Introduction
More than 90% of cases of head and neck cancer,
including oral cancer, originate from the squamous epithelia; this
type of cancer is referred to as head and neck squamous cell
carcinoma (HNSCC). HNSCC is the sixth most common type of cancer,
and a significant cause of cancer-associated mortality worldwide
(1). The biological
characteristics of HNSCC cells are highly diverse (2), and the molecular background that
contributes to the heterogeneity of this cancer has yet to be
elucidated, despite recent developments in molecular genetics. For
the treatment of HNSCC, combined surgery, radiation and
chemotherapy is often performed. However, particularly in cases
with metastatic lesions, treatment is very complex, and the overall
survival rate is poor. Therefore, the identification of key
cancer-associated genes is required, which may contribute to the
pathogenesis, progression and metastasis of HNSCC, and may be
considered a therapeutic target.
As a specific cancer-associated gene candidate, our
previous study led us to focus on the Dickkopf-related protein 3
(DKK3) gene, which is a member of the Dickkopf WNT signaling
pathway inhibitor family. This protein family comprises secreted
proteins with two distinct cysteine-rich domains, which antagonize
the Wnt ligand, thus functioning as negative regulators of
oncogenic Wnt signaling (3).
However, the DKK3 protein does not antagonize the Wnt protein;
however, its constitutional function remains unclear. Previous
studies have indicated that DKK3 possesses a tumor suppressor
function; it has been reported that DKK3 expression is decreased in
cancer (4,5), and that it may induce cancer cell
apoptosis when overexpressed by adenovirus-mediated gene transfer
(6,7).
Interestingly, the expression pattern and putative
function of DKK3 in HNSCC differ from those in other types of
cancer. Our previous study demonstrated that HNSCC tissue samples
and cell lines predominantly express DKK3 gene and protein, and
that its expression increases from low level in the cytoplasmic
membrane to high cytoplasmic expression accompanied by progression
of epithelial dysplasia, which is the precursor lesion of HNSCC
(8). In addition, patients with
HNSCC and high DKK3 protein expression exhibit significantly
shorter disease-free survival and metastasis-free survival
(9). Furthermore, small
interfering RNA-mediated downregulation of DKK3 gene expression in
HNSCC-derived cell lines results in significantly decreased
cellular invasion and migration (10). Our previous study also demonstrated
that DKK3 overexpression in a HNSCC cell line results in
significantly elevated cellular proliferation, migration and
invasion, as well as in vivo tumor growth, together with
increased phosphorylation of protein kinase B (Akt) and cyclin D1
(CCND1), and c-myc mRNA expression (11). These results led to the hypothesis
that DKK3 may specifically exert oncogenic functions in HNSCC.
The present study, using lentivirus-mediated short
hairpin (sh)RNA, established a stable DKK3 knockdown cell line
(HSC-3 shDKK3), and aimed to investigate the precise effects of
DKK3 loss-of-function on HNSCC cells, and the detailed mechanism.
Supporting our hypothesis, the results revealed that DKK3 knockdown
reduced the malignancy of HNSCC cells.
Materials and methods
Cell lines
The human tongue cancer-derived cell line, HSC-3,
was used in this study, which was purchased from RIKEN Bioresource
Center (Tsukuba, Japan). Using this cell line as a parent cell
line, stable DKK3 knockdown and control cell lines were generated
[HSC-3 shDKK3 and HSC-3 scrambled shRNA (shScr), respectively]. The
cell lines were maintained in Dulbecco's modified Eagle's medium
(DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), supplemented
with 10% fetal bovine serum (FBS; Nichirei Biosciences, Inc.,
Tokyo, Japan). For HSC-3 shDKK3 and HSC-3 shScr cells, 10
µg/µl puromycin (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan) was additionally supplemented.
Generation of the HSC-3 shDKK3 cell
line
For lentivirus-mediated shRNA targeting of DKK3, a
DKK3 shRNA expressing vector [DKK3-Human, four unique 29mer shRNA
constructs cloned into a lentiviral green fluorescent protein (GFP)
vector; cat. no. TL313463; OriGene Technologies, Inc., Rockville,
MD, USA] and a control vector (non-effective 29-mer shScr cassette
cloned into a pGFP-C-shLenti Vector; cat. no. TR30021; OriGene
Technologies, Inc.) were purchased and used according to the
manufacturer's protocol. Briefly, 2.5×106 293T cells
(RIKEN Bioresource Center) were seeded in a 100-mm dish in growth
medium (DMEM supplemented with 10% FBS). The next day, 5 µg
vectors containing shRNA constructs and 6 µg packaging
plasmid (Lentivpak packaging kit; cat. no. TR30037; OriGene
Technologies, Inc.) were transfected into the cells using MegaTran
1.0 (OriGene Technologies, Inc.); the cells were incubated at 37°C
in an atmosphere containing 5% CO2 for 12 h. The
supernatant containing viral particles was subsequently collected.
HSC-3 cells were seeded in 12-well plate and the cells reached 60%
confluence in 24 h. The next day, the medium was replaced with
complete medium containing 8 µg/µl polybrene
(Sigma-Aldrich; Merck KGaA) and 200 µl viral solution was
added to the cells and incubated for 4 h at 37°C in an atmosphere
containing 5% CO2. The cells were then cultivated in
growth medium for 4 days. After passage into a 60-mm dish, the
cells were incubated in growth medium supplemented with 10
µg/µl puromycin. The knockdown efficacy was confirmed
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blotting. The experimental protocol was
approved by the Recombinant DNA Experiments Safety committee of
Kawasaki Medical School (No. 13-14; Kurashiki, Japan).
RT-qPCR
Cells were cultured in 100-mm dishes or 6-well
plates, and total RNA was extracted from cells using ISOGEN (Nippon
Gene Co., Ltd., Tokyo, Japan) or Nucleospin RNA®
(Macherey-Nagel GmbH, Düren, Germany). cDNA was synthesized using
ReverTra Ace® (Toyobo Life Science, Osaka, Japan),
according to the manufacturer's protocol. RT-qPCR was conducted
using THUNDERBIRD® qPCR mix (Toyobo Life Science) on a
StepOnePlus PCR system (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Gene expression was quantified and normalized to the
RPL30 housekeeping gene (12). The
primer sequences were as follows: DKK3, forward
5′-CAGGCTTCACAGTCTGGTGCTTG-3′, reverse
5′-ACATTGTTTCCATCTCCTCCCCTC-3′; RPL30, forward
5′-ACAGCATGCGGAAAATACTAC-3′, reverse 5′-AAAGGAAAATTTTGCAGGTTT-3′;
and CCND1, forward 5′-CTTCCTCTCCAAAATGCCAG-3′ and reverse
5′-AGCGTGTGAGGCGGTAGTAG-3′ (11).
The PCR conditions were as follows: 95°C for 1 min, followed by 40
cycles at 95°C for 15 sec and 60°C for 45 sec, and a final
extension step at 95°C for 15 sec. Absolute quantification was used
for analyses as previously described (13).
Transfection of DKK3-expressing
plasmid
An expression plasmid for full-length DKK3 was
prepared in our previous experiment (11). HSC-3 cells were seeded at
1.5×106 in a 100-mm dish, and the next day, 12 µg
plasmids were transfected into the cells using Turbofectin 8.0™
(OriGene Technologies, Inc.) at 37°C overnight in an atmosphere
containing 5% CO2. As a control for transfection, a
pCS2+ empty vector (Addgene, Cambridge, MA, USA) was
used.
Western blotting
The cell lines (HSC-3, HSC-3 shScr and HSC-3 shDKK3)
were maintained until they became 100% confluent. Subsequently,
cells were lysed in immunoprecipitation buffer [20 mM Tris-HCl (pH
8.0), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100]. Protein
concentration was quantified using the DC™ Protein Assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and 10 µg protein
was used for western blotting. Briefly, proteins were boiled in
Laemmli's buffer for 3 min, loaded onto an e-PAGEL®
precast gel (ATTO Corporation, Tokyo, Japan) and blotted onto
polyvinylidene difluoride (PVDF) membranes. After blocking
non-specific binding by soaking the PVDF membranes in PVDF Blocking
Reagent for Can Get Signal® (Toyobo Life Science) at
room temperature for 1 h, the membranes were incubated with primary
antibodies at 4°C overnight. The following primary antibodies were
used in this study: DKK3 (1:10,000; cat. no. ab186409, Abcam,
Cambridge, MA, USA), β-catenin (1:1,000; cat. no. 8480S),
phosphorylated (p)-β-catenin (Ser675) (1:1,000; cat. no. 4176S),
glycogen synthase kinase (GSK)-3β (1:1,000; cat. no. 12456S),
p-GSK3β (Ser9) (1:1,000; cat. no. 5558S), transforming growth
factor (TGF)-β (1:1,000; cat. no. 3711S), Akt (1:1,000; cat. no.
4685S), p-Akt (Ser473) (1:1,000; cat. no. 9271S), c-Jun (1:1,000;
cat. no. 9165S), p-c-Jun (Ser63) (1:1,000; cat. no. 9261S), c-Jun
N-terminal kinase (JNK) (1:1,000; cat. no. 9258S), p-JNK
(Thr183/Tyr182) (1:1,000; cat. no. 9251S), phosphoinositide
3-kinase (PI3K) p110α (1:1,000; cat. no. 4249S),
3-phosphoinositide-dependent protein kinase-1 (PDK1) (1:1,000; cat.
no. 5662S), p-PDK1 (Ser241) (1:1,000; cat. no. 3061S), mechanistic
target of rapamycin (mTOR) (1:1,000; cat. no. 2972S), p-mTOR
(Ser2448) (1:1,000; cat. no. 5536S), p38 MAPK (1:1,000; cat. no.
9212S), p-p38 MAPK (Thr180/Tyr182) (1:1,000; cat. no. 9211S),
p44/p42 MAPK (1:1,000; cat. no. 9102S), p-p44/p42 MAPK
(Thr202/Tyr182) (1:1,000; cat. no. 9101S), DEP domain-containing
mTOR-interacting protein (DEPTOR) (1:1,000; cat. no. 11816S),
β-actin (1:50,000; cat. no. 5057S) (Cell Signaling Technology, Inc.
