Introduction
Polyamines are organic polycations implicated in
several physiological functions, such as DNA synthesis, cellular
proliferation, differentiation and the response to abiotic and
biotic stresses (1-3). Several studies have emphasized the
important activities displayed by polyamines. Among these,
polyamines seem to play a role in the regulation of the translation
elongation process by modulating the Ser/Thr kinases involved in
the phosphorylation of translation elongation factors, in the
regulation of ion channel gating and in the modulation of oxidative
processes (4-8). In an interesting review, it was
reported by Madeo et al (9)
that the natural polyamine, spermidine, exerted prominent
cardioprotective and neuroprotective effects, and prevented stem
cell senescence. Moreover, spermidine displays other pleiotropic
effects that include anti-inflammatory properties, antioxidant
functions, the enhancement of mitochondrial metabolic function and
respiration, as well as improved proteostasis and chaperone
activity. A very recent study demonstrated a novel role of
polyamines in the maintenance of genome integrity via
homology-directed DNA repair (10). Therefore, naturally occurring
polyamines, such as putrescine, spermidine and spermine are found
in a wide variety of organisms from bacteria to plants and animals.
Their levels are tightly regulated through several processes,
including biosynthesis, catabolism, feedback regulation of
expression and excretion from cells.
However, the dysregulation of polyamine metabolism
is a frequent event in various pathological conditions, including
cancer, inflammation, stroke, neurodegeneration, diabetes and renal
failure (11,12). In particular, high amounts of
polyamines and polyamine biosynthesis enzymes are strongly
associated with rapidly growing tumors, including breast, colon,
prostate and gastric cancers (13,14).
Moreover, polyamines and their metabolites, such as diacetylated
derivatives of spermine and spermidine, in urine and plasma, have
also been considered as possible specific markers of neoplastic
cell proliferation (15).
Polyamines can regulate gene expression by altering the DNA and RNA
structure. Several studies have demonstrated that polyamines also
regulate oncogene expression and function through transcriptional
and post-transcriptional processes (4,16-18).
Given that cancer and polyamines appear to be tightly linked, the
modulation of polyamine biosynthesis and catabolism has been
considered as a promising target for both cancer chemoprevention
and chemotherapy.
Polyamines are substrates of amine oxidases, a class
of enzymes present in numerous living systems. These enzymes are
important for the catabolism of polyamines. Enzymatic oxidation
products of polyamines generated by amine oxidases, such as
aldehyde(s) and H2O2, can induce several
biological events.
Maize polyamine oxidase (ZmPAO), one of the
best-characterized plant polyamine oxidases purified from maize, is
a secretory glycoprotein with a non-covalently bound
flavinadenin-dinucleotide (FAD) as a cofactor in a ratio of 1 mol
of FAD per mol of the enzyme (19,20)
(Table I). ZmPAO is an
extracellular enzyme and is predominantly abundant in primary and
secondary cell walls of several tissues (21). Several studies have suggested that
ZmPAO activity is associated with cell wall stiffening and
differentiation through the peroxidase-catalyzed cross-linking, and
lignification of the cell wall (22-24).
Not only that, since several lines of evidence suggest that
H2O2 biosynthesis in the cell wall acts as a
trigger to induce programmed cell death and cellular defense
response (24), the accumulation
of ZmPAO in the cell walls may be associated with the particular
physiological event.
 | Table IStructural properties of ZmPAO and
BSAO. |
Table I
Structural properties of ZmPAO and
BSAO.
| ZmPAO | BSAO |
|---|
| Source | Maize, cell
wall | Calf, serum |
| Cofactor | FAD | Cu2+,
TPQ |
| Molecular mass | 53 kDa | 170 kDa |
| Structure | Monomeric
glycoprotein | Homodimeric
glycoprotein |
| Catabolic
pathway | Terminal polyamine
catabolism | Back-conversion
pathway catabolism |
Amine oxidase from bovine serum (BSAO) is a 170 kDa
homodimeric glycoprotein, and each subunit contains a
2,4,5-trihydroxyphenylalanine quinone (TPQ) cofactor and a copper
ion coordinated by three histidine residues. The primary amino
group of the aminopropyl moiety, such as spermine, spermidine and
benzylamine, are preferentially deaminated by bovine serum amine
oxidase (BSAO) compared to putrescine (25,26).
BSAO catalyzes the oxidative deamination of spermine and spermidine
with a ‘ping-pong’ mechanism, producing spermidine and putrescine,
respectively, in addition to H2O2, ammonia,
and corresponding aldehyde. Since the formed dialdehydes are
unstable, they are likely to be converted to acrolein by
spontaneous β-elimination (27,28).
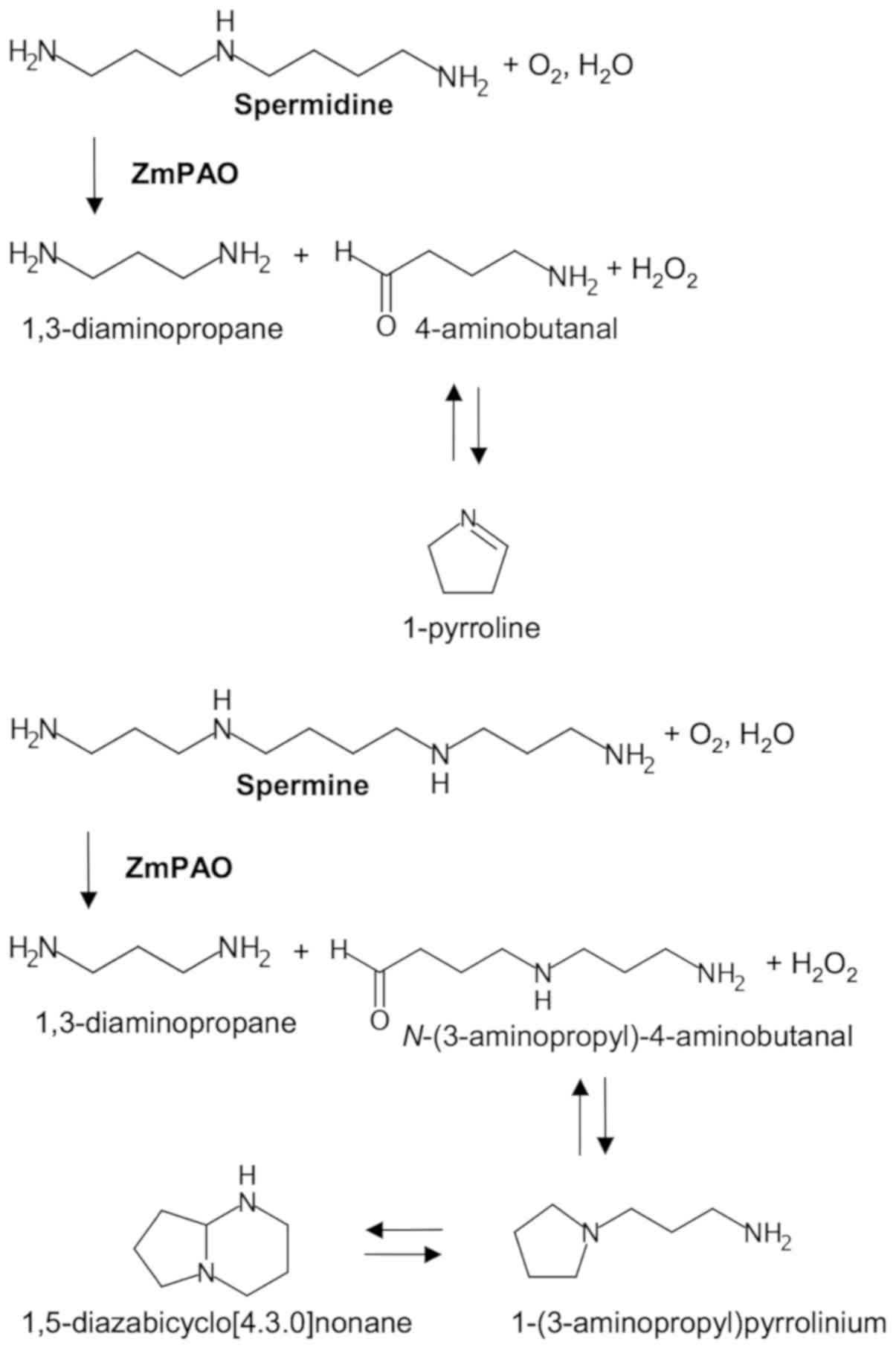
By contrast, ZmPAO oxidizes the C6 carbon
on the endo side of the N5 nitrogens of spermine and
spermidine, generating N-(3-aminopropyl)-4-aminobutanal and
4-aminobutanal, respectively, in addition to 1,3-diaminopropane and
H2O2 (29,30).
N-(3-aminopropyl)-4-aminobutanal and 4-aminobutanal arising
from polyamine oxidation spontaneously cyclize to form
1-(3-aminopropyl) pyrrolinium and 1-pyrroline, respectively
(Fig. 1) (19,31).
The former compound is mainly present in the bicyclic form of
1,5-diazabicyclo[4.3.0]nonane in the leaves of various cereals, and
the latter can be further metabolized to γ-aminobutyric acid (GABA)
by aldehyde dehydrogenase (19,32,33).
Oxidized polyamines are known to have toxic effects on a variety of
bacteria and viruses. These aminoaldehyde(s) in mice have exhibited
a consistent inhibitory effect on Leishmania infantum, the
etiological agent of visceral leishmaniasis; in particular,
4-aminobutanal exhibited a lower ED50 value (0.14 mg/kg)
than N-(3-aminopropyl)-4-aminobutanal (31). In vitro cultivation assays
on Leishmania infantum promastigotes have also shown that
the aminoaldehydes exert a significant inhibitory effect on the
vitality and growth of these parasites (34).
Multidrug resistance/resistant (MDR) is the most
commonly exploited mechanism through which cancer eludes
chemotherapy and is crucial for cancer metastasis and recovery. MDR
is defined as the resistance of the cancer cells to one
chemotherapeutic drug accompanied by simultaneous resistance to a
variety of structurally and mechanistically unrelated drugs
(35,36). MDR cancer cells exhibit a reduced
intracellular accumulation of chemotherapeutic drugs by pumping
them out of the cells. Resistance to chemotherapy and molecularly
targeted therapies is a significant impediment to successful cancer
treatment. Therefore, the search for an innovative therapeutic
strategy against MDR tumors is ongoing.
