Introduction
Acute lymphoblastic leukemia (ALL) is the most
common childhood malignancy (1).
Chromosomal translocations are the hallmark of pediatric ALL, and
generate fusion genes encoding chimeric transcription factors. The
translocation t(17;19)(q22;p13) that results in the fusion gene
E2A-HLF (2,3) defines a rare subtype of ALL that
accounts for ~1% of pediatric B-cell precursor ALL cases and is
associated with a very poor prognosis (4,5).
The immunoglobulin enhancer-binding factor
(E2A) gene encodes two proteins, E12 and E47, which are
members of the basic helix-loop-helix (bHLH) family of
transcriptions factors, and it is required for proper B-cell
development (6-8). Hepatic leukemia factor (HLF)
encodes a transcription factor of the basic leucine zipper (bZIP)
family containing a proline- and acidic amino acid-rich (PAR)
domain, which enables it to form either homodimers or heterodimers
with other PAR protein family members (2,9,10).
In the E2A-HLF fusion protein, the two transactivation domains, AD1
and AD2, of E2A are fused to the bZIP DNA-binding and dimerization
domain of HLF (3). The expression
of the E2A-HLF fusion gene results in transcriptional
reprogramming with dedifferentiation in pre-leukemic cells
(11). E2A-HLF promotes the
anchorage-independent growth of murine fibroblasts (12). Human leukemic cells expressing
E2A-HLF rapidly undergo apoptosis when programmed to express a
dominant-negative mutant of E2A-HLF. In addition, the conditional
expression of E2A-HLF prevents apoptosis induced by cytokine
withdrawal in interleukin (IL)-3-dependent mouse Ba/F3 cells
(13). However, B-cell
progenitor-specific conditional E2A-HLF knock-in mice
exhibit hyposplenia and lymphopenia, whereas hematopoietic
stem/progenitor cell (HSPC)-specific ones are embryonically lethal
(14). The E2A-HLF fusion likely
requires additional events to cause leukemia, since immunoglobulin
enhancer and promoter-driven E2A-HLF transgenic and knock-in mice
exhibit maturation arrest and apoptosis in cells expressing
E2A-HLF (14-16). Numerous molecules downstream of
E2A-HLF or cooperative with E2A-HLF have since been identified,
including transcription factor Lim domain only 2 (LMO2), which is
involved in T-cell ALL (17,18);
snail family transcriptional repressor 2 (19); nuclear factor, interleukin 3
regulated (20); Groucho-related
genes (21); Annexin VIII and
sushi-repeat protein upregulated in leukemia, which have
paraneoplastic roles in E2A-HLF-expressing leukemia (22); Zfp521 (23); survivin (24); and death receptors DR4/DR5
(25).
Drosophila eyes absent homolog 2 protein
belongs to the eyes absent (Eya) family of proteins and acts as a
transcriptional co-activator. Eya proteins serve a critical role in
fly eye development and are also involved in numerous processes,
including organ development, innate immunity, and DNA damage
repair. Eya proteins have threonine phosphatase and transactivation
activity in the N-terminal domain and tyrosine phosphatase and
protein-interacting activity in the C-terminal domain. Eya proteins
are located in the cytoplasm and are translocated into the nucleus
following binding to Six protein for transactivation (26-28).
Human EYA transcriptional coactivator and phosphatase 2
(EYA2) is located on chromosome 20q13 (29). EYA2 was reported to be
upregulated in ovarian and breast cancer and astrocytoma (30-32).
Eya2 was also reported to promote metastasis of breast cancer cells
(31) and to promote the
proliferation and invasion of human astrocytoma cells (32). By contrast, silencing of
EYA2 promotes tumor growth in pancreatic adenocarcinoma,
indicating the tumor suppressive function of EYA2 (33). Eya2 is differentially
expressed in mouse long-term hematopoietic stem cells (34) and confers an aberrant self-renewal
capacity in HSPCs (35). In
addition, Eya2 is critically involved in leukemogenesis via zinc
finger and BTB domain containing 16-retinoic acid receptor-α
(PLZF-RARA) resulting from t(11;17)(q23;q21) in patients
with acute promyelocytic leukemia (35).
To identify effective therapeutic targets in
leukemia with E2A-HLF, it is important to clarify the
molecular mechanism underlying E2A-HLF-mediated leukemogenesis. The
present study investigated the upregulation of Eya2 by
E2A-HLF through promotor binding. It was also demonstrated that
Eya2 has a crucial role in the aberrant self-renewal capacity
conferred by E2A-HLF. Eya2 knockdown experiments via
retrovirally-expressed short hairpin (sh)RNA revealed the critical
involvement of Eya2 in immortalizing HSPCs. The present findings
therefore identified Eya2 as one of the key players downstream of
oncogenic E2A-HLF.
Materials and methods
Mice
Mice were kept under standardized (temperature,
22-24°C; humidity, 45-65%; 12 h light/12 h dark cycle; free access
to food and water) and specific pathogen-free conditions until
sacrifice. For purification of mouse HSPCs, bone marrow cells were
harvested from one to two female C57BL/6N mice (8-12 weeks old;
body weight, 17-21 g) which were purchased from Japan SLC, Inc.
(Hamamatsu, Japan), for each experiment (n=13 in total). All animal
studies were approved by the Animal Care Committees of Mie
University (Tsu, Japan).
Reagents
G418 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and puromycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) were used at final concentrations of 1 mg/ml
and 1 µg/ml, respectively, for drug selection.
Construction of the plasmids and
retroviral vectors
The retroviral vectors used in this study,
pMYs-IRES-Neomycinr (pMYs-IN) and pMXsU6-Kusabira Orange
(KO), were previously described (36,37).
The pMYs-E2A-HLF-IN vector was also described previously (36). To produce the E2A-pre-B-cell
leukemia transcription factor 1 (E2A-PBX1) (38) fusion fragment, a portion of
PBX1 encoding amino acid residues 89-430 (39) generated by polymerase chain
reaction (PCR) using cDNA derived from K562 cells (provided by Dr.
Toshio Kitamura, The Institute of Medical Science, The University
of Tokyo, Tokyo, Japan), was inserted into pMYs-E2A-HLF-IN to
replace that of HLF. E2A-HLF mutants were prepared as
previously reported (40). Mutants
lacking the AD1 domain, AD2 domain, bZIP domain, and a part of the
basic region, respectively, were generated by site-directed
mutagenesis using PCR with the wild-type E2A-HLF construct
as a template, followed by cloning in a series of pMYs retroviral
vectors (37). The E2A-HLF
mutants were as follows: i) ∆AD1, which lacks 426 bp (Met-1 to
Gly-142); ii) ∆AD2, which lacks the 405 bp PvuII-NaeI
restriction fragment (Leu-278 to Ala-412) in the E2A
transactivation region; iii) ∆bZIP, which lacks 132 bp (Try-508 to
Ala-551) in the bZIP domain of HLF; and iv) ∆509-518, which lacks
30 bp (Ala-509 to Ala-518) in the basic region of HLF. For the
chromatin immunoprecipitation (ChIP) analysis, E2A-HLF was fused
with the FLAG epitope tag at the N-terminus in pMYs-E2A-HLF-IN. PCR
for construction was performed with Phusion High-Fidelity DNA
Polymerase (New England Biolabs Inc., Ipswich, MA, USA), according
to the manufacturer's protocol. Each insert fragment in the plasmid
was validated by DNA sequence analysis. For the reporter assay,
E2A-HLF and E2A-PBX1 cDNAs were subcloned into
pcDNA3.1+ expression vector (Invitrogen; Thermo Fisher Scientific,
Inc.), respectively.
Purification of mouse HSPCs
Mouse HSPCs were purified as described (36). In brief, bone marrow mononuclear
cells (BMMNCs) were prepared from 8- to 12-week-old C57BL/6N mice.
Using a MACS cell separation system (Miltenyi Biotec, Inc., Auburn,
CA, USA), Lin-depleted cells were isolated from BMMNCs, and
c-Kit+Sca−1+Lin− (KSL)
cells were purified from Lin-depleted cells using a FACSAria
operated with FACSDiVa version 6.1.3 software (BD Biosciences, San
Jose, CA, USA).
Retrovirus production and
transduction
Plat-E packaging cells (41) were plated at a density of
4.5×105/ml and trans-fected the following day with
retroviral constructs using Polyethylenimine 'Max' (Polysciences,
Inc., Warrington, PA, USA), according to the manufacturer's
protocol. Retroviral supernatants of transfected Plat-E cells were
harvested 48 h post-transfection following two medium changes. KSL
and immortalized cells were transduced with retroviruses using
RetroNectin (Takara Bio Inc., Otsu, Japan) for 48-72 h at 37°C, as
previously described (36).
