Introduction
The epidermal growth factor receptor (EGFR) family
consists of four members: EGFR, human epidermal growth factor
receptor (HER)2, HER3 and HER4. Structurally, the EGFR family
consists of an extracellular ligand binding domain, a single
transmembrane-spanning region, and an intracellular region
containing the kinase domain (1).
Overexpression of the EGFR protein has been detected in 9-62% of
cases of human ovarian cancer in previous studies, and the
differences in frequencies in these studies likely reflect the use
of different antibodies and cutoffs for overexpression (2). EGFR gene amplification or protein
overexpression occurs across all epithelial ovarian cancer
histological subtypes, and increased expression of EGFR has been
associated with high tumor grade, a high cell proliferation index
and poor patient outcome (1,3). It
was shown in a previous study (4)
on human ovarian carcinoma cells and the expression of EGFR that
EGFR regulates cell adhesion proteins that may enhance cell growth
and invasiveness.
For solid tumor growth, tumor angiogenesis is
essential, and the passage of carcinoma cells through the basic
membrane and the infiltration of adjacent tissues are key stages in
the development of ovarian cancer (4). Therefore, angiogenesis is an
important process for the creation of blood and lymphatic vessels,
which sustain the growth of the tumor (5). It is known, that VEGF-R2 acts as a
receptor for VEGF-A during neo-vascularization (6).
The prognostic value of the overexpression of EGFR
has been associated with contradictory results. A poor prognosis
was reported in a previous study (3) on a population of 106 patients with
International Federation of Gynecology and Obstetrics (FIGO) stage
I-II disease, and from a study (7)
on 398 patients with FIGO stages I-IV epithelial ovarian cancer.
However, among studies that showed no prognostic effect of EGFR
status, two included a large number of patients with epithelial
ovarian cancer, including 80 patients at FIGO stage III (8) and 93 patients at FIGO stages III-IV
(9). The results from studies
concerning the prognostic value of VEGF-R2 in ovarian cancer have
also been associated with contradictory results (5,6).
In a previous study (10), VEGF-R2 status was significantly
(P=0.011) associated with type II tumors. Furthermore, recurrent
disease occurred more frequently (P=0.049) in a subgroup of
patients with VEGF-R2-negative tumors. In a survival analysis,
patients from the subgroup with VEGF-R2-positive tumors had a
significantly higher 5-year disease-free survival (DFS) rate of
90%, compared to 66% in the subgroup of patients with
VEGF-R2-negative tumors. The objective of the present study was to
investigate the prognostic value of the growth factor receptor EGFR
and the angiogenesis regulator VEGF-R2, and examine their
association with clinicopatho logical factors, recurrent disease
and DFS rates in 131 patients with epithelial ovarian cancer at
FIGO stages I-II.
Materials and methods
Study population
In the Uppsala-Örebro Medical Region during the
5-year period between 1st January 2000 and 31st December 2004, a
total of 140 consecutive patients with FIGO stage I-II epithelial
ovarian cancer, who underwent primary surgery and post-surgical
chemotherapy, were recruited to the present study. All tissue
samples were collected with the patients' informed consent and were
in compliance with the Declaration of Helsinki (11), and were used in accordance with the
Swedish Biobank Legislation and Ethical Review Act approved by the
Uppsala Ethical Review Board (Uppsala, Sweden; decision ref.
UPS-03-477). Of the 140 patients, a total of 131 patients who
agreed to participate in the study were included. There were 131
available tumors for the analysis of EGFR and there were 130
available tumors for the analysis of VEGF-R2.
The primary surgery was performed at nine surgical
gynecological departments. The staging procedure was performed at
the time of primary surgery. According to the European Organization
for the Research and Treatment of Cancer surgical staging (12), modified surgical staging was
undertaken in 34 (26%) of the 131 cases and, according to the same
guidelines, surgical staging was regarded as minimal or inadequate
in the remaining 77 (74%) patients.
The characteristics of the patients are summarized
in Table I, including the age,
body mass index (BMI), performance status of the patients (World
Health Organization), FIGO stage, serous/non-serous histology and
type of ovarian tumor (type I and type II). All patients had
post-surgical chemotherapy 4-6 weeks following primary surgery,
most commonly with paclitaxel (175 mg/m2) and
carboplatin (AUC=5) at 3-week intervals, usually for four courses
(n=105), or single-drug carboplatin for four to six courses (n=26).
The mean follow-up time was 65 months (range 5-110 months). The
definition of survival was taken as the date of confirmed
histological diagnosis following primary surgery to the date of
recurrence, the patient succumbing to mortality, or their final
visit.
 | Table IPatient characteristics.. |
Table I
Patient characteristics..
| Characteristic | n (%) |
|---|
| Median age
(years) | 59.0 (range
25-84) |
| BMI | |
| BMI ≤25 | 69 (53.9) |
| BMI >25 | 59 (46.1) |
| WHO performance
status | |
| 0 | 37 (28.2) |
| 1 | 66 (50.4) |
| 2 | 21 (16.0) |
| 3 | 6 (4.6) |
| FIGO stage | |
| IA | 39 (29.7) |
| IB | 6 (4.6) |
| IC | 66 (50.4) |
| II | 20 (15.3) |
|
Histopathologya | |
| Serous ovarian
tumors | 51 (39.2) |
| Non-serous ovarian
tumors | 78 (60.8) |
| Mucinous | 20 (25.6) |
| Endometrioid | 42 (53.8) |
| Clear cell | 16 (20.5) |
| Types of ovarian
tumorsb | |
| Type I tumors | 79 (65.8) |
| Low-grade (G1)
serous | 14 |
| Mucinous
(G1+G2+G3) | 20 |
| Low-grade
endometrioid (G1+G2) | 29 |
| Clear cell | 16 |
| Type II tumors | 52 (34.2) |
| High-grade (G2+G3)
serous | 37 |
| High-grade (G3)
endometrioid | 13 |
| Anaplastic | 2 |
Sampling and tissue microarray
construction of ovarian cancer tissue
Paraffin-embedded tumor tissue from primary surgery
was used. Following staining with hematoxylin and eosin, the tumors
were classified and graded by a single pathologist. The tissue
microarrays were constructed as described previously (13). Briefly, tumor tissues were embedded
in paraffin and 5-µm sections stained with hematoxylin and
eosin were obtained to select representative areas for biopsies.
