
|
Wang R, Li Y, Hou Y, Yang Q, Chen S, Wang
X, Wang Z, Yang Y, Chen C, Wang Z, et al: The
PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal
transition in gemcitabine resistance hepatoma cells. Oncotarget.
6:7000–7010. 2015.PubMed/NCBI
|
|
30
|
Ye CG, Yeung JH, Huang GL, Cui P, Wang J,
Zou Y, Zhang XN, He ZW and Cho CH: Increased glutathione and
mitogen-activated protein kinase phosphorylation are involved in
the induction of doxorubicin resistance in hepatocellular carcinoma
cells. Hepatol Res. 43:289–299. 2013. View Article : Google Scholar
|
|
31
|
Ye CG, Wu WK, Yeung JH, Li HT, Li ZJ, Wong
CC, Ren SX, Zhang L, Fung KP and Cho CH: Indomethacin and SC236
enhance the cytotoxicity of doxorubicin in human hepatocellular
carcinoma cells via inhibiting P-glycoprotein and MRP1 expression.
Cancer Lett. 304:90–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun J, Yeung CA, Co NN, Tsang TY, Yau E,
Luo K, Wu P, Wa JC, Fung KP, Kwok TT, et al: Clitocine reversal of
P-glycoprotein associated multi-drug resistance through
down-regulation of transcription factor NF-κB in R-HepG2 cell line.
PLoS One. 7:e407202012. View Article : Google Scholar
|
|
33
|
Jin S and Scotto KW: Transcriptional
regulation of the MDR1 gene by histone acetyltransferase and
deacetylase is mediated by NF-Y. Mol Cell Biol. 18:4377–4384. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zou Z, Zou R, Zong D, Shi Y, Chen J, Huang
J, Zhu J, Chen L, Bao X, Liu Y, et al: miR-495 sensitizes MDR
cancer cells to the combination of doxorubicin and taxol by
inhibiting MDR1 expression. J Cell Mol Med. 21:1929–1943. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng R and Dong L: Knockdown of
microRNA-127 reverses adriamycin resistance via cell cycle arrest
and apoptosis sensitization in adriamycin-resistant human glioma
cells. Int J Clin Exp Pathol. 8:6107–6116. 2015.PubMed/NCBI
|
|
36
|
Ma H, Yue X, Gao L, Liang X, Yan W, Zhang
Z, Shan H, Zhang H, Spear BT and Ma C: ZHX2 enhances the
cytotoxicity of chemotherapeutic drugs in liver tumor cells by
repressing MDR1 via interfering with NF-YA. Oncotarget.
6:1049–1063. 2015. View Article : Google Scholar :
|
|
37
|
Saxena M, Stephens MA, Pathak H and
Rangarajan A: Transcription factors that mediate
epithelial-mesenchymal transition lead to multidrug resistance by
upregulating ABC transporters. Cell Death Dis. 2:e1792011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chao HM, Huang HX, Chang PH, Tseng KC,
Miyajima A and Chern E: Y-box binding protein-1 promotes
hepatocellular carcinoma-initiating cell progression and
tumorigenesis via Wnt/β-catenin pathway. Oncotarget. 8:2604–2616.
2017. View Article : Google Scholar
|