Several in vitro studies have been performed
to investigate whether the oxidative products of polyamines exert
cytotoxic effects against human cancer cells. An MDR human colon
adenocarcinoma cell line (LoVo DX), selected from the parental
drug-sensitive cell line (LoVo WT) by continuous exposure to
doxorubicin (DOX), has been shown to express high levels of
membrane transporter protein P-glycoprotein (P-gp) which can
transport chemotherapeutic drugs outside (37), suggesting that the resistance to
anticancer agents in colon adenocarcinoma cells is attributed to
overexpression of P-gp. Previous studies have shown that enzymatic
oxidation products of exogenous spermine, catalyzed by BSAO, exert
a greater cytotoxic effect on LoVo DX cells than on LoVo WT
(38,39). Other studies have demonstrated that
human melanoma M14 MDR cells (M14 ADR2), overexpressing P-gp, as
well as LoVo DX, are also more sensitive to spermine metabolites
oxidized by BSAO than the parental M14 cells (M14 WT) (40-42).
The antitumor efficacy of BSAO has also been evaluated in
vivo. BSAO, immobilized on a poly(ethylene glycol) (PEG)
biocompatible matrix, was previously directly injected into
subcutaneous B16 melanoma tumors in C57BL mice, leading to
decreased tumor growth (43,44).
Given that intratumor polyamine concentrations are increased in
multiple tumors, these findings raise the possibility of using
oxidation products of polyamines formed by amine oxidases to
preferentially kill cancer cells and even MDR cancer cells.
Therefore, this study investigated the potential of
ZmPAO to induce cancer cell death by the oxidative products of
polyamines. ZmPAO was selected for use in this study due to its
higher affinities and higher catalytic efficiencies for polyamine
substrates than BSAO (45-48). Moreover, ZmPAO has a lower
molecular weight (53 kDa) than BSAO (170 kDa), which may be an
advantage when the enzyme is conjugated to biocompatible carrier
molecules for efficient delivery to tumor sites in vivo.
Moreover, the expression system in the culture medium of Pichia
pastoris for the production of recombinant ZmPAO, a monomeric
enzyme, unlike BSAO that is a homodimeric enzyme, has been already
established, which has shown high expression levels of recombinant
ZmPAO (45). The catalytic
parameters of the recombinant enzyme are similar to those of native
enzyme (45). It seems easy to
alter the DNA sequence to adapt the enzyme to drug delivery system
or improve the enzyme features. In addition, ZmPAO is involved in
the terminal polyamine catabolism that does not produce polyamines,
while BSAO is implicated in a polyamine back-conversion pathway
that converts spermine to spermidine, and then to putrescine
(25,29). ZmPAO delivery to the tumor
microenvironment in vivo may cause polyamine depletion,
leading to growth inhibition.
In this study, the involvement of
H2O2 and aldehydes derived from spermine and
spermidine oxidized by purified ZmPAO in causing cytotoxicity to
LoVo WT and its MDR cells was investigated in vitro. It was
also examined whether the cytotoxicity induced by the treatment
with ZmPAO and spermine is associated with apoptosis. Moreover, the
involvement of mitochondria in cell death induced by ZmPAO/spermine
enzymatic systems was also assessed.
Materials and methods
Reagents
Spermine tetrahydrochloride and spermidine
trihydrochloride were obtained from Fluka (Buchs, Switzerland).
Fetal bovine serum (FBS), ribonuclease A (RNase A), catalase,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1), verapamil, bovine serum albumin (BSA), penicillin,
streptomycin and propidium iodide (PI) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Aldehyde dehydrogenase (ALDH)
and NAD+ were obtained from Boehringer-Mannheim
(Mannheim, Germany). Commercial H2O2 was
purchased from Baker Analyzed Reagent (J.T. Baker, Deventer, The
Netherlands). The Annexin V-FITC apoptosis detection kit was
obtained from Enzo Life Sciences (Farmingdale, NY, USA). Ham’s F-12
medium was purchased from Gibco/Thermo Fischer Scientific (Waltham,
MA, USA). All cell culture flasks and dishes were obtained from
Corning (Corning, NY, USA).
Purification of ZmPAO and BSAO
ZmPAO was purified using SP-sepharose ion exchange
chromatography from maize shoot grown in the dark at 25°C as
described previously (49). The
sample was concentrated by vacuum dialysis through a dialysis
membrane with a 12-14,000 Da cut-off, which was dialyzed against 50
mM sodium phosphate buffer (pH 5.3) for storage. BSAO was purified
by a combination of ion-exchange and affinity chromatographies as
previously described (50,51). The BSAO purification factor was
approximately 1,600-fold and a single band was obtained on 6%
SDS-PAGE gel. The protein concentrations for BSAO and ZmPAO were
measured by the absorbance at 280 and 450 nm using an absorption
extinction coefficient of 1.74 liters g−1
cm−1 and 0.213 liters g−1 cm−1,
respectively. For all the experiments performed,
6.58×10−3 U/ml of enzyme were used. The enzyme activity
of BSAO was assayed as the amount of benzaldehyde formed per min
(µmol/min) from benzylamine at 250 nm (ε = 12,500
M−1 cm−1) in 0.1 M sodium phosphate buffer
(pH 7.2) at 25°C. 3,5-Dichloro-2-hydroxybenzene-sulfonic acid
(DCHBS) is oxidized by horseradish peroxidase (HRP) in the presence
of H2O2 to a semiquinone radical form, which
generates a pink quinoneimine with 4-aminoantipyrine (AAP). For
ZmPAO, the enzymatic activity was determined by measuring the
accumulation of the pink quinononeimine dye (ε515 =
26,000 M−1 cm−1) produced from AAP and DCHBS
in 0.1 M sodium phosphate buffer (pH 7.4) at 25°C, as previously
described (52).
Determination of catalytic properties of
ZmPAO and BSAO
The enzyme catalytic properties were
spectrophotometrically determined by measuring the amount of
H2O2 formed during the oxidation of the
polyamines, spermine or spermidine. In the presence of AAP and
DCHBS, the measurements were performed in 0.2 M sodium phosphate
buffer (pH 6.0) at 25°C using spermine or spermidine as a substrate
to detect ZmPAO activity, or in PBS (pH 7.4) at 37°C using spermine
as a substrate for ZmPAO or BSAO activity. The pink adduct produced
by the oxidation of both substrates was measured
spectrophotometrically. The kinetic constants: Affinity constant
(Km), catalytic constant (kcat)
and specificity constant
(kcat/Km) values were
calculated using Lineweaver-Burk plots for each enzyme.
Cell cultures
The LoVo cell line was isolated from a metastatic
nodule by Dolfini et al (53); its MDR variant, LoVo DX, and a
gastric adenocarcinoma cell line (AGS) were used in this study. The
MDR cell line, LoVo DX, was obtained by the prolonged culture of
drug-sensitive parental LoVo WT cells in medium containing DOX
(Adriblastina; Pharmacia & Upjohn, Milan, Italy) as previously
described by Grandi et al (54). LoVo DX cells are also resistant to
other drugs, such as etoposide and vincristine (39,53).
The LoVo cells were a kind gift from Professor E. Dolfini
(University of Milan, Milan, Italy). The AGS cell line (homo
sapiens gastric adenocarcinoma) was obtained from the American
Type Culture Collection (ATCC® CRL-1739™; ATCC,
Manassas, VA, USA). All cell lines were grown in Ham’s F-12 medium
containing L-glutamine supplemented with 10% FBS, 1% minimum
essential medium (MEM) vitamins, 1% MEM non-essential amino acid,
penicillin (100 U/ml) and streptomycin (100 µg/ml) and were
incubated in a humidified atmosphere of 5% CO2 in a
water-jacketed incubator at 37°C. For each passage, exponentially
growing LoVo and AGS cells were harvested with 10 mM EDTA in PBS
and then by further addition of 0.25% trypsin solution in PBS. The
trypsin activity was quenched by the addition of complete F-12
medium.
Cell viability assay
The cytotoxic effect induced by polyamine
metabolites on human tumor cells was evaluated using a plating
clonogenic assay. Cell viability assays were carried out using
subconfluent cells that had been incubated in fresh medium for 24 h
at 37°C. Cells were detached with 10 mM EDTA in PBS and then by
addition of 0.25% trypsin in PBS. The harvested cells were washed
with PBS containing 1% BSA (PBS-1% BSA), centrifuged at 400 × g for
2 min at 25°C, and resuspended in PBS supplemented with 1% BSA.
Freshly harvested cells (105/ml) were incubated at 37°C
for different periods of time, up to 60 min, in the presence of the
following reagents, used alone or in combination: Several
concentrations of polyamine, spermine or spermidine, BSAO or ZmPAO
(6.58×10−3 U/ml), catalase (242 U/ml) from bovine liver,
ALDH (EC 1.2.1.5) from yeast (0.4 U/ml) and NAD+ (1.8
µg/ml). Spermine and spermidine were freshly prepared prior
to each experiment in water or phosphate buffer, respectively, and
if present, added last, to initiate the enzymatic reaction.
Following incubation at 37°C for different periods of time, up to
60 min, cells were washed twice in PBS-1% BSA, centrifuged at 400 ×
g for 2 min at 25°C, and resuspended in 1 ml PBS-1% BSA. AGS, LoVo
WT and LoVo DX cells were subsequently plated in tissue
culture-coated Petri dishes and incubated at 37°C until colonies
were formed (13 or 18 days, respectively). The colonies were washed
with PBS, fixed with 70% ethanol, stained with methylene blue at
25°C for 5 min and counted manually. Control plating efficiencies
were >85% for the AGS and LoVo WT and 80% for the LoVo DX cells,
corresponding to 9.1×104±1.0×104 and
8.5×104±1.0×104 number of cells,
respectively. The percentage of colony-forming cells was determined
as the ratio between the mean number of colonies in the treated and
control samples.
Measurements ‘in situ’ of mitochondrial
membrane potential (Δψm)
The changes in Δψm in the LoVo cells were
assayed using the lipophilic cationic probe, JC-1 dye, as
previously described (39). The
LoVo WT and LoVo DX cells were harvested with EDTA/trypsin, washed
with PBS-1% BSA, centrifuged at 400 × g for 2 min at 25°C, and
resuspended in PBS-1% BSA, as described above. The cells were
treated with various concentrations of H2O2
or spermine in the presence of ZmPAO or BSAO for 1 h at 37°C.
