Introduction
Molecular imaging of cancer-associated targets
facilitates the diagnosis and stratification of patients for
targeted treatment. In patients with breast cancer, overexpression
of human epidermal growth factor receptor 2 (HER2) is a predictor
of poor prognosis. HER2 is an established therapeutic target in
breast and gastroesophageal cancer (1,2).
Anti-HER2 therapeutic agents including the monoclonal antibody
trastuzumab, trastuzumab-DM1 conjugates and the tyrosine kinase
inhibitor lapatinib, have been demonstrated to significantly
improve the survival of patients with breast and gastric cancer
(1,3-5).
However, only ~20% of breast tumors have a sufficiently high level
of HER2 for successful targeting (6). Therefore, accurate determination of
the HER2 level in tumors is critical for making a decision about
targeted therapy.
The current clinical method of evaluating HER2
expression is biopsy sampling followed by immunohistochemistry
and/or fluorescence in situ hybridization analysis (7). The principal disadvantage of
biopsy-based diagnostics is the morbidity associated with the
invasiveness of the procedure, which limits the number of samples
taken; the expression in only a few metastases may thus be
determined. Heterogeneity of HER2 expression and discrepancies in
expression between the primary tumor and metastases make the
accurate determination of HER2 expression in disseminated disease
challenging (8-10).
Molecular imaging is a non-invasive method for the
global detection of HER2 expression that may overcome the
limitations of current procedures. Therapeutic antibodies
radiolabeled with γ- or positron-emitters may be repurposed for
single photon emission computed tomography (SPECT) or positron
emission tomography (PET) imaging with lower translational costs,
since the safety and toxicity profiles of approved antibodies are
well defined. However, the major problem with using antibodies to
image tumors is the low contrast, due to their slow accumulation
and long half-lives. Small engineered scaffold proteins (ESPs) are
promising targeting probes for molecular imaging due to their
potentially high affinities for targets and rapid clearance from
the blood and normal tissues (11). Various ESPs, including affibody
molecules (12), ABD-derived
affinity proteins (ADAPTs) (13),
fibronectin domains (14),
knottins (15) and anticalins
(16), have demonstrated high
sensitivity of radionuclide imaging in preclinical studies.
Affibody molecules labeled with gallium-68 have been successfully
used for whole-body quantification of HER2 expression using
PET/computed tomography (CT) imaging in the clinic (17).
Designed ankyrin repeat proteins (DARPins) are small
ESPs selected for their high-affinity binding to numerous
cancer-associated targets. However, the number of studies
concerning their potential for imaging is limited. DARPins are
built of tightly packed repeat modules of 33 amino acids (18). Their generally high stability,
solubility and aggregation resistance have made them important
tools in a number of research applications. Clinical trials
assessing the efficacy and safety of an anti-VEGF DARPin in
patients with macular degeneration have reported promising results
(19). DARPin G3 (14.5 kDa) is a
variant that binds to domain IV of HER2 with picomolar affinity
(20). Biparatopic G3-based
DARPins have demonstrated efficient growth suppression of
HER2-expressing xenografts and lack of toxicity at high doses (up
to 60 mg/kg) in preclinical studies (21,22),
and are currently being evaluated in a clinical trial (23). DARPin G3 labeled with indium-111,
technetium-99m and radioiodine has demonstrated efficient tumor
targeting with a favorable biodistribution profile (20,24).
High-contrast molecular imaging is achieved when the
uptake of an imaging probe in tumors is several folds higher
compared with the uptake in healthy tissues. Our previous study
indicated that the internalization of anti-HER2 DARPins in tumors
is relatively slow; however, internalization in excretory organs
(the liver and kidneys) is rapid (25). A comparison of residualizing and
non-residualizing labels for DARPins demonstrated that the use of
non-residualizing labels (labels producing lipophilic catabolites
that leak from cells following internalization and lysosomal
proteolysis) resulted in the rapid removal of radiocatabolites from
the liver and kidneys, providing decreased activity in these organs
and increased contrast.
Radioisotopes of iodine provide versatile
non-residualizing labels for preclinical studies (iodine-125) and
clinical SPECT (iodine-123) and PET (iodine-124) imaging.
Radioiodination of proteins may be performed using a number of
labeling strategies. Direct labeling using chloramine-T is a robust
and straightforward method. However, electrophilic oxidative
radioiodination of tyrosines provides random attachment of the
radionuclide to a protein. Modification of tyrosines in the binding
site may negatively influence the affinity. For example, in the
case of anti-HER2 affibody molecules, this method was not
applicable as they lost binding specificity following direct
radioiodination (26). Indirect
labeling using a bifunctional linker facilitates site-specific
attachment of the label with control over the position and number
of labels per protein. The choice of a label and labeling method
may influence the biodistribution of the labeled protein and the
re-distribution of radiocatabolites. Indirect labeling has been
reported to reduce the accumulation of radiocatabolites in organs
with expression of sodium-iodide symporters, including the salivary
glands, thyroid and stomach (27,28).
Therefore, the selection of an optimal strategy may lead to a
substantial improvement in imaging contrast.
The goal of the present study was to select a
labeling method for radioiodination of DARPin G3, providing the
best imaging contrast. Direct and indirect radioiodination methods
were compared. Direct labeling of G3 has been previously reported
by Goldstein et al (24).
For site-specific labeling, a cysteine was introduced through a
triglycine spacer -GGGC at the C terminus of G3. As the DARPin
scaffold does not contain cysteines, the thiol group of the
engineered cysteine was used for site-specific maleimidethiol
coupling. A maleimide derivative of tyramine,
[(4-hydroxyphenyl)ethyl] maleimide (HPEM), was used as a
bifunctional linker in this study.
Materials and methods
General materials and instruments
The molecular weight of the DARPins was measured by
liquid chromatography-electrospray ionization-mass spectrometry on
a 6520 Accurate Q-TOF LC/MS (Agilent Technologies, Inc., Santa
Clara, CA, USA) with a mass range of 250-2,500 m/z and positive
ionization mode. The method was divided into three segments: 0-3
min, 3-7 min and 7-10 min. The parameters were set as follows:
Nitrogen gas temperature, 350°C for all segments; nebulizer
pressure, 15 psi, 25 psig and 40 psig; and drying gas flow rate, 5
l/min, 5 l/min and 9 l/min. Iodine radioisotopes
[124I]NaI, [125I]NaI and [131I]
NaI were purchased from PerkinElmer Sverige AB (Upplands Väsby,
Sweden). Instant thin-layer chromatography (iTLC) analysis was
performed using iTLC silica gel strips (Varian Medical Systems,
Palo Alto, CA, USA). The activity distribution was measured using a
Cyclone storage phosphor system and analyzed using OptiQuant image
analysis software (version 2.5) (both from PerkinElmer, Waltham,
MA, USA). Size-exclusion chromatography was performed using NAP-5
columns (GE Healthcare, Chicago, IL, USA). Radio-high performance
liquid chromatography (HPLC) analysis was performed using Hitachi
Chromaster HPLC systems with radioactivity detector and Vydac RP
C18 column (300 Å; 3×150 mm; 5-µm) at room temperature
(20°C). The sample quantity used for analysis was 5 µl.
Solvent A was 0.1% trifluoroacetic acid (TFA) in H2O;
solvent B was 0.1% TFA in acetonitrile. The flow rate was 1 ml/min,
with a 5% B to 80% B gradient over 20 min. Activity was measured
using an automated γ-spectrometer with an NaI(TI) detector (1480
Wizard; PerkinElmer Wallac Oy, Turku, Finland). SKOV3, BT474, DU145
and A431 cells were purchased from the American Type Culture
Collection and were cultured in RPMI-1640 medium (Biochrom GmbH,
Berlin, Germany) supplemented with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 2 mM L-glutamine,
100 IU/ml penicillin and 100 µg/ml streptomycin in a
humidified incubator with 5% CO2 at 37°C, unless stated
otherwise.
Protein production
DARPins G3-H6 and G3-GGGC were produced
in Escherichia coli strain BL21(DE3) (Novagen; EMD
Millipore, Billerica, MA, USA). The genes for DARPin
G3-H6 and G3-GGGC were deduced from a DARPin G3 amino
acid sequence deposited in the Protein Data Bank (PDB) database
(https://www.rcsb.org; PDB accession no. 2JAB),
taking into account the codon usage in highly expressed E.
coli genes. The amino acid sequence encoded by the DARPin
G3-H6 gene was as follows: MDLGKKLLEAARAGQDDEVRILMANGA
DVNAKDEYGLTPLYLATAHGHLEIVEVLLKNGADVNA
VDAIGFTPLHLAAFIGHLEIAEVLLKHGADVNAQDKFG
KTAFDISIGNGNEDLAEILQKLNGSHHHHHH. The gene was cloned into the
plasmid vector pET39b (Novagen; EMD Millipore) between restriction
sites NdeI and HindIII. The amino acid sequence of G3
containing a three glycine spacer and a cysteine at the C-terminus
(G3-GGGC) was as follows: DLGKKLLEAARAGQDDEVRILMANGADVNAKDEYGL
TPLYLATAHGHLEIVEVLLKNGADVNAVDAIGFTPLHL
AAFIGHLEIAEVLLKHGADVNAQDKFGKTAFDISIGNG NEDLAEILQKLNGGGGC. The
DARPin G3-GGGC gene was fused to the 3′-terminus of the small
ubiquitin related modifier (SUMO) gene (29) by overlapping polymerase chain
reaction (30). The procedure was
performed in two steps. First, the DNA fragments containing the
SUMO and DARPin G3-GGGC genes were amplified via PCR using primers
T7dir (5′-GCGAAATTAATACGACTCACTATAGGG-3′) and Sur
(5′-GCCACCAATCTGCTCAC-3′) for the SUMO gene and primers SG
(5′-GTGAGCAGATTGGTGGCGACCTGGGCA AGAAACTG-3′) and T7rev
(5′-GGGTTATGCTAGTTATTG CTCAGC-3′) for the DARPin G3-GGGC gene. The
primers Sur and SG contained the complementary sequences
(underlined). Second, the fragments were fused by PCR using the
primer pair T7dir and T7rev. PCR reactions were performed with the
thermostable polymerase Tersus (Evrogen JSC, Moscow, Russia),
following the conditions recommended by the supplier. The junction
between two genes encoded the following amino acid sequence
(SUMO)-QIGG†DLGKK-(DARPin G3-GGGC). The 5′-terminus of the SUMO
gene was extended with the coding sequence GHHHHHHGS. The hybrid
SUMO-DARPin G3-GGGC gene was cloned into the pET39b plasmid vector
between restriction sites NdeI and HindIII. Briefly,
E. coli was grown in autoinduction ZYM-5052 medium prepared
according to Studier (31)
containing 100 µg//ml kanamycin at 25°C. The cells were
harvested by centrifugation at 10,000 × g at 4°C for 20 min, and
resuspended in lysis buffer [200 mM Tris-HCl, 500 mM sucrose, 1 mM
EDTA (pH 8.0), 1 mM PMSF and 60 µg/ml lysozyme]. The
suspension was diluted 2-fold with distilled water and incubated at
room temperature for 30 min. Cells were broken on ice using a Vibra
Cell ultrasonic liquid processor VCX130 (Sonics & Materials,
Inc., Newtown, CT, USA). The cellular debris were pelleted at
70,000 × g at 4°C for 30 min. After addition of imidazole (30 mM)
and NaCl (500 mM), the supernatant was filtered through a 0.22
µm membrane and applied onto a HisTrap HP 1 ml column (GE
Healthcare) equilibrated with 20 mM NaPi (pH 7.5), 500 mM NaCl and
30 mM imidazole. The bound proteins were eluted with a linear
30-500 mM imidazole gradient. The DARPin G3-H6 solution,
diluted 5-fold with 25 mM Tris-Cl (pH 8.0), was loaded onto a MonoQ
10/100 GL column (GE Healthcare) equilibrated with the same buffer.
