Introduction
Cellular senescence is usually defined as a cellular
state characterized by specific metabolic, epigenetic, genetic and
phenotypical changes culminating in the inability of cells to
proliferate (1). These changes
rely on substantial modifications of their secretome, transcriptome
and proteome (2,3). Typical characteristics of cellular
senescence include a flat and large morphology, the presence of
vacuoles, positive staining for the senescence-associated
β-galactosidase marker, and the increased expression of specific
cyclin-dependent kinase inhibitors, including p16Ink4a,
p21Waf1 (p21) and p27Kip1 (4). Senescent cells are often
multinucleated, and their nuclei are often larger compared with
those in proliferating cells (5).
Furthermore, senescent cells exhibit changes in the chromatin
structure with the appearance of senescence-associated
heterochromatin foci (6).
To date, four different types of senescence have
been distinguished: Stress-induced premature senescence (SIPS),
replicative senescence (RS), oncogene-induced senescence and
oncogene-invalidation-induced senescence (7). The different types of stimuli
inducing senescence engage common and specific effector pathways
and result in senescent phenotypes that, consequently, share
general and specific markers (8).
Replicative senescence was first described in 1961 by Hayflick and
Moorhead (9) as limited
proliferating potential observed in primary human fibroblasts .
This type of senescence may be induced by diverse stimuli,
including damage to telomeric DNA, resulting in the activation of
the DNA damage response (10,11).
Compared with RS, SIPS operate independently of telomere attrition
and is induced by oxidative stress in addition to by numerous
pharmacological drugs (e.g. doxorubicin), bacterial toxins
(12), immunomodulatory cytokines
[including interferon γ (IFNγ) and tumour necrosis factor α (TNFα)]
or small synthetic and natural compounds (13-15).
Senescent cells secrete a specific pattern of
cytokines, chemokines, growth factors and proteases (16,17)
that constitute the senescence-associated secretory phenotype
(SASP). The typical SASP components are immunomodulatory and
inflammatory cytokines, including interleukin (IL)-6, IL-8, IL-1,
chemokines [e.g. growth-regulated oncogene α (GROα)] and growth
factors (e.g. insulin-like growth factor) (8,18,19).
It has been demonstrated that SASP is able to reinforce the
senescence programme and influence the tissue and tumour
microenvironment, affecting tumour and immune cells (1,20).
Senescent cells may induce senescence in cells in their surrounding
environment via paracrine effects, an effect known as ‘bystander’
senescence (17,21,22).
It is also known that senescence-associated cytokines trigger and
maintain the senescence phenotype in an autocrine manner (23,24).
Cytokines that are produced by senescent cells may also mediate the
impact of ionizing radiation on senescence; in vivo
experiments indicated the presence of DNA damage in tissues distant
from the irradiated field resembling the radiation- linked
‘bystander effect’ (25,26).
In the present study, comparative analysis was
performed by evaluating the effects of two distinct senescence
inductors: Docetaxel (DTX) and a combination of immunomodulatory
cytokines, IFNγ and TNFα (27). It
was previously demonstrated that DTX is able to induce senescence
in TC-1 and TRAMP-C2 tumour cell lines (28). However, the tumour growth of
proliferating murine TC-1 cancer cells in syngeneic B6 was
accelerated by the co-administration of TC-1 or TRAMP-C2 prostate
cancer cells made senescent by treatment with DTX, or by
lethally-irradiated cells. IFNγ and TNFα have been described as
potential senescence inducers in vivo in certain tumour cell
lines (27). However, further
phenotyping and mechanistic studies of DTX and for IFNγ and TNFα
combined treatment are required in order to understand how tumour
cell senescence may serve a function in cancer control and
development.
The aim of the present study was to compare the cell
phenotypes resulting from two different methods of senescence
induction, DTX and IFNγ + TNFα, in two distinct murine tumour cell
lines, TC-1 and B16. Furthermore, the present study evaluated the
ability of culture medium to induce SASP-associated ‘bystander’
senescence.
Materials and methods
Cell culture and mice
The TC-1 cell line is generated by the in
vitro co-transfection of murine lung C57BL/6 cells with human
papillomavirus type 16 (HPV16) E6/E7 and activated human Ha-Ras
oncogenes (29). The B16F10 (B16)
murine melanoma cell line is syngeneic in C57BL/6 mice (30). The two cell lines were obtained for
the present study from American Type Culture Collection (Manassas,
VA, USA). The two cell types were cultured in RPMI-1640 medium
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) supplemented with
10% foetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), and antibiotics (gentamicin and nystatin)
in standard conditions (5% CO2, 37°C and 95% relative
humidity). C57Bl/6NCrl (B6) male mice (weight ~25 g; 7-8 weeks
old), were obtained from AnLab, s.r.o. (Prague, Czech Republic) and
maintained in specific pathogen-free conditions. The total number
of the mice used in the study was 112. The mice were housed and
assayed under a controlled temperature of 22±2°C, humidity of 55±5%
and a 12:12-h light:dark cycle with ad libitum access to rodent
chow (Altromin-1310 breeding diet for rats and mice; Altromin
Spezialfutter GmbH & Co. KG, Lage, Germany) and water
(autoclaved, UV disinfected). All experiments were performed
according to the EU Directive 2010/63/EU on the protection of
animals used for scientific purposes (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm).
Experimental protocols were ethically approved by the Institutional
Animal Care Committee of the Institute of Molecular Genetics
(Prague, Czech Republic).
Induction of ‘primary’ premature
senescence
TC-1 and B16 cells were cultured in fresh RPMI-1640
medium for 24 h, following which the medium was removed and
replaced with medium containing either recombinant IFNγ (50 U/ml;
R&D Systems, Inc., Minneapolis, MN, USA) and TNFα (5 ng/ml;
PeproTech, Inc., Rocky Hill, NJ, USA) or 7.5 µM DTX (Actavis
Generics, Dublin, Ireland). The doses of DTX and IFNγ + TNFα were
optimized to induce senescence but not apoptosis, as reported
previously (17,28). To induce senescence, TC-1 and B16
tumour cells were cultured in the medium containing DTX or IFNγ +
TNFα for 4 days at 37°C. IFNγ and TNFα were added to the culture
medium each day of the treatment. In this experiment B16 tumour
cells were washed following the 4-day treatments and then cultured
in fresh medium until day 7 of cultivation. The cell number was
counted on days 4 and 7 using an automated cell counter
(Countess®; Invitrogen; Thermo Fisher Scientific,
Inc.).
Induction of ‘bystander’ senescence
Medium conditioned by medium from senescent or
‘parental’ tumour cells (control) was used to provoke bystander
senescence. First, primary senescence was induced by the
cultivation of tumour cells in the RPMI-1640 medium containing
either DTX or recombinant IFNγ and TNFα for 4 days at 37°C. The
medium was then replaced with fresh medium and cells were
cultivated for another 24 h to prepare senescence-conditioned
medium [defined as DTX senescent medium (SM) or IFNγ + TNFα medium
(IFNγ + TNFα M)]. The medium was then used for the induction of
‘bystander’ senescence. Tumour cells were cultured for 4 days in
fresh medium at 37°C mixed with the senescent medium at a ratio of
1:1. As a control, conditioned medium from untreated ‘parental’
tumour cells was used [defined as tumour medium (TM)].
Senescence-associated
(SA)-β-galactosidase staining
Senescent cells were visualised by estimation of
senescence-associated β-galactosidase activity using the Senescence
β-galactosidase Staining kit (Cell Signaling Technology, Inc.,
Danvers, MA, USA) according to the manufacturer’s protocol. Cell
culture images were obtained using an inverted fluorescence
microscope Leica DMI6000 with total internal reflection
fluorescence illumination at a magnification of x20 (Leica
Microsystems GmbH, Wetzlar, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA samples from TC-1 and B16 cell lines were
isolated using RNeasy Plus mini kit (Qiagen Sciences, Inc.,
Gaithersberg, MD, USA) according to the manufacturer’s protocol.
cDNA was synthesized with random hexamer primers using the
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The temperature profile for RT was
42°C for 30 min, 99°C for 5 min and 10°C for 5 min. RT-qPCR was
performed in an LC480II system (Roche Applied Science, Penzberg,
Germany) using SYBR Select Master mix containing SYBR Green dye
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The samples
underwent a denaturation step (95°C for 6 min), followed by 42
amplification cycles (95°C for 30 sec, 60°C for 50 sec and 72°C for
70 sec), melting step (95°C for 1 min, 65°C for 1 min and 95°C
continuous acquisition) and cooling (37°C for 1 min). The relative
quantity of cDNA was estimated by the 2−ΔΔCq
method (31). The following
primers were purchased from East Port Praha s.r.o. (Prague, Czech
Republic): β-actin (ACTB) forward, 5′-CATTGCTGACAGGATGCAGAAGG-3′
and reverse, 5′-TGCTGGAAGGTGGACAGTGAGG-3′; p21 forward,
5′-CAGATCCACAGCGATATCCA-3′ and reverse, 5′-ACGGGACCGAAGAGACAAC-3′.
The final concentration of the primers used was 1 µM. Fold
changes in transcript levels were calculated relative to β-actin,
which was used as the endogenous reference gene control. The
relative expression in the control group was normalized to 1. All
samples were run in triplicate.
Enzyme-linked immunosorbent assay
(ELISA)
The protein levels of murine GROα (cat no. DY453;
R&D Systems, Inc.) and IL-6 (cat no. 555240; BD Biosciences,
San Diego, CA, USA) were detected in the supernatants of
non-senescent and senescent TC-1 and B16 cells using
high-sensitivity ELISA kits. Supernatants were prepared by 4-day
cell treatments at 37°C followed by a medium change and another 24
h of cell cultivation (1.5×106 cells/5 ml) in fresh
medium. Experiments were performed according to the manufacturer’s
protocols.
Estimation of DNA replication
B16 and TC-1 cells were driven to ‘primary’ or
‘bystander’ senescence as described above. DNA replication was
estimated by 5-ethynyl-2′-deoxyuridine (EdU) incorporation with
Click-iT Plus EdU Alexa Fluor 647 Flow Cytometry Assay kit
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer’s protocol. On day 4 of the treatments, cells were
incubated with 10 µM EdU for 24 h at 37°C. For the Click-iT
reaction, cells were washed once with PBS and detached using
trypsin. Furthermore, the cells were washed twice in PBS and
resuspended in fresh PBS. EdU incorporation was measured using a BD
FACSVerse™ flow cytometer (BD Biosciences) and the data were
analysed using FlowJo 10 software (FlowJo LLC, Ashland, OR,
USA).
