Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently
the fourth leading cause of cancer-related mortality in the United
States and is projected to become the second leading cause by 2030
(1). Pancreatic cancer is
associated with a dismal prognosis, largely due to late disease
presentation, aggressive local invasion with early metastatic
spread and fairly resistant malignancy with a poor response to
conventional chemotherapy and radiotherapy (2). An increased risk of pancreatic cancer
has been associated with several environmental, biological and
genetic factors. For example, exposure to certain classes of
organic solvents, smoking, radiation, diabetes, chronic
pancreatitis, prior gastric surgery and specific gene polymorphisms
has been associated with the incidence of pancreatic cancer
(3,4). Despite a significant enhancement in
our understanding of the genetic makeup of this disease and
considerable advancements being made in the use of targeted
therapy, the management of pancreatic cancer continues to be one of
the greatest therapeutic challenges. Recent studies have documented
the significance of dietary factors in the treatment and/or
prevention of pancreatic cancer (5-12).
Resveratrol is a polyphenolic compound that is
naturally found in many plant species (13). Due to several of its properties,
such as anti-aging, anti-carcinogenic, anti-inflammatory and
antioxidant properties, resveratrol is regarded as a beneficial
agent for the treatment of chronic diseases and for increasing
longevity in humans (14-17). In pre-clinical and animal models of
pancreatic cancer, resveratrol has been shown to inhibit cell
proliferation, metastasis and angiogenesis, and to induce apoptosis
(6,8,18,19).
Furthermore, resveratrol sensitizes cancer cells and exerts
synergistic effects with chemotherapy and radiotherapy to provide
an enhanced advantage to conventional therapeutics (14,20-22).
Recent human clinical trials have demonstrated that resveratrol is
safe and reasonably well-tolerated at doses of up to 5 g/day
(23). However, the major
limitation of its use in the management of human ailments is
primarily due to its unfavorable pharmacokinetics
(PK)/pharmacodynamics (PD) profile. This is in part due to its poor
oral bioavailability; i.e., a low aqueous solubility and an
extensive pre-systemic metabolism. Thus, in the present study, we
used the resveratrol derivative, triacetyl-resveratrol (TCRV),
which is more stable and provides a better PK/PD profile than
parent resveratrol.
The hedgehog (Hh) signaling pathway is highly
conserved from Drosophila to humans. It plays a crucial role
in embryonic development and cell fate decisions (24). The sonic hedgehog (Shh) canonical
pathway is initiated by the binding of the Hh ligand to
transmembrane receptor patched 1 (PTCH1), which relieves its
repression on Smoothened (Smo), a G-protein coupled receptor. Thus,
Smo activation leads to the transmission of the Hh signal through
the protein complex, which results in nuclear translocation and in
the activation of the glioma-associated oncogene homolog (Gli1),
and in the degradation of the repressor form, Gli3 (25). Therefore, the activation of Shh
signaling through Smo can either occur through the stimulation of
Hh protein or the loss of PTCH1 activity. Upon the activation and
translocation of Gli to the nucleus, Gli acts as a transcriptional
regulator of its target genes and regulates the expression of Gli1,
PTCH1, Bcl-2, Cyclin D1, Snail and vascular endothelial growth
factor (VEGF) (26). Gli1 and Gli2
function as transcriptional activators, whereas Gli3 functions as a
repressor (27). The Hh signaling
pathway also plays a crucial role in adult tissue homeostasis
(28). In adulthood, the Hh
signaling pathway is usually repressed; however, its activity is
maintained in certain stem cell populations to promote tissue
renewal and regeneration. Several studies have implicated the
aberrant activation of Hh signaling pathway in various human
malignancies, such as medulloblastoma, rhabdomyosarcoma,
hepatocellular carcinoma, pancreatic cancer, digestive tract
cancer, colon cancer, breast cancer, small-cell lung carcinoma,
basal cell carcinoma and prostate cancer (29-38).
Furthermore, the transcriptional activation of Gli1/2 enhances cell
proliferation and cell cycle progression, whereas the inhibition of
Gli1/2 attenuates cell proliferation and induces apoptosis
(32,39,40).
MicroRNAs (miRNAs or miRs) are short noncoding RNAs
that are evolutionarily conserved and can modulate gene expression
at the post transcriptional level via sequence-specific
interactions with the cognate mRNA targets (41). In mammalian cells, miRNAs regulate
gene silencing through both degradations of the mRNA and inhibition
of translation. Based on array data, miRNAs have been estimated to
regulate ~30% of the human genome (41). Various types of cancer, including
pancreatic cancer have been identified to exhibit a deregulation of
miRNA expression (42,43). Indeed, a recent study identified
that miRNAs can function as classical oncogenes or tumor suppressor
genes (44). Furthermore, miRNAs
can also regulate cancer progression and metastasis in various
types of human cancer (45). More
importantly, miRNA expression has been associated with the
recurrence, development of metastases and/or the survival of
patients through various clinical trials (45). miRNAs are significantly
dysregulated in pancreatic cancer, suggesting the potential role of
miRNAs as key regulators of pancreatic carcinogenesis. However, at
least to the best of our knowledge, there are no studies available
to date examining the effects of TCRV on epithelial-mesenchymal
transition (EMT)-related miRNAs.
The aim of the present study was to elucidate the
molecular mechanisms through which the resveratrol derivative,
TCRV, inhibits the growth and EMT in pancreatic cancer cells and
induces apoptosis. Our data demonstrate that TCRV inhibits the
growth and induces the apoptosis of pancreatic cancer cells. TCRV
inhibits EMT by the induction of E-cadherin expression and the
inhibition of the expression of the EMT-associated transcription
factors, Snail, Slug, and Zeb1 and N-cadherin. The inhibitory
effects of TCRV on EMT are presumably exerted through the
suppression of the Shh pathway and the upregulation of miR-200.
These data indicated that TCRV may be used in the treatment and/or
prevention of pancreatic cancer.
Materials and methods
Reagents and cell culture conditions
TCRV (trans-3,5,4′-triacetylstilbene,
3,5,4′-Tri-O-acetyl resveratrol) was purchased from LKT
Laboratories, Inc. (St. Paul, MN, USA). Recombinant human sonic
hedgehog (Shh) protein was purchased from R&D Systems
(Minneapolis, MN, USA). Gli1 cDNA (pReceiver) was purchased from
GeneCopoeia (Rockville, MD, USA). Plasmids expressing scrambled
(Human pre-miRNA Scramble Negative Control Expression Lentivector,
pCDH-CMVMCH-EF1α-CopGFP), anti-miR-200a, b and c (MirZip™
anti-miRNA Expression Lentivector) and ZEB1 3′UTR construct
(contains miR-200 family sites, MiR-Selection Fire-Ctx Lentivector)
were purchased from System Biosciences (Palo Alto, CA, USA). The
pancreatic cancer cells (AsPC-1 and PANC-1) and the human
pancreatic normal ductal epithelial (HPNE) cells were purchased
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). Dulbecco’s modified Eagle’s medium with 10% fetal bovine
serum with antibiotics was used to grow the cancer cells. HPNE
cells were grown as per the recommendations of ATCC.
Lentiviral particle production and
transduction
Lentivirus was produced by the triple transfection
of packaging 293T cells (American Type Culture Collection) with
plasmid, lentiviral vector and lipofectamine 2000/Plus reagent,
(Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA) as per the
manufacturer’s instructions. Viral supernatants were collected and
concentrated using PEG-it virus precipitation solution [System
Biosciences (SBI)] by centrifugation (1,500 × g for 30 min at 4°C)
and concentrated 100-fold to produce virus stocks with titers of
1×108 to 1×109 infectious units per ml.
Titers were determined on 293T cells. The pancreatic cancer cells
were transduced with lentiviral particles in the presence of 6
µg/ml polybrene (Thermo Fisher Scientific).
Apoptosis assay
Fluorescence-activated cell sorting (FACS) analysis
was one of the methods used to determine cell apoptosis. In brief,
the cultured cells were trypsinized, washed with PBS and
resuspended in 200 µl PBS and incubated with 10 µl
RNAase (10 mg/ml) at 37°C. Following incubation of the cells for 30
min, 50 µl propidium iodide solution was added, and tge
induction of cell apoptosis was measured by flow cytometry (Accuri
C6 flow cytometer; BD Biosciences, San Jose, CA, USA). The
induction of apoptosis was further confirmed by another method
using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP
nick-end labeling (TUNEL) and performed according to the
manufacturer’s instructions (Life Technologies, Grand Island, NY,
USA).
Caspase-3 activity assay
The cells (1×104 cells per well) were
seeded in 96-well plates and treated with various concentrations of
TCRV (0-20 µM) for 36 h. At the end of the incubation
period, caspase-3 activity was measured using a colorimetric assay
kit as per the manufacturer’s instructions (Sigma-Aldrich, St.
Louis, MO, USA). The caspase-3 colorimetric assay kit was based on
the hydrolysis of acetyl-Asp-Glu-Val-Asp p-nitroanilide
(Ac-DEVD-pNA) by caspase-3, resulting in the release of the
p-nitroaniline (pNA) moiety. The concentration of pNA released from
the substrate was calculated from the absorbance values at 405 nm
(BioTek Synergy Multimode Microplate reader; Thermo Fisher
Scientific).
Colony formation assay
The cells (100 cells per well) were seeded in 6-well
plates and treated with various concentrations of TCRV (1, 5, 10
and 20 µM). At the end of 21 days, colonies were fixed with
10% neutral-buffered formalin solution for 15-30 min, and stained
with crystal violet (0.5% w/v; Sigma-Aldrich) at room temperature
for 30 min. The plates were washed with dH2O and allowed
to dry. Colonies containing >50 individual cells were counted
using a stereo-microscope (Olympus, New Orleans, LA, USA).
