Introduction
Malignant melanoma (MM) is one of the most
aggressive types of malignant tumor, and is responsible for the
majority of skin cancer-associated mortalities (1,2).
Extracellular matrix metalloproteinase inducer, also known as
Basigin and cluster of differentiation 147 (CD147), is a highly
glycosylated type-I transmembrane protein of the immunoglobulin
superfamily (3). A previous study
revealed that CD147 served a key role in cellular apoptosis.
Inhibition of CD147 expression by short hairpin (sh)RNA increased
the chemosensitivity of oral squamous cell carcinoma cells,
specifically a multidrug-resistant cell line, by downregulating the
expression of the anti-apoptotic gene X-linked inhibitor of
apoptosis (4). Recently,
propranolol was reported to induce the apoptosis of vascular
endothelial cells by decreasing the expression levels of CD147 in
the treatment of infantile hemangiomas (5). Overexpression of CD147 also
facilitates the induction of apoptosis of Jurkat T and Chinese
hamster ovary cells via methotrexate treatment (6); however, the mechanism underlying the
effects of CD147 on apoptosis in melanoma cells remains
unclear.
The phosphatase and tensin homolog
(PTEN)/phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)
signaling pathway is a pivotal pathway in melanomagenesis, and is
involved in a variety of biological processes, including cell death
and survival, cell proliferation, angiogenesis and autophagy
(7,8). Under physiological conditions, the
activation of PI3K facilitates transfer of a phosphoryl group to
form phosphatidylinositol (3,4,5)-trisphosphate (PIP3); PTEN,
characterized by phosphatase activity, negatively regulates this
procedure (9). PIP3 acts as a
secondary messenger to activate the downstream signaling pathway;
as a consequence, AKT and its substrate mechanistic target of
rapamycin (mTOR), are activated and are able to induce the
synthesis of proteins involved in cell survival, proliferation and
apoptosis (10). In melanoma, the
PTEN gene has been observed to possess deletions in ~30% of
sporadic cases (with loss of the corresponding protein in 5-20% of
primary melanomas) and in ~40% of melanoma cell lines (11,12).
Therefore, PTEN is a key molecule associated with the pathogenesis
of melanoma.
Insulin-like growth factor-binding protein 2
(IGFBP2) belongs to the IGF-binding protein family, containing six
members (IGFBP1-6) with a high affinity of IGF1 and IGF2. Previous
studies (13,14) have revealed that IGFBP2 could
associate with IGFs to inhibit binding to the receptor, thereby
attenuating IGF-induced tumorigenesis; however, accumulating
evidence has demonstrated that IGFBP2 exhibits oncogenic effects,
including the suppression of apoptosis, and facilitating cell
growth and migration (15), which
are independent of the ability of IGFBP2 to associate with
IGFs.
The aim of the present study was to investigate the
role of CD147 in melanoma cell apoptosis by examining the effects
of CD147 knockdown on IGFBP2 expression in melanoma cells and the
activity of the AKT/mTOR signaling pathway to determine whether the
CD147/IGFBP2 axis serves a key role in melanoma cell apoptosis. In
addition, the present study investigated the underlying
mechanism.
Materials and methods
Cell culture and lentiviral
infection
The MM cell lines, A375 and SK-MEL-28, (American
Type Culture Collection, Manassas VA, USA) were stored in our
laboratory (Hunan Key Laboratory of Skin Cancer and Psoriasis,
Xiangya Hospital, Central South University, Changsha, China), and
cultured in high-glucose Dulbecco's modified Eagles medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Gibco; hermo Fisher
Scientific, Inc., Waltham, MA, USA) and antibiotics (1%
penicillin-streptomycin). The cells were maintained at 37°C in an
incubator under 5% CO2.
For lentiviral packaging as previously established
(16), briefly, 293T cells were
stored in our laboratory, and transfected with vectors containing
an shRNA targeting CD147 (shRNA-CD147-C1, forward sequence
5′-GATCCCCGTCGTCAGAACACATCAACTTCAAGAGAGTTGATGTGTTCTGACGACTTTTTGGAAA-3′,
reverse sequence:
5′-AGCTTTTCCAAAAAGTCGTCAGAACACATCAACTCTCTTGAAGTTGATGTGTTCTGACGACGGG-3′
or shRNA-CD147-C2, forward sequence:
5′-GATCCCCTGACAAAGGCAAGAACGTCTTCAAGAGAGACGTTCTTGCCTTTGTCATTTTTG
GAAA-3′, reverse sequence: 5′-AGCTTTTCCAAAAATGACAAAGG
CAAGAACGTCTCTCTTGAAGACG TTCTTGCC TTTGTCAGGG-3′, AgeI and
EcoRI restriction sites) or a negative control
(pLKO.1-sh-Mock, sequence: 5′-AGAAGT GTAGCATGCAGATTACT ATTG
AGCCTTATCGGAC TTGACGTCAGTA GTCAACACTCTC-3′), and packaging vectors
(psPAX2 and pMD2-G) using TurboFest transfection reagent (Thermo
Fisher Scientific, Inc.) for 24 h. Then, the supernatant fraction
containing lentiviral particles was collected at 48 and 72 h,
respectively; transduction with A375 and SK-MEL-28 cells was
conducted using 10 μg/ml Polybrene (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany, cat. no. H9268) and 1 ml lentiviral
fraction in 6-well plate. Each cell line was infected for three
wells. The medium (10% FBS DMEM) was replaced with fresh medium
containing 1 μg/ml puromycin for stable cell selection
following 16 h post-transfection. In the current study,
pLKO.1-sh-CD147-C2 vector was used for subsequent analysis.
LY294002, a PI3K signaling pathway inhibitor, was
purchased from Sigma-Aldrich (Merck KGaA), dissolved in DMSO and
diluted to a final concentration of 50 μg/ml; LY294002 was
applied to cells at room temperature for 24 h; 0 μg/ml
LY294002 was used as the control.
Flow cytometry and transmission electron
microscopy
Cellular apoptosis was determined by flow cytometry,
according to the manufacturer's protocols of an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit (C1062,
(Beyotime Institute of Biotechnology, Beijing, China). Briefly, a
total of 5×105 cells were harvested and collected,
resuspended in 195 μl binding buffer (included in kit), and
stained with 5 μl Annexin V-fluorescein isothiocyanate
conjugate and 10 μl prop-idium iodide solution at room
temperature for 20 min. The stained cells were then analyzed using
a FACSCanto II flow cytometer using BD FACSDiva™ software v 6.0 (BD
Biosciences, San Jose, CA, USA).
Transmission electron microscopy was performed
according to previous our study (4), briefly, cells were first fixed with
2% paraformaldehyde and 2% glutaraldehyde, and then with 2% osmium
tetroxide for ≤2 h. Fixed specimens were dehydrated, embedded and
sliced with an ultra-microslicing microtome (LKB-III, LKB
Instruments, Victoria, Australia). Samples were then examined by
transmission electron microscopy (H-7500, magnification, ×5,000,
Hitachi, Ltd., Tokyo, Japan).
Human apoptosis assay
Detection of apoptotic proteins was performed using
the RayBio® human apoptosis array G1 kit (cat. no. AAH-APO-G1;
RayBioTech, Inc., Guangzhou, China). The assay conducted in the
present study detects 43 different apoptotic proteins, as well as
GAPDH as a loading control. The assays for A375-sh-Mock and
A375-sh-CD147 were performed using 6-well glass slides, according
to the manufacturer's protocols. SK-MEL-28 cells were used as
confirmation. Then, the assay slides were analyzed by Genomax
Technologies (Singapore) using a microarray scanner (G2505C,
Agilent Technologies Inc., Santa Clara, CA, USA). Data obtained
from scanning were exported for analysis using Agilent feature
extraction software version 10.5 (Agilent Technologies Inc). and
the relative fold changes in the expression profile of each protein
were calculated; two independent experiments (n=4) were performed
to confirm the results.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting
For qPCR, total cellular RNA was extracted using
TRizol reagent (Thermo Fisher Scientific, Inc.). A total of 3
μg RNA was used as a template for RT (cat. no. R233-01,
Vazyme, Piscataway, NJ, USA), the procedure of RT comprised two
steps, the first at 42°C for 2 min, then at 50°C 15 min, followed
by the second step, final at 85°C for 5 sec to terminate the
reaction. qPCR was performed using the ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Each 20-μl PCR
reaction mixture contained 1 μl cDNA product, 2 μl
specific forward/universal primer mix, and 10 μl SYBRGreen
2X Universal PCR Master Mix (cat. no. Q141-02, Vazyme). The
thermocycling conditions of qPCR were: Denaturation at 95°C for 5
min, then extension at 60°C for 40 cycles. The IGFBP2 primers used
were as 5′-AGAAGGTCACTGAGCAGCAC-3′ (forward), and
5′-GAGGTTGTACAGGCCATGCT-3′ (reverse). β-actin (forward,
5′-GTCATCACCATTGGCAATGAG-3′ and reverse,
5′-CGTCACACTTCATGATGGAGTT-3′) were determined as a control by using
the 2−ΔΔCq analysis method (17). Triplicate determination was
repeated 3 times.