Flow cytometry
Cell size and granularity of 20,000 cells was
evaluated by analysing the side scattering (SSC) and forward
scattering (FSC) of the unstained cells. FSC intensity is
associated with the cell size, whereas SSC corresponds with the
cell refractive index that depends on the cell granularity
(32). The data were presented as
FSC-A and SSC-A plots, where -A, also known as the pulse area,
represents the integral of the height and width of the pulse. Pulse
area is considered to be more accurate when compared with the pulse
height (-H) value only. Cell size and granularity were measured
using a BD FACSVerse™ flow cytometer (BD Biosciences). Cell
autofluorescence was measured in the APC channel. Data were
analysed using FlowJo 10 software (FlowJo LLC), as described
below.
Immunofluorescence staining
TC-1 and B16 (control and treated) cells were grown
on glass coverslips coated with 0.01% poly-L-lysine solution
(Sigma-Aldrich; Merck KGaA) for 15 min at room temperature.
Senescence was induced by DTX or IFNγ + TNFα, as described above.
On day 4 of the treatment, cells were fixed with 4% formaldehyde
and permeabilized with 0.1% Triton X-100 in two consecutive steps,
each for 15 min at room temperature. Subsequently, the cells were
washed once with PBS, blocked in 10% FBS/PBS for 30 min at room
temperature, stained with diluted primary antibodies at 1:100 for 1
h at room temperature and washed twice with PBS/0.1% Tween-20.
Following washing with PBS, cells were incubated with diluted
secondary antibody at 1:500 for 1 h at room temperature. To
counterstain nuclei, coverslips were mounted in Mowiol containing
4′,6-diamidine-2-phenylindole at room temperature (Sigma-Aldrich;
Merck KGaA). Cells were examined using a fluorescence microscope at
a magnification of x63 (Leica DMI6000; Leica Microsystems GmbH).
The antibodies used were as follows: Phospho-Ser139 of histone H2A
histone family, member X (γH2AX) rabbit monoclonal antibody (cat
no. 9718; Cell Signaling Technology, Inc.) and goat anti-rabbit
immunoglobulin G antibody conjugated with Alexa 488 (cat no.
A11034; Invitrogen; Thermo Fisher Scientific, Inc.).
Western blotting
TC-1 and B16 (control as well as treated) cells were
washed twice with PBS and harvested with sample lysis buffer (20 mM
HEPES, 50 mM NaCl, 1% mM EDTA, 0.1% Triton X-100 and 10% glycerol
in double distilled water) supplemented with a cOmplete™ ULTRA
Tablets, mini, EASYpack Protease Inhibitor сocktail (cat no.
05892970001; Roche Diagnostics GmbH, Mannheim, Germany) and
PhosSTOP phosphatase inhibitor cocktail (cat no. 04906837001; Roche
Diagnostics GmbH). Concentration of proteins was determined by the
bicinchoninic acid method (Pierce; Thermo Fisher Scientific, Inc.).
DTT (100 mM) and 0.01% bromphenol blue was added to lysates prior
to separation by 12% SDS-PAGE. Equal amounts of protein (35
µg) were loaded into each well. Proteins were
electrotransferred onto a nitrocellulose membrane using wet
transfer. The membrane was blocked in 10% nonfat dry milk diluted
in 0.1% Tween/PBS for 1 h at room temperature, and detected by
specific antibodies combined with horseradish peroxidase-
conjugated secondary antibody (anti-rabbit; cat. no. 1706515;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was
incubated with primary antibodies (anti-p21 and anti-GAPDH)
overnight at 4°C and secondary antibody for 1 h at room
temperature. Peroxidase activity was detected by SuperSignal West
Dura Extended Duration Substrate (cat. no. 34075; Pierce; Thermo
Fisher Scientific, Inc.). GAPDH was used as a loading control. The
following primary antibodies were used: Anti-mouse p21 rabbit
monoclonal antibody (1:1,000; cat. no. ab109199; Abcam, Cambridge,
UK) and anti-mouse GAPDH rabbit monoclonal antibody (1:1,000; cat.
no. 2118S; Cell Signaling Technology, Inc.). Protein signals were
detected by developing the blots with X-ray film (Agfa Healthcare
Corporation, Greenville, SC, USA) on X-ray film processor (Optimax
2010, Protec GmbH, Ottobrunn, Bavaria, Germany). X-ray films then
were scanned (Epson Scan Perfection V700 Photo, Japan) and final
data were edited by Adobe Photoshop CS6 (Adobe Inc., version
13).
In vivo experiments
B6 mice (8 per group) were transplanted on day 0
subcutaneously (s.c.) with control TC-1 or B16 cells
(3×104), DTX-induced senescent TC-1/DTX or B16/DTX cells
(in two doses: 3×104 and 3×105), IFNγ +
TNFα-treated TC-1/IFNγ + TNFα or B16/IFNγ + TNFα cells
(3×104 each). In the case of induction of ‘bystander’
senescence, B6 mice (8 per group) were transplanted on day 0 s.c.
with control TC-1 and B16 cells (3×104), ‘bystander’
senescent TC-1/SM or B16/SM cells (in two doses: 3×104
and 3×105). Mice were observed twice a week and the size
of the tumours was recorded. Two diameters of the tumours (largest
diameter and perpendicular) were measured with a calliper and the
tumour size was expressed as the tumour area (cm2) by
the following formula: Tumour area (cm2) = largest
diameter (cm) × perpendicular diameter (cm). The maximum tumour
size in one direction was 1.8 cm. Mice were sacrificed by cervical
dislocation and CO2 asphyxiation.
Statistical analysis
For the statistical analyses of the in vitro
experiments, statistical significance was determined by a
two-tailed analysis of variance test and subsequently by Bonferroni
multiple comparisons as a post-test using GraphPad Prism 5.04
(GraphPad Software, Inc., La Jolla, CA, USA). All experiments were
performed in three independent replicates. For the evaluation of
in vivo experiments, analysis of variance from the Number
Cruncher Statistical System v.10 (NCSS, LLC, Kaysville, UT, USA)
statistical package was utilized. The data were presented as the
mean ± standard deviation in the figures. P<0.05 was considered
to indicate a statistically significant difference.
Results
DTX and IFNγ + TNFα-mediated senescence
induction in mouse tumour cell lines TC-1 and B16
First, the impact of DTX and IFNγ in combination
with IFNγ + TNFα in terms of senescence induction on two tumour
cell lines, TC-1 (Fig. 1) and B16
(Fig. 2) was examined. The two
cell lines were sensitive to 7.5 µM DTX treatment and became
senescent subsequent to 4 days of incubation, as characterized by
increased SA-β-galactosidase activity and typical phenotypic and
morphological changes of the cells (Figs. 1A and 2A). In comparison with the DTX treatment,
no indicators of senescence were observed in TC-1 cells following
incubation with IFNγ + TNFα (Fig.
1C-E). IFNγ + TNFα-treated B16 cells were larger, flattened and
elongated (spindle-shaped) compared with the controls (Fig. 2A). Additionally, the cellular
senescent phenotype was confirmed by FACS measurement of the size
and granularity of TC-1 and B16 cells. In the two tumour cell
lines, a significant >5-fold increase of SSC and FSC high-gated
cells was detected [statistical analysis from three independent
experiments: TC-1 13.2±1.92 vs. TC-1/DTX 85.33±1.99 (P<0.001);
B16 15.87±1.88 vs. B16/DTX 94.93±2.65 (P<0.01); the numbers
correspond to the percentage of gated cells, Figs. 1B and 2B] following DTX treatment compared with
the control. A 4-fold increase of SSC and FSC high-gated cells was
detected following IFNγ + TNFα treatment in B16 but not in TC-1
cells [TC-1 13.2.33±1.92 vs. TC-1/IFNγ + TNFα 8.8±0.64 (P>0.05);
B16 15.87±1.88 vs. B16/IFNγ + TNFα 64.23±7.70 (P<0.05); Figs. 1B and 2B]. As senescent cell autofluorescence is
considered to be a marker of senescence, in the present study,
autofluorescence was evaluated in TC-1 and B16 cells following DTX
treatment (Figs. 1C and 2C). In comparison with DTX-treated cells,
TC-1 cells following IFNγ + TNFα treatment did not exhibit
increased auto- fluorescence, compared with the control cells;
whereas B16/IFNγ + TNFα-treated cells exhibited higher
autofluorescence compared with the control or DTX-treated cells. An
increase in p21 gene expression, typical of cell stress/senescence,
was detected by RT-qPCR in DTX-treated TC-1 and B16 cells (Figs. 1D and 2D) and was significant in B16 cells
(P<0.01), but not in IFNγ + TNFα-treated B16 and TC-1 cells.
Immunoblotting detection of mouse p21 indicated increased protein
expression in DTX-treated TC-1 and B16 cells and also a moderate
increase in IFNγ + TNFα-treated B16 cells, but not in TC-1 cells
(Figs. 1E and 2E).
