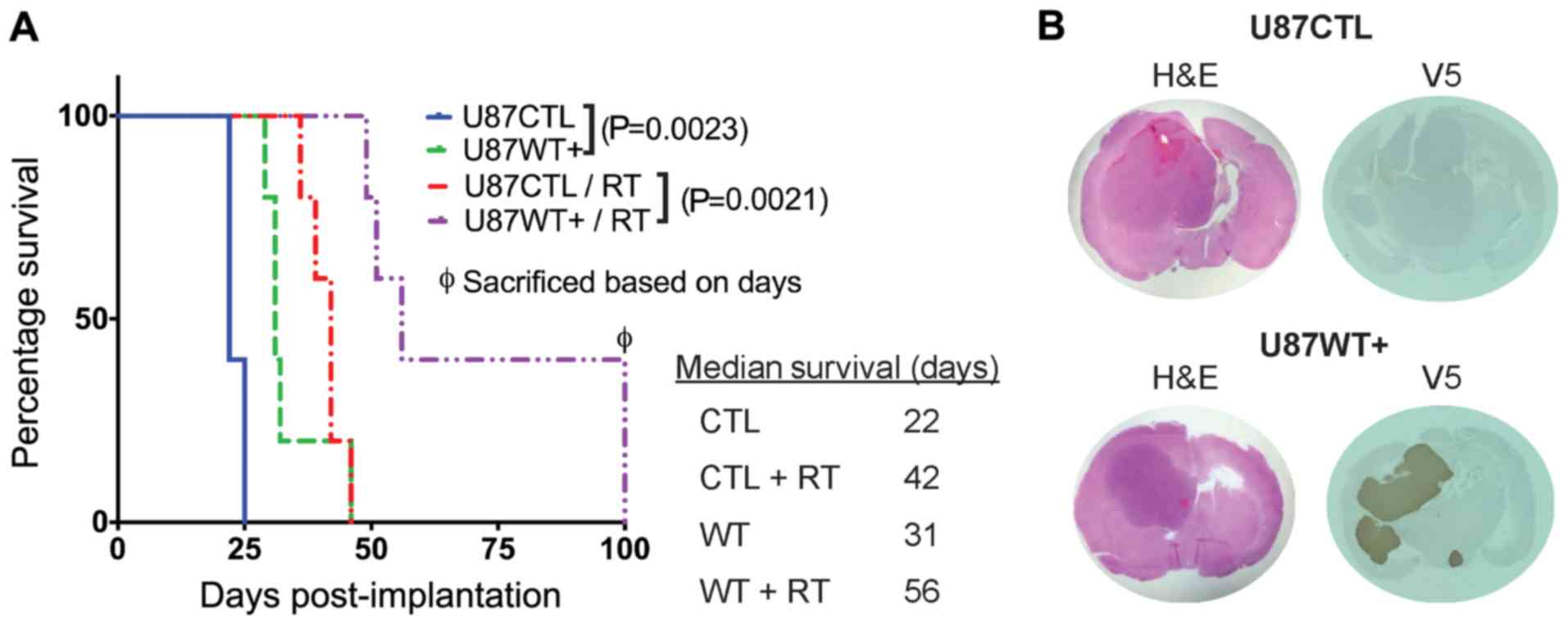
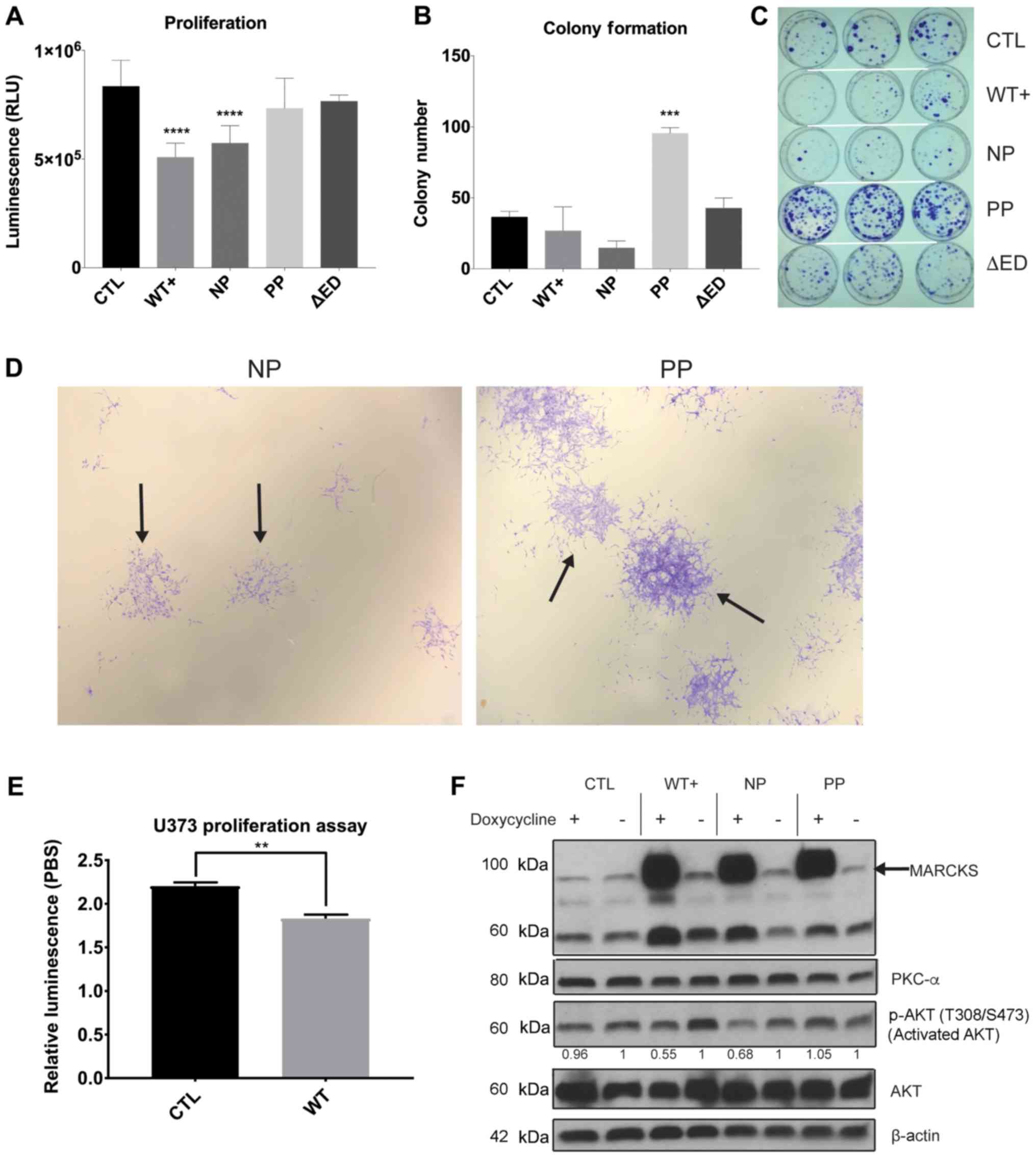
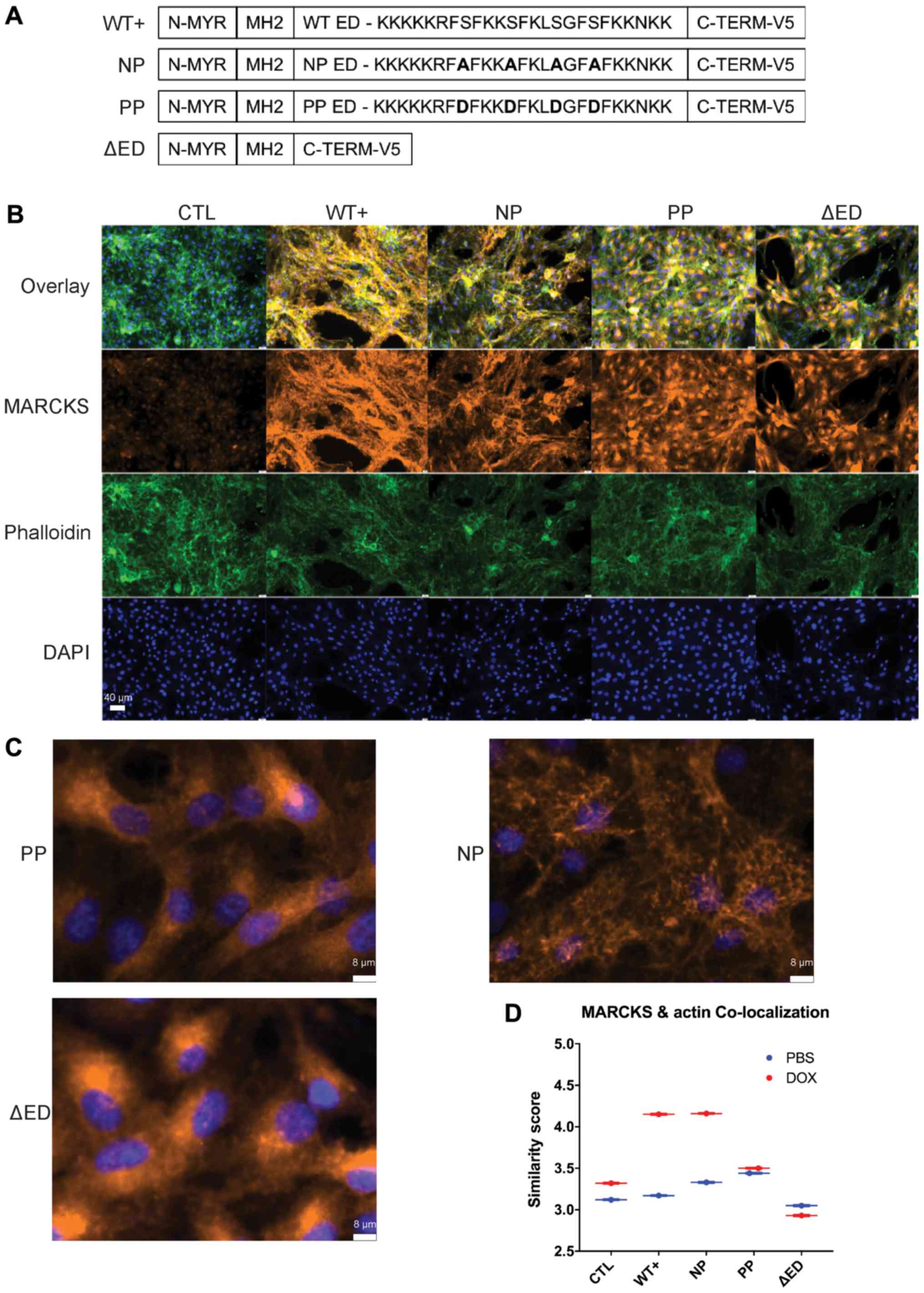
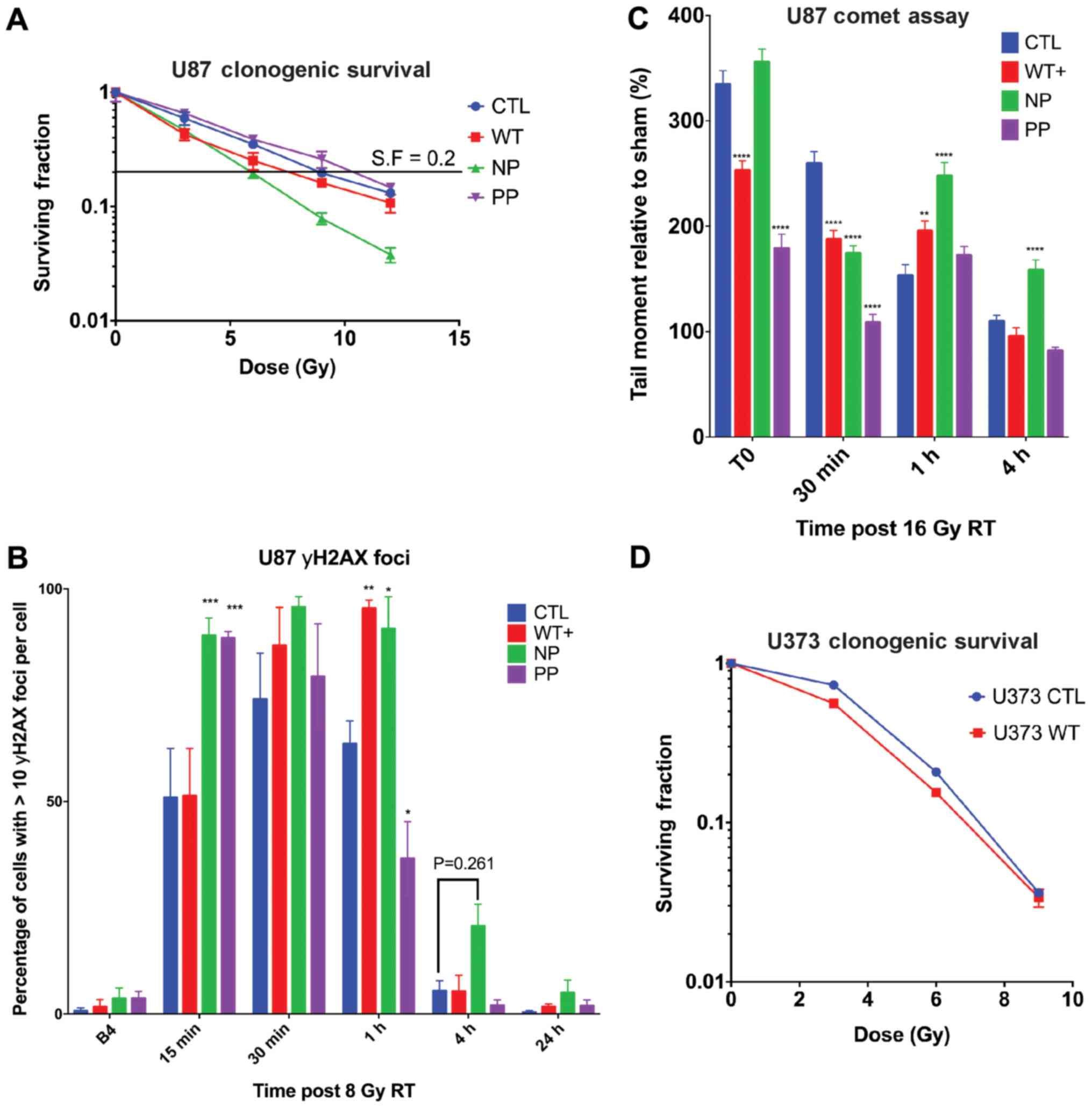
|
1
|
Li YM, Suki D, Hess K and Sawaya R: The
influence of maximum safe resection of glioblastoma on survival in
1229 patients: Can we do better than gross-total resection? J
Neurosurg. 124:977–988. 2016. View Article : Google Scholar
|
|
2
|
Johnson DR and O’Neill BP: Glioblastoma