The core tissue biopsy specimens (diameter, 0.6 mm) were obtained
from these regions of individual donor paraffin blocks and
precisely arrayed into a new recipient paraffin block using a
custom built instrument. Two tissue core specimens (diameter, 0.6
mm) from all 131 ovarian carcinomas were arranged in three
recipient paraffin blocks. A single pathologist (T.S.) verified all
hematoxylin and eosin-stained sections and the presence of tumor
tissue on the arrayed samples using a Nikon Eclipse Ni microscope
(Nikon Corporation, Tokyo, Japan). The tissue microarray
construction was performed at the Department of Pathology,
University Hospital MAS (Malmö, Sweden).
Immunohistochemistry (IHC) and
interpretation
From each multi-tissue block, 5-µm-thick
sections were cut and placed on coated slides, and dried overnight
at 37°C. The sections were pre-treated by heat-induced epitope
retrieval in target retrieval solution (Dako, Glostrup, Denmark; pH
6.0), or EDTA buffer (pH 9.0), for 7+7 min in a microwave oven
(99°C). Blocking with peroxidase was performed for 5 min. The
slides were counterstained for 2 min with hematoxylin. The
following monoclonal primary antibodies were, used: For EGFR, the
monoclonal mouse primary antibody EGFR 113 (dilution 1:40)
(Novocastra; Leica Biosystems GmbH, Nussloch, Germany) was used,
and for VEGF-R2, the polyclonal mouse antibody Flk-1 (dilution
1:40) was used (Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
as described in a previous study (10). Using the REAL Envision detection
system (Dako), the immunos-tainings were performed in an
Autostainer automated machine (Dako). The IHC analyses and
interpretation were performed at the Department of Pathology,
Halmstad Medical Central Hospital (Halmstad, Sweden). The IHC
staining was interpreted by I.S. and T.S. No information was
available on the specific diagnosis or prognosis of the individual
cases at the time of evaluation. Of the 131 tumor samples, staining
was successful in 131 tumor samples for EGFR, and in 130 available
tumor samples for VEGF-R2. A semi-quantitative analysis (14) was performed and the staining was
graded as negative, +, ++, and +++ for EGFR and VEGF-R2, and both
of the markers were dichotomized into negative and positive (+,++
and +++) cases (15). Positive
staining for EGFR was characterized by distinct staining of the
cytoplasmic membrane, whereas staining for VEGF-R2 was confined to
the cytoplasm and the membrane of the tumor cells.
Statistical analysis
Pearson's χ2 test was used to assess
proportional differences in univariate analyses. The survival
curves were generated using the Kaplan-Meier technique and
differences between these curves were, tested with the log rank
test or χ2 test. The logistic regression model was, used
for bivariate and multivariate analyses, with recurrent disease as
the endpoint. Furthermore, the univariate and multivariate Cox
regression model was, used, with DFS as the endpoint. All tests
were two-sided, and P≤0.05 was, considered to indicate a
statistically significant difference. The STATISTICA 13.2
(StatSoft, Inc., Tulsa, OK, USA) statistical package was, used for
analyses.
Results
Background characteristics
The Patients' characteristics are, presented in
Table I. The study population was
divided into 79 type I tumors (65.8%) and 52 type II tumors
(34.2%). The majority of the patients (84.3%) had stage I disease
and the majority (66%) of the tumors were classified as type I
tumors. A primary cure was, achieved in all 131 patients. The total
number of recurrences in the complete cohort was 34/131 (26%), and
22 of these patients (67%) succumbed to the disease. Recurrent
disease was significantly associated with FIGO grade (P=0.030),
FIGO sub stage (P=0.0005), adequate surgical staging (P=0.033) and
residual disease (P=0.001). In the entire cohort, the 5-year DFS
rate was 68%, the disease-specific survival rate was 76%, and the
overall survival rate was 71%. The protein expression status
(positive/negative) of the growth factor receptor EGFR and the
angiogenesis regulator VEGF-R2 (Table
II) was, compared in addition to specific clinical and
pathological factors. However, no correlation between the protein
expression of EGFR and VEGF-R2 was detected (P=0.164).
 | Table IIStatus of protein expression of EGFR
and VEGF-R2 in tumors, vs. clinical and pathological features
(n=131). |
Table II
Status of protein expression of EGFR
and VEGF-R2 in tumors, vs. clinical and pathological features
(n=131).
| Feature | EGFR, n
(%)+ | |
EGFR− |
VEGF-R2+ | |
VEGF-R2− |
|---|
| Number | 31 (24) | | 100 (77) | 100 (77) | | 30 (23) |
| Age (mean,
years) | 61 | | 57 | 59 | | 58 |
| P-value
(t-test) | | 0.140 | | | 0.620 | |
|
Histopathologya | | | | | | |
| Serous | 10 (32) | | 41 (42) | 41 (42) | | 9 (30) |
| Non-serous | 21 (68) | | 57 (58) | 57 (58) | | 21 (70) |
| P-value
(χ2) | | 0.342 | | | 0.245 | |
| Tumor grade | | | | | | |
| G1+G2 | 24 (77) | | 51 (51) | 56 (56) | | 19 (63) |
| G3 | 7 (23) | | 49 (49) | 44 (44) | | 11 (37) |
| P-value
(χ2) | | 0.009 | | | 0.476 | |
| Type of tumor | | | | | | |
| Type I | 23 (74) | | 56 (56) | 54 (54) | | 24 (80) |
| T ype II | 8 (26) | | 44 (44) | 46 (46) | | 6 (20) |
| P-value
(χ2) | | 0.070 | | | 0.011 | |
| FIGO stage | | | | | | |
| IA-IB | 14 (45) | | 31 (31) | 34 (34) | | 11 (37) |
| IC | 15 (48) | | 51 (51) | 49 (49) | | 16 (53) |
| II | 2 (7) | | 18 (18) | 17 (17) | | 3 (10) |
| P-value
(χ2) | | 0.175 | | | 0.647 | |
| Recurrent
disease | | | | | | |
| Without | 26 (84) | | 71 (71) | 78 (78) | | 18 (60) |
| With | 5 (16) | | 29 (29) | 22 (22) | | 12 (40) |
| P-value
(χ2) | | 0.153 | | | 0.049 | |
EGFR status
Positive expression of EGFR was identified as
distinct staining of the cytoplasmic membrane, and positive
expression of EGFR was observed in 31 (24%) of the 131 available
tumors. There were no significant differences in mean age between
the groups of patients with EGFR-positive and EGFR-negative tumors
(61 years, vs. 57 years; P=0.140) across the cohort of patients.
The EGFR-status (Table II) was
not-associated with serous/non-serous tumors, FIGO stage or
recurrent disease. By contrast, the EGFR status was associated with
tumor grade. The EGFR-positive tumors were predominantly of a lower
grade (G1+G2), compared with the EGFR-negative tumors, which were
more frequently of a high grade (G3). All 16 tumors with clear cell
histology were classified as high grade (G3) tumors. Furthermore, a
trend was observed (P=0.070), that EGFR-positive tumors more were
frequently type I tumors. EGFR status was not significantly
associated with BMI (dichotomized; P=0.478).
