Introduction
Malignant melanoma, which is derived from
melanocytes, is a highly aggressive skin cancer. The incidence of
malignant melanoma continues to increase worldwide (1). Postoperative adjuvant therapy is
important in order to prevent tumor recurrence and metastasis
(2). Various modalities, including
immunological therapy, chemotherapy and radiation have been
investigated as adjuvant therapies; however, the effects of
advanced melanoma treatment remain unsatisfactory. Recent advances
have demonstrated that immunotherapies using immune checkpoint
inhibitors, including anti-cytotoxic T lymphocyte antigen-4 and
anti-programmed death-1 antibodies, or molecularly-targeted agents,
such as BRAF and MEK inhibitors, can markedly alter
the effects of melanoma therapy. Of note, these agents exhibit only
a transient benefit in the majority of cases (3-5) and
patients suffer from the rapid onset of resistance (6). In addition, the financial cost is
considerably high and government finances are restricted. Thus,
there is an urgent need for novel agents or the novel application
of current agents to overcome this aggressive malignancy (7).
Temozolomide (3-methyl-4-oxo-3,4-dihydro-imidazo
[5,1-d][1,2,3,5]tetrazine-8-carboxamide; TMZ), is an oral
alkylating agent employed for the treatment of metastatic melanoma
and malignant glioma (8). TMZ, as
compared with dacarbazine (DTIC), is very well tolerated and
possesses an advantage in terms of improving the quality of life of
patients with metastatic melanoma (9). DTIC and TMZ are the most commonly
used drugs as first-line therapy for patients with wild-type
BRAF tumors in Europe (10). The effect of TMZ has been
hypothesized to be dependent on the tumoral expression of
O6-methylguanine-DNA transferase (MGMT), and low
expression of MGMT has been correlated with a high likelihood of
the increased sensitivity to TMZ (11,12).
MGMT may be a key determinant of cellular resistance to methylating
drugs; however, there is no significant association between MGMT
expression and the therapeutic response in melanoma (13-15).
Interferons (IFNs) are cytokines; there are two
groups of IFNs: type I (IFN-α, IFN-β, IFN-τ and IFN-ω) and type II
(IFN-γ) (16). IFN-α2b and
pegylated IFN-α2b have been approved in numerous countries as an
adjuvant therapy for malignant melanoma (17). On the other hand, IFN-β is known to
exhibit pleiotropic biological activities, including antiviral,
antiproliferative, antiangiogenetic and immunomodulatory effects
(18). IFN-β also acts as a drug
sensitizer to enhance toxicity against a variety of neoplasias when
administered in combination with alkylating agents (19). IFN-β exhibits greater growth
inhibitory and proapoptotic effects than IFN-α2 (20).
TMZ in combination with IFN-α2 has been investigated
in metastatic melanomas (21-25).
Treatment with a combination of TMZ and IFN-α can increase the
median survival of patients with metastatic melanomas (26); however, a combination treatment
with TMZ and IFN-β has rarely been investigated in malignant
melanomas (15). In the present
study, TMZ and IFN-β was applied to determine whether this
combination can exert an antitumor activity on malignant
melanomas.
Materials and methods
Materials
Natural-type of IFN-β (Toray Industries, Tokyo,
Japan), TMZ (Tokyo Chemical Industry, Tokyo, Japan) and
O6-benzylguanine (O6-BG; Abcam, Cambridge,
UK) were used for the experiments.
Cell lines
Human malignant melanoma A375, CRL-1579, G361, MeWo
and SK-MEL-28 cells were purchased from the American Type Culture
Collection (Manassas, VA, USA). Cells were routinely cultured in
Dulbecco’s modified Eagle’s medium (Nissui Pharmaceutical, Tokyo,
Japan) supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) using plastic culture flasks
(Corning Inc., Corning, NY, USA) in a humidified incubator at 37°C
with an atmosphere containing 5% CO2.
Quantification of MGMT mRNA by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Quantification of the MGMT gene expression was
performed via the RT-qPCR method as described previously (27). Complementary DNA was synthesized
from 1 µg total RNA isolated from five melanoma cell lines
using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA) with a
random primer, 40 units M-MLV reverse transcriptase (both from
Invitrogen; Thermo Fisher Scientific, Inc.), 0.5 mM dNTP, 24 units
RNase inhibitor (both from Takara Bio, Inc., Otsu, Japan), 10
µM DTT (Invitrogen; Thermo Fisher Scientific, Inc.) and 5X
RT buffer at 37°C for 60 min. The qPCR mixture was prepared using a
TaqMan Universal Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.), 120 nM of each primer and 2.5 µl of each
cDNA sample. The primers were as follows: MGMT, forward, 5′-CCT GGC
TGA ATG CCT ATT TCC-3′ and reverse, 5′-GAT GAG GAT GGG GAC AGG
ATT-3′; and 200 nM probe, 5′-CGA GCA GTG GGA GGA GCA ATG AGA-3′.
The qPCR conditions were as follows: initial denaturation at 95°C
for 10 min; denaturation 45 cycles at 95°C for 30 sec, annealing at
60°C for 30 sec, elongation at 72°C for 30 sec; and final
extension, 72°C for 7 min, using a real-time PCR system (ABI PRISM
7900HT Sequence Detection System; Applied Biosystems; Thermo Fisher
Scientific, Inc.) as previously reported (28). Glyceraldehyde-3-phosphatase
dehydrogenase (GADPH) mRNA expression levels were employed
as the quantitative internal control. Standard curves for
MGMT and GADPH mRNA were generated using 10-fold
serially diluted standard plasmid clones (pCR2.1-TOPO TA Vector;
Thermo Fisher Scientific, Inc.) containing MGMT or GAPDH PCR
products as templates as previously reported (28,29).
The amount of each mRNA expression level was calculated from the
relevant standard curve. For precise quantification, the
MGMT mRNA expression levels of each cell line was normalized
based on expression of GADPH. The expression levels were
calculated using the equations by comparing the threshold cycles
(30).
Growth inhibitory effect
The growth inhibition of malignant melanoma cells by
TMZ alone, or a combination of TMZ and IFN-β was evaluated by
counting the number of cells as previously reported (27). Each well was seeded with
1×104 cells in 24-well plates and cultured for 24 h at
37°C. The cells were incubated with fresh medium containing
0.1-1,000 µM of TMZ, or medium containing a combination of
0.1, 1, 10, 100 or 1,000 µM of TMZ and 10 IU/ml of IFN-β;
the cells were cultured for 72 h at 37°C. The latter incubation
condition (10 IU/ml of IFN-β) was selected as this condition
represents a clinically relevant concentration of IFN-β (31,32).
For the depletion of the MGMT activity, 10 µM of
O6-BG was added to the cells at 1 h prior to drug
treatment (15). The cells were
trypsinized at room temperature for 5 min with 0.25% Trypsin-EDTA
solution (Invitrogen; Thermo Fisher Scientific, Inc.) and counted
using a ZI Coulter Counter® (Beckman Coulter, Inc.,
Brea, CA, USA). The experiments were repeated at least four times
at each concentration.
Cell cycle distribution analysis
The cells were plated at 2×105 cells in a
6-well plate and incubated for 24 h until attachment to the plate
was observed. Following drug treatment, the cells were harvested
using a trypsin-EDTA solution at 8 and 24 h, and fixed in ice-cold
70% ethanol overnight. The fixed cells were treated with 0.5% RNase
A (Roche Diagnostics GmbH, Mannheim, Germany) and stained with 1
µg/ml propidium iodide (PI) for 30 min at room temperature.
The fluorescence was measured with a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) at a wavelength of 610 nm
(FL3). The DNA histograms were analyzed using FlowJo software
(version 10.2) (FlowJo LLC, Ashland, OR, USA).
Determination of apoptotic cells
Based on the results for the growth inhibitory and
MGMT mRNA expression analyses, A375 and CRL-1579 cells were
employed in the further experiments. Apoptosis was detected by dual
staining at room temperature for 10 min with Annexin V and
Propidium Iodide (PI) using Annexin V Alexa Fluor® 488
conjugate (Thermo Fisher Scientific, Inc.) and PI (Miltenyi Biotec,
Inc., Cambridge, CA, USA). A375 and CRL-1579 cells were treated
with TMZ alone, or with a combination of TMZ and IFN-β for 24, 66
and 72 h at room temperature; adherent and floating cells were then
harvested and washed with cold PBS(−) and re-suspended in 1X
binding buffer (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
Cells (1×106) were treated with 5 µl of Annexin V
Alexa Fluor 488 conjugate and 10 µl of PI solution, and
immediately analyzed by flow cytometry according to the
manufacturer’s protocols. Flow cytometry was performed with a
FACSCalibur flow cytometer and apoptosis was analyzed using Flowjo
software.