Following 30 min of incubation at 37°C, verapamil was further added
to the solution at a final concentration of 100 µM, in order
to inhibit the P-gp-mediated efflux of JC-1 in the LoVo DX cells.
Exposure to verapamil significantly increased the fluorescence
intensity of JC-1 in the LoVo DX cells, while it did not affect
fluorescence in LoVo WT cells. Subsequently, the cells were stained
with 2.5 µg/ml of JC-1 during the final 15 min of treatment
at 37°C. The detached cells were washed with cold PBS-1% BSA and
the cells were then resuspended in cold PBS. The samples were
analyzed by a BD Accuri C6 flow cytometer (BD Biosciences, San
Jose, CA, USA). JC-1 was excited using an argon laser at a
wavelength of 488 nm (using a BD Accuri C6 flow cytometer). The
emitted green (JC-1 monomer) and red (JC-1 aggregate) fluorescence
were detected at the FL-1 channel (533/30 nm) and FL-2 channel
(585/40 nm), respectively. At least 10,000 events/sample were
acquired in log mode using an Accuri C6 flow cytometer. The ratio
of red (FL2)/green (FL1) fluorescence intensity was used to
represent the Δψm. As positive controls, LoVo WT and
LoVo DX cells were treated with exogenous
H2O2 solution for 60 min at 37°C and then
labeled, as described above.
Determination of apoptotic cell death by
Annexin V-FITC staining
To detect phosphatidylserine residue exposure on the
surface of plasma membrane of tumor cells in the initial step of
apoptosis, an Annexin V-FITC apoptosis detection kit was used as
previously described by Van Engeland et al (55). LoVo WT and LoVo DX cells
(5×105/ml) were treated with several concentrations of
spermine and ZmPAO for 1 h at 37°C, as described above for cell
viability assay. Cell suspensions were seeded in a 6-well plate
containing culture medium supplemented with FBS. Following
incubation at 37°C for 48 h, the cells were detached, washed with
PBS, centrifuged at 400 × g for 2 min at 25°C and then stained with
1 µg/ml of Annexin V-FITC and with 1 µg/ml of PI for
10 min at room temperature in the dark. Annexin V-FITC and PI
fluorescence were measured on the FL-1 channel (533/30 nm) and the
FL-3 channel (>670 nm), respectively, with excitation at 488 nm.
A minimum of 10,000 events/sample was acquired.
Cell cycle analysis
Cell cycle distribution was analyzed by labeling the
cells with PI. The assays were carried out as previously described
by Nicoletti et al (56).
Spermine- and ZmPAO-treated and untreated LoVo WT and LoVo DX cells
(5×105/ml), as described above in Annexin V-FITC
labeling, were harvested, washed twice with cold PBS and
centrifuged at 400 × g for 2 min at 4°C. The pellet was fixed in
70% ethanol at −20°C for 1 h. After washing twice with PBS, the
cells were resuspended in PBS containing 100 µg/ml RNase A
and 40 µg/ml PI. Following incubation at 37°C for 1 h, the
cells were subsequently analyzed by flow cytometry using the FL-3
channel (>670 nm) with the acquisition of 10,000
events/sample.
Scanning and transmission electron
microscopy analyses
Scanning electron microscopy (SEM) was performed to
investigate the ultrastructural features of the cell surface. A
total of 3×105 cells were plated on glass coverslips
contained in a 6-well plate and allowed to adhere overnight. The
cells were grown to near confluence and in Ham’s F-12 FBS-free
medium containing 1% BSA, cells were incubated with ZmPAO and 18
µM spermine, 18 µM H2O2, or
only medium for 60 min at 37°C. The cells were washed with F-12
medium and 0.1 M cacodylate buffer and then fixed in 2.5%
glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3), supplemented
with 2% sucrose, at 4°C for 1 h. The samples were rinsed in 0.1 M
cacodylate twice and then post-fixed with 1% osmium tetroxide
(OsO4) in 0.1 M cacodylate buffer (pH 7.3) at room
temperature for 30 min. After washing in distilled water for 1 h,
the cells were dehydrated through graded ethanol concentrations
(from 30% to absolute alcohol). The specimens were sputter-coated
in a vacuum with an electrically conductive 5 nm thick layer of
gold-palladium. Finally, the prepared samples were examined with a
Hitachi S4000 field emission scanning electron microscope (Hitachi
Ltd., Tokyo, Japan) operating at 6-8 kV a field emission scanning
electron microscope.
For transmission electron microscopy (TEM)
observation, LoVo WT and DX cells were harvested as described
above. After washing with PBS-1% BSA, the cells were incubated for
60 min at 37°C in the absence or presence of 21 µM spermine,
or 21 µM of spermine and ZmPAO in Ham’s F-12 FBS-free medium
containing 1% BSA. The cells were washed with F-12 medium twice and
fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3),
added with 2% sucrose, at room temperature for 1 h. The samples
were washed with 0.1 M cacodylate buffer twice, post-fixed with 1%
osmium tetroxide (OsO4) solution in 0.2 M cacodylate
buffer (pH 7.3) at room temperature for 2 h and rinsed in the same
buffer. Cells were then embedded in small blocks of 1% agar of
approximately 5×5×1 mm in size, dehydrated in ascending series of
ethanol concentrations (from 30% to absolute alcohol), immersed in
propylene oxide for solvent substitution, embedded in Epon 812
(Agar Scientific, Stansted, UK) and sectioned by a Reichert-Jung
Ultracut E ultra-microtome. Semithin sections (1 µm thick)
were stained with Toluidine Blue, examined by light microscopy
(Zeiss Axioskop) and photographed using a digital camera (Leica
DFC230). Ultrathin sections (60-80 nm) were cut with a diamond
knife, mounted on copper grids and contrasted with saturated uranyl
acetate followed by lead citrate (SIC, Rome, Italy). They were
examined and photographed using a Zeiss EM 10 and a Philips TEM
CM100 Electron Microscopes operating at 80 kV (FEI Co., Eindhoven,
The Netherlands).
Statistical analysis
The data are presented as the means ± SEM or means ±
SD. Statistical analysis was performed using one-way ANOVA with the
Tukey’s post hoc test in GraphPad Prism 8.0.2 (GraphPad Software).
A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Purification and characterization of
ZmPAO
ZmPAO purification was performed on 10-day-old maize
seedlings. The kinetic parameters of the purified ZmPAO were
determined and compared with those of BSAO, isolated from bovine
plasma (Table II). ZmPAO
exhibited similar Km, kcat and
kcat/Km values for both
substrates, spermine and spermidine, at 25°C and pH 6.0. The
Km value for the oxidation of spermine by ZmPAO
decreased from 17.4 µM at 25°C and pH 6.0 to 2.8 µM
at 37°C and pH 7.4. The kcat and
kcat/Km values increased from
37.9 S−1 and 2.2 S−1 µM−1
measured at 25°C and pH 6.0 to 72.5 S−1 and 26.2
S−1 µM−1 determined at 37°C and pH
7.4, respectively. The enzyme exhibited a higher affinity for
spermine than BSAO at 37°C and pH 7.4. The affinity of ZmPAO was
approximately 10-fold greater than that of BSAO (2.8 vs. 28.1
µM) and the specificity constant of ZmPAO was >200-fold
higher than that of BSAO (26.2 S−1
µM−1 vs. 0.1 S−1
µM−1).
 | Table IIKinetic parameters of ZmPAO and
BSAO. |
Table II
Kinetic parameters of ZmPAO and
BSAO.
| Enzyme | Substrate | Tm (°C) | pH |
Km (µM) | Specific activity
(U/mg pro.) |
kcat
(S−1) |
kcat/Km
(S−1 µM−1) |
|---|
| ZmPAO | SPM | 25°C | 6.0 | 17.4 | 42.9 | 37.9 | 2.2 |
| ZmPAO | SPD | 25°C | 6.0 | 24.9 | 48.0 | 42.4 | 1.7 |
| ZmPAO | SPM | 37°C | 7.4 | 2.8 | 82.0 | 72.5 | 26.2 |
| BSAO | SPM | 37°C | 7.4 | 28.1 | 1.2 | 3.3 | 0.1 |
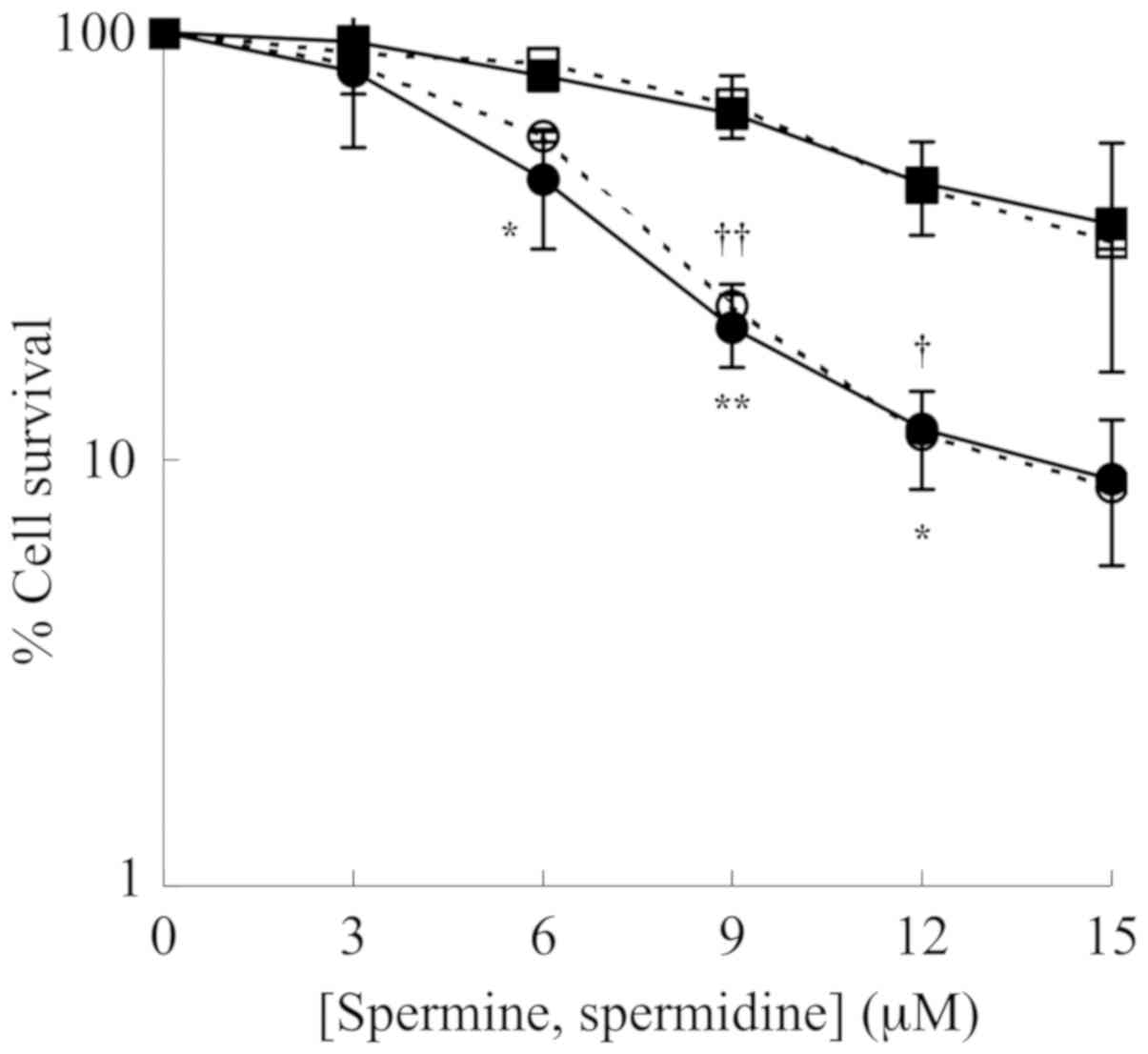
Cytotoxic effect induced by ZmPAO in the
presence of polyamine on human tumor cell cultures
The cytotoxic effect caused by the oxidation
products of polyamines, spermine and spermidine, generated by the
enzymatic reaction catalyzed by ZmPAO (Fig. 1) was examined on LoVo WT and LoVo
DX cells. The cells were treated with various concentrations of
spermine or spermidine in presence of ZmPAO for 1 h at 37°C. A
clonogenic assay revealed that the viability of the LoVo WT and
LoVo DX cells gradually decreased with the polyamine concentration
of up to 15 µM (Fig. 2). It
was also observed that the treatments significantly reduced the
viability of the LoVo DX cells when compared with the LoVo WT ones.