For western blotting, cells were harvested and lysed
with radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Total proteins were
quantified via a Bradford protein assay (Beyotime Institute of
Biotechnology). The protein samples (30 μg/sample) were
separated by 10% SDS-PAGE and transferred onto a polyvinylidene
difluoride membrane. The membranes were incubated with primary
antibodies at 4°C in 5% non-fat dried milk in TBS buffer for
overnight. The membranes were washed three times with TBS buffer
and incubated with horseradish peroxidase secondary antibodies
(cat. nos. AS003 and AS014, 1:5,000) from ABclonal Biotech Co.,
Ltd. (Wuhan, China) at room temperature for 1 h. The blots were
detected using enhanced chemiluminescent reagents Clarity Max™
Western ECL Blotting Substrates (cat. no. 1705062) (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocols. The following primary antibodies were
used: Anti-CD147 (cat. no. sc-21746, 1:1,000; Santa Cruz
Biotechnology, Inc.), and anti-IGFBP2 (cat. no. ab109284, 1:1,000;
Abcam), anti-PTEN (cat. no. ab32199, 1:1,000; Abcam),
phosphorylated (p)-AKT (cat. no. 9271, 1:1,000), AKT (cat. no.
9272, 1:1,000), p-mTOR (cat. no. 2971, 1:1,000) and mTOR (cat. no.
2972, 1:1,000) and cle-PARP (cat. no. 9532S, 1:1,000) were
purchased from Cell Signaling Technology, Inc., Danvers, MA, USA).
β-actin (cat. no. sc-517582, 1:1,000; Santa Cruz Biotechnology,
Inc.) or GAPDH (sc-47724, 1:1,000; Santa Cruz Biotechnology, Inc.)
was used as an internal reference. The band intensity was
quantified by using Bio-Rad Image Lab™ software version 6.1
(Bio-Rad Laboratories, Inc.).
Tumor xenograft mice
Tumor xenograft mouse models were previously
established by our laboratory (18) and the animal study was approved by
the Ethics Committee of Xiangya Hospital, Central South University
(Changsha, China). All animal experiments were conducted in
accordance with the Guidelines of The National Institutes of Health
Guide for the Care and Use of Laboratory Animals (19). Briefly, A375 cells stably
transduced with sh-CD147 or sh-Mock lentiviruses, were harvested,
washed with 1X PBS buffer, re-suspended in cold serum-free
high-glucose DMEM, and then subcutaneously injected
(5×106/0.15 ml cells) into the right flank of
4-6-week-old male BALB/c nude mice (Shanghai SLAC Laboratory Animal
Co., Ltd., Shanghai, China) and 5 mice for each group. All mice
were exposed to a 12 h light/dark cycle, supplied with free food
and drinking water under specific-pathogen free condition. The
tumors were measured using calipers and the tumor volumes were
calculated using the following formula: Length x width x height x
0.52. The animals were sacrificed 35 days following tumor cell
inoculation, and the tumor tissues were collected and fixed in 10%
buffered formalin, embedded in paraffin, sectioned at 5 μm
and stained with H&E based on our previous study (18), or subjected to immunohistochemical
analysis.
Immunohistochemical analysis
Tumor tissues were obtained from A375-sh-CD147 and
A375-sh-Mock nude mouse xeno-grafts. A human melanoma tissue array
was purchased from Alenabio (Xi'an, China), including 128 primary
melanomas and 64 metastatic melanomas (cat. nos. M1004 and M1004a).
In addition, we also collected 15 paraffin-embedded specimens with
a clinical diagnosis of nevus from the Department of Dermatology,
Xiangya Hospital (Changsha, China) from 2015 to 2017, the average
age was 35.13±12.61 years-old, and the sex ratio was 1.14 (8
male):1 (7 female). Immunohistochemical staining was performed
using a biotin-streptavidin horseradish peroxidase detection kit
(OriGene Technologies, Inc., Beijing, China), according to the
manufacturer's protocols. Briefly, the slides were heated at 60°C
for 2 h, dewaxed in turpentine and rehydrated in a graded ethanol
series and washed with 1X PBS. Following antigen retrieval, and
inactivation of endogenous peroxidase activity with 3%
H2O2, the slides were incubated with CD147
(1:100) or IGFBP2 antibodies (1:100) at 4°C in a humidified chamber
overnight. The secondary antibody conjugated with biotin from the
detection kit (OriGene Technologies, Inc.) was applied for 1 h at
room temperature. Horseradish peroxidase-streptavidin (OriGene
Technologies, Inc.) was added to the slides for 30 min, then the
slides were developed in 3,3-diaminobenzidine (DAB) and
counterstained with hematoxylin, and mounted in neutral balsam. For
semi-quantitative analysis, the positive areas (%) in each image
were quantified using Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). The cut-off values of
expression for partial staining in <10% tumor cells was
considered as weak, staining in 10-40% tumor cells was considered
moderate, and staining in >40% tumor cells as strong
expression.
A terminal deoxynucleotidyl-transferase-mediated
dUTP nick end labeling (TUNEL) assay of the tumor tissues was
performed using a TUNEL system (Promega Corporation, Madison, WI,
USA), according to the manufacturer's protocols. Briefly, the
slides were fixed in 4% formaldehyde in PBS for 15 min at room
temperature, the procedure of permeabilization and equilibration
was performed, and TdT reaction mix was added to the slides and
incubated for 60 min at 37°C in a humidified chamber. The slides
were washed with PBS, then DAB staining at room temperature and
developed until there is a light brown background under microscope
detection, then mounting were conducted. Semi-quantitation of
TUNEL-positive cells was performed using Image-Pro Plus 6.0
software.
Statistical analysis
All data are presented as the mean ± standard
deviation from independent samples analyzed in three replicates.
SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA) was used for
analysis. A Student's t-test or one-way analysis of variance was
used to determine statistical differences. P<0.05 was considered
to indicate a statistically significant difference.
Results
Inhibition of CD147 promotes apoptosis in
melanoma cell lines
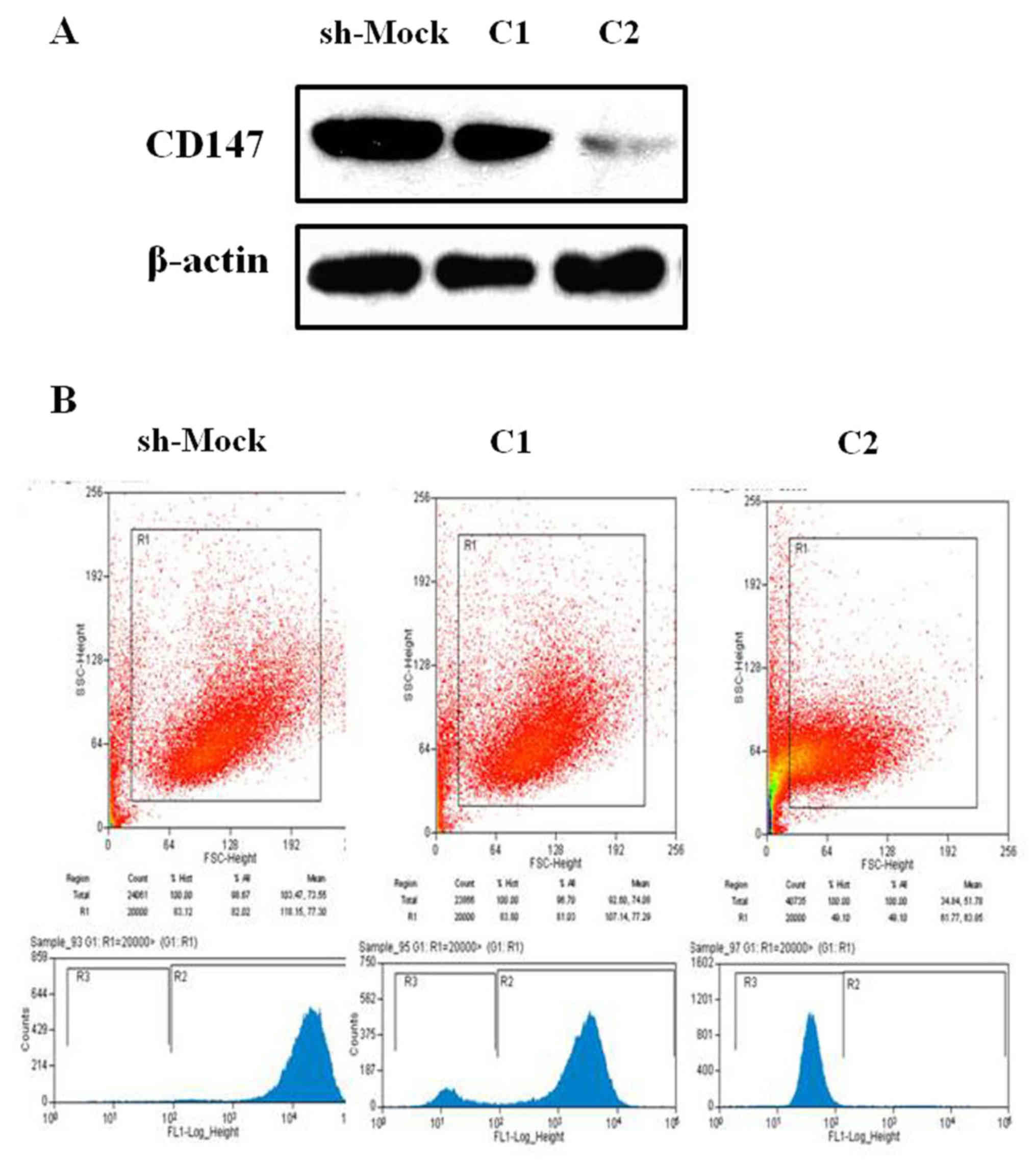
In the present study, two stable melanoma cell lines
with CD147 downregulation (referred to as C1 and C2) were
generated. The protein expression levels of CD147 were notably
decreased in sh-CD147-C2-transfected A375 cells compared with the
sh-Mock-transfected cells (Fig.