VEGF-R2 status
Positive expression of VEGF-R2 was confined to the
membrane and cytoplasm, and positive expression of VEGF-R2 was
observed in 100 (77%) of the available 130 tumors, as shown in
Table II. Furthermore, the
association between VEGF-R2 status in tumors, and clinical and
pathological features, was, presented in an earlier study (10).
EGFR VEGF-R2 status
As presented in Table
III, the differences in clinical and pathological variables
with concomitant EGFR and VEGF-R2 status in four subgroups were
limited to tumor grade (G1+G2 / G3) and type (I/II). The
EGFR-positive tumors were predominantly of a lower grade (G1+G2)
with/without concomitant VEGF-R2-positive expression. Furthermore,
VEGF-R2-positive tumors with concomitant EGFR-positive or
EGFR-negative expression were usually type II tumors. There were
different outcomes in the four subgroups of patients (n=130) in
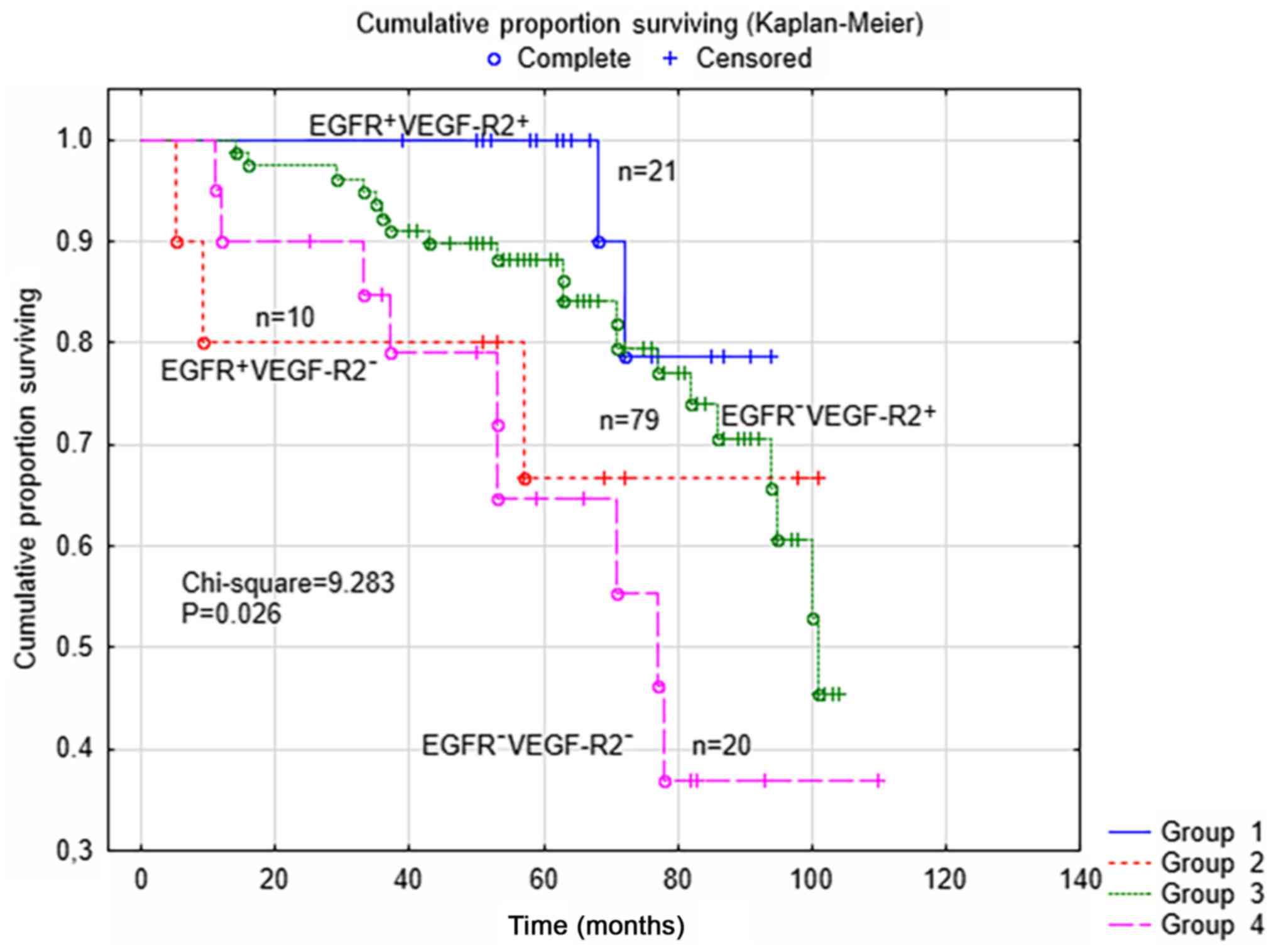
terms of the EGFR and VEGF-R2 status of the tumors, as shown in
Fig. 1. In the survival analysis
(P=0.026; χ2=9.283), it was shown that the patients
(n=21) in the subgroup with concomitant positive expression of EGFR
and VEGF-R2 had 5-year DFS rates of 100%. However, no differences
between the four subgroups were found according to different
surgical staging (P=0.640) or the type of post-surgical treatment,
(paclitaxel and carboplatin, vs. single drug carboplatin;
P=0.198).
 | Table IIIStatus of protein expression in
tumors of concomitant EGFR and VEGF-R2, vs. clinical and
pathological features (n=130). |
Table III
Status of protein expression in
tumors of concomitant EGFR and VEGF-R2, vs. clinical and
pathological features (n=130).
