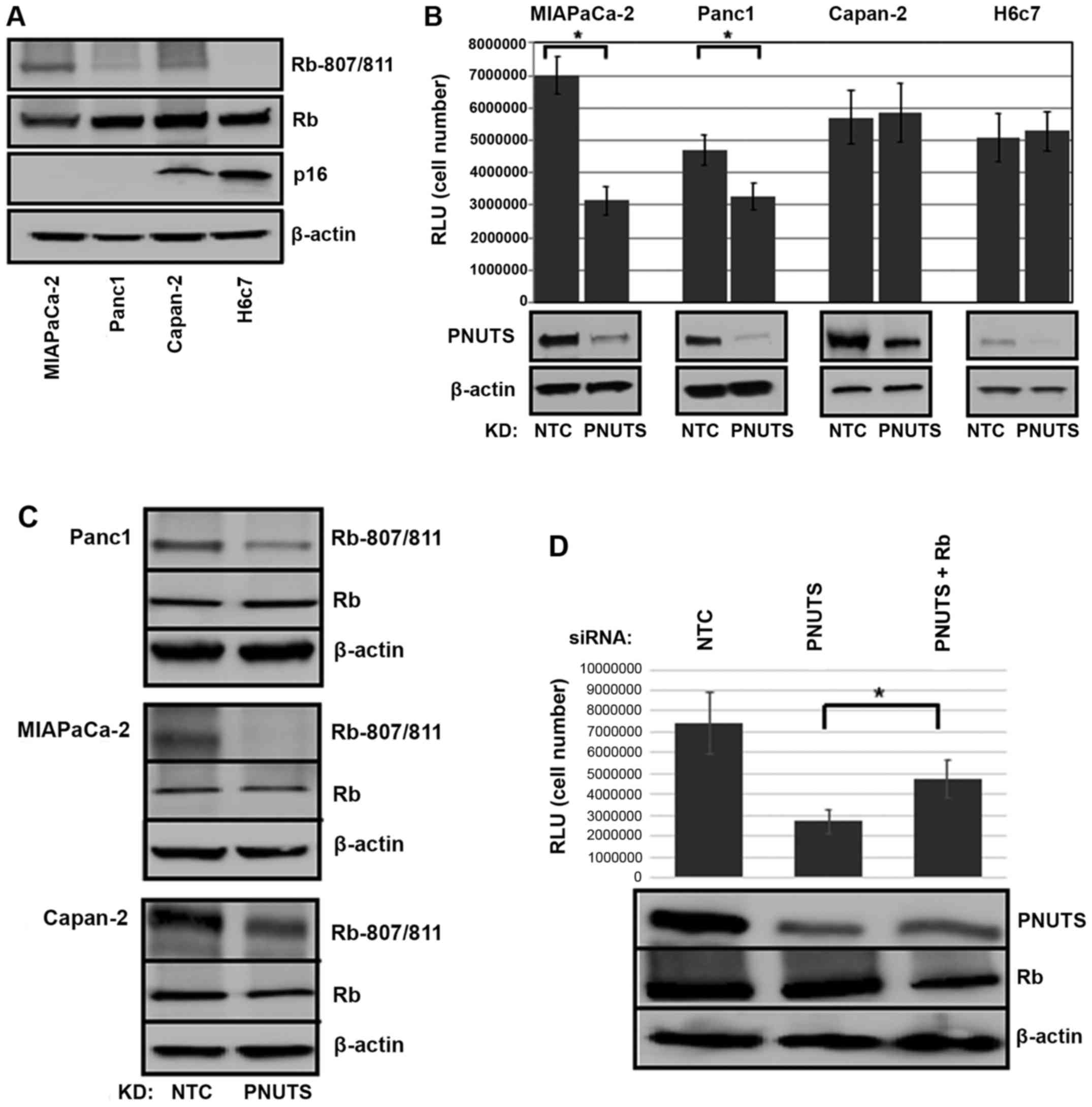
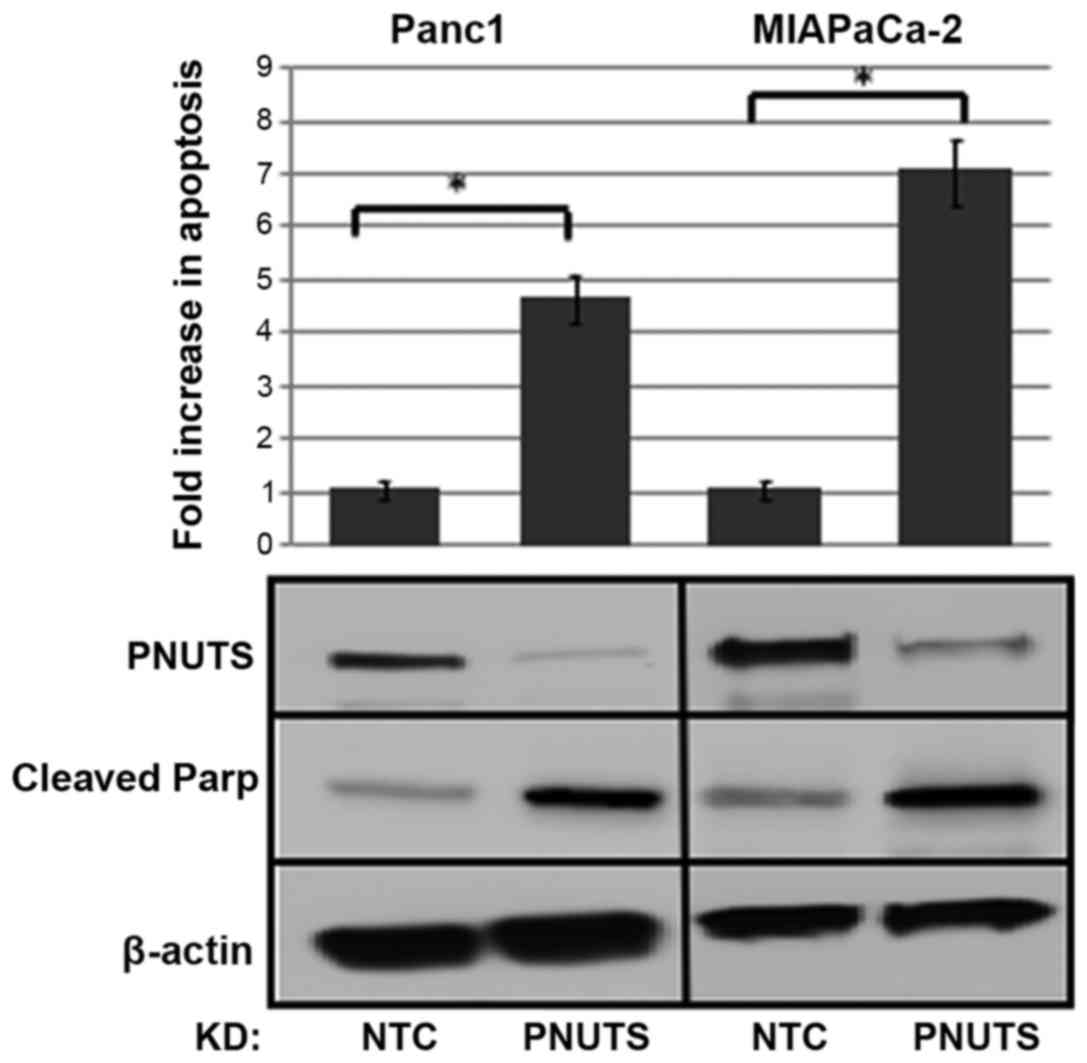
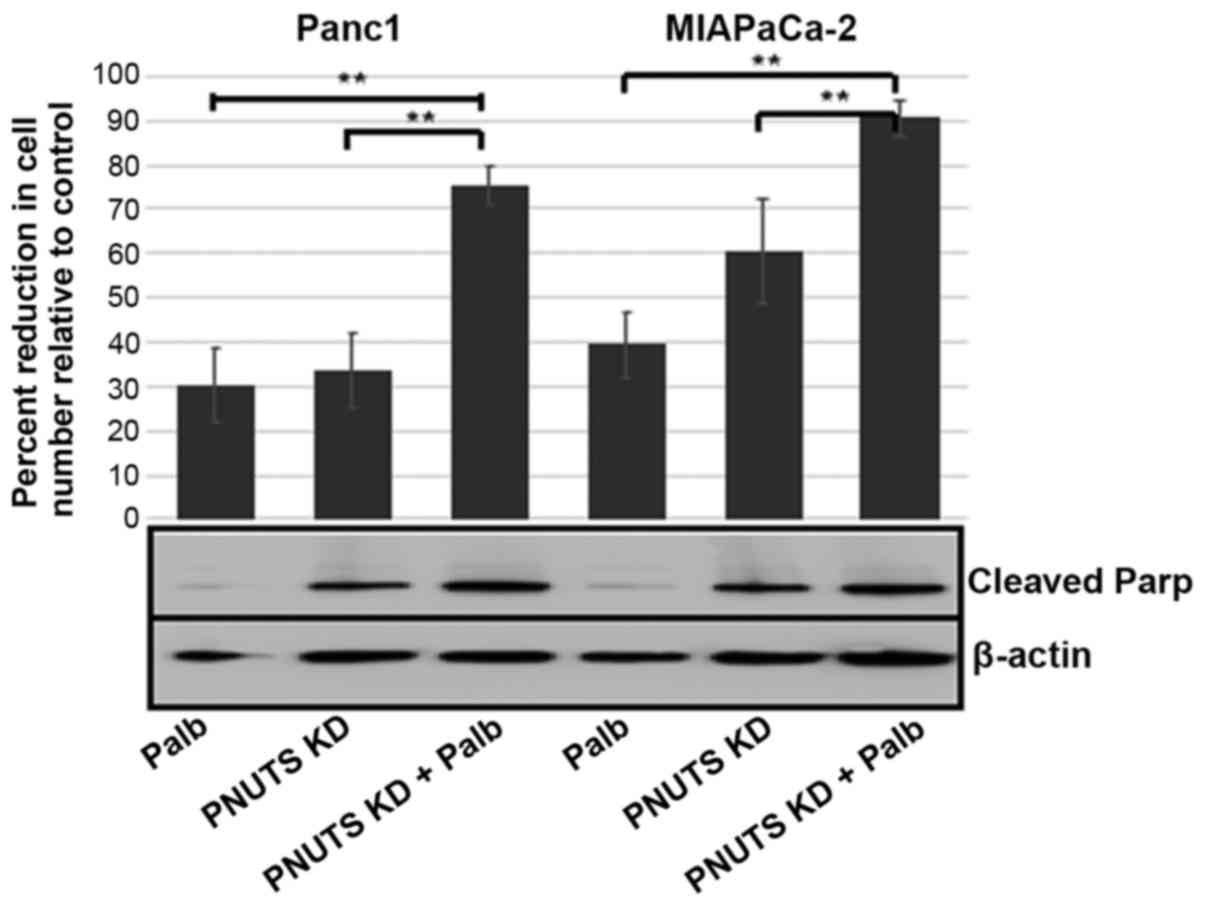
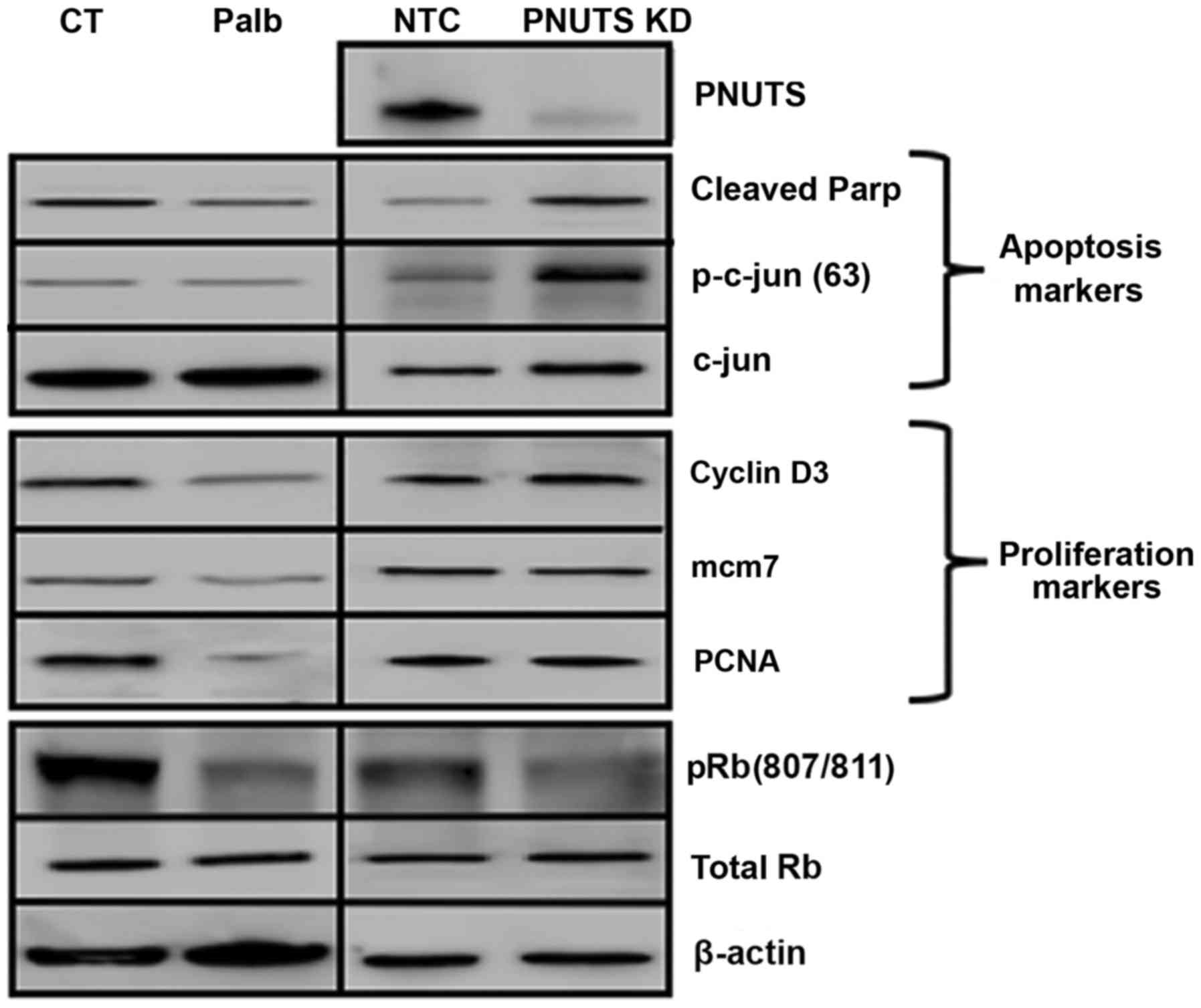
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar
|
|
2
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar
|
|
3
|
Philip PA and Lutz MP: Targeting Epidermal
Growth factor receptor-related signaling pathways in pancreatic
