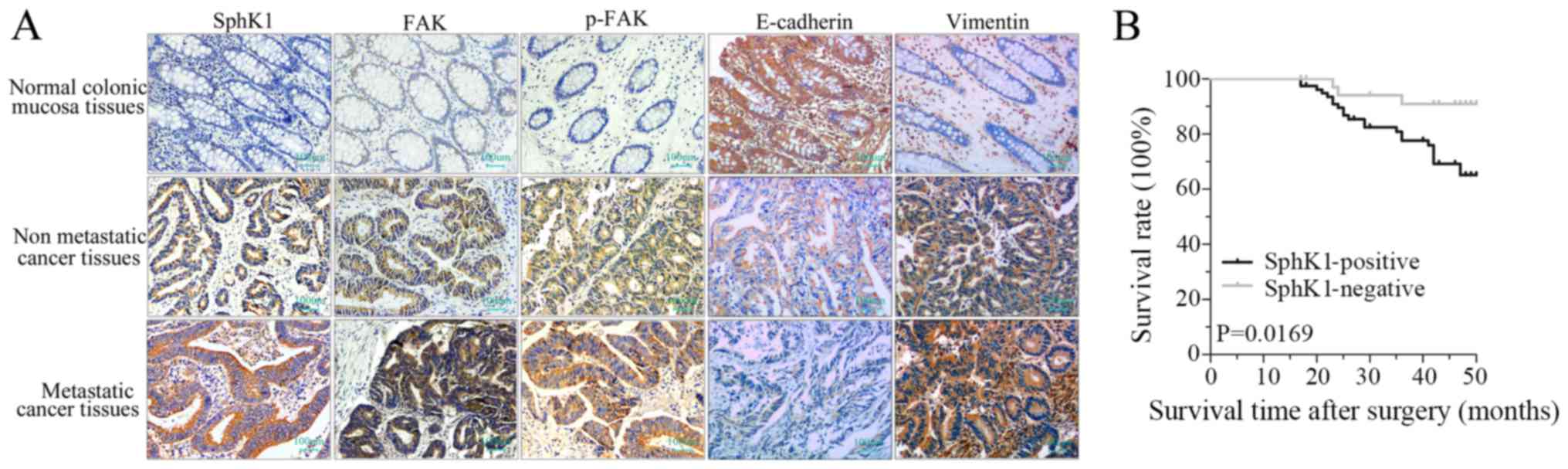
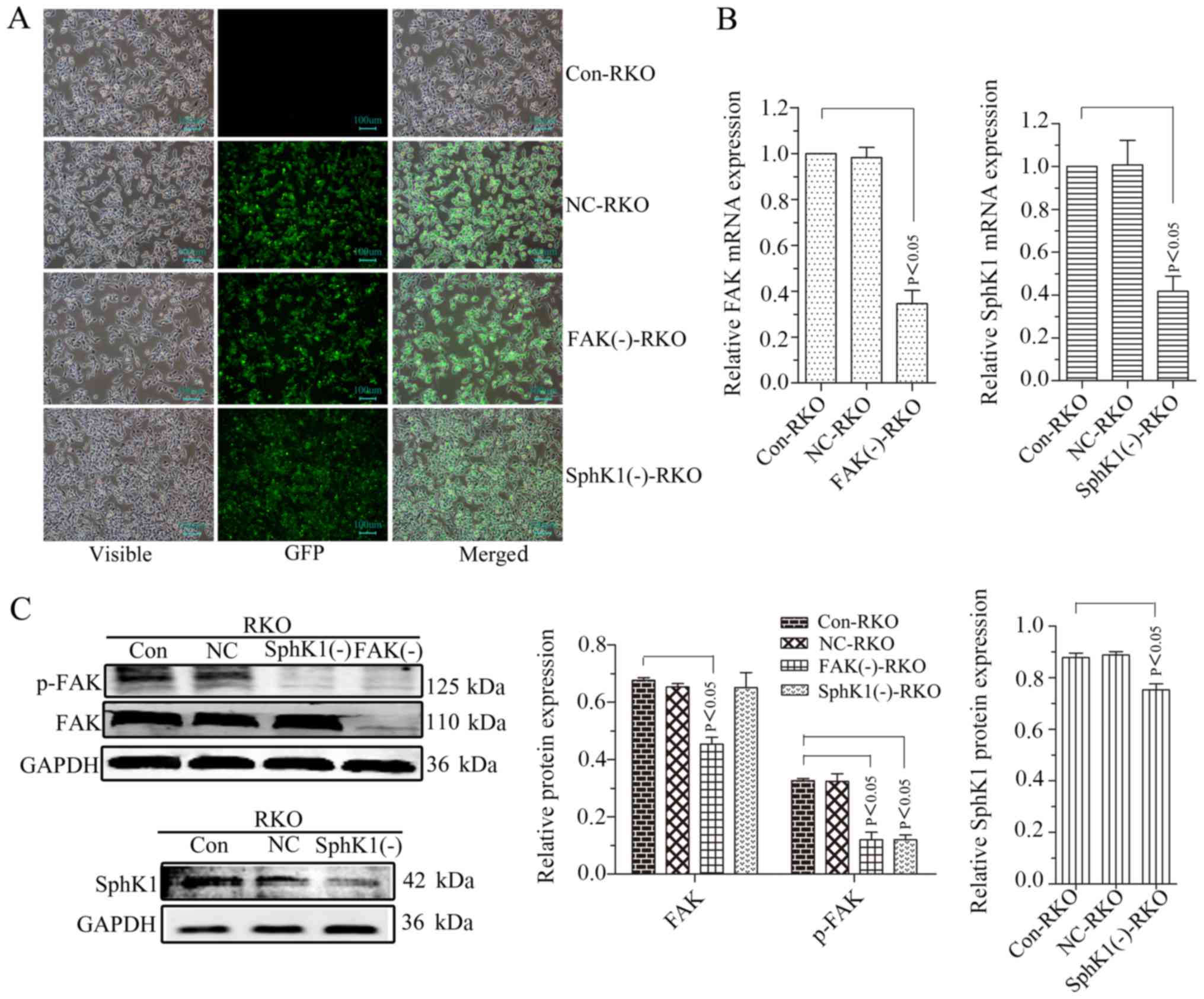
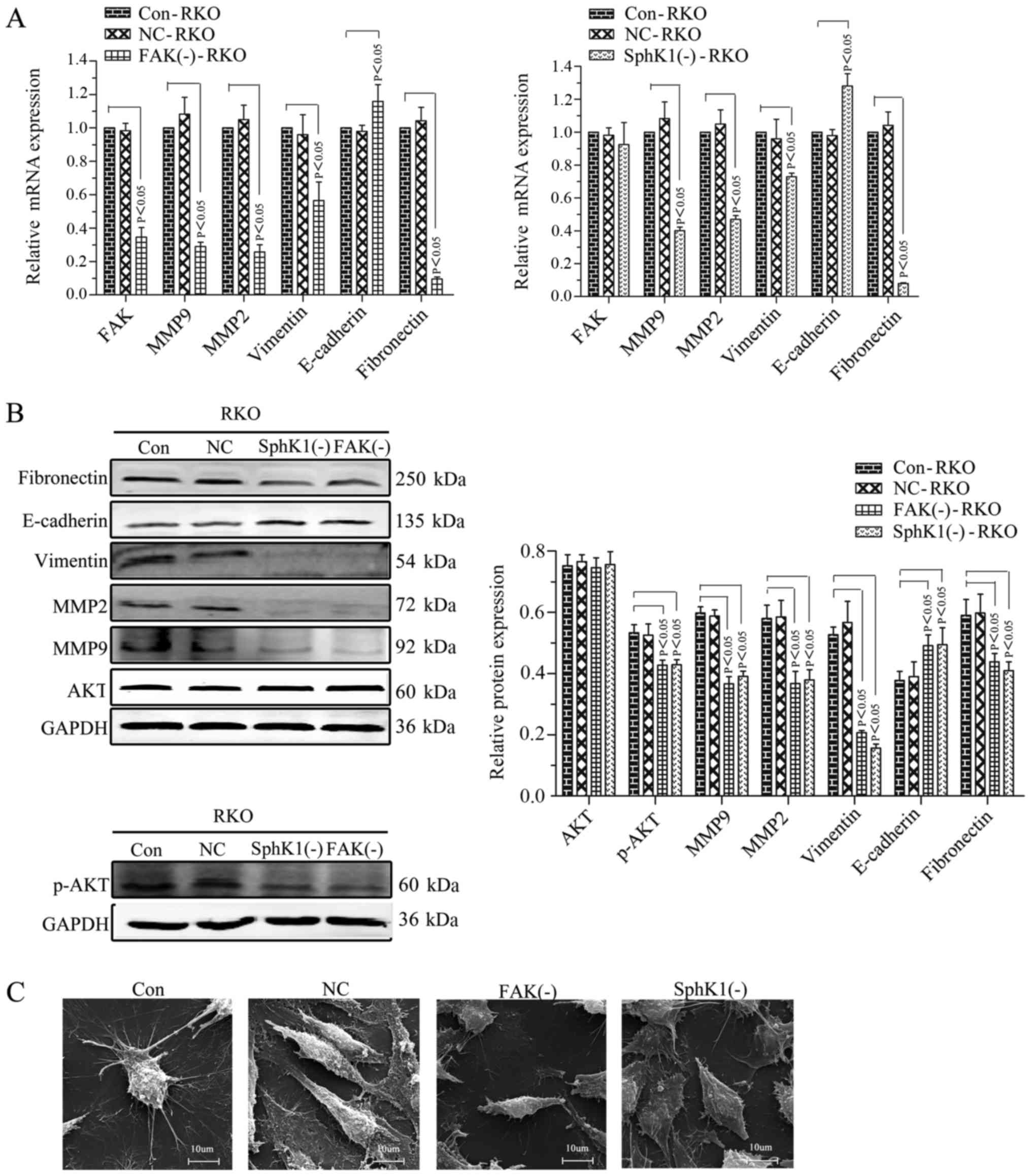
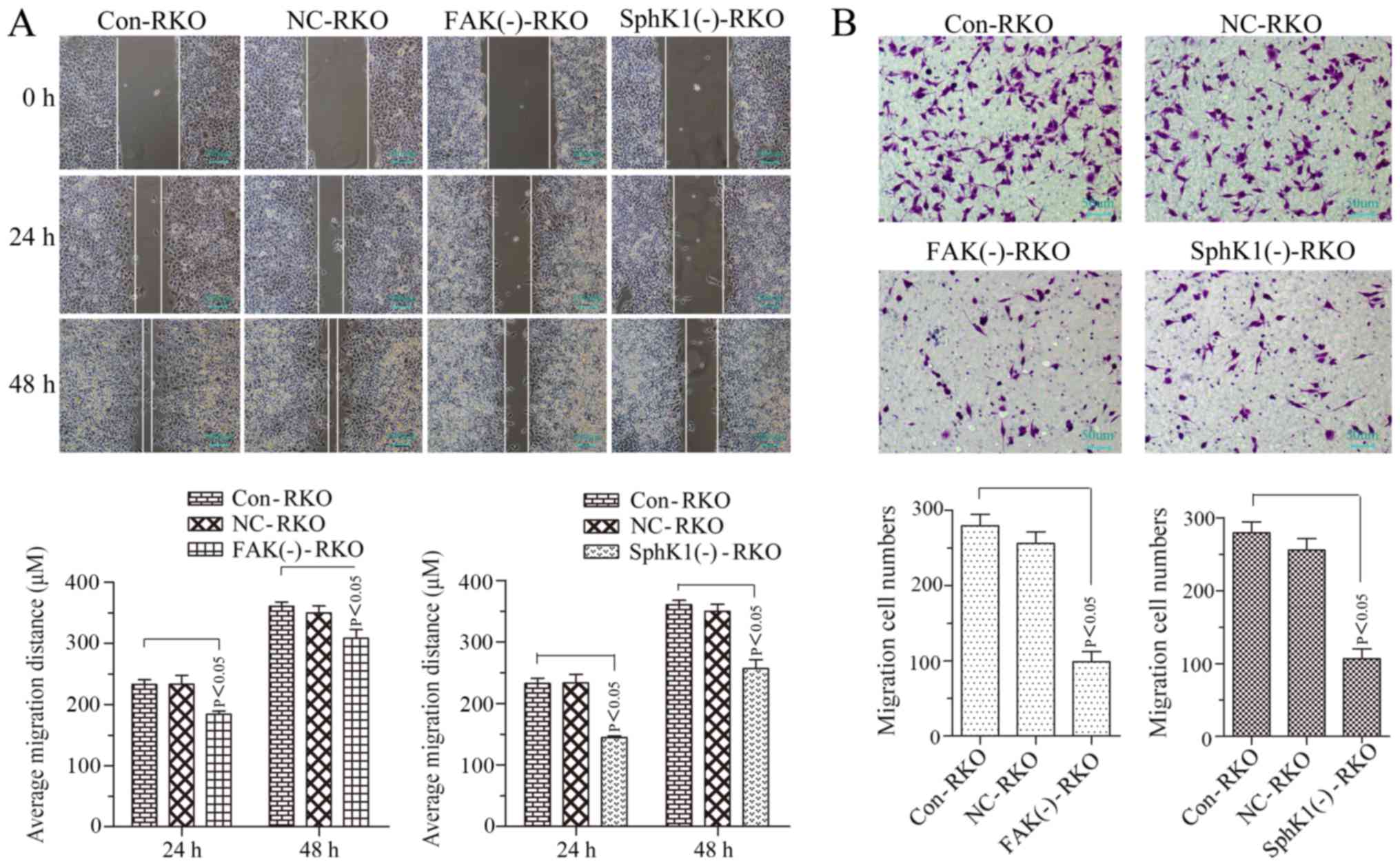
|
1
|
Jie D, Zhongmin Z, Guoqing L, Sheng L, Yi
Z, Jing W and Liang Z: Positive expression of LSD1 and negative
expression of E-cadherin correlate with metastasis and poor
prognosis of colon cancer. Dig Dis Sci. 58:1581–1589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Wu H, Li W, Yin L, Guo S, Xu X,
Ouyang Y, Zhao Z, Liu S, Tian Y, et al: Downregulated miR-506
expression facilitates pancreatic cancer progression and
chemoresistance via SPHK1/Akt/NF-KB signaling. Oncogene.
