Introduction
Extracellular vesicles (EVs) represent a series of
spherical membranous particles that are secreted from cells into
the extracellular microenvironment (1). EVs enclose and transfer molecular
cargoes, including proteins, nucleic acids, lipids and other
metabolites, derived from the donor cells and deliver them to
recipient cells (2). Based on
intracellular origin and size, EVs are frequently classified into
three clusters, termed apoptotic bodies, microvesicles and exosomes
(3). Exosomes are small membranous
vesicles between 30-150 nm in diameter, and are derived from late
endosomes and multivesicular bodies (4). Exosomes function as a cell-to-cell
communicator by naturally transferring macromolecules from producer
to recipient cells (1). This
communication system consists of multiple steps, including an
exosomes release, local or long range transport, and exosomes
capture and internalization, and finally results in the changes of
recipient cell behavior, and modification of physiological and
pathological processes (5-7). Exosomes uptake by target cells
involves a variety of mechanisms, including clathrin- or
caveolin-dependent endocytosis, macropinocytosis, phagocytosis,
lipid raft-mediated internalization and membrane fusion (8,9).
Routes and mechanisms of exosomes uptake largely depend on surface
molecules and glycoproteins on the membrane of the vesicle and the
recipient cell, and normally, >1 route is involved in the
internalization of exosomes (8). A
number of pharmacological inhibitors, including heparin,
wortmannin, dynasore, chlorpromazine, amiloride and omeprazole,
have been frequently used to block an uptake route of specific
exosomes (8). Heparin blocks the
binding of heparin sulphate proteoglycans, which are exhibited on
membrane particles and lipoproteins, to the plasma membrane, and
thus inhibits endocytosis (10).
Dynasore is a specific inhibitor of dynamin 2, which is required
for clathrin-mediated and caveolin-dependent endocytosis (11). Wortmannin inhibits membrane
insertion by suppressing phosphoinositide 3-kinases (PI3Ks),
eventually resulting in the inhibition of phagocytic processes,
including phagosomes formation (12). Chlorpromazine can inhibit the
formation of clathrin-coated pits by targeting multiple receptors,
including dopamine, serotonin and histamine receptors, and thus
serves as an inhibitor of clathrin-mediated endocytosis (8,13).
Amiloride, an inhibitor targeting the sodium/proton exchanger, is
known to specifically inhibit macropinosome formation by reducing
submembranous pH and preventing Rac family small GTPase 1/cell
division cycle 42 signaling (14,15).
Omeprazole, a proton pump inhibitor, has been demonstrated to
inhibit the fusion of the EV membrane with the cell plasma membrane
by inducing both acidification of the cell cytosol and acidic
vesicle retention within the cells (16). The intrinsic properties, including
low toxicity and immunogenicity, high biocompatibility and
biodegradability, and nanometric size, make exosomes highly
suitable as drug carriers (17,18),
and identification of specific routes for their uptake will enable
researchers to develop a more effective delivery system.
Multiple myeloma (MM), also known as plasma cell
myeloma, is a hematological cancer that starts with the
accumulation of uncontrolled monoclonal plasma cells in bone marrow
(BM) (19). During MM progression,
MM cells interact with other BM-derived cells, particularly with BM
stromal cells (BMSCs), to support their own survival and growth
(20). The interplay between them
performs a crucial function in MM pathogenesis and this interaction
is mediated by direct cell-to-cell contacts, soluble factors and
exosomes (21). Additionally, the
interaction of MM cell-derived exosomes with the bystander cells in
the BM microenvironment enhances angiogenesis and
immunosuppression, which favors MM progression (22,23).
The positive roles of exosomes-mediated communication between BMSCs
and MM cells in MM progression was demonstrated by indicating that
BMSC-derived exosomes promote MM cell survival, proliferation,
migration and even drug resistance, and that MM cell-derived
exosomes enhance pro-tumoral activity of BMSCs through the delivery
of microRNAs (miRNAs) (24,25).
Judging by the important functions of exosomes in MM cell-BMSC
communication, blockade of this mediator may facilitate MM
treatment. Nonetheless, the detailed mechanisms involved in
exosomes-mediated communication, particularly in exosomes
internalization, remain unknown.
The routes and mechanisms of exosomes uptake
strongly determine the ability of exosomes to transport their
content and to elicit a biological response in recipient cells
(26,27). In the present study, the endocytic
routes of uptake of MM cell-derived exosomes by BMSCs were
determined and the effects of specific uptake inhibitors on MM
exosomes-induced changes in BMSCs were assessed.
Materials and methods
Materials and reagents
Human MM cell lines H929, MM1S, RPMI8226 and U266
were purchased from China Center for Type Culture Collection
(Wuhan, China). Human BM was collected for BMSC culture from a
healthy donor (41 years old; male) in December 2017 at the
Department of Hematology, The Third Affiliated Hospital of Sun
Yat-Sen University after informed consent was obtained. Ethical
approval was obtained from Ethical Committee for Clinical Medicine
Research of The Third Affiliated Hospital of Sun Yat-Sen University
in Guangzhou (Guangzhou, China). Primary antibodies against
flotillin-1 (cat. no. 18634T; 1:1,000), calreticulin (cat. no.
12238T; 1:1,000), heat shock protein 70 (HSP70; cat. no. 4872T;
1:1,000), GAPDH (cat. no. 5174S; 1:1,000), Alix (cat. no. 2171S;
1:1,000), phosphor-(p-) extracellular signal-regulated kinase 1/2
(ERK1/2; cat. no. 4370T; 1:1,000), ERK1/2 (cat. no. 4695T;
1:1,000), p-c-Jun N-terminal kinase (JNK; cat. no. 9255S; 1:1,000),
JNK2 (cat. no. 9258S; 1:1,000), p-signal transducer and activator
of transcription 1 (STAT1; cat. no. 7649T; 1:1,000), STAT1 (cat.
no. 9172T; 1:1,000), p-STAT3 (cat. no. 9138S; 1:1,000) and STAT3
(cat. no. 4904S; 1:1,000), as well as a horseradish peroxidase
(HRP)-conjugated anti-mouse IgG antibody (cat. no. 7076S; 1:2,000)
and HRP-conjugated anti-rabbit IgG antibody (cat. no. 7074S;
1:2,000), were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-human cluster of differentiation 63 (CD63;
cat. no. 10628D; 1:250) and antitumor susceptibility 101 (TSG101;
cat. no. sc-7964; 1:200) antibodies were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA) and Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), respectively. For inhibition
experiments, heparin, wortmannin, dynasore, chlorpromazine,
amiloride, and omeprazole were purchased from Selleck Chemicals
(Houston, TX, USA).
Cell culture
H929, MM1S and RPMI8226 cells were maintained in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (FBS; Excell Bio, Shanghai, China). U266
cells were cultured in RPMI-1640 supplemented with 15% of FBS. All
these four MM cell lines were used for all experiments and cultured
at 37°C. The BM mononuclear cells were isolated via a Lymphoprep
(Stemcell Technologies, Inc., Vancouver, BC, Canada) gradient and
were cultured in OriCell Human MSC Culture medium (Cyagen
Biosciences, Inc., Santa Clara, CA, USA) at 37°C. After 24 h,
non-adherent cells were removed and adherent cells were
continuously cultured in OriCell Human MSC Culture medium. Human
BMSCs were used within 5 passages. All culture media were
supplemented with 100 U/ml penicillin and 0.1 mg/ml
streptomycin.
Exosomes isolation, purification,
quantification and nanoparticle tracking analysis
Exosomes were isolated as described previously
(24). MM cells were cultured in
the serum-free RPMI-1640 medium at 37°C for 24 h and the
conditioned medium (CM) was collected and then centrifuged for 5
min at 2,000 × g at room temperature to remove cells and large
debris. Subsequently, CM was passed through 0.22 µm filters
and concentrated to 2 ml in a 100K MWCO Advance Centrifugal Device
(Pall Corporation, New York, NY, USA) for enriching exosomes.