survival in the United States before and during the temozolomide
era. J Neurooncol. 107:359–364. 2012. View Article : Google Scholar
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mao H, Lebrun DG, Yang J, Zhu VF and Li M:
Deregulated signaling pathways in glioblastoma multiforme:
Molecular mechanisms and therapeutic targets. Cancer Invest.
30:48–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ceccarelli M, Barthel FP, Malta TM,
Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh
A, Pagnotta SM, et al: TCGA Research Network: Molecular Profiling
Reveals Biologically Discrete Subsets and Pathways of Progression
in Diffuse Glioma. Cell. 164:550–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Wu C, Chen N, Gu H, Yen A, Cao L,
Wang E and Wang L: PI3K/Akt/mTOR signaling pathway and targeted
therapy for glioblastoma. Oncotarget. 7:33440–33450.
2016.PubMed/NCBI
|
|
7
|
Jhanwar-Uniyal M, Amin AG, Cooper JB, Das
K, Schmidt MH and Murali R: Discrete signaling mechanisms of mTORC1
and mTORC2: Connected yet apart in cellular and molecular aspects.
Adv Biol Regul. 64:39–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Denley A, Gymnopoulos M, Kang S, Mitchell
C and Vogt PK: Requirement of
phosphatidylinositol(3,4,5)trisphosphate in phosphatidylinositol
3-kinase-induced oncogenic transformation. Mol Cancer Res.