Transwell migration assay
Transwell migration assay was performed as
previously described (46). In
brief, non-coated membrane inserts (24-well insert; pore size, 8
µm; Corning Costar, Inc., New York, NY, USA) were used to
plate 1×105 pancreatic cancer cells in the top chamber.
The cells were then allowed to migrate towards the serum-containing
DMED medium in the lower chamber. Following incubation for 24 h,
the cells that had migrated through the membrane were fixed with
methanol for 15-30 min, and stained with 0.1% crystal violet (2
mg/ml; Sigma-Aldrich) at room temperature for 30 min. The number of
cells that migrated through the membrane were evaluated under a
light microscope (Olympus) and counted.
Transwell invasion assay
Transwell invasion assay was performed as previously
described (46). In brief,
Matrigel (60 µg; BD Biosciences) was freshly coated in the
top chamber of the membrane inserts (24-well insert; pore size, 8
µm; Corning Costar, Inc.). A total of 1×105
pancreatic cancer cells in DMEM without serum or growth factors
were plated in the top chamber before the invasion assay. In the
lower chamber, DMEM supplemented with serum was used as a
chemoattractant. Following incubation for 48 h, the upper chamber
was cleared up of non-invading cells using a cotton swab. The cells
that had invaded on to the lower surface of the membrane were then
fixed with methanol for 15-30 min, and stained with 0.1% crystal
violet (2 mg/ml; Sigma-Aldrich) at room temperature for 30 min. The
number of cells that invaded through the membrane were evaluated
under a light microscope (Olympus) and counted.
Motility assay
Scratch motility assay was used to monitor the
horizontal movement of cells as previously described (46). The cells were seeded in a 6-well
plate to form a monolayer for 24 h, and then a scratch was made
using a pipette tip through the established monolayer giving rise
to an in vitro wound. The cells were then washed twice with
PBS, and wells were replaced with media with or without TCRV.
Movement of cells from the confluent sides of the monolayer to the
scratch area as single cells was monitored. They were viewed under
a microscope in 4 separate areas each day until the width of the
wound healing gap is filled in the untreated control wells. Three
replicate wells from a 6-well plate were used for each experimental
condition.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cultured cells using
an RNeasy Mini kit (Qiagen, Valencia, CA, USA). Briefly, cDNA was
synthesized by reverse transcription reaction using a high capacity
cDNA reverse transcription kit (Applied Biosystems, Foster City,
CA, USA). Signaling molecule specific primers used to generate the
PCR products were designed using NCBI/Primer-BLAST (https://www.ncbi.nlm.nih. gov/tools/primer-blast). The
gene amplification was quantified by qPCR using an ABI 7300
Sequence Detection System in the presence of SYBR-Green (Thermo
Fisher Scientific). The following gene-specific primers were used:
PTCH1 forward, 5′-TGA CCT AGT CAG GCT GGA AG-3′ and reverse, 5′-GAA
GGA GAT TAT CCC CCT GA-3′; Gli1 forward, 5′-CTG GAT CGG ATA GGT GGT
CT-3′ and reverse, 5′-CAG AGG TTG GGA GGT AAG GA-3′; Gli2 forward,
5′-GCC CTT CCT GAA AAG AAG AC-3′ and reverse, 5′-CAT TGG AGA AAC
AGG ATT GG-3′; Snail forward, 5′ACC CCA CAT CCT TCT CAC TG-3′ and
reverse, 5′-TAC AAA AAC CCA CGC AGA CA-3′; Zeb1 forward, 5′-GCA CAA
CCA AGT GCA GAA GA-3′ and reverse, 5′-CAT TTG CAG ATT GAG GCT
GA-3′; E-cadherin forward, 5′-TGC TCT TGC TGT TTC TTC GG-3′ and
reverse, 5′-TGC CCC ATT CGT TCA AGT AG-3′; N-cadherin forward,
5′-TGG ATG GAC CTT ATG TTG CT-3′ and reverse, 5′-AAC ACC TGT CTT
GGG ATC AA-3′; GAPDH forward, 5′-GAG TCA ACG GAT TTG GTC GT-3′ and
reverse, 5′-TTG ATT TTG GAG GGA TCT CG-3′; Cyclin D1 forward,
5′-TTC AAA TGT GTG CAG AAG GA-3′ and reverse, 5′-GGG ATG GTC TCC
TTC ATC TT-3′; Bcl-2 forward, 5′-AGATGGGAACACTGGTGGAG-3′ and
reverse, 5′-CTTCCCCAAAAGAAATGCAA-3′; miR-200a forward,
5′-CAUCUUACCGGACAGUGCUGGA-3′ and reverse,
5′-CATCTTACCGGACAGTGCTGGA-3′; miR-200b forward,
5′-CAUCUUACUGGGCAGCAUUGGA-3′ and reverse,
5′-CATCTTACTGGGCAGCATTGG-3′; and miR200c
forward, 5′-CCCTCGTCTTACCCAGCAGT-3 and reverse,
5′-CCATCATTACCCGGCAGTAT-3′.
Target sequences were amplified under the following
reaction conditions: 95°C for 10 min, followed by 40 cycles of 95°C
for 15 sec and 60°C for 1 min. GAPDH was used as an endogenous
normalization control. The experiments were performed in
triplicate, and the results were calculated using the ΔΔCt method.
The n-fold change in mRNA expression was determined according to
the 2−ΔΔCt method (47).
Gli reporter assay
Gli reporter activity was measured as previously
described (34). Briefly, the
pancreatic cancer cells were transduced with lentivirus expressing
luciferase reporter construct, and stable cells were selected.
cop-GFP and luciferase genes were cloned downstream of Gli-response
element, containing 4 Gli binding motifs (pGreen
Fire1-4xGli-mCMV-EF1-Neo). The transduced pancreatic cancer cells
(5-10,000 cells per well) were seeded in 12-well plates for the
transcription assay and treated with or without TCRV (0-20
µM) for up to 48 h. Following incubation, the cells were
harvested and analyzed for either green fluorescence or luciferase
reporter activity (Promega Corporation, Madison, WI, USA).
Statistical analysis
All results are presented as the means ± SD that
were calculated for each experimental group with replicates. ANOVA,
followed by Bonferroni’s multiple comparison tests using PRISM
statistical analysis software (GraphPad Software, Inc., San Diego,
CA, USA) was used to analyze the differences between groups.
Statistically significant differences among groups were considered
at P<0.05.
Results
TCRV induces the apoptosis and inhibits
the colony formation of human pancreatic cancer cells, but has no
effects on HPNE cells
We first measured the effects of TCRV on the
apoptosis of, caspase-3 activity in and the colony formation of
AsPC-1 and PANC-1 human pancreatic cancer cells (Fig. 1). Colony formation assay is
regarded as a gold standard and is used to measure the effects of
cytotoxic agents on the clonogenic survival of cancer cells in
vitro. TCRV (1, 5, 10 and 20 µM) induced apoptosis and
caspase-3 activity, and inhibited the colony formation of both the
AsPC-1 and PANC-1 cells in a dose-dependent manner (Fig. 1A-H). We selected these
concentrations of TCRV based on our previously published studies on
resveratrol in pancreatic cancer (6,8,18,19).
These data suggest that TCRV may be used as an anticancer
agent.
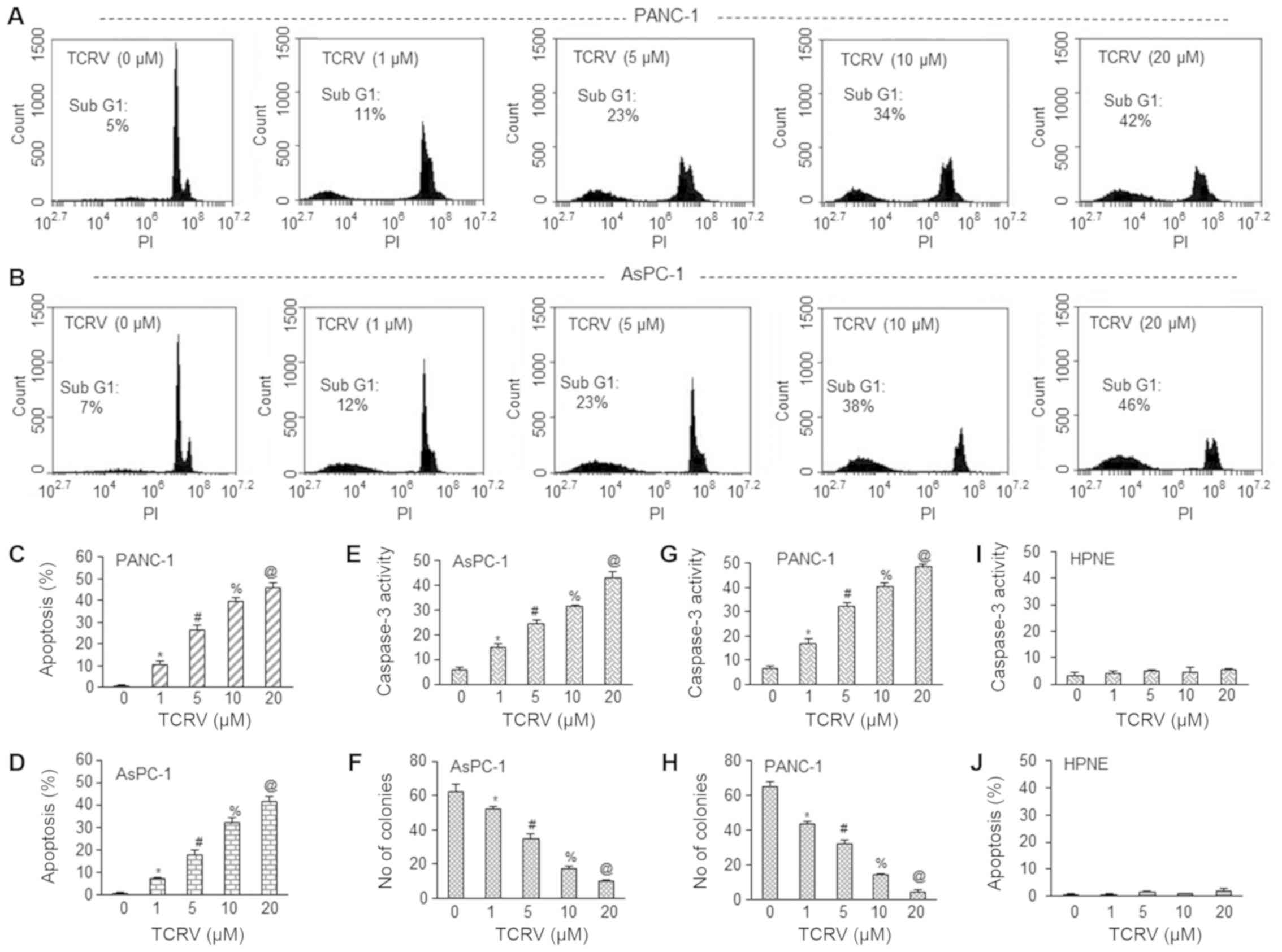 | Figure 1TCRV induces the apoptosis of,
activates caspase-3 in and inhibits the colony formation of
pancreatic cancer cells. (A-D) AsPC-1 and PANC-1 pancreatic cancer
cells were treated with TCRV (0-20 µM) for 48 h, and
apoptosis was measured. Data represent the means ± SD (n=4). The
symbols *, #, % and @ indicate significant differences compared to
the respective control (P<0.05). (E and G) AsPC-1 and PANC-1
pancreatic cancer cells were treated with TCRV (0-20 µM) for
36 h, and caspase-3 activity was measured as per the manufacturer’s
instructions (Sigma-Aldrich). Data represent the means ± SD (n=4).