Danvers, MA, USA) and p-PI3K p85 (Y467)/p55 (Y199) (1:1,000; cat.
no. BS4605; Bioworld Technology, Inc., St. Louis Park, MN, USA).
Subsequently, the membranes were washed in Tris-buffered saline
(TBS) containing 0.1% Tween-20, and were incubated with a secondary
antibody (1:50,000; cat. no. 111-036-003; Jackson ImmunoReaserch
Laboratories, Inc., West Grove, PA, USA) for 1 h at room
temperature. Antibodies were diluted in Can Get Signal®
(Toyobo Life Science). Proteins were visualized using the enhanced
chemiluminescence prime western blotting detection system (GE
Healthcare Life Sciences, Little Chalfont, UK).
Immunocytochemistry
Cells were fixed in PBS containing 4%
paraformaldehyde for 10 min at room temperature. After three washes
with PBS, cells were immunohistochemically stained with anti-DKK3
(1:200; cat. no. ab186409, Abcam) overnight at 4°C. After washing,
the cells were incubated with Alexa Fluor®
594-conjugated goat anti-rabbit secondary antibodies (1:500; cat.
no. A11037; Thermo Fisher Scientific, Inc.) for 60 min at room
temperature. Cell nuclei were stained with 1 µg/ml DAPI
(cat. no. 342-07431; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) for 60 min at room temperature. Fluorescent images
were captured using a BZ-X710 All-in-One Fluorescence Microscope
(Keyence Corporation, Osaka, Japan).
Cell proliferation assay
To assess the effects of DKK3 knockdown on cell
proliferation, an MTT assay was performed using the
TACS® Cell Proliferation Assay kit (Trevigen,
Gaithersburg, MD, USA). HSC-3, HSC-3 shScr and HSC-3 shDKK3 cells
were seeded into a 96-well microplate at 1.0×103
cells/100 µl/well and were cultured for 24 h. MTT reagent
was added to the cells and incubated for 4 h at 37°C in an
atmosphere containing 5% CO2, resulting in the formation
of formazan crystals. Subsequently, detergent agent included in the
kit was added and absorbance was measured at 570 nm. Data were
acquired on days 1, 3, 5 and 7.
Migration assay
The cell migration assay was performed using an
Ibidi Culture-Insert (Ibidi GmbH, Munich, Germany). HSC-3, HSC-3
shScr and HSC-3 DKK3 cells were suspended in DMEM supplemented with
10% FBS (1.0×106 cells/ml); 70 µl cell suspension
was transferred to each well of the Culture-Insert in a 6-well
plate. After 24-h incubation at 37°C in an atmosphere containing 5%
CO2, the Culture-Insert was removed using sterilized
tweezers. Time-lapse photography was captured using a BZ-X700
microscope (Keyence Corporation). The area was measured using
ImageJ software version 1.51 (http://rsb.info.nih.gov/ij/; National Institutes of
Health, Bethesda, MD, USA).
Invasion assay
BioCoat™ Matrigel® Invasion Chambers
(Corning Life Sciences, Bedford, MA, USA) were used to conduct an
invasion assay, according to the manufacturer's protocol. Cells
were harvested and suspended in serum-free DMEM at
2.5×105 cells/ml; 500 µl cell suspension was
added into the upper chambers. After 24-h incubation at 37°C in an
atmosphere containing 5% CO2, the chambers were fixed
and stained with Diff-Quik Stain™ (Lab Aids Pty Ltd., North
Narrabeen, NSW, Australia) and mounted on a glass slide. Cell
number was counted under an optical microscope (AxioSkop 2 plus;
Carl Zeiss AG, Oberkochen, Germany) and relative cellular invasion
(invasion index) was calculated according to the manufacturer's
protocol.
Xenograft model and histological
evaluation
Cells were suspended in PBS at 4.0×106
cells/150 µl and were subcutaneously injected into the
dorsal area of 5-week-old, male BALB/cAJcl-nu/nu nude mice (CLEA
Japan, Inc., Tokyo, Japan). The animals had free access to food and
water and were housed at 22°C (60-70% humidity), under a 12-h
light/dark cycle. The average body weight of the mice in each group
was 22.70±0.34 g (HSC-3), 21.87±0.60 g (HSC-3 shScr) and 22.00±0.77
g (HSC-3shDKK3). A total of 30 mice were used in this study
(n=10/experimental group), which were divided into three groups: i)
Injected with HSC-3 cells, ii) injected with HSC-3 shScr, and iii)
injected with HSC-3 shDKK3 cells. Tumor volume (V) was measured and
calculated using the following formula: V = 4/3π × L/2 ×
(W/2)2, where L, longest diameter and W, diameter
perpendicular to L. A total of 4 weeks post-injection, the mice
were sacrificed and tumors were collected for histological
evaluation. This study was performed in accordance with the
Guidelines for Animal Experiments at Kawasaki Medical School, and
the animal protocol for this study was approved by the Animal Care
and Use Committee of Kawasaki Medical School (no. 15-052, 2015).
Tissues were then fixed in 10% neutral buffered formalin for 8 h at
room temperature, embedded in paraffin, sectioned at 4 µm
and stained with hematoxylineosin (HE). For HE staining,
4-µm sections were stained with 0.1% hematoxylin at room
temperature for 10 min. Following incubation with 1% hydrochloric
acid in ethanol for 3 sec, the sections were stained with 0.5%
eosin for 30 sec. In addition, tissues sections underwent
immunohistochemistry (IHC) to detect Ki-67 (1:50; cat. no. M7240;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). Ki-67
positive cells were counted and Ki-67 labeling index was
calculated. Briefly, antigen retrieval was conducted by heating the
samples in Target Retrieval Solution, Citrate pH 6, (cat. no.
S2369; Dako; Agilent Technologies, Inc.) using a pressure cooker
for 15 min. IHC was performed using Vectastain Elite ABC
Rabbit immunoglobulin G kit (cat. no. PK-6101; Vector Laboratories,
Inc., Burlingame, CA, USA). Following antigen retrieval, the
sections were incubated in methanol containing 0.3%
H2O2 to block endogenous biotin for 30 min at
room temperature. The sections were rinsed twice in TBS (5
min/rinse), and were then blocked with normal goat serum, from the
kit, diluted in TBS (1:75) for 30 min at room temperature. The
primary antibody was diluted in TBS (1:50) and the sections were
incubated with it overnight at 4°C. The secondary antibody, from
the kit, was diluted in TBS and normal goat serum (TBS:normal
serum:secondary antibody, 1:75:200), and the sections were
incubated with it for 30 min at room temperature. After three
washes in TBS (5 min/wash), the sections were incubated with
avidinbiotin complex, from the kit, for 30 min at room temperature.
Diaminobenzidine (cat. no. 343-00901; Dojindo Molecular
Technologies, Inc.) was used to identify peroxidase activity.
Finally, the sections were counterstained with Mayer's hemalum
(cat. no. 109249; Merck KGaA) and were examined under an optical
microscope (AxioSkop 2 plus; Carl Zeiss AG).
Microarray analysis and pathway
analysis
Expression profiles were compared between HSC-3 and
HSC-3 shDKK3 cells, and between HSC-3 shScr and HSC-3 shDKK3 cells.
Labeling, hybridization, scanning and data processing were carried
out using Toray 3D-Gene® (Human Oligo chip 25k, cat. no.
TRT-XR126; Toray Industries, Inc., Tokyo, Japan), according to
manufacturer's protocol. Minimum information about a microarray
experiment (MIAME)-compliant array data, including raw data, were
deposited in the Gene Expression Omnibus, National Center for
Biotechnology Information with accession number GSE107403
(https://www.ncbi.nlm.nih.gov/geo/;
currently private until November 30, 2018). Pathway analysis of the
microarray data was performed using the Database for Annotation,
Visualization and Integrated Discovery Bioinformatics Resources 6.8
(14,15).
Bioinformatics analysis of RNA expression
data from The Cancer Genome Atlas (TCGA)
TCGA dataset is a large cancer dataset that contains
high-throughput sequencing data for protein-coding genes. In the
present study, TCGA was used to investigate the association between
DKK3 mRNA expression and overall survival. TCGA dataset was
obtained from cBioPortal (http://www.cbioportal.org) (16,17)
and The Human Protein Atlas (https://www.proteinatlas.org) (18). The data used for analyses were mRNA
expression status of DKK3 in HNSCC [Head and Neck Squamous Cell
Carcinoma (TCGA, Provisional), n=530], pancreatic cancer
[Pancreatic Adenocarcinoma (TCGA, PanCancer Atlas), n=184], renal
cancer [Kidney Renal Clear Cell Carcinoma (TCGA, Provisional),
n=538 and Kidney Renal Papillary Cell Carcinoma (TCGA,
Provisional), n=293] and prostate cancer [Prostate Adenocarcinoma
(TCGA, PanCancer Atlas), n=494], and
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(PIK3CA), Akt1, β-catenin and DEPTOR in HNSCC. The fragments per
kilobase of exon per million reads mapped (FPKM) units were used
for mRNA expression amount, and the FPKM cut-off value was
determined based on The Human Protein Atlas. Associations were
analyzed using the Kaplan-Meier method with log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Statistical analysis
All values are presented as the means ± standard
deviation. Significant differences were determined using two-tailed
Student's t-test with Bonferroni correction. All analyses were
conducted using R version 3.5.1 (The R Foundation for Statistical
Computing, Vienna, Austria; http://www.r-project.org/). P<0.05 was considered
to indicate a statistically significant difference. All experiments
were independently conducted at least three times.