cancer. Pancreas. 44:1046–1052. 2015. View Article : Google Scholar
|
|
4
|
Yang ZY, Yuan JQ, Di MY, Zheng DY, Chen
JZ, Ding H, Wu XY, Huang YF, Mao C and Tang JL: Gemcitabine plus
erlotinib for advanced pancreatic cancer: A systematic review with
meta-analysis. PLoS One. 8:e575282013. View Article : Google Scholar :
|
|
5
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
National Cancer Institute of Canada Clinical Trials Group:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
6
|
Troiani T1, Martinelli E, Capasso A,
Morgillo F, Orditura M, De Vita F and Ciardiello F: Targeting EGFR
in pancreatic cancer treatment. Curr Drug Targets. 13:802–810.
2012. View Article : Google Scholar
|
|
7
|
Nedaeinia R, Avan A, Manian M, Salehi R
and Ghayour- Mobarhan M: EGFR as a potential target for the
treatment of pancreatic cancer: Dilemma and controversies. Curr
Drug Targets. 15:1293–1301. 2014. View Article : Google Scholar
|
|
8
|
Zhao C, Li H, Lin HJ, Yang S, Lin J and
Liang G: Feedback activation of STAT3 as a cancer drug-resistance
mechanism. Trends Pharmacol Sci. 37:47–61. 2016. View Article : Google Scholar
|
|
9
|
Lee H-J, Zhuang G, Cao Y, Du P, Kim HJ and
Settleman J: Drug resistance via feedback activation of Stat3 in
oncogene-addicted cancer cells. Cancer Cell. 26:207–221. 2014.