1A). In addition, the results of the flow cytometry also
suggested that CD147 expression was markedly inhibited in melanoma
A375 cells, particularly within sh-CD147-C2-transfected cells
(Fig. 1B).
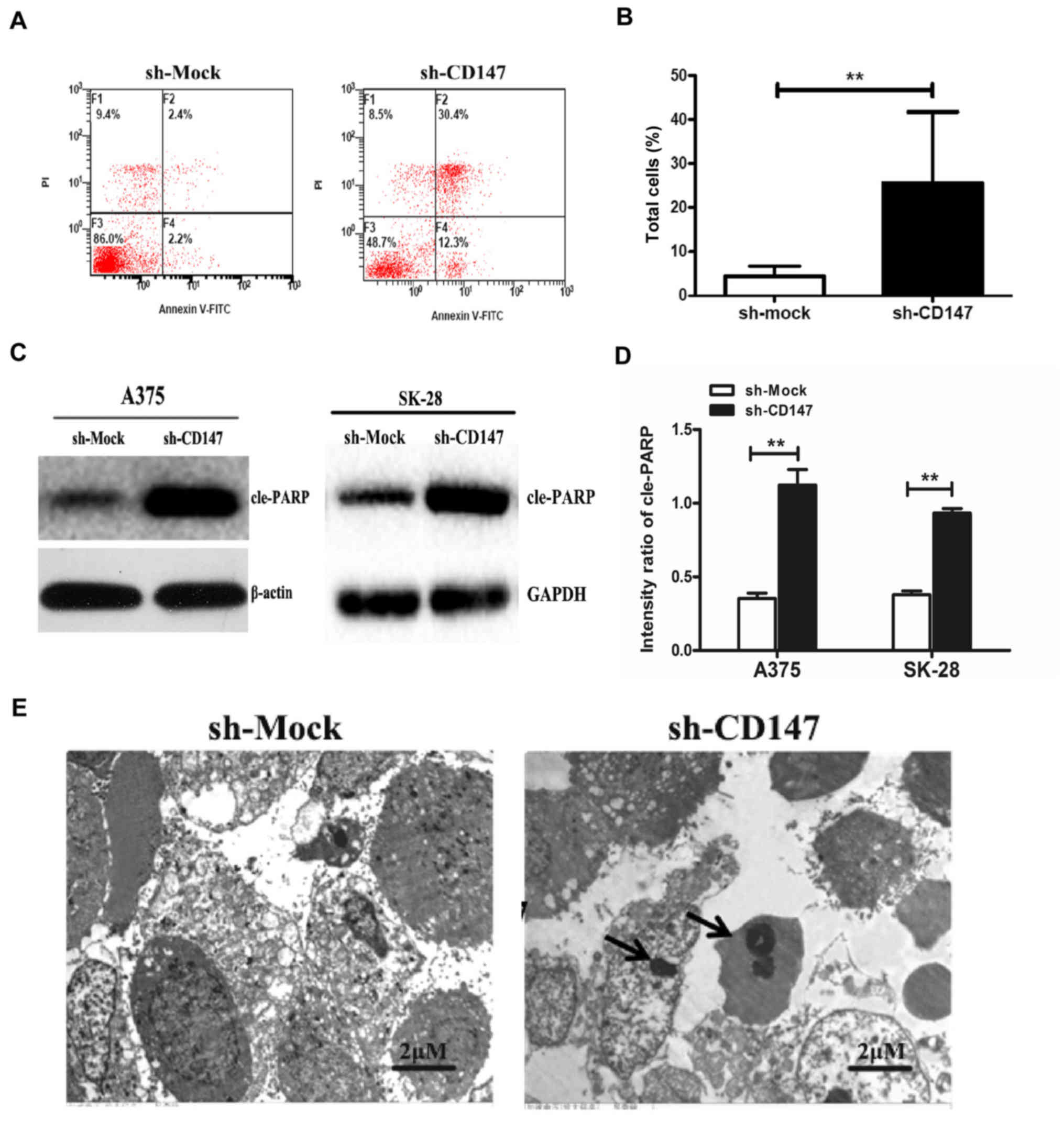
In addition, the rate of apoptosis was determined by
flow cytometry. The results demonstrated that the apoptotic rate of
A375-sh-CD147 cells was 22.24±4.07%, which was significantly higher
compared with that of the control group (5.08±1.28%, P<0.01;
Fig. 2A and B). Furthermore, the
expression of the apoptosis-associated protein, cleaved poly
(ADP-ribose) polymerase (cle-PARP), was also significantly
increased in A375-sh-CD147 and SK-MEL-28-sh-CD147 cells compared
with the control cells (P<0.01; Fig. 2C and D).
The morphology of A375 cells was evaluated by
transmission electron microscopy. As presented in Fig. 2E, compared with sh-Mock-transfected
cells, A375-sh-CD147 cells were small and round. The membranes were
bulging and ballooning. Typical apoptotic morphological
characteristics, including condensation and fragmentation of the
nuclei, as well as bleb-bing of the membrane, were also observed.
Collectively, these findings demonstrated that knockdown of CD147
expression significantly induced apoptosis in melanoma cell
lines.
High-throughput screening to determine
the apoptosis-related proteins associated with CD147 in melanoma
cells
In the present study, the total apoptosis-associated
proteins in A375-sh-Mock- and A375-sh-CD147-transfected cells were
investigated via a human apoptosis antibody array. Then, 43
apoptosis-associated proteins in the array were detected by
comparing the fluorescence spectra and the corresponding
fluorescence values (Fig. 3A).
Finally, 9 apoptosis-associated proteins were selected, which
exhibited significantly different expression compared with the
control group. Among these proteins, CD40 ligand (CD40L), p53,
cellular inhibitor of apoptosis 2 (cIAP-2), Survivin, tumor
necrosis factor (TNF)-related apoptosis-inducing ligand 2
(TRAILR-2) and p21 were >1.5-fold higher in A375-sh-CD147 cells,
and three proteins, including heat shock protein 60 (Hsp60),
IGFBP-6 and IGFBP-2, had decreased by 0.178- to 0.606-fold
(Table I). As IGFBP2 expression
exhibited the most significant change (0.178-fold) by statistical
analysis, IGFBP2 was selected for the subsequent analysis.
