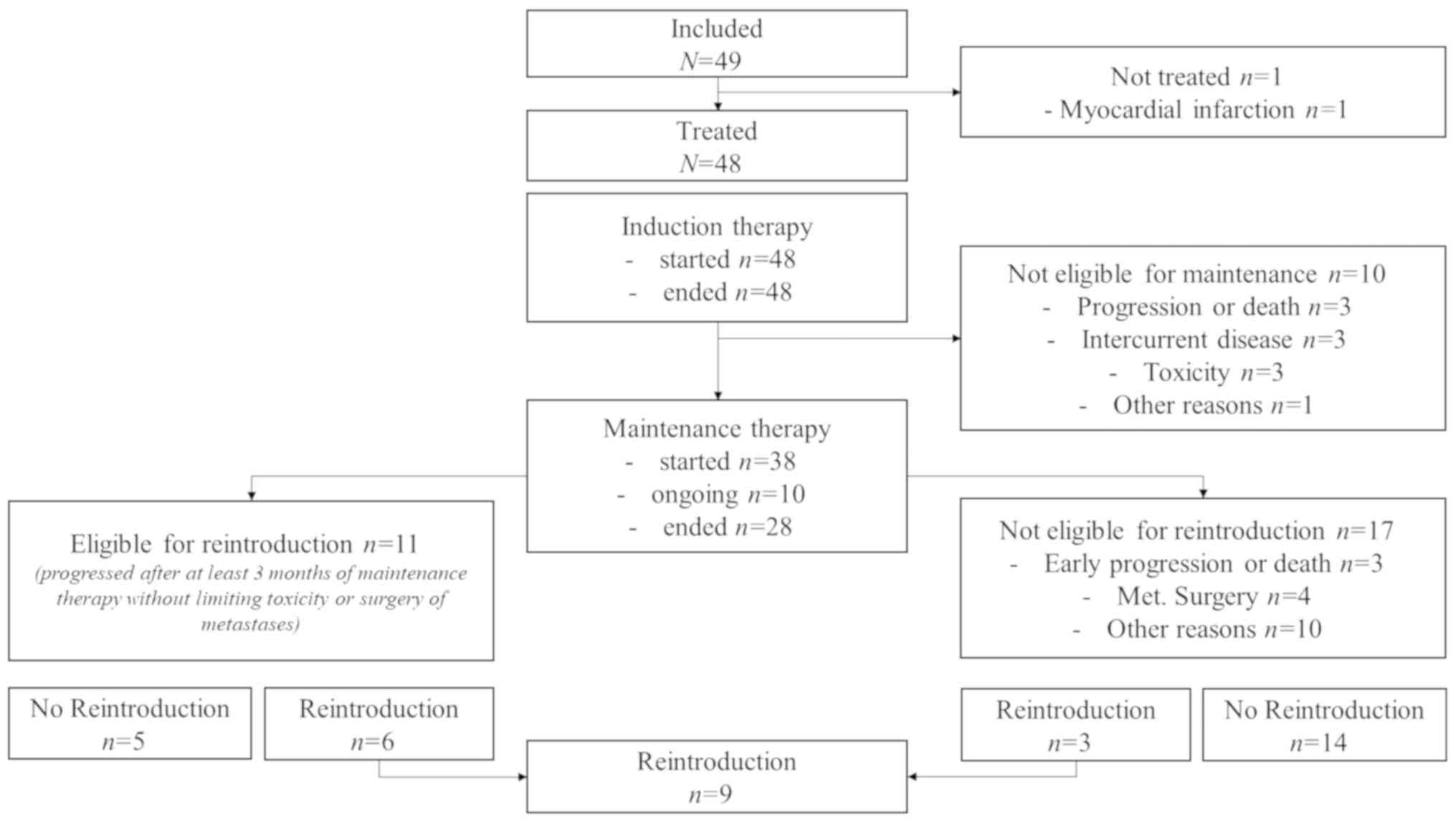
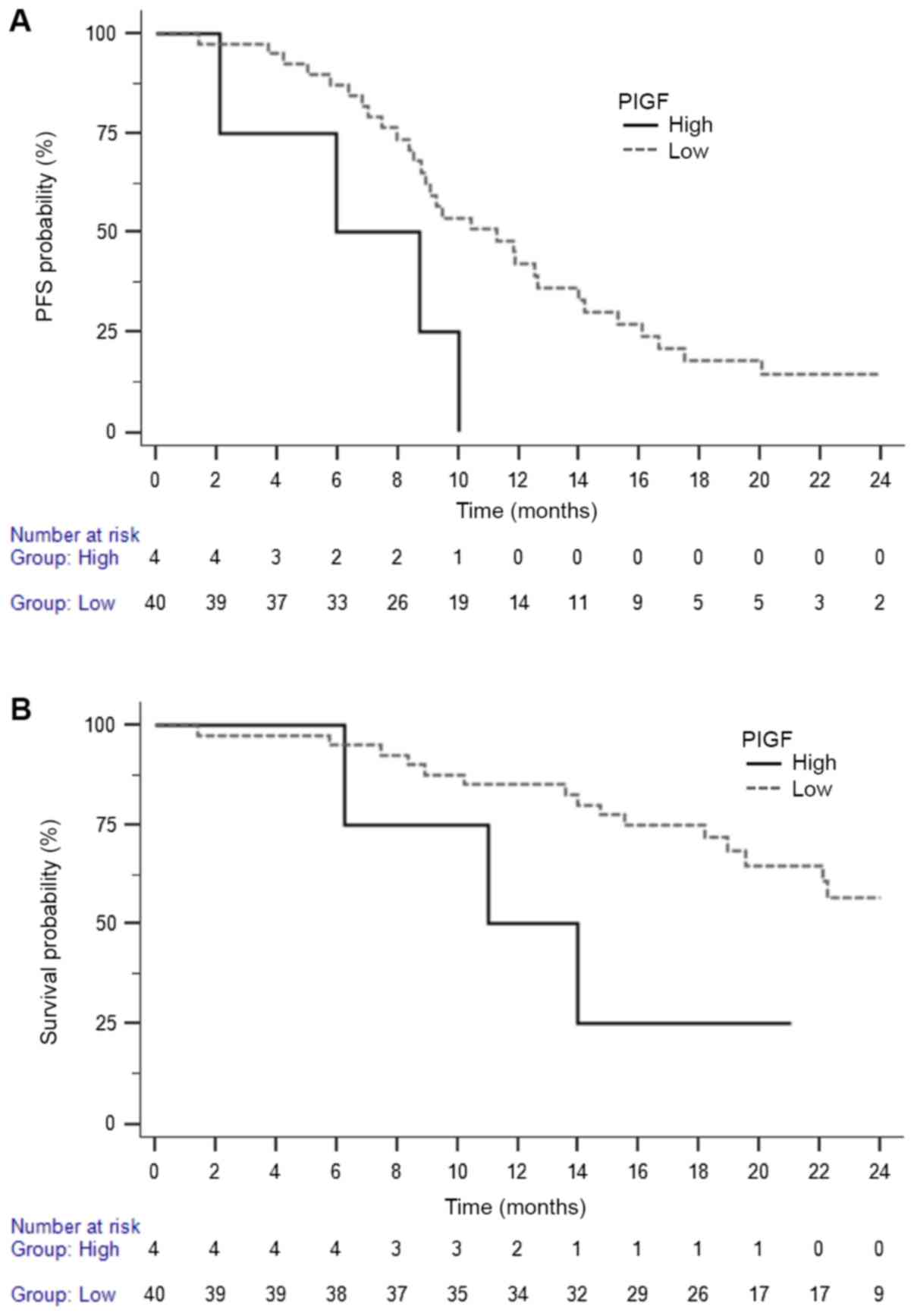
|
1
|
American Cancer Society: Cancer facts and
figures. 2015, http://www.cancer.org/research/cancerfactsstatistics/cancerfacts-figures2015/index,
Accessed, May 14, 2018.