View Article : Google Scholar
|
|
10
|
Ioannou N, Seddon AM, Dalgleish A,
Mackintosh D, Solca F and Modjtahedi H: Acquired resistance of
pancreatic cancer cells to treatment with gemcitabine and
HER-inhibitors is accompanied by increased sensitivity to STAT3
inhibition. Int J Oncol. 48:908–918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schutte M, Hruban RH, Geradts J, Maynard
R, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schwarte-Waldhoff
I, Schmiegel W, et al: Abrogation of the Rb/p16 tumor-suppressive
pathway in virtually all pancreatic carcinomas. Cancer Res.
57:3126–3130. 1997.
|
|
12
|
Hustinx SR, Leoni LM, Yeo CJ, Brown PN,
Goggins M, Kern SE, Hruban RH and Maitra A: Concordant loss of MTAP
and p16/CDKN2A expression in pancreatic intraepithelial neoplasia:
Evidence of homozygous deletion in a noninvasive precursor lesion.
Mod Pathol. 18:959–963. 2005. View Article : Google Scholar
|
|
13
|
Fukushima N, Sato N, Ueki T, Rosty C,
Walter KM, Wilentz RE, Yeo CJ, Hruban RH and Goggins M: Aberrant
methylation of preproenkephalin and p16 genes in pancreatic
intraepithelial neoplasia and pancreatic ductal adenocarcinoma. Am
J Pathol. 160:1573–1581. 2002. View Article : Google Scholar
|
|
14
|
Gerdes B, Ramaswamy A, Kersting M, Ernst
M, Lang S, Schuermann M, Wild A and Bartsch DK: p16(INK4a)
alterations in chronic pancreatitis-indicator for high-risk lesions
for pancreatic cancer. Surgery. 129:490–497. 2001. View Article : Google Scholar
|
|
15
|
Makohon-Moore A and Iacobuzio-Donahue CA:
Pancreatic cancer biology and genetics from an evolutionary
perspective. Nat Rev Cancer. 16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Notta F, Chan-Seng-Yue M, Lemire M, Li Y,
Wilson GW, Connor AA, Denroche RE, Liang SB, Brown AM, Kim JC, et
al: A renewed model of pancreatic cancer evolution based on genomic
rearrangement patterns. Nature. 538:378–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rabien A, Sanchez-Ruderisch H, Schulz P,
Otto N, Wimmel A, Wiedenmann B and Detjen KM: Tumor suppressor
p16INK4a controls oncogenic K-Ras function in human pancreatic
cancer cells. Cancer Sci. 103:169–175. 2012. View Article : Google Scholar
|
|
18
|
Chang Z, Ju H, Ling J, Zhuang Z, Li Z,
Wang H, Fleming JB, Freeman JW, Yu D, Huang P, et al: Cooperativity
of oncogenic K-ras and downregulated p16/INK4A in human pancreatic
tumorigenesis. PLoS One. 9:e1014522014. View Article : Google Scholar :
|
|
19
|
Li J, Poi MJ and Tsai MD: Regulatory
mechanisms of tumor suppressor P16(INK4A) and their relevance to
cancer. Biochemistry. 50:5566–5582. 2011. View Article : Google Scholar
|
|
20
|
Franco J, Witkiewicz AK and Knudsen ES:
CDK4/6 inhibitors have potent activity in combination with pathway
selective therapeutic agents in models of pancreatic cancer.
Oncotarget. 5:6512–6525. 2014. View Article : Google Scholar
|
|
21
|
Heilmann AM, Perera RM, Ecker V, Nicolay
BN, Bardeesy N, Benes CH and Dyson NJ: CDK4/6 and IGF1 receptor
inhibitors synergize to suppress the growth of p16INK4A-deficient
pancreatic cancers. Cancer Res. 74:3947–3958. 2014. View Article : Google Scholar
|
|
22
|
Liu F and Korc M: Cdk4/6 inhibition
induces epithelial-mesenchymal transition and enhances invasiveness
in pancreatic cancer cells. Mol Cancer Ther. 11:2138–2148. 2012.
