Introduction
Colorectal cancer (CRC) is the third most common
type of cancer in men and women and is the fourth leading cause of
cancer-associated mortality (1).
The incidence of CRC is rapidly increasing in China, which is a
serious threat to the health of the population (2). Colorectal adenocarcinoma accounts for
98% of cancer types among newly diagnosed cases. Advances in
surgical technology and conception of treatment have been made, and
optimization of treatment for patients with colorectal
adenocarcinoma has led to an increase of survival rate at 5 and 10
years (3). However, metastasis
remains the main cause of mortality and poor prognosis (4-6). The
metastases produced by carcinomas are a result of a complex
succession of the invasion-metastasis cascade (7). The fact that cancer cells gain cell
motility and invasiveness is considered a critical step
contributing to the metastasis of cancer (8). However, the mechanisms by which tumor
cells become metastatic remain to be fully elucidated.
Rho guanine nucleotide exchange factor 7 (ARHGEF7),
a guanine nucleotide exchange factor for Rho GTPases, functions in
cell migration, attachment and cell spread (9-11).
ARHGEF7 is implicated in cytoskeleton remodelling, which is
important in cell migration (12,13).
In other types of tumor, studies have shown that ARHGEF7 affects
the motility of cells in vitro and invasion in vivo
(14,15). Specially, it has been reported that
the ARHGEF7 gene frequently exhibits high- level genetic
amplification in metastatic lesions compared with primary sites in
colorectal adenocarcinoma by SNP array (16). These data indicate that ARHGEF7 may
be involved in colorectal adenocarcinoma metastasis. However, few
investigations have focused on the role of ARHGEF7 in the
metastasis of colorectal adenocarcinoma. Metastasis is the main
contributor to the poor prognosis of patients (17). ARHGEF7 may be a prognostic
biomarker and associated with colorectal adenocarcinoma
metastasis.
The present study aimed to examine the expression of
ARHGEF7 in colorectal adenocarcinoma. The role of ARHGEF7 in
colorectal adenocarcinoma metastasis and its underlying molecular
mechanism were examined using a series of in vitro and in
vivo assays. Finally, whether the expression of ARHGEF7 is
clinically relevant in patients with colorectal adenocarcinoma was
determined according to ReMARK guidelines for the reporting of
prognostic biomarkers in cancer (18).
Materials and methods
Colorectal adenocarcinoma samples
The present study was approved by the Ethics
Committee of the Institutional Review Boards of the First
Affiliated Hospital of Nanchang University (Nanchang, China) and
Jiangxi Pingxiang People’s Hospital (Pingxiang, China). Prior
informed consent was obtained from all participants, and the study
was performed in accordance with the Declaration of Helsinki and
current ethical guidelines. Firstly, 30 pairs of frozen fresh
colorectal adenocarcinoma tumor tissues and corresponding
nontumorous colorectal tissues (NCTs) from 30 patients were
collected, and another five matched liver metastatic nodules
(LMNs), tumor tissues and NCTs from five patients were collected
following surgical resection at the Department of General Surgery,
the First Affiliated Hospital of Nanchang University between July
2016 and January 2017. These tissues were used to screen the mRNA
and protein expression of ARHGEF7. Secondly, another two sets of
samples were used for prognostic analysis according to ReMARK
guidelines for reporting prognostic biomarkers in cancer (18). Formalin-fixed, paraffin-embedded
paired colorectal adenocarcinoma samples (including tumors and
NCTs) obtained from 180 patients undergoing radical surgical
resection at the Department of General Surgery, the First
Affiliated Hospital of Nanchang University between January 2007 and
December 2009 were designated as the training set. Another sample
cohort containing 150 samples, including tumors and NCTs, from
patients who underwent resection between July 2007 and July 2010 at
the Department of General Surgery, Jiangxi Pingxiang People’s
Hospital was designated as the validation set. The inclusion
criteria for the samples enrolled in the study were as follows:
Collection from patients with sporadic CRC, histopathologically
diagnosed as adenocarcinoma by hematoxylin and eosin (H&E)
staining; had completed clinicopathologic and follow-up data; were
without neoadjuvant chemotherapies or distant metastasis prior to
surgery. Patients with hereditary CRC were excluded from the
study.
Prognostic evaluation
All patients were regularly followed-up by trained
and experienced researchers. The follow-up period was defined as
the interval between the date of surgery and that of the patient’s
mortality or distant metastasis or the last follow-up. The median
follow-up was 62.3 months (range 6.0-100.0 months) for the training
set and 60.0 months (range 6.4-100.0 months) for the validation
cohort. Patient mortality from other causes were treated as
censored cases. Following surgery, all patients had regular
clinical examination, serial monitoring of carcinoembryonic antigen
(CEA) levels at 1-month interval, had computed tomography (CT) or
magnetic resonance imaging (MRI) scan at a 3-month interval, and
had investigation by colonoscopy at the 1-year interval. Recurrence
or metastasis was diagnosed by clinical examination, serial CEA
level, and CT or MRI or positron emission tomography-CT.
Disease-free survival (DFS) was defined as the length of time
following resection during which a patient survived without signs
of recurrence or metastasis. Overall survival (OS) was defined as
the interval between surgery and mortality, or between surgery and
the last observation for surviving patients. Data for conventional
clinical and pathological variables were also collected for
analysis, including sex, age, serum CEA level, tumor
differentiation, tumor site, tumor size, tumor grade, lymphatic
vessel/vessel/neuron infiltration, mesenteric tumor deposit
formation, and tumor-node-metastatsis (TNM) stage. The follow-up
data were regularly updated in the database for each patient.
Patients alive at the end of follow up or those who succumbed to
mortality from causes without sign of recurrence or metastasis were
censored.
Cell lines
The FHC normal colorectal mucosal cell line, and the
HCT116, HT-29, SW480, SW620, LoVo cell lines were purchased from
the American Type Culture Collection (Manassas, VA, USA). Short
tandem repeat (STR) DNA fingerprinting was used to authenticate all
cell lines prior to commencement of the study. All cell lines were
routinely cultured with RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA), and
maintained in a 5% CO2 humidified incubator at 37°C.
Vector construction and transfection
The lentiviral vector (LV) encoding short hairpin
RNAs (shRNAs) for ARHGEF7 knockdown, and the LV encoding the
ARHGEF7 gene were purchased from OriGene Technologies, Inc.
(Rockville, MD, USA). The sequences of four shRNAs for ARHGEF7
knockdown were as follows: ARHGEF7-shRNA-Seq1, sense,
5′-ACAAGGTCCTCAGTTCCTTAGTGACTCTA-3′; ARHGEF7-shRNA-Seq2, sense,
5′-CCACCATAAAGCCTCATTCAGTGCCATCT-3′; ARHGEF7-shRNA-Seq3, sense,
5′-CCTGAACGGAAGCCTTCAGATGAGGAGTT-3′; and ARHGEF7-shRNA-Seq4, sense,
5′-TACGGCCATTGCAGACCAGTGAGAAGTTA-3′. The LoVo cells were
transfected with LVs encoding the shRNAs, and the HCT116 cells were
transfected with LVs encoding the human ARHGEF7 gene. An empty
vector was used as the negative control and was designated as
LV-control. The LV vectors were transfected into the CRC cells with
an appropriate multiplicity of infection of 50. At 48 h
post-transfection, 3.0 µg/ml puromycin (OriGene
Technologies, Inc.) was added, and the cells were incubated for 2
weeks to select the stably transfected cells. The overexpression or
downregulated expression of ARHGEF7 was confirmed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses. The inhibitory efficiencies of the four
shRNAs were validated and ARHGEF7-shRNA-Seq2 was used for
subsequent experiments due to highly effective inhibition of the
expression of ARHGEF7 in LoVo cells.
RT-qPCR analysis
Total RNA was extracted from the cell lines
(~1×107 cells) or fresh frozen tumor specimens (50-100
mg) using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer’s protocol. The RNA was then
reverse transcribed to obtain cDNA using the universal cDNA
synthesis kit (Toyobo Co., Ltd., Osaka, Japan) according to the
manufacturer’s protocol. The aliquots of double-stranded cDNA were
subjected to RT-qPCR. RT-qPCR analysis was performed using the
SYBR®-Green Realtime PCR Master Mix assay kit (Toyobo
Co., Ltd.) according to the manufacturer’s protocol; the assay was
performed on a PRISM 7300 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The cycling parameters
were as follows: Initial denaturation at 95°C for 2 min, followed
by 95°C for 15 sec, 55-60°C for 15 sec and 72°C for 15 sec for 50
cycles, and a final extension step at 72°C for 5 min. The primers
for ARHGEF7 were as follows: Forward, 5′-CGCAAACCTGAACGGAAGC CTT-3′
and reverse, 5′-GTTTTGGCGCTGGTGCAGTAAG-3′. GAPDH was used as a
control using the following primers: Forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
The results were analyzed using the 2-ΔΔCq method with
the following formula: ΔΔCq = ΔCqTumor - ΔCqNCT; ΔCq =
CqARHGEF7-CqGAPDH (19).
Western blot analysis
Total proteins were extracted using RIPA lysis
buffer. Protein concentration was then determined using the
bicinchoninic acid method, and equal quantities of protein (20-60
µg) from each sample were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred
onto PVDF membranes (EMD Millipore, Bedford, MA, USA). The blotted
membranes were blocked with 5% skimmed milk for 30 min at 25°C and
incubated with primary antibodies (mouse anti-ARHGEF7; 1:400; cat.
no. sc-136035; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
then an appropriate HRP-conjugated goat anti-mouse secondary
antibody (1;1,000; cat. no. 04-18-06; KPL, Inc., Gaithersburg, MD,
USA) in order. Bands were detected with enhanced chemiluminescence
regents (Thermo Fisher Scientific, Inc.). β-actin protein was also
determined using the specific mouse anti-β-actin antibody (1:1,000;
cat. no. A1978, Sigma; EMD Millipore) as a loading control. Protein
expression levels were quantified using BandScan software 4.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and defined as the
ratio of target protein relative to β-actin.