| Feature |
EGFR+/VEGF-R2+ n
(%) |
EGFR+/VEGF-R2− n
(%) | |
EGFR−/VEGF-R2− n
(%) |
EGFR−/VEGF-R2− n
(%) |
|---|
| Number | 21 (21) | 10 (7) | | 79 (61) | 20 (15) |
|
Histopathologya | | | | | |
| Serous | 6 (29) | 4 (40) | | 35 (45) | 5 (25) |
| Non-serous | 15 (71) | 6 (60) | | 42 (55) | 15 (75) |
| P-value
(χ2) | | | 0.266 | | |
| Tumor grade | | | | | |
| G1+G2 | 15 (71) | 9 (90) | | 40 (51) | 10 (50) |
| G3 | 6 (29) | 1 (10) | | 39 (49) | 10 (50) |
| P-value
(χ2) | | | 0.047 | | |
| Type of tumors | | | | | |
| Type I | 15 (71) | 8 (80) | | 39 (49) | 16 (80) |
| Type II | 6 (29) | 2 (20) | | 40 (51) | 4 (20) |
| P-value
(χ2) | | | 0.019 | | |
| FIGO stage | | | | | |
| IA-IB | 10 (48) | 4 (40) | | 24 (30) | 7 (35) |
| IC | 9 (43) | 6 (60) | | 40 (51) | 10 (50) |
| II | 2 (9) | 0 (00) | | 15 (19) | 3 (15) |
| P-value
(χ2) | | | 0.413 | | |
| Recurrent
disease | | | | | |
| Without | 19 (90) | 7 (70) | | 59 (75) | 11 (55) |
| With | 2 (10) | 3 (30) | | 20 (25) | 9 (45) |
| P-value
(χ2) | | | 0.079 | | |
Serous tumors
For the serous tumors (n=51) the EGFR status of the
tumors was associated with tumor grade (P=0.018); 9/10 (90%) of
serous tumors with EGFR-positive expression were of low grade
(G1+G2) compared with 20/41 (49%) of the tumors with EGFR-negative
expression. The VEGF-R2 status of the tumors was not associated
with any of the variables shown in Table II, nor with BMI
(dichotomized).
Patients who had EGFR-positive tumors of type I
(n=79) were older (62 vs. 55-years; P=0.039) than the patients with
EGFR-negative tumors. For patients with type I tumors (serous low
grade), recurrent disease was significantly associated (P=0.008)
with VEGF-R2-negative expression, however, no further differences
in clinical or pathological features were detected for the type of
tumor, according to VEGF-R2 status, in the present study.
Non-serous tumors
For the non-serous tumors (n=78) the EGFR status of
the tumors was associated with age; patients with EGFR-positive
tumors were older (62 vs. 55-years; P=0.039) than patients with
EGFR-negative tumors. The EGFR status of the tumors was not
associated with BMI (dichotomized; P= 0.641).
The EGFR status of non-serous tumors was associated
with recurrent disease (P=0.027), as shown in Table IV. Only one (5%) patient had
recurrent disease in the subgroup of 21 patients with EGFR-positive
tumors, compared with 16 (28%) of the 57 patients with
EGFR-negative tumors. Furthermore, the VEGF-R2 status of the
non-serous tumors (Table IV) was
associated with the type of tumor (P=0.016) and with recurrent
disease (P=0.034); only nine (16%) patients had recurrent disease
in the subgroup of 57 patients with VEGF-R2-positive tumors,
compared with 8/21 (38%) patients with VEGF-R2-negative tumors. In
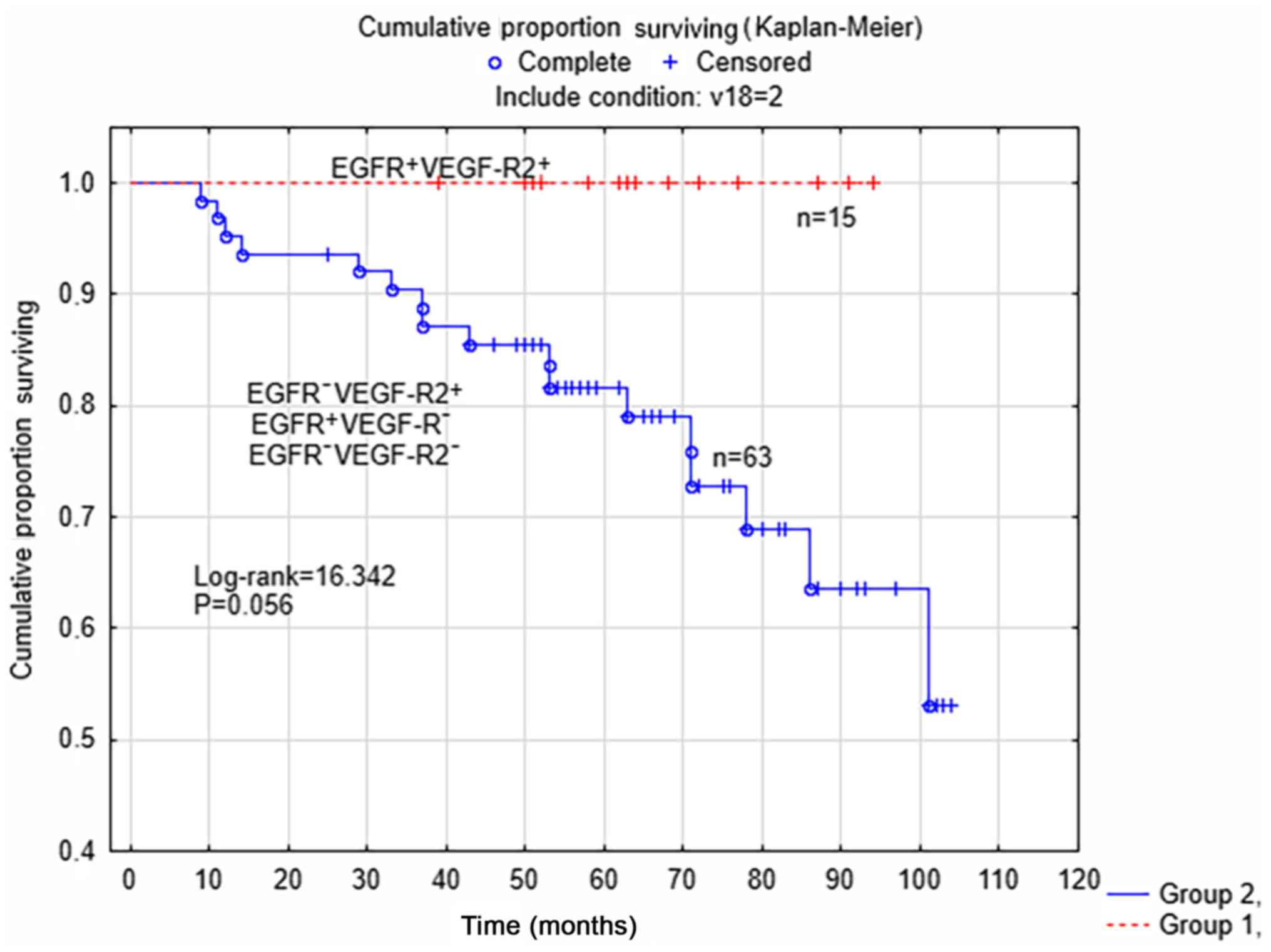
the survival analysis limited to patients with non-serous tumors
(Fig. 2), there was a trend for
improved survival rate (P=0.056; log rank=16.342) in the subgroup
of patients with tumors with concomitant positive expression of
EGFR and VEGF-R2 (n=15), compared with the other three subgroups
(n=63). There were no differences between the two subgroups of
patients according to surgical staging (P=0.471) or post-surgical
treatment (P=0.228).