Concentrated CM was washed with 10 ml PBS once using a concentrator
and passed again through a 0.22 µm filter. Exosomes were
precipitated with the ExoQuick-TC exosomes precipitation solution
(System Biosciences, Palo Alto, CA, USA). The exosomes were
resuspended in a serum-free RPMI-1640 medium, and the protein
concentration was quantified with a Bicinchoninic Acid (BCA)
Protein Assay kit (Thermo Fisher Scientific, Inc.). Particle size
distribution of isolated exosomes was determined via a Zetaview
Nanoparticle Tracking Analyzer (Particle Metrix GmbH, Meerbusch,
Germany).
Western blot analysis
Cells and exosomes were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) and mixed with the Protease Inhibitor
Cocktail (Beyotime Institute of Biotechnology). Protein
concentrations were detected with a BCA Protein Assay kit. Lysates
were mixed with loading buffer (Beyotime Institute of
Biotechnology) and then heated to 95°C for 10 min. Every sample was
loaded onto 8 (for STAT1 detection) or 10% (for other protein
detection) polyacrylamide gels (Beyotime Institute of
Biotechnology) and subjected to electrophoresis. Subsequently, the
proteins were transferred to polyvinylidene fluoride membranes
(Beyotime Institute of Biotechnology), and the membrane was blocked
in 5% non-fat milk for 1 h at room temperature. It has been
demonstrated that non-fat milk did not affect the phosphorylation
levels of the target proteins, including STAT1, STAT3 and ERK1/2,
analyzed in the present study, as indicated in other studies
(28-30). The membranes were incubated with
primary antibodies for 2 h at room temperature and then with
secondary antibodies for 2 h at room temperature. Protein bands
were visualized and documented using an Enhanced Chemiluminescent
Western Blotting solution (Beyotime Institute of Biotechnology) and
GelView 6000 Pro (Guangzhou Biolight Equipment Co., Ltd.,
Guangzhou, China), respectively.
Cell viability assays
Human BMSCs were seeded at a density of 2,000
cells/well in a 96-well plate and cultured in OriCell Human MSC
Culture medium at 37°C. After 24 h, the medium was replaced with
non-serum Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C overnight. Exosomes and/or
inhibitors, including heparin, wortmannin, dynasore,
chlorpromazine, amiloride and omeprazole were added to BMSCs for 4
or 48 h at 37°C. Cell viability was measured and recorded using a
Cell Titer Glo Luminescent Viability Assay kit (Promega
Corporation, Madison, WI, USA) and a Varioskan Flash Multimode
Reader (Thermo Fisher Scientific, Inc.).
Labeling of exosomes with DIO
Membrane fluorescent dye DIO (Beyotime Institute of
Biotechnology) was added to a H929 exosomes suspension at a final
concentration of 10 µM and was incubated for 30 min at 37°C.
An Exosome Spin Column (Thermo Fisher Scientific, Inc.) was
employed to remove free DIO. Subsequently, 10 µM DIO
solutions were also processed via an Exosome Spin Column, and the
flow-through served as a DIO control.
Confocal microscopy analysis
Human BMSCs were cultured in 24-well plates at a
density of 50,000 cells/well in OriCell Human MSC Culture medium at
37°C. DIO-labeled H929 exosomes and/or inhibitors were added to
BMSCs for 4 h at 37 or 4°C. Subsequently, the medium was discarded,
and the cells were washed once with 1 ml serum-free DMEM/F12 medium
to remove excess exosomes. For labeling the cell nucleuses and
lysosomes, Hoechst 33342 (Beyotime Institute of Biotechnology) and
Lyso-Tracker Red (Beyotime Institute of Biotechnology) were added
and incubated with human BMSCs for 1 h at 37°C. Fluorescent images
were obtained using a TCS SP8 confocal microscope at ×200
magnification (Leica Microsystems GmbH, Wetzlar, Germany).
Flow cytometry analysis
Following incubation with DIO-labeled H929 exosomes
and/or inhibitors, BMSCs were trypsinized and collected. The mean
and median fluorescent intensity in these cells were measured on a
BD Accuri C6 cytometer (BD Biosciences; Becton Dickinson and
Company, Franklin Lakes, NJ, USA) and analyzed using FlowJo V10
software (FlowJo LLC, Ashland, OR, USA).
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was employed to analyze the results and generate
histograms presenting the data as the mean values ± standard
deviation. One-way analysis of variance with Tukey's post hoc test
was performed to determine the statistical significance of
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
MM cell-derived exosomes increase BMSC
viability
MM cell-derived exosomes were first isolated from
the CM of four human MM cell lines, RPMI8226, H929, MM1S and U266.
The sizes of exosomes were determined by nanoparticle tracking
analysis, and a size distribution of 50-200 nm for the four types
of exosomes was observed (Fig.
1A). A number of well-established markers of exosomes,
including HSP70, Alix, TSG101, CD63 and flotillin-1 were detected
in these exosomes, whereas an intracellular contaminant,
calreticulin, was absent (Fig.
1B). To assess the effect of MM cell-derived exosomes on BMSC
viability, BMSCs were cultured with exosomes isolated from the four
cell lines at different final concentrations. These exosomes
dose-dependently promoted cell viability (Fig. 1C). Additionally, MM cell-derived
exosomes increased the phosphorylation of STAT1, STAT3 and ERK1/2,
while phosphorylation of JNK was increased only slightly (Fig. 1D), indicating that these exosomes
can affect multiple pathways in BMSCs.