Results
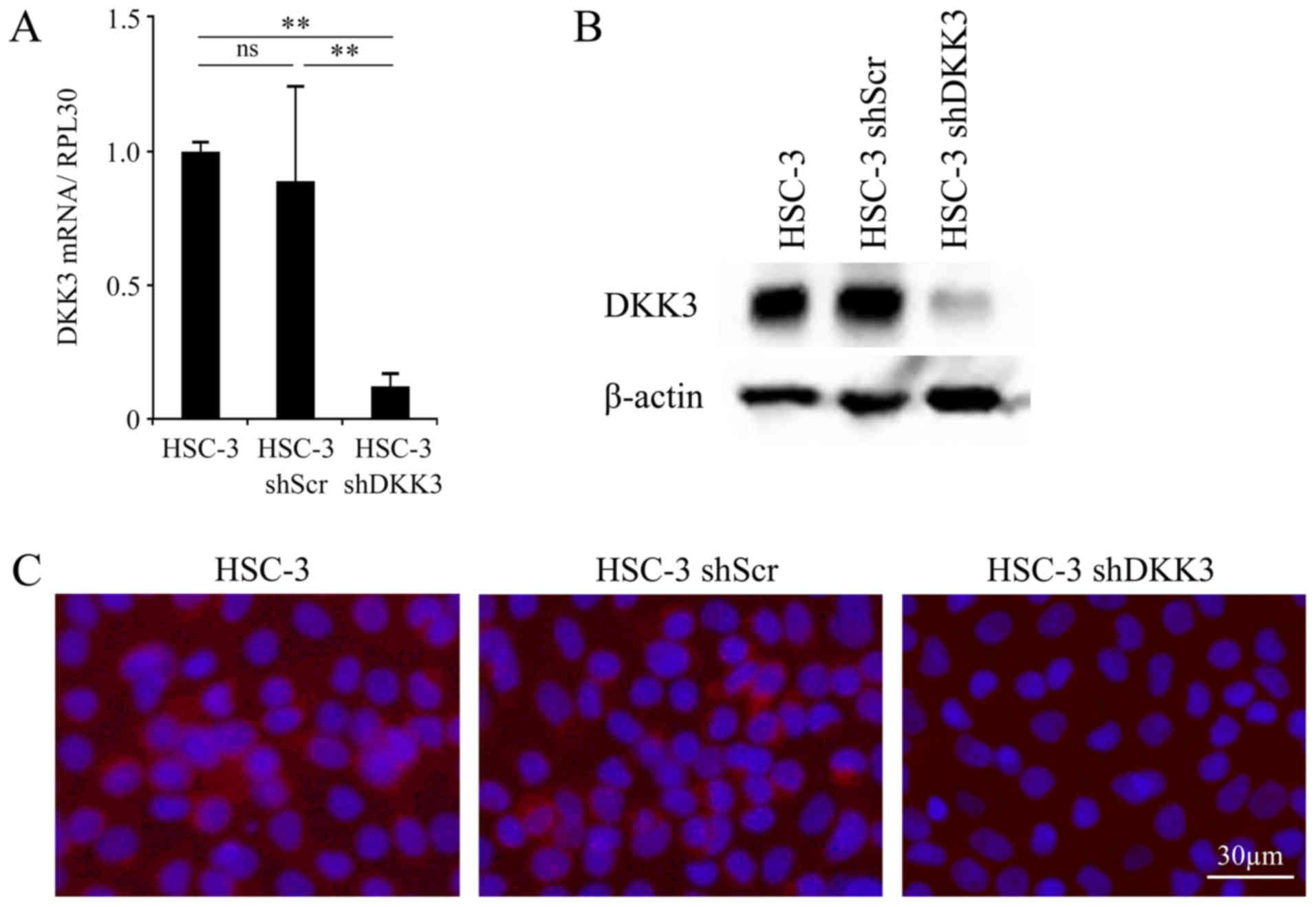
Evaluation of DKK3 knockdown by
lentivirus-mediated shRNA in HSC-3 cells
The cells were maintained in DMEM with or without
the selection pressure of puromycin. Subsequently, after the cells
reached 100% confluence, mRNA and protein expression levels were
detected by RT-qPCR and western blotting, respectively. As shown in
Fig. 1A, the mRNA expression
levels of DKK3 were significantly decreased in HSC-3 shDKK3 cells
compared with in HSC-3 (P<0.001) and HSC-3 shScr (P<0.001)
cells. There was no significant difference in DKK3 mRNA expression
between the HSC-3 and HSC-3 shScr cells (P=0.362). Similarly, the
protein expression levels of DKK3 were decreased in HSC-3sh DKK3
cells compared with in HSC-3 and HSC-3 shScr cells; however, there
was no difference between HSC-3 and HSC-3 shScr cells (Fig. 1B). The results of
immunocytochemistry were concordant with those of western blotting;
the protein expression levels of DKK3 were decreased in HSC-3
shDKK3 cells (Fig. 1C).
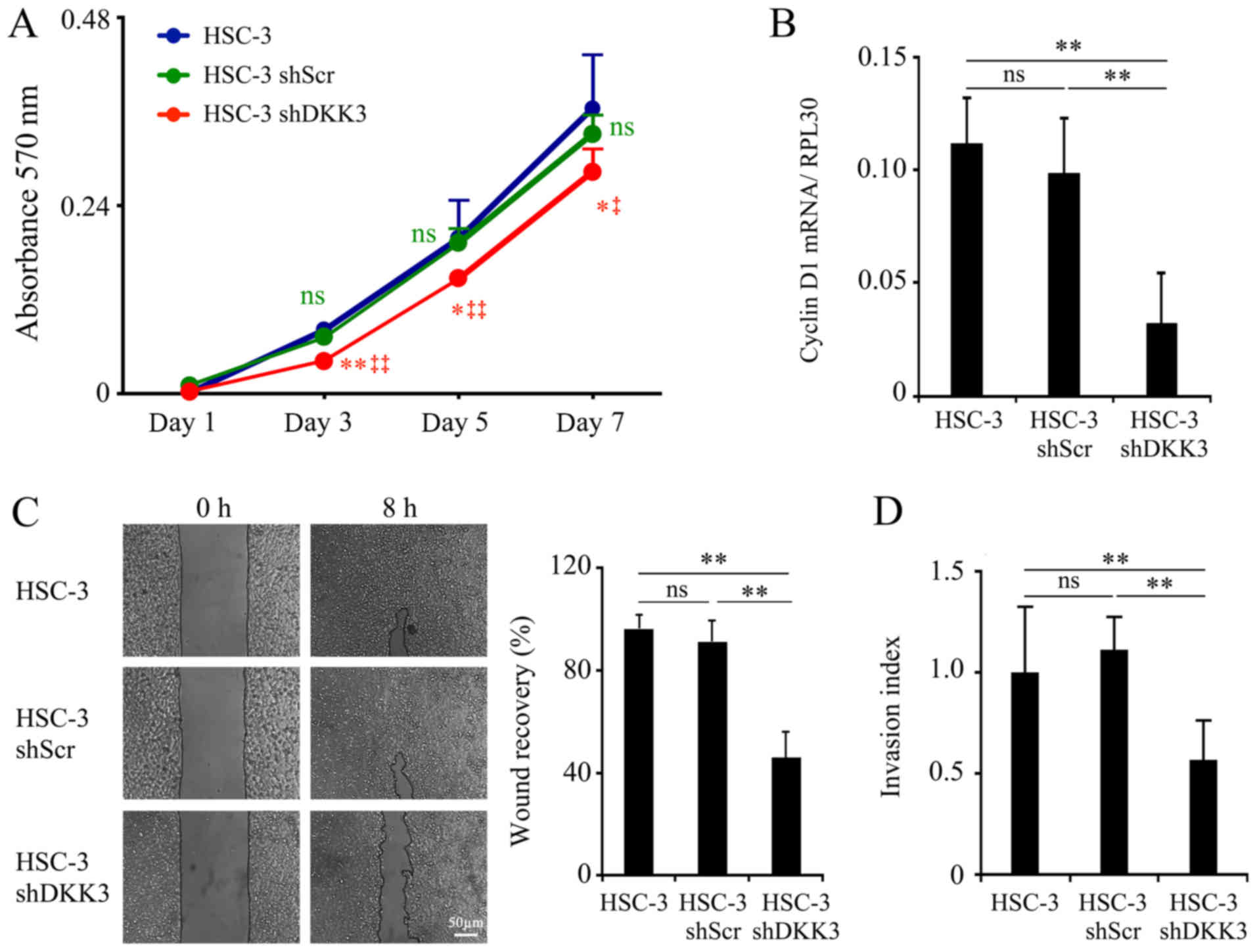
DKK3 knockdown decreases cellular
proliferation, migration and invasion in vitro
After confirming DKK3 knockdown, its effects on
in vitro cellular proliferation, migration and invasion were
determined. With regards to cellular proliferation, HSC-3 shDKK3
cells exhibited reduced proliferation, which was significant on day
3 (vs. HSC-3, P<0.01; vs. HSC-3 shScr, P<0.001), and
sustained on day 5 (vs. HSC-3, P=0.035; vs. HSC-3 shScr,
P<0.001) and day 7 (vs. HSC-3, P=0.022; vs. HSC-3 shScr,
P=0.015). There was no significance detected between HSC-3 and
HSC-3 shScr cells at any time point (day 3, P=0.058; day 5,
P=0.800; day 7, P=0.302, respectively; Fig. 2A). Alongside decreased cellular
proliferation, the mRNA expression levels of CCND1 were
significantly decreased in HSC-3 shDKK3 cells (vs. HSC-3,
P<0.001; vs. HSC-3 shScr, P<0.001), whereas there was no
significance detected between HSC-3 and HSC-3 shScr cells (P=0.093;
Fig. 2B).
Cellular migration was decreased in HSC-3 shDKK3
cells compared with in HSC-3 (P<0.001) and HSC-3 shScr cells
(P<0.001), whereas there was no significance between HSC-3 and
HSC-3 shScr cells (P=0.181; Fig.
2C). In addition, cellular invasion was diminished in HSC-3
shDKK3 cells (vs. HSC-3, P<0.001; vs. HSC-3 shScr, P<0.001).
