Introduction
R-spondins consist of a signal peptide, furin
repeats and a thrombospondin type 1 repeat (TSR1) domain (1-4).
They enhance Wnt/β-catenin signaling by acting as ligands of
leucine-rich repeat-containing G protein-coupled receptor (LGR)
4-6, E3 ubiquitin ligase zinc and ring finger 3 (ZNRF3), and its
homolog, ring finger 43 (RNF43) (2,5-8).
Wnt/β-catenin signaling regulates several developmental events,
including embryo morphogenesis, formation of muscle, and limb
patterning (9-12). Conversely, Wnt/β-catenin signaling
is associated with various diseases, including cancer; thus,
abnormal Wnt/β-catenin signaling contributes to tumor cell
progression in vivo and in vitro (9,13).
R-spondin2 (Rspo2), a member of the R-spondin family
of proteins, has crucial functions in development through the
Wnt/β-catenin signaling pathway. Rspo2 is essential for normal
laryngeal-tracheal, lung, and limb morphogenesis, and Rspo2
deficiency results in immediate death following birth (14,15).
Additionally, Rspo2 is important for cell differentiation and has
been demonstrated to have a role in the mineralization of
osteoblasts in osteoarthritis (16), myogenic differentiation, and
hypertrophic myotube formation (17). Notably, Rspo2 is also involved in
cancer progression. There are two contrasting aspects regarding the
function of Rspo2 in cancer progression: It has suppressive
activity in colorectal cancer, but promoting in hepatocellular
carcinoma and pancreatic cancer (18-20).
However, both these opposite functions are regulated by
Wnt/β-catenin signaling. Therefore, it is important to examine the
association between Wnt/β-catenin signaling and Rspo2 in various
types of cancer.
C-mannosylation is a protein modification in
which an α-mannose attaches to the indole C2 carbon of the first
tryptophan residue of the consensus sequence Trp-Xaa-Xaa-Trp/Cys,
via a C-C linkage (21,22). This unique type of glycosylation
was first identified in human ribonuclease 2 (23). C-mannosylated substrates
have been reported for ~25 proteins (24), and have been demonstrated to occur
in various mammalian cells, including human, monkey, pig, mouse and
hamster (25). The majority of
C-mannosylated proteins contain the TSR1 domain (26) or are type 1 cytokine receptors that
contain a Trp-Ser-Xaa-Trp-Ser motif (27,28).
C-mannosylation affects protein secretion, intracellular
localization, protein folding, and protein-protein interactions,
similar to other forms of glycosylations (29-32);
however, the association between disease and C-mannosylation
is not fully understood. C-mannosyltransferase dpy-19
(DPY19) was first identified quite recently as a
C-mannosyltransferase in Caenorhabditis elegans
(33). Four homologs of DPY19
exist in mammals, and human dpy-19 like
C-mannosyltransferase (DPY19L) 3 and mouse DPY19L1 and
DPY19L3 have been identified as mammalian
C-mannosyltransferases (30,34).
We previously reported that both Rspo1 and Rspo3 are
C-mannosylated at two conserved Trp residues (30,35).
Although these modifications regulate secretion and Wnt/β-catenin
signaling activity in HT1080 cells, the association between
tumorigenesis and C-mannosylation is not yet fully
understood. Furthermore, among the R-spondin family proteins, only
Rspo2 has been reported to have a suppressive effect on frizzled
class receptor 7 (Fzd7) levels, resulting in a reduction in
colorectal cancer cell migration (36). Thus, it is important to investigate
the role of Rspo2 in additional tumor cell lines. In the present
study, several types of malignant tumor cell lines were used in
order to elucidate the role of C-mannosylation on Rspo2
function in cancer. These included the pancreatic cancer PANC1
cells, in which Rspo2 acts as an enhancer, as well as the breast
cancer MDA-MB-231, lung cancer A549 and fibrosarcoma HT1080
cells.
The current study examined whether Rspo2 was
C-mannosylated. Using liquid chromatography-tandem mass
spectrometry (LC-MS/MS) analysis, Rspo2 was determined to be
C-mannosylated at Trp150 and Trp153,
and these modifications were demonstrated to alter its secretory
levels and intracellular trafficking. In addition,
C-mannosylation of Rspo2 regulated the agonistic activity of
Rspo2 in the Wnt/β-catenin signaling pathway and on cancer cell
migration. The present data indicated that the
C-mannosylation of Rspo2 may serve as a potential novel
therapeutic target for cancer.
Materials and methods
Cell culture
HT1080 human fibrosarcoma (Japanese Collection of
Research Bioresources Cell Bank, Osaka, Japan), 293T (RIKEN
BioResource Center, Tsukuba, Japan), A549 human lung adenocarcinoma
(RIKEN BioResource Center) (37-40),
PANC1 human pancreatic adenocarcinoma (RIKEN BioResource Center)
and MDA-MB-231 human breast adenocarcinoma cell lines, which was
gifted by Professor Masakazu Toi (Kyoto University Graduate School
of Medicine, Kyoto, Japan), were all cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Nissui Pharmaceutical Co., Ltd,
Tokyo, Japan) supplemented with 6% (v/v) fetal bovine serum (Cosmo
Bio Co. Ltd., Tokyo, Japan), 100 U/ml penicillin G, 100 mg/l
kanamycin, 600 mg/l L-glutamine, and 2.25 g/l NaHCO3 at
37°C in a humidified incubator with 5% CO2. The LoVo
human colon adenocarcinoma cell line was cultured in RPMI-1640
medium (Nissui Pharmaceutical Co., Ltd) supplemented with 10% (v/v)
fetal bovine serum, 105 U/ml penicillin G, 105 mg/l kanamycin, 314
mg/l L-glutamine, and 2.25 g/l NaHCO3 at 37°C in a
humidified incubator with 5% CO2.
Plasmid construction
Total RNA was extracted from THP1 cells using RNA
extraction buffer (38% (w/w) Phenol, 0.8 M guanidine thiocyanate,
0.4 M ammonium thiocyanate, 0.1 M sodium acetate, 5% (v/v)
glycerol), and cDNA was prepared from 2 µg of total RNA with
the High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The resulting cDNA was
used for polymerase chain reaction (PCR) amplification with
PrimeSTAR Max DNA Polymerase (Takara Bio Inc., Shiga, Japan). The
sequences of the primers used to amplify the human Rspo2 cDNA,
were: 5′-GAACCCTCCAGTTCCTAGACTTTGA GAGGCGTCTC-3′ (forward) and
5′-ATTGGTTAGCTCTGT CTGTAGCTAGGAAGACGCTG-3′ (reverse). PCR was
performed for 35 cycles with an annealing temperature of 60°C. The
PCR product contained a 5′ untranslated region (UTR), the coding
sequence for the Rspo2 gene, and a 3′UTR. To obtain the coding
region of the Rspo2 gene, a second PCR was performed with the same
reagents/conditions as the first PCR, and using the following
primers: 5′-TTTTCTCGAGATGCAGTTTCGCCTTTTCTCCTTTG-3′ (forward) and
5′-TTTTGCGGCCGCTTGGTTAGCTCTGTCTGTAGC-3′ (reverse). The obtained
cDNA was subcloned into pCI-neo (Promega Corporation, Madison, WI,
USA) and CSII-CMV-MCS-IRES2-Bsd vectors (RIKEN BioResource Center).
To introduce the C-terminal myc-his6 tag, PCR was performed with
primers that had Myc and his6 codons. The sequences of the tags
were as follows: Myc, 5′-GAACAAAAACTCATCTCAGAAGAGGATCTG-3′; and
his6, 5′-CATCATCACCATCACCAT-3′. Certain tryptophan residues in
Rspo2 were substituted with alanine residues by PCR site-directed
mutagenesis using the overlap extension technique with PrimeSTAR
Max DNA Polymerase. The sequences of primers that were used for the
mutagenesis, the number of cycles, and the annealing temperatures
were as follows: W150A, 5′-GTGAAGTTGGTCATGCGAGCGAATGGGGAA-3′
(forward) and 5′-GTTCCCCATTCGCTCGCATGACCAACTTCA-3′ (reverse), 35
cycles, 63°C; and W153A, 5′-GAGCGAAGCGGGAACTTG-3′ (forward) and
5′-CAAGTTCCCGCTTCGCTC-3′ (reverse), 35 cycles, 63°C. The resulting
cDNAs were cloned into the XhoI/NotI restriction
sites of pCI-neo and CSII-CMV-MCS-IRES2-Bsd vectors for mammalian
cell expression.
Green fluorescent protein (GFP) cDNA was amplified
from pAcGFP1-N1 (Takara Bio Inc.) with PrimeSTAR Max DNA Polymerase
and subcloned into CSII-CMV-MCS-IRES2-Bsd. The sequences of the
gene-specific primers, the number of cycles, and the annealing
temperature were as follows: 5′-TT
TTCTCGAGATGGTGAGCAAGGGCGCCGAGCTG-3′ (forward) and
5′-TTTTGCGGCCGCTCACTTGTACAGCTCATCCATGC-3′ (reverse), 35 cycles,
63°C. The resulting cDNA was cloned into the
XhoI/NotI restriction sites of CSII-CMV-MCS-IRES2-Bsd
for mammalian cell expression.