35:5501–5514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Long J, Xie Y, Yin J, Lu W and Fang S:
SphK1 promotes tumor cell migration and invasion in colorectal
cancer. Tumour Biol. 37:6831–6836. 2016. View Article : Google Scholar
|
|
4
|
Zheng XD, Zhang Y, Qi XW, Wang MH, Sun P,
Zhang Y and Jiang J: Role of Sphk1 in the malignant transformation
of breast epithelial cells and breast cancer progression. Indian J
Cancer. 51:524–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su YJ, Huang JA, Liu SQ, Huang JX, Zhong
YY, Tang GD and Jiang HX: The expression and clinical significance
of SphK1 and nuclear factor-KB p65 in human colon carcinoma.
Zhonghua Nei Ke Za Zhi. 51:220–224. 2012.PubMed/NCBI
|
|
6
|
Liu SQ, Su YJ, Qin MB, Mao YB, Huang JA
and Tang GD: Sphingosine kinase1 promotes tumor progression and
confers malignancy phenotypes of colon cancer by regulating the
focal adhesion kinase pathway and adhesion molecules. Int J Oncol.
42:617–626. 2013. View Article : Google Scholar
|
|
7
|
Cai G, Wu D, Wang Z, Xu Z, Wong KB, Ng CF,
Chan FL and Yu S: Collapsin response mediator protein-1 (CRMP1)
acts as an invasion and metastasis suppressor of prostate cancer
via its suppression of epithelial-mesenchymal transition and
remodeling of actin cytoskeleton organization. Oncogene.
36:546–558. 2017. View Article : Google Scholar
|
|
8
|
Leonel C, Borin TF, de Carvalho Ferreira
L, Moschetta MG, Bajgelman MC, Viloria-Petit AM and de Campos
Zuccari DA: Inhibition of epithelial-mesenchymal transition and
metastasis by combined TGFbeta knockdown and metformin treatment in
a canine mammary cancer xenograft model. J Mammary Gland Biol
Neoplasia. 22:27–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singla M, Kumar A, Bal A, Sarkar S and
Bhattacharyya S: Epithelial to mesenchymal transition induces stem
cell like phenotype in renal cell carcinoma cells. Cancer Cell Int.
