Introduction
Prostate cancer is the second most commonly
diagnosed cancer in men worldwide and the fifth leading cause of
cancer mortality (1). Currently,
radical prostatectomy and radiotherapy represent ‘salvage
therapies’ for individuals with localized disease. Unfortunately,
treatment options for men with metastatic prostate cancer are not
curative. While hormone therapy i.e. androgen deprivation therapy
reduces tumor progression, relapse frequently occurs following
surgical or chemical castration. Over a decade, docetaxel is the
first-line systemic chemotherapy used for metastatic
castration-resistant prostate cancer (mCRPC) (2).
Docetaxel is a taxane chemotherapeutic agent that
sensitizes cancer cells to apoptosis by binding to β-tubulin and
prevents its depolarization blocking cells in the G2/M phase of the
cell cycle (3). By stabilizing the
microtubules, docetaxel can inhibit a key driver of mCRPC, androgen
receptors signaling (4,5). Although docetaxel demonstrated
overall survival benefit, the majority of mCRPC patients eventually
become refractory to this chemotherapy and carries a poor prognosis
(6).
A number of previous studies have shed light into
the underlying mechanisms that mediate acquired resistance to
docetaxel (6-10). Notably, clusterin (CLU), an
important stress-induced chaperone (when overexpressed), is one
characterized way that confers docetaxel resistance in mCRPC
(11). CLU, also known as
Apolipoprotein J, is an ATP-independent glycoprotein present in all
human tissues and fluids (12). It
exists in at least two forms with different subcellular
localization and antagonistic functions: The soluble clusterin
(sCLU) and the nuclear clusterin (nCLU) form, reported to serve
distinct roles in cancer, protumoral and antitumoral, respectively
(13). The sCLU is the most
predominant form. It is a heterodimeric protein comprising two
subunits (α and β) of ~40 kDa each. This form bears endoplasmic
reticulum signal peptide sequence that directs the protein to the
endoplasmic reticulum and then to the Golgi apparatus where it
undergoes various post translational modifications during
maturation. The mature protein (80 kDa) is then cleaved by a
furin-like convertase to produce the two subunits (14). Elevated levels of sCLU support a
cytoprotective role through the inhibition of pro-apoptotic
signaling pathways, in cells challenged with different therapeutic
agents allowing them to mediate resistance to treatment
induced-apoptosis (15,16). Several preclinical studies have
been performed investigating the inhibition of the level of CLU and
have exhibited enhanced chemosensitivity of human prostate cancer
cells to treatment-induced apoptosis and delay tumor progression
(17,18). This has led to the clinical
development of OGX-011 (custirsen), a second-generation antisense
oligonucleotide inhibitor of CLU (19,20).
The results of two phase II studies of custirsen in combination
with docetaxel or second-line chemotherapy in men with mCRPC
reported reduced CLU expression within tumor cells, as well as
lowered serum CLU levels which are correlated with improved
survival (21-23).
An alternative splicing of CLU mRNA generates nCLU
(55 kDa) that lacks the endoplasmatic reticulum signal peptide
sequence and localizes in the nucleus. In contrast, the cytotoxic
activity of nCLU in prostate cancer cells is acknowledged. Evidence
for an antitumoral role of nCLU is demonstrated through the
promotion of cell cycle arrest and antimetastatic activity in
prostate cancer cells by decreasing their motility and progression
(24,25). Besides sCLU and nCLU, other
intracellular non-secreted CLU isoforms are expressed within
stressed cells but at a very low level (26).
Cytokines, including growth differentiation
factor-15 (GDF-15), a member of the transforming growth factor
(TGF) superfamily, have been reported to be substantially induced
in prostate cancer cells exposed to docetaxel and mitoxanthrone
chemotherapy and to contribute to tumor cell therapy resistance
(27,28). Increased serum GDF-15 levels
following one cycle of docetaxel regimen was associated with a
shorter overall survival suggesting that GDF-15 could predict for
early docetaxel resistance (28).
Notably, GDF-15 has been reported to exert also a proapoptotic
function in prostate cancer cells (29).
There are now novel therapeutic agents approved in
the treatment of mCRPC following docetaxel failure, including
cabazitaxel, a second-generation of taxane (30). Nevertheless, there is a growing
interest in developing novel therapeutic approaches to overcome
resistance to docetaxel and to provide better disease control in
mCRPC. Previous preclinical studies have demonstrated the efficacy
of nitric oxide (NO) donor therapy for the treatment of prostate
cancer (31,32). Many NO donors have been
demonstrated to be potent chemosensitiser and/or radiosensitiser
against a wide variety of human tumor cells (33-36).
It is now well documented that NO exerts dual activities in cancer:
Protumoral or antitumoral. NO donors-induced anti-tumoral
activities in prostate cancer cells are due to their ability to
simultaneously inhibit cell survival, cell growth pathways and
sensitize tumor cells to apoptosis. In prostate cancer cells,
apoptosis can be positively regulated by NO through the
S-nitrosylation and inhibition of nuclear factor-kappa B (NF-κB)
and subsequent regulated resistant factors such as Yin Yang 1 (YY1)
and B cell lymphoma-2 (BCL2)/BCL-extra large (BCL-XL) (32). In addition, a direct role for NO
with the S-nitrosylation of YY1 has been evidenced in reversing
tumor necrosis factor-related apoptosis-inducing ligand-resistant
prostate cancer cells (37).
Furthermore, the therapeutic efficacy of NO donors in the
inhibition of epithelial-to-mesenchymal transition phenotype and
metastasis has been demonstrated in metastatic human prostatic
cancer cells (38,39).
In this present study the sensitivity of
docetaxel-resistant human prostate cancer cells to the NO donor
glyceryl trini-trate (GTN) and the regulation of resistant markers
were examined to explore novel therapeutic strategies for targeting
mCRPC.
Materials and methods
Cell culture
The human mCRPC cell line DU145 was obtained from
the American Type Culture Collection (Manassas, VA, USA). The PC3
AG and docetaxel-resistant derivative PC3-D12 cell lines were
kindly provided by Professor B. Watson (University College Dublin,
Dublin, Ireland) (40). The
docetaxel-resistant DU145 (DU145-DR) cell line was established
within the team. The DU145 parental cells were seeded in T25 flasks
(2×106 cells) and treated twice a week with increasing
doses (0.01, 0.1, 0.5, 0.75, 1, 5 and 10 nM) of docetaxel (Sanofi
S.A., Paris, France). Doses were increased at intervals of 2-3
weeks, dependent on the rate of cell proliferation. Following each
step, when the cells stopped proliferating and exhibited a modified
morphology, as observed via microscopy, docetaxel treatment was
ceased immediately and the cells were placed in complete medium.
All cells were cultured in Dulbecco's modified Eagle's medium
(DMEM)-4.5 g/l glucose supplemented with 10% fetal calf serum (FCS)
(both from Dominique Dutscher SAS, Brumath, France) at 37°C in a
humid atmosphere of 5% CO2. All cell lines were
mycoplasma free.
Viability test
Cells were seeded in 96-well plates (2,000
cells/well) in complete DMEM medium (100 µl/well). Following
24 h, the DMEM medium was replaced with Opti-MEM™ medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for DU145 lines
and Opti-MEM™ supplemented with 1% FCS for PC3 cell lines. The
cells were treated with docetaxel (0.1, 0.2, 0.4, 0.8, 1.6, 3.2,
6.4, 12.8, 25.6, 51.2, 102.4, 204.8, 409.6 or 819.2 nM) or GTN (25,
50, 100, 200 or 400 µM; Merck KGaA, Darmstadt, Germany)
supplemented or not with 5 ng/ml of human recombinant GDF-15
(R&D Systems, Inc., Minneapolis, MN, USA). Following 72 h of
treatment, cell viability was evaluated by adding MTS (Promega
Corporation, Madison, WI, USA). Absorbance (abs) was read following
4 h at 490 nm. The following formula was used to obtain the
percentage of cell viability: % viable cells =
[(abssample-absblank)/(abscontrol-absblank)]
×100. Half maximal inhibitory concentration (IC50)
calculations were determined using GraphPad Prism 6 software
(GraphPad Software, Inc., La Jolla, CA, USA).
Cell transfection
Cells were seeded in 6-well plates (DU145 and
DU145-DR at 3×105 cells/well; PC3 AG and PC3-D12 at
2×105 cells/well) in complete medium and incubated at
37°C the day prior to transfection. DU145 and DU145-DR cells were
transfected with 50 nM small interfering RNA (siRNA) control and
SmartPool anti-human CLU-siRNA (cat. no. L-019513-00-0005; GE
Healthcare Dharmacon, Inc., Lafayette, CO, USA) using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) as a transfection
reagent. The transfection of PC3 AG and PC3-D12 cell lines was
carried out with 5 nM siRNA using Dharmafect 2 (GE Healthcare
Dharmacon, Inc.) as a transfection reagent. At 5 h following
transfection, the medium was replaced and GTN treatments at 100
µM were performed. Cells were incubated for 72 h.