The symbols *, #, % and @ indicate significant differences compared
to the respective control (P<0.05). (F and H) AsPC-1 and PANC-1
pancreatic cancer cells were treated with TCRV (0-20 µM).
After 3 weeks of treatment, colonies were fixed with 10%
neutral-buffered formalin and stained with crystal violet. Colonies
containing >50 individual cells were counted. Data represent the
means ± SD (n=4). The symbols *, #, % and @ indicate significant
differences compared to the respective control (P<0.05). (I and
J) Human pancreatic normal ductal epithelial (HPNE) cells were
treated with TCRV (0-20 µM). Apoptosis and caspase-3
activity were measured for 48 and 36 h, respectively. Data
represent the means ± SD (n=4). TCRV, triacetyl resveratrol. |
We then examined the ability of TCRV to induce
apoptosis and activate caspase-3 in HPNE cells. As shown in
Fig. 1I-J, TCRV had no effect on
apoptosis and caspase-3 activity in HPNE cells. Overall, our
results demonstrated that TCRV induced apoptosis and inhibited the
growth of AsPC-1 and PANC-pancreatic cancer 1 cells, but did not
affect normal HPNE cells (Fig. 1I and
J).
TCRV inhibits pancreatic cancer cell
motility, migration and invasion
EMT an is essential process through which cancer
cells to invade and migrate through the basement membrane. The EMT
program is highly conserved and is implicated in the dissemination
of primary epithelial cancer cells (48). Metastasis involves an increase in
the motility and invasiveness of cancer cells. Metastatic cancer
cells acquire motility by losing cell to cell adhesion, which is
characterized by the loss of E-cadherin and an increase in the
expression of its transcriptional repressors, Zeb1, Zeb2, Twist,
Snail and Slug (49). In this
study, we thus examined the effects of TCRV on the motility,
migration and invasion of AsPC-1 and PANC-pancreatic cancer 1
cells. TCRV inhibited the motility, migration and invasion of both
pancreatic cancer cell lines (Fig.
2-D). To examine the molecular mechanisms underlying the EMT
process, we measured the expression of E-cadherin, N-cadherin and
Vimentin by RT-qPCR. TCRV enhanced the expression of E-cadherin and
inhibited the expression of N-cadherin and Vimentin in both the
AsPC-1 and PANC-1 cell lines (Fig. 2E
and G). These results thus suggest that the early metastasis of
pancreatic cancer cells can be inhibited by TCRV through a
‘cadherin switch’ and the inhibition of Vimentin. Since the cell
motility, migration and invasion of pancreatic cancer cells was
inhibited by TCRV, we then examined the effects of TCRV on the
regulation of the EMT-associated transcription factors, Snail, Slug
and Zeb1 (Fig. 2F and H). The
RT-qPCR data indicated that TCRV inhibited the expression of the
EMT factors, Snail, Slug and Zeb1. Taken together, these data
suggest that TCRV modulates the expression of cadherins, Vimentin,
and that of the EMT transcription factors, Snail, Slug and Zeb1,
and can thus can regulate the early metastasis of pancreatic
cancer.
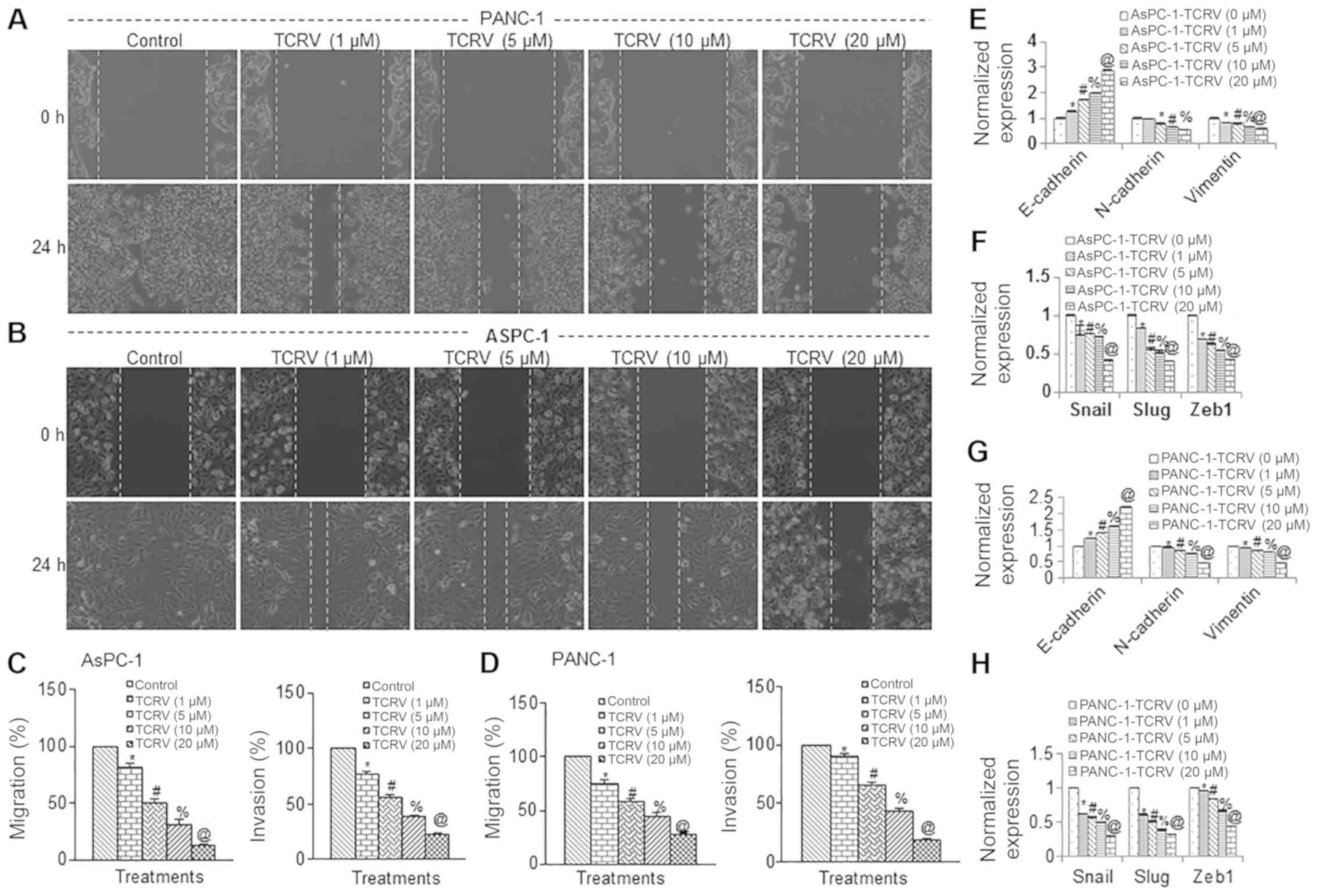 | Figure 2TCRV inhibits pancreatic cancer cell
invasion and migration, and modulates the expression of genes
related to epithelial-mesenchymal transition. (A and B) Motility
assay. Photomicrographs demonstrating the results of the in
vitro motility of AsPC-1 and PANC-1 cells after TCRV treatment.
Pancreatic cancer cells were grown in monolayer, scratched and
treated with or without TCRV for 48 h. (C and D) Transwell
migration assay. Pancreatic cancer cells were plated in the top
chamber of the Transwell plate and treated with TCRV (0-20
µM) for 24 h. Cells that had migrated to the lower chambered
were fixed with methanol, stained with crystal violet and counted.