Myeloid immortalization assay
Myeloid immortalization assays via serial replating
were performed as previously described (36). In brief, every 5-7 days, colonies
were counted, followed by replating of the harvested cells
(1×104 cells/dish) in methylcellulose culture medium
MethoCult™ M3234 (STEMCELL Technologies, Inc., Vancouver, BC,
Canada) supplemented with 25 ng/ml mouse stem cell factor (SCF)
(Miltenyi Biotec, Inc.), 10 ng/ml each of mouse IL-3 (Miltenyi
Biotec, Inc.), human IL-6 (Miltenyi Biotec, Inc.), and mouse
granulocyte macrophage-colony stimulating factor (GM-CSF) (Miltenyi
Biotec, Inc.).
To evaluate the effects of knockdown of Eya2
in the immortalized cells, the Eya2-depleted cells were plated in
the same methylcellulose culture medium as that used in the
immortalization assays. Relative colony-forming units (CFUs) were
calculated as a percentage of the colony numbers compared with the
corresponding controls (normalized to 100%) in each experiment
following culture for 5-7 days.
Fluorescence-activated cell sorting
(FACS) analysis
An immunophenotypical analysis was performed using a
FACSCalibur and BD Cell Quest Pro version 5.2.1 software (BD
Biosciences), as previously described (42).
Gene silencing by RNA interference
The target sequences against the Eya2 and
luciferase genes were 5′-GTGTTTCAG AGACAATCAT-3′ (shE09) and
5′-GCCTTATGCCGCCAT CTTG-3′ (shE12), and 5′-GGCTATGAAGAGATACGCC-3′
(shLuc). The shE09 and shE12 were two out of twelve sequences
(shE01-shE12) designed for Eya2 knockdown, which exerted the
most efficient effects (35). To
design a short hairpin structure, a loop sequence
(5′-CTTCAAGAGAG-3′) was used (35). Short hairpin RNA (shRNA)
sequence(s) against Eya2 or luciferase were cloned
into the retroviral vector pMXsU6-KO (36). Each 9 µg DNA of pMXsU6-KO
derivative was transfected into Plat-E cells (41) in a 10 cm dish to produce
retroviruses, as described above. The filtered culture supernatant
of Plat-E cells containing retroviruses 48 h post-transfection was
used for transduction of E2A-HLF-immortalized mouse KSL
cells (2.5×105) for 48 h at 37°C using RetroNectin
(Takara Bio, Inc.) in liquid culture containing SCF, IL-3, IL-6,
and GM-CSF. The shRNA-transduced cells were then subjected to
sorting by KO expression on the FACSAria operated by FACSDiVa
version 6.1.3 software. The sorted KO+ cells were
cultured for 5-7 days for a colony-forming assay as previously
described (36).
ChIP
ChIP was performed as previously described (36). In brief, the chromatin prepared
from FLAG-tagged E2A-HLF-immortalized cells was precipitated
using Dynabeads anti-Mouse IgG (Invitrogen; Thermo Fisher
Scientific, Inc.) preincubated with a mouse monoclonal anti-FLAG
(cat. no. M2; Sigma-Aldrich; Merck KGaA), a mouse monoclonal
anti-RNA polymerase II (cat. no. CTD4H8; EMD Millipore, Billerica,
MA, USA), or a mouse IgG1 antibody (BioLegend, Inc., San Diego, CA,
USA). The purified DNA in the precipitates was subjected to
quantitative polymerase chain reaction (qPCR).
Reverse transcription (RT), qPCR, RT-qPCR
and RT-PCR
Total RNA was extracted using TRI Reagent LS
(Molecular Research Center, Inc., Cincinnati, OH, USA). RT was
performed with random hexamers using SuperScript II reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) as
previously described (36). The
qPCR analyses were performed using the KOD SYBR qPCR Mix (for ChIP
products; Toyobo Life Science, Osaka, Japan) or
PowerSYBR® Green PCR Master Mix (for cDNA) on a
StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) (36).
For the RT-qPCR analysis, PCR was performed for 40 cycles (94°C for
15 sec, 57°C for 15 sec and 72°C for 34 sec) in a total volume of
12 µl containing appropriately diluted cDNA. Following
quantification of the expression of samples using the
quantification cycle (2−ΔΔCT) method (43) and performing normalization relative
to β-2-microglobulin (B2m), the relative expression was
calculated. The sequences of the primers used (Eya1,
Eya2, Eya3, Eya4, and B2m) have been
previously described (35,36). For the ChIP-qPCR analysis, PCR was
performed for 40 cycles (98°C for 10 sec, 60°C for 10 sec and 68°C
for 34 sec) in a total volume of 20 µl. When the CT values
of the ChIP products had been measured and normalized to those of
the corresponding input samples using the 2−ΔΔCT method,
the percentages of samples relative to the input were calculated.
The primer sets (Eya2c-4) for ChIP-qPCR were described
previously (35). The promoter
region of hemoglobin subunit β1 was used as a negative control.
RT-PCR was performed as previously described (42). In brief, to detect the transcripts
of E2A-HLF, standard PCR amplification of cDNA was performed
for 30 cycles (98°C for 10 sec and 68°C for 40 sec) using LA Taq
(Takara Bio Inc.). The primers specific for E2A-HLF were as
follows: E2A S4, 5′-GATAGAAGACCA CCTGGACGAG-3′; E2A S2,
5′-GTGAGGACTACGGCA GGGAT-3′; HLF AS1, 5′-CCAGCTCCTTCCTCAAGTCAG-3′;
and HLF ASx, 5′-gaaagaattcaCAGGGGCCCGTGCCTGG-3′ (small letters,
non-related sequence). To detect transcripts of B2m, PCR
amplification of cDNA was performed for 26 cycles (94°C for 30 sec,
60°C for 30 sec and 72°C for 30 sec) using Quick TaqR HS DyeMix
(Toyobo Life Science). The primers specific for B2m were as
previously described (44).
Together with 1 kb DNA ladder (New England Biolabs, Inc.) as a DNA
size marker, the PCR products were electrophoresed on a 1%
Tris-Acetate-EDTA agarose (Nacalai Tesque, Inc., Kyoto, Japan) gel,
subsequently stained with ethidium bromide (VWR International,
Radnor, PA, USA), and were visualized with an ultraviolet
transilluminator system, Gel Doc 2000 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's
recommendation.
Western blot analyses
The expression of transgenes in transfected Plat-E
cells was examined by western blot analysis, as previously
described (45). In brief, the
transfected cells were harvested with lysis buffer supplemented
with protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) and 2
mM phenylmethylsulfonyl fluoride. The lysates were mixed with an
equal volume of 2X SDS sample buffer and boiled for 5 min. Western
blot analyses of the samples obtained from the cells transfected
with pMYs-IN, pMYs-E2A-HLF-IN, pMYs-E2A-PBX1-IN, pMYs-∆AD1-IN,
pMYs-∆AD2-IN, pMYs-∆bZIP-IN, and pMYs-∆509-518-IN were performed
using mouse monoclonal anti-E2A (Yae) (at 1:1,000 dilution for 1 h
at room temperature; cat. no. sc-416; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and anti-α-tubulin (at 1:10,000 dilution for
1 h at room temperature; cat. no. T-5168; Sigma-Aldrich; Merck
KGaA) antibodies, followed by reaction with a horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibody (at
1:5,000 dilution for 1 h at room temperature; cat. no. 330; Medical
& Biological Laboratories Co., Ltd., Nagoya, Japan).
Luciferase reporter constructs and
assays
To generate pGL3-mP, a minimal promotor (mP)
sequence (derived from pGL 4.23; Promega Corporation, Madison, WI,
USA) was inserted between the NheI and HindIII sites
in the pGL3-Basic Vector (Promega Corporation). The putative
promotor region of Eya2 (-2219 to -226, relative to the ATG
of the translation initiation site) was inserted upstream of the mP
sequence in pGL3-mP to generate pGL3-E2pro3. pGL3-E2pro3CSmut with
the sequence CTATTCTAGT instead of CTTACCTAGT, a putative
DNA-binding sequence of E2A-HLF that is similar to the consensus
sequence (CS) GTTACGTAAT (9), was
generated by PCR-mediated mutagenesis. All constructs for
appropriate insertions were confirmed by DNA sequence analyses.
Human leukemic K562 cells (2.5×106) were resuspended in
400 µl K-PBS (NaCl, 30.8 mM; KCl, 120.7 mM;
Na2HPO4, 8.1 mM;
KH2PO4, 1.46 mM) containing reporter (6.6
µg pGL3-E2pro3 or pGL3-E2pro3CSmut), equimolar amounts of
effector (pcDNA-E2A-HLF, pcDNA-E2A-PBX1 or empty vector), and 0.5
µg internal control (phTK-RL; Promega Corporation) plasmid
DNA. The reporter-to-effector molar ratio of the DNA was 2:1. These
cells were electroporated at 170 V and 950 µF in a 4 mm
cuvette using Gene Pulser Xcell (Bio-Rad Laboratories, Inc.), and
were incubated in the culture medium for 48 h. Cells were harvested
post-incubation and lysed in 100 µl lysis buffer (Promega
Corporation). The activity of firefly and Renilla
luciferases in each lysate was measured sequentially using a Dual
Luciferase Assay System (Promega Corporation) on a luminometer
(TD-20/20; Turner Designs, Sunnyvale, CA, USA), according to the
manufacturer's protocol.