Immunohistochemistry (IHC)
The detailed IHC procedures were performed as
described previously (20). The
paraffin- embedded tissues were sectioned into 4-µm slides.
These slides were dewaxed, tissues were rehydrated, and antigen
retrieval was performed using microwave-pretreated EDTA buffer (1
mM; pH 8.0) for 10 min. Following blocking, the slides were
incubated for ARHGEF7 antibody (mouse anti-ARHGEF7 1:200; cat. no.
sc-136035; Santa Cruz Biotechnology, Inc.) at 4°C overnight. The
slides were incubated with biotin-labeled secondary and
streptavidin-peroxidase (cat. no. SP-9002; Zhongshan Goldenbridge
Biotechnology Co., Ltd., Beijing, China) for 30 min at 37°C. The
samples were developed using 3,3′-diaminobenzidine substrate (cat.
no. ZLI-9018) and counterstained with hematoxylin (cat. no.
ZLI-9609) (both from Zhongshan Goldenbridge Biotechnology Co.,
Ltd.). Negative
controls were without primary antibody incubation
during the procedure. The staining was determined in a blinded
manner by two independent pathologists. The final immunostaining
score (IS) was defined by the consistency of the grading by two
pathologists. The expression levels of ARHGEF7 were scored based on
staining intensity (SI) and percentage of positive cells (PP) using
the IS as described previously (21). The SI was classified into four
grades: 0, negative; 1, weak; 2, moderate; 3, strong. The PP was
defined into five categories: 0, 0% positive cells; 1, 0-25%
positive cells; 2, 25-50% positive cells; 3, 50-75% positive cells,
and 4, 75-100% positive cells. IS = SI x PP.
Cell proliferation and colony formation assays
Methyl thiazolyl tetrazolium (MTT) assays were used
to determine the level of cell proliferation. For the MTT assays,
5×103 cells were seeded into each well of 96-well
plates. Three repeated wells for each group were detected every
time. Fresh medium (100 µl; 0.5 mg/ml) containing MTT
(Sigma; EMD Millipore) was added into each well and incubated at
37°C for 4 h. The medium was then replaced with 100 µl DMSO
and shaken at room temperature for 10 min. The absorbance was
measured at 570 nm. For the colony formation assays, 500 cells were
seeded into 35-mm dishes (Corning Incorporated, Corning, NY, USA)
and cultured in 5% CO2 for 2 weeks at 37°C. The number
of colonies per dish was counted following staining with crystal
violet (Beyotime Institute of Biotechnology, Jiangsu, China). Only
positive colonies (diameter >40 µm) in the dishes were
counted by inverted microscope (TE-2000S; Nikon Corporation, Tokyo,
Japan) and compared (22). These
experiments were performed in triplicate.
Transwell assays
Transwell migration and invasion assays were used to
determine cell motility and invasion ability separately. For the
Transwell invasion assays, the upper chamber of the insert was
plated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). For
the Transwell migration assays, the upper chamber of the insert was
without Matrigel. Briefly, following preincubation with Mitomycin-C
(10 µg/ml) for 1 h at 37°C to suppress cell proliferation,
~1×105 cells in serum- free medium were placed into the
upper chamber of the insert. Following incubation in 5%
CO2 at 37°C for 24 h, the cells in the upper chamber
were removed with cotton swabs, fixed in 20% methanol, and then
stained with a solution containing
0.1% crystal violet (Beyotime Institute of
Biotechnology). The number of cells that adhered to the lower
membrane of the inserts was counted. For each experimental group,
the assays were performed in triplicate, and five random fields of
view were selected for analysis.
Adhesion assay
Cell-extracellular matrix (ECM) adhesion and
cell-cell adhesion assays were used to analyze the adhesive ability
of the cells. For the cell-ECM adhesion assay, a 96-well plate was
coated with fibronectin at 37°C for 1 h and washed twice with
washing buffer (0.1% BSA in DMEM; HyClone; GE Healthcare Life
Sciences). The plates were blocked in blocking buffer (0.5% BSA in
DMEM) at 37°C in a CO2 incubator for 60 min. The cells
(100 µl) at a density of ~1×105/ml were added
into each well of a 96-well plate and cultured at 37°C. Five wells
for each group were detected at 60, 90 or 120 min. The medium was
entirely removed, and unbound cells were washed away with PBS.
Fresh medium (100 µl; 0.5 mg/ml) containing MTT (Sigma; EMD
Millipore) was added into each well and incubated at 37°C for 4 h.
The medium was then replaced with 100 µl of DMSO and shaken
at room temperature for 10 min. The absorbance was measured at 570
nm. For the cell-cell adhesion assay, subconfluent cell layers
(~70-80%) were rinsed twice with Ca2- and
Mg2-free PBS and detached by incubation in HBSS
containing 1 mmol/l EDTA at 37°C for 20 min. Subsequently, single
cells (100 µl) at density of ~1×105/ml were added
into a 96-well plate (Costar; Corning Incorporated) with a fully
confluent single cell layer, and cultured at 37°C for 0-120 min.
The unbound cells were washed from the wells, and were collected
and quantified using an inverted microscope (TE-2000S; Nikon
Corporation). The adhesion rate was determined by counting
representative aliquots from each sample on a hematocytometer. The
percentage of adhesion was quantified at 60, 90 or 120 min as:
N0-Nt/N0 × 100, where
Nt is the total number of unbound cells at the
incubation time t, and N0 is the total number of
cells.
Cellular cytoskeleton analysis
Rhodamine-conjugated phalloidin was used to analyze
cell cytoskeleton. The cells grown on cover slides were fixed, and
then incubated with rhodamine-conjugated phalloidin (1:200; cat.
no. CA1610; Solarbio Science and Technology Co., Ltd., Beijing,
China). Following staining with DAPI (1:200; cat. no. C1002;
Beyotime Institute of Biotechnology), images of the slides were
captured using an inverted fluorescence microscope (TE-2000S; Nikon
Corporation).
GTPase activity assays
In brief, the cells were grown to ~80% confluence in
regular culture medium, were serum-starved for 24 h and were
stimulated with 10% FBS for 5 min. GTP-bound Ras-related C3
botulinum toxin substrate 1 (Rac1), cell division cycle 42 (Cdc42)
and total protein were detected using G-LISA Rac1 and G-LISA Cdc42
Activation Assay Biochem kits (Cytoskeleton, Inc., Denver, CO, USA)
according to the manufacturer’s protocol.
Metastatic assays in an in vivo
orthotopic model
The mice were provided by the Animal Institute of
Nanchang University and were housed under specific pathogen-free
conditions: Temperature, 25°C; relative humidity, ~40%; lighting,
10 h/day with fluorescent lights. The mice received ad
libitum access to sterilized food and water. The experiments
were performed according to the protocols approved by the Medical
Experimental Animal Care Commission. For the in vivo
metastatic assays, the orthotopic model in mice was constructed
(23). Briefly, 1×106
cells were injected into the subserosal layer of the sigmoid colon
of male BALB/c nude mice weighing ~16-20 g (5 weeks old). The mice
were sacrificed following 7 weeks of cell inoculation. Following
necropsy, tumors growing in the colon and peritoneum were excised.
All livers and macroscopically enlarged mesenteric lymph nodes were
harvested, fixed with 10% phosphate- buffered neutral formalin,
sectioned serially, and stained with H&E for defining the
presence of metastatic disease.
Statistical analysis
All data were analyzed using the statistical
software SPSS 18.0 for Windows (SPSS, Inc., Chicago, IL, USA). The
differences between groups were analyzed using Student’s t-test
between two groups or by one-way analysis of variance in more than
two groups when the variance was homogeneous. If the variance was
not homogeneous, the differences between groups were analyzed using
the Mann-Whitney U test between two groups or Kruskal-Wallis H test
in more than two groups. χ2 analysis was used to analyze
the correlation between the expression of ARHGEF7 and
clinicopathologic features, and the presence of metastasis between
two groups. Survival curves were constructed with the Kaplan-Meier
method and compared using the log-rank test. The Cox proportional
hazards regression model was established to identify independent
factors for OS and DFS rates of patients. Receiver operating
characteristics (ROC) curves were constructed to assess
sensitivity, specificity, and respective areas under the curves
with 95% CI. The cut-off value was assessed by the highest Youden
index. All tests were two-tailed and P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of ARHGEF7 is significantly
increased and associated with metastasis in colorectal
adenocarcinoma
Firstly, the expression of ARHGEF7 in colorectal
adenocarcinoma cell lines was detected. Compared with the FHC
normal colorectal mucosal cell line, ARHGEF7 mRNA and protein were
expressed at high levels in the colorectal adenocarcinoma cell
lines, including the HCT116, HT-29, SW480, SW620 and LoVo cell
lines (Fig. 1A and B). The
expression of ARHGEF7 was also determined in 30 paired colorectal
adenocarcinoma samples. RT-qPCR analysis revealed that the mRNA
expression of ARHGEF7 was markedly higher in tumor tissues than in
corresponding NCTs (P<0.001; Fig.
1C). Western blot analysis also showed that the protein
expression of ARHGEF7 was markedly higher in tumor tissues than in
NCTs (P<0.001; Fig. 1D).