Conversely, HSC-3 shScr cells did not exhibit a significant
difference compared with HSC-3 cells (P=0.112; Fig. 2D).
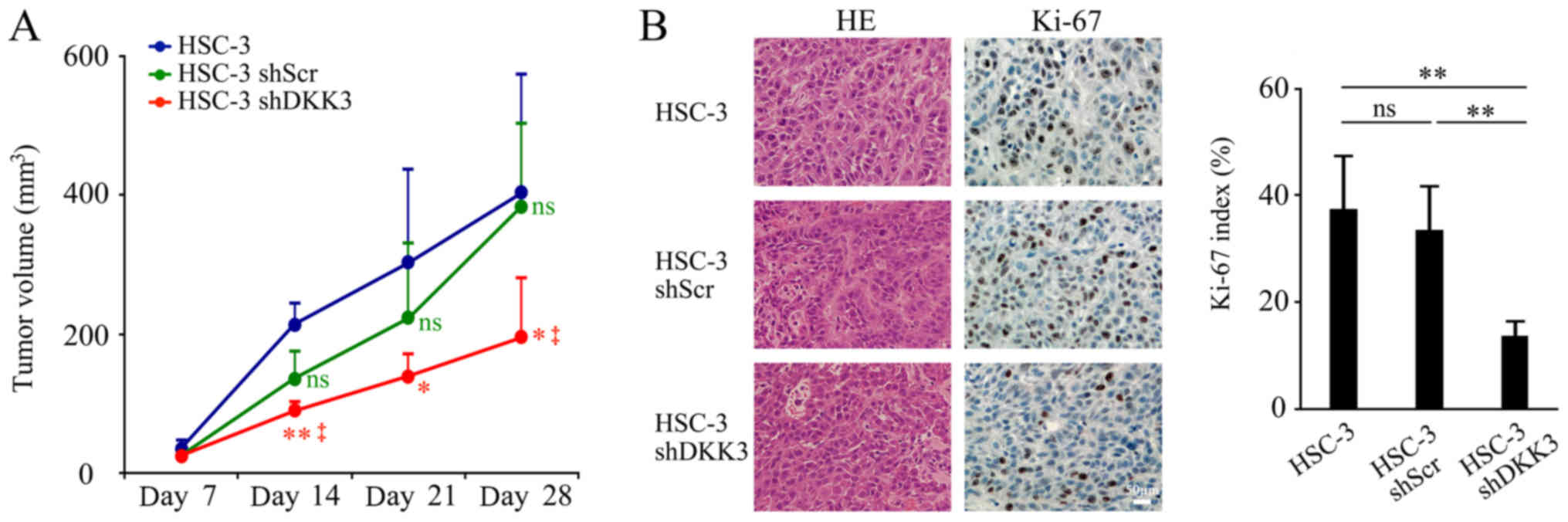
DKK3 knockdown negatively affects in vivo
tumor growth
To determine whether the in vitro results
were replicable in in vivo experiments, HSC-3, HSC-3 shScr
and HSC-3 shDKK3 cells were subcutaneously injected into nude mice.
Tumor volume was observed from day 7 post-injection and gradually
increased. On day 14 post-injection, tumor volume in the HSC-3 DKK3
group was significantly smaller than that in the HSC-3 (P=0.002) or
HSC-3 shScr (P=0.022) groups. On day 21, tumor volume in the HSC-3
shDKK3 group was significantly smaller than that of HSC-3 cells
(P=0.030), but was not significantly different when compared with
HSC-s shScr cells (P=0.070). However, on day 28, the endpoint of
the experiment, it was significantly smaller again (vs. HSC-3,
P=0.021; vs. HSC-3 shScr, P=0.011, respectively). There was no
significant difference between HSC-3 and HSC-3 shScr cells on any
experimental day (day 7, P=0.146; day 14, P=0.055; day 21, P=0.459;
day 28, P=0.814; Fig. 3A). After
28 days, the mice were sacrificed and tissues were collected for
histological analyses. HE-stained sections exhibited no differences
in cancer cell morphology or invasive behavior among the groups.
The injected cells formed a local tumor mass, and did not exhibit
distant or lymph node metastasis. The Ki-67 index was significantly
lower in the HSC-3 shDKK3 group compared with in the HSC-3
(P=0.0017) and HSC-3 shScr (P=0.0012) groups, whereas there was no
significance between the HSC-3 and HSC-3 shScr groups (P=0.514;
Fig. 3B).
Microarray pathway analyses in DKK3
knockdown cells
Microarray analyses were performed to investigate
gene expression alterations in HSC-3 shDKK3 cells. The results
revealed that 2,026 and 2,029 genes were downregulated in HSC-3
shDKK3 cells, compared with in parental HSC-3 cells and HSC-3 shScr
cells, respectively. Of these genes, 1,437 genes were commonly
downregulated compared with in both HSC-3 and HSC-3 shScr cells. In
addition, 862 and 2,925 genes were upregulated in HSC-3 shDKK3
cells, compared with in HSC-3 and HSC-3 shScr cells, respectively,
of which 595 genes were commonly upregulated.
Pathway analyses were performed to determine the
specific pathways, which may contribute to the reduced malignant
properties in DKK3 knockdown cells. Firstly, the 1,437 commonly
downregulated genes in HSC-3 shDKK3 cells were investigated, and 32
pathways were obtained, including 'RNA transport', 'Ribosome
biogenesis in eukaryotes', 'Cell cycle', 'Endocytosis', 'p53
signaling pathway', 'Hepatitis B', 'Bladder cancer', 'Viral
carcinogenesis', 'Colorectal cancer' and 'RIG-I-Like receptor
signaling pathway', etc. The genes associated with these pathways
are listed in Table I.
 | Table IPathways associated with the
downregulated genes in HSC-3 shDKK3 cells. |
Table I
Pathways associated with the
downregulated genes in HSC-3 shDKK3 cells.
| Term | Genes | P-value |
|---|
| RNA transport | FXR1, NMD3, RANBP2,
THOC2, UPF2, UPF3A, UPF3B, EIF1AY, EIF2S2, EIF5B, XPO1, XPOT, FMR1,
MAGOHB, NXT2, NUP153, NUP35, NUP54, PHAX, PNN, RPP40, SUMO1,
TPR |
4.00×10−5 |
| Ribosome biogenesis
in eukaryotes | XRN1, FCF1, GNL2,
GNL3, MPHOSPH10, NMD3, NOP58, RIOK2, WDR43, WDR75, AK6, EFL1, XPO1,
NXT2, RPP40 |
9.00×10−5 |
| Protein processing
in endoplasmic reticulum | BAG2, DNAJA1,
DNAJB11, DNAJC10, DNAJC3, EDEM3, NGLY1, SEC24A, SEC63, UGGT2, YOD1,
ATF6B, ERO1B, HSP90AA1, HSPA1A, MAN1A2, MBTPS2, MAPK8, NFE2L2,
PLAA, UBE2D1 |
2.70×10−4 |
| RIG-I-like receptor
signaling pathway | CXCL8, DDX3X,
DDX58, TBK1, TANK, ATG12, ATG5, CHUK, IFNE, MAPK8, MAP3K7,
SIKE1 |
6.20×10−4 |
| Hepatitis B | CXCL8, DDX3X,
DDX58, E2F3, FAS, KRAS, RB1, TBK1, ATF6B, CASP3, CHUK, CCND1,
CCNE2, MAPK8, MAP2K4, PIK3R1, TLR3, TGFBR1 |
8.40×10−4 |
| Endocytosis | ARFGEF1, ARAP2,
ASAP1, ASAP2, ACAP2, KIAA1033, RAB11FIP2, RAB5A, SMURF2, VPS26A,
WWP1, CAPZA1, CAPZA2, CHMP4C, EEA1, HSPA1A, KIF5B, PRKCI, RABEP1,
SNX2, SNX4, SPG20, TGFBR1, USP8, VPS36, VTA1, ZFYVE16 |
1.00×10−3 |
| Fanconi anemia
pathway | ATR, BRIP1, BRCA2.
POLI, POLK, FANCB, FANCM, REV3L, RMI1, USP1 FANCM, REV3L, RMI1,
USP1 |
1.10×10−3 |
| Pathways in
cancer | APC, BRCA2, CXCL8,
E2F3, FAS, KRAS, RB1, ROCK1, ROCK2, SOS2, BIRC2, BIRC3, CASP3,
CCDC6, CHUK, CUL2, CC-ND1, CCNE2, FZD3, FZD6, HSP90AA1, HDAC2,
HIF1A, ITGA2, ITGB1, LAMA3, LPAR1, LPAR6, MAPK8, MSH2, PIK3R1,
PTGS2, PRKACB, TGFBR1, TPR |
1.10×10−3 |
| NOD-like receptor
signaling pathway | CXCL8, SUGT1,
BIRC2, BIRC3, CHUK, ERBIN, HSP90AA1, MAPK8, MAP3K7, RIPK2 |
1.40×10−3 |
| Signaling pathways
regulating pluripotency of stem cells | APC, BMI1, JAK2,
KRAS, KLF4, SKIL, SMARCAD1, BMPR1A, FZD3, FZS6, INHBA, IL6ST,
PIK3R1, PCGF5, PCGF6, RIF1, ZFHX3 |
1.60×10−3 |
| Spliceosome | DDX42, DDX46,
RBM25, THOC2, U2SURP, CRNKL1, HSPA1A, HNRNPA3, MAGOHB, PLRG1,
PRPF18, PRPF38B, PRPF40A, SRSF10, SRSF5, SF3B1 |
2.50×10−3 |
|
| Small cell lung
cancer | E2F3, RB1, BIRC2,
BIRC3, CHUK, CCND1, CCNE2, ITGA2, ITGB1, LAMA3, RIK3R1, PTGS2 |
3.20×10−3 |
| mRNA surveillance
pathway | HBS1L, PCF11, RNMT,
UPF2, UPF3A, UPF3B, CPSF2, MAGOHB, NXT2, PNN, PAPOLA, PPP1CC |
5.40×10−3 |
|
| Pancreatic
cancer | BRCA2, E2F3, KRAS,
RB1, CHUK, CCND1, MAPK8, RIK3R1, TGFBR1 |
1.50×10−2 |
| Non-homologous
end-joining | LIG4, MRE11, RAD50,
XRCC4 |
2.40×10−2 |
| Chronic myeloid
leukemia | E2F3, KRAS, RB1,
SOS2, CHUK, CCND1, HDAC2, PIK3R1, TGFBR1 |
2.60×10−2 |
| Colorectal
cancer | APC, KRAS, CASP3.