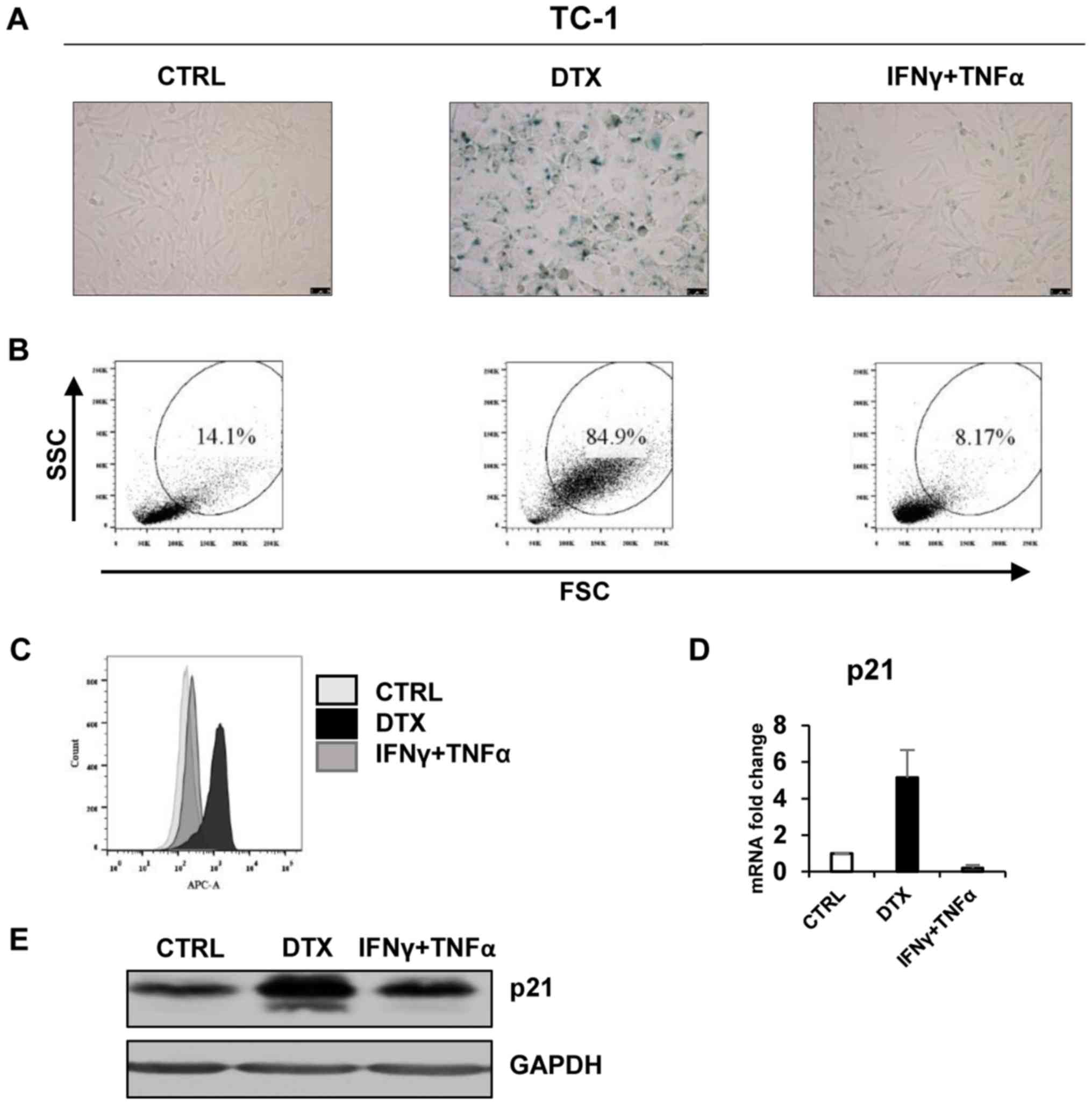 | Figure 1DTX induces senescence in TC-1 cells.
(A) Senescence-associated β-galactosidase activity in TC-1 cells
treated with DTX or IFNγ + TNFα for 4 days. (B) The size and
granularity of control or IFNγ + TNFα-treated senescent TC-1 cells
was determined by forward and side scatter flow cytometry analysis.
(C) Autofluorescence of the TC-1 control cells is presented in
light grey, DTX-treated in black and IFNγ + TNFα-treated in grey.
(D) Reverse transcription-quantitative polymerase chain reaction
quantification of p21 in control, DTX- and IFNγ + TNFα-treated TC-1
cells. (E) Immunoblotting detection of mouse p21 in control, DTX-
and IFNγ + TNFα-treated TC-1 cells harvested on day 4. GAPDH was
used as a loading control. Representative results from at least
three independent experiments are presented. Data are presented as
the mean ± standard deviation. CTRL, control cells; DTX, docetaxel;
IFNγ, interferon γ; TNFα, tumour necrosis factor α; FSC, forward
scattering; SSC, side scattering; p21, p21Waf1. |
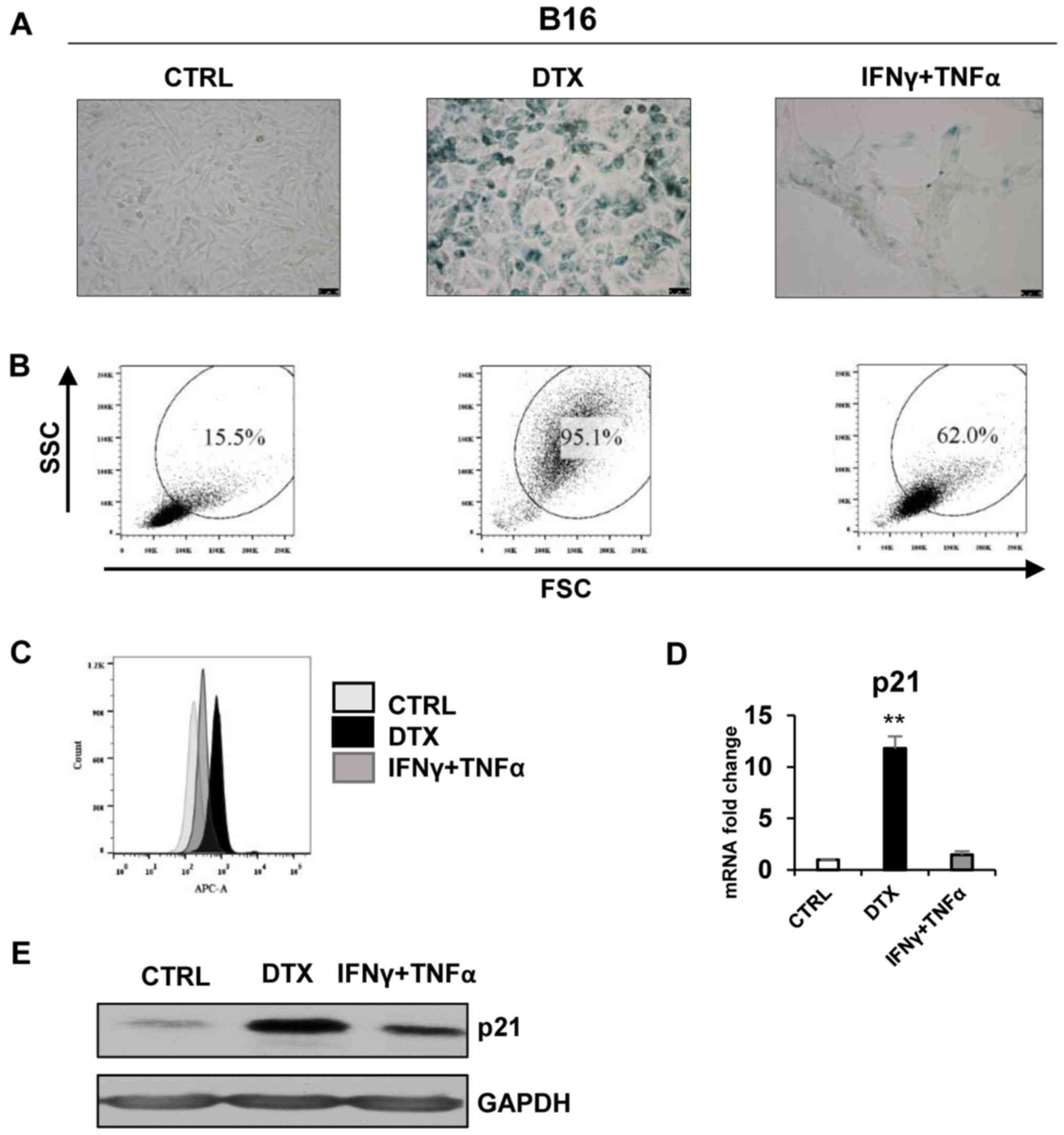 | Figure 2DTX induces senescence in the B16
cell line. (A) Senescence-associated β-galactosidase activity in
B16 cells treated with DTX or IFNγ + TNFα for 4 days. (B) The size
and granularity of control or IFNγ + TNFα-treated, senescent B16
cells was determined by forward and side scatter flow cytometry
analysis. (C) Autofluorescence of the B16 control cells is
presented in light grey, DTX-treated in black and IFNγ +
TNFα-treated in grey. (D) Reverse transcription-quantitative
polymerase chain reaction quantification of p21 in control, DTX-
and IFNγ + TNFα-treated B16 cells. (E) Immunoblotting detection of
mouse p21 in control, DTX- and IFNγ + TNFα-treated B16 cells
harvested on day 4. GAPDH was used as a loading control.
Representative results from at least three independent experiments
are presented. Data are presented as the mean ± standard deviation.
**P<0.01 vs. CTRL. CTRL, control cells; DTX,
docetaxel; IFNγ, interferon γ; TNFα, tumour necrosis factor α; FSC,
forward scattering; SSC, side scattering; p21, p21Waf1;
B16, B16F10 cell line. |
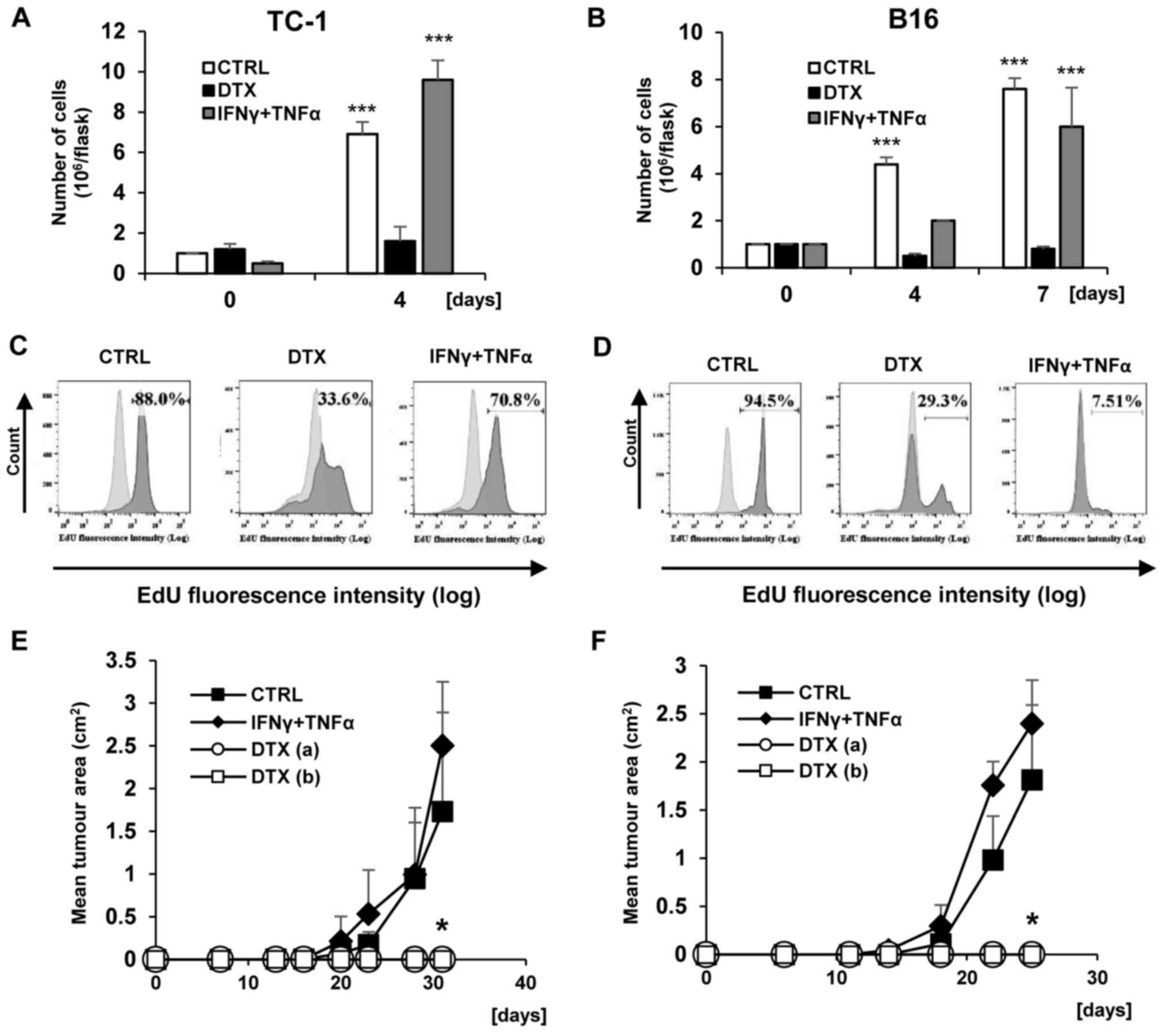
Furthermore, TC-1 and B16 tumour cell proliferation
was evaluated in vitro at different time points (day 4 and
7) following DTX and IFNγ + TNFα treatments. Following 4 days of
treatments in TC-1 cells (Fig.