Establishment of Rspo2-overexpressing
cell lines
HT1080 cells were grown in a 6-well plate at 50-60%
confluence and transfected with 2 µg pCI-neo vectors using
the Lipofectamine 3000 transfection kit (Thermo Fisher Scientific,
Inc.), according to the manufacturer’s protocol, in order to
establish stable cell lines that expressed wild-type or mutant
Rspo2-myc-his6. At 72 h post-transfection, cells were treated with
400 µg/ml G418 (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) for selection of stable cells. Clonal cells that expressed
high levels of myc-his6-tagged wild-type and double mutant
W150A/W153A (termed herein 2WA) Rspo2 were designated
HT1080-Rspo2-MH and HT1080-Rspo2/2WA-MH cells, respectively. Mixed
population of W150A-expressing cells were designated
HT1080-Rspo2/W150A-MH. Cells that were transfected with the empty
pCI-neo vector were termed HT1080-neo (41).
To establish Rspo2-overexpressing A549, PANC1, MDA-
MB-231, and LoVo cell lines, the CSII-CMV-MCS-IRES2-Bsd plasmids
were transfected into 293T cells using a Lentivirus High Titer
Packaging Mix (Takara Bio Inc.) for lentivirus production. After 6
h of transfection, the cells were washed, and fresh medium was
added. After an additional 48 h of culture, the conditioned media,
containing lentivirus, were collected, and each cell line was
infected with the lentivirus media. Following infection, A549,
PANC1, MDA-MB-231 and LoVo cells were selected with 20, 10, 7.5 and
15 µg/ml Blasticidin S (Wako Pure Chemical Industries,
Ltd.), respectively. Cells that expressed high levels of
myc-his6-tagged wild-type and 2WA mutant Rspo2, and GFP which was
used for control expression, were used for experiments.
Rspo2-overexpressing A549 cells were followed by limiting dilution
method to establish clonal cells.
Western blotting
For the western blot analysis, a slightly modified
version of a previously described method was used (42-44).
Cells were cultured, and then lysed in buffer (50 mM Tris-HCl pH
7.5, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium
deoxycholate, and 1 mM PMSF) at 4°C with sonication. The lysates
were centrifuged at 15,300 × g for 10 min, and the amount of
protein in each lysate was measured by Coomassie Brilliant Blue
(CBB) G-250 staining (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Loading buffer (350 mM Tris-HCl pH 6.8, 30% glycerol, 0.012%
bromophenol blue, 6% SDS, and 30% 2-mercaptoethanol) was added to
each lysate. The samples were then boiled for 5 min and
electrophoresed on 12.5% SDS-polyacrylamide gels. Proteins were
transferred to polyvinylidene fluoride membranes and, following
blocking with 5% skim milk at room temperature for 30 min,
immunoblotted with anti-c-Myc (9E10 hybridoma culture supernatant;
Developmental Studies Hybridoma Bank, Iowa City, IA, USA) or
anti-α-tubulin (cat. no. T5168; Merck KGaA, Darmstadt, Germany).
Signals were detected with enhanced chemiluminescence using Western
Lightning Plus-ECL reagent (PerkinElmer, Inc., Waltham, MA, USA) or
Immobilon Western Chemiluminescent HRP substrate (Merck KGaA), and
exposed to RX-U films (Fujifilm, Tokyo, Japan) in a dark room. The
9E10 hybridoma culture supernatant was diluted 1:50 in a washing
buffer (19.8 mM Tris-HCl pH 7.6, 137 mM NaCl, and 0.1% Tween-20)
containing 5% skim milk.
Detection of secreted Rspo2
Cells were washed with PBS twice, and then cultured
in serum-free DMEM for 24 h with or without 50 µg/ml soluble
heparin. The conditioned media was collected, and cell lysates were
prepared as described above. The conditioned media was concentrated
using Ni-nitrilotriacetic acid (Ni-NTA) agarose (Roche Diagnostics,
Mannheim, Germany) for 2 h at 4°C. Then, the Ni-NTA agarose was
collected, washed with PBS twice, and eluted with buffer I (900 mM
NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM
KH2PO4, and 500 mM imidazole). After being eluted from the Ni-NTA
agarose, the amount of protein in each sample of conditioned media
was estimated from the total amount of protein in each set of cell
lysates by CBB staining. Based on the inverse ratio of each cell
lysate concentration, additional buffer I was added to each sample
to establish uniform concentrations among the conditioned media.
Loading buffer was added to the conditioned media and cell lysates,
and boiled for 5 min. Then, the proteins were separated by 12.5%
SDS-PAGE and analyzed by immunoblotting with anti-c-Myc and
anti-α-tubulin antibodies, as aforementioned.
Purification of recombinant Rspo2 for
LC-MS
To purify recombinant Rspo2, a slightly modified
version of a previously described method was used (35). Clonal HT1080 or A549 cells that
expressed high levels of wild-type Rspo2-MH were established and
cultured for 24 h in serum-free medium that contained 25
µl/ml heparin sepharose 6 fast flow (GE Healthcare Life
Sciences, Little Chalfont, UK). Then, the heparin sepharose was
collected, washed with PBS twice, and eluted with buffer II (900 mM
NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM
KH2PO4, and 5 mM imidazole). The eluted
samples were concentrated using Ni-NTA agarose for 2 h at 4°C. The
Ni-NTA agarose was collected, washed with buffer II, and eluted
with buffer I by incubating for 5 min at room temperature followed
by boiling for 5 min. The eluted samples were further concentrated
on a Vivaspin 500 (3000 MWCO; Sartorius AG, Göttingen, Germany).
The eluates were electrophoresed on an SDS-polyacrylamide gel, and
the protein bands were visualized by CBB staining.
LC-MS
To identify the C-mannosylation sites, an
ultrasensitive Q-Exactive nanoLC-MS/MS system was used. Purified
Rspo2 samples were subjected to 12.5% SDS-PAGE. After CBB staining,
the visible band was excised and de-stained. Following reduction
and S-carboxymethylation with iodoacetic acid, in-gel digestion was
performed using trypsin (TPCK-treated; Worthington Biochemical,
Worthington, OH, USA) and an endoproteinase Asp-N
(sequencing-grade; Roche Diagnostics, Basel, Switzerland). The
digestion mixture was separated on a nanoflow LC (Easy nLC; Thermo
Fisher Scientific, Inc.) using a nano-electrospray ionization spray
column (NTCC analytical column; C18, φ75 µm × 100 mm,
3 µm; Nikkyo Technology Co., Ltd., Tokyo, Japan) with a
linear gradient of 0-66% buffer B (100% acetonitrile and 0.1%
formic acid) and a flow rate of 300 nl/min over 20 min, coupled
on-line to a Q-Exactive mass spectrometer (Thermo Fisher
Scientific, Inc.) that was equipped with a nanospray ion source
that had no gas assistance. The mass spectrometer was operated in
positive-ion mode, and the MS/MS spectra were acquired using an
inclusion list that contained double or triple charged peptide ions
with or without the C-mannosylation
(146EVGHWSEWGTCSR158 unmodified,
m/z=796.3333 or 531.2247; mono-C-mannosylation,
m/z=877.3598 or 585.2423; di-C-mannosylations,
m/z=958.3862 or 639.2599). The MS/MS chromatograms of the y5
ion (m/z=581.2350±10 ppm) of the listed peptides and their
MS/MS spectra were constructed using Qual Browser Thermo Xcalibur
3.1.66.10 (Thermo Fisher Scientific, Inc.).
Immunofluorescence
Cells were grown on coverslips in a 6-well plate,
washed twice with PBS, fixed in 4% paraformaldehyde for 10 min, and
permeabilized with 0.1% Triton X-100 for 10 min at room
temperature. After being blocked with 3% bovine serum albumin for
30 min, the cells were incubated with anti-c-Myc at room
temperature for 1 h. Alexa Fluor488-conjugated anti-mouse
immunoglobulin (Ig)G (cat. no. A11029; Thermo Fisher Scientific,
Inc.) was used as the secondary antibody at room temperature for 1
h. To determine the localization of the Golgi apparatus and the
endoplasmic reticulum (ER), cells were incubated with a rabbit
polyclonal anti-Golgi reassembly stacking protein 1 (GRASP65; cat.
no. sc-30093; Santa Cruz Biotechnology, Inc., Dallas, TX., USA) and
a rabbit polyclonal anti-calnexin (cat. no. 2433; Cell Signaling
Technology, Inc., Danvers, MA, USA) antibody at room temperature
for 1 h each. Alexa Fluor568-conjugated anti-rabbit IgG (cat. no.
A11036; Thermo Fisher Scientific, Inc.) was used as the secondary
antibody at room temperature for 1 h. After being washed twice with
PBS, the cells were incubated with 2 µg/ml Hoechst 33258
(Polysciences, Inc., Warrington, PA, USA) for 10 min to stain the
nuclei. Cells were then washed again three times with PBS and
observed under a FLUOVIEW FV10i confocal laser-scanning microscope
(Olympus Corporation, Tokyo, Japan). The 9E10 hybridoma culture
supernatant towards c-Myc was diluted 1:4, while the anti-GRASP65
and anti-calnexin antibodies were diluted 1:100 in PBS containing
3% bovine serum albumin. The secondary antibodies were diluted
1:500 in PBS containing 3% bovine serum albumin.
Luciferase reporter assay
To assess Wnt/β-catenin signaling activity, a
TOP/FOP luciferase reporter system was used. Each cell
(4×104 cells/well) was seeded in 24-well plates and
cultured overnight. Using the Lipofectamine 3000 transfection kit,
the cells were transiently transfected with 800 ng canonical Wnt
signaling reporter Super 8×TopFlash or mutant reporter Super
8×FopFlash (cat. nos. 12456 and 12457; Addgene, Watertown, MA, USA)
(45) and 20 ng phRL-TK (Promega
Corporation) in the presence of 30% Wnt3a-conditioned medium from
L-Wnt3a cells (cat. no. CRL-2647; American Type Culture Collection,
Manassas, VA, USA), as previously described (46). After 24 h, the cells were lysed,
and firefly and Renilla luciferase activities were measured using
infinite 200Pro multifunctional microplate reader (Tecan Group
Ltd., Männedorf, Switzerland). TOPFlash and FOPFlash activities
were normalized to that of Renilla.