View Article : Google Scholar
|
|
23
|
Witkiewicz AK, Borja NA, Franco J, Brody
JR, Yeo CJ, Mansour J, Choti MA, McCue P and Knudsen ES: Selective
impact of CDK4/6 suppression on patient-derived models of
pancreatic cancer. Oncotarget. 6:15788–15801. 2015. View Article : Google Scholar
|
|
24
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and Letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nelson DA, Krucher NA and Ludlow JW: High
molecular weight protein phosphatase type 1 dephosphorylates the
retinoblastoma protein. J Biol Chem. 272:4528–4535. 1997.
View Article : Google Scholar
|
|
26
|
Bollen M, Peti W, Ragusa MJ and Beullens
M: The extended PP1 toolkit: Designed to create specificity. Trends
Biochem Sci. 33:113–121. 2003.
|
|
27
|
Kolupaeva V and Janssens V: PP1 and PP2A
phosphatases - cooperating partners in modulating retinoblastoma
protein activation. FEBS J. 280:627–643. 2013. View Article : Google Scholar
|
|
28
|
Allen PB, Kwon YG, Nairn AC and Greengard
P: Isolation and characterization of PNUTS, a putative protein
phosphatase 1 nuclear targeting subunit. J Biol Chem.
273:4089–4095. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kreivi JP, Trinkle-Mulcahy L, Lyon CE,
Morrice NA, Cohen P and Lamond AI: Purification and
characterisation of p99, a nuclear modulator of protein phosphatase
1 activity. FEBS Lett. 420:57–62. 1997. View Article : Google Scholar
|
|
30
|
Udho E, Tedesco VC, Zygmunt A and Krucher
NA: PNUTS (phosphatase nuclear targeting subunit) inhibits
retinoblastoma-directed PP1 activity. Biochem Biophys Res Commun.
297:463–467. 2002. View Article : Google Scholar
|
|
31
|
Choy MS, Hieke M, Kumar GS, Lewis GR,
Gonzalez-DeWhitt KR, Kessler RP, Stein BJ, Hessenberger M, Nairn
AC, Peti W, et al: Understanding the antagonism of retinoblastoma
protein dephosphorylation by PNUTS provides insights into the PP1
regulatory code. Proc Natl Acad Sci USA. 111:4097–4102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoekstra E, Peppelenbosch MP and Fuhler
GM: Meeting report Europhosphatase 2015: Phosphatases as drug
targets in cancer. Cancer Res. 76:193–196. 2016. View Article : Google Scholar
|
|
33
|
Kim YM, Watanabe T, Allen PB, Kim YM, Lee
SJ, Greengard P, Nairn AC and Kwon YG: PNUTS, a protein phosphatase
1 (PP1) nuclear targeting subunit. Characterization of its PP1- and
RNA-binding domains and regulation by phosphorylation. J Biol Chem.
278:13819–13828. 2003. View Article : Google Scholar
|
|
34
|
Krucher NA, Rubin E, Tedesco VC, Roberts
MH, Sherry TC and De Leon G: Dephosphorylation of Rb (Thr-821) in
response to cell stress. Exp Cell Res. 312:2757–2763. 2006.
View Article : Google Scholar
|
|
35
|
De Leon G, Sherry TC and Krucher NA:
Reduced expression of PNUTS leads to activation of Rb-phosphatase
and caspase- mediated apoptosis. Cancer Biol Ther. 7:833–841. 2008.
View Article : Google Scholar
|
|
36
|
Kavela S, Shinde SR, Ratheesh R,
Viswakalyan K, Bashyam MD, Gowrishankar S, Vamsy M, Pattnaik S, Rao
S, Sastry RA, et al: PNUTS functions as a proto-oncogene by
sequestering PTEN. Cancer Res. 73:205–214. 2013. View Article : Google Scholar
|
|
37
|
Egger JV, Lane MV, Antonucci LA, Dedi B
and Krucher NA: Dephosphorylation of the Retinoblastoma protein
(Rb) inhibits cancer cell EMT via Zeb. Cancer Biol Ther.
17:1197–1205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pino MS, Shrader M, Baker CH, Cognetti F,
Xiong HQ, Abbruzzese JL and McConkey DJ: Transforming growth factor
alpha expression drives constitutive epidermal growth factor
receptor pathway activation and sensitivity to gefitinib (Iressa)
in human pancreatic cancer cell lines. Cancer Res. 66:3802–3812.