|
|
2
|
Chibaudel B, Tournigand C, Bonnetain F,
Richa HM, André T and de Gramont A: Therapeutic strategy in
unresectable metastatic colorectal cancer: An updated review. Ther
Adv Med Oncol. 7:153–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loupakis F, Cremolini C, Masi G, Lonardi
S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi
R, et al: Initial therapy with FOLFOXIRI and bevacizumab for
metastatic colorectal cancer. N Engl J Med. 371:1609–1618. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tournigand C, Cervantes A, Figer A, Lledo
G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, et
al: OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with
oxaliplatin in a stop-and-Go fashion in advanced colorectal
cancer--a GERCOR study. J Clin Oncol. 24:394–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simkens LH, van Tinteren H, May A, ten
Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec
Z, van der Torren AM, et al: Maintenance treatment with
capecitabine and bevacizumab in metastatic colorectal cancer
(CAIRO3): A phase 3 randomised controlled trial of the Dutch
Colorectal Cancer Group. Lancet. 385:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomized trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chibaudel B, Bonnetain F, Shi Q, Buyse M,
Tournigand C, Sargent DJ, Allegra CJ, Goldberg RM and de Gramont A:
Alternative end points to evaluate a therapeutic strategy in
advanced colorectal cancer: Evaluation of progression-free
survival, duration of disease control, and time to failure of
strategy--an Aide et Recherche en Cancerologie Digestive Group
Study. J Clin Oncol. 29:4199–4204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
15
|
National Canceer Institute: Common
terminology criteria for adverse events 9CTCAE), v4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf,
Accessed May 14, 2018.
|
|
16
|
EORTC Quality of Life Group: EORTC QLQ-C30
(version 3) Brussels: EORTC Quality of Life Group. 1995, http://groups.eortc.be/qol/sites/default/files/img/slider/specimen_qlq-c30_english.pdf,
Accessed May 14, 2018.
|
|
17
|
Chibaudel B, Bonnetain F, Tournigand C,
Bengrine-Lefevre L, Teixeira L, Artru P, Desramé J, Larsen AK,
André T, Louvet C, et al: Simplified prognostic model in patients
with oxaliplatin-based or irinotecan-based first-line chemotherapy
for metastatic colorectal cancer: A GERCOR study. Oncologist.
16:1228–1238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simon R: Optimal two-stage designs for
phase II clinical trials. Control Clin Trials. 10:1–10. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaplan EL and Meier P: Non parametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
21
|
Chibaudel B, Maindrault-Goebel F, Lledo G,
Mineur L, André T, Bennamoun M, Mabro M, Artru P, Carola E, Flesch
M, et al: Can chemotherapy be discontinued in unresectable
metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin
Oncol. 27:5727–5733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Folprecht G, Pericay C, Saunders MP,
Thomas A, Lopez Lopez R, Roh JK, Chistyakov V, Höhler T, Kim JS,
Hofheinz RD, et al: Oxaliplatin and 5-FU/folinic acid (modified
FOLFOX6) with or without aflibercept in first-line treatment of
patients with metastatic colorectal cancer: The AFFIRM study. Ann
Oncol. 27:1273–1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tournigand C, Lledo G, Delord J, André T,
Maindrault-Goebel F, Louvet C, Scheithauer W and de Gramont A:
Modified Folfox7/bevacizumab or modified Xelox/bevacizumab with or
without erlotinib in first-line metastatic colorectal cancer:
Results of the feasability phase of the DREAM-OPTIMOX3 study
(GERCOR). J Clin Oncol. 25(Suppl. 18): 40972007.
|
|
24
|
Koopman M, Simkens Lieke HJ, Ten Tije AJ,
Creemers GJ, Loosveld OJ, De Jongh FE, Erdkamp F, Erjavec Z, van
der Torren AME, et al: Maintenance treatment with capecitabine and
Bevacizumab versus observation after induction treatment with
chemotherapy and Bevacizumab in metastatic colorectal cancer
(mCRC): The phase Iii Cairo3 Study of the Dutch Colorectal Cancer
Group (DCCG). J Clin Oncol. 31(Suppl): 35022013.
