Introduction
Hepatocellular carcinoma (HCC) is one of the
deadliest malignancies worldwide. It is also the third most common
cancer in males (accounting for 12.72% of cancer incidence) and the
fifth most common in females (accounting for 5.68% of cancer
incidence) in China. The mortality rate of HCC is ~25%, primarily
due to a high rate of recurrence (1). Chemotherapy drugs can effectively
control the progression of HCC. However, tumor cells that are
insensitive to these drugs remain present in the liver, which may
subsequently lead to HCC recurrence (2). These cells may belong to a stem
cell-like population known as tumor-initiating cells (TICs)
(3). Although stem cell-like
properties, including self-renewal, tumorigenicity and
dedifferentiation, are closely associated with secondary tumor
growth in HCC (4), the
contribution of TICs to tumor angiogenesis has not been
elucidated.
Growing evidence suggests an association between
TICs and angiogenesis (5).
Previous studies have reported that CD24(+) tumorigenic cells,
which are involved in initiating, maintaining and expanding tumor
growth, have TIC properties and angiogenic potential (6). As a marker of angiogenic endothelial
cells, CD13 has recently been indicated to be a biomarker of human
liver cancer stem cells, and a fusion protein composed of
CD13-targeting peptide Asn-Gly-Arg was able to reduce endothelial
tube formation (7). CD133(+)
cancer stem cells exhibit increased tumor-initiating potential and
tumor-endothelial cell interaction (8). In a HCC xenograft mouse model,
low-dose metronomic gemcitabine significantly slowed tumor growth,
and decreased levels of cancer stem-like cells and epithelial
progenitor cells, which are dependent on the vascular
microenvironment (9). These cancer
stem-like cells or TIC-enriched cells of HCC are identified by
different markers, including Kruppel like factor 5 (KLF5) (10), SRY-box 9 (SOX9) (11), SOX12 (12) and aldehyde dehydrogenase (13), and have similar characteristics,
namely, self-renewal, sphere formation and long-term growth with no
differentiation. This sphere-derived population of stem-like cells
has been widely used to investigate TICs (13,14),
as the spheroid culturing of cancer and stromal cells promotes
their stem-like properties and accelerates the density of vascular
formations (15).
A secreted enzyme, lysyl oxidase (LOX), reportedly
contributes to tumor angiogenesis (16,17).
Vascular endothelial growth factor (VEGF) results in
phosphorylation of AKT, ERK and JNK pathways and active p65, which
was demonstrated to be inhibited by LOX silencing (18). LOX has been reported to be a
predictor of less favorable outcomes and may regulate the
expression of VEGF via p38 mitogen-activated protein kinase (MAPK)
signaling (19). It has also been
reported modulating the expression of heat shock-induced factor-1α
(HIF-1α)/LOX pathway attenuates the metastatic potential of liver
cancer stem cells (20).
Therefore, the importance of angiogenesis in cancer development and
recurrence is well established, as an adequate blood supply is
essential for tumor growth dependent or independent of TIC
properties. Additionally, LOX is involved in the mechanistic target
of rapamycin kinase (21),
transforming growth factor-β (22)
and phosphatidylinositol 3-kinase (PI3K) (23) signaling axis, mediates
collagen-linking in tumor microenvironments, and associated with
the activity of integrin β1 (24)
and CD44 (25). Notably, HIF-1α,
integrin β1 and CD44 have essential roles in embryonic stem cells
and TICs. Thus, LOX may be required to create a permissive niche
environment for tumorigenesis, with particular relevance for TICs.
Consequently, LOX may be a potential therapeutic target for HCC via
effects on TICs and angiogenesis (19).
In the current study, it was identified that LOX has
a bridge role of stem cell-like properties, acting to promote
angiogenesis upon endothelial cell (EC) proliferation and tube
formation. Furthermore, LOX, which is secreted from TICs, enhanced
angiogenesis via activation of the VEGF signaling pathway.
Furthermore, anti-angiogenesis therapy is a general strategy
against HCC (26,27), such as using sorafenib (28,29)
or rageferinib (30). The effect
of combination therapy of a LOX inhibitor and sorafenib on EC
proliferation was determined. Considering all of the above, the
findings suggest that TICs secrete LOX to promote tumor
angiogenesis, which is blocked by sorafenib in combination with a
LOX inhibitor.
Materials and methods
Cell lines and culture conditions
Human umbilical vein endothelial cells (hUVECs) were
obtained from the cell bank at the Chinese Academy of Sciences
(Shanghai, China). The HEP cell line was established from patient
HCC tissue from Peking University Cancer Hospital (PUCH; Beijing,
China), as previously described (31). The HCC cell line HuH-7 (cat. no.
JCRB0403) was purchased from the Health Science Research Resources
Bank (Osaka, Japan). Cells were cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; cat. no. 10099-141;
Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Invitrogen) at 37°C in a humidified
incubator under an atmosphere of 95% air plus 5% CO2.
After culture for 48 h with in conditioned medium (CD CHO medium;
cat. no. 10743011; FBS-free, 1% penicillin/streptomycin;
Invitrogen; Thermo Fisher Scientific, Inc.), the supernatant from
each group of cells was harvested and concentrated 10-fold using a
Millipore concentration tube (10 kDa nominal molecular weight
limit; EMD Millipore, Billerica, MA, USA) according to the
manufacturer’s protocol.
HCC tissue was obtained from a 47-old male patient
that underwent HCC resection of his right-half liver surgery at
PUCH in February 2004. The use was approved by the Ethics Committee
of PUCH (no. 2014KT-09). All studies involving human samples
adhered to the principles of the Declaration of Helsinki in
accordance with the National Institutes of Health guidelines with
patient consent (31).
Sequencing and bioinformation
analysis
Total RNAs extracted from cultured cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) were
sequenced by the Illumina Hiseq X10 platform (Illumina, Inc., San
Diego, CA, USA) to obtain raw reads. Filtered clean reads had
equality distributed base composition and mass. The quality of
sequencing outcomes was subjected to subsequent bioinformatics
analysis. The upregulated differentially expressed genes was
clustered by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment analysis, which performed using the Database for
Annotation, Visualization and Integrated Discovery (DAVID;
david.ncifcrf.gov/). The protein-protein interaction network of
upregulated differentially expressed genes was constructed using
the Search Tool for the Retrieval of Interacting Genes database
(string-db.org/) followed by highest confidence (score, >0.9).
High relational proteins were analyzed and illustrated with
biological pathway using FunRich software version 3 (funrich.org/).
The mRNA-sequencing expression profiles and clinical data from HCC
samples were downloaded from The Cancer Genome Atlas (TCGA;
cancergenome.nih. gov/), and then analyzed gene transcript per
million between normal and tumor tissues using web resource UALCAN
(ualcan.path.uab.edu.) which access to publicly available cancer
transcriptome data.
Sphere formation
Each well contained 6,000 single cells that were
plated in Ultra Low Attachment 6-well plates (Corning Life Science,
Acton, MA, USA) and cultured in 2 ml 1:1 mix of 2% methylcellulose
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and Dulbecco’s
modified Eagle’s medium/F12 (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with B27 (1:50), 20 ng/ml epidermal growth
factor, 20 ng/ml basic fibroblast growth factor and 10 ng/ml
hepatocyte growth factor (Invitrogen). The spheres over 100 μm in
diameter were harvested under a stereomicroscope (Olympus
Corporation, Tokyo, Japan) following incubation at 37°C and in 5%
CO2 for 2-3 weeks. The cell lines induced to form
spheres were termed ‘HEP-sph’ and ‘HuH-7-sph’, compared with the
parental control.
