Introduction
Pancreatic cancer is an intractable disease with a
5-year survival rate of <5% (1). Additionally, even if pancreatic
cancer tumors are identified at an early stage without symptoms,
the prognosis of the patients and recurrence of the tumors tend to
be worse and higher, respectively, than other cancers (2). Surgical resection is considered in
15-20% of patients due to late-stage diagnosis (3). Therefore, chemotherapy and radiation
therapy are treatment modalities used for patients with pancreatic
cancer who cannot undergo surgical resection (4). However, these therapeutic strategies
do not fully improve the survival rate and prognosis in pancreatic
patients (2). In spite of its
limitations, radiotherapy is still being used for the treatment of
patients with pancreatic cancer (1).
In recent years, high-energy radiation therapies
using X-rays, gamma rays and proton beams are being used to improve
the therapeutic efficiency of various cancer patients (5). Among these, X-ray radiation therapy
has been demonstrated to be the most common tool to treat cancer
patients (5). However, physical
and molecular biological damage in normal adjacent tissues close to
tumor tissues are further induced by X-ray radiation (6,7).
Therefore, X-ray radiation may not be a useful therapeutic tool to
treat pancreatic cancer with high dose radiation. In contrast,
proton beam (PB) therapy is a newly proposed radiation therapeutic
tool that can irradiate the target tumors with high-dose energy,
minimizing injuries to normal adjacent tissues (8). Clinically, PB therapy has been used
to treat intractable cancers, including brain, eye, neck and liver
cancers (9,10).
During radiation therapy, cell death occurs in tumor
tissues via cell cycle arrest induced by radiation-mediated DNA
damage (11). However, cell death
is determined by radiosensitivity, which depends on the type of
cancer and cancer cell (12).
Radiosensitivity is an important measurement in radiation therapy
to determine treatment efficiency (13). However, to date, there is no
diagnostic tool for evaluating radiosensitivity.
Several studies have revealed that the expression of
DNA repair protein RAD51 homolog 1 (RAD51), a DNA repair protein,
and survivin, a member of protein family responsible for the
inhibition of apoptosis, affect radioresistance (14,15).
RAD51 is a key protein that repairs double-stranded DNA damage;
increased RAD51 expression has been observed in various cancers
(16). A number of studies have
suggested that RAD51 is a potent target for cancer therapy,
demonstrating that radiation- and chemo-sensitivities were induced
by the inhibition of RAD51 expression (16-21).
Survivin serves a role in regulating cell cycle, cell protection
and cell division, inhibiting apoptosis by blocking caspase
(22,23), and has been reported as a
radioresistance factor in pancreatic cancer (24). In addition, several investigations
have suggested that survivin may be a potent target for cancer
treatment due to its overexpression in various human tumors
(25,26).
In the current study, the effects of PB irradiation
were observed on the cell survival of two human pancreatic cell
lines: Capan-1, which has been demonstrated to express high levels
of cyclooxygenase (COX)-2, and Panc-1, which has been revealed to
express low levels of COX-2 (27).
Several investigations determined an association between
radiosensitivity and COX-2 expression (28,29).
Therefore, the effects of PB on cell survival were assessed in the
two pancreatic cancer cell lines to confirm whether COX-2
expression level correlated with radiosensitivity. Additionally,
the regulation of survivin and RAD51 expression by PB therapy was
investigated, as they are factors that influence
radioresistance.
Materials and methods
Cell culture
Human pancreatic cancer cell lines, Capan-1 and
Panc-1, were purchased from the Korean Cell Line Bank (Seoul,
Korea). Capan-1 and Panc-1 cells were cultured in Roswell Park
Memorial Institute-1640 medium and Dulbecco's modified Eagle's
medium supplemented with 1% antimycotic/antibiotic solution (all
Welgene, Inc., Gyeongsan, Korea) and 10% heat-inactivated fetal
bovine serum (American Type Culture Collection, Manassas, VA, USA)
at 37°C in a 5% CO2 atmosphere.
PB irradiation
PB irradiation was performed using a 100 MeV proton
accelerator in Korea Multi-Purpose Accelerator Complex at Korean
Atomic Energy Research Institute (Gyeongju, Korea). The cells were
irradiated with PB at a dose of 2, 4, 8 or 16 Gy with Bragg peaks
width of 6 cm (30).
Cell cytotoxicity assay
A total of 5,000 cells/well were seeded onto 96-well
plates. The cells were further cultured for 72 h at 37°C after PB
irradiation. Then, 20 µl of 5 mg/ml MTT reagent (Sigma
Aldrich; Merck KGaA, Darmstadt, Germany) was added in each well and
further incubated at 37°C for 4 h to generate formazan. Insoluble
formazan was dissolved with dimethyl sulfate (Duksan Pure Chemicals
Co., Ltd., Ansan, Korea) and was colorimetrically analyzed by
measuring optical density at 540 nm using Spectramax M2 (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) and RT-
quantitative (q)PCR
The cells were irradiated with a PB and cultured for
an additional 72 h. Then, the cells were trypsinized and collected
by centrifugation at room temperature for 3 min with 1,000 × g.
Total RNA extraction was performed with the easy-BLUE™ Total RNA
extraction kit (iNtRON Biotechnology Inc., Sungnam, Korea)
according to the manufacturer's protocol. Total RNA (1 µg)
was used for cDNA synthesis using dNTP (final concentration, 0.5
mM), Goscript™ Reverse Transcriptase (both Promega Corporation,
Madison, WI, USA) and Random Primer pd(N)9 (Takara Bio, Inc., Otsu,
Japan). The reaction was performed in 1X Goscript reaction buffer
containing 2 mM MgCl2 (Promega Corporation).
Amplification of synthesized cDNA was performed as previously
described (31). The primer
sequences for target genes were as follows: COX-2, forward
5'-TTCACGCATCAGTTTTTCAA-'3 and reverse 5'-ACAGCAAACCGTAGATGCTC-3'
(32); survivin, forward
5'-ATTTGAATCGCGGGACCCGTTG-3' and reverse
5'-TGGCTCGTTCTCAGTGGGGCAGT-3' (33); GAPDH, forward
5'-ATCCCATCACCATCTTCCAG-3' and reverse 5'-TTCTAGACGGCAGGTCAGGT-3'
(34). All PCR primers were
synthesized from Bioneer Corporation (Daejeon, Korea). For
semi-quantitative RT-PCR analysis, PCR amplicons were synthesized
with Dream Taq polymerase (Promega Corporation) and the
thermocycling conditions were as follows: For COX-2, pre-heating
for 10 min at 95°C, 35 cycles of 95°C for 30 sec, 60°C for 30 sec
and 72°C for 45 sec, and final extension for 10 min at 72°C; for
GAPDH, pre-heating for 10 min at 95°C, 25 cycles of 95°C for 30
sec, 60°C for 30 sec and 72°C for 30 sec, and final extension for
10 min at 72°C. The amplicons were then electrophoresed on 1%
agarose gels containing EtBr (Amresco, LLC, Solon, OH, USA) and the
bands were visualized with a UV transilluminator. Bands densities
were analyzed with Scion Image software (Alpha 4.0.3.2; Scion
Corporation, Frederick, MD, USA). qPCR analyses were performed with
Q Green Sybr Green Master Mix Kit (Cellsafe Co., Ltd., Yongin,
Korea) using Eco™ Real-Time PCR (Illumina, Inc., San Diego, CA,
USA). The thermocycling conditions were as follows: pre-heating for
5 min at 95°C, 45 cycles of 95°C for 10 sec, 60°C for 15 sec and
72°C for 20 sec. Relative mRNA expression was automatically
determined using Eco™ software v3.1.7 (Illumina, Inc.) via the
2-ΔΔCq method (35).