|
|
25
|
Arnold D, Graeven U, Lerchenmuller C,
Killing B, Depenbusch R, Steffens C, Salah-Eddin Al-Batran S-E,
Lange T, Dietrich G, Jan Stoehlmacher J, et al: Maintenance
strategy with fluoro-pyrimidines (FP) plus Bevacizumab (Bev), Bev
alone, or no treatment, following a standard combination of FP,
oxaliplatin (Ox), and Bev as first-line treatment for patients with
metastatic colorectal cancer (mCRC): A phase III non-inferiority
trial (AIO KRK 0207). J Clin Oncol. 32(Suppl. 5): 35032014.
View Article : Google Scholar
|
|
26
|
Koeberle D, Betticher DC, von Moos R,
Dietrich D, Brauchli P, Baertschi D, Matter K, Winterhalder R,
Borner M, Anchisi S, et al: Bevacizumab continuation versus no
continuation after first-line chemotherapy plus bevacizumab in
patients with metastatic colorectal cancer: A randomized phase III
non-inferiority trial (SAKK 41/06). Ann Oncol. 26:709–714. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tournigand C, Chibaudel B, Samson B,
Scheithauer W, Vernerey D, Mésange P, Lledo G, Viret F, Ramée JF,
Tubiana-Mathieu N, et al: Bevacizumab with or without erlotinib as
maintenance therapy in patients with metastatic colorectal cancer
(GERCOR DREAM; OPTIMOX3): A randomised, open-label, phase 3 trial.
Lancet Oncol. 16:1493–1505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chibaudel B, Tournigand C, Samson B,
Scheithauer W, Mesange P, Lledo G, Viret FJ, Ramée JF,
Tubiana-Mathieu N, Dauba J, et al: Bevacizumab-erlotinib as
maintenance therapy in metastatic colorectal cancer. Final results
of the GERCOR DREAM study. Ann Oncol. 25(Suppl. 4): iv167–iv209.
2014. View Article : Google Scholar
|
|
29
|
Tabernero J, Van Cutsem E, Lakomý R,
Prausová J, Ruff P, van Hazel GA, Moiseyenko VM, Ferry DR,
McKendrick JJ, Soussan-Lazard K, et al: Aflibercept versus placebo
in combination with fluorouracil, leucovorin and irinotecan in the
treatment of previously treated metastatic colorectal cancer:
Prespecified subgroup analyses from the VELOUR trial. Eur J Cancer.
50:320–331. 2014. View Article : Google Scholar
|
|
30
|
Schmoll HJ, Cunningham D, Sobrero A,
Karapetis CS, Rougier P, Koski SL, Kocakova I, Bondarenko I, Bodoky
G, Mainwaring P, et al: Cediranib with mFOLFOX6 versus beva-cizumab
with mFOLFOX6 as first-line treatment for patients with advanced
colorectal cancer: A double-blind, randomized phase III study
(HORIZON III). J Clin Oncol. 30:3588–3595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Díaz-Rubio E, Gómez-España A, Massutí B,
Sastre J, Abad A, Valladares M, Rivera F, Safont MJ, Martínez de
Prado P, Gallén M, et al Spanish Cooperative Group for the
Treatment of Digestive Tumors: First-line XELOX plus bevacizumab
followed by XELOX plus bevacizumab or single-agent bevacizumab as
maintenance therapy in patients with metastatic colorectal cancer:
The phase III MACRO TTD study. Oncologist. 17:15–25. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA and Benson AB III;
Eastern Cooperative Oncology Group Study E3200: Bevacizumab in
combination with oxaliplatin, fluorouracil, and leucovorin
(FOLFOX4) for previously treated metastatic colorectal cancer:
Results from the Eastern Cooperative Oncology Group Study E3200. J
Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|