Wound-healing assay
HT1080 and A549 cells (2×105 cells/well)
were seeded into 24-well plates and cultured for 24 h to ~90%
confluence, and then wounded using a yellow pipette tip. After
being washed twice with PBS to remove floating cells, the cells
were cultured in serum-free DMEM for 12 h (HT1080) or 96 h (A549).
Photographs were captured of four independent areas, and the
migrated areas were quantified using ImageJ 1.51 software (National
Institutes of Health, Bethesda, MD, USA) (47).
Transwell migration assay
To assess migration, 6.5-mm transwell inserts with
8.0 µm pore polycarbonate membranes were used (cat. no.
3422; Corning, Inc., Corning, NY, USA). Cells were washed twice
with serum-free DMEM to remove serum and seeded into the upper
chambers at 2×104 cells/chamber; the lower chambers were
filled with growth medium. The cells were incubated for 3 h, and
then non-migrated cells on the upper surfaces of the membranes were
carefully removed with a cotton swab. Migrated cells were fixed and
stained using Diff-Quick solution (Sysmex Corporation, Kobe,
Japan). Photographs were capture from four independent areas and
migration was quantified as the average number of migrated cells
per area (48).
Statistical analysis
Data were expressed as the mean ± standard deviation
of three independent experiments. Statistical analyses were
performed using one-way analysis of variance with Tukey’s post-hoc
test. Analysis was performed with GraphPad Prism 8 statistical
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
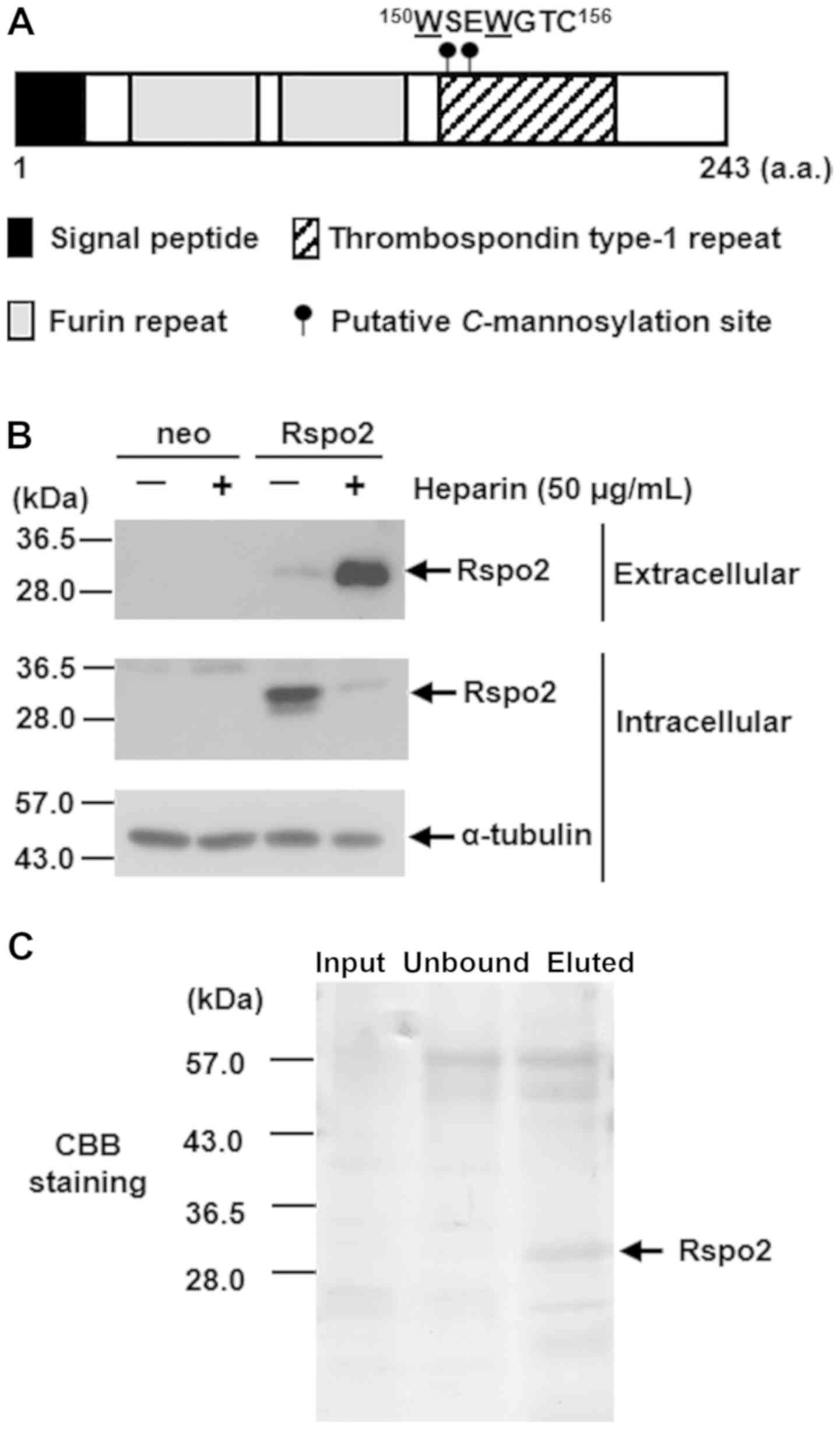
Rspo2 is C-mannosylated at
Trp150 and Trp153
Human Rspo2 has two consecutive
C-mannosylation consensus sequence sites from
Trp150 (W150-S-E-W153-G-T-C) in
the TSR1 domain (Fig. 1A). To
determine whether these tryptophans are C-mannosylated, a
Rspo2-overexpressing HT1080 clonal cell line was established
(HT1080-Rspo2-MH). Because the R-spondin family proteins bind to
cell surface heparan sulfate proteoglycans (14,49),
to confirm that Rspo2 is secreted in the conditioned medium, cells
were treated with heparin. As a result, the levels of secreted
Rspo2 were increased (Fig. 1B).
Therefore, sequential affinity chromatography was performed using
heparin sepharose and Ni-NTA agarose to purify the recombinant
Rspo2 (Fig. 1C).
The resulting purified sample was digested with
trypsin and Asp-N, and the subsequent mixture of peptides was
subjected to LC-MS. LC-MS analysis indicated a triply charged form
of the peptide 146EVGHWSEWGTCSR158,
corresponding to un-, mono-, and di-mannosylated forms (Fig. 2A). The expected values of
m/z for each peptide are 531.22, 585.24 and 639.26,
respectively, and each peptide was detected at the expected
m/z, suggesting that Rspo2 exists in un-, mono-, and
di-mannosylated forms. In addition, to identify the
C-mannosylation sites of Rspo2, LC-MS/MS analysis was
performed on the aforementioned peptides (Fig. 2B). The un- and mono-mannosylated
peptides had the same spectral pattern until the y8 ions. This
result suggested that one mannose was modified in
146EVGHW150. Based on previous studies, there
is no indication that any hexose modification occurs in those
peptides, except at Trp150. Thus, it is most likely that
Trp150 is mannosylated. Then, the di-mannosylated
peptide was identified from the y1 ion to the y5 ion by LC-MS/MS
analysis, indicating that two mannoses are modified in
146EVGHWSEW153. Considering that un- and
mono-mannosylated peptides were identified from the y1 ion to the
y8 ion, it was concluded that Trp153 was modified. In
addition, Trp150 was likely modified by one mannose
residue; one- and two-hexose glycosylation at Glu, Val, Gly, His,
and Ser have not been reported. Furthermore, to verify whether
Rspo2 is also C-mannosylated in other cell types, a
Rspo2-overexpressing clonal cell line was generated in A549 cells
(A549-Rspo2-MH) and recombinant Rspo2 was purified. As presented in
Fig. S1, Rspo2 was also
C-mannosylated in A549 cells. Altogether, Rspo2 was
demonstrated to exist in three forms: un-mannosylated,
Trp150-mannosylated, and Trp150- and
Trp153-mannosylated. Therefore, it was concluded that
both Trp150 and Trp153 were
C-mannosylation sites of Rspo2 in human tumor cells.