Transfection of LOX expression plasmid
and identification
A lentivirus overexpressing LOX was constructed
using the pLenti6-Blast lentivirus vector containing a full long
cDNA clone of LOX from GeneCards (GCID GC05M122063) using
restriction endonuclease BamH1 and Xho1 (New England
BioLabs, Inc., Ipswich, MA, USA). Lentiviral packaging was
performed by transfecting 106 293FT cells with each 3 µg
plasmid of the ViraPower Packaging Mix (Invitrogen; Thermo Fisher
Scientific, Inc.) using Lipofectamine® 2000. HEP and
HuH-7 cells were infected using recombinant lentiviruses and vector
empty control, respectively (10 multiplicity of infection), and
selected stably infected cells by 5 µg/ml blasticidin
resistance according to manufacturer’s protocol as previously
described (32). The cell lines
infected with LOX and the vector control were termed ‘HEP-LOX’ and
‘HuH-7-LOX’; the controls were HEP-C and HuH-7-C. LOX mRNA and
protein levels were analyzed using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
Western blot analysis
Cells were suspended in an appropriate volume of
lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% NP-40, 0.25%
sodium deoxycholate and 0.1% SDS) supplemented with 1 mM
phenylmethylsulfonyl fluoride, phosphatase inhibitor cocktail and
Complete Mini Protease Inhibitor Cocktail (Roche Diagnostics GmbH,
Mannheim, Germany). Following protein quantitation using
bicinchoninic acid assay, 30 mg HEP, HEP-sph, HuH-7, and HuH-7-sph
cells protein lysate were resolved by SDS-PAGE on 10% gels and
electroblotted onto nitrocellulose membranes. Following blocking
using 5% fat-free milk at room temperature for 1 h, the membranes
were incubated with primary antibodies specific to LOX, VEGF,
CD105, phospho (p)-extracellular signal-regulated kinase (ERK),
voltage-dependent calcium channel subunit α2δ1 (α2δ1), Nanog, POU
domain, class 5 transcription factor 1 (OCT4), SOX2 and actin
(Table I) at room temperature for
1 h, and corresponding secondary horseradish peroxidase
(HRP)-conjugated goat anti-rabbit (cat. no. 111-035-003) or
anti-mouse antibodies (cat. no. 115-035-003; dilution 1:50,000;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at
room temperature for 1 h. Immobilon™ Western Chemiluminescent HRP
substrate (Merck KGaA, Darmstadt, Germany) was used to detect the
immuno-complexes.
 | Table IInformation for antibodies used in WB
and IHC. |
Table I
Information for antibodies used in WB
and IHC.
| Name | Supplier | Catalogue
number | Species | Dilution |
|---|
| α2δ1 | Abcam | ab2864 | Mouse monoclonal
IgG | WB 1:2,000 |
| LOX | Abcam | ab31238 | Rabbit polyclonal
IgG | WB 1:2,000 |
| Nanog | Abcam | ab109250 | Rabbit monoclonal
IgG | WB 1:2,000 |
| OCT4 | Abcam | ab18976 | Rabbit polyclonal
IgG | WB 1:2,000 |
| SOX2 | Epitomics;
Abcam | ab97959 | Rabbit polyclonal
IgG | WB 1:2,000 |
| VEGF | Abcam | ab53465 | Rabbit polyclonal
IgG | WB 1:2,000; IHC
1:200 |
| CD105 | Abcam | ab11414 | Mouse monoclonal
IgG | IHC 1:200 |
| ERK1/2 | Cell Signaling
Technology, Inc. | 9102 | Rabbit polyclonal
IgG | WB 1:2,000 |
| p-ERK1/2 | Cell Signaling
Technology, Inc. | 9101 | Rabbit polyclonal
IgG | WB 1:2,000 |
| Actin | Roche
Diagnostics | 1378996 | Mouse monoclonal
IgG | WB 1:10,000 |
RT-qPCR analysis
Total RNA was extracted from HEP, HEP-sph, HuH-7 and
HuH-7-sph cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and cDNA was subsequently synthesized from 2
µg total RNA using the MultiScribe™ Reverse Transcriptase at
37°C for 50 min (Invitrogen; Thermo Fisher Scientific, Inc.).
RT-qPCR was performed on an ABI7500 PCR machine using the SYBR
Green PCR Master Mix (Toyobo Life Science, Osaka, Japan) and
specific primers for each gene were designed and validated by using
PrimerDepot (primerdepot.nci.nih.gov; Table II). GAPDH was used as an internal
control. PCR using 40 cycles of 95°C for 15 sec and 60°C for 1 min
was performed in duplicate. The relative gene expression for each
sample was calculated using the formula 2(-ΔCq) =
2[Cq (GAPDH) - Cq (target)] (33).
 | Table IIPrimer sequences. |
Table II
Primer sequences.
| Gene | Sense | Anti-sense |
|---|
| BMI1 |
5′-AGCAGCAATGACTGTGATGC-3′ |
5′-CAGTCTCAGGTATCAACCAG-3′ |
| KLF4 |
5′-AAGCCAAAGAGGGGAAGAC-3′ |
5′-CATCTGAGCGGGCGAATTTC-3′ |
| LOX |
5′-GTTCCAAGCTGGCTACTC-3′ |
5′-GGGTTGTCGTCAGATAC-3′ |
| Nanog |
5′-TGCCTCACACGGAGACTGTC-3′ |
5′-TGCTATTCTTCGGCCAGTTG-3′ |
| OCT-4 |
5′-GACAACAATGAAAATCTTCAGGAGA-3′ |
5′-CTGGCGCCGGTTACAGAACCA-3′ |
| SOX2 |
5′-ACATGAACGGCTGGAGCAAC-3′ |
5′-AGGAAGAGGTAACCACAGGG-3′ |
| VEGF |
5′-CTACCTCCACCATGCCAAGT-3′ |
5′-AGCTGCGCTGATAGACATCC-3′ |
LOX activity assay
LOX activity in the culture medium from HCC cells
was measured using an Amplite™ Fluorimetric Lysyl Oxidase Assay kit
(AAT Bioquest Inc., Sunnyvale, CA, USA) following the protocols
provided by the manufacturers as described as reported (34).
ELISA
LOX secretion was measured by ELISA (eBioscience;
Thermo Fisher Scientific, Inc.), according to the manufacturer’s
instructions (35). Cell culture
supernatant samples were collected following the periods of
incubation with HEP-C, HEP-LOX, HuH-7-C and HuH-7-LOX cultured with
or without 100 µmol/l β-aminopropionitrile (BAPN). Standards
and samples were added to the appropriate microtiter plate wells,
which were pre-coated with an antibody specific to LOX. Following
addition of reagents to every well and incubation at 37°C for 2 h,
a color change was detected using iMark™ microplate reader (BioRad
Laboratories, Inc., Waltham, MA, USA) at a wavelength of 450±2 nm.
The concentration of LOX in every sample was determined by
comparing the optical density of the samples to a standard
curve.
Cell proliferation assay
Aliquots of 1×105 cells/ml hUVECs were
seeded in a 96-well microplate. After incubation at 37°C under 5%
CO2 for 24 h, cells were treated with the culture
supernatant of HEP, HEP-sph, HuH-7, and HuH-7-sph cells, with or
without BAPN for further 48 h cultivation using no-phenol-red CHO
medium (Invitrogen; Thermo Fisher Scientific, Inc.). Then, cells
were exposed to MTS method using CellTiter 96 one solution cell
proliferation assay kit (Promega Corporation, Madison, WI, USA) for
2 h and measured spectrophotometrically at a wavelength of 450±2
nm.
Tube formation assay
The tube formation assay was performed as previously
described (36). Initially, 4
mg/ml Matrigel dissolved in PBS was added into 96-well plates (50
µl/well) and the plates were incubated at 37°C for 30 min to
allow gel formation. Subsequently, 1×105 cells/ml hUVECs
in the logarithmic growth phase were starved and plated into each
well with medium containing the culture supernatant of HEP,
HEP-sph, HuH-7 and HuH7-sph cells with or without BAPN. After
incubation for 72 h, six randomly chosen fields per well were
captured, the 2-dimensional organization of the cells was imaged
using IncuCyte® image observation (Essen Bioscience, Ann
Arbor, MI, USA), and the network growth area of blood vessels was
calculated using ImageJ v1.41 (rsb. info.nih.gov/ij/) (37).
Experimental proliferation on chick
chorioallantoic membrane assay (CAM)
Ten-day-old chicken eggs obtained from Vital River
Laboratories Co., Ltd. (Beijing, China) following fertilization
were windowed in the shell over a chorioallantoic membrane vein.
Briefly, the area of the outer eggshell where prominent blood
vessels are located close to the inner shell surface was swabbed
with 70% ethanol and a small window was cut through the eggshell
with a hobby-grinding wheel. Cells (5×106) in a total
volume of 30 µl RPMI-1640 medium were transplanted gently to
the CAM and reagents covered the surface of the tumor following the
xenograft for 24 h. The window was sealed using sterile adhesive
plaster, and the embryo was allowed to develop for an additional 7
days in a humidified forced-draft egg incubator (37°C). The embryo
was then sacrificed. Tumors growing on the CAM were dissected and
weighed to evaluate growth rate in vivo.
