Introduction
Colorectal cancer (CRC) is the third most common
type of cancer in western countries and the third most common cause
of cancer-related mortality (1).
The median overall survival (OS) of patients with previously
untreated with unresectable advanced CRC ranges from 25 to 30
months, when combining molecular targeted therapies and
chemotherapy (2).
Standard first-line therapy is doublet or
triplet-chemotherapy combined with targeting agents, including
either the monoclonal antibody, bevacizumab, that inhibits
angiogenesis through vascular endothelial growth factor (VEGF)-A or
the monoclonal antibodies, cetuximab and panitumumab, which inhibit
the epidermal growth factor receptor (EGFR) pathway (3-7); the
latter option is restricted to approximately half the patients
harboring wild-type RAS in their tumor (8). Oxaliplatin combined with 5-FU
(FOLFOX) is one of most commonly used first-line treatment
combinations (9). This regimen is
optimized with the oxaliplatin stop-and-go strategy (OPTIMOX),
which consists of 6 cycles as induction therapy followed by
maintenance with fluoropyrimidine without oxaliplatin and later, at
progression, reintroduction of the full regimen. Maintenance
therapy reduces the frequency and severity of the cumulative
neuropathy observed with oxaliplatin (10). Bevacizumab with fluoropyrimidine is
considered as a standard for maintenance therapy (11).
Aflibercept is a recombinant fusion protein
consisting of the extracellular domains VEGFR1 and VEGFR2 fused to
the Fc portion of human immunoglobulin G1. Aflibercept binds VEGF-A
and VEGF-B with high affinity (Kd <1 pM) and placental growth
factor (PlGF) with lower affinity (Kd 39 pM), leading to the
blockade of tumor angiogenesis and vascular permeability. The
combination of aflibercept to the standard FOLFIRI regimen in
patients with metastatic CRC (mCRC) has been shown to improve OS
[primary endpoint, 12.1-13.5 months; hazard ratio (HR), 0.82;
P=0.003], progression-free survival (PFS, 4.7-6.9 months; HR, 0.76;
P<0.001), and the objective response rate (ORR, 11.1-19.8%;
P<0.001) (12). This effect was
observed whether or not patients had received prior bevacizumab
therapy.
The aim of this study was to evaluate the efficacy
and safety of the aflibercept and an oxaliplatin-based
chemotherapeutic regimen combination in first-line therapy in order
to determine whether aflibercept has the potential to challenge
bevacizumab in the first-line treatment of mCRC.
Patients and methods
Study population
The main patient inclusion criteria were as follows:
an age ≥18 years, an Eastern Cooperative Oncology Group performance
status (ECOG PS) of 0 to 2, histologically or cytologically
confirmed unresectable mCRC and no prior treatment for metastatic
disease.
Study design and treatment schedule
This was a prospective, single-arm, multicenter
phase II study. All patients provided written inform consent before
enrollment. The study was carried out in accordance with the
declaration of Helsinki and Good Clinical Practice guidelines. This
study was approved by the Ethics Committee (CPP Ile de France VI
Groupe Hospitalier Pitié Salpêtrière PARIS) of our institution.
Patients received intravenously modified FOLFOX7
with aflibercept as induction therapy every 2 weeks for 6 cycles as
follows: Aflibercept 4 mg/kg, oxaliplatin 100 mg/m2,
folinic acid 400 mg/m2 and 5-FU 3,000 mg/m2.
In patients without progression or non-amenable to surgery,
induction therapy was followed by maintenance therapy with
aflibercept and fluoropyrimidine (either 5-FU or capecitabine)
until disease progression or limiting toxicity. Dose postponements
or reductions were permitted to manage treatment-related adverse
events.
Endpoints
The primary endpoint was PFS, defined as the time
from the date of inclusion to the date of progression or death
(from any cause). Patients alive without documented objective
progressive disease (PD) at the time of the final analysis were
censored at the date of their final objective tumor assessment. OS
was defined as the time from the date of inclusion to the date of
patient death (from any cause) or to the last date the patient was
known to be alive. Patients still alive at the time of the analysis
were censored using the date of final news. The duration of disease
control (DDC) was defined as the sum of PFS of each active
treatment course (13).
The ORR was defined as the proportion of patients
having either complete response (CR) or partial response (PR)
according to RECIST version 1.1 (14). The optimal ORR was defined as the
optimal response recorded from the beginning of treatment until
treatment failure, taking as reference for PD the smallest
measurements recorded since the beginning of treatment. The early
response rate was evaluated at the first disease evaluation (i.e.,
2 months). The disease control rate (DCR) was defined as the
percentage of patients who achieved CR, PR, or stable disease
(SD).
The reintroduction rate was defined as the number of
patients who received reintroduction of oxaliplatin after disease
progression during aflibercept-based maintenance therapy. The
absolute reintroduction rate was calculated for all included
patients and the relative reintroduction rate was calculated for
patients eligible to reintroduction, excluding patients having
progressed during induction therapy, amenable to surgery or having
a residual sensory neuropathy grade >1. The curative surgery
rate was assessed globally and per sequence of therapy.
Toxicity was evaluated according to the US National
Cancer Institute's Common Terminology Criteria for Adverse Events
(NCI CTCAE) version 4.03 (15).
Health-related quality of life (HRQoL) assessments were performed
at baseline, and every 2 months thereafter, using the Quality of
Life Questionnaire Core 30 (QLQ-C30) (French version) (16). The survival prognosis was assessed
through the GERCOR prognostic model (17), using two-baseline (pre-treatment)
parameters: ECOG PS and serum lactate dehydrogenase levels.
Sample size
According to Simon's Minimax two-stage design
(18) with a two-sided 5% type I
error, a power of 80%, and a 15% improvement in PFS rate at 6-month
from 70% (H0, considered as uninteresting to pursue any further
investigation) to 85% (H1, considered as promising to warrant
further investigation), it was required that we enroll 49 patients,
including a 5% drop-out. If >16 patients were free of
progression or death at 6 months from inclusion among the first 23
evaluable patients (stage 1), the trial could be pursued to the
second stage with further 26 patients. If at least 40 patients were
free of progression or death among the 49 included patients (stage
2), treatment could be considered as promising for further
evaluation.
Statistical analysis
The primary analysis of efficacy used the
intent-to-treat (ITT) population, i.e., including all recruited
patients regardless of their eligibility. The confirmative analysis
was conducted in the ITT population of eligible patients and in the
per-protocol (PP) population comprising all patients who have
received at least 2 cycles of the allocated treatment and without
any major protocol deviations. The safety analysis included all
patients who received at least one dose of any study drug.
Follow-up and survival were estimated using the reverse
Kaplan-Meier method (19) and
Kaplan-Meier method (20),
respectively, and were described using median with 95% confidence
interval (CI). A linear mixed effects model (repeated measures of
variance) was used as to analyze longitudinal changes of HRQoL at
baseline, and every 2 months. All patients who completed at least
one baseline HRQoL assessment were included. Qualitative variables
were described using percentage and means (SD), and continuous
variables using medians (minimum-maximum). Fisher's exact test was
used for comparison of proportions. The log-rank test was used to
compare survival curves, and Cox proportional-hazards regression
was used to analyze the effect of several risk factors on survival.
The cut-off date for statistical analysis was December, 2015.