GAPDH was used as the internal control.
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay
lysis buffer (Biosesang, Seongnam, Korea) containing protease
inhibitor cocktail and phosphatase inhibitor cocktail (GenDEPOT,
LLC, Barker, TX, USA). Whole cell lysates were centrifuged at
13,000 × g for 20 min at 4°C and stored at −80°C until to use. The
protein concentration was measured with the bicinchoninic acid
method. Total protein (20 µg/lane) was subjected to SDS-PAGE
on 12 or 15% gel and transferred to polyvinylidenefluoride (PVDF)
membrane (Pall Life Science, Port Washington, NY, USA). The
membranes were blocked with 5% non-fat dry milk in Tris-buffered
saline (TBS)-Tween (50 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20) for
1 h at room temperature. The primary antibodies were diluted at
1:3,000 in 5% non-fat dry milk or 1% bovine serum albumin (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) in TBS-Tween solution
and were probed for overnight at 4°C onto PVDF membrane. Then,
secondary antibodies were diluted at 1:5,000 in TBS and the
reaction was performed at room temperature for 1 h. The bands for
target proteins were visualized with handmade chemiluminascent
substrate [100 mM Tris (pH 8.5; BioShop Canada, Inc., Burlington,
ON, Canada), 1.25 mM luminol, 198 µM coumaric acid and 0.01%
hydrogen peroxide (all Sigma Aldrich; Merck KGaA)] and photographed
using Luminescent Image Analyzer LAS-4000 (Fujifilm Corporation,
Tokyo, Japan). The bands were densitometrically analyzed with Scion
Image software (Alpha 4.0.3.2). The primary antibodies against
RAD51 (cat. no. sc-8349) and β-actin (cat. no. sc-69879) were
purchased from Santa Cruz Biotechnology, Inc., and antibodies for
histone 2AX (H2A.X; cat. no. 7631), phospho-H2A.X (cat. no. 9718),
cyclin-dependent kinase inhibitor 1 (p21; cat. no. 2947), poly
(ADP-ribose) polymerase (PARP; cat. no. 9542), caspase-7 (cat. no.
9492), caspase-3 (cat. no. 9662), signal transducer and activator
of transcription 3 (STAT3; cat. no. 4904), phospho-STAT3 (cat. no.
9145) and survivin (cat. no. 2808) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. NCI1460KR) and
anti-mouse (cat. no. sc-2005) immunoglobulin G antibodies were
bought from Thermo Scientific Fisher Scientific, Inc. and Santa
Cruz Biotechnology, Inc., respectively.
Statistical analysis
Statistical significance was determined using the
Student's t-test or a one-way analysis of variance followed by the
Tukey's post hoc test. Statistical analyses were conducted using
SPSS V20.0 software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. P<0.05 indicated
that the difference between groups was statistically
significant.
Results
Cell viability decreases in Capan-1 cells
following PB irradiation
The inhibition of pancreatic cancer cell growth by a
COX-2 inhibitor, celecoxib, was demonstrated in a previous
investigation (36). Shin et
al (29) demonstrated that
celecoxib treatment enhanced radiosensitivity in various cancer
cells, suggesting that COX-2 expression is closely associated with
radiosensitivity. Therefore, the change of cell viability by PB
irradiation was assessed in the two pancreatic cancer cells,
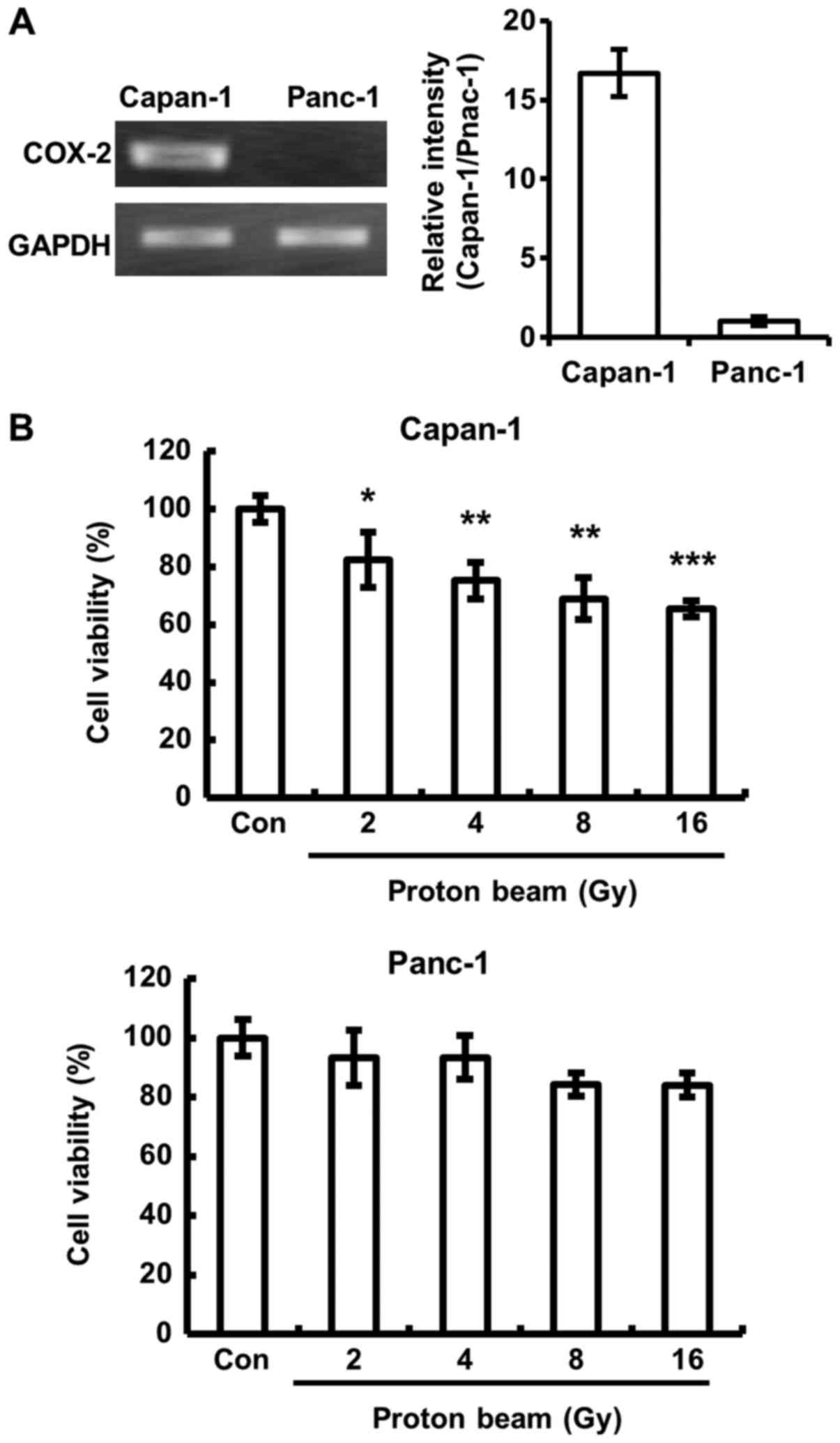
Capan-1 cells, which expressed high levels of COX-2 mRNA, and
Panc-1 cells, which expressed low level of COX-2 mRNA (Fig. 1A). Unexpectedly, it was
demonstrated that there was a significantly lower cell viability
following PB irradiation in Capan-1 cells compared with control
cells (Fig. 1B). No significant
differences were identified in the cell viability of Panc-1
cells.