Animal samples and
immunofluorescence
In order to establish a tumor-bearing mouse model,
HEP, HEP-sph, HuH-7 and HuH-7-sph cells were mixed with 100
µl equal volume plain RPMI-1640 and Matrigel (10 mg/ml; BD
Biosciences; Becton, Dickinson and Company, Franklin, Lakes, NJ,
USA). The cell suspension was subcutaneously injected into the back
of six female 4-6 week old NOD/SCID mice (18 g; Vital River
Laboratories Co., Ltd.). Animals were housed under individual
ventilated cages system at 22±2°C and 40-60% atmosphere with
alternating 12 h light/dark cycle. Food and sterile water was
freely available and changed every three days. Tumor formation was
monitored every week. At ~10 weeks later, tumors were frozen
immediately in liquid nitrogen and stored at -80°C. All animal
experiments were approved by and conformed to the regulatory
standards of Peking University Cancer Hospital on Laboratory
Animals Care and Use in accordance with the National Institutes of
Health Guide (Guide for the Care and Use of Laboratory Animals)
(38). For immunofluorescence
staining, frozen tumor tissues were sectioned (4-µm-thick)
using a cryostat and fixed in methanol for 30-40 sec. Following
blocking with 5% non-fat milk in PBS at room temperature for 1 h,
slides were incubated with an antibody specific to LOX at 4°C
overnight, followed by a reaction with fluorescein
isothiocyanate-goat-anti-rabbit IgG. Nuclei were stained with DAPI
at 0.5 µg/ml at room temperature for 5 min. All the
specimens were mounted in 90% glycerol/PBS with 2.5%
1,4-diazabicyclo (2, 2, 2)
octane and examined using a Leica SP5 confocal microscope (Leica
Microsystems GmbH, Wetzlar, Germany). For hematoxylin and eosin
(H&E) staining, the tissues were fixed in 4% paraformaldehyde
and dehydrated through a serial alcohol gradient, and embedded in
paraffin wax blocks. following sectioning of 5-µm-thick
slides and rehydration in xylene and ethanol, H&E were used to
stain the tissues.
Statistical analysis
Experiments were performed independently in
triplicate, and data are presented as the mean ± standard
deviation. Statistical significance was determined using the
unpaired two-sided Student’s t-test for independent samples with
Excel 2010 software (Microsoft Corporation, Redmond, WA, USA). In
case of multiple tests, one-way analysis of variance followed by
Tukey’s multiple comparison test was applied. Correlation was
performed using two-tailed Spearman’s test using GraphPad Prism 5.0
version (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
TIC-enriched cells contribute to HCC
angiogenesis
TIC-enriched cells drive tumor formation and
processing because of their stem cell characteristics (4) and, in particular, their capacity for
self-renewal. Sphere cells are a group of stem-like cells, as
previously reported and shown in Fig.
1A. The expression of a panel of proteins associated with TICs,
including OCT4, Nanog, SOX2 and novel liver cancer stem cell marker
α2δ1, was validated by western blot (Fig. 1B) and RT-qPCR (Fig. 1C). In a mouse model, a large number
of vessels and capillaries surrounded tumor tissues and were deep
inside xenograft tumors, which were produced from the
sphere-derived cells (Fig. 1D).
These properties of angiogenic morphology fundamentally distinguish
TICs from tumor cells. To explore the association between TICs and
tumor angiogenesis, immunohistochemical staining of CD105 and VEGF
was performed on the tumor slides, and histopathological H&E
staining (Fig. 1E). Elevated
expression of CD105 and VEGF was present in sphere-derived tumors.
Furthermore, the CM from cultured HuH-7 sphere cells significantly
increased tube formation among hUVECs (Fig. 1F). The branching of hUVEC tube
networks was calculated using ImageJ software (Fig. 1G). The findings indicated that CM
from culturing sphere cells induced hUVEC proliferation and tube
formation. These results indicate that sphere-derived cells and
their secreted substrate could directly activate angiogenesis with
HCC tumorigenesis.
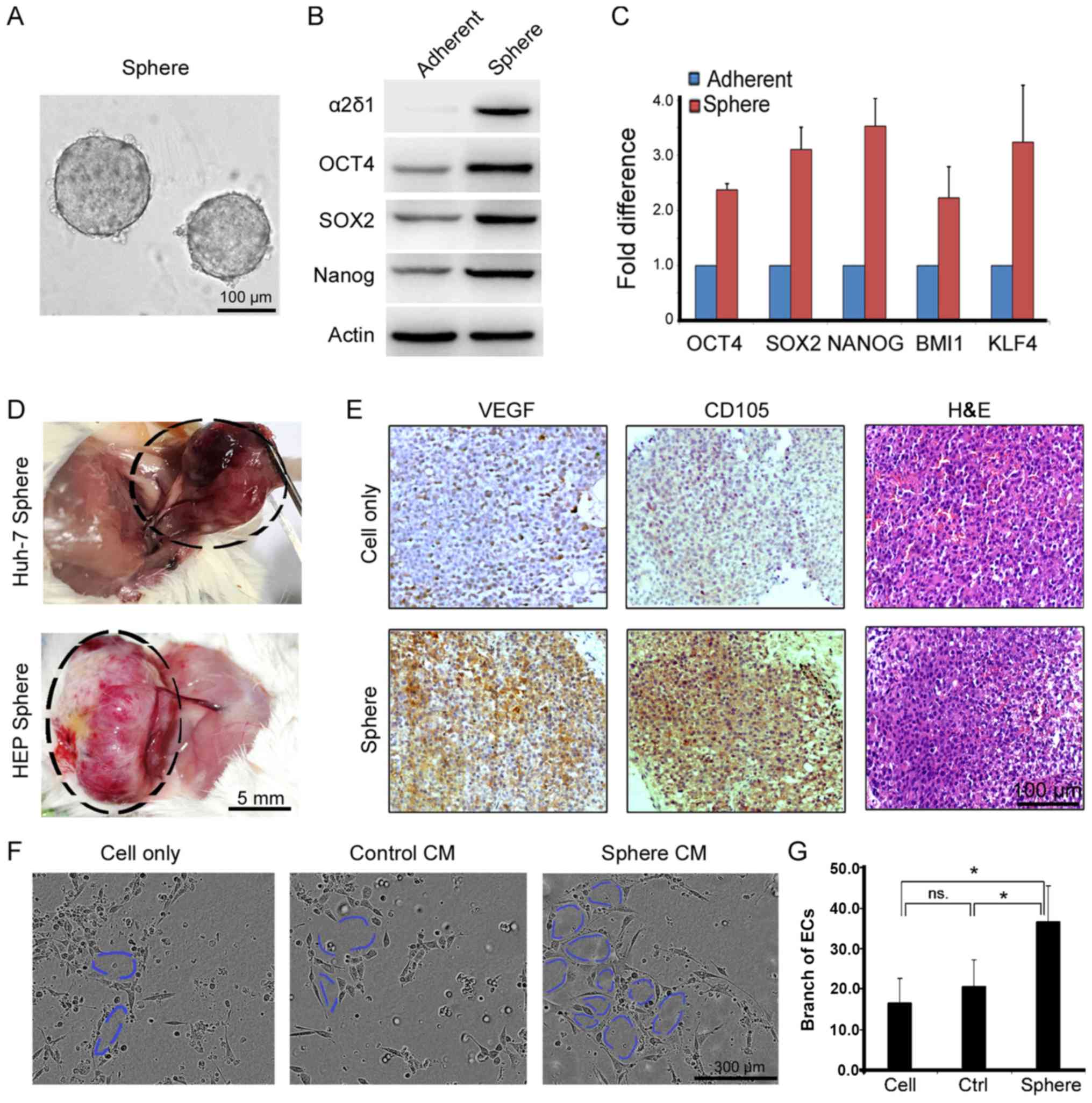 | Figure 1Sphere-derived cells contribute to
HCC angiogenesis. (A) Sphere formation of cells is performed using
serum-free medium supplied with B27/epidermal growth
factor/fibroblast growth factor. (B) Stem-associated protein was
validated by western blot and (C) RNA levels are detected by
reverse transcription-quantitative polymerase chain reaction.
Transplanted spheres into NOD/SCID mice. (D) A large number of
vessels and capillaries are available surrounding tumor tissues,
which grow up from sphere-derived cells of HuH-7 (maximum tumor
diameter 1.32 cm) and HEP (maximum tumor diameter 1.67 cm). (E)
Immunohistochemical staining of VEGF and CD105 are performed on
tumor slides form HuH-7 cell and sphere derived transplantations,
and histopathological H&E staining. (F) Tube formation of human
ECs using CM from Huh-7 sphere-derived cells. (G) Measurement of EC
tube branches. *P<0.05 using one-way analysis of variance. α2δ1,
voltage-dependent calcium channel subunit α2δ1; OCT4, POU domain,
class 5 transcription factor 1; SOX2, SRY-box 2; BMI1, BMI1
proto-oncogene, polycomb ring finger; KLF4, Kruppel like factor 4;
VEGF, vascular endothelial growth factor; H&E, hematoxylin and
eosin; CM, conditioned media; ECs, endothelial cells; ns, not
significant. |
LOX activates angiogenesis signaling by
sequencing and bioinformation analysis
To identify the characteristics of TIC-enriched cell
populations, sequencing analysis comparing sphere cells and
non-sphere cells from patient primary culture cells was performed
to confirm which genes were up- or downregulated during the
angiogenic response. Notably, >70 percent of 1,232
differentially regulated genes were highlighted in hUVECs via
analysis by KEGG, which reportedly affect cell signal pathways or
functional behaviors of hUVECs (Fig.