Circulating biomarkers
The plasma concentration of 31 biomarkers (3
panels), including cytokines, growth factors, or soluble receptors
was determined using multiplexing immunoassays on a
Biorad®Bioplex platform. PlGF and neuropillin 1 levels
were determined by enzyme-linked immunosorbent assays (ELISA;
R&D Systems, Minneapolis, MN, USA). The samples and standards
were prepared in duplicate according to the manufacturer's
protocol. Plates were incubated for 2 h, washed 4 times, and
incubated with enzyme-conjugated antibodies for an additional 2 h
at room temperature. The wells were then washed 4 times and
substrate was added for 20 min also at room temperature, in the
dark. Finally, stop solution was added to each well, and the
absorptions at 450 nm were determined using a luminometer plate
reader. Plasma markers were evaluated at baseline, and before each
induction therapy infusion, for a total of 7 time points.
Results
Study conduct
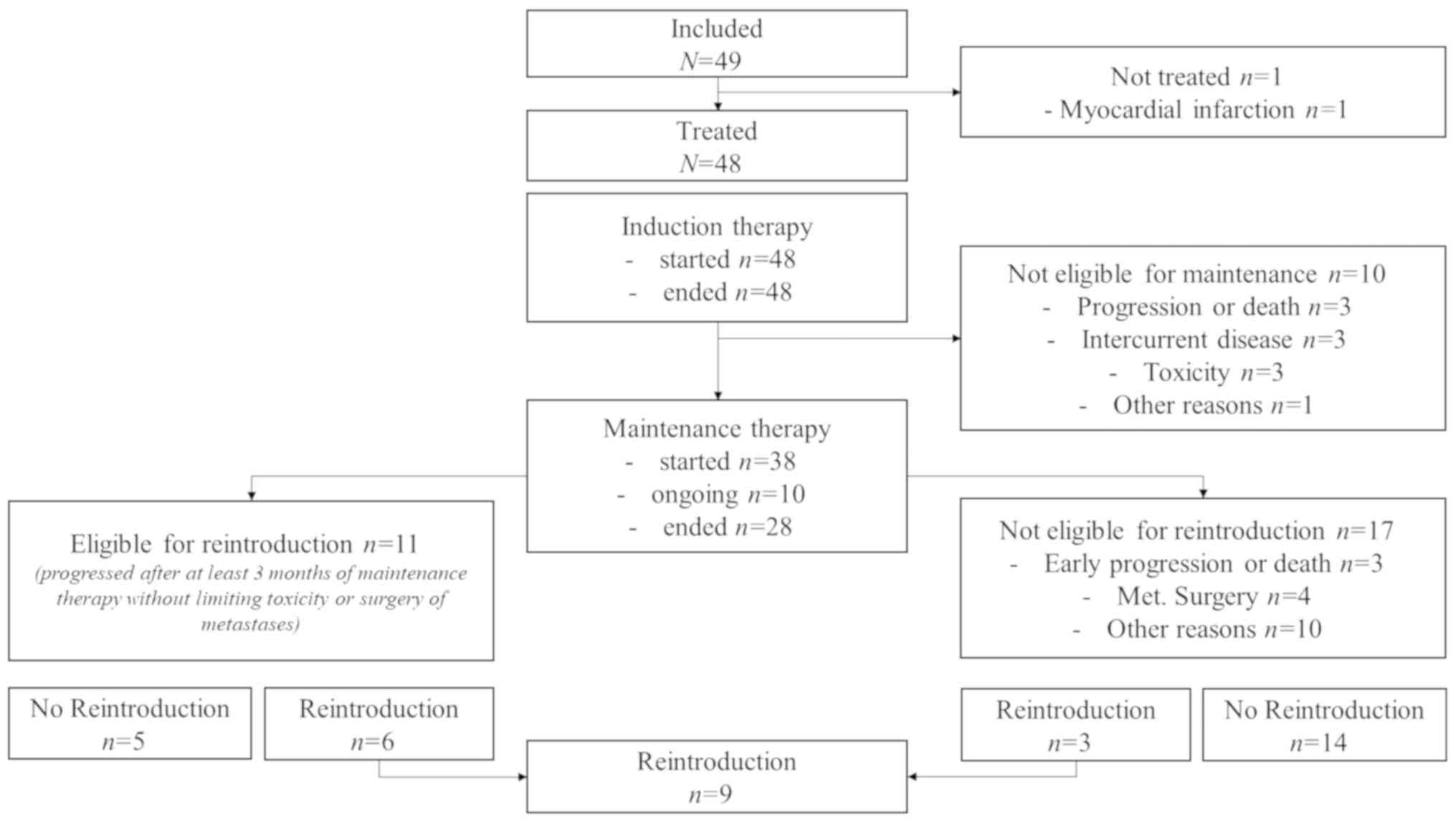
From May, 2013 to May, 2014, 49 patients were
included in 9 French centers (Fig.
1). In total, 23 (46.9%) and 26 (53.1%) patients were included
in the Simon's stage 1 and stage 2, respectively.
Patient characteristics
The patient and tumor baseline characteristics are
presented in Table I. The median
age was 62.9 years, ranging from 32 to 86 years. In total, 20
(40.8%) patients were 70 years or older, 19 (38.8%) had a medical
history of hypertension, and 18 (36.7%) had liver-limited
metastatic disease. According to the GERCOR prognostic model, 13
(26.5%) patients were at high-risk for death at study entry.
 | Table IPatient and tumor baseline
characteristics. |
Table I
Patient and tumor baseline
characteristics.
| Baseline
characteristics | No. of patients
(n=49) | % |
|---|
| Sex |
| Male | 26 | 53.1 |
| Female | 23 | 46.9 |
| Age, years |
| <70 | 30 | 61.2 |
| ≥70 | 19 | 38.8 |
| ECOG performance
status |
| 0 | 23 | 46.9 |
| 1 | 22 | 44.9 |
| 2 | 4 | 8.2 |
| Number of
metastatic organ sites |
| 1 | 26 | 53.1 |
| ≥2 | 23 | 46.9 |
| Metastatic
disease |
| Liver | 37 | 75.5 |
| Lung | 16 | 32.6 |
| Node | 15 | 30.6 |
| Peritoneal | 8 | 16.3 |
| Primary tumor
sidedness |
| Right | 20 | 40.8 |
| Left | 29 | 59.2 |
| Initial disease
stage |
| I-III
(metachronous) | 6 | 12.2 |
| IV
(synchronous) | 43 | 87.8 |
| Prior primary tumor
resection |
| Yes | 20 | 40.8 |
| No | 29 | 59.2 |
| Prior adjuvant
chemotherapy |
| Yes | 5 | 10.2 |
| No | 44 | 89.8 |
| RAS
mutational status |
| Wild-type | 18 | 36.7 |
| Mutated | 27 | 55.1 |
| Unknown | 4 | 8.2 |
| White blood cell
count |
|
<10,000/mm3 | 38 | 77.5 |
|
≥10,000/mm3 | 11 | 22.5 |
| Platelet count |
| ≤1 x ULN | 39 | 79.6 |
| >1 x ULN | 10 | 20.4 |
| Lactate
dehydrogenase level |
| ≤1 x ULN | 19 | 38.8 |
| >1 x ULN | 26 | 53.1 |
| Missing data | 4 | 8.2 |
| Alkaline
phosphatase level |
| ≤1xULN | 31 | 63.3 |
| >1xULN | 18 | 36.7 |
| Carcinoembryonic
antigen level |
| ≤1 x ULN | 10 | 20.4 |
| >1 x ULN | 28 | 57.1 |
| Missing data | 1 | 2.0 |
| GERCOR prognostic
score |
| Low-risk | 8 | 16.3 |
|
Intermediate-risk | 24 | 49.0 |
| High-risk | 13 | 26.5 |
| Missing data | 4 | 8.2 |
Treatment administration
One patient did not receive study treatment due to
myocardial infarction.
