Introduction
Breast cancer is the most common invasive cancer and
is a leading cause of cancer-associated mortality in women
(1,2). Breast cancer is a heterogeneous and
complex disease that may be classified into different subtypes
based on the estrogen receptor (ER), progesterone receptor (PR) and
human epidermal growth factor receptor 2 (HER2) expression status.
Triple-negative breast cancer (TNBC) is an aggressive subtype of
breast cancer characterized by a lack of ER, PR and HER2 expression
(3). TNBC is associated with early
metastasis, high grade disease, a high tumor proliferation rate,
drug resistance and poor prognosis (4,5). Due
to a lack of molecular targets for drugs, TNBC therapies are
restricted to conventionally available chemotherapies, including
endocrine therapies and molecular targeted treatments (6). Patients with TNBC are not able to
benefit from the currently available targeted therapies, including
hormone therapies and HER2-based therapies (7). Therefore, effective therapeutic
approaches for TNBC are urgently required. There is additionally a
critical requirement to identify the responsible genes of TNBC and
to identify novel therapeutic strategies for patients with TNBC
(8,9).
Forkhead box M1 (FOXM1) is a member of the fork
head/winged-helix family of proteins, which are involved in
numerous biological processes, including cell differentiation, cell
proliferation, cell cycle progression, DNA damage repair, tissue
homeostasis, angiogenesis and apoptosis (10). FOXM1 is overexpressed in various
human malignancies, including breast cancer (11). A complete understanding of the
regulation and role of FOXM1 in cancer may demonstrate its
application as a biomarker for cancer diagnosis and a target for
treatment (12). The expression of
FOXM1 in breast cancer molecular subtypes and its interaction with
associated genes have been described in recent studies; eukaryotic
elongation factor 2 kinase and integrin β1 have been determined to
be involved in the FOXM1 network (13-15).
However, the therapeutic role of the FOXM1-associated pathway in
TNBC remains unclear.
In the present study, the role of FOXM1 in TNBC was
examined using bioinformatics analysis, including the Cancer
Landscapes online tool, the Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING) database and Database for
Annotation, Visualisation and Integrated Discovery (DAVID). The
present results were additionally verified by in vitro and
in vivo experiments. Furthermore, it was demonstrated that a
FOXM1 inhibitor significantly suppressed the growth of MDA-MB-231
breast cancer cells in vitro and in vivo. These
results provide a better understanding of the regulatory mechanisms
of FOXM1 and suggest that FOXM1 may be a novel molecular
therapeutic target for TNBC.
Materials and methods
Drugs and treatments
Thiostrepton, a specific inhibitor of FOXM1, was
purchased from Abcam (Cambridge, UK; cat. no. ab143458) and was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) to generate a 50 µM stock solution and
stored at −20°C. For all working doses, the stock solution of
Thiostrepton was diluted with culture medium. To suppress
MDA-MB-231 cell growth in vitro, the cells were treated with
4 and 10 µM Thiostrepton for 48 h. The negative control
group was treated with an equal volume of DMSO.
Antibodies
Anti-polo like kinase 1 (PLK1) and anti-zinc finger
E-box-binding homeobox 1 (ZEB1) antibodies were obtained from
Boster Biological Technology (Pleasanton, CA, USA; cat. no.
P00182-1) and Santa Cruz Biotechnology, Inc. (Dallas, TX, USA;
H102; cat. no. sc-25388), respectively. Anti-cyclin-dependent
kinase 1 (CDK1; cat. no. 9116), anti-G2/mitotic-specific cyclin-B1
(CCNB1; cat. no. 4138) were purchased from Cell Signaling
Technology, Inc. Anti-filamentous actin (F-actin) antibodies were
purchased from Abcam (Cambridge, UK, cat. no. ab205).
Anti-proliferation marker protein Ki-67 (Ki-67; cat. no. ZA-0502)
and anti-vimentin antibodies (cat. no. ZA-0511), in addition to
biotin-conjugated goat anti-rabbit immunoglobulin G (IgG) secondary
antibody (cat. no. TA130016) were obtained from OriGene
Technologies, Inc. (Beijing, China).
Cell lines and cell culture
The MDA-MB-231 cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA). The cells
were maintained in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck
KGaA) and were cultured in a humidified incubator at 37°C with 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from MDA-MB-231 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer’s protocol. For RT-qPCR, the
Qiagen One Step RT-PCR kit (Qiagen GmbH, Heidelberg, Germany; cat.
no. 210212) was used. Subsequently, 2 µg total RNA was used
as the template for RTqi. cDNA was synthesized from total RNA
according to the following steps: One cycle at 42°C for 1 h and
70°C for 15 min. RT-qPCR was performed using SYBR-Green Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Reactions for
RT-qPCR assays were run in a CFX96™ PCR cycler (Bio-Rad
Laboratores, Inc., Hercules, CA, USA) according to the following
steps: 5 min preheating and denaturation at 95°C; 40 cycles at 95°C
for 15 sec; 53-58°C for 1 min; and a final extension step at 72°C
for 10 min. GAPDH was used to normalize the relative mRNA
expression levels. The specificity of the RT-qPCR primers was
confirmed using melt curve analysis and electrophoresis on 1.2%
agarose gels stained with GelRed fluorescent dye (Biotium, Inc.,
Freemont, CA, USA). Data analysis was performed using the
2−ΔΔCq method (16).
The primer sequences were as follows: FOXM1 forward,
5′-CCTTCTGGACCATTCACCCC-3′ and reverse, 5′-TCACCGGGAACTGGATAGGT-3′;
cyclin B2 (CCNB2) forward, 5′-AGTTCCAGTTCAACCCACCAA-3′ and reverse,
5′-TTGCAGAGCAAGGCATCAGA-3′; cyclin A2 (CCNA2 forward,
5′-CTCTACACAGTCACGGGACAAAG-3′ and reverse,
5′-CTGTGGTGCTTTGAGGTAGGTC-3′; centrosomal protein 55 (CEP55)
forward, 5′-TCGACCGTCAACATGTGCAGCA-3′ and reverse,
5′-GGCTCTGTGATGGCAAACTCATG-3′; checkpoint kinase 1 (CHEK1) forward,
5′-ATCAACTCATGGCAGGGGTG-3′ and reverse, 5′-TCCAGCGAGCATTGCAGTAA-3′;
PLK1 forward, 5′-AGCCCCTCACAGTCCTCAATA-3′ and reverse,
5′-TGTCCGAATAGTCCACCCAC-3′; GAPDH forward,
5′-GGTGGTCTCCTCTGACTTCAACA-3′ and reverse,
5′-GTTGCTGTAGCCAAATTCGTTGT-3′.
Immunohistochemistry
Tumor tissues were fixed with 10% formaldehyde for
24 h at room temperature, embedded in paraffin and cut into 4
µm thick sections. For immunohistochemistry, the primary
Ki-67 antibody (1:100) was used according to the manufacturer’s
protocol. Each section was blocked with 3% hydrogen peroxide for 10
min at 37°C. The sections were incubated with the primary antibody
overnight at 4°C. Subsequently, the sections were incubated with
biotin-conjugated goat anti-rabbit IgG secondary antibodies at a
dilution of 1:100 for 20 min at room temperature. The staining was
observed using a Nikon microscope (Nikon Corporation, Tokyo, Japan;
magnification, ×40)
Immunofluorescence staining
After 48 h of treatment with Thiostrepton,
MDA-MB-231 cells were washed with PBS and fixed in 4%
paraformaldehyde for 30 min at room temperature. PBS with Tween-20
and 5% bovine serum albumin (Cell Signaling Technology, Inc.,
Danvers, MA, USA) was used to block the washed cells for 30 min at
37°C. The cells were incubated with primary antibodies at 4°C
overnight and were subsequently stained with a fluorescent
secondary antibody (Alexa-Fluor™ 594 donkey anti-rabbit
IgG; 1:1,000; cat. no. A-21207; Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 30 min in the dark. DAPI (cat. no.
C1006; Beyotime Institute of Biotechnology, Haimen, China) was used
to stain the nucleus for 15 min at room temperature. Immunopositive
cells were observed under a fluorescence microscope. The sections
were observed under a Nikon ECLIPSE 80i fluorescent microscope
(Nikon Corporation; magnification, ×40) and the images were
captured using a Nikon digital camera DXM1200 (Nikon
Corporation).
Real-time cell proliferation assay
(RTCA)
Cell proliferation was determined by an RTCA assay.