2006. View Article : Google Scholar
|
|
39
|
Buck E, Eyzaguirre A, Haley JD, Gibson NW,
Cagnoni P and Iwata KK: Inactivation of Akt by the epidermal growth
factor receptor inhibitor erlotinib is mediated by HER-3 in
pancreatic and colorectal tumor cell lines and contributes to
erlotinib sensitivity. Mol Cancer Ther. 5:2051–2059. 2006.
View Article : Google Scholar
|
|
40
|
Ali S, Banerjee S, Ahmad A, El-Rayes BF,
Philip PA and Sarkar FH: Apoptosis-inducing effect of erlotinib is
potentiated by 3,3'-diindolylmethane in vitro and in vivo using an
orthotopic model of pancreatic cancer. Mol Cancer Ther.
7:1708–1719. 2008. View Article : Google Scholar
|
|
41
|
Deer EL, Gonzalez-Hernandez J, Coursen JD,
Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ: Phenotype
and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435.
2010. View Article : Google Scholar :
|
|
42
|
Gradiz R, Silva HC, Carvalho L, Botelho MF
and Mota-Pinto A: MIA PaCa-2 and PANC-1 - pancreas ductal
adenocarcinoma cell lines with neuroendocrine differentiation and
somatostatin receptors. Sci Rep. 6:216482016. View Article : Google Scholar
|
|
43
|
Loukopoulos P, Kanetaka K, Takamura M,
Shibata T, Sakamoto M and Hirohashi S: Orthotopic transplantation
models of pancreatic adenocarcinoma derived from cell lines and
primary tumors and displaying varying metastatic activity.
Pancreas. 29:193–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Furukawa T, Duguid WP, Rosenberg L,
Viallet J, Galloway DA and Tsao MS: Long-term culture and
immortalization of epithelial cells from normal adult human
pancreatic ducts transfected by the E6E7 gene of human papilloma
virus 16. Am J Pathol. 148:1763–1770. 1996.PubMed/NCBI
|
|
45
|
Macdonald JI and Dick FA:
Posttranslational modifications of the retinoblastoma tumor
suppressor protein as determinants of function. Genes Cancer.
3:619–633. 2012. View Article : Google Scholar
|
|
46
|
De Leon G, Cavino M, D'Angelo M and
Krucher NA: PNUTS knockdown potentiates the apoptotic effect of
Roscovitine in breast and colon cancer cells. Int J Oncol.
36:1269–1275. 2010.PubMed/NCBI
|
|
47
|
Mohammed A, Janakiram NB, Li Q, Madka V,
Ely M, Lightfoot S, Crawford H, Steele VE and Rao CV: EGFR
inhibitor gefitinib prevents progression of pancreatic lesions to
carcinoma in a conditional LSL-KrasG12D/+ transgenic mice model.
Cancer Prev Res (Phila). 3:1417–1426. 2010. View Article : Google Scholar
|
|
48
|
Lin Y, Wang X and Jin H: EGFR-TKI
resistance in NSCLC patients: Mechanisms and strategies. Am J
Cancer Res. 4:411–435. 2014.
|
|
49
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar
|
|
50
|
Jackman D, Pao W, Riely GJ, Engelman JA,
Kris MG, Jänne PA, Lynch T, Johnson BE and Miller VA: Clinical
definition of acquired resistance to epidermal growth factor
receptor tyrosine kinase inhibitors in non-small-cell lung cancer.
J Clin Oncol. 28:357–360. 2010. View Article : Google Scholar
|
|
51
|
Scholz A, Heinze S, Detjen KM, Peters M,
Welzel M, Hauff P, Schirner M, Wiedenmann B and Rosewicz S:
Activated signal transducer and activator of transcription 3
(STAT3) supports the malignant phenotype of human pancreatic
cancer. Gastroenterology. 125:891–905. 2003. View Article : Google Scholar
|
|
52
|
Toyonaga T, Nakano K, Nagano M, Zhao G,
Yamaguchi K, Kuroki S, Eguchi T, Chijiiwa K, Tsuneyoshi M and
Tanaka M: Blockade of constitutively activated Janus kinase/signal
transducer and activator of transcription-3 pathway inhibits growth
of human pancreatic cancer. Cancer Lett. 201:107–116. 2003.