To establish the DU145-DR fluorescent cell line,
DU145-DR cells were stably transfected with 2.5 µg/µl
pCMV-DsRed plasmid (Clontech Laboratories, Inc., Mountainview, CA,
USA), supplemented with 60 µl Superfect (Qiagen GmbH,
Hilden, Germany). The positive cells were selected using Geniticin
(750 µg/ml, determined as the minimum effective
concentration to kill non-resistant cells; Gibco; Thermo Fisher
Scientific, Inc.) and sorted by flow cytometry following 2 weeks of
selection.
Western blotting
Western blotting was performed to examine the
expression of clusterin and pro-GDF-15. Cells were treated with the
indicated concentrations of GTN in presence or absence of human
recombinant GDF-15. Cells were lysed in boiling buffer (150 mM
NaCl, 150 mM TrisHCl pH 7.4, 1% SDS and 1 mM sodium orthovanadate)
supplemented with protease inhibitor cocktail (Roche Diagnostics,
Basel, Switzerland). The viscosity of the samples was reduced by
several passages through a 26-gauge needle. Proteins concentrations
were measured using the DC™ protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The proteins (from
whole-cell extracts or cell supernatants) were separated by 12%
SDS-PAGE and transferred to nitrocellulose membrane (Bio-Rad
Laboratories, Inc.). After blocking non-specific binding sites
overnight at 4°C by 5% non-fat milk in PBS with Tween-20 0.1%, the
membranes were incubated with the appropriate primary antibody:
Monoclonal mouse anti-human clusterin (MAB29372; 1:1,000)
polyclonal goat anti-human GDF-15 (BAF940; 1:1,000) (both from
R&D Systems, Inc.), monoclonal mouse anti-human β-actin
(SC-47778; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) or monoclonal mouse anti-human heat shock cognate 70 (HSC70;
SC-7298; 1:1,000; Santa Cruz Biotechnology, Inc.) overnight at 4°C,
then with the secondary antibody peroxidase AffiniPure goat
anti-mouse IgG (H+L; 115-035-003; 1:5,000; Jackson ImmunoResearch
Europe, Ltd., Newmarket, UK) or streptavidin protein Dylight 800
(1:10,000; Thermo Fischer Scientific, Inc.) for 1 h at room
temperature. The level of protein expression was analyzed using the
Odyssey imaging system (LI-COR Biosciences, Lincoln, NE, USA) or
the Molecular Imager Chemi Doc™ XRS+ (Bio-Rad Laboratories, Inc.).
β-actin or HSC70 were used as loading control for cell extracts,
and Ponceau red (Sigma-Aldrich; Merck KGaA) staining was used as
the loading control for cell supernatants. Densitometric analyses
of protein levels were performed using ImageJ 1.52a software
(National Institutes of Health, Bethesda, MD, USA).
Zebrafish model
All zebrafish experiments were conducted according
to the French and European Union guidelines concerning laboratory
animal handling. The animal procedures described were reviewed and
approved by the local Ethics Committee (C2EA ‘Comité d'Ethique en
Expérimentation Animale’, Grand Campus Dijon, registered no. 105 by
the national Ethics Committee CNREEA ‘Comité National de Réflexion
Ethique sur l'Expérimentation Animale’). In the present study a
transgenic zebrafish line fli1a: Enhanced Green Fluorescent
Protein (EGFP) expressing EGFP in endothelial cells under the
fli1a promoter was used (Zebrafish International Resource
Center, University of Oregon, Eugene, OR, USA). This model allows
the following of eventual neovascularization at the yolksac level
following a microinjection of tumor cells (41). Adult zebrafish (15 males and 15
females) were maintained in a recirculating aquaculture system
(Müller & Pfleger GmbH & Co. KG, Rockenhausen, Germany)
with a temperature range of 26-28°C, and a 14/10-h light/dark cycle
as previously described (41).
They were fed twice a day with dried flake food. The mean ranges
for conductivity and pH in the system were 600-700 µS and
6.0-8.0, respectively. At 2 days prior to the xenotransplantation,
males and females were placed in the same tanks and mating was
triggered by light stimuli the following day. Eggs were collected
in sourcing water a few hours following the fertilization, counted,
sorted and up to 50 eggs placed at 28°C per Petri dish.
Xenotransplantation and treatment
procedure
Zebrafish larvae were dechorionized by pronase (1
mg/ml; Roche Diagnostics) for 20 min prior to micro-injection and
arrayed on a Petri dish. The injection of DsRed expressing DU145-DR
cells was carried out under a fluorescence magnifying glass (Leica
MZFLII) using micro-injectors (Eppendorf Femtojet) (20-100 tumor
cells/injection) as described previously (41,42).
The injected larvae were incubated at 32°C in saturated humid
conditions. At 1 day following the injection, the larvae were
anesthetized with 0.17 mg/ml tricaine (Sigma-Aldrich; Merck KGaA)
and sorted according to their red fluorescence using a Leica
MZFLIII fluorescence stereomicroscope bearing appropriated filters
with 12.5:1 zoom leading to a maximum magnification of ×800 with
10× micro objectives (Leica Microsystems, Inc., Buffalo Grove, IL,
USA). The positive larvae were then treated with 10 µM GTN
for 5 days. Non-positive larvae were euthanized with tricaine (0.3
mg/ml).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from zebrafish larvae using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and 1 µg
RNA was reverse transcribed using Moloney murine leukemia virus
reverse transcriptase with random hexamers (both from Promega
Corporation) according to manufacturer's protocol. cDNA was
quantified by qPCR on a 7500 Fast system (Life Technologies; Thermo
Fisher Scientific, Inc.) using the standard SYBR-Green PCR Master
mix detection protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). Standard reaction volume was 20 µl and contained 10
µl SYBR-Green mix, 2 µl cDNA template and 125 nM of
primers. The thermocycling conditions were composed of an
activation step at 50°C for 2 min, a denaturation step at 95°C for
10 min, followed by 45 cycles of denaturation at 95°C for 15 sec
and primer annealing/extension at 60°C for 1 min. The final step
was a 60°C incubation for 1 min. All reactions were performed in
triplicate. The mRNA abundance was calculated according to the
2−ΔΔCq method analysis (43). Expression of all genes was
normalized respective to human L32 and zebrafish actin expression
levels. The primers used were as follows: Human clusterin, forward,
5′-CCG CAA AAA GCA CCG GGA GGA-3′ and reverse, 5′-GGG CTG CAG CTC
ATC TTG GGG-3′; zebrafish clusterin, forward, 5′-AAG AGG AAG AAT
CAA AGC AGG TGT-3′ and reverse, 5′-GTA GAG GAG AAA CAG CCC CAG-3′;
human cyclin D1, forward, 5′-CCC TGA CAG TCC CTC CTC T-3′ and
reverse, 5′-GAA GGG GGA AAG AGC AAA G-3′; human L32, forward,
5′-TGT CCT GAA TGT GGT CAC CTG-3′ and reverse, 5′-CTG CAG TCT CCT
TGC ACA CCT-3′; and zebrafish actin, forward, 5′-CCC AGA CAT CAG
GGA GTG AT-3′ and reverse, 5′-CAC AAT ACC GTG CTC AAT GG-3′.