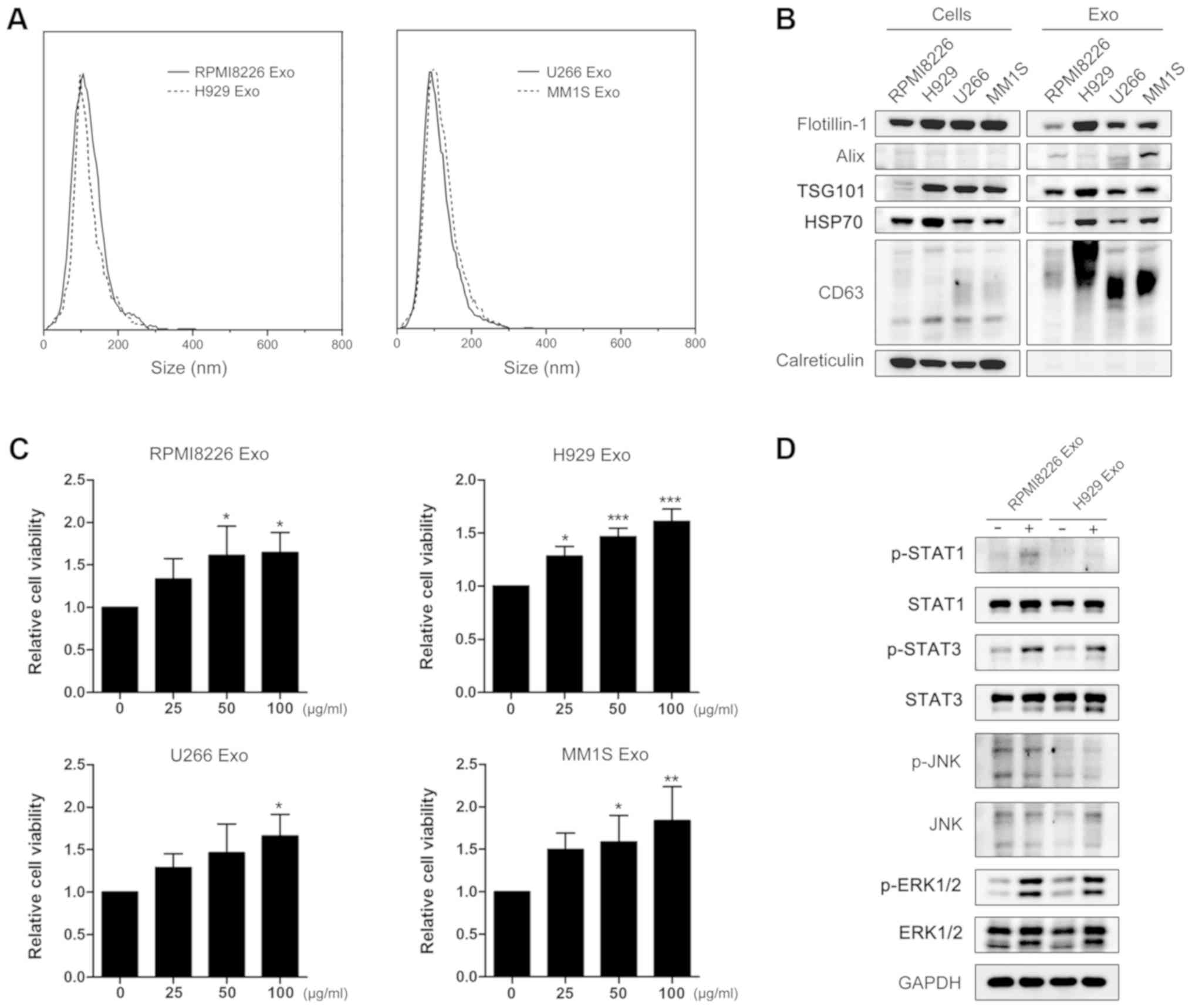 | Figure 1Multiple myeloma cell-derived
exosomes increase human BMSC viability and activate multiple
pathways. (A) Size distribution of exosomes isolated from RPMI8226,
H929, U266 and MM1S cell lines was determined by nanoparticle
tracking analysis. (B) Western blot analysis of exosomes-specific
markers flotillin-1, Alix, TSG101, HSP70 and CD63, as well as a
negative marker (calreticulin), in RPMI8226, H929, U266 and MM1S
cell lysates and their exosomes. (C) Human BMSCs in serum-free
RPMI-1640 medium were treated with RPMI8226, H929, U266 or MM1S
cell-derived exosomes at a concentration of 25, 50 or 100
µg/ml for 48 h, and then the cell viability was determined.
The fold changes were measured by comparing the viability of
exosomes-treated cells with that of untreated cells. (D) Human
BMSCs were incubated with 100 µg/ml RPMI8226 or H929-derived
exosomes for 6 h; subsequently, total and phosphorylated proteins
STAT1, STAT3, JNK and ERK1/2 were quantified by western blot
analysis. The GAPDH protein served as a loading control. Mean
values ± standard deviation for 3 independent experiments are
depicted. *P<0.05, **P<0.01 and
***P<0.001, compared with the control group. BMSCs,
bone marrow stromal cells; Exo, exosomes; Cells, cell lysates; p,
phosphor; STAT, signal transducer and activator of transcription 1;
JNK, c-Jun N-terminal kinase; ERK1/2, extracellular
signal-regulated kinase 1/2; CD63, cluster of differentiation;
TSG101, tumor susceptibility 101; HSP70, heat shock protein 70. |
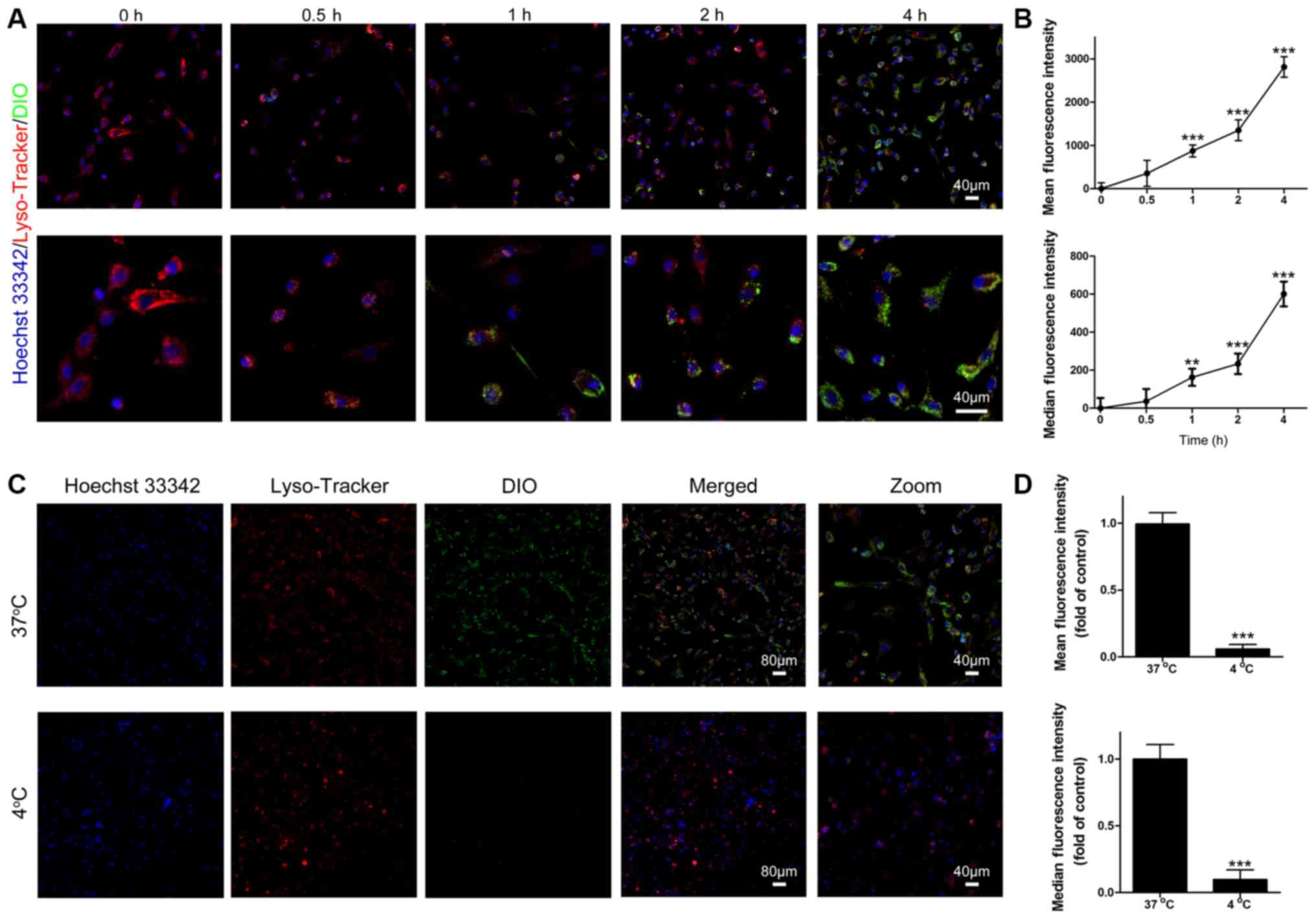
Uptake of MM cell-derived exosomes by
BMSCs is energy-dependent
To determine internalization of exosomes by BMSCs,
the green dye DIO was used to fluorescently label the membrane of
purified MM cell-derived exosomes, and BMSCs were visualized by
staining lysosomes with Lyso-Tracker Red. Following incubation with
labeled exosomes, an increasing green fluorescent signal was
observed in BMSCs over time, as revealed by confocal microscopy
analysis (Fig. 2A) and flow
cytometry (Fig. 2B). At 4 h after
incubation, internalization of a large number of exosomes was
observed in almost all BMSCs (Fig.
2A). To test whether the uptake of exosomes by BMSCs is an
active process or passive membrane fusion, BMSCs were incubated
with labeled exosomes at 4°C for 4 h. Internalization of MM
cell-derived exosomes was inhibited almost completely at 4°C
(Fig. 2C and D), indicating that
this internalization is mediated by active endocytic processes and
is energy-dependent.