18:572018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu CC, Cai DL, Sun F, Wu ZH, Yue B, Zhao
SL, Wu XS, Zhang M, Zhu XW, Peng ZH, et al: fermt1 mediates
epithelial-mesenchymal transition to promote colon cancer
metastasis via modulation of β-catenin transcriptional activity.
Oncogene. 36:1779–1792. 2017. View Article : Google Scholar
|
|
11
|
Harner-Foreman N, Vadakekolathu J,
Laversin SA, Mathieu MG, Reeder S, Pockley AG, Rees RC and Boocock
DJ: A novel spontaneous model of epithelial-mesenchymal transition
(EMT) using a primary prostate cancer derived cell line
demonstrating distinct stem-like characteristics. Sci Rep.
7:406332017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiu LY, Hsin IL, Yang TY, Sung WW, Chi
JY, Chang JT, Ko JL and Sheu GT: The ERK-ZEB1 pathway mediates
epithelial-mesenchymal transition in pemetrexed resistant lung
cancer cells with suppression by vinca alkaloids. Oncogene.
36:242–253. 2017. View Article : Google Scholar
|
|
13
|
Yang Y, Zhang J, Yan Y, Cai H, Li M, Sun
K, Wang J, Liu X, Wang J and Duan X: Low expression of Rap1GAP is
associated with epithelial-mesenchymal transition (EMT) and poor
prognosis in gastric cancer. Oncotarget. 8:8057–8068. 2017.
|
|
14
|
Lv QL, Chen SH, Zhang X, Sun B, Hu L, Qu
Q, Huang YT, Wang GH, Liu YL, Zhang YY, et al: Upregulation of long
noncoding RNA zinc finger antisense 1 enhances
epithelial-mesenchymal transition in vitro and predicts poor
prognosis in glioma. Tumour Biol. 39:10104283176950222017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ling HH, Kuo CC, Lin BX, Huang YH and Lin
CW: Elevation of YAP promotes the epithelial-mesenchymal transition
and tumor aggressiveness in colorectal cancer. Exp Cell Res.
350:218–225. 2017. View Article : Google Scholar
|
|
16
|
Taliaferro-Smith L, Oberlick E, Liu T,
McGlothen T, Alcaide T, Tobin R, Donnelly S, Commander R, Kline E,
Nagaraju GP, et al: FAK activation is required for IGFIR-mediated
regulation of EMT, migration, and invasion in mesenchymal triple
negative breast cancer cells. Oncotarget. 6:4757–4772. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang L, Huang Z, Fan Y, He L, Ye M, Shi
K, Ji B, Huang J, Wang Y and Li Q: FOXC1 promotes proliferation and
epithelial-mesenchymal transition in cervical carcinoma through the
PI3K-AKT signal pathway. Am J Transl Res. 9:1297–1306.
2017.PubMed/NCBI
|
|
18
|
Xu H, Li M, Zhou Y, Wang F, Li X, Wang L
and Fan Q: S100A4 participates in epithelial-mesenchymal transition
in breast cancer via targeting MMP2. Tumour Biol. 37:2925–2932.
2016. View Article : Google Scholar
|
|
19
|
Asuthkar S, Nalla AK, Gondi CS, Dinh DH,
Gujrati M, Mohanam S and Rao JS: Gadd45a sensitizes medulloblastoma
cells to irradiation and suppresses MMP-9-mediated EMT.
Neuro-oncol. 13:1059–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baquero P, Jiménez-Mora E, Santos A, Lasa
M and Chiloeches A: TGFβ induces epithelial-mesenchymal transition
of thyroid cancer cells by both the BRAF/MEK/ERK and Src/FAK
pathways. Mol Carcinog. 55:1639–1654. 2016. View Article : Google Scholar
|
|
21
|
Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu
L, Tang X, Du YE, Hu P and Liu M: Twist induces
epithelial-mesenchymal transition and cell motility in breast
cancer via ITGB1-FAK/ILK signaling axis and its associated
downstream network. Int J Biochem Cell Biol. 71:62–71. 2016.