ZmPAO catalyzes the conversion of spermine and spermidine to
N-(3-aminopropyl)-4-aminobutanal and 4-aminobutanal,
respectively, in addition to the formation of
H2O2 and 1,3-diaminopropane. No significant
differences in the viability of the cells treated with spermine and
those treated in the presence of spermidine were observed between
the LoVo WT and LoVo DX cell lines (Fig. 2). Likewise, in the human gastric
cancer cell line, AGS, treatment with each polyamine in the
presence of ZmPAO (6.58×10−3 U/ml) was effective in
inhibiting cell growth in a dose-dependent manner and the cell
survival rates were nearly the same for both the treatments (data
not shown). This suggested that either the cytotoxicity induced by
the treatments was mainly due to H2O2, or
both aldehydes produced by the enzymatic reactions could provoke
the same level of cytotoxicity.
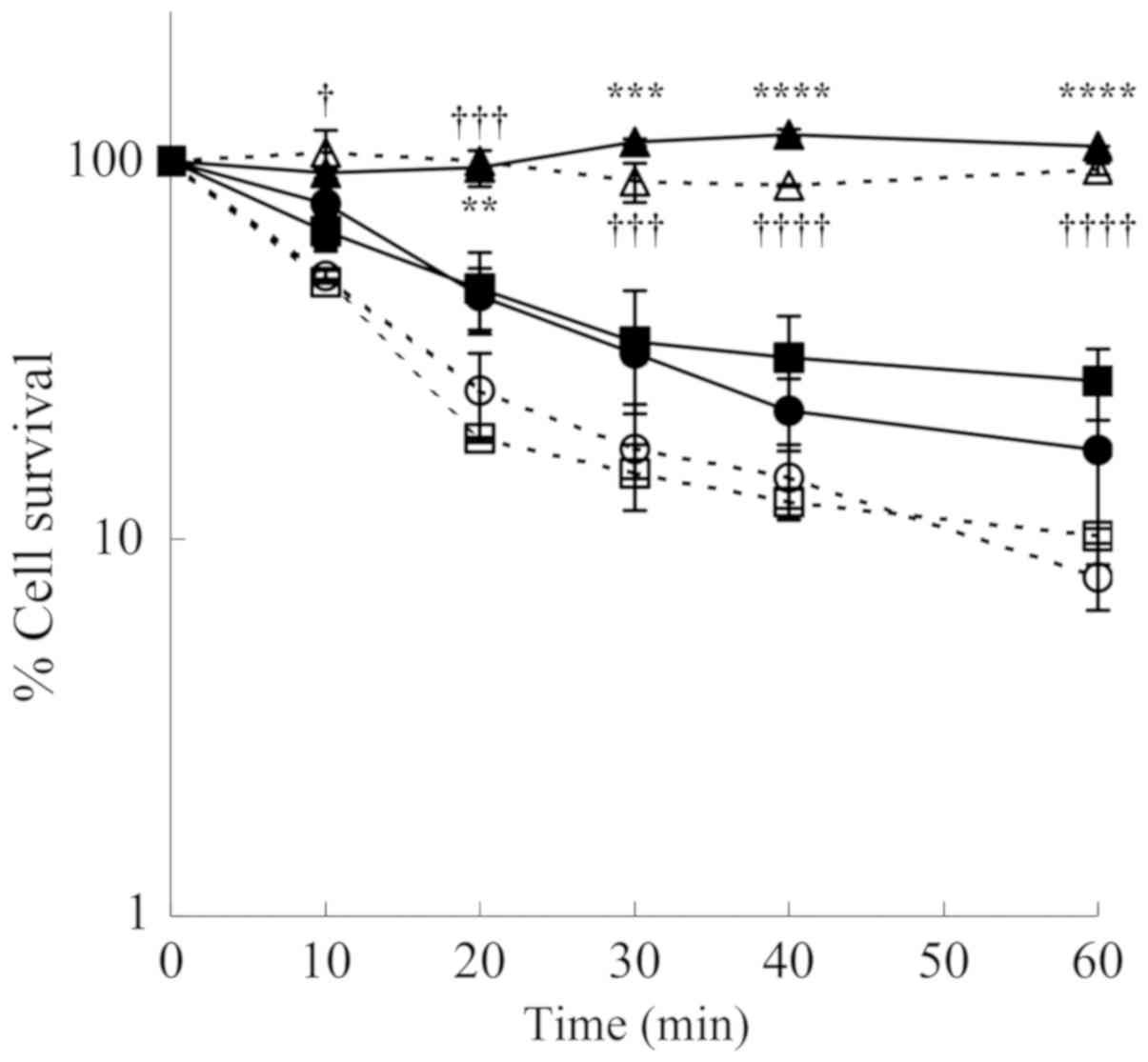
To determine the cytotoxic effect caused by
H2O2 and aldehydes formed from spermine, the
LoVo cells were treated with spermine and ZmPAO in the presence of
the scavengers, catalase and ALDH. Both the LoVo WT and LoVo DX
cells treated with 12 µM of exogenous spermine and ZmPAO
exhibited a cytotoxic effect in a time-dependent manner. The
cytotoxicity was completely prevented by the addition of catalase,
while it was not affected by the presence of ALDH (Fig. 3). These results demonstrated that
the aldehyde produced from spermine was not responsible for the
cytotoxicity. The cytotoxicity induced by ZmPAO and exogenous
spermidine was also completely inhibited in the presence of
catalase in both the LoVo WT and LoVo DX cells (data not shown),
suggesting that under these experimental conditions, the
cytotoxicity was only due to H2O2.
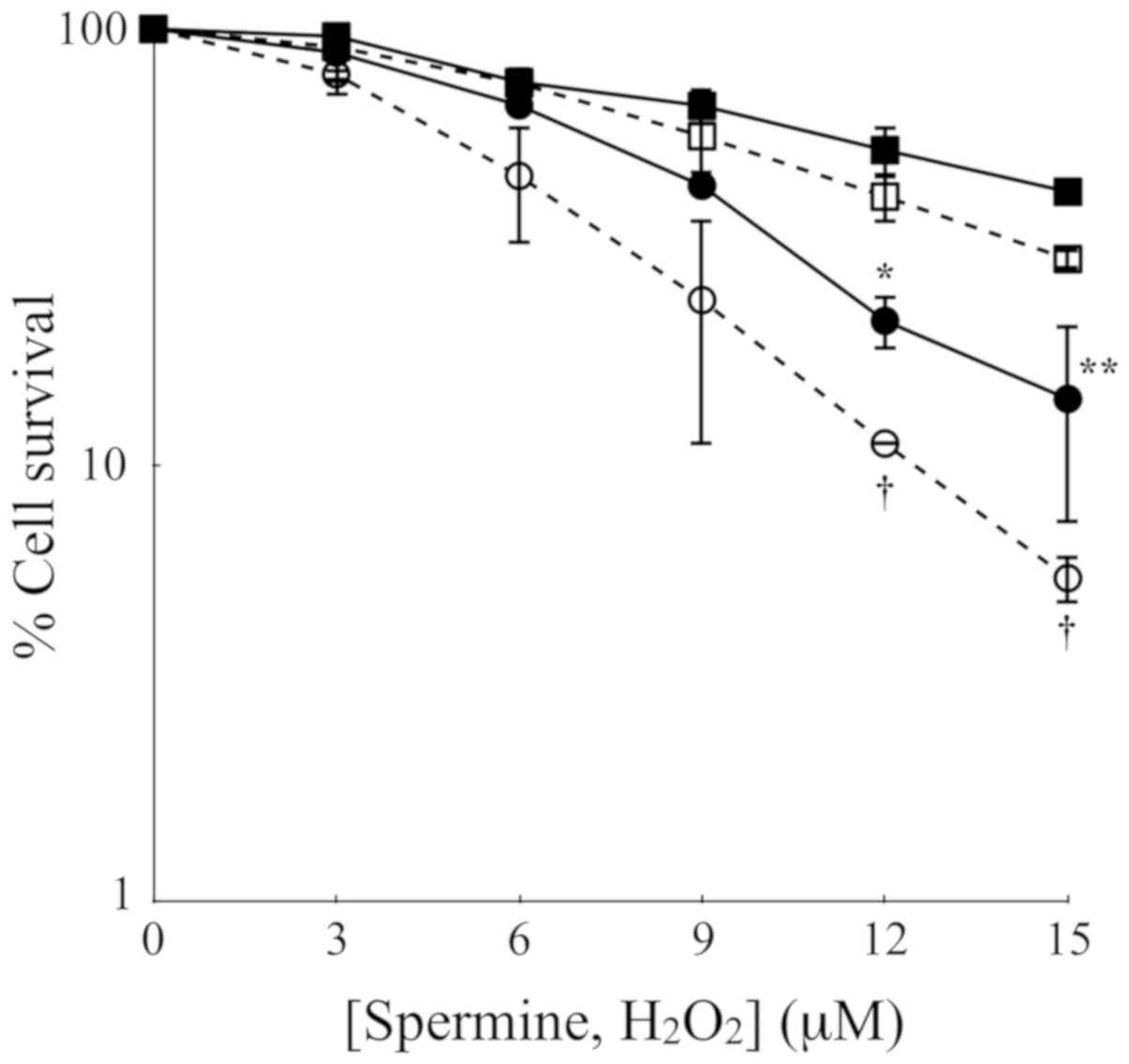
To further assess the cytotoxicity of polyamine
metabolites, H2O2 and aldehydes, the LoVo
cells were treated with increasing concentrations of exogenous
H2O2 or spermine and the ZmPAO enzymatic
system for 60 min. The results of clonogenic assay revealed that in
both cell lines, exogenous H2O2 exerted a
slightly greater cytotoxicity than the same molar concentration of
exogenous spermine enzymatically oxidized by ZmPAO (Fig. 4). Treatment of the LoVo WT and LoVo
DX cells with 15 µM of H2O2 at 37°C
for 60 min decreased the viability to 29.7 and 5.5%, respectively,
while the exposure of both cell lines to the same molar
concentration of spermine in the presence of ZmPAO reduced the
viability to 42.4 and 14.2%, respectively (Fig. 4).