Statistical analyses
Analyzed data are presented as mean ± standard
deviation of three independent experiments. Comparisons of bar
charts between two groups were performed using an unpaired
one-tailed Student's t-test. As for the statistical comparisons of
more than two groups, the assumption of homogeneity of variance was
first tested using Bartlett's test. In cases where the variances
were not equal at the 0.05 level, statistical differences were
analyzed using a Friedman test as a non-parametric analysis of
variance, followed by Steel's test as a post hoc multiple
comparison test. All statistical tests were performed using R
software version 3.1.2 (https://www.r-project.org/).
Results
Eya2 upregulation in E2A-HLF-immortalized
cells
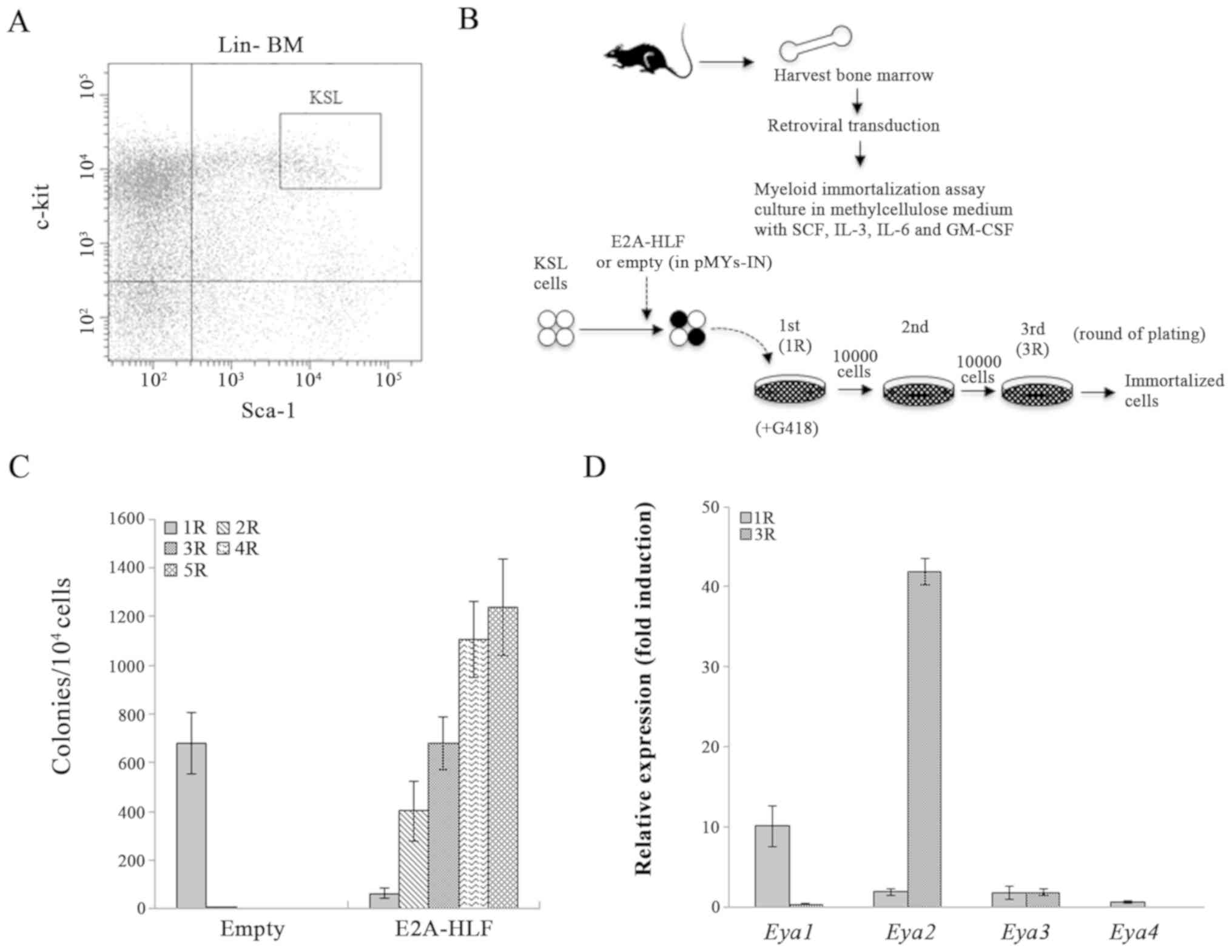
KSL cells were sorted from Lin-depleted bone marrow
cells, retrovirally transduced with the E2A-HLF fusion
oncogene, and subjected to a myeloid immortalization assay.
E2A-HLF transduction immortalized KSL cells (Fig. 1A–C). Since it was recently revealed
that Eya2 is important for inducing aberrant self-renewal activity
in HSPCs (35), the expression of
the Eya family genes Eya1, Eya2, Eya3,
and Eya4 was analyzed in E2A-HLF-transduced
colony-forming cells. An RT-qPCR analysis revealed that Eya2
was exclusively highly expressed among these genes following serial
replating (Fig. 1D). Although the
Eya1 expression was increased compared with that of
Eya2 in the first-round colonies, the Eya1 expression
was much lower compared with that of Eya2 in the third-round
colonies. Eya3 and Eya4 were not highly expressed in
either the first- or third-round colonies. These results suggested
that an E2A-HLF fusion product induced upregulation of Eya2
in HSPCs, which may be associated with aberrant self-renewal
activity in E2A-HLF-immortalized HSPCs.
E2A-HLF upregulates Eya2 expression in
the aberrant self-renewal program
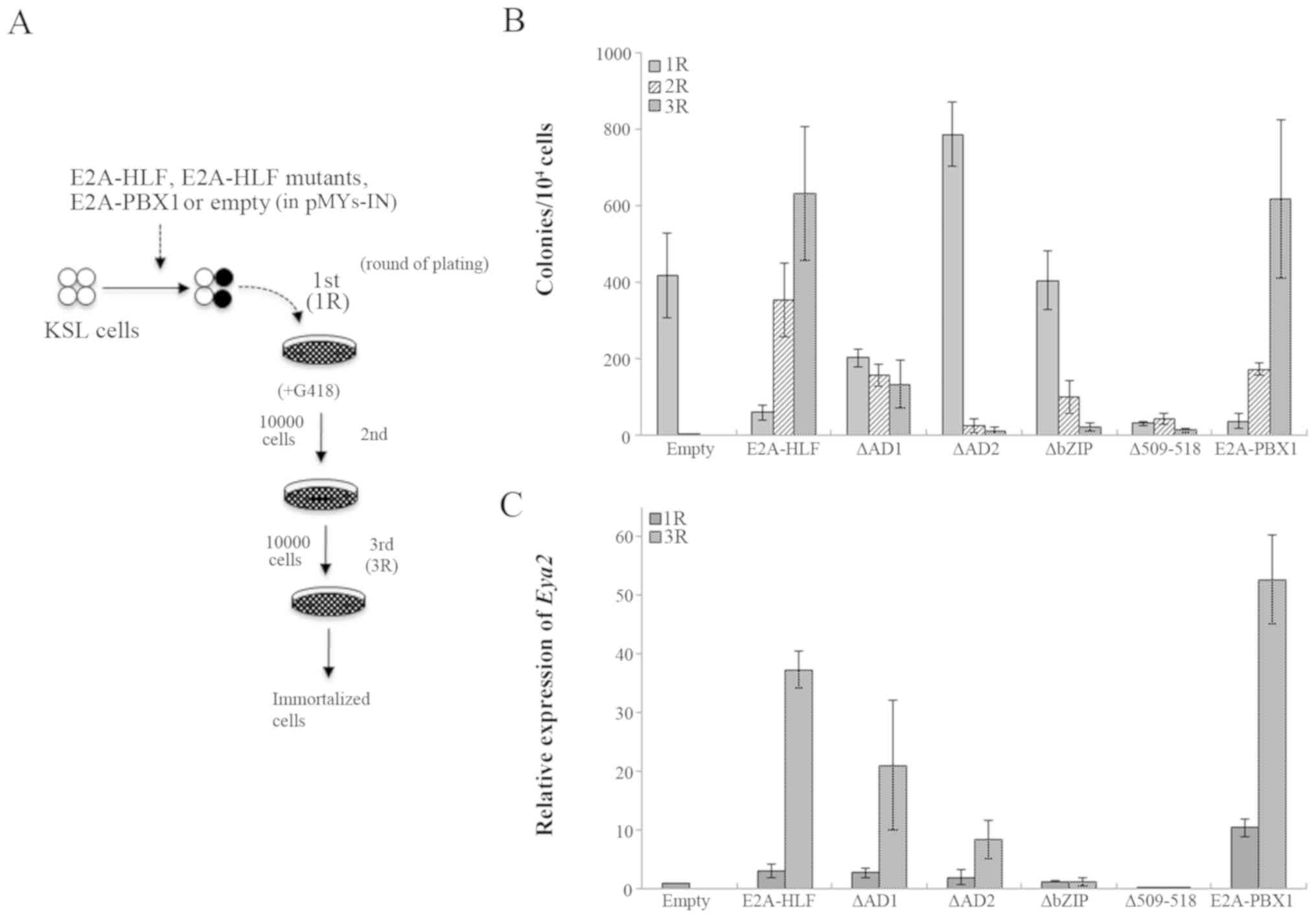
To investigate the molecular mechanism underlying
the upregulation of Eya2 by E2A-HLF in HSPCs, mouse KSL
cells were retrovirally transduced with E2A-HLF and its
mutants (Fig. 2A) and subjected to
myeloid immortalization assays. The immortalized cells exhibited an
immunophenotype of myeloid lineage with high expression of c-kit
(Fig. 2B). Initial plating showed
similar colony morphologies and reflected the transduction
efficiency. In serial replating, E2A-HLF-transduced cells
yielded and maintained increased numbers of dense colonies, whereas
E2A-HLF mutant-transduced cells formed loose colonies that
were smaller in size compared with those with E2A-HLF at the
third round (Figs. 2C and D, and
3A) and failed to be immortalized
(data not shown). A western blot analysis indicated similar
expression of each mutant protein to that of wild-type E2A-HLF
(Fig. 3B). The expression of the
chimeric transcripts of E2A-HLF and E2A-HLF mutants
was detected by RT-PCR of total RNA extracted from the transduced
colonies at the first- and third-round plating, respectively.