In total, 3,000 cells (100 µl/well) were seeded into an
E-Plate. After a 24-h incubation, the cells were treated with 0.5,
1, 2, 5, 10, 20 and 40 µM Thiostrepton for 72 h at 37°C.
Cell proliferation was automatically monitored in each well using
the xCELLigence system (ACEA Biosciences, Inc., San Diego, CA, USA)
and was expressed as the Cell Index (CI). CI is regarded as an
indicator of cell proliferation. The CI values were automatically
calculated using RTCA software (version 2.0; ACEA Biosciences,
Inc.) and were recorded every 15 min for 96 h. Data analysis was
performed using RTCA software.
Tumor xenograft growth in nude mice
In total, 20 BALB/C nude mice (female; 5-6 weeks of
age; weighing 20-25 g) were purchased from the Chinese Academy of
Medical Science Cancer Institute (Beijing, China). The mice were
housed in a specific-pathogen-free grade animal center at standard
temperature (23±1°C) and humidity (45-55%) conditions, and a
standard 12-h dark/12-h light cycle. The mice were given chow and
water ad libitum. Tumor xenografts were generated via the
subcutaneous injection of 2×106 MDA-MB-231 cells into
the right front leg of the mice (n=8 mice/group). Tumor xenografts
were classified into two groups: The negative control (NC) group or
the treatment with Thiostrepton (50 mg/kg, every other day) group.
Tumor sizes were measured every other day using micrometer
calipers, and tumor volumes were calculated according to the
following formula: Tumor volume (mm3) = 0.5 × D ×
d2, where d and D represent the shortest and the longest
diameters, respectively. It was possible to measure the tumor sizes
with a caliper due to the subcutaneous location of the tumors. All
tumors were measured by one investigator to prevent observational
differences. Day 35 was selected as the humane endpoint to
terminate the present study, based on the tumor size. On the
35th day, all mice were sacrificed by CO2
inhalation. CO2 was delivered in a predictable and
regulated method at a low flow rate of 10-30% volume
displacement/min. Animal mortalities were confimed by trained
personnel, who recognized the arrest of vital signs in the animals.
The tumor tissues were paraffin-embedded for immunohistochemical
analysis of Ki-67. Bioluminescent imaging was used to detect
intra-cranial tumor growth on the 35th day. Animal
studies were conducted according to the recommendations outlined in
the Guide for the Care and Use of Laboratory Animals in the
Weatherall report (17). Animal
experiments were approved by the Committee on the Ethics of Animal
Experiments of Hebei University (Baoding, China).
Breast invasive carcinoma (BRCA)
microarray data
The Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov) data were downloaded from
the University of California Santa Cruz (UCSC) Cancer Genome
Browser (https://genome-cancer.ucsc.edu/proj/site/hgHeatmap/).
The Agilent custom arrays (Agilent G4502A_07_3 array; n=597) were
used in the study (18). The UCSC
Cancer Genome Browser is a suite of web-based tools used for the
visualization, integration and analysis of cancer genomics, and
associated clinical data.
Cancer Landscapes analysis
Cancer Landscapes is an online-based statistical
network model developed by Professor Sven Nelander from Uppsala
University (Uppsala, Sweden), which provides high-performance
statistical network modeling of multiple human cancer. The network
model was constructed using data from Cancer Landscapes (www.cancerlandscapes.org; version beta).
Analysis of differentially expressed
genes (DEGs)
Total RNA was isolated from the MDA-MB-231 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer’s protocol. The
RNA-Sequecncing data were generated with Illumina sequencing
technology (Bejing Genomics Institute, Shenzhen, China), as
previously described (19). The
DEGs between different groups were identified using Bioconductor
edgeR (version 3.12.0) (20). A
Student’s t-test was performed to identify the DEGs between the
Thiostrepton group and control group. P<0.01 and
|log2 fold change|>2 were selected as the cut-off
criterion. Hierarchical clustering were performed using DEG
expression values through the MultiExperiment Viewer software
version 4.9 (http://mev.tm4.org/#/welcome) (21). DAVID (version 6.7; http://david.ncifcrf.gov/) was used to detect Kyoto
Encyclopedia of Genes and Genomes (KEGG; https://www.genome.jp/kegg/) pathways (22). P<0.05 was selected as the
cut-off criterion for significantly enriched KEGG pathways.
STRING protein networks tool
To construct a protein-protein interaction (PPI)
network, the STRING database (https://string-db.org/) was used with the cut-off
criterion of combined score >0.7.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Experiments were performed and
repeated three times with similar results. All data are presented
as the mean ± standard deviation. Student’s t-test and one-way
analysis of variance followed by Dunnett’s post hoc test were used
to evaluate differences among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Position of FOXM1 in a breast cancer gene
network
The key role of FOXM1 in breast cancer was
identified in a Cancer Landscapes analysis. A network model was
constructed using multidimensional perspective data from breast
cancer and ovarian cancer, which are the two most common tumor
types in females. An overview of the gene interaction networks is
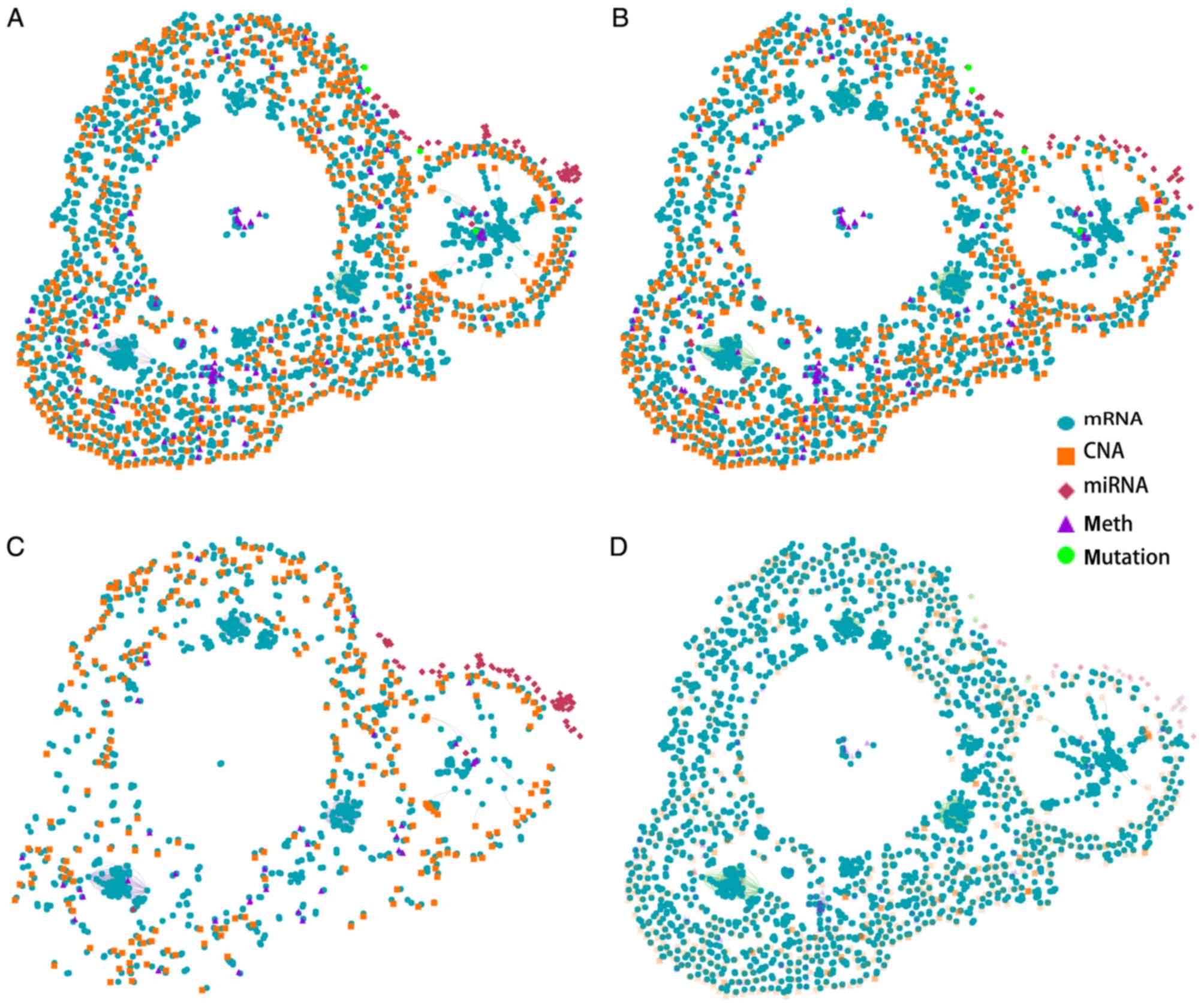
presented in Fig. 1A, which
demonstrated that DNA copy number aberrations (CNAs) and mRNA
alterations are characteristic features of these networks. The
overlapping genes suggested that the two types of cancer share
specific network genes, which suggests that certain gene functions
are evolutionarily conserved between the two cancer types. The gene
interaction network contains three primary clusters that
demonstrate strong similarities in gene expression patterns in the
two types of tumors. A previous study demonstrated that basal-like
breast cancer and serous ovarian carcinoma are the most similar in
terms of genomic mutations and copy number and that these two
difficult-to-treat cancer types may share driver mutation events
and common therapeutic approaches (18). The present results further
suggested that the overlapping genes identified in the network are
strongly associated with these tumors. These results suggested that
breast and ovarian cancer may have similar molecular pathogenic
mechanisms.