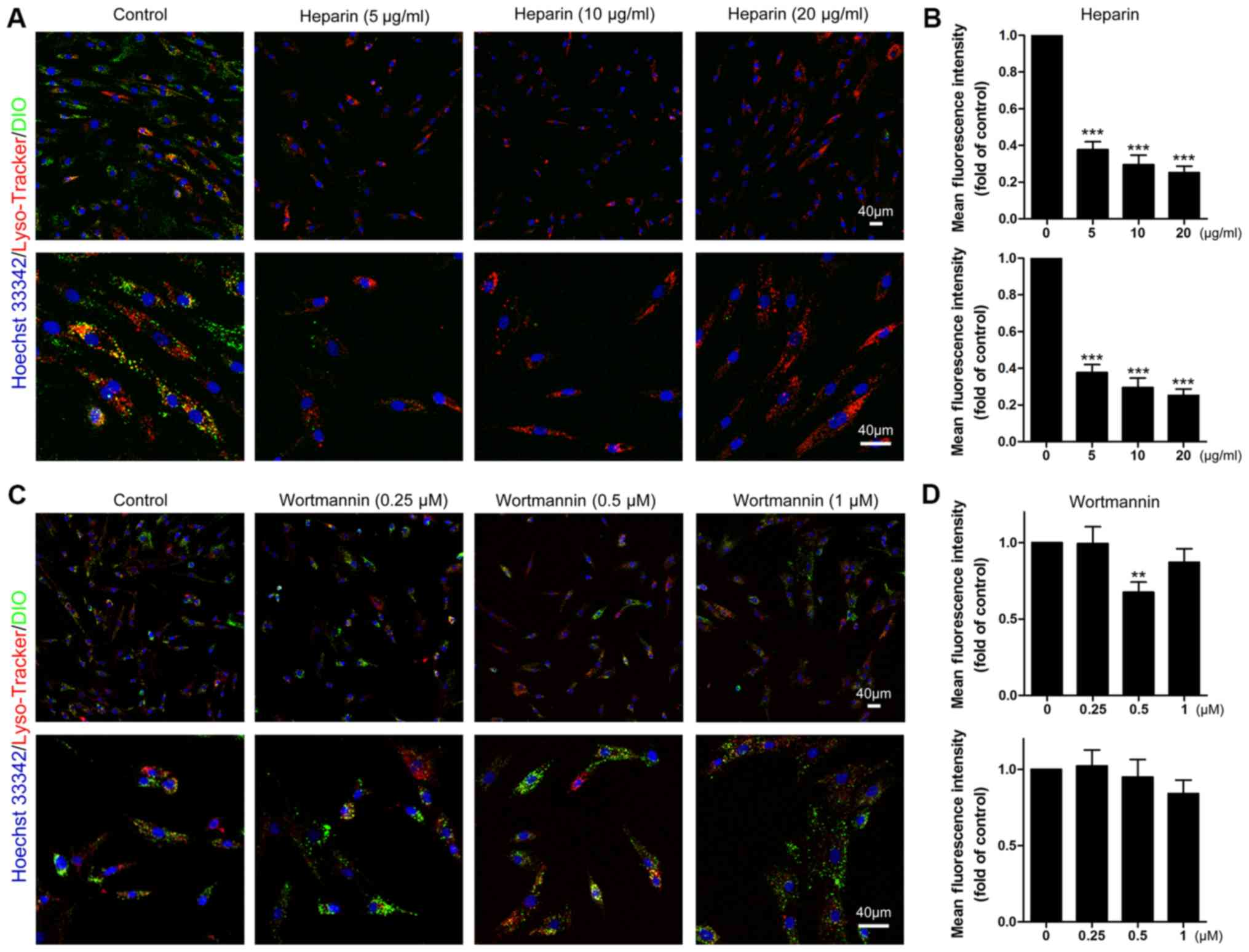
The endocytosis inhibitor decreases
internalization of MM cell-derived exosomes
Exosomes deliver a cargo and affect target cells
through membrane fusion, endocytosis and macropinocytosis. To
identify the pathway involved in the uptake of MM cell-derived
exosomes by BMSCs, heparin, an endocytosis inhibitor (10), and wortmannin, a phagocytosis
blocker (31), were employed. In
the presence of heparin, the internalization of MM cell-derived
exosomes was notably suppressed (Fig.
3A), and the mean fluorescence intensity was significantly
decreased by ~78% in the presence of 20 µg/ml heparin
(Fig. 3B), pointing to the
involvement of endocytic mechanisms requiring cytoskeletal
remodeling. In contrast, notable inhibition was not observed in the
presence of wortmannin (Fig. 3C),
and the mean and median fluorescence intensity underwent a slight
decrease when the highest concentration of wortmannin was applied
(Fig. 3D). Although the mean
fluorescence intensity decreased by 30% in the presence of 0.5
µM wortmannin, the median value did not change
significantly. Since wortmannin primarily targets PI3Ks, these data
indicated that internalization of MM cell-derived exosomes is
independent of PI3K-involved macropinocytosis.
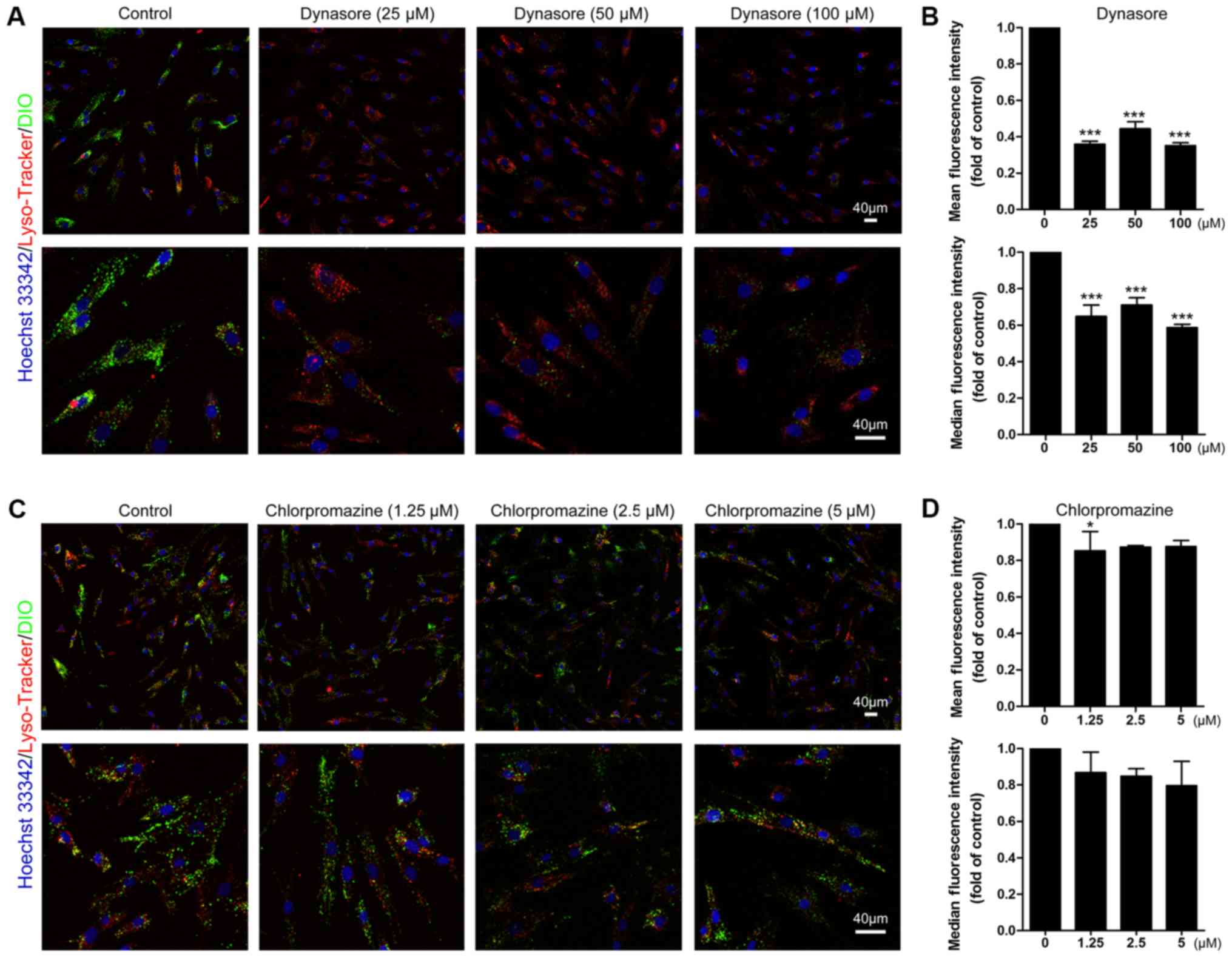
Internalization of MM cell-derived
exosomes is independent of clathrin-dependent endocytosis
To identify the specific endocytic processes that
contribute to the internalization of MM cell-derived exosomes,
BMSCs were treated with pharmacological inhibitors known to
interfere with clathrin- or caveolin-dependent endocytosis. Dynamin
2 is a GTPase required for clathrin- and caveolin-dependent
endocytosis (11), and its
specific inhibitor, dynasore, notably reduced the internalization
of exosomes in the present study even at the lowest concentration
(25 µM) applied (Fig. 4A).