Comparison of cytotoxicity to LoVo cells
induced by ZmPAO and BSAO in the presence of exogenous
spermine
The cytotoxic effects of spermine metabolites
generated by ZmPAO were compared with those induced by BSAO on the
LoVo cells. As shown in Fig. 5,
the viability of the LoVo WT and LoVo DX cells incubated up to 60
min at 37°C in the presence of 12 µM spermine and ZmPAO or
BSAO was examined. The metabolites formed by BSAO/spermine
enzymatic system induced a more significant cytotoxicity than that
produced by ZmPAO/spermine during the whole duration of the
incubation period. After 60 min of incubation, treatment with ZmPAO
and BSAO in the presence of spermine reduced the viability of the
LoVo WT cells to 39.1 and 5.3%, respectively. The LoVo DX cells
were found to be more sensitive to the treatment in the presence of
both enzymes. The viability of the LoVo DX cells treated with ZmPAO
and BSAO in the presence of spermine decreased to 15.1 and 0.4%,
respectively.
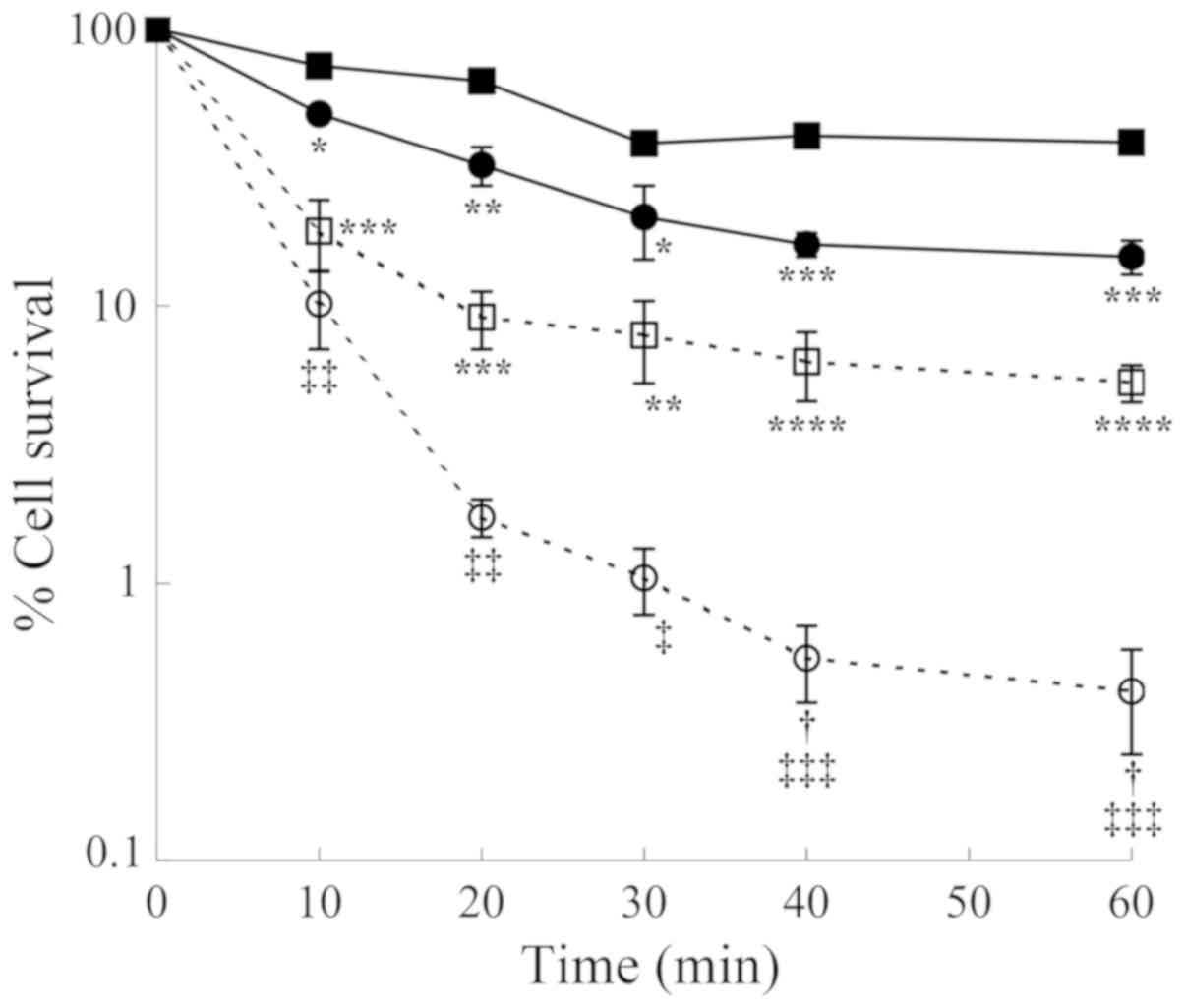 | Figure 5Comparison of cytotoxic effects
induced by both purified ZmPAO and BSAO in the presence of spermine
on LoVo cells. LoVo WT (squares) and LoVo DX (circles) cells were
incubated with ZmPAO (solid symbols) or BSAO (open symbols) in the
presence of 12 µM spermine up to 60 min at 37°C. Each point
represents the mean ± SEM of 2 independent experiments, with 2 to 5
plates per experiment. Where not visible, error bars are smaller
than the symbols. Data are shown on a logarithmic scale. Data were
analyzed by one-way ANOVA, followed by Tukey’s post hoc test.
*P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001 vs. LoVo WT cells incubated with ZmPAO
and spermine; †P<0.05 vs. LoVo WT cells incubated
with BSAO and spermine; ‡P<0.05,
‡‡P<0.01 and ‡‡‡P<0.001 vs. LoVo DX
cells incubated with ZmPAO and spermine. ZmPAO, maize polyamine
oxidase; BSAO, bovine serum amine oxidase; LoVo WT cells, LoVo
wild-type cells; LoVo DX cells, LoVo multidrug-resistant cells. |
Analysis of apoptosis induction by flow
cytometry
The loss of phospholipid asymmetry and the
appearance of phosphatidylserine residues on the outer layer of the
plasma membrane is an early signal of cell death, namely apoptosis.
Annexin V is a Ca2+-dependent phospholipid-binding
protein with a high affinity for phosphatidylserine. In this study,
to detect externalized phosphatidylserine, Annexin V-FITC/PI
staining was performed in the LoVo WT and LoVo DX cells. The
results of flow cytometric analysis are shown in Fig. 6. Treatment with 12 and 24 µM
of exogenous spermine in the presence of purified ZmPAO increased
the percentage of the total apoptotic cells (Fig. 6A, lower and upper right quadrants)
to 26.0 and 42.3%, respectively, compared with the untreated LoVo
WT cells (8.7%) (Fig. 6A, left
panels). On the contrary, the percentage of apoptotic cells was
higher in the LoVo DX cells than the LoVo WT cells following
treatment with 12 and 24 µM of exogenous spermine (Fig. 6A, right panels, 49.2 and 57.6%,
respectively, and Fig. 6B). An
increase in the number of apoptotic cells following treatment was
also detectable in the AGS cells, resulting in 32.2 and 58.0%
Annexin V-positive cells in the treated cells with 24 and 48
µM of spermine in the presence of ZmPAO, respectively (data
not shown).