Cytokine array analysis
Cell culture supernatants from DU145, DU145-DR, PC3
AG and PC3-D12 were analyzed using RayBio® human
cytokine array C3 and C9 according to the manufacturer's
recommendation (RayBiotech, Inc., Norcross, GA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of the indicated number of experiments. Significant differences
were evaluated using Student's t-test, one-way analysis of variance
with a post-hoc Tukey's test or two-way analysis of variance with a
post-hoc Bonferroni's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Docetaxel-resistant human prostatic
cancer cells are sensitive to GTN
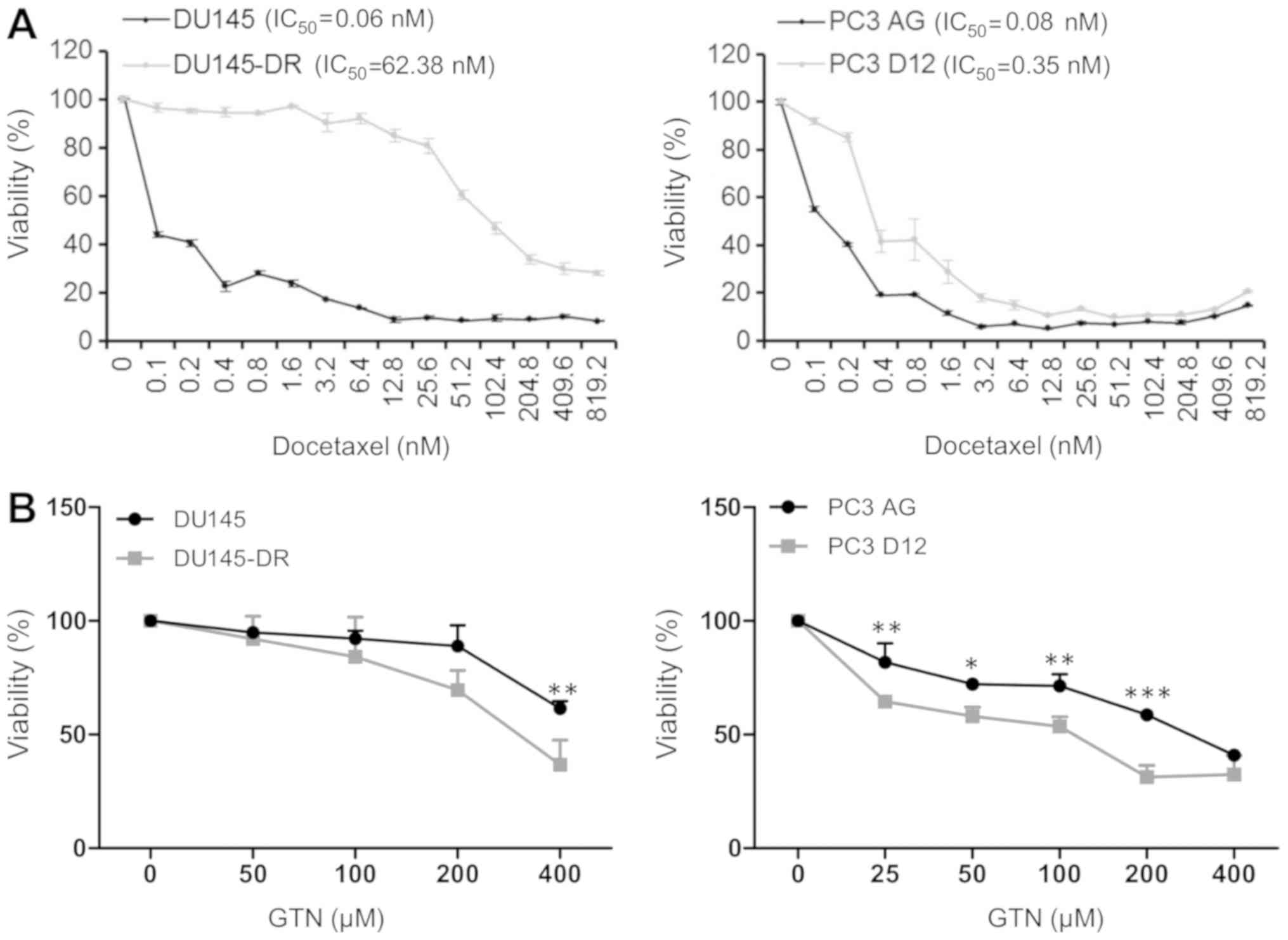
In the current study, two human prostatic cancer
cell lines were utilized, both chemosensitive (DU145 and PC3AG) and
chemoresistant (DU145-DR and PC3-D12) to docetaxel. In order to
generate DU145-DR cells, DU145 cells were treated with increasing
concentration of docetaxel. The cell viability in the
chemosensitive and chemoresistant cells to docetaxel was first
assayed by MTS assay in the presence of a range of docetaxel
concentrations. In response to increasing concentrations of
docetaxel, an increased survival rate was observed in prostatic
resistant cancer cell lines DU145-DR and PC3-D12 in comparison with
the parental cells, which confirmed the docetaxel resistant
phenotype of DU145-DR and PC3-D12. DU145-DR and PC3 D12 cells
demonstrated a greater resistance to docetaxel compared with their
parental counterparts (DU145 DR IC50=62.38 nM vs. DU145
IC50=0.06 nM; PC3D12 IC50=0.35 nM vs.
PC3-AG=0.08 nM; Fig. 1A).
Notably, when compared with the parental cell lines,
DU145-DR and PC3-D12 cell lines chemoresistant to docetaxel
exhibited increased sensitivity to the antiproliferative effect of
GTN used at various concentrations (ranging from 25-400 µM)
over a time course of 72 h (Fig.
1B).
GTN-induced cytoxicity is attributable to
differential regulation of CLU
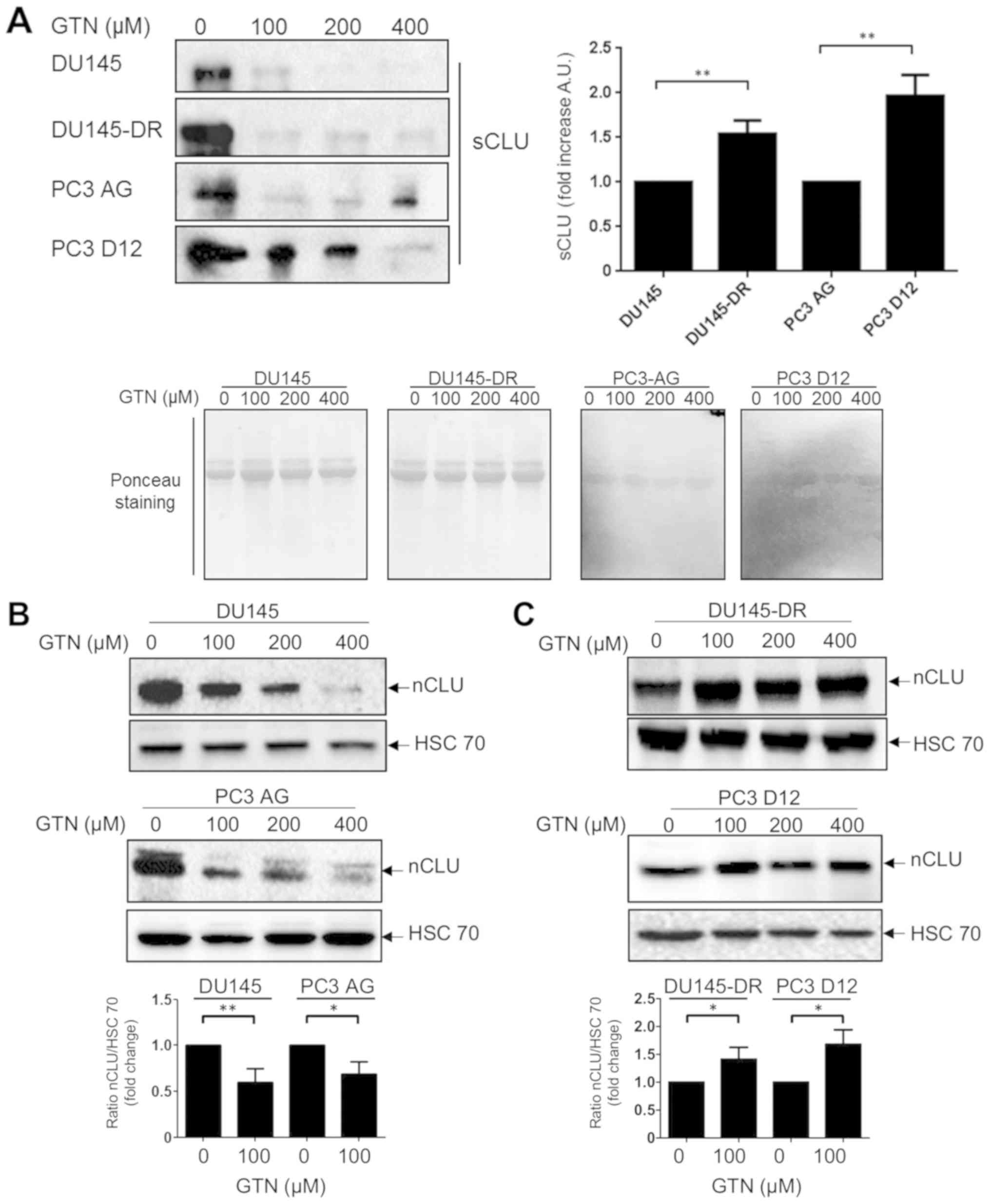
Having demonstrated that docetaxel-resistant cell
lines were more prone to GTN cytotoxicity, it was attempted to
identify the molecular mechanisms underlying this effect. The level
of clusterin, a key docetaxel resistant marker in human prostatic
cancer, was investigated. The basal levels of the secretory form of
clusterin (sCLU; anti-apoptotic function) in the supernatant from
docetaxel-resistant and parental mCRPC cell lines were evaluated.