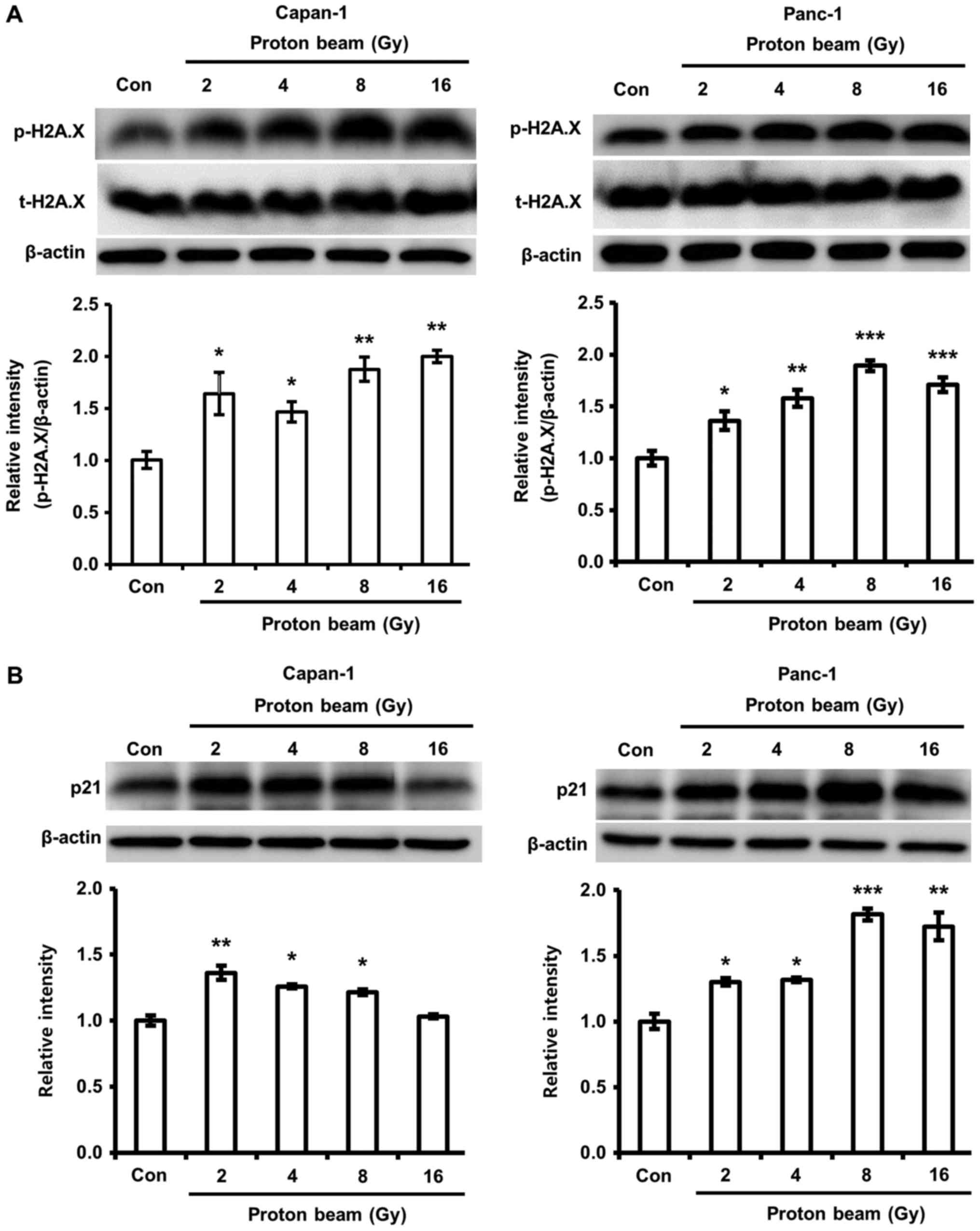
PB irradiation increases the expression
of phosphorylation of H2A.X and the expression of p21
Cell death and cell cycle arrest by PB irradiation
have been determined to be closely linked with DNA damage in
various cancer cells (37). As
shown in Fig. 1B, higher
cytotoxicity following PB was observed in Capan-1, but not Panc-1
cells. Therefore, the association between cytotoxicity and DNA
damage was examined through the change of phosphorylation of H2A
histone family, member X (H2A.X), a histone protein phosphorylated
by DNA damage. The phosphorylation of H2A.X in Capan-1 and Panc-1
cells was significantly increased by PB irradiation compared with
control cells (Fig. 2A). This
result suggests that DNA damage by PB irradiation increased with
dosage in Capan-1 and Panc-1 cells. Additionally, the effect of PB
irradiation on cell cycle arrest was investigated. p21 protein
expression levels in the two cells were increased by PB compared
with control cells, indicating the occurrence of cell cycle arrest
(Fig. 2B). However, 16 Gy of PB
irradiation did not enhance p21 protein expression in Capan-1
cells.