2A). Upregulated genes were clustered for protein-protein
interactions using STRING network analysis V 10.5 (Fig. 2B). A total of 684 genes were then
selected by clustering interaction using the FunRich functional
enrichment analysis tool (39).
The majority of genes were associated with angiogenesis, such as
platelet-derived growth factor (PDGF) receptors, the integrin
family, the epidermal growth factor receptor, and components of the
VEGF network and IGF pathway (Fig.
2C). Furthermore, a heat map of the relevant pathways of
angiogenesis is shown in Fig. 2D.
Some of the subunits of hepatocyte growth factor (HGF),
erythropoietin producing hepatocyte A4 (EPHA4), tumor necrosis
factor (TNF), PI3K and LOX were greatly increased in sphere-derived
cells. Furthermore, analysis of TCGA database by UALCAN (http://ualcan.path.uab.edu/) (40) revealed that the mRNA levels of LOX
and Ras GTPase-activating protein 4 expression were higher in HCC
tissues (370 cases) than in normal controls (50 cases), but HGF,
EPHA4, TNF, MAPK8 and others were not increased.
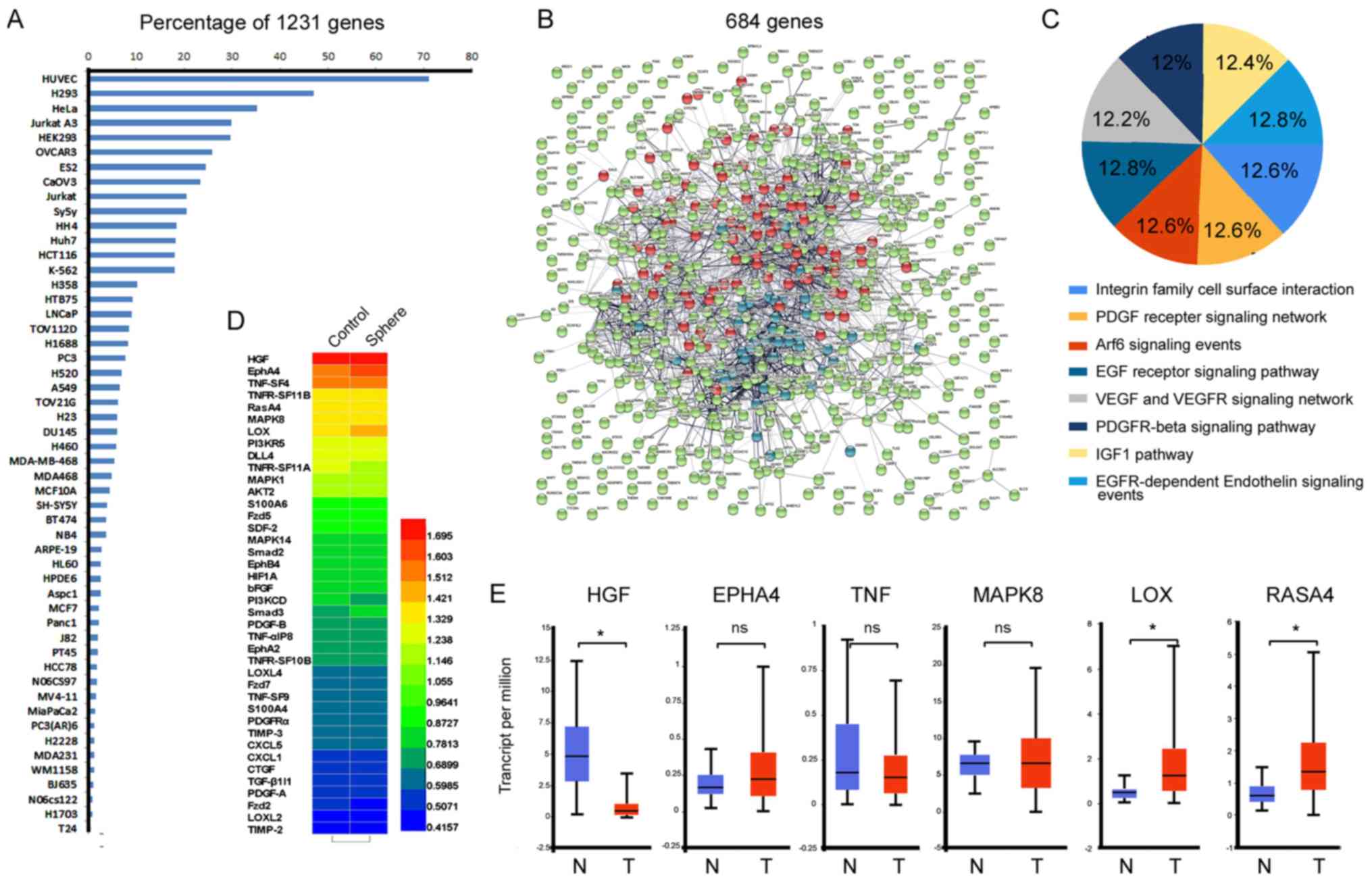 | Figure 2Sequencing and bioinformatics
analysis. Sequencing analysis was performed to compare
sphere-derived and Petri dish cells from patient primary cultures.
(A) A total of 1,232 differentially regulated genes of HEP-sph
cells were detected and Kyoto Encyclopedia of Genes and Genomes was
used to cluster cell lines highly related to these genes. (B) Of
the 1,232 genes, 684 were clustered by the Markov Cluster Algorithm
with an inflation parameter (number: 3) using the STRING database
with different average node degrees. Red, >1.0; blue, >0.5,
<1.0; green, <0.5. (C) Angiogenesis response molecular
signaling analysis of these 684 genes using the FunRich analysis
tool. (D) The heat map presented relevant angiogenesis pathway
genes and their scores among these 684 genes using Gene Set
Enrichment Analysis website. (E) Some subunits of HGF, TNF, PI3K,
LOX and EPHA4 were highly increased in tumors compared with their
normal tissues based on The Cancer Genome Atlas data using UALCAN.