RT-PCR using primers E2A S4 and HLF AS1 in E2A-HLF, ∆AD1 and
∆509-518 generated 497, 497 and 467 bp products, respectively,
while that using primers E2A S4 and HLF ASx in ∆bZIP generated a
426 bp product, and that using primers E2A S2 and HLF AS1 in ∆AD2
generated a 696 bp product (Fig.
3C).
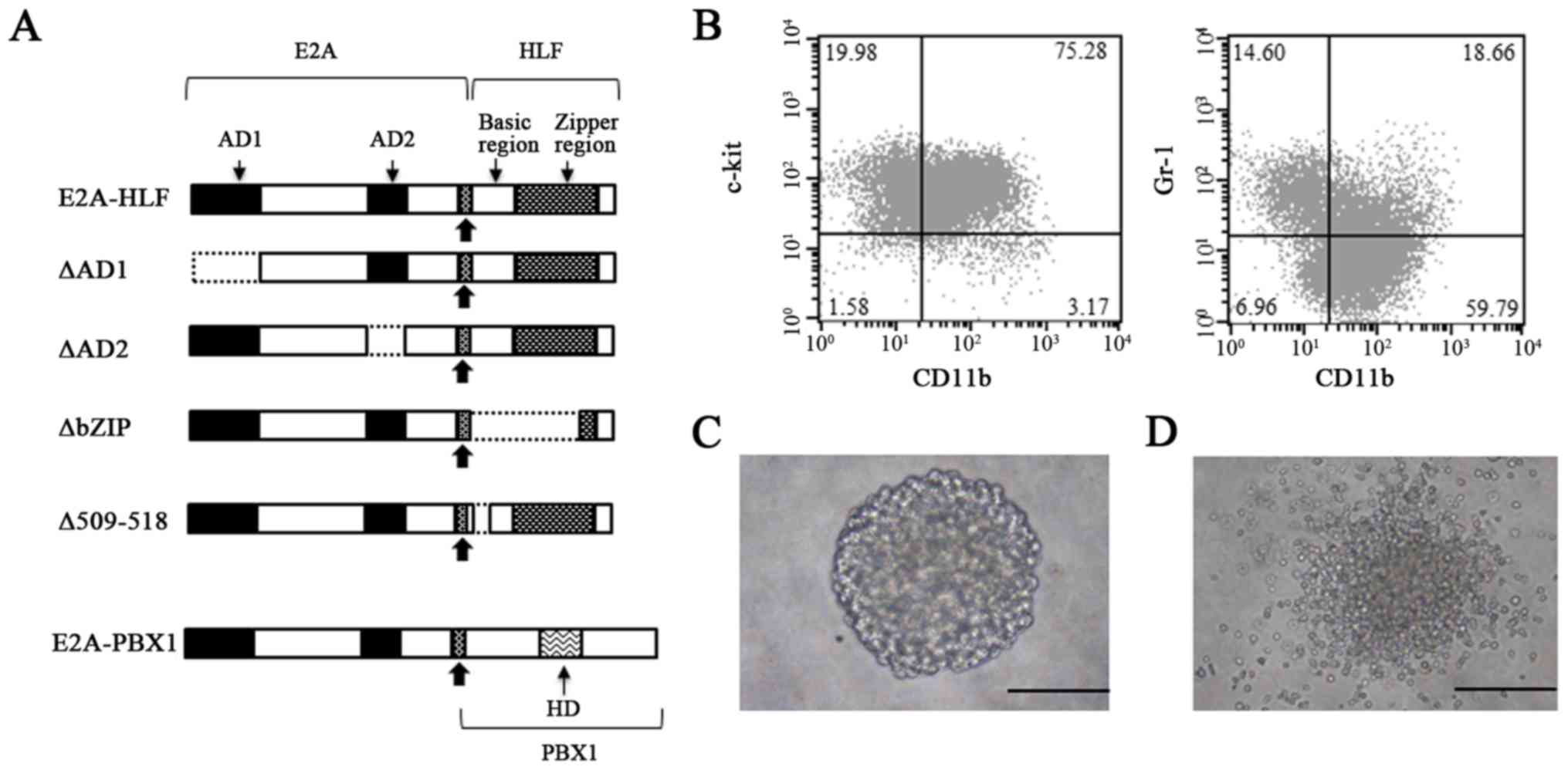 | Figure 2A schematic representation of the
chimeric proteins E2A-HLF, E2A-HLF mutants, and E2A-PBX1 used in
this study, and the characterization of the cells immortalized by
E2A-HLF. (A) The chimeric oncoproteins E2A-HLF and E2A-PBX1
produced by the t(17;19) and t(1;19) translocations, respectively,
retain the N-terminal transactivation domains of E2A. The E2A
C-terminus including the bHLH DNA-binding and protein dimerization
domain is replaced with the bZIP DNA-binding and dimerization
domain of the HLF protein and a region of PBX1 containing a
homeobox DNA-binding domain, respectively. Bold arrows indicate the
fusion sites. The proportions of each domain are not to scale in
this representation, in order to facilitate comparisons. (B)
Immunophenotype of the cells immortalized by retroviral
transduction of E2A-HLF. The typical morphology of the
colonies generated by (C) E2A-HLF and (D) ∆509-518 at the end of
the third round is presented. Colonies generated by the E2A-HLF
mutant were smaller and less dense compared with those generated by
the wild-type. Scale bar, 200 µm. E2A-HLF, immunoglobulin
enhancer-binding factor/hepatic leukemia factor; PBX1, pre-B-cell
leukemia transcription factor 1; CD, cluster of differentiation; AD
activation domain; HD, homeodomain. |
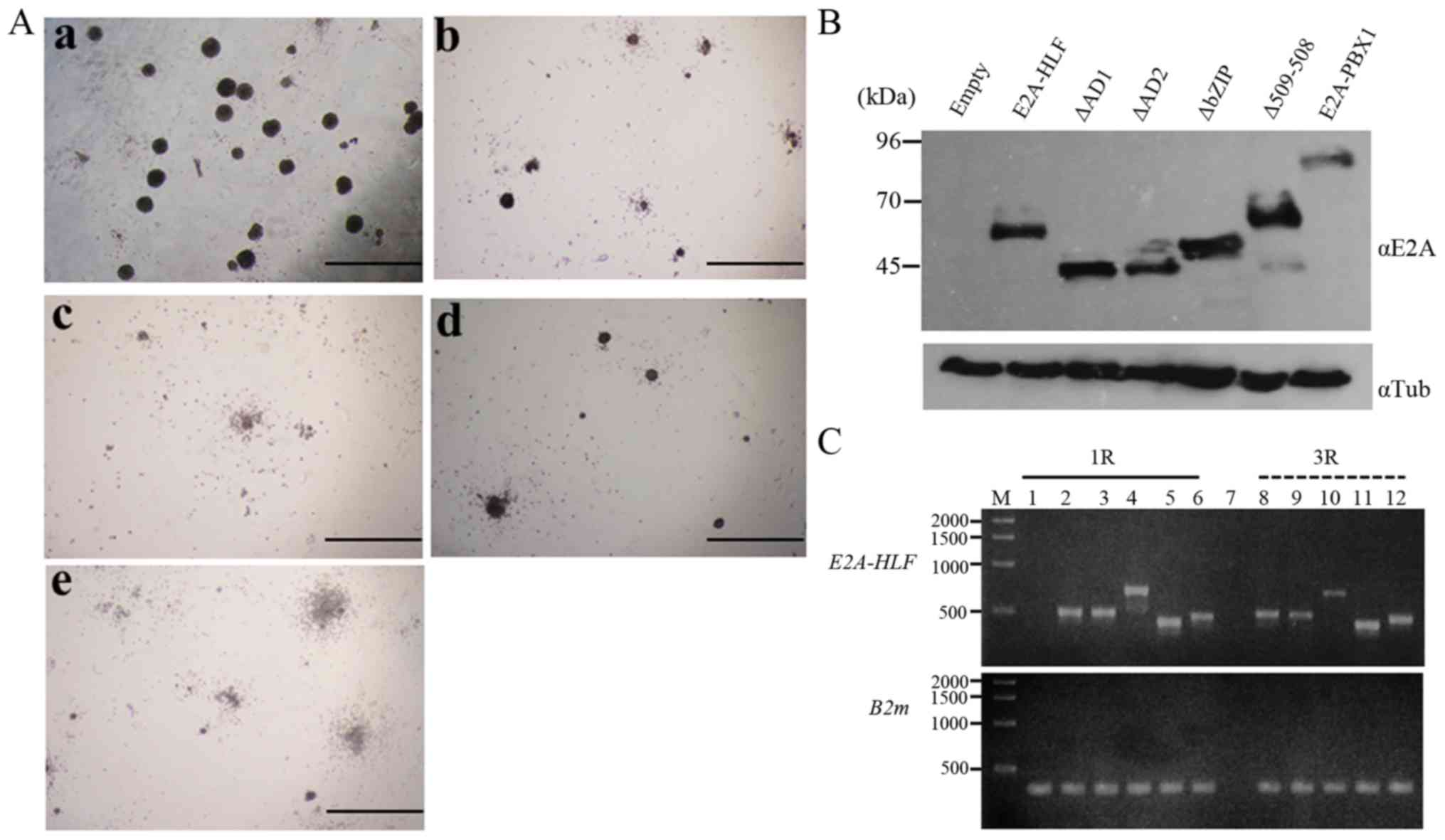 | Figure 3Colony formation in the
methylcellulose assay by E2A-HLF and E2A-HLF mutants and the
expression of these proteins. (A) Colonies generated by E2A-HLF and
its mutants at the end of the third round of plating. Colonies
generated by the mutants exhibited an altered morphology. (Aa)
E2A-HLF; (Ab) ∆AD1; (Ac) ∆AD2; (Ad) ∆bZIP; (Ae) ∆509-518. Colonies
were viewed with an Olympus CKX41 microscope. Scale bar, 200
µm. (B) The expression of wild-type and mutant E2A-HLF
chimeric proteins was assessed by western blot analysis. Lysates
extracted from Plat-E cells (41)
transfected with empty vector (pMYs-IN), pMYs-E2A-HLF-IN, E2A-HLF
mutants (pMYs-∆AD1-IN, pMYs-∆AD2-IN, pMYs-∆bZIP-IN, and
pMYs-∆509-518-IN), and pMYs-E2A-PBX1-IN were blotted with the
anti-E2A antibody (αE2A), followed by reprobing with αTub antibody
as an internal control. (C) The expression of E2A-HLF by
reverse transcription-polymerase chain reaction in the cells from
the first- and third-round cultures. B2m was used as an internal
standard. M, 1 kb DNA ladder; lane 1, mock (pMYs-IN); lane 2,
E2A-HLF; lane 3, ∆AD1; lane 4, ∆AD2; lane 5,
∆bZIP; lane 6, ∆509-518; lane 7, negative control
(water instead of cDNA mixture); lane 8, E2A-HLF; lane 9,
∆AD1; lane 10, ∆AD2; lane 11, ∆bZIP; lane 12,
∆509-518. E2A-HLF, immunoglobulin enhancer-binding
factor/hepatic leukemia factor; PBX1, pre-B-cell leukemia