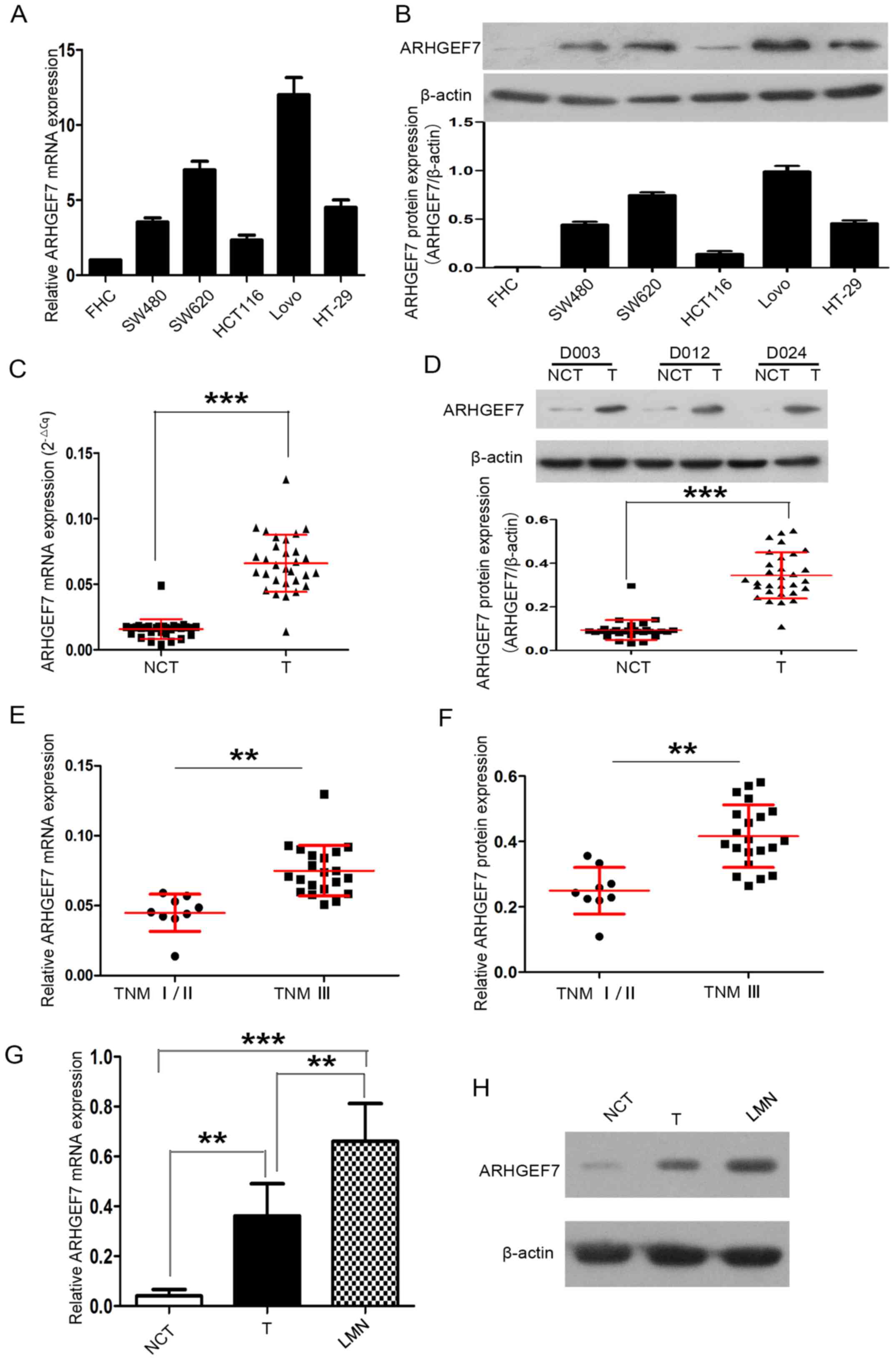 | Figure 1ARHGEF7 is overexpressed in
colorectal adenocarcinoma and associated with metastasis. ARHGEF7
was significantly upregulated in colorectal adenocarcinoma cell
lines. (A) RT-qPCR analysis of ARHGEF7 mRNA showed that, compared
with the FHC normal colorectal mucosal cell line, mRNA expression
of ARHGEF7 was elevated in HCT116, HT-29, SW480, SW620, LoVo
colorectal adenocarcinoma cell lines. (B) Western blot results
showed ARHGEF7 protein was overexpressed in HCT116, HT-29, SW480,
SW620 and LoVo cells relative to FHC cells. Expression of ARHGEF7
was significantly upregulated in colorectal adenocarcinoma tissues.
(C) RT-qPCR was used to analyze mRNA expression of ARHGEF7 in
colorectal adenocarcinoma T tissues (n=30) and corresponding NCTs
(n=30). RT-qPCR results showed that the mRNA expression level of
ARHGEF7 was significantly higher in T tissues than in NCTs. (D)
Western blot results showed that the expression of ARHGEF7 was
higher in T tissues than in NCTs. Upper panel: Representative
western blot of randomly selected samples (D003, D012 and D024)l;
lower panel: Scatter plot for ARHGEF7 expression in T tissues and
NCTs. (E) mRNA and (F) protein expression of ARHGEF7 in colorectal
adenocarcinoma tissues from advanced stage (TNM III) patients were
significantly higher than that in those from early stage patients
(TNM I/II). Expression of ARHGEF7 progressively increased from
NCTs, to T, to LMNs from the same patient (n=5). (G) RT-qPCR
analysis of ARHGEF7 mRNA in NCTs, T tissues and LMNs. P=0.003 NCT
vs. T; P<0.001 NCT vs. LMN; P=0.005 T vs. LMN (P-values
determined by analysis of variance and further SNK test). (H)
Representative images of western blot results showed that
expression of ARHGEF7 progressively increased from NCTs, to T, to