View Article : Google Scholar
|
|
22
|
Xue X, Wang X, Liu Y, Teng G, Wang Y, Zang
X, Wang K, Zhang J, Xu Y, Wang J, et al: SchA-p85-FAK complex
dictates isoform-specific activation of Akt2 and subsequent
PCBPl-mediated post-transcriptional regulation of TGFβ-mediated
epithelial to mesenchymal transition in human lung cancer cell line
A549. Tumour Biol. 35:7853–7859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv C, Yang S, Chen X and Zhu X:
MicroRNA-21 promotes bone mesenchymal stem cells migration in vitro
by activating PI3K/Akt/MMPs pathway. J Clin Neurosci. 46:156–162.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin M and Liu S, Li A, Xu C, Tan L, Huang
J and Liu S: NIK- and IKKβ-binding protein promotes colon cancer
metastasis by activating the classical NF-KB pathway and MMPs.
Tumour Biol. 37:5979–5990. 2016. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
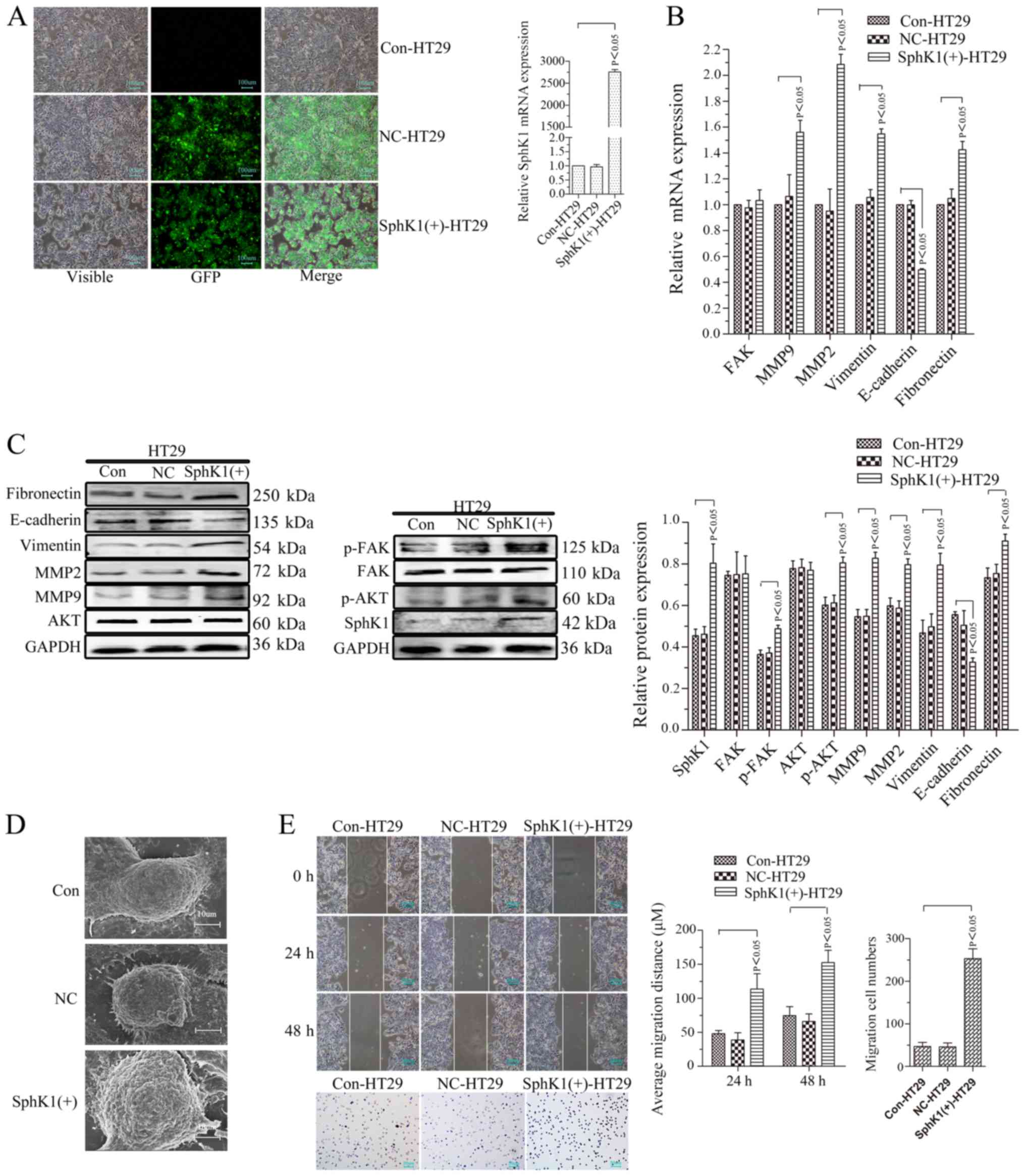
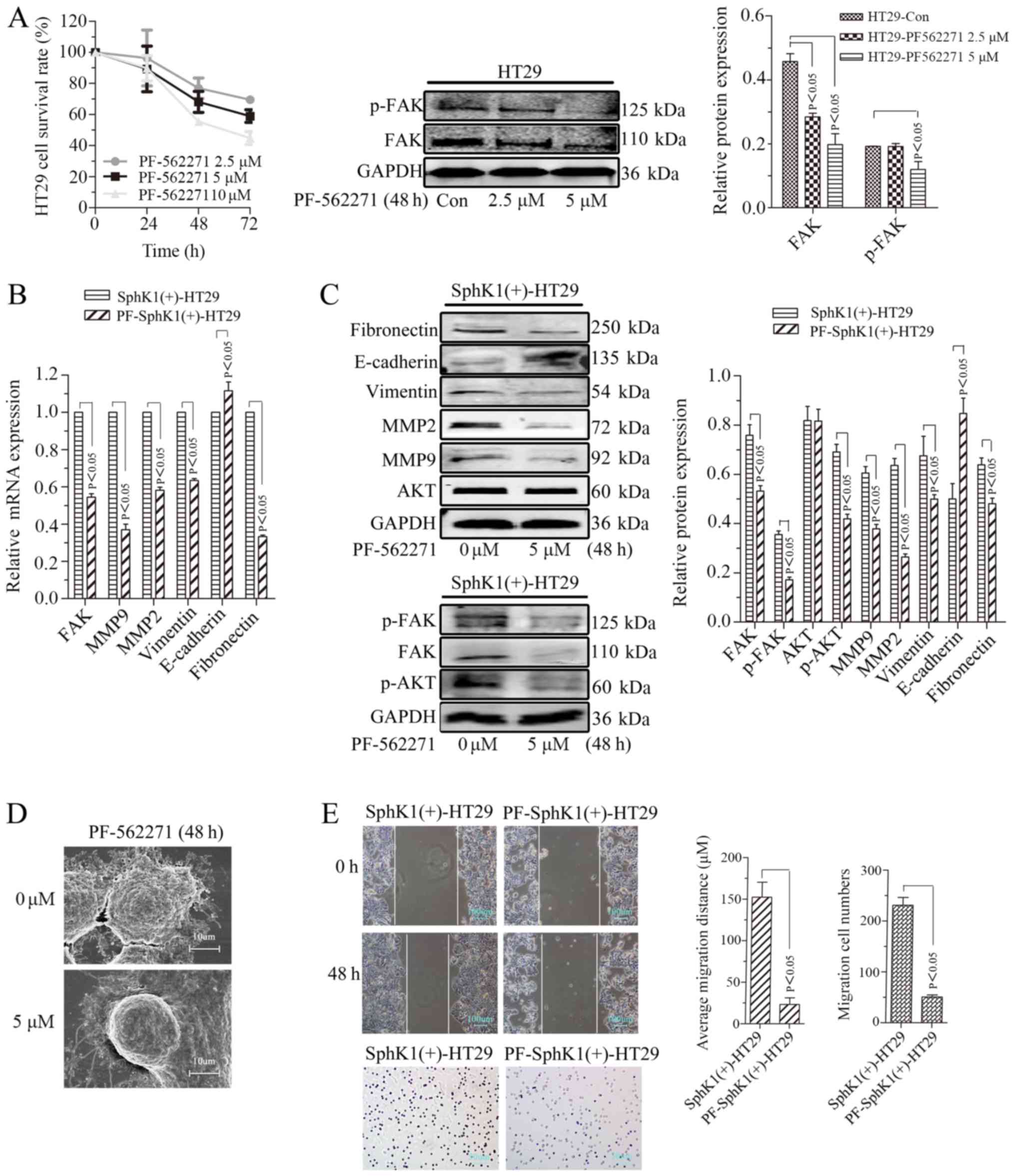
Xu CY, Liu SQ, Qin MB, Zhuge CF, Qin L,
Qin N, Lai MY and Huang JA: SphK1 modulates cell migration and
EMT-related marker expression by regulating the expression of p-FAK
in colorectal cancer cells. Int J Mol Med. 39:1277–1284. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S, Zhou Y, Zheng X, Wu X, Liang Y, Wang
S and Zhang Y: Sphk1 promotes breast epithelial cell proliferation
via NF-K B-p65-mediated cyclin D1 expression. Oncotarget.
