Introduction
Rho guanine nucleotide exchange factor 4 (ARHGEF4),
which is also referred to as ASEF, has been identified as a
Rac1-specific guanine nucleotide exchange factor (GEF) that
interacts with adenomatous polyposis coli (APC) (1). ARHGEF4 is a Dbl family GEF that
contains an Src homology 3 (SH3) domain followed by the Dbl
homology and pleckstrin homology domains that are characteristic of
Dbl family GEFs, which specifically activate members of the Rho
family of GTPases (2). APC binds
to the NH2-terminal APC-binding region of ARHGEF4 via
its armadillo repeat domain and enhances the GEF activity of
ARHGEF4 against Rac1 and Cdc42, thereby regulating the
reorganization of the actin cytoskeleton, cell morphology,
adhesion, and migration (3–5).
Truncated mutant APC present in colorectal cancer cells strongly
enhance the constitutive GEF activity of ARHGEF4, which upregulates
the expression of matrix metalloproteinase 9 via the c-Jun
NH2-terminal kinase (JNK) pathway, and causes decreased
cell-cell adhesion and aberrant migratory properties (6–8).
Collectively, these reports demonstrated that ARHGEF4 is associated
with motility and invasiveness via its GEF activity.
The majority of patients with pancreatic ductal
adenocarcinoma (PDAC) progress to either locally invasive or
metastatic disease (9); however,
the detailed mechanism by which PDAC cells invade and metastasize
remains unknown. The present authors previously reported that
insulin-like growth factor-2 mRNA-binding protein 3 (IGF2BP3) and
IGF2BP3-bound mRNAs are localized to cytoplasmic RNA granules that
accumulate in cell protrusions of PDAC cells (10,11).
IGF2BP3-bound transcripts, including ARHGEF4, are preferentially
translated in cell protrusions and induce the formation of cell
protrusions; consequently, IGF2BP3 promotes invasiveness and
metastasis (10). These findings
indicate that IGF2BP3 contributes to translational regulation only
in the cell protrusions and that ARHGEF4 translated in the
protrusions is associated with cell invasion and metastasis.
The present study aimed to examine the ARHGEF4
expression in tissue samples of PDAC patients and its association
with clinicopathologic characteristics and survival, and to
evaluate the mechanism of ARHGEF4 in the control of PDAC cell
motility and invasion. It was demonstrated that ARHGEF4 induces the
formation of cell protrusions by increasing phosphorylated
extracellular signal-regulated kinase (ERK)1/2 and glycogen
synthase kinase-3 (GSK-3)α/β, resulting in an increase in motility
and invasiveness in PDAC cells.
Materials and methods
Antibodies
Anti-ARHGEF4 (55213-1-AP) antibody was purchased
from Proteintech Group, Inc. (Chicago, IL, USA). Anti-GSK-3α/β
(sc7291), anti-myc (sc40) and anti-GAPDH (sc32233) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Anti-ERK1/2 (4697) and anti-phosphorylated ERK1/2 (Thr204/Tyr187;
5726) antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Anti-phosphorylated GSK-3α (Ser9; 75814)
and anti-phosphorylated GSK-3β (Tyr216; 75745) antibodies were
purchased from Abcam (Cambridge, MA, USA). Anti-caspase-8 antibody
(LS-C344789) was purchased from LifeSpan BioSciences, Inc.
(Seattle, WA, USA). The JLA20 anti-actin antibody (MABT219) was
purchased from Merck KGaA (Darmstadt, Germany).
Primary human PDAC samples
Tumor tissues were obtained from 102 patients who
underwent surgical treatment for pancreatic intraepithelial
neoplasm (PanIN, n=2) and PDAC (n=100) and received surgical
resection during 1999-2014 at the Departments of Surgery at Kochi
Medical School Hospital (Nankoku, Japan) and Matsuyama Shimin
Hospital (Matsuyama, Japan), as published previously (12). Postoperative follow-up consisted of
physical examination, measurement of serum sialyl Lewis (a) blood
group antigen (CA19-9), which is the clinical standard PDAC tumor
biomarker, and computed tomography at 3–4-month intervals at Kochi
Medical School Hospital (Nankoku, Japan) and Matsuyama Shimin
Hospital (Matsuyama, Japan). The disease status of each patient was
determined at the date of the final follow up in 2014. If patients
succumbed during follow-up, PanIN- or PDAC-associated death was
considered an outcome event. Medical records of the 102 patients
provided information regarding sex, age, tumor diameter, histology,
Union for International Cancer Control (UICC) tumor-node-metastasis
(TNM) stage, venous invasion, lymphatic invasion and postoperative
survival time. Tumors were classified according to the
classification of pancreatic carcinoma of the Japan Pancreas
Society (JPS) (13) and the UICC
TNM classification (14). The JPS
staging system is adequate for analysis of resected PDAC cases, and
the UICC staging system is more suitable for analyses of both
resected and non-resected PDAC patients (15). It is recommended to record the
clinical and pathological information according to both JPS and
UICC staging systems (15). The
present study was approved by the Ethical Review Board of Kochi
Medical School and Matsuyama Shimin Hospital prior to patient
recruitment. In conformity with the principles of the Declaration
of Helsinki, written informed consent was acquired from each
patient prior to initiation.
Immunohistochemical staining
Immunohistochemistry was carried out, as published
previously (12). Tissue sections
from normal pancreas, brain, lung, liver and kidney were purchased
from BioChain Institute, Inc. (Hayward, CA, USA).
Evaluation of ARHGEF4 staining
A total of 10 random microscopic fields (original
magnification, ×400) per slide were evaluated by two independent
observers (SN and MF) who were blinded to clinical and outcome
data. ARHGEF4 expression levels were classified semi-quantitatively
based on the total combined scores of positive-staining tumor cell
percentage (1, <50% reacting cells; 2, 50–80% reacting cells; 3,
>80%) and staining intensity (1, weaker than the intensity of
surface staining in the islet of Langerhans; 2, equal to the
intensity of the islet of Langerhans; 3, stronger than the
intensity of the islet of Langerhans). A total immunohistochemical
score was calculated by summing the percentage score and the
intensity score. ARHGEF4 expression was classified into two groups
based on total score (low group, 2–3; high group, 4–6) in
accordance with previous studies (12,16).
Cell culture
The human PDAC cell line S2-013, a subline of
SUIT-2, was obtained from Dr T. Iwamura (Miyazaki Medical College,
Miyazaki, Japan) (17). The human
PDAC cell line PANC-1 was purchased from the American Type Culture
Collection (Manassas, VA, USA). All cells were maintained in
Dulbecco’s modified Eagle’s medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal calf serum
(FCS; Gibco, Thermo Fisher Scientific, Inc.) at 37°C. In selected
experiments, cells were incubated at 37°C with 30 µM U0126
(Sigma-Aldrich; Merck KGaA), a specific mitogen-activated protein
kinase kinase (MEK) 1/2 inhibitor, for 24 h or with 20 µM
SB216763 (Sigma-Aldrich; Merck KGaA), a specific GSK-3 inhibitor,
for 48 h.
Confocal immunofluorescence
microscopy
Immunocytochemistry was performed as previously
detailed (18,19). Briefly, S2-013 and PANC-1 cells
incubated on fibronectin-coated glass coverslips for 5 h at 37°C
were fixed with 4% paraformaldehyde for 30 min and permeabilized
with 0.1% Triton X-100 for 2 min at room temperature. Cells were
then incubated with anti-ARHGEF4, anti-myc, anti-phosphorylated
ERK1/2 or anti-caspase-8 primary antibodies (all 1:100) for 1 h at
room temperature, followed by incubation with Alexa488- or
Alexa594-conjugated secondary antibodies at 1:500 for 1 h at room
temperature (A32723 and R37117, respectively; Molecular Probes,
Thermo Fisher Scientific, Inc.). Each specimen was visualized using
a Zeiss LSM 510 META microscope (Carl Zeiss AG, Oberkochen,
Germany) at ×80 magnification. Z-stacks of confocal images
(approximately 10 stacks/cell) were taken using a Zeiss LSM 510
META microscope at ×80 magnification.
S2-013 and PANC-1 cells that formed cell protrusions
were counted by two blinded individuals (SN and TS). Four
independent visual fields were counted via microscopic observation
to count the number of cells that formed cell protrusions. Data are
derived from three independent experiments.
Small interfering (si)RNA treatment
A single mixture with four different small
interfering RNA (siRNA) oligonucleotides targeting ARHGEF4
(FlexiTube GeneSolution GS50649) was purchased from Qiagen, Inc.
(Valencia, CA, USA) and a single mixture with four different
scrambled negative control siRNA oligonucleotides (37007) was
obtained from Santa Cruz Biotechnology, Inc.; S2-013 and PANC-1
cells were transfected with 80 pmol of each siRNA mixture in siRNA
transfection reagent (31985-062; HiPerfect Transfection Reagent;
Qiagen, Inc.) following the manufacturer’s protocol. Following
incubation for 48h at 37°C, total cell lysates were extracted, and
immunoblotting was carried out to evaluate the effects of siRNA
treatment.
ARHGEF4-rescue construct
Total RNA were extracted from S2-013 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer’s recommendations, and reverse
transcribed to single-stranded cDNAs using SuperScript III
First-Strand Synthesis system for RT-PCR (18080-051; Invitrogen,
Thermo Fisher Scientific, Inc.) and oligo(dT)12-18 primer for 50
min at 50°C. Polymerase chain reaction (PCR) amplification was
performed using an AmpliTaq Gold DNA Polymerase kit (N8080241;
Invitrogen, Thermo Fisher Scientific, Inc.) and the primer
sequences for ARHGEF4 were forward,
5’-CCAAGCTTATGCCCTGGGAAGAAC CAGC-3’ and reverse,
5’-CCGCTCGAGTTGCTGGCTTG GCTTTGTGG-3’. All reactions were comprised
of initial denaturation at 94°C for 2 min followed by 28 cycles at
94°C for 30 sec, 58°C for 30 sec, and 72°C for 1 min, on a Takara
PCR Thermal Cycler Dice (Takara Bio, Inc., Otsu, Japan). The
product was then inserted into the HindIII and XhoI
sites of pCMV6-Entry vector (Origene Technologies, Inc., Rockville,
MD, USA) bearing a C-terminal myc-DDK-tag. Transient transfection
of resultant ARHGEF4-rescue construct was carried out with
X-tremeGENE HP DNA transfection reagent (Roche Applied Science,
Penzberg, Germany) at room temperature. The transfected cells were
assayed, typically 2 days following transfection.
Immunoblotting analysis of cell
lysates
Total cell lysates were extracted using lysis buffer
[Tris-HCl (pH 7.4), sodium dodecyl sulfate (SDS), mercaptoethanol
and glycerol]. Protein concentrations were determined using Takara
Bradford Protein Assay kit (T9310A; Takara Bio, Inc.), and an equal
amount of protein (10 µg) was separated on a 4–20% gradient
SDS-PAGE (TEFCO, Tokyo, Japan). Proteins were transferred using a
Trans-Blot Turbo RTA Mini LF PVDF Transfer kit (170-4274) to a
TransBlot Turbo Mini-size LF polyvinylidene difluoride membrane
(both from Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
blocked with 5% non-fat dry milk in Tris-buffered saline [10 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% Tween-20] for 1 h at room
temperature. The membranes were then incubated with anti-ARHGEF4,
anti-myc, anti-ERK1/2, anti-phosphorylated ERK1/2, anti-GSK-3α/β,
anti-phosphorylated GSK-3α, anti-phosphorylated GSK-3β,
anti-caspase-8 or anti-GAPDH primary antibodies at dilutions of
1:1,000 in 5% non-fat dry milk in Tris-buffered saline overnight at
4°C. Following incubation with appropriate secondary antibodies
conjugated with horseradish peroxidase (sc2004, sc2005; Santa Cruz
Biotechnology, Inc.) at dilutions of 1:2,000 for 1 h at room
temperature, the immunoreactive bands were visualized using the ECL
Plus kit (GE Healthcare, Chicago, IL, USA) according to the
manufacturer’s instructions.