Matrigel invasion assay. AsPC-1 and PANC-1 cells were plated onto
the Matrigel-coated membrane in the top chamber of the Transwell
and treated with TCRV (0-20 µM) for 48 h. Cells that had
invaded to the lower chambered were fixed with methanol, stained
with crystal violet and counted. Data represent the means ± SD
(n=4). The symbols *, #, % and @ indicate significant differences
compared to the respective control (P<0.05). (E-H) Pancreatic
cancer cells were treated with TCRV (0-20 µM) for 36 h. At
the end of the incubation period, the expression of E-cadherin,
N-cadherin, Vimentin, Snail, Slug and Zeb1 was measured by RT-qPCR.
Data represent the means ± SD (n=4). The symbols *, #, % and @
indicate significant differences compared to the respective control
(P<0.05). TCRV, triacetyl resveratrol. |
TCRV inhibits EMT through the modulation
of miR-200 and Zeb1 in pancreatic cancer
In defining the underlying molecular mechanisms of
action of TCRV, we further explored the involvement of miRNAs that
can regulate the gene expression of target mRNAs through either
their degradation or the repression of translation. It has been
shown that miR-200 inhibits the expression of Zeb1 by directly
targeting the 3′UTR’s of Zeb1 mRNA (50). In this study, we found that the
loss of Zeb1 expression due to the miR-200-mediated downregulation
of Zeb1 was associated with a significant reduction in the invasive
phenotype of cancer cells. The binding sites of miR-200a, miR-200b,
and miR-200c on the 3′UTR of Zeb1 were identified (data not shown).
To examine the role of the miR-200 family in the regulation of EMT,
we thus measured the expression of miR-200 family members (Fig. 3A). TCRV induced the expression of
miR-200a, miR-200b and miR-200c in the AsPC-1 cells. We thus
hypothesized that if the miR-200 family mediates the effects of
TCRV, then the inhibition of miR-200 should abolish the biological
effects of TCRV. The overexpression of anti-miR-200a/b/c in the
AsPC-1 cells counteracted the inhibitory effects of TCRV on cell
migration and invasion (Fig. 3B and
C).
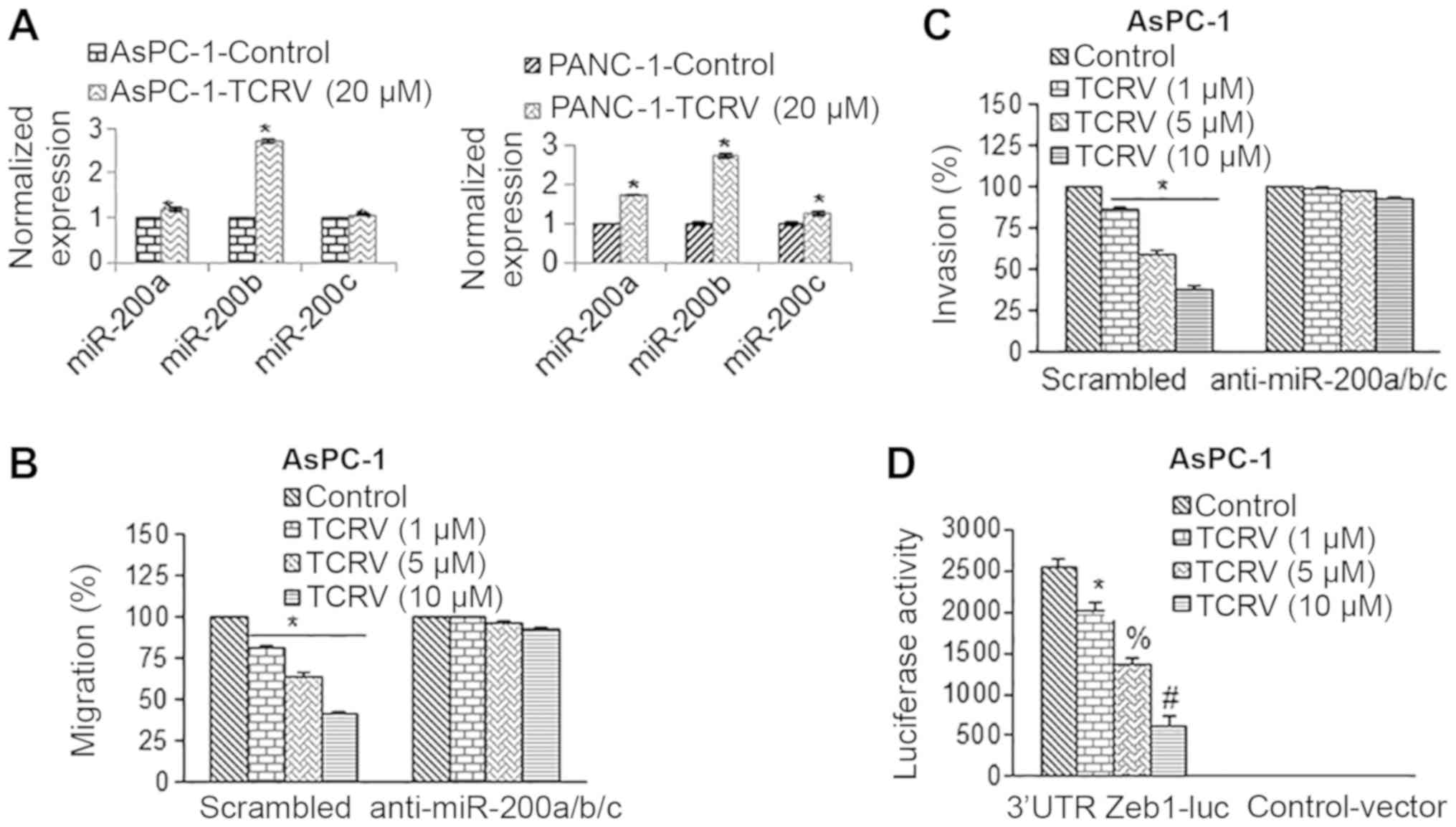 | Figure 3TCRV inhibits cell migration and
invasion by upregulating miR-200 and inhibiting Zeb1. (A) AsPC-1
and PANC-1 cells pancreatic cancer were treated with TCRV (10
µM) for 36 h. At the end of the incubation period, RNA was
extracted, and the expression of miR-200a, miR-200b and miR-200c
was measured by RT-qPCR. Data represent the means ± SD (n=4). The
symbol * indicates a significant difference compared to the
respective control (P<0.05). (B and C) AsPC-1 cells were
transduced with scrambled, or anti-miR-200a, anti-miR-200b and
anti-miR-200c lentiviral particles. Transduced cells were treated
with TCRV (0-10 µM) to measure cell migration and invasion
for 24 and 48 h, respectively. Data represent the mean ± SD (n=4).
The symbol * indicates a significant difference compared to the
respective control (P<0.05). (D) TCRV inhibits 3’UTR Zeb1
luciferase reporter activity. AsPC-1 cells were transduced with
either 3’UTR-Zeb1 luciferase reporter construct or control vector.
Following transduction, the cells were treated with TCRV (0-10
µM) for 36 h, and Zeb1 reporter activity was measured. Data
represent the means ± SD (n=4). The symbols *, # and % indicate
significant differences compared to the respective control
(P<0.05). TCRV, triacetyl resveratrol. |
Since miR-200 transcriptionally targets Zeb1, we
further evaluated the 3′UTR Zeb1-luciferase activity in the AsPC-1
cells upon treatment with TCRV (Fig.
3D). In the AsPC-1 cells treated with TCRV, the 3′UTR
Zeb1-luciferase activity was inhibited. By comparison, the cells
transduced with the control vector (with no miR-200 binding sites)
had no luciferase activity. These data suggest that TCRV inhibits
Zeb1 through miR-200 family members.
TCRV inhibits the transcriptional
activity of Gli and its downstream targets in pancreatic cancer
cells
We then examined the effect of TCRV on the Shh-Gli
pathway. To this end, the effects of TCRV on Gli transcriptional
activity were evaluated using a reporter assay. A Gli-dependent
luciferase reporter construct was used to transduce the AsPC-1 and
PANC-1 cells followed by treatment with TCRV for 36 h. As shown in
Fig. 4A and D, the Gli
transcriptional activity was inhibited in both the AsPC-1 and
PANC-1 cells in a dose-dependent manner upon treatment with TCRV.
These data suggest that the pro-apoptotic activities of TCRV are
exerted through the inhibition of the Shh pathway.