The gene networks of the two tissue types were
separated (Fig. 1B and C). More
mRNA alterations and CNAs were identified in the breast cancer gene
network compared with the ovarian cancer gene network. Furthermore,
the two types of cancer genes were identified to be
tissue-specific. A recent study identified that certain cancer
genes are only involved in the development of specific cancer
types; however, are rarely identified in other types of cancer
(23). This bias is affected by
environmental factors and cellular processes. The present study
provides a better understanding of the gene interaction networks in
the two tissue types.
FOXM1 is a typical proliferation-associated
transcription factor, and is essential for cancer initiation and
progression (24). To obtain a
thorough understanding of the regulation and function of FOXM1 in
breast cancer, breast cancer mRNA networks were constructed
(Fig. 1D). The results suggested
that FOXM1 serves a key role in mRNA networks and in the
progression of breast cancer. As FOXM1 is the most important member
of the gene network, a further analysis of FOXM1 in breast cancer
was conducted.
Expression of FOXM1 and its regulated
gene network in breast cancer
FOXM1 was observed to be highly expressed in breast
cancer; however, further research was required to determine the
different expression patterns and functions of FOXM1 in breast
cancer subtypes. To identify the functions and gene networks
associated with FOXM1 in breast cancer, a BRCA gene expression
dataset from TCGA was used. Using the FOXM1 transcriptional network
extracted from the UCSC Cancer Browser website, the expression of
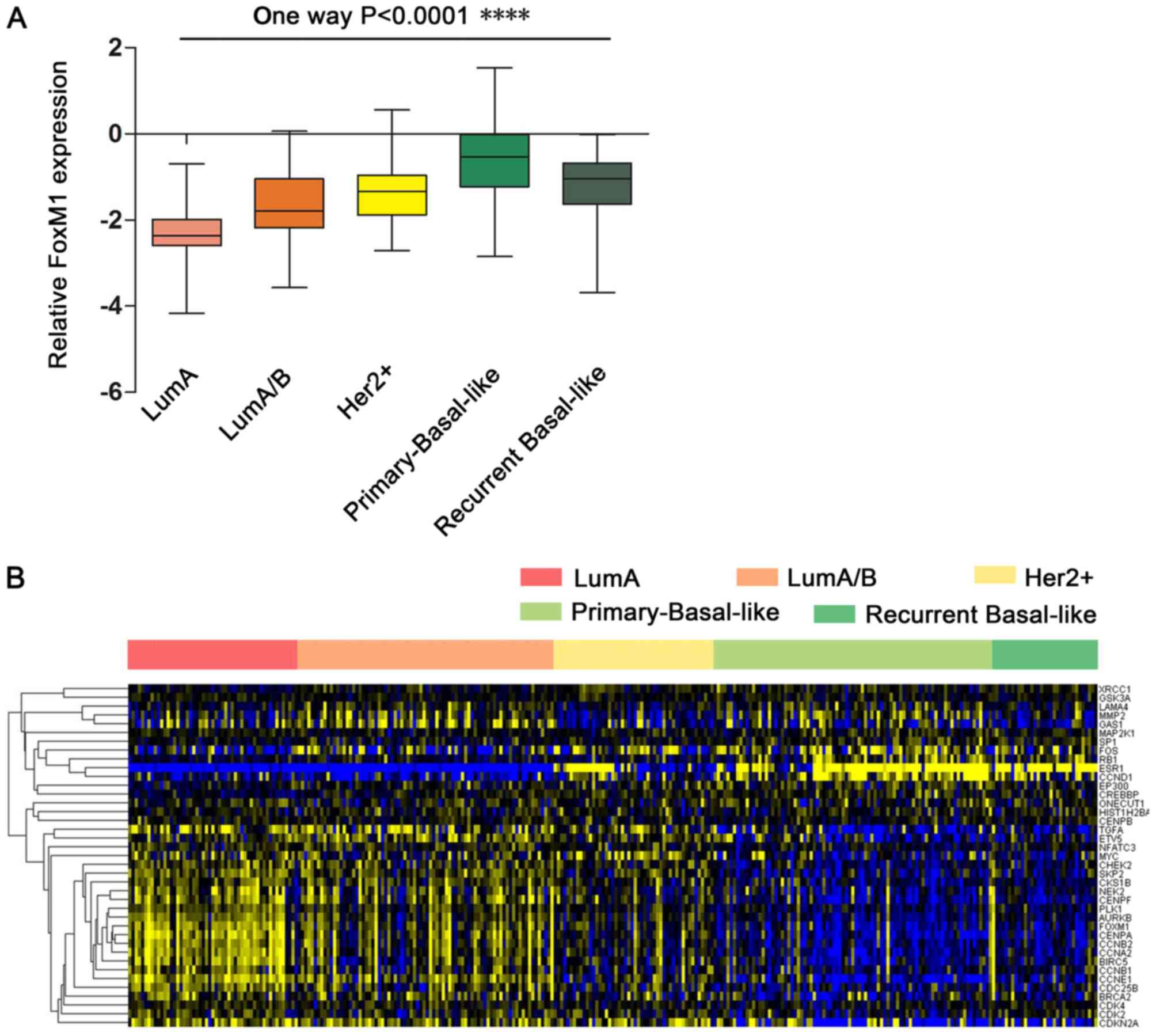
FOXM1 in different breast cancer subtypes was analyzed. A gene
expression profile analysis revealed that the breast cancer
subtypes are associated with the expression of ER, PR and HER2. As
presented in Fig. 2A, the
expression of FOXM1 is low in the luminal A (ER+/PR+/HER2−) and
luminal A/B (ER+/PR+/HER2+/−) subtypes, which are
characteristically ER- and PR-positive. In contrast, the HER2+
subtype (ER−/PR−/HER2+) demonstrated slightly increased expression
of FOXM1. Notably, it was observed that primary and recurrent
tumors of the basal-like breast cancer subtype (typically
characterized by ER−/PR−/HER2- status) exhibited stronger
expression of FOXM1. Furthermore, FOXM1 expression was slightly
decreased in recurrent breast cancer compared with primary breast
cancer. These results suggested that the differential FOXM1
expression across the various subtypes of breast cancer is
associated with the ER, PR and HER2 status. ER/PR may function with
HER2 to contribute to differential FOXM1 expression in breast
cancer subtypes. ER−/PR−/HER2− status (characteristic of the
basal-like or TNBC subtypes) is more positively associated with
FOXM1 expression compared with the other breast cancer
subtypes.
To identify the functions of FOXM1 regulatory
networks in breast cancer, the expression of FOXM1 and
FOXM1-regulated genes was further analyzed. A heatmap demonstrated
that 38 genes are directly associated with FOXM1 in breast cancer,
including coregulators (RB transcriptional corepressor 1) and
target genes (Fig. 2B). The
signature of 38 FOXM1-regulated genes downregulated by treatment
with Thiostrepton was validated by previously published data
(25).
FOXM1 expression was increased in the basal-like
breast cancer subtype compared with the other breast cancer
subtypes. Furthermore, FOXM1 target genes were upregulated in the
basal-like breast cancer subtype and were associated with FOXM1
expression. Genes with expression that was highly associated with
FOXM1 expression in basal-like breast cancer were further
investigated. Two principal groups of cell cycle-associated genes
have been defined; those that demonstrated peak expression in S
phase (G1/S) and those whose expression peaked in
mitosis (G2/M) (26).