Transwell motility assay
A Transwell motility assay was carried out, as
detailed previously (10). Cells
(3.0×104) were added to the upper chamber of BD BioCoat
Control Culture inserts (24-well plates, 8-µm pore size; BD
Biosciences, San Jose, CA, USA). A total of 200 µl
serum-free Dulbecco’s modified Eagle’s medium (Gibco; Thermo Fisher
Scientific, Inc.) was added to the top well and 700 µl
medium containing 5% FCS to the bottom well. Following incubation
for 12 h at 37°C, the bound cells were stained with 0.1% crystal
violet solution for 10 min at room temperature, and four
independent visual fields were examined via light microscopy (BX41;
Olympus Corporation, Tokyo, Japan) at x100 magnification to count
the number of cells that had migrated to the bottom chamber. The
assay was conducted three times independently.
Matrigel invasion assay
A Matrigel invasion assay was carried out, as
published previously (10). The
invasion capabilities of PDAC cells were examined using a
two-chamber invasion assay kit (24-well plates, 8-µm pore
size membrane coated with a layer of Matrigel extracellular matrix
proteins; BD Biosciences) according to the manufacturer’s
instructions. The bound cells were stained with 0.1% crystal violet
solution for 10 min at room temperature, and four independent
visual fields were examined via light microscopy (BX41; Olympus
Corporation) at ×100 magnification to count the number of cells
that had moved to the bottom chamber. The assay was conducted three
times independently.
Phospho-kinase array assay
The Proteome Profiler Human Phospho-Kinase Array kit
(ARY003) was purchased from R&D Systems, Inc. (Minneapolis, MN,
USA) and screened according to the manufacturer’s protocol, as
published previously (20).
Subcellular fractionation
S2-013 and PANC-1 cells were treated with 100
µM cytochalasin D (22144-77-0; Fermentek, Ltd., Jerusalem,
Israel) for 12 h at 37°C, and then incubated on fibronectin for 5 h
at 37°C. The cells were extracted for 10 min on ice in lysis buffer
[100 mM PIPES (pH 6.8), 1 mM EGTA, 1 mM MgCl2, 1% Triton
X-100 (Nacalai Tesque, Inc., Kyoto, Japan)] containing 2 µM
phalloidin, protease inhibitor cocktail tablets (Roche Applied
Science), and phosphatase inhibitor cocktail (Nacalai Tesque,
Inc.). Following centrifugation at 10,000 × g for 5 min at 4°C, the
soluble fraction was removed, and the residual insoluble fraction
was washed once with lysis buffer and scraped with sample buffer
[50 mM Tris-HCL (pH 6.8), 2% SDS, 0.02% bromophenol blue, 1%
β-mercaptoethanol and 10% glycerol]. Equal amounts of soluble and
insoluble fractions (10 µg) were subjected to a 4–20%
gradient SDS-PAGE (TEFCO) and analyzed by immunoblotting using
anti-ARHGEF4 and anti-actin antibodies with dilution at
1:1,000.
Statistical analysis
For immunohistochemical analysis, statistical
analyses were performed using R (version 3.3.3; The R Foundation,
Wien, Austria), as published previously (12). Cumulative survival rates were
calculated using the Kaplan-Meier method and were compared using
the log-rank test (Mantel-Cox). Survival rates are expressed as the
median value and interquartile range (IQR). Univariate Cox
regression analysis was performed to determine the prognostic
significance of individual clinicopathological factors. Cox
proportional hazard models were used for multivariate analysis of
independent factors for overall survival. For the in vitro
experiments, statistical significance was evaluated by using
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
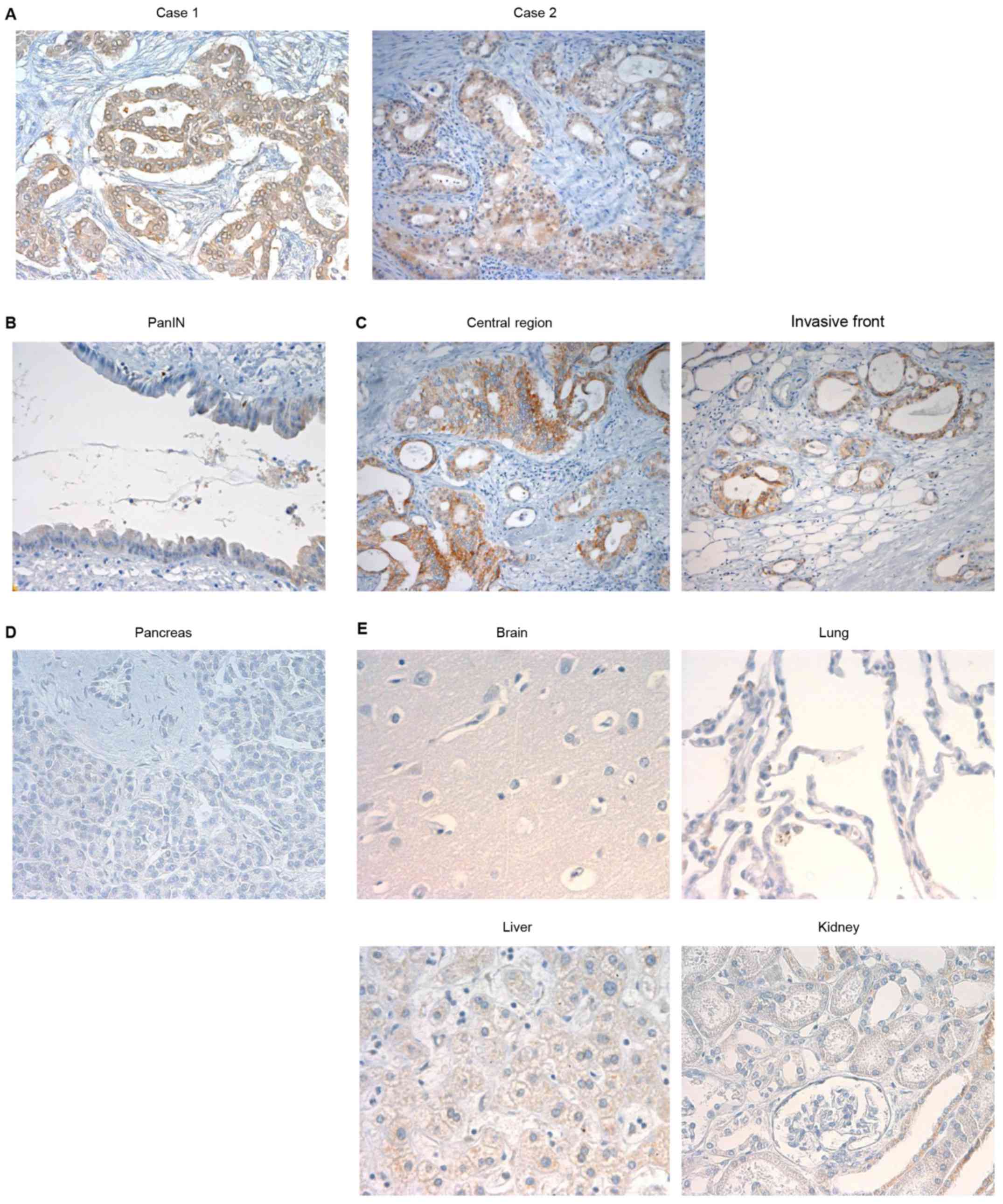
ARHGEF4 expression in PDAC samples
The potential significance of ARHGEF4 protein
expression was examined in surgical specimens from 102 patients
with PanIN (n=2) and PDAC (n=100) by immunohistochemical analysis.
PDAC is thought to arise from precursor lesions, PanIN (21). ARHGEF4 demonstrated positive
immunoreactivity in all 102 cases, and the specimens were
classified into low-expressing (62.7%) and high-expressing (37.3%)
ARHGEF4 groups (Table I). ARHGEF4
was localized in the cytoplasm of PDAC cells (Fig. 1A), whereas ARHGEF4 was
low-expressing in PanIN cells, indicating that PanINs do not
contain ARHGEF4 (Fig. 1B). The
level of ARHGEF4 expression at the invasive front of the tumor was
not increased when compared with the central region of the tumor
(Fig. 1C). ARHGEF4
immunoreactivity was not observed in normal pancreatic ducts
(Fig. 1D). In addition, normal
brain, lung, liver and kidney were not stained by the anti-ARHGEF4
antibody (Fig. 1E).
 | Table ISummary of characteristics in 102
patients with pancreatic cancer. |
Table I
Summary of characteristics in 102
patients with pancreatic cancer.
| Characteristic | Patients, n | % of total |
|---|
| Age at surgery,
years | | |
| 40–50 | 4 | 3.9 |
| 50–60 | 17 | 16.7 |
| 60–70 | 32 | 31.4 |
| 70–80 | 41 | 40.2 |
| >80 | 8 | 7.8 |
| Sex | | |
| Male | 56 | 54.9 |
| Female | 46 | 45.1 |
| Stagea | | |
| 0 | 2 | 2.0 |
| IA | 4 | 3.9 |
| IB | 8 | 7.8 |
| IIA | 32 | 31.4 |
| IIB | 50 | 49.0 |
| III | 2 | 2.0 |
| IV | 4 | 3.9 |
| Primary
tumora | | |
| Tis | 2 | 2.0 |
| T1 | 6 | 5.9 |
| T2 | 15 | 14.6 |
| T3 | 77 | 75.5 |
| T4 | 2 | 2.0 |
| Regional lymph
nodesa | | |
| N0 | 46 | 45.1 |
| N1 | 56 | 54.9 |
| Distant
metastasisa | | |
| M0 | 98 | 96.1 |
| M1 | 4 | 3.9 |
| Histologyb | | |
| PanIN | 2 | 2.0 |
| Well | 31 | 30.4 |
| Moderate | 57 | 55.8 |
| Poor | 12 | 11.8 |
| Venous
invasionb | | |
| v0 | 57 | 55.4 |
| v1 | 31 | 30.7 |
| v2 | 11 | 10.9 |
| v3 | 3 | 3.0 |
| Lymphatic
invasionb | | |
| ly0 | 43 | 42.6 |
| ly1 | 34 | 33.6 |
| ly2 | 21 | 19.9 |
| ly3 | 4 | 3.9 |
| ARHGEF4
expression | | |
| Low | 64 | 62.7 |
| High | 38 | 37.3 |
Association among ARHGEF4 expression,
clinicopathological characteristics and survival
The associations between ARHGEF4 expression and
clinicopathological factors are presented in Table II. No significant associations
were noted between ARHGEF4 expression and any clinicopathological
parameters.
 | Table IICorrelation between ARHGEF4
expression and clinicopathological parameters. |
Table II
Correlation between ARHGEF4
expression and clinicopathological parameters.