The bound proteins were eluted with a linear 0-1 M NaCl gradient.
The fractions were analyzed by 15% reducing SDS-PAGE. Protein
concentration was determined by UV spectroscopy using
ε280 =2,560 M−1 cm−1.
The in-house-produced SUMO hydrolase (ULP1)
(29) was added to the
SUMO-G3-GGGC solution at a molar ratio of 1:100 (enzyme:
substrate). The solution was incubated at 6°C overnight, diluted
5-fold with 20 mM NaPi (pH 7.5) and applied to a HisTrap HP 1 ml
column equilibrated with the same buffer. The flow-through eluate
was collected, 2-mercaptoethanol was added to a final concentration
of 50 mM and the protein sample was loaded onto Mono Q 10/100 GL
column equilibrated with 20 mМ NaPi, 50 mM 2-mercaptoethanol (рН
7.5). The bound protein was eluted with a linear 0-1 M NaCl
gradient. The fractions containing DARPin G3-GGGC were pooled and
concentrated with an Amicon Ultra-15 centrifugal filter (Merck
KGaA). The centrifugation was performed at 4,000 × g at 4°C for 20
min. The resultant protein solution was sterilized by filtration
through a 0.22 µm membrane. Protein concentration was
determined by UV spectroscopy using ε280=2,980
M−1 cm−1.
Radiolabeling and stability
Direct radioiodination of G3-H6 with
iodine-125, iodine-131 or iodine-124 using the chloramine-T method
was performed, as described previously for DARPin 9_29 (25).
Site-specific labeling of G3-GGGC with iodine-125
was performed in three steps using maleimide-cysteine conjugation.
In the first step, reduction of G3-GGGC by dithiothreitol (DTT) was
performed to ensure the availability of cysteine for conjugation.
To a solution of G3-GGGC (550 µg; 41 nmol) in degassed PBS
(45 µl), 1,000-fold molar excess of DTT (4.1 µl of 1
M solution; 632 µg; 4.1 nmol) was added. Following
incubation at 40°C for 1 h, G3-GGGC was purified using a NAP-5
size-exclusion column, pre-equilibrated with degassed 0.2 M
NH4OAc (pH 6.0).
In the second step, HPEM was labeled with iodine-125
using the chloramine-T method. To a solution of HPEM (5 µg;
23 nmol) in MeOH containing 1% CH3COOH (10 µl),
[125I]NaI (16 µl; 40-60 MBq) and chloramine-T (5
µl of 8 mg/ml in H2O; 40 µg; 142 nmol)
were added. Following incubation at room temperature for 5 min,
sodium metabisulfite was added (5 µl of 12 mg/ml in
H2O; 60 µg; 316 nmol). The labeling yield was
determined by radio-TLC analysis, which was performed using silica
plates on an aluminum support in ethyl acetate. The radiolabeled
HPEM had Rf=0.8, while the free radioiodine remained at the
application point.
In the third step, the purified G3-GGGC (550
µg; 41 nmol; 900 µl) was added to the
[125I]I-HPEM (5 µg; 23 nmol) in a 1.8:1 molar
ratio and incubated at 40°C for 1 h. The radiolabeled
[125I]I-HPEM-G3-GGGC was purified using a NAP-5 column,
pre-equilibrated and eluted with PBS. The labeling yield was
determined by radio-iTLC analysis in a 4:1 acetone: water
system.
The in vitro stability test was performed by
incubating [125I]I-HPEM-G3-GGGC and
[125I]I-G3-H6 with a 5,000-fold molar excess
of KI in PBS at room temperature for 3 h (control samples were
incubated in PBS). Samples were analyzed by iTLC in 4:1
acetone:water system.
To evaluate the binding affinity of G3-GGGC, it was
labeled with [125I]I using direct iodination, as
described previously (25). To
prevent the formation of dimers via disulfide bonds, the
radiolabeled [125I]I-G3-GGGC (40 µg; 3 nmol) was
reduced by DTT (46 µg; 300 nmol) and purified using the
NAP-5 column. The terminal cysteine of [125I] I-G3-GGGC
(11 µg; 0.8 nmol) was capped by alkylation with
iodoacetamide (IAA; 74 µg; 400 nmol) at 40°C for 30 min. The
radiolabeled [125I]I-G3-GGGC-IAA was purified using the
NAP-5 column, pre-equilibrated and eluted with PBS.
Binding specificity and cellular processing assays.
In vitro studies were performed using cell lines with high
HER2 expression, including SKOV3 (1.6×106
receptors/cell) (32) and BT474
(1.2×106 receptors/cell) (33), and cells with low HER2 expression,
including DU145 (5×104 receptors/cell) (34) and A431 (1.5×105
receptors/cell) (35). Cells were
seeded in 3 cm Petri dishes (~106 cells/dish), and three
dishes were used for each group.
Binding specificity to HER2 was evaluated as
described previously (25). Two
sets of dishes were used for each cell line. A 100-fold excess of
non-labeled DARPin G3-H6 (100 nM) was added to the first
group of cells to saturate the HER2 receptors, and medium only was
added to the second group. After 30 min, radiolabeled
125I[I]-G3-H6 or
[125I]I-HPEM-G3-GGGC were added to each group at 1 nM
concentration. After 1 h in a humidified incubator at 37°C, the
cell medium was collected, cells were washed with 1 ml of fresh
medium and 1 ml of 1 M NaOH was added to lyse the cells. After 30
min of incubation, the cell lysate was collected. The radioactivity
in each fraction was measured to calculate the percentage of
cell-bound radioactivity. The average number of cells per dish at
the time of assay was calculated and the value of cell-bound
radioactivity was calculated per 106 cells. Cellular
retention and the processing of radiolabeled proteins by SKOV3
cells was studied during continuous incubation via an acid-wash
method (25). The cells
(~1×106 cells/dish) were seeded in three dishes for each
time point. Radiolabeled DARPins (1 nM) were added to the cells and
incubated at 37°C in a humidified incubator. At 1, 2, 4, 8 and 24 h
post-addition, the medium was collected from one set of dishes and
the cells were washed once with serum-free media (1 ml). To collect
the membrane-bound DARPins, the cells were treated with 0.2 M
glycine buffer containing 4 M urea, pH 2.0 (1 ml) on ice for 5 min,
the buffer was collected, and the cells were washed once with the
same buffer (1 ml). To collect the internalized DARPins, the cells
were treated with 1 M NaOH (1 ml) for 30 min, following which the
cells were collected and washed with an additional 1 ml. The
activity in every fraction was measured. The percentage of
cell-associated activity was calculated. The maximum value of
cell-associated activity in each dataset (individually for
[125I]I-HPEM-G3-GGGC and for
[125I]I-G3-H6) was taken as 100% and the data
were normalized to this.
Affinity measurements using
LigandTracer
The binding kinetics of radiolabeled DARPins
[125I]I-G3-H6, [125I]I-G3-GGGC-IAA
and [125I]I-HPEM-G3-GGGC to living SKOV3 cells was
measured using LigandTracer (Ridgeview Instruments AB, Vänge,
Sweden) as described previously (36). Kinetics of binding to and
dissociation from cells were recorded at room temperature in real
time. Increasing concentrations of radiolabeled DARPins (0.5 and 2
nM) were added to cells followed by replacement of the medium and
measurements of retention in the dissociation phase. TraceDrawer
Software (version 1.7.1; Ridgeview Instruments AB) was used to
calculate the dissociation constants based on the association and
dissociation rates.
Animal studies
The animal experiments were planned and performed in
accordance with national legislation on laboratory animal
protection. The animal studies were approved by the local ethics
committee for animal research in Uppsala, Sweden (Uppsala
djurgörsöketiska nämnd), decision no. C4/2016.
Female BALB/c nu/nu mice (n=14) of 6 weeks old, with
an average weight at arrival of 16-17 g, were supplied from Scanbur
A/S (Karlslunde, Denmark). Mice were housed in standard conditions
at 22°C, 48% humidity, with a 12/12 h light/dark cycle. Standard
laboratory food and water were provided ad libitum. Mice had
an adaptation period of 1 week prior to the start of the
experimental procedures. For the implantation of tumors,
107 of SKOV3 cells with high HER2 expression or
5×106 of A431 cells with low HER2 expression in 100
µl media were subcutaneously injected in the right hind leg.
The experiments were performed two and a half weeks after
implantation. The average animal weight at the time of sacrifice
was 19±0 g in the SKOV3 group, 18±1 g in the A431 group. The
average tumor weight in the biodistribution studies was 0.08±0.03 g
for SKOV3 xenografts and 0.16±0.06 g for A431 xenografts. The
maximum tumor diameter (in the imaging studies) was 1.0 cm in the
SKOV3 group and 1.1 cm in the A431 group. The tumor volume was
<1 cm3. No multiple tumors were observed. For the
comparative biodistribution of [131I]I-G3-H6
and [125I]I-HPEM-G3-H6-GGGC, a dual-label
approach was used. The mice were intravenously injected with a
mixture of [131I]I-G3-H6 and
[125I]I-HPEM-G3-H6-GGGC in 100 µl of
1% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in PBS per
mouse (27 kBq for [131I]I-G3-H6, 20 kBq for
[125I]I-HPEM-G3-H6-GGGC). In addition, the
biodistribution of [125I]I-G3-H6 was studied
in SKOV-3-bearing BALB/c nu/nu mice with Na/I-symporters blocked by
supplementation of drinking water with 1% KI 3 days prior to the
experiment. The injected protein amount was adjusted to 4 µg
by non-labeled G3-H6. At 4 h post-injection (pi) mice
were anesthetized by an intraperitoneal injection of ketamine and
xylazine solution and sacrificed by heart puncture. The dose of
ketamine was 250 mg/kg, and the dose of xylazine was 25 mg/kg. The
average volume of blood collected by cardiac puncture with a
heparinized syringe was 0.7±0.2 ml. The salivary glands, lungs,
liver, spleen, stomach (without contents), kidneys, tumor, samples
of muscle and bone from the contralateral leg to the tumor
implantation site, the gastrointestinal tract with contents and the
tail were harvested. Organs were weighed and activity was measured
using an automated γ-spectrometer. The percentage of injected dose
per gram of sample (%ID/g) was calculated. Data for the intestines
with contents and carcass were calculated as %ID per whole sample.