3A), a significant increase in the number of tumour cells was
observed in the control and IFNγ + TNFα-treated groups compared
with day 0 (P<0.001), whereas no cell proliferation was detected
in DTX. In B16 cells, a loss of proliferation was detected
following DTX treatment for 4 days, but not following IFNγ + TNFα
treatment, where the number of proliferative cells was increased
compared with day 0 (P<0.01) but did not reach the number in the
control group (Fig. 3B). In
addition, the medium containing DTX and IFNγ + TNFα was removed and
the cells were cultured in fresh medium until day 7. Then, the
number of proliferating B16 cells was examined. It was identified
that the number of tumour cells pretreated with IFNγ + TNFα was
comparable to the control group, and was significantly increased
compared with day 0 (P<0.01). In the case of DTX pretreated B16
cells, the cell cycle remained arrested.
To evaluate the loss of proliferation associated
with senescence development, the discontinuation of DNA replication
was assayed by the detection of EdU incorporation into DNA. Only
limited subsets of EdU-positive cells were observed in TC-1 and B16
cell populations following cultivation with DTX, as measured by
FACS analysis (Fig. 3C and D).
Proliferative arrest was also detected in IFNγ + TNFα-treated B16
cells, but not in IFNγ + TNFα-treated TC-1 cells. Average
percentages (from three measurements) of EdU-positive cells and the
differences between experimental groups were as follows: TC-1
82.9±4.50 vs. TC/DTX 35.2±5.28 (P<0.05); TC-1 82.9±4.50 vs.
TC-1/IFNγ + TNFα 75.3±7.11 (P>0.05); B16 94.73±1.96 vs. B16/DTX
26.43±7.80 (P<0.01) and B16 94.73±1.96 vs. B16/IFNγ + TNFα
7.95±0.42 (P<0.001).
To verify the in vitro cessation of
proliferation in senescent cells, the growth of senescent tumour
cells was evaluated in vivo. B6 mice were injected with
DTX-induced senescent cells in the same dose as a control testing
dose for proliferating cells (3×104) and a 10-fold dose
(3×105). Mice were also injected with the cells that
underwent treatment with IFNγ + TNFα (3×104). No tumour
growth was observed following the injection of DTX-treated
senescent TC-1 and B16 cells (P<0.05 compared with the controls
injected with untreated cells and harbouring growing tumours). On
the other hand, TC-1, as expected, in addition to B16 cells, did
not exhibit tumour growth arrest in vivo following treatment
with immunostimulatory cytokines. The differences in tumour growth
rates were not significant compared with the growth of tumours
following the injection of mice with proliferating tumour cells.
This indicates that following treatment with IFNγ + TNFα, B16 cells
underwent an only temporary loss of proliferation (Fig. 3E and F).
Induction of cellular senescence is
associated with DNA damage response
The presence of DNA damage foci positive for γH2AX,
a factor participating in DNA double-strand break sensing and
repair, was investigated by immunofluorescent staining. In the
majority of the DTX-treated TC-1 (Fig.
4A) and B16 cells (Fig. 4B) an
increase of γH2AX foci was detected with persistent DNA damage
response. Following treatment with IFNγ + TNFα, only a small number
of TC-1 and B16 cells exhibited a mild increase in γH2AX foci
compared with the control cells. Quantification analysis indicated
an increase in the number of micronuclei in TC-1 and B16 cells
treated with DTX compared with control cells, with a significantly
greater percentage of TC-1 cells with 1 or 2 micronuclei
(P<0.001) and B16 cells with 2 (P<0.01) or 3 or more
micronuclei (P<0.001) compared with the control cells (Fig. 4C and D). A significantly greater
percentage of TC-1 cells treated with IFNγ + TNFα had 1 micronuclei
per cell compared with control cells (P<0.05; Fig. 4C). More than three micronuclei were