 | Table IVStatus of protein expression in
tumors of EGFR and VEGF-R2 vs. clinical and pathological features
in non-serous tumors (n=78). |
Table IV
Status of protein expression in
tumors of EGFR and VEGF-R2 vs. clinical and pathological features
in non-serous tumors (n=78).
| Feature | EGFR+ n
(%) | EGFR− n
(%) | VEGF-R2+
n (%) | VEGF-R2−
n (%) |
|---|
| Number | 21 (27) | 57 (73) | 57 (73) | 21 (27) |
| Histopathology | | | | |
| Mucinous | 4 (19) | 16 (28) | 13 (23) | 7 (33) |
| Endometroid | 13 (62) | 29 (51) | 34 (60) | 8 (38) |
| Clear cell | 4 (19) | 12 (21) | 10 (17) | 6 (29) |
| P-value
(χ2) | 0.649 | | 0.234 | |
| Tumor grade | | | | |
| G1+G2 | 5 (24) | 12 (21) | 13 (23) | 4 (19) |
| G3 | 16 (76) | 45 (79) | 44 (77) | 17 (81) |
| P-value
(χ2) | 0.739 | | 0.721 | |
| Type of tumor | | | | |
| Type I | 19 (90) | 46 (81) | 44 (77) | 21 (100) |
| Type II | 2 (10) | 11 (19) | 13 (23) | 0 (00) |
| P-value
(χ2) | 0.304 | | 0.016 | |
| FIGO stage | | | | |
| IA-IB | 10 (48) | 20 (35) | 21 (37) | 9 (43) |
| IC | 9 (43) | 26 (46) | 25 (44) | 10 (48) |
| II | 2 (9) | 11 (19) | 11 (19) | 2 (9) |
| P-value
(χ2) | 0.464 | | 0.584 | |
| Recurrent
disease | | | | |
| Without | 20 (95) | 41 (72) | 48 (84) | 13 (62) |
| With | 1 (5) | 16 (28) | 9 (16) | 8 (38) |
| P-value
(χ2) | 0.027 | | 0.034 | |
Histological subtypes of non-serous
tumors
In a separate univariate analysis limited to tumors
with endometrioid histology (n=42), it was found that recurrent
disease was associated with EGFR-negative expression of tumors
(P=0.023). Among the 42 patients, recurrent disease was present in
9/29 (31%) patients with EGFR-negative tumors, whereas none of the
13 patients with EGFR-positive endometrioid tumors had recurrent
disease.
Multivariate analysis
The results for univariate and multivariate Cox
analysis with DFS as the endpoint, and logistic regression with
recurrent disease as the endpoint, across the entire cohort of
patients are, shown in s V and VI, respectively. In the first
analysis (Table V), FIGO stage and
VEGF-R2 status were significant and independent prognostic factors
for DFS. With recurrent disease as the endpoint in a multivariate
logistic regression analysis, FIGO stage, type of tumor (I/II) and
VEGF-R2 status were all independent predictive factors (Table VI).
 | Table VCox analysis (univariate and
multivariate) with disease-free survival as endpoint (n=130
patients). |
Table V
Cox analysis (univariate and
multivariate) with disease-free survival as endpoint (n=130
patients).
| Variable | Univariate analysis
| Multivariate
analysis
| P-value |
|---|
| HR | 95% CI | HR | 95% CI |
|---|
| Age | 1.016 | 0.986-1.046 | 1.014 | 0.983-1.046 | 0.360 |
| Stage (I/II) | 3.318 | 1.655-6.654 | 3.602 | 1.753-7.403 | <0.005 |
| Type (I/II) | 1.908 | 0.969-3.758 | 1.799 | 0.899-3.597 | 0.096 |
|
EGFRa | 0.641 | 0.247-1.663 | 0.643 | 0.242-1.709 | 0.376 |
|
VEGF-R2b | 0.436 | 0.215-0.883 | 0.322 | 0.153-0.153 | 0.003 |
 | Table VIPredictive factors for recurrent
disease via univariate and multivariate logistic regression
analysis (n=130). |
Table VI
Predictive factors for recurrent
disease via univariate and multivariate logistic regression
analysis (n=130).
| Variable | Univariate analysis
| Multivariate
analysis
| P value |
|---|
| HR | 95% CI | HR | 95% CI |
|---|
| Age | 1.013 | 0.981-1.047 | 1.020 | 0.982-1.061 | 0.289 |
| Stage (I/II) | 7.959 | 2.801-22.617 | 9.750 | 3.056-31.104 | <0.001 |
| Type (I/II) | 2.456 | 1.099-5.490 | 2.994 | 1.109-8.804 | 0.028 |
| EGFRa | 0.470 | 0.163-1.358 | 0.484 | 0.144-1.630 | 0.237 |
| VEGF-R2b | 0.423 | 0.175-1.018 | 0.175 | 0.057-0.537 | 0.002 |
In the univariate analysis, the results for age and
FIGO stage (I/II) listed in Table
VI are, also presented in Table
V of a previous study (10),
as the same study population was included in both. For the subgroup
of patients with non-serous tumors (n=78), the same analyses were
performed and the results are shown in Tables VII and VIII. In the multivariate Cox analysis
DFS as the endpoint, only FIGO stage and type (I/II) of tumor were
significant and independent prognostic factors for DFS. However, in
the logistic regression with recurrent disease as the endpoint
(Table VIII), FIGO stage, type
(I/II) of tumor, EGFR status and VEGF-R2 status were all
significant and independent predictive factors for recurrent
disease.
 | Table VIICox analysis (univariate and
multivariate) with disease-free survival as endpoint for patients
with non-serous tumors (n=78). |
Table VII
Cox analysis (univariate and
multivariate) with disease-free survival as endpoint for patients
with non-serous tumors (n=78).