transcription factor 1; αTub, α-tubulin; R, round of plating;
B2m, β-2-microglobulin. |
∆AD1 and ∆AD2 mutants lack each transactivation
domain of E2A to abolish the transactivation ability while
retaining DNA-binding and dimerization abilities, while ∆bZIP lacks
the basic region and the leucine zipper domain of HLF to abolish
DNA-binding and dimerization abilities, and ∆509-518 lacks a part
of the basic region of HLF to abolish DNA-binding ability (Fig. 2A). Wild type E2A-HLF
transduction immortalized KSL cells in serial colony replating
(Figs. 1C, and 4A and B). Consistent with a previous
report demonstrating that the two transactivation domains of E2A
and the leucine zipper dimerization domain of HLF are essential for
the transforming potential of the E2A-HLF in NIH3T3 cells (12), ∆AD2- and ∆bZIP-transduced cells
failed to form colonies in serial replating. In addition,
∆509-518-transduced cells also failed to form colonies (Fig. 4B). ∆AD1-transduced cells formed
some colonies at the third round, although they lost the ability at
the fifth round (data not shown). Transduction of E2A-PBX1,
a fusion oncogene generated by t(1;19)(q23;p13) (38), also induced immortalization of KSL
cells (Fig. 4B). RT-qPCR analyses
demonstrated that Eya2 was highly expressed in the
transduced cells with wild-type E2A-HLF or E2A-PBX1.
The mutant ∆AD1- and ∆AD2-transduced cells exhibited elevated
Eya2 expression, albeit to a lesser extent compared with the
wild-type. However, ∆bZIP- or ∆509-518-transduced cells showed no
induction of Eya2 expression (Fig. 4C). It is noteworthy that the
colony-forming ability of E2A-HLF and its mutants roughly
correlated with the level of Eya2 expression. These results
suggested that the two AD domains of E2A are required for complete
activity of Eya2 transactivation, while the DNA-binding and
dimerization abilities of HLF are indispensable for Eya2
transactivation in KSL-derived cells.
Eya2 is critical for the E2A-HLF-mediated
myeloid immortalization of HSPCs
To corroborate the involvement of Eya2 in myeloid
immortalization by E2A-HLF, Eya2 was depleted in
E2A-HLF-immortalized cells using shRNA expression (Fig. 5). The expression of E2A-HLF
chimeric transcripts was not affected following shRNA transduction
according to RT-PCR analysis of total RNA extracted from
shRNA-transduced cells (Fig. 5B).
shE09 and shE12 exhibited effective depletion of endogenous
Eya2 expression in the E2A-HLF-immortalized cells (Fig. 5C), and Eya2 knockdown by
shE09 and shE12 reduced the clonogenicity to ~40 and 70% of that in
the control at the first round of plating, respectively (Fig. 5D). It was not possible to perform
rescue experiments on Eya2-knockdown HSPCs that were
immortalized with E2A-HLF by forced expression of
Eya2 without the shRNA target sequence, likely due to the
genotoxic stress induced by Eya2 overexpression, as the
forced expression of Eya2 resulted in markedly reduced
colony-forming efficiency in E2A-HLF-immortalized cells
(data not shown).