Spectra of standards and samples were recorded. For the
dual-isotope study, the activities of iodine-125 and iodine-131
were calculated by integration of the counts in the energy ranges
5-100 keV and 150-500 keV, respectively. The data were corrected
for dead time of the γ-spectrometer, background and spillover of
iodine-131 counts into the iodine-125 energy window.
PET and SPECT imaging was performed to obtain visual
confirmation of the ex vivo biodistribution measurements. A
total of 3 days before the imaging, the drinking water was
supplemented with 1% potassium iodide.
The SPECT study was performed using
125I-labeled G3-H6. Although 131I
is also potentially suitable for SPECT imaging, it emits high
energy γ quanta (364 and 637 keV) (37). This requires a specialized
high-energy whole-body collimator, which was not available in the
device used in the present study. Therefore, the low-energy γ
emitter 125I was used as the label.
A mouse bearing SKOV3 xenografts with high HER2
expression was injected with [125I]I-G3-H6 (7
µg; 19.7 MBq). The SPECT imaging was performed using
nanoScan SPECT/CT (Mediso Medical Imaging Systems Ltd., Budapest,
Hungary) at 1, 2 and 4 h post-injection. The acquisition time was
15 min. CT scans were acquired using the following parameters:
X-ray energy peak of 50 keV; 670 µA; 480 projections; and
5.26 min acquisition time. A mouse bearing A431 xenografts with low
HER2 expression was injected with
[125I]I-G3-H6 (7 µg, 19.7 MBq). The
imaging was performed at 4 h post-injection using the same settings
as those for the mouse bearing the SKOV-3 xenograft. SPECT raw data
were reconstructed using Tera-Tomo™ 3D SPECT reconstruction
technology (version 3.00.020.000; Mediso Medical Imaging Systems
Ltd.): Normal dynamic range; 48 iterations; 1 subset. The area
corresponding to the activity in the urinary bladder was removed
from the SPECT image following reconstruction to facilitate better
visualization of tumor uptake. CT data were reconstructed using
Filter Back Projection in Nucline 2.03 Software (Mediso Medical
Imaging Systems Ltd.). SPECT and CT files were fused using Nucline
2.03 Software and are presented as maximum intensity projections
(MIP) in the RGB color scale.
Whole-body PET imaging was performed using nanoScan
PET/MRI (Mediso Medical Imaging System). A mouse bearing SKOV3
xenografts was injected with [124I] I-G3-H6
(7 µg; 4.7 MBq) and imaged at 4 h pi. The PET scan was
performed for 90 min; subsequently, the CT scan was performed using
nanoScan SPECT/CT (Mediso Medical Imaging Systems Ltd.) using the
same bed position as for the PET scan. The CT scan was acquired
using the same parameters as for the SPECT/CT images and the CT
data were reconstructed in the same way. PET data were
reconstructed using the Tera- 3D reconstruction engine. PET and CT
files were fused using Nucline 2.03 Software and are presented as
maximum intensity projections (MIP) in the RGB color scale.
Statistical analysis of the data
The in vitro specificity and cell processing
data are presented as the mean ± standard deviation of three
samples. The data were analyzed using an unpaired two-tailed t-test
to find the significant differences. A paired two-tailed t-test was
performed using GraphPad Prism (version 7.02; GraphPad Software,
Inc., La Jolla, CA, USA) for analysis of the biodistribution data
from the dual-label study to find significant differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Radiolabeling and stability
A total of two methods for the radio-iodination of
DARPin G3 variants were used: Direct labeling of G3-H6
and indirect labeling of G3-GGGC using a HPEM linker. The data
concerning the isolated radiochemical yields and radiochemical
purity of the conjugates are presented in Table I.
 | Table ILabeling of designed ankyrin repeat
proteins G3-H6 and G3-GGGC using direct and indirect
radioiodination. |
Table I
Labeling of designed ankyrin repeat
proteins G3-H6 and G3-GGGC using direct and indirect
radioiodination.
| Labeling
method | Labeled
protein | Radionuclide | Radiochemical
yield, % | Radiochemical
purity, % |
|---|
| Direct random
labeling |
G3-H6 |
125I | 98±1 (n=3) | 99±1 (n=3) |
|
G3-H6 |
124I | 87 (n=1) | 99 (n=1) |
|
G3-H6 |
131I | 99 (n=1) | 99 (n=1) |
| Indirect
site-specific labeling | G3-GGGC (conjugated
to HPEM) |
125I | 31±1 (n=2, overall
yield) | 95±0 (n=2) |
Direct labeling of G3-H6 with iodine-125
was performed as described previously for DARPin 9_29 (22), with a high radiochemical yield.
Size-exclusion chromatography on a NAP-5 column provided
radiolabeled proteins with a radiochemical purity >98%. For the
biodistribution studies and imaging, direct iodination of
G3-H6 with iodine-131 and iodine-124 was performed using
the same labeling protocol with good radiochemical yields.
Purification using a NAP-5 column provided
[131I]I-G3-H6 and
[131I]I-G3-H6 with 99% radiochemical
purity.
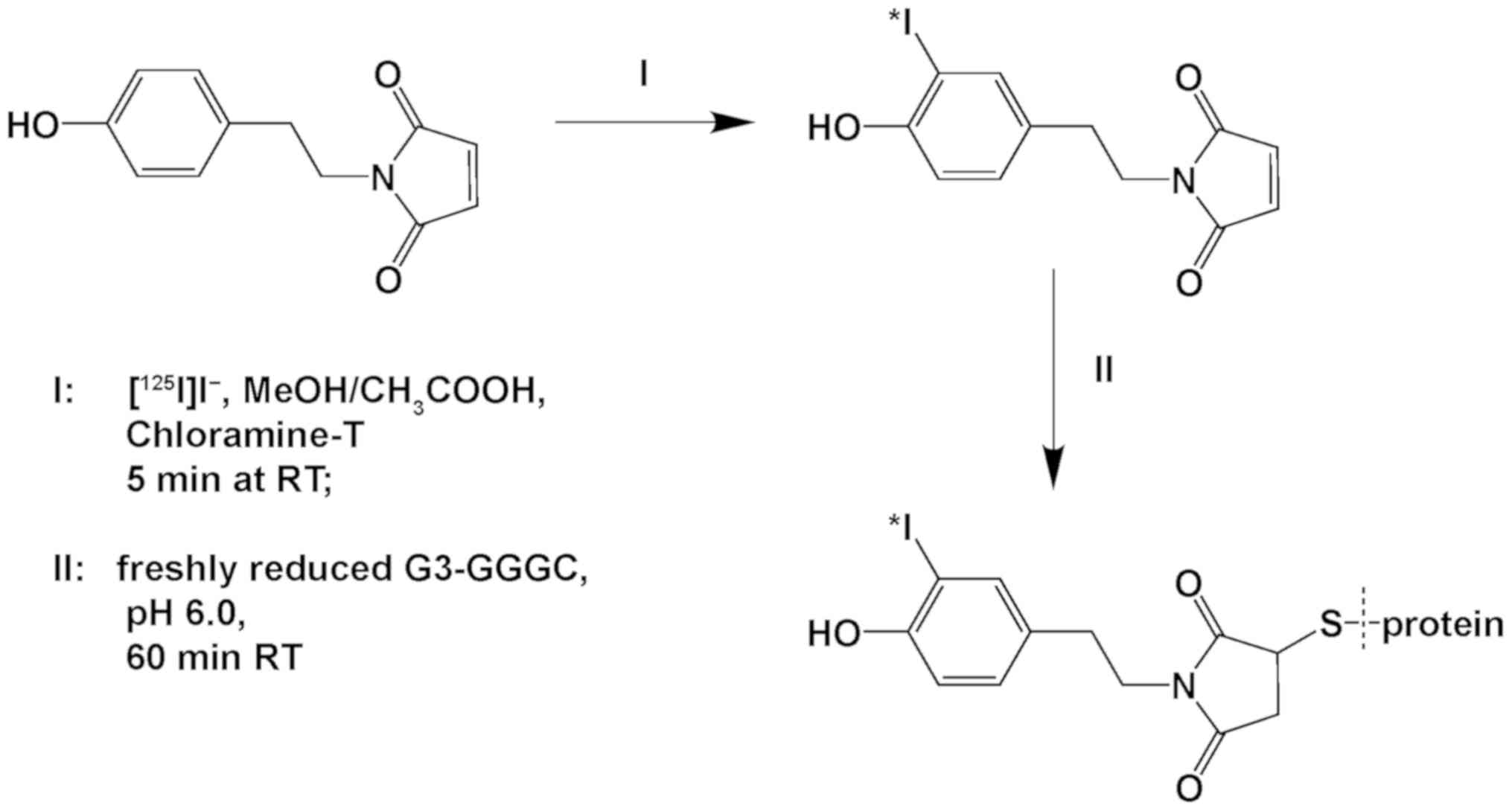
Indirect labeling was performed as a one-pot
procedure in two steps without intermediate purification (Fig. 1). First, a bifunctional linker with
an activated phenolic ring and a maleimide group (HPEM) was
iodinated, with a resulting radiochemical yield of 95±3%.
Subsequently, [125I]I-HPEM was conjugated to G3-GGGC
with an overall radiochemical yield of 31±1%. The radiolabeled
conjugate was purified on a NAP-5 column with radiochemical purity
of 95±0%. Specific activity of 33 kBq/µg was achieved.
Radio-HPLC analysis of
[125I]I-G3-H6 and
[125I]I-HPEM-G3-GGGC illustrated a single peak at 14.5
min. Incubation of [125I]I-G3-H6 and
[125I]I-HPEM-G3-GGGC with a 5,000-fold molar excess of
cold iodide did not demonstrate any measurable release of the
labeled compound compared with the PBS control (Table II).
 | Table IIIn vitro stability of
[125I]I-HPEM-G3-GGGC and
[125I]I-G3-H6. |
Table II
In vitro stability of
[125I]I-HPEM-G3-GGGC and
[125I]I-G3-H6.