7:1132–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramos AR, Elong Edimo W and Erneux C:
Phosphoinositide 5-phosphatase activities control cell motility in
glioblastoma: Two phosphoinositides PI(4,5)P2 and PI(3,4)P2 are
involved. Adv Biol Regul. 67:40–48. 2018. View Article : Google Scholar
|
|
10
|
Fong LWR, Yang DC and Chen CH:
Myristoylated alanine-rich C kinase substrate (MARCKS): A multirole
signaling protein in cancers. Cancer Metastasis Rev. 36:737–747.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan Y, Zhu Q, Huang D, Zhao S, Jan Lo L
and Peng J: An equation to estimate the difference between
theoretically predicted and SDS PAGE-displayed molecular weights
for an acidic peptide. Sci Rep. 5:133702015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramsden JJ: MARCKS: A case of molecular
exaptation. Int J Biochem Cell Biol. 32:475–479. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arbuzova A, Schmitz AA and Vergères G:
Cross-talk unfolded: MARCKS proteins. Biochem J. 362:1–12. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brudvig JJ and Weimer JM: X MARCKS the
spot: Myristoylated alanine-rich C kinase substrate in neuronal
function and disease. Front Cell Neurosci. 9:4072015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tapp H, Al-Naggar IM, Yarmola EG, Harrison
A, Shaw G, Edison AS and Bubb MR: MARCKS is a natively unfolded
protein with an inaccessible actin-binding site: Evidence for
long-range intramolecular interactions. J Biol Chem. 280:9946–9956.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arbuzova A, Wang J, Murray D, Jacob J,
Cafiso DS and McLaughlin S: Kinetics of interaction of the
myristoylated alanine-rich C kinase substrate, membranes, and
calmodulin. J Biol Chem. 272:27167–27177. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartwig JH, Thelen M, Rosen A, Janmey PA,
Nairn AC and Aderem A: MARCKS is an actin filament crosslinking
protein regulated by protein kinase C and calcium-calmodulin.
Nature. 356:618–622. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yarmola EG, Edison AS, Lenox RH and Bubb
MR: Actin filament cross-linking by MARCKS: Characterization of two
actin-binding sites within the phosphorylation site domain. J Biol
Chem. 276:22351–22358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Arbuzova A, Hangyás-Mihályné G and
McLaughlin S: The effector domain of myristoylated alanine-rich C
kinase substrate binds strongly to phosphatidylinositol
4,5-bispho-sphate. J Biol Chem. 276:5012–5019. 2001. View Article : Google Scholar
|
|
20
|
Zhang W, Crocker E, McLaughlin S and Smith
SO: Binding of peptides with basic and aromatic residues to bilayer
membranes: Phenylalanine in the myristoylated alanine-rich C kinase
substrate effector domain penetrates into the hydrophobic core of
the bilayer. J Biol Chem. 278:21459–21466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thelen M, Rosen A, Nairn AC and Aderem A:
Regulation by phosphorylation of reversible association of a
myristoylated protein kinase C substrate with the plasma membrane.
Nature. 351:320–322. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanabe A, Kamisuki Y, Hidaka H, Suzuki M,
Negishi M and Takuwa Y: PKC phosphorylates MARCKS Ser159 not only
directly but also through RhoA/ROCK. Biochem Biophys Res Commun.
345:156–161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Denisov G, Wanaski S, Luan P, Glaser M and
McLaughlin S: Binding of basic peptides to membranes produces
lateral domains enriched in the acidic lipids phosphatidylserine
and phosphatidylinositol 4,5-bisphosphate: An electrostatic model
and experimental results. Biophys J. 74:731–744. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakaoka T, Kojima N, Ogita T and Tsuji S:
Characterization of the phosphatidylserine-binding region of rat
MARCKS (myristoylated, alanine-rich protein kinase C substrate).
Its regulation through phosphorylation of serine 152. J Biol Chem.