It was observed that the level of sCLU was significantly higher in
docetaxel-resistant cells compared with parental cells (Fig. 2A). Notably, a reduction in sCLU
protein expression was observed in all cell lines upon GTN
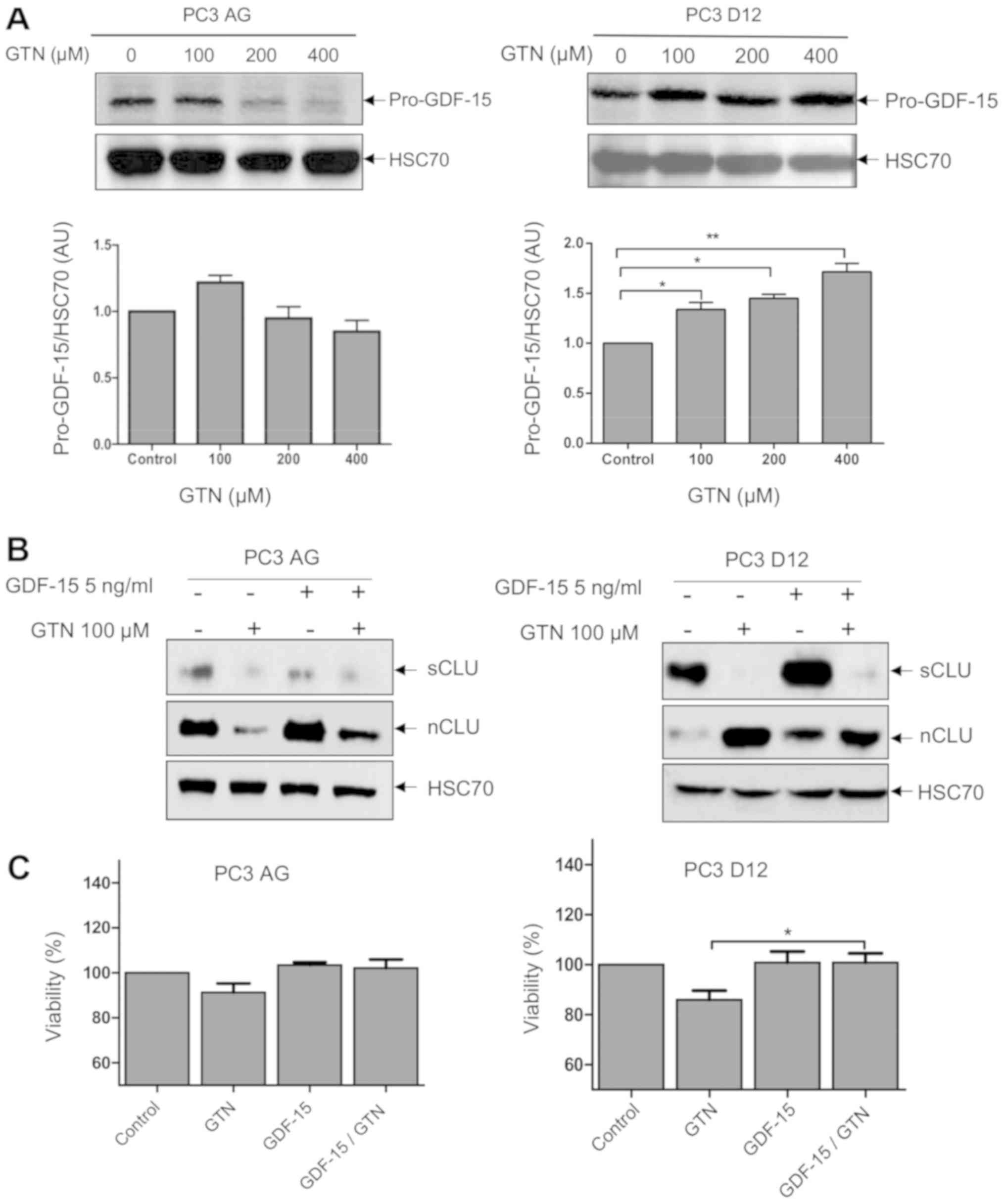
treatment at 100 µM for 72 h, except for the PC3-D12 cells,
in which a reduction of the level of expression was observed only
with higher concentrations of GTN (400 µM). The nCLU protein
expression level was then evaluated. It was observed that GTN
induced a significant reduction in the levels of nCLU compared with
control in the parental cells (Fig.
2B) and an increase in the levels of nCLU compared with control
in the resistant cell lines (Fig.
2C), in accordance with their sensitivity to GTN-induced
cytotoxicity (Fig. 1B). These
results may suggest that GTN regulates the balance of proapoptotic
and antiapoptotic levels of expression of CLU, thereby favoring
death over survival.
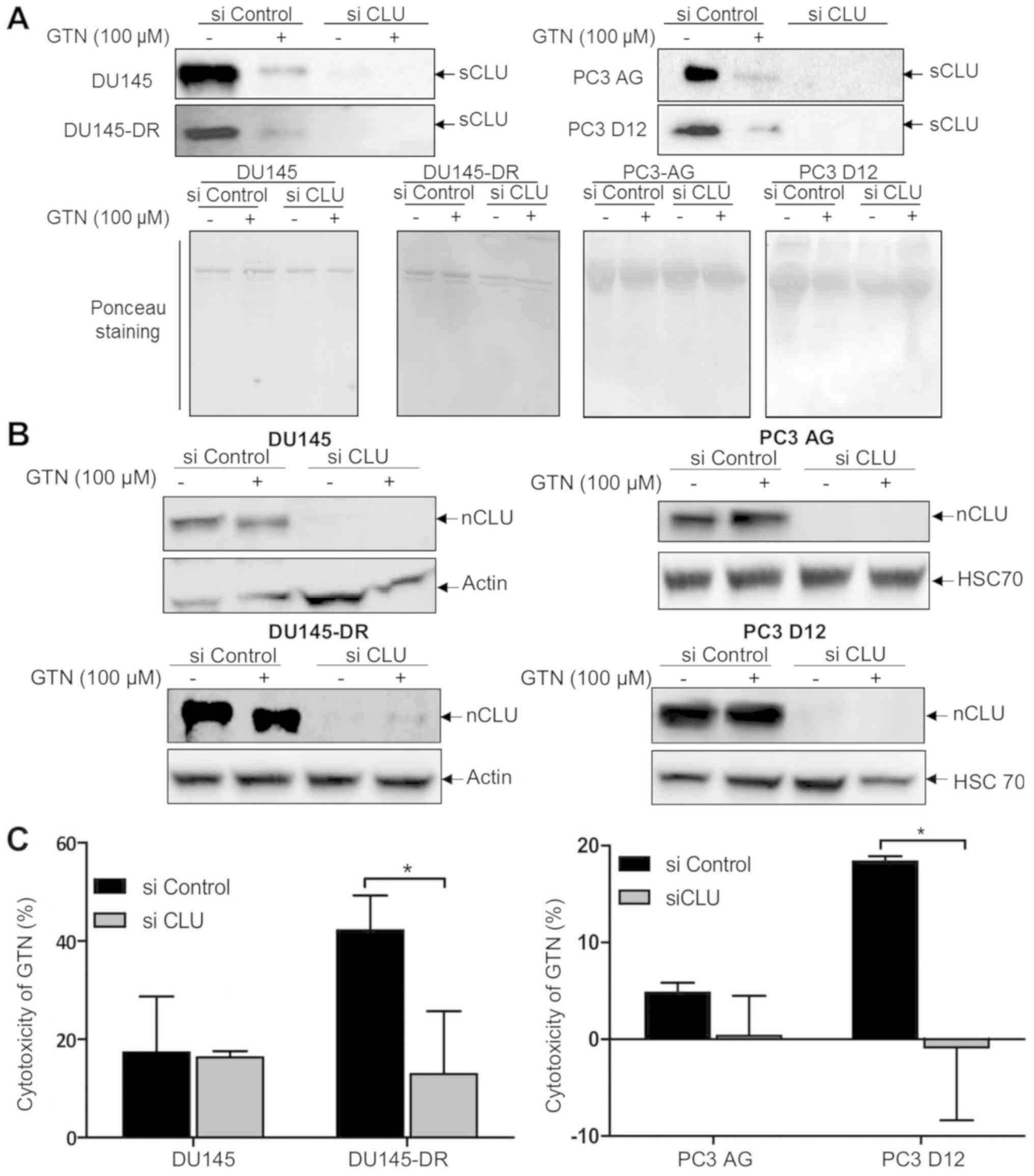
The biological involvement of the modulation of CLU
expression in GTN-induced cytotoxicity for docetaxel-resistant cell
lines was then examined. Thus, whether CLU silencing (sCLU and
nCLU) could affect the cytotoxic effects of GTN in the four human
prostate cancer cell lines was explored. As presented in Fig. 3A and B, western blotting indicated
that sCLU and nCLU levels of expression were dramatically decreased
with CLU siRNA compared with controls, both in the absence or
presence of GTN (even though the transfection reagent increases the
amount of CLU). Furthermore, sensitive cells (DU145 and PC3-AG
cells) transfected with CLU siRNA and then treated with GTN did not
exhibit any marked changes in cytotoxicity compared with the
control (cells transfected with control siRNA). In accordance with
our previous findings (Fig. 1B),
docetaxel-resistant control cells (control siRNA) were more
sensitive to GTN-induced cytotoxicity than the parental control
cells (control siRNA). However, clusterin silencing significantly
reduced the cytotoxic effect of GTN in both DU145-DR and PC3-D12
docetaxel-resistant cells compared with the control cells to reach
a similar level of cytotoxicity observed in sensitive cells
(Fig. 3C).