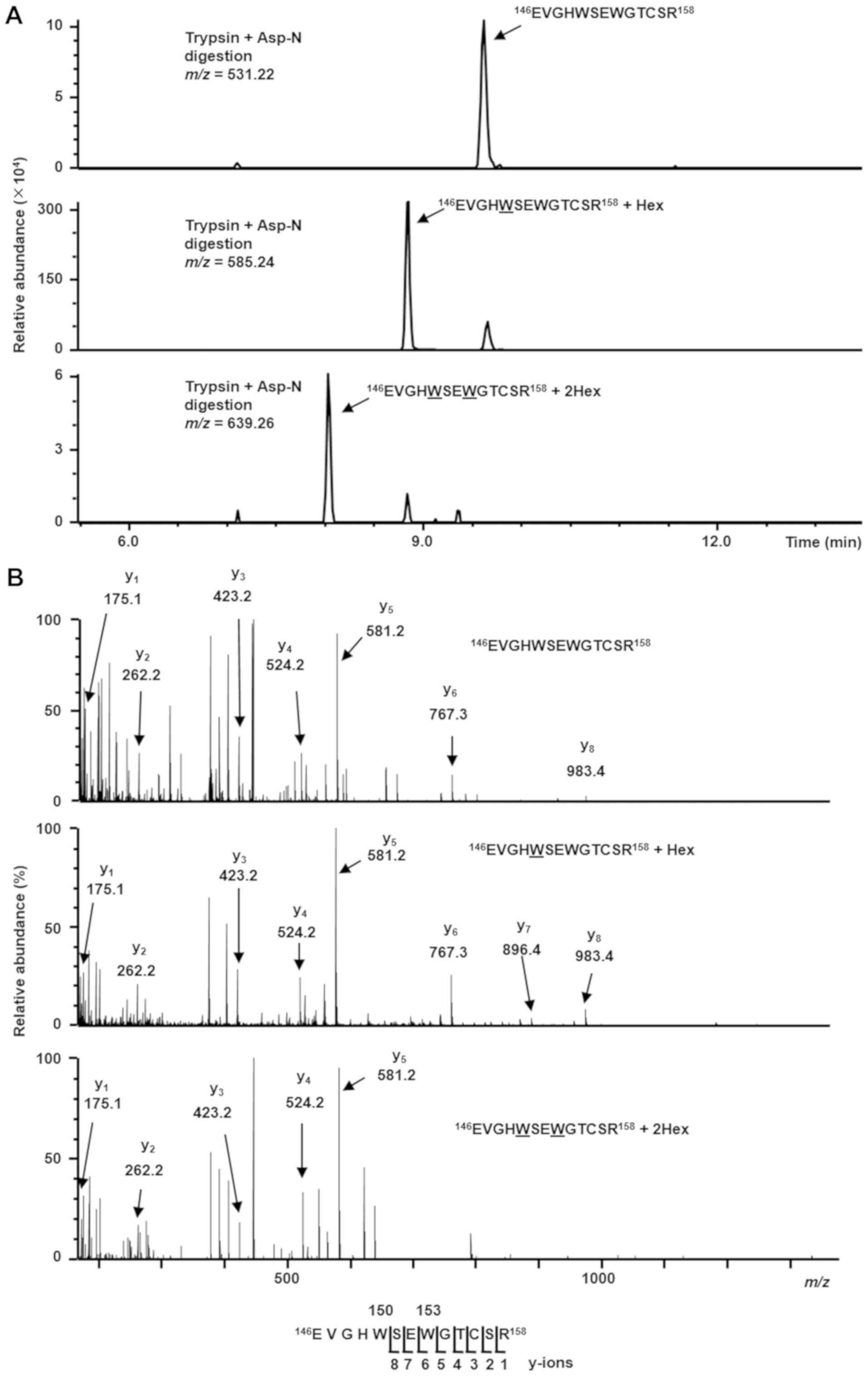 | Figure 2Trp150 and
Trp153 of Rspo2 are C-mannosylated. (A) Purified
recombinant Rspo2 protein samples were digested with trypsin and
Asp-N, and the resulting peptides were analyzed by targeted MS/MS.
According to the inclusion list of triple protonated ions of un-,
mono-, and di-mannosylated
146EVGHWSEWGTCSR158 peptides
(m/z=531.22, 585.24 and 639.26, respectively), MS/MS spectra
were obtained, and selected ion chromatograms of y5 ions
(m/z=581.24) of these parent ions were drawn. (B) MS/MS
spectra of the triple-charged un-, mono-, and di-mannosylated
146EVGHWSEWGTCSR158 peptides
(m/z=531.22, 585.24 and 639.26, respectively). The indicated
y-ions were detected as single charged ions. Un-mannosylated-
(top), only Trp150-mannosylated- (middle), and both
Trp150- and Trp153-mannosylated- (bottom)
peptides were observed. Trp, tryptophan; Rspo2, R-spondin2; MS/MS,
tandem mass spectroscopy. |
C-mannosylation of Rspo2 regulates its
secretion and intracellular trafficking in HT1080 cells
Glycoproteins often regulate their secretion by
glycosylation. Because Rspo2 is secreted into the extracellular
environment, we examined whether C-mannosylation of Rspo2
regulates its secretion. To this end, a
C-mannosylation-defective mutant Rspo2-overexpressing HT1080
clonal cell line was generated (HT1080-Rspo2/2WA-MH) by
substituting the two Trps, Trp150 and Trp153,
with Ala. This cell line was confirmed by western blot analysis
(Fig. 3A).
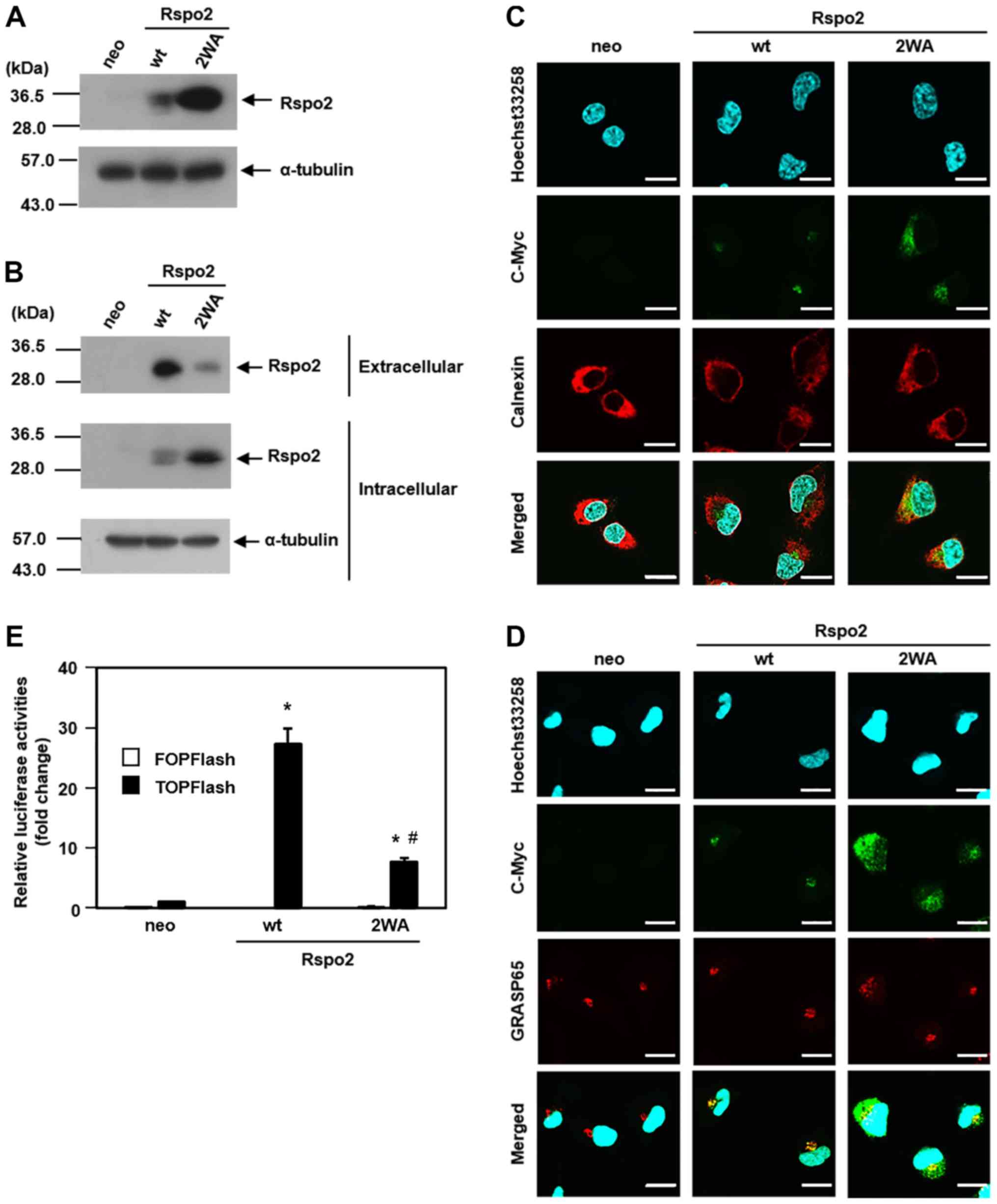 | Figure 3Rspo2 functions are regulated by
C-mannosylation. (A) A C-mannosylation-defective
mutant form of Rspo2 was generated (2WA) and overexpressed in the
HT1080 cell line (HT1080-Rspo2/2WA-MH). Control HT1080-neo (neo),
HT1080-Rspo2-MH (wt), and HT1080-Rspo2/2WA-MH (2WA) cells were
lysed, and each cell lysate was electrophoresed and immunoblotted
with anti-c-Myc and anti-α-tubulin. (B) Effect of
C-mannosylation on Rspo2 secretion. Each cell line was
cultured in serum-free medium with 50 µg/ml heparin. After
24 h, each batch of cell lysates and conditioned media were
collected, and the samples were electrophoresed and immunoblotted
with anti-c-Myc or anti-α-tubulin. (C) Effect of
C-mannosylation on the intracellular trafficking of Rspo2.