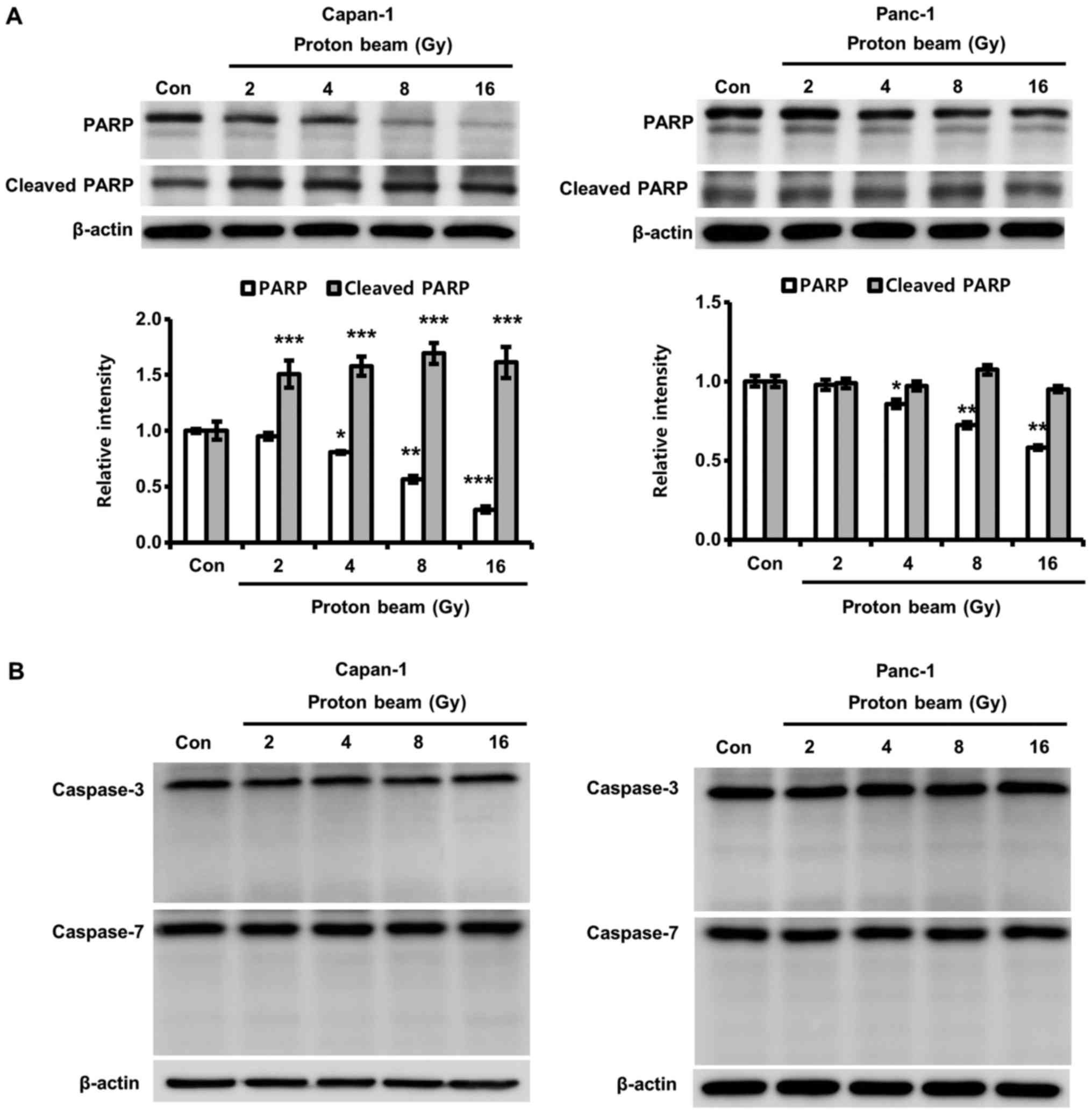
PB induces PARP cleavage in a
caspase-3-independent manner
PARP serves an important role in the repair of
damaged DNA caused by a variety of cellular stresses (38). Also, cleaved PARP (~89 kDa) is
known as a marker for apoptotic cell death (39). Therefore, PARP cleavage by PB
irradiation was explored to determine whether Capan-1 cell death by
PB was due to apoptosis. Cleaved PARP significantly increased with
PB irradiation, while the intact form of PARP was significantly
decreased with PB irradiation in Capan-1 cells compared with
control cells (Fig. 3A). However,
in spite of significant decreases in PARP due to PB irradiation in
Panc-1 cells compared with control cells, PARP cleavage was not
detected in Panc-1 cells. Cleaved PARP is primarily produced by
caspase-3 (40). Therefore, the
authors of the current study postulated that the decrease of PARP
in Panc-1 cells is caused by the down-regulation of
caspase-3-independent cleavage. Based the results, the effects of
PB irradiation on caspase-3 activation was investigated to confirm
whether the cleavage of PARP was mediated in a caspase-3-dependent
manner. As shown in Fig. 3B, the
cleaved forms of caspase-3 and -7 were not detected in either cell
line.
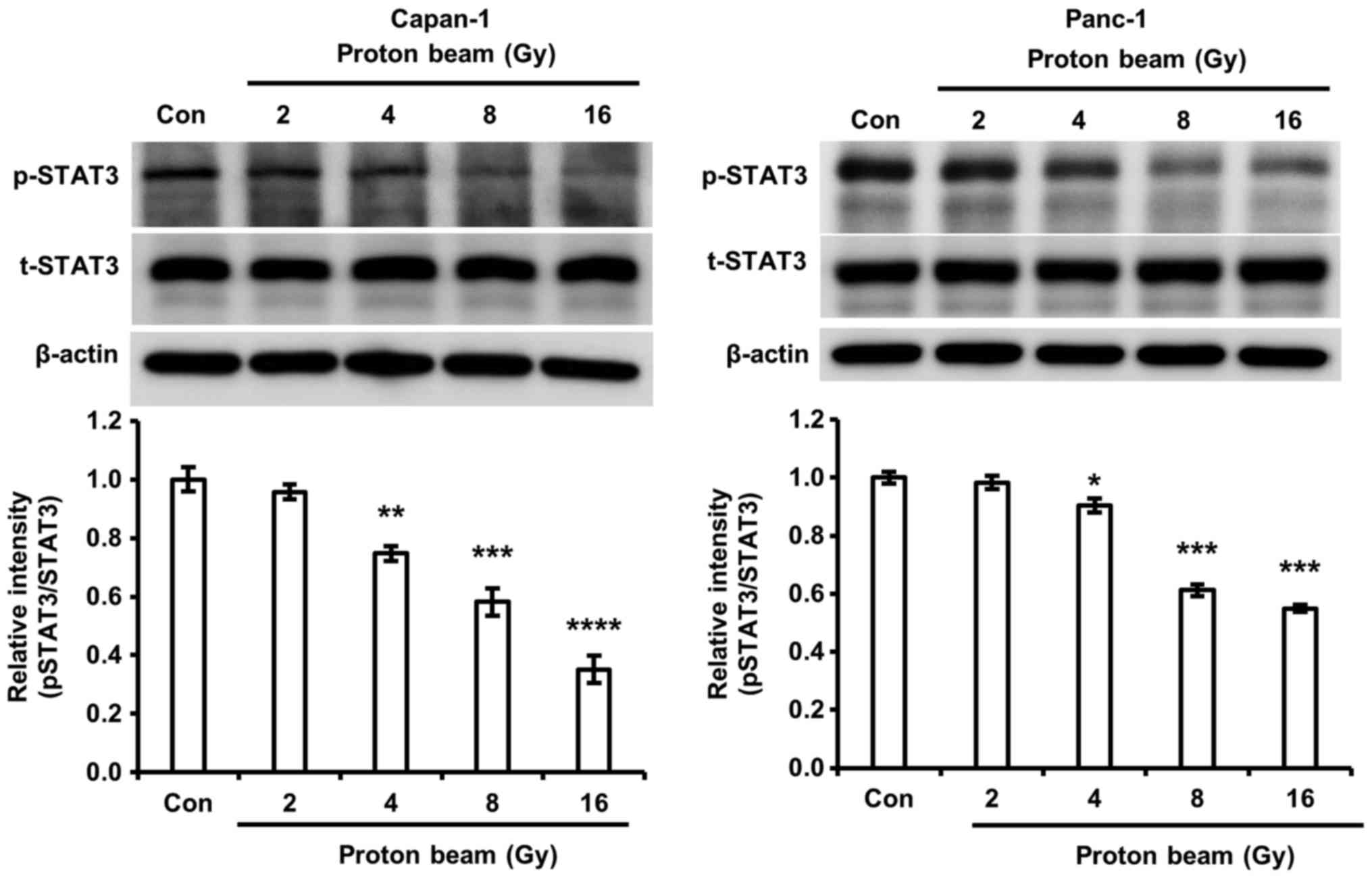
PB irradiation decreases STAT3
phosphorylation
The importance of STAT3 on cell cycle arrest and
apoptosis in chemotherapy and radiotherapy was reported by previous
investigations (41,42). Li et al (43) revealed that the down-regulation of
STAT3 by short hairpin RNA led to an increase of radiosensitivity.