Induction therapy
A total of 48 (97.9%) patients received at least one
treatment dose, and 46 (93.8%) received at least 2 cycles of the
full therapy. A total of 268 cycles of induction therapy were
administered with a mean number of 5.6 cycles per patient. In
total, 19/268 (7.1%) cycles were postponed.
Maintenance therapy
Following induction therapy, 10 (20.8%) patients did
not receive the planned maintenance therapy with fluoropyrimidine
and aflibercept due to limiting toxicity (n=4), progression or
death (n=3), or interrupted administration of aflibercept for
>21 days (n=2), or investigator decision (n=1). Among the 38
(79.2%) patients who received maintenance therapy
(fluorouracile-based, n=37; capecitabine-based, n=1), 10 (26.3%)
patients were still on maintenance therapy. A total of 415 cycles
of maintenance therapy were administered, with a mean number of
10.9 cycles per patient. In total, 48/415 (11.6%) cycles were
postponed. The median duration of maintenance therapy was 5.5
months (95% CI, 3.7-9.9).
Reintroduction
At the time of analysis, 11 patients were eligible
for oxaliplatin reintroduction and 6 patients received an
oxaliplatin reintroduction. Three other patients had an unplanned
reintroduction of FOLFOX-aflibercept after surgery of metastasis
(n=2) or an early progression (n=1).
Efficacy
Progression-free survival
At Simon's stage 1 (n=23), 17 (73.9%; 95% CI,
56.0-91.9) patients were alive without disease progression at 6
months. In the ITT population (n=49), 33 (67.4%; 95% CI, 54.2-80.5)
patients were alive without disease progression at 6 months, 12
(24.5%) patients were considered as failure (5 patients had RECIST
progression, 4 patients had clinical progression, and 3 patients
died), and 4 (8.2%) patients were not evaluated for other reasons
(no tumor measure, patient decision, surgery of the primary tumor
and investigator's decision). Following a median follow-up of 22.5
months (95% CI, 20.9-24.5), the median PFS was 9.3 months (95% CI,
8.3-12.5). The 6-month and 1-year PFS rates were 79.1 and 36.1%,
respectively. The median PFS from the beginning of maintenance
therapy (n=38) was 7.4 months (95% CI, 5.9-9.5). Patients with
prior hypertension or high systolic blood pressure (≥140 mmHg) at
study entry had a significantly shorter PFS (HR, 2.37 and 2.61,
respectively) than the other subgroups (Table II).
 | Table IIProgression-free survival in the ITT
population. |
Table II
Progression-free survival in the ITT
population.
| Parameter | No. | Events | Median
(months) | 95% CI | Hazard ratio | 95% CI | P-value |
|---|
| All patients | 49 | 23 | 9.5 | 8.7-12.6 | | | |
| Age (years) | | | | | | | |
| <65 | 25 | 9 | 11.9 | 9.3-12.6 | ref | | |
| ≥65 | 24 | 14 | 8.8 | 7.0-9.9 | 1.86 | 0.82-4.21 | 0.136 |
| Tumor response | | | | | | | |
| CR or PR | 29 | 13 | 9.9 | 8.8-12.6 | ref | | |
| SD or PD | 20 | 10 | 9.5 | 5.0-11.0 | 1.71 | 0.71-4.15 | 0.191 |
| Body mass index
(kg/m2) | | | | | | | |
| <25 | 29 | 12 | 9.5 | 8.7-11.9 | ref | | |
| ≥25 | 20 | 11 | 9.1 | 7.0-11.0 | 1.81 | 0.76-4.29 | 0.148 |
| Systolic blood
pressure (mmHg) | | | | | | | |
| <140 | 34 | 13 | 12.6 | 8.7-12.6 | ref | | |
| ≥140 | 13 | 8 | 8.7 | 5.7-11.0 | 2.61 | 0.87-7.74 | 0.023 |
| Diastolic blood
pressure (mmHg) | | | | | | | |
| <90 | 40 | 18 | 9.1 | 8.3-12.6 | ref | | |
| ≥90 | 7 | 3 | 11.0 | 5.7-11.0 | 0.90 | 0.28-2.93 | 0.866 |
| Prior
hypertension | | | | | | | |
| No | 30 | 10 | 11.9 | 9.3-12.6 | ref | | |
| Yes | 19 | 13 | 8.8 | 6.8-9.9 | 2.37 | 1.00-5.56 | 0.033 |
| Number of
metastatic sites | | | | | | | |
| 1 | 26 | 12 | 9.5 | 8.7-12.6 | ref | | |
| >1 | 23 | 11 | 8.8 | 6.4-9.9 | 1.36 | 0.59-3.15 | 0.455 |
| Liver
involvement | | | | | | | |
| No | 12 | 3 | - | - | ref | | |
| Yes | 37 | 20 | 9.3 | 8.7-12.6 | 2.86 | 1.17-6.97 | 0.074 |
| ECOG PS | | | | | | | |
| 0 | 23 | 10 | 11.0 | 7.6-12.6 | ref | | |
| 1-2 | 26 | 13 | 9.5 | 8.7-11.9 | 1.26 | 0.56-2.86 | 0.562 |
| Sex | | | | | | | |
| Male | 23 | 11 | 9.1 | 7.7-12.6 | ref | | |
| Female | 26 | 12 | 9.5 | 8.3-11.9 | 0.96 | 0.42-2.18 | 0.922 |
| KRAS exon 2
mutation status | | | | | | | |
| Mutated | 25 | 10 | 9.9 | 8.7-11.0 | ref | | |
| Wild-type | 20 | 11 | 9.5 | 7.7-12.6 | 1.12 | 0.48-2.65 | 0.784 |
| Weight (kg) | | | | | | | |
| <70 | 27 | 10 | 9.5 | 8.7-9.5 | ref | | |
| ≥70 | 22 | 13 | 9.9 | 6.4-11.9 | 1.41 | 0.62-3.19 | 0.406 |
Overall survival
At the time of analysis, 26 (53.1%) patients were
alive. The median follow-up was 10.9 months (95% CI, 9.9-12.0). The
median OS was 22.2 months (95% CI, 18.2-24.7). The 6-month and
1-year survival rates were 91.8 and 79.6%, respectively.
Tumor response
A total of 45/49 (91.8%) patients were evaluated,
and 4 (8.2%) patients were not evaluable for tumor response (2
patients with early death, 1 with gastrointestinal perforation, and
1 patient was not treated). The ORR (CR or PR) was observed in 29
(59.2%) of the 49 patients in the ITT population, and in 28 (60.9%)
of the 46 patients in the PP population (Table III).
 | Table IIITumor response in the ITT and PP
populations. |
Table III
Tumor response in the ITT and PP
populations.
| Response | Intent-to-treat
population (n=49), no. (%) | Per protocol
population (n=46), no. (%) |
|---|
| Optimal response
rate | | |
| Complete
response | 2 (4.1) | 2 (4.3) |
| Partial
response | 27 (55.1) | 26 (56.5) |
| Stable
disease | 15 (30.6) | 15 (30.4) |
| Progressive
disease | 1 (2.0) | 1 (2.2) |
| Not evaluable | 4 (8.2) | 2 (4.3) |
| Objective response
rate | 29 (59.2) | 28 (60.9) |
| Disease control
rate | 44 (89.8) | 43 (93.5) |
Salvage surgery
A total of 6 (8.4%) patients had liver surgery
during maintenance therapy for the resection of 2 to 7 lesions per
patient with a maximum tumor size of 15 to 55 mm. The percentage of
necrosis ranged between 50 and 100%. Of the 4 patients who
underwent salvage surgery, 1 patient had a complete pathological
response and 1 patient had <1% viable residual tumor cells. A R0
resection was achieved in 1 patient and R1 in 3 patients.