Taken together, these findings indicate that the
cytotoxic effect of GTN in sensitive cells is not dependent of
clusterin expression. On the contrary, the cytotoxic effect of GTN
in docetaxel-resistant cells is dependent of clusterin expression.
Although the present study cannot distinguish the role of sCLU and
nCLU (siRNA CLU indifferently targets sCLU and nCLU), these results
suggest that GTN cytotoxicity may be most likely dependent of the
nuclear form of the clusterin, upregulated by GTN treatment in
docetaxel-resistant cells.
GTN modulates the level of human
clusterin in vivo
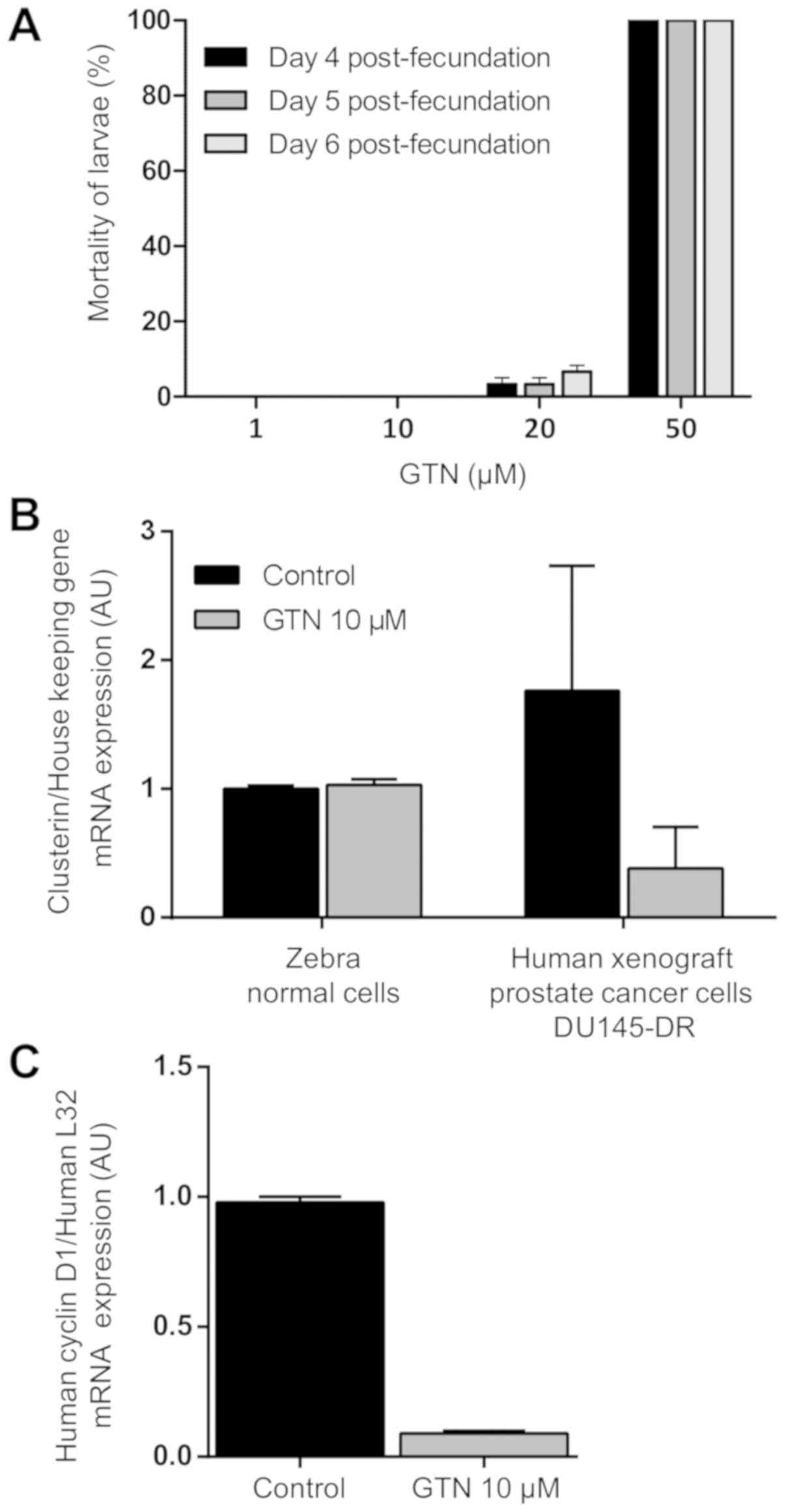
The role of GTN in modulating the level of clusterin
expression in human prostatic cancer cells in vivo was then
investigated by experimental approaches in zebrafish. The tolerance
of zebrafish larvae (4-6 days post-fertilization) to GTN was tested
and mortality rate was recorded. All larvae treated with 1 and 10
µM GTN during the 4, 5 and 6 days post-fertilization
exposure sets survived. For the larvae exposed to 20 µM GTN,
the mortality rates were 3.3% in the 4 and 5 days
post-fertilization and 6.6% in the 6 days post-fertilization group.
All zebrafish larvae exposed to 50 µM died in each stage of
larval development tested (Fig.
4A). The maximum tolerated dose for GTN selected for further
studies was 10 µM, corresponding to 100% zebrafish larvae
survival. A DU145-DR xenograft model in zebrafish was then
generated, and the effects of GTN on the level of clusterin
transcription were assessed in vivo. Following GTN
treatment, it was observed that the level of zebrafish clusterin
gene expression remains unchanged compared with controls, whereas
the level of human clusterin gene expression in human tumor cells
was reduced (Fig. 4B). The level
of expression of human cyclin D1 upon GTN treatment was also
measured. Following 5 days post-xenotransplantation exposure of
GTN, the level of expression of cyclin D1 gene (key cell cycle
regulator) was decreased (Fig.
4C), which suggests a reduction in the amount of clusterin in
DU145-DR cells and the inhibition of proliferation status of these
tumor cells in vivo.
GTN and GDF-15 modulate the level of
CLU
As cytokines such as TGF-β can regulate the level of
expression of CLU (44), the
presence of cytokines in the docetaxel-resistant and sensitive
cellular model (using a cytokine array, data not shown) was
investigated to better delineate the role of CLU in cancer cell
sensitivity to GTN. A signal was detectable for GDF-15, a member of
TGF-β family, that lead us to evaluate the impact of GTN on GDF-15
regulation. Following GTN exposure, the intracellular form of
GDF-15 (pro-GDF-15, a precursor form of GDF-15) is differentially
regulated between sensitive and docetaxel-resistant prostate cancer
cells. It is significantly increased in docetaxel-resistant cells
with the lower concentration of GTN (100 µM) compared with
control, but not in parental cells (Fig. 5A). DU145 and DU145-DR cells were
not used here due to an undetectable level of the intracellular
form of GDF-15 (data not shown).
A GDF-15 recombinant protein was used to elucidate
whether it could affect the level of expression of sCLU and nCLU in
PC3 AG and PC3 D12 cell lines. Cells were treated with recombinant
GDF-15 or GTN or the combination of both. Notably, GDF-15 induces
sCLU and nCLU levels of expression mainly in PC3 D12
docetaxel-resistant cells. However, when GTN is added to GDF-15
treatment, the level of sCLU is completely reduced and the level of
nCLU is increased compared with GDF-15 treatment alone (Fig. 5B). Meanwhile, the effect of GTN,
GDF-15 and GTN/GDF-15 was tested on the viability of PC3 AG and PC3
D12 cells. Although no significant effect was observed on PC3 AG
viability under these conditions, recombinant GDF-15 along with GTN
in docetaxel-resistant PC3 D12 cells significantly abrogates the
GTN-induced decrease in viability in these cells (Fig. 5C). This effect is in accordance
with a lower amount of nCLU in comparison with GTN treatment alone
(Fig. 5B). Conversely, although
recombinant GDF-15 treatment resulted in an increase in nCLU
(pro-apoptotic) in PC3 cell lines, GDF-15 treatment did not result
in an increase in cytotoxicity. Due to its various biological
functions and role in other signaling pathways, its cytotoxicity
could have been counteracted.