Furthermore, the enhancement of apoptosis and cell cycle arrest by
the inhibition of STAT3 signaling was demonstrated in colorectal
cancer cells (42). Therefore, the
authors of the current study postulated that STAT3 signaling may be
associated with cell cycle arrest and/or cell death in Capan-1 and
Panc-1 cells. Phosphorylated STAT3 was significantly reduced by PB
irradiation in Capan-1 and Panc-1 cells compared with control cells
(Fig. 4). The decrease of
phosphorylated STAT3 by PB irradiation corresponded with an
increase of H2A.X phosphorylation and p21 expression in the Capan-1
and Panc-1 cells.
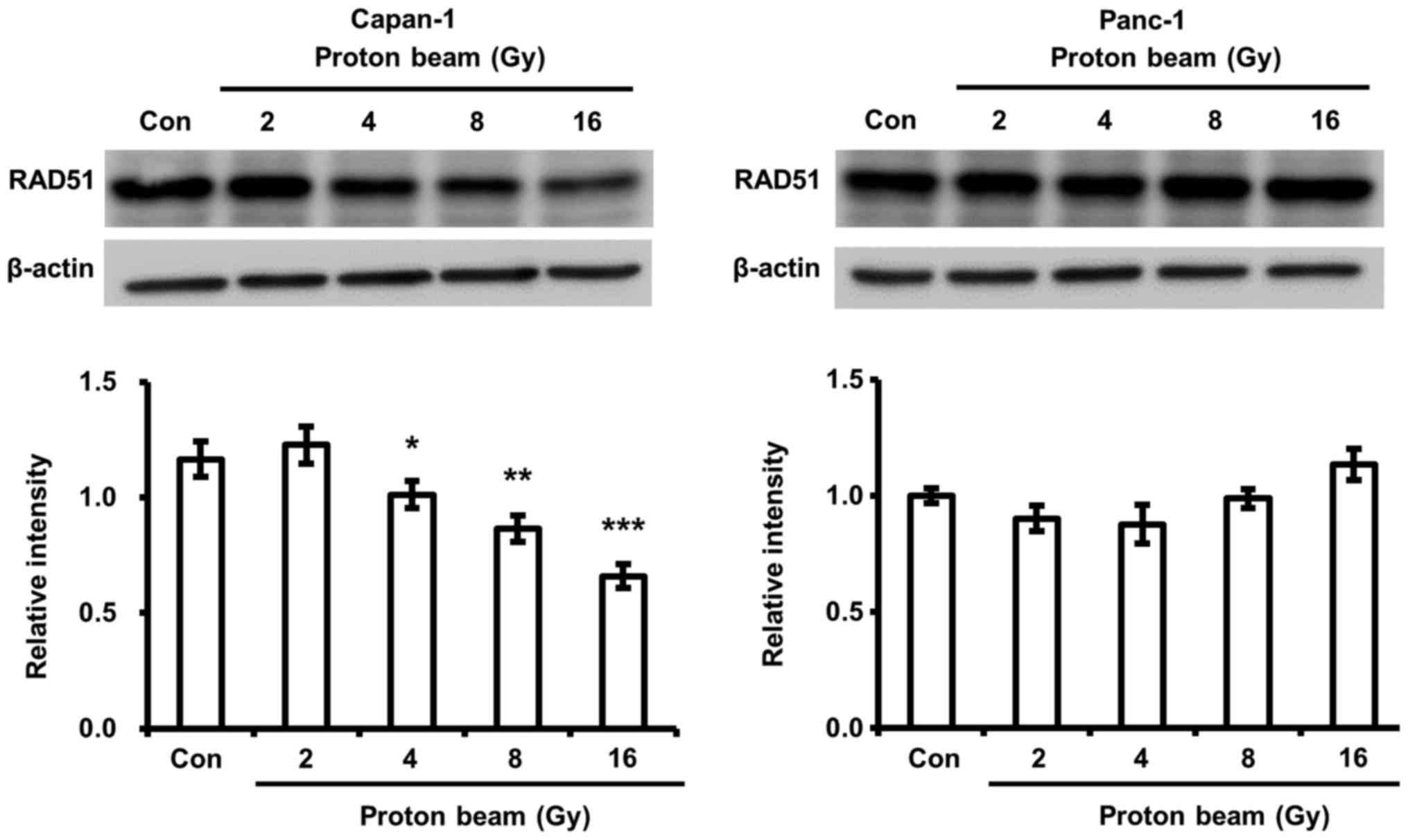
PB irradiation decreases RAD51 expression
in Capan-1 cells
The fate of DNA-damaged cancer cells by chemotherapy
and radiotherapy is determined by whether DNA damage is repaired
(44,45). Therefore, the activities of DNA
repair enzymes have been demonstrated to be closely associated with
cell death and cell cycle arrest (45). RAD51, a homologous recombination
repair enzyme, serves an important role in the repair of
radiation-induced DNA damage and has been implicated as a
radiosensitivity determinant (46). The change in RAD51 expression by PB
was surveyed. It was demonstrated that a significant decrease of
RAD51 protein expression by PB irradiation was identified in
Capan-1 cells compared with control cells, but not in Panc-1 cells
(Fig. 5). This finding corresponds
to the change of cell viability by PB irradiation in Capan-1 and
Panc-1 cells.