LMNs from the same patient. **P<0.01;
***P<0.001. ARHGEF7, Rho guanine nucleotide exchange
factor 7; T, tumor; NCT nontumorous tissues; TNM,
tumor-node-metastasis; LMN, liver metastatic nodule; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
Subsequently, 30 primary tumors of different TNM
stages were analyzed. The expression of ARHGEF7 in colorectal
adenocarcinoma tissues was significantly higher in advanced stage
patients (TNM stage III) than in early stage patients (TNM stage
I/II) (P<0.01; Fig. 1E and F).
The expression of ARHGEF7 was further analyzed in LMNs, primary
tumors and corresponding NCTs from the same patient (n=5), and the
results showed that the expression of ARHGEF7 was progressively
increased from NCT to tumor to LMN at the mRNA (Fig. 1G) and protein (Fig. 1H) levels. The LMNs had the highest
expression of ARHGEF7, whereas NCTs had the lowest expression
(Fig. 1G and H). Taken together,
these data confirmed that the expression of ARHGEF7 was
significantly elevated in colorectal adenocarcinoma tissues and may
be associated with metastasis in colorectal adenocarcinoma.
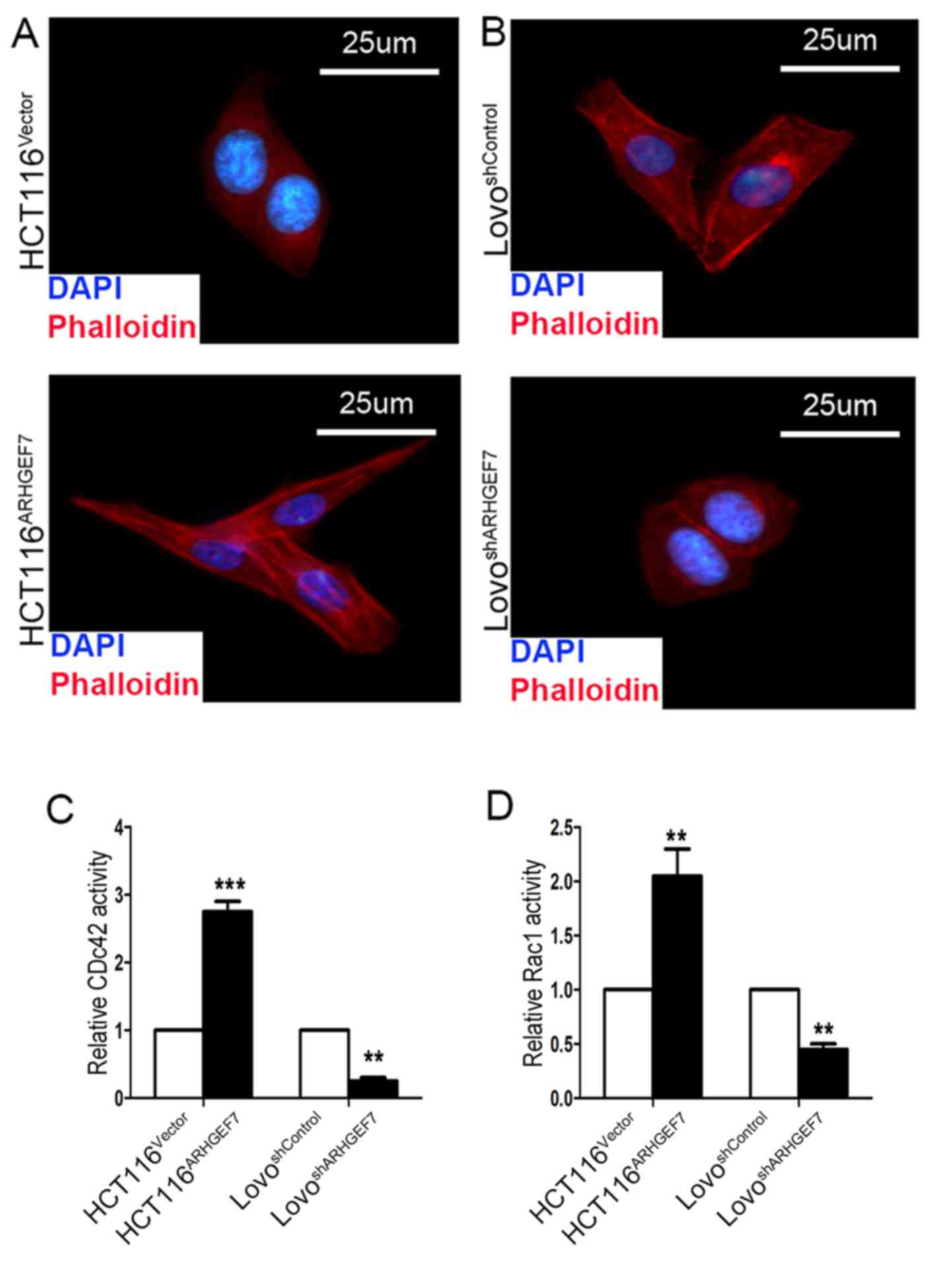
ARHGEF7 promotes colorectal
adenocarcinoma cell motility and invasion in vitro
To determine the role of ARHGEF7 in the metastasis
of colorectal adenocarcinoma, ARHGEF7 was overexpressed in HCT116
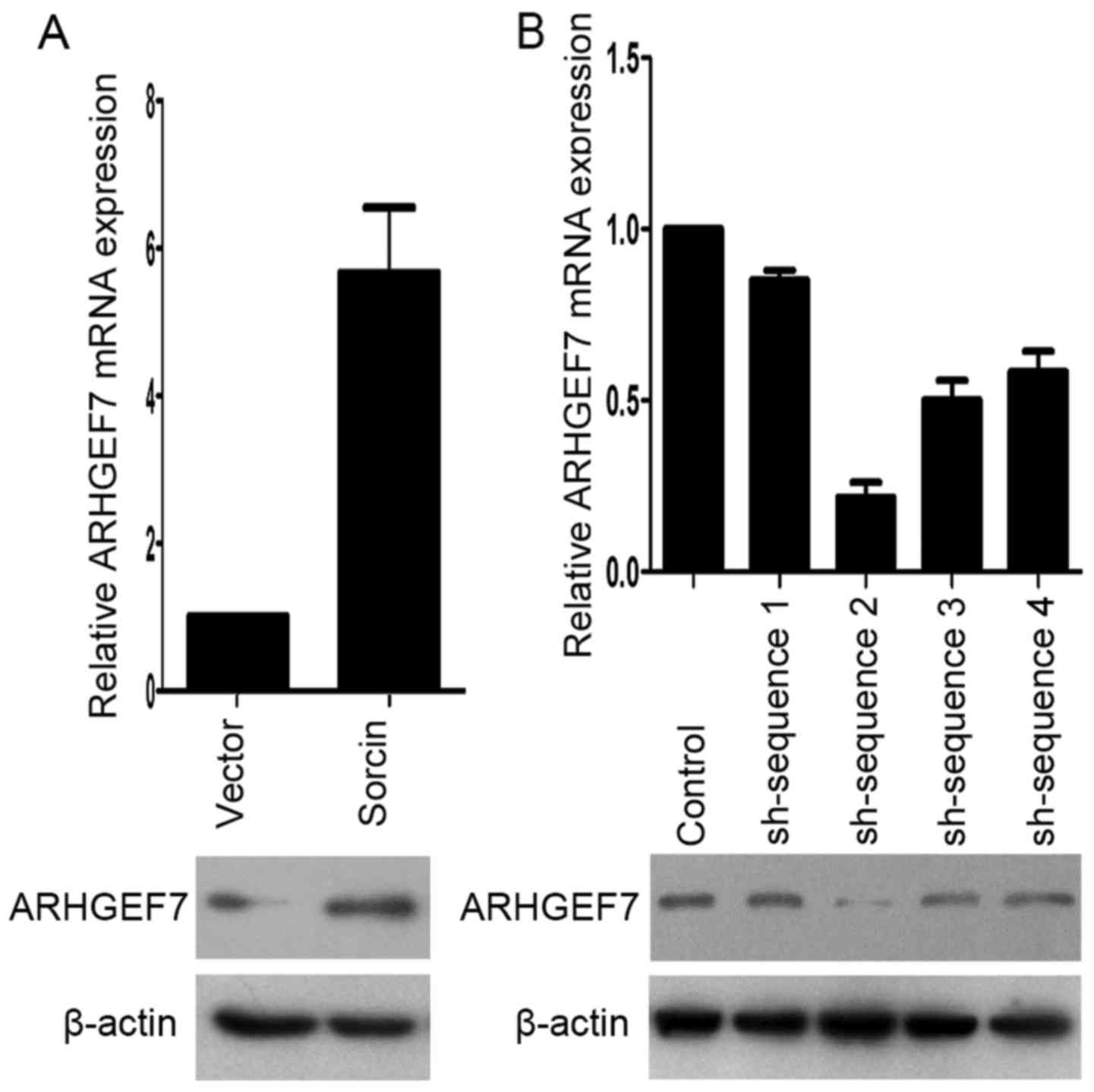
cells (Fig. 2A) and was stably
knocked down in LoVo cells (Fig.
2B) according to the expression level of ARHGEF7 and biological
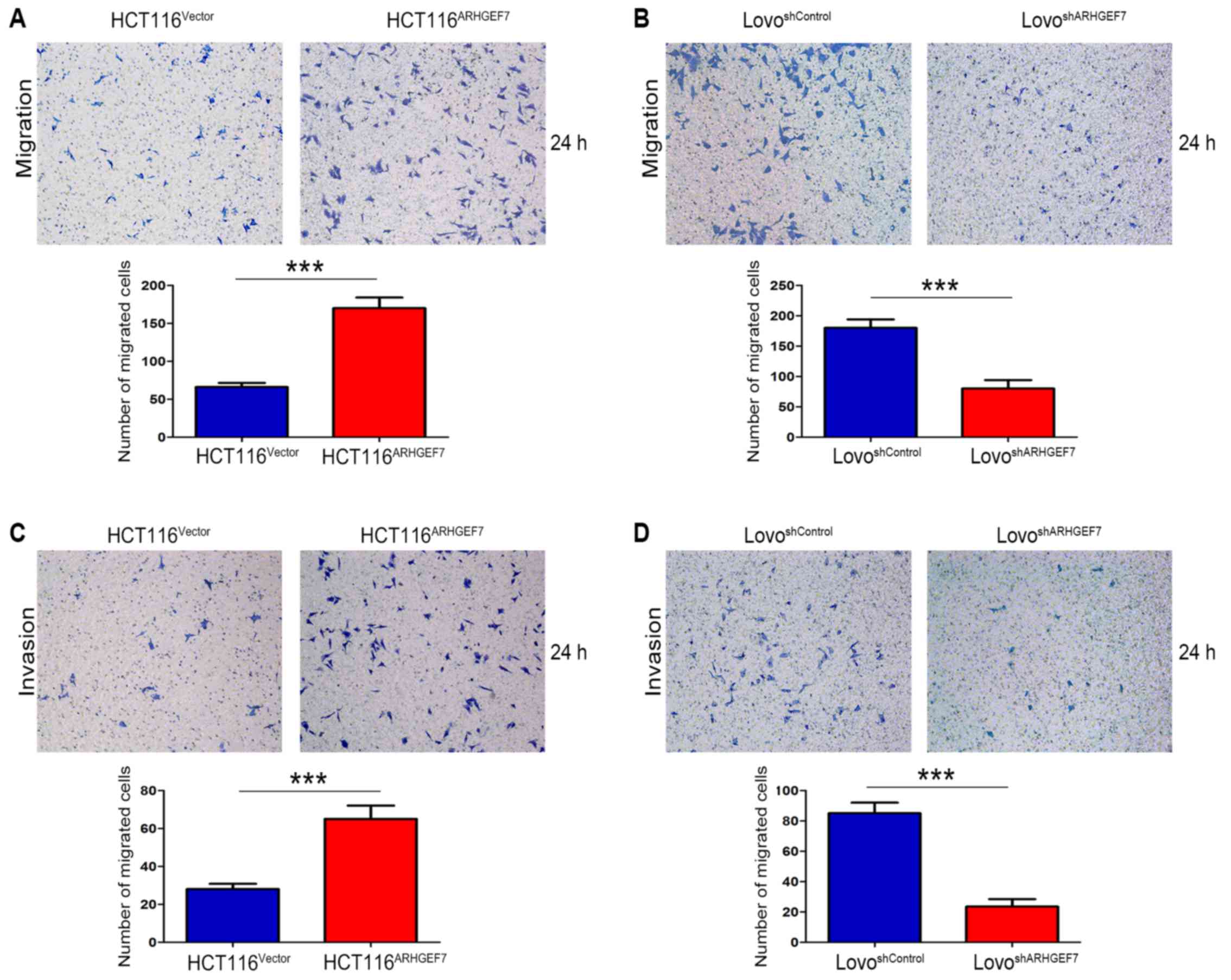
characteristics of CRC cell lines. The Transwell migration assays
showed that the overexpression of ARHGEF7 significantly increased
motility of HCT116 cells (Fig.
3A), whereas the downregulation of ARHGEF7 decreased the
motility of LoVo cells (Fig. 3B).