Each cell line was fixed and stained with Hoechst33258 (blue),
anti-c-Myc (green), and anti-calnexin (red). The samples were
observed under a confocal laser-scanning microscope and
representative photographs were captured under identical
magnification (magnification, ×60), microscope conditions and
linear adjustment. Scale bar, 20 µm. (D) Effect of
C-mannosylation on the intracellular trafficking of Rspo2. Each
cell line was fixed and stained with Hoechst33258 (blue),
anti-c-Myc (green), and anti-GRASP65 (red). The samples were
observed under a confocal laser-scanning microscope and
representative photographs were captured under identical
magnification (magnification, ×60), microscope conditions and
linear adjustment. Scale bar, 20 µm. (E) Effect of
C-mannosylation on Rspo2-mediated enhancement of Wnt
signaling. Each cell line was transfected with TOPFlash or FOPFlash
in the presence of 30% L-Wnt3a cell-conditioned medium. After 24 h,
luciferase activities were measured and normalized to
Renilla luciferase. *P<0.05 compared
with neo; #P<0.05 compared with wt. Rspo2,
R-spondin2; wt, wild-type; GRASP65, Golgi reassembly stacking
protein 1. |
Firstly, western blotting was performed to compare
secretory levels between wild-type and 2WA Rspo2. The results
indicated that the secretory levels of the
C-mannosylation-defective 2WA mutant Rspo2 decreased
compared with wild-type Rspo2 (Fig.
3B). Because secretory proteins must be able to move from the
ER to the Golgi apparatus (50),
it was hypothesized that the defect in C-mannosylation of
Rspo2 may have affected its intracellular localization, resulting
in less secretion and increased accumulation of Rspo2/2WA in the
cell lysate. Using antibodies against calnexin, an ER marker, and
GRASP65, a marker for the Golgi apparatus, immunofluorescence
staining was performed in order to examine the localization of
wild-type and 2WA localization in the ER or Golgi apparatus. As
expected, whereas wild-type Rspo2 localized to the Golgi apparatus,
Rspo2/2WA accumulated in the ER (Fig.
3C and D). These data indicated that C-mannosylation of
Rspo2 was important for its transportation from the ER to the Golgi
apparatus.
C-mannosylation of Rspo2 mediates
agonistic activity in Wnt/β-catenin signaling in HT1080 cells
R-spondin proteins, including Rspo2, have been
reported to be agonists of Wnt/β-catenin signaling (2,4-8).
Because N-glycosylation of Wnt3a is necessary for its
function and subsequent signaling (51), it was examined whether
C-mannosylation of Rspo2 affects its agonistic activity in
the Wnt/β-catenin signaling pathway. To measure Wnt/β-catenin
signaling activity, a luciferase reporter assay was used. TOPFlash,
which has seven tandem repeats of the TCF/LEF binding motifs
upstream of the firefly luciferase gene, was transfected into each
Rspo2-overexpressing HT1080 clonal cell line, and luciferase
activity was measured. Transfected cells were cultured in
Wnt3a-containing medium for 24 h. Although both wild-type and 2WA
Rspo2-overexpressing cell lines displayed increased TOPFlash
activity compared with control cells, the activity in wild-type
cells was increased by 27.3-fold compared with only 7.8-fold in 2WA
cells (Fig. 3E). Thus, the
C-mannosylation-defective Rspo2/2WA had reduced TOPFlash
activity compared with the wild-type Rspo2.
Effect of C-mannosylation on Rspo2
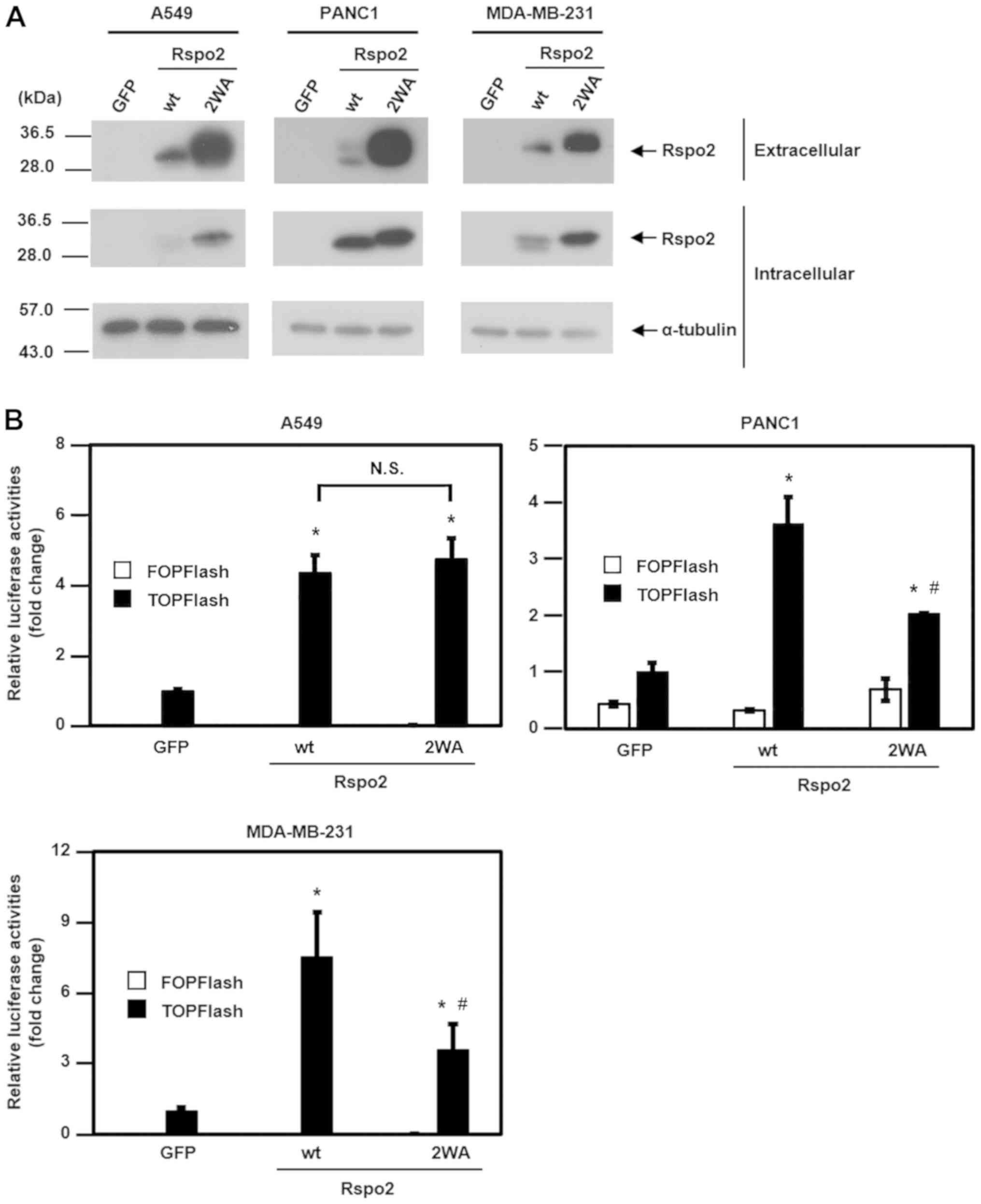
functions in other human tumor cell lines
To confirm the role of C-mannosylation on
Rspo2 functions in cancer, three additional human tumor cell lines
were used, namely A549, PANC1 and MDA-MB-231. First, wild-type and
2WA mutant Rspo2-overexpressing A549, PANC1 and MDA-MB-231 cell
lines were established, and then the secretory levels of these
proteins were assessed. Notably, the secretory levels of the
C-mannosylation-defective 2WA mutant Rspo2 were increased
compared with wild-type Rspo2 in A549, PANC1 and MDA-MB-231 cells
(Fig. 4A). Next, the effect of
C-mannosylation on Rspo2-mediated activation of
Wnt/β-catenin signaling was examined in these cell lines. The
results demonstrated that the TOPFlash activity of 2WA mutant
Rspo2-overexpressing cells was reduced compared with wild-type
Rspo2-overexpressing cells in PANC1 and MDA-MB-231 cell lines
(Fig. 4B), similar to the
aforementioned results in HT1080 cells. No difference in TOPFlash
activity was observed, however, in Rspo2/wild-type- and
Rspo2/2WA-overexpressing A549 cells (Fig. 4B). Therefore, the present results
suggested that C-mannosylation may be important for the
Wnt/β-catenin signaling-enhancing activity of Rspo2 in HT1080,
PANC1 and MDA-MB-231 cells.
C-mannosylation of Rspo2 regulates cancer
cell migration
Because, Rspo2 contributes to cancer progression
(18-20), it was next examined whether
C-mannosylation of Rspo2 may regulate cancer cell migration
in vitro. Confluent Rspo2-overexpressing HT1080 clonal cells
were subjected to wound-healing assays, and 12 h post-scratching,
wound closure areas were quantified. As presented in Fig. 5A, the overexpression of wild-type
Rspo2 significantly increased cell migration compared with control
cells, whereas overexpression of 2WA mutant Rspo2 had little effect
on migration. Similar results were obtained with transwell
migration assays (Fig. 5B).