Nevertheless this inhibition was not dose-dependent (Fig. 4B). Chlorpromazine, an inhibitor of
clathrin-mediated endocytosis (13), did not induce significant decreases
in the exosomes uptake by BMSCs except for 1.25 µM
chlorpromazine that decreased the mean exosomes uptake by 20%, as
revealed by confocal microscopy (Fig.
4C) and flow cytometry (Fig.
4D). These data indicated that caveolin-dependent endocytosis
is the primary endocytic process that contributes to the uptake of
MM cell-derived exosomes by BMSCs.
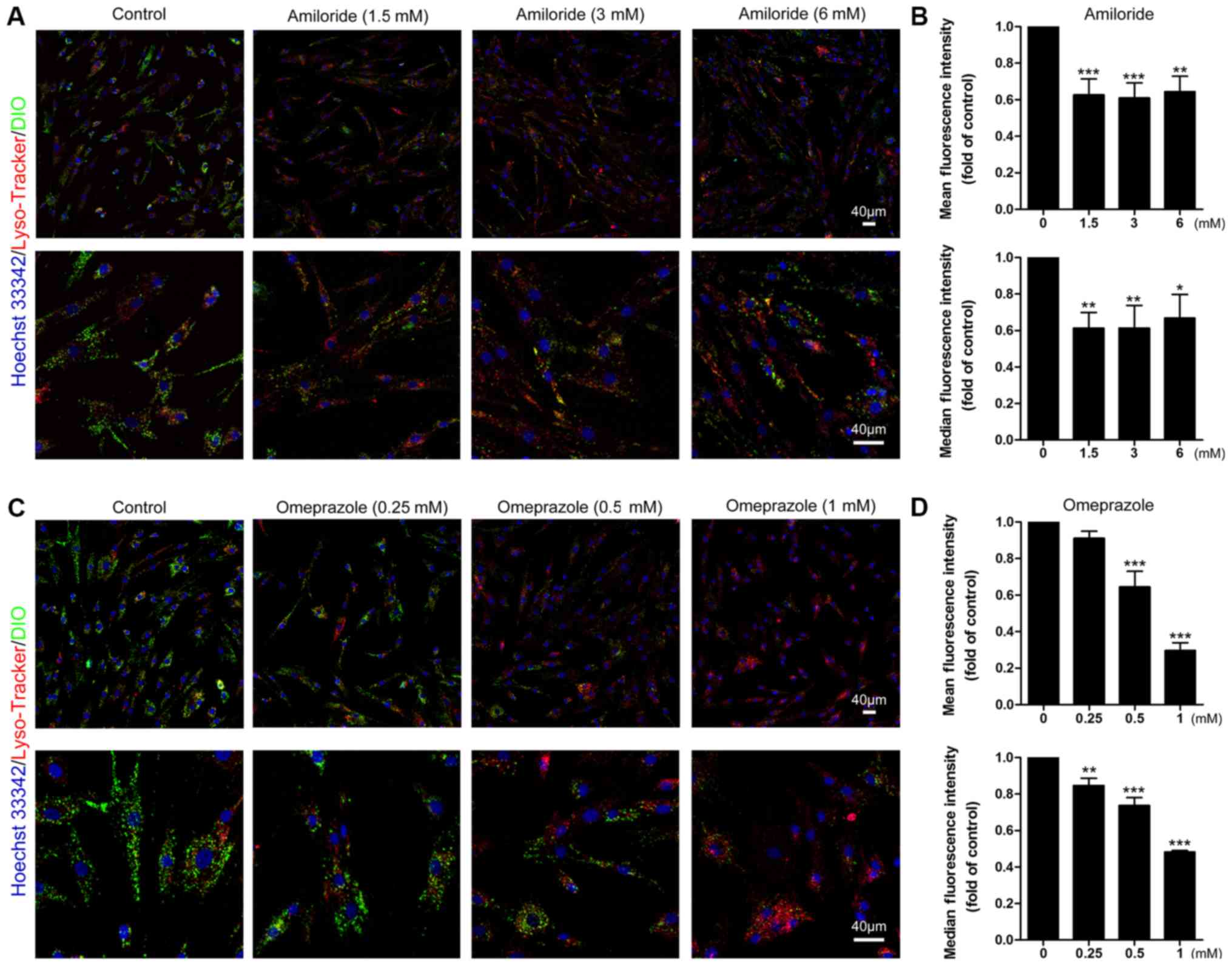
Macropinocytosis and membrane fusion are
partially involved in the uptake of MM cell-derived exosomes
Other pathways, including macropinocytosis and
membrane fusion, are also routes of EV uptake, and thus, the
effects of their inhibitors on the uptake of MM cell-derived
exosomes by BMSCs were determined. An inhibitor targeting the
sodium/proton exchanger, amiloride, dose-independently depressed
exosomes internalization, and the exosomes uptake was reduced by
~40% (Fig. 5A and B). Omeprazole,
a proton pump inhibitor, dose-dependently attenuated exosomes
uptake, and 1 mM omeprazole decreased the mean fluorescence
intensity in MSCs by up to 70%, indicating strong inhibition
(Fig. 5C and D). These results
validate the involvement of macropinocytosis and membrane fusion in
the uptake of MM cell-derived exosomes by BMSCs.
Endocytosis inhibitors suppress the
effects of MM cell-derived exosomes on BMSCs
Although a reduction in exosomes internalization was
observed in BMSCs treated with the inhibitors of endocytosis,
macropinocytosis or membrane fusion, these inhibitors may influence
the viability of BMSCs and therefore decrease exosomes uptake.
Accordingly, the changes in BMSC viability following treatment with
different inhibitors for 4 h at the concentration used in uptake
experiments were evaluated (Fig.
6A). Heparin and dynasore did not impair BMSC viability,
implying that the reduction of exosomes uptake by these two
inhibitors is not caused by a decrease in BMSC viability. However,
omeprazole and the highest dose of amiloride (6 mM) significantly
inhibited BMSC viability within 4 h. Nonetheless, 1.5-3.0 mM
amiloride, which reduced exosomes internalization, did not decrease
BMSC viability, indicating that the inhibition of exosomes uptake
induced by amiloride in this range of concentrations was not a
result of impaired BMSC viability. Additionally, heparin and
dynasore suppressed the phosphorylation of STAT1, STAT3, and ERK1/2
in the presence of RPMI8226 or H929 exosomes, compared with
treatment with RPMI8226 or H929 exosomes only (Fig. 6B–D), indicating that the
suppression of exosomes uptake by these two inhibitors can
attenuate the exosomes-induced functional changes in BMSCs.