*P<0.05 using a two-sided Student’s t-test. PDGF,
platelet-derived growth factor; Arf6, ADP ribosylation factor 6;
EGF, epidermal growth factor; VEGF, vascular endothelial growth
factor; VEGFR, VEGF receptor; PDGFR, platelet-derived growth factor
receptor; IGF1, insulin-like growth factor 1; EGFR, EGF receptor;
HGF, hepatocyte growth factor; EPHA4, ephrin type-A receptor 4;
TNF, tumor necrosis factor; MAPK8, mitogen-activated protein kinase
8; LOX, lysyl oxidase; RASA4, Ras GTPase-activating protein 4. |
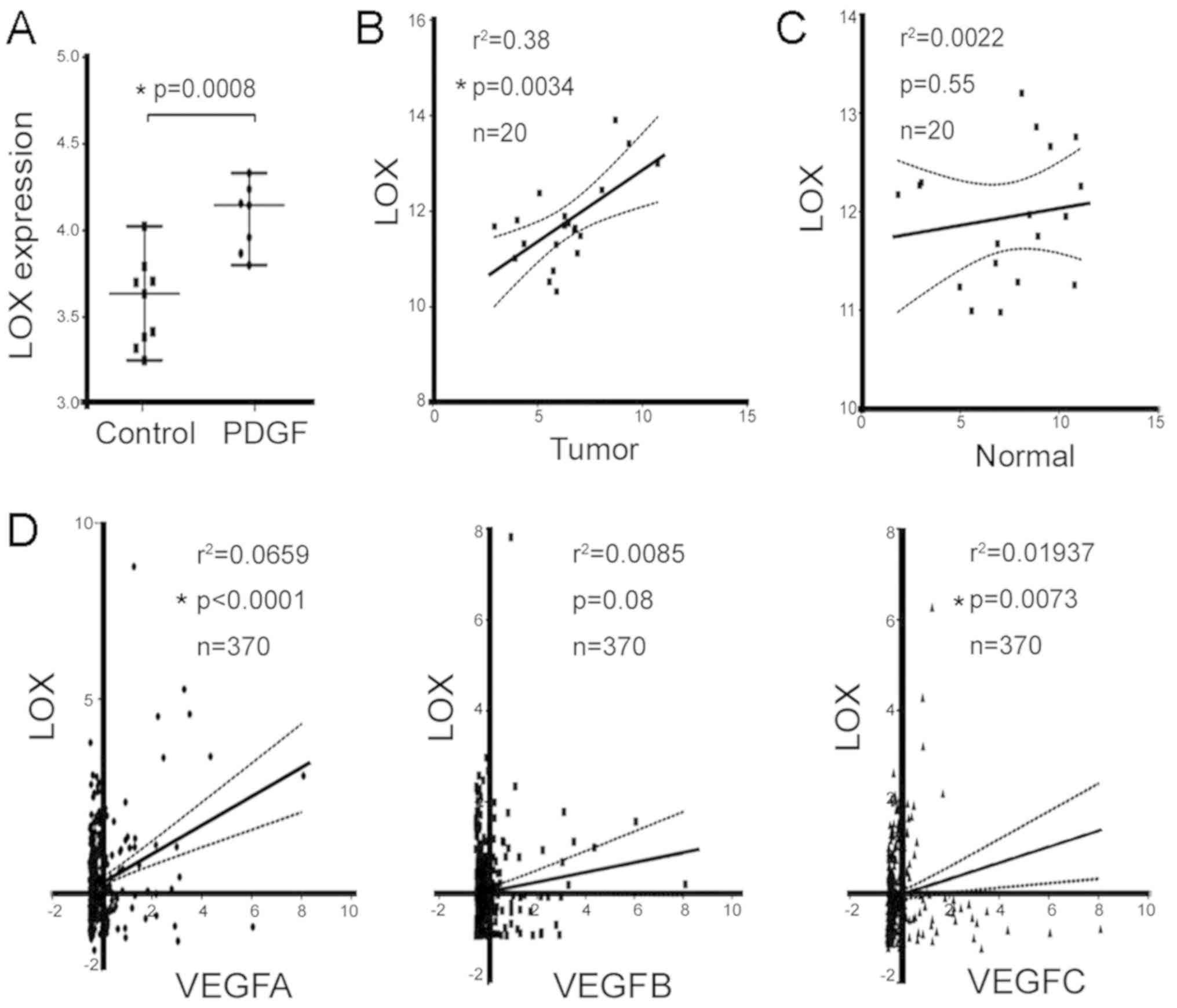
LOX is an activator of angiogenesis, and
LOX associated with PDGF and VEGF
The significantly elevated LOX mRNA was identified
from the Gene Expression Omnibus (GEO) profile of a PDGF-driven
genetic mouse model (GDS5320:10458894; Fig. 3A). The highly upregulated LOX
indicates that it may have a critical role in mediating tumor
angiogenesis in a mouse model. Another GEO database with human
clinical samples (GDS4887) (41)
was analyzed, which revealed significant correlations between the
mRNA level of LOX and VEGF were in the tumor tissues of patients
with HCC (P=0.0034; Fig. 3B);
however, there was no significant correlation in normal tissues
(P=0.55; Fig. 3C). In the TCGA
database, LOX and VEGFA (r2=0.066, P<0.001), LOX and
VEGFB (r2=0.008, P=0.08); LOX and VEGFC
(r2=0.02, P=0.0073) were significantly correlated
(Fig. 3D). These results suggest
that LOX may be involved in stimulating tumor angiogenesis, which
is associated with sphere-derived HCC cells.
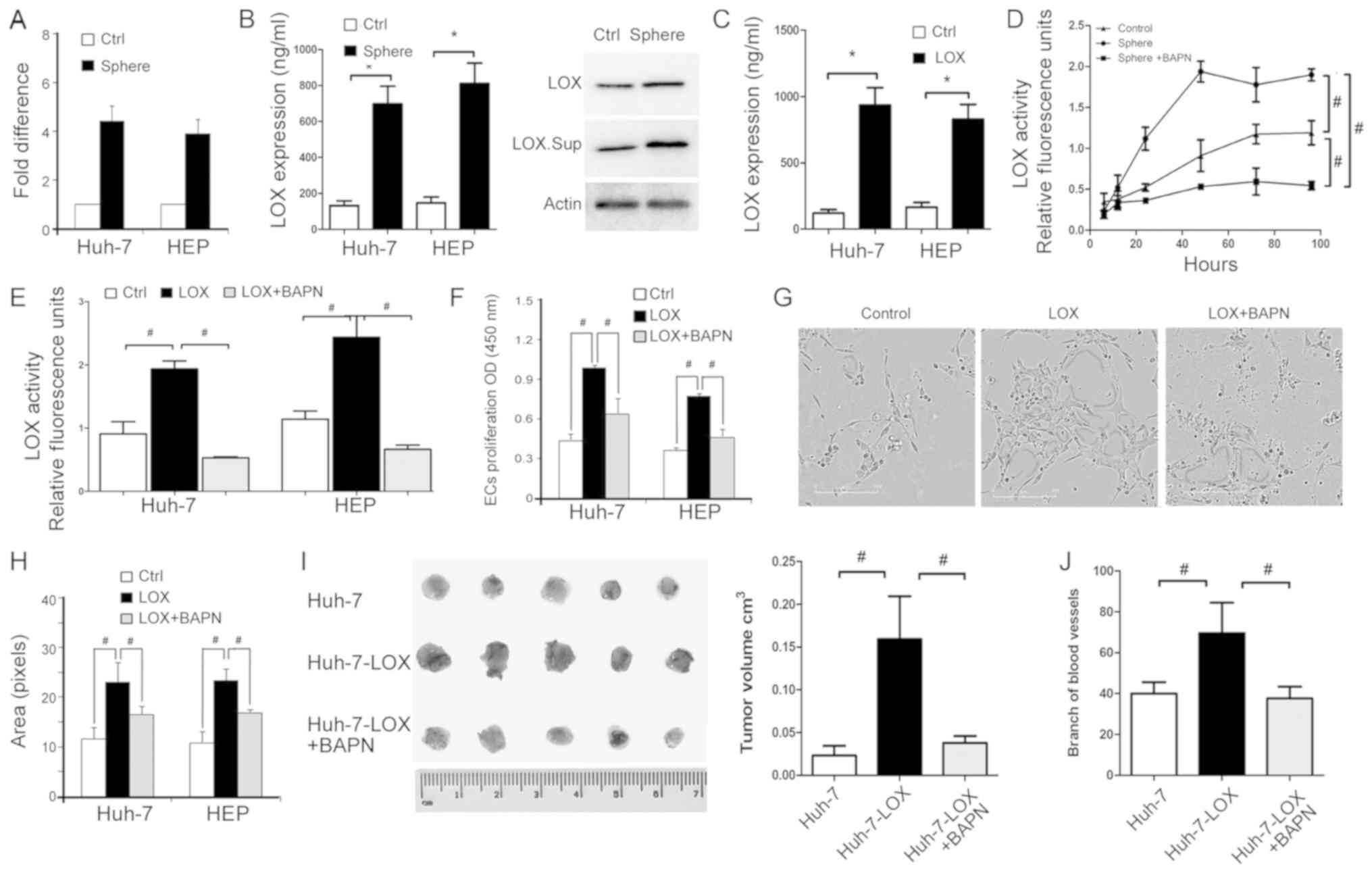
TIC-enriched HCC cells express high LOX
levels
It has been reported that LOX is upregulated in
tumors and their associated endothelial cells (42), but seldom in TICs. In the current
study, RT-qPCR demonstrated that LOX mRNA is highly
expressed in sphere-derived cells, including HEP-sph and HuH7-sph
cells (Fig. 4A). The protein level
of extracellular secreted LOX was determined using an ELISA and
western blot analysis (Fig. 4B).
LOX secretion in was increased in the sphere-derived cells compared
with the parental control cell lines, HuH-7 and HEP cells.
Additionally, LOX secretion was increased in HuH-7 and HEP cells
overexpressing LOX (Fig. 4C).
BAPN, a LOX inhibitor, binds to the active site of LOX. BAPN was
used to inhibit the catalytic activity of LOX in sphere cells and
LOX-overexpressing cells (Fig. 4D and
E). The inhibition was detected using a fluorometric lysyl
oxidase assay kit used to measure the release of active LOX from
cells. These data indicate that LOX secretion and activity may be
important in TIC-enriched HCC cells.
TIC-enriched cells enhance EC
proliferation and tube formation via LOX
Proliferation and tube formation of ECs was
performed to identify whether TIC-enriched cells mediate
angiogenesis via effects on LOX. CM of LOX-overexpressing cells
significantly promoted EC viability compared with the control,
while BAPN reversed this effect (Fig.
4F). The area of capillary-like structures in
LOX-overexpressing cells was larger than in the control cell group,
and BAPN suppressed EC tube formation (Fig. 4G and H). Furthermore, a CAM assay
was used to assess tumor formation and the intensity of
microvessels around tumor sites. BAPN significantly reduced the
volume of LOX-HuH-7 tumors on the CAM and reduced the branching of
microvessels that supported tumor proliferation (Fig. 4I and J). These data suggest that
sphere-derived cells regulate EC proliferation and tube formation
via LOX secretion and activity.