CCND1, MAPK8, MSH2, PIK3R1, TGFBR1 |
3.40×10−2 |
| TNF signaling
pathway | FAS, ATF6B, BIRC2,
BIRC3, CASP3, CHUK, MAPK8, MAP2K4, MAP3K7, PIK3R1, PTGS2 |
3.80×10−2 |
| Shigellosis | CXCL8, ROCK1,
ROCK2, ATG5, CHUK, ITGB1, MAPK8, RIPK2 |
3.90×10−2 |
| Proteoglycans in
cancer | FAS, KRAS, ROCK1,
ROCK2, SOS2, CASP3, CCND1, FZD3, FZD6, HIF1A, ITGA2, ITGB1, PIK3R1,
PRKACB, PPP1CC, RDX, RPS6KB1 |
4.10×10−2 |
| Cell cycle | ATR, E2F3, RB1,
TTK, CCND1, CCNE2, CCNH, CDK1, HDAC2, ORC3, STAG2, SMC3 |
4.50×10−2 |
| p53 signaling
pathway | ATR, FAS, CASP3,
CCND1, CCNE2, CDK1, PMAIP1, ZMAT3 |
4.90×10−2 |
| Epithelial cell
signaling in Helicobacter pylori infection | ADAM10, ADAM17,
ATP6V1C1, CXCL8, CASP3, CHUK, MAPK8, MAP2K4 |
4.90×10−2 |
| Epstein-Barr virus
infection | CD58, DDX58, RB1,
TBK1, CHUK, CDK1, XPO1, HSPA1A, HDAC2, MAPK8, MAP2K4, MAP3K7,
PIK3R1, PSMC6, PRKACB, RBPJ |
5.20×10−2 |
| MAPK signaling
pathway | FAS, KRAS, RASA1,
RAPGEF2, SOS2, TAOK1, CASP3, CHUK, HSPA1A, IL1A, LAMTOR3, MAPK8,
MAP2K4, MAP3K2, MAP3K7, MAP4K3, PRKACB, RPS6KA3, STK3, TGFBR1 |
5.20×10−2 |
| Homologous
recombination | BRCA2, MRE11,
RAD50, RAD54B, NBN |
5.40×10−2 |
| Toxoplasmosis | JAK2, BIRC2, BIRC3,
CASP3, CHUK, HSPA1A, ITGB1, LAMA3, MAPK8, MAP3K7, PIK3R1 |
7.00×10−2 |
| Prostate
cancer | E2F3, KRAS, RB1,
SOS2, CHUK, CCND1, CCNE2, HSP90AA1, PIK3R1 |
7.20×10−2 |
| Herpes simplex
infection | DDX58, FAS, JAK2,
SP100, TBK1, CASP3, C5, CHUK, CDK1, HCFC2, MAPK8, MAP3K7, PPP1CC,
SRSF5, TLR3 |
7.30×10−2 |
| HTLV-I
infection | APC, ATR, E2F3,
ETS1, KRAS, MYBL1, RB1, ATF1, CHUK, CCND1, DLG1, XPO1, FZD3, FZD6,
MAP2K4, NFYB, PI3KR1, PRKACB, TGFBR1 |
8.80×10−2 |
| Viral
carcinogenesis | DDX3X, KRAS, RB1,
SP100, ATF6B, CASP3, CCND1, CCNE2, CDK1, DLG1, HDAC2, IL6ST,
PMAIP1, PIK3R1, PRKACB, RBPJ |
8.80×10−2 |
| Influenza A | CXCL8, DDX58,
DNAJC3, FAS, JAK2, TBK1, XPO1, HSPA1A, IL1A, MAPK8, MAP2K4, NXT2,
PIK3R1, TLR3 |
9.40×10−2 |
As for upregulated genes, pathway analysis of the
595 genes revealed 18 pathways. The majority of the upregulated
genes in HSC-3 shDKK3 cells were involved in metabolic pathways,
and did not include genes which are thought to be important to
cancer proliferation, migration and invasion (data not shown).
Therefore, this study focused on the pathways associated with the
commonly downregulated genes. The downregulated genes included some
important genes, including those involved in the PI3K/mTOR/Akt
pathway (phosphoinositide-3-kinase regulatory subunit 1), MAPKs
(MSPK8, MAPK kinase-4, MAPK kinase kinase-7 and MAPK kinase kinase
kinase-3), cell cycle-associated genes (CCND1, cyclin E2 and
cyclin-dependent kinase-1), chemokines [C-X-C motif chemokine
ligand 8 (CXCL8)] and oncogenes (KRAS proto-oncogene, GTPase, BMI1
proto-oncogene, polycomb ring finger, MYB proto-oncogene like 1,
SKI like proto-oncogene).
Based on these data, the present study investigated
the PI3K/mTOR/Akt, MAPK and Wnt signaling pathways using western
blotting, since these pathways are thought to be closely associated
with HNSCC proliferation, migration and invasion, or DKK/Wnt
signaling.
DKK3 knockdown affects signaling
pathways
To identify the signaling pathways in which
alterations in DKK3 expression are involved western blotting was
performed. Proteins associated with the Wnt/β-catenin,
PI3K/mTOR/Akt and MAPK pathways, including JNK were detected. These
signaling pathways were chosen based on our previous study, which
revealed that DKK3 overexpression in HNSCC cells elevates
phosphorylation of Akt and c-Jun (12). The results revealed that DKK3
knockdown did not affect phosphorylation of β-catenin (Ser675) or
GSK-3β (Ser9), thus suggesting that DKK3 might not function via the
Wnt/β-catenin pathway in HNSCC cells. In addition, DKK3 knockdown
resulted in only a slight alteration in TGF-β expression (Fig. 4A).
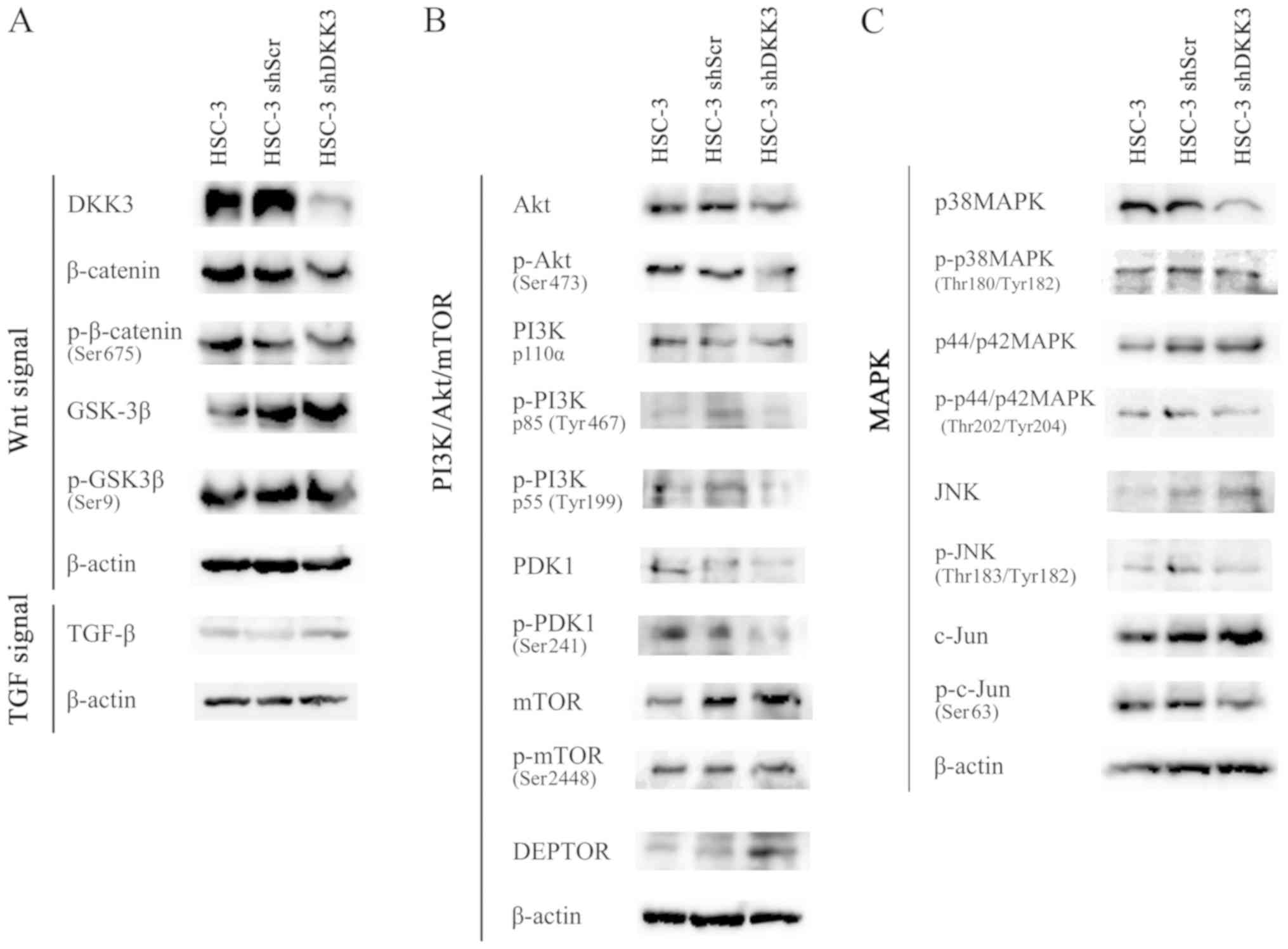 | Figure 4Western blotting revealed alterations
in protein expression and phosphorylation status. (A) DKK3
knockdown did not affect phosphorylation of β-catenin (Ser675) or
GSK-3β (Ser9). (B and C) Phosphorylation of Akt (Ser473) was
decreased, together with the expression of p-PI3K p85 (Tyr467),
p-PI3K p55 (Tyr199), p-PDK1 (Ser241), p38 MAPK and p-p38 MAPK
(Thr180/Tyr182), in HSC-3 shDKK3 cells. (B) DEPTOR expression was
increased, and phosphorylation of mTOR (Ser2448) was decreased in
HSC-3 shDKK3 cells. (C) c-Jun (Ser63) or p-JNK (Thr183/Tyr182)
expression was not altered. Akt, protein kinase B; DEPTOR, DEP
domain-containing mTOR-interacting protein; DKK3, Dickkopf-related
protein 3; GSK-3β, glycogen synthase kinase-3β; JNK, c-Jun
N-terminal kinase; MAPK, mitogen-activated protein kinase; mTOR,
mechanistic target of rapamycin; p, phosphorylated; PDK1,
phosphoinositide 3-kinase; PI3K, phosphoinositide 3-kinase; Scr,
scrambled; sh/shRNA, short hairpin RNA; TGF-β, transforming growth
factor-β. |
Corresponding to our previous data, the results
revealed that phosphorylation of Akt (Ser473) was decreased in
HSC-3 shDKK3 cells, which may be due to the decreased
phosphorylation of PI3K and PDK1. Intriguingly, phosphorylation of
mTOR (Ser2448) was slightly decreased, whereas the expression of
DEPTOR was increased, which is a structural element of the mTOR
complex (mTORC) that functions as a negative regulator of mTORC
(Fig. 4B). The expression levels
of p38 MAPK were markedly decreased in HSC-3 shDKK3 cells, and
phosphorylation of c-Jun (Ser63) and p38 MAPK (Thr180/Tyr182) were
slightly decreased in HSC-3 shDKK3 cells (Fig. 4C). The slight decrease in p-p38MAPK
(Thr180/Tyr182) may be affected by the decreased expression of
total p38 MAPK. From these results, it was hypothesized that DKK3
may stimulate certain cell surface receptors and drive
PI3K/Akt/mTOR and MAPK signaling pathways, and/or facilitate DEPTOR
degradation and activate Akt via mTOR.