| Test solution | DARPin-associated
activity, %
|
|---|
[125I]I-HPEM-G3-GGGC
|
[125I]I-G3-H6
|
|---|
| 1 h | 3 h | 1 h | 3 h |
|---|
| PBS (control) | 97±1 | 97±1 | 99±0 | 99±0 |
| 5,000X KI | 98±0 | 95±2 | 99±0 | 99±0 |
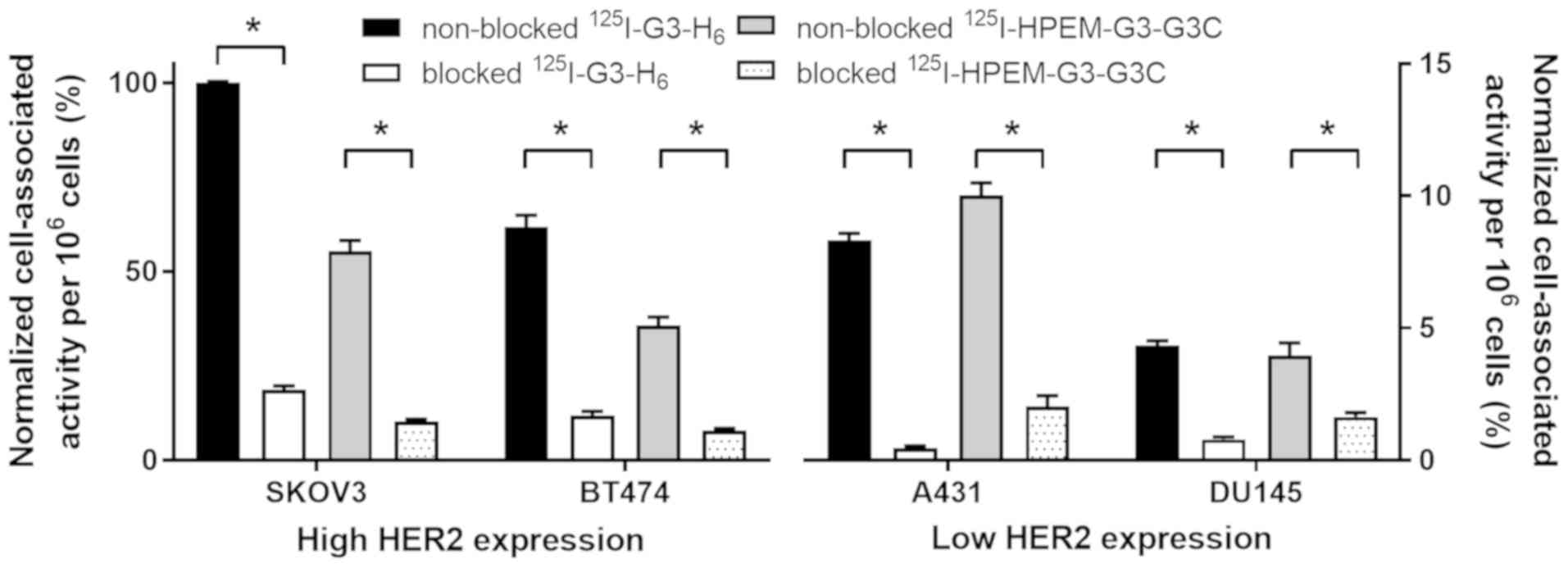
In vitro studies. Binding specificity of
[125I]I-G3-H6 and
[125I]I-HPEM-G3-GGGC to HER2 was evaluated in cancer
cell lines possessing different levels of HER2 expression (Fig. 2). Saturable character binding of
the two radiolabeled DARPins to HER2 demonstrated specificity.
Cell-bound activity was proportional to the level of HER2
expression in cells, and was higher for SKOV3 and BT474 compared
with A431 and DU145 cells.
The binding kinetics of
[125I]I-G3-H6, [125I]I-G3-GGGC-IAA
and [125I]I-HPEM-G3-GGGC to HER2-expressing SKOV3 cells
was measured using LigandTracer. The binding of all labeled G3
variants to living cells was best fitted to a 1:2 interaction
model, as previously published for the anti-HER2 DARPin 9_29
(25). Two types of interactions
were observed: A high affinity interaction in the picomolar range,
and a low affinity interaction in the single digit nanomolar range
(Table III). The same types of
binding interactions have been previously observed for DARPin 9_29
(25). All three variants had
similar values for the high affinity interaction, and the
[125I]I-G3-GGGC-IAA had a slightly lower dissociation
constant for the second interaction. These data confirmed that
[125I]I-G3-H6 had the same binding affinity
to HER2 as [125I]I-HPEM-G3-GGGC, and that the direct
iodination had no adverse effect on binding.
 | Table IIIDissociation equilibrium constants
(KD) for the interaction between radiolabeled designed
ankyrin repeat proteins and human epidermal growth factor receptor
2-expressing SKOV3 cells. |
Table III
Dissociation equilibrium constants
(KD) for the interaction between radiolabeled designed
ankyrin repeat proteins and human epidermal growth factor receptor
2-expressing SKOV3 cells.
| KD1
(pM) | KD2
(nM) |
|---|
|
[125I]I-HPEM-G3-GGGC (n=3) | 99±5 | 3.5±0.4 |
|
[125I]I-G3-GGGC-IAA (n=2) | 146±20 | 1.5±0.4 |
|
[125I]I-G3-H6
(n=3) | 163±41 | 3.9±1.5 |
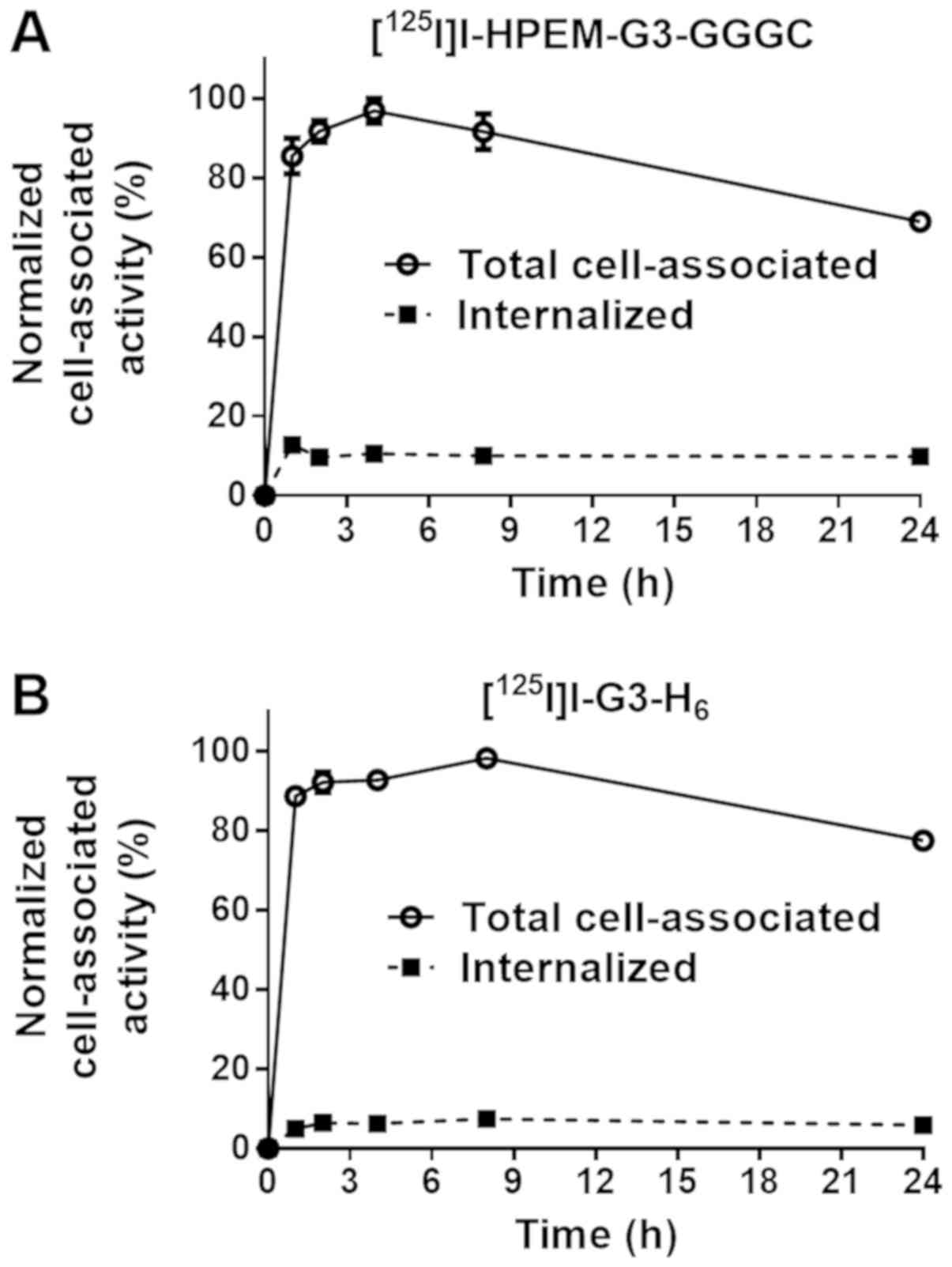
The processing of radiolabeled DARPins by
HER2-expressing SKOV3 cells is illustrated in Fig. 3. The pattern of processing was
typical for a non-residualizing label with a low internalized
fraction for the two proteins. The decrease in cell-associated
activity following maximal accumulation was due to the release of
radiocatabolites from cells. Cell-associated activity was >85%
of the maximum between 1 and 8 h post-addition.
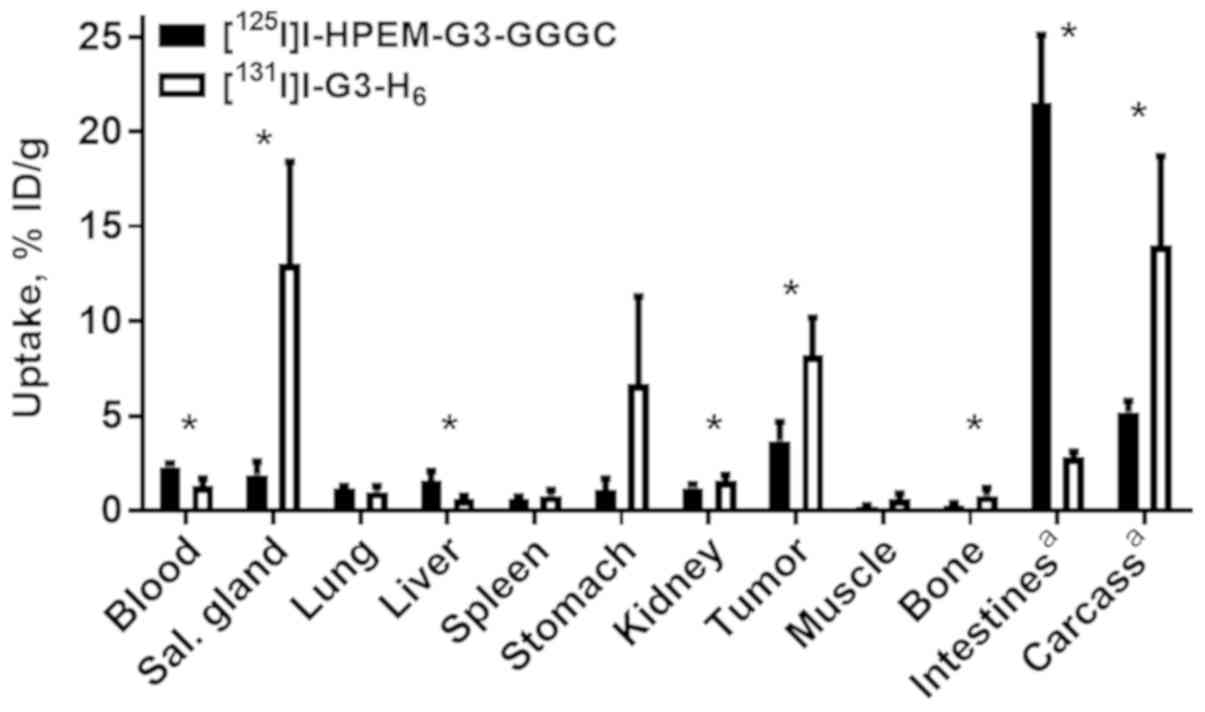
Animal studies
To study the biodistribution of radio-iodinated
proteins side-by-side in vivo, a dual isotope approach was
used. HPEM-G3-GGGC was labeled with iodine-125 and G3-H6
was labeled with iodine-131. Biodistribution and tumor targeting
were studied in BALB/C nu/nu mice bearing HER2-expressing SKOV3
xenografts at 4 h pi (Fig. 4). The
two radiolabeled DARPins had fast clearance from the blood and low
retention in excretory organs. Notably,
[125I]I-HPEM-G3-GGGC demonstrated 2-fold lower
accumulation in tumors compared with
[131I]I-G3-H6 (3.7±1.0 vs. 8.2±2.0 %ID/g;
P=0.003; paired t-test). Besides lower tumor uptake, a low level of
iodine-125 radiocatabolites was observed in organs with expression
of Na/I-symporters (salivary glands, stomach) compared with
iodine-131. Accumulation of iodine-125 activity in the intestines
was approximately seven times higher compared with that for
iodine-131.