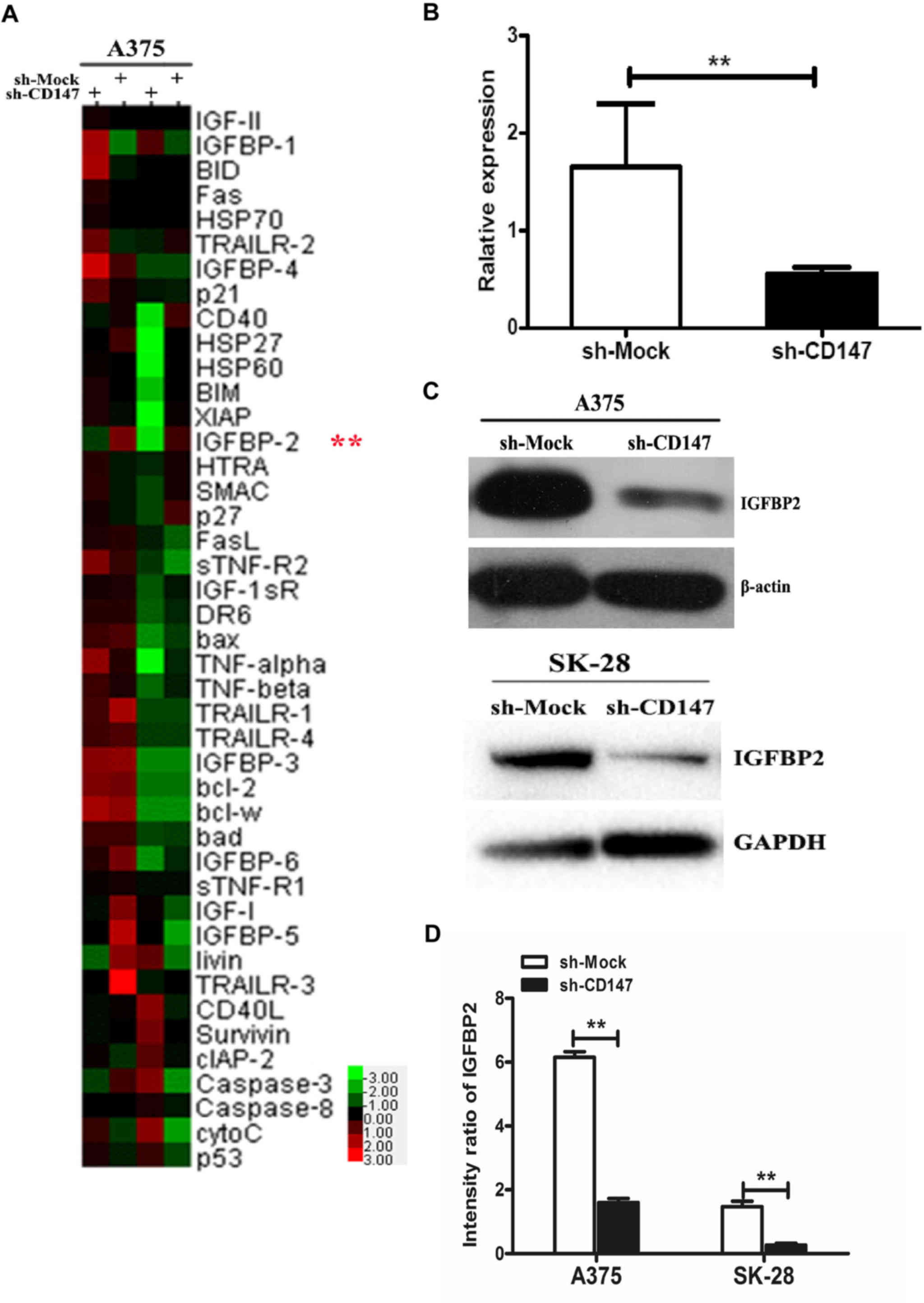 | Figure 3IGFBP2 is an apoptosis-associated
protein in sh-CD147 melanoma cells. (A) Cluster analysis of the
expression of 43 apoptosis-associated proteins in sh-CD147- and
sh-Mock-transfected A375 cells. Green presented downregulation and
red indicated higher protein expression relative to the mean
expression of all samples. (B) Relative mRNA expression of IGFBP2
in sh-Mock- and sh-CD147-transfected A375 cells. (C and D)
Quantitation of the protein expression levels of IGFBP2 in sh-Mock-
and sh-CD147-transfected A375 and SK-28 cells was performed via
western blotting using ImageJ software. n=3 for each experiment.
**P<0.01 vs. sh-Mock. Bcl-2, B-cell lymphoma 2;
Bcl-w, Bcl-2-like protein 2; Bad, Bcl-2-associated agonist of cell
death; BID, BH3 interacting-domain death agonist; BIM, Bcl-2-like
protein 11; CD147, cluster of differentiation 147; CD40L, CD40
ligand; cIAP-2, cellular inhibitor of apoptosis 2; cytoC,
cytochrome c; FasL, Fas ligand; HSP, heat shock protein;
HTRA, HtrA serine peptidase; IGFBP2, insulin-like growth
factor-binding protein 2; sh, short hairpin RNA; SMAC, second
mitochondria-derived activator of caspases; TNF, tumor necrosis
factor; sTNF-R2, soluble TNF receptor 2; TRAILR, TNF-related
apoptosis-inducing ligand receptor; XIAP, X-linked inhibitor of
apoptosis protein. |
 | Table IDifferential expression of
apoptosis-associated proteins in A375-sh-CD147 cells compared to
A375-sh-Mock control cells. |
Table I
Differential expression of
apoptosis-associated proteins in A375-sh-CD147 cells compared to
A375-sh-Mock control cells.
| Gene | Average intensity
| Ratio |
|---|
| Control | sh-CD147 |
|---|
| CD40L | 280.75 | 572.75 | 2.040 |
| p53 | 2,457.00 | 4,910.00 | 1.998 |
| cIAP-2 | 137.75 | 269.00 | 1.953 |
| Survivin | 5,983.50 | 10,351.75 | 1.730 |
| TRAILR-2 | 138.75 | 218.25 | 1.573 |
| p21 | 37,326.00 | 56,199.00 | 1.506 |
| Hsp60 | 3,183.75 | 1,930.50 | 0.606 |
| IGFBP6 | 855.75 | 450.50 | 0.526 |
| IGFBP2 | 1,425.25 | 254.25 | 0.178a |
Consistent with the results from the human apoptosis
assay, IGFBP2 mRNA by qPCR assay and protein expression was
significantly reduced in A375-sh-CD147 cells compared with sh-Mock
cells (Fig. 3B–D). In addition,
downregulation of IGFBP2 expression induced by sh-CD147 was
investigated in SK28-sh-CD147 melanoma cells (Fig. 3C and D). Collectively, the findings
demonstrated that CD147 was positively associated with IGFBP2 in
melanoma cells.
CD147 regulates IGFBP2 expression via the
PTEN/PI3K/AKT signaling pathway
To investigate the regulatory mechanism associated
with CD147 and IGFBP2, whether CD147 interacted with IGFBP2 in
melanoma cells was determined; however, no interaction between
CD147. Providing that IGFBP2 is a downstream molecule in the
PTEN/PI3K/AKT signaling pathway, the AKT-mTOR signaling pathway was
investigated. The results demonstrated that p-AKT and p-mTOR
expression was significantly decreased when CD147 expression was
downregulated in A375 and SK-28 cells compared with in
sh-Mock-transfected cells (Fig.
4A–C); however, the expression of the phosphatase PTEN was
significantly increased in A375-sh-CD147 and SK-MEL-28-sh-CD147
cells compared with in sh-Mock-transfected cells (Fig. 4D). Importantly, it was observed
that LY294002, a direct inhibitor of the PI3K signaling pathway,
significantly decreased IGFBP2 protein expression in sh-Mock A375
and sh-Mock SK-MEL-28 cells compared with the untreated groups
(Fig. 4E and F). The results of
the present study indicated that CD147 upregulated the expression
of IGFBP2 by activating the PTEN/PI3K/AKT signaling pathway in
melanoma cells.