| Variable | Univariate analysis
| Multivariate
analysis
| P value |
|---|
| HR | 95% CI | HR | 95% CI |
|---|
| Age | 1.016 | 0.977-1.056 | 1.023 | 0.977-1.072 | 0.317 |
| Stage (I/II) | 4.315 | 1.654-11.254 | 4.749 | 1.729-13.041 | 0.002 |
| Type (I/II) | 2.732 | 1.005-7.427 | 6.516 | 1.584-26.800 | 0.009 |
|
EGFRa | 0.205 | 0.027-1.558 | 0.239 | 0.031-1.840 | 0.169 |
|
VEGF-R2b | 0.244 | 0.090-0.662 | 0.076 | 0.018-0.319 | 0.319 |
 | Table VIIIPredictive factors for recurrent
disease (univariate and multivariate logistic regression analysis)
for patients with non-serous tumors (n=78). |
Table VIII
Predictive factors for recurrent
disease (univariate and multivariate logistic regression analysis)
for patients with non-serous tumors (n=78).
| Variable | Univariate analysis
| Multivariate
analysis
| P-value |
|---|
| HR | 95% CI | HR | 95% CI |
|---|
| Age | 1.015 | 0.971-1.060 | 1.063 | 0.992-1.139 | 0.075 |
| Stage (I/II) | 10.133 | 2.649-38.759 | 75.965 | 4.212-1369.81 | 0.003 |
| Type (I/II) | 3.682 | 1.043-12.995 | 43.836 | 2.088-919.998 | 0.013 |
| EGFRa | 0.131 | 0.016-1.096 | 0.050 | 0.003-0.766 | 0.028 |
| VEGF-R2b | 0.298 | 0.094-0.942 | 0.008 | 0.0002-0.229 | 0.004 |
Discussion
In the present study, the EGFR status alone was
associated with tumor grade across the cohort of 131 patients, all
in FIGO-stage I-II epithelial ovarian cancer, but not with any
other clinical or pathological feature or with survival rate.
However, recurrent disease was associated with EGFR-negative tumors
in the subgroup of patients with non-serous tumors (n=78), and EGFR
status was a significant and independent predictive factor for
recurrent disease in a multivariate logistic regression analysis
for non-serous tumors. The VEGF-R2 status was associated with tumor
type and recurrent disease; positive staining for VEGF-R2 was, more
frequently detected in type II tumors in the entire cohort and in
the subgroup of non-serous tumors. The VEGF-R2 status was,
according to a multivariate analysis, a prognostic factor for DFS
rate in the whole cohort of patients, and also an independent
predictive factor for recurrent disease in the whole cohort and the
subgroup with non-serous tumors. There were different outcomes in
the four subgroups of patients following analysis of the
concomitant EGFR and VEGF-R2 status of tumors and in survival
analysis; the subgroup of patients (n=21) with tumors exhibiting
concomitant positive expression of EGFR and VEGF-R2 had 5-year DFS
rates of 100% across the whole cohort and in survival analysis
limited to patients with non-serous tumors (n=78) in the subgroup
of patients with tumors with concomitant positive expression of
EGFR and VEGF-R2, compared with the other three subgroups. There
were no differences between the subgroups of patients in the entire
cohort nor those with non-serous tumors, according to staging at
primary surgery or post-surgical treatment. Therefore, the
different outcomes between the subgroups with respect to EGFR
status, VEGF-R2 status and concomitant EGFR and VEGF-R2 status,
were most likely explained by their own biological properties.
The EGFR staining was characterized by distinct
staining of the cytoplasmic membrane, and positive expression of
EGFR was detected in 31/131 (24%) tumors in the present study. The
differences in frequencies of 9–62% for positive staining of the
EGFR protein in previous studies of human ovarian cancer may
reflect the use of different antibodies and cutoffs for
overexpression (2). In a previous
study on ovarian cancer, positive staining for EGFR was detected in
37/106 (34.9%) patients at FIGO stages I-II, and multivariate
analysis revealed the EGFR status of the tumors was an independent
and significant prognostic factor (3). The overexpression of EGFR, according
to IHC, was present in 39.4% of the 218 patients with available IHC
data in a phase III randomized European Organization for Research
and Treatment of Cancer-Gynecological Cancer Group study (16) comparing erlotinib with observations
in patients with no evidence of disease progression following
first-line platinum-based chemotherapy; the expression of EGFR in
tumors was not validated as a poor prognostic marker. It was
concluded that, although the EGFR pathway appears to be important
in ovarian cancer tumor development, how this pathway may be used
for therapeutic benefit remained unclear. By contrast, the results
from a meta-analysis (17) of
EGFR, including 2,471 patients in 15 studies, showed a significant
association between overexpression and poor patient outcome [HR
1.65 (95% CI 1.25-2.19)]. In a review article entitled Targeting
the EGF Receptor for Ovarian Cancer Therapy (18), it was concluded that the overall
clinical impact of targeting EGFR and its dimers in ovarian cancer,
either with monoclonal antibodies or via inhibition of the tyrosine
kinase domain, has been modest in unselected women with advanced or
recurrent ovarian cancer. Furthermore, two separate groups have
shown an inverse correlation between EGFR and survival rate in
ovarian cancer (19).
In another study (20), it was reported that ligand-induced
downregulation of EGFR in the CaOV3 ovarian cancer cell line was
possible without tyrosine kinase activity. The downregulation of
EGFR without the induction of mitogenic signals, by priming ovarian
cancer cells with EGF and EGFR inhibitor PD153035 prior to
chemotherapy, was observed in cancer cells that were expected to
exhibit increased sensitivity to Taxol-induced cell death.
Therefore, it was hypothesized that, by priming with EGFR
inhibitors and EGF, certain pathways that lead to cell
proliferation and survival can be inhibited by downregulating EGFR.
This priming procedure, by sensitizing ovarian cancer cells, was
considered to result in improved chemotherapeutic outcome from
paclitaxel. This hypothesis may explain the favorable prognostic
effect of the positive EGFR status of ovarian tumors on outcomes in
the present study, although 105/131 (80%) of patients received
post-surgical paclitaxel. However, no differences in the
post-surgical treatments (paclitaxel and carboplatin vs. single
drug carboplatin) were found between the two subgroups in the
present study. Gavalas et al (4) reported that paclitaxel appeared to
have an antiangiogenic effect due to possible increased uptake by
endothelial cells in the tumor.
Positive VEGF-R2 staining was observed in 100/130
(77%) tumors in the present study. This was in line with findings
from a study by Nishida et al (6), in which positive staining for VEGF-R2
was detected by IHC in 60/80 (75%) ovarian tumors from patients at
FIGO stages I-IV. However, in this previous study, the high
expression of VEGF-R2 in tumors was associated with poorer DFS
compared with tumors with negative or low expression of VEGF-R2. In
another study (5) of 76 cases of
ovarian cancer tumor, a high expression of VEGF-R2 did not have any
effect on progression-free or overall survival rates. However, high
expression levels of VEGF-R2 were found in 17/17 (100%) ovarian
tumors at FIGO stages I-II, but only in 39/59 tumors (66%) at FIGO
stages III-IV. Furthermore, it was reported in a study of 128
patients at FIGO stages I-IV, that patients with high serum levels
of VEGF-R2 had improved prognosis, compared with those with low
levels of VEGF-R2 (21).