Western blot analysis
Soluble protein lysates of subconfluent melanoma
cells were obtained using radioimmunoprecipitation buffer (Wako
Pure Chemical Industries, Ltd.) containing protease inhibitors
(Complete Mini, EDTA-free; Roche Diagnostics GmbH) for 20 min on
ice. Following centrifugation at 20,613 × g for 1 h at 4°C, the
protein content of the separated supernatants was determined by
using a Bicinchoninic Acid assay kit (Pierce; Thermo Fisher
Scientific, Inc.). The proteins (50 µg) were loaded and
separated by 12.5% polyacrylamide gel electrophoresis and then
transferred onto nitrocellulose membranes for 30 min at 15 V with a
Bio-Rad Trans Blot® (both from Bio-Rad Laboratries,
Inc., Hercules, CA, USA). The membrane was treated with blocking
buffer (1% skimmed milk) for 1 h at room temperature, and
subsequently incubated with primary antibodies in fresh blocking
buffer for 24 h at 4°C. Following rinsing the membrane with washing
buffer (PBS/0.05% Tween-20), the membranes were incubated with a
secondary antibody conjugated with horseradish peroxidase (HRP) for
1 h at 37°C. The primary antibodies employed were all specific
anti-mouse monoclonal antibodies: Anti-Fas (1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-caspase-3 (1:500),
anti-caspase-8 (1:1,000), anti-caspase-9 (1:1,000) (both from Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-p53 (1:500),
anti-phosphorylated (p)-p53 (1:500), anti-p21 (1:500), anti-B-cell
lymphoma 2-associated X protein (Bax; 1:500) (all from Santa Cruz
Biotechnology, Inc.), anti-microtubule-associated protein
light-chain 3 (LC3) antibody (1:1,000), anti-autophagy regulated
gene 5/12 complex (Atg5/Atg12 complex) antibody (1:1,000) (both
from Medical & Biological Laboratories Co., Ltd., Aichi, Japan)
and β-actin (1:2,000; Wako Pure Chemical Industries, Ltd.). The
secondary antibody was an HRP-conjugated anti-mouse IgG
(Sigma-Aldrich; Darmstadt, Germany) for 1 h at room temperature.
The transferred membrane was washed three times with washing buffer
and visualized using LAS-4000 (GE Healthcare, Chicago, IL, USA)
following treatment with ECL Prime Western Blotting Detection
Reagent according to the manufacturer’s protocols (GE
Healthcare).
Statistical analysis
All studies were repeated 2-3 times. One-way
analysis of variance conducted indicated demonstrated a significant
difference between the group and the Tukey-Kramer test was used for
multiple comparisons. Data were expressed as the means ± standard
error. P<0.05 was considered to indicate a statistically
significant difference. Data analyses were performed using SPSS
Statistics version 21.0 (IBM Corporation, Armonk, NY, USA).
Results
Quantitative MGMT mRNA expression of
melanoma cell lines
An important mechanism of resistance to methylating
agents, including DTIC and TMZ is DNA repair mediated by the
damage-reversal suicide enzyme, MGMT (33). The absolute values for MGMT
mRNA in A375, G361, MeWo and SK-MEL-28 were 9.8×103
copies/µg RNA, 7.3×103 copies/µg RNA,
1.5×104 copies/µg RNA and 7.8×103
copies/µg RNA, respectively. In contrast, such expression
was not detected in CRL-1579 (data not shown). These findings
indicated that the A375, G361, MeWo, and SK-MEL-28 cell lines
expressed MGMT, whereas the CRL-1579 cell line did not.
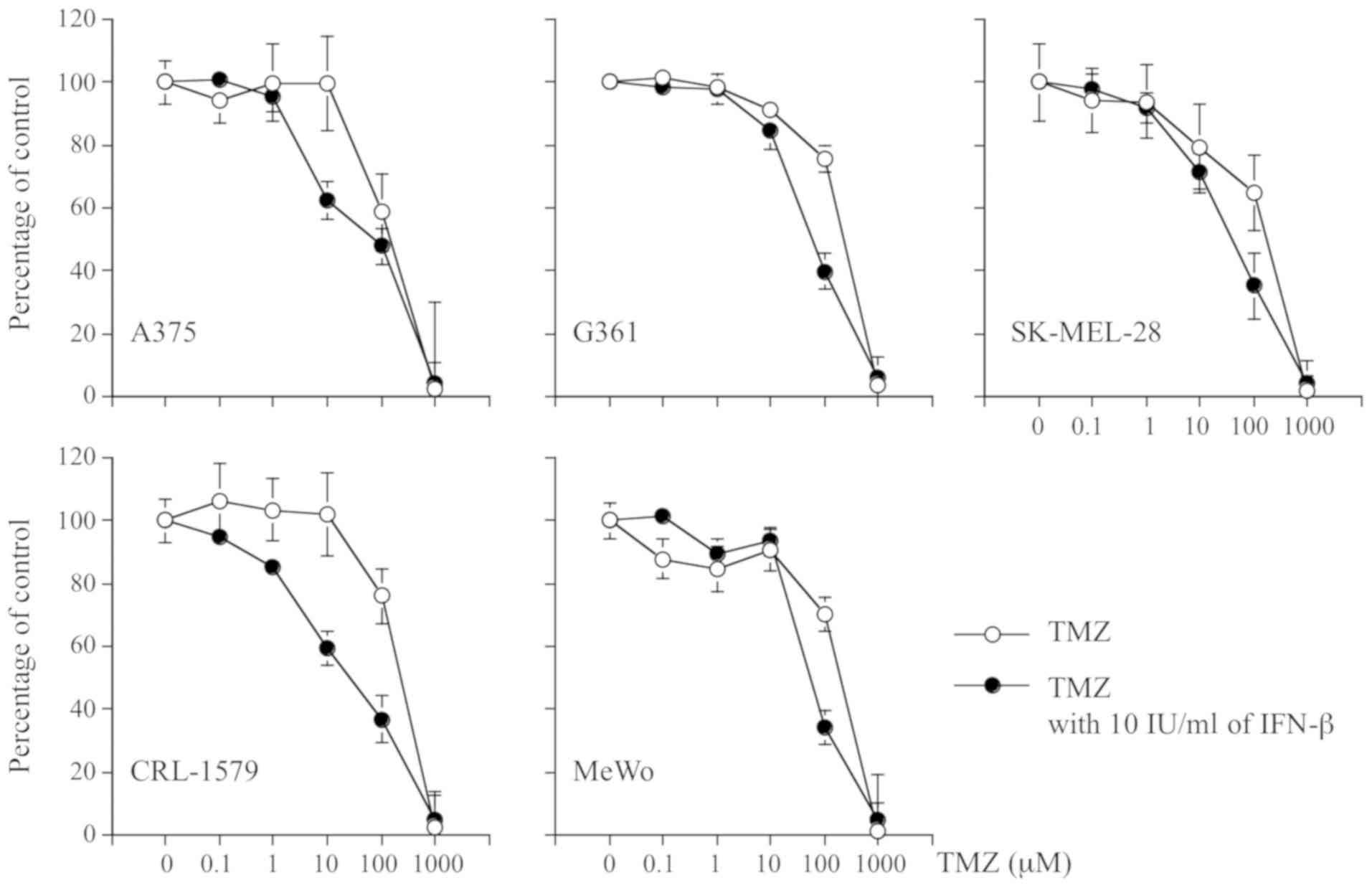
Antitumor efficacy of TMZ, and the
combination of TMZ and IFN-β
To assess the anti-tumor effects of drug treatments,
we treated the 5 melanoma cell lines with 0-1,000 µM of TMZ
alone, or with 0-1,000 µM of TMZ plus 10 IU/ml of IFN-β;
cells were cultured for 72 h. The growth of all 5 cell lines was
inhibited by TMZ alone, and with a combination of TMZ and IFN-β;
however, the sensitivity of each of the cell lines varied. The cell
growth inhibitory effect of TMZ combined with IFN-β, compared with
TMZ alone, exhibited a dose-dependent pattern, particularly in A375
and CRL-1579 cells (Fig. 1).
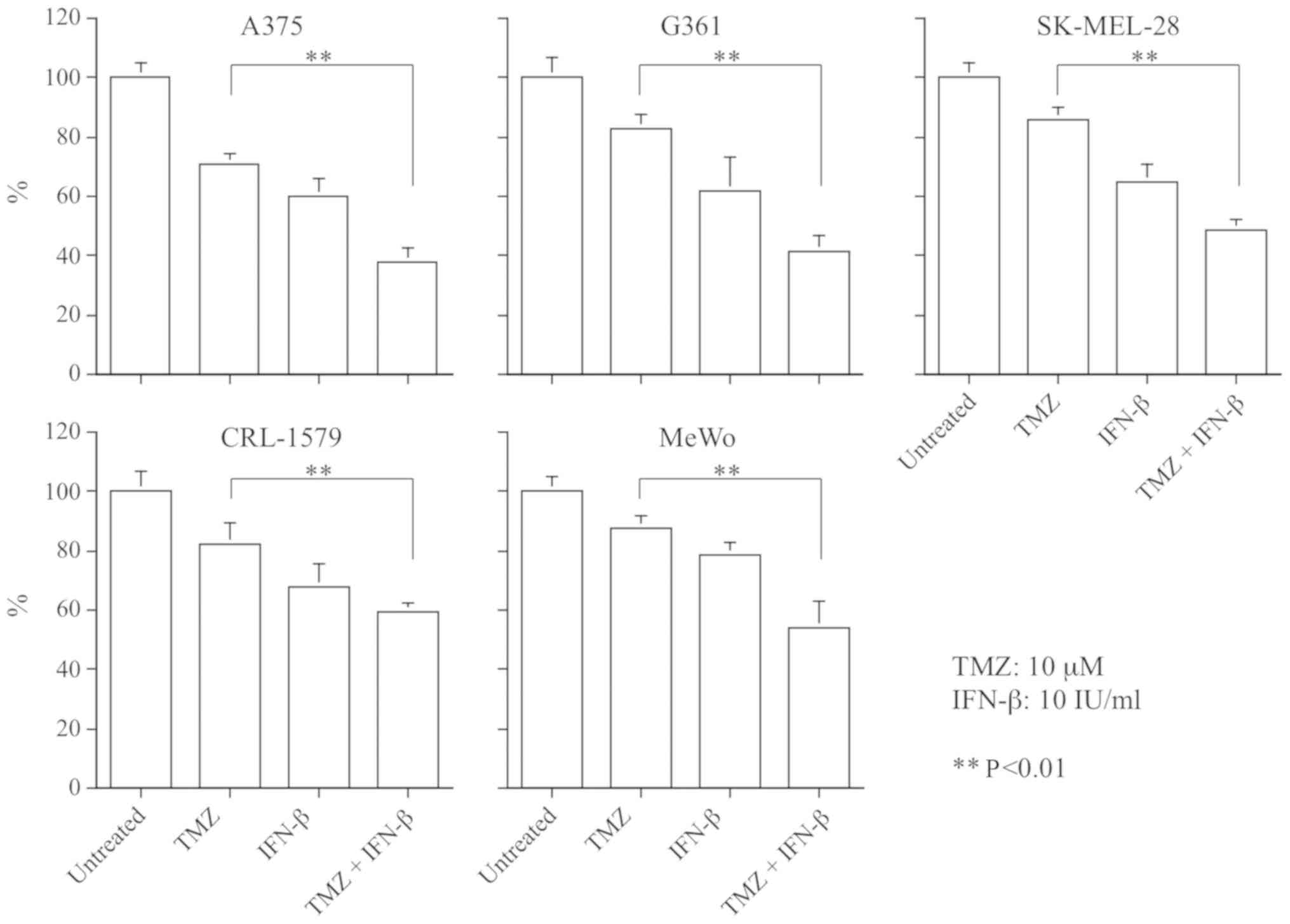
Growth inhibitory effects
The concentration of TMZ applied was set at 10
µM, as this level of TMZ demonstrated growth inhibition in
not only MGMT-proficient cells, but also MGMT-deficient cells
(Fig. 2). Additionally, this
condition represents a clinically applicable dose of TMZ. MGMT is a
key determinant of cellular resistance to methylating agents, such
as DTIC and TMZ (27). Based on
the aforementioned results for the quantification of MGMT
mRNA expression and anti-proliferative efficacy, A375 and CRL-1579
cell lines were selected for further experiments.