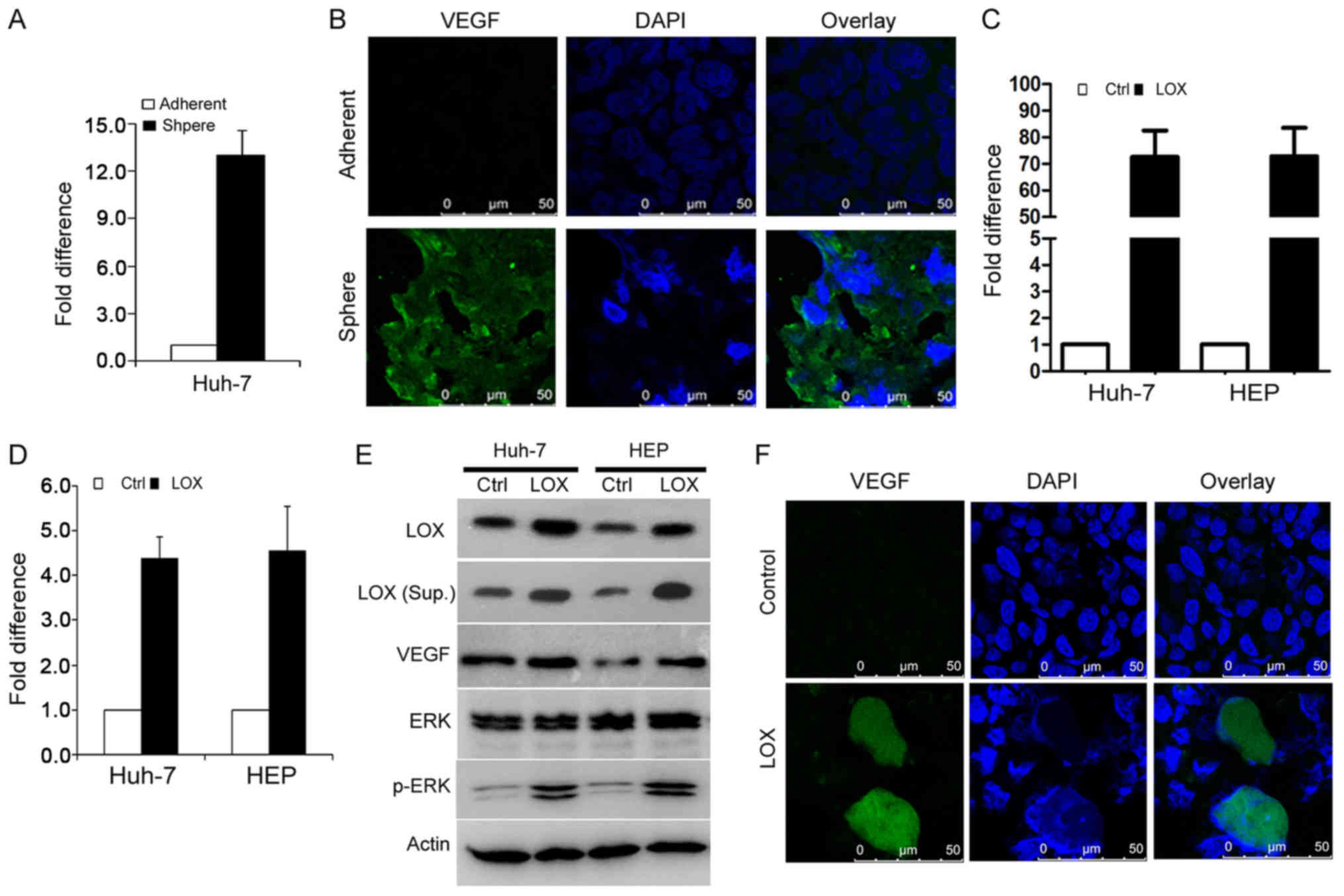
LOX stimulates TIC angiogenesis via VEGF
expression
The findings of the current study have demonstrated
that TIC-enriched cells enhance angiogenesis by secreting LOX. The
expression of pro-angiogenesis factors at the molecular level and
the response to tumor angiogenesis was investigated. As VEGF has an
essential role in tumor angiogenesis, RT-qPCR and western blot
analyses were performed to detect VEGF expression, comparing
sphere-derived cells and parental control cells. The VEGF mRNA
level was significantly upregulated in sphere cells compared with
adherent cells (Fig. 5A). Similar
results were validated using immunofluorescence staining (Fig. 5B). Furthermore, cells expressing
LOX (Fig. 5C) were assessed to
detect mRNA and protein levels of VEGF, ERK and p-ERK (Fig. 5D and E) and were validated using an
immunofluorescence assay (Fig.
5F). It was demonstrated that LOX overexpression enhances VEGF
expression in TIC-enriched cells.
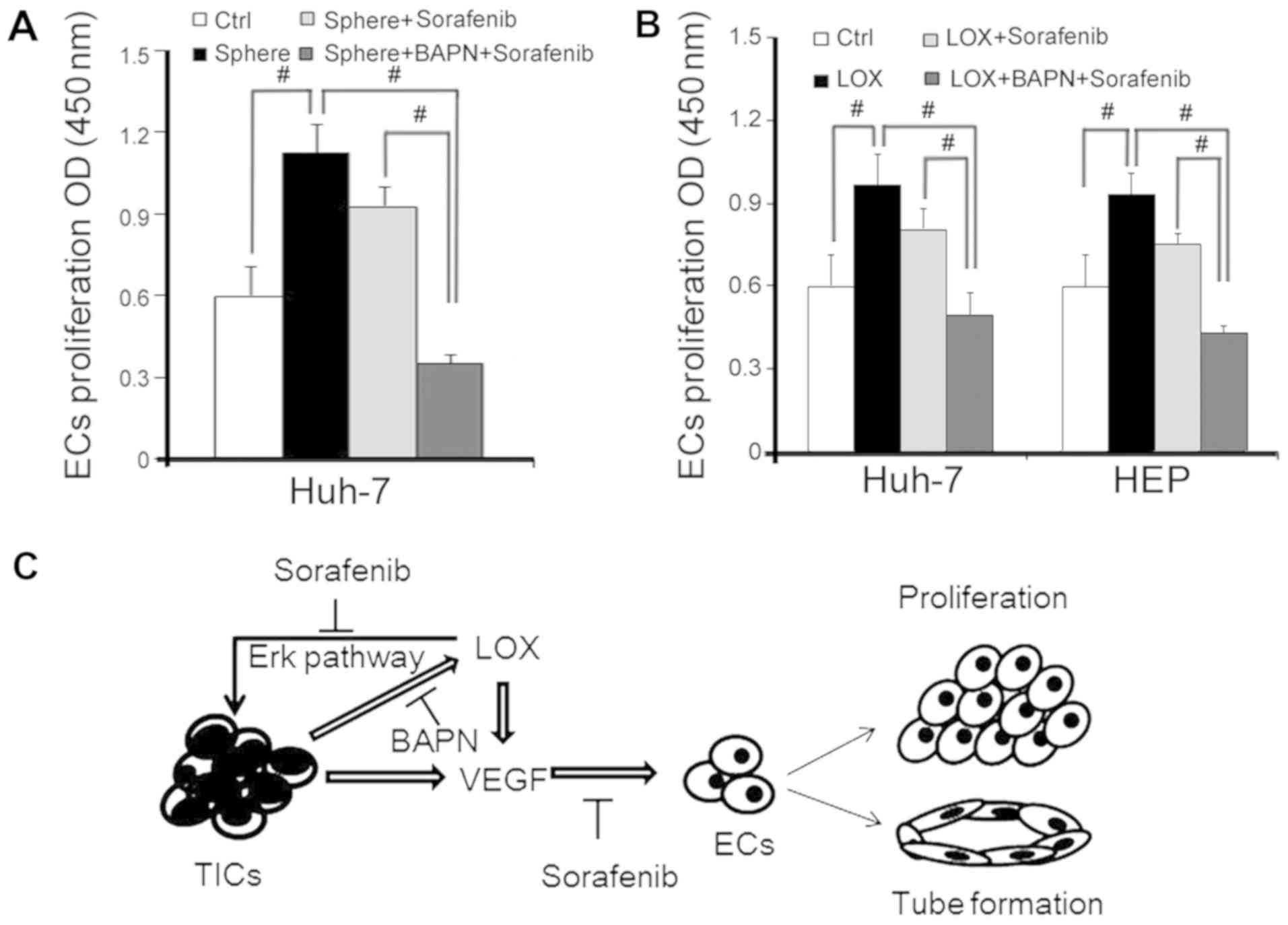
Combination treatment of LOX inhibitor
and sorafenib inhibits EC proliferation
Sorafenib is a drug widely used to treat HCC tumor
angiogenesis (27). The
therapeutic effects of sorafenib combined with the LOX inhibitor
revealed that the functional behavior of angiogenesis is associated
with ECs via cell proliferation. EC proliferation was enhanced by
CM from HEP-sph and rescued by sorafenib or combination with BAPN
(Fig. 6A). The same response was
also observed in cells overexpressing LOX (Fig. 6B). These data suggest that ECs were
suppressed by sorafenib, and that BAPN may improve the inhibition
of sorafenib and exhibit an anti-angiogenesis function when LOX is
overexpressed.