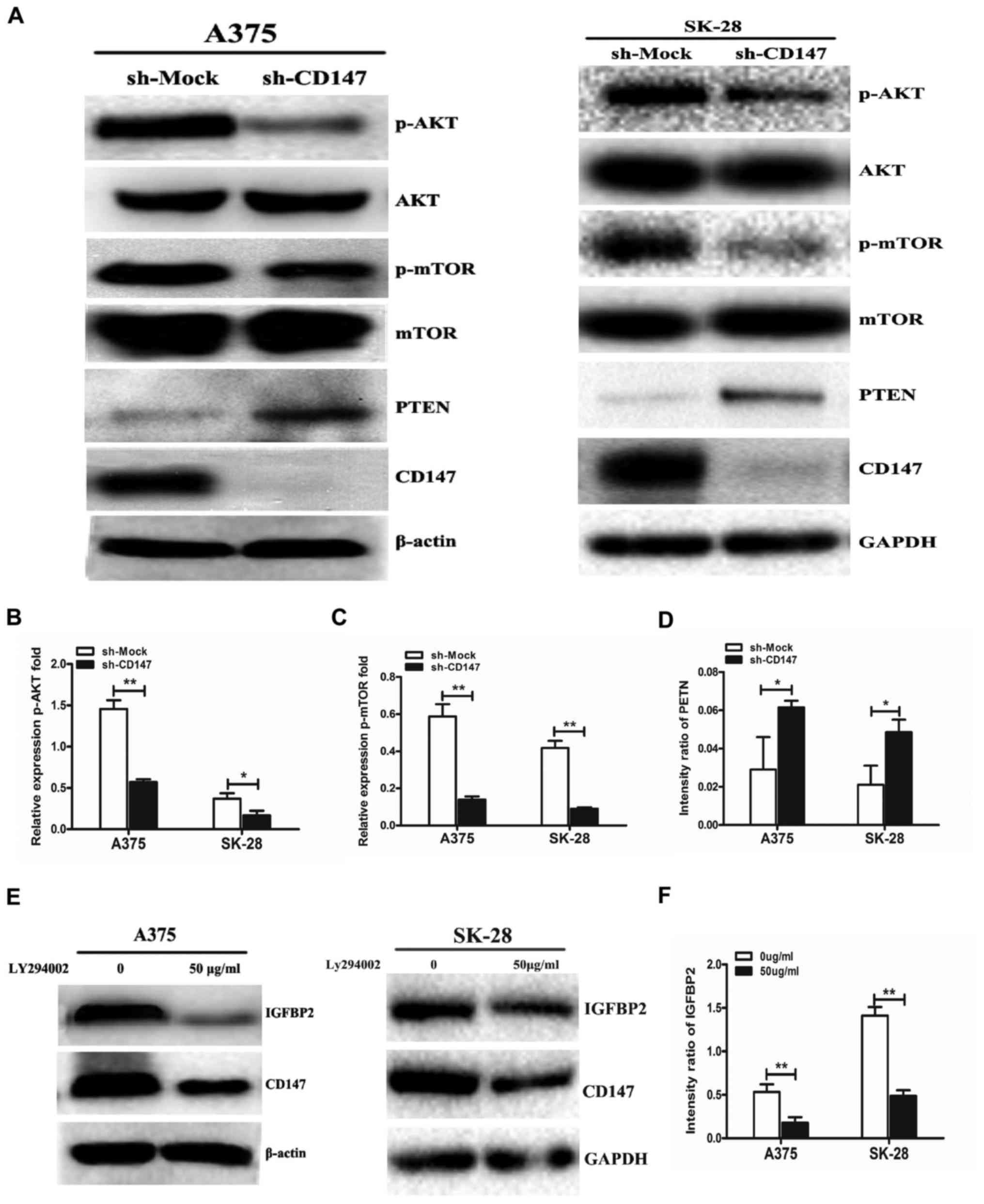 | Figure 4CD147 regulates IGFBP2 expression via
the PTEN/PI3K/AKT signaling pathway. (A) Cell lysates of sh-CD147
and sh-Mock A375 and SK-MEL-28 cells were subjected to western
blotting to detect p-AKT, p-mTOR and PTEN expression. Quantitation
of (B) p-AKT, (C) p-mTOR and (D) PTEN expression was performed
using ImageJ software. (E) Sh-Mock A375 and SK-MEL-28 cells were
cultured with 50 μg/ml LY294002, a PI3K signaling pathway
inhibitor, for 24 h. IGFBP2 and CD147 expression was detected by
western blotting and (F) quantitative analysis of IGFBP2 expression
was performed using ImageJ software. n=3 for each experiment.
*P<0.05, **P<0.01 vs. sh-Mock or 0
μg/ml LY294002. AKT, protein kinase B; CD147, cluster of
differentiation 147; m-TOR; p, phosphorylated; PI3K,
phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog;
sh, short hairpin RNA. |
In vivo experiments show that CD147
combined with IGFBP2 mediates apoptosis in melanoma
In the present study, a xenograft mouse model was
successfully generated by inoculating melanoma A375-sh-CD147 and
A375-sh-Mock cells into mice. As expected, after 35 days following
inoculation of the melanoma cells, it was observed that the average
tumor volume in the A375-sh-CD147 group was notably lower compared
with that in the sh-Mock group (Fig.
5A). The pathological characteristics of malignant melanoma
were confirmed via HE staining (Fig.
5B).
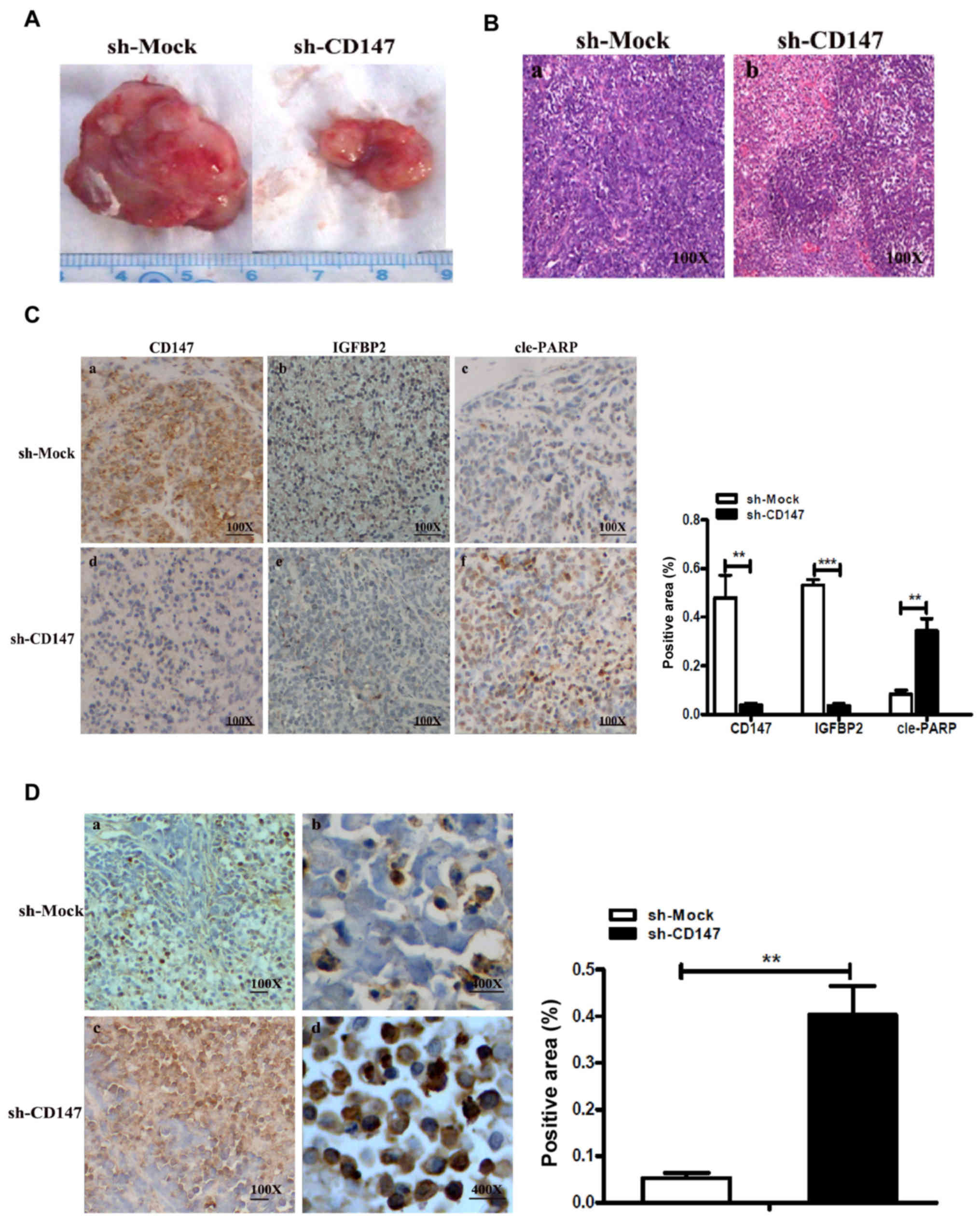 | Figure 5CD147 with IGFBP2 mediates the
apoptosis of melanoma cells in vivo. (A) Tumor volume in the
xenograft mouse model generated by inoculating melanoma sh-CD147
A375 and sh-Mock A375 cells. **P<0.05 or
***P<0.01 vs. sh-Mock. (B) Pathological
characteristics of xenograft mouse tumors were observed by HE
staining. Magnification, ×100. (C) Representative
immunohistochemical staining for CD147, IGFBP2 or cle-PARP in
serial sections of sh-CD147-injected xenograft mouse tumors or
sh-Mock tumors. Magnification, ×100. The area positive for CD147,
IGFBP2 and cleaved PARP- in sh-CD147 or sh-Mock cell-injected
xenograft mouse tumors was determined by using Image-Pro Plus 6.0
software. (D) Representative TUNEL assay in sh-CD147-injected
xenograft mouse tumors and sh-Mock tumors; the apoptosis-positive
regions are indicated in brown-yellow. The histogram indicated the
TUNEL-positive area (%) (right). Magnifications, ×100 or ×400.