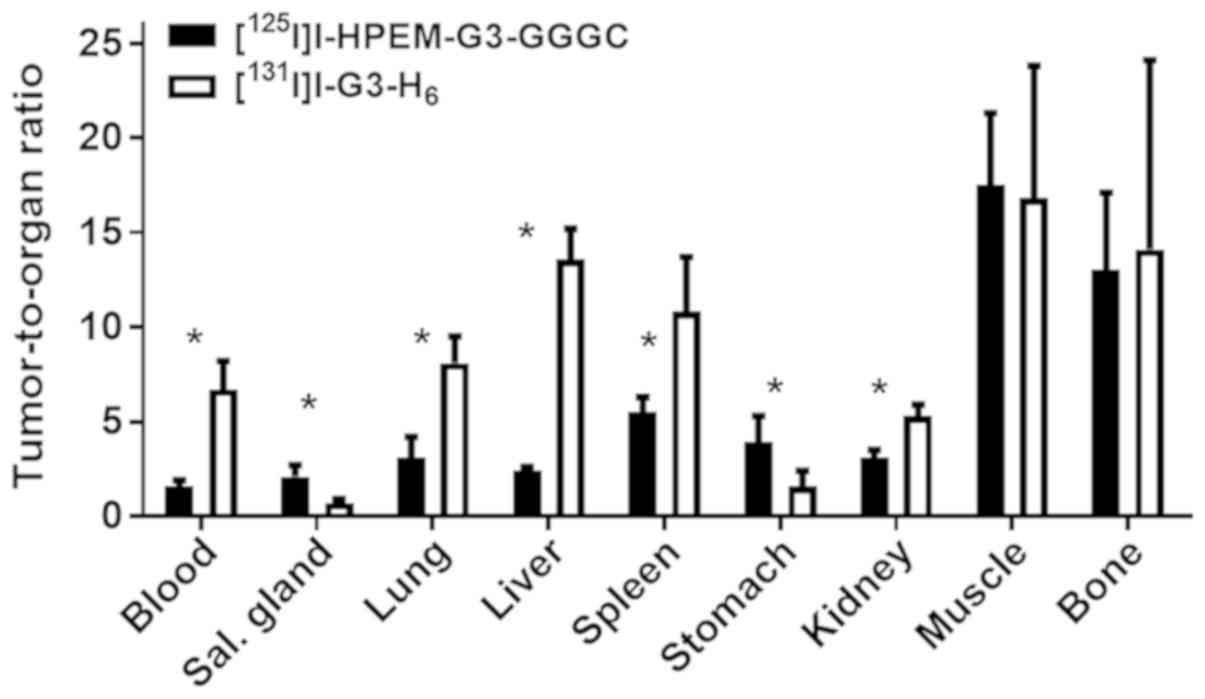
High tumor accumulation of
[131I]I-G3-H6 resulted in significantly
(P<0.05, determined by paired t test) higher tumor-to-organ
ratios for the majority of organs, apart from organs expressing
sodium-iodide symporters, muscles and bones, in comparison with
[125I]I-HPEM-G3-GGGC (Fig.
5).
The present study further investigated the
biodistribution of [125I]I-G3-H6 in
SKOV3-bearing BALB/c nu/nu mice when the Na/I-symporters were
blocked by cold KI. The uptake of [125I]
I-G3-H6 in the tumor and kidneys was at the same level
as the uptake of [131I]I-G3-H6 (P>0.05;
unpaired t-test). The blocking of Na/I-symporters resulted in a
marked decrease in uptake in the salivary glands and stomach. There
was a significant (P<0.05; unpaired t-test) improvement of
tumor-to-organ ratios for every organ, except the kidneys and bone
(Table IV).
 | Table IVBiodistribution and tumor-to-organ
ratios of [125I]I-G3-H6 (Na/I-symporters were
blocked by cold KI) and [131I]I-G3-H6 (no
blockade of Na/I-symporters) at 4 h post-injection in BALB/C nu/nu
mice bearing SKOV3 xenografts. |
Table IV
Biodistribution and tumor-to-organ
ratios of [125I]I-G3-H6 (Na/I-symporters were
blocked by cold KI) and [131I]I-G3-H6 (no
blockade of Na/I-symporters) at 4 h post-injection in BALB/C nu/nu
mice bearing SKOV3 xenografts.
| Location | Uptake of
[125I]I-G3-H6, % ID/g | Tumor-to-organ
ratio for [125I]I-G3-H6 | Uptake of
[131I]I-G3-H6, % ID/g | Tumor-to-organ
ratio for [131I]I-G3-H6 |
|---|
| Blood | 0.3±0.1a | 37±13a | 1.3±0.4 | 7±2 |
| Salivary
glands | 0.23±0.08a | 45±14a | 13.0±5.4 | 0.7±0.2 |
| Lung | 0.4±0.1a | 29±8a | 1.0±0.3 | 8±1 |
| Liver | 0.16±0.05a | 48±22a | 0.6±0.2 | 14±2 |
| Spleen | 0.22±0.07a | 48±13a | 0.8±0.3 | 11±3 |
| Stomach | 0.6±0.2a | 18±7a | 6.7±4.6 | 2±1 |
| Kidney | 1.5±0.4 | 6.7±1.4 | 1.6±0.3 | 5.3±0.6 |
| Muscle | 0.19±0.03a | 53±9a | 0.6±0.3 | 17±7 |
| Bone | 0.4±0.2 | 33±25 | 0.8±0.4 | 14±10 |
| Tumor | 9±3 | – | 8±2 | – |
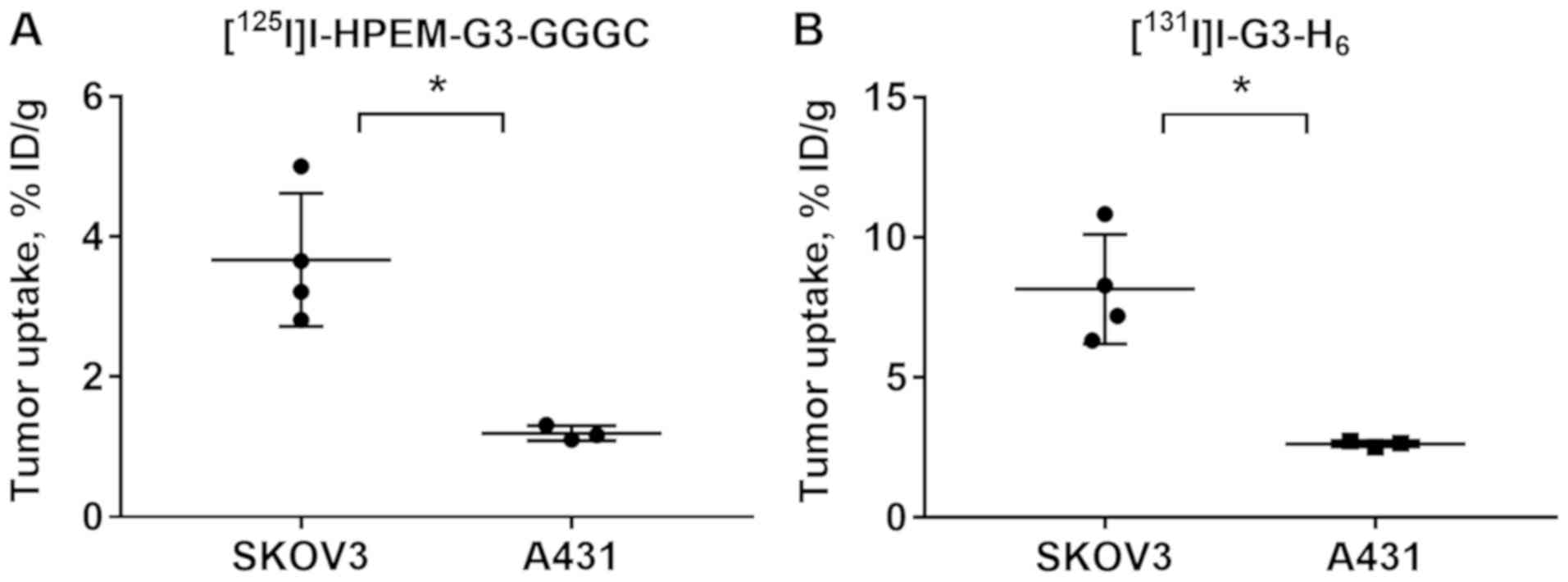
Specificity of HER2 targeting by radioiodinated
DARPins was confirmed in BALB/C nu/nu mice bearing A431 xenografts
with a low level of HER2 expression (Fig. 6). The tumor uptake of
[125I]I-HPEM-G3-GGGC and
[131I]I-G3-H6 was significantly (P=0.007 and
P=0.005, respectively; unpaired t-test) lower in A431 xenografts
compared with SKOV3 xenografts.