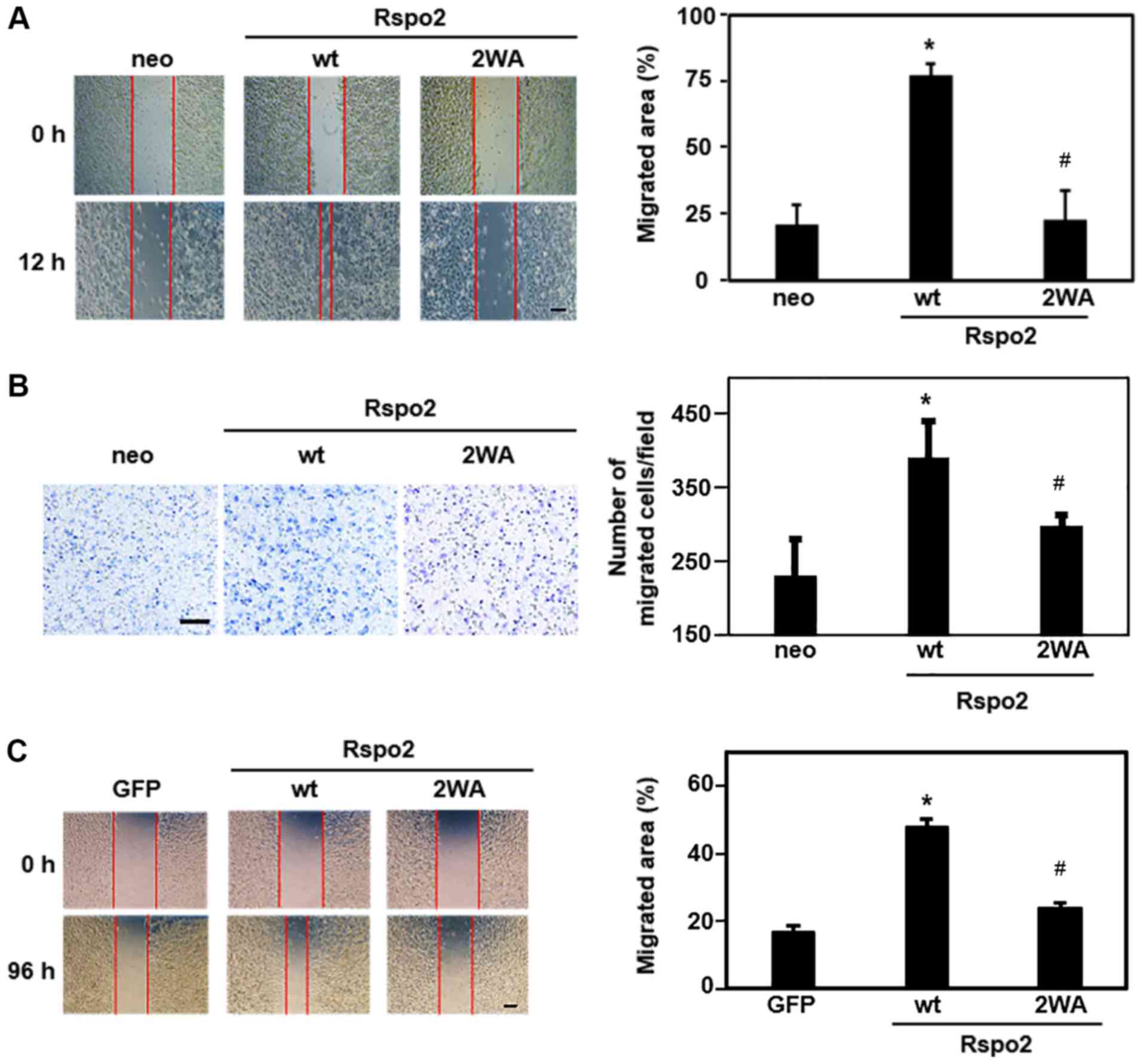 | Figure 5Effect of C-mannosylation on
Rspo2-mediated cancer cell migration. (A) Wound-healing assay of
Rspo2-overexpressing HT1080 cells. Cells were scratched and
photographed under an inverted microscope at 0 and 12 h
post-wounding. Photographs were captured at four independent areas.
Representative images and quantitative analysis of the relative
area of migration are shown. Scale bar, 100 µm. (B)
Transwell migration assay of Rspo2-overexpressing HT1080 cells.
Cells were seeded into transwell chambers and cultured for 3 h.
After 3 h, non-migrated cells were removed with a cotton swab, and
migrated cells were visualized by Diff-Quick staining. Stained
cells were observed by inverted microscopy, and photographs were
captured at four independent areas (magnification, ×10).
Representative images and quantitative analysis are shown. Scale
bar, 100 µm. (C) Wound-healing assay of Rspo2-overexpressing
A549 cells. Cells were scratched and photographed by inverted
microscopy at 0 and 96 h post-wounding. Photographs were captured
at four independent areas. Representative images and quantitative
analysis of the relative area of migration are shown. Scale bar,
100 µm. *P<0.05 compared with control (neo or
GFP); #P<0.05 compared with wt. Rspo2, R-spondin2;
wt, wild-type; GFP, green fluorescent protein. |
To confirm that C-mannosylation of Rspo2 may
mediate migration of other cancer cell lines, Rspo2-overexpressing
A549 clonal cells were used. Using a wound-healing assay, cell
migration ability was demonstrated to be increased in
A549-Rspo2/wild-type, compared with control cells, but was
unchanged in A549-Rspo2/2WA cells (Fig. 5C). These results indicate that
C-mannosylation of Rspo2 regulated the migration of HT1080
and A549 cells. In addition, the present findings demonstrated that
a defect in the C-mannosylation of Rspo2 abrogated its
ability to mediate cancer cell migration.
C-mannosylation of Rspo2 at
Trp150 is important for Wnt/β-catenin signaling
activation
Although the present results demonstrated that Rspo2
is C-mannosylated at Trp150 and
Trp153, Rspo2 was observed in un-mannosylated (4%),
Trp150-mannosylated (94%), and Trp150- and
Trp153-mannosylated (2%) forms (Fig. 2A). Therefore, it can by
hypothesized that single C-mannosylation at
Trp150 may be more important for Rspo2 functions,
compared with the di-mannosylated form. Therefore,
Trp150-C-mannosylation-defective mutant
Rspo2-overexpressing HT1080 cells were generated (HT1080
Rspo2/W150A-MH) and compared with the other Rspo2-overexpressing
HT1080 cells for their ability to activate Wnt/β-catenin signaling.
The results demonstrated that Rspo2/W150A had reduced TOPFlash
activity compared with the wild-type Rspo2, but exhibited
comparable activity with the Rspo2/2WA mutant (Fig. S2). These results suggested that
C-mannosylation at Trp150 may be more important
than Trp153 for Rspo2 functions.
Discussion
C-mannosylation is a unique type of
glycosylation that contributes to proper secretion, protein
folding, and protein-protein interactions (29-32).
Recently, Hendee et al (52) reported that the
C-mannosylated Trp42 of ADAMTS like 1 protein is
often mutated to Arg in a human pedigree that is affected by eye
diseases, such as developmental glaucoma. Their study is the first
to suggest an association between abnormal C-mannosylation
and disease; however, because there are fewer reports on
C-mannosylation and C-mannosylated proteins than
other types of glycosylation, the function of
C-mannosylation, particularly in cancer progression, is
unknown.
The present study focused on Rspo2, which has two
putative C-mannosylation sites in the TSR1 domain, and
determined that Trp150 and Trp153 were
C-mannosylated by LC-MS/MS. C-mannosylation affected
the extracellular secretion of Rspo2 and its intracellular
trafficking from the ER to the Golgi apparatus (Figs. 3B-D and 4A). Furthermore, it regulated the
agonistic activity of Wnt/β-catenin signaling and cancer cell
migration (Figs. 3E, 4B and 5). Although an increase in mass due to
the attachment of mannose and the characteristic cross-ring
cleavage (53) was not detected in
HT1080 cells (Fig. 2B), MS/MS
spectra of Rspo2 purified from Rspo2-overexpressing A549 cells
exhibited hexose attachment and characteristic cross-ring cleavage
(Fig. S1). In the
mono-mannosylated peptide, addition of 162 kDa (one mannose
residue) was detected in the y9 ion, but not in the y7 and y8 ions
(Fig. S1), clearly indicating
that this modification occurred at Trp150. Furthermore,
in the di-mannosylated peptide, one hexose attachment and a
characteristic cross-ring cleavage were detected in the y8 ion
(Fig. S1), but not in the y1-y5
ions (data not shown). These results indicated that one
C-mannosylated amino acid residue existed among
Ser151, Glu152 and Trp153. Based
on previous studies, it is most likely that Trp153 was
C-mannosylated. Furthermore, since Trp150 was
C-mannosylated at the mono-mannosylated peptide, the other
hexose attachment in the di-mannosylated peptide had most likely
occurred at Trp150. The possibility of other
glycosylations may be excluded for the following three reasons:
N-mannosylation is observed only in insects and fungus
(54,55); O-mannosylation at Ser/Thr
residues is further elongated by some saccharides; and
O-glucosylation and O-galactosylation have not been
reported in the residues of 146EVGHW150, and
these glycosylations occur primarily in Notch protein and collagen,
respectively (56). Thus, it was
concluded from the present results that the Trp150 and
Trp153 residues of Rspo2 were C-mannosylated.
Next, the present study examined whether
C-mannosylation affected the functions of Rspo2 by
establishing wild-type Rspo2-overexpressing cells and
C-mannosylation-defective mutant Rspo2/2WA-overexpressing
cells. Because Rspo2 is a secreted protein and a ligand that
activates Wnt/β-catenin signaling (2,5-8), it
was determined whether C-mannosylation alters its secretion.
The results demonstrated that secretion of Rspo2/2WA was reduced in
HT1080 cells, but increased in A549, PANC1 and MDA-MB-231 cells
(Figs. 3B and 4A). These findings prompted us to examine
its intracellular localization. Because C-mannosylation
occurs in the ER lumen (57,58),
it was hypothesized that changes in glycosylation may affect the
intracellular trafficking of Rspo2. As expected, a deficiency in
C-mannosylation of Rspo2 resulted in its accumulation in the
ER in HT1080 cells (Fig. 3C and
D), suggesting that C-mannosylation was required for
intracellular trafficking from the ER to the Golgi apparatus. In
addition, the intracellular mutant Rspo2 expression levels were
significantly increased compared with the wild-type. As Rspo2 bound
to the cell surface (secreted Rspo2) is dissociated by heparin, it
is presumed that these greater levels were also caused by its
accumulation in the ER. However, the effect of
C-mannosylation on the intracellular trafficking of Rspo2 in
the other cell lines examined, namely A549, PANC1 and MDA-MB-231
cells, was similar to the HT1080 cells (data not shown), which was
inconsistent with the secretory effects. Since Rspo2 has an
opposite function on cancer progression depending on the type of
cancer (18-20), it is possible that Rspo2 secretion
may be different in the different tumor cell lines, and that the
effect of C-mannosylation may be dependent on the type of
tumor cell line. Further studies on the effects of
C-mannosylation on the secretion of Rspo2 in additional cell
lines, and a deeper understanding of the mechanisms involved, will
be required in the future.