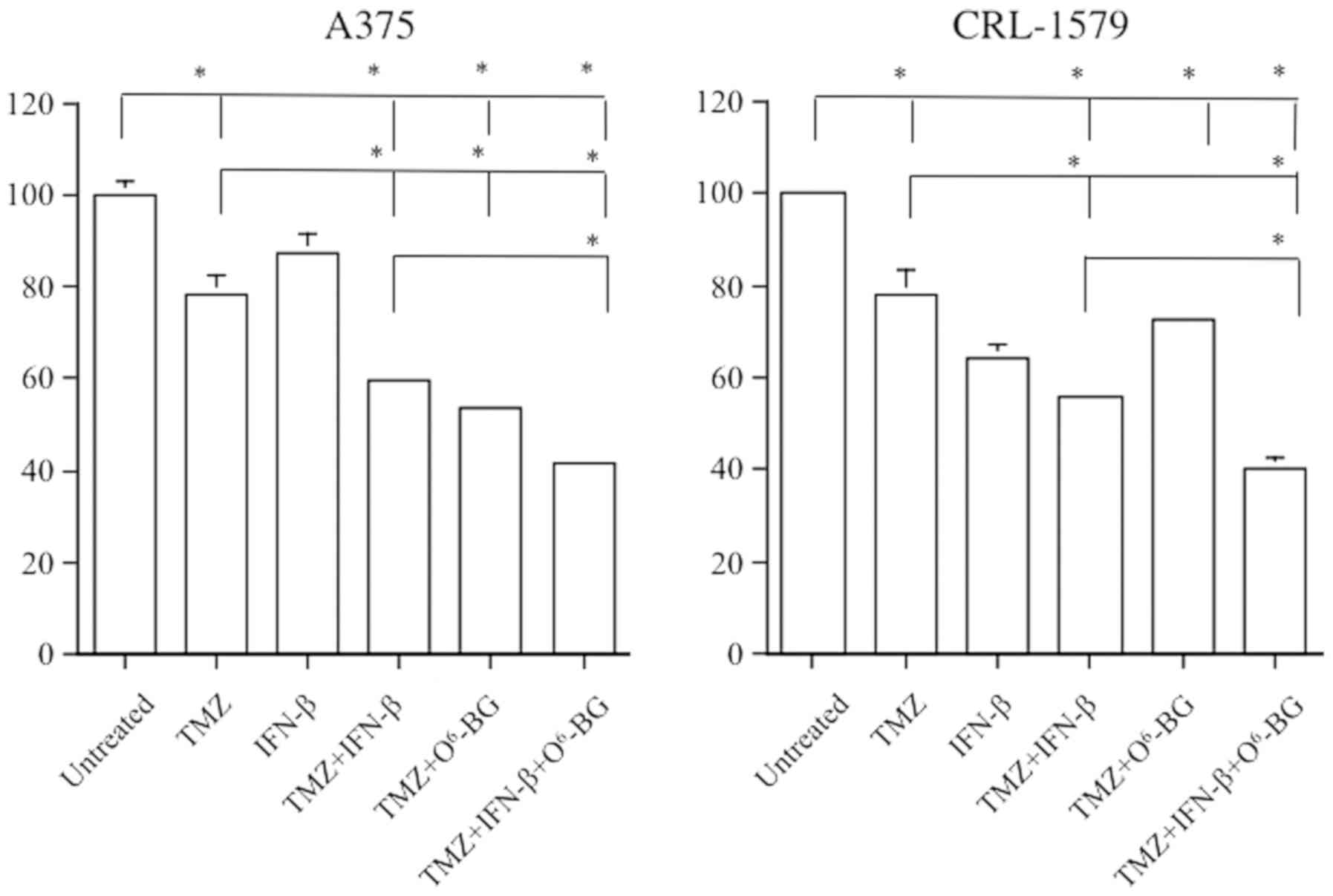
O6-BG is a modulating agent that induces
the inactivation and depletion of MGMT, which is the DNA repair
protein, by serving as a substrate and transferring its benzyl
group to the active site of MGMT (34). To examine whether the expression of
MGMT is responsible for the efficacy of growth inhibition,
experiments with A375 and CRL-1579 cells were performed under the
specific MGMT inhibitor O6-BG pretreatment conditions
for the depletion of residual MGMT activity. In A375 cells,
addition of O6-BG prior to TMZ treatment significantly
induced growth inhibition of the melanoma cells compared with TMZ
treatment alone. Furthermore, inactivation of MGMT via
O6-BG significantly sensitized A375 cells to combined
treatment with TMZ and IFN-β compared with combined treatment
alone. These findings indicated that O6-BG potentiated
the anti-proliferative effects of TMZ and that IFN-β induced by
notable sensitization to TMZ when MGMT was depleted with the
specific MGMT inhibitor O6-BG in A375 cells. Conversely,
in CRL-1579 cells, there was no significant difference in the
growth inhibitory effects between TMZ alone, and combined treatment
of TMZ and O6-BG; however, the addition of IFN-β led to
significantly decreased cell viability under pretreatment with
O6-BG compared with combination treatment of CRL-1579
cells (Fig. 3).
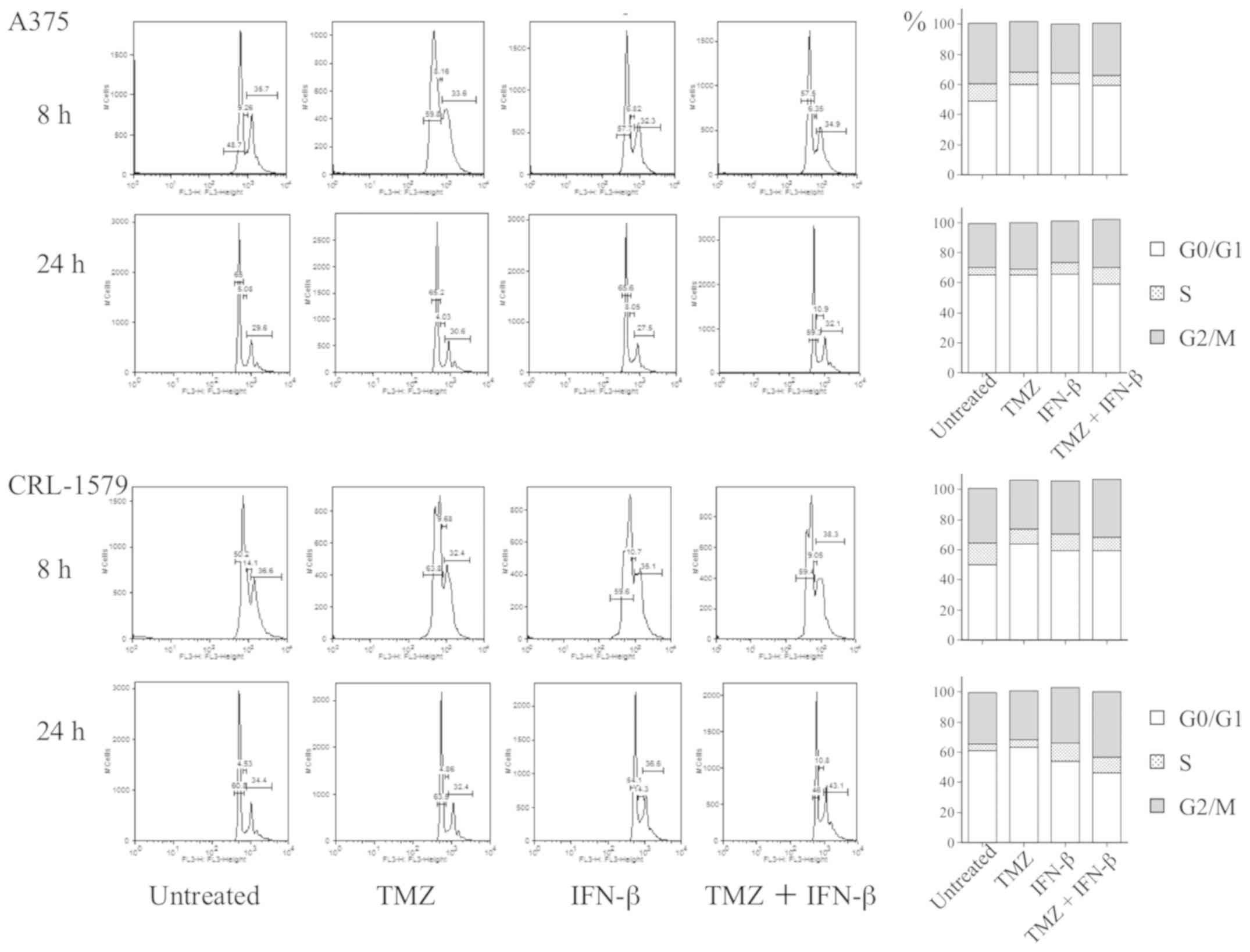
Cell cycle analysis distribution
We performed DNA flow cytometry analysis to
investigate whether cell cycle arrest could be induced in malignant
melanoma cells by TMZ alone, and with a combination of TMZ and
IFN-β. DNA histograms and the proportions of each cell cycle phase
are presented in Fig. 4. We
obtained equivalent results from repeating the analyses in
triplicate; however statistical significance was not observed. In
A375 cells, TMZ alone and combination treatment notably increased
the number of cells in G0/G1 phase and
decreased the abundance of G2/M phase cells at 8 h.