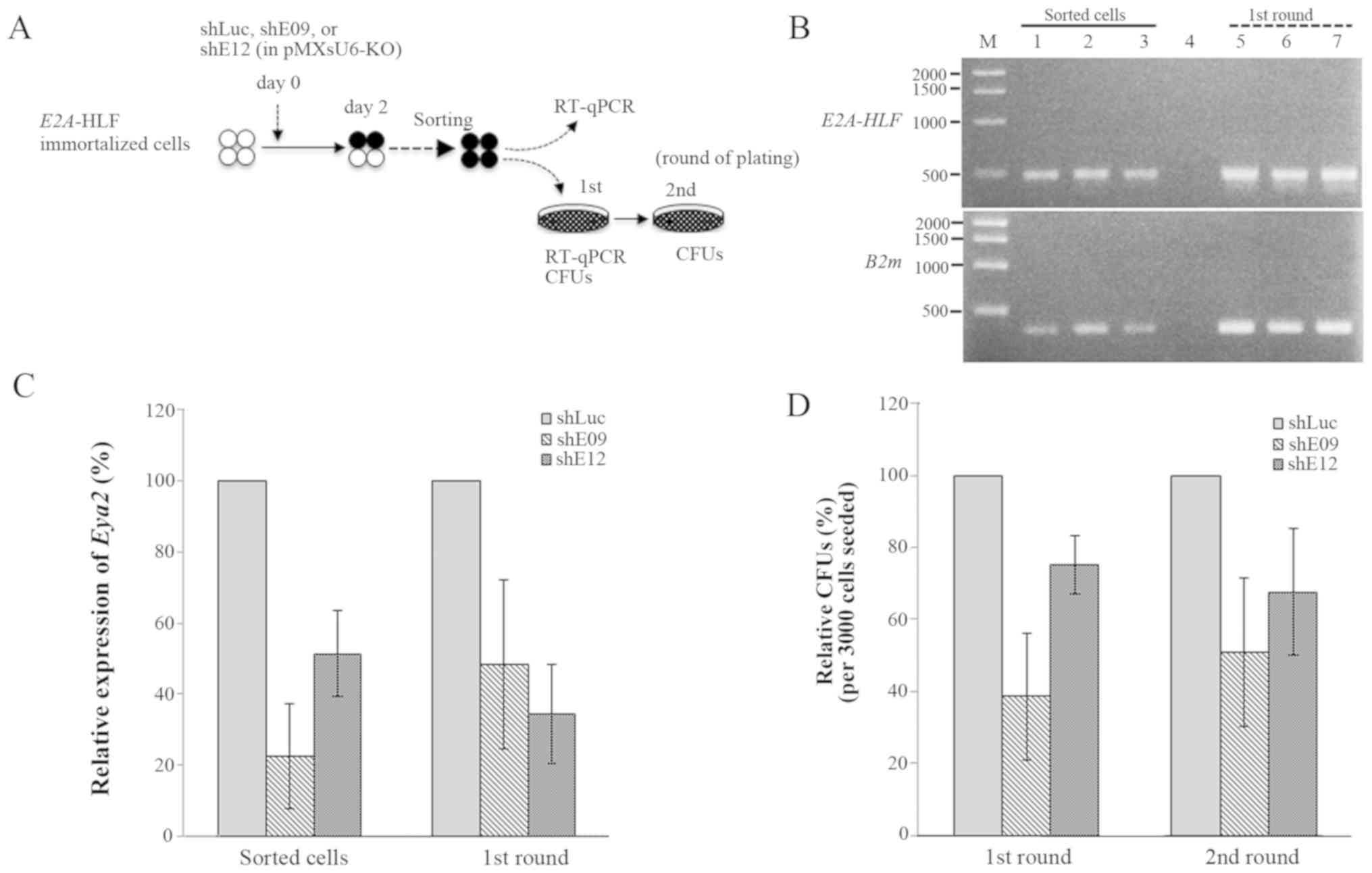 | Figure 5Suppressive effects of Eya2 depletion
on the clonogenicity of E2A-HLF-immortalized KSL cells. (A)
Experimental strategy for the analysis of
E2A-HLF-immortalized cells with Eya2 depletion by retroviral
transduction of the shRNA/KO co-expresser in pMXsU6-KO. (B) The
expression of E2A-HLF by RT-PCR in the sorted cells and
colony-forming cells at the end of the first-round of plating.
B2m was used as an internal standard. M, 1 kb DNA ladder;
lane 1, shLuc; lane 2, shE09; lane 3, shE12; lane 4, negative
control, lane 5, shLuc; lane 6, shE09; lane 7, shE12. (C) The
expression of Eya2 by RT-qPCR in the cells sorted from
shRNA-transduced E2A-HLF-immortalized cells and subsequent
colony-forming cells at the end of the first round of plating. (D)
Relative CFUs of the cells at the end of the first and second round
of plating. Bar graphs illustrate the mean ± standard deviation of
three independent experiments. CFU, colony-forming unit; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; sh,
short hairpin; Luc, luciferase; E2A-HLF, immunoglobulin
enhancer-binding factor/hepatic leukemia factor; PBX1, pre-B-cell
leukemia transcription factor 1; Eya2, EYA transcriptional
coactivator and phosphatase 2; B2m, β-2-microglobulin; KO,
Kusabira-Orange. |
E2A-HLF upregulates the Eya2 expression
by binding to the promoter region\
It was previously observed that, in
Plzf-mediated immortalization, Plzf binds to the putative
Eya2 promoter region around exon 1c of Eya2 (35), in accordance with public data
(46) from ChIP sequencing.
Therefore, a ChIP-qPCR analysis of FLAG-tagged
E2A-HLF-immortalized HSPCs with the primers around exon 1c
was performed (Fig. 6). The
analyses indicated increased binding of E2A-HLF to the Eya2
promoter region in the immortalized cells, accompanied by RNA pol
II binding signals (Fig. 6A).
These results suggested that E2A-HLF drives the aberrant expression
of Eya2 in HSPCs by promoter binding. Subsequently, the
effect of E2A-HLF expression on Eya2 promoter activity was
confirmed using a reporter assay. The luciferase assay in K562
cells demonstrated that E2A-HLF and E2A-PBX1 activated the reporter
gene expression through the Eya2 promoter region (Fig. 6C), and transactivation through the
Eya2 promoter region was significantly reduced by the
mutation of the putative E2A-HLF binding consensus sequence
(Fig. 6B and D). Taken together,
these findings suggested that E2A-HLF upregulates Eya2
transcription through the E2A-HLF binding consensus sequence.
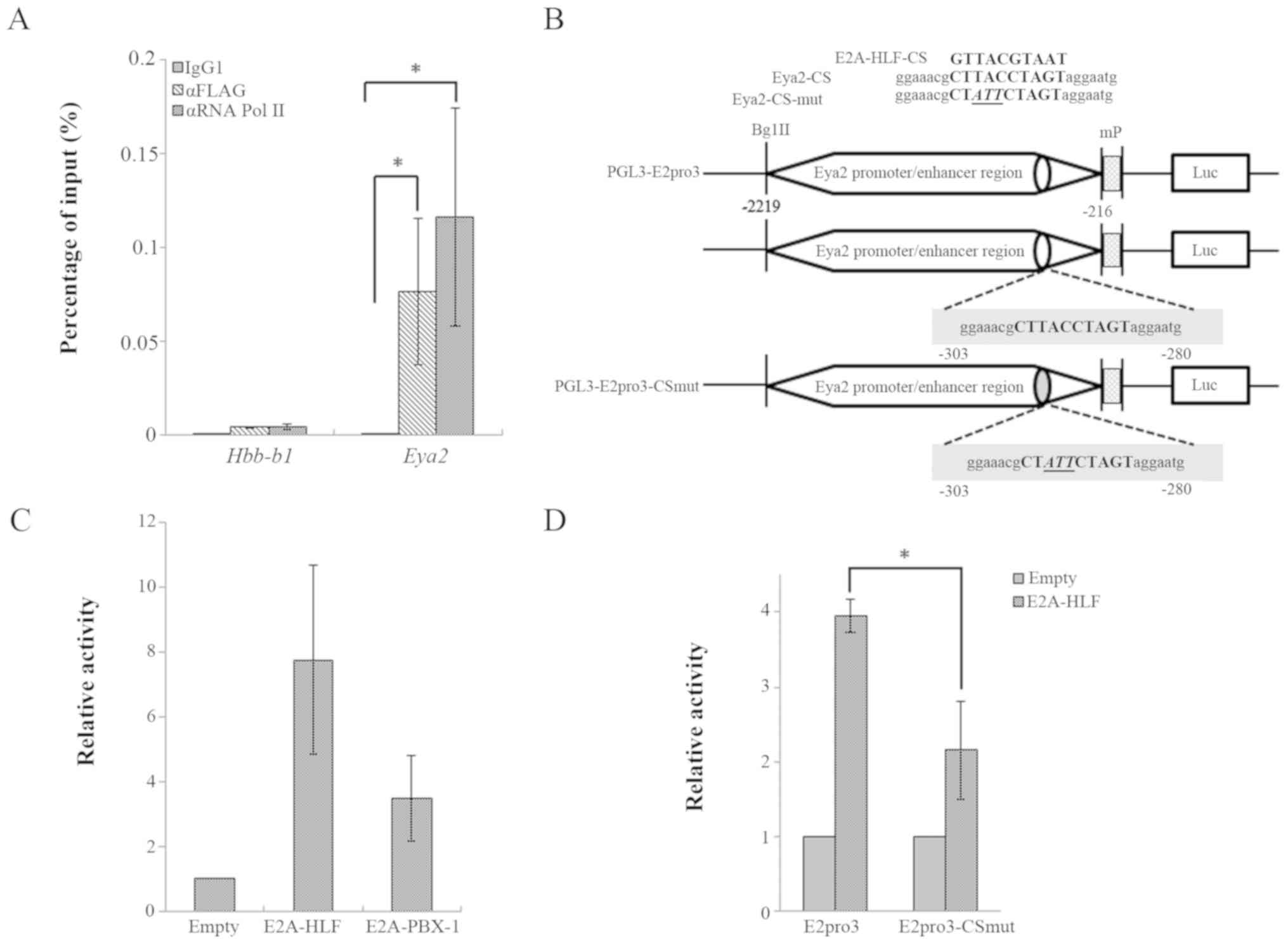 | Figure 6E2A-HLF upregulates Eya2
expression by binding to the promoter region. (A) A ChIP-qPCR
analysis on the Eya2 promoter region in
E2A-HLF-immortalized mouse hematopoietic stem/progenitor
cells. The relative binding activity of E2A-HLF (detected by the
anti-Flag antibody) and RNA polymerase II around exon 1c of
Eya2 in KSL cells immortalized by FLAG-tagged E2A-HLF is
presented. The promoter region of Hbb-b1 was examined as a
negative control. Statistical analysis was performed using a
Friedman test, followed by a Steel's test for post hoc
nonparametric multiple comparisons. (B) A schematic representation
of two reporter constructs for the luciferase assay. Mutations in
the consensus sequence (9) are
underlined. The position of the 5′end of the protein coding
sequence in exon 2 of mouse Eya2 is numbered +1. The CS of
E2A-HLF binding is located within the amplified region by
ChIP-qPCR. (C) The effects of E2A-HLF and E2A-PBX1 on the
Eya2 promoter activity in the luciferase assay in K562
cells. (D) A luciferase assay using a wild-type Eya2 and a
mutated reporter construct containing a putative E2A-HLF-binding
CS. Statistical analysis was performed using a t-test.