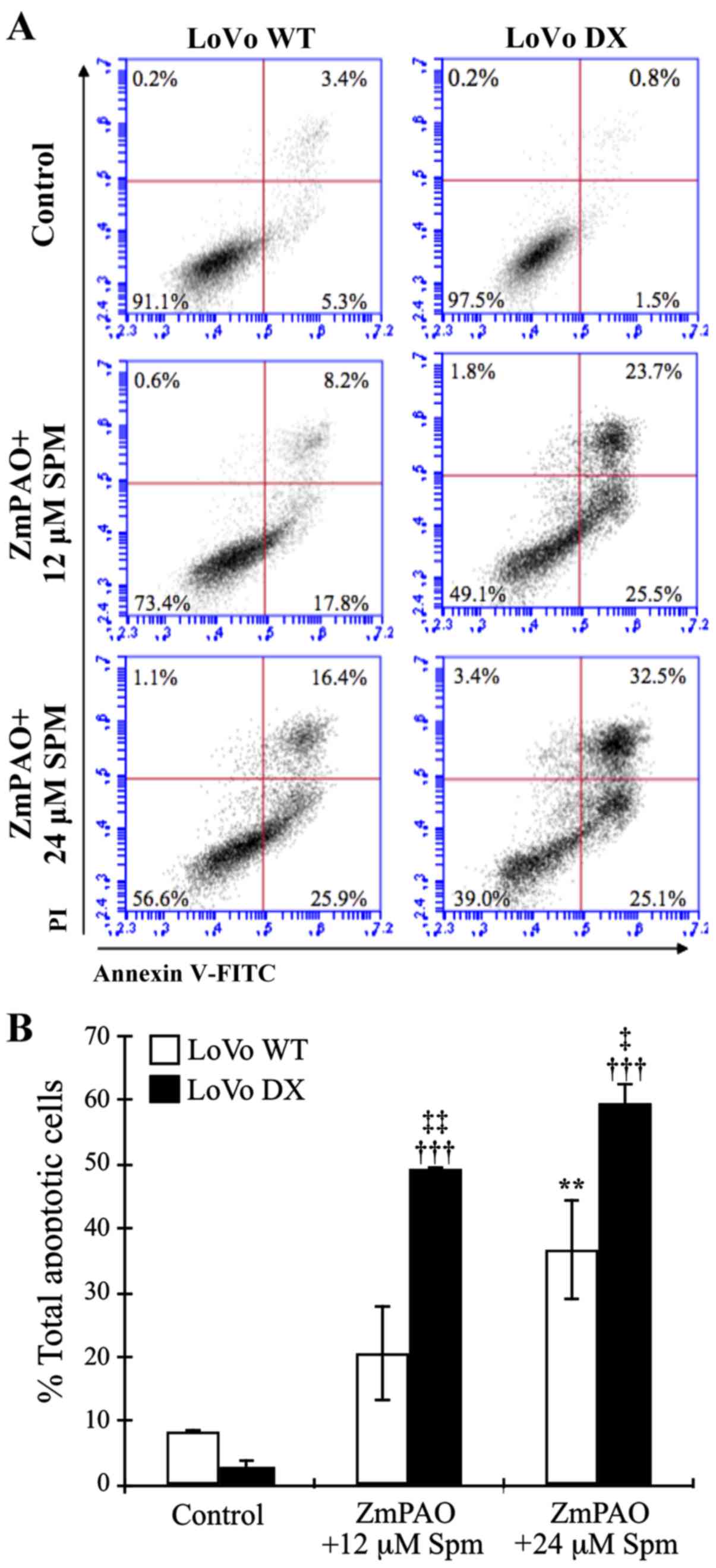 | Figure 6Flow cytometric analysis of apoptosis
of LoVo cells after double labeling with Annexin V-FITC and PI.
LoVo cells were incubated with 12 or 24 µM spermine in the
presence of ZmPAO for 60 min at 37°C. At 24 h after the end of the
treatment, and incubation at 37°C, cells were analyzed by flow
cytometry. (A) Representative Annexin V-FITC and PI flow cytometry
dot plots of LoVo WT and LoVo DX are shown. The x-axis represents
FITC staining, and the y-axis represents PI staining. The
percentage of cells displaying Annexin V-FITC positive/PI-negative
(early apoptosis), Annexin V-FITC positive/PI-positive (late
apoptosis or dead), Annexin V-FITC negative/PI-positive (necrosis)
and double negative cells (viable cells) is indicated. The dot
plots have been obtained from 1 out of 2 independent experiments,
performed in the same experimental conditions, which gave similar
results. (B) Each bar represents the mean ± SD of total apoptotic
cells of 2 independent experiments. Data were analyzed by one-way
ANOVA, followed by Tukey’s post hoc test. **P<0.01
vs. control LoVo WT cells; †††P<0.001 vs. control
LoVo DX cells; ‡P<0.05 and ‡‡P<0.01 vs.
LoVo WT cells incubated with ZmPAO and spermine. ZmPAO, maize
polyamine oxidase; LoVo WT cells, LoVo wild-type cells; LoVo DX
cells, LoVo multidrug-resistant cells. |
To confirm the involvement of apoptotic cell death
induced by ZmPAO/spermine, flow cytometric analysis using PI
staining was performed to analyze the cell cycle status. The
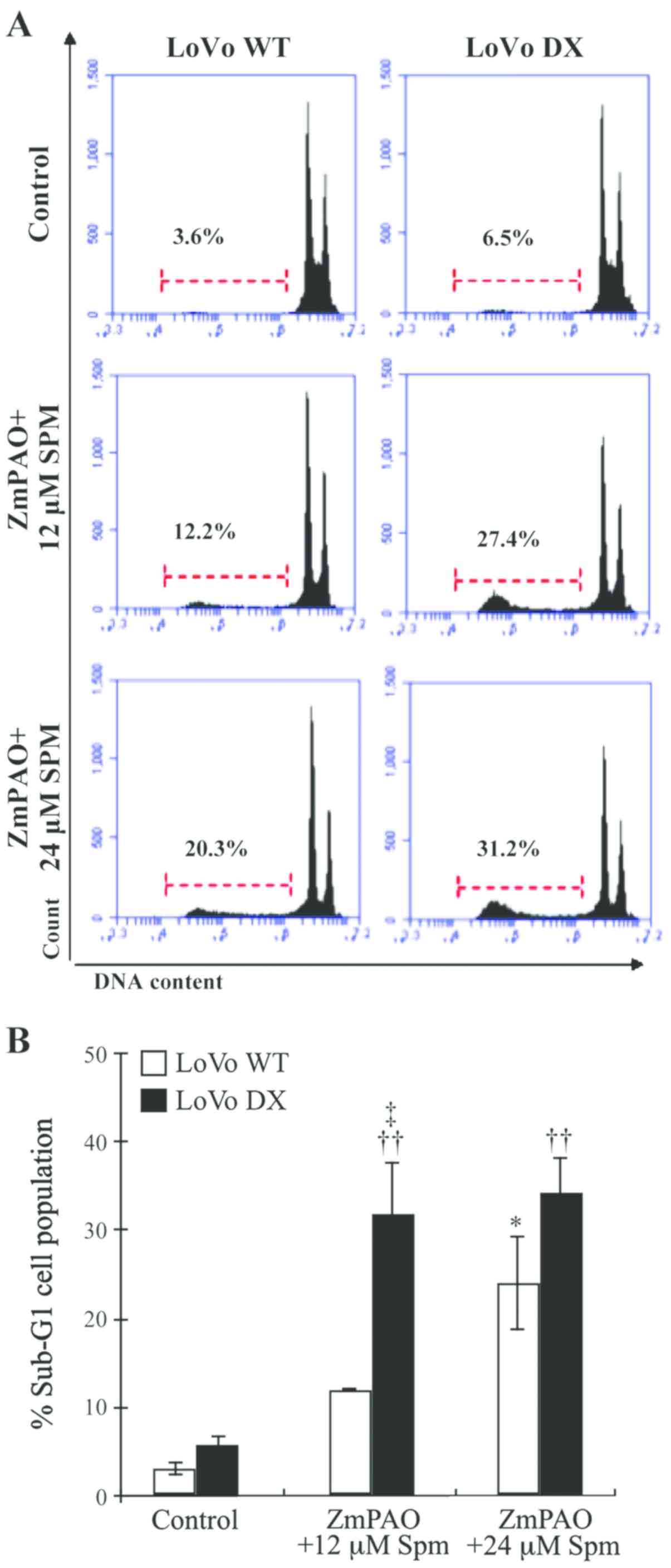
apoptotic cells that undergo DNA fragmentation exhibit sub-G1 DNA
contents. Exposure of the LoVo WT and DX cells to exogenous
spermine and ZmPAO induced a significant increase in the sub-G1
apoptotic cell population, compared to that observed in the
untreated control cells (Fig. 7).
The percentage of treated LoVo DX cells in the sub-G1 phase was
higher than the percentage of LoVo WT cells in the same phase,
which is consistent with the results of the Annexin V-FITC/PI
staining assay (Fig. 6). These
results confirm the cytotoxicity induced by spermine enzymatic
oxidation products and suggest the involvement of an apoptotic
mechanism.
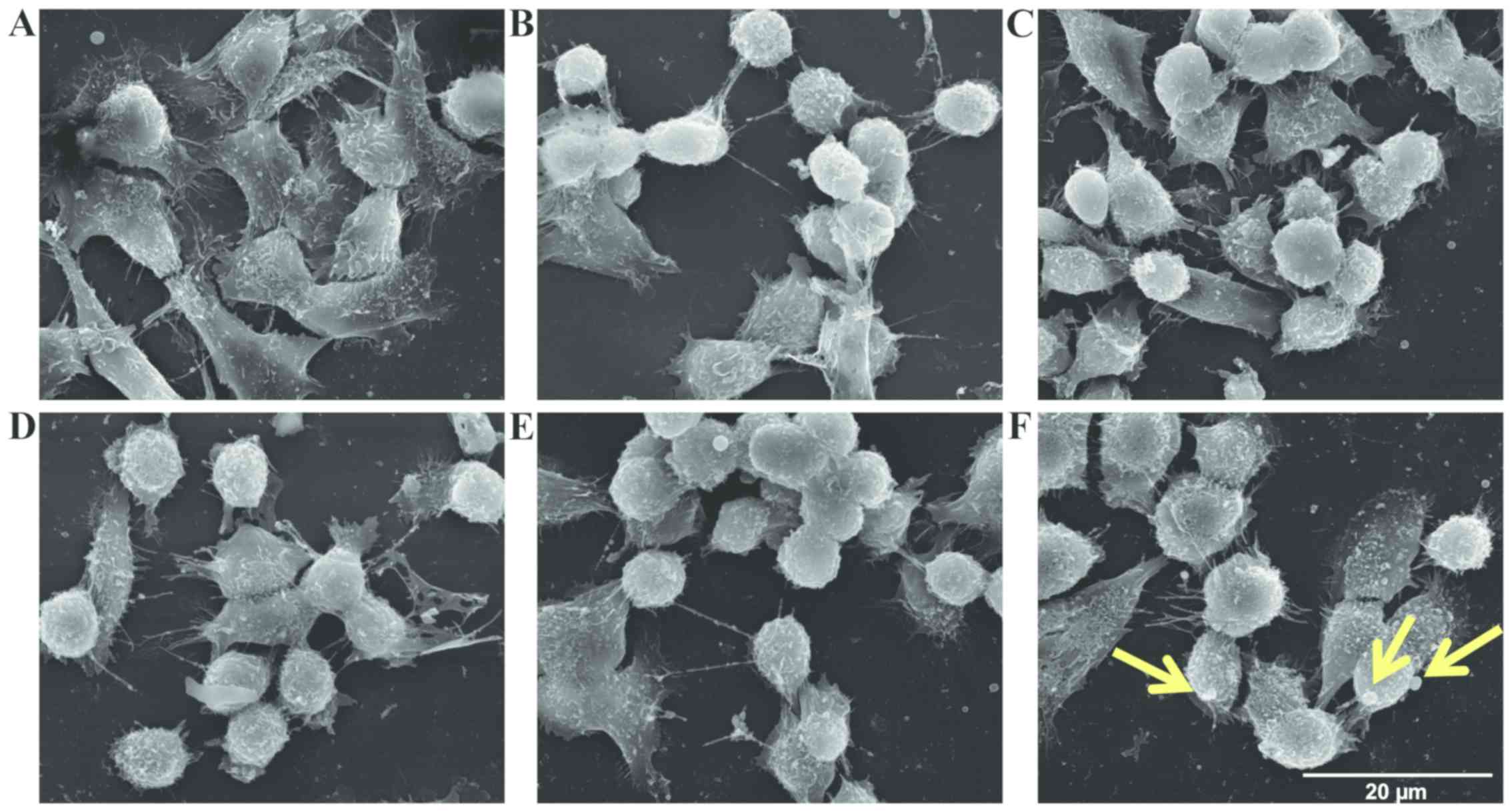
Morphological and ultrastructural changes
observed by SEM and TEM analyses
To further investigate the mechanisms underlying
spermine metabolite-mediated cytotoxicity, the morphological
changes and the ultrastructural modifications induced by treatment
of the LoVo cells with ZmPAO and spermine were examined by SEM and
TEM. The LoVo WT and LoVo DX control cells exhibited numerous
randomly distributed microvilli (Fig.