Safety
The most common (≥10%) treatment-related grade 3-4
adverse events were hypertension (23%), fatigue (15%), neutro-penia
(12%), neuropathy (12%) and stomatitis (10%; Table IV). The majority of events
occurred during induction therapy and decreased following the
termination of oxaliplatin, apart from fatigue and stomatitis.
Severe (grade 3 or 4) hypertension occurred in 11 (22.9%) patients,
mainly during induction therapy (n=10/11, 90.9%), and was reversed
in most cases before maintenance therapy. In total, 26 (54.2%) and
22 (45.8%) patients had treatment-related hypertension grade 0-1
and 2-4, respectively (Table V).
Patients with grade 2-4 hypertension were more frequently women
(P=0.081), had more frequently high systolic blood pressure at
study entry (P=0.001), had a higher number of metastatic sites
involved (P=0.008), and had more treatment-induced proteinuria
(P=0.016). There were 3 (6.1%; 95% CI, -0.6-12.8) treatment-related
deaths due to stroke in the context of hypertension (n=1),
pulmonary embolism (n=1) and neutropenic sepsis (n=1).
 | Table IVA summary of the adverse events by
System Organ Class. |
Table IV
A summary of the adverse events by
System Organ Class.
| NCI CTCAE | Whole
strategya (n=48)
| Induction (n=48)
| Maintenance (n=28)
|
|---|
| Any grade no.
(%) | Grade 3-4 no.
(%) | Any grade no.
(%) | Grade 3-4 no.
(%) | Any grade no.
(%) | Grade 3-4 no.
(%) |
|---|
| Neutrophil count
decreased | 18 (37) | 6 (12) | 18 (37) | 5 (10) | 3 (11) | 1 (4) |
| Platelet count
decreased | 21 (44) | 2 (4) | 19 (40) | 2 (4) | 7 (25) | 0 (0) |
| Anemia | 29 (60) | 1 (2) | 27 (56) | 1 (2) | 11 (39) | 0 (0) |
| Febrile
neutropenia | 1 (6) | 1 (6) | 1 (6) | 1 (6) | 0 (0) | 0 (0) |
| Nausea | 35 (73) | 0 (0) | 32 (67) | 0 (0) | 14 (50) | 0 (0) |
| Vomiting | 20 (42) | 1 (2) | 18 (37) | 1 (2) | 2 (7) | 0 (0) |
| Mucositis oral | 35 (73) | 5 (10) | 29 (60) | 2 (4) | 16 (57) | 3 (11) |
| Diarrhea | 27 (56) | 2 (4) | 23 (48) | 2 (4) | 10 (36) | 0 (0) |
| Peripheral sensory
neuropathy | 43 (90) | 6 (12) | 43 (90) | 4 (8) | 20 (71) | 2 (7) |
| Palmar-plantar
erythrodysesthesia syndrome | 17 (35) | 4 (8) | 11 (23) | 1 (2) | 13 (46) | 4 (14) |
| Alopecia | 11 (23) | 5 (10)b | 7 (15) | 3 (6)b | 7 (25) | 2 (7)b |
| Fatigue | 33 (69) | 7 (15) | 30 (62) | 5 (10) | 15 (31) | 3 (11) |
| Hypertension | 26 (54) | 11 (23) | 26 (54) | 10 (21) | 14 (50) | 2 (7) |
| Venous
thromboembolic event | 1 (2) | 1 (2) | 1 (2) | 1 (2) | 0 (0) | 0 (0) |
| Arterial
thromboembolic event | 2 (4) | 2 (4) | 2 (4) | 2 (4) | 0 (0) | 0 (0) |
| Proteinuria | 17 (35) | 3 (6) | 9 (19) | 1 (2) | 11 (29) | 2 (7) |
| Gastrointestinal
perforation | 2 (4) | 2 (4) | 1 (2) | 1 (2) | 1 (4) | 1 (4) |
| Hemorrhage | 9 (19) | 1 (2) | 5 (10) | 1 (2) | 5 (18) | 0 (0) |
| Fistula | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) |
 | Table VPatient baseline characteristics and
clinical outcomes according to the occurrence of hypertension
during study treatment. |
Table V
Patient baseline characteristics and
clinical outcomes according to the occurrence of hypertension
during study treatment.
| Characteristic | Grade 0-1
hypertension (n=26), no. (%) | Grade 2-4
hypertension (n=22), no. (%) | P-value |
|---|
| Sex | | | |
| Male | 17 (65.4) | 8 (36.4) | 0.081 |
| Female | 9 (34.6) | 14 (63.6) | |
| Age (years) | | | |
| <70 | 15 (57.7) | 14 (63.6) | 0.771 |
| ≥70 | 11 (42.3) | 8 (36.4) | |
| Prior history of
hypertension | | | |
| No | 18 (69.2) | 12 (54.5) | 0.375 |
| Yes | 8 (30.8) | 10 (45.5) | |
| Prior history of
arterial TEE | | | |
| No | 26 (100.0) | 20 (90.0) | 0.205 |
| Yes | 0 (0.0) | 2 (9.1) | |
| Prior history of
venous TEE | | | |
| No | 26 (100.0) | 22 (100.0) | 1.000 |
| Yes | 0 (0.0) | 0 (0.0) | |
| Baseline systolic
blood pressure (mmHg) | | | |
| <120 | 8 (30.8) | 3 (13.6) | 0.001a |
| 120-139 | 15 (57.7) | 7 (31.8) | |
| 140-159 | 2 (7.7) | 9 (40.9) | |
| >160 | 0 (0.0) | 2 (9.1) | |
| Missing | 1 (3.8) | 1 (4.5) | |
| Baseline diastolic