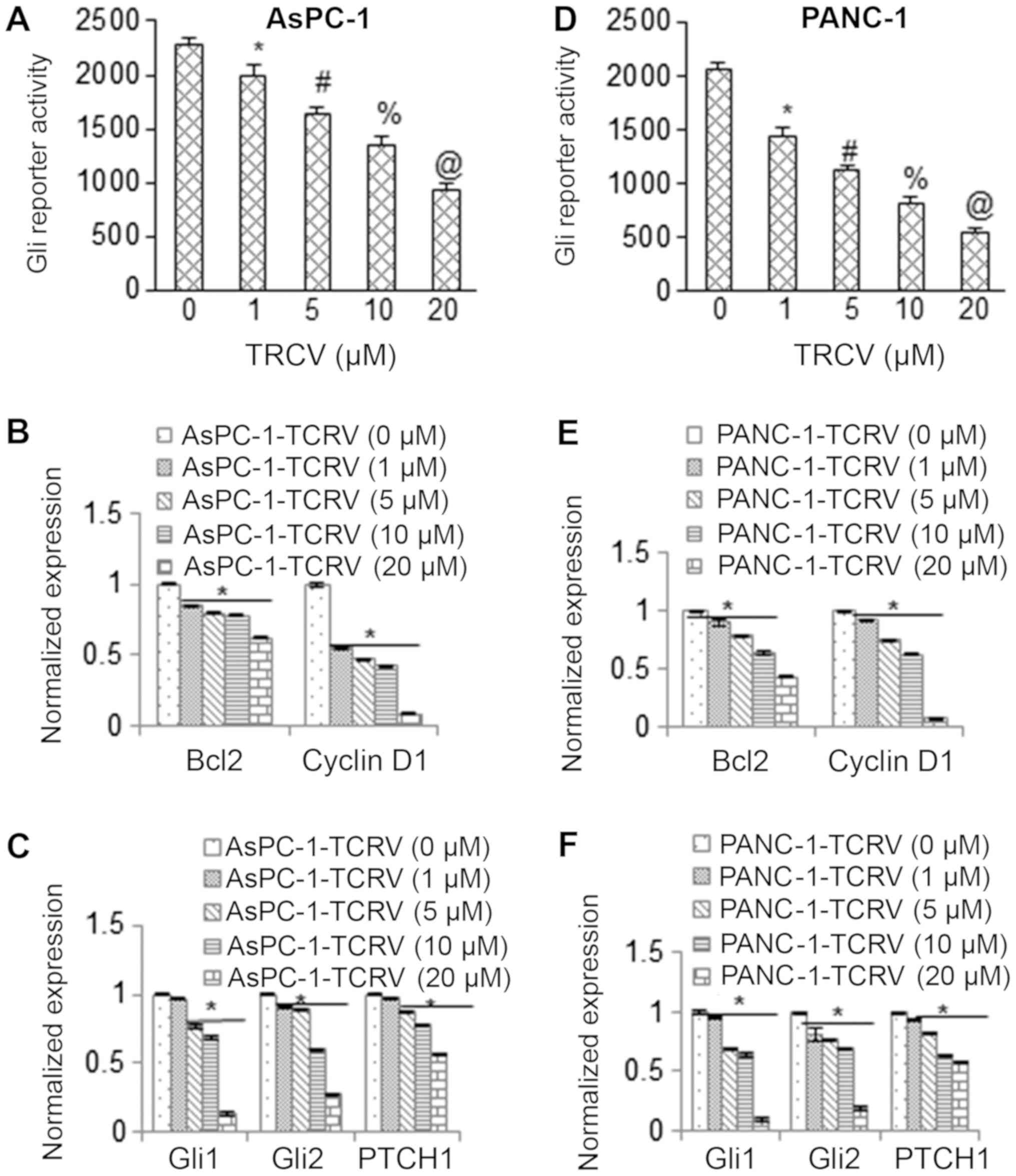 | Figure 4TCRV inhibits Gli reporter activity
and Gli target genes. (A) Inhibition of Gli reporter activity in
AsPC-1 pancreatic cancer cells by TCRV. AsPC-1 cells were
transduced with lentiviral particles expression Gli reporter
construct and treated with TCRV (0-20 µM) for 36 h, and Gli
reporter activity was measured. Data represent the means ± SD
(n=4). The symbols *, #, % and @ indicate significant differences
compared to the respective control (P<0.05). (B and C) AsPC-1
cells were treated with TCRV (0-20 µM) for 36 h. At the end
of the incubation period, the expression of Bcl-2, cyclin D1, Gli1,
Gli2 and Patched1 (PTCH1) was measured by RT-qPCR. Data represent
the means ± SD (n=4). The symbol * indicates a significant
difference compared to the respective control (P<0.05). (D)
Inhibition of Gli reporter activity in PANC-1 cells by TCRV. PANC-1
cells were transduced with lentiviral particles expressing Gli
reporter and treated with TCRV (0-10 µM) for 36 h, and
reporter activity was measured. Data represent the means ± SD
(n=4). The symbols *, #, % and @ indicate significant differences
compared to the respective control (P<0.05). (E and F) PANC-1
cells were treated with TCRV (0-20 µM) for 36 h. At the end
of the incubation period, the expression of Bcl-2, cyclin D1, Gli1,
Gli2 and PTCH1 was measured by RT-qPCR. Data represent the means ±
SD (n=4). The symbol * indicates a significant difference compared
to the respective control (P<0.05). TCRV, triacetyl
resveratrol. |
We then measured the effects of TCRV on the
expression of Gli target genes in AsPC-1 and PANC-1 cells by
RT-qPCR. TCRV inhibited the expression of Bcl-2 and Cyclin D1 in
the AsPC-1 and PANC-1 cells (Fig. 4B
and E). Similarly, TCRV inhibited the gene expression levels of
Gli effectors (Gli1 and Gli2) and the receptor (PTCH1) of the Shh
pathway in both cell lines (Fig. 4C
and F). Taken together, these results suggest that TCRV
suppresses the Shh pathway and its target genes to regulate
pancreatic cancer cell growth.
The inhibitory effects of TCRV on
apoptosis and the epithelial-mesenchymal transition are mediated by
modulation of Sonic hedgehog pathway
To further confirm the role of the Shh pathway in
mediating the effects of TCRV on apoptosis, invasion and migration,
we took a dual approach. Firstly, we used the Shh protein to
activate the Shh pathway (Fig. 5).
To this end, we pretreated the AsPC-1 cells with Shh protein (100
nM) for 2 h, followed by treatment with TCRV (0-20 µM) for
36-48 h to measure apoptosis, invasion and migration. As shown in
Fig. 5A-C, TCRV induced the
apoptosis, and inhibited invasion and migration of AsPC-1 cells.
Pretreatment of the cells with Shh protein abolished the effects of
TCRV on apoptosis, invasion and migration. Since Shh protein
induces Gli transcription, we further evaluated the effects of Shh
pathway activation on the Gli reporter activity (Fig. 5D). Treatment with TCRV (0-20
µM) inhibited Gli reporter activity in the AsPC-1 cells in a
dose-dependent manner. Furthermore, pretreatment of the AsPC-1
cells with Shh protein abolished the inhibitory effects of TCRV on
Gli reporter activity. Therefore, these data suggest the role of
the Shh-Gli pathway in mediating the biological effects of
TCRV.
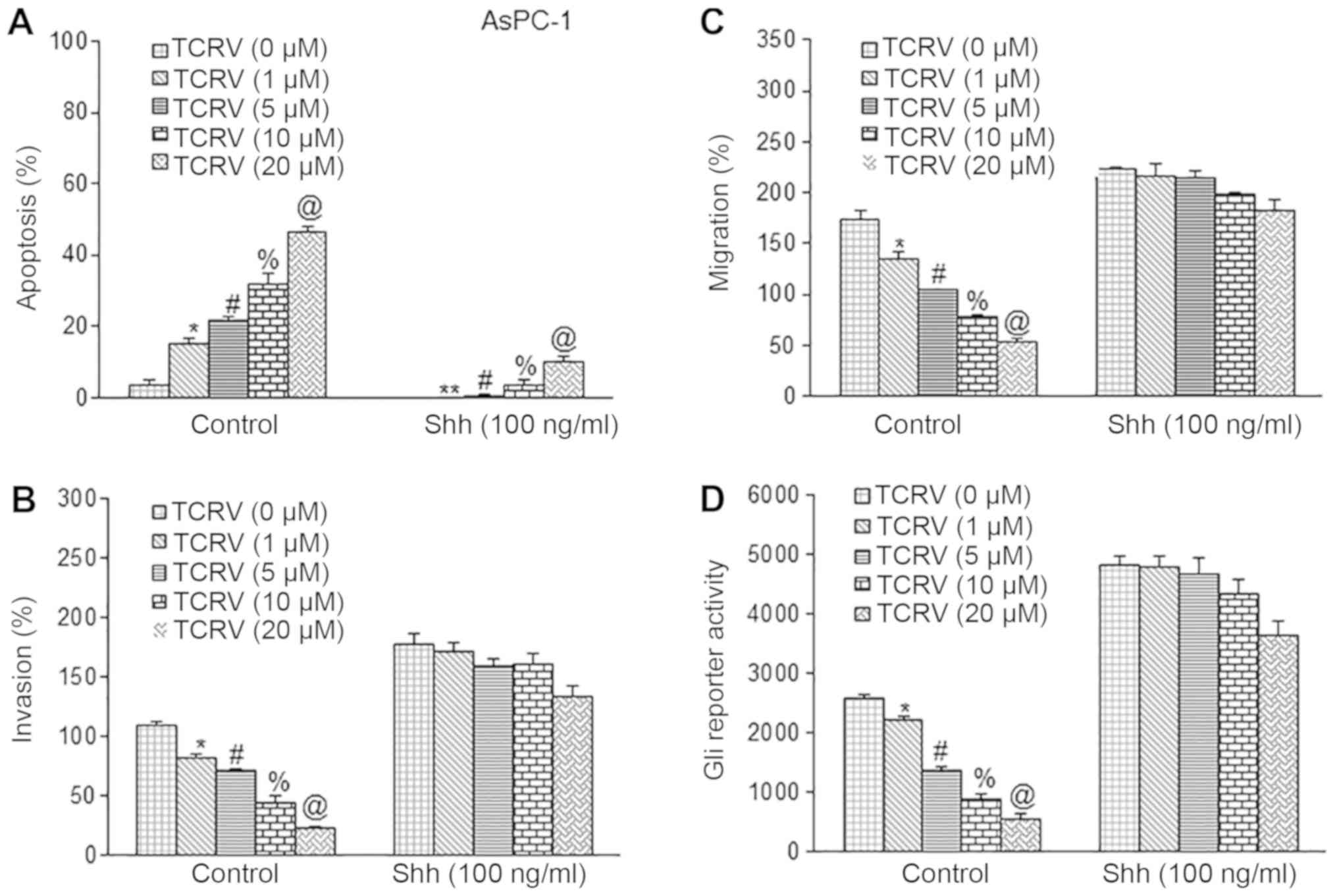 | Figure 5Sonic hedgehog protein counteracts
the biological effects of TCRV. (A) AsPC-1 cells were pretreated
with Shh protein (100 nM) for 2 h followed by TCRV (0-20 µM)
for 48 h. At the end of the incubation period, apoptosis was
measured by TUNEL assay. Data represent the means ± SD. The symbols
*, #, % and @ indicate significant differences compared to the
respective control (P<0.05). (B) Matrigel invasion assay. AsPC-1
cells were pretreated with Shh protein (100 nM) for 2 h followed by
TCRV (0-20 µM) for 48 h. At the end of the incubation
period, cell invasion was measured as described in Material and
methods. Data represent the means ± SD (n=4). The symbols *, #, %
and @ indicate significant differences compared to the respective
control (P<0.05). (C) Transwell migration assay. AsPC-1 cells
were pretreated with Shh protein (100 nM) for 2 h followed by TCRV
(0-20 µM) for 48 h. At the end of the incubation period,
cell migration was measured as described in the Material and
methods. Data represent the means ± SD (n=4). The symbols *, #, %
and @ indicate significant differences compared to the respective
control (P<0.05). (D) Shh counteracts the inhibitory effects of
TCRV on Gli reporter activity. AsPC-1 cells were transduced with
lentiviral particles expressing Gli reporter construct. Transduced
cells were pretreated with Shh protein (100 nM) for 2 h, followed
by TCRV (0-20 µM) for 48 h. At the end of the incubation
period, Gli reporter activity was measured. Data represent the
means ± SD (n=4). The symbols *, #, % and @ indicate significant
differences compared to the respective control (P<0.05). TCRV,
triacetyl resveratrol. |
In the second approach, we hyperactivated the Shh
pathway by overexpressing Gli1 (Fig.