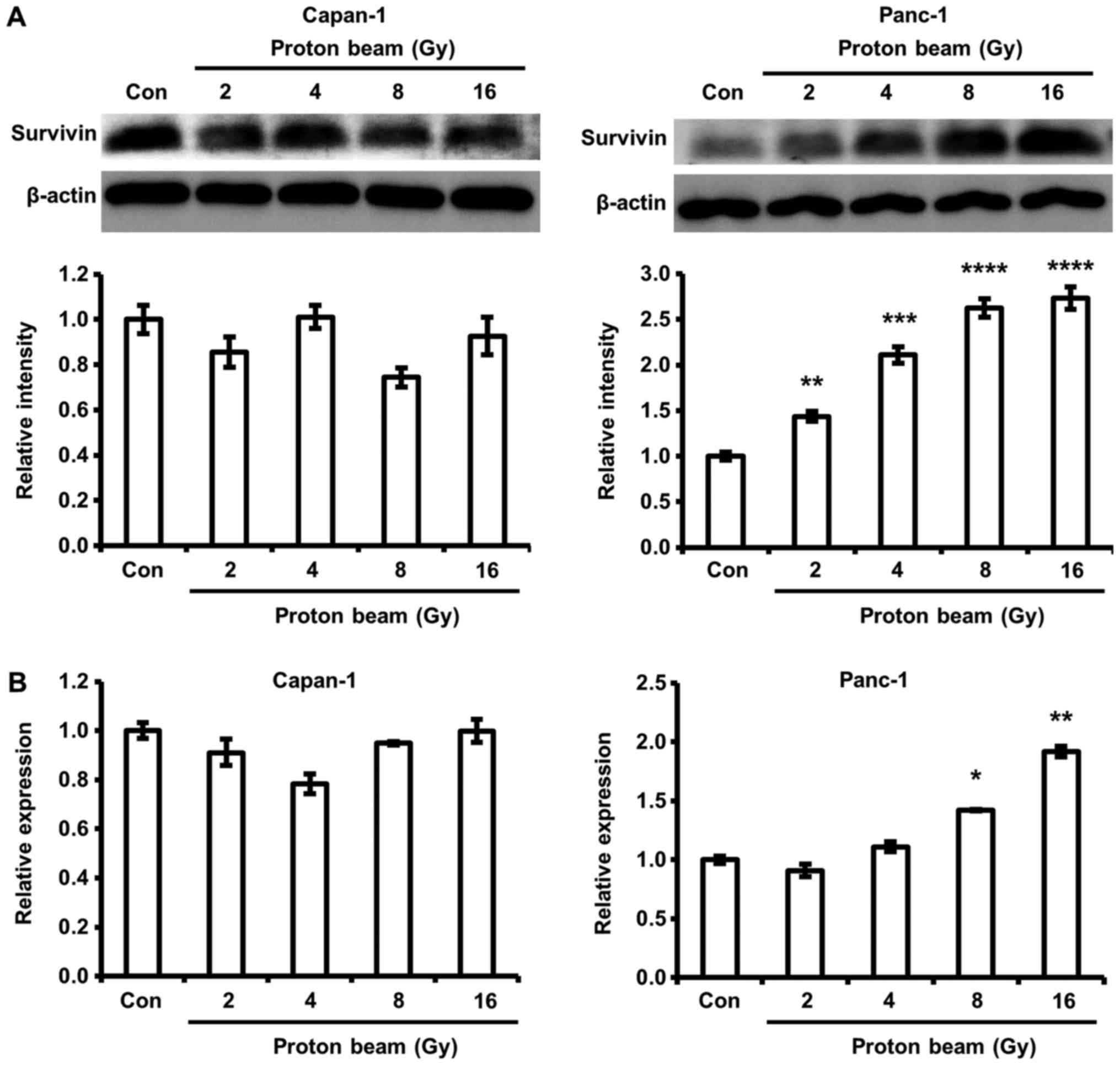
PB irradiation increases survivin protein
and mRNA expression in Panc-1 cells
A variety of survival factors participate in
determining whether cells die or survive from chemotherapy and
radiotherapy. Survivin is one of the survival factors that has been
considered as a determinant of cell sensitivity to radiation in
pancreatic cancer cells (27).
Therefore, change in survivin expression by PB irradiation was
investigated in Capan-1 and Panc-1 cells. PB significantly
increased survivin gene and protein expression in Panc-1 cells
compared with control cells (Fig.
6). However, the survivin gene and protein in Capan-1 cells
were not significantly increased by PB irradiation.
Discussion
Previous studies suggested that the
radiosensitivities of several cancer cells were enhanced by
celecoxib, a selective COX-2 inhibitor (28,29).
Additionally, several experiments demonstrated that COX-2
expression levels were associated with radioresistance in various
cancer cells (47-49). These results imply that COX-2 may
act as a determinant of radiosensitivity for cancer cells. The
present investigation revealed that Capan-1 cells were more
sensitive to PB irradiation than Panc-1 cells within 72 h. Also,
the expression level of COX-2 in Capan-1 cells was higher than that
of Panc-1 cells. Consequentially, this implies that the COX-2
expression level was not associated with radiosensitivity to PB
irradiation. Therefore, the present investigation demonstrated, at
least to some degree, that COX-2 may not act as a determinant of
radiosensitivity for PB irradiation in Capan-1 and Panc-1 human
pancreatic cancer cells in the first 72 h after irradiation.
As mentioned earlier, STAT3 signaling has been
revealed to be associated with radiosensitivity, cell cycle arrest
and apoptosis induced by anti-cancer drugs and radiation (41-43).
The current study determined that an increase in H2A.X
phosphorylation and a decrease of STAT3 phosphorylation occurred
simultaneously when the cells were irradiated with a PB. Chen et
al (50) revealed that the
inhibition of phosphorylated STAT3 was linked with an increase of
H2A.X phosphorylation, indicating DNA damage. Furthermore, Wen
et al revealed that apoptosis was enhanced with the
phosphorylation of H2A.X by mammalian STE20-like kinase 1 (51). These findings indicate that
apoptotic cell death induced by DNA damage was increase by an
induction of H2A.X phosphorylation, suggesting that the cell death
of Capan-1 cells by PB irradiation is regulated by STAT3-mediated
H2A.X phosphorylation. However, the regulation of STAT3-mediated
H2A.X phosphorylation did not affect the viability of Panc-1 cells
in the first 72 h after irradiation.
DNA damage-mediated cell death is primarily
regulated by caspase-3 dependent apoptosis; the process is
accompanied by PARP fragmentation (52). In the present study, the authors
observed the induction of PARP cleavage in Capan-1 cells by PB
irradiation. However, increases in cleaved caspase-3 and -7 were
not detected in Capan-1 cells. The loss of PARP function by
fragmentation has been demonstrated to cause cell death (40). Therefore, Capan-1 cell cell death
by PB irradiation is mediated by PARP fragmentation in a
caspase-3-independent manner.
Additionally, the current study revealed a decrease
in PARP expression by PB irradiation in Panc-1 cells without the
induction of cell death and PARP fragmentation. Powel et al
(53) revealed that PARP
inhibitors act as tumor-specific radiosensitizers in pre-clinical
and clinical studies; implying that radiosensitivity should be
increased by a down-regulation of PARP expression. Furthermore,
hyperradiosensitivity was elicited by PARP silencing in HeLa cells
(54). However, the association
between radiosensitivity and a decrease in PARP expression in
Capan-1 and Panc-1 was not identified in the present study.
Therefore, the present study suggests that PARP may not act as a
determinant of radiosensitivity for PB irradiation, at least in
Capan-1 and Panc-1 cells in the first 72 h after irradiation.
Yu et al (55) previously revealed that a novel
STAT3 activation inhibitor induced cell cycle arrest and apoptosis
in HL-60 and K562 human myeloid leukemia cell lines. Those results
implied that increased p21 expression, a cell cycle arrest marker
protein, is correlated with the inhibition of STAT3
phosphorylation. In the current study, the authors observed that
increases in P21 expression by PB irradiation were detected in the
two cells investigated. The increase corresponded with a decrease
of STAT3 phosphorylation. Cell cycle arrest has been demonstrated
to be closely associated with DNA damage (52). Therefore, an increase in H2A.X
phosphorylation is linked with the induction of p21 expression. A
close association between an increase in H2A.X phosphorylation and
p21 expression was identified in the current study. Furthermore,
the changes were coupled with decreased STAT3 phosphorylation,
except 16 Gy PB-irradiated Capan-1 cells. These results suggest
that PB irradiation should induce cell death and/or cell cycle
arrest through the regulation of STAT3 signaling, regardless of
radiosensitivity.