270:12147–12151. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rohrbach TD, Shah N, Jackson WP, Feeney
EV, Scanlon S, Gish R, Khodadadi R, Hyde SO, Hicks PH, Anderson JC,
et al: The effector domain of MARCKS is a nuclear localization
signal that regulates cellular PIP2 levels and nuclear PIP2
localization. PLoS One. 10:e01408702015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bickeböller M, Tagscherer KE, Kloor M,
Jansen L, Chang-Claude J, Brenner H, Hoffmeister M, Toth C,
Schirmacher P, Roth W, et al: Functional characterization of the
tumor-suppressor MARCKS in colorectal cancer and its association
with survival. Oncogene. 34:1150–1159. 2015. View Article : Google Scholar
|
|
27
|
Brooks G, Brooks SF and Goss MW: MARCKS
functions as a novel growth suppressor in cells of melanocyte
origin. Carcinogenesis. 17:683–689. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanada S, Kakehashi A, Nishiyama N, Wei M,
Yamano S, Chung K, Komatsu H, Inoue H, Suehiro S and Wanibuchi H:
Myristoylated alanine-rich C-kinase substrate as a prognostic
biomarker in human primary lung squamous cell carcinoma. Cancer
Biomark. 13:289–298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Manenti S, Malecaze F, Chap H and Darbon
JM: Overexpression of the myristoylated alanine-rich C kinase
substrate in human choroidal melanoma cells affects cell
proliferation. Cancer Res. 58:1429–1434. 1998.PubMed/NCBI
|
|
30
|
Chen CH, Cheng CT, Yuan Y, Zhai J, Arif M,
Fong LW, Wu R and Ann DK: Elevated MARCKS phosphorylation
contributes to unresponsiveness of breast cancer to paclitaxel
treatment. Oncotarget. 6:15194–15208. 2015.PubMed/NCBI
|
|
31
|
Chen CH, Fong LWR, Yu E, Wu R, Trott JF
and Weiss RH: Upregulation of MARCKS in kidney cancer and its
potential as a therapeutic target. Oncogene. 36:3588–3598. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Micallef J, Taccone M, Mukherjee J, Croul
S, Busby J, Moran MF and Guha A: Epidermal growth factor receptor
variant III-induced glioma invasion is mediated through
myristoylated alanine-rich protein kinase C substrate
overexpression. Cancer Res. 69:7548–7556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jarboe JS, Anderson JC, Duarte CW, Mehta
T, Nowsheen S, Hicks PH, Whitley AC, Rohrbach TD, McCubrey RO, Chiu
S, et al: MARCKS regulates growth and radiation sensitivity and is
a novel prognostic factor for glioma. Clin Cancer Res.
18:3030–3041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rohrbach TD, Jones RB, Hicks PH, Weaver
AN, Cooper TS, Eustace NJ, Yang ES, Jarboe JS, Anderson JC and
Willey CD: MARCKS phosphorylation is modulated by a peptide mimetic
of MARCKS effector domain leading to increased radiation
sensitivity in lung cancer cell lines. Oncol Lett. 13:1216–1222.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bradley JD, Kataoka Y, Advani S, Chung SM,
Arani RB, Gillespie GY, Whitley RJ, Markert JM, Roizman B and
Weichselbaum RR: Ionizing radiation improves survival in mice
bearing intracranial high-grade gliomas injected with genetically
modified herpes simplex virus. Clin Cancer Res. 5:1517–1522.
1999.PubMed/NCBI
|
|
37
|
Arbuzova A, Murray D and McLaughlin S:
MARCKS, membranes, and calmodulin: Kinetics of their interaction.
Biochim Biophys Acta. 1376:369–379. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cameron AJ, Procyk KJ, Leitges M and
Parker PJ: PKC alpha protein but not kinase activity is critical
for glioma cell proliferation and survival. Int J Cancer.
123:769–779. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li HF, Kim JS and Waldman T:
Radiation-induced Akt activation modulates radioresistance in human
glioblastoma cells. Radiat Oncol. 4:432009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rohrbach TD, Jarboe JS, Anderson JC,
Trummell HQ, Hicks PH, Weaver AN, Yang ES, Oster RA, Deshane JS,
Steele C, et al: Targeting the effector domain of the myristoylated
alanine rich C-kinase substrate enhances lung cancer radiation
sensitivity. Int J Oncol. 46:1079–1088. 2015. View Article : Google Scholar
|
|
41
|
Naidu MD, Mason JM, Pica RV, Fung H and
Peña LA: Radiation resistance in glioma cells determined by DNA
damage repair activity of Ape1/Ref-1. J Radiat Res (Tokyo).
51:393–404. 2010. View Article : Google Scholar
|
|
42
|
Moon SH, Kim DK, Cha Y, Jeon I, Song J and
Park KS: PI3K/Akt and Stat3 signaling regulated by PTEN control of
the cancer stem cell population, proliferation and senescence in a
glioblastoma cell line. Int J Oncol. 42:921–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peltier J, O’Neill A and Schaffer DV:
PI3K/Akt and CREB regulate adult neural hippocampal progenitor
proliferation and differentiation. Dev Neurobiol. 67:1348–1361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Golding SE, Morgan RN, Adams BR, Hawkins
AJ, Povirk LF and Valerie K: Pro-survival AKT and ERK signaling
from EGFR and mutant EGFRvIII enhances DNA double-strand break
repair in human glioma cells. Cancer Biol Ther. 8:730–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Toulany M, Lee KJ, Fattah KR, Lin YF,
Fehrenbacher B, Schaller M, Chen BP, Chen DJ and Rodemann HP: Akt
promotes post-irradiation survival of human tumor cells through
initiation, progression, and termination of DNA-PKcs-dependent DNA
double-strand break repair. Mol Cancer Res. 10:945–957. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Toulany M and Rodemann HP:
Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of
tumor cell responsiveness to radiation. Semin Cancer Biol.