Discussion
Angiogenesis is the process by which new capillaries
sprout or split from pre-existing vessels through the activation,
proliferation, migration and channelization of ECs (43). Angiogenesis has an essential role
in tumor growth and metastasis as newly formed capillaries are
required to provide nutrients and remove waste from tumor tissues
(44). Targeting tumor blood
vessel growth can suppress neoangiogenesis and temporarily
normalize tumor vessel structure, which may enhance tumor
sensitivity to anti-cancer therapy. Multifactorial contributions of
angiogenesis have been reported for decades, and the association
between angiogenesis and matrix-modifying enzymes (45), which are required for remodeling of
the extracellular matrix (ECM), is a new topic in cancer research.
LOX is a collagen cross-linking enzyme, which has an essential role
in creating a niche permissive for tumorigenesis in remodeling the
vascular extracellular matrix during angiogenesis (46). The current study demonstrated that
LOX was highly expressed in TICs and regulates the level of VEGF.
Thus, there may be a link between TICs and angiogenesis mediated by
LOX secretion and VEGF response in hUVECs (Fig. 6C).
As it has been demonstrated that sphere-derived
tumor cells are TIC-enriched, two types of sphere-derived cells,
HEP-sph and HuH7-sph, were generated. Gene and protein expression
in liver cancer cells was analyzed, and it was demonstrated that
stem-associated gene expression, including OCT4, Nanog, SOX2 and
KLF4, were increased in the sphere cells than in the parental
adherent cells. By analyzing bioinformatics data from multiple
databases, LOX was identified as a candidate that is upregulated in
TIC-enriched cells and downregulated in non-TIC cells. Therefore,
the CM of cultured sphere-derived cells and LOX-overexpressing
cells was collected to investigate the effect of TICs on
hUVECs.
The current study revealed that LOX has a critical
role in EC proliferation and contributes to angiogenesis in HCC,
which can be induced by TIC enrichment. The evidence indicated that
capillary vessels were observed adjacent to tumor tissues produced
from sphere-derived cells. Additionally, GEO analysis and ELISA
assays demonstrated that LOX was associated with TIC and VEGF.
These results illustrated that LOX may be involved in mediating
TIC-inducing angiogenesis. Thus, the tumor cell response to
angiogenesis in cells overexpressing LOX was evaluated.
Overexpressing LOX in cells promoted EC viability and tube
formation, which indicated that LOX could be important for
angiogenesis induced by TICs. Furthermore, a LOX inhibitor
attenuated the effectiveness of sphere-derived cell CM on ECs,
demonstrating that EC behaviors are inhibited by BAPN. By contrast,
LOX increased ERK phosphorylation to promote HCC progression
(33). Recently, Nareshkumar et
al (47) reported that the
pro-peptide domain of LOX (2.5 µg/ml) repressed angiogenesis
in hUVECs by reducing the phosphorylation status of focal adhesion
kinase and ERK. These results led the current study to investigate
the mechanism of how TIC affects angiogenesis via LOX.
Immunofluorescence demonstrated that VEGF expression levels were
high in sphere-derived cells, which represented neovascularization
in these tissues. Finally, BAPN increased the anti-angiogenesis
effects of sorafenib on EC viability and tube formation.
In summary, the findings of the present study
indicate that as an ECM remodeling enzyme secreted by HCC TICs, LOX
may promote angiogenesis by regulating VEGF expression. Although
the function and mechanism of LOX in endothelial cells requires
further investigation, these observations provide evidence that
TICs promote tumor angiogenesis via LOX overexpression, and that a
LOX inhibitor in combination with sorafenib may be a strategy for
HCC therapy focused on angiogenesis.
Funding
This study was supported by grants from the ‘863’
Project (grant nos. 2014AA021606 and 2015AA020403); National
Natural Science Foundation of China (grant nos. 81372594 and
81460360 and 81872025); Beijing Natural Science Foundation (grant
no. 7182030); Natural Science Foundation of Xinjiang Uygur
Autonomous Region (grant no. 2015211C126); Capital Funds for Health
Improvement and Research 2018-2-1022.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
WZ and BW supervised this study. MY, JL and ZL
conceived the experiments and analyzed the data. BW analyzed data
using the public database. MY, JL, FW, ZT and BM performed
experiments, and MY, JL and WZ wrote the manuscript.
Ethics approval and consent to
participate
All studies involving human samples adhered to the
principles of the Declaration of Helsinki in accordance with the
National Institutes of Health guidelines of patient consent. The
acquisition and use of HCC tissue was approved by the Ethics
Committee of PUCH. All animal experiments were approved by and
conformed to the regulatory standards of Peking University Cancer
Hospital on Laboratory Animals Care and Use in accordance with the
National Institutes of Health Guide (Guide for the Care and Use of
Laboratory Animals) (38).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Thanks are given to Dr Bin Dong (Department of
Central Laboratory, Peking University Cancer Hospital and
Institute, Beijing, China) for providing help with H&E
staining.
References
|
1
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Gilabert M, Bruix J and Raoul
JL: Treatment of intermediate-stage hepatocellular carcinoma. Nat
Rev Clin Oncol. 11:525–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao W, Wang L, Han H, Jin K, Lin N, Guo
T, Chen Y, Cheng H, Lu F, Fang W, et al: 1B50-1, a mAb raised
against recurrent tumor cells, targets liver tumor-initiating cells
by binding to the calcium channel α2δ1 subunit. Cancer Cell.
23:541–556. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Markowska A, Sajdak S, Markowska J and
Huczynski A: Angiogenesis and cancer stem cells: New perspectives
on therapy of ovarian cancer. Eur J Med Chem. 142:87–94. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zimmerer RM, Ludwig N, Kampmann A,
Bittermann G, Spalthoff S, Jungheim M, Gellrich NC and Tavassol F:
CD24+ tumor-initiating cells from oral squamous cell carcinoma
induce initial angiogenesis in vivo. Microvasc Res. 112:101–108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng YB, Gong JH, Liu XJ, Li Y and Zhen
YS: A CD13-targeting peptide integrated protein inhibits human
liver cancer growth by killing cancer stem cells and suppressing
angiogenesis. Mol Carcinog. 56:1395–1404. 2017. View Article : Google Scholar
|
|
8
|
Kumar D, Kumar S, Gorain M, Tomar D, Patil
HS, Radharani NNV, Kumar TVS, Patil TV, Thulasiram HV and Kundu GC:
Notch1-MAPK Signaling Axis Regulates CD133+ Cancer Stem
Cell-Mediated Melanoma Growth and Angiogenesis. J Invest Dermatol.
136:2462–2474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi SY, Ruan J, Zhao L, Ke Y and Li XN:
Metronomic gemcitabine targeted tumor vascular microenvironment
decreases the population of CD133(+) cells in hepatocarcinoma
xenografts. Cancer Biomark. 14:427–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maehara O, Sato F, Natsuizaka M, Asano A,
Kubota Y, Itoh J, Tsunematsu S, Terashita K, Tsukuda Y, Nakai M, et
al: A pivotal role of Krüppel-like factor 5 in regulation of cancer
stem-like cells in hepatocellular carcinoma. Cancer Biol Ther.
16:1453–1461. 2015. View Article : Google Scholar
|
|
11
|
Richtig G, Aigelsreiter A, Schwarzenbacher
D, Ress AL, Adiprasito JB, Stiegelbauer V, Hoefler G, Schauer S,
Kiesslich T, Kornprat P, et al: SOX9 is a proliferation and stem
cell factor in hepatocellular carcinoma and possess widespread
prognostic significance in different cancer types. PLoS One.
12:e01878142017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zou S, Wang C, Liu J, Wang Q, Zhang D, Zhu
S, Xu S, Kang M and He S: Sox12 Is a Cancer Stem-Like Cell Marker
in Hepatocellular Carcinoma. Mol Cells. 40:847–854. 2017.PubMed/NCBI
|
|
13
|
Mirshahidi S, Simental A, Lee SC, De
Andrade Filho PA, Peterson NR, Cao W, Necochea-Campion R, Yang H,
Duerksen-Hughes P and Yuan X: Subpopulations of cancer stem cells
found in papillary thyroid carcinoma. Exp Cell Res. 362:515–524.