Previous studies (22,23)
on various anti VEGF/VEGF receptor therapies have shown that these
agents, when used in combination with chemotherapy, significantly
improve survival and response rates in patients. A large number of
studies have shown that the inhibition of VEGF or its receptor
VEGF-R2 normalizes the tumor vasculature and increases oxygen
tension or improves drug penetration. The combination of VEGF
targeted agents with chemotherapy may explain the increased
neovascular damage (24). A
phenomenon termed 'evasive resistance', which is observed in tumors
following anti VEGF therapy, has been detected, and the suggested
mechanisms for the acquisition of this resistance include the
induction of angiogenic factors other than VEGF. By using an
antibody targeting VEGF-R2 in an animal experiment, it has been
shown that vascular regression and tumor reduction occur first,
followed by the induction of angiogenesis, leading to tumor
regrowth (25,26). These observations may explain why
the positive staining of VEGF-R2 in ovarian tumors in the present
study had a favorable prognostic effect on survival rates.
According to observations from a study on the regulation of
angiogenesis (27), it is
suggested that vasohibin-1, which acts alone to inhibit multiple
different angiogenic factors, may be a more effective inhibitor of
angiogenesis than inhibitors which focus on VEGF alone (27).
In a survival analysis of the four subgroups of
patients in the present study, with respect to the concomitant EGFR
and VEGF-R2 status of the tumors, the patients in the subgroup of
tumors with concomitant positive expression of EGFR and VEGF-R2 had
a DFS rate of 100% at 5-years. According to the same analysis, the
poorest outcome was found for patients belonging to the subgroup of
tumors with concomitant negative expression of EGFR and VEGF-R2,
with a DFS rate of 64% at 5-years. However, the main findings from
the present study were limited to non-serous tumors and, in further
a survival analysis on patients with non-serous tumors (n=78),
there was a trend for improved survival rate in the subgroup of
patients with concomitant positive expression of EGFR and VEGF-R2,
compared with survival rates in the other three subgroups. All 15
patients with non-serous tumors (10/15 patients had endometroid
tumors) with concomitant EGFR and VEGF-R2-positive expression had
5-year survival rates of 100% and were alive 8 years following
diagnosis of the primary tumor. A previous study (28) was designed as phase II trial to
evaluate the clinical activity and target modulation of vandetanib,
designed to inhibit VEGF-R2 and EGFR in women with recurrent and
mainly platinum-resistant ovarian cancer. However, 300 mg daily
monotherapy with vandetanib had no significant clinical benefit in
this disease setting. Proteomic analysis of paired biopsies
detected phosphorylated-EGFR and phosphorylated-VEGFR2 in ovarian
tumor tissues, but only phosphorylated-EGFR was, measurably
inhibited by vandetanib. Apart from targeting the VEGF pathway,
novel strategies aim to influence other molecular factors that are
involved in tumor angiogenesis (8). In the present study, positive
staining for VEGF-R2 in ovarian tumors led to positive results for
progression free survival. Furthermore, in a survival analysis
comparing four subgroups following analysis of the status of EGFR
and VEGF-R2, the subgroup of patients with concomitant positive
expression of EGFR and VEGF-R2 had a 5-year DFS rate of 100%.
In a multivariate logistic regression analysis with
recurrent disease as the endpoint, FIGO stage, type (I/II) of tumor
and VEGF-R2 status were all independent predictive factors for the
entire cohort of patients. In a further multivariate logistic
regression analysis with recurrent disease as the endpoint for
patients belonging to the subgroup of non-serous tumors (n=78), the
FIGO-stage, type (I/II) of tumor, EGFR status and VEGF-R2 status
were all significant and independent predictive factors. However,
in a multivariate Cox analysis with DFS as the endpoint, in the
group of patients with non-serous tumors, only the FIGO-stage and
type (I/II) of tumor were significant and independent prognostic
factors. The different outcomes of variables between the two forms
of multivariate analysis reflect the fact that prognostic factors,
but not predictive factors, are dependent of the time interval
between diagnosis and analysis.
The limitations of the present study correspond to
the relatively limited number of patients included and the method
of semi-quantitative analysis used for interpretation, wherein all
markers were, dichotomized into negative and positive groups.
Preclinical and clinical observations have shown that the process
of angiogenesis remains to be fully, elucidated. Therefore, the
first concept underlying antiangiogenic therapy was the destruction
of tumor vessels; it transpired that, paradoxically, antiangiogenic
drugs normalized the vasculature and, as result, offered an
improvement in chemotherapeutic delivery. Several trials of
anti-angiogenic agents in the front-eline treatment of ovarian
cancer have shown positive results for progression-free survival.
However, the impact on overall survival rates remains to be fully
elucidated. Therefore, one of the challenges in the investigation
of ovarian cancer is to identify novel biomarkers for
angiogenesis.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IS, TS and HÅ were involved in conceptualization,
formal analysis and methodology; they also provided resources, and
validation and visualization of the data. IS was involved in data
curation and project administration. IS and TS contributed toward
the investigation. IS and HÅ supervised the study, and wrote and
edited the manuscript.
Ethics approval and consent to
participate
All tissue samples were collected with the patients'
informed consent and were in compliance with the Helsinki
Declaration (11), and used in
accordance with the Swedish Biobank Legislation and Ethical Review
Act approved by the Uppsala Ethical Review Board (Uppsala, Sweden;
decision ref. UPS-03-477).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siwak DR, Carey M, Hennessy BT, Nguyen CT,
McGahren Murray MJ, Nolden L and Mills GB: Targeting the epidermal
growth factor receptor in epithelial ovarian cancer: Current
knowledge and future challenges. J Oncol. 2010:5689382010.
View Article : Google Scholar
|
|
2
|
Sheng Q and Liu J: The therapeutic
potential of targeting the EGFR family in epithelial ovarian
cancer. Br J Cancer. 104:1241–1245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skirnisdottir I, Sorbe B and Seidal T: The
growth factor receptors HER-2/neu and EGFR, their relationship, and
their effects on the prognosis inearly stage (FIGO I-II) epithelial
ovarian carcinoma. Int J Gynecol Cancer. 11:119–129. 2001.