6). Either empty vector or Gli1 cDNA was used to transduce
AsPC-1 cells followed by treatment with various doses of TCRV (0-20
µM). TCRV induced the apoptosis, and inhibited the invasion
and migration of AsPC-1 cells transduced with empty vector
(Fig. 6A-C). By contrast, the
overexpression of Gli with Gli1 cDNA resulted in the inhibition of
TCRV-induced apoptosis, and further abrogated the effects of TCRV
on the invasion and migration of AsPC-1 cells. We then examined the
influence of Shh pathway activation by Gli1 overexpression on the
inhibitory effects of TCRV on Gli reporter activity. As shown in
Fig. 6D, the results suggested
that TCRV indeed inhibited Gli reporter activity in AsPC-1/vector
cells. However, the overexpression of Gli1 abolished the inhibitory
effects of TCRV on Gli reporter activity in AsPC-1/Gli1 cells.
These data confirm our findings that the Shh-Gli pathway is
required for the anti-metastatic and pro-apoptotic effects of TCRV
on pancreatic cancer cells.
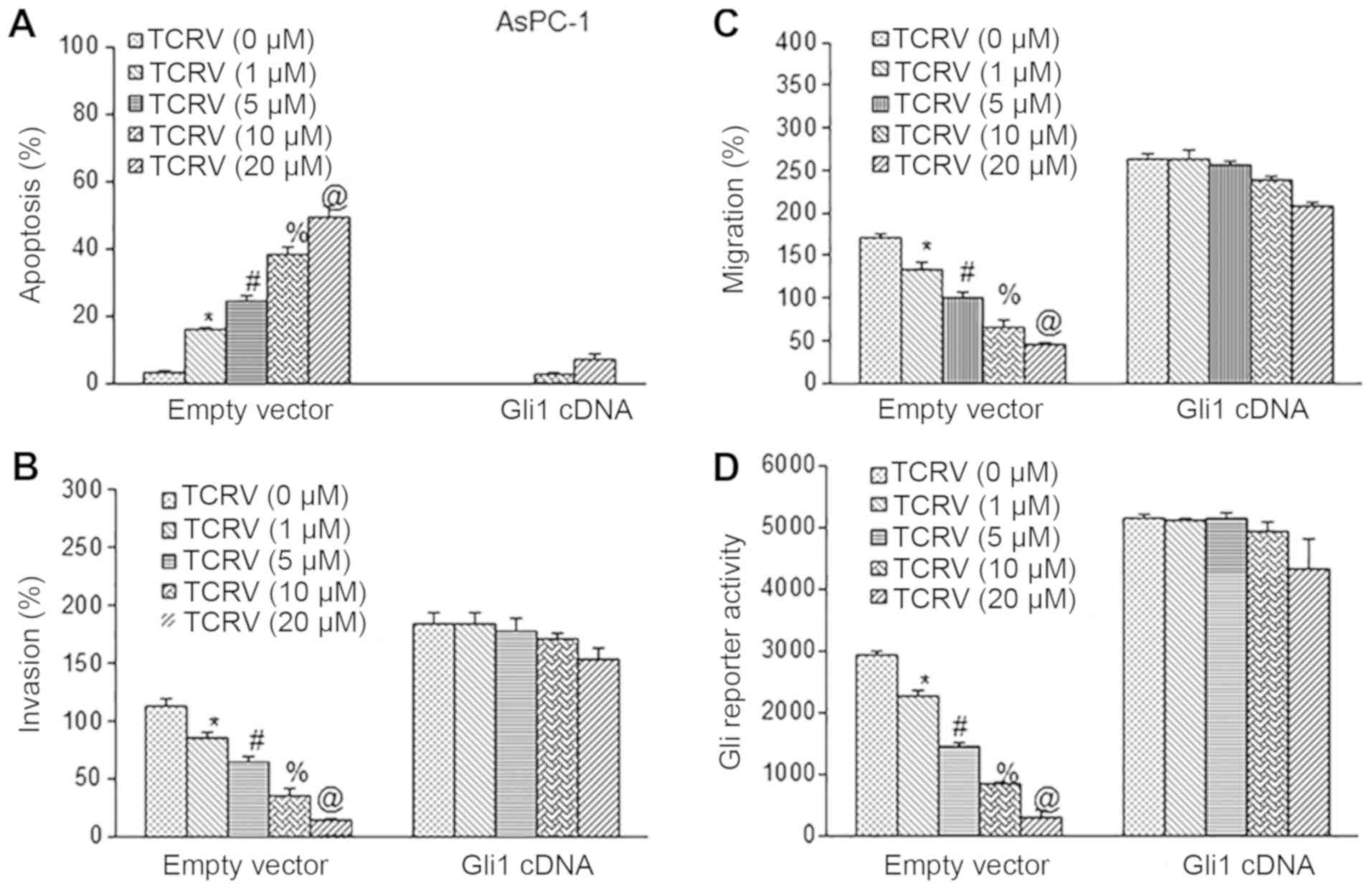 | Figure 6Overexpression of Gli counteracts the
biological effects of TCRV. (A) AsPC-1 cells were transiently
transfected with either empty vector or Gli1 cDNA, and treated with
TCRV (0-20 µM) for 48 h. At the end of the incubation
period, apoptosis was measured by TUNEL assay. Data represent the
means ± SD (n= 4). The symbols *, #, % and @ indicate significant
differences compared to the respective control (P<0.05). (B)
Matrigel invasion assay. AsPC-1 cells were transiently transfected
with either empty vector or Gli1 cDNA, and treated with TCRV (0-20
µM) for 48 h. At the end of the incubation period, cell
invasion was measured. Data represent the means ± SD (n=4). The
symbols *, #, % and @ indicate significant differences compared to
the respective control (P<0.05). (C) Transwell migration assay.
AsPC-1 cells were transiently transfected with either empty vector
or Gli1 cDNA and treated with TCRV (0-20 µM) for 48 h. At
the end of the incubation period, cell migration was measured. Data
represent the means ± SD (n=4). The symbols *, #, % and @ indicate
significant differences compared to the respective control
(P<0.05). (D) Gli1 counteracts the inhibitory effects of TCRV on
Gli reporter activity. AsPC-1 cells were co-transfected with either
empty vector or Gli1 cDNA along with Gli-luciferase reporter
construct. Cells were treated with TCRV (0-20 µM) for 48 h.
At the end of the incubation period, Gli reporter activity was
measured. Data represent the means ± SD (n=4). The symbols *, #, %
and @ indicate significant differences compared to the respective
control (P<0.05). TCRV, triacetyl resveratrol. |
Discussion
In the current study, to the best of our knowledge,
we demonstrate for the first time that TCRV inhibits pancreatic
cancer growth by inducing the apoptosis of, and inhibiting the
colony formation and EMT in pancreatic cancer cells, whereas it has
no effect on HPNE cells. TCRV modulated the cadherin switch and
inhibits EMT by downregulating the EMT-associated transcription
factors, Snail, Slug and Zeb1. Furthermore, the upregulation of the
miR-200 family by TCRV inhibited Zeb1 transcriptional activity and
pancreatic cancer cell invasion and migration. Finally, the
biological effects of TCRV on apoptosis and EMT were found to be
regulated via the inhibition of the Shh pathway and its target
genes.
Resveratrol is a naturally occurring polyphenolic
compound with a wide spectrum of beneficial biological activities
in human health by acting as an antioxidant, neuroprotector,
cardioprotector and anticancer agent (13). TCRV is derived from resveratrol by
esterase activity and exhibits better bioactivity than resveratrol
in cell culture models. The therapeutic properties of TCRV are
superior to those of resveratrol due to the following reasons: i)
It is much more lipid-soluble and absorbs lipid membranes at a much
more rapid rate; ii) it decreases exposure to the enzymes
responsible for glucuronidation and sulfation; iii) its acetyl
groups resist breakdown better than hydroxy groups; and iv) its
acetyl groups enhance binding with proteins/substrates (51,52).
Based on these biochemical properties, TCRV is an attractive and
effective candidate for the treatment and prevention of pancreatic
cancer.