8A and D). In particular, the LoVo WT control cells exhibited a
typical polygonal shape (Fig. 8A).
Treatment with spermine alone 18 µM did not affect the
morphological aspect of either the LoVo WT or LoVo DX cells (data
not shown). Following incubation with exogenous
H2O2 or ZmPAO and spermine, rounded cells
were observed in the LoVo WT cells (Fig. 8B and C). The LoVo DX cells appeared
to be affected by treatment with 18 µM
H2O2 alone (Fig.
8E) and, in particular, following treatment with ZmPAO and
spermine, morphological alterations were more evident, in that tiny
blebs appeared on the surface and numerous cells tended to detach
from the substrate (Fig. 8F).
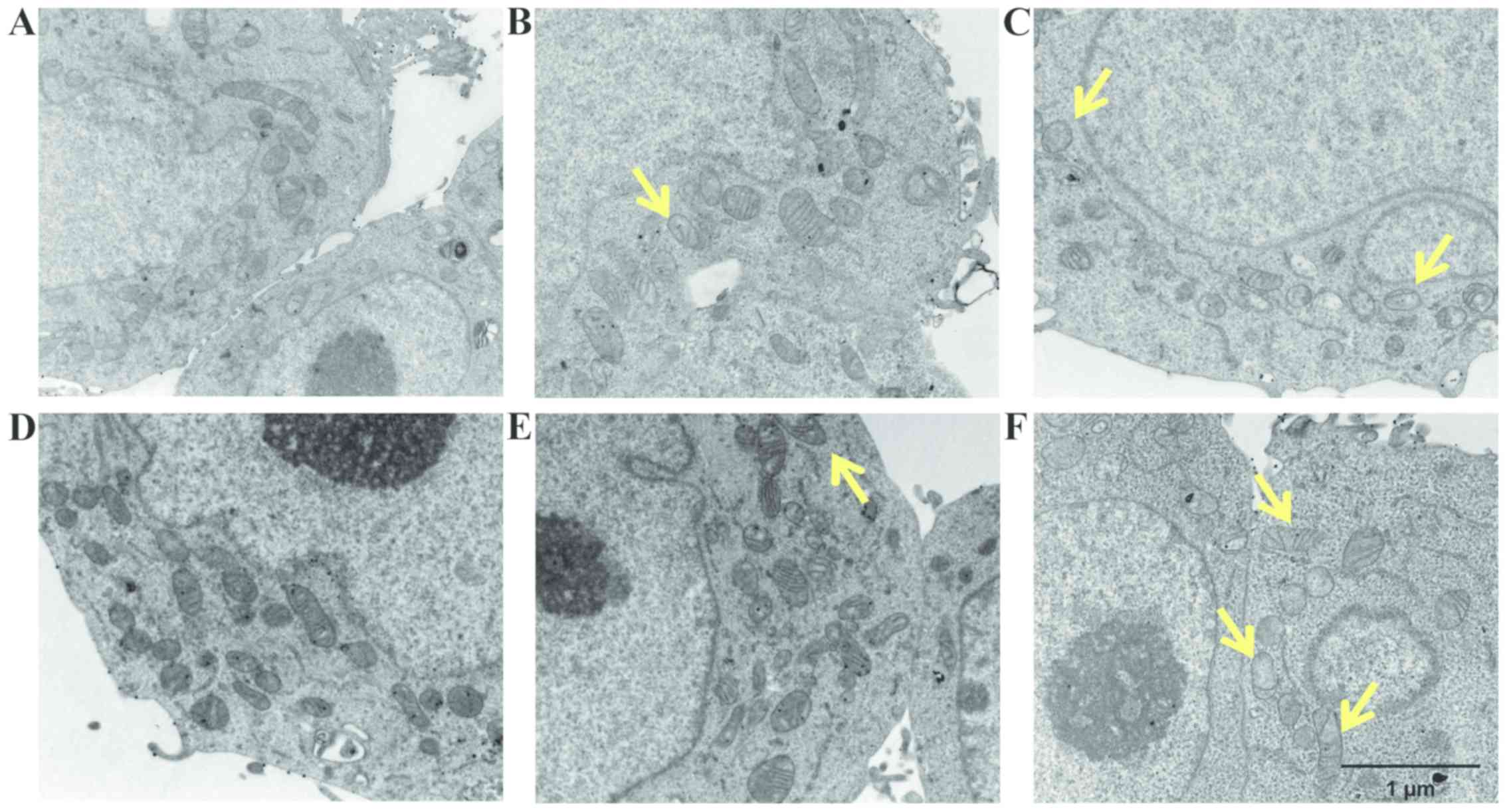
Analyses by TEM were also performed to clarify which subcellular
organelles are the targets involved during the treatment. Both the
LoVo WT and LoVo DX cells which were left untreated exhibited a
well-preserved ultrastructure of cytoplasmic organelles and
mitochondria (Fig. 9A and D).
Treatment with spermine alone did not induce any consistent
ultrastructural changes in both cell lines (Fig. 9B and E). By contrast, following
treatment with ZmPAO and spermine, the LoVo WT cells exhibited
mitochondrial alterations, which were considered to be a typical
ultrastructural feature of apoptosis (Fig. 9C). Moreover, treatment with the
enzymatic system induced marked mitochondrial alterations in the
LoVo DX cells (Fig. 9F). Almost
all mitochondria possessed abnormal vacuolated cristae.
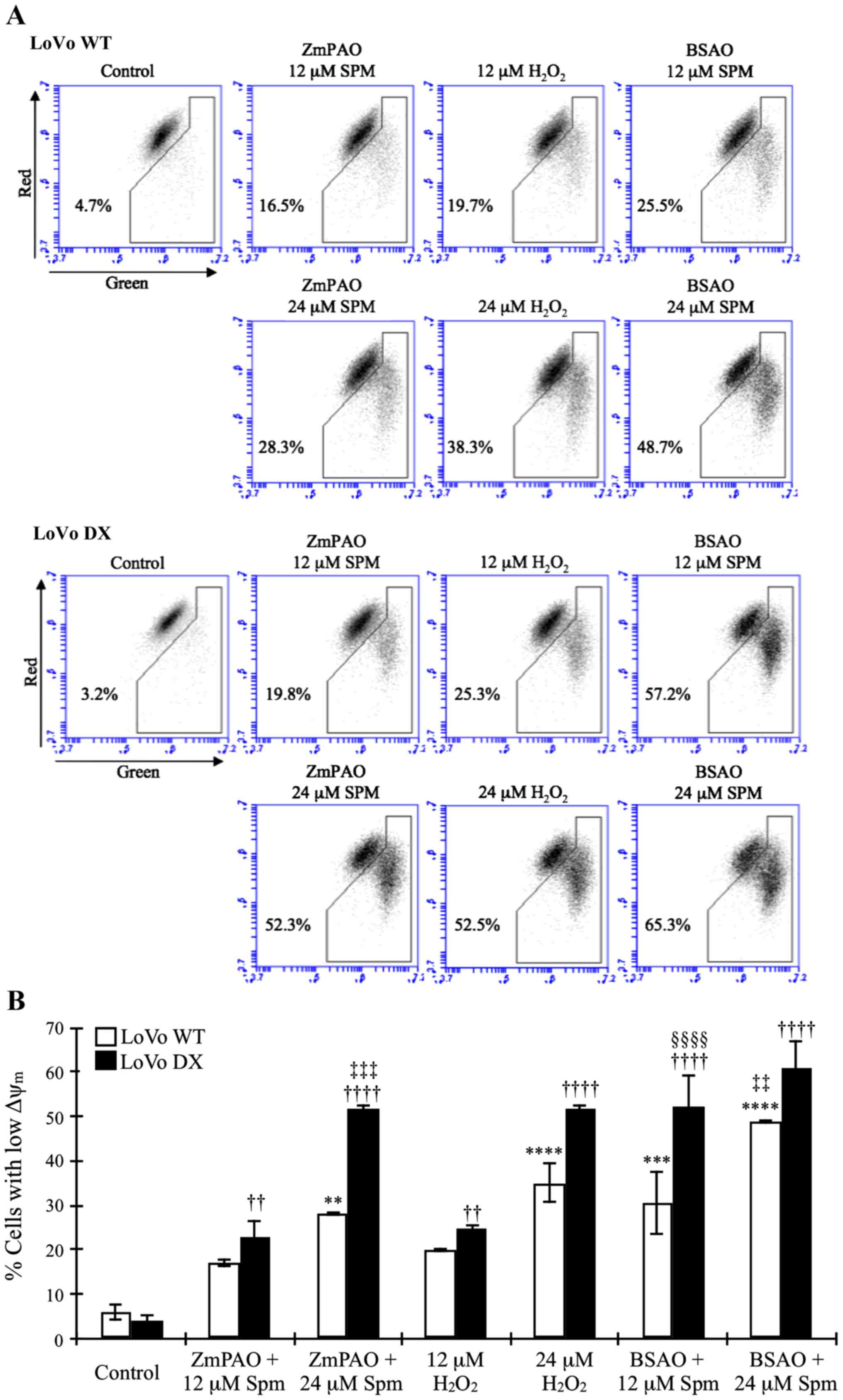
Cytofluorimetric analysis of
mitochondrial membrane potential
To investigate the mechanisms through which the
oxidative products of spermine generated by ZmPAO induced cell
death, we examined the loss of Δψm using a flow
cytometric analysis of the control and treated cells loaded with
the mitochondrial probe, JC-1. In healthy cells, membrane-permeable
JC-1 dye spontaneously accumulates in the mitochondria and forms
aggregates known as J-aggregates that emit red fluorescence after
excitation. By contrast, in apoptotic cells, with a low
Δψm, JC-1 remains in the cytoplasm as monomers, which
emit green fluorescence. Therefore, the loss of Δψm is
indicated by a decrease in the ratio of red/green fluorescence
intensity. As shown in Fig. 10,
in the LoVo WT and DX cells, the exposure to spermine in the
presence of ZmPAO induced an evident mitochondrial membrane
depolarization. Treatment with spermine increased the green
fluorescence intensity and concomitantly decreased the red
fluorescence intensity in a dose-dependent manner. The
ZmPAO/spermine-induced Δψm dissipation was more evident
in the LoVo DX cells than in the LoVo WT ones following treatment
with 24 µM spermine (52.3 and 28.3%, respectively). We also
examined the ability of spermine with BSAO and exogenous
H2O2 at the same concentrations to induce
mitochondrial membrane depolarization in both cell lines.