View Article : Google Scholar
|
|
4
|
Gavalas NG, Liontos M, Trachana SP,
Bagratuni T, Arapinis C, Liacos C, Dimopoulos MA and Bamias A:
Angiogenesis-related pathways in the pathogenesis of ovarian
cancer. Int J Mol Sci. 14:15885–15909. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klasa-Mazurkiewicz D, Jarząb M, Milczek T,
Lipińska B and Emerich J: Clinical significance of VEGFR-2 and
VEGFR-3 expression in ovarian cancer patients. Pol J Pathol.
62:31–40. 2011.PubMed/NCBI
|
|
6
|
Nishida N, Yano H, Komai K, Nishida T,
Kamura T and Kojiro M: Vascular endothelial growth factor C and
vascular endothelial growth factor receptor 2 are related closely
to the prognosis of patients with ovarian carcinoma. Cancer.
101:1364–1374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lassus H, Sihto H, Leminen A, Joensuu H,
Isola J, Nupponen NN and Butzow R: Gene amplification, mutation,
and protein expression of EGFR and mutations of ERBB2 in serous
ovarian carcinoma. J Mol Med (Berl). 84:671–681. 2006. View Article : Google Scholar
|
|
8
|
Maj E, Papiernik D and Wietrzyk J:
Antiangiogenic cancer treatment: The great discovery and greater
complexity (Review). Int J of Oncol. 49:1773–1784. 2016. View Article : Google Scholar
|
|
9
|
Elie C, Geay JF, Morcos M, Le Tourneau A,
Girre V, Broët P, Marmey B, Chauvenet L, Audouin J, Pujade-Lauraine
E, et al GINECO Group: Lack of relationship between EGFR-1
immunohistochemical expression and prognosis in a multicentre
clinical trial of 93 patients with advanced primary ovarian
epithelial cancer (GINECO group). Br J Cancer. 91:470–475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Skirnisdottir I, Seidal T and Åkerud H:
The relationship of the angiogenesis regulators VEGF-A, VEGF-R1 and
VEGF-R2 to p53 status and prognostic factors in epithelial ovarian
carcinoma in FIGO-stages I-II. Int J Oncol. 48:998–1006. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
World Medical Association: WMA Declaration
of Helsinki Ethical Principles for Medical Research Involving Human
Subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects.
|
|
12
|
Trimbos JB, Vergote I, Bolis G, Vermorken
JB, Mangioni C, Madronal C, Franchi M, Tateo S, Zanetta G, Scarfone
G, et al EORTC-ACTION collaborators; European Organisation for
Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian
Neoplasm: Impact of adjuvant chemotherapy and surgical staging in
early-stage ovarian carcinoma: European Organisation for Research
and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm
trial. J Natl Cancer Inst. 95:113–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kononen J, Bubendorf L, Kallioniemi A,
Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G
and Kallioniemi OP: Tissue microarrays for high-throughput
molecular profiling of tumor specimens. Nat Med. 4:844–847. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seidal T, Balaton AJ and Battifora H:
Interpretation and quantification of immunostains. Am J Surg
Pathol. 25:1204–1207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Köbel M, Kalloger SE, Boyd N, McKinney S,
Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al:
Ovarian carcinoma subtypes are different diseases: Implications for
biomarker studies. PLoS Med. 5:e2322008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Despierre E, Vergote I, Anderson R, Coens
C, Katsaros D, Hirsch FR, Boeckx B, Varella-Garcia M, Ferrero A,
Ray-Coquard I, et al European Organisation for Research and
Treatment of Cancer-Gynaecological Cancer Group (EORTC-GCG); Groupe
d'Investigateurs Nationaux pour les Etudes des Cancers de l'Ovaire
(GINECO); Austrian Arbeitsgemeinschaft für Gynäkologische Onkologie
(A-AGO); National Cancer Research Institute (NCRI); Australia New
Zealand Gynaecological Oncology Group (ANZGOG); Mario Negri
Gynecologic Oncology group (MaNGO): Epidermal growth factor
receptor (EGFR) pathway biomarkers in the randomized phase III
trial of erlotinib versus observation in ovarian cancer patients
with no evidence of disease progression after first-line
platinum-based chemotherapy. Target Oncol. 10:583–596. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Graeff P, Crijns APG, Ten Hoor KA, Klip
HG, Hollema H, Oien K, Bartlett JM, Wisman GB, de Bock GH, de Vries
EG, et al: The ErbB signalling pathway: Protein expression and
prognostic value in epithelial ovarian cancer. Br J Cancer.
99:341–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeineldin R, Muller CY, Stack MS and
Hudson LG: Targeting the EGF receptor for ovarian cancer therapy. J
Oncol. 2010:4146762010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Hu W and Sood AK: Prognostic
biomarkers in ovarian cancer. Cancer Biomark. 8:231–251. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao C, Lu S, Sowa A, Kivlin R, Amaral A,
Chu W, Yang H, Di W and Wan Y: Priming with EGFR tyrosine kinase
inhibitor and EGF sensitizes ovarian cancer cells to respond to
chemotherapeutical drugs. Cancer Lett. 266:249–262. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Komatsu H, Oishi T, Itamochi H, Shimada M,
Sato S, Chikumi J, Sato S, Nonaka M, Sawada M, Wakahara M, et al:
Serum vascular endothelial growth factor-A as a prognostic
biomarker for epithelial ovarian cancer. Int J Gynecol Cancer.
27:1325–1332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burger RA: Role of vascular endothelial
growth factor inhibitors in the treatment of gynecologic
malignancies. J Gynecol Oncol. 21:3–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang C, Hess K, Jardim DL, Gagliato Dde M,
Tsimberidou AM, Falchook G, Fu S, Janku F, Naing A, Piha-Paul S, et
al: Synergy between VEGF/VEGFR inhibitors and chemotherapy agents
in the phase I clinic. Clin Cancer Res. 20:5956–5963. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis (review). J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar
|
|
25
|
Casanovas O, Hicklin DJ, Bergers G and
Hanahan D: Drug resistance by evasion of antiangiogenic targeting
of VEGF signaling in late-stage pancreatic islet tumors. Cancer
Cell. 8:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi Y, Saga Y, Koyanagi T, Takei Y,
Machida S, Taneichi A, Mizukami H, Sato Y, Matsubara S and Fujiwara
H: The angiogenesis regulator vasohibin-1 inhibits ovarian cancer
growth and peritoneal dissemination and prolongs host survival. Int
J Oncol. 47:2057–2063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Annunziata CM, Walker AJ, Minasian L, Yu
M, Kotz H, Wood BJ, Calvo K, Choyke P, Kimm D, Steinberg SM, et al:
Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no
clinical activity as monotherapy for recurrent ovarian cancer and
no detectable modulation of VEGFR2. Clin Cancer Res. 16:664–672.
2010. View Article : Google Scholar : PubMed/NCBI
|