The survival of DNA-damaged cancer cells is
determined by whether cancer cells can repair damage to DNA
(44,45). The current study revealed that the
viability of Capan-1 cells was decreased and not in Panc-1 cells,
and the difference was associated with a decrease of RAD51
expression. The result suggests that the weakening of homologous
recombination repair activity mediated by the down-regulation of
RAD51 expression causes higher cytotoxicity in Capan-1 cells
following PB irradiation, but not Panc-1. Furthermore, lower
radiosensitivity of Panc-1 was associated with an increase in
survivin expression by PB irradiation. RAD51 and survivin are known
to be dependent on radiosensitivity. Studies have demonstrated that
the expression levels of RAD51 and survivin were associated with
radiosensitivity (27,46,55).
However, a correlation between radiaosensitivity and changes in
RAD51 and survivin expression has not been identified in previous
investigations. In the present study, it was revealed that the
inhibition of RAD51 protein expression by PB was associated with an
increase of cell death in Capan-1 cells. The results demonstrate
that radiosensitivities of Capan-1 and Panc-1 cells following PB
therapy may be determined by whether a PB leads to the reduction of
RAD51 expression and/or the induction of survivin expression.
Taken together, although many previous studies
reported the involvement of COX-2, PARP, RAD51, and survivin in
radiosensitivity, the current study demonstrated that
radiosensitivity in early stages (<72 h) of PB treatment may be
predominantly determined by the regulation of RAD51 and/or survivin
expression, at least in Capan-1 and Panc-1 cells. However, the
regulation of COX-2 and PARP expression levels did not determine
radiosensitivity. The current investigation demonstrates that
changes in RAD51 and survivin expression are important roles in
determining radiosensitivity and implies that a specialized
strategy is necessary for improving the efficacy of PB therapy in
patients with pancreatic cancer.
Funding
The current study was supported by the National
Research Foundation of Korea grant funded by the Ministry of
Science, ICT and Future Planning (Grant no. 2016M2B2A4911420).
Availability of data and materials
All the original data generated for this manuscript
are available upon request.
Authors' contributions
KSL and KSN designed the experiment. MGL and KSL
performed experiments. MGL, KSL and KSN analyzed the data and wrote
the manuscript. All authors confirmed and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Bailey P, Chang DK, Nones K, Johns AL,
Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC,
et al Australian Pancreatic Cancer Genome Initiative: Genomic
analyses identify molecular subtypes of pancreatic cancer. Nature.
531:47–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De La Cruz MS, Young AP and Ruffin MT:
Diagnosis and management of pancreatic cancer. Am Fam Physician.
89:626–632. 2014.PubMed/NCBI
|
|
3
|
Souchek JJ, Baine MJ, Lin C, Rachagani S,
Gupta S, Kaur S, Lester K, Zheng D, Chen S, Smith L, et al:
Unbiased analysis of pancreatic cancer radiation resistance reveals
cholesterol biosynthesis as a novel target for radiosensitisation.
Br J Cancer. 111:1139–1149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rossi ML, Rehman AA and Gondi CS:
Therapeutic options for the management of pancreatic cancer. World
J Gastroenterol. 20:11142–11159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gianfaldoni S, Gianfaldoni R, Wollina U,
Lotti J, Tchernev G and Lotti T: An overview on radiotherapy: From
its history to its current applications in dermatology. Open Access
Maced J Med Sci. 5:521–525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rycaj K and Tang DG: Cancer stem cells and
radioresistance. Int J Radiat Biol. 90:615–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stone HB, Coleman CN, Anscher MS and
McBride WH: Effects of radiation on normal tissue: Consequences and
mechanisms. Lancet Oncol. 4:529–536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suit H and Urie M: Proton beams in
radiation therapy. J Natl Cancer Inst. 84:155–164. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Habrand JL, Schlienger P, Schwartz L,
Pontvert D, Lenir-Cohen-Solal C, Helfre S, Mammar H, Haie-Meder C,
Ferrand R and Mazal A: Clinical applications of proton therapy.
Bull Cancer Radiother. 83(Suppl): 207s–211s. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeung RH, Chapman TR, Bowen SR and
Apisarnthanarax S: Proton beam therapy for hepatocellular
carcinoma. Expert Rev Anticancer Ther. 17:911–924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ross GM: Induction of cell death by
radiotherapy. Endocr Relat Cancer. 6:41–44. 1999. View Article : Google Scholar
|
|
12
|
Maeda J, Froning CE, Brents CA, Rose BJ,
Thamm DH and Kato TA: Intrinsic Radiosensitivity and Cellular
Characterization of 27 Canine Cancer Cell Lines. PLoS One.
11:e01566892016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peltenburg LT: Radiosensitivity of tumor
cells Oncogenes and apoptosis. Q J Nucl Med. 44:355–364. 2000.
|
|
14
|
Gildemeister OS, Sage JM and Knight KL:
Cellular redistribution of Rad51 in response to DNA damage: Novel
role for Rad51C. J Biol Chem. 284:31945–31952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greve B, Sheikh-Mounessi F, Kemper B,
Ernst I, Götte M and Eich HT: Survivin, a target to modulate the
radiosensitivity of Ewing's sarcoma. Strahlenther Onkol.
188:1038–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sak A, Stueben G, Groneberg M, Böcker W
and Stuschke M: Targeting of Rad51-dependent homologous
recombination: Implications for the radiation sensitivity of human
lung cancer cell lines. Br J Cancer. 92:1089–1097. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du LQ, Wang Y, Wang H, Cao J, Liu Q and
Fan FY: Knockdown of Rad51 expression induces radiation- and
chemo-sensitivity in osteosarcoma cells. Med Oncol. 28:1481–1487.
2011. View Article : Google Scholar
|
|
18
|
Graeser M, McCarthy A, Lord CJ, Savage K,
Hills M, Salter J, Orr N, Parton M, Smith IE, Reis-Filho JS, et al:
A marker of homologous recombination predicts pathologic complete
response to neoadjuvant chemotherapy in primary breast cancer. Clin
Cancer Res. 16:6159–6168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagathihalli NS and Nagaraju G: RAD51 as a
potential biomarker and therapeutic target for pancreatic cancer.
Biochim Biophys Acta. 1816:209–218. 2011.PubMed/NCBI
|
|
20
|
Taki T, Ohnishi T, Yamamoto A, Hiraga S,
Arita N, Izumoto S, Hayakawa T and Morita T: Antisense inhibition
of the RAD51 enhances radiosensitivity. Biochem Biophys Res Commun.
223:434–438. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tennstedt P, Fresow R, Simon R, Marx A,
Terracciano L, Petersen C, Sauter G, Dikomey E and Borgmann K:
RAD51 overexpression is a negative prognostic marker for colorectal
adenocarcinoma. Int J Cancer. 132:2118–2126. 2013. View Article : Google Scholar
|
|
22
|
Capalbo G, Dittmann K, Weiss C, Reichert
S, Hausmann E, Rödel C and Rödel F: Radiation-induced survivin
nuclear accumulation is linked to DNA damage repair. Int J Radiat
Oncol Biol Phys. 77:226–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
24
|
Asanuma K, Moriai R, Yajima T, Yagihashi
A, Yamada M, Kobayashi D and Watanabe N: Survivin as a
radioresistance factor in pancreatic cancer. Jpn J Cancer Res.