According to the present data, numerous FOXM1-associated genes were
expressed in S phase (G1/S), including
G1/S-specific cyclin E-1, cyclin-dependent kinase 2,
M-phase inducer phosphatase 2 (CDC25B), S-phase kinase-associated
protein 2, breast cancer type 1 susceptibility protein and breast
cancer type 2 susceptibility protein. Other specific genes were
critical for G2-M progression, including CCNB2, CCNB1,
CCNA2, PLK1, aurora kinase B, serine/threonine-protein kinase 2,
CHEK2, CDC25B, baculoviral IAP repeat containing protein 5,
centromere protein A, centromere protein E and centromere protein
F. This observation emphasized the role of FOXM1 as a core gene in
the enhanced proliferation signature in breast cancer subtypes.
Other genes that are known to be associated with cancer, including
matrix metalloproteinase 2, are associated with the role of FOXM1
in metastasis. These results demonstrated the core function of
FOXM1 within its regulatory network. Increased FOXM1 expression
demonstrated good predictive ability for the diagnosis of TNBC.
Differential gene expression profiling in
breast cancer cell lines treated with Thiostrepton
To investigate the function of FOXM1 in TNBC in
vitro, RNA-sequencing technology was used to analyze RNA
expression following treatment of the MDA-MB-231 breast cancer cell
line with Thiostrepton. Thiostrepton, a specific inhibitor of
FOXM1, was previously demonstrated to induce cell death via a
decrease in FOXM1 expression in breast cancer cell lines (27,28).
MDA-MB-231 cells were treated with 4 µM and 10 µM
Thiostrepton for 48 h. Following treatment, RNA was extracted and
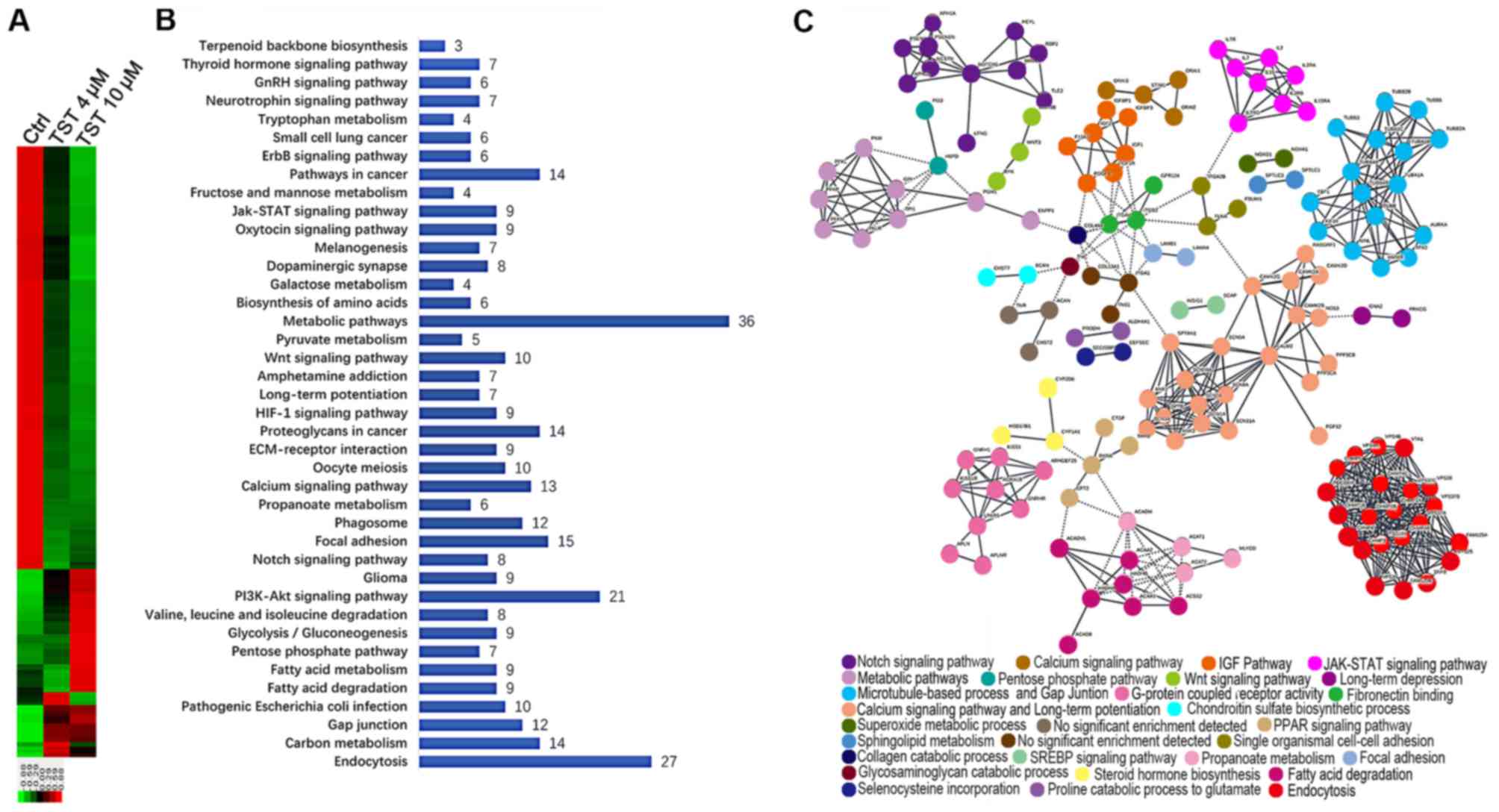
RNA-sequencing analysis was performed. In total, 5,888
significantly differentially expressed genes (DEGs) were identified
in MDA-MB-231 cells treated with 10 µl/ml Thiostrepton
compared with the control group. Among these genes, 4,200 genes
were downregulated and 1,688 genes were upregulated (Fig. 3A). A comparative analysis of cells
treated with 4 µl/ml Thiostrepton identified 662 DEGs, of
which 165 DEGs were upregulated and 497 DEGs were downregulated.
These results suggest that treatment with Thiostrepton altered gene
expression in MDA-MB-231 cells in a dose-dependent manner. This
observation further emphasizes the important role of FOXM1 in the
regulation of gene networks in TNBC.
To identify the biologically meaningful pathways
affected by a FOXM1 inhibitor, KEGG pathway enrichment analyses
were performed. Specifically, the 248 downregulated DEGs that were
common to the 4 and 10 µM Thiostrepton treatment groups were
selected for further analysis. The top 10 significantly enriched
pathways included the following: ‘Metabolic pathways’,
‘Endocytosis’, ‘PI3K-Akt signaling pathway’, ‘Focal adhesion’,
‘Pathways in cancer’, ‘Proteoglycans in cancer’, ‘Carbon
metabolism’, ‘Calcium signaling pathway’, ‘Phagosome’ and ‘Gap
junction’ (Fig. 3B). Metabolic
pathways were considered significantly enriched pathways. FOXM1
serves a key role in metabolic pathways in TNBC. These results
suggested that FOXM1 was highly associated with multiple biological
processes in the network.
Determination of PPI networks is a useful method for
assessing functional associations among genes that exhibit
collective mRNA level differential expression in disease (29,30).
The observed dysregulation of PPIs suggested an important mechanism
of FOXM1 in TNBC. To obtain a global view of the interactions
between proteins in Thiostrepton-treated cells, a PPI network was
constructed using STRING. STRING provides a critical assessment and
integration of protein-protein interactions, including direct
(physical) and indirect (functional) associations (31). Among the number of clusters
presented in Fig. 3C, known and
predicted interactions arose from the majority of proteins in the
network. Proteins, including neurogenic locus notch homolog protein
1 (NOTCH-1), insulin-like growth factor 1 (IGF1), cytokine receptor
common subunit γ (IL2RG), tubulin β (TUBB), calmodulin 2, integrin
β3, sodium channel protein type 5 subunit α and charged
multivesicular body protein were observed in the hub positions
(proteins with multiple edges) in the PPI network. NOTCH-1 is a
critical regulator of the development of human breast cancer
(32). The knockdown of NOTCH-1 is
therapeutically effective in ER α-negative breast cancer (33). FOXM1 is a downstream target of
NOTCH1 signaling (34). IGF1 has
significant growth-promoting activity and serves an important role
in the development, progression and metastasis of breast cancer
(35). The IL2RG protein is
required for T-cell proliferation and other activities crucial to
the regulation of the immune response (36). TUBB is a principal constituent of
microtubules that binds two molecules of guanosine triphosphate,
one at an exchangeable site on the β chain and the other at a
non-exchangeable site on the α chain (37). FOXM1 is essential for the migration
of mesenchymal cells and directly induces integrin-β3 expression
(38). These results emphasized
the multiple important functions of FOXM1 in breast cancer
progression at the proteome level.