DKK3 overexpression in HSC-3 shDKK3
cells
A DKK3- expressing plasmid was transfected into
HSC-3 shDKK3 cells and its effects were assessed, in order to
confirm whether exogenous overexpression of DKK3 cancels the
negative effects of DKK3 knockdown on the malignant properties of
cancer cells. Transfection with the plasmid successfully elevated
DKK3 expression and significantly increased cancer cell
proliferation on day 7 (vs. HSC-3 shDKK3, P=0.004; vs. HSC-3 shDKK3
+ empty vector, P<0.001), migration (vs. HSC-3 shDKK3, P=0.010;
vs. HSC-3 shDKK3 + empty vector, P=0.037) and invasion (vs. HSC-3
shDKK3, P<0.001; vs. HSC-3 shDKK3 + empty vector, P<0.001).
Conversely, the empty vector did not affect proliferation on day 7
(P=0.095), migration (P=0.352) or invasion (P=0.063) compared with
the HSC-3 shDKK3 group (Fig.
5A–D).
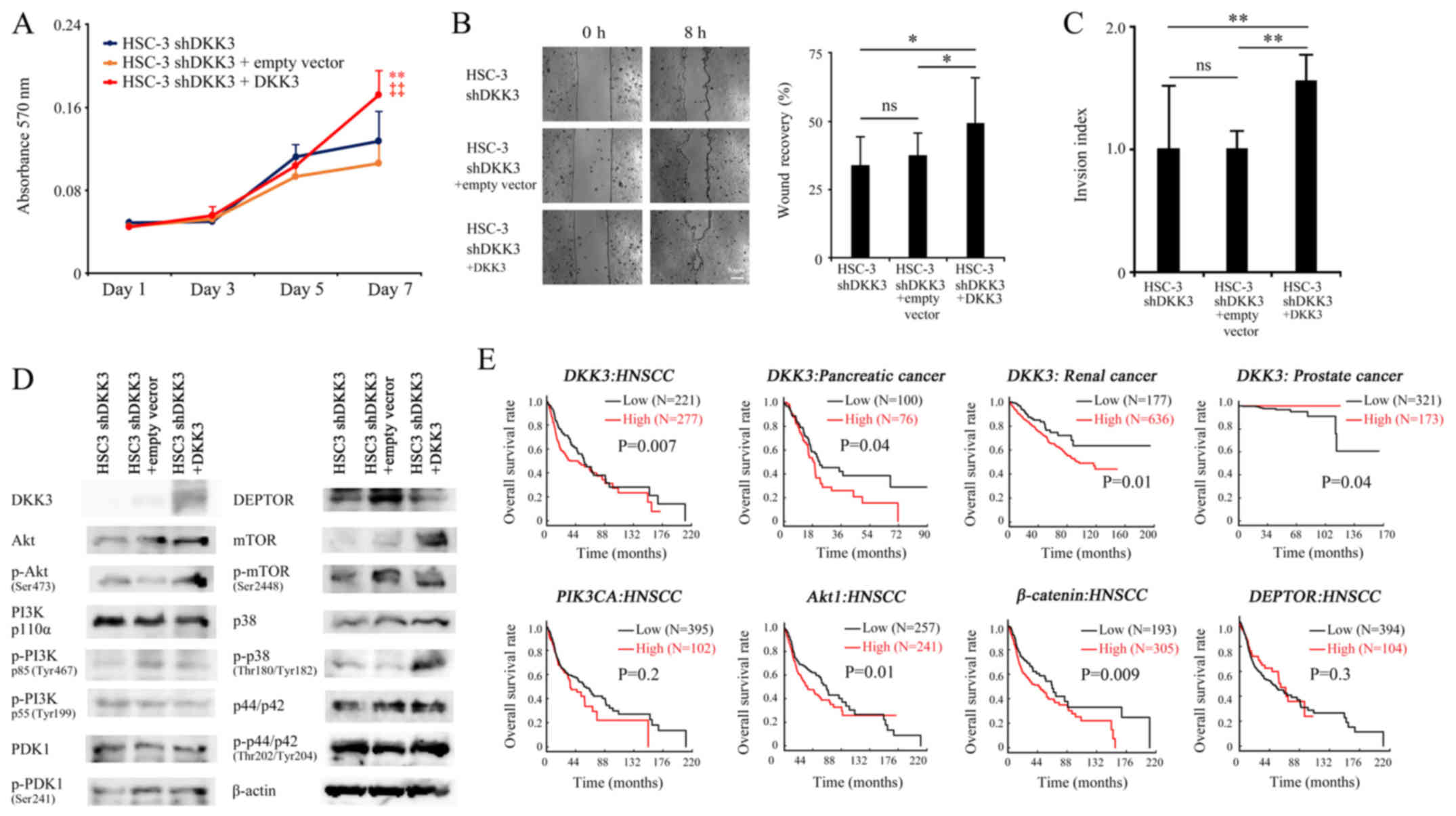 | Figure 5Effects of transfection with a
DKK3-expressing plasmid on HSC-3 shDKK3 cells. (A–C) DKK3
overexpression significantly rescued cellular proliferation,
migration and invasion, (D) elevated phosphorylation of Akt
(Ser473) and p38 mitogen-activated protein kinase (Thr180/Tyr204),
and decreased expression of DEPTOR. Scale bar, 50 µm.
*P<0.05, **P<0.01 vs. HSC-3 cells;
‡‡P<0.01 vs. HSC-3 shScr cells. (E) Kaplan-Meier
analyses based on The Cancer Genome Atlas database revealed that
high DKK3 expression was associated with poorer OS in HNSCC,
pancreatic cancer and renal cancer, whereas, in prostate cancer,
high DKK3 expression was associated with a better OS. Although not
significant, the data revealed that high mRNA expression levels of
PIK3CA and low mRNA expression levels of DEPTOR may be attributed
to a poorer OS, whereas high mRNA expression levels of Akt1 and
β-catenin were significantly associated with poorer prognosis. Akt,
protein kinase B; DEPTOR, DEP domain-containing mTOR-interacting
protein; DKK3, Dickkopf-related protein 3; HNSCC, head and neck
squamous cell carcinoma; mTOR, mechanistic target of rapamycin; ns,
not significant; OS, overall survival; p, phosphorylated; PDK1,
phosphoinositide 3-kinase; PI3K, phosphoinositide 3-kinase; PIK3CA,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α;
Scr, scrambled; sh/shRNA, short hairpin RNA. |
Western blotting revealed that DKK3 overexpression
in HSC-3 shDKK3 cells rescued the expression of DKK3, and elevated
phosphorylation of Akt (Ser473) and p38 MAPK (Thr180/Tyr204). In
addition, DKK3 overexpression decreased DEPTOR and, in turn,
increased expression of mTOR. However, phosphorylation of PI3K and
PDK1 was not altered in response to DKK3 overexpression (Fig. 5D).
Association between DKK3 and
PI3K/Akt/mTOR signaling molecules, and cancer prognosis
To evaluate whether DKK3 expression serves a
prognostic role in cancer, the mRNA expression levels of DKK3 were
analyzed in HNSCC, pancreatic cancer, renal cancer and prostate
cancer using TCGA data. The results revealed that high DKK3
expression was associated with poorer overall survival (OS) in
HNSCC, pancreatic cancer and renal cancer; however, in prostate
cancer, high DKK3 expression was associated with a better OS.