Data from the ex vivo measurements were
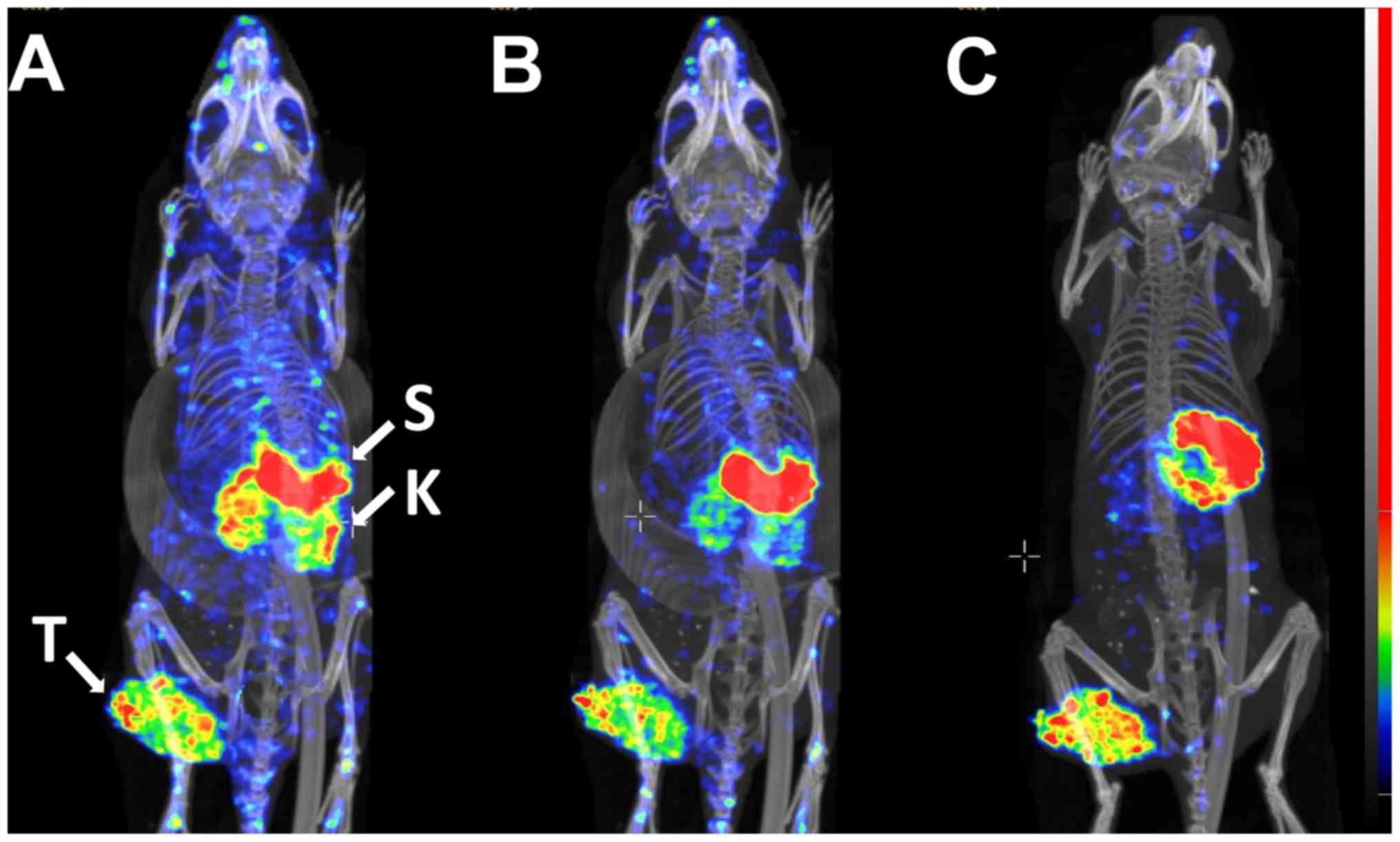
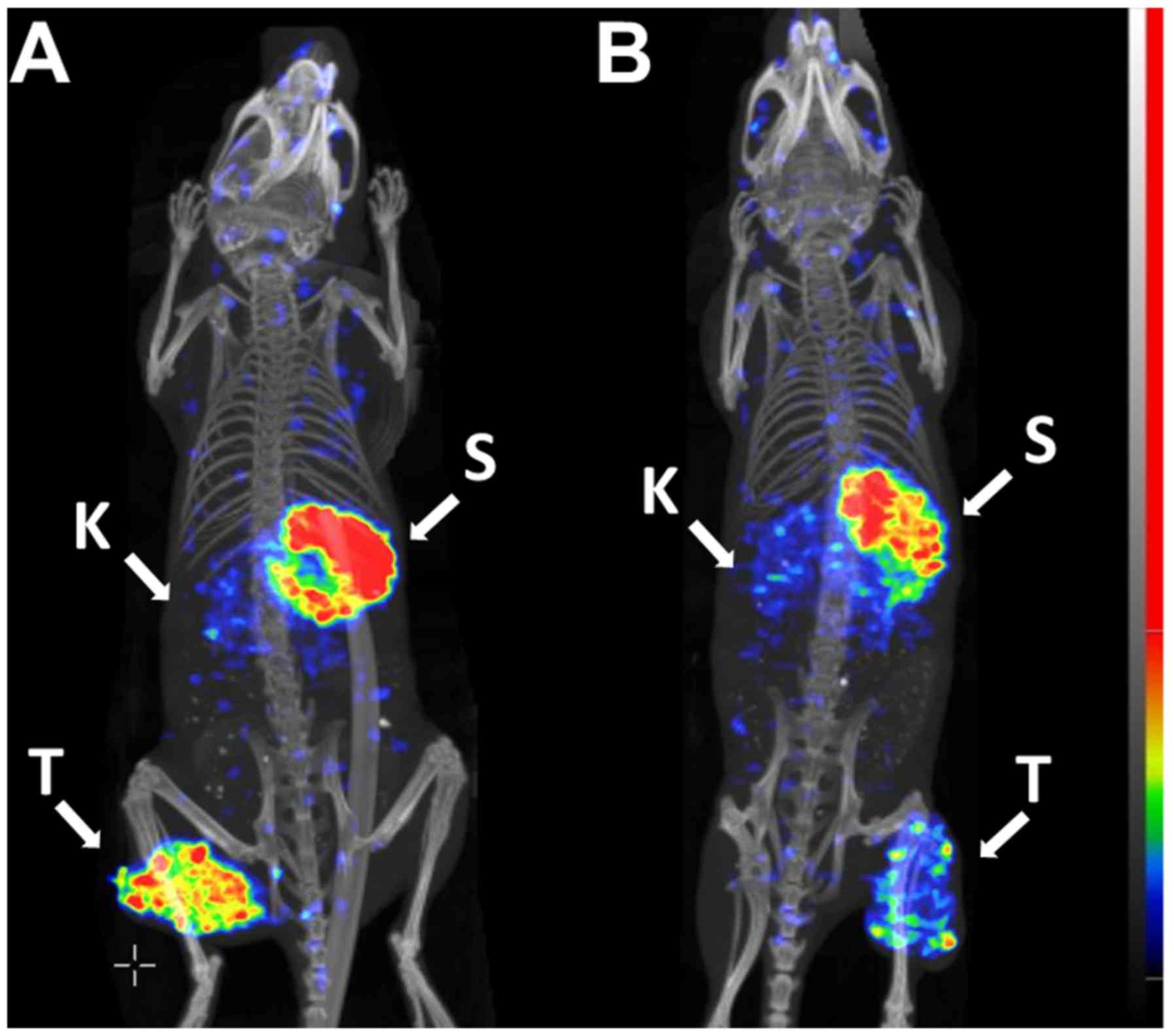
confirmed by experimental imaging (Figs. 7-9). The SPECT/CT data revealed a high
activity accumulation in SKOV-3 xenografts with already-high HER2
expression at 1 h following injection (Fig. 7A). Besides the tumor, appreciable
activity accumulation was visualized in the kidneys and stomach.
Activity uptake in other tissues was lower compared with that in
the tumor at this time point. At the later time points, 2 h after
injection (Fig. 7B) and 4 h after
injection (Fig. 7C), the high
activity uptake in the tumor remained, but the activity in the
kidneys was considerably reduced. The activity in other normal
tissues was also reduced, which resulted in a noticeable increase
in the imaging contrast. The activity in the stomach remained high
during the whole imaging experiment. Subsequent to imaging, the
gastrointestinal tract was excised, and the activity was measured.
The highest activity accumulation was observed in the stomach
contents (data not shown). The uptake in the A431 xenografts with
low HER2 expression (Fig. 8B) was
lower compared with that in the SKOV3 xenograft, as expected
(Fig. 8A), while the uptake in
other tissues followed the same pattern as in the mouse with the
SKOV3 xenograft.
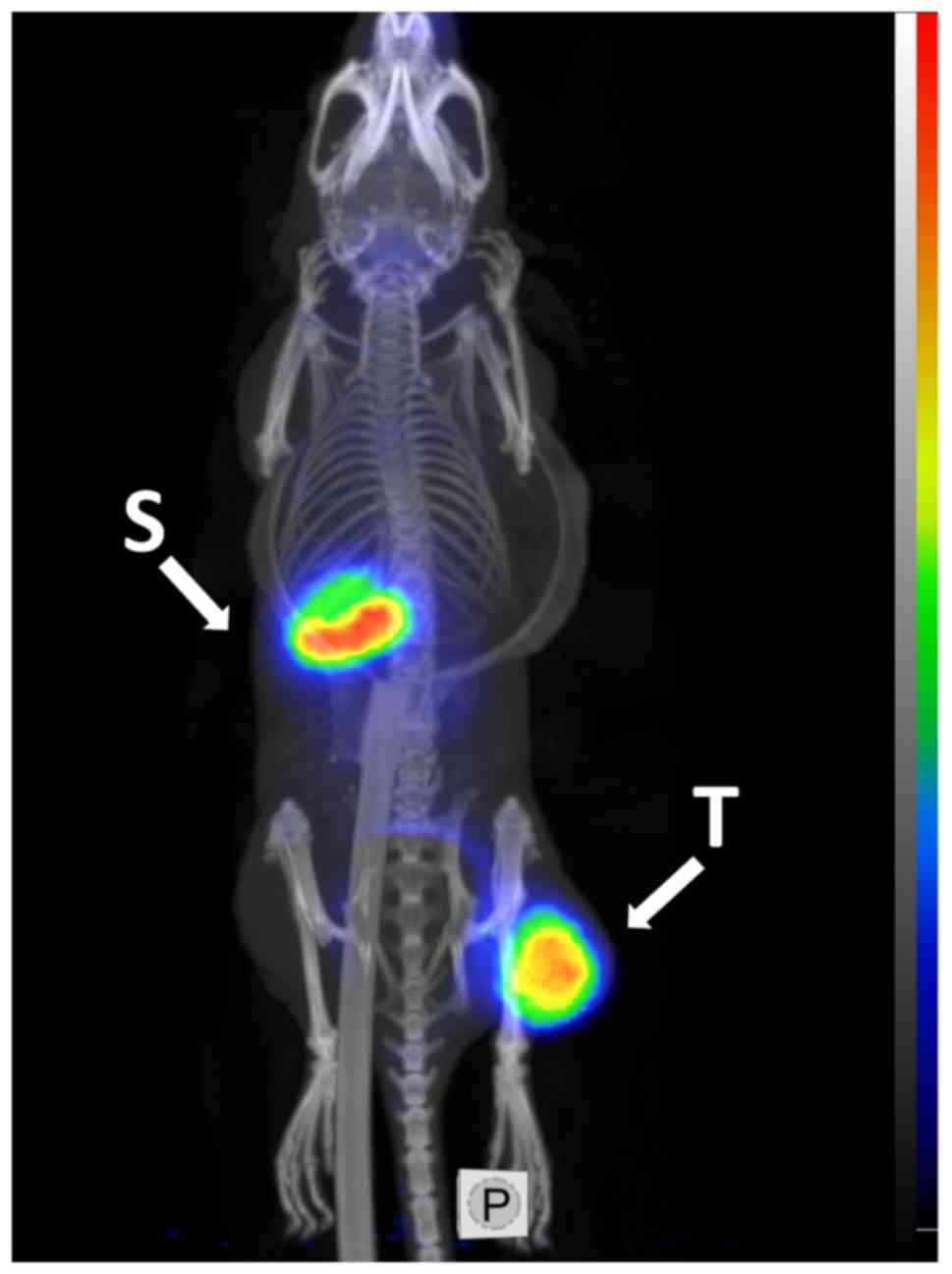
To demonstrate the feasibility of PET imaging of
HER2-expressing xenografts using the best-performing variant,
G3-H6 was labeled with the positron emitter iodine-124.