In summary, through the comprehensive analysis of
global pathway regulation in TNBC in response to Thiostrepton, the
underlying mechanisms of FOXM1 in the network were
demonstrated.
Thiostrepton inhibits TNBC cell growth in
vitro
To investigate the effects of Thiostrepton on TNBC
cell growth in vitro, MDA-MB-231 cells were treated with
increasing concentrations of Thiostrepton and cell proliferation
was measured via RTCA. MDA-MB-231 cells were used in the present
study and were treated with Thiostrepton for 80 h at concentrations
that ranged between 1 and 40 µM. The RTCA results
demonstrated that the proliferation of MDA-MB-231 cells was
suppressed in a dose-dependent manner compared with the negative
control group (Fig. 4A). qPCR
analysis demonstrated that FOXM1 mRNA expression levels decreased
in a dose-dependent manner in Thiostrepton-treated MDA-MB-231 cells
(Fig. 4B). To examine the role of
FOXM1 and its possible target genes in cell proliferation, qPCR was
used to detect the expression of cell cycle-associated genes,
including CCNA2, PLK1, CCNB2, CEP55 and CHEK1. As presented in
Fig. 4B, it was observed that the
expression of these genes decreased in a dose-dependent manner in
Thiostrepton-treated MDA-MB-231 cells from 4 µM
Thiostrepton. These results suggested that Thiostrepton exerted an
anti-tumor effect in TNBC cells. Immunofluorescence staining
additionally demonstrated that CDK1, PLK1 and CCNB1 expression
decreased upon treatment with 10 µM Thiostrepton for 48 h
(Fig. 4C). These results suggested
that Thiostrepton inhibited cell proliferation by suppressing the
expression of cell cycle-associated factors at the mRNA and protein
expression levels. These results demonstrated that FOXM1 is
functionally essential for the proliferation of TNBC cells in
vitro.
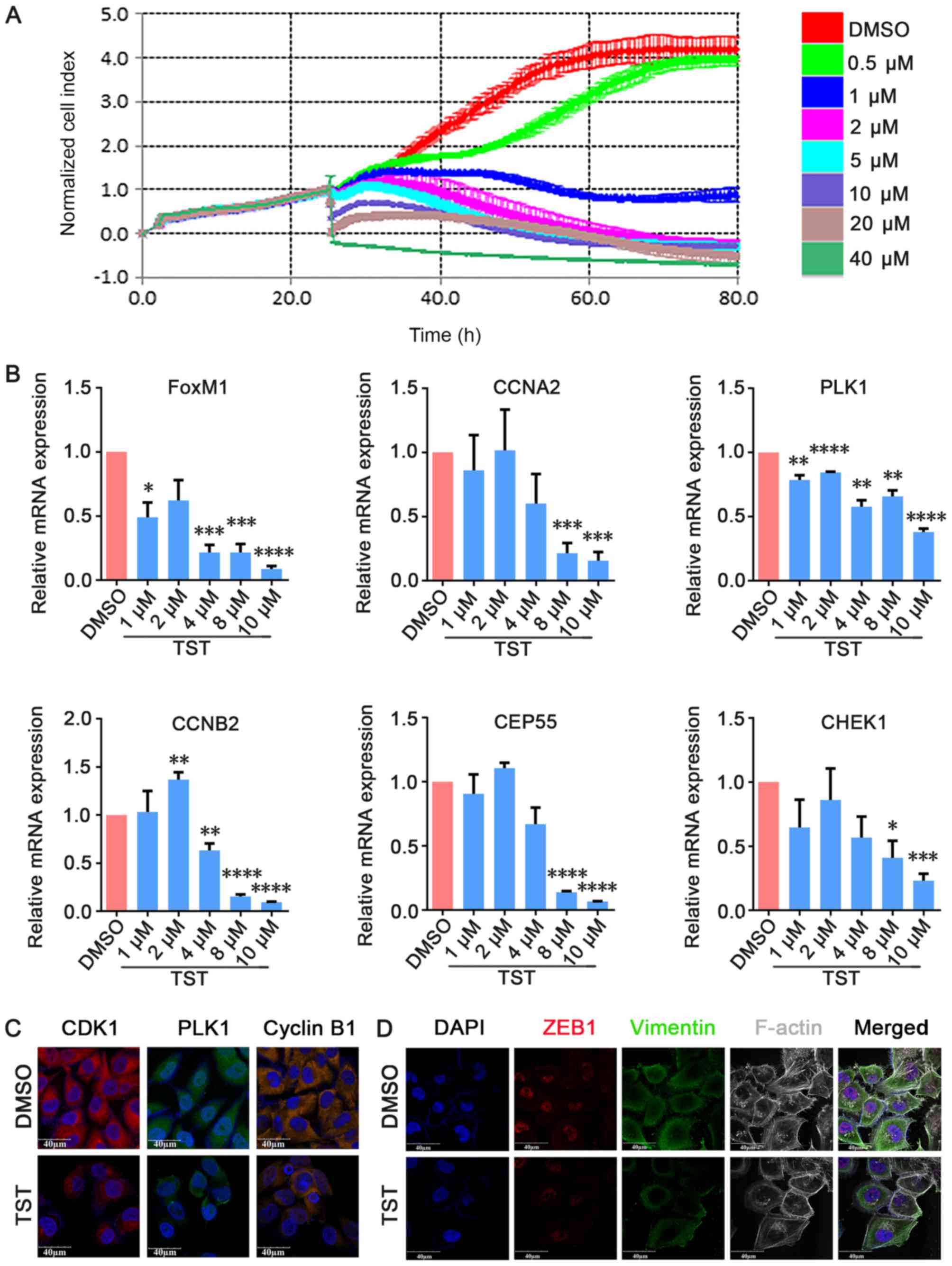 | Figure 4TST inhibits MDA-MB-231 cell growth
in vitro. (A) MDA-MB-231 cells were treated with TST at the
indicated doses. Cell viability was determined by real-time cell
proliferation assay. (B) Cells were treated with 10 µmol/l
TST for 48 h. The mRNA expression levels of FOXM1, CCNA2, PLK1,
CHEK1, CEP55 and CCNB2 were determined by quantitative polymerase
chain reaction. (C) Immunofluorescence staining demonstrated that
CDK1, PLK1 and cyclin B1 expression decreased upon treatment. (D)
Triple immunofluorescence staining demonstrated that ZEB1
co-localized with vimentin and F-actin, and decreased upon
treatment. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. respective
DMSO. TST, Thiostrepton; DMSO, dimethyl sulfoxide; FOXM1, forkhead
box M1; CCNA2, cyclin A2; PLK1, polo like kinase 1; CHEK1,
checkpoint kinase 1; CEP55, centrosomal protein 55; CCNB2, cyclin
B2; ZEB1, zinc finger E-box-binding homeobox 1; F-actin,
filamentous actin; CDK1, cyclin-dependent kinase 1. |
FOXM1 serves an important role in the regulation of
the epithelial-mesenchymal transition (EMT). EMT is an important
feature of TNBC, and a previous study demonstrated that FOXM1
enhances EMT in breast cancer cells (39). Treatment with Panepoxydone, a
nuclear factor-κB inhibitor, downregulated FOXM1 and resulted in a
reversal of EMT (40). However,
the molecular mechanisms of FOXM1 in EMT and the establishment of
distant metastasis remain unclear. To analyze the inhibitory effect
of treatment with Thiostrepton on FOXM1-driven EMT, triple
immunofluorescence staining of EMT-associated factors (vimentin,
F-actin and ZEB1) was performed following treatment with 10
µM Thiostrepton for 48 h. ZEB1 influences EMT in breast
cancer cells by inhibiting E-cadherin repressors (41). Vimentin is a mesenchymal marker
that is expressed during EMT (42). Rearrangement of the F-actin
cytoskeleton is a crucial event during EMT (43). ZEB1, vimentin and F-actin
co-localized and were decreased in MDA-MB-231 cells upon treatment
with Thiostrepton (Fig. 4D). These
results suggested that Thiostrepton may inhibit EMT in TNBC cells
in vitro and that inhibition of FOXM1 may induce EMT
reversal.
Thiostrepton reduces tumorigenesis in
TNBC in vivo
A number of previous studies demonstrated that FOXM1
is involved in tumorigenesis and promotes cell proliferation by
targeting downstream genes (44,45).