detected in B16 cells only (Fig.
4D).
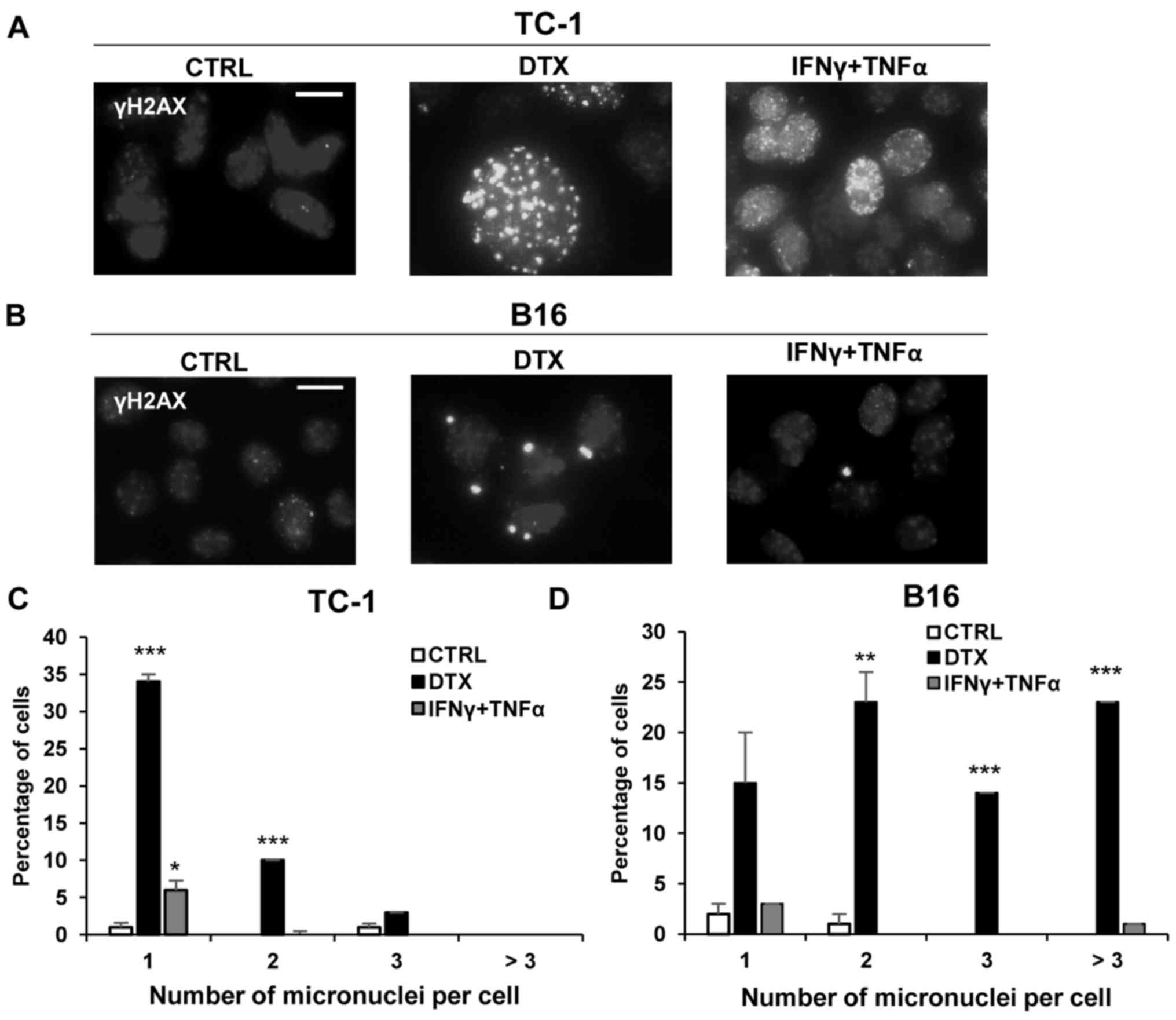 | Figure 4DNA damage detection in TC-1 and B16
tumour cell lines. To detect DNA damage, control, DTX- or IFNγ +
TNFα-treated (A) TC-1 and (B) B16 cells were stained with
phosphoSer139 H2A histone family, member X antibody and mounted
with Mowiol containing 4’,6-diamidine-2-phenylindole. Scale bar, 20
µm. Percentage of cells with 1, 2, 3 or more micronuclei in
(C) TC-1 and (D) B16 cells treated with DTX or IFNγ + TNFα was
quantified. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and
***P<0.001 vs. CTRL. A total of 100 cells were
analysed in each experimental group. CTRL, control cells; DTX,
docetaxel; IFNγ, interferon γ; TNFα, tumour necrosis factor α; B16,
B16F10 cell line. |
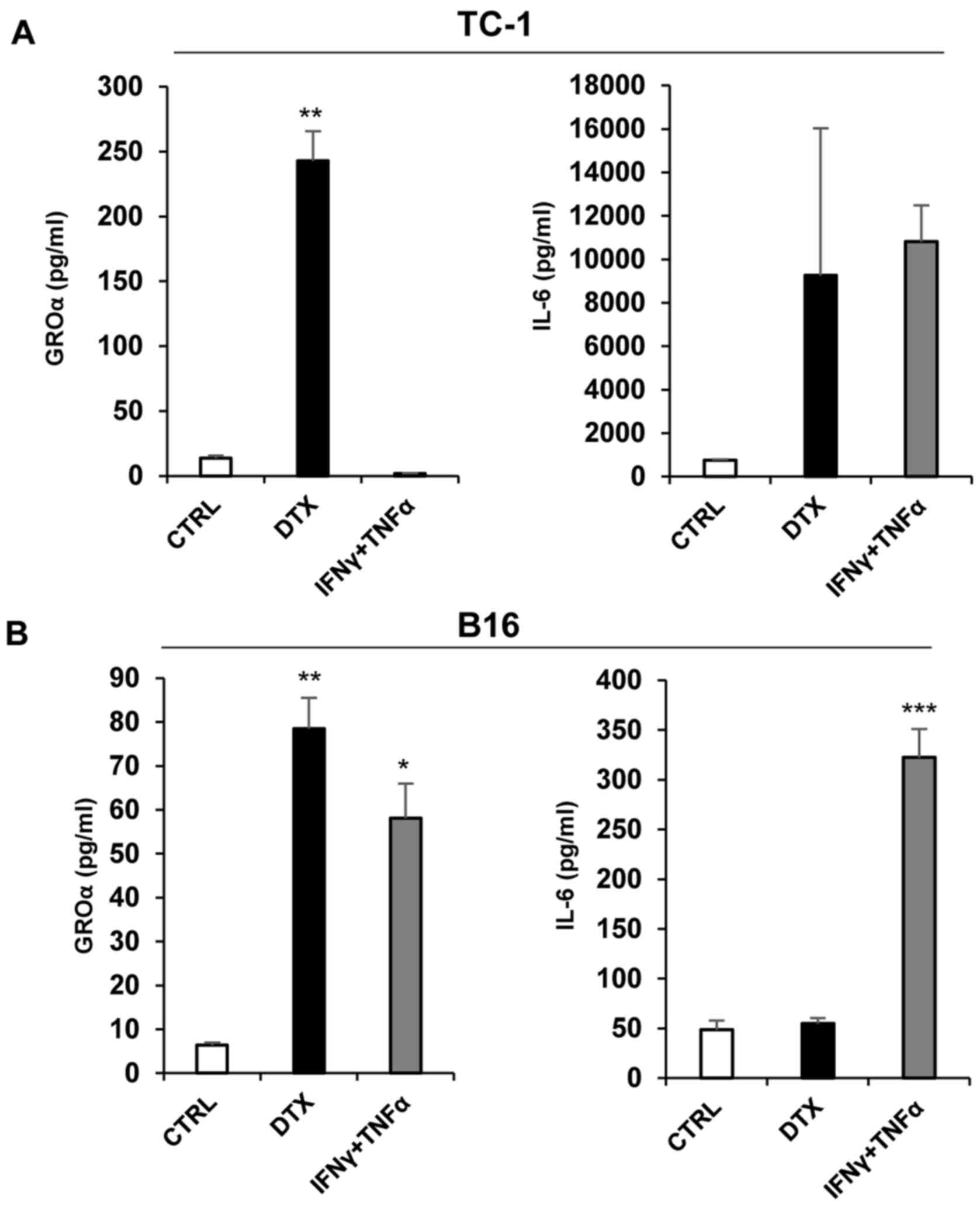
Senescent cells produced IL-6 and GROα
cytokines
To analyse the production of selected cytokines
(GROα and IL-6) by senescent tumour cells, supernatants were
prepared and analysed by ELISA. A significant increase in secreted
GROα was observed following DTX treatment in TC-1 cells compared
with control cells (P<0.01; Fig.
5A). Significantly higher levels of IL-6 (P<0.001) and GROα
(P<0.05) were detected in IFNγ + TNFα-treated B16 cells compared
with control cells, while the secretion of IL-6 following DTX
treatment was not established, although the levels of GROα in B16
cells following DTX treatment were significantly increased compared
with the control cells (P<0.01) (Fig. 5B). Notably, IL-6 levels in B16
cells were substantially lower, as compared with TC-1 cells. IL-6
was also induced in TC-1 cells upon IFNγ + TNFα treatment that did
not induce genotoxic stress. This was expected considering that
IL-6 is regulated through nuclear factor-κB, which is activated by
TNFα (33)
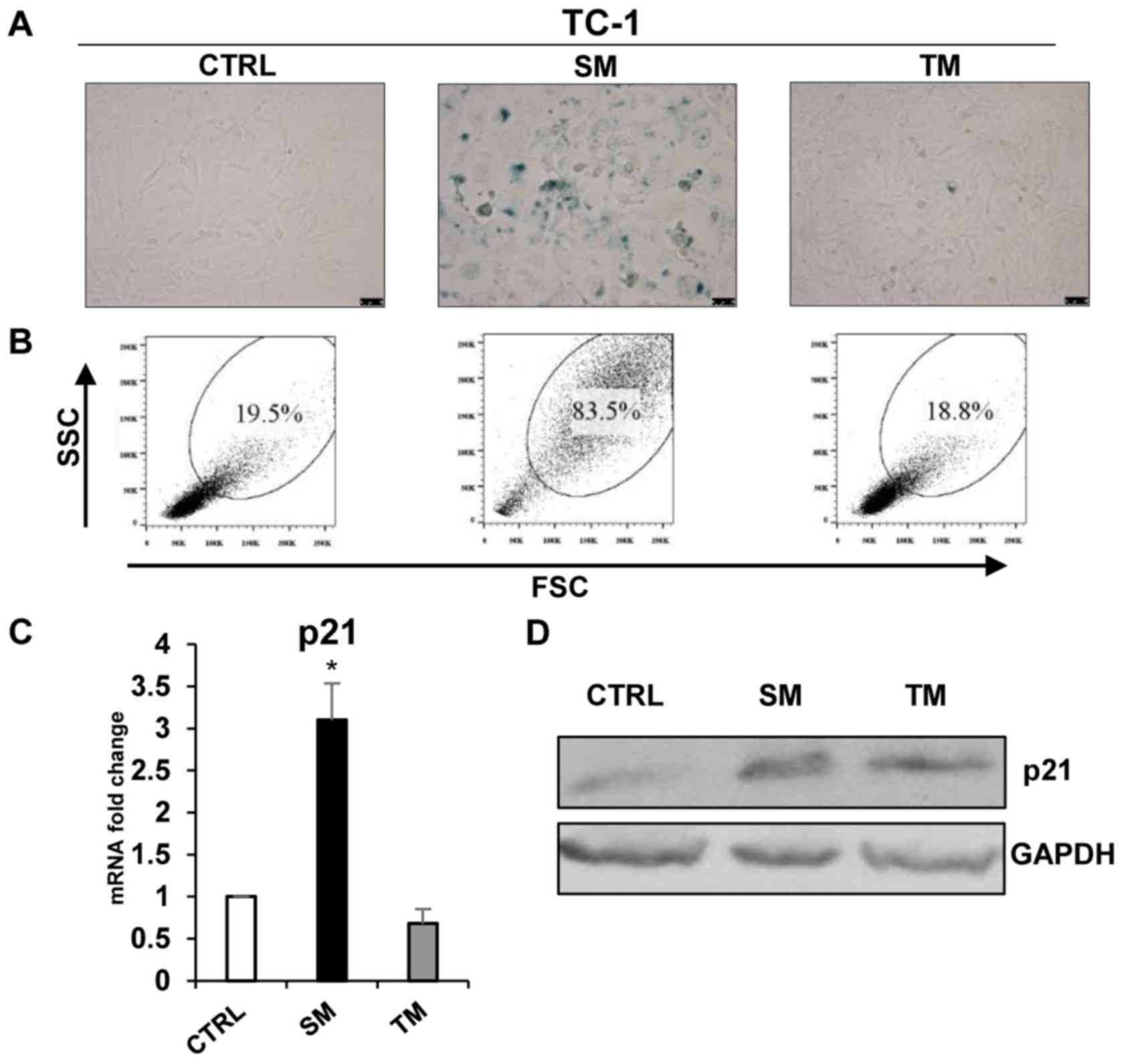
Conditioned medium from DTX-treated
senescent tumour cell culture was able to induce ‘bystander’
senescence in TC-1 and B16 cell lines
To analyse the ‘bystander’ phenomenon of SASP in
tumour cells that were driven to senescence, murine TC-1 and B16
cells were exposed to culture media partly enriched with
conditioned media (1:1) from TC-1 and B16 cells that were driven to
senescence by cultivation with 7.5 µM DTX (SM). In the case
of B16 cells, medium from IFNγ + TNFα-treated cells was also used
(IFNγ + TNFα medium). IFNγ + TNFα medium from TC-1 cells was not
tested since there was no proliferation arrest of ‘primary’
senescence following this treatment. For comparison, medium from
parental untreated cells was also used (TM). The presence of
senescent cells in cultures exposed to these media was assessed
using established markers of cellular senescence. After 4 days of
exposure, the culture medium conditioned by DTX-treated cells
resulted in the increased activity of SA-β-galactosidase in TC-1
and B16 cells (Figs. 6A and
7A) and the increased size and
granularity of the tumour cells analysed by flow cytometry
(Figs. 6B and 7B). The mean percentages (from three
measurements) of SSC and FSC high-gated cells were as follows: TC-1
19.83±1.90 vs. TC-1/SM 83.27±0.59 (P<0.001); TC-1 19.83±1.90 vs.
TC-1/TM 19.24.3±1.49 (P>0.05); B16 13.83±0.57 vs. B16/SM
93.77±3.17 (P<0.001); B16 13.83±0.57 vs. B16/TM 18.2±1.3
(P>0.05) and B16 13.83±0.57 vs. B16/IFNγ + TNFα medium 4.84±0.47
(P>0.05). Significantly elevated levels of p21 in SM-treated
cells (P<0.05; Figs. 6C and D,
7C and D) as compared with fresh medium and medium from untreated
tumour cells were identified. By contrast, the medium conditioned
with medium from IFNγ + TNFα-treated cells was unable to induce
‘bystander’ senescence. Generally, the patterns of these senescent
markers observed in ‘bystander’ cells were similar to those in
senescent cells.