MicroPET imaging with [124I]I-G3-H6, clearly
visualized the HER2-expressing SKOV-3 xenograft at 4 h pi (Fig. 9). Low accumulation of activity was
observed in other organs, with the exception of the stomach
contents in the upper abdomen.
Discussion
In the present study, direct and indirect labeling
methods for the radioiodination of DARPin G3 were compared in order
to select an imaging probe with the best biodistribution and tumor
targeting properties.
Direct radioiodination of proteins using
chloramine-T is a straightforward, fast and robust labeling method
that provides high radiochemical yields and high specific
activities. However, the number of labels and their position in the
protein is not controlled. Indirect radioiodination using
bifunctional linkers allows for site-specific attachment of labels
and provides well-defined conjugates. The selection of linkers
allows for the optimization of biodistribution properties,
including intracellular retention and excretion of
radiocatabolites. Additionally, the accumulation of
radiocatabolites in organs with expression of Na/I-symporters
(thyroid, salivary glands and stomach) is generally reduced with
indirect iodination (27,28).
The bifunctional HPEM linker bearing an activated
phenolic ring and a maleimide group is suitable for ‘one-pot’
radiohalogenation and conjugation to reactive thiol groups on
proteins. The authors of the present study have previously
demonstrated that site-specific radiobromination of affibody
molecules using HPEM reduces their retention in the kidneys more
than 7-fold in comparison with another linker, N-succinimidyl
4-bromobenzoate (38). In a
different study, the use of a [125I]I-HPEM label was
even more favorable for decreasing the renal radioactivity of
another engineered scaffold protein, ADAPT (39). This attractive feature of HPEM led
to its evaluation for site-specific radioiodination of DARPin G3,
with the goal of improving the excretion and clearance of
radioiodine catabolites in vivo.
The two labeling approaches used in the present
study provided radiolabeled G3 variants with reasonably high
radiochemical yields and satisfactory radiochemical purities. The
stability of the label was high following direct and indirect
labeling.
A HER2-binding saturation assay demonstrated the
binding specificity of the two radiolabeled DARPins
[125I] I-HPEM-G3-GGGC and
[125I]I-G3-H6 to HER2-expressing cells.
Cell-bound activity was proportional to the HER2 expression level
in the studied cell lines for the two conjugates. Each variant had
an equally high affinity to HER2-expressing cells in vitro.
These results demonstrated that the indirect labeling method did
not alter the binding specificity and affinity of G3-GGGC to
HER2.
For the two labeled variants, the pattern of
cellular processing was similar and characteristic for a
non-residualizing label. Proteins labeled with non-residualizing
labels produce lipophilic radiocatabolites that diffuse from cells
following internalization and intracellular degradation (40). Following maximal accumulation, the
decrease in cell-associated activity was due to the release of
radiocatabolites from cells. High cell-associated activity up to 8
h post-addition indicated the relevance of using these variants for
clinical imaging.
HER2-mediated tumor targeting was confirmed for the
two radiolabeled DARPins in mice bearing xenografts with different
levels of HER2 expression. A significantly lower uptake of DARPins
was observed in A431 xenografts (low HER2 expression) in comparison
with SKOV3 xenografts (high HER2 expression).
Comparative biodistribution studies in mice bearing
SKOV3 xenografts demonstrated the high accumulation of
[125I]I-HPEM-G3-GGGC in the intestines, which suggests
rapid hepatobiliary clearance of the conjugate. Despite the similar
size and affinity of the two G3 variants to HER2,
[125I]I-HPEM-G3-GGGC demonstrated 2-fold lower
accumulation in tumors compared with
[131I]I-G3-H6. These results suggested that
[125I]I-HPEM-G3-GGGC was quickly removed from the blood
circulation via sequestration in the liver. This, in turn, likely
led to low bioavailability and less of the conjugate being
delivered to the tumor.
An increased liver uptake has previously been
observed for the radiobrominated HPEM-affibody conjugate in
comparison with an N-succinimidyl 4-bromobenzoate affibody;
however, the overall level of uptake was quite low (1.32±0.31 vs.
0.22±0.03 %IA/g, respectively) (38). In the case of ADAPT, the C-terminal
placement of [125I]I-HPEM provided a better tumor uptake
compared with N-terminal placement, although it also led to higher
liver retention (1.6±0.8 vs. 0.6±0.3 %IA/g at 4 h pi, respectively)
(39).
Peptide-based chelators containing three glycines
GGG at the C terminus of affibody molecules labeled with
technetium-99m (41) and
rhenium-188 (42) provide
non-residualizing labels with high tumor uptake and low retention
in normal organs. On the other hand, the N-terminal placement of
GGG in the affibody molecule labeled with technetium-99m results in
a high level of hepatobiliary excretion (43). It was therefore hypothesized that
the C-terminal placement of GGG in DARPin G3-GGGC would provide low
retention of activity in excretory organs and organs with
expression of sodium-iodide symporters. The uptake of activity in
the salivary glands and stomach was indeed lower for
[125I]I-HPEM-G3-GGGC compared with
[125I]I-G3-H6. However, it is possible that
the combination of the GGG amino acid sequence and the HPEM linker
at the C terminus of G3 led to increased local hydrophobicity of
the conjugate and the primarily hepatobiliary excretion of the
labeled protein.
The use of direct radioiodination for labeling of
G3-H6 provided significantly higher tumor-to-blood and
tumor-to-organ ratios for the lungs and liver, which are important
metastatic sites, compared with indirect labeling. The re-uptake of
radiocatabolites in the salivary glands, thyroid and stomach was
blocked to a large extent by cold iodide, as demonstrated by
microSPECT/CT imaging using [125I]I-G3-H6 and
by microPET/CT imaging using [124I]I-G3-H6.
Blocking of Na/I-symporters further improved the tumor-to-organ
ratios for G3-H6. Notably, the blocking of the
Na/I-symporter in the stomach was not complete at the doses of KI
used in the present study. It was previously demonstrated in rats
that the administration of ‘cold’ iodide suppressed the
accumulation of 131I in the thyroid, but not in the
gastric juices, which suggested a lower affinity of gastric
symporters to iodide compared with those of the thyroid (44). G3-H6 labeled with
iodine-124 provided high-contrast PET images of HER2 expression in
human xenografts in mice shortly following injection. Direct
radioiodination of DARPin G3-H6 is a straightforward and
well-established method applicable to a number of radioiodine
isotopes, including 123I and 124I. Iodine-123
has a half-life of 13.2 h and emits 159 keV (84%) γ rays. This
emission energy is ideal for modern γ cameras and allows for the
use of low energy high resolution collimators. Iodine-124 is a
positron emitter that offers quantitative PET imaging with high
spatial resolution for improved diagnostic accuracy of cancer.
In conclusion, direct and indirect site-specific
methods provided stable labeling of DARPins with preserved capacity
for binding HER2 in vitro and in vivo. However, an
appreciable level of hepatobiliary excretion of
[125I]I-HPEM-G3-GGGC was observed in vivo, which
hampered efficient tumor targeting. The use of direct
radioiodination is therefore the preferred approach for labeling
DARPin G3-H6 with iodine-123 and iodine-124 for further
clinical SPECT and PET imaging of HER2 expression.
Funding
The present study was financially supported by
grants from the Swedish Cancer Society (grant nos. CAN 2015/350 and
2017/425), the Swedish Research Council (grant nos. 2015-02353 and
2015-02509), the Swedish Agency for Innovation VINNOVA (grant no.
2016-04060), RFBR grant nos. 17-00-00121 (komfi), 18-04-00365 А and
18-34-00899 mol_a for the protein engineering and purification, the
State Contract of Russian Federation no. 14.N08.11.0163, and by
Tomsk Polytechnic University CE Program.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors’ contributions
AV participated in the study design, conjugation
and labeling chemistry development, in vitro and in
vivo studies, data treatment and interpretation, and drafting
of the first version of the manuscript. AS and EK performed the
production and purification of proteins. RG and JL performed the
biochemical and biophysical characterization of proteins. BM, JG,
SR and AO participated in planning and performing in vivo
experiments, including imaging, data treatment and interpretation.
SD participated in the molecular design of the probes, and
supervised the production and purification of proteins, the
biochemical and biophysical characterization, and coordinated the
project. VT participated in the study design, labeling chemistry
development, in vivo studies, data treatment and
interpretation, and coordinating of the work at the Uppsala site.
All co-authors revised the manuscript and approved the final
version.
Ethics approval and consent to
participate
The animal experiments were planned and performed
in accordance with Swedish national legislation on laboratory
animal protection and were approved by the local Ethics Committee
for Animal Research in Uppsala, Sweden.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mr. Joshua Gentry
for proof-reading the paper.
Abbreviations:
|
DARPin
|
designed ankyrin repeat protein
|
|
HER2
|
human epidermal growth factor
receptor 2
|
|
HPEM
|
[(4-hydroxyphenyl) ethyl]
maleimide
|
|
PET
|
positron emission tomography
|
|
CT
|
computed tomography
|
|
SPECT
|
single photon emission computed
tomography
|
|
HPLC
|
high performance liquid
chromatography
|
References
|
1
|
Giordano SH, Temin S, Kirshner JJ,
Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Gonzalez-Angulo
AM, Krop I, Levinson J, et al: American Society of Clinical
Oncology: Systemic therapy for patients with advanced human
epidermal growth factor receptor 2-positive breast cancer: American
Society of Clinical Oncology clinical practice guideline. J Clin
Oncol. 32:2078–2099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM,
Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, et
al: HER2 screening data from ToGA: Targeting HER2 in gastric and
gastro-esophageal junction cancer. Gastric Cancer. 18:476–484.
2015. View Article : Google Scholar
|
|
3
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gianni L, Pienkowski T, Im YH, Roman L,
Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J,
Im SA, et al: Efficacy and safety of neoadjuvant pertuzumab and
trastuzumab in women with locally advanced, inflammatory, or early
HER2-positive breast cancer (NeoSphere): A randomised multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:25–32. 2012. View Article : Google Scholar
|
|
5
|
de Azambuja E, Holmes AP, Piccart-Gebhart
M, Holmes E, Di Cosimo S, Swaby RF, Untch M, Jackisch C, Lang I,
Smith I, et al: Lapatinib with trastuzumab for HER2-positive early
breast cancer (NeoALTTO): Survival outcomes of a randomised,
open-label, multicentre, phase 3 trial and their association with
pathological complete response. Lancet Oncol. 15:1137–1146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al American Society of Clinical Oncology: College
of American Pathologists: Recommendations for human epidermal
growth factor receptor 2 testing in breast cancer: American Society
of Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foukakis T, Åström G, Lindström L,
Hatschek T and Bergh J: When to order a biopsy to characterise a
metastatic relapse in breast cancer. Ann Oncol. 23(Suppl 10):
x349–x353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Houssami N, Macaskill P, Balleine RL,
Bilous M and Pegram MD: HER2 discordance between primary breast
cancer and its paired metastasis: Tumor biology or test artefact?