A recent study demonstrated that FOXM1 overexpression was
correlated with larger tumor size, lymph node metastasis, advanced
tumor stage and lymph-vascular invasion (46). To investigate the role of FOXM1 in
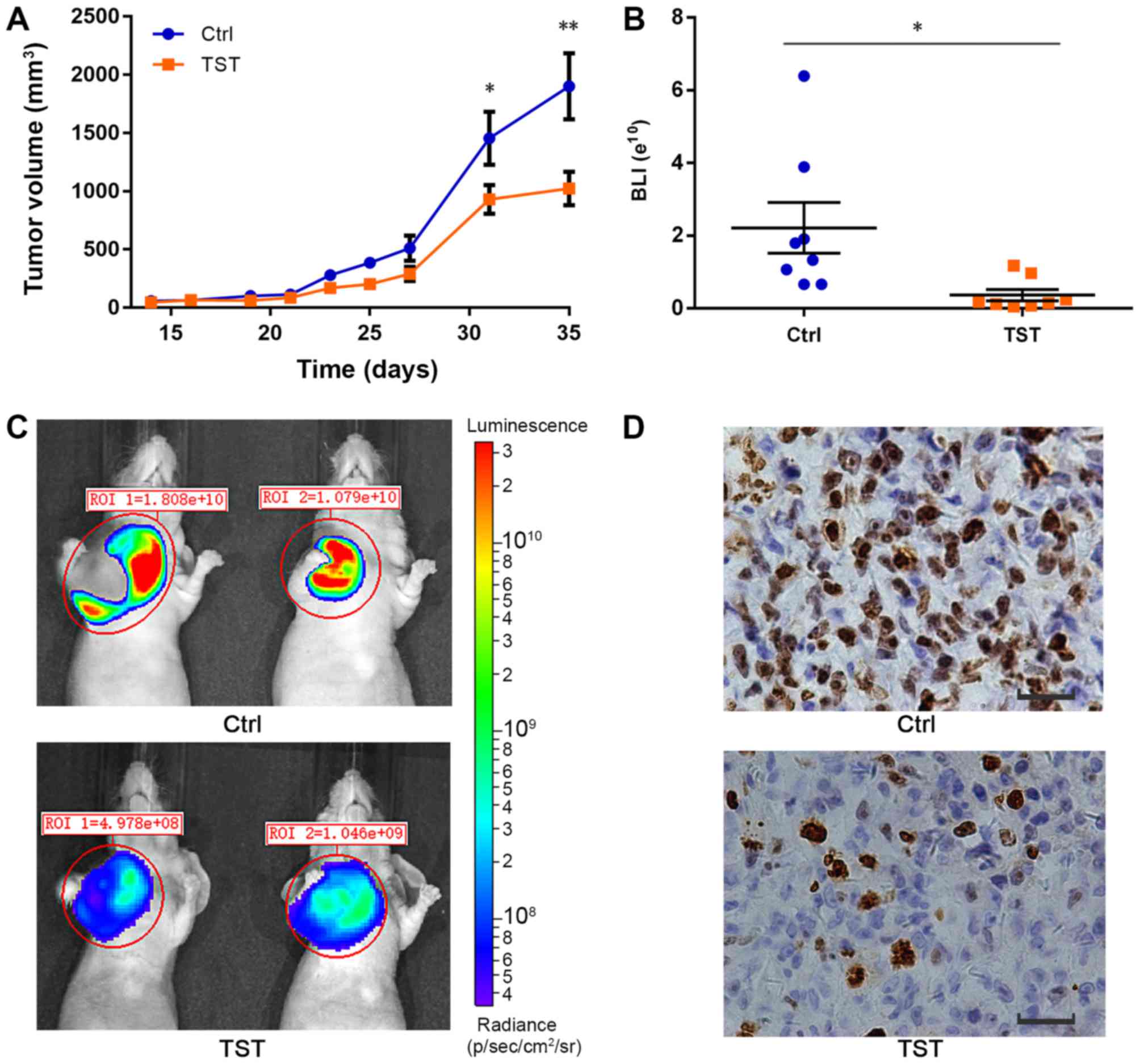
TNBC tumorigenesis, MDA-MB-231 breast cancer cell xenografts were
established in mice. The tumor volume in each animal at the defined
time points was measured during the procedure. At day 35, the
average tumor volume in the control mice group was 1,900.8±801.7
mm3, whereas the tumor volume in the treated group was
1,023.4±410.6 mm3. The average tumor volume was
significantly decreased in the treated group compared with the
control group (P<0.01; Fig.
5A). Thiostrepton suppressed tumor growth in vivo
(Fig. 5A-C). Furthermore, using
immunohistochemistry, the expression of Ki-67, a protein associated
with cell proliferation, was detected. The expression of Ki-67 was
decreased following treatment with Thiostrepton compared with the
control treatment (Fig. 5D). These
results suggested that although Thiostrepton may inhibit tumor
proliferation, it was not able to completely inhibit tumor growth
in vivo. Other mechanisms may contribute to the
tumorigenesis of TNBC. These results provide a better understanding
of the important role served by FOXM1 in TNBC tumorigenesis. In the
present study, the formation of one small tumor was observed in the
liver of a mouse in the control group (data not shown). No distant
metastases were observed in the treatment group. Therefore,
Thiostrepton slightly inhibits the metastasis of TNBC tumors in
vivo.
Discussion
In the present study, it was identified that FOXM1
serves a central role in breast cancer gene networks, which provide
a rich resource for the study of the molecular mechanisms of FOXM1.
Using an mRNA network, FOXM1 was identified as the most important
member of the gene network. Further experiments were used to
validate this hypothesis. FOXM1 was examined at the gene expression
level to better elucidate the molecular mechanisms of FOXM1 in a
complex gene interaction network. These results suggested that
targeting FOXM1 or its network members may be a potential
therapeutic strategy for TNBC.
FOXM1 is overexpressed in breast cancer, including
TNBC (47,48). Although multiple molecular
characteristics of TNBC have been previously identified, the
molecular mechanisms of FOXM1 in TNBC have not been fully
elucidated (49). Breast cancer is
a heterogeneous disease. Different breast cancer subtypes exhibit
different histopathological features, biological features,
treatment responses and prognoses (50). In the present study, FOXM1
expression patterns were examined in different subtypes of breast
cancer. FOXM1 and its associated regulatory network was most
markedly expressed in the TNBC subtype compared with other breast
cancer subtypes. This provides a better understanding of the highly
aggressive nature of TNBC. It was additionally observed that FOXM1
expression was slightly decreased in recurrent breast cancer
compared with primary breast cancer. This genetic disparity becomes
highly relevant when the application of targeted molecular
therapies for primary and recurrent tumors is considered (51). Genomic discordance may result in
differences in therapeutic response. The present study suggested
that FOXM1 may be a potential therapeutic target for primary breast
tumors and recurrent breast cancer. Patients with primary breast
tumors may experience more therapeutic benefits compared with
patients with recurrent tumors. However, additional studies are
required to validate this.
The present study examined the role of FOXM1 and
aimed to map global gene networks in TNBC cell lines, and to
clarify their association with the biological function of FOXM1. It
was identified that FOXM1 regulates certain cell cycle genes and
affects TNBC proliferation, which is consistent with previous
studies (52,53). The pathway analysis demonstrated
that FOXM1 is highly associated with multiple biological processes
in the network. The present study confirmed previous results and
further established that FOXM1 has numerous functions in TNBC in
addition to cell cycle regulation. FOXM1 regulates a generalized
gene network. ‘Metabolic pathways’, ‘Endocytosis’ and ‘PI3K-Akt
signaling pathway’ were the top three enriched pathways following
treatment with Thiostrepton. There are a number of different
aspects of the inhibitory effects of Thiostrepton. Metabolic
pathways were considerably enriched among the pathways. Notably,
tumor metabolism is associated with the tumor microenvironment and
tumor progression (54). Recently,
it was demonstrated that FOXM1 expression is associated with
glucose metabolism in cancer cells (55). FOXM1 promotes the reprogramming of
glucose metabolism in human hepatocellular carcinoma cells and is
considered a novel transcriptional regulator of glycolysis
(55). The present results
suggested that treatment with Thiostrepton may downregulate
metabolic processes in MDA-MB-231 cells. It was additionally
identified that IGF1 and its interaction network are consistent
with the pathway enrichment analysis. IGF1 has high
growth-promoting activity. IGF1 has an important role in breast
cancer development, progression and metastasis (56). The present study suggested that
FOXM1 serves an important role in the regulation of metabolism
during TNBC progression. Further studies are required to elucidate
the mechanisms underlying this regulation.