In the majority of cancers, aberrant Hh pathway
activation is associated with poor treatment outcomes or the
development of chemoresistance. The deregulation of the Shh pathway
has been associated with the mutation of the Kras oncogene and has
been shown to be an early event in pancreatic carcinogenesis
(53-55). Furthermore, hedgehog signaling
plays a functional role in the tumor microenvironment by regulating
myofibroblast differentiation and inducing the stroma-derived
growth-promoting molecules (56).
Human pancreatic adenocarcinoma is characterized by precursor
lesions (PanIN) which contain multiple genetic mutations among
which activating K-ras mutations and the overexpression in
HER-2/neu along with the aberrant expression of Shh have been
demonstrated to occur early in progression. In Pdx-Shh mice, the
pancreata have been shown to develop abnormal tubular structures,
PanIN-1 and -2 (57). We have
recently demonstrated that the aberrant activation of the Shh
pathway components is expressed in human pancreatic cancer stem
cells and pancreatic cancer cell lines, which could be inhibited by
various chemopreventive agents such as sulforaphane, resveratrol
and EGCG (5,8,33,58,59).
It is thus imperative from these data that the activation of Shh
signaling is an early event and the maintenance of Shh signaling
plays a critical role in pancreatic cancer proliferation and
progression.
Metastasis is a leading cause of cancer-related
mortality which involves the degradation of the extracellular
matrix and the invasion of the local and distant tissues by cancer
cells (2). The most critical step
in invasion and metastases is attributed to the process of EMT, in
which transformed sessile epithelial cells acquire a motile
mesenchymal phenotype characterized by cadherin switch, loss of
homotypic adhesion and cell polarity. Thus, the dissemination of
cells from the primary tumor followed by their re-establishment in
a secondary site is required for sustained metastatic growth, and
EMT confers a metastatic ability on carcinoma. In the present
study, TCRV induced apoptosis and inhibited EMT in pancreatic
cancer cells. This may be due to the heterogeneous characteristics
of the cell population; i.e., some cells are likely to be sensitive
to apoptosis, while other surviving cells may undergo EMT. Since
TCRV induced apoptosis, it may have affected cell migration and EMT
characteristics. Furthermore, growth arrest in response to TCRV
treatment may lead to apoptosis.
Zeb1 and Zeb2 overexpression in several primary
tumors have been significantly associated with a poorer prognosis
and have been inversely correlated with E-cadherin (60). Thus, EMT-associated transcription
factors and regulators Snail, Slug, SNAI3, Zeb1, Zeb2, KLF8 and
Twist1/2 repress CDH1 gene encoding E-cadherin. In the present
study, we demonstrated a key and essential role of the Shh-Gli
pathway in the promotion of pancreatic cancer metastasis. We
demonstrated that TCRV suppressed cell motility, invasion and
migration through the inhibition of EMT. TCRV significantly
inhibited the expression of the EMT-associated transcription
factors Zeb1, Snail and Slug. Additionally, the depletion of EMT
regulators decreased the expression of N-Cadherin and Vimentin and
further increased the expression level of E-Cadherin in pancreatic
cancer cells, suggesting a potential beneficial role of TCRV in
early metastasis. Our gene expression analyses were based on mRNA
levels. We did not measure protein expression by western blot
analysis, which may be considered a limitation of this study.
Nevertheless, the mRNA levels on EMT-related genes has been shown
to correspond with the protein expression in our other study
(11).
During metastasis, cancer cells acquire multiple
molecular traits to counteract anoikis, migrate, invade,
proliferate and survive in distant/unrelated microenvironments
(61). Several studies have
highlighted that miRNAs play a significant role in the regulation
of metastasis (62-64). In the current study, we
demonstrated that TCRV inhibited EMT in pancreatic cancer cells via
the upregulation of miR-200a, miR-200b, and miR-200c, and the
inhibition of Zeb1 expression. In support of our study, the role of
miR200 in regulation of EMT has been demonstrated (65). Furthermore, miR-200 does not play
any role in cell death or apoptosis. For this reason, we did not
measure apoptosis under conditions of miR-200 knockdown. In this
study, TCRV induced apoptosis through the activation of caspase-3.
Furthermore, Zeb1 has been shown to be a direct target of miR-200
family members (50). Zeb1 links
EMT activation and stemness maintenance and this regulates
tumorigenesis. Furthermore, since the loss of miR-200 expression is
a late event in the progression of pancreatic cancer, it can be
concluded that it may be relevant to the development of distant
metastases. Thus, our study indicates that targeting the
Zeb1-miR-200 feedback loop may provide the basis for a potential
treatment strategy for pancreatic cancer.
The ability of TCRV to inhibit the Shh-Gli pathway
was confirmed by two approaches; i.e., the use of Shh protein and
the overexpression of by transfection with Gli1 cDNA. Using both
the approaches, the hyperactivation of the Shh pathway counteracted
the inhibitory effects of TCRV on apoptosis, cell migration and
invasion. This study confirmed that the Shh pathway was involved in
mediating the biological effects of TCRV. Similarly, we have
recently demonstrated that resveratrol suppressed the pluripotency
maintaining factors (Nanog, Sox-2, c-Myc and Oct-4) to mediate the
inhibition of the self-renewal capacity of pancreatic cancer stem
cells (8). Moreover, the ability
of resveratrol to attenuate the self-renewal capacity of pancreatic
cancer stem cells was further enhanced by the inhibition of Nanog
by RNAi (8). Further studies are
required to demonstrate the exact role of TCRV in human pancreatic
cancer.
In conclusion, we in this study, we demonstrated
that TCRV selectively inhibited human pancreatic cancer growth by
inducing apoptosis without having any negative impact on HPNE
cells, and this is suggestive of its potential use in the treatment
and/or prevention of pancreatic cancer. Furthermore, we
demonstrated that TCRV inhibited pancreatic cancer cell invasion
and migration by upregulating the miR-200 family and inhibiting the
transcription and expression of Zeb1. The biological effects of
TCRV were exerted through the inhibition of the Shh pathway. Since
the activation of the Shh pathway is associated with pancreatic
malignancy, TCRV alone or in combination with standard
chemotherapeutic or targeted drugs effecting tumor microenvironment
may be tested in clinical trials for the management of pancreatic
cancer in the future.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors’ contributions
JF performed the experiments, analyzed the data and
wrote the manuscript. SS, AS, SKS and RKS designed the study,
contributed the reagents and/or provided scientific inputs. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that no competing interests
exist.
Acknowledgments
The authors would like to thank the laboratory
members for the critical reading of the manuscript.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D: Molecular epidemiology of pancreatic
cancer. Cancer J. 7:259–265. 2001.PubMed/NCBI
|
|
4
|
Gold EB and Goldin SB: Epidemiology of and
risk factors for pancreatic cancer. Surg Oncol Clin N Am. 7:67–91.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li SH, Fu J, Watkins DN, Srivastava RK and
Shankar S: Sulforaphane regulates self-renewal of pancreatic cancer
stem cells through the modulation of Sonic hedgehog-GLI pathway.
Mol Cell Biochem. 373:217–227. 2013. View Article : Google Scholar
|
|
6
|
Roy SK, Chen Q, Fu J, Shankar S and
Srivastava RK: Resveratrol inhibits growth of orthotopic pancreatic
tumors through activation of FOXO transcription factors. PLoS One.
6:e251662011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shankar S, Ganapathy S, Hingorani SR and
Srivastava RK: EGCG inhibits growth, invasion, angiogenesis and
metastasis of pancreatic cancer. Front Biosci. 13:440–452. 2008.
View Article : Google Scholar
|
|
8
|
Shankar S, Nall D, Tang SN, Meeker D,
Passarini J, Sharma J and Srivastava RK: Resveratrol inhibits
pancreatic cancer stem cell characteristics in human and KrasG12D
transgenic mice by inhibiting pluripotency maintaining factors and
epithelial-mesenchymal transition. PLoS One. 6:e165302011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shankar S, Suthakar G and Srivastava RK:
Epigallocatechin-3-gallate inhibits cell cycle and induces
apoptosis in pancreatic cancer. Front Biosci. 12:5039–5051. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srivastava RK, Tang SN, Zhu W, Meeker D
and Shankar S: Sulforaphane inhibits self-renewal capacity of
pancreatic cancer stem cells and synergizes with quercetin. Front
Biosci (Elite Ed). 3:515–528. 2011. View
Article : Google Scholar
|
|
11
|
Verma RK, Yu W, Shrivastava A, Shankar S
and Srivastava RK: α-Mangostin-encapsulated PLGA nanoparticles
inhibit pancreatic carcinogenesis by targeting cancer stem cells in
human, and transgenic (Kras(G12D), and Kras(G12D)/tp53R270H) mice.
Sci Rep. 6:327432016. View Article : Google Scholar
|
|
12
|
Zhao M, Tang SN, Marsh JL, Shankar S and
Srivastava RK: Ellagic acid inhibits human pancreatic cancer growth
in Balb c nude mice. Cancer Lett. 337:210–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shankar S, Singh G and Srivastava RK:
Chemoprevention by resveratrol: Molecular mechanisms and
therapeutic potential. Front Biosci. 12:4839–4854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shankar S, Siddiqui I and Srivastava RK:
Molecular mechanisms of resveratrol
(3,4,5-trihydroxy-transstilbene) and its interaction with
TNF-related apoptosis inducing ligand (TRAIL) in
androgen-insensitive prostate cancer cells. Mol Cell Biochem.
304:273–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang M and Pezzuto JM: Cancer
chemopreventive activity of resveratrol. Drugs Exp Clin Res.
25:65–77. 1999.PubMed/NCBI
|
|
16
|
Marques FZ, Markus MA and Morris BJ: The
molecular basis of longevity, and clinical implications. Maturitas.