2018. View Article : Google Scholar
|
|
14
|
Jin B, Wang W, Meng XX, Du G, Li J, Zhang
SZ, Zhou BH and Fu ZH: Let-7 inhibits self-renewal of
hepatocellular cancer stem-like cells through regulating the
epithelial-mesenchymal transition and the Wnt signaling pathway.
BMC Cancer. 16:8632016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park IS, Chung PS and Ahn JC:
Adipose-derived stem cell spheroid treated with low-level light
irradiation accelerates spontaneous angiogenesis in mouse model of
hindlimb ischemia. Cytotherapy. 19:1070–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ribeiro AL, Kaid C, Silva PBG, Cortez BA
and Okamoto OK: Inhibition of Lysyl Oxidases Impairs Migration and
Angiogenic Properties of Tumor-Associated Pericytes. Stem Cells
Int. 2017:49720782017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie Q, Xie J, Tian T, Ma Q, Zhang Q, Zhu B
and Cai X: Hypoxia triggers angiogenesis by increasing expression
of LOX genes in 3-D culture of ASCs and ECs. Exp Cell Res.
352:157–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bae WJ, Yi JK, Park J, Kang SK, Jang JH
and Kim EC: Lysyl oxidase-mediated VEGF-induced differentiation and
angio-genesis in human dental pulp cells. Int Endod J. 51:335–346.
2018. View Article : Google Scholar
|
|
19
|
Zhu J, Huang S, Wu G, Huang C, Li X, Chen
Z, Zhao L and Zhao Y: Lysyl Oxidase Is Predictive of Unfavorable
Outcomes and Essential for Regulation of Vascular Endothelial
Growth Factor in Hepatocellular Carcinoma. Dig Dis Sci.
60:3019–3031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tse AP, Sze KM, Shea QT, Chiu EY, Tsang
FH, Chiu DK, Zhang MS, Lee D, Xu IM, Chan CY, et al: Hepatitis
transactivator protein X promotes extracellular matrix modification
through HIF/LOX pathway in liver cancer. Oncogenesis. 7:442018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao Y, Xiao Q, Ma H, Li L, Liu J, Feng Y,
Fang Z, Wu J, Han X, Zhang J, et al: LKB1 inhibits lung cancer
progression through lysyl oxidase and extracellular matrix
remodeling. Proc Natl Acad Sci USA. 107:18892–18897. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pickup MW, Laklai H, Acerbi I, Owens P,
Gorska AE, Chytil A, Aakre M, Weaver VM and Moses HL: Stromally
derived lysyl oxidase promotes metastasis of transforming growth
factor-β-deficient mouse mammary carcinomas. Cancer Res.
73:5336–5346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kucharzewska P, Christianson HC, Welch JE,
Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain
E, Bengzon J and Belting M: Exosomes reflect the hypoxic status of
glioma cells and mediate hypoxia-dependent activation of vascular
cells during tumor development. Proc Natl Acad Sci USA.
110:7312–7317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuura S, Mi R, Koupenova M, Eliades A,
Patterson S, Toselli P, Thon J, Italiano JE Jr, Trackman PC,
Papadantonakis N, et al: Lysyl oxidase is associated with increased
thrombosis and platelet reactivity. Blood. 127:1493–1501. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pietras A, Katz AM, Ekström EJ, Wee B,
Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT and
Holland EC: Osteopontin-CD44 signaling in the glioma perivascular
niche enhances cancer stem cell phenotypes and promotes aggressive
tumor growth. Cell Stem Cell. 14:357–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berretta M, Rinaldi L, Di Benedetto F,
Lleshi A, De Re V, Facchini G, De Paoli P and Di Francia R:
Angiogenesis Inhibitors for the Treatment of Hepatocellular
Carcinoma. Front Pharmacol. 7:4282016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montella L, Addeo R, Caraglia M and Del
Prete S: Latest developments in targeted therapy for hepatocellular
carcinoma. Expert Rev Anticancer Ther. 10:1635–1646. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Liu YC, Sung YC, Ramjiawan RR, Lin
TT, Chang CC, Jeng KS, Chang CF, Liu CH, Gao DY, et al: Overcoming
sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted
nanoparticles to co-deliver MEK-inhibitors. Sci Rep. 7:441232017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao JJ, Shi ZY, Xia JF, Inagaki Y and Tang
W: Sorafenib-based combined molecule targeting in treatment of
hepatocellular carcinoma. World J Gastroenterol. 21:12059–12070.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trojan J and Waidmann O: Role of
regorafenib as second-line therapy and landscape of investigational
treatment options in advanced hepatocellular carcinoma. J
Hepatocell Carcinoma. 3:31–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu XL, Xing BC, Han HB, Zhao W, Hu MH, Xu
ZL, Li JY, Xie Y, Gu J, Wang Y, et al: The properties of
tumor-initiating cells from a hepatocellular carcinoma patient’s
primary and recurrent tumor. Carcinogenesis. 31:167–174. 2010.
View Article : Google Scholar
|
|
32
|
Su B, Zhao W, Shi B, Zhang Z, Yu X, Xie F,
Guo Z, Zhang X, Liu J, Shen Q, et al: Let-7d suppresses growth,
metastasis, and tumor macrophage infiltration in renal cell
carcinoma by targeting COL3A1 and CCL7. Mol Cancer. 13:2062014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Liu C, Guo C, Wang W, Zhu P, Li W, Mi Y,
Myatt L and Sun K: Inhibition of Lysyl Oxidase by Cortisol
Regeneration in Human Amnion: Implications for Rupture of Fetal
Membranes. Endocrinology. 157:4055–4065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng T, Sun X, Wu J, Wang M, Eisenberg RA
and Chen Z: Increased serum levels of tumor necrosis factor
receptor-associated factor 1 (TRAF1) correlate with disease
activity and autoantibodies in rheumatoid arthritis. Clin Chim
Acta. 462:103–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shu Q, Li W, Li H and Sun G: Vasostatin
inhibits VEGF-induced endothelial cell proliferation, tube
formation and induces cell apoptosis under oxygen deprivation. Int
J Mol Sci. 15:6019–6030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giacomini A, Ackermann M, Belleri M,
Coltrini D, Nico B, Ribatti D, Konerding MA, Presta M and Righi M:
Brain angioar-chitecture and intussusceptive microvascular growth
in a murine model of Krabbe disease. Angiogenesis. 18:499–510.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
39
|
Pathan M, Keerthikumar S, Chisanga D,
Alessandro R, Ang CS, Askenase P, Batagov AO, Benito-Martin A,
Camussi G, Clayton A, et al: A novel community driven software for
functional enrichment analysis of extracellular vesicles data. J
Extracell Vesicles. 6:13214552017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A Portal for Facilitating Tumor Subgroup Gene
Expression and Survival Analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hodo Y, Honda M, Tanaka A, Nomura Y, Arai
K, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Sakai A, et al:
Association of interleukin-28B genotype and hepatocellular
carcinoma recurrence in patients with chronic hepatitis C. Clin
Cancer Res. 19:1827–1837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Osawa T, Ohga N, Akiyama K, Hida Y,
Kitayama K, Kawamoto T, Yamamoto K, Maishi N, Kondoh M, Onodera Y,
et al: Lysyl oxidase secreted by tumour endothelial cells promotes
angiogenesis and metastasis. Br J Cancer. 109:2237–2247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu J, Guo W, Xu B, Ran F, Chu M, Fu H and
Cui J: Angiogenesis inhibition and cell cycle arrest induced by
treatment with Pseudolarix acid B alone or combined with
5-fluorouracil. Acta Biochim Biophys Sin (Shanghai). 44:490–502.
2012. View Article : Google Scholar
|
|
44
|
Bisacchi D, Benelli R, Vanzetto C, Ferrari
N, Tosetti F and Albini A: Anti-angiogenesis and angioprevention:
Mechanisms, problems and perspectives. Cancer Detect Prev.
27:229–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li M, Li M, Yin T, Shi H, Wen Y, Zhang B,
Chen M, Xu G, Ren K and Wei Y: Targeting of cancer associated
fibroblasts enhances the efficacy of cancer chemotherapy by
regulating the tumor microenvironment. Mol Med Rep. 13:2476–2484.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Semenza GL: Cancer-stromal cell
interactions mediated by hypoxia-inducible factors promote
angiogenesis, lymphangio-genesis, and metastasis. Oncogene.
32:4057–4063. 2013. View Article : Google Scholar
|
|
47
|
Nareshkumar RN, Sulochana KN and Coral K:
Inhibition of angiogenesis in endothelial cells by Human Lysyl
oxidase propeptide. Sci Rep. 8:104262018. View Article : Google Scholar : PubMed/NCBI
|