Insights through meta-analysis. Breast Cancer Res Treat.
129:659–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilking U, Karlsson E, Skoog L, Hatschek
T, Lidbrink E, Elmberger G, Johansson H, Lindström L and Bergh J:
HER2 status in a population-derived breast cancer cohort:
Discordances during tumor progression. Breast Cancer Res Treat.
125:553–561. 2011. View Article : Google Scholar
|
|
11
|
Krasniqi A, D’Huyvetter M, Devoogdt N,
Frejd FY, Sörensen J, Orlova A, Keyaerts M and Tolmachev V:
Same-day imaging using small proteins: Clinical experience and
translational prospects in oncology. J Nucl Med. 59:885–891. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orlova A, Magnusson M, Eriksson TL,
Nilsson M, Larsson B, Höidén-Guthenberg I, Widström C, Carlsson J,
Tolmachev V, Ståhl S, et al: Tumor imaging using a picomolar
affinity HER2 binding affibody molecule. Cancer Res. 66:4339–4348.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garousi J, Lindbo S, Nilvebrant J, Åstrand
M, Buijs J, Sandström M, Honarvar H, Orlova A, Tolmachev V and
Hober S: ADAPT, a novel scaffold protein-based probe for
radionuclide imaging of molecular targets that are expressed in
disseminated cancers. Cancer Res. 75:4364–4371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hackel BJ, Kimura RH and Gambhir SS: Use
of (64)Cu-labeled fibronectin domain with EGFR-overexpressing tumor
xenograft: Molecular imaging. Radiology. 263:179–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang L, Tu Y, Kimura RH, Habte F, Chen H,
Cheng K, Shi H, Gambhir SS and Cheng Z: 64Cu-labeled divalent
cystine knot peptide for imaging carotid atherosclerotic Plaques. J
Nucl Med. 56:939–944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Terwisscha van Scheltinga AG, Lub-de Hooge
MN, Hinner MJ, Verheijen RB, Allersdorfer A, Hülsmeyer M, Nagengast
WB, Schröder CP, Kosterink JG, de Vries EG, et al: In vivo
visualization of MET tumor expression and anticalin biodistribution
with the MET-specific anticalin 89Zr-PRS-110 P ET tracer. J Nucl
Med. 55:665–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sörensen J, Velikyan I, Sandberg D,
Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M,
Lubberink M, Olofsson H, et al: Measuring HER2-receptor expression
in metastatic breast cancer using [68Ga]ABY-025 Affibody PET/ CT.
Theranostics. 6:262–271. 2016. View Article : Google Scholar
|
|
18
|
Binz HK, Stumpp MT, Forrer P, Amstutz P
and Plückthun A: Designing repeat proteins: Well-expressed, soluble
and stable proteins from combinatorial libraries of consensus
ankyrin repeat proteins. J Mol Biol. 332:489–503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
The European Union Clinical Trials
Register [Internet]: EudraCT Number 2011-002526-43. Single and
Repeat Dose Study of the Safety and Efficacy of AGN-150998 in
Patients with Exudative Age-related Macular Degeneration.
https://www.clinicaltrial-sregister.eu/ctr-search/search?query=2011-002526-43
Accessed January 30, 2012.
|
|
20
|
Zahnd C, Kawe M, Stumpp MT, de Pasquale C,
Tamaskovic R, Nagy-Davidescu G, Dreier B, Schibli R, Binz HK,
Waibel R, et al: Efficient tumor targeting with high-affinity
designed ankyrin repeat proteins: Effects of affinity and molecular
size. Cancer Res. 70:1595–1605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raguin O, Leblanc L, Collin B, Oudot A,
Mirjolet JF, Fiedler U and Dolado I: Biodistribution and antitumor
efficacy study of novel Her2 targeting DARPins. Cancer Res.
74(Suppl 19): Abstract 5442. 2014. View Article : Google Scholar
|
|
22
|
Fiedler U, Metz C, Zitt C, Bessey R, Béhé
M, Blanc A, Schibli R, Dolado I, Herbst J, Dawson KM and
Kiemle-Kallee J: Pre-clinical antitumor activity, tumor
localization, and pharmacokinetics of MP0274, an apoptosis
inducing, biparatopic HER2-targeting DARPin. Cancer Res. 77(Suppl
4): Abstract P4-21-18. 2017. View Article : Google Scholar
|
|
23
|
ClinicalTrials.gov [Internet]. Bethesda
(MD): National Library of Medicine (US). Identifier NCT03084926.
First-in-human Study to Investigate Safety, Blood Levels and
Activity of MP0274 in Cancer Patients With HER2-positive Solid
Tumors. Accessed December 18, 2018. https://clinicaltrials.gov/ct2/show/NCT03084926.
|
|
24
|
Goldstein R, Sosabowski J, Livanos M,
Leyton J, Vigor K, Bhavsar G, Nagy-Davidescu G, Rashid M, Miranda
E, Yeung J, et al: Development of the designed ankyrin repeat
protein (DARPin) G3 for HER2 molecular imaging. Eur J Nucl Med Mol
Imaging. 42:288–301. 2015. View Article : Google Scholar :
|
|
25
|
Vorobyeva A, Bragina O, Altai M, Mitran B,
Orlova A, Shulga A, Proshkina G, Chernov V, Tolmachev V and Deyev
S: Comparative evaluation of radioiodine and technetium-labeled
DARPin 9_29 for radionuclide molecular imaging of HER2 expression
in malignant tumors. Contrast Media Mol Imaging.
2018.6930425:2018.
|
|
26
|
Steffen AC, Wikman M, Tolmachev V, Adams
GP, Nilsson FY, Ståhl S and Carlsson J: In vitro characterization
of a bivalent anti-HER-2 affibody with potential for
radionuclide-based diagnostics. Cancer Biother Radiopharm.
20:239–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zalutsky MR and Narula AS: A method for
the radiohalogenation of proteins resulting in decreased thyroid
uptake of radioiodine. Int J Rad Appl Instrum. 38:1051–1055. 1987.
View Article : Google Scholar
|
|
28
|
Rea DW, Ultee ME, Belinka BA Jr, Coughlin
DJ and Alvarez VL: Site-specifically radioiodinated antibody for
targeting tumors. Cancer Res. 50(Suppl 3): 857s–861s.
1990.PubMed/NCBI
|
|
29
|
Malakhov MP, Mattern MR, Malakhova OA,
Drinker M, Weeks SD and Butt TR: SUMO fusions and SUMO-specific
protease for efficient expression and purification of proteins. J
Struct Funct Genomics. 5:75–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heckman KL and Pease LR: Gene splicing and
mutagenesis by PCR-driven overlap extension. Nat Protoc. 2:924–932.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Studier FW: Protein production by
auto-induction in high density shaking cultures. Protein Expr
Purif. 41:207–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tolmachev V, Tran TA, Rosik D, Sjöberg A,
Abrahmsén L and Orlova A: Tumor targeting using affibody molecules:
Interplay of affinity, target expression level, and binding site
composition. J Nucl Med. 53:953–960. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McLarty K, Cornelissen B, Scollard DA,
Done SJ, Chun K and Reilly RM: Associations between the uptake of
111In-DTPA-trastuzumab, HER2 density and response to trastuzumab
(Herceptin) in athymic mice bearing subcutaneous human tumour
xenografts. Eur J Nucl Med Mol Imaging. 36:81–93. 2009. View Article : Google Scholar
|
|
34
|
Malmberg J, Tolmachev V and Orlova A:
Imaging agents for in vivo molecular profiling of disseminated
prostate cancer: Cellular processing of [(111)In]-labeled
CHX-A″DTPA-trastuzumab and anti-HER2 ABY-025 Affibody in prostate
cancer cell lines. Exp Ther Med. 2:523–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Björkelund H, Gedda L, Barta P, Malmqvist
M and Andersson K: Gefitinib induces epidermal growth factor
receptor dimers which alters the interaction characteristics with
125I-EGF. PLoS One. 6:e247392011. View Article : Google Scholar :
|
|
36
|
Tolmachev V, Orlova A and Andersson K:
Methods for radio-labelling of monoclonal antibodies. Methods Mol
Biol. 1060:309–330. 2014. View Article : Google Scholar
|
|
37
|
Wyszomirska A: Iodine-131 for therapy of
thyroid diseases. Physical and biological basis Nucl Med Rev Cent
East Eur. 15:120–123. 2012.
|
|
38
|
Mume E, Orlova A, Larsson B, Nilsson A-S,
Nilsson FY, Sjöberg S and Tolmachev V: Evaluation of
((4-hydroxyphenyl) ethyl)maleimide for site-specific
radiobromination of anti-HER2 affibody. Bioconjug Chem.
16:1547–1555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lindbo S, Garousi J, Mitran B, Altai M,
Buijs J, Orlova A, Hober S and Tolmachev V: Radionuclide tumor
targeting using ADAPT scaffold proteins: Aspects of label
positioning and residualizing properties of the label. J Nucl Med.
59:93–99. 2018. View Article : Google Scholar
|
|
40
|
Shih LB, Thorpe SR, Griffiths GL, Diril H,
Ong GL, Hansen HJ, Goldenberg DM and Mattes MJ: The processing and
fate of antibodies and their radiolabels bound to the surface of
tumor cells in vitro: A comparison of nine radiolabels. J Nucl Med.
35:899–908. 1994.PubMed/NCBI
|
|
41
|
Wållberg H, Orlova A, Altai M,
Hosseinimehr SJ, Widström C, Malmberg J, Ståhl S and Tolmachev V:
Molecular design and optimization of 99mTc-labeled recombinant
affibody molecules improves their biodistribution and imaging
properties. J Nucl Med. 52:461–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Altai M, Honarvar H, Wållberg H, Strand J,
Varasteh Z, Rosestedt M, Orlova A, Dunås F, Sandström M, Löfblom J,
et al: Selection of an optimal cysteine-containing peptide-based
chelator for labeling of affibody molecules with (188)Re. Eur J Med
Chem. 87:519–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Engfeldt T, Orlova A, Tran T, Bruskin A,
Widström C, Karlström AE and Tolmachev V: Imaging of
HER2-expressing tumours using a synthetic Affibody molecule
containing the 99mTc-chelating mercaptoacetyl-glycyl-glycyl-glycyl
(MAG3) sequence. Eur J Nucl Med Mol Imaging. 34:722–733. 2007.
View Article : Google Scholar
|
|
44
|
Halmi NS and Stuelke RG: Comparison of
thyroidal and gastric iodide pumps in rats. Endocrinology.
64:103–109. 1959. View Article : Google Scholar : PubMed/NCBI
|