blood pressure (mmHg) | | | |
| <80 | 12 (46.2) | 13 (59.1) | 0.686a |
| 80-89 | 10 (38.5) | 4 (18.2) | |
| 90-99 | 3 (11.5) | 2 (9.1) | |
| ≥100 | 0 (0.0) | 2 (9.1) | |
| Missing | 1 (3.8) | 1 (4.5) | |
| Weight (kg) | | | |
| <70 | 16 (61.5) | 11 (50.0) | 0.561 |
| ≥70 | 10 (38.5) | 11 (50.0) | |
| Body mass index
(kg/m2) | | | |
| <25 | 18 (69.2) | 11 (50.0) | 0.239 |
| ≥25 | 8 (30.8) | 11 (50.0) | |
| Number of
metastatic sites | | | |
| 1 | 19 (73.1) | 7 (31.8) | 0.008 |
| >1 | 7 (26.9) | 15 (68.2) | |
| Liver
involvement | | | |
| No | 7 (26.9) | 5 (22.7) | 1.000 |
| Yes | 19 (73.1) | 17 (77.3) | |
| KRAS exon 2
mutation status | | | |
| Wild-type | 10 (38.5) | 9 (40.9) | 1.000 |
| Mutated | 13 (50.0) | 12 (54.5) | |
| Unknown | 3 (11.5) | 1 (4.5) | |
| Time to
metastasis | | | |
| Metachronous | 3 (11.5) | 3 (13.6) | 1.000 |
| Synchronous | 23 (88.5) | 19 (86.4) | |
| ECOG performance
status | | | |
| 0 | 11 (42.3) | 11 (50.0) | 0.772 |
| 1 | 13 (50.0) | 9 (40.0) | |
| 2 | 2 (7.7) | 2 (9.1) | |
| Symptoms | | | |
| No | 16 (61.5) | 17 (77.3) | 0.351 |
| Yes | 10 (38.5) | 5 (22.7) | |
| Creatinine
level | | | |
| ≤1 x ULN | 25 (96.2) | 20 (90.9) | 0.587 |
| >1 x ULN | 1 (3.8) | 2 (9.1) | |
| Clearance of
creatinine (ml/min/m2) | | | |
| ≥90 | 14 (53.8) | 10 45.5) | 0.147 |
| <90 | 12 (46.2) | 12 (54.5) | |
| Aspartate
aminotransferase level | | | |
| ≤1 x ULN | 15 (57.7) | 18 (81.8) | 0.241 |
| >1 x ULN | 11 (42.3) | 6 (27.3) | |
| Alanine
aminotransferase level | | | |
| ≤1xULN | 20 (76.9) | 17 (77.3) | 1.000 |
| >1xULN | 6 (23.1) | 5 (22.7) | |
| Lactate
dehydrogenase level | | | |
| ≤1 x ULN | 10 (38.5) | 9 (40.9) | 1.000 |
| >1 x ULN | 13 (50.0) | 12 (54.5) | |
| Missing | 1 (3.8) | 1 (4.5) | |
| Carcinoembryonic
antigen level | | | |
| ≤1 x ULN | 6 (23.1) | 5 (22.7) | 1.000 |
| >1 x ULN | 20 (76.9) | 17 (77.3) | |
| Placenta growth
factor level | | | |
| Low | 11 (36.7) | 9 (62.3) | 0.256 |
| High | 19 (63.3) | 5 (35.7) | |
| Treatment outcomes,
efficacy | | | |
| Tumor response (CR
or PR) | | | |
| No | 12 (46.2) | 7 (31.8) | 0.382 |
| Yes | 14 (53.8) | 15 (68.2) | |
| Treatment outcomes,
safety | | | |
| Arterial TEE | | | |
| No | 25 (96.2) | 21 (95.5) | 1.000 |
| Yes | 1 (3.8) | 1 (4.5) | |
| Hemorrhage | | | |
| No | 23 (88.5) | 16 (72.7) | 0.267 |
| Yes | 3 (11.5) | 6 (27.3) | |
| Proteinuria | | | |
| No | 21 (80.8) | 10 (45.5) | 0.016 |
| Yes | 5 (19.2) | 12 (54.5) | |
| On treatment
death | | | |
| No | 24 (92.3) | 22 (100.0) | 0.493 |
| Yes | 2 (7.7) | 0 (0.0) | |
| Serious adverse
events reported | | | |
| No | 12 (46.2) | 7 (31.8) | 0.382 |
| Yes,
treatment-related | 8 (30.8) | 8 (36.4) | |
| Yes,
non-treatment-related | 6 (23.1) | 7 (31.8) | |
Health-related quality of life
A total of 47 (95.9%) patients filled the baseline
HRQoL questionnaire. In total, 10 patients with no follow-up
measure had a lower baseline HRQoL level than other patients. The
median time until definitive deterioration or death varied from 5.6
months (99% CI, 2.0-10.3) for physical functioning to 8.9 months
(99% CI, 3.9-14.1) for emotional functioning. For sensitivity
analysis, all medians for targeted dimensions were <5 months. An
abnormal monocyte level was associated with a shorter time until
the definitive deterioration of emotional functioning or death
(HR=3.7; 99% CI, 1.1-12.0).
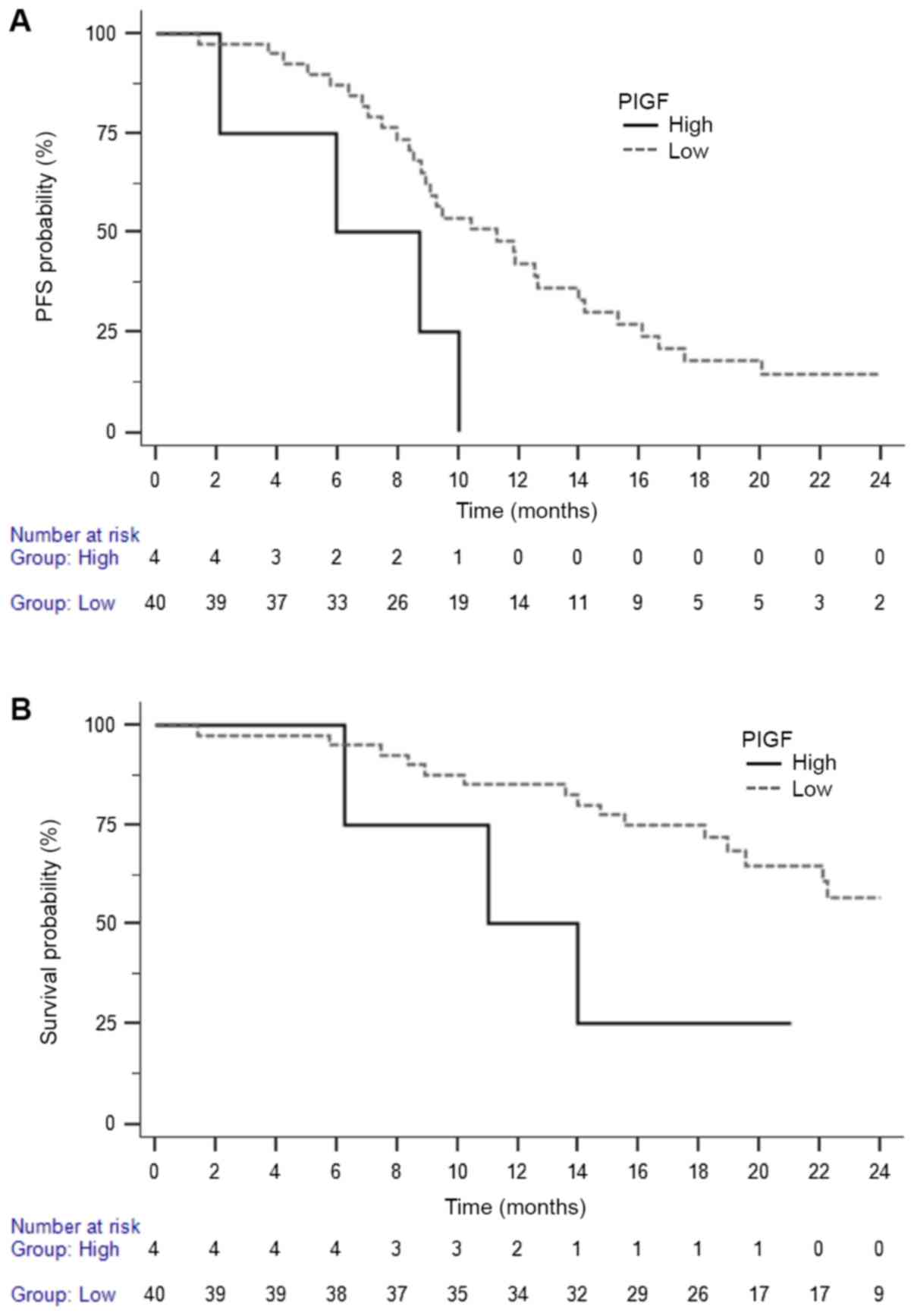
Circulating biomarkers
The exposure to aflibercept with FOLFOX was
associated with an increase in the levels of soluble (s)VEGFR1 and
PlGF after the first infusion. High baseline levels of sVEGFR2,
sEGFR, G-CSF, prolactin and low baseline levels of VEGFA and
migration inhibitory factor (MIF) were associated with a higher
response rate. High baseline levels of PlGF predict a poor PFS and
OS (Table VI and Fig. 2). There was a trend for an
association between the high on-treatment PlGF level and the
occurrence of grade 2-4 diarrhea (P=0.086), but not with
hypertension (P=0.256).