Thiostrepton, a natural product originally isolated
from Streptomyces azureus, has attracted increasing attention in
the field of breast cancer therapy due to its potential anti-cancer
activity as a FOXM1 inhibitor (57). In the present study, the FOXM1 gene
was investigated; MDA-MB-231 cells were treated with 4 and 10
µl/ml Thiostrepton, and the expression profiles were
subsequently analyzed by RNA-sequencing. KEGG and STRING analyses
provided a clear description of DEGs following treatment with
Thiostrepton. The variety of pathways suggested an extensive impact
of Thiostrepton on breast cancer cells. It is crucial to identify
the specific mechanism of FOXM1, and in the present study, the
function of FOXM1 in the cell cycle was identified. The
FOXM1-associated genes were defined by the UCSC Cancer Browser. All
these genes were associated with the cell cycle, and a number of
them were inhibited by treatment with Thiostrepton, which was
confirmed by real-time PCR and RNA-sequencing. Taken together,
these data suggested that FOXM1 was able to influence multiple
aspects of breast cancer cells and that cell cycle-associated
pathways are a key mechanism affected by FOXM1. In the present
study, Thiostrepton exhibited anti-cancer activity in TNBC in
vivo and in vitro. Although Thiostrepton may inhibit
tumor proliferation, it is not able to completely inhibit tumor
growth and metastasis in vivo. Therefore, there may be other
mechanisms that contribute to the tumorigenesis of TNBC. One of the
leading causes of resistance to small molecule inhibitors is
cross-talk between dysregulated survival pathways (58). Recent studies demonstrated that
targeting multiple components of different pathways with a
combination of specific inhibitors is more effective compared with
treatment with a single agent alone (59,60).
A previous study observed that the combined targeting of
cyclooxygenase-2 and FOXM1 causes inhibition of invasion and
migration, reduction in cell viability and induction of apoptosis
in colorectal cancer cells (61).
Due to the complicated molecular mechanisms of FOXM1 in TNBC, a
combined therapy using multiple targeting agents for TNBC treatment
is highly recommended. This may provide more effective therapeutic
strategies for TNBC.
In conclusion, the present study provides a better
understanding of the molecular mechanisms of FOXM1 within a complex
gene interaction network. FOXM1 serves a critical role in the
regulatory network in TNBC. FOXM1 may be a promising molecular
therapeutic target for TNBC.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Hebei
Provincial Top Level Talents Funded Projects of China (grant no.
CY201601) and the Hebei Province Technical Innovation Guidance
Funded Projects of China (grant no. 18247792D).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors’ contributions
BZ and YT conceived and designed the experiments. YT
was responsible for writing the manuscript. QW conducted the
bioinformatics analyses. YX, XQ, SZ, YW and YY performed the
experiments and analyzed the data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Animal studies were conducted according to the
recommendations outlined in the Guide for the Care and Use of
Laboratory Animals in the Weatherall report. Animal experiments
were approved by the Committee on the Ethics of Animal Experiments
of Hebei University (Baoding, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khongkow P, Gomes AR, Gong C, Man EP,
Tsang JW, Zhao F, Monteiro LJ, Coombes RC, Medema RH, Khoo US, et
al: Paclitaxel targets FOXM1 to regulate KIF20A in mitotic
catastrophe and breast cancer paclitaxel resistance. Oncogene.
35:990–1002. 2016. View Article : Google Scholar
|
|
3
|
Borin TF, Angara K, Rashid MH, Achyut BR
and Arbab AS: Arachidonic acid metabolite as a novel therapeutic
target in breast cancer metastasis. Int J Mol Sci. 18:pii: E2661.
2017. View Article : Google Scholar
|
|
4
|
Arnold KM, Pohlig RT and Sims-Mourtada J:
Co-activation of Hedgehog and Wnt signaling pathways is associated
with poor outcomes in triple negative breast cancer. Oncol Lett.
14:5285–5292. 2017.PubMed/NCBI
|
|
5
|
McGuire A, Lowery AJ, Kell MR, Kerin MJ
and Sweeney KJ: Locoregional recurrence following breast cancer
surgery in the trastuzumab era: A systematic review by subtype. Ann
Surg Oncol. 24:3124–3132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu G, Dustin D and Fuqua SA: Targeted
therapy for breast cancer and molecular mechanisms of resistance to
treatment. Curr Opin Pharmacol. 31:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Xiao Y, Wei W, Guo JX, Liu YC,
Huang XH, Zhang RX, Wu YJ and Zhou J: Clinical efficacy of
administering oxaliplatin combined with S-1 in the treatment of
advanced triple-negative breast cancer. Exp Ther Med. 10:379–385.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reaz S, Tamkus D and Andrechek ER: Using
gene expression data to direct breast cancer therapy: Evidence from
a preclinical trial. J Mol Med (Berl). 96:111–117. 2018. View Article : Google Scholar
|
|
9
|
Guo GC, Wang JX, Han ML, Zhang LP and Li
L: microRNA-761 induces aggressive phenotypes in triple-negative
breast cancer cells by repressing TRIM29 expression. Cell Oncol
(Dordr). 40:157–166. 2017. View Article : Google Scholar
|
|
10
|
Lam EW and Gomes AR: Forkhead box
transcription factors in cancer initiation, progression and
chemotherapeutic drug response. Front Oncol. 4:3052014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abdeljaoued S, Bettaieb I, Nasri M, Adouni
O, Goucha A, El Amine O, Boussen H, Rahal K and Gamoudi A:
Overexpression of FOXM1 Is a Potential Prognostic Marker in Male
Breast Cancer. Oncol Res Treat. 40:167–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lam AK, Ngan AW, Leung MH, Kwok DC, Liu
VW, Chan DW, Leung WY and Yao KM: FOXM1b, which is present at
elevated levels in cancer cells, has a greater transforming
potential than FOXM1c. Front Oncol. 3:112013.PubMed/NCBI
|
|
13
|
Hamurcu Z, Kahraman N, Ashour A and
Ozpolat B: FOXM1 transcriptionally regulates expression of integrin
β1 in triple-negative breast cancer. Breast Cancer Res Treat.
163:485–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JJ, Lee HJ, Son BH, Kim SB, Ahn JH,
Ahn SD, Cho EY and Gong G: Expression of FOXM1 and related proteins
in breast cancer molecular subtypes. Int J Exp Pathol. 97:170–177.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamurcu Z, Ashour A, Kahraman N and
Ozpolat B: FOXM1 regulates expression of eukaryotic elongation
factor 2 kinase and promotes proliferation, invasion and
tumorgenesis of human triple negative breast cancer cells.
Oncotarget. 7:16619–16635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US); Washington, DC:
1996
|
|
18
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Li W, Liu X, Ma H, Tu Z and Dai Y:
Analysis of microRNA expression profile by small RNA sequencing in
Down syndrome fetuses. Int J Mol Med. 32:1115–1125. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang M, Li H, Li Y, Ruan Y and Quan C:
Identification of genes and pathways associated with MDR in
MCF-7/MDR breast cancer cells by RNA-seq analysis. Mol Med Rep.
17:6211–6226. 2018.PubMed/NCBI
|
|
21
|
Eisen MB, Spellman PT, Brown PO and
Botstein D: Cluster analysis and display of genome-wide expression
patterns. Proc Natl Acad Sci USA. 95:14863–14868. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C, Chen N, Huang K, Jiang M, Liang H,
Sun Z, Tian J and Wang D: Identifying hub genes and potential
mechanisms associated with senescence in human annulus cells by
gene expression profiling and bioinformatics analysis. Mol Med Rep.