Following treatment for 24 h, a combination of TMZ and IFN-β
increased the population of S and the G2/M phase cells
compared with the untreated control. In CRL-1579 cells, TMZ alone
and combination treatment increased the cell population in
G0/G1 phase and decreased that in
G2/M phase at 8 h. Following treatment for 24 h, a
combination of TMZ and IFN-β increased the abundance of cells in
G2/M and S phase. These findings indicated that TMZ
treatment, and a combination of TMZ and IFN-β could induce
G0/G1 arrest in melanoma cell lines at 8 h;
however, the combined treatment induced G2/M arrest in a
pattern which differed from that of TMZ alone, particularly in
CRL-1579 cells.
Western blot analysis of cell
cycle-associated proteins
We hypothesized that TMZ-induced
G0/G1 arrest and combined-treatment-induced
G2/M arrest in melanoma cells were associated with
alterations in the expression of proteins that serve a key role in
cell cycle regulation. p53 and p21 have important roles in cellular
growth. Thus, we analyzed protein expression associated with the
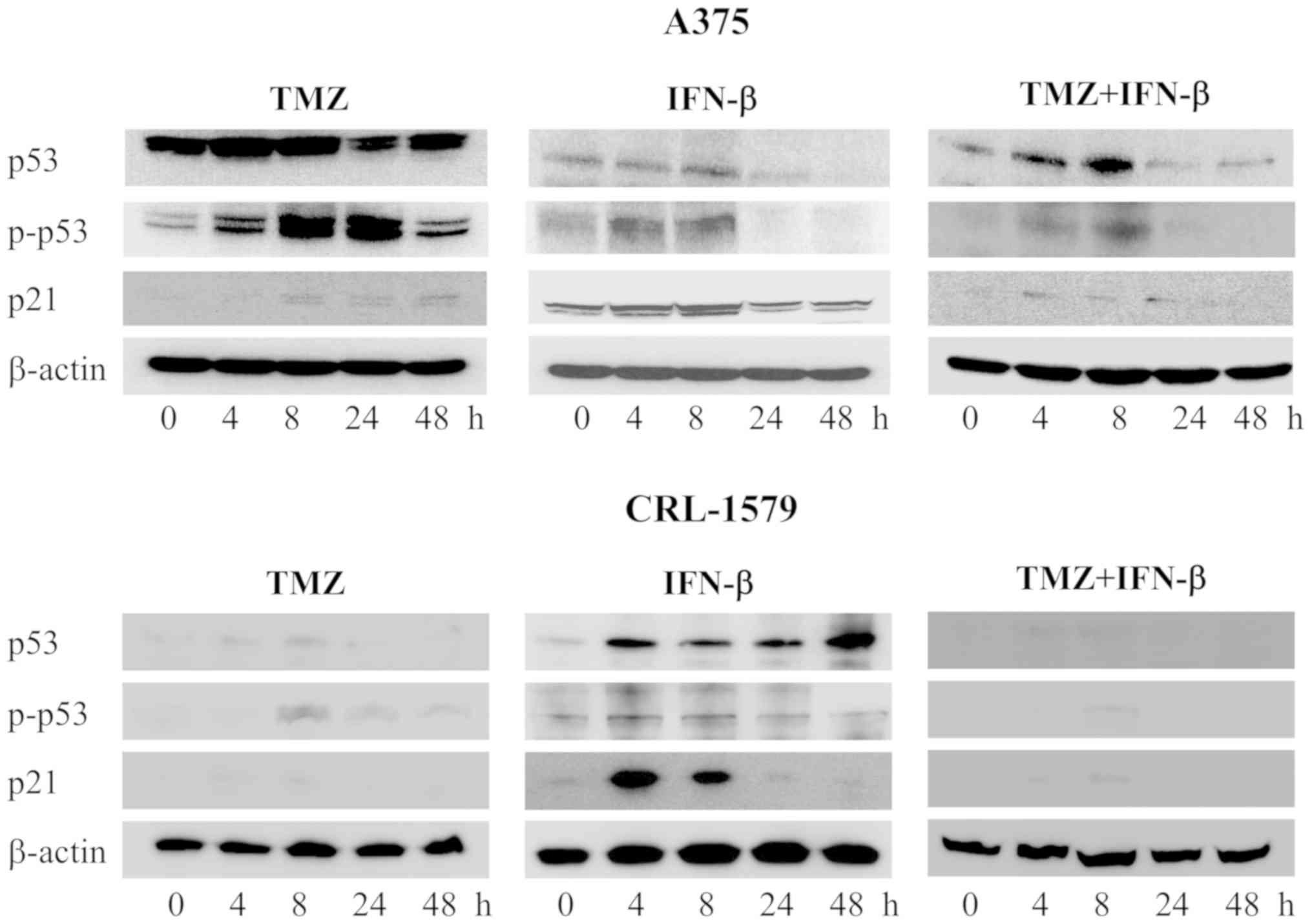
cell cycle mediated by p53 using western blot analysis (Fig. 5). In A375 cells, the expression
levels of p53, and p-p53 were increased after 4 h and were further
upregulated after 24 h following treatment with TMZ alone. The p21
expression levels were increased after 8-48 h. Following combined
treatment with TMZ and IFN-β, the expression levels of p53, p-p53
and p21 were increased after 4-24 h. Similar results were obtained
for CRL-1579 cells; however, p53, p-p53, and p21 were notably
induced in CRL-1579 cells than in A375 cells following combined
treatment with TMZ and IFN-β at 4 and 8 h. p21 was observed to show
maximum expression after 4 or 8 h.