 | Table VIAssociation between baseline
circulating biomarker levels and progression-free survival and
overall survival. |
Table VI
Association between baseline
circulating biomarker levels and progression-free survival and
overall survival.
| Biomarker | Cut-off
(pg/ml) | No. | HR PFS | 95% CI | P-value | HR OS | 95% CI | P-value |
|---|
| Angiopoietin 1 | >7,000 | 21 | | | | | | |
| <7,000 | 23 | 0.73 | 0.38-1.43 | 0.361 | 1.32 | 0.54-3.24 | 0.547 |
| Angiopoietin 2 | >2,700 | 22 | | | | | | |
| <2,700 | 23 | 1.39 | 0.72-2.70 | 0.319 | 1.47 | 0.60-3.60 | 0.403 |
| Eotaxin | >120 | 23 | | | | | | |
| <120 | 21 | 0.94 | 0.48-1.82 | 0.851 | 0.66 | 0.27-1.63 | 0.378 |
| FGF | >800 | 21 | | | | | | |
| <800 | 23 | 1.40 | 0.71-2.73 | 0.332 | 1.47 | 0.60-3.61 | 0.403 |
| Follistatin | >800 | 20 | | | | | | |
| <800 | 24 | 0.90 | 0.46-1.77 | 0.778 | 0.68 | 0.27-1.68 | 0.392 |
| G-CSF | >250 | 22 | | | | | | |
| <250 | 22 | 1.20 | 0.62-2.33 | 0.584 | 0.99 | 0.40-2.44 | 0.991 |
| HER1 (EGFR) | >28,000 | 22 | | | | | | |
| <28,000 | 22 | 0.76 | 0.39-1.49 | 0.427 | 0.96 | 0.38-2.37 | 0.925 |
| HER2 | >7,300 | 22 | | | | | | |
| <7,300 | 22 | 0.47 | 0.23-0.94 | 0.019 | 0.53 | 0.21-1.36 | 0.195 |
| HGF | >1,700 | 22 | | | | | | |
| <1,700 | 22 | 0.64 | 0.33-1.26 | 0.192 | 0.42 | 0.17-1.07 | 0.074 |
| ICAM1 (CD54) | >115,000 | 23 | | | | | | |
| <115,000 | 22 | 1.60 | 0.82-3.11 | 0.159 | 0.78 | 0.31-1.91 | 0.579 |
| IL6Rα | >24,000 | 22 | | | | | | |
| <24,000 | 21 | 0.50 | 0.25-0.98 | 0.034 | 0.53 | 0.21-1.34 | 0.194 |
| IL8 | >30 | 21 | | | | | | |
| <30 | 23 | 0.88 | 0.45-1.72 | 0.710 | 1.77 | 0.72-4.34 | 0.220 |
| Leptin | >5,900 | 22 | | | | | | |
| <5,900 | 22 | 0.98 | 0.50-1.89 | 0.950 | 1.06 | 0.43-2.62 | 0.890 |
| MIF | >6,000 | 22 | | | | | | |
| <6,000 | 22 | 0.71 | 0.36-1.39 | 0.289 | 0.58 | 0.23-1.44 | 0.233 |
| NRP1 | >500,000 | 22 | | | | | | |
| <500,000 | 21 | 1.02 | 0.52-2.00 | 0.953 | 0.59 | 0.23-1.48 | 0.264 |
| Osteopontin | >145,000 | 20 | | | | | | |
| <145,000 | 23 | 0.66 | 0.33-1.32 | 0.194 | 0.99 | 0.39-2.49 | 0.977 |
| PDGF | >1,000 | 21 | | | | | | |
| <1,000 | 22 | 0.66 | 0.33-1.31 | 0.221 | 0.57 | 0.22-1.43 | 0.233 |
| PECAM1 | >7,300 | 21 | | | | | | |
| <7,300 | 23 | 0.63 | 0.32-1.22 | 0.160 | 0.41 | 0.16-1.00 | 0.058 |
| PlGF | >20 | 4 | | | | | | |
| <20 | 40 | 0.32 | 0.06-1.74 | 0.021 | 0.31 | 0.04-2.23 | 0.044 |
| Prolactin | >7,000 | 23 | | | | | | |
| <7,000 | 20 | 0.93 | 0.48-1.83 | 0.840 | 0.62 | 0.25-1.57 | 0.322 |
| SCF | >400 | 22 | | | | | | |
| <400 | 21 | 1.12 | 0.57-2.22 | 0.740 | 0.82 | 0.32-2.07 | 0.668 |
| SDF1α | >135 | 20 | | | | | | |
| (CXCL12) | <135 | 24 | 0.84 | 0.43-1.63 | 0.598 | 0.80 | 0.32-1.97 | 0.625 |
| SPD | >9,600 | 22 | | | | | | |
| <9,600 | 22 | 1.69 | 0.86-3.31 | 0.106 | 2.10 | 0.85-5.19 | 0.106 |
| Tenascin C | >10,000 | 22 | | | | | | |
| <10,000 | 22 | 0.62 | 0.32-1.23 | 0.152 | 0.40 | 0.16-1.00 | 0.047 |
| TIE2 | >20,000 | 16 | | | | | | |
| <20,000 | 27 | 0.70 | 0.34-1.46 | 0.299 | 0.49 | 0.19-1.28 | 0.129 |
| VCAM1 | >1,300,000 | 23 | | | | | | |
| <1,300,000 | 21 | 0.92 | 0.48-1.79 | 0.812 | 0.70 | 0.28-1.72 | 0.438 |
| VEGF-A | >0 | 17 | | | | | | |
| 0 | 27 | 0.66 | 0.32-1.39 | 0.232 | 0.67 | 0.26-1.74 | 0.379 |
| VEGF-C | >800 | 18 | | | | | | |
| <800 | 26 | 1.72 | 0.89-3.35 | 0.098 | 1.41 | 0.57-3.47 | 0.469 |
| VEGFR1 | >1,300 | 22 | | | | | | |
| <1,300 | 21 | 0.98 | 0.50-1.92 | 0.957 | 1.06 | 0.42-2.67 | 0.901 |
| VEGFR2 | >7,000 | 22 | | | | | | |
| <7,000 | 21 | 0.96 | 0.49-1.89 | 0.905 | 1.10 | 0.43-2.80 | 0.840 |
| VEGFR3 | >2,250 | 21 | | | | | | |
| <2,250 | 23 | 1.74 | 0.89-3.40 | 0.093 | 1.52 | 0.62-3.74 | 0.361 |
Discussion
VELVET was the first phase II study evaluating
aflibercept with an oxaliplatin stop-and-go strategy in patients
with previously untreated and unresectable mCRC. The targeted 85%
6-month PFS rate was not reached in the ITT population: The
absolute rate and the Kaplan-Meier estimates of 6-month PFS were 67
and 79%, respectively. The ORR was 59% and median PFS and OS were
9.3 months and 22.2 months, respectively. The maintenance rate
(79%) was higher than in previous oxaliplatin stop-and-go studies
(10,21,27).