CD147, cluster of differentiation 147; cle-PARP, cleaved poly
(ADP-ribose) polymerase; IGFBP2, insulin-like growth factor-binding
protein 2; sh, short hairpin RNA; TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling. |
In addition, it was reported that the expression
levels of CD147 and IGFBP2 in the A375-sh-CD147-inoculated group
were significantly decreased compared with the control group; CD147
was mainly expressed in the cell membranes in tumor tissue, while
IGFBP2 was mainly located in the cytoplasm (Fig. 5C). The expression levels of the
apoptosis-associated protein, cle-PARP, were significantly
increased compared with the control group. Therefore, it was
suggested that CD147 could upregulate the expression of IGFBP2
in vivo, and CD147 induced melanoma cell apoptosis via the
regulation of IGFBP2 expression.
Additionally, the apoptotic area in tumor tissues
from the xenograft mouse model was determined from a TUNEL assay as
Fig. 5D. The results revealed that
the positive expression rate was significantly higher in the
A375-sh-CD147 cell group compared with the control. The results of
the present study indicated that the expression levels of IGFBP2
and CD147 were positively associated in vivo, and the
inhibition of CD147 may induce the apoptosis of MM cells via the
regulation of IGFBP2 expression.
CD147 and IGFBP2 overexpression in
melanoma tissues
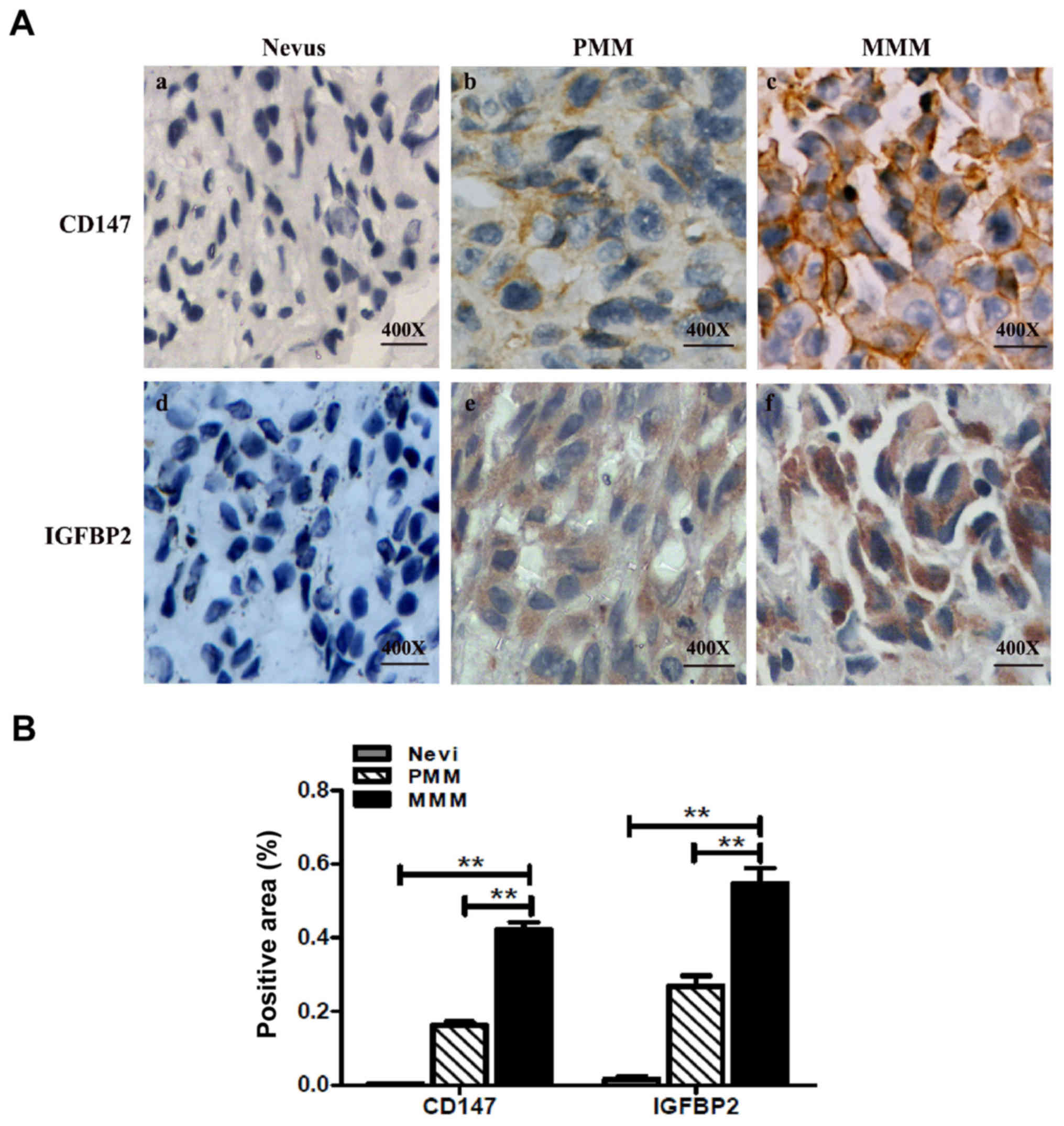
To further investigate the association between CD147
and IGFBP2, the expression of CD147 and IGFBP2 in 15
paraffin-embedded specimens with a clinical diagnosis of nevus was
determined; the results revealed low expression levels of CD147 and
IGFBP2 (Fig. 6A). In addition, an
MM tissue microarray was employed, which contained 128 cases of
primary MM (PMM) and 64 cases of metastatic MM (MMM). The
expression of CD147 and IGFBP2 in two adjacent microarray tissues
were observed; the expression of CD147 and IGFBP2 was higher
compared with in the nevus tissue (Fig. 6A and B). The semi-quantitation
results revealed that area of positive expression of CD147 in PMM
and MMM were 78.1 and 93.8% respectively, which was significantly
higher than that of the nevus tissue (Table II). Similar findings were observed
with IGFBP2 expression in MM; the area of positive expression of
IGFBP2 were 80.5 and 90.6% in PMM and metastatic MM, respectively,
which was significantly higher than the nevus tissue (Table II). Importantly, the expression
levels of CD147 and IGFBP2 were positively associated in human MM
(Table II).
 | Table IIRelative expression of CD147 and
IGFBP2 in PMM and MMM. |
Table II
Relative expression of CD147 and
IGFBP2 in PMM and MMM.
| A, CD147
expression |
|---|
|
|---|
| Tumor type | Cases | Weak | Moderate | Strong | Positive rate
(%) |
|---|
| PMM | 128 | 28 | 68 | 32 | 78.1 |
| MMM | 64 | 4 | 11 | 49 | 93.8a |
| B, IGFBP2
expression |
|---|
|
|---|
| Tumor type | Cases | Weak | Moderate | Strong | Positive rate
(%) |
|---|
| PMM | 128 | 25 | 75 | 28 | 80.5 |
| MMM | 64 | 6 | 18 | 40 | 90.6a |
Collectively, these findings indicate that CD147
regulated the expression of IGFBP2 in MM, and may participate in
tumor occurrence and development. Therefore, the CD147/IGFBP2 axis
may serve an important role in the transformation process of
pigmented moles to MM.
Discussion
CD147 serves a key role in facilitating tumor cell
invasiveness and metastasis by regulating matrix metalloproteinase
(MMP) expression, including MMP-1, MMP-2, MMP-9 and A membrane-type
1-MMP, in adjacent fibroblasts or cancer cells (18,20).
For example, in hepatocellular carcinoma (HCC), CD147 is highly
upregulated and inhibition of CD147 significantly inhibited HCC
cell proliferation and metastasis via MMP production (21). In addition, it has been
demonstrated that CD147 is upregulated in various
multidrug-resistant cancer cells, including breast cancer, HCC and
ovarian cancer cells (22).
Suppression of CD147 increased the sensitivity of SKOV3 and OVCAR3
ovarian cells to paclitaxel treatment (23). In spermatogenesis, knockdown of
CD147 promoted apoptosis in spermatocytes in a p53-independent
manner (24). Recently, novel
evidence has demonstrated that suppressing CD147 inhibited TNF
receptor-associated factor 2 expression, leading to alterations in
canonical and non-canonical nuclear factor-κB activity, which may
increase apoptosis in spermatocytes (25). CD147 has been demonstrated to
induce endoplasmic reticulum stress to attenuate apoptosis and
increase chemoresistance by increasing the transcriptional levels
of binding immunoglobulin protein in HCC (26).