35:180–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fan QW and Weiss WA: Inhibition of
PI3K-Akt-mTOR signaling in glioblastoma by mTORC1/2 inhibitors.
Methods Mol Biol. 821:349–359. 2012. View Article : Google Scholar :
|
|
48
|
Westhoff MA, Karpel-Massler G, Brühl O,
Enzenmüller S, La Ferla-Brühl K, Siegelin MD, Nonnenmacher L and
Debatin KM: A critical evaluation of PI3K inhibition in
Glioblastoma and Neuroblastoma therapy. Mol Cell Ther. 2:322014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Opel D, Westhoff MA, Bender A, Braun V,
Debatin KM and Fulda S: Phosphatidylinositol 3-kinase inhibition
broadly sensitizes glioblastoma cells to death receptor- and
drug-induced apoptosis. Cancer Res. 68:6271–6280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Trovò L, Ahmed T, Callaerts-Vegh Z, Buzzi
A, Bagni C, Chuah M, Vandendriessche T, D’Hooge R, Balschun D and
Dotti CG: Low hippocampal PI(4,5)P(2) contributes to reduced
cognition in old mice as a result of loss of MARCKS. Nat Neurosci.
16:449–455. 2013. View Article : Google Scholar
|
|
51
|
Wang J, Gambhir A, Hangyás-Mihályné G,
Murray D, Golebiewska U and McLaughlin S: Lateral sequestration of
phos-phatidylinositol 4,5-bisphosphate by the basic effector domain
of myristoylated alanine-rich C kinase substrate is due to
nonspecific electrostatic interactions. J Biol Chem.
277:34401–34412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Glaser M, Wanaski S, Buser CA, Boguslavsky
V, Rashidzada W, Morris A, Rebecchi M, Scarlata SF, Runnels LW,
Prestwich GD, et al: Myristoylated alanine-rich C kinase substrate
(MARCKS) produces reversible inhibition of phospho-lipase C by
sequestering phosphatidylinositol 4,5-bisphosphate in lateral
domains. J Biol Chem. 271:26187–26193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vadlakonda L, Pasupuleti M and Pallu R:
Role of PI3K-AKT-mTOR and Wnt Signaling Pathways in Transition of
G1-S Phase of Cell Cycle in Cancer Cells. Front Oncol. 3:852013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jarboe JS, Anderson JC, Duarte CW, Mehta
T, Nowsheen S, Hicks PH, Whitley AC, Rohrbach TD, McCubrey RO, Chiu
S, et al: MARCKS regulates growth and radiation sensitivity and is
a novel prognostic factor for glioma. Clin Cancer Res.
18:3030–3041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Masaki T, Tokuda M, Yoshida S, Nakai S,
Morishita A, Uchida N, Funaki T, Kita Y, Funakoshi F, Nonomura T,
et al: Comparison study of the expressions of myristoylated
alanine-rich C kinase substrate in hepatocellular carcinoma, liver
cirrhosis, chronic hepatitis, and normal liver. Int J Oncol.
26:661–671. 2005.PubMed/NCBI
|
|
56
|
Chen CH, Statt S, Chiu CL, Thai P, Arif M,
Adler KB and Wu R: Targeting myristoylated alanine-rich C kinase
substrate phosphor-ylation site domain in lung cancer. Mechanisms
and therapeutic implications. Am J Respir Crit Care Med.
190:1127–1138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li H, Chen G, Zhou B and Duan S: Actin
filament assembly by myristoylated alanine-rich C kinase
substrate-phosphati-dylinositol-4,5-diphosphate signaling is
critical for dendrite branching. Mol Biol Cell. 19:4804–4813. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nairn AC and Aderem A: Calmodulin and
protein kinase C cross-talk: The MARCKS protein is an actin
filament and plasma membrane cross-linking protein regulated by
protein kinase C phosphorylation and by calmodulin. Ciba Found
Symp. 164:145–161. 1992.PubMed/NCBI
|
|
59
|
Sundaram M, Cook HW and Byers DM: The
MARCKS family of phospholipid binding proteins: Regulation of
phospholipase D and other cellular components. Biochem Cell Biol.