Similar results were observed in the Transwell invasion assays
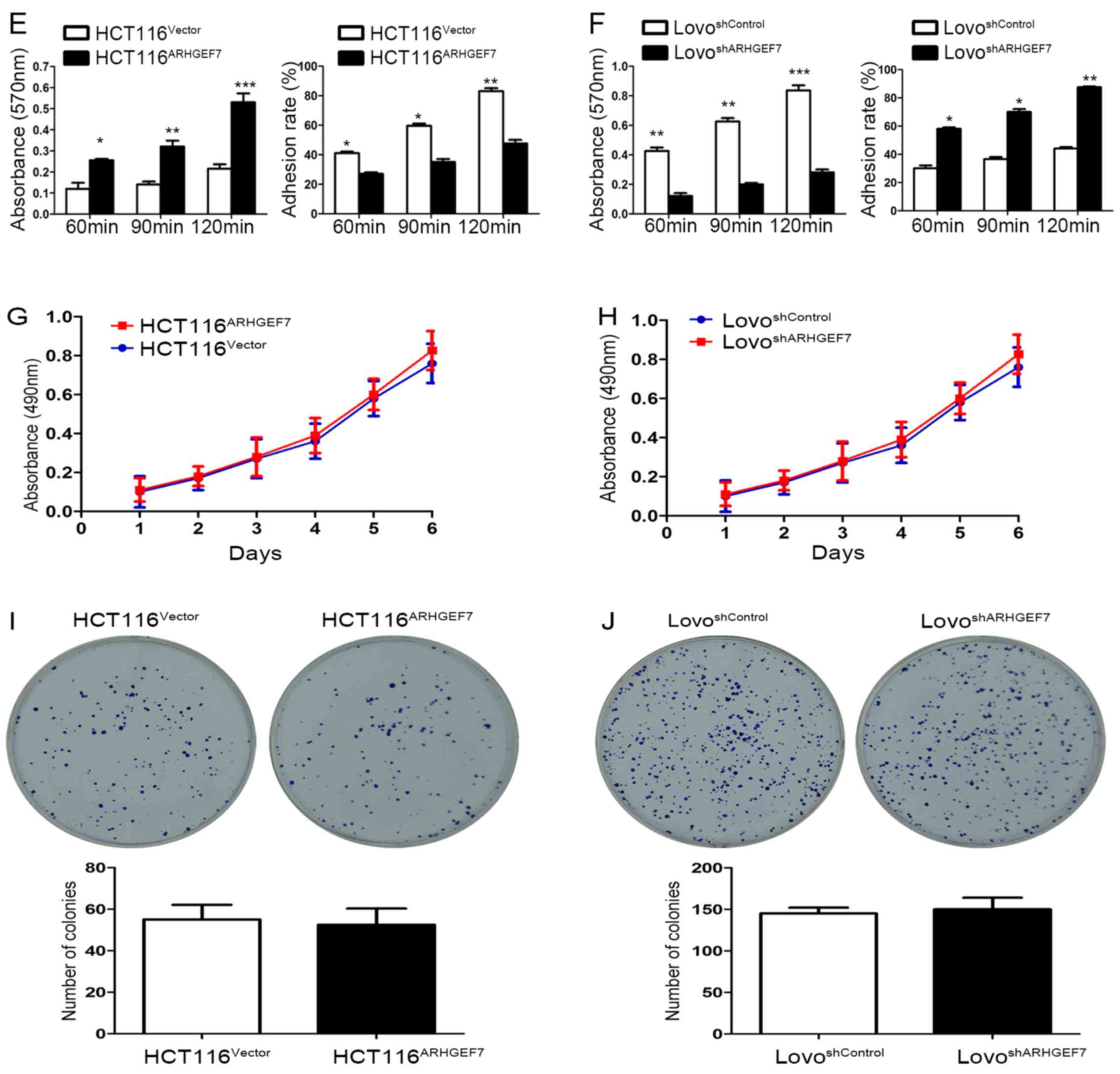
(Fig. 3C and D). Adhesion assays
showed that ARHGEF7 knockdown suppressed cell-ECM adhesion and
enhanced cell-cell adhesion (Fig.
3E and F), whereas the overexpression of ARHGEF7 increased
cell-ECM adhesion and inhibited cell-cell adhesion (Fig. 3E and F). Subsequently, the effect
of ARHGEF7 on the proliferation of CRC cells was examined. The MTT
assays showed that the overexpression of ARHGEF7 had minimal effect
on HCT116 cell proliferation (Fig.
3G). Similarly, the downregulation of ARHGEF7 did not affect
LoVo cell proliferation (Fig. 3H).
The colony formation assays also showed that the capacity of colony
formation of HCT116ARHGEF7 cells was almost equal to
that of HCT116Vector cells (Fig. 3I). The colony formation capacity of
the LoVoshSocrin cells was similar to that of the
LoVoshControl cells (Fig.
3J). These data indicated that ARHGEF7 was involved in
promoting colorectal adenocarcinoma cell motility and invasion
in vitro.
ARHGEF7 enhances the invasion and
metastasis of colorectal adenocarcinoma cells in vivo
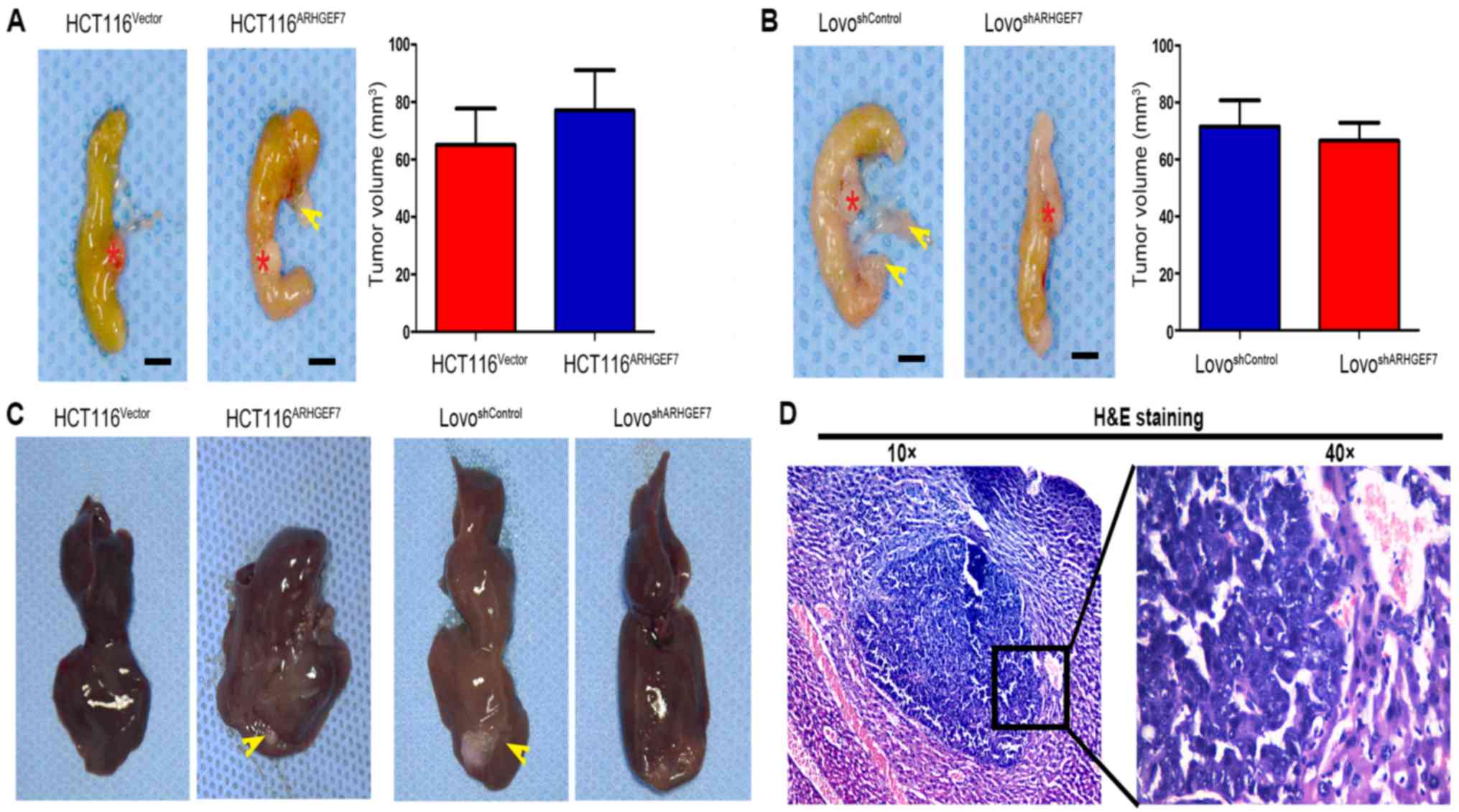
The role of ARHGEF7 in colorectal adenocarcinoma was
further evaluated using in situ xenograft metastasis mice
models. Following 7 weeks of cell inoculation, the tumor volume of
orthotopic mice colon tumors was analyzed. Compared with orthotopic
implantation of HCT116Vector cells, mice with
implantation of HCT116ARHGEF7 cells had the similar
tumor incidence (Table I) and
tumor volume in the colon (P>0.05; Fig. 4A). However, mice with implantation
of HCT116ARHGEF7 cells had increased peritoneum
incidence of regional mesenteric lymph node metastasis (P=0.031;
Fig. 4A; Table I) and liver metastasis (P=0.038;
Fig. 4C and D; Table I) than mice with implantation of
HCT116Vector cells. In the mice implanted with LoVo
cells, the tumor incidence and volume of orthotopic colon tumors
derived from the LoVoshSocrin cells was not
significantly smaller than that of orthotopic colon tumors derived
from LoVoshControl cells (P>0.05; Fig. 4B; Table I). However, mice receiving
implantation of LoVoshSocrin cells showed lower
incidence of regional mesenteric lymph node metastasis (P=0.010;
Fig. 4B; Table I) and liver metastasis (P=0.031;
Fig. 4C and D; Table I) than the control. Taken together,
these results demonstrated that ARHGEF7 promoted colorectal
adenocarcinoma metastasis in vivo.
 | Table IHuman colon cancer cells implanted in
the colon of nude mice. |
Table I
Human colon cancer cells implanted in
the colon of nude mice.
| Group | Tumor
incidence | P-value | Incidence of lymph
node metastasis | P-value | Hepatic
metastasis | P-value |
|---|
|
HCT116Vector | 6/8 | 0.450 | 0/8 | 0.031 | 0/8 | 0.038 |
|
HCT116ARHGEF7 | 8/8 | | 5/8 | | 4/8 | |
|
LoVoshControl | 8/8 | 0.500 | 8/8 | 0.010 | 8/8 | 0.031 |
|
LoVoshARHGEF7 | 7/8 | | 2/8 | | 3/8 | |
ARHGEF7 promotes colorectal
adenocarcinoma metastasis by regulating actin cytoskeleton
rearrangements
The above results showed that ARHGEF7 promoted
colorectal adenocarcinoma metastasis by facilitating cell motility,
which is associated with cytoskeletal reorganization by
polymerization of filamentous actin (F-actin) (24). Therefore, the present study
investigated the effect of ARHGEF7 on regulating the actin
cytoskeleton in CRC cell motility by staining F-actin. F-actin
immunofluorescence staining was used to analyze the cytoskeleton.
The immunofluorescence analysis showed that, compared with the
HCT116Vector cells, the appearance of F-actin fibers was
present in the HCT116ARHGEF7 cells, which had an
increase in filopodia and lamellipodia (Fig. 5A). By contrast, the
LoVoshARHGEF7 cells exhibited loosely organized F-actin
and shrinkable parallel bundles stress fibers relative to the
LoVoshControl cells (Fig.