Wnt/β-catenin signaling is activated by the
interaction between Rspo2, LGR4-6 and ZNRF3/RNF43 (2,5-8).
Because it was observed that a defect in the C-mannosylation
of Rspo2 decreased its agonistic activity in the Wnt/β-catenin
signaling pathway (Fig. 3E and
4B), it is likely that the binding
of Rspo2 to its receptors was also reduced. It is possible that the
significant decrease in Wnt/β-catenin signaling in Rspo2/2WA may be
due to the lower secretion in HT1080 cells (Fig. 3B). However, whereas the secretory
levels of Rspo2/2WA were increased in PNAC1 and MDA-MB-231 cells
(Fig. 4A), their TOPFlash
activities were reduced compared with Rspo2/wild-type cells
(Fig. 4B). These results suggested
that C-mannosylated Rspo2 had stronger activity compared
with the C-mannosylation-defective Rspo2. Furthermore,
C-mannosylation of Rspo1 has been reported to enhance
Wnt/β-catenin signaling, regardless of its secretory level
(30). Further experiments will be
required in the future to confirm the effects of
C-mannosylation in the secreted form of Rspo2, by using
purified wild-type and mutant Rspo2. The present results,
nonetheless, indicated that C-mannosylation of Rspo2 may
have contributed to its agonistic activity in the Wnt/β-catenin
signaling pathway, more than to its regulation of secretion.
Rspo2 is involved in cancer progression as an
enhancer and a suppressor (18-20).
The present results demonstrated that Rspo2 enhanced cell migration
in two distinct cancer cell lines: Human lung adenocarcinoma A549
and human fibrosarcoma HT1080 (Fig.
5). By contrast, Dong et al (36) reported that Rspo2 has a suppressive
effect on cancer progression in human colorectal cancer LoVo cells.
Thus, to confirm these results, a Rspo2-overexpressing LoVo cell
lines was generated and the results demonstrated that
overexpression of Rspo2 suppressed the migration of LoVo cells
(data not shown), similar to the previous report (36). These findings suggested that Rspo2
may have opposing functions on cancer progression. Subsequently, it
was examined whether C-mannosylation was associated with
migration. Migration was impeded in Rspo2/2WA-overexpressing cells
(Fig. 5), suggesting that the
C-mannosylation of Rspo2 enhanced cell migration in certain
tumor cell lines. To further elucidate the role of
C-mannosylation on Rspo2 and cancer progression, in
vivo studies will be required in the future.
Finally, as the majority (94%) of all secreted
Rspo2 was demonstrated to have a C-mannosylation at
Trp150, it was hypothesized that this modification had
an important effect on Rspo2 functions. Regarding a
Trp153-C-mannosylation-defective mutant Rspo2,
substitution of Trp153 to Ala would result in the
disruption of the consensus sequence of
Trp150-C-mannosylation. In addition, because
Cys156 is predicted to form a disulfide bond, replacing
this Cys to break the consensus sequence against Trp153
may induce other effects independent of the C-mannosylation
at Trp153. Therefore, only a Rspo2/W150A mutant was
constructed and overexpressed in HT1080 cells and its effects on
Wnt/β-catenin agonistic activity were compared with the
Rspo2/wild-type- and Rspo2/2WA-overexpressing HT1080 cells. As
expected, Rspo2/W150A and Rspo2/2WA-expressing cells had
significantly reduced agonistic activity. This corresponded with
the results regarding the role of Rspo3 C-mannosylation
previously reported (35). Because
Rspo1-4 have a conserved W-X-X-W-X-X-C sequence, these results
suggested that C-mannosylation at the first Trp residue of
the above sequence may have a common important role in R-spondin
family proteins.
In conclusion, the present study demonstrated that
Rspo2 is C-mannosylated at Trp150 and
Trp153 and that this modification regulated certain
functions of Rspo2. Wnt/β-catenin signaling is often abnormally
activated in cancer progression, and cell migration is one of the
features of tumor malignancy that is associated with cancer
metastasis. Thus, the present findings indicated that the
C-mannosylation of Rspo2 may serve as a novel therapeutic
target and diagnostic marker in cancer.
Supplementary Materials
Funding
This study was supported by a Grant-in-Aid for
Young Scientists (grant no. JP17K15094), a Grant-in-Aid for
Scientific Research (grant no. JP18K06137), and the Amano Institute
of Technology.
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors’ contributions
HM, KK, YN and SS designed the study. HM and KK
performed the cell-based functional analysis. TS and ND performed
the LC-MS analysis. HM, YN and SS wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations
Abbreviations:
|
CBB
|
coomassie brilliant blue
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium
|
|
ER
|
endoplasmic reticulum
|
|
LGR
|
leucine-rich repeat containing G
protein-coupled receptor
|
|
Ni-NTA
|
Ni-nitrilotriacetic acid
|
|
PBS
|
phosphate-buffered saline
|
|
RNF43
|
ring finger 43
|
|
Rspo2
|
R-spondin2
|
|
TSR1
|
thrombospondin type 1 repeat
|
|
ZNRF3
|
zinc and ring finger 3
|
Acknowledgments
We thank Dr Randall T Moon (University of
Washington School of Medicine, Seattle, WA, USA) for providing
Super 8× TopFlash and Super 8× FopFlash (45).
References
|
1
|
Kamata T, Katsube K, Michikawa M, Yamada
M, Takada S and Mizusawa H: R-spondin, a novel gene with
thrombospondin type 1 domain, was expressed in the dorsal neural
tube and affected in Wnts mutants. Biochim Biophys Acta.
1676:51–62. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kazanskaya O, Glinka A, del Barco
Barrantes I, Stannek P, Niehrs C and Wu W: R-Spondin2 is a secreted
activator of Wnt/β-catenin signaling and is required for Xenopus
myogenesis. Dev Cell. 7:525–534. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Lau WB, Snel B and Clevers HC: The
R-spondin protein family. Genome Biol. 13:2422012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KA, Zhao J, Andarmani S, Kakitani M,
Oshima T, Binnerts ME, Abo A, Tomizuka K and Funk WD: R-Spondin
proteins: A novel link to β-catenin activation. Cell Cycle.
5:23–26. 2006. View Article : Google Scholar
|
|
5
|
Carmon KS, Gong X, Lin Q, Thomas A and Liu
Q: R-spondins function as ligands of the orphan receptors LGR4 and
LGR5 to regulate Wnt/β-catenin signaling. Proc Natl Acad Sci USA.
108:11452–11457. 2011. View Article : Google Scholar
|
|
6
|
de Lau W, Barker N, Low TY, Koo BK, Li
VSW, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de
Wetering M, et al: Lgr5 homologues associate with Wnt receptors and
mediate R-spondin signalling. Nature. 476:293–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glinka A, Dolde C, Kirsch N, Huang YL,
Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM and Niehrs C:
LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and
Wnt/PCP signalling. EMBO Rep. 12:1055–1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao HX, Xie Y, Zhang Y, Charlat O, Oster
E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, et al: ZNRF3
promotes Wnt receptor turnover in an R-spondin-sensitive manner.
Nature. 485:195–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar
|
|
10
|
Cisternas P, Henriquez JP, Brandan E and
Inestrosa NC: Wnt signaling in skeletal muscle dynamics:
Myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol.
49:574–589. 2014. View Article : Google Scholar
|
|
11
|
van Amerongen R and Berns A: Knockout
mouse models to study Wnt signal transduction. Trends Genet.
22:678–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawakami Y, Rodriguez Esteban C, Raya M,
Kawakami H, Martí M, Dubova I and Izpisúa Belmonte JC:
Wnt/β-catenin signaling regulates vertebrate limb regeneration.
Genes Dev. 20:3232–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nusse R and Clevers H: Wnt/β-Catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bell SM, Schreiner CM, Wert SE, Mucenski
ML, Scott WJ and Whitsett JA: R-spondin 2 is required for normal
laryngeal-tracheal, lung and limb morphogenesis. Development.
135:1049–1058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aoki M, Kiyonari H, Nakamura H and Okamoto
H: R-spondin2 expression in the apical ectodermal ridge is
essential for outgrowth and patterning in mouse limb development.
Dev Growth Differ. 50:85–95. 2008. View Article : Google Scholar
|
|
16
|
Abed É, Chan TF, Delalandre A,
Martel-Pelletier J, Pelletier JP and Lajeunesse D: R-spondins are
newly recognized players in osteoarthritis that regulate Wnt
signaling in osteoblasts. Arthritis Rheum. 63:3865–3875. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han XH, Jin YR, Seto M and Yoon JK: A
WNT/β-catenin signaling activator, R-spondin, plays positive
regulatory roles during skeletal myogenesis. J Biol Chem.
286:10649–10659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu C, Qiu S, Lu L, Zou J, Li WF, Wang O,
Zhao H, Wang H, Tang J, Chen L, et al: RSPO2-LGR5 signaling has
tumour-suppressive activity in colorectal cancer. Nat Commun.