*P<0.05. Bar graphs illustrate the mean ± standard
deviation of three independent experiments. ChIP, chromatin
immunoprecipitation; Eya2, EYA transcriptional coactivator
and phosphatase 2; mut, mutant; E2A-HLF, immunoglobulin
enhancer-binding factor/hepatic leukemia factor; PBX1, pre-B-cell
leukemia transcription factor 1; Luc, luciferase; Hbb-b1,
hemoglobin subunit β1; Eya2, EYA transcriptional coactivator and
phosphatase 2; qPCR, quantitative polymerase chain reaction; CS,
consensus sequence. |
Discussion
To clarify the mechanism underlying E2A-HLF-mediated
leukemogenesis, the present study examined the mechanism whereby
E2A-HLF leads to the transformation of HSPCs, and explored
therapeutic targets for novel treatments.
Since E2A-HLF alone is reportedly unable to
immortalize mouse hematopoietic cells in vitro under
lymphoid conditions on irradiated stromal cells, unless the
anti-apoptotic molecule BCL-2 is forcibly co-expressed (47), the present study employed a myeloid
condition to assess the transforming potential of E2A-HLF on mouse
HSPCs in vitro, where E2A-HLF expression alone is
sufficient to immortalize mouse HSPCs. It was observed that E2A-HLF
binds to the promotor of Eya2 to elevate Eya2
expression in HSPCs where endogenous Eya2 is preferentially
expressed (34). The present study
also identified the cis-acting element that resembles the
previously reported DNA-binding consensus sequence (10) within the Eya2 promoter
region in transactivation by E2A-HLF. It was recently reported that
the forced expression of Eya2 using a retroviral vector
induces aberrant self-renewal in mouse HSPCs (35). The suppressive effect induced by
Eya2 depletion on the clonogenicity of
E2A-HLF-immortalized mouse HSPCs in the present study
suggested the involvement of Eya2 in the aberrant self-renewal
capacity of E2A-HLF-immortalized HSPCs.
Previous studies revealed the anti-apoptotic
activity of E2A-HLF in IL-3-dependent murine pro-B cells through
the AD1 and AD2 transactivation domains of E2A, but not the bZIP
domain (13,19,40).
However, the molecular mechanism underlying the function of E2A-HLF
on the self-renewal and/or cell proliferation of hematopoietic
cells is poorly understood. E2A-HLF was previously demonstrated to
transactivate the LMO2 oncogene through the AD1 and AD2
domains of E2A and the basic region of HLF (17). Similar to this finding, the present
study revealed that the two transactivation domains of E2A and the
basic region of HLF are essential for the E2A-HLF-mediated
transformation of mouse HSPCs. The present study focused on Eya2 as
one of the key molecules downstream of E2A-HLF. Eya2, as an
oncogenic molecule, has been demonstrated to be involved in solid
tumors with a poor prognosis (30-32).
It was recently observed that Eya2 is critical for leukemogenesis
by PLZF-RARA (35). The
present study further indicated that Eya2 is involved in
E2A-HLF-mediated leukemogenesis. The same may be true for
E2A-PBX1, which also activated Eya2 expression in the
present study. Therefore, Eya2 may be involved more generally in
leukemogenesis, and further studies using clinical samples may be
helpful to verify this hypothesis. Of note, Eya2 knockout
mice (48) appear to have no
severe abnormalities, suggesting that Eya2 may be a potential
target of molecular therapy without major adverse effects for
certain subtypes of leukemia.
In conclusion, the present study demonstrated the
critical role of Eya2 in the E2A-HLF-mediated transformation
of mouse HSPCs in vitro.
Funding
This study was supported by Grants-in-Aid from the
Ministry of Education, Culture, Sports, Science, and Technology in
Japan (to TN, grant nos. Basic-B, 26293247 and Basic-B, 17H04227;
to RO, grant no. Basic-C 15K09452), the Japan Leukemia Research
Fund (to TN), and Takeda Science Foundation (to TN).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
RO and TN designed the research. BDM and RO
performed the experiments. BDM, RO, and TN analyzed the results.
BDM and TN wrote the manuscript.
Ethics approval and consent to
participate
All animal studies were approved by the Animal Care
Committees of Mie University (Tsu, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mr. Brian Quinn for
linguistic assistance.
References
|
1
|
Pui CH, Gajjar AJ, Kane JR, Qaddoumi IA
and Pappo AS: Challenging issues in pediatric oncology. Nat Rev
Clin Oncol. 8:540–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inaba T, Roberts WM, Shapiro LH, Jolly KW,
Raimondi SC, Smith SD and Look AT: Fusion of the leucine zipper
gene HLF to the E2A gene in human acute B-lineage leukemia.
Science. 257:531–534. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hunger SP, Ohyashiki K, Toyama K and
Cleary ML: Hlf, a novel hepatic bZIP protein, shows altered
DNA-binding properties following fusion to E2A in t(17;19) acute
lymphoblastic leukemia. Genes Dev. 6:1608–1620. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raimondi SC, Privitera E, Williams DL,
Look AT, Behm F, Rivera GK, Crist WM and Pui CH: New recurring
chromosomal translocations in childhood acute lymphoblastic
leukemia. Blood. 77:2016–2022. 1991.PubMed/NCBI
|
|
5
|
Inukai T, Hirose K, Inaba T, Kurosawa H,
Hama A, Inada H, Chin M, Nagatoshi Y, Ohtsuka Y, Oda M, et al:
Hypercalcemia in childhood acute lymphoblastic leukemia: Frequent
implication of parathyroid hormone-related peptide and E2A-HLF from
translocation 17;19. Leukemia. 21:288–296. 2007. View Article : Google Scholar
|
|
6
|
Greenbaum S and Zhuang Y: Identification
of E2A target genes in B lymphocyte development by using a gene
tagging-based chromatin immunoprecipitation system. Proc Natl Acad
Sci USA. 99:15030–15035. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Massari ME and Murre C: Helix-loop-helix
proteins: Regulators of transcription in eucaryotic organisms. Mol
Cell Biol. 20:429–440. 2000. View Article : Google Scholar
|
|
8
|
Quong MW, Romanow WJ and Murre C: E
protein function in lymphocyte development. Annu Rev Immunol.
20:301–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hunger SP, Li S, Fall MZ, Naumovski L and
Cleary ML: The proto-oncogene HLF and the related basic leucine
zipper protein TEF display highly similar DNA-binding and
transcriptional regulatory properties. Blood. 87:4607–4617.
1996.PubMed/NCBI
|
|
10
|
Hunger SP, Brown R and Cleary ML:
DNA-binding and transcriptional regulatory properties of hepatic
leukemia factor (HLF) and the t(17;19) acute lymphoblastic leukemia
chimera E2A-HLF. Mol Cell Biol. 14:5986–5996. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fischer U, Forster M, Rinaldi A, Risch T,
Sungalee S, Warnatz HJ, Bornhauser B, Gombert M, Kratsch C, Stütz
AM, et al: Genomics and drug profiling of fatal TCF3-HLF-positive
acute lymphoblastic leukemia identifies recurrent mutation patterns
and therapeutic options. Nat Genet. 47:1020–1029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshihara T, Inaba T, Shapiro LH, Kato JY
and Look AT: E2A-HLF-mediated cell transformation requires both the
trans-activation domains of E2A and the leucine zipper dimerization
domain of HLF. Mol Cell Biol. 15:3247–3255. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inaba T, Inukai T, Yoshihara T, Seyschab
H, Ashmun RA, Canman CE, Laken SJ, Kastan MB and Look AT: Reversal
of apoptosis by the leukaemia-associated E2A-HLF chimaeric
transcription factor. Nature. 382:541–544. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duque-Afonso J, Smith KS and Cleary ML:
Conditional expression of E2A-HLF induces B-cell precursor death
and myeloproliferative-like disease in knock-in mice. PLoS One.