| Parameter | ARHGEF4 expression
| P-value |
|---|
Low
| High
|
|---|
| % of total | Patients, n | % of total | Patients, n |
|---|
| Stagea | | | | | 0.912 |
| 0 | 3.2 | 2 | 0.0 | 0 | |
| IA | 4.7 | 3 | 2.6 | 1 | |
| IB | 9.3 | 6 | 5.3 | 2 | |
| IIA | 28.1 | 18 | 36.9 | 14 | |
| IIB | 48.4 | 31 | 50.0 | 19 | |
| III | 1.6 | 1 | 2.6 | 1 | |
| IV | 4.7 | 3 | 2.6 | 1 | |
| Primary
tumora | | | | 0.595 | |
| Tis | 3.2 | 2 | 0.0 | 0 | |
| T1 | 7.7 | 5 | 2.6 | 1 | |
| T2 | 12.5 | 8 | 18.4 | 7 | |
| T3 | 75.0 | 48 | 76.4 | 29 | |
| T4 | 1.6 | 1 | 2.6 | 1 | |
| Regional lymph
nodesa | | | | | 1 |
| N0 | 45.3 | 29 | 47.4 | 18 | |
| N1 | 54.7 | 35 | 52.6 | 20 | |
| Distant
metastasisa | | | | | 1 |
| M0 | 95.3 | 61 | 97.4 | 37 | |
| M1 | 4.7 | 3 | 2.6 | 1 | |
| Histologyb | | | | | 0.117 |
| PanIN | 3.2 | 2 | 0.0 | 0 | |
| Well | 34.3 | 22 | 23.6 | 9 | |
| Moderate | 56.2 | 36 | 55.3 | 21 | |
| Poor | 6.3 | 4 | 21.1 | 8 | |
| Venous
invasionb | | | | | 0.173 |
| v0+v1 | 87.5 | 56 | 84.2 | 32 | |
| v2+v3 | 12.5 | 8 | 15.8 | 6 | |
| Lymphatic
invasionb | | | | | 0.636 |
| ly0+ly1 | 73.4 | 47 | 78.9 | 30 | |
| ly2+ly3 | 26.6 | 17 | 21.1 | 8 | |
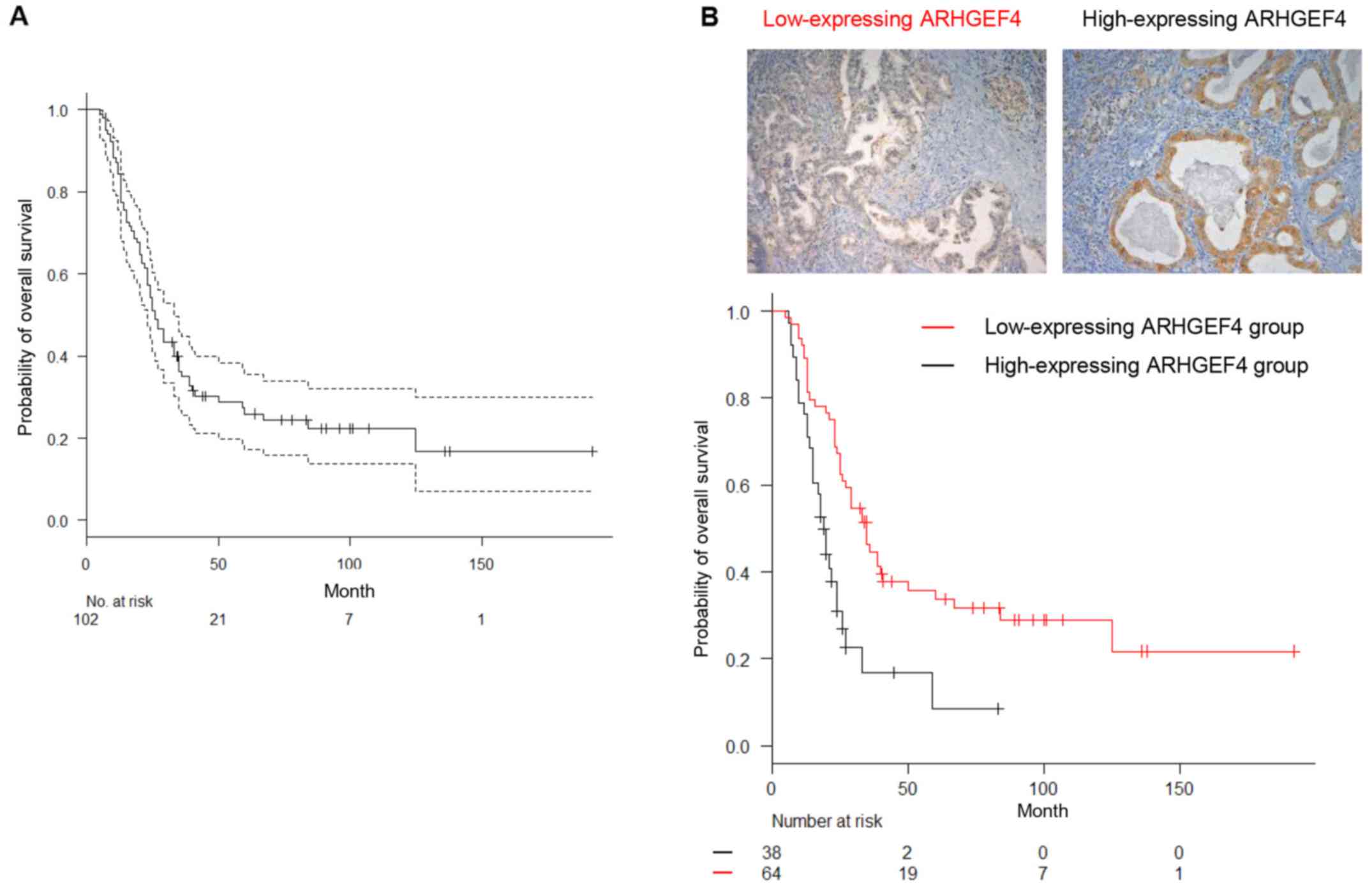
Subsequently, the correlation between ARHGEF4
expression and PDAC patient prognosis was examined. The follow-up
period for the 102 PDAC survivors ranged from 18–192 months
(median, 64.0 months). The overall survival time for PDAC patients
with high ARHGEF4 expression was significantly shorter than that of
PDAC patients with low ARHGEF4 expression on Kaplan-Meier curves
(P<0.001; Fig. 2). In relation
to clinical outcome, univariate and multivariate analyses revealed
that stage III and IV and high ARHGEF4 expression were independent
predictors of worse overall survival (Table III). These results suggested that
ARHGEF4 participated in PDAC progression.
 | Table IIIUnivariate and multivariate analysis
of prognostic factors for overall survival. |
Table III
Univariate and multivariate analysis
of prognostic factors for overall survival.
| Parameter | Overall survival
|
|---|
Univariate
| Multivariate
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Stagea | | | | |
| 0+IA+IB | 1.0
(reference) | 0.549 | 1.0
(reference) | |
| IIA | 1.159
(0.714–1.881) | 0.196 | 4.638
(1.593–13.50) | 0.004 |
| IIB | 1.356
(0.854–2.151) | 0.010 | 5.446
(1.911–15.52) | 0.001 |
| III+IV | 3.035
(1.301–7.081) | | 13.71
(3.748–50.18) | <0.001 |
| Age | 1.021
(0.995–1.048) | 0.110 | 1.015
(0.989–1.043) | 0.255 |
| Sex | 1.107
(0.696–1.761) | 0.666 | 1.192
(0.742–1.913) | 0.468 |
| ARHGEF4
expression | 0.345
(0.213–0.559) | <0.001 | 0.411
(0.239–0.706) | 0.001 |
| Diameter of primary
tumor | 1.338
(1.176–1.524) | <0.001 | | |
| Histologyb | 1.383
(0.846–2.261) | 0.196 | | |
| Lymphatic
invasionb (ly0+ly1 or
ly2+ly3) | 1.269
(0.751–2.145) | 0.373 | | |
| Venous
invasionb (v0+v1 or v2+v3) | 1.928
(1.034–3.593) | 0.038 | | |
| Intrapancreatic
nerve invasionb (n0+n1 or
n2+n3) | 1.500
(0.947–2.377) | 0.083 | | |
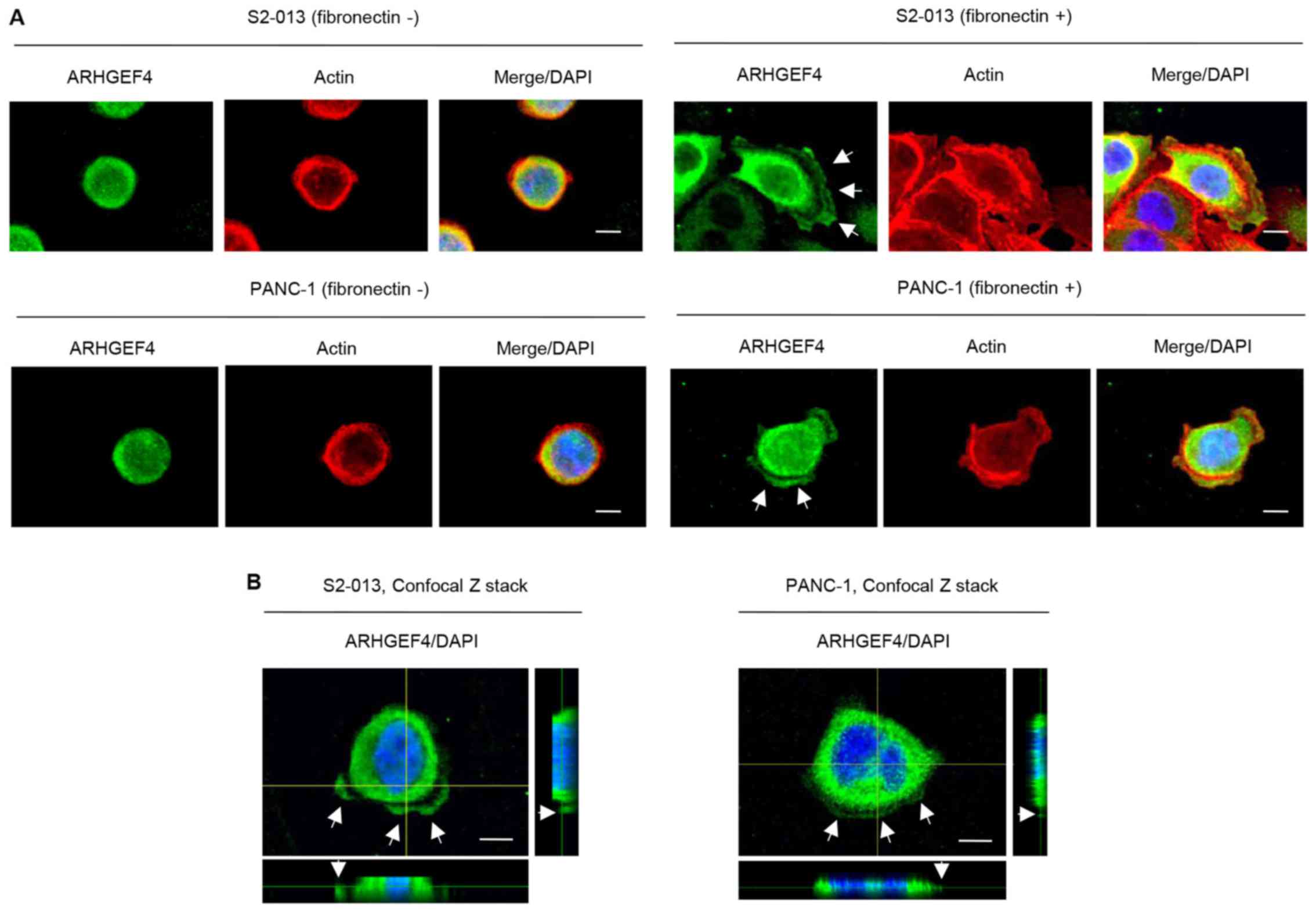
Subcellular localization of ARHGEF4 in
PDAC cells
Immunocytochemistry was used to investigate the
subcellular localization of ARHGEF4 in moderately differentiated
PDAC cells (line S2-013) (17) and
cells from a poorly differentiated PDAC line (PANC-1) (22). Fibronectin induces the formation of
cell protrusions at the leading edge of PDAC cells (23,24).
There were fewer cell protrusions formed by S2-013 and PANC-1 cells
when the cells were cultured without fibronectin than when the
cells are grown on fibronectin (12,20).
In S2-013 and PANC-1 cells grown on fibronectin, ARHGEF4 was mainly
present in the cytoplasm of cell bodies, and ARHGEF4 was also
localized in cell protrusions containing many peripheral actin
structures (Fig. 3A). ARHGEF4,
which accumulates in cell protrusions, accumulated in abundance in
the S2-013 and PANC-1 cells cultured on fibronectin compared with
the corresponding cells that were not cultured on fibronectin
(Fig. 3A). S2-013 cells grown on
fibronectin exhibited intracellular expression of ARHGEF4 in cell
protrusions in Z stack panels (Fig.
3B).