7:80579–80585. 2016.PubMed/NCBI
|
|
28
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu K, Li MM, Shen J, Liu F, Cao JY, Jin S
and Yu Y: Interleukin-17-induced EMT promotes lung cancer cell
migration and invasion via NF-KB/ZEB1 signal pathway. Am J Cancer
Res. 5:1169–1179. 2015.
|
|
30
|
Dai S, Zhang J, Huang S, Lou B, Fang B, Ye
T, Huang X, Chen B and Zhou M: HNRNPA2B1 regulates the
epithelial-mesenchymal transition in pancreatic cancer cells
through the ERK/snail signalling pathway. Cancer Cell Int. 17(12):
2017
|
|
31
|
Park J and Schwarzbauer JE: Mammary
epithelial cell interactions with fibronectin stimulate
epithelial-mesenchymal transition. Oncogene. 33:1649–1657. 2014.
View Article : Google Scholar :
|
|
32
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer–observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar
|
|
33
|
Kessler BE, Sharma V, Zhou Q, Jing X, Pike
LA, Kerege AA, Sams SB and Schweppe RE: FAK expression, not kinase
activity, is a key mediator of thyroid tumorigenesis and
protumorigenic processes. Mol Cancer Res. 14:869–882. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee BY, Timpson P, Horvath LG and Daly RJ:
FAK signaling in human cancer as a target for therapeutics.
Pharmacol Ther. 146:132–149. 2015. View Article : Google Scholar
|
|
35
|
Zhu L, Wang Z, Lin Y, Chen Z, Liu H, Chen
Y, Wang N and Song X: Sphingosine kinase 1 enhances the invasion
and migration of non-small cell lung cancer cells via the AKT
pathway. Oncol Rep. 33:1257–1263. 2015. View Article : Google Scholar
|
|
36
|
Bugide S, Gonugunta VK, Penugurti V,
Malisetty VL, Vadlamudi RK and Manavathi B: HPIP promotes
epithelial-mesenchymal transition and cisplatin resistance in
ovarian cancer cells through PI3K/AKT pathway activation. Cell
Oncol (Dordr). 40:133–144. 2017. View Article : Google Scholar
|
|
37
|
Fu QF, Liu Y, Fan Y, Hua SN, Qu HY, Dong
SW, Li RL, Zhao MY, Zhen Y, Yu XL, et al: Alpha-enolase promotes
cell glycolysis, growth, migration, and invasion in non-small cell
lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol.
8:222015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Y, Qiu S, Qian L, Tian Y, Chen Y, Bi
L and Chen W: OCF can repress tumor metastasis by inhibiting
epithelial-mesenchymal transition involved in PTEN/PI3K/AKT pathway
in lung cancer cells. PLoS One. 12:e01740212017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu SQ, Huang JA, Qin MB, Su YJ, Lai MY,
Jiang HX and Tang GD: Sphingosine kinase 1 enhances colon cancer
cell proliferation and invasion by upregulating the production of
MMP-2/9 and uPA via MAPK pathways. Int J Colorectal Dis.
27:1569–1578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su Y, Gao L, Teng L, Wang Y, Cui J, Peng S
and Fu S: Id1 enhances human ovarian cancer endothelial progenitor
cell angiogenesis via PI3K/Akt and NF-kB/MMP-2 signaling pathways.
J Transl Med. 11:1322013. View Article : Google Scholar
|
|
41
|
Yuan H, Yang P, Zhou D, Gao W, Qiu Z, Fang
F, Ding S and Xiao W: Knockdown of sphingosine kinase 1 inhibits
the migration and invasion of human rheumatoid arthritis
fibroblast-like synoviocytes by downregulating the PI3K/AKT
activation and MMP-2/9 production in vitro. Mol Biol Rep.
41:5157–5165. 2014. View Article : Google Scholar : PubMed/NCBI
|