65:87–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marzetti E, Wohlgemuth SE, Anton SD,
Bernabei R, Carter CS and Leeuwenburgh C: Cellular mechanisms of
cardioprotection by calorie restriction: state of the science and
future perspectives. Clin Geriatr Med. 25:715–732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harikumar KB, Kunnumakkara AB, Sethi G,
Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S
and Aggarwal BB: Resveratrol, a multitargeted agent, can enhance
antitumor activity of gemcitabine in vitro and in orthotopic mouse
model of human pancreatic cancer. Int J Cancer. 127:257–268.
2010.
|
|
19
|
Oi N, Jeong CH, Nadas J, Cho YY, Pugliese
A, Bode AM and Dong Z: Resveratrol, a red wine polyphenol,
suppresses pancreatic cancer by inhibiting leukotriene
A4 hydrolase. Cancer Res. 70:9755–9764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shankar S, Chen Q, Siddiqui I, Sarva K and
Srivastava RK: Sensitization of TRAIL-resistant LNCaP cells by
resveratrol (3,4′,5-tri-hydroxystilbene): Molecular mechanisms and
therapeutic potential. J Mol Signal. 2:72007. View Article : Google Scholar
|
|
21
|
Delmas D, Jannin B and Latruffe N:
Resveratrol: Preventing properties against vascular alterations and
ageing. Mol Nutr Food Res. 49:377–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fulda S and Debatin KM:
Resveratrol-mediated sensitisation to TRAIL-induced apoptosis
depends on death receptor and mitochondrial signalling. Eur J
Cancer. 41:786–798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel KR, Scott E, Brown VA, Gescher AJ,
Steward WP and Brown K: Clinical trials of resveratrol. Ann N Y
Acad Sci. 1215:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saqui-Salces M and Merchant JL: Hedgehog
signaling and gastrointestinal cancer. Biochim Biophys Acta.
1803:786–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kinzler KW, Ruppert JM, Bigner SH and
Vogelstein B: The GLI gene is a member of the Kruppel family of
zinc finger proteins. Nature. 332:371–374. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kasper M, Schnidar H, Neill GW, Hanneder
M, Klingler S, Blaas L, Schmid C, Hauser-Kronberger C, Regl G,
Philpott MP, et al: Selective modulation of Hedgehog/GLI target
gene expression by epidermal growth factor signaling in human
keratinocytes. Mol Cell Biol. 26:6283–6298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruiz i Altaba A, Sánchez P and Dahmane N:
Gli and hedgehog in cancer: Tumours, embryos and stem cells. Nat
Rev Cancer. 2:361–372. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramalho-Santos M, Melton DA and McMahon
AP: Hedgehog signals regulate multiple aspects of gastrointestinal
development. Development. 127:2763–2772. 2000.PubMed/NCBI
|
|
29
|
Taylor MD, Liu L, Raffel C, Hui CC,
Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, et
al: Mutations in SUFU predispose to medulloblastoma. Nat Genet.
31:306–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tostar U, Malm CJ, Meis-Kindblom JM,
Kindblom LG, Toftgård R and Undén AB: Deregulation of the hedgehog
signalling pathway: A possible role for the PTCH and SUFU genes in
human rhabdomyoma and rhabdomyosarcoma development. J Pathol.
208:17–25. 2006. View Article : Google Scholar
|
|
31
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berman DM, Karhadkar SS, Maitra A, Montes
De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman
JR, Watkins DN, et al: Widespread requirement for Hedgehog ligand
stimulation in growth of digestive tract tumours. Nature.
425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rodova M, Fu J, Watkins DN, Srivastava RK
and Shankar S: Sonic hedgehog signaling inhibition provides
opportunities for targeted therapy by sulforaphane in regulating
pancreatic cancer stem cell self-renewal. PLoS One. 7:e460832012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh BN, Fu J, Srivastava RK and Shankar
S: Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits
pancreatic cancer stem cell characteristics: Molecular mechanisms.
PLoS One. 6:e273062011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Magistri P, Battistelli C, Strippoli R,
Petrucciani N, Pellinen T, Rossi L, Mangogna L, Aurello P, D’Angelo
F, Tripodi M, et al: SMO inhibition modulates cellular plasticity
and invasiveness in colorectal cancer. Front Pharmacol. 8:9562018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Wang X, Xie C, Zhu J, Meng Y, Chen
Y, Li Y, Jiang Y, Yang X, Wang S, et al: Sonic hedgehog and
Wnt/β-catenin pathways mediate curcumin inhibition of breast cancer
stem cells. Anticancer Drugs. 29:208–215. 2018.PubMed/NCBI
|
|
37
|
Morgan H, Olivero C and Patel GK:
Identification of human cutaneous basal cell carcinoma cancer stem
cells. Methods Mol Biol. Apr 20–2018.(Epub ahead of print).
View Article : Google Scholar
|
|
38
|
Tong W, Qiu L, Qi M, Liu J, Hu K, Lin W,
Huang Y and Fu J: GANT-61 and GDC-0449 induce apoptosis of prostate
cancer stem cells through a GLI-dependent mechanism. J Cell
Biochem. 119:3641–3652. 2018. View Article : Google Scholar
|
|
39
|
Wang K, Pan L, Che X, Cui D and Li C: Gli1
inhibition induces cell-cycle arrest and enhanced apoptosis in
brain glioma cell lines. J Neurooncol. 98:319–327. 2010. View Article : Google Scholar
|
|
40
|
Tsuda N, Ishiyama S, Li Y, Ioannides CG,
Abbruzzese JL and Chang DZ: Synthetic microRNA designed to target
glioma-associated antigen 1 transcription factor inhibits division
and induces late apoptosis in pancreatic tumor cells. Clin Cancer
Res. 12:6557–6564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cho WC: MicroRNAs in cancer - from
research to therapy. Biochim Biophys Acta. 1805:209–217. 2010.
|
|
43
|
Guo S, Fesler A, Wang H and Ju J: microRNA
based prognostic biomarkers in pancreatic Cancer. Biomark Res.
6:182018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar
|
|
45
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs - the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fu J, Rodova M, Roy SK, Sharma J, Singh
KP, Srivastava RK and Shankar S: GANT-61 inhibits pancreatic cancer
stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice
xenograft. Cancer Lett. 330:22–32. 2013. View Article : Google Scholar
|
|
47
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
48
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar
|
|
50
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hsieh TC, Wong C, John Bennett D and Wu
JM: Regulation of p53 and cell proliferation by resveratrol and its
derivatives in breast cancer cells: An in silico and biochemical
approach targeting integrin αvβ3. Int J Cancer. 129:2732–2743.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hsieh TC, Huang YC and Wu JM: Control of
prostate cell growth, DNA damage and repair and gene expression by
resveratrol analogues, in vitro. Carcinogenesis. 32:93–101. 2011.
View Article : Google Scholar
|
|
53
|
Fritz S, Fernández-del Castillo C, Iafrate
AJ, Mino-Kenudson M, Neyhard N, LaFemina J, Stirman A, Warshaw AL
and Thayer SP: Novel xenograft and cell line derived from an
invasive intraductal papillary mucinous neoplasm of the pancreas
give new insights into molecular mechanisms. Pancreas. 39:308–314.
2010. View Article : Google Scholar
|
|
54
|
Nissim S, Idos GE and Wu B: Genetic
markers of malignant transformation in intraductal papillary
mucinous neoplasm of the pancreas: A meta-analysis. Pancreas.
41:1195–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nolan-Stevaux O, Lau J, Truitt ML, Chu GC,
Hebrok M, Fernández-Zapico ME and Hanahan D: GLI1 is regulated
through Smoothened-independent mechanisms in neoplastic pancreatic
ducts and mediates PDAC cell survival and transformation. Genes
Dev. 23:24–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lauth M and Toftgård R: Hedgehog signaling
and pancreatic tumor development. Adv Cancer Res. 110:1–17. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernándezdel
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang SN, Fu J, Nall D, Rodova M, Shankar S
and Srivastava RK: Inhibition of sonic hedgehog pathway and
pluripotency maintaining factors regulate human pancreatic cancer
stem cell characteristics. Int J Cancer. 131:30–40. 2012.
View Article : Google Scholar :
|
|
59
|
Tang SN, Singh C, Nall D, Meeker D,
Shankar S and Srivastava RK: The dietary bioflavonoid quercetin
synergizes with epigallocathechin gallate (EGCG) to inhibit
prostate cancer stem cell characteristics, invasion, migration and
epithelial-mesenchymal transition. J Mol Signal. 5:142010.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S, et al:
Epithelial-mesenchymal transition and mesenchymal-epithelial
transition via regulation of ZEB-1 and ZEB-2 expression in
pancreatic cancer. J Surg Oncol. 105:655–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pantel K, Alix-Panabières C and Riethdorf
S: Cancer microme-tastases. Nat Rev Clin Oncol. 6:339–351. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Baffa R, Fassan M, Volinia S, O’Hara B,
Liu CG, Palazzo JP, Gardiman M, Rugge M, Gomella LG, Croce CM, et
al: MicroRNA expression profiling of human metastatic cancers
identifies cancer gene targets. J Pathol. 219:214–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.
|
|
64
|
Nalls D, Tang SN, Rodova M, Srivastava RK
and Shankar S: Targeting epigenetic regulation of miR-34a for
treatment of pancreatic cancer by inhibition of pancreatic cancer
stem cells. PLoS One. 6:e240992011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
O’Brien SJ, Carter JV, Burton JF, Oxford
BG, Schmidt MN, Hallion JC and Galandiuk S: The role of the miR-200
family in epithelial-mesenchymal transition in colorectal cancer: A
systematic review. Int J Cancer. 142:2501–2511. 2018. View Article : Google Scholar
|




