Together, these results demonstrate that GTN
promotes a differential regulation of the level of sCLU and nCLU in
favor of a cytotoxic effect in docetaxel-resistant PC3 D12 cells
which appears to be associated with the level of GDF-15.
Discussion
In an attempt to overcome resistance to
docetaxel-based therapy in mCRPC, the effect of the NO donor GTN
was studied in docetaxel-resistant human prostatic cancer cells
(PC3-D12 and DU145-DR) in comparison with the docetaxel-sensitive
parental counterpart. GTN was revealed to be more effective in
inhibiting cell viability in docetaxel-resistant cells than in
docetaxel-sensitive cells. It was observed that GTN regulates the
level of expression of two known markers of resistance in mCRPC,
CLU and GDF-15. More precisely, a differential regulation of
soluble and nuclear CLU isoforms in docetaxel sensitive and
resistant prostate cancer cells by GTN was observed. Also, GTN
modulated CLU isoforms in favor of a cytotoxic effect associated
with increased nCLU pro-death isoform and decreased sCLU
cytoprotective isoform in docetaxel resistant cells. Notably,
targeting CLU expression by siRNA abrogated the ability of GTN to
sensitize docetaxel-resistant cells. Altogether an association
between the levels of nCLU expression and GTN-induced cytotoxicity
was demonstrated.
These findings support the hypothesis that the
response of docetaxel-resistant mCRPC cells to GTN is governed by
the balance between the pro-death and cytoprotective isoforms of
the CLU protein. These findings suggest that docetaxel resistance
is mediated to a certain extent by sCLU and that GTN can overcome
docetaxel resistance. CLU is a key contributor in mediating
survival in chemoresistant cancer cells. A number of studies have
demonstrated the cytoprotective role of sCLU against docetaxel in
prostate cancers and multiple chemotherapeutic agents in a wide
range of late-stage tumors (45,46).
Mechanistically, a previous report highlighted the importance of
protein kinase B (Akt) pathway activation, responsible for sCLU
induction in docetaxel resistance in prostate cancer cells
(15). Notably, an inhibitory
effect of NO donors S-Nitroso-N-Acetyl-D,L-Penicillamine and
S-nitrosoglutathion on Akt pathway activation through
S-nitrosylation of the kinase at cysteine 224 has been described
(47). Notably, the present study
demonstrated in vivo, in a xenograft zebrafish model, the
ability of GTN to specifically regulate the level of human
clusterin expression in cancer cells and most likely the growth
in vivo of xenografted human DU145-DR cells. The use of
xenograft human prostate cancer cell models have already been
revealed to be a useful tool to develop functional cancer models
using PC3 cells (42) or to
evaluate docetaxel treatment in DU145-xenografted zebrafish
(48).
Altogether these findings suggest that GTN could act
as a negative transcriptional regulator of sCLU, but the exact
mechanism remains unclear. Given that the Akt pathway is commonly
activated in several human cancers it is reasonable to speculate
that NO donors may be exploited for counteracting sCLU-dependent
docetaxel resistance. Furthermore, Zhou et al (16) demonstrated that acquired resistance
to docetaxel in prostate cancer cells was linked to sCLU induction
triggered by High Mobility Group Box 1 (HMGB1) produced from dying
cells in a HMGB1/Toll Like Receptor 4 - Receptor for Advanced
Glycation End-products/sCLU pathway. This pathway involves the
activation of the transcription factor NF-κB to promote sCLU gene
expression. Notably, it has been demonstrated that NF-κB activity
can be inhibited by S-nitrosylation of critical thiol in both p65
and p50 subunits (49,50).
The inhibition of sCLU in prostate cancer and its
impact on the cytotoxic effect of various chemotherapeutic agents
has been extensively investigated in preclinical studies and
provided rationale for the use of CLU antisense inhibitor OGX011
(or custirsen) as a therapeutic target (51-55).
Following encouraging results of phase II clinical studies, two
phase III clinical trials for custirsen in combination with
docetaxel or cabazitaxel and prednisone for patients with mCRPC but
no survival benefit was reported in patients treated with the
combination compared with patients treated with chemotherapeutic
agents alone (56,57). The disappointing result from phase
III clinical trials may suggest that a therapeutic strategy
directed against sCLU alone is not sufficient and that a
therapeutic strategy that would module the two isoforms (sCLU and
nCLU) in favor of an antitumoral response may present an
interesting strategy.
The nuclear sub-localization of CLU (nCLU) was
demonstrated to promote pro-apoptotic signaling in many cells. Kim
et al (58) demonstrated
that nCLU mediates apoptosis by sequestering BCL-XL, thereby
releasing Bax which triggers mitochondria cytochrome c
release and activation of caspase-3. The current study demonstrates
that GTN treatment enhances the level of nCLU which is correlated
with GTN-induced cytotoxicity. The present study is, to the best of
our knowledge, the first report on GDF-15-mediated regulation of
CLU, and the findings suggest an implication of GDF-15 in the
regulatory mechanism of nCLU by GTN. The regulation of CLU by other
cytokines, including TGF-β1 and interleukin 24, has also been
reported, but the mechanism of action remains largely unknown
(59,60).
A number of preclinical and clinical studies have
demonstrated the role of GTN as an anti-cancer agent for prostate
cancer. A phase II study of GTN in patients with prostate cancer
has revealed an inhibitory effect on prostate-specific antigen
progression following primary treatment failure (surgery or
radiotherapy) (61). Notably, it
was demonstrated that GTN attenuates hypoxia-induced hypoxic cells
and enhances the in vitro (hypoxic PC3 cells) and in
vivo antitumor effect of doxorubicin in mice bearing PC3
prostate tumor xenograft model (31). Furthermore, the NO donor
DETANONOate was demonstrated to sensitize prostate cancer cells to
cisplatin both in vitro and in vivo (32). Therefore, novel approaches for
combination therapy strategies (chemotherapy/NO donor) are required
for the effective treatment of prostate cancer.
Recently, transcriptomic signatures associated with
docetaxel-resistant mCRPC cells were analyzed with the goal of
identifying putative new therapeutic target to overcome docetaxel
resistance. RNA sequencing analysis revealed upregulation of genes
associated with cancer stem cells-like characteristics (62). Further studies would be required to
determine whether NO could directly or indirectly affect these
targets and molecular pathways to overcome docetaxel
resistance.
Taken together, the present results demonstrate that
NO donors, such as GTN, may be an interesting therapeutic agent to
disrupt the resistant pathways mediated by docetaxel. NO can
interfere with multiple signaling pathways involved in resistance
to drugs and many NO donors have been demonstrated to sensitize
cancer cells to various chemotherapeutic agents (63). In accordance with the literature,
the present data support a key role of CLU in cancer
chemoresistance with sCLU being overexpressed following intensive
exposure of docetaxel. Thus, as GTN differentially modulates the
two CLU isoforms (sCLU and nCLU), it makes GTN a promising
therapeutic agent to combine with other chemotherapies.
Funding
This project was supported by ‘Région Bourgogne’. SB
is a recipient of a PhD fellowship from Ligue Contre le Cancer.
Availability of data and materials
The dataset generated from cytokine arrays are not
publicly available to fully exploit data for future research
project and publication. All other data generated or analyzed
during this study are included in this article.
Authors' contributions
SB and LM carried out the experiments and
contributed to the interpretation of the results. LD carried out
experiments with support from LM. SB and LM designed the figures.
SR was involved in the establisment of docetaxel-resistant cells.
SB wrote the material and methods section. VL conceived, designed,
supervised the study and analysed data. JFJ contributed to the
elaboration of the project with NI underlining clinical challenges.
SP partially co-supervised the work and took the lead in writing
the manuscript with support from VL. AB contributed to the
interpretation of the results, proposed experiments and reviewed
the manuscript. All authors provided critical feedback and helped
shape the research, analysis and manuscript.
Ethics approval and consent to
participate
The animal procedures described in the present study
were reviewed and approved by the local Ethics Committee of the
University of Burgundy.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors thank Professor B. Watson (University
College Dublin, Dublin, Ireland) for the generous gift of PC3 AG
and PC3 D12 cells and Professor J. Chluba (Université de Bourgogne
Franche-Comté, Dijon, France) who kindly provided access to the
zebrafish core facility.