In the OPTIMOX1 and OPTIMOX2 studies (10,21)
a similar oxaliplatin stop-and-go strategy without anti-angio-genic
agent led to a response rate of 59.2% and median PFS <9 months.
In the AFFIRM randomized phase II study (22), 236 patients with unresectable mCRC
were randomized between first-line FOLFOX (n=117) and
FOLFOX-aflibercept (n=119) until progression. That study was
conducted in Europe, Asia and Australia, regions with different
clinical guidelines for the treatment of mCRC. The 1-year PFS rate
(primary endpoint) was similar in both groups (21.2 versus 25.8%).
There was no significant improvement in efficacy endpoints with the
addition of aflibercept to chemotherapy (ORR, 45.9 versus 49.1%;
median PFS, 8.8 versus 8.5 months; and median OS, 22.3 versus 19.5
months) and in salvage surgery rate (5.1 versus 5.0%). In the
NO16966 study (4) the addition of
bevacizumab to an oxaliplatin-based chemotherapy (FOLFOX or XELOX)
led to an improvement in PFS (primary endpoint) from 8.0 to 9.5
months (HR, 0.83; P=0.002). This benefit was greater when patients
were censored at the time of drug discontinuation (‘on-treatment
PFS’; HR, 0.63). The median PFS in patients who received
FOLFOX4-bevacizumab was 9.4 months. The ORR was similar whether
patients received chemotherapy with (47%) or without (49%)
bevacizumab. The oxaliplatin-based stop-and-go strategy with
bevacizumab was previously evaluated in several randomized phase
III trials (11,23-26).
Among 700 patients enrolled in the DREAM study (27), 429 (61.3%) received an induction
therapy with modified FOLFOX7 plus bevacizumab, using the same dose
of oxaliplatin (100 mg/m2) than in the present study,
although a lower dose of 5-FU infusion. In those patients, the ORR
was 52.2% and the median PFS was 9.4 months (28). Thus, the addition of aflibercept to
an oxaliplatin stop-and-go strategy in patients with unresectable
mCRC seems to increase PFS to the same degree as bevacizumab (from
<9 to 9.5 months) and to slightly increase the tumor ORR
(Table VII). This effect may
also be associated with higher doses of 5-FU infusion.
 | Table VIISummary of treatment regimens and
outcomes of studies evaluating FOLFOX with or without
antiangiogenic agent. |
Table VII
Summary of treatment regimens and
outcomes of studies evaluating FOLFOX with or without
antiangiogenic agent.
| Antiangiogenic
agent | None
| Bevacizumab
| Aflibercept
|
|---|
| | |
|---|
| Study (ref.) | NO16966 (4) | OPTIMOX1 (10) | OPTIMOX1 (10) | OPTIMOX2 (21) | NO16966 (4) | HORIZON III
(30) | DREAMa (28) | AFFIRM (22) | VELVET (29) |
| No. of
patients | 351 | 309 | 311 | 98 | 699 | 713 | 429 | 119 | 49 |
| Administration | Continuously | Continuously | Stop-and-go | Stop-and-go
(maintenance) | Continuously | Continuously | Stop-and-go | Continuously | Stop-and-go |
| Chemotherapeutic
regimen | FOLFOX4 | FOLFOX4 | FOLFOX7 | mFOLFOX7 | FOLFOX4 | mFOLFOX6 | mFOLFOX7 | mFOLFOX6 | mFOLFOX7 |
| Oxaliplatin dose
(mg/m2) | 85 | 85 | 130 | 100 | 85 | 85 | 100 | 85 | 100 |
| 5-FU infusion dose
(mg/m2) | 2,400 | 2,400 | 2,400 | 3,000 | 2,400 | 2,400 | 2,400 | 2,400 | 3,000 |
| 5-FU bolus | Yes | Yes | No | No | Yes | Yes | No | Yes | No |
| Objective response
rate (%) | 49.0a | 58.5 | 59.2 | 59.2 | 47.0a | 47.3 | 52.2 | 49.1 | 59.2 |
| PFS (months) | 8.6 | 9.0 | 8.7 | 8.6 | 9.4 | 10.3 | 9.4 | 8.5 | 9.5 |
| OS (months) | 20.3 | 19.3 | 21.2 | 23.8 | 21.2 | 21.3 | 25.6 | 19.5 | 22.2 |
In the present study, the frequency of severe (grade
3 or 4) hypertension (23%) was similar to that reported in the
VELOUR trial (19%) (29), although
lower than described in the AFFIRM study (36%) (22). When adding bevacizumab to an
oxaliplatin-based chemotherapy in patients with advanced mCRC, the
incidence of grade 3-4 hypertension ranges between 4 and 6%
(4,30-32).
In this study, this adverse event occurred mainly during induction
therapy, and was reversed in most cases before maintenance therapy.
Of note, a high systolic blood pressure (≥140 mmHg) at study entry
was associated with shorter PFS and a higher frequency of treatment
induced grade 2-4 hypertension.
The exposure to aflibercept with FOLFOX was
associated with an increase in PlGF levels after the first
infusion. When trapping circulating PlGF, aflibercept inhibits the
binding to VEGF receptors 1 and 2, thus increasing the circulating
PlGF level.
Despite the statistically negative result of this
study, but given the high response rate, OPTIMOX-aflibercept may be
an active first-line treatment strategy in patients with previously
untreated and unresectable mCRC, providing strict monitoring of
blood pressure and immediate management of hypertension during
therapy. Further trials evaluating this combination should provide
early safety analysis.
Funding
This study was supported by Sanofi. Sanofi did not
have any role in the study design, collection, analysis and
interpretation of the data, the writing of the manuscript and the
decision to submit the study for publication.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on request.
Authors' contributions
BC and ADG were responsible for the conception and
design of the study. CT, BC, TA, WS, and ADG recruited the
patients. BC, JBB, TA, DA, JDe, GD, CLe, CLo, CT, VL, JDa, GL, MLG,
OD, NBH, AM, AKL, and ATR collected the data. BC, FB, and AdG
analyzed the data. CT and AdG interpreted the data. BC and AdG
wrote the manuscript. All authors have edited, read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was carried out in accordance with the
declaration of Helsinki and Good Clinical Practice guidelines. All
patients provided written informed consent. This study was approved
by the Ethics Committee of our institution (CPP Ile de France VI
Groupe Hospitalier Pitié Salpêtrière PARIS).
Patient consent for publication
Not applicable.
Competing interests
BC reported personal fees from Roche Pharma AG,
Amgen, Sanofi and Menarini. JBB reported personal fees from Amgen,
Bayer, Celgène, Merck Serono, Roche, Sanofi and Roche. TA reported
personal fees from BMS, Roche, MSD Oncology, Sanofi, Novartis,
Servier, Amgen, Lilly, Xbiotec, Mundipharma and Yacult. All
remaining authors have declared no competing interests.
Acknowledgments
The authors would like to thank the patients who
participated in this study, their families, the coordinating staff
in GERCOR (particularly Ms. Attia Malika) and all the following
investigators: Dr Artru Pascal (Hôpital Privé Jean Mermoz, Lyon,
France), Dr Savinelli Francesco, and Dr Sverdlin Robert (Hôpital
Privé Saint-Joseph, Paris, France). The authors also acknowledge
the assistance of Benetkiewicz Magdalena in manuscript preparation.
Her work was funded by GERCOR.