In the present study, it was reported that knockdown
of CD147 significantly increased melanoma cell apoptosis. Using a
xenograft mouse model, it was observed that knockdown of CD147
attenuated tumor cell growth via the induction of cellular
apoptosis in vivo, suggesting that CD147 may serve an
important role in melanoma apoptosis in vitro and in
vivo. Importantly, via the human apoptosis array, we identified
that the expression of 9 apoptosis-associated proteins, including
CD40L, Hsp60, IGFBP2, IGFBP6, Survivin, TRAILR-2, cIAP-2, p21 and
p53, was markedly altered following the suppression of CD147
expression. Furthermore, the present study reported the
downregulation of IGFBP2 expression following CD147 knockdown.
The IGF axis is crucial for biological functions,
including cell growth and apoptosis. The IGF-induced signaling
pathway constitutes several components, including the two ligands
(IGF-I and IGF-II), corresponding receptors (IGF-1 and -2R) and
IGFBPs, which serve key roles in modulating the activity of the IGF
signaling pathway (27). IGFBP2
may associate with IGF to form a complex, which can readily
translocate via the endothelial barrier, and reach local tissues to
interact with integrin and elements of the extracellular matrix
(28). Therefore, IGFBP2 acts as a
container that harbors IGF in the local tissues (29). IGFBP2 has been reported to be
overexpressed in tumors, including glioma, prostate, breast and
lung cancers (30). It was
reported that ectopic expression of IGFBP2 attenuated
camptothecin-induced apoptosis, while inhibition of IGFBP2
increased sensitivity to camptothecin-induced death of NCI-H522
cells (31). Chen et al
(24) demonstrated that IGFBP2 is
highly expressed in lung cancer cells compared with in normal
epithelial tissues, and intracellular IGFBP2 inhibited apoptosis
via the regulation of caspase-3 activation.
The PI3K signaling pathway-specific inhibitor
LY294002 decreased IGFBP2 expression in the present study.
Furthermore, p-AKT, p-mTOR and IGFBP2 expression levels were
significantly decreased, whereas PTEN expression was significantly
increased in CD147-knockdown melanoma cells. The findings of the
present study revealed that the PTEN/PI3K/AKT signaling pathway may
be associated with IGFBP2 expression in melanoma cells. PTEN is
able to catalyze the dephos phorylation of PIP3, which is a key
secondary messenger for downstream signaling pathway activation.
Providing the frequent loss or inactivation of PTEN function in
tumors, accumulating PIP3 may recruit proteins with pleckstrin
homology domains to the cell membrane, including
phosphoinositide-dependent kinase-1 and AKT (32). Consequently, PDK1 could directly
phosphorylate and activate AKT (33,34);
activated AKT isoforms (AKT1, AKT2 and AKT3) may induce a variety
of malignant phenotypes, including cell proliferation, cell death,
angiogenesis and cellular metabolism by activating downstream
molecules, including glycogen synthase kinase 3, forkhead box O,
B-cell lymphoma 2 (Bcl-2)-associated antagonist of cell death,
mouse double minute 2 homolog and p27 (35-37).
Importantly, AKT also activates the mTOR complex 1 (mTORC1) via the
phosphorylation and inhibition of proline-rich AKT substrate 40,
which is a negative regulator of mTORC1 (38,39).
Activation of following PTEN inacti vation promotes the translation
of specific mRNAs and the synthesis of proteins involved in cell
proliferation (40). Based on the
findings of the present study, it was hypothesized that inhibition
of CD147 in melanoma cells could increase PTEN activation, and that
the phosphatase activity of PTEN may decrease the intracellular
p-AKT levels, promoting the apoptosis of melanoma cells.
Interestingly, evidence has demonstrated that IGFBP2
is the most significantly altered molecule following PTEN loss and
PI3K/AKT activation. The expression of IGFBP2 was observed to be
upregulated in PTEN−/− cancers (41). Inhibition of PI3K/AKT activation by
LY294002 was observed to significantly inhibit IGFBP2 expression,
which suggests that the PTEN/PI3K/AKT signaling pathway may
regulate IGFBP2 expression in prostate cancer and glioblastoma
(42). In addition, inhibiting the
activation of PI3K/AKT reduced the expression of IGFBP2 via the
specificity protein-1 transcription factor in MCF-7 breast cancer
cells (43). Consistent with these
results, the present study also reported that the inhibition of
PI3K/AKT by LY294002 attenuated IGFBP2 expression in melanoma
cells. Therefore, the underlying mechanism of CD147-regulated
expression of PTEN or PI3K/AKT activation requires further
investigation to provide novel insight into the role of CD147 in
melanoma.
Collectively, the results of the present study
demonstrated that CD147 serves a key role in melanoma cell
apoptosis, which may be mediated via IGFBP2 and the PTEN/PI3K/AKT
signaling pathway. In addition, the expression of IGFBP2 and CD147
was upregulated and exhibited a positive association in melanoma
clinical tissues, suggesting that CD147 may be a potential
therapeutic target in chemotherapy or a target for the prevention
of melanoma.
Acknowledgments
We are very grateful to Dr Minxue Shen who kindly
gave us advice for data statistics.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 81402263), China
National Funds for Distinguished Young Scientists (grant no.
81225013), the Major International (Regional) Joint Research
Program of China (grant no. 1620108024), the Special Foundation For
State Major Basic Research Program of China (grant no. 81430075),
the Strategy-Oriented Special Project of Central South University
in China (grant no. ZLXD2017003); the National Natural Science
Foundation of China (grant no. 81572679) and the National Natural
Science Foundation of China (grant no. 81673046).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SZ, LW and YK performed all of experiments the
research and wrote the manuscript. JS, ZL, JL, JZ and FL analyzed
and interpreted the patient data. YW, WC, YH, JT, JZ and XX
critically revised the manuscript for important intellectual
information. CP and XC made substantial contributions to the
concenption and design of the present study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xiangya Hospital, Central South University (Changsha,
China). Informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zimmer L, Haydu LE, Menzies AM, Scolyer
RA, Kefford RF, Thompson JF, Schadendorf D and Long GV: Incidence
of new primary melanomas after diagnosis of stage III and IV
melanoma. J Clin Oncol. 32:816–823. 2014. View Article : Google Scholar
|
|
2
|
Arnold M, Holterhues C, Hollestein LM,
Coebergh JW, Nijsten T, Pukkala E, Holleczek B, Tryggvadóttir L,
Comber H, Bento MJ, et al: Trends in incidence and predictions of
cutaneous melanoma across Europe up to 2015. J Eur Acad Dermatol
Venereol. 28:1170–1178. 2014. View Article : Google Scholar
|
|
3
|
Yin H, Shao Y and Chen X: The effects of
CD147 on the cell proliferation, apoptosis, invasion, and
angiogenesis in glioma. Neurol Sci. 38:129–136. 2017. View Article : Google Scholar
|
|
4
|
Kuang YH, Chen X, Su J, Wu LS, Liao LQ, Li
D, Chen ZS and Kanekura T: RNA interference targeting the CD147
induces apoptosis of multi-drug resistant cancer cells related to
XIAP depletion. Cancer Lett. 276:189–195. 2009. View Article : Google Scholar
|
|
5
|
Xie W, Xie H, Liu F, Li W, Dan J, Mei Y,
Dan L, Xiao X, Li J and Chen X: Propranolol induces apoptosis of
human umbilical vein endothelial cells through downregulation of
CD147. Br J Dermatol. 168:739–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao S, Kuang YH and Chen X:
Overexpression of CD147 inhibits Chinese Hamster Ovary cells
apoptosis due to inducing methotrexate efflux. J Dermatol. 39:170.
2012.
|
|
7
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lian C, Guo Y, Zhang J, Chen X and Peng C:
Targeting CD147 is a Novel Strategy for Antitumor Therapy. Curr
Pharm Des. 23:4410–4421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stocker H, Andjelkovic M, Oldham S,
Laffargue M, Wymann MP, Hemmings BA and Hafen E: Living with lethal
PIP3 levels: Viability of flies lacking PTEN restored by a PH
domain mutation in Akt/PKB. Science. 295:2088–2091. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abraham J: PI3K/AKT/mTOR pathway
inhibitors: The ideal combination partners for breast cancer
therapies? Expert Rev Anticancer Ther. 15:51–68. 2015. View Article : Google Scholar
|
|
11
|
Bucheit AD, Chen G, Siroy A, Tetzlaff M,
Broaddus R, Milton D, Fox P, Bassett R, Hwu P, Gershenwald JE, et
al: Complete loss of PTEN protein expression correlates with
shorter time to brain metastasis and survival in stage IIIB/C
melanoma patients with BRAFV600 mutations. Clin Cancer Res.