References
|
1
|
Wong MCS, Hamilton W, Whiteman DC, Jiang
JY, Qiao Y, Fung FD, Wang HH, Chiu PW, Ng EK, Wu JC, et al: Global
incidence and mortality of oesophageal cancer and their correlation
with socioeconomic indicators temporal patterns and trends in 41
countries. Sci Rep. 8:45222018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hurwitz M: Chemotherapy in prostate
cancer. Curr Oncol Rep. 17:442015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGrogan BT, Gilmartin B, Carney DN and
McCann A: Taxanes, microtubules and chemoresistant breast cancer.
Biochim Biophys Acta. 1785:96–132. 2008.
|
|
4
|
Zhu ML, Horbinski CM, Garzotto M, Qian DZ,
Beer TM and Kyprianou N: Tubulin-targeting chemotherapy impairs
androgen receptor activity in prostate cancer. Cancer Res.
70:7992–8002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Darshan MS, Loftus MS, Thadani-Mulero M,
Levy BP, Escuin D, Zhou XK, Gjyrezi A, Chanel-Vos C, Shen R, Tagawa
ST, et al: Taxane-induced blockade to nuclear accumulation of the
androgen receptor predicts clinical responses in metastatic
prostate cancer. Cancer Res. 71:6019–6029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotte SJ: Addressing taxane resistance in
metastatic castration-resistant prostate cancer: A focus on
chaperone proteins. Future Oncol. 13:369–379. 2017. View Article : Google Scholar
|
|
7
|
Magadoux L, Isambert N, Plenchette S,
Jeannin JF and Laurens V: Emerging targets to monitor and overcome
docetaxel resistance in castration resistant prostate cancer
(Review). Int J Oncol. 45:919–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galletti G, Leach BI, Lam L and Tagawa ST:
Mechanisms of resistance to systemic therapy in metastatic
castration-resistant prostate cancer. Cancer Treat Rev. 57:16–27.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao YS, Qiu WS, Yao RY, Zhang Q, Zhuang
LK, Zhou F, Sun LB and Yue L: miR-141 confers docetaxel
chemoresistance of breast cancer cells via regulation of EIF4E
expression. Oncol Rep. 33:2504–2512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zu S, Ma W, Xiao P, Cui Y, Ma T, Zhou C
and Zhang H: Evaluation of docetaxel-sensitive and
docetaxel-resistant proteomes in PC-3 cells. Urol Int. 95:114–119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyake H, Muramaki M, Furukawa J,
Kurahashi T and Fujisawa M: Serum level of clusterin and its
density in men with prostate cancer as novel biomarkers reflecting
disease extension. Urology. 75:454–459. 2010. View Article : Google Scholar
|
|
12
|
Zoubeidi A, Chi K and Gleave M: Targeting
the cytoprotective chaperone, clusterin, for treatment of advanced
cancer. Clin Cancer Res. 16:1088–1093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trougakos IP, Djeu JY, Gonos ES and
Boothman DA: Advances and challenges in basic and translational
research on clusterin. Cancer Res. 69:403–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rohne P, Prochnow H, Wolf S, Renner B and
Koch-Brandt C: The chaperone activity of clusterin is dependent on
glycosylation and redox environment. Cell Physiol Biochem.
34:1626–1639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong B, Sallman DA, Gilvary DL, Pernazza
D, Sahakian E, Fritz D, Cheng JQ, Trougakos I, Wei S and Djeu JY:
Induction of clusterin by AKT - role in cytoprotection against
docetaxel in prostate tumor cells. Mol Cancer Ther. 9:1831–1841.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Chen X, Gilvary DL, Tejera MM,
Eksioglu EA, Wei S and Djeu JY: HMGB1 induction of clusterin
creates a chemoresistant niche in human prostate tumor cells. Sci
Rep. 5:150852015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muhammad LA and Saad F: The role of
clusterin in prostate cancer: Treatment resistance and potential as
a therapeutic target. Expert Rev Anticancer Ther. 15:1049–1061.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tellez T, Garcia-Aranda M and Redondo M:
The role of clusterin in carcinogenesis and its potential utility
as therapeutic target. Curr Med Chem. 23:4297–4308. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lamoureux F, Thomas C, Yin MJ, Kuruma H,
Beraldi E, Fazli L, Zoubeidi A and Gleave ME: Clusterin inhibition
using OGX-011 synergistically enhances Hsp90 inhibitor activity by
suppressing the heat shock response in castrate-resistant prostate
cancer. Cancer Res. 71:5838–5849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higano CS: Potential use of custirsen to
treat prostate cancer. OncoTargets Ther. 6:785–797. 2013.
View Article : Google Scholar
|
|
21
|
Chi KN, Hotte SJ, Yu EY, Tu D, Eigl BJ,
Tannock I, Saad F, North S, Powers J, Gleave ME, et al: Randomized
phase II study of docetaxel and prednisone with or without OGX-011
in patients with metastatic castration-resistant prostate cancer. J
Clin Oncol. 28:4247–4254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saad F, Hotte S, North S, Eigl B, Chi K,
Czaykowski P, Wood L, Pollak M, Berry S, Lattouf JB, et al Canadian
Uro-Oncology Group: Randomized phase II trial of Custirsen
(OGX-011) in combination with docetaxel or mitoxantrone as
second-line therapy in patients with metastatic castrate-resistant
prostate cancer progressing after first-line docetaxel: CUOG trial
P-06c. Clin Cancer Res. 17:5765–5773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blumenstein B, Saad F, Hotte S, Chi KN,
Eigl B, Gleave M and Jacobs C: Reduction in serum clusterin is a
potential therapeutic biomarker in patients with
castration-resistant prostate cancer treated with custirsen. Cancer
Med. 2:468–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moretti RM, Mai S, Montagnani Marelli M,
Rizzi F, Bettuzzi S and Limonta P: Molecular mechanisms of the
antimetastatic activity of nuclear clusterin in prostate cancer
cells. Int J Oncol. 39:225–234. 2011.PubMed/NCBI
|
|
25
|
Scaltriti M, Santamaria A, Paciucci R and
Bettuzzi S: Intracellular clusterin induces G2-M phase arrest and
cell death in PC-3 prostate cancer cells. Cancer Res. 64:6174–6182.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prochnow H, Gollan R, Rohne P, Hassemer M,
Koch-Brandt C and Baiersdörfer M: Non-secreted clusterin isoforms
are translated in rare amounts from distinct human mRNA variants
and do not affect Bax-mediated apoptosis or the NF-κB signaling
pathway. PLoS One. 8:e753032013. View Article : Google Scholar
|
|
27
|
Huang CY, Beer TM, Higano CS, True LD,
Vessella R, Lange PH, Garzotto M and Nelson PS: Molecular
alterations in prostate carcinomas that associate with in vivo
exposure to chemotherapy: Identification of a cytoprotective
mechanism involving growth differentiation factor 15. Clin Cancer
Res. 13:5825–5833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao L, Lee BY, Brown DA, Molloy MP, Marx
GM, Pavlakis N, Boyer MJ, Stockler MR, Kaplan W, Breit SN, et al:
Identification of candidate biomarkers of therapeutic response to
docetaxel by proteomic profiling. Cancer Res. 69:7696–7703. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shim M and Eling TE: Protein kinase
C-dependent regulation of NAG-1/placental bone morphogenic
protein/MIC-1 expression in LNCaP prostate carcinoma cells. J Biol
Chem. 280:18636–18642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patel SA and Hoffman-Censits J:
Cabazitaxel in the treatment of metastatic castration-resistant
prostate cancer: Patient selection and special considerations.
OncoTargets Ther. 10:4089–4098. 2017. View Article : Google Scholar
|
|
31
|
Frederiksen LJ, Sullivan R, Maxwell LR,
Macdonald-Goodfellow SK, Adams MA, Bennett BM, Siemens DR and
Graham CH: Chemosensitization of cancer in vitro and in vivo by
nitric oxide signaling. Clin Cancer Res. 13:2199–2206. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huerta-Yepez S, Baritaki S, Baay-Guzman G,
Hernandez-Luna MA, Hernandez-Cueto A, Vega MI and Bonavida B:
Contribution of either YY1 or BclXL-induced inhibition by the
NO-donor DETANONOate in the reversal of drug resistance, both in
vitro and in vivo. YY1 and BclXL are overexpressed in prostate
cancer. Nitric Oxide. 29:17–24. 2013. View Article : Google Scholar
|
|
33
|
Huerta S, Baay-Guzman G, Gonzalez-Bonilla
CR, Livingston EH, Huerta-Yepez S and Bonavida B: In vitro and in
vivo sensitization of SW620 metastatic colon cancer cells to
CDDP-induced apoptosis by the nitric oxide donor DETANONOate:
Involvement of AIF. Nitric Oxide. 20:182–194. 2009. View Article : Google Scholar
|
|
34
|
Gao X, Saha D, Kapur P, Anthony T,
Livingston EH and Huerta S: Radiosensitization of HT-29 cells and
xenografts by the nitric oxide donor DETANONOate. J Surg Oncol.