Activation of apoptosis in melanoma cells
induced by TMZ alone, and a combination of TMZ and IFN-β
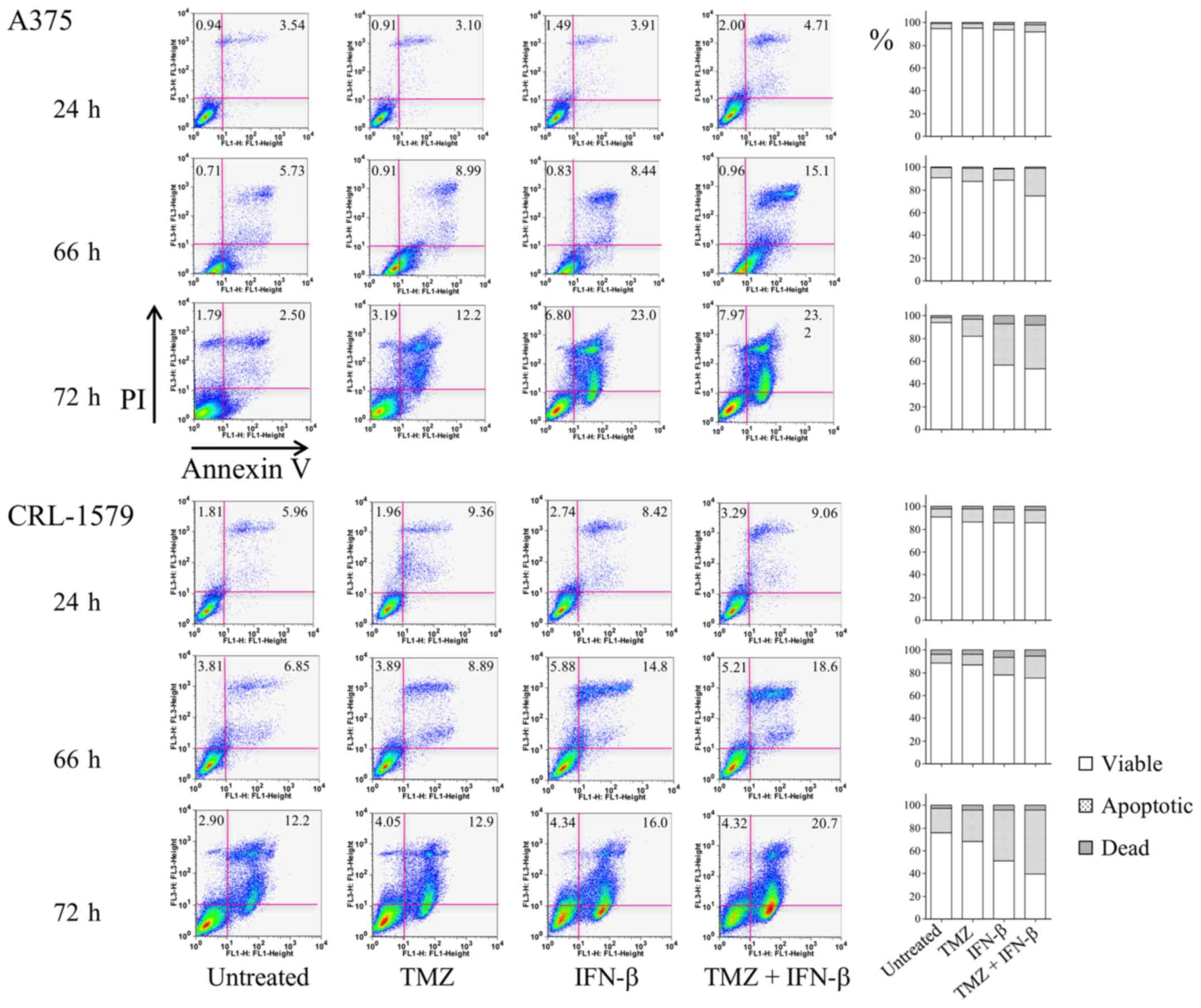
The induction of apoptosis by TMZ and IFN-β in human
melanoma cells was examined by Annexin V/PI double staining and
measured using flow cytometry. A375 and CRL-1579 cells were treated
with TMZ alone, IFN-β alone, or a combination of TMZ and IFN-β.
A375 cells treated with TMZ alone for 72 h exhibited a 14.5%
increase in Annexin V/PI staining. Following treatment with a
combination of TMZ and IFN-β for 72 h, the staining intensity
increased to 38.7% (Fig. 6).
Conversely, CRL-1579 cells pretreated with TMZ alone for 72 h
exhibited a 27.5% increase in Annexin V/PI positivity. Following
treatment with a combination of TMZ and IFN-β for 72 h, 56.3% of
the cell were positively stained (Fig.
6).
Protein expression of
apoptosis-associated protein is induced by TMZ alone or in
combination with IFN-β
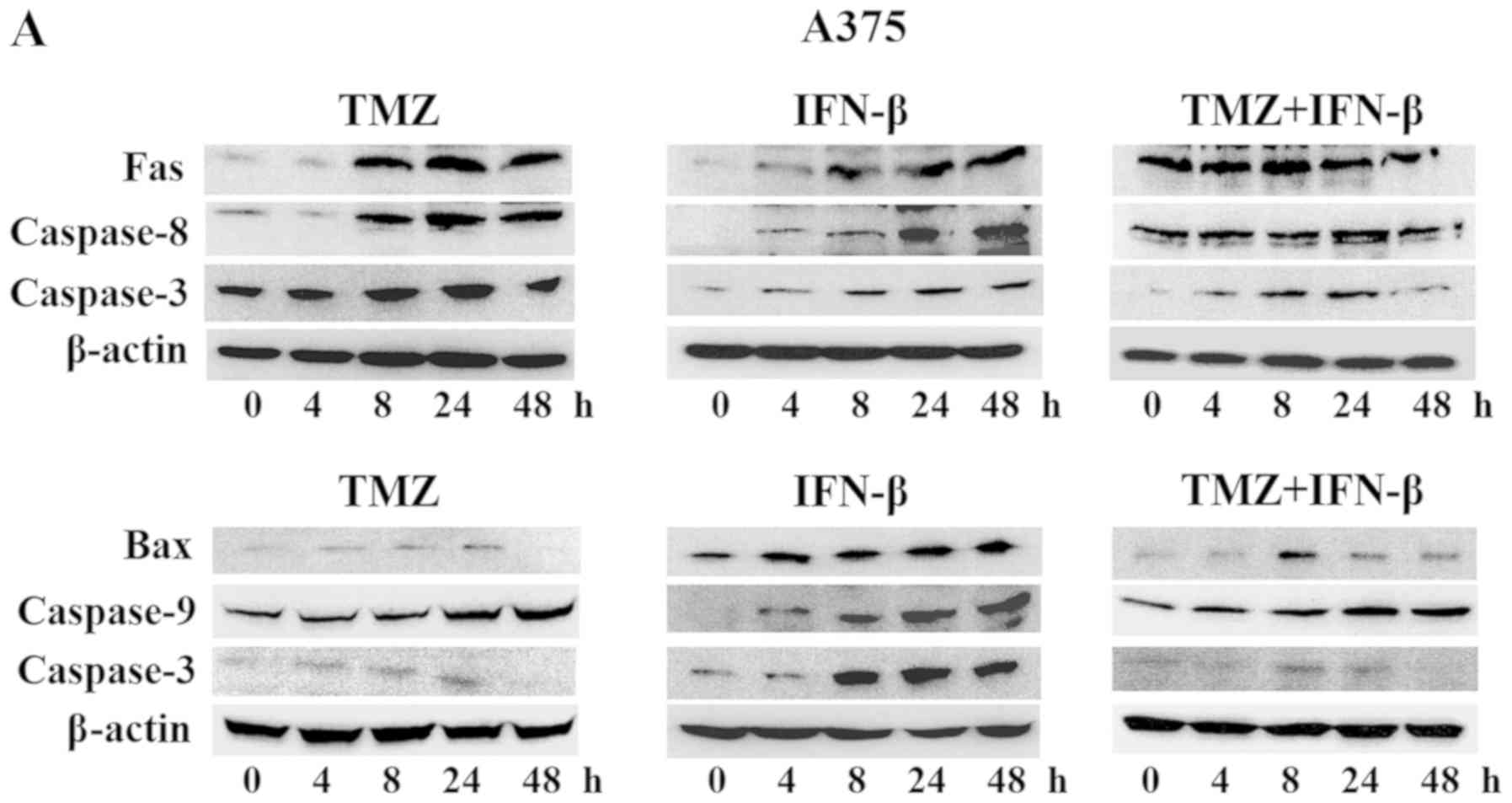
We assessed several apoptosis-associated proteins by
western blot analysis in order to examine the mechanisms underlying
the anti-apoptotic effect of TMZ and IFN-β. The intrinsic
mitochondrial pathway associated with apoptosis was analyzed, and
the extrinsic apoptotic pathway mediated by Fas was also
investigated. In melanoma cells treated with TMZ alone, the
expression levels of p53 and p-p53 were activated and the levels of
Bax, and caspases-9 and -3 were also increased (Fig. 7A and B). In cells treated with a
combination of TMZ and IFN-β, the findings were similar to those
for the cells treated with TMZ alone; however, the duration of
activation following treatment with TMZ and IFN-β appeared to be
earlier compared with TMZ alone. Conversely, the expression of
caspases-8 and -3 in melanoma cells treated with a combination of
TMZ and IFN-β were upregulated compared with in melanoma cells
exposed to TMZ alone after 4 h. This suggests activation of the
extrinsic apoptoic pathway mediated by caspase-8 (Fig. 7A and B). Based on these results,
IFN-β may synergize with TMZ and the combination treatment may
potentiate cell death via the intrinsic mitochondrial and the
extrinsic signaling pathways.