References
|
1
|
American Cancer Society: Cancer facts and
figures. 2015, http://www.cancer.org/research/cancerfactsstatistics/cancerfacts-figures2015/index,
Accessed, May 14, 2018.
|
|
2
|
Chibaudel B, Tournigand C, Bonnetain F,
Richa HM, André T and de Gramont A: Therapeutic strategy in
unresectable metastatic colorectal cancer: An updated review. Ther
Adv Med Oncol. 7:153–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tournigand C, Cervantes A, Figer A, Lledo
G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, et
al: OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with
oxaliplatin in a stop-and-Go fashion in advanced colorectal
cancer--a GERCOR study. J Clin Oncol. 24:394–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simkens LH, van Tinteren H, May A, ten
Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec
Z, van der Torren AM, et al: Maintenance treatment with
capecitabine and bevacizumab in metastatic colorectal cancer
(CAIRO3): A phase 3 randomised controlled trial of the Dutch
Colorectal Cancer Group. Lancet. 385:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomized trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chibaudel B, Bonnetain F, Shi Q, Buyse M,
Tournigand C, Sargent DJ, Allegra CJ, Goldberg RM and de Gramont A:
Alternative end points to evaluate a therapeutic strategy in
advanced colorectal cancer: Evaluation of progression-free
survival, duration of disease control, and time to failure of
strategy--an Aide et Recherche en Cancerologie Digestive Group
Study. J Clin Oncol. 29:4199–4204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
15
|
National Canceer Institute: Common
terminology criteria for adverse events 9CTCAE), v4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf,
Accessed May 14, 2018.
|
|
16
|
EORTC Quality of Life Group: EORTC QLQ-C30
(version 3) Brussels: EORTC Quality of Life Group. 1995, http://groups.eortc.be/qol/sites/default/files/img/slider/specimen_qlq-c30_english.pdf,
Accessed May 14, 2018.
|
|
17
|
Chibaudel B, Bonnetain F, Tournigand C,
Bengrine-Lefevre L, Teixeira L, Artru P, Desramé J, Larsen AK,
André T, Louvet C, et al: Simplified prognostic model in patients
with oxaliplatin-based or irinotecan-based first-line chemotherapy
for metastatic colorectal cancer: A GERCOR study. Oncologist.
16:1228–1238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simon R: Optimal two-stage designs for
phase II clinical trials. Control Clin Trials. 10:1–10. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaplan EL and Meier P: Non parametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
21
|
Chibaudel B, Maindrault-Goebel F, Lledo G,
Mineur L, André T, Bennamoun M, Mabro M, Artru P, Carola E, Flesch
M, et al: Can chemotherapy be discontinued in unresectable
metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin
Oncol. 27:5727–5733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Folprecht G, Pericay C, Saunders MP,
Thomas A, Lopez Lopez R, Roh JK, Chistyakov V, Höhler T, Kim JS,
Hofheinz RD, et al: Oxaliplatin and 5-FU/folinic acid (modified
FOLFOX6) with or without aflibercept in first-line treatment of
patients with metastatic colorectal cancer: The AFFIRM study. Ann
Oncol. 27:1273–1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tournigand C, Lledo G, Delord J, André T,
Maindrault-Goebel F, Louvet C, Scheithauer W and de Gramont A:
Modified Folfox7/bevacizumab or modified Xelox/bevacizumab with or
without erlotinib in first-line metastatic colorectal cancer:
Results of the feasability phase of the DREAM-OPTIMOX3 study
(GERCOR). J Clin Oncol. 25(Suppl. 18): 40972007.
|
|
24
|
Koopman M, Simkens Lieke HJ, Ten Tije AJ,
Creemers GJ, Loosveld OJ, De Jongh FE, Erdkamp F, Erjavec Z, van
der Torren AME, et al: Maintenance treatment with capecitabine and
Bevacizumab versus observation after induction treatment with
chemotherapy and Bevacizumab in metastatic colorectal cancer
(mCRC): The phase Iii Cairo3 Study of the Dutch Colorectal Cancer
Group (DCCG). J Clin Oncol. 31(Suppl): 35022013.
|
|
25
|
Arnold D, Graeven U, Lerchenmuller C,
Killing B, Depenbusch R, Steffens C, Salah-Eddin Al-Batran S-E,
Lange T, Dietrich G, Jan Stoehlmacher J, et al: Maintenance
strategy with fluoro-pyrimidines (FP) plus Bevacizumab (Bev), Bev
alone, or no treatment, following a standard combination of FP,
oxaliplatin (Ox), and Bev as first-line treatment for patients with
metastatic colorectal cancer (mCRC): A phase III non-inferiority
trial (AIO KRK 0207). J Clin Oncol. 32(Suppl. 5): 35032014.
View Article : Google Scholar
|
|
26
|
Koeberle D, Betticher DC, von Moos R,
Dietrich D, Brauchli P, Baertschi D, Matter K, Winterhalder R,
Borner M, Anchisi S, et al: Bevacizumab continuation versus no
continuation after first-line chemotherapy plus bevacizumab in
patients with metastatic colorectal cancer: A randomized phase III
non-inferiority trial (SAKK 41/06). Ann Oncol. 26:709–714. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tournigand C, Chibaudel B, Samson B,
Scheithauer W, Vernerey D, Mésange P, Lledo G, Viret F, Ramée JF,
Tubiana-Mathieu N, et al: Bevacizumab with or without erlotinib as
maintenance therapy in patients with metastatic colorectal cancer
(GERCOR DREAM; OPTIMOX3): A randomised, open-label, phase 3 trial.
Lancet Oncol. 16:1493–1505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chibaudel B, Tournigand C, Samson B,
Scheithauer W, Mesange P, Lledo G, Viret FJ, Ramée JF,
Tubiana-Mathieu N, Dauba J, et al: Bevacizumab-erlotinib as
maintenance therapy in metastatic colorectal cancer. Final results
of the GERCOR DREAM study. Ann Oncol. 25(Suppl. 4): iv167–iv209.
2014. View Article : Google Scholar
|
|
29
|
Tabernero J, Van Cutsem E, Lakomý R,
Prausová J, Ruff P, van Hazel GA, Moiseyenko VM, Ferry DR,
McKendrick JJ, Soussan-Lazard K, et al: Aflibercept versus placebo
in combination with fluorouracil, leucovorin and irinotecan in the
treatment of previously treated metastatic colorectal cancer:
Prespecified subgroup analyses from the VELOUR trial. Eur J Cancer.
50:320–331. 2014. View Article : Google Scholar
|
|
30
|
Schmoll HJ, Cunningham D, Sobrero A,
Karapetis CS, Rougier P, Koski SL, Kocakova I, Bondarenko I, Bodoky
G, Mainwaring P, et al: Cediranib with mFOLFOX6 versus beva-cizumab
with mFOLFOX6 as first-line treatment for patients with advanced
colorectal cancer: A double-blind, randomized phase III study
(HORIZON III). J Clin Oncol. 30:3588–3595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Díaz-Rubio E, Gómez-España A, Massutí B,
Sastre J, Abad A, Valladares M, Rivera F, Safont MJ, Martínez de
Prado P, Gallén M, et al Spanish Cooperative Group for the
Treatment of Digestive Tumors: First-line XELOX plus bevacizumab
followed by XELOX plus bevacizumab or single-agent bevacizumab as
maintenance therapy in patients with metastatic colorectal cancer:
The phase III MACRO TTD study. Oncologist. 17:15–25. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA and Benson AB III;
Eastern Cooperative Oncology Group Study E3200: Bevacizumab in
combination with oxaliplatin, fluorouracil, and leucovorin
(FOLFOX4) for previously treated metastatic colorectal cancer:
Results from the Eastern Cooperative Oncology Group Study E3200. J
Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|