91:1204–1209. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yip-Schneider MT, Barnard DS, Billings SD,
Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS
and Sweeney CJ: Cyclooxygenase-2 expression in human pancreatic
adenocarcinomas. Carcinogenesis. 21:139–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garg H, Suri P, Gupta JC, Talwar GP and
Dubey S: Survivin: A unique target for tumor therapy. Cancer Cell
Int. 16:492016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy. Expert Opin Ther Targets.
12:463–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jalalabdi Y, Shirazi A, Ghavam-Nasiri MR,
Davood AA and Sardari D: The role of celecoxib as a Cox-2 inhibitor
increasing the radioswensitivity of tumor tissue. Br J Med Med Res.
8:123–139. 2015. View Article : Google Scholar
|
|
29
|
Shin YK, Park JS, Kim HS, Jun HJ, Kim GE,
Suh CO, Yun YS and Pyo H: Radiosensitivity enhancement by
celecoxib, a cyclo-oxygenase (COX)-2 selective inhibitor, via
COX-2-dependent cell cycle regulation on human cancer cells
expressing differential COX-2 levels. Cancer Res. 65:9501–9509.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwon YS, Lee KS, Chun SY, Jang TJ and Nam
KS: Suppressive effects of a proton beam on tumor growth and lung
metastasis through the inhibition of metastatic gene expression in
4T1 orthotopic breast cancer model. Int J Oncol. 49:336–342. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chun SY, Kwon YS, Nam KS and Kim S:
Lapatinib enhances the cytotoxic effects of doxorubicin in MCF-7
tumorspheres by inhibiting the drug efflux function of ABC
transporters. Biomed Pharmacother. 72:37–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Degner SC, Kemp MQ, Bowden GT and
Romagnolo DF: Conjugated linoleic acid attenuates cyclooxygenase-2
transcriptional activity via an anti-AP-1 mechanism in MCF-7 breast
cancer cells. J Nutr. 136:421–427. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zaffaroni N, Pennati M, Colella G, Perego
P, Supino R, Gatti L, Pilotti S, Zunino F and Daidone MG:
Expression of the anti-apoptotic gene survivin correlates with
taxol resistance in human ovarian cancer. Cell Mol Life Sci.
59:1406–1412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guttilla IK, Phoenix KN, Hong X, Tirnauer
JS, Claffey KP and White BA: Prolonged mammosphere culture of MCF-7
cells induces an EMT and repression of the estrogen receptor by
microRNAs. Breast Cancer Res Treat. 132:75–85. 2012. View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Xu XF, Xie CG, Wang XP, Liu J, Yu YC, Hu
HL and Guo CY: Selective inhibition of cyclooxygenase-2 suppresses
the growth of pancreatic cancer cells in vitro and in vivo. Tohoku
J Exp Med. 215:149–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alan Mitteer R, Wang Y, Shah J, Gordon S,
Fager M, Butter PP, Jun Kim H, Guardiola-Salmeron C,
Carabe-Fernandez A and Fan Y: Proton beam radiation induces DNA
damage and cell apoptosis in glioma stem cells through reactive
oxygen species. Sci Rep. 5:139612015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Herceg Z and Wang ZQ: Functions of
poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity
and cell death. Mutat Res. 477:97–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Casao A, Mata-Campuzano M, Ordás L,
Cebrián-Pérez JA, Muiño-Blanco T and Martínez-Pastor F: Cleaved
PARP-1, an Apoptotic Marker, can be Detected in Ram Spermatozoa.
Reprod Domest Anim. 50:688–691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Poli V and Camporeale A: STAT3-Mediated
Metabolic Reprograming in Cellular Transformation and Implications
for Drug Resistance. Front Oncol. 5:1212015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,
2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces
tumor cell invasion in colorectal cancer cells. Neoplasia.
10:287–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li X, Wang H, Lu X and Di B: Silencing
STAT3 with short hairpin RNA enhances radiosensitivity of human
laryngeal squamous cell carcinoma xenografts in vivo. Exp Ther Med.
1:947–953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang Q, Pan Q, Li C, Xu Y, Wen C and Sun
F: NRAGE is involved in homologous recombination repair to resist
the DNA-damaging chemotherapy and composes a ternary complex with
RNF8-BARD1 to promote cell survival in squamous esophageal
tumorigenesis. Cell Death Differ. 23:1406–1416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Borràs-Fresneda M, Barquinero JF, Gomolka
M, Hornhardt S, Rössler U, Armengol G and Barrios L: Differences in
DNA Repair Capacity, Cell Death and Transcriptional Response after
Irradiation between a Radiosensitive and a Radioresistant Cell
Line. Sci Rep. 6:270432016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhong X, Luo G, Zhou X, Luo W, Wu X, Zhong
R, Wang Y, Xu F and Wang J: Rad51 in regulating the
radiosensitivity of non-small cell lung cancer with different
epidermal growth factor receptor mutation status. Thorac Cancer.
7:50–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ,
Chang SK, Park HC, Suh CO, Park TK and Kim BS: Overexpression of
cyclooxygenase-2 is associated with a poor prognosis in patients
with squamous cell carcinoma of the uterine cervix treated with
radiation and concurrent chemotherapy. Cancer. 95:531–539. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin F, Luo J, Gao W, Wu J, Shao Z, Wang Z,
Meng J, Ou Z and Yang G: COX-2 promotes breast cancer cell
radioresistance via p38/MAPK-mediated cellular anti-apoptosis and
invasiveness. Tumour Biol. 34:2817–2826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Terakado N, Shintani S, Yano J, Chunnan L,
Mihara M, Nakashiro K and Hamakawa H: Overexpression of
cyclooxy-genase-2 is associated with radioresistance in oral
squamous cell carcinoma. Oral Oncol. 40:383–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen L, Fu L, Kong X, Xu J, Wang Z, Ma X,
Akiyama Y, Chen Y and Fang J: Jumonji domain-containing protein 2B
silencing induces DNA damage response via STAT3 pathway in
colorectal cancer. Br J Cancer. 110:1014–1026. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wen W, Zhu F, Zhang J, Keum YS, Zykova T,
Yao K, Peng C, Zheng D, Cho YY, Ma WY, et al: MST1 promotes
apoptosis through phosphorylation of histone H2AX. J Biol Chem.
285:39108–39116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Borges HL, Linden R and Wang JY: DNA
damage-induced cell death: Lessons from the central nervous system.
Cell Res. 18:17–26. 2008. View Article : Google Scholar
|
|
53
|
Powell C, Mikropoulos C, Kaye SB, Nutting
CM, Bhide SA, Newbold K and Harrington KJ: Pre-clinical and
clinical evaluation of PARP inhibitors as tumour-specific
radiosensitisers. Cancer Treat Rev. 36:566–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Godon C, Cordelières FP, Biard D, Giocanti
N, Mégnin-Chanet F, Hall J and Favaudon V: PARP inhibition versus
PARP-1 silencing: Different outcomes in terms of single-strand
break repair and radiation susceptibility. Nucleic Acids Res.
36:4454–4464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yu ZY, Huang R, Xiao H, Sun WF, Shan YJ,
Wang B, Zhao TT, Dong B, Zhao ZH, Liu XL, et al: Fluacrypyrim, a
novel STAT3 activation inhibitor, induces cell cycle arrest and
apoptosis in cancer cells harboring constitutively-active STAT3.
Int J Cancer. 127:1259–1270. 2010. View Article : Google Scholar : PubMed/NCBI
|