Protein expression associated with
autophagy is induced by TMZ alone or in combination with IFN-β
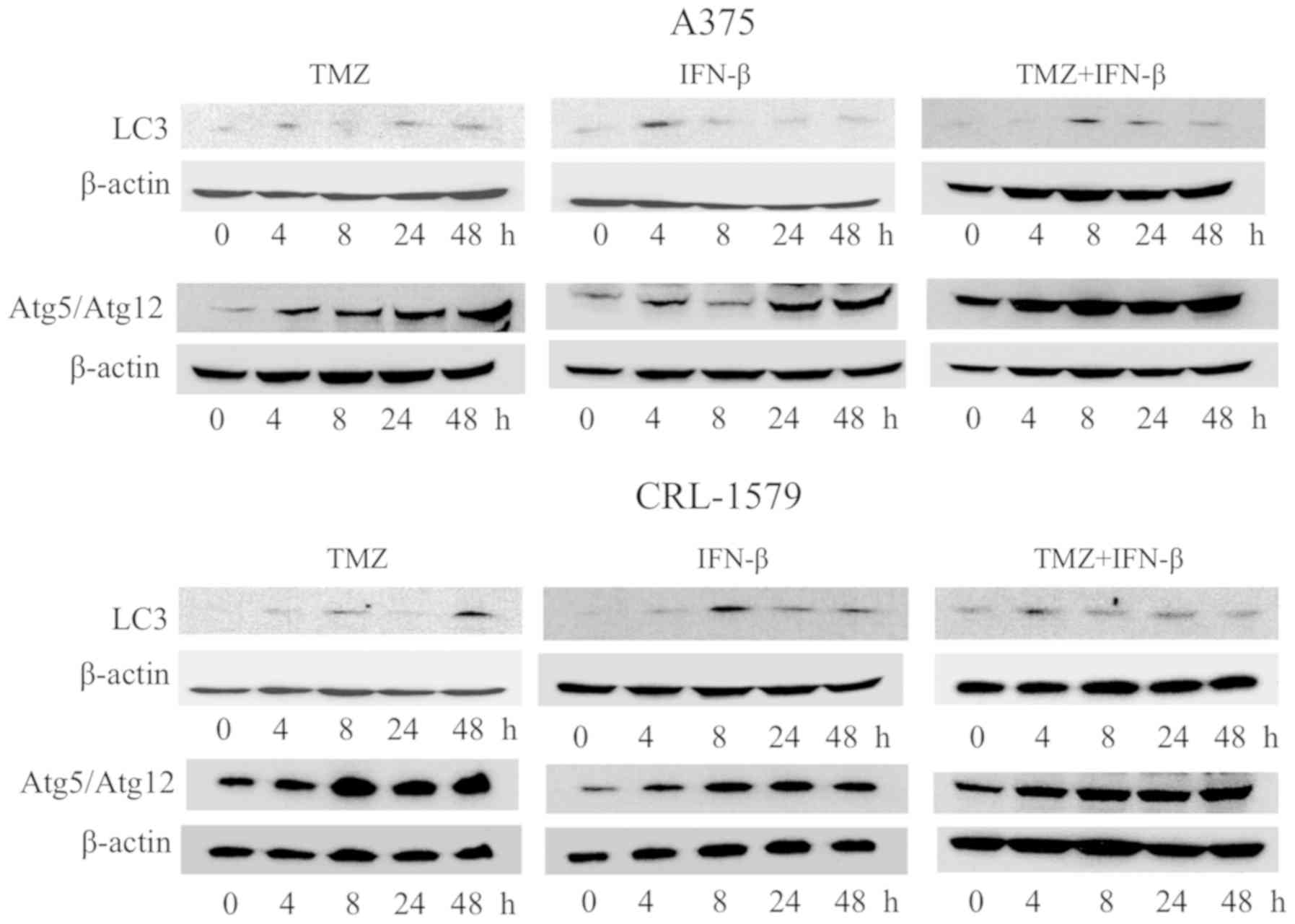
We examined whether TMZ alone or in combination with
IFN-β can induce autophagy in melanoma cells. The expression levels
of LC3 protein and Atg5/Atg12 complex proteins were elevated
following TMZ treatment at 4 h in A375 and CRL-1579 cells. These
protein levels were clearly increased and activated relatively soon
after treatment with TMZ and IFN-β in A375 and CRL-1579 cells
(Fig. 8). These findings indicated
that the induction of autophagy by TMZ and IFN-β may promote this
process in melanoma cells.
Discussion
In the present study, we found that the
antiproliferative properties in melanoma cells may be ascribed to
inhibition of cell growth, and the induction of apoptosis and
autophagy.
The effects of methylating agents have been
hypothesized to be dependent on tumoral expression of MGMT.
Overexpression of MGMT protects against alkylation-induced cell
death; low expression of MGMT is correlated with a high likelihood
of a response to methylating agents (12,35).
Melanomas tend to express low levels of MGMT (36,37),
which could explain why melanomas respond to methylating drugs,
including DTIC and TMZ, but not to other anticancer drugs. MGMT can
determine the clinical outcome in melanoma therapy with methylating
drugs; however, conflicting data have been reported (33). No significant association was
observed between MGMT immunoexpression and the response to TMZ
(13,14). The present study demonstrated that
A375 cells were more sensitized to TMZ than CRL-1579 cells
following depletion of MGMT with O6-BG. In addition,
these two cell lines exhibited a notably increased sensitivity to
combined treatment with TMZ and IFN-β than TMZ alone. These
findings were consistent with those reported Roos et al
(15), that is, IFN-β increased
sensitization effects of cells to TMZ, when depleting MGMT with
O6-BG. The addition of IFN-β led to decreases in cell
viability when depleted with O6-BG; analysis of
MGMT-deficient cells suggested that O6-BG may have
increased cell death sensitivity (38), and that IFN-β did not affect MGMT
(15).
The G2/M checkpoint serves an important
role in the action of TMZ in melanomas (39,40).
We revealed that IFN-β sensitized melanoma cells to TMZ to induce
G2/M arrest. In addition, combination treatment of TMZ
and IFN-β was associated with alterations in the expression of
proteins that serve a key role in the regulation of the cell
cycle.
The biological outcomes of p53 activity include the
inhibition of cell cycle progression, senescence, differentiation,
acceleration of DNA repair and apoptosis (41). p21 and cyclin-dependent kinase
inhibitor 1 are activated by p53 and induces cell cycle arrest in
G0/G1 phase. In the present study, the
expression levels of p21 as investigated by western blot analysis
increased following combined treatment of TMZ and IFN-β treatment
of A375 and CRL-1579 cells. The data suggested that one of the
mechanisms underlying the antitumor effects this particular
treatment combination is cell cycle arrest in melanoma cells. p53
inhibits cell cycle progression and has been demonstrated to be
functionally inactivated in a variety of human cancers (42). IFN-α/β signaling affects the p53
responses in tumor suppression and antiviral defense (43). The sensitization effect of IFN-β in
TMZ-treated melanoma cells is dependent on p53 (15), in which p21 is directly activated
(44). In addition to growth
inhibition, G2/M cell cycle arrest as a result of p21
activation following treatment with TMZ plus IFN-β was observed in
the present study. As p21 has been observed to be associated with
the induction of apoptosis (45,46),
upregulation of p21 in melanoma cells may affect the induction of
apoptosis. IFN-β induced apoptosis, which was linked to the
activation of Bax gene expression (47,48).
The present study investigated apoptotic cell death
via Annexin V/PI staining. There are several distinct processes
associated with cell death which include not only apoptosis and
necrosis, but also necroptosis, autophagy and ferroptosis (49). In the present study, there appeared
to be a discrepancy between the results of growth inhibition and
apoptosis analyses. Different methods were conducted and different
cell process were investigated; however, an antitumor effect was
observed following combination treatment. We also investigated
numerous apoptosis-related proteins by western blot analysis. The
proportion of Annexin V-positive melanoma cells increased
irrespective of the treatment in a time-dependent manner; however,
the proportion of apoptotic cells were higher for combination
treatment with TMZ and IFN-β than TMZ alone in A375 and CRL-1579
cells. Compared with A375 cells, the number of apoptotic cells was
notable increased in CRL-1579 cells following combined treatment.
IFN-β in combination with TMZ led to a high degree of early
apoptotic responses, particularly in CRL-1579 cells by flow
cytometric analysis. A key regulator of apoptosis is the
mitochondrial intrinsic signaling pathway and another is the
Fas/cluster of differentiation 95/Apo-1 extrinsic signaling
pathway. In the present study, p53 and its downstream effector Bax,
were upregulated in A375 and CRL-1579 cells treated with TMZ alone.
Notable increases in apoptosis were observed following the combined
treatment of TMZ + IFN-β. Regarding caspases, we reported increases
in the expression of caspases-9 and -3. During the investigation of
the intrinsic and extrinsic pathways, the expression of caspase-3
differed, that is, the expression of caspase-3 was upregulated in
studying the extrinsic and intrinsic pathways following combination
treatment of CRL-1579, and that caspase-3 was upregulated in A375
cells. Unknown factors may have affected caspase-3 or cell
variation may be a contributing cause for differing observations;
however, the underlying mechanism requires further investigation.
Melanomas express numerous extrinsic death receptors, Fas, tumor
necrosis factor (TNF) and TNF-related apoptosis-inducing ligand
(7). Activation of the apoptotic
pathway may occur via Fas-L/FasR and activation of downstream
caspase-8 (50,51). In the present study, a combination
of TMZ and IFN-β induced Fas expression and its proximal downstream
caspase-8 expression, thereby activating the Fas/CD95/Apo-1
extrinsic apoptotic signaling pathway. Roos et al (15) have reported that the induction of
Fas/CD95/Apo-1 by TMZ and the upregulation of procaspase-8 are
required for the sensitization observed during combination
therapy.
We demonstrated that malignant melanoma cells
undergo apoptosis following the treatments with TMZ and IFN-β. In
addition, the present study revealed that CRL-1579 cells exhibited
a higher degree of apoptosis compared with A375 cells, which
indicated that alkylation-induced cell death may be largely
attributable to apoptosis and O6-methyl-guanine
(O6MeG) acted as an inducer in the toxic response of TMZ
(36,52,53).
The apoptotic process investigated in our study required p53, and
O6MeG may lead to the apoptosis of A375 and CRL-1579
cells with dependence on p53. These findings suggested that IFN-β
may promote the apoptosis induced by TMZ in melanoma cells.
Recent evidence has demonstrated that autophagy can
often occur with apoptosis during the process of programmed cell
death (30). In the present study,
it was observed that TMZ alone and in combination with IFN-β
induced not only apoptosis, but also autophagy in melanoma cells.
Therefore, IFN-β may markedly induce autophagy when combined with
TMZ. Autophagy was originally designated as a process of protein
recycling (54). Autophagosome
nucleation is mediated by Belin 1 (Atg6). Subsequently, the
Atg5/Atg12 complex and LC3 (Atg8) are required for the elongation
of autophagosomes (55). The
abundance of LC3-II is increased via the conversion of LC3-Ⅰ
(56). Autophagy serves complex
roles in cancer, which can promote or inhibit tumorigenesis
(54). Functional crosstalk exists
between autophagy and apoptosis (57), and autophagy can occur with
apoptosis in the process of programmed cell death (53). Autophagic degradation of active
caspase-8 has been associated with the crosstalk between autophagy
and apoptosis (58). IFN-β also
regulates the adaptive immune response and the innate immune
response by inducing C-X-C motif chemokine ligand 10 or Toll-like
receptor 3 (59). These processes
require further investigation and the effects of IFN-dependent
innate immunity in cancer are to be determined in the future.