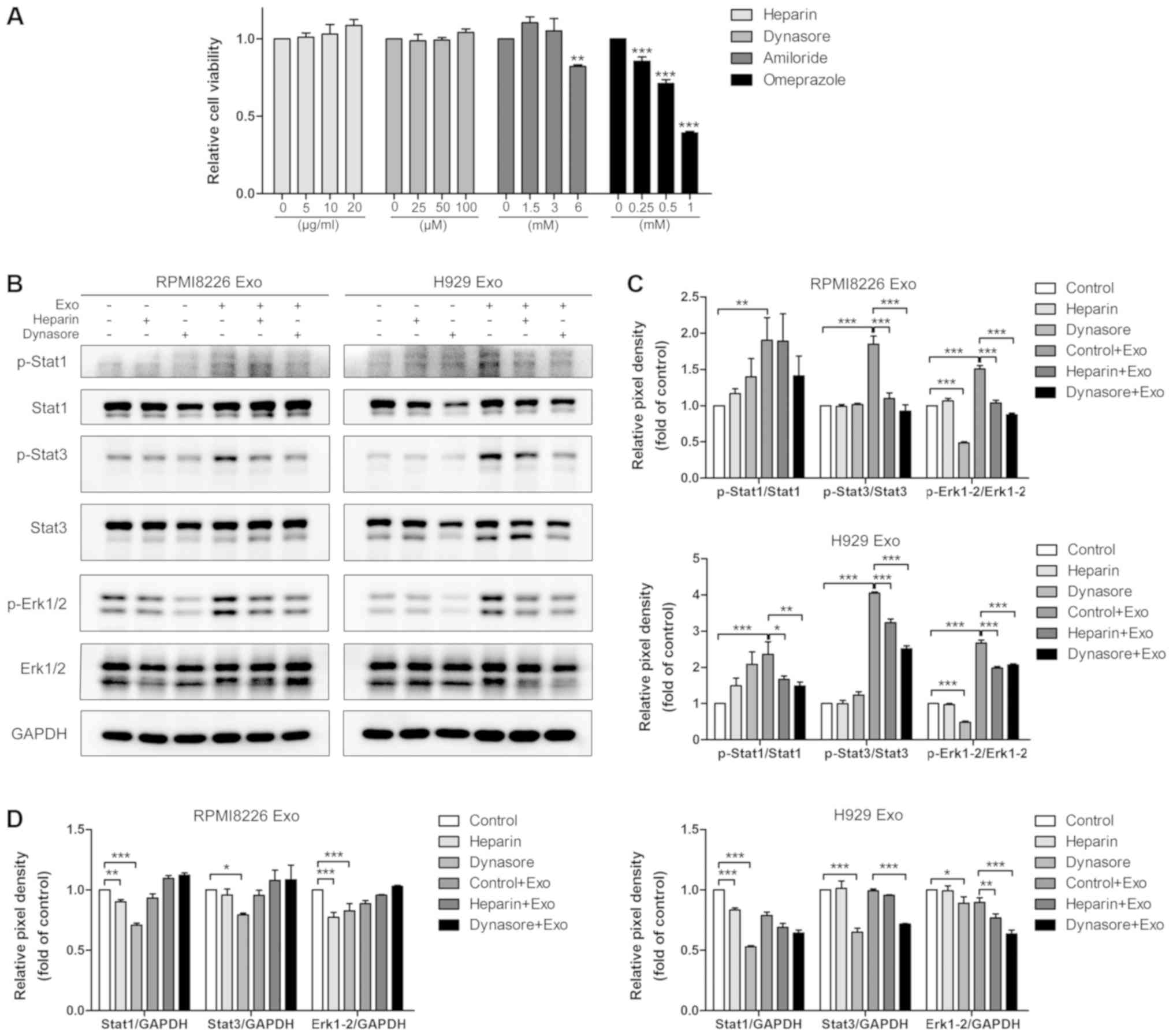 | Figure 6Endocytosis inhibitors partially
suppress the effects of multiple myeloma cell-derived exosome on
BMSCs. (A) Human BMSCs were incubated with heparin, dynasore,
amiloride or omeprazole at the indicated final concentration for
4.5 h, and cell viability was examined. **P<0.01 and
***P<0.001, compared with the group without inhibitor
treatment. (B) Human BMSCs were pre-treated with heparin (10
µg/ml) or dynasore (50 µM) for 30 min and incubated
with 100 µg/ml RPMI8226 or H929 cell-derived exosomes for 6
h. Subsequently, total and phosphorylated STAT1, STAT3 and ERK1/2
were quantified via western blot analysis. The GAPDH protein was
included as a loading control. The pixel density of phosphorylated
and total STAT1, STAT3 and ERK1/2 was quantified. (C)
Phosphorylated proteins were normalized to their total protein
amounts, and (D) total amounts of proteins were normalized to GAPDH
quantities. *P<0.05; **P<0.01; and
***P<0.001. BMSCs, bone marrow stromal cells; Exo,
exosomes; p, phosphor; STAT, signal transducer and activator of
transcription 1; ERK1/2, extracellular signal-regulated kinase
1/2. |
Discussion
Accumulating evidence highlights EVs as crucial
mediators in cell-to-cell communication during tumor progression,
and a number of studies revealed that exosomes mediate the
communication between BMSCs and MM cells, and promote the formation
of a microenvironment with enhanced angiogenesis and
immunosuppression (22,32). Thus, blocking this communication
may facilitate the treatment of MM, while the routes and mechanisms
of the uptake of EVs involved in the MM cell-BMSC interaction
remain unknown. Large macrovesicles (200-1,000 nm size) are also
produced by tumor cells and meditate the communication between the
tumor cells and stromal cells (21,33).
Unlike homogeneous exosomes, the shape of these vesicles is more
irregular and inhomogeneous, resulting in difficulty in identifying
them (34). Additionally, the
release of large EVs is not constitutive and can be regulated by
various stimuli and conditions, including starvation and cytokine
stimulation (35). Thus, the
present study primarily investigates the function of a subtype of
homogeneous EVs, exosomes. In the present study, pharmacological
inhibition of endocytic pathways was conducted and it was
demonstrated that routes of uptake of MM cell-derived exosomes by
BMSCs include caveolin-dependent endocytosis, macropinocytosis and
membrane fusion. Furthermore, specific inhibitors suppressed the
functional changes in BMSCs induced by the exosomes-mediated MM
cell-to-BMSC communication. These data are indicative of a possible
use of endocytic inhibitors as enhancers of MM treatment.
Previously, it was demonstrated that murine MM
cell-derived exosomes carrying a variety of soluble factors,
including monocyte chemoattractant protein 1, macrophage
inflammatory protein 1a and stromal cell-derived factor 1, can
increase the viability of murine BMSCs and activate a number
pro-survival or pro-proliferation pathways, including STAT3, JNK
and p53, in BMSCs (22). To
validate this observation in human cells, exosomes from four human
MM cell lines were isolated, and all these exosomes promoted the
viability of human BMSCs isolated from a healthy donor under
starvation conditions. Similarly, MM cell-derived exosomes
activated the STAT1, STAT3 and ERK1/2 pathways, which have been
demonstrated to be associated with cell survival and proliferation
(36-38). Additionally, our previous study
indicated that miRNAs enclosed in MM cell-derived exosomes enhance
the pro-tumoral activity of BMSCs (25), and these results highlight the
involvement of exosomes in the functional regulation of target
cells.