5B). ARHGEF7 is an identified guanine nucleotide exchange
factor for Cdc42/Rac (10), which
is involved in cell migration by the extension and maintenance of
filopodia (25,26). The effect of ARHGEF7 on the
GTP/GDP-binding status of Rac1 and Cdc42 was determined. The
results showed that GTP-Cdc42 and GTP-Rac1 activation was
significantly increased in the ARHGEF7-overexpressing HCT116 cells
(Fig. 5C and D). By contrast, in
the LoVo cells, basal Rac1 and Cdc42 activities were marked reduced
by ARHGEF7 knockdown (Fig. 5C and
D). Therefore, these data indicated that ARHGEF7 may be associated
with colorectal adenocarcinoma metastasis by regulating actin
cytoskeleton rearrangements.
High expression of ARHGEF7 correlates
with aggressive clinicopathological characteristics and predicts
poor prognosis in patients with colorectal adenocarcinoma
It has been demonstrated that ARHGEF7 is involved in
colorectal adenocarcinoma metastasis, which is the main causative
factor for poor prognosis in colorectal adenocarcinoma. Therefore,
the present study further determined whether the expression of
ARHGEF7 was clinically relevant to colorectal adenocarcinoma
according to ReMARK guidelines for reporting prognostic biomarkers
in cancer (18). An IHC method was
used to assay two sets of colorectal adenocarcinoma samples from
two independent cohorts, including training and validation cohorts
(Fig. 6). The clinicopathologic
characteristics of the patients in these two sets are shown in
Table II. Based on the training
cohort, the results showed that the expression of ARHGEF7 was
mainly located in the cytoplasm (Fig.
7A), and the expression level of ARHGEF7 was significantly
higher in tumor tissues than in adjacent NCTs (Fig. 7B). An ROC curve for metastasis was
utilized to determine the cut-off value according to the results of
the IHC staining analysis (Fig.
7C). The highest Youden index was the cut-off value, defined as
4, thus an IS of 4 was selected as a cut-off value for low
expression of ARHGEF7. In the training cohort, a high expression of
ARHGEF7 was found in 60.0% (108/180) of the tumor tissues, compared
with only 20% (36/180) of the adjacent NCTs (P<0.001; Table III). A high expression of ARHGEF7
was significantly correlated with lymph node, mesenteric and
distant metastasis (Table IV).
Following analysis with the Kaplan-Meier method and log- rank test,
patients with colorectal adenocarcinoma with a high expression of
ARHGEF7 had either shorter DFS (P<0.001; Fig. 7D) or shorter OS (P=0.003; Fig. 7E), compared with patients with a
low expression of ARHGEF7. Finally, to determine whether high
expression of ARHGEF7 was an independent prognostic factor for
colorectal adenocarcinoma, univariate analysis was first performed
followed by multivariate Cox proportional hazards analysis. The
data showed a high expression of ARHGEF7 as an independent risk
factor for DFS (HR 3.541, 95% CI 1.959-6.400, P<0.001; Table V) and OS (HR 2.050, 95% CI
1.157-3.633, P=0.012; Table V) in
colorectal adenocarcinoma.
 | Table IIClinicopathologic characteristics of
patients with colorectal adenocarcinoma in the training cohort and
validation cohort. |
Table II
Clinicopathologic characteristics of
patients with colorectal adenocarcinoma in the training cohort and
validation cohort.
| Clinicopathologic
variable | Training cohort (n)
(n=180) | Validation cohort
(n) (n=150) | P-value |
|---|
| Sex | | | |
| Female | 75 | 65 | 0.760 |
| Male | 105 | 85 | |
| Age (years) | | | |
| ≤60 | 89 | 70 | 0.615 |
| >60 | 91 | 80 | |
| CEA (ng/ml) | | | |
| ≤5 | 110 | 98 | 0.429 |
| >5 | 70 | 52 | |
| Tumor
differentiation | | | |
| I/II | 83 | 67 | 0.454 |
| III/IV | 97 | 83 | |
| Tumor site | | | |
| Colon | 78 | 64 | 0.903 |
| Rectum | 102 | 86 | |
| Tumor size | | | |
| ≤5 cm | 94 | 88 | 0.241 |
| >5 cm | 86 | 62 | |
| pT stage | | | |
| T1/T2 | 56 | 34 | 0.086 |
| T3/T4 | 124 | 116 | |
| pN stage | | | |
| N0 | 85 | 63 | 0.342 |
| N+ | 95 | 87 | |
|
Lymphatic/microvascular/ | | | |
| nerve invasion | | | |
| Negative | 81 | 61 | 0.429 |
| Positive | 99 | 89 | |
| Mesenteric
tumor | | | |
| deposit
formation | | | |
| Negative | 114 | 91 | 0.619 |
| Positive | 66 | 59 | |
| Distant
metastasis | | | |
| Negative | 98 | 83 | 0.872 |
| Positive | 82 | 67 | |
 | Table IIIImmunohistochemical analysis of the
protein expression of ARHGEF7 in tumor tissues and NCTs from the
training and validation cohort. |
Table III
Immunohistochemical analysis of the
protein expression of ARHGEF7 in tumor tissues and NCTs from the
training and validation cohort.
| Training cohort
| Validation cohort
|
|---|
ARHGEF7 expression
| P-valuea | ARHGEF7 expression
| P-valuea |
|---|
| Type | High | Low | High | Low |
|---|
| Tumor | 108 | 72 | <0.001 | 93 | 57 | <0.001 |
| NCT | 36 | 144 | | 45 | 105 | |
 | Table IVCorrelations between expression of
ARHGEF7 in CRC tissues and clinicopathologic variables of patients
with CRC in the training cohort. |
Table IV
Correlations between expression of
ARHGEF7 in CRC tissues and clinicopathologic variables of patients
with CRC in the training cohort.
| Clinicopathologic
variable | n | ARHGEF7 expression
| P-value |
|---|
| Low (n=72) | High (n=108) |
|---|
| Sex | | | | |
| Female | 75 | 35 | 40 | 0.123 |
| Male | 105 | 37 | 68 | |
| Age (years) | | | | |
| ≤60 | 89 | 38 | 51 | 0.465 |
| >60 | 91 | 34 | 57 | |
| CEA (ng/ml) | | | | |
| ≤5 | 110 | 49 | 61 | 0.119 |
| >5 | 70 | 23 | 47 | |
| Tumor
differentiation | | | | |
| I/II | 83 | 43 | 40 | 0.003 |
| III/IV | 97 | 29 | 68 | |
| Tumor site | | | | |
| Colon | 78 | 31 | 47 | 0.951 |
| Rectum | 102 | 41 | 61 | |
| Tumor size | | | | |
| ≤5 cm | 94 | 40 | 54 | 0.465 |
| >5 cm | 86 | 32 | 54 | |
| pT stage | | | | |
| T1/T2 | 56 | 33 | 23 | 0.001 |
| T3/T4 | 124 | 39 | 85 | |
| pN stage | | | | |
| N0 | 85 | 47 | 38 | <0.001 |
| N+ | 95 | 25 | 70 | |
|
Lymphatic/microvascular/nerve
invasion | | | | |
| Negative | 81 | 42 | 39 | 0.003 |
| Positive | 99 | 30 | 69 | |
| Mesenteric tumor
deposit formation | | | | |
| Negative | 114 | 54 | 60 | 0.008 |
| Positive | 66 | 18 | 48 | |
| Distant
metastasis | | | | |
| Negative | 98 | 51 | 47 | <0.001 |
| Positive | 82 | 21 | 61 | |
 | Table VCox proportional hazard regression
analyses for DFS and OS in the training cohort. |
Table V
Cox proportional hazard regression
analyses for DFS and OS in the training cohort.
| Variable | n | DFS
| OS
|
|---|
Univariate analysis
| Multivariate
analysis
| Univariate analysis
| Multivariate
analysis
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
| Female | 75 | Reference | | | | Reference | | | |
| Male | 105 | 1.051
(0.609-1.814) | 0.298 | | NA | 1.176
(0.739-2.833) | 0.271 | | NA |
| Age (years) |
| ≤60 | 89 | Reference | | | | Reference | | | NA |
| >60 | 91 | 1.192
(0.715-1.987) | 0.180 | | NA | 1.207
(0.572-2.137) | 0.170 | | |
| CEA (ng/ml) |
| ≤5 | 110 | Reference | | Reference | | Reference | | Reference | |
| >5 | 70 | 2.782
(1.948-4.083) | 0.003 | 1.615
(1.271-2.052) | 0.039 | 2.064
(1.356-3.142) | 0.008 | 1.432
(1.083-2.348) | 0.043 |
| Tumor
differentiation |
| I/II | 83 | Reference | | Reference | | Reference | | Reference | |
| III/IV | 97 | 1.521
(1.034-2.416) | 0.045 |
1.007(0.634-1.629) | 0.102 | 1.773
(1.349-3.082) | 0.039 | 1.275
(0.802-2.027) | 0.218 |
| Tumor site |
| Colon | 78 | Reference | | | | Reference | | | |
| Rectum | 102 | 1.206
(0.803-1.811) | 0.127 | | NA | 1.216
(0.835-2.238) | 0.103 | | NA |
| Tumor size |
| ≤5 cm | 94 | Reference | | Reference | | Reference | | Reference | |
| >5 cm | 86 | 2.033
(1.080-3.824) | 0.028 | 1.594
(0.914-2.780) | 0.073 | 2.078
(1.123-3.845) | 0.026 | 1.602
(0.923-2.781) | 0.057 |
| pT stage |
| T1/T2 | 56 | Reference | | Reference | | Reference | | Reference | |
| T3/T4 | 124 | 2.175
(1.532-4.782) | 0.012 | 1.876
(1.068-3.296) | 0.024 | 2.179
(1.723-3.835) | 0.010 | 1.832
(1.214-2.907) | 0.035 |
| pN stage |
| N0 | 85 | Reference | | Reference | | Reference | | Reference | |
| N+ | 95 | 4.309
(2.908-6.385) | <0.001 | 2.524
(1.715-3.715) | 0.001 | 4.204
(2.841-6.221) | <0.001 | 1.918
(1.534-3.235) | 0.022 |
|
Lymphatic/microvascular/nerveinvasion |
| Negative | 81 | Reference | | Reference | | Reference | | Reference | |
| Positive | 99 | 3.034
(1.790-5.145) | 0.005 | 2.175
(1.532-4.782) | 0.010 | 4.914
(3.009-8.025) | <0.001 | 3.190
(1.209-3.967) | 0.001 |
| Mesenteric tumor
deposit formation |
| Negative | 114 | Reference | | Reference | | Reference | | Reference | |
| Positive | 66 | 4.057
(2.485-6.623) | <0.001 | 2.323
(1.219-4.427) | 0.007 | 4.309
(2.908-6.385) | <0.001 | 2.875
(1.733-4.769) | 0.006 |
| ARHGEF7
expression |
| Low | 72 | Reference | | Reference | | Reference | | Reference | |
| High | 108 | 5.854
(3.193-10.733) | <0.001 | 3.541
(1.959-6.400) | <0.001 | 3.377
(2.135-5.342) | 0.003 | 2.050
(1.157-3.633) | 0.012 |
To validate the clinical significance of ARHGEF7 in
colorectal adenocarcinoma, IHC assays of another cohort of CRC
samples from the validation cohort showed that the expression level
of ARHGEF7 was significantly higher in the tumor tissues than in
the adjacent NCTs (Fig. 7B). A
high expression of ARHGEF7 was found in 62.0% (93/150) of the tumor
tissues, compared with only 30% (45/150) of the NCTs (P<0.001;
Table III) from the validation
cohort. A high expression of ARHGEF7 was significantly correlated
with lymph node, mesenteric and distant metastasis (Table VI). Analysis with the Kaplan-Meier
method and log-rank test showed that patients with colorectal
adenocarcinoma with a high expression of ARHGEF7 had either shorter
DFS (P=0.003; Fig. 7F) or shorter
OS (P=0.002; Fig. 7H), compared
with those with a low expression of ARHGEF7. Finally, Cox
proportional hazards analysis showed that a high expression of
ARHGEF7 was an independent risk factor for DFS (HR 3.128, 95% CI
2.536-3.858, P=0.011; Table VII)
and OS (HR 2.801, 95% CI 1.503-5.219, P=0.015; Table VII) in colorectal adenocarcinoma.
Collectively, these data suggested that ARHGEF7 was a potent
prognosticator in addition to promoting colorectal adenocarcinoma
metastasis.
 | Table VICorrelations between the expression
of ARHGEF7in CRC tissues and clinicopathologic variables of
patients with CRC in the validation cohort. |
Table VI
Correlations between the expression
of ARHGEF7in CRC tissues and clinicopathologic variables of
patients with CRC in the validation cohort.
| Clinicopathologic
variable | n | ARHGEF7 expression
| P-value |
|---|
| Low (n=57) | High (n=93) |
|---|
| Sex |
| Female | 65 | 29 | 36 | 0.144 |
| Male | 85 | 28 | 57 | |
| Age (years) |
| ≤60 | 70 | 30 | 40 | 0.139 |
| >60 | 80 | 27 | 53 | |
| CEA (ng/ml) |
| ≤5 | 98 | 38 | 60 | 0.788 |
| >5 | 52 | 19 | 33 | |
| Tumor
differentiation |
| I/II | 67 | 37 | 30 | <0.001 |
| III/IV | 83 | 20 | 63 | |
| Tumor site |
| Colon | 64 | 23 | 41 | 0.653 |
| Rectum | 86 | 34 | 52 | |
| Tumor size |
| ≤5 cm | 88 | 34 | 54 | 0.848 |
| >5 cm | 62 | 23 | 39 | |
| pT stage |
| T1/T2 | 34 | 24 | 10 | <0.001 |
| T3/T4 | 116 | 33 | 83 | |
| pN stage |
| N0 | 63 | 31 | 32 | 0.016 |
| N+ | 87 | 26 | 61 | |
|
Lymphatic/microvascular/ |
| nerve invasion | | | | |
| Negative | 61 | 40 | 21 | <0.001 |
| Positive | 89 | 17 | 72 | |
| Mesenteric tumor
deposit formation |
| Negative | 91 | 35 | 56 | 0.885 |
| Positive | 59 | 22 | 37 | |
| Distant
metastasis |
| Negative | 83 | 39 | 44 | 0.012 |
| Positive | 67 | 18 | 49 | |
 | Table VIICox proportional hazard regression
analyses for DFS and OS in the validation cohort. |
Table VII
Cox proportional hazard regression
analyses for DFS and OS in the validation cohort.
| Variable | n | DFS
| OS
|
|---|
Univariate analysis
| Multivariate
analysis
| Univariate analysis
| Multivariate
analysis
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
| Female | 65 | Reference | | | | Reference | | | |
| Male | 85 | 1.316
(0.782-1.894) | 0.141 | | NA | 1.617
(0.883-2.308) | 0.190 | | NA |
| Age (years) |
| ≤60 | 70 | Reference | | | | Reference | | | |
| >60 | 80 | 1.196
(0.616-2.322) | 0.342 | | NA | 1.104
(0.804-1.623) | 0.239 | | NA |
| CEA (ng/ml) |
| ≤5 | 98 | Reference | | Reference | | Reference | | Reference | |
| >5 | 52 | 1.702
(1.301-2.227) | 0.010 | 1.201
(1.009-1.430) | 0.047 | 1.875
(1.290-2.725) | 0.008 | 1.287
(1.100-1.505) | 0.039 |
| Tumor
differentiation |
| I/II | 67 | Reference | | Reference | | Reference | | Reference | |
| III/IV | 83 | 1.349
(1.072-1.698) | 0.037 | 1.064
(0.804-1.801) | 0.094 | 1.381
(1.012-2.783) | 0.044 | 1.109
(0.803-1.532) | 0.141 |
| Tumor site |
| Colon | 64 | Reference | | | | Reference | | | |
| Rectum | 86 | 1.332
(0.680-2.609) | 0.108 | | NA | 1.206
(0.660-2.153) | 0.204 | | NA |
| Tumor size |
| ≤5 cm | 88 | Reference | | Reference | | Reference | | Reference | |
| >5 cm | 62 | 1.603
(1.108-2.319) | 0.042 | 1.180
(0.904-1.540) | 0.089 | 1.506
(1.041-2.198) | 0.036 | 1.145
(0.937-1.399) | 0.109 |
| pT stage |
| T1/T2 | 34 | Reference | | Reference | | Reference | | Reference | |
| T3/ T4 | 116 | 3.054
(1.625-5.154) | 0.007 | 1.795
(1.187-2.753) | 0.031 | 3.634
(2.327-5.675) | 0.004 | 1.837
(1.351-2.985) | 0.027 |
| pN stage |
| N0 | 63 | Reference | | Reference | | Reference | | Reference | |
| N+ | 87 | 4.725
(3.051-7.317) | <0.001 | 3.314
(2.191-5.013) | 0.005 | 4.982
(3.087-8.040) | <0.001 | 3.526
(2.354-5.282) | 0.006 |
|
Lymphatic/microvascular/nerve
invasion |
| Negative | 61 | Reference | | Reference | | Reference | | Reference | |
| Positive | 89 | 3.004
(2.172-7.624) | 0.007 | 1.934
(1.072-3.489) | 0.044 | 3.582
(2.723-4.712) | 0.009 | 2.036
(1.288-4.732) | 0.20 |
| Mesenteric tumor
deposit formation | | | | | | | | | |
| Negative | 91 | Reference | | | | | | | |
| Positive | 59 | 3.904
(2.605-5.851) | <0.001 | Reference | 0.019 | Reference | <0.001 | Reference | 0.008 |
| ARHGEF7 | | | | 2.524
(1.715-3.715) | | 3.972
(1.433-6.587) | | 2.832
(2.054-3.905) | |
| Low | 57 | Reference | | | | | | | |
| High | 93 | 3.834
(1.972-7.624) | 0.003 | Reference | 0.011 | Reference | 0.002 | Reference | 0.015 |
Discussion
Colorectal adenocarcinoma is a frequently
life-threatening disease with heterogeneous outcomes (27). Surgical resection remains the most
important therapy used for patients with colorectal adenocarcinoma
(3). However, cancer metastasis
presents an obstacle in improving the clinical outcome, which
affects up to 60% of patients with colorectal adenocarcinoma
(28). The detailed molecular
mechanism of metastasis remains to be fully elucidated. The present
study revealed that the expression of ARHGEF7 was increased in
colorectal adenocarcinoma tissues compared with that in NCTs.
Increased expression of ARHGEF7 was found to be positively
correlated with the metastatic potential of colorectal
adenocarcinoma cells, suggesting that ARHGEF7 was involved in
colorectal adenocarcinoma metastasis.
To define the role of ARHGEF7 in metastasis, a
serial of in vitro assays showed that that overexpression of
ARHGEF7 in colorectal adenocarcinoma cells significantly enhanced
cell migration and invasion, whereas the knockdown of ARHGEF7 in
colorectal adenocarcinoma cells significantly decreased cell
migration and invasion. Furthermore, the in vivo assays
showed that the overexpression of ARHGEF7 in colorectal
adenocarcinoma cells facilitated tumor metastasis, whereas the
knockdown of ARHGEF7 in colorectal adenocarcinoma cells
significantly inhibited tumor metastasis. Although the effect of
ARHGEF7 on the proliferation of colorectal adenocarcinoma cells was
not observed, the results were consistent with the involvement of
ARHGEF7 in cell migration and metastasis in other malignancies
(14,29), suggesting that ARHGEF7 possesses
oncogenic properties in promoting metastasis in colorectal
adenocarcinoma.
Cancer metastasis occurs via a process involving
abnormal cell migration (30).
Cell migration, a dynamic physical process, is controlled by the
cytoskeletal system, which includes the dynamics of actin
organization (30). A previous
study showed that ARHGEF7 had a direct regulatory role in promoting
dense actin networks to direct cell migration (29). In the present study, the
immunofluorescences analysis showed that ARHGEF7 promoted the
polymerization of F-actin in colorectal adenocarcinoma cells and
facilitated the formation of filopodia and lamellipodia. The
results further showed that the activation of GTP-Cdc42 and
GTP-Rac1 was significantly increased in the ARHGEF7-overexpressing
colorectal adenocarcinoma cells. By contrast, basal Rac1 and Cdc42
activity was markedly reduced by ARHGEF7 knockdown in colorectal
adenocarcinoma cells, supporting the hypothesis that ARHGEF7 serves
as a GEF protein with activity towards Rac1 and Cdc42 in cell
migration (26).
The prognosis for patients with metastatic disease
remains poor (31), therefore, the
identification of more potent molecular biomarkers for identifying
patient subgroups with high-risk metastasis is urgently required.
The present study also suggested that ARHGEF7 may be a promising
prognostic biomarkers associated with survival rates. The
prognostic significance of the expression of ARHGEF7 was validated
in two independent cohorts according to ReMARK guidelines for
reporting prognostic biomarkers in cancer (18). It was found that a high expression
of ARHGEF7 was significantly correlated with metastasis, and
shorter DFS or shorter OS. A high expression of ARHGEF7 was found
to be an independent prognostic factor in colorectal
adenocarcinoma. However, complex pathways contribute to colorectal
adenocarcinoma progression, and the prediction of efficacy of
ARHGEF7 and outcome in colorectal adenocarcinoma requires
validation for clinical use.
In conclusion, the present study identified the
frequently increased expression of ARHGEF7 in colorectal
adenocarcinoma tissues and this expression pattern was associated
with colorectal adenocarcinoma metastasis. Furthermore, it was
demonstrated that ARHGEF7 promoted metastasis by regulating actin
cytoskeleton rearrangements. Finally, it was shown that a high
expression of ARHGEF7 was clinically relevant, with aggressive
clinicopathological characteristics and poor prognosis in patients
with colorectal adenocarcinoma, when consulting ReMARK guidelines
for reporting prognostic biomarkers in cancer. Collectively, the
data indicated that ARHGEF7 was important in colorectal
adenocarcinoma metastasis and may be an independent potential
prognostic marker for predicting clinical outcome in colorectal
adenocarcinoma.
Funding
This study was supported by the Natural Science
Foundation of Jiangxi, China (grant no. 20142BAB205055) and also
partly supported by the National Natural Science Foundation of
China (grant no. 81702922).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
TL, XL and BX designed the experiments. XL, LD, JL,
ZW, DL, SL, HD, GH, CT and CX performed experiments and analyzed
data. TL, BX, XL and LD provided patient samples and collected
data. TL, XL, JL, ZW wrote and revised the paper.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Institutional Review Boards of the First Affiliated Hospital of
Nanchang University and Jiangxi Pingxiang People’s Hospital, and
was performed in accordance with the Declaration of Helsinki and
current ethical guidelines. Prior informed consent was obtained
from all participants.
Patient consent for publication
Patients provided written informed consent for
publication.
Competing interests
The authors confirm that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Jian Lei
(Department of Pathology, Affiliated Cancer Hospital of Xiangya
School of Medicine, Central South University) for providing
technical support for histology.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
4
|
Kobayashi H, Ueno H, Hashiguchi Y and
Mochizuki H: Distribution of lymph node metastasis is a prognostic
index in patients with stage III colon cancer. Surgery.
139:516–522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reddy GK: Prognostic significance of
increasing lymph node count in stage III colorectal cancer. Clin
Colorectal Cancer. 5:403–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenberg R, Engel J, Bruns C, Heitland W,
Hermes N, Jauch KW, Kopp R, Pütterich E, Ruppert R, Schuster T, et
al: The prognostic value of lymph node ratio in a population-based
collective of colorectal cancer patients. Ann Surg. 251:1070–1078.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh CG, Manser E, Zhao ZS and Ng CP: Lim
L. Beta1PIX, the PAK-interacting exchange factor, requires
localization via a coiled-coil region to promote microvillus-like
structures and membrane ruffles. J Cell Sci. 114:4239–4251.
2001.PubMed/NCBI
|
|
10
|
Lee S, Eom M, Lee SJ, Kim S, Park H and
Park D: βPix-enhanced p38 Activation by
Cdc42/Rac/PAK/MKK3/6-mediated Pathway. J Biol Chem.
276:25066–25072. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuo JC, Han X, Hsiao CT, Yates JR III and
Waterman CM: Analysis of the myosin-II-responsive focal adhesion
proteome reveals a role for β-Pix in negative regulation of focal
adhesion maturation. Nat Cell Biol. 13:383–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Campa F, Machuy N, Klein A and Rudel T: A
new interaction between Abi-1 and betaPIX involved in
PDGF-activated actin cytoskeleton reorganisation. Cell Res.
16:759–770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heidary Arash E, Song KM, Song S, Shiban A
and Attisano L: Arhgef7 promotes activation of the Hippo pathway
core kinase Lats. EMBO J. 33:2997–3011. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Han M, Whetsell W Jr, Wang J, Rich
J, Hallahan D and Han Z: Tax-interacting protein 1 coordinates the
spatiotemporal activation of Rho GTPases and regulates the
infiltrative growth of human glioblastoma. Oncogene. 33:1558–1569.
2014. View Article : Google Scholar :
|
|
15
|
Hsu YH, Lin WL, Hou YT, Pu YS, Shun CT,
Chen CL, Wu YY, Chen JY, Chen TH and Jou TS: Podocalyxin EBP50
ezrin molecular complex enhances the metastatic potential of renal
cell carcinoma through recruiting Rac1 guanine nucleotide exchange
factor ARHGEF7. Am J Pathol. 176:3050–3061. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muñoz-Bellvis L, Fontanillo C,
González-González M, Garcia E, Iglesias M, Esteban C, Gutierrez ML,
Abad MM, Bengoechea O, De Las Rivas J, et al: Unique genetic
profile of sporadic colorectal cancer liver metastasis versus
primary tumors as defined by high- density single-nucleotide
polymorphism arrays. Mod Pathol. 25:590–601. 2012. View Article : Google Scholar
|
|
17
|
Zhang Y, Davis C, Shah S, Hughes D, Ryan
JC, Altomare D and Peña MM: IL-33 promotes growth and liver
metastasis of colorectal cancer in mice by remodeling the tumor
microenvironment and inducing angiogenesis. Mol Carcinog.
56:272–287. 2017. View Article : Google Scholar :
|
|
18
|
McShane LM, Altman DG, Sauerbrei W, Taube
SE, Gion M and Clark GM; Statistics Subcommittee of the NCI-EORTC
Working Group on Cancer Diagnostics: Reporting recommendations for
tumor marker prognostic studies (REMARK). J Natl Cancer Inst.
97:1180–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
20
|
Lei X, Liang Y, Chen J, Xiao S, Lei J, Li
J, Duanmu J, Jiang Q, Liu D, Tang C, et al: Sorcin predicts poor
prognosis and promotes metastasis by facilitating
epithelial-mesenchymal transition in hepatocellular carcinoma. Sci
Rep. 7:100492017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Su, Chen SQ, Chen J, Chen J, He F,
Huang C, Wu D, Lin W, Huang LW, et al: A positive feedback loop
between mesenchymal-like cancer cells and macrophages is essential
to breast cancer metastasis. Cancer Cell. 25:605–620. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
García-Echeverría C, Pearson MA, Marti A,
Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG,
Cozens R, et al: In vivo antitumor activity of NVP-AEW541-A novel,
potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell.
5:231–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yokoi K, Thaker PH, Yazici S, Rebhun RR,
Nam DH, He J, Kim SJ, Abbruzzese JL, Hamilton SR and Fidler IJ:
Dual inhibition of epidermal growth factor receptor and vascular
endothelial growth factor receptor phosphorylation by AEE788
reduces growth and metastasis of human colon carcinoma in an
orthotopic nude mouse model. Cancer Res. 65:3716–3725. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Insall RH and Machesky LM: Actin dynamics
at the leading edge: From simple machinery to complex networks. Dev
Cell. 17:310–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reddy PN, Radu M, Xu K, Wood J, Harris CE,
Chernoff J and Williams DA: p21-activated kinase 2 regulates HSPC
cytoskeleton, migration, and homing via CDC42 activation and
interaction with β-Pix. Blood. 127:1967–1975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
ten Klooster JP, Jaffer ZM, Chernoff J and
Hordijk PL: Targeting and activation of Rac1 are mediated by the
exchange factor beta-Pix. J Cell Biol. 172:759–769. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guinney J, Dienstmann R, Wang X, de
Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda
G, Angelino P, et al: The consensus molecular subtypes of
colorectal cancer. Nat Med. 21:1350–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sahani DV, Bajwa MA, Andrabi Y, Bajpai S
and Cusack JC: Current status of imaging and emerging techniques to
evaluate liver metastases from colorectal carcinoma. Ann Surg.
259:861–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu HW, Chen YQ, Huang CM, Liu CY, Chiou A,
Wang YK, Tang MJ and Kuo JC: β-PIX controls intracellular
viscoelasticity to regulate lung cancer cell migration. J Cell Mol
Med. 19:934–947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pang B, Wu N, Guan R, Pang L, Li X, Li S,
Tang L, Guo Y, Chen J, Sun D, et al: Overexpression of RCC2
enhances cell motility and promotes tumor metastasis in lung
adenocarcinoma by inducing epithelial-mesenchymal transition. Clin
Cancer Res. 23:5598–5610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanoff HK, Sargent DJ, Campbell ME, Morton
RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC
and Goldberg RM: Five-year data and prognostic factor analysis of
oxaliplatin and irinotecan combinations for advanced colorectal
cancer. J Clin Oncol. 26:5721–5727. 2008. View Article : Google Scholar : PubMed/NCBI
|