17:3465–3472. 2018.
|
|
23
|
Polak P, Karlić R, Koren A, Thurman R,
Sandstrom R, Lawrence M, Reynolds A, Rynes E, Vlahoviček K,
Stamatoyannopoulos JA, et al: Cell-of-origin chromatin organization
shapes the mutational landscape of cancer. Nature. 518:360–364.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zona S, Bella L, Burton MJ, Nestal de
Moraes G and Lam EW: FOXM1: An emerging master regulator of DNA
damage response and genotoxic agent resistance. Biochim Biophys
Acta. 1839.1316–1322. 2014.
|
|
25
|
Sanders DA, Ross-Innes CS, Beraldi D,
Carroll JS and Balasubramanian S: Genome-wide mapping of FOXM1
binding reveals co-binding with estrogen receptor alpha in breast
cancer cells. Genome Biol. 14:R62013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fischer M and Müller GA: Cell cycle
transcription control: DREAM/MuvB and RB-E2F complexes. Crit Rev
Biochem Mol Biol. 52:638–662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwok JMM, Myatt SS, Marson CM, Coombes RC,
Constantinidou D and Lam EW: Thiostrepton selectively targets
breast cancer cells through inhibition of forkhead box M1
expression. Mol Cancer Ther. 7:2022–2032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang N, Zhou TC, Lei XX, Wang C, Yan M,
Wang ZF, Liu W, Wang J, Ming KH, Wang BC, et al: Inhibition of
Sonic Hedgehog Signaling Pathway by Thiazole Antibiotic
Thiostrepton Attenuates the CD44+/CD24-Stem-Like Population and
Sphere-Forming Capacity in Triple-Negative Breast Cancer. Cell
Physiol Biochem. 38:1157–1170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chuang HY, Lee E, Liu YT, Lee D and Ideker
T: Network-based classification of breast cancer metastasis. Mol
Syst Biol. 3:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chowdhury SA, Nibbe RK, Chance MR and
Koyutürk M: Subnetwork state functions define dysregulated
subnetworks in cancer. J Comput Biol. 18:263–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43(D1): D447–D452. 2015. View Article : Google Scholar
|
|
32
|
Li L, Zhao F, Lu J, Li T, Yang H, Wu C and
Liu Y: Notch-1 signaling promotes the malignant features of human
breast cancer through NF-κB activation. PLoS One. 9:e959122014.
View Article : Google Scholar
|
|
33
|
Rizzo P, Miao H, D’Souza G, Osipo C, Song
LL, Yun J, Zhao H, Mascarenhas J, Wyatt D, Antico G, et al:
Cross-talk between notch and the estrogen receptor in breast cancer
suggests novel therapeutic approaches. Cancer Res. 68:5226–5235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi
AS, Kong D, Wojewoda C, Miele L and Sarkar FH: Downregulation of
Notch-1 is associated with Akt and FoxM1 in inducing cell growth
inhibition and apoptosis in prostate cancer cells. J Cell Biochem.
112:78–88. 2011. View Article : Google Scholar
|
|
35
|
Christopoulos PF, Msaouel P and
Koutsilieris M: The role of the insulin-like growth factor-1 system
in breast cancer. Mol Cancer. 14:432015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suzuki S, Iwamoto M, Saito Y, Fuchimoto D,
Sembon S, Suzuki M, Mikawa S, Hashimoto M, Aoki Y, Najima Y, et al:
Il2rg gene-targeted severe combined immunodeficiency pigs. Cell
Stem Cell. 10:753–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kopp S, Slumstrup L, Corydon TJ, Sahana J,
Aleshcheva G, Islam T, Magnusson NE, Wehland M, Bauer J, Infanger
M, et al: Identifications of novel mechanisms in breast cancer
cells involving duct-like multicellular spheroid formation after
exposure to the Random Positioning Machine. Sci Rep. 6:268872016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Malin D, Kim IM, Boetticher E, Kalin TV,
Ramakrishna S, Meliton L, Ustiyan V, Zhu X and Kalinichenko VV:
Forkhead box F1 is essential for migration of mesenchymal cells and
directly induces integrin-beta3 expression. Mol Cell Biol.
27:2486–2498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xue J, Lin X, Chiu WT, Chen YH, Yu G, Liu
M, Feng XH, Sawaya R, Medema RH, Hung MC, et al: Sustained
activation of SMAD3/SMAD4 by FOXM1 promotes TGF-β-dependent cancer
metastasis. J Clin Invest. 124:564–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arora R, Yates C, Gary BD, McClellan S,
Tan M, Xi Y, Reed E, Piazza GA, Owen LB and Dean-Colomb W:
Panepoxydone targets NF-kB and FOXM1 to inhibit proliferation,
induce apoptosis and reverse epithelial to mesenchymal transition
in breast cancer. PLoS One. 9:e983702014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Roslan S, Johnstone CN, Wright JA,
Bracken CP, Anderson M, Bert AG, Selth LA, Anderson RL, Goodall GJ,
et al: MiR-200 can repress breast cancer metastasis through
ZEB1-independent but moesin-dependent pathways. Oncogene.
33:4077–4088. 2014. View Article : Google Scholar
|
|
42
|
Park MY, Kim KR, Park HS, Park BH, Choi
HN, Jang KY, Chung MJ, Kang MJ, Lee DG and Moon WS: Expression of
the serum response factor in hepatocellular carcinoma: Implications
for epithelial-mesenchymal transition. Int J Oncol. 31:1309–1315.
2007.PubMed/NCBI
|
|
43
|
Shankar J and Nabi IR: Correction: Actin
cytoskeleton regulation of epithelial mesenchymal transition in
metastatic cancer cells. PLoS One. 10:e01327592015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang C, Chen H, Tan G, Gao W, Cheng L,
Jiang X, Yu L and Tan Y: FOXM1 promotes the epithelial to
mesenchymal transition by stimulating the transcription of Slug in
human breast cancer. Cancer Lett. 340:104–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Halasi M and Gartel AL: Targeting FOXM1 in
cancer. Biochem Pharmacol. 85:644–652. 2013. View Article : Google Scholar
|
|
46
|
Song X, Fiati Kenston SS, Zhao J, Yang D
and Gu Y: Roles of FoxM1 in cell regulation and breast cancer
targeting therapy. Med Oncol. 34:412017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ahn H, Sim J, Abdul R, Chung MS, Paik SS,
Oh YH, Park CK and Jang K: Increased expression of forkhead box M1
is associated with aggressive phenotype and poor prognosis in
estrogen receptor-positive breast cancer. J Korean Med Sci.
30:390–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wierstra I: FOXM1 (Forkhead box M1) in
tumorigenesis: Overexpression in human cancer, implication in
tumorigenesis, oncogenic functions, tumor-suppressive properties,
and target of anticancer therapy. Adv Cancer Res. 119:191–419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bayraktar R, Ivan C, Bayraktar E,
Kanlikilicer P, Kabil NN, Kahraman N, Mokhlis HA, Karakas D,
Rodriguez-Aguayo C, Arslan A, et al: Dual Suppressive Effect of
miR-34a on the FOXM1/eEF2-Kinase Axis Regulates Triple-Negative
Breast Cancer Growth and Invasion. Clin Cancer Res. 24:4225–4241.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: Prognostic and therapeutic implications.
World J Clin Oncol. 5:412–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Krøigård AB, Larsen MJ, Thomassen M and
Kruse TA: Molecular concordance between primary breast cancer and
matched metastases. Breast J. 22:420–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wierstra I and Alves J: FOXM1, a typical
proliferation-associated transcription factor. Biol Chem.
388:1257–1274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lv C, Zhao G, Sun X, Wang P, Xie N, Luo J
and Tong T: Acetylation of FOXM1 is essential for its
transactivation and tumor growth stimulation. Oncotarget.
7:60366–60382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer’s Achilles’ heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shang R, Pu M, Li Y and Wang D: FOXM1
regulates glycolysis in hepatocellular carcinoma by transactivating
glucose transporter 1 expression. Oncol Rep. 37:2261–2269. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lei T and Ling X: IGF-1 promotes the
growth and metastasis of hepatocellular carcinoma via the
inhibition of proteasome-mediated cathepsin B degradation. World J
Gastroenterol. 21:10137–10149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hegde NS, Sanders DA, Rodriguez R and
Balasubramanian S: The transcription factor FOXM1 is a cellular
target of the natural product thiostrepton. Nat Chem. 3:725–731.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rahman MA, Amin AR and Shin DM:
Chemopreventive potential of natural compounds in head and neck
cancer. Nutr Cancer. 62:973–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tolcher AW, Peng W and Calvo E: Rational
Approaches for Combination Therapy Strategies Targeting the MAP
Kinase Pathway in Solid Tumors. Mol Cancer Ther. 17:3–16. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang N, Wang C, Wang Z, Zona S, Lin SX,
Wang X, Yan M, Zheng FM, Li SS, Xu B, et al: FOXM1 recruits nuclear
Aurora kinase A to participate in a positive feedback loop
essential for the self-renewal of breast cancer stem cells.
Oncogene. 36:3428–3440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ahmed M, Hussain AR, Siraj AK, Uddin S,
Al-Sanea N, Al-Dayel F, Al-Assiri M, Beg S and Al-Kuraya KS:
Co-targeting of Cyclooxygenase-2 and FoxM1 is a viable strategy in
inducing anticancer effects in colorectal cancer cells. Mol Cancer.
14:1312015. View Article : Google Scholar : PubMed/NCBI
|