82:191–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Adan A, Kiraz Y and Baran Y: Cell
proliferation and cytotoxicity sssays. Curr Pharm Biotechnol.
17:1213–1221. 2016. View Article : Google Scholar
|
|
61
|
Posimo JM, Unnithan AS, Gleixner AM, Choi
HJ, Jiang Y, Pulugulla SH and Leak RK: Viability assays for cells
in culture. J Vis Exp. 83:506452014.
|
|
62
|
Yu D, Makkar G, Dong T, Strickland DK,
Sarkar R and Monahan TS: MARCKS signaling differentially regulates
vascular smooth muscle and endothelial cell proliferation through a
KIS-, p27kip1-dependent mechanism. PLoS One. 10:e01413972015.
View Article : Google Scholar
|
|
63
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
64
|
Borràs M, Armengol G, De Cabo M,
Barquinero JF and Barrios L: Comparison of methods to quantify
histone H2AX phosphorylation and its usefulness for prediction of
radiosensi-tivity. Int J Radiat Biol. 91:915–924. 2015. View Article : Google Scholar
|
|
65
|
Thinon E, Serwa RA, Broncel M, Brannigan
JA, Brassat U, Wright MH, Heal WP, Wilkinson AJ, Mann DJ and Tate
EW: Global profiling of co- and post-translationally
N-myristoylated proteomes in human cells. Nat Commun. 5:49192014.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
67
|
Lott K and Cingolani G: The importin β
binding domain as a master regulator of nucleocytoplasmic
transport. Biochim Biophys Acta. 1813:1578–1592. 2011. View Article : Google Scholar
|
|
68
|
Vargova J, Vargova K, Dusilkova N, Kulvait
V, Pospisil V, Zavadil J, Trneny M, Klener P and Stopka T:
Differential expression, localization and activity of MARCKS
between mantle cell lymphoma and chronic lymphocytic leukemia.
Blood Cancer J. 6:e4752016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Herhaus L, Perez-Oliva AB, Cozza G,
Gourlay R, Weidlich S, Campbell DG, Pinna LA and Sapkota GP: Casein
kinase 2 (CK2) phosphorylates the deubiquitylase OTUB1 at Ser16 to
trigger its nuclear localization. Sci Signal. 8:ra352015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gao Y and Wang HY: Casein kinase 2 Is
activated and essential for Wnt/beta-catenin signaling. J Biol
Chem. 281:18394–18400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tu X, Joeng KS, Nakayama KI, Nakayama K,
Rajagopal J, Carroll TJ, McMahon AP and Long F: Noncanonical Wnt
signaling through G protein-linked PKCdelta activation promotes
bone formation. Dev Cell. 12:113–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jin BY, Lin AJ, Golan DE and Michel T:
MARCKS protein mediates hydrogen peroxide regulation of endothelial
permeability. Proc Natl Acad Sci USA. 109:14864–14869. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen WC, McBride WH, Iwamoto KS, Barber
CL, Wang CC, Oh YT, Liao YP, Hong JH, de Vellis J and Shau H:
Induction of radioprotective peroxiredoxin-I by ionizing
irradiation. J Neurosci Res. 70:794–798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mesiwala AH, Farrell L, Santiago P, Ghatan
S and Silbergeld DL: The effects of hydrogen peroxide on brain and
brain tumors. Surg Neurol. 59:398–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xu XH, Deng CY, Liu Y, He M, Peng J, Wang
T, Yuan L, Zheng ZS, Blackshear PJ and Luo ZG: MARCKS regulates
membrane targeting of Rab10 vesicles to promote axon development.
Cell Res. 24:576–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yu D, Makkar G, Strickland DK, Blanpied
TA, Stumpo DJ, Blackshear PJ, Sarkar R and Monahan TS:
Myristoylated alanine-rich protein kinase substrate (MARCKS)
regulates small GTPase Rac1 and Cdc42 activity and is a critical
mediator of vascular smooth muscle cell migration inintimal
hyperplasia formation. J Am Heart Assoc. 4:e0022552015. View Article : Google Scholar
|
|
77
|
Patel AP, Tirosh I, Trombetta JJ, Shalek
AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT,
Martuza RL, et al: Single-cell RNA-seq highlights intratumoral
heterogeneity in primary glioblastoma. Science. 344:1396–1401.
2014. View Article : Google Scholar : PubMed/NCBI
|