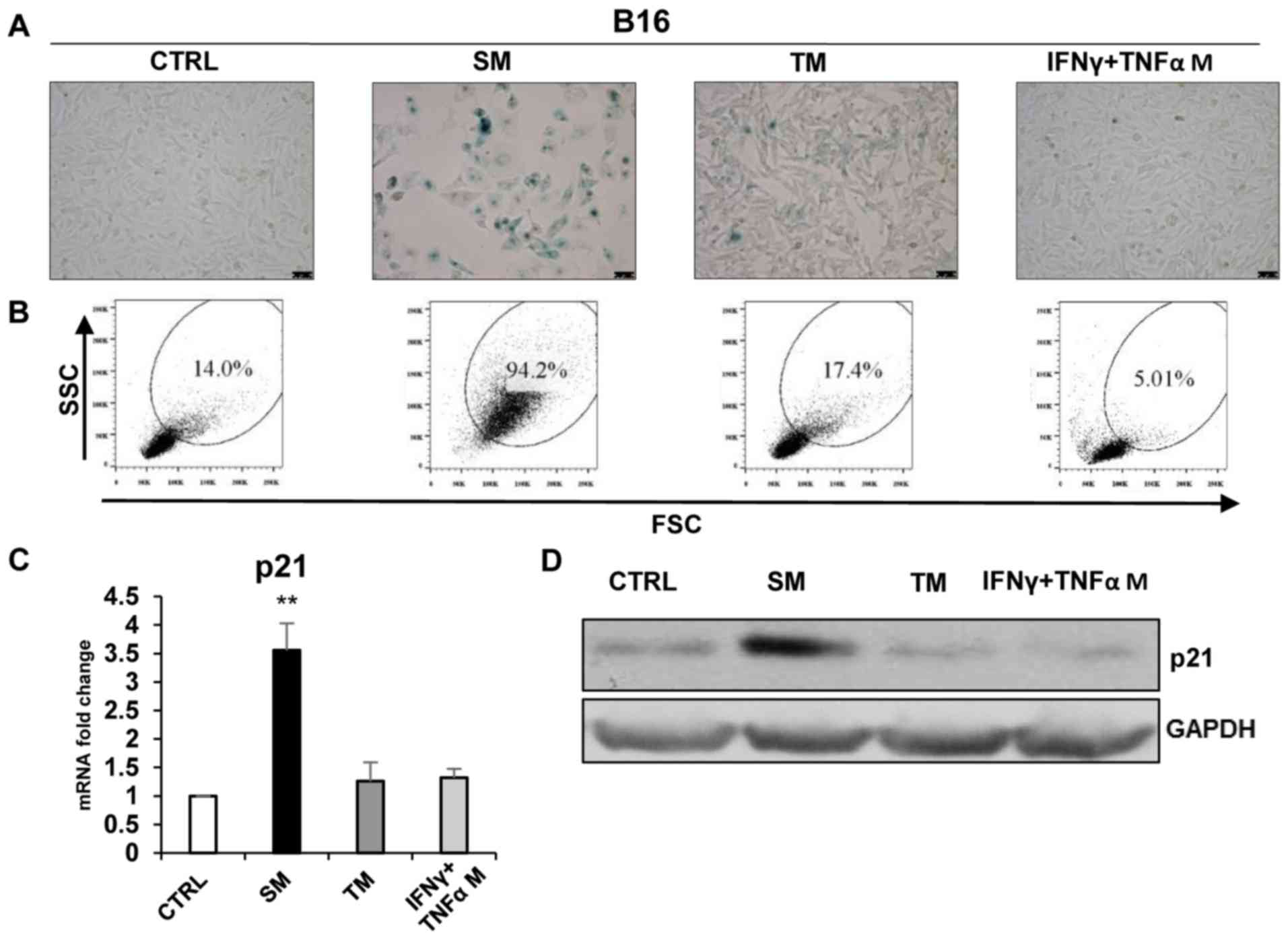 | Figure 7Induction of ‘bystander’ senescence
in B16 tumour cells. (A) Senescence-associated β-galactosidase
activity in B16 cells cultured for 4 days in the medium from
DTX-treated cells (SM), IFNγ + TNFα-treated cells or proliferating
cell medium (TM). (B) The size and granularity of control and
senescent B16 cells was determined by forward and side scatter flow
cytometry analysis. (C) Expression of p21 in B16 cells cultured for
4 days in different media (reverse transcription-quantitative
polymerase chain reaction). (D) Immunoblotting detection of mouse
p21 in B16 cells harvested on day 4 after cultivation in different
media. GAPDH was used as a loading control. Data are presented as
the mean ± standard deviation. **P<0.01. CTRL,
control cells; SM, senescence medium; TM, tumour medium; FSC,
forward scattering; SSC, side scattering; p21, p21Waf1;
B16, B16F10 cell line; IFNγ, interferon γ; TNFα, tumour necrosis
factor α. |
‘Bystander’ senescent cells exhibit
proliferation arrest
The number of proliferating TC-1 and B16 cells was
determined during cultivation with SM and TM. The pattern of
results was similar to that directly following DTX and IFNγ + TNFα
treatments. There was no proliferation of TC-1 and B16 cells
cultivated with SM, whereas following the cultivation of B16 cell
lines with TM, a significant loss of proliferation compared with
the control day 0 was observed (P<0.001; Fig. 8A and B). In addition, TC-1 cells
cultivated with TM proliferated in the same manner as the control
proliferative cells. Similar to ‘primary’ senescent cells, the
arrest of DNA replication in ‘bystander’ senescent cells was tested
by incorporation of EdU (Fig. 8C
and D). Decreased incorporation of EdU in the cells cultured in
senescent medium compared with tumour medium from untreated cells
was observed. The mean percentages (from three measurements) of
EdU-positive cells and the differences between experimental groups
were following: TC-1 84.03±4.00 vs. TC/SM 26.93±3.28 (P<0.001);
TC-1 84.03±4.00 vs. TC-1/TM 68.67±2.46 (P>0.05); B16 93.67±2.46
vs. B16/SM 10.8±1.3 (P<0.001) and B16 93.67±2.46 vs. B16/TM
54.5±5.65 (P<0.05). Next, the proliferative arrest of TC-1 and
B16 cells cultivated with SM was also confirmed in vivo. For
this purpose, B6 mice were injected with two doses of ‘bystander’
senescent cells, the same dose as the testing dose
(3×104) and a 10-fold dose (3×105). Notably,
no tumour growth was observed in B6 mice following the injection of
‘bystander’ senescent TC-1 and B16 cells (Fig. 8E and F; P<0.05 compared with the
controls injected with untreated cells and harbouring growing
tumours). B16 cells treated with conditioned media from the IFNγ +
TNFα cells were not tested since no morphological changes or
increased p21Waf1 expression were observed.
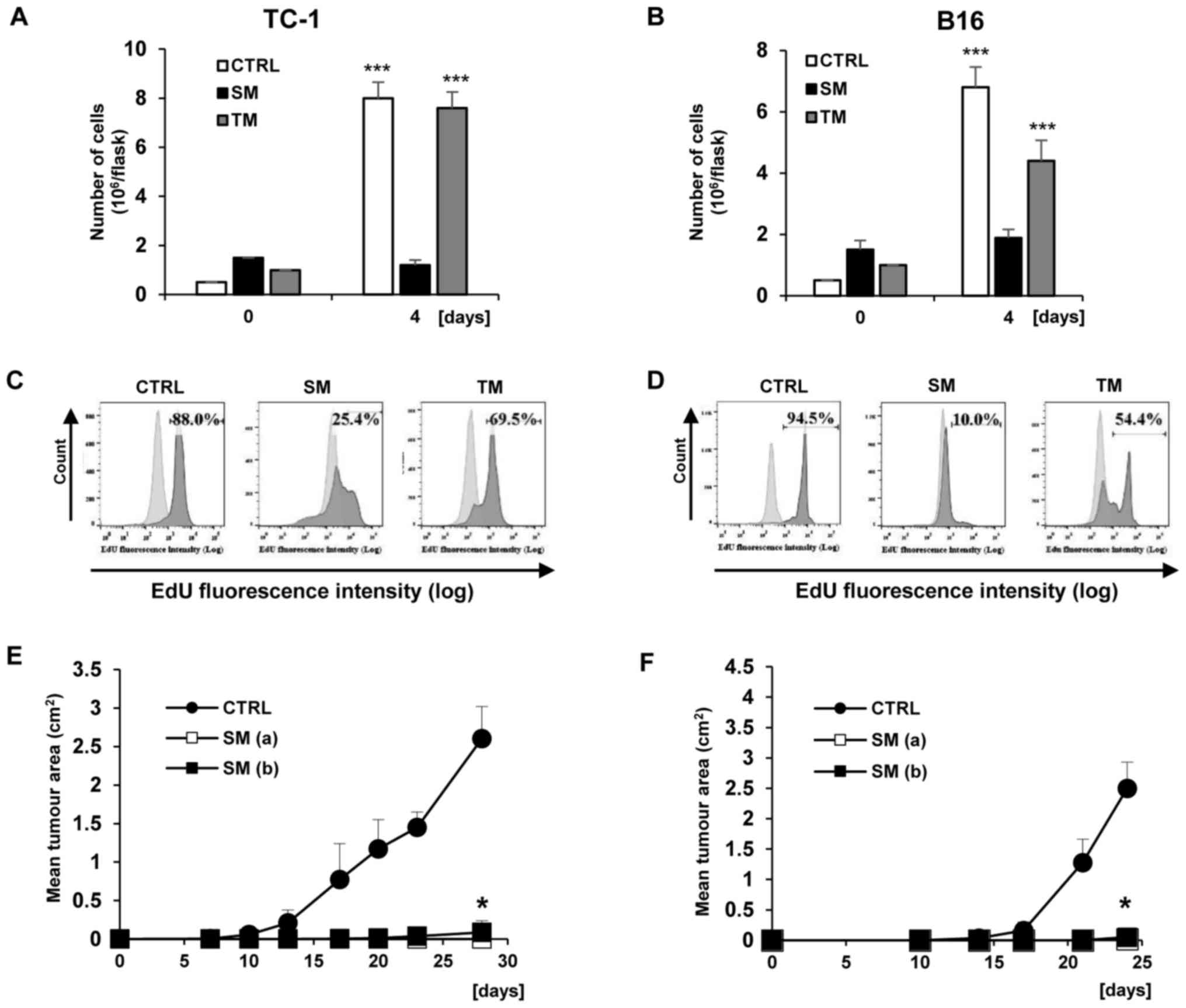 | Figure 8Analysis of TC-1 and B16 cell
proliferation during ‘secondary’ induction. (A) TC-1 and (B) B16
cells were seeded in 25 cm2 cell culture flasks in
triplicate and treated with SM and TM. Cell proliferation was
determined by counting the cell number on day 4. Data are presented
as the mean ± standard deviation. ***P<0.001 vs. day
0. For EdU incorporation: Cells were driven to senescence by
cultivation for 4 days in the medium from DTX-treated cells (SM),
proliferating cell medium (TM) and in fresh RPMI medium (CTRL), and
then incubated with 10 µM EdU for 2 h. Click-iT reaction was
performed on fixed cells and FACS analysis was performed to
determine the fraction of proliferating (C) TC-1 and (D) B16 cells
in the treated and control samples. Mice (8 per group) were
transplanted subcutaneously on day 0 with (E) TC-1 and (F) B16
cells (3×104), with SM (3×104) [B16,
TC-1/DTX(a)] or with SM at a density of 3×105 [B16,
TC-1/DTX(b)] of tumour cells and the tumour growth was monitored.