H2O2 induced a dose-dependent increase in
green fluorescence at the same level or more as spermine with ZmPAO
(Fig. 10). Moreover, treatment
with 12 µM spermine with BSAO already led to a larger
increase in green fluorescence than that obtained with ZmPAO. In
the presence of 24 µM spermine with BSAO, the increase was
higher in both the LoVo WT and LoVo DX cells.
Discussion
In this study, we investigated, for the first time,
at least to the best of our knowledge, the potential of using
polyamine oxidase ZmPAO as an antitumor agent in the presence of
polyamines. Polyamines are degraded by polyamine oxidase, producing
H2O2 and the corresponding aldehyde. In
previous studies, it was hypothesized that these reactive
metabolites, formed by BSAO/spermine enzymatic system, can induce
cytotoxicity selectively in tumor cells, when enzymatically
generated by endogenous polyamines. Moreover, the enzymes amine
oxidases may further cause the depletion of polyamine levels in the
tumor microenvironment in vivo, resulting in growth
inhibition (39-44). Therefore, the aim of this study was
to further improve our knowledge of polyamine-derived cytotoxic
metabolites in inducing cell death. The study was first carried out
in vitro to examine the cytotoxicity of
H2O2 and aldehyde(s) formed by
ZmPAO-catalyzed reaction. ZmPAO, a FAD-dependent enzyme, was used
due to both its higher catalytic efficiency and the lower molecular
weight compared to BSAO, which are important aspects for the
delivery of these enzymes into tumor cells, as a new anticancer
therapy. Due to the limited success of chemotherapy for tumors, the
majority of investigations have focused on the cellular and
molecular mechanisms responsible for the onset of MDR (35,39).
Currently, resistance to drugs in tumor cells is one of the most
major obstacles in the treatment of cancer. Hence, there is a
demand for alternative therapeutic strategies. This study was
therefore performed on LoVo human colon adenocarcinoma cells, a
sensitive cell line (WT) and its MDR counterpart (DX), to examine
the cytotoxic effects induced by ZmPAO in the presence of
polyamines.
ZmPAO purified from the maize shoot exhibited a
high affinity to spermine and spermidine and a higher specificity
constant than BSAO (Table II).
These features can be an advantage to sustain sufficient enzymatic
activity when the enzyme is conjugated to biocompatible carrier
nanoparticles. ZmPAO is relatively stable in storage and has the
highest stability at acidic pH values (57). A previous study showed that the
highest ZmPAO activity was found at pH 6.5 for the amine substrate
(45). Due to switching the energy
metabolism toward glycolysis that promotes the formation of lactic
acid and reduces the ability to remove tumor-derived protons by
poor perfusion, it is well known that solid tumors tend to be
acidic compared to normal tissues (58). The acidic conditions may thus
enhance the catalytic activity of ZmPAO, particularly in the tumor
microenvironment.
In this study, the effect of cell growth inhibition
induced by polyamine metabolites oxidized by ZmPAO on LoVo WT and
LoVo DX cells was also investigated. Since the efficacy of
therapeutic agents depends on its long-term effect on cancer cells,
we examined the effects of the treatments on in vitro
tumorigenic capacity by employing clonogenic assay, which is widely
considered to be the most valid method for the evaluation of tumor
cell sensitivity to anticancer drugs. As reported above, in
previous studies, it was demonstrated that
H2O2 and aldehyde(s) derived from
BSAO-catalyzed oxidation of spermine can induce cytotoxicity,
greater in both LoVo DX and M14 ADR2 cancer cells than in their
sensitive LoVo WT and M14 WT counterparts (39,40).
Consistent with previous observations, in this study, exogenous
spermine and ZmPAO treatment reduced the number of colonies in a
spermine dose- and time-dependent manner in LoVo cells (Figs. 2 and 3). It was also found that treatment
further decreased the viability of the LoVo DX cells when compared
with that of the LoVo WT cells. Moreover, there no marked
difference in cell viability was observed following treatment of
both the LoVo WT and DX cells with spermine and spermidine,
suggesting that the aminoaldehydes,
N-(3-aminopropyl)-4-aminobutanal and 4-aminobutanal, derived
from spermine and spermidine, respectively (Fig. 1), do not exert any cytotoxic
effect. This effect was mainly due to H2O2.
Similar results were also obtained with the AGS cells (data not
shown), indicating that the effect of cell growth inhibition
induced by polyamines and ZmPAO was not cell-specific.
Further colony formation assay revealed that
cytotoxicity induced by spermine metabolites in both the LoVo WT
and DX cells was completely prevented by the addition of exogenous
catalase, an enzyme that decomposes H2O2,
while it was not affected by the presence of exogenous
NAD+-dependent ALDH, an enzyme that oxidizes aldehydes
to the corresponding carboxylic acids (Fig. 3). This result supports the
hypothesis that H2O2 is mainly responsible
for the cytotoxicity. The addition of catalase also protected the
AGS cells from oxidative products of spermine and spermidine
catalyzed by ZmPAO (data not shown).
Unexpectedly, a slightly higher cytotoxicity was
observed when both the LoVo WT and DX cell were treated with
exogenous H2O2 than with the same
concentration of spermine in the presence of ZmPAO (Fig. 4). Although ZmPAO theoretically
produces one molecule of H2O2 from spermine,
in addition to N-(3-aminopropyl)-4-aminobutanal and
1,3-diaminopropane, the stoichiometric amount of
H2O2 actually generated by ZmPAO/spermine
enzymatic reaction might be lower than that of exogenous
H2O2 when directly exposed, as reported in a
previous study for BSAO (59).
In this study, a clonogenic assay also revealed
that the cytotoxicity induced by ZmPAO and spermine was less than
that caused by BSAO and spermine (Fig.
5). The greater cytotoxicity induced by BSAO was probably due
to the presence of acrolein, another toxic metabolite only formed
by the BSAO/spermine enzymatic system (28).
For clinical applications, the increase in the
efficiency of the in situ generation of polyamine
metabolites is fundamental. Several studies have demonstrated that
an increase in the incubation temperature from 37 to 42°C enhances
the cytotoxicity in both LoVo and M14 cells exposed to BSAO and
spermine; the cytotoxic effect has been shown to be higher in both
MDR cell lines than in their drug-sensitive counterparts (40,60,61).
Other studies have demonstrated that pre-treatment of the cells
with MDL 72527, a lysosomotropic compound, sensitizes several tumor
cell lines to the subsequent exposure to BSAO and spermine
(40,41,62).
In addition, chloroquine, another lysosomotropic agent, was
recently reported to enhance apoptotic and non-apoptotic cell death
induced by H2O2 and other spermine
metabolites in M14 cells, particularly in ADR2 cells (42,63).
It has also been observed that the ectopic expression of
recombinant ZmPAO in the nucleus of MCF-7 human breast cancer cells
confers a greater growth sensitivity to etoposide, a potent DNA
topoisomerase II inhibitor widely used for cancer therapy (64), suggesting that ZmPAO may also be a
potential tool which may be used to enhance efficacy of
anti-proliferative agents. Therefore, a further investigation of
drug combinations with the aim of enhancing the induction of cell
death by polyamine metabolites appears to be necessary.
In this study, Annexin V-FITC and PI staining assay
revealed that treatment with spermine and ZmPAO increased the
percentage of apoptotic cells in a spermine dose-dependent manner,
and the proportion of apoptotic cells was significantly higher in
the LoVo DX cells than the LoVo WT ones (Fig. 6), indicating that treatment with
spermine and ZmPAO is able to induce cell death through apoptosis.
The apoptotic process was also detectable in the AGS cells (data
not shown). During the initial phases of apoptosis, cell shrinkage
and the membrane remains intact, resulting in a decrease in forward
light scatter and an increase in side light scatter determined by
flow cytometry. In this study, apoptotic cells were detected and
distinguished from necrosis on the basis of the scatter parameters
following treatment with ZmPAO and spermine on both cell lines
(data not shown). The nature of apoptotic cell death was also
demonstrated by the increase in the sub-G1 hypodiploid cell
population (Fig. 7).
In this study, SEM and TEM observations also
confirmed the occurrence of apoptosis in the cancer cells following
treatment with the enzymatic system (Figs. 8 and 9). Spermine metabolites catalyzed by
ZmPAO induced morphological alterations, such as plasma membrane