View Article : Google Scholar
|
|
53
|
Corcoran RB, Contino G, Deshpande V,
Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA
and Bardeesy N: STAT3 plays a critical role in KRAS-induced
pancreatic tumorigenesis. Cancer Res. 71:5020–5029. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fofaria NM and Srivastava SK: STAT3
induces anoikis resistance, promotes cell invasion and metastatic
potential in pancreatic cancer cells. Carcinogenesis. 36:142–150.
2015. View Article : Google Scholar
|
|
55
|
Wu X, Tang W, Marquez RT, Li K, Highfill
CA, He F, Lian J, Lin J, Fuchs JR, Ji M, et al: Overcoming
chemo/radio-resistance of pancreatic cancer by inhibiting STAT3
signaling. Oncotarget. 7:11708–11723. 2016.
|
|
56
|
Zhang X, Ren D, Wu X, Lin X, Ye L, Lin C,
Wu S, Zhu J, Peng X and Song L: miR-1266 contributes to pancreatic
cancer progression and chemoresistance by the STAT3 and NF-kB
signaling pathways. Mol Ther Nucleic Acids. 11:142–158. 2018.
View Article : Google Scholar
|
|
57
|
Finn RS, Dering J, Conklin D, Kalous O,
Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al: PD
0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially
inhibits proliferation of luminal estrogen receptor-positive human
breast cancer cell lines in vitro. Breast Cancer Res. 11:R772009.
View Article : Google Scholar
|
|
58
|
Dean JL, Thangavel C, McClendon AK, Reed
CA and Knudsen ES: Therapeutic CDK4/6 inhibition in breast cancer:
Key mechanisms of response and failure. Oncogene. 29:4018–4032.
2010. View Article : Google Scholar
|
|
59
|
Yang C, Li Z, Bhatt T, Dickler M, Giri D,
Scaltriti M, Baselga J, Rosen N and Chandarlapaty S: Acquired CDK6
amplification promotes breast cancer resistance to CDK4/6
inhibitors and loss of ER signaling and dependence. Oncogene.
36:2255–2264. 2017. View Article : Google Scholar
|
|
60
|
Guri Y and Hall MN: mTOR signaling confers
resistance to targeted cancer drugs. Trends Cancer. 2:688–697.
2016. View Article : Google Scholar
|
|
61
|
Franco J, Balaji U, Freinkman E,
Witkiewicz AK and Knudsen ES: Metabolic reprogramming of pancreatic
cancer mediated by CDK4/6 inhibition elicits unique
vulnerabilities. Cell Rep. 14:979–990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang J, Xu K, Liu P, Geng Y, Wang B, Gan
W, Guo J, Wu F, Chin YR, Berrios C, et al: Inhibition of Rb
phosphorylation leads to mTORC2 mediated activation of AKT. Mol
Cell. 62:929–942. 2016. View Article : Google Scholar
|
|
63
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar
|
|
65
|
Mittnacht S: The retinoblastoma
protein--from bench to bedside. Eur J Cell Biol. 84:97–107. 2005.
View Article : Google Scholar
|
|
66
|
Sherr CJ and McCormick F: The RB and p53
pathways in cancer. Cancer Cell. 2:103–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Harbour JW and Dean DC: The Rb/E2F
pathway: Expanding roles and emerging paradigms. Genes Dev.
14:2393–2409. 2000. View Article : Google Scholar
|
|
68
|
Thangavel C, Boopathi E, Liu Y, Haber A,
Ertel A, Bhardwaj A, Addya S, Williams N, Ciment SJ, Cotzia P, et
al: RB loss promotes prostate cancer metastasis. Cancer Res.
77:982–995. 2017. View Article : Google Scholar :
|
|
69
|
Arima Y, Hayashi H, Sasaki M, Hosonaga M,
Goto TM, Chiyoda T, Kuninaka S, Shibata T, Ohata H, Nakagama H, et
al: Induction of ZEB proteins by inactivation of RB protein is key
determinant of mesenchymal phenotype of breast cancer. J Biol Chem.
287:7896–7906. 2012. View Article : Google Scholar
|