The experiment was repeated two times with similar results.
*P<0.05 [TC-1 vs. TC-1/SM (a or b)]; [B16 or B16 vs.
B16/SM (a or b); analysis of variance]. CTRL, control cells; SM,
senescence medium; TM, tumour medium; B16, B16F10 cell line; DTX,
docetaxel. |
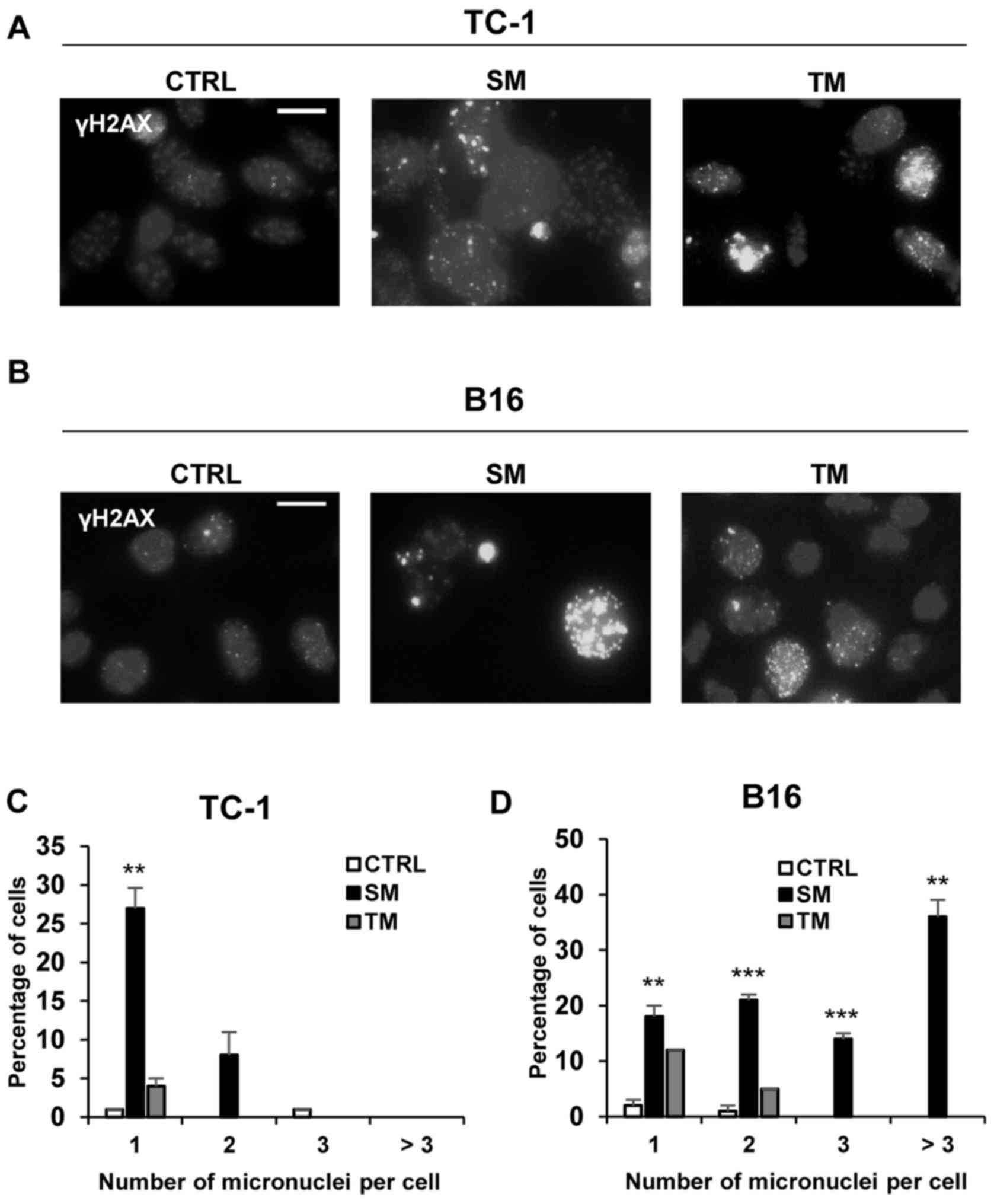
DNA damage response in ‘bystander’
senescent cells
It was investigated whether the conditioned
senescent medium may induce DNA damage in a ‘bystander’ manner in
TC-1 and B16 cells. Notably, there was an increase in the number of
γH2AX foci following incubation of the cells with senescent medium
(Fig. 9A and B). Quantification
analysis indicated an increase in the number of micronuclei in the
cells treated with SM in TC-1 and B16 cell lines compared with
control cells (Fig. 9C and D).
There was a significant increase in the percentage of TC-1 cells
treated with SM with 1 micronucleus per cell compared with the
control cells (P<0.01); and a significant increase in the
percentage of B16 cells treated with SM with 1, 2, 3 or more
micronuclei per cell compared with the control cells (P<0.01).
TM medium resulted in an increase only in the group with 1
micronucleus per cell in the B16 cell line (Fig. 9D). This increase may be explained
by the fact that TM, conditioned control cell medium, was obtained
from the proliferating cancer cells culture. The secretome of
proliferating tumour cells also harbors cytokines, chemokines and
other soluble agents that may be genotoxic (1), and their concentration when the cells
are cultured in the TM medium may be higher when compared with the
cells cultured in fresh medium, although no senescence induction
was observed in the present study. When compared with ‘primary’
senescence, the pattern of DNA damage in ‘bystander’ senescence was
similar (Figs. 4 and 9). This indicated that ‘bystander’
senescence is induced by the DNA damage response pathway.
Discussion
The present study provides insight into the effects
of two known inducers of cell stress and premature cellular
senescence in a number of cell lines: DTX as a chemotherapeutic
agent and a combination of Th1 cytokines, IFNγ and TNFα (28,34).
The differences in the senescence-associated phenotype between the
cells that underwent these two treatments were compared. For the
experiments, two murine cell lines were employed: TC-1 expressing
human HPV16 E6 and E7 oncoproteins, and B16 melanoma cell lines.
DTX has been demonstrated to induce senescence in TC-1 cells. p53
and retinoblastoma protein (pRb) are inactivated in unperturbed
TC-1 cells by the presence of E3 ubiquitin ligases E6 and E7
(35), respectively, so it is
unclear how proliferation arrest is mediated. p53 and pRb may be
reactivated in TC-1 cells by the suppression of E6 and E7, which
may be downregulated by genotoxic stress (35). This is analogous to HeLa cells
exposed to genotoxic senescence-inducing conditions (36).
The present study particularly focused on the
capability of cells to induce ‘bystander’ senescence through their
secretome. The phenotype and biological behaviours of senescent
cells correspond with the particular agents that induce cellular
stress and subsequent premature senescence. These effects may be
distinct in various cell lineages, reflecting the presence or
absence of intact crucial signalling pathways.
DTX is a microtubule-stabilizing taxane that is
widely used clinically for the treatment of breast and prostate
cancer types and small cell carcinoma of the lung (37). The present study characterized the
phenotype of DTX-induced senescent cells. DTX has already been
described to induce cellular senescence in several tumour cell
lines in a limited number of studies (38,39).
DNA damage following DTX treatment was previously described in MCF7
cells (40) and in
p53-non-functional MDA-MB-231 cells (41). However, the effects of DTX in terms
of senescence induction remain not fully described or understood.
The presence of micronuclei along with γH2AX foci in DTX-treated
cells in the present study indicates at the generation of DNA
double-strand breaks prior to or during mitosis, resulting in the
activation of persisting cell cycle checkpoints and the development
of senescence in daughter cells. A previous study demonstrated the
therapeutic effects of DTX on TC-1 cells in several experiments
(42) and, notably, demonstrated
that DTX induced senescence in the TC-1 cells (and TRAMP-C2 cells),
which accelerated tumour growth when co-cultured with proliferating
tumour cells (28). The present
study provides further insight and, notably, demonstrates the
capacity of SASP from DTX-induced cells to induce ‘bystander’
senescence. In two murine cell lines, DTX induced cellular stress
and proliferation arrest, accompanied by the increased production
of IL-6 and GROα, typical (although not exclusively) components of
SASP. Concurrent with previously published results, the results of
the present study demonstrate that DTX is capable of inducing
stable senescence in different tumour cell lines with the capacity
to induce genotoxic stress and senescence in neighbouring
cells.
It has been previously demonstrated that IFNγ or
IFNγ + TNFα induce cellular stress and proliferation
arrest/senescence, or even apoptosis, in certain cell lineages
(27). This is associated with the
induction of the transforming growth factor-β signalling pathway
and subsequent induction of NADPH oxidase 4 protein and oxidative
stress (17). The results of the
present study indicated that the reason why the TC-1 cell line did
not undergo cell arrest was due to a lack of oxidative stress.
Previously, it has been demonstrated that the cytostatic effects of
IFNγ on B16 cells (B16 cells expressing the ubiquitination-based
cell cycle indicator) were associated with G1 arrest, mediated by
the induction of p27 (43).
The present study evaluated the effects of IFNγ +
TNFα treatment on B16 cells. The data demonstrated a cytostatic
effect, as opposed to the true senescence-inducing effect of IFNγ +
TNFα in B16 cells. Notably, unlike TC-1 cells, IFNγ + TNFα-treated
B16 cells produced elevated amounts of GROα, a principle component
of SASP that serves a role in senescence induction and maintenance
in a paracrine manner (1).
However, the treated B16 cells injected into mice resulted in
tumour growth. Furthermore, the cells did not produce SASP capable
of inducing ‘bystander’ senescence, suggesting that other
components or higher concentrations of IL-6 and GROα are required.
This indicates a lack of paracrine cytokine loop components
contributing to senescence maintenance (44).
In conclusion, the results of the present study
indicate that DTX induces senescence in TC-1 and B16 cells.
Furthermore, in B16 cells, IFNγ + TNFα treatment induces a
reversible proliferation arrest, as opposed to true senescence,
despite the fact that this treatment induced certain senescence
markers. TC-1 cells were indicated to be resistant to IFNγ + TNFα
treatment. These results suggest that each senescent inducer must
therefore be studied in the context of a specific cell type.