MM cell-derived exosomes can be time-dependently
engulfed by BMSCs, and this process requires energy, in line with
the observation reported in another study (39). Exosomes utilize native mechanisms
of uptake, which consist of numerous endocytic pathways, including
phagocytosis, clathrin- and caveolin-mediated endocytosis, to
functionally deliver their cargo (8). Internalization of extracellular
material is primarily subdivided into two subtypes, termed
phagocytosis and pinocytosis (40). Large particles are primarily
internalized through phagocytosis, and this process is generally
restricted to phagocytes (41). In
contrast, pinocytosis, including clathrin-dependent endocytosis,
clathrin-independent endocytosis and macropinocytosis, can be
conducted by all cell types to engulf relatively small vesicles and
extracellular molecules (42,43).
Heparin functions as a soluble analogue of heparin sulphate
proteoglycans and inhibits endocytosis (44). In the present study, heparin
decreased the uptake of MM cell-derived exosomes by ~78%,
indicating that endocytosis is the dominant route. Heparin has been
reported to significantly inhibit the internalization of exosomes
or EVs in numerous cell types, including glioblastoma multiform
primary tumor cells, bladder cancer cells and human cervical
carcinoma cells (39,44). These studies highlight endocytosis
as a primary route of exosomes entry into recipient cells.
Endocytosis can be categorized into clathrin-,
flotillin-1-, RhoA- and caveolin-dependent endocytosis (45). Dynamin 2, a GTPase, is recruited to
nascent clathrin-coated pits and is required during membrane
fission and a release of clathrin-coated vesicles (46,47).
Dynamin 2 activity also promotes the assembly and expansion of
caveolar vesicles (48).
Suppression of dynamin 2 by a specific inhibitor (dynasore) can
strongly reduce the internalization of exosomes (39,49).
Indeed, dynasore significantly decreased the uptake of MM
cell-derived exosomes by ~65% in the present study. The inhibitory
effect of heparin was stronger than dynasore, indicating that other
types of endocytosis are also involved in exosomes uptake.
Treatment with chlorpromazine reduces uptake of EVs by ovarian
cancer and phagocytic recipient cells (31,50).
By contrast, this inhibitor did not decrease the internalization of
MM cell-derived exosomes in the present study, indicating that
clathrin-mediated endocytosis does not contribute significantly to
the aforementioned process. Thus, caveolin-dependent endocytosis is
the primary endocytic pathway for the uptake of MM cell-derived
exosomes by BMSCs.
Phagocytosis, macropinocytosis and membrane fusion
also contribute to EV internalization. Although wortmannin has been
reported to significantly inhibit EV uptake (31,39),
the present study did not reveal notable inhibitory effects on the
uptake of MM cell-derived exosomes by BMSCs when wortmannin was
used, indicating the absence of phagocytosis in this process. The
first step of macropinocytosis is the formation of
invagination-based membrane ruffles, and then they are pinched off
into the intercellular space (8).
Unlike phagocytosis, macropinocytosis does not require the direct
contact with internalized materials but requires
Na+/H+ exchanger activity (14,51).
Abrogation of macropinocytosis by amilorde, a
Na+/H+ exchanger inhibitor, reduces the EV
internalization by microglia (49). The present results also uncovered
an inhibitory effect of amilorde on the uptake of MM cell-derived
exosomes by BMSCs, although this effect is not stronger than that
induced by heparin and dynasore. These data indicate that
caveolin-dependent endocytosis contributes more to the uptake than
macropinocytosis. Direct fusion of the EV membrane with the cell
membrane is another mechanism underlying exosomes uptake (3). Proton pump inhibitors have been
demonstrated to decrease exosomes fusion with melanoma cells by
targeting sodium reabsorption (16). Strong inhibition of exosomes uptake
was observed in the present study in BMSCs treated with 1 mM
omeprazole, a proton pump inhibitor, whereas it decreased BMSC
viability by 60%. Lower concentrations of omeprazole still
decreased BMSC viability, although omeprazole can inhibit exosomes
uptake, indicating that this inhibition may be primarily caused by
impaired cell viability instead of inhibition of membrane fusion.
Furthermore, endocytosis inhibitors heparin and dynasore suppressed
and delayed the phosphorylation of STAT1, STAT3, and ERK1/2 induced
by MM cell-derived exosomes following a brief blockade. These
results indicate that an endocytosis inhibitor can attenuate
exosomes uptake and suppress exosomes-induced changes of
intercellular pathways. These observations are indicative of
potential usefulness of these inhibitors for blocking MM cell-BMSC
communication. Nevertheless, further studies that reveal the
specific ligand-receptor pairs responsible for exosomal uptake, as
well as in vivo studies investigating the minimum effective
dose range, are required in the future to improve the understanding
of the uptake process and determine the blocking efficiency.
In conclusion, endocytosis was identified as the
primary route of uptake of MM cell-derived exosomes by BMSCs and it
was demonstrated that macropinocytosis and membrane fusion are also
partially involved in exosomes internalization. These observations
will facilitate the development of modification-based approaches to
the enhancement of exosomes uptake and thus improve the efficiency
of exosomes-mediated cargo or drug delivery. Additionally, the
present data indicated that the blockade of the endocytosis pathway
can suppress the exosomes-induced alteration in target BMSCs,
underscoring the importance of this route in MM cell-BMSC
communication. Furthermore, blocking the communication between MM
cells and BMSCs may be another option for improving MM treatment
due to this communication being demonstrated to be critical for MM
progression.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81700203), Medical
Scientific Research Foundation of Guangdong Province, China
(project no. A2018031) and startup funding issued by Guangzhou
Medical University and Bureau of Education of Guangzhou.
Availability of data and materials
All data and materials in this study are available
from corresponding author on reasonable request.
Authors' contributions
JW conceived the idea and supervised the
experiments. YZ and CT implemented the experiments with the
assistance of JZ. JW, YZ and CT analyzed the data and wrote the
manuscript with suggestions from all the authors. All the authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from Ethical Committee
for Clinical Medicine Research of The Third Affiliated Hospital of
Sun Yat-Sen University (Guangzhou, China). Informed consent was
obtained from participate prior to sample collection.
Patient consent for publication
Consent for publication was obtained from the
participants.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mr. Yonghua Liao at
School of Basic Medical Sciences, Guangzhou Medical University
(Guangzhou, China) for expert technical assistance.
References
|
1
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camussi G, Deregibus MC, Bruno S, Grange
C, Fonsato V and Tetta C: Exosome/microvesicle-mediated epigenetic
reprogramming of cells. Am J Cancer Res. 1:98–110. 2011.PubMed/NCBI
|
|
3
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: Important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
EL Andaloussi S, Mäger I and Breakefield
XO: Extracellular vesicles: Biology and emerging therapeutic
opportunities. Nat Rev Drug Discov. 12:347–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mulcahy LA, Pink RC and Carter DR: Routes
and mechanisms of extracellular vesicle uptake. J Extracell
Vesicles. 3:32014. View Article : Google Scholar
|
|
9
|
O'Donoghue EJ and Krachler AM: Mechanisms
of outer membrane vesicle entry into host cells. Cell Microbiol.
18:1508–1517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christianson HC, Svensson KJ, van
Kuppevelt TH, Li JP and Belting M: Cancer cell exosomes depend on
cell-surface heparan sulfate proteoglycans for their
internalization and functional activity. Proc Natl Acad Sci USA.
110:17380–17385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ehrlich M, Boll W, Van Oijen A, Hariharan
R, Chandran K, Nibert ML and Kirchhausen T: Endocytosis by random
initiation and stabilization of clathrin-coated pits. Cell.