The results of the present study suggested that the
clinical therapeutic efficacy of TMZ may be enhanced by the
combined treatment of TMZ and IFN-β in malignant melanomas.
Combination treatment of TMZ and IFN-β suppressed cell
proliferation, and enhanced apoptosis and autophagy in melanoma
cells. The present study did not investigate the chemoresistance of
melanoma cells treated with TMZ and IFN-β; the underlying mechanism
remains unknown. On the contrary, our data demonstrated that one of
the possible immunomodulatory effects of IFN-β may be that IFN-β
acts as a sensitizer of malignant melanomas to enhance the toxicity
of TMZ. Experiments using TMZ and IFN-β were conducted in the
present study as it has been reported that IFN-β exerts notable
inhibitory effects on melanoma cells to IFN-α (60-62).
Tus, this treatment may be applied in the treatment of melanoma;
however, future studies should be conducted.
Funding
This work was supported in part by Grants-in-Aid for
Scientific Research from the Japan Society for the Promotion of
Science (grant no. 16K10772) and in part by the Health Sciences
Research Institute, Inc. (Yokohama, Japan) to the Division of
Companion Diagnostics, Department of Pathology and Microbiology,
Nihon University School of Medicine.
Availability of data and materials
All data analysed during this study are included in
this article.
Authors’ contributions
KM and ES performed the experimental design, most of
the experiments and analysis, drafted the manuscript. HH was
involved in the conception and design of the study, analyzed the
data and contributed to the writing of the manuscript. YO (Ochiai)
and TH also conducted experiments and analyzed the data. YO
(Okamoto), TU, TN and SA supervised the study and proofread the
manuscript. AY contributed to the writing of the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors are grateful to Mr. Hiroyuki Satake and
Mr. Nobuo Miyazaki, Toray Industries Inc. (Tokyo, Japan), for their
invaluable discussions. The authors would also like to thank Ms.
Miyuki Yuda, Division of Anatomical Science, Department of
Functional Morphology, Nihon University School of Medicine (Tokyo,
Japan), for her excellent technical assistance. Some parts of this
study have been included in the Japanese-language thesis for Kotaro
Makita’s Ph.D. degree at Nihon University School of Medicine
(Tokyo, Japan).
References
|
1
|
Cancer Facts and Figures 2009. American
Cancer Society; Atlanta: 2009
|
|
2
|
Coit DG, Andtbacka R, Anker CJ, Bichakjian
CK, Carson WE III, Daud A, Dimaio D, Fleming MD, Guild V, Halpern
AC, et al: Melanoma, version 2.2013: featured updates to the NCCN
guidelines. J Natl Compr Canc Netw. 11:395–407. 2013.
|
|
3
|
Kainthla R, Kim KB and Falchook GS:
Dabrafenib for treatment of BRAF-mutant melanoma. Pharmgenomics
Pers Med. 7:21–29. 2013.
|
|
4
|
Jang S and Atkins MB: Which drug, and
when, for patients with BRAF-mutant melanoma? Lancet Oncol.
14:e60–e69. 2013.
|
|
5
|
Jang S and Atkins MB: Treatment of
BRAF-mutant melanoma: The role of vemurafenib and other therapies.
Clin Pharmacol Ther. 95:24–31. 2014.
|
|
6
|
Homet B and Ribas A: New drug targets in
metastatic melanoma. J Pathol. 232:134–141. 2014.
|
|
7
|
Nihal M, Wu J and Wood GS: Methotrexate
inhibits the viability of human melanoma cell lines and enhances
Fas/Fas-ligand expression, apoptosis and response to
interferon-alpha: Rationale for its use in combination therapy.
Arch Biochem Biophys. 563:101–107. 2014.
|
|
8
|
Yoshino A, Tashiro S, Ogino A, Yachi K,
Ohta T, Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Sano E, et
al: Gene expression profiles predicting the response to IFN-β and a
combination of temozolomide and IFN-β in malignant gliomas. Int J
Oncol. 39:529–542. 2011.
|
|
9
|
Li RH, Hou XY, Yang CS, Liu WL, Tang JQ,
Liu YQ and Jiang G: Temozolomide for treating malignant melanoma. J
Coll Physicians Surg Pak. 25:680–688. 2015.
|
|
10
|
Harries M, Malvehy J, Lebbe C, Heron L,
Amelio J, Szabo Z and Schadendorf D: Treatment patterns of advanced
malignant melanoma (stage III-IV) - A review of current standards
in Europe. Eur J Cancer. 60:179–189. 2016.
|
|
11
|
Losa M, Mazza E, Terreni MR, McCormack A,
Gill AJ, Motta M, Cangi MG, Talarico A, Mortini P and Reni M:
Salvage therapy with temozolomide in patients with aggressive or
metastatic pituitary adenomas: Experience in six cases. Eur J
Endocrinol. 163:843–851. 2010.
|
|
12
|
Zuhur SS, Tanik C, Karaman Ö, Velet S, Çil
E, Öztürk FY, Özkayalar H, Müslüman AM and Altuntaş Y: MGMT
immu-noexpression in growth hormone-secreting pituitary adenomas
and its correlation with Ki-67 labeling index and cytokeratin
distribution pattern. Endocrine. 40:222–227. 2011.
|
|
13
|
Bush ZM, Longtine JA, Cunningham T, Schiff
D, Jane JA Jr, Vance ML, Thorner MO, Laws ER Jr and Lopes MB:
Temozolomide treatment for aggressive pituitary tumors: Correlation
of clinical outcome with O(6)-methylguanine meth-yltransferase
(MGMT) promoter methylation and expression. J Clin Endocrinol
Metab. 95:E280–E290. 2010.
|
|
14
|
Raverot G, Sturm N, de Fraipont F, Muller
M, Salenave S, Caron P, Chabre O, Chanson P, Cortet-Rudelli C,
Assaker R, et al: Temozolomide treatment in aggressive pituitary
tumors and pituitary carcinomas: A French multicenter experience. J
Clin Endocrinol Metab. 95:4592–4599. 2010.
|
|
15
|
Roos WP, Jöst E, Belohlavek C, Nagel G,
Fritz G and Kaina B: Intrinsic anticancer drug resistance of
malignant melanoma cells is abrogated by IFN-β and valproic acid.
Cancer Res. 71:4150–4160. 2011.
|
|
16
|
Sano E, Tashiro S, Tadakuma H, Takei T,
Ueda T and Tsumoto K: Type 1 IFN inhibits the growth factor
deprived apoptosis of cultured human aortic endothelial cells and
protects the cells from chemically induced oxidative cytotoxicity.
J Cell Biochem. 113:3823–3834. 2012.
|
|
17
|
Herndon TM, Demko SG, Jiang X, He K,
Gootenberg JE, Cohen MH, Keegan P and Pazdur R: U.S. food and drug
administration of patients with melanoma. Oncologist. 17:1323–1328.
2012.
|
|
18
|
Baron S, Tyring SK, Fleischmann WR Jr,
Coppenhaver DH, Niesel DW, Klimpel GR, Stanton GJ and Hughes TK:
The interferons Mechanisms of action and clinical applications.
JAMA. 266:1375–1383. 1991.
|
|
19
|
Yoshida J, Kajita Y, Wakabayashi T and
Sugita K: Long-term follow-up results of 175 patients with
malignant glioma: Importance of radical tumour resection and
postoperative adjuvant therapy with interferon, ACNU and radiation.
Acta Neurochir (Wien). 127:55–59. 1994.
|
|
20
|
Chawla-Sarkar M, Leaman DW and Borden EC:
Preferential induction of apoptosis by interferon (IFN)-beta
compared with IFN-alpha2: Correlation with TRAIL/Apo2L induction in
melanoma cell lines. Clin Cancer Res. 7:1821–1831. 2001.
|
|
21
|
García M, del Muro XG, Tres A, Crespo C,
Valladares M, López JJ, Rifà J, Pérez X, Filipovich E and
Germà-Lluch JR: Phase II multicentre study of temozolomide in
combination with interferon alpha-2b in metastatic malignant
melanoma. Melanoma Res. 16:365–370. 2006.
|
|
22
|
Hwu WJ, Panageas KS, Menell JH, Lamb LA,
Aird S, Krown SE, Williams LJ, Chapman PB, Livingston PO, Wolchok
JD, et al: Phase II study of temozolomide plus pegylated
interferon-alpha-2b for metastatic melanoma. Cancer. 106:2445–2451.
2006.
|
|
23
|
Quirt I, Verma S, Petrella T, Bak K and
Charette M: Temozolomide for the treatment of metastatic melanoma:
A systematic review. Oncologist. 12:1114–1123. 2007.
|
|
24
|
Guillot B, Khamari A, Cupissol D, Delaunay
M, Bedane C, Dreno B, Picot MC and Dereure O: Temozolomide
associated with PEG-interferon in patients with metastatic
melanoma: A multicenter prospective phase I/II study. Melanoma Res.