Funding
The present study was supported by the Czech Science
Foundation (grant nos. 15-24769S and 15-03379S), the Academy of
Sciences of the Czech Republic (grant no. RVO 68378050), the
ministry of Education (grant no. LM2015040 to the Czech Centre for
Phenogenomics), the Youth and Sports and European Regional
Development Fund and Smartbrain (project DiaNa21).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
OS and RM performed the molecular studies, in
vivo assays, statistical analysis and drafted the manuscript.
JB performed fluorescence-activated cell sorting measuring and
analysis. BM helped with the molecular studies and statistical
analysis. MR and ZH designed the study and helped to draft the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed according to the EU
Directive 2010/63/EU on the protection of animals used for
scientific purposes. Experimental protocols were ethically approved
by the Institutional Animal Care Committee of the Institute of
Molecular Genetics (Prague, Czech Republic).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mrs Renáta Turečková
(Institute of Molecular Genetics of the Czech Academy of Sciences,
Czech Republic) for skilful technical assistance and Dr Šárka
Takáčová (Institute of Molecular Genetics of the Czech Academy of
Sciences, Czech Republic) for editorial assistance.
References
|
1
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz
DP, Goldstein J, Nelson PS, Desprez PY and Campisi J:
Senescence-asso ciated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS Biol. 6:2853–2868. 2008. View Article : Google Scholar
|
|
3
|
Benvenuti S, Cramer R, Bruce J, Waterfield
MD and Jat PS: Identification of novel candidates for replicative
senescence by functional proteomics. Oncogene. 21:4403–4413. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bringold F and Serrano M: Tumor
suppressors and oncogenes in cellular senescence. Exp Gerontol.
35:317–329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cristofalo VJ, Lorenzini A, Allen RG,
Torres C and Tresini M: Replicative senescence: A critical review.
Mech Ageing Dev. 125:827–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salama R, Sadaie M, Hoare M and Narita M:
Cellular senescence and its effector programs. Genes Dev.
28:99–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pluquet O, Pourtier A and Abbadie C: The
unfolded protein response and cellular senescence. A review in the
theme: Cellular mechanisms of endoplasmic reticulum stress
signaling in health and disease. Am J Physiol Cell Physiol.
308:C415–C425. 2015. View Article : Google Scholar
|
|
8
|
Kojima H, Inoue T, Kunimoto H and Nakajima
K: IL-6STAT3signaling and premature senescence. JAK-STAT.
2:e257632013. View Article : Google Scholar
|
|
9
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
d’Adda di Fagagna F, Reaper PM,
Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G,
Carter NP and Jackson SP: A DNA damage checkpoint response in
telomere-initiated senescence. Nature. 426:194–198. 2003.
View Article : Google Scholar
|
|
11
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blazkova H, Krejcikova K, Moudry P, Frisan
T, Hodny Z and Bartek J: Bacterial intoxication evokes cellular
senescence with persistent DNA damage and cytokine signalling. J
Cell Mol Med. 14:357–367. 2010. View Article : Google Scholar
|
|
13
|
Cairney CJ, Bilsland AE, Evans TR, Roffey
J, Bennett DC, Narita M, Torrance CJ and Keith WN: Cancer cell
senescence: A new frontier in drug development. Drug Discov Today.
17:269–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pascal T, Debacq-Chainiaux F, Chrétien A,
Bastin C, Dabée AF, Bertholet V, Remacle J and Toussaint O:
Comparison of replicative senescence and stress-induced premature
senescence combining differential display and low-density DNA
arrays. FEBS Lett. 579:3651–3659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davalos AR, Coppe JP, Campisi J and
Desprez PY: Senescent cells as a source of inflammatory factors for
tumor progression. Cancer Metastasis Rev. 29:273–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hubackova S, Krejcikova K, Bartek J and
Hodny Z: IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA
damage response are shared features of replicative,
oncogene-induced, and drug-induced paracrine ‘bystander
senescence’. Aging (Albany NY). 4:932–951. 2012. View Article : Google Scholar
|
|
18
|
Calcinotto A and Alimonti A: Aging tumour
cells to cure cancer: ‘pro-senescence’ therapy for cancer. Swiss
Med Wkly. 147:w143672017.
|
|
19
|
Ortiz-Montero P, Londoño-Vallejo A and
Vernot JP: Senescence- associated IL-6 and IL-8 cytokines induce a
self- and cross-reinforced senescence/inflammatory milieu
strengthening tumorigenic capabilities in the MCF-7 breast cancer
cell line. Cell Commun Signal. 15:172017. View Article : Google Scholar
|
|
20
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nelson G, Wordsworth J, Wang C, Jurk D,
Lawless C, Martin- Ruiz C and von Zglinicki T: A senescent cell
bystander effect: Senescence-induced senescence. Aging Cell.
11:345–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hodny Z, Hubackova S and Bartek J:
Cytokines shape chemotherapy-induced and ‘bystander’ senescence.
Aging (Albany NY). 2:375–376. 2010. View Article : Google Scholar
|
|
23
|
Kuilman T, Michaloglou C, Vredeveld LC,
Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ and Peeper DS:
Oncogene-induced senescence relayed by an interleukin-dependent
inflammatory network. Cell. 133:1019–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozturk M, Arslan-Ergul A, Bagislar S,
Senturk S and Yuzugullu H: Senescence and immortality in
hepatocellular carcinoma. Cancer Lett. 286:103–113. 2009.
View Article : Google Scholar
|
|
25
|
Koturbash I, Rugo RE, Hendricks CA, Loree
J, Thibault B, Kutanzi K, Pogribny I, Yanch JC, Engelward BP and
Kovalchuk O: Irradiation induces DNA damage and modulates
epigenetic effectors in distant bystander tissue in vivo. Oncogene.
25:4267–4275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou H, Randers-Pehrson G, Waldren CA,
Vannais D, Hall EJ and Hei TK: Induction of a bystander mutagenic
effect of alpha particles in mammalian cells. Proc Natl Acad Sci
USA. 97:2099–2104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Braumüller H, Wieder T, Brenner E, Aßmann
S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M,
Griessinger C, et al: T-helper-1-cell cytokines drive cancer into
senescence. Nature. 494:361–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simova J, Sapega O, Imrichova T, Stepanek
I, Kyjacova L, Mikyskova R, Indrova M, Bieblova J, Bubenik J,
Bartek J, et al: Tumor growth accelerated by chemotherapy-induced
senescent cells is suppressed by treatment with IL-12 producing
cellular vaccines. Oncotarget. 7:54952–54964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin KY, Guarnieri FG, Staveley-O’Carroll
KF, Levitsky HI, August JT, Pardoll DM and Wu TC: Treatment of
established tumors with a novel vaccine that enhances major
histocompatibility class II presentation of tumor antigen. Cancer
Res. 56:21–26. 1996.PubMed/NCBI
|
|
30
|
Overwijk WW and Restifo NP: B16 as a mouse
model for human melanoma. Curr Protoc Immunol Chapter: 20 Unit.
20:12001.
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Ramirez JM, Bai Q, Péquignot M, Becker F,
Kassambara A, Bouin A, Kalatzis V, Dijon-Grinand M and De Vos J:
Side scatter intensity is highly heterogeneous in undifferentiated
pluripotent stem cells and predicts clonogenic self-renewal. Stem
Cells Dev. 22:1851–1860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YH, Lin JX and Vilcek J:
Interleukin-6 induction by tumor necrosis factor and interleukin-1
in human fibroblasts involves activation of a nuclear factor
binding to a kappa B-like sequence. Mol Cell Biol. 10:3818–3823.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hubackova S, Kucerova A, Michlits G,
Kyjacova L, Reinis M, Korolov O, Bartek J and Hodny Z: IFNγ induces
oxidative stress, DNA damage and tumor cell senescence via
TGFβ/SMAD signaling-dependent induction of Nox4 and suppression of
ANT2. Oncogene. 35:1236–1249. 2016. View Article : Google Scholar
|
|
35
|
Mukherjee S, Debata PR, Hussaini R,
Chatterjee K, Baidoo JN, Sampat S, Szerszen A, Navarra JP, Fata J,
Severinova E, et al: Unique synergistic formulation of curcumin,
epicatechin gallate and resveratrol, tricurin, suppresses HPV E6,
eliminates HPV+ cancer cells, and inhibits tumor
progression. Oncotarget. 8:60904–60916. 2017.PubMed/NCBI
|
|
36
|
Novakova Z, Hubackova S, Kosar M,
Janderova-Rossmeislova L, Dobrovolna J, Vasicova P, Vancurova M,
Horejsi Z, Hozak P, Bartek J, et al: Cytokine expression and
signaling in drug- induced cellular senescence. Oncogene.
29:273–284. 2010. View Article : Google Scholar
|
|
37
|
Ringel I and Horwitz SB: Studies with RP
56976 (taxotere): A semisynthetic analogue of taxol. J Natl Cancer
Inst. 83:288–291. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schwarze SR, Fu VX, Desotelle JA, Kenowski
ML and Jarrard DF: The identification of senescence-specific genes
during the induction of senescence in prostate cancer cells.
Neoplasia. 7:816–823. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Di Mitri D, Toso A, Chen JJ, Sarti M,
Pinton S, Jost TR, D’Antuono R, Montani E, Garcia-Escudero R,
Guccini I, et al: Tumour-infiltrating Gr-1+ myeloid cells
antagonize senescence in cancer. Nature. 515:134–137. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hernández-Vargas H, Palacios J and
Moreno-Bueno G: Molecular profiling of docetaxel cytotoxicity in
breast cancer cells: Uncoupling of aberrant mitosis and apoptosis.
Oncogene. 26:2902–2913. 2007. View Article : Google Scholar
|
|
41
|
Hernández-Vargas H, Palacios J and
Moreno-Bueno G: Telling cells how to die: Docetaxel therapy in
cancer cell lines. Cell Cycle. 6:780–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mikyšková R, Štěpánek I, Indrová M,
Bieblová J, Šímová J, Truxová I, Moserová I, Fučíková J, Bartůňková
J, Špíšek R, et al: Dendritic cells pulsed with tumor cells killed
by high hydrostatic pressure induce strong immune responses and
display therapeutic effects both in murine TC-1 and TRAMP-C2 tumors
when combined with docetaxel chemotherapy. Int J Oncol. 48:953–964.
2016. View Article : Google Scholar
|
|
43
|
Kakimi K, Matsushita H, Hosoi A, Miyai M
and Ohara O: CTLs regulate tumor growth via cytostatic effects
rather than cytotoxicity: A few T cells can influence the growth of
many times more tumor cells. OncoImmunology. 4:e9704642014.
View Article : Google Scholar
|
|
44
|
Bartek J, Hodny Z and Lukas J: Cytokine
loops driving senescence. Nat Cell Biol. 10:887–889. 2008.
View Article : Google Scholar : PubMed/NCBI
|