118:591–605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stephens L, Ellson C and Hawkins P: Roles
of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell
Biol. 14:203–213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang LH, Rothberg KG and Anderson RG:
Mis-assembly of clathrin lattices on endosomes reveals a regulatory
switch for coated pit formation. J Cell Biol. 123:1107–1117. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Swanson JA: Shaping cups into phagosomes
and macropi-nosomes. Nat Rev Mol Cell Biol. 9:639–649. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koivusalo M, Welch C, Hayashi H, Scott CC,
Kim M, Alexander T, Touret N, Hahn KM and Grinstein S: Amiloride
inhibits macropinocytosis by lowering submembranous pH and
preventing Rac1 and Cdc42 signaling. J Cell Biol. 188:547–563.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parolini I, Federici C, Raggi C, Lugini L,
Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A,
et al: Microenvironmental pH is a key factor for exosome traffic in
tumor cells. J Biol Chem. 284:34211–34222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ha D, Yang N and Nadithe V: Exosomes as
therapeutic drug carriers and delivery vehicles across biological
membranes: Current perspectives and future challenges. Acta Pharm
Sin B. 6:287–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Batrakova EV and Kim MS: Using exosomes,
naturally-equipped nanocarriers, for drug delivery. J Control
Release. 219:396–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lemaire M, Deleu S, De Bruyne E, Van
Valckenborgh E, Menu E and Vanderkerken K: The microenvironment and
molecular biology of the multiple myeloma tumor. Adv Cancer Res.
110:19–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manier S, Sacco A, Leleu X, Ghobrial IM
and Roccaro AM: Bone marrow microenvironment in multiple myeloma
progression. J Biomed Biotechnol. 2012:1574962012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Faict S, Maes K, De Bruyne E, Van
Valckenborgh E, Schots R, Vanderkerken K and Menu E: Extracellular
vesicle cross-talk in the bone marrow microenvironment:
Implications in multiple myeloma. Oncotarget. 7:38927–38945.
2016.PubMed/NCBI
|
|
22
|
Wang J, De Veirman K, Faict S, Frassanito
MA, Ribatti D, Vacca A and Menu E: Multiple myeloma exosomes
establish a favourable bone marrow microenvironment with enhanced
angiogenesis and immunosuppression. J Pathol. 239:162–173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, De Veirman K, De Beule N, Maes K,
De Bruyne E, Van Valckenborgh E, Vanderkerken K and Menu E: The
bone marrow microenvironment enhances multiple myeloma progression
by exosome-mediated activation of myeloid-derived suppressor cells.
Oncotarget. 6:43992–44004. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Hendrix A, Hernot S, Lemaire M, De
Bruyne E, Van Valckenborgh E, Lahoutte T, De Wever O, Vanderkerken
K and Menu E: Bone marrow stromal cell-derived exosomes as
communicators in drug resistance in multiple myeloma cells. Blood.
124:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Veirman K, Wang J, Xu S, Leleu X, Himpe
E, Maes K, De Bruyne E, Van Valckenborgh E, Vanderkerken K, Menu E,
et al: Induction of miR-146a by multiple myeloma cells in
mesenchymal stromal cells stimulates their pro-tumoral activity.
Cancer Lett. 377:17–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuhorn IS, Kalicharan R and Hoekstra D:
Lipoplex-mediated transfection of mammalian cells occurs through
the cholesterol-dependent clathrin-mediated pathway of endocytosis.
J Biol Chem. 277:18021–18028. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uhrig S, Coutelle O, Wiehe T, Perabo L,
Hallek M and Büning H: Successful target cell transduction of
capsid-engineered rAAV vectors requires clathrin-dependent
endocytosis. Gene Ther. 19:210–218. 2012. View Article : Google Scholar
|
|
28
|
Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY,
Shin SW, An JJ, Kim MS, Choi SY, Sun W, et al: Enhanced
hypothalamic leptin signaling in mice lacking dopamine D2
receptors. J Biol Chem. 285:8905–8917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gawecka JE, Young-Robbins SS, Sulzmaier
FJ, Caliva MJ, Heikkilä MM, Matter ML and Ramos JW: RSK2 protein
suppresses integrin activation and fibronectin matrix assembly and
promotes cell migration. J Biol Chem. 287:43424–43437. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacGibeny MA, Koyuncu OO, Wirblich C,
Schnell MJ and Enquist LW: Retrograde axonal transport of rabies
virus is unaffected by interferon treatment but blocked by emetine
locally in axons. PLoS Pathog. 14:e10071882018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ,
Chang LF, Zhou Q and Sui SF: Cellular internalization of exosomes
occurs through phagocytosis. Traffic. 11:675–687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Umezu T, Tadokoro H, Azuma K, Yoshizawa S,
Ohyashiki K and Ohyashiki JH: Exosomal miR-135b shed from hypoxic
multiple myeloma cells enhances angiogenesis by targeting
factor-inhibiting HIF-1. Blood. 124:3748–3757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Théry C, Ostrowski M and Segura E:
Membrane vesicles as conveyors of immune responses. Nat Rev
Immunol. 9:581–593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raimondo F, Morosi L, Chinello C, Magni F
and Pitto M: Advances in membranous vesicle and exosome proteomics
improving biological understanding and biomarker discovery.
Proteomics. 11:709–720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Orozco AF and Lewis DE: Flow cytometric
analysis of circulating microparticles in plasma. Cytometry A.
77:502–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meissl K, Macho-Maschler S, Müller M and
Strobl B: The good and the bad faces of STAT1 in solid tumours.
Cytokine. 89:12–20. 2017. View Article : Google Scholar
|
|
37
|
Wingelhofer B, Neubauer HA, Valent P, Han
X, Constantinescu SN, Gunning PT, Müller M and Moriggl R:
Implications of STAT3 and STAT5 signaling on gene regulation and
chromatin remodeling in hematopoietic cancer. Leukemia.
32:1713–1726. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanimura S and Takeda K: ERK signalling as
a regulator of cell motility. J Biochem. 162:145–154. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Costa Verdera H, Gitz-Francois JJ,
Schiffelers RM and Vader P: Cellular uptake of extracellular
vesicles is mediated by clathrin-independent endocytosis and
macropinocytosis. J Controll Release. 266:100–108. 2017. View Article : Google Scholar
|
|
40
|
Doherty GJ and McMahon HT: Mechanisms of
endocytosis. Annu Rev Biochem. 78:857–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rosales C and Uribe-Querol E:
Phagocytosis: A fundamental process in immunity. BioMed Res Int.
2017:90428512017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaksonen M and Roux A: Mechanisms of
clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 19:313–326.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rosendale M and Perrais D: Imaging in
focus: Imaging the dynamics of endocytosis. Int J Biochem Cell
Biol. 93:41–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Franzen CA, Simms PE, Van Huis AF, Foreman
KE, Kuo PC and Gupta GN: Characterization of uptake and
internalization of exosomes by bladder cancer cells. BioMed Res
Int. 2014:6198292014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mayor S and Pagano RE: Pathways of
clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 8:603–612.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Taylor MJ, Lampe M and Merrifield CJ: A
feedback loop between dynamin and actin recruitment during
clathrin-mediated endocytosis. PLoS Biol. 10:e10013022012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marks B, Stowell MH, Vallis Y, Mills IG,
Gibson A, Hopkins CR and McMahon HT: GTPase activity of dynamin and
resulting conformation change are essential for endocytosis.
Nature. 410:231–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Parton RG and Simons K: The multiple faces
of caveolae. Nat Rev Mol Cell Biol. 8:185–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fitzner D, Schnaars M, van Rossum D,
Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK and Simons
M: Selective transfer of exosomes from oligodendrocytes to
microglia by macropinocytosis. J Cell Sci. 124:447–458. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Escrevente C, Keller S, Altevogt P and
Costa J: Interaction and uptake of exosomes by ovarian cancer
cells. BMC Cancer. 11:1082011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kerr MC and Teasdale RD: Defining
macropinocytosis. Traffic. 10:364–371. 2009. View Article : Google Scholar : PubMed/NCBI
|