5:31492014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin X, Yi H, Wang L, Wu W, Wu X and Yu L:
R-spondin 2 promotes proliferation and migration via the
Wnt/β-catenin pathway in human hepatocellular carcinoma. Oncol
Lett. 14:1757–1765. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ilmer M, Boiles AR, Regel I, Yokoi K,
Michalski CW, Wistuba II, Rodriguez J, Alt E and Vykoukal J: RSPO2
enhances canonical Wnt signaling to confer stemness-associated
traits to susceptible pancreatic cancer cells. Cancer Res.
75:1883–1896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krieg J, Hartmann S, Vicentini A, Gläsner
W, Hess D and Hofsteenge J: Recognition signal for C-mannosylation
of Trp-7 in RNase 2 consists of sequence Trp-x-x-Trp. Mol Biol
Cell. 9:301–309. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Julenius K: NetCGlyc 1.0: Prediction of
mammalian C-mannosylation sites. Glycobiology. 17:868–876. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hofsteenge J, Müller DR, de Beer T,
Löffler A, Richter WJ and Vliegenthart JFG: New type of linkage
between a carbohydrate and a protein: C-glycosylation of a specific
tryptophan residue in human RNase Us. Biochemistry. 33:13524–13530.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niwa Y and Simizu S: C-mannosylation:
Previous studies and future research perspectives. Trends Glycosci
Glycotechnol. 30:E231–E238. 2018. View Article : Google Scholar
|
|
25
|
Krieg J, Gläsner W, Vicentini A, Doucey
MA, Löffler A, Hess D and Hofsteenge J: C-Mannosylation of human
RNase 2 is an intracellular process performed by a variety of
cultured cells. J Biol Chem. 272:26687–26692. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morishita S, Suzuki T, Niwa Y, Dohmae N
and Simizu S: Dpy-19 like 3-mediated C-mannosylation and expression
levels of RPE-spondin in human tumor cell lines. Oncol Lett.
14:2537–2544. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Otani K, Niwa Y, Suzuki T, Sato N,
Sasazawa Y, Dohmae N and Simizu S: Regulation of granulocyte
colony-stimulating factor receptor-mediated granulocytic
differentiation by C-mannosylation. Biochem Biophys Res Commun.
498:466–472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sasazawa Y, Sato N, Suzuki T, Dohmae N and
Simizu S: C-Mannosylation of thrombopoietin receptor (c-Mpl)
regulates thrombopoietin-dependent JAK-STAT signaling. Biochem
Biophys Res Commun. 468:262–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goto Y, Niwa Y, Suzuki T, Dohmae N,
Umezawa K and Simizu S: C-mannosylation of human hyaluronidase 1:
Possible roles for secretion and enzymatic activity. Int J Oncol.
45:344–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niwa Y, Suzuki T, Dohmae N and Simizu S:
Identification of DPY19L3 as the C-mannosyltransferase of
R-spondin1 in human cells. Mol Biol Cell. 27:744–756. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perez-Vilar J, Randell SH and Boucher RC:
C-Mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology.
14:325–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ihara Y, Manabe S, Ikezaki M, Inai Y,
Matsui ISL, Ohta Y, Muroi E and Ito Y: C-Mannosylated peptides
derived from the thrombospondin type 1 repeat interact with Hsc70
to modulate its signaling in RAW264.7 cells. Glycobiology.
20:1298–1310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Buettner FF, Ashikov A, Tiemann B, Lehle L
and Bakker H: C. elegans DPY-19 is a C-mannosyltransferase
glycosylating thrombospondin repeats. Mol Cell. 50:295–302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shcherbakova A, Tiemann B, Buettner FFR
and Bakker H: Distinct C-mannosylation of netrin receptor
thrombospondin type 1 repeats by mammalian DPY19L1 and DPY19L3.
Proc Natl Acad Sci USA. 114:2574–2579. 2017. View Article : Google Scholar
|
|
35
|
Fujiwara M, Kato S, Niwa Y, Suzuki T,
Tsuchiya M, Sasazawa Y, Dohmae N and Simizu S: C-mannosylation of
R-spondin3 regulates its secretion and activity of Wnt/β-catenin
signaling in cells. FEBS Lett. 590:2639–2649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong X, Liao W, Zhang L, Tu X, Hu J, Chen
T, Dai X, Xiong Y, Liang W, Ding C, et al: RSPO2 suppresses
colorectal cancer metastasis by counteracting the Wnt5a/Fzd7-driven
nonca-nonical Wnt pathway. Cancer Lett. 402:153–165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsuchiya M, Niwa Y and Simizu S:
N-glycosylation of R-spondin1 at Asn137 negatively regulates its
secretion and Wnt/β-catenin signaling-enhancing activity. Oncol
Lett. 11:3279–3286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miyazaki S, Sasazawa Y, Mogi T, Suzuki T,
Yoshida K, Dohmae N, Takao K and Simizu S: Identification of
seco-clavi-lactone B as a small-molecule actin polymerization
inhibitor. FEBS Lett. 590:1163–1173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miyazaki I, Simizu S, Ichimiya H, Kawatani
M and Osada H: Robust and systematic drug screening method using
chemical arrays and the protein library: Identification of novel
inhibitors of carbonic anhydrase II. Biosci Biotechnol Biochem.
72:2739–2749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kawahara R, Niwa Y and Simizu S: Integrin
β1 is an essential factor in vasculogenic mimicry of human cancer
cells. Cancer Sci. 109:2490–2496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Niwa Y, Suzuki T, Dohmae N and Simizu S:
O-Fucosylation of CCN1 is required for its secretion. FEBS Lett.
589:3287–3293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yasukagawa T, Niwa Y, Simizu S and Umezawa
K: Suppression of cellular invasion by glybenclamide through
inhibited secretion of platelet-derived growth factor in ovarian
clear cell carcinoma ES-2 cells. FEBS Lett. 586:1504–1509. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Simizu S, Umezawa K, Takada M, Arber N and
Imoto M: Induction of hydrogen peroxide production and Bax
expression by caspase-3(-like) proteases in tyrosine kinase
inhibitor-induced apoptosis in human small cell lung carcinoma
cells. Exp Cell Res. 238:197–203. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsuki W, Miyazaki S, Yoshida K, Ogura A,
Sasazawa Y, Takao KI and Simizu S: Synthesis and evaluation of
biological activities of vibsanin A analogs. Bioorg Med Chem Lett.
27:4536–4539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Veeman MT, Slusarski DC, Kaykas A, Louie
SH and Moon RT: Zebrafish prickle, a modulator of noncanonical
Wnt/Fz signaling, regulates gastrulation movements. Curr Biol.
13:680–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Willert K, Brown JD, Danenberg E, Duncan
AW, Weissman IL, Reya T, Yates JR III and Nusse R: Wnt proteins are
lipid-modified and can act as stem cell growth factors. Nature.
423:448–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Komai K, Niwa Y, Sasazawa Y and Simizu S:
Pirin regulates epithelial to mesenchymal transition independently
of Bcl3-Slug signaling. FEBS Lett. 589:738–743. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ishida K, Wierzba MK, Teruya T, Simizu S
and Osada H: Novel heparan sulfate mimetic compounds as antitumor
agents. Chem Biol. 11:367–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nam JS, Turcotte TJ, Smith PF, Choi S and
Yoon JK: Mouse cristin/R-spondin family proteins are novel ligands
for the Frizzled 8 and LRP6 receptors and activate
β-catenin-dependent gene expression. J Biol Chem. 281:13247–13257.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hirschberg K, Miller CM, Ellenberg J,
Presley JF, Siggia ED, Phair RD and Lippincott-Schwartz J: Kinetic
analysis of secretory protein traffic and characterization of golgi
to plasma membrane transport intermediates in living cells. J Cell
Biol. 143:1485–1503. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Komekado H, Yamamoto H, Chiba T and
Kikuchi A: Glycosylation and palmitoylation of Wnt-3a are coupled
to produce an active form of Wnt-3a. Genes Cells. 12:521–534. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hendee K, Wang LW, Reis LM, Rice GM, Apte
SS and Semina EV: Identification and functional analysis of an
ADAMTSL1 variant associated with a complex phenotype including
congenital glaucoma, craniofacial, and other systemic features in a
three-generation human pedigree. Hum Mutat. 38:1485–1490. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang LW, Leonhard-Melief C, Haltiwanger RS
and Apte SS: Post-translational modification of thrombospondin
type-1 repeats in ADAMTS-like 1/punctin-1 by C-mannosylation of
tryptophan. J Biol Chem. 284:30004–30015. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li JS, Cui L, Rock DL and Li J: Novel
glycosidic linkage in Aedes aegypti chorion peroxidase: N-mannosyl
tryptophan. J Biol Chem. 280:38513–38521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brunner F, Wirtz W, Rose JKC, Darvill AG,
Govers F, Scheel D and Nürnberger T: A β-glucosidase/xylosidase
from the phytopathogenic oomycete, Phytophthora infestans.
Phytochemistry. 59:689–696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Moremen KW, Tiemeyer M and Nairn AV:
Vertebrate protein glycosylation: Diversity, synthesis and
function. Nat Rev Mol Cell Biol. 13:448–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Doucey MA, Hess D, Cacan R and Hofsteenge
J: Protein C-mannosylation is enzyme-catalysed and uses
dolichyl-phosphate-mannose as a precursor. Mol Biol Cell.
9:291–300. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Anand M, Rush JS, Ray S, Doucey MA, Weik
J, Ware FE, Hofsteenge J, Waechter CJ and Lehrman MA: Requirement
of the Lec35 gene for all known classes of
mono-saccharide-P-dolichol-dependent glycosyltransferase reactions
in mammals. Mol Biol Cell. 12:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|