10:e01432162015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Honda H, Inaba T, Suzuki T, Oda H, Ebihara
Y, Tsuiji K, Nakahata T, Ishikawa T, Yazaki Y and Hirai H:
Expression of E2A-HLF chimeric protein induced T-cell apoptosis,
B-cell maturation arrest, and development of acute lymphoblastic
leukemia. Blood. 93:2780–2790. 1999.PubMed/NCBI
|
|
16
|
Smith KS, Rhee JW, Naumovski L and Cleary
ML: Disrupted differentiation and oncogenic transformation of
lymphoid progenitors in E2A-HLF transgenic mice. Mol Cell Biol.
19:4443–4451. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirose K, Inukai T, Kikuchi J, Furukawa Y,
Ikawa T, Kawamoto H, Oram SH, Göttgens B, Kiyokawa N, Miyagawa Y,
et al: Aberrant induction of LMO2 by the E2A-HLF chimeric
transcription factor and its implication in leukemogenesis of
B-precursor ALL with t(17;19). Blood. 116:962–970. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Boer J, Yeung J, Ellu J, Ramanujachar
R, Bornhauser B, Solarska O, Hubank M, Williams O and Brady HJ: The
E2A-HLF oncogenic fusion protein acts through Lmo2 and Bcl-2 to
immortalize hematopoietic progenitors. Leukemia. 25:321–330. 2011.
View Article : Google Scholar
|
|
19
|
Inukai T, Inoue A, Kurosawa H, Goi K,
Shinjyo T, Ozawa K, Mao M, Inaba T and Look AT: SLUG, a
ces-1-related zinc finger transcription factor gene with
antiapoptotic activity, is a downstream target of the E2A-HLF
oncoprotein. Mol Cell. 4:343–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeung J, O'Sullivan E, Hubank M and Brady
HJ: E4BP4 expression is regulated by the t(17;19)-associated
oncoprotein E2A-HLF in pro-B cells. Br J Haematol. 125:560–567.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dang J, Inukai T, Kurosawa H, Goi K, Inaba
T, Lenny NT, Downing JR, Stifani S and Look AT: The E2A-HLF
oncoprotein activates Groucho-related genes and suppresses Runx1.
Mol Cell Biol. 21:5935–5945. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurosawa H, Goi K, Inukai T, Inaba T,
Chang KS, Shinjyo T, Rakestraw KM, Naeve CW and Look AT: Two
candidate downstream target genes for E2A-HLF. Blood. 93:321–332.
1999.
|
|
23
|
Yamasaki N, Miyazaki K, Nagamachi A,
Koller R, Oda H, Miyazaki M, Sasaki T, Honda ZI, Wolff L, Inaba T,
et al: Identification of Zfp521/ZNF521 as a cooperative gene for
E2A-HLF to develop acute B-lineage leukemia. Oncogene.
29:1963–1975. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okuya M, Kurosawa H, Kikuchi J, Furukawa
Y, Matsui H, Aki D, Matsunaga T, Inukai T, Goto H, Altura RA, et
al: Up-regulation of survivin by the E2A-HLF chimera is
indispensable for the survival of t(17;19)-positive leukemia cells.
J Biol Chem. 285:1850–1860. 2010. View Article : Google Scholar :
|
|
25
|
Zhang X, Inukai T, Hirose K, Akahane K,
Kuroda I, Honna-Oshiro H, Kagami K, Goi K, Nakamura K, Kobayashi M,
et al: Oncogenic fusion E2A-HLF sensitizes t(17;19)-positive acute
lymphoblastic leukemia to TRAIL-mediated apoptosis by upregulating
the expression of death receptors. Leukemia. 26:2483–2493. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bonini NM, Leiserson WM and Benzer S: The
eyes absent gene: Genetic control of cell survival and
differentiation in the developing Drosophila eye. Cell. 72:379–395.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonini NM, Leiserson WM and Benzer S:
Multiple roles of the eyes absent gene in Drosophila. Dev Biol.
196:42–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tadjuidje E and Hegde RS: The Eyes Absent
proteins in development and disease. Cell Mol Life Sci.
70:1897–1913. 2013. View Article : Google Scholar :
|
|
29
|
Zimmerman JE, Bui QT, Steingrímsson E,
Nagle DL, Fu W, Genin A, Spinner NB, Copeland NG, Jenkins NA, Bucan
M, et al: Cloning and characterization of two vertebrate homologs
of the Drosophila eyes absent gene. Genome Res. 7:128–141. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Yang N, Huang J, Buckanovich RJ,
Liang S, Barchetti A, Vezzani C, O'Brien-Jenkins A, Wang J, Ward
MR, et al: Transcriptional coactivator Drosophila eyes absent
homologue 2 is up-regulated in epithelial ovarian cancer and
promotes tumor growth. Cancer Res. 65:925–932. 2005.PubMed/NCBI
|
|
31
|
Farabaugh SM, Micalizzi DS, Jedlicka P,
Zhao R and Ford HL: Eya2 is required to mediate the pro-metastatic
functions of Six1 via the induction of TGF-β signaling,
epithelial-mesenchymal transition, and cancer stem cell properties.
Oncogene. 31:552–562. 2012. View Article : Google Scholar
|
|
32
|
Wen Z, Liang C, Pan Q and Wang Y: Eya2
overexpression promotes the invasion of human astrocytoma through
the regulation of ERK/MMP9 signaling. Int J Mol Med. 40:1315–1322.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vincent A, Hong SM, Hu C, Omura N, Young
A, Kim H, Yu J, Knight S, Ayars M, Griffith M, et al: Epigenetic
silencing of EYA2 in pancreatic adenocarcinomas promotes tumor
growth. Oncotarget. 5:2575–2587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Forsberg EC, Prohaska SS, Katzman S,
Heffner GC, Stuart JM and Weissman IL: Differential expression of
novel potential regulators in hematopoietic stem cells. PLoS Genet.
1:e282005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ono R, Masuya M, Ishii S, Katayama N and
Nosaka T: Eya2, a Target Activated by Plzf, Is Critical for
PLZF-RARA-Induced Leukemogenesis. Mol Cell Biol. 37:e00585–e16.
2017. View Article : Google Scholar :
|
|
36
|
Ono R, Masuya M, Nakajima H, Enomoto Y,
Miyata E, Nakamura A, Ishii S, Suzuki K, Shibata-Minoshima F,
Katayama N, et al: Plzf drives MLL-fusion-mediated leuke-mogenesis
specifically in long-term hematopoietic stem cells. Blood.
122:1271–1283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kitamura T, Koshino Y, Shibata F, Oki T,
Nakajima H, Nosaka T and Kumagai H: Retrovirus-mediated gene
transfer and expression cloning: Powerful tools in functional
genomics. Exp Hematol. 31:1007–1014. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hunger SP, Galili N, Carroll AJ, Crist WM,
Link MP and Cleary ML: The t(1;19)(q23;p13) results in consistent
fusion of E2A and PBX1 coding sequences in acute lymphoblastic
leukemias. Blood. 77:687–693. 1991.PubMed/NCBI
|
|
39
|
LeBrun DP: E2A basic helix-loop-helix
transcription factors in human leukemia. Front Biosci. 8:s206–s222.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Inukai T, Inaba T, Ikushima S and Look AT:
The AD1 and AD2 transactivation domains of E2A are essential for
the antiapoptotic activity of the chimeric oncoprotein E2A-HLF. Mol
Cell Biol. 18:6035–6043. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Morita S, Kojima T and Kitamura T: Plat-E:
An efficient and stable system for transient packaging of
retroviruses. Gene Ther. 7:1063–1066. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ono R, Nakajima H, Ozaki K, Kumagai H,
Kawashima T, Taki T, Kitamura T, Hayashi Y and Nosaka T:
Dimerization of MLL fusion proteins and FLT3 activation synergize
to induce multiple-lineage leukemogenesis. J Clin Invest.
115:919–929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
44
|
Nosaka T, Morita S, Kitamura H, Nakajima
H, Shibata F, Morikawa Y, Kataoka Y, Ebihara Y, Kawashima T, Itoh
T, et al: Mammalian twisted gastrulation is essential for
skeleto-lymphogenesis. Mol Cell Biol. 23:2969–2980. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nosaka T, Kawashima T, Misawa K, Ikuta K,
Mui AL and Kitamura T: STAT5 as a molecular regulator of
proliferation, differentiation and apoptosis in hematopoietic
cells. EMBO J. 18:4754–4765. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
ENCODE Project Consortium: An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Smith KS, Rhee JW and Cleary ML:
Transformation of bone marrow B-cell progenitors by E2a-Hlf
requires coexpression of Bcl-2. Mol Cell Biol. 22:7678–7687. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grifone R, Demignon J, Giordani J, Niro C,
Souil E, Bertin F, Laclef C, Xu PX and Maire P: Eya1 and Eya2
proteins are required for hypaxial somitic myogenesis in the mouse
embryo. Dev Biol. 302:602–616. 2007. View Article : Google Scholar
|