The present study also associated the association
between PI3K/Akt/mTOR signaling molecules and HNSCC. Although not
significant, the data revealed that high mRNA expression levels of
PIK3CA and low mRNA expression levels of DEPTOR may be attributed
to a poorer OS. Importantly, high mRNA expression levels of Akt1
and β-catenin were significantly associated with poorer prognosis
(Fig. 5E).
Discussion
HNSCC is common malignancy worldwide, which is
characterized by highly diverse biological behavior. It is widely
accepted that HNSCC occurs as a result of cumulative
genetic/epigenetic abnormalities in cancer-associated genes.
However, to date, a limited number of genes have been reported as
candidate somatic drivers for HNSCC, including tumor protein p53
(19), Akt1, APC, CCND1,
fibroblast growth factor receptor 3 (FGFR3), NOTCH1,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha (PI3KCA), and phosphatase and tensin homolog (PTEN) (20-24).
In addition, the HNSCC-specific cancer-associated genes/pathways
are not well illustrated.
We previously aimed to detect specific HNSCC-
associated genes by genome wide loss of heterozygosity analyses
(25,26), and focused on DKK3. DKK family
members can bind to lipoprotein receptor-related protein 5/6
(LRP5/6), by the β-propeller domain via a cysteine rich domain
(C2), and negatively regulate oncogenic Wnt signaling (27). However, due to the differences in
amino acid sequences in C2, DKK3 cannot bind to LRP5/6 (28); therefore, DKK3 is not thought to
modulate oncogenic Wnt signaling.
Our previous studies have illustrated unique and
specific expression of DKK3 in HNSCC (8-11),
and have reported that DKK3 exerts oncogenic functions in HNSCC.
Intriguingly, it has been demonstrated that DKK3 overexpression in
HNSCC cells results in significantly increased cellular malignant
properties in vitro and in vivo (11). To confirm the oncogenic roles of
DKK3, a stable knockdown of DKK3 was generated and the effects of
DKK3 loss-of-function were analyzed in HSC-3 cells. Compared with
in our previous gain-of-function experiment, DKK3 knockdown
resulted in decreased cellular proliferation, migration, invasion,
and tumor cell growth and proliferation in vivo. In
addition, microarray and pathway analyses suggested involvement of
the PI3K/mTOR/Akt and MAPK pathways, thus indicating that DKK3 may
modulate cancer cell malignant properties via the PI3K/mTOR/Akt and
MAPK pathways.
A recent study regarding gene alterations and
mutations suggested that the PI3K/Akt/mTOR and MAPK kinase-MAPK
signaling pathways may be targeted in HNSCC (29). PI3K/Akt/mTOR signaling is a
critical pathway for cell growth, survival and metabolism, and its
functional activation is frequently detected in HNSCC cells and
tissue samples (30-33). Activated PI3K results in activation
of Akt via direct phosphorylation of Akt (Ser473) (34). Phosphatidylinositol (3,4,5)-trisphosphate may also indirectly
activate Akt via recruiting PDK1 protein. Akt is a serine/threonine
kinase that regulates numerous cellular responses, including cell
proliferation, cell growth, protein synthesis and apoptosis.
Activated Akt can activate mTORC1 and mTORC2, and promote cell
growth and inhibition of apoptosis. The present data revealed that
DKK3 knockdown decreased phosphorylation of Akt (Ser473), which was
accompanied by decreased phosphorylation of PI3K (both p85 and p55)
and PDK1. These findings indicated that DKK3 may modulate PI3K/Akt
signaling both directly and indirectly. Generally, PI3K/Akt
signaling is activated by stimulation from an activated receptor
tyrosine kinase or G-protein coupled receptor. The present data
suggested the existence of a specific cell membrane receptor for
DKK3; however, no such receptor has been identified yet.
The present study explored another possible
mechanism. Akt is also directly activated by mTORC2 (34). DEPTOR, which is a component of
mTORC2, is an endogenous regulator that directly interacts with the
mTOR signaling pathway; overexpression of DEPTOR downregulates the
activity of mTORC1 and mTORC2 (35). The present data revealed that
DEPTOR expression was increased in HSC-3 shDKK3 cells, and DKK3
overexpression in HSC-3 shDKK3 cells decreased DEPTOR
expression.
Supporting the present data, the tumor suppressor
role of DEPTOR has been reported in several malignancies, including
squamous cell carcinoma (SCC). For example, Ji et al
reported that DEPTOR functions as a tumor suppressor, which
suppresses tumor cell growth of esophageal SCC, and downregulation
of DEPTOR expression predicts a poor prognosis (36). Recently, Baldassarri et al
reported that DEPTOR is under-expressed in normal and tumor tissues
in non-smoking female patients with lung SCC (37). With regards to the mechanism
underlying how DKK3 modulates Akt via mTOR, Gao et al
reported that stability of DEPTOR is controlled by the β-transducin
repeats-containing proteins (TrCP) E3 ubiquitin ligase, and that
failure to degrade DEPTOR by degron mutation or β-TrCP depletion
leads to reduced activity of mTOR (38). In addition, β-TrCP is known to
interact with intracellular DKK3 to block nuclear translocation of
β-catenin via β-catenin re-distribution (39,40).
Taken together, DKK3 may interact with β-TrCP and degrade DEPTOR,
thus resulting in mTORC2 activation and consequent Akt
phosphorylation. Therefore, DKK3 knockdown may result in increased
DEPTOR expression, and decreased phosphorylation of mTOR and
Akt.
To highlight the possible AKT/PI3K activation in
HNSCC and to confirm whether DKK3 serves oncogenic roles in other
types of cancer, TCGA tissue database was used. The results
demonstrated that high mRNA expression levels of DKK3 were an
unfavorable prognostic marker for HNSCC, pancreatic cancer and
renal cancer, despite previous reports suggesting the tumor
suppressor role of DKK3. Conversely, high DKK3 mRNA expression was
associated with a favorable prognosis in prostate cancer, which is
concordant with a previous report (6), thus suggesting that DKK3 may possess
tumor- or tissue-specific roles. Furthermore, high PIK3CA, Akt1 and
β-catenin mRNA expression, and low DEPTOR mRNA expression, were
associated with a poor prognosis.
Moreover, microarray and pathway analyses also
suggested other possible mechanisms associated with DKK3
expression, which may modulate the malignant potential of HNSCC
cells. For example, CXCL8, also known as interleukin 8, is a
chemokine secreted by macrophages, epithelial cells and endothelial
cells, which functions as a neutrophil chemotactic factor. It has
been reported that CXCL8 expression is high in HNSCC, contributing
to cancer cell invasion (41), and
that CXCL8 secreted by tumor-associated macrophages and cancer
cells enhances cancer cell migration and invasion in esophageal SCC
(42). In addition, high
expression of Rho-associated protein kinase-1 is associated with
lymph node metastasis in laryngeal SCC (43). Another important gene is
hypoxia-inducible factor 1α, of which high expression may be
associated with invasion of oral SCC (44). These alterations in the gene
expression profile of DKK3 knockdown cells may also partially
explain the decreased malignant potential of these cancer
cells.
In conclusion, the present study was the first, to
the best of our knowledge, to demonstrate the oncogenic function of
DKK3, and to report the possible molecular mechanism by which DKK3
activates Akt in HNSCC cells. The results indicated that DKK3 may
be considered a promising therapeutic target for the treatment of
HNSCC.
Funding
The present study was supported by a Grant-in-Aid
for Young Scientists (B) (grant no. 25861742 to NK) and a
Grant-in-Aid for Scientific Research (C) (grant no. 16K11470 to NK)
from MEXT KAKENHI; Research Project grants from Kawasaki Medical
School (grant nos. 25-S-3, 26-B-34 and 27-B-063 to NK), and grants
from the KAWASAKI Foundation for Medical Science and Medical
Welfare (grant no. 2013-06 to NK), the Wesco Scientific Promotion
Foundation (grant no. 2016-39 to NK) and the Takeda Science
Foundation (grant no. 2018047114 to NK).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article. The microarray data are
available in the GEO (accession no. GSE107403; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107403).
Authors' contributions
NK conceived and designed the experiments, and
prepared the paper. NK and SIN performed the experiments. NK, SIN,
AY, MY and SF analyzed the data. SF revised the manuscript and
figures, and approved the article. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Animal studies were conducted according to the
recommendations outlined in the Guidelines for Animal Experiments
at Kawasaki Medical School. Animal experiments were approved by the
Animal Care and Use Committee of Kawasaki Medical School.
Patient consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sarode GS, Sarode SC, Maniyar N, Anand R
and Patil S: Oral cancer databases: a comprehensive review. J Oral
Pathol Med. 47:547–556. 2018. View Article : Google Scholar
|
|
2
|
Ali J, Sabiha B, Jan HU, Haider SA, Khan
AA and Ali SS: Genetic etiology of oral cancer. Oral Oncol.
70:23–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Veeck J and Dahl E: Targeting the Wnt
pathway in cancer: The emerging role of Dickkopf-3. Biochim Biophys
Acta. 1825:18–28. 2012.
|
|
4
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows down-regulation in human
immortalized cells and human tumor-derived cell lines. Biochem
Biophys Res Commun. 268:20–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katase N and Nohno T: DKK3 (dickkopf 3
homolog (Xenopus laevis)). Atlas Genet Cytogenet Oncol Haematol.
17:678–686. 2013.
|
|
6
|
Edamura K, Nasu Y, Takaishi M, Kobayashi
T, Abarzua F, Sakaguchi M, Kashiwakura Y, Ebara S, Saika T,
Watanabe M, et al: Adenovirus-mediated REIC/Dkk-3 gene transfer
inhibits tumor growth and metastasis in an orthotopic prostate
cancer model. Cancer Gene Ther. 14:765–772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawasaki K, Watanabe M, Sakaguchi M,
Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH,
Kumon H, et al: REIC/Dkk-3 overexpression downregulates
P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces
apoptosis in breast cancer. Cancer Gene Ther. 16:65–72. 2009.