18:141–146. 2008.
|
|
25
|
Spieth K, Kaufmann R, Dummer R, Garbe C,
Becker JC, Hauschild A, Tilgen W, Ugurel S, Beyeler M, Bröcker EB,
et al: Temozolomide plus pegylated interferon alfa-2b as first-line
treatment for stage IV melanoma: A multicenter phase II trial of
the Dermatologic Cooperative Oncology Group (DeCOG). Ann Oncol.
19:801–806. 2008.
|
|
26
|
Ridolfi R, Romanini A, Sileni VC, Michiara
M, Guida M, Biasco G, Poletti P, Amaducci L, Leoni M and Ravaioli
A: Temozolomide and interferon-alpha in metastatic melanoma: A
phase II study of the Italian Melanoma Intergroup. Melanoma Res.
14:295–299. 2004.
|
|
27
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N and Sano
E: Effect of IFN-beta on human glioma cell lines with temozolomide
resistance. Int J Oncol. 35:139–148. 2009.
|
|
28
|
Tanaka S, Oka H, Fujii K, Watanabe K,
Nagao K and Kakimoto A: Quantitation of
O6-methylguanine-DNA methyltransferase gene messenger
RNA in gliomas by means of real-time RT-PCR and clinical response
to nitrosoureas. Cell Mol Neurobiol. 25:1067–1071. 2005.
|
|
29
|
Tanaka S, Kobayashi I, Utsuki S, Oka H,
Fujii K, Watanabe T, Nagashima T and Hori T:
O6-methylguanine-DNA methyl-transpherase gene expression
in gliomas by means of real-time quantitative RT-PCR and clinical
response to nitrosoureas. Int J Cancer. 103:67–72. 2003.
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
31
|
Ostermann S, Csajka C, Buclin T, Leyvraz
S, Lejeune F, Decosterd LA and Stupp R: Plasma and cerebrospinal
fluid population pharmacokinetics of temozolomide in malignant
glioma patients. Clin Cancer Res. 10:3728–3736. 2004.
|
|
32
|
Higuchi Y and Hashida M: Pharmacokinetics
of interferon. Clin All-Round. 52:2499–2505. 2003.In Japanese.
|
|
33
|
Naumann SC, Roos WP, Jöst E, Belohlavek C,
Lennerz V, Schmidt CW, Christmann M and Kaina B: Temozolomide- and
fotemustine-induced apoptosis in human malignant melanoma cells:
Response related to MGMT, MMR, DSBs, and p53. Br J Cancer.
100:322–333. 2009.
|
|
34
|
Friedman HS, Keir S, Pegg AE, Houghton PJ,
Colvin OM, Moschel RC, Bigner DD and Dolan ME:
O6-benzylguanine-mediated enhancement of chemotherapy.
Mol Cancer Ther. 1:943–948. 2002.
|
|
35
|
Kaina B, Fritz G, Mitra S and Coquerelle
T: Transfection and expression of human
O6-methylguanine-DNA methyltransferase (MGMT) cDNA in
Chinese hamster cells: The role of MGMT in protection against the
genotoxic effects of alkylating agents. Carcinogenesis.
12:1857–1867. 1991.
|
|
36
|
Chen JM, Zhang YP, Wang C, Sun Y, Fujimoto
J and Ikenaga M: O6-methylguanine-DNA methyltransferase
activity in human tumors. Carcinogenesis. 13:1503–1507. 1992.
|
|
37
|
Kaina B, Ziouta A, Ochs K and Coquerelle
T: Chromosomal instability, reproductive cell death and apoptosis
induced by O6-methylguanine in Mex−,
Mex+ and methylation-tolerant mismatch repair
compromised cells: Facts and models. Mutat Res. 381:227–241.
1997.
|
|
38
|
Liu L, Markowitz S and Gerson SL: Mismatch
repair mutations override alkyltransferase in conferring resistance
to temozolomide but not to 1,3-bis(2-chloroethyl)nitrosourea.
Cancer Res. 56:5375–5379. 1996.
|
|
39
|
Hirose Y, Berger MS and Pieper RO: p53
effects both the duration of G2/M arrest and the fate of
temozolomide-treated human glioblastoma cells. Cancer Res.
61:1957–1963. 2001.
|
|
40
|
Beaumont KA, Hill DS, Daignault SM, Lui
GYL, Sharp DM, Gabrielli B, Weninger W and Haass NK: Cell cycle
phase-specific drug resistance as an escape mechanism of melanoma
cells. J Invest Dermatol. 136:1479–1489. 2016.
|
|
41
|
Natsume A, Ishii D, Wakabayashi T, Tsuno
T, Hatano H, Mizuno M and Yoshida J: IFN-beta down-regulates the
expression of DNA repair gene MGMT and sensitizes resistant glioma
cells to temozolomide. Cancer Res. 65:7573–7579. 2005.
|
|
42
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997.
|
|
43
|
Takaoka A, Hayakawa S, Yanai H, Stoiber D,
Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, et al:
Integration of interferon-alpha/beta signalling to p53 responses in
tumour suppression and antiviral defence. Nature. 424:516–523.
2003.
|
|
44
|
Zhang X, Fang P, Zhao Z, Ding X, Xie F,
Wang Y and Li C: Antitumorigenic effect of damnacanthal on melanoma
cell viability through p53 and NF-κB/caspase-3 signaling pathways.
Oncol Lett. 16:6039–6044. 2018.
|
|
45
|
el-Deiry WS, Harper JW, O’Connor PM,
Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill
DE, Wang Y, et al: WAF1/CIP1 is induced in p53-mediated
G1 arrest and apoptosis. Cancer Res. 54:1169–1174.
1994.
|
|
46
|
Giandomenico V, Vaccari G, Fiorucci G,
Percario Z, Vannuchi S, Matarrese P, Malorni W, Romeo G and
Affabris GR: Apoptosis and growth inhibition of squamous carcinoma
cells treated with interferon-alpha, IFN-beta and retinoic acid are
associated with induction of the cyclin-dependent kinase inhibitor
p21. Eur Cytokine Netw. 9:619–631. 1998.
|
|
47
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995.
|
|
48
|
Wittnebel S, Jalil A, Thiery J, DaRocha S,
Viey E, Escudier B, Chouaib S and Caignard A: The sensitivity of
renal cell carcinoma cells to interferon alpha correlates with
p53-induction and involves Bax. Eur Cytokine Netw. 16:123–127.
2005.
|
|
49
|
Florean C, Song S, Dicato M and Diederich
M: Redox biology of regulated cell death in cancer: A focus on
necroptosis and ferroptosis. Free Radic Biol Med. 134:177–189.
2019.
|
|
50
|
Peter ME, Kischkel FC, Hellbardt S,
Chinnaiyan AM, Krammer PH and Dixit VM: CD95
(APO-1/Fas)-associating signalling proteins. Cell Death Differ.
3:161–170. 1996.
|
|
51
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997.
|
|
52
|
Tominaga Y, Tsuzuki T, Shiraishi A, Kawate
H and Sekiguchi M: Alkylation-induced apoptosis of embryonic stem
cells in which the gene for DNA-repair, methyltransferase, had been
disrupted by gene targeting. Carcinogenesis. 18:889–896. 1997.
|
|
53
|
Meikrantz W, Bergom MA, Memisoglu A and
Samson L: O6-alkylguanine DNA lesions trigger apoptosis.
Carcinogenesis. 19:369–372. 1998.
|
|
54
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004.
|
|
55
|
Ling YH, Aracil M, Zou Y, Yuan Z, Lu B,
Jimeno J, Cuervo AM and Perez-Soler R: PM02734 (elisidepsin)
induces caspase-independent cell death associated with features of
autophagy, inhibition of the Akt/mTOR signaling pathway, and
activation of death-associated protein kinase. Clin Cancer Res.
17:5353–5366. 2011.
|
|
56
|
Chen WL, Pan L, Kinghorn AD, Swanson SM
and Burdette JE: Silvestrol induces early autophagy and apoptosis
in human melanoma cells. BMC Cancer. 167:172016.
|
|
57
|
Koga S, Hirohata S, Kondo Y, Komata T,
Takakura M, Inoue M, Kyo S and Kondo S: A novel telomerase-specific
gene therapy: Gene transfer of caspase-8 utilizing the human
telomerase catalytic subunit gene promoter. Hum Gene Ther.
11:1397–1406. 2000.
|
|
58
|
Hou W, Han J, Lu C, Goldstein LA and
Rabinowich H: Autophagic degradation of active caspase-8: A
crosstalk mechanism between autophagy and apoptosis. Autophagy.
6:891–900. 2010.
|
|
59
|
Sistigu A, Yamazaki T, Vacchelli E, Chaba
K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et
al: Cancer cell-autonomous contribution of type I interferon
signaling to the efficacy of chemotherapy. Nat Med. 20:1301–1309.
2014.
|
|
60
|
Johns TG, Mackay IR, Callister KA, Hertzog
PJ, Devenish RJ and Linnane AW: Antiproliferative potencies of
interferons on melanoma cell lines and xenografts: Higher efficacy
of interferon beta. J Natl Cancer Inst. 84:1185–1190. 1992.
|
|
61
|
Horikoshi T, Fukuzawa K, Hanada N, Ezoe K,
Eguchi H, Hamaoka S, Tsujiya H and Tsukamoto T: In vitro
comparative study of the antitumor effects of human
interferon-alpha, beta and gamma on the growth and invasive
potential of human melanoma cells. J Dermatol. 22:631–636.
1995.
|
|
62
|
Köpf J, Hanson C, Delle U, Weimarck A and
Stierner U: Action of interferon alpha and beta on four human
melanoma cell lines in vitro. Anticancer Res. 16:791–798. 1996.
|