100:149–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaliyaperumal K, Sharma AK, McDonald DG,
Dhindsa JS, Yount C, Singh AK, Won JS and Singh I:
S-Nitrosoglutathione-mediated STAT3 regulation in efficacy of
radiotherapy and cisplatin therapy in head and neck squamous cell
carcinoma. Redox Biol. 6:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Selvendiran K, Bratasz A, Tong L, Ignarro
LJ and Kuppusamy P: NCX-4016, a nitro-derivative of aspirin,
inhibits EGFR and STAT3 signaling and modulates Bcl-2 proteins in
cisplatin-resistant human ovarian cancer cells and xenografts. Cell
Cycle. 7:81–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huerta-Yepez S, Vega M, Escoto-Chavez SE,
Murdock B, Sakai T, Baritaki S and Bonavida B: Nitric oxide
sensitizes tumor cells to TRAIL-induced apoptosis via inhibition of
the DR5 transcription repressor Yin Yang 1. Nitric Oxide. 20:39–52.
2009. View Article : Google Scholar
|
|
38
|
Bonavida B and Baritaki S: Inhibition of
epithelial-to-mesen-chymal transition (EMT) in cancer by nitric
oxide: Pivotal roles of nitrosylation of NF-κB, YY1 and Snail. For
Immunopathol Dis Therap. 3:125–133. 2012. View Article : Google Scholar
|
|
39
|
Baritaki S, Huerta-Yepez S, Sahakyan A,
Karagiannides I, Bakirtzi K, Jazirehi A and Bonavida B: Mechanisms
of nitric oxide-mediated inhibition of EMT in cancer: Inhibition of
the metastasis-inducer Snail and induction of the
metastasis-suppressor RKIP. Cell Cycle. 9:4931–4940. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Girard FP, Byrne J, Downes M, Fanning D,
Desgrandchamps F, Fitzpatrick JM and Watson RW: Detecting soluble
clusterin in in-vitro and in-vivo models of prostate cancer.
Neoplasma. 57:488–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yousfi N, Pruvot B, Lopez T, Magadoux L,
Franche N, Pichon L, Salvadori F, Solary E, Garrido C, Laurens V,
et al: The impact of tumor nitric oxide production on VEGFA
expression and tumor growth in a zebrafish rat glioma xenograft
model. PLoS One. 10:e01204352015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu W, Foster BA, Richards M, Bondioli KR,
Shah G and Green CC: Characterization of prostate cancer cell
progression in zebrafish xenograft model. Int J Oncol. 52:252–260.
2018.
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
44
|
Shiota M, Zardan A, Takeuchi A, Kumano M,
Beraldi E, Naito S, Zoubeidi A and Gleave ME: Clusterin mediates
TGF-β-induced epithelial-mesenchymal transition and metastasis via
Twist1 in prostate cancer cells. Cancer Res. 72:5261–5272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lourda M, Trougakos IP and Gonos ES:
Development of resistance to chemotherapeutic drugs in human
osteosarcoma cell lines largely depends on up-regulation of
clusterin/apolipo-protein J. Int J Cancer. 120:611–622. 2007.
View Article : Google Scholar
|
|
46
|
Zhang H, Kim JK, Edwards CA, Xu Z,
Taichman R and Wang CY: Clusterin inhibits apoptosis by interacting
with activated Bax. Nat Cell Biol. 7:909–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yasukawa T, Tokunaga E, Ota H, Sugita H,
Martyn JA and Kaneki M: S-nitrosylation-dependent inactivation of
Akt/protein kinase B in insulin resistance. J Biol Chem.
280:7511–7518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang B, Shimada Y, Kuroyanagi J,
Nishimura Y, Umemoto N, Nomoto T, Shintou T, Miyazaki T and Tanaka
T: Zebrafish xenotransplantation model for cancer stem-like cell
study and high-throughput screening of inhibitors. Tumour Biol.
35:11861–11869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kelleher ZT, Potts EN, Brahmajothi MV,
Foster MW, Auten RL, Foster WM and Marshall HE: NOS2 regulation of
LPS-induced airway inflammation via S-nitrosylation of NF-{kappa}B
p65. Am J Physiol Lung Cell Mol Physiol. 301:L327–L333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Marshall HE and Stamler JS: Inhibition of
NF-kappa B by S-nitrosylation. Biochemistry. 40:1688–1693. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wilson MR and Zoubeidi A: Clusterin as a
therapeutic target. Expert Opin Ther Targets. 21:201–213. 2017.
View Article : Google Scholar
|
|
52
|
Chun YJ: Knockdown of clusterin expression
increases the in vitro sensitivity of human prostate cancer cells
to paclitaxel. J Toxicol Environ Health A. 77:1443–1450. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
García-Aranda M, Téllez T, Muñoz M and
Redondo M: Clusterin inhibition mediates sensitivity to
chemotherapy and radiotherapy in human cancer. Anticancer Drugs.
28:702–716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Trougakos IP, So A, Jansen B, Gleave ME
and Gonos ES: Silencing expression of the clusterin/apolipoprotein
j gene in human cancer cells using small interfering RNA induces
spontaneous apoptosis, reduced growth ability, and cell
sensitization to genotoxic and oxidative stress. Cancer Res.
64:1834–1842. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Al Nakouzi N, Wang CK, Beraldi E, Jager W,
Ettinger S, Fazli L, Nappi L, Bishop J, Zhang F, Chauchereau A, et
al: Clusterin knockdown sensitizes prostate cancer cells to taxane
by modulating mitosis. EMBO Mol Med. 8:761–778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Beer TM, Hotte SJ, Saad F, Alekseev B,
Matveev V, Fléchon A, Gravis G, Joly F, Chi KN, Malik Z, et al:
Custirsen (OGX-011) combined with cabazitaxel and prednisone versus
cabazitaxel and prednisone alone in patients with metastatic
castration-resistant prostate cancer previously treated with
docetaxel (AFFINITY): A randomised, open-label, international,
phase 3 trial. Lancet Oncol. 18:1532–1542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chi KN, Higano CS, Blumenstein B, Ferrero
JM, Reeves J, Feyerabend S, Gravis G, Merseburger AS, Stenzl A,
Bergman AM, et al: Custirsen in combination with docetaxel and
prednisone for patients with metastatic castration-resistant
prostate cancer (SYNERGY trial): A phase 3, multicentre,
open-label, randomised trial. Lancet Oncol. 18:473–485. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim N, Yoo JC, Han JY, Hwang EM, Kim YS,
Jeong EY, Sun CH, Yi GS, Roh GS, Kim HJ, et al: Human nuclear
clusterin mediates apoptosis by interacting with Bcl-XL through
C-terminal coiled coil domain. J Cell Physiol. 227:1157–1167. 2012.
View Article : Google Scholar
|
|
59
|
Peix L, Evans IC, Pearce DR, Simpson JK,
Maher TM and McAnulty RJ: Diverse functions of clusterin promote
and protect against the development of pulmonary fibrosis. Sci Rep.
8:19062018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bhutia SK, Das SK, Kegelman TP, Azab B,
Dash R, Su ZZ, Wang XY, Rizzi F, Bettuzzi S, Lee SG, et al:
mda-7/IL-24 differentially regulates soluble and nuclear clusterin
in prostate cancer. J Cell Physiol. 227:1805–1813. 2012. View Article : Google Scholar
|
|
61
|
Siemens DR, Heaton JP, Adams MA, Kawakami
J and Graham CH: Phase II study of nitric oxide donor for men with
increasing prostate-specific antigen level after surgery or
radiotherapy for prostate cancer. Urology. 74:878–883. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cajigas-Du Ross CK, Martinez SR,
Woods-Burnham L, Durán AM, Roy S, Basu A, Ramirez JA,
Ortiz-Hernández GL, Ríos-Colón L, Chirshev E, et al: RNA sequencing
reveals upreg-ulation of a transcriptomic program associated with
stemness in metastatic prostate cancer cells selected for taxane
resistance. Oncotarget. 9:30363–30384. 2018.PubMed/NCBI
|
|
63
|
Plenchette S, Romagny S, Laurens V and
Bettaieb A: NO and cancer: Itinerary of a double agent. Med Sci
(Paris). 32:625–633. 2016.In French. View Article : Google Scholar
|