View Article : Google Scholar
|
|
8
|
Fujii M, Katase N, Lefeuvre M, Gunduz M,
Buery RR, Tamamura R, Tsujigiwa H and Nagatsuka H: Dickkopf (Dkk)-3
and β-catenin expressions increased in the transition from normal
oral mucosal to oral squamous cell carcinoma. J Mol Histol.
42:499–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katase N, Lefeuvre M, Gunduz M, Gunduz E,
Beder LB, Grenman R, Fujii M, Tamamura R, Tsujigiwa H and Nagatsuka
H: Absence of Dickkopf (Dkk)-3 protein expression is correlated
with longer disease-free survival and lower incidence of metastasis
in head and neck squamous cell carcinoma. Oncol Lett. 3:273–280.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katase N, Lefeuvre M, Tsujigiwa H, Fujii
M, Ito S, Tamamura R, Buery RR, Gunduz M and Nagatsuka H: Knockdown
of Dkk-3 decreases cancer cell migration and invasion independently
of the Wnt pathways in oral squamous cell carcinoma-derived cells.
Oncol Rep. 29:1349–1355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katase N, Nishimatsu SI, Yamauchi A,
Yamamura M, Terada K, Itadani M, Okada N, Hassan NMM, Nagatsuka H,
Ikeda T, et al: DKK3 overexpression increases the malignant
properties of head and neck squamous cell carcinoma cells. Oncol
Res. 26:45–58. 2018. View Article : Google Scholar
|
|
12
|
de Jonge HJ, Fehrmann RS, de Bont ES,
Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te
Meerman GJ and ter Elst A: Evidence based selection of housekeeping
genes. PLoS One. 2:e8982007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katase N, Terada K, Suzuki T, Nishimatsu S
and Nohno T: miR-487b, miR-3963 and miR-6412 delay myogenic
differentiation in mouse myoblast-derived C2C12 cells. BMC Cell
Biol. 16:132015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar
|
|
15
|
Huang W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar :
|
|
16
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBio-Portal. Sci Signal. 6:pp. pl12013,
View Article : Google Scholar
|
|
17
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357:pp. eaan25072017, View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pickering CR, Zhang J, Yoo SY, Bengtsson
L, Moorthy S, Neskey DM, Zhao M, Ortega Alves MV, Chang K, Drummond
J, et al: Integrative genomic characterization of oral squamous
cell carcinoma identifies frequent somatic drivers. Cancer Discov.
3:770–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma V, Nandan A, Sharma AK, Singh H,
Bharadwaj M, Sinha DN and Mehrotra R: Signature of genetic
associations in oral cancer. Tumour Biol. 39:10104283177259232017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khayer N, Zamanian-Azodi M, Mansouri V,
Ghassemi-Broumand M, Rezaei-Tavirani M, Heidari MH and Rezaei
Tavirani M: Oral squamous cell cancer protein-protein interaction
network interpretation in comparison to esophageal adenocarcinoma.
Gastroenterol Hepatol Bed Bench. 10:118–124. 2017.PubMed/NCBI
|
|
22
|
Upadhyay P, Gardi N, Desai S, Chandrani P,
Joshi A, Dharavath B, Arora P, Bal M, Nair S and Dutt A: Genomic
characterization of tobacco/nut chewing HPV-negative early stage
tongue tumors identify MMP10 asa candidate to predict metastases.
Oral Oncol. 73:56–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nayak S, Goel MM, Makker A, Bhatia V,
Chandra S, Kumar S and Agarwal SP: Fibroblast growth factor (FGF-2)
and its receptors FGFR-2 and FGFR-3 may be putative biomarkers of
malignant transformation of potentially malignant oral lesions into
oral squamous cell carcinoma. PLoS One. 10:e01388012015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang KY, Tsai SY, Chen SH, Tsou HH, Yen
CJ, Liu KJ, Fang HL, Wu HC, Chuang BF, Chou SW, et al: Dissecting
the EGFR-PI3K-AKT pathway in oral cancer highlights the role of the
EGFR variant III and its clinical relevance. J Biomed Sci.
20:432013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katase N, Gunduz M, Beder L, Gunduz E,
Lefeuvre M, Hatipoglu OF, Borkosky SS, Tamamura R, Tominaga S,
Yamanaka N, et al: Deletion at Dickkopf (dkk)-3 locus (11p15.2) is
related with lower lymph node metastasis and better prognosis in
head and neck squamous cell carcinomas. Oncol Res. 17:273–282.
2008. View Article : Google Scholar
|
|
26
|
Katase N, Gunduz M, Beder LB, Gunduz E, Al
Sheikh Ali M, Tamamura R, Yaykasli KO, Yamanaka N, Shimizu K and
Nagatsuka H: Frequent allelic loss of Dkk-1 locus (10q11.2) is
related with low distant metastasis and better prognosis in head
and neck squamous cell carcinomas. Cancer Invest. 28:103–110. 2010.
View Article : Google Scholar
|
|
27
|
Chen L, Wang K, Shao Y, Huang J, Li X,
Shan J, Wu D and Zheng JJ: Structural insight into the mechanisms
of Wnt signaling antagonism by Dkk. J Biol Chem. 283:23364–23370.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujii Y, Hoshino T and Kumon H: Molecular
simulation analysis of the structure complex of C2 domains of DKK
family members and β-propeller domains of LRP5/6: Explaining why
DKK3 does not bind to LRP5/6. Acta Med Okayama. 68:63–78. 2014.
|
|
29
|
Van Waes C and Musbahi O: Genomics and
advances towards precision medicine for head and neck squamous cell
carcinoma. Laryngoscope Investig Otolaryngol. 2:310–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang SQ, Wang X, Zheng K, Liu KS, Wang SX
and Xie CH: Simultaneous targeting PI3K and PERK pathways promotes
cell death and improves the clinical prognosis in esophageal
squamous carcinoma. Biochem Biophys Res Commun. 493:534–541. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Valera JC, Zhao X, Chen Q and
Silvio Gutkind J: mTOR co-targeting strategies for head and neck
cancer therapy. Cancer Metastasis Rev. 36:491–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simpson DR, Mell LK and Cohen EE:
Targeting the PI3K/AKT/mTOR pathway in squamous cell carcinoma of
the head and neck. Oral Oncol. 51:291–298. 2015. View Article : Google Scholar
|
|
33
|
Li SH, Chien CY, Huang WT, Luo SD, Su YY,
Tien WY, Lan YC and Chen CH: Prognostic significance and function
of mammalian target of rapamycin in tongue squamous cell carcinoma.
Sci Rep. 7:81782017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rogers SJ, Box C, Harrington KJ, Nutting
C, Rhys-Evans P and Eccles SA: The phosphoinositide 3-kinase
signalling pathway as a therapeutic target in squamous cell
carcinoma of the head and neck. Expert Opin Ther Targets.
9:769–790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peterson TR, Laplante M, Thoreen CC,
Sancak Y, Kang SA, Kuehl WM, Gray NS and Sabatini DM: DEPTOR is an
mTOR inhibitor frequently overexpressed in multiple myeloma cells
and required for their survival. Cell. 137:873–886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji YM, Zhou XF, Zhang J, Zheng X, Li SB,
Wei ZQ, Liu T, Cheng DL, Liu P, Song K, et al: DEPTOR suppresses
the progression of esophageal squamous cell carcinoma and predicts
poor prognosis. Oncotarget. 7:14188–14198. 2016.PubMed/NCBI
|
|
37
|
Baldassarri M, Fallerini C, Cetta F,
Ghisalberti M, Bellan C, Furini S, Spiga O, Crispino S, Gotti G,
Ariani F, et al: Omic approach in non-smoker female with lung
squamous cell carcinoma pinpoints to germline susceptibility and
personalized medicine. Cancer Res Treat. 50:356–365. 2018.
View Article : Google Scholar :
|
|
38
|
Gao D, Inuzuka H, Tan MK, Fukushima H,
Locasale JW, Liu P, Wan L, Zhai B, Chin YR, Shaik S, et al: mTOR
drives its own activation via SCF(βTrCP)-dependent degradation of
the mTOR inhibitor DEPTOR. Mol Cell. 44:290–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee EJ, Jo M, Rho SB, Park K, Yoo YN, Park
J, Chae M, Zhang W and Lee JH: Dkk3, downregulated in cervical
cancer, functions as a negative regulator of beta-catenin. Int J
Cancer. 124:287–297. 2009. View Article : Google Scholar
|
|
40
|
Leonard JL, Leonard DM, Wolfe SA, Liu J,
Rivera J, Yang M, Leonard RT, Johnson JPS, Kumar P, Liebmann KL, et
al: The Dkk3 gene encodes a vital intracellular regulator of cell
proliferation. PLoS One. 12:e01817242017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Warner KA, Miyazawa M, Cordeiro MM, Love
WJ, Pinsky MS, Neiva KG, Spalding AC and Nör JE: Endothelial cells
enhance tumor cell invasion through a crosstalk mediated by CXC
chemokine signaling. Neoplasia. 10:131–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hosono M, Koma YI, Takase N, Urakawa N,
Higashino N, Suemune K, Kodaira H, Nishio M, Shigeoka M, Kakeji Y,
et al: CXCL8 derived from tumor-associated macrophages and
esophageal squamous cell carcinomas contributes to tumor
progression by promoting migration and invasion of cancer cells.
Oncotarget. 8:106071–106088. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang J, He X, Ma Y, Liu Y, Shi H, Guo W
and Liu L: Overexpression of ROCK1 and ROCK2 inhibits human
laryngeal squamous cell carcinoma. Int J Clin Exp Pathol.
8:244–251. 2015.PubMed/NCBI
|
|
44
|
Lin PY, Yu CH, Wang JT, Chen HH, Cheng SJ,
Kuo MY and Chiang CP: Expression of hypoxia-inducible factor-1
alpha is significantly associated with the progression and
prognosis of oral squamous cell carcinomas in Taiwan. J Oral Pathol
Med. 37:18–25. 2008. View Article : Google Scholar
|