20:5527–5536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong Y, Richards JA, Gupta R, Aung PP,
Emley A, Kluger Y, Dogra SK, Mahalingam M and Wajapeyee N: PTEN
functions as a melanoma tumor suppressor by promoting host immune
response. Oncogene. 33:4632–4642. 2014. View Article : Google Scholar
|
|
13
|
Zhang W and Fuller G: IGFBP2 as a brain
tumor oncogene. Cancer Biol Ther. 6:995–996. 2007.PubMed/NCBI
|
|
14
|
Sztefko K, Hodorowicz-Zaniewska D, Popiela
T and Richter P: IGF-I, IGF-II, IGFBP2, IGFBP3 and acid-labile
subunit (ALS) in colorectal cancer patients before surgery and
during one year follow up in relation to age. Adv Med Sci.
54:51–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moore LM, Holmes KM, Smith SM, Wu Y,
Tchougounova E, Uhrbom L, Sawaya R, Bruner JM, Fuller GN and Zhang
W: IGFBP2 is a candidate biomarker for Ink4a-Arf status and a
therapeutic target for high-grade gliomas. Proc Natl Acad Sci USA.
106:16675–16679. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng W, Su J, Wu L, Yang D, Long T, Li D,
Kuang Y, Li J, Qi M, Zhang J, et al: CD147 promotes melanoma
progression through hypoxia-induced MMP2 activation. Curr Mol Med.
14:163–173. 2014. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Chen X, Lin J, Kanekura T, Su J, Lin W,
Xie H, Wu Y, Li J, Chen M and Chang J: A small interfering
CD147-targeting RNA inhibited the proliferation, invasiveness, and
metastatic activity of malignant melanoma. Cancer Res.
66:11323–11330. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Council NR: Guide for the Care and Use of
Laboratory Animals. The National Academies Press; Washington, DC:
1996
|
|
20
|
Su J, Gao T, Jiang M, Wu L, Zeng W, Zhao
S, Peng C and Chen X: CD147 silencing inhibits tumor growth by
suppressing glucose transport in melanoma. Oncotarget.
7:64778–64784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gou X, Tang X, Kong DK, He X, Gao X, Guo
N, Hu Z, Zhao Z and Chen Y: CD147 is increased in HCC cells under
starvation and reduces cell death through upregulating p-mTOR in
vitro. Apoptosis. 21:110–119. 2016. View Article : Google Scholar
|
|
22
|
Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q,
Yang JM and Xu ZD: Up-regulation of CD147 and matrix
metalloproteinase-2, -9 induced by P-glycoprotein substrates in
multidrug resistant breast cancer cells. Cancer Sci. 98:1767–1774.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou W, Yang H, Hou X, Zhang W, Chen B and
Xin X: Inhibition of CD147 gene expression via RNA interference
reduces tumor cell invasion, tumorigenicity and increases
chemosensitivity to paclitaxel in HO-8910 pm cells. Cancer Lett.
248:211–218. 2007. View Article : Google Scholar
|
|
24
|
Chen H, Fok KL, Jiang X, Jiang J, Chen Z,
Gui Y, Chan HC and Cai Z: CD147 regulates apoptosis in mouse
spermatocytes but not spermatogonia. Hum Reprod. 27:1568–1576.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Fok KL, Cai Z, Chen H and Chan HC:
CD147 regulates extrinsic apoptosis in spermatocytes by modulating
NFκB signaling pathways. Oncotarget. 8:3132–3143. 2017.
|
|
26
|
Tang J, Guo YS, Zhang Y, Yu XL, Li L,
Huang W, Li Y, Chen B, Jiang JL and Chen ZN: CD147 induces UPR to
inhibit apoptosis and chemosensitivity by increasing the
transcription of Bip in hepatocellular carcinoma. Cell Death
Differ. 19:1779–1790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reynolds CM, Perry JK and Vickers MH:
Manipulation of the growth hormone-insulin-like growth factor
(GH-IGF) axis: A treatment strategy to reverse the effects of early
life developmental programming. Int J Mol Sci. 18:182017.
View Article : Google Scholar
|
|
28
|
Podlutsky A, Valcarcel-Ares MN, Yancey K,
Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE,
Csiszar A and Ungvari Z: The GH/IGF-1 axis in a critical period
early in life determines cellular DNA repair capacity by altering
transcriptional regulation of DNA repair-related genes:
Implications for the developmental origins of cancer. Geroscience.
39:147–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sehgal P, Kumar N, Praveen Kumar VR, Patil
S, Bhattacharya A, Vijaya Kumar M, Mukherjee G and Kondaiah P:
Regulation of protumorigenic pathways by insulin like growth factor
binding protein2 and its association along with β-catenin in breast
cancer lymph node metastasis. Mol Cancer. 12:632013. View Article : Google Scholar
|
|
30
|
Chua CY, Liu Y, Granberg KJ, Hu L,
Haapasalo H, Annala MJ, Cogdell DE, Verploegen M, Moore LM, Fuller
GN, et al: IGFBP2 potentiates nuclear EGFR-STAT3 signaling.
Oncogene. 35:738–747. 2016. View Article : Google Scholar
|
|
31
|
Biernacka KM, Uzoh CC, Zeng L, Persad RA,
Bahl A, Gillatt D, Perks CM and Holly JM: Hyperglycaemia-induced
chemore-sistance of prostate cancer cells due to IGFBP2. Endocr
Relat Cancer. 20:741–751. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu H, Goel V and Haluska FG: PTEN
signaling pathways in melanoma. Oncogene. 22:3113–3122. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen X, Xi G, Radhakrishnan Y and Clemmons
DR: PDK1 recruitment to the SHPS-1 signaling complex enhances
insulin-like growth factor-i-stimulated AKT activation and vascular
smooth muscle cell survival. J Biol Chem. 285:29416–29424. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harris TK: PDK1 and PKB/Akt: Ideal targets
for development of new strategies to structure-based drug design.
IUBMB Life. 55:117–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Wang SJ, Han ZH, Li YQ, Xue JH,
Gao DF, Wu XS and Wang CX: PI3K/AKT signaling pathway plays a role
in enhancement of eNOS activity by recombinant human angiotensin
converting enzyme 2 in human umbilical vein endothelial cells. Int
J Clin Exp Pathol. 7:8112–8117. 2014.
|
|
36
|
Li H, Zeng J and Shen K: PI3K/AKT/mTOR
signaling pathway as a therapeutic target for ovarian cancer. Arch
Gynecol Obstet. 290:1067–1078. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goschzik T, Gessi M, Denkhaus D and
Pietsch T: PTEN mutations and activation of the PI3K/Akt/mTOR
signaling pathway in papillary tumors of the pineal region. J
Neuropathol Exp Neurol. 73:747–751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv D, Liu J, Guo L, Wu D, Matsumoto K and
Huang L: PRAS40 deregulates apoptosis in Ewing sarcoma family
tumors by enhancing the insulin receptor/Akt and mTOR signaling
pathways. Am J Cancer Res. 6:486–497. 2016.PubMed/NCBI
|
|
39
|
Havel JJ, Li Z, Cheng D, Peng J and Fu H:
Nuclear PRAS40 couples the Akt/mTORC1 signaling axis to the
RPL11-HDM2-p53 nucleolar stress response pathway. Oncogene.
34:1487–1498. 2015. View Article : Google Scholar
|
|
40
|
Bhattacharya K, Maiti S and Mandal C: PTEN
negatively regulates mTORC2 formation and signaling in grade IV
glioma via Rictor hyperphosphorylation at Thr1135 and direct the
mode of action of an mTORC1/2 inhibitor. Oncogenesis. 5:e2272016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mehrian-Shai R, Chen CD, Shi T, Horvath S,
Nelson SF, Reichardt JK and Sawyers CL: Insulin growth
factor-binding protein 2 is a candidate biomarker for PTEN status
and PI3K/Akt pathway activation in glioblastoma and prostate
cancer. Proc Natl Acad Sci USA. 104:5563–5568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahani N, Karimi Arzenani M, Shirkoohi R,
Rokouei M, Alipour Eskandani M and Nikravesh A: Expression of
insulin-like growth factor binding protein-2 (IGFBP-2) gene in
negative and positive human cytomegalovirus glioblastoma multiforme
tissues. Med Oncol. 31:8122014. View Article : Google Scholar
|
|
43
|
Mireuta M, Darnel A and Pollak M: IGFBP-2
expression in MCF-7 cells is regulated by the PI3K/AKT/mTOR pathway
through Sp1-induced increase in transcription. Growth Factors.
28:243–255. 2010. View Article : Google Scholar : PubMed/NCBI
|