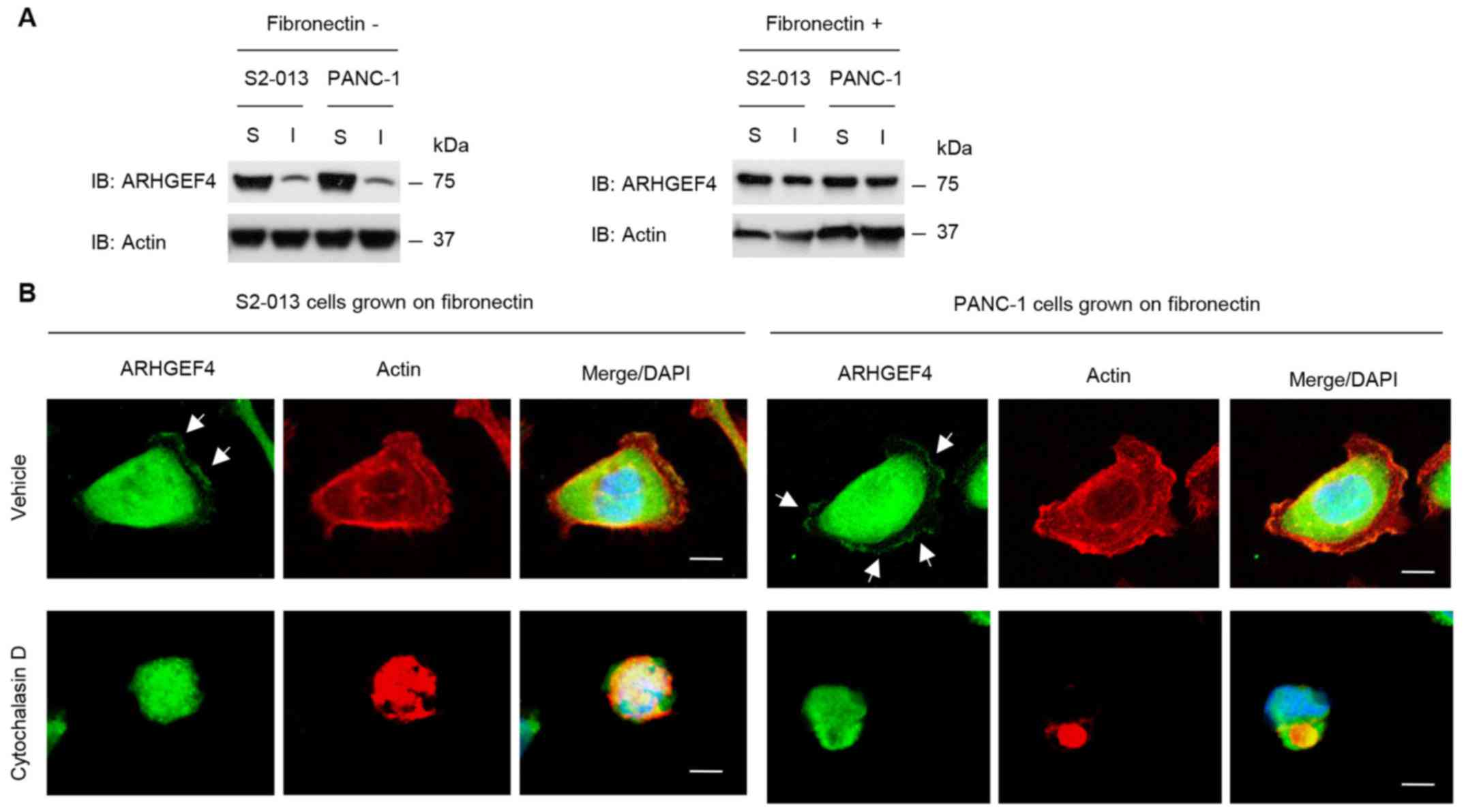
Localization of ARHGEF4 in cell
protrusions
To quantify the subcellular distribution of ARHGEF4
in S2-013 and PANC-1 cells cultured with or without fibronectin,
cell lysates were fractionated into detergent-soluble and
-insoluble (cytoskeletal) fractions. The amounts of ARHGEF4 and
actin in the insoluble fraction increased in response to culture on
fibronectin (Fig. 4A), which
indicates that ARHGEF4 translocated from the cytosol to the actin
cytoskeleton following culture on fibronectin.
To examine the effects of alteration of actin
cytoskeleton dynamics on the subcellular distribution of ARHGEF4,
S2-013 and PANC-1 cells were treated with the actin depolymerising
agent, cytochalasin D. Peripheral actin structures were reduced in
the fibronectin-stimulated S2-013 and PANC-1 cells exposed to 100
µM cytochalasin D for 12 in the fibronectin-stimulated
non-treated cells, and ARHGEF4 was present in the cytoplasm of cell
bodies in the cells (Fig. 4B). In
the non-treated S2-013 and PANC-1 grown on fibronectin, ARHGEF4 was
accumulated in the cell protrusions (Fig. 4B).
Roles of ARHGEF4 in cell motility and
invasion of PDAC cells
To determine whether ARHGEF4 was associated with
motility and invasiveness of PDAC cells, ARHGEF4 expression in
S2-013 and PANC-1 cells was transiently suppressed by
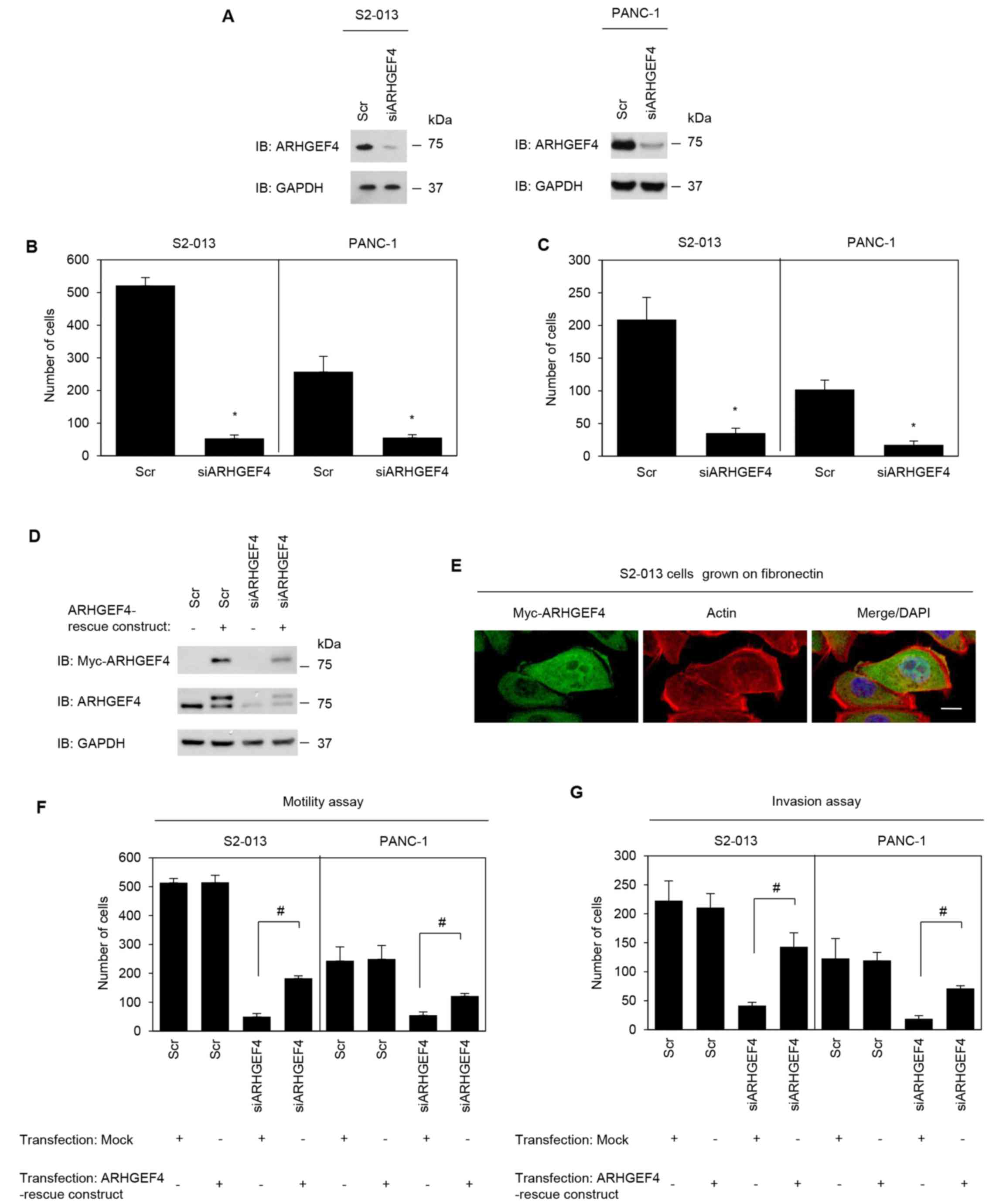
ARHGEF4-specific siRNA (Fig.
5A). Suppression of ARHGEF4 significantly inhibited cell
motility (Fig. 5B) and invasion
(Fig. 5C) in S2-013 and PANC-1
cells. An ARHGEF4-rescue construct was transiently transfected into
scrambled control-siRNA and ARHGEF4-siRNA transfected S2-013
cells, and expression of myc-tagged ARHGEF4 was confirmed (Fig. 5D). The exogenous ARHGEF4 from the
rescue construct was localized in the cytoplasm of cell bodies and
in cell protrusions, similar to endogenous ARHGEF4 (Fig. 5E). ARHGEF4-siRNA transfected
S2-013 and PANC-1 cells expressing the rescue construct could
rescue cell motility and invasiveness inhibited following ARHGEF4
silencing (Fig. 5F and G). These
results indicated that ARHGEF4 promoted PDAC cell motility and
invasion.
Roles of ARHGEF4 in forming cell
protrusions
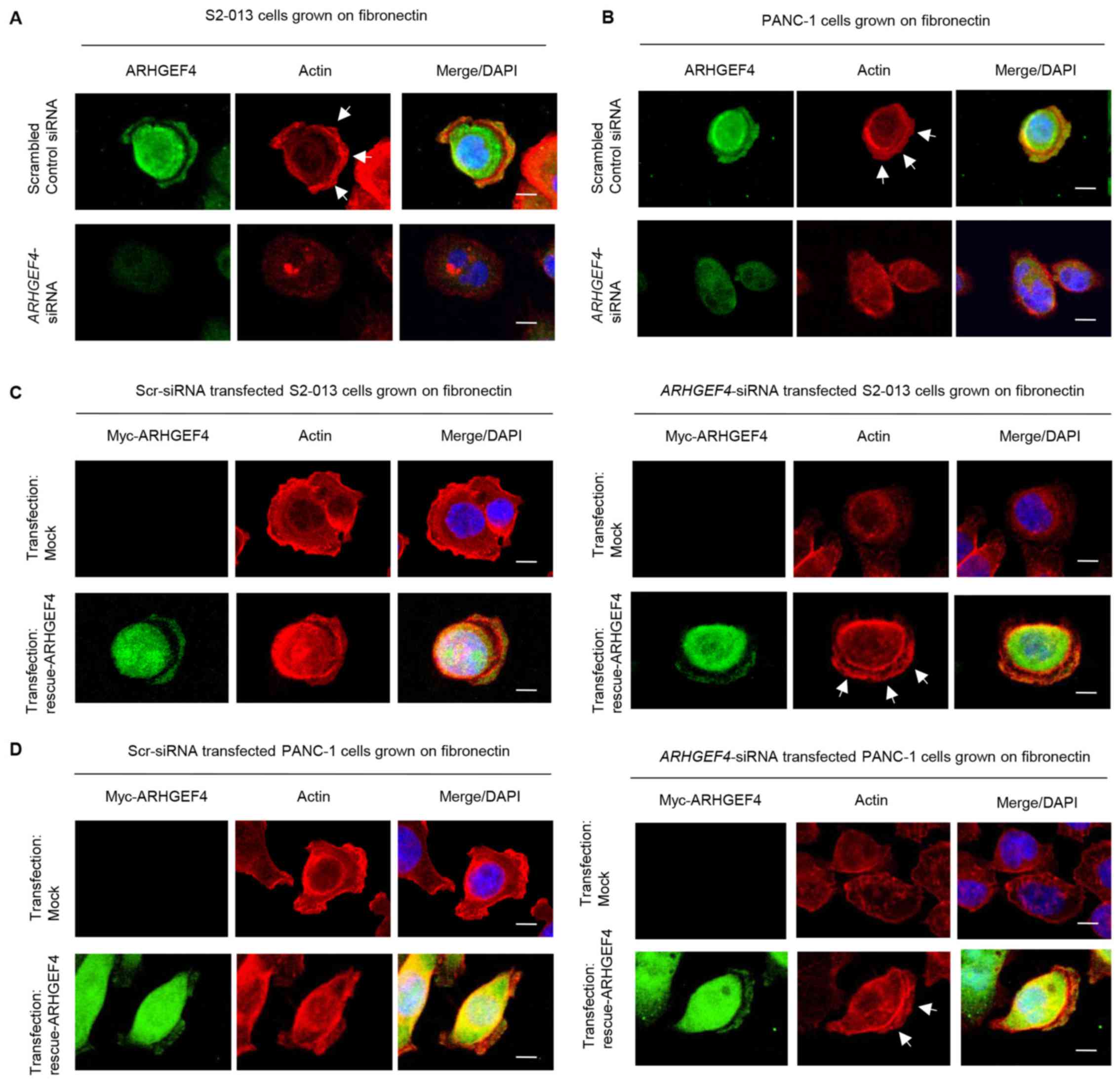
Peripheral actin structures in membrane ruffles of
scrambled control-siRNA and ARHGEF4-siRNA transfected S2-013
and PANC-1 cells cultured on fibronectin were then analyzed.
Peripheral actin structures in cell protrusions were less abundant
in ARHGEF4-siRNA transfected S2-013 and PANC-1 cells
compared with control-siRNA transfected S2-013 and PANC-1 cells
(S2-013, Fig. 6A; PANC-1, Fig. 6B). Transfection of an
ARHGEF4-rescue construct into ARHGEF4-siRNA transfected
S2-013 and PANC-1 cells rescued the decrease in peripheral actin
structures at the protrusions caused by the ARHGEF4-siRNA
(S2-013, Fig. 6C; PANC-1, Fig. 6D).
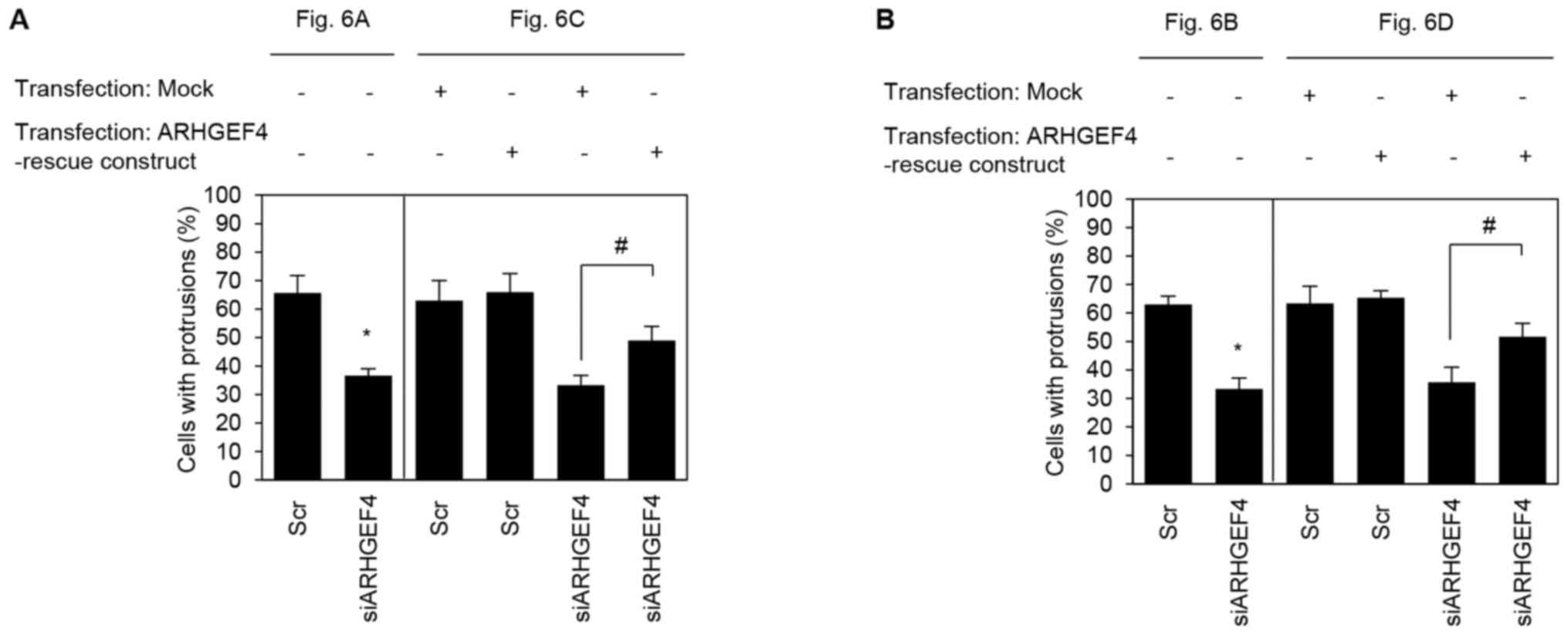
To quantify the degree of inhibition caused by
suppression of ARHGEF4, the induction of membrane protrusions was
observed. In ARHGEF4-siRNA transfected S2-013 and PANC-1
cells cultured on fibronectin, suppression of ARHGEF4 inhibited the
formation of protrusions compared with control-siRNA transfected
S2-013 and PANC-1 cells (S2-013, Fig.
7A; PANC-1, Fig. 7B).
Transfection of an ARHGEF4-rescue construct into
ARHGEF4-siRNA transfected S2-013 and PANC-1 cells rescued
the decrease in the protrusions caused by the ARHGEF4-siRNA
(Fig. 7). These results indicated
that ARHGEF4 regulates the rearrangement of peripheral actin to
induce the formation of additional membrane protrusions.
Links of ARHGEF4 with associated cell
signaling pathways
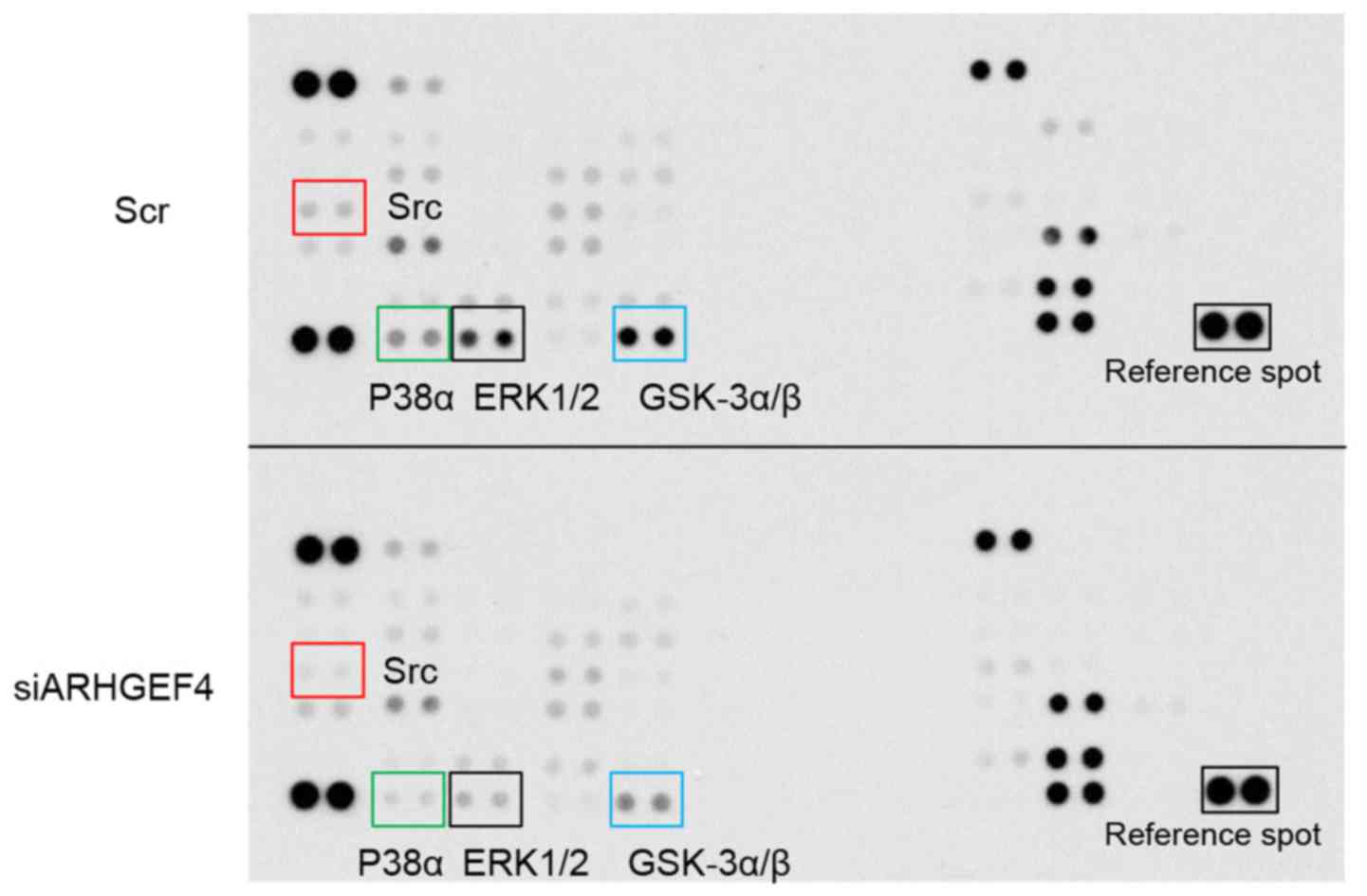
To determine whether ARHGEF4 could regulate the
activity of phosphoproteins, the intracellular signaling pathways
were further analyzed in the scrambled control-siRNA transfected
S2-013 cells and ARHGEF4-siRNA transfected S2-013 cells grown on
fibronectin (Fig. 8). Of the 38
kinases investigated in a commercially available human
phosphoprotein array kit, suppression of ARHGEF4 markedly
downregulated the activity of Src, ERK1/2, p38α and GSK-3α/β.
Association of ARHGEF4 with ERK1/2 in the
formation of cell protrusions
To evaluate the role of the ERK1/2 pathway on the
ARHGEF4-mediated formation of cell protrusions, the actin
cytoskeletal structures of the scrambled control-siRNA transfected
S2-013 and PANC-1 cells as well as the ARHGEF4-siRNA
transfected S2-013 and PANC-1 cells grown on fibronectin in the
absence or presence of the MEK inhibitor, U0126 were examined.
Inhibition of MEK activity by treatment with U0126 has previously
been reported to decrease downstream kinase target ERK1/2
phosphorylation, confirming the inhibition of the MEK signaling
pathway (25). Treatment of S2-013
cells grown on fibronectin with U0126 inhibited ERK1/2 activity
(Fig. 9A). The suppression of
ARHGEF4 partially decreased ERK1/2 activity, and similarly, ERK1/2
phosphorylation was inhibited by U0126 at the concentration of 30
µM (Fig. 9A).
Immunocytochemistry demonstrated that treatment with U0126
decreased phosphor-ylated ERK1/2 localized to the cytoplasm of the
cell bodies, and inhibited peripheral actin rearrangement in S2-013
cells grown on fibronectin (Fig.
9B). Notably, phosphorylated ERK1/2 was also present at cell
protrusions of non-treated S2-013 cells (Fig. 9B). Additionally, suppression of
ARHGEF4 decreased phosphorylated ERK1/2 localized to the cytoplasm
of the cell bodies and peripheral actin filaments in the cell
protrusions when compared with those in S2-013 cells transfected
with scrambled control-siRNA (Fig.
9C). Transfection of an ARHGEF4-rescue construct into S2-013
and PANC-1 cells in which ARHGEF4 had been suppressed and ERK1/2
phosphorylation had been blocked by U0126 did not result in a
marked increase in peripheral actin filaments in the cell
protrusions when compared with those in cells transfected with the
mock control vector (S2-013, Fig.
9D; PANC-1, Fig. 9E). These
data indicated that the ERK pathway was largely responsible for the
ARHGEF4-mediated formation of membrane ruffles in PDAC cells.
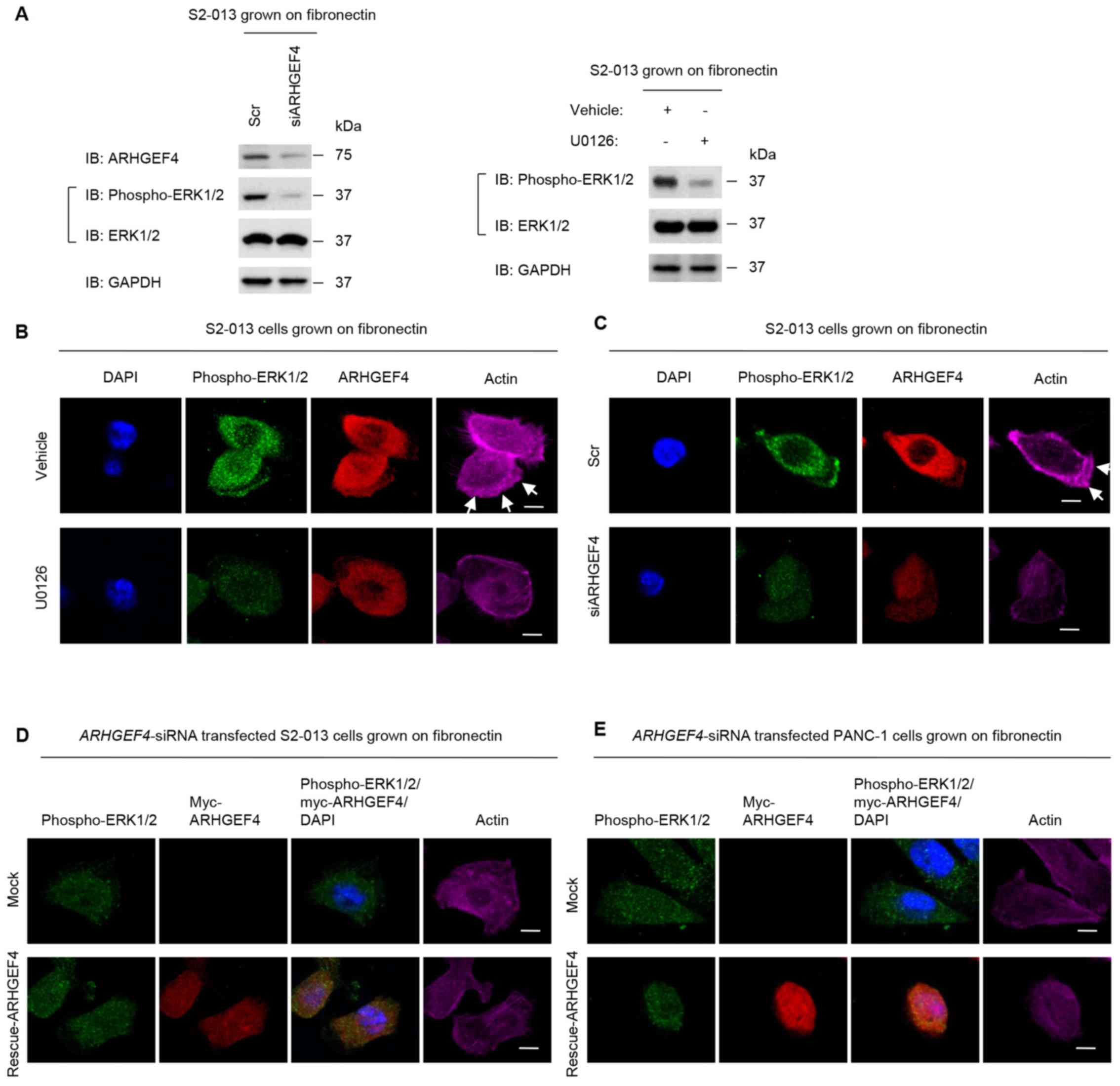 | Figure 9Association of ARHGEF4 with ERK1/2 in
peripheral actin rearrangements. (A) S2-013 cells were incubated on
fibronectin with or without U0126 treatment. Additionally, Scr
transfected S2-013 cells and siARHGEF4 transfected S2-013 cells
were grown on fibronectin. Western blotting of steady-state levels
was performed using antibodies against ARHGEF4, ERK1/2 and
phospho-ERK1/2. Data are representative of three independent
experiments. (B) Confocal immunofluorescence microscopic images.
S2-013 cells were incubated on fibronectin with or without U0126
treatment. The cells were immunocytochemically stained with
anti-phospho-ERK1/2 antibody (green), anti-ARHGEF4 antibody (red)
and phalloidin (violet). (C) Confocal immunofluorescence
microscopic images. Scr transfected S2-013 cells and siARHGEF4
transfected S2-013 cells incubated on fibronectin were
immunocytochemically stained with anti-phospho-ERK1/2 antibody
(green), anti-ARHGEF4 antibody (red) and phalloidin (violet).
Confocal immunofluorescence microscopic images. A myc-tagged
ARHGEF4-rescue construct was transfected into (D) S2-013 and (E)
PANC-1 cells that had been transfected with siARHGEF4; 48 h later,
the cells were incubated on fibronectin with or without U0126
treatment. Cells were stained with anti-phospho-ERK1/2 antibody
(green), anti-myc antibody (red) and phalloidin (violet). Blue
staining indicates DAPI staining. Arrows indicate cell protrusions
in which the peripheral actin structures are increased. Scale bars,
10 µM. ARHGEF4, rho guanine nucleotide exchange factor 4;
ERK, extracellular signal-regulated kinase; phospho-,
phosphorylated; siRNA, small interfering RNA; Scr, negative control
scrambled siRNAs; siARHGEF4, siRNA oligonucleotides targeting
ARHGEF4. |
Association of ARHGEF4 with GSK-3α/β in
the formation of cell protrusions
The association of ARHGEF4 and GSK-3α/β with the
formation of membrane ruffles was evaluated. GSK-3 activity was
downregulated using the GSK-3 inhibitor SB216763. GSK suppression
increases the expression levels of cleaved caspase-8, cleaved
caspase-3, and cleaved poly(ADP-ribose) polymerase (26,27).
SB216763 was potent in markedly reducing GSK-3α/β activity, as
visualized by an increased level of cleaved caspase-8 in S2-013
cells grown on fibronectin (Fig.
10A). Cleaved caspase-8 was also increased by the suppression
of ARHGEF4 in S2-013 cells, compared with scrambled control-siRNA
transfected cells (Fig. 10A).
Immunocytochemistry demonstrated that treatment with SB216763
increased caspase-8 in the cytoplasm, and inhibited peripheral
actin rearrangement in S2-013 cells grown on fibronectin (Fig. 10B), similar to
ARHGEF4-siRNA transfected S2-013 cells (Fig. 10C). Transfection of an
ARHGEF4-rescue construct into S2-013 and PANC-1 cells in which
ARHGEF4 had been suppressed and GSK-3α/β phosphorylation had been
blocked by SB216763 did not result in an increase in peripheral
actin filaments when compared with those in cells transfected with
the mock-control vector (S2-013, Fig.
10D; PANC-1, Fig. 10E). These
results indicated that ARHGEF4 serves a role in promoting
peripheral actin cytoskeletal rearrangements by increasing the
levels of phosphorylated GSK-3α/β.
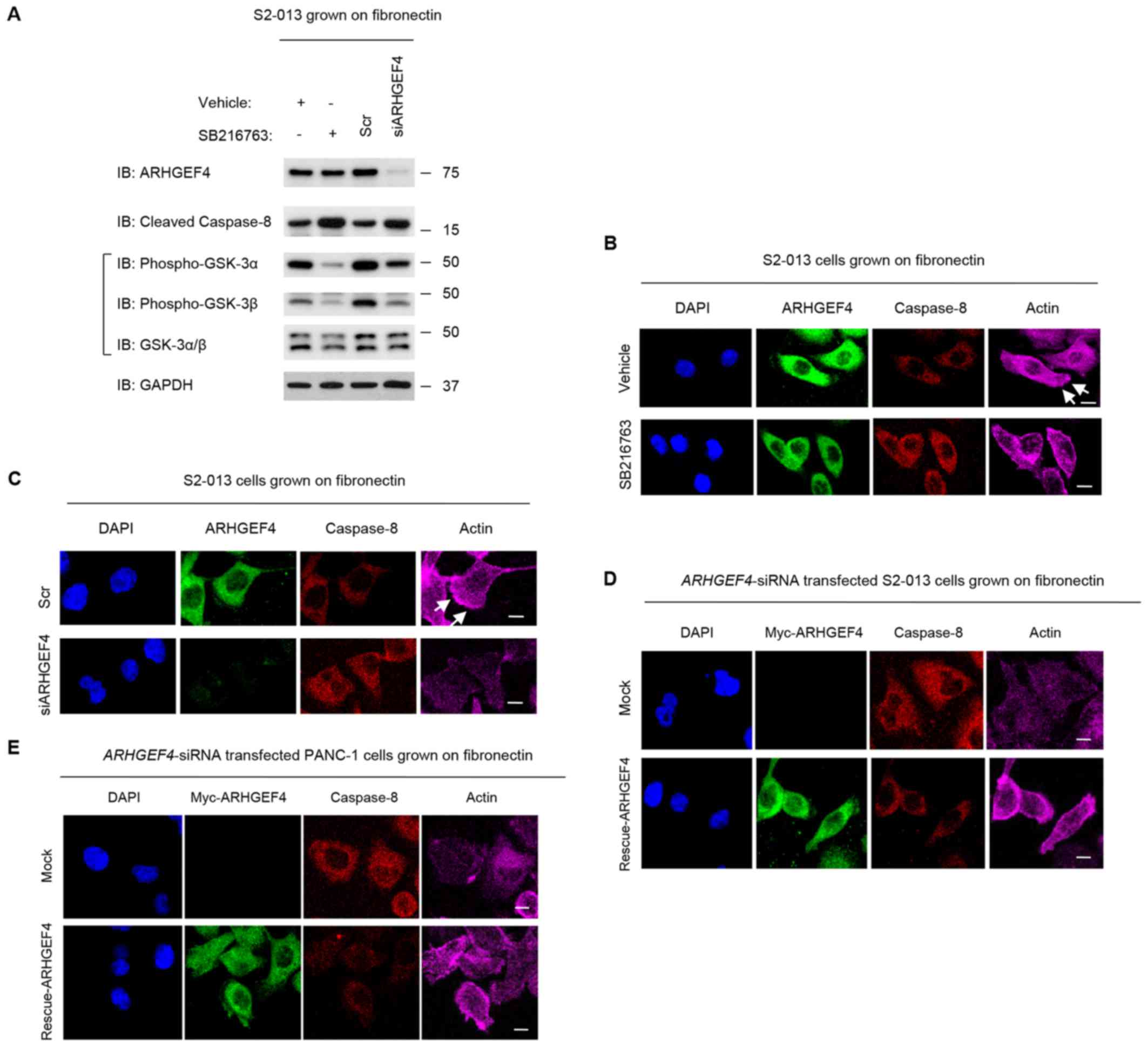 | Figure 10Association of ARHGEF4 with GSK-3α/β
in forming cell protrusions. (A) S2-013 cells were incubated on
fibronectin with or without SB216763 treatment. Additionally, Scr
transfected S2-013 cells and siARHGEF4 transfected S2-013 cells
were grown on fibronectin. Western blotting of steady-state levels
was performed using anti-ARHGEF4, anti-GSK-3α/β and anti-caspase-8
antibodies. Data are representative of three independent
experiments. (B) Confocal immunofluorescence microscopic images.
S2-013 cells were incubated on fibronectin with or without SB216763
treatment. The cells were immunocytochemically stained with
anti-ARHGEF4 antibody (green), anti-caspase-8 antibody (red) and
phalloidin (violet). (C) Confocal immunofluorescence microscopic
images. Scr transfected S2-013 cells and siARHGEF4 transfected
S2-013 cells incubated on fibronectin were immunocytochemically
stained with anti-ARHGEF4 antibody (green), anti-caspase-8 antibody
(red) and phalloidin (violet). Confocal immunofluorescence
microscopic images. A myc-tagged ARHGEF4-rescue construct was
transfected into (D) S2-013 and (E) PANC-1 cells that had been
transfected with siARHGEF4; 48 h later, the cells were incubated on
fibronectin with or without SB216763 treatment. Cells were stained
with anti-myc antibody (green), anti-caspase-8 antibody (red) and
phalloidin (violet). Blue staining indicates DAPI staining. Arrows
indicate cell protrusions in which the peripheral actin structures
are increased. Scale bars, 10 µM. ARHGEF4, rho guanine
nucleotide exchange factor 4; GSK, glycogen synthase kinase; siRNA,
small interfering RNA; Scr, negative control scrambled siRNAs;
siARHGEF4, siRNA oligonucleotides targeting ARHGEF4. |
Association of ARHGEF4 with ERK1/2 and
GSK-3α/β in the cell motility and invasion of PDAC cells
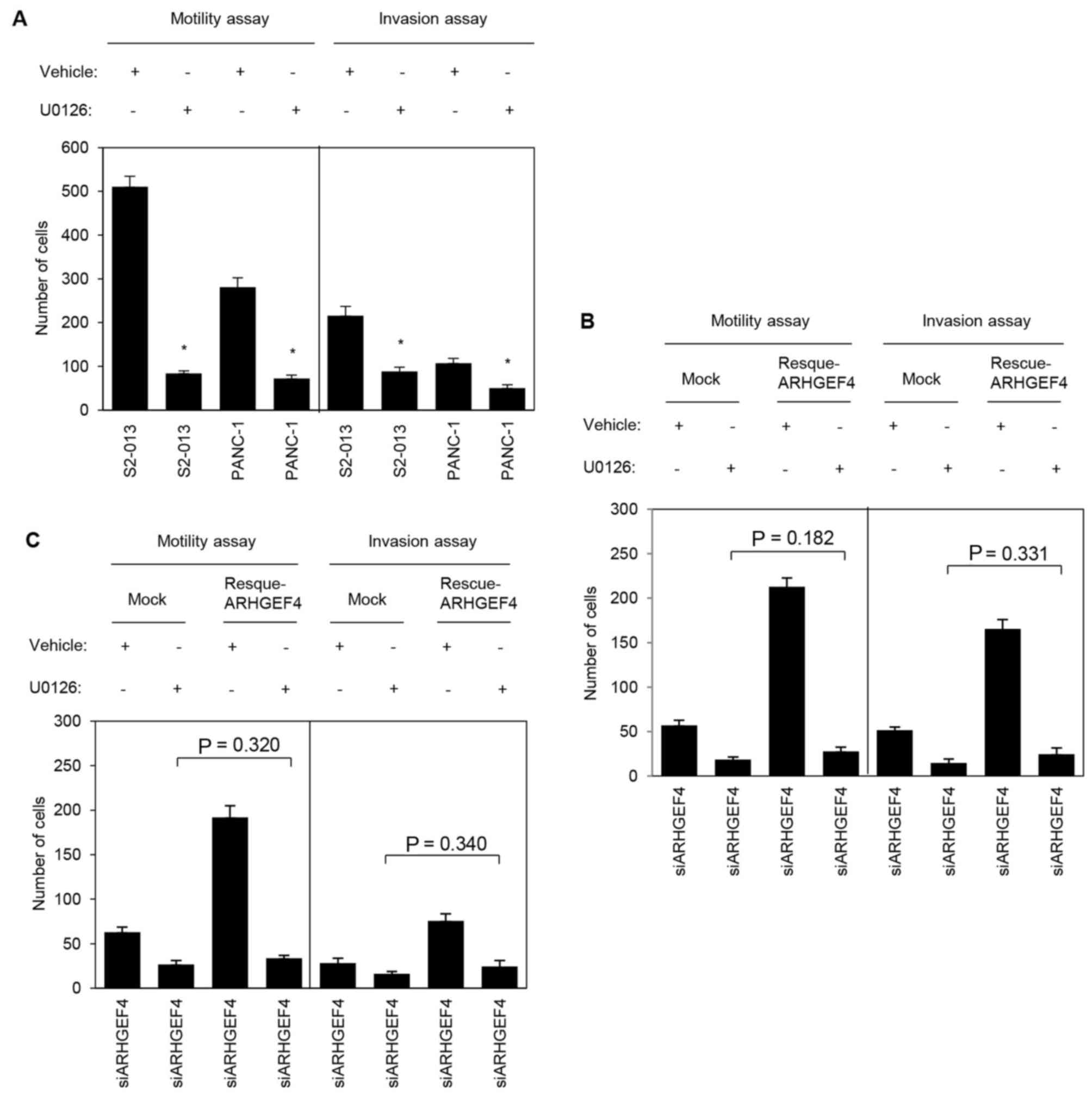
To evaluate whether ERK1/2 is necessary for ARHGEF4
to promote cell motility and invasion, motility and two-chamber
invasion assays were performed using S2-013 and PANC-1 cells with
or without U0126 pretreatment. S2-013 and PANC-1 cells pretreated
with U0126 demonstrated significantly decreased cell motility and
invasion when compared with the corresponding non-treated cells
(Fig. 11A). Transfection of an
ARHGEF4-rescue construct into S2-013 and PANC-1 cells in which
ARHGEF4 had been suppressed and ERK1/2 had been inactivated did not
significantly abrogate the changes to cell motility and
invasiveness caused by the suppression of ARHGEF4 and
inactivation of ERK1/2 (S2-013, Fig.
11B; PANC-1, Fig. 11C). These
results indicated that ERK1/2 was necessary for the
ARHGEF4-associated promotion of motility and invasiveness.
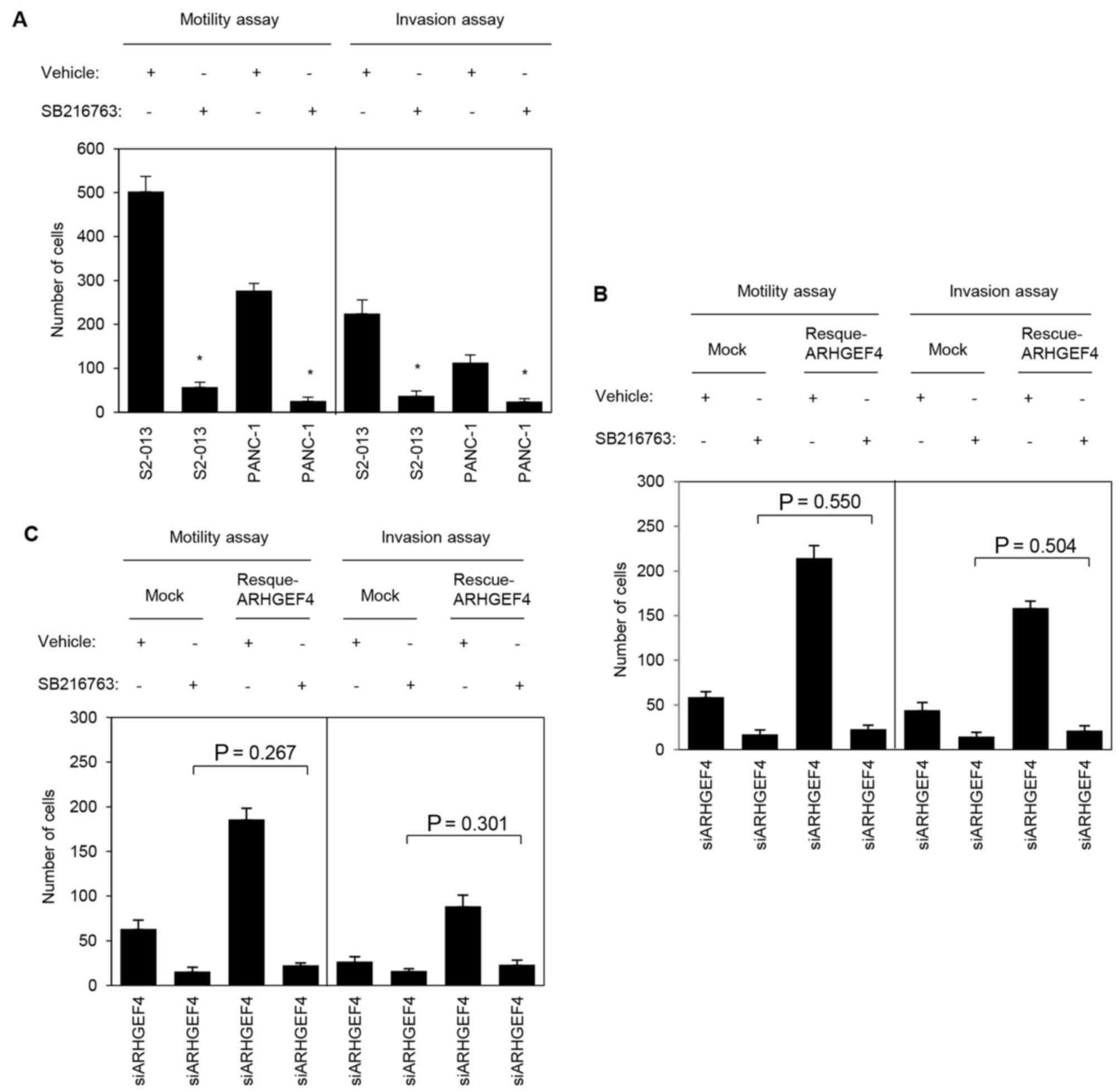
To evaluate the association of ARHGEF4 and GSK-3α/β
with motility and invasiveness, motility and two-chamber invasion
assays were performed using S2-013 and PANC-1 cells with or without
SB216763 pretreatment. S2-013 and PANC-1 cells pretreated with
U0126 demonstrated significantly decreased cell motility and
invasion when compared with the corresponding non-treated cells
(Fig. 12A). Transfection of an
ARHGEF4-rescue construct into S2-013 and PANC-1 cells in which
ARHGEF4 had been suppressed and GSK-3α/β had been inactivated did
not significantly abrogate the changes to cell motility and
invasiveness caused by the suppression of ARHGEF4 and inactivation
of GSK-3α/β (S2-013, Fig. 12B;
PANC-1, Fig. 12C). These results
indicated that GSK-3α/β was necessary for the ARHGEF4-associated
promotion of motility and invasiveness.
Discussion
In the present study, it was demonstrated that high
ARHGEF4 expression is closely associated with poor prognosis of
patients with PDAC. PDAC is one of the deadliest of all solid
malignancies, with a 5-year survival rate of 8%, due to its ability
to extensively invade surrounding tissues and to metastasize at an
early stage (28,29). Multivariate Cox regression analysis
indicated that high ARHGEF4 expression was an independent predictor
of worse overall survival in a model including other
clinicopathological factors. Table
II demonstrated that there was no statistically significant
relationship between ARHGEF4 expression and any of the other
clinicopathological factors; this may be attributable to the short
overall survival rate of the high-expressing ARHGEF4 group, in
which the median survival time and 3-year survival rate were 24
months and 26.3%, respectively. In vitro motility and
invasion assays demonstrated that ARHGEF4 promoted cell motility
and invasion through an increase in cell protrusions in PDAC cells.
These results indicate that ARHGEF4 may be a useful marker of poor
prognosis of PDAC patients that is functionally associated with
cell motility and invasion.
ARHGEF4 is a GEF for Rac1 and a link between APC and
G-protein signaling (1,3). ARHGEF4 transduces a Wnt signal to
Rac1, thereby regulating the actin cytoskeleton and cell migration
(4). The crystal structure of
ARHGEF4 has been determined and it was demonstrated that the SH3
domain in the NH2-terminal regulatory domain serves a
crucial role in the auto-inhibition of GEF activity (3). Epidermal growth factor stimulation
phosphorylates ARHGEF4 at Tyr94 within the APC-binding region in a
Src kinase-dependent manner; however, no increase in GEF activity
upon ARHGEF4 tyrosine phosphorylation or upon the introduction of a
phosphomimetic amino acid into Tyr94 of ARHGEF4 has been observed
(30). This finding suggested that
ARHGEF4 may serve different roles besides being a GEF, and it was
demonstrated that ARHGEF4 knockdown inactivated Src, ERK1/2, p38α
and GSK-3α/β in S2-013 cells. As such, in the present study, the
investigation and discussion was limited to only how ARHGEF4
promotes the motility and invasiveness through ERK1/2 and GSK-3α/β,
and the GEF activity of ARHGEF4 was not subjected to further
investigation.
Src is found at the crossroads of several signaling
pathways, including Ras/Raf/ERK1/2 (31–33),
and it promotes cell proliferation, survival, invasion, migration
and angiogenesis. The p38 and GSK-3α/β pathways promote the
proliferation, migration, and invasion of lung cancer cells
(34). In PDAC cells, an
antioxidant defense enzyme, Prdx1, is associated with the formation
of membrane protrusions through the modulation of p38-MAPK
activity, which in turn promotes cell invasion (35). Inhibition of GSK-3 impairs PDAC
growth (36), thus providing a
rationale for assessing the potential clinical utility of GSK3
inhibitors in PDAC patients (37).
These findings indicated that ARHGEF4-associated actin remodeling
and cell motility and invasiveness may be modulated through the
regulation of multiple pathways, including Src, MAPK-ERK1/2,
p38-MAPK and WNT-GSK, in PDAC cells. Future studies should evaluate
the ARHGEF4-associated Src, MAPK-ERK1/2, p38-MAPK, and WNT-GSK
pathways that coordinate the actin cytoskeletal remodeling that is
required for cell spreading, motility, and invasion.
In conclusion, ARHGEF4 promotes cell motility and
invasion, and may be a useful marker for predicting the outcome of
patients with PDAC. Mechanistically, the present study provides the
first evidence, to the best of our knowledge, that ARHGEF4-mediated
phosphorylation of ERK1/2 and GSK-3α/β may regulate the formation
of cell protrusions, resulting in the promotion of PDAC cell
motility and invasion. In addition, the data presented herein
indicated that the i) inhibition of ARHGEF4, ii) inhibition of
ERK1/2 and/or GSK-3α/β pathways that associate with ARHGEF4, or
iii) some combination thereof may be effective for development of
new therapeutic strategies, as any such therapy would inhibit the
ARHGEF4-mediated cell motility and invasion of PDAC cells.
Funding
The present study was supported by a Grant-in-Aid
for Scientific Research (KAKENHI; grant nos. 24591013, 15K14396 and
17K09463).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
KT conceived and designed the experiments. SN and KT
performed the experiments. KT, MF and TS coordinated the research
and analyzed the data. MF and SN performed pathological analyses.
KT wrote the manuscript with contributions from all authors. TS
supervised laboratorial processes. KT obtained financial support.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Board of Kochi Medical School and Matsuyama Shimin Hospital
(Nankoku, Japan) prior to patient recruitment. Written informed
consent was acquired from each patient prior to initiation.
Patient consent for publication
Written informed consent was acquired from each
patient prior to initiation.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Makiko Tsuboi,
Ms. Miki Nishigawa, Ms. Rieko Takahashi and Mr. Shunichi Manabe for
their technical assistance. The authors would also like to thank Dr
Masahiko Sakaguchi for statistical analysis of the data.
References
|
1
|
Kawasaki Y, Senda T, Ishidate T, Koyama R,
Morishita T, Iwayama Y, Higuchi O and Akiyama T: Asef, a link
between the tumor suppressor APC and G-protein signaling. Science.
289:1194–1197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiesen S, Kübart S, Ropers HH and
Nothwang HG: Isolation of two novel human RhoGEFs, ARHGEF3 and
ARHGEF4, in 3p13-21 and 2q22. Biochem Biophys Res Commun.
273:364–369. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawasaki Y, Sato R and Akiyama T: Mutated
APC and Asef are involved in the migration of colorectal tumour
cells. Nat Cell Biol. 5:211–215. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akiyama T and Kawasaki Y: Wnt signalling
and the actin cytoskeleton. Oncogene. 25:7538–7544. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitin N, Betts L, Yohe ME, Der CJ, Sondek
J and Rossman KL: Release of autoinhibition of ASEF by APC leads to
CDC42 activation and tumor suppression. Nat Struct Mol Biol.
14:814–823. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawasaki Y, Tsuji S, Sagara M, Echizen K,
Shibata Y and Akiyama T: Adenomatous polyposis coli and Asef
function downstream of hepatocyte growth factor and
phosphatidylinositol 3-kinase. J Biol Chem. 284:22436–22443. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawasaki Y, Tsuji S, Muroya K, Furukawa S,
Shibata Y, Okuno M, Ohwada S and Akiyama T: The adenomatous
polyposis coli-associated exchange factors Asef and Asef2 are
required for adenoma formation in Apc(Min/+)mice. EMBO Rep.
10:1355–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawasaki Y, Jigami T, Furukawa S, Sagara
M, Echizen K, Shibata Y, Sato R and Akiyama T: The adenomatous
polyposis coli-associated guanine nucleotide exchange factor Asef
is involved in angiogenesis. J Biol Chem. 285:1199–1207. 2010.
View Article : Google Scholar :
|
|
9
|
DiMagno EP, Reber HA and Tempero MA;
American Gastroenterological Association: AGA technical review on
the epidemiology, diagnosis, and treatment of pancreatic ductal
adenocarcinoma. Gastroenterology. 117:1464–1484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taniuchi K, Furihata M, Hanazaki K, Saito
M and Saibara T: IGF2BP3-mediated translation in cell protrusions
promotes cell invasiveness and metastasis of pancreatic cancer.
Oncotarget. 5:6832–6845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taniuchi K, Furihata M and Saibara T:
KIF20A-mediated RNA granule transport system promotes the
invasiveness of pancreatic cancer cells. Neoplasia. 16:1082–1093.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuboi M, Taniuchi K, Furihata M, Naganuma
S, Kimura M, Watanabe R, Shimizu T, Saito M, Dabanaka K, Hanazaki
K, et al: Vav3 is linked to poor prognosis of pancreatic cancers
and promotes the motility and invasiveness of pancreatic cancer
cells. Pancreatology. 16:905–916. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Japan Pancreatic Society: Classification
of Pancreatic Carcinoma. 2nd English edition: Kanehara & Co.;
Tokyo: 2003
|
|
14
|
Sobin LH, Gospodarowicz MK and Witteknd C:
TNM classification of malignant tumors. 7th edition.
Wiley-Blackwell; New York, NY: pp. 132–135. 2009
|
|
15
|
Kondo S: Japanese pancreas society staging
systems for pancreatic cancer In Pancreatic Cancer. Springer; New
York, NY: pp. 1035–1050. 2010
|
|
16
|
Taniuchi K, Furihata M, Naganuma S,
Dabanaka K, Hanazaki K and Saibara T: BCL7B, a predictor of poor
prognosis of pancreatic cancers, promotes cell motility and
invasion by influencing CREB signaling. Am J Cancer Res. 8:387–404.
2018.
|
|
17
|
Iwamura T, Katsuki T and Ide K:
Establishment and characterization of a human pancreatic cancer
cell line (SUIT-2) producing carcinoembryonic antigen and
carbohydrate antigen 19-9. Jpn J Cancer Res. 78:54–62.
1987.PubMed/NCBI
|
|
18
|
Taniuchi K, Nishimori I and Hollingsworth
MA: Intracellular CD24 inhibits cell invasion by
posttranscriptional regulation of BART through interaction with
G3BP. Cancer Res. 71:895–905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taniuchi K, Furihata M, Naganuma S,
Dabanaka K, Hanazaki K and Saibara T: PODXL, linked to poor
prognosis of pancreatic cancers, promotes cell invasion via binding
to gelsolin. Cancer Sci. 107:1430–1442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanouchi A, Taniuchi K, Furihata M,
Naganuma S, Dabanaka K, Kimura M, Watanabe R, Kohsaki T, Shimizu T,
Saito M, et al: CCDC88A, a prognostic factor for human pancreatic
cancers, promotes the motility and invasiveness of pancreatic
cancer cells. J Exp Clin Cancer Res. 35:1902016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andea A, Sarkar F and Adsay VN:
Clinicopathological correlates of pancreatic intraepithelial
neoplasia: A comparative analysis of 82 cases with and 152 cases
without pancreatic ductal adenocarcinoma. Mod Pathol. 16:996–1006.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deer EL, González-Hernández J, Coursen JD,
Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ: Phenotype
and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taniuchi K, Furihata M, Iwasaki S, Tanaka
K, Shimizu T, Saito M and Saibara T: RUVBL1 directly binds actin
filaments and induces formation of cell protrusions to promote
pancreatic cancer cell invasion. Int J Oncol. 44:1945–1954. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taniuchi K, Yokotani K and Saibara T: BART
inhibits pancreatic cancer cell invasion by Rac1 inactivation
through direct binding to active Rac1. Neoplasia. 14:440–450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yip-Schneider MT and Schmidt CM: MEK
inhibition of pancreatic carcinoma cells by U0126 and its effect in
combination with sulindac. Pancreas. 27:337–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao X, Zhang L, Thrasher JB, Du J and Li
B: Glycogen synthase kinase-3beta suppression eliminates tumor
necrosis factor-related apoptosis-inducing ligand resistance in
prostate cancer. Mol Cancer Ther. 2:1215–1222. 2003.PubMed/NCBI
|
|
27
|
Min KJ, Han MA, Kim S, Park JW and Kwon
TK: Osthole enhances TRAIL-mediated apoptosis through
downregulation of c-FLIP expression in renal carcinoma Caki cells.
Oncol Rep. 37:2348–2354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahrendt SA and Pitt HA: Surgical
management of pancreatic cancer. Oncology (Williston Park).
16:725–734. 736–738. 7407432002.
|
|
29
|
Rescher U and Gerke V: Annexins - unique
membrane binding proteins with diverse functions. J Cell Sci.
117:2631–2639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itoh RE, Kiyokawa E, Aoki K, Nishioka T,
Akiyama T and Matsuda M: Phosphorylation and activation of the Rac1
and Cdc42 GEF Asef in A431 cells stimulated by EGF. J Cell Sci.
121:2635–2642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farkas A, Szatmári E, Orbók A, Wilhelm I,
Wejksza K, Nagyoszi P, Hutamekalin P, Bauer H, Bauer HC, Traweger
A, et al: Hyperosmotic mannitol induces Src kinase-dependent
phosphorylation of beta-catenin in cerebral endothelial cells. J
Neurosci Res. 80:855–861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bowman T, Broome MA, Sinibaldi D, Wharton
W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA and
Jove R: Stat3-mediated Myc expression is required for Src
transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci
USA. 98:7319–7324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levin VA: Basis and importance of Src as a
target in cancer. Cancer Treat Res. 119:89–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He Y, Wang L, Liu W, Zhong J, Bai S, Wang
Z, Thomas DG, Lin J, Reddy RM, Ramnath N, et al: MAP3K3 expression
in tumor cells and tumor-infiltrating lymphocytes is correlated
with favorable patient survival in lung cancer. Sci Rep.
5:114712015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taniuchi K, Furihata M, Hanazaki K,
Iwasaki S, Tanaka K, Shimizu T, Saito M and Saibara T:
Peroxiredoxin 1 promotes pancreatic cancer cell invasion by
modulating p38 MAPK activity. Pancreas. 44:331–340. 2015.
View Article : Google Scholar :
|
|
36
|
Wilson W III and Baldwin AS: Maintenance
of constitutive IkappaB kinase activity by glycogen synthase
kinase-3alpha/ beta in pancreatic cancer. Cancer Res. 68:8156–8163.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyashita K, Nakada M, Shakoori A,
Ishigaki Y, Shimasaki T, Motoo Y, Kawakami K and Minamoto T: An
emerging strategy for cancer treatment targeting aberrant glycogen
synthase kinase 3 beta. Anticancer Agents Med Chem. 9:1114–1122.
2009. View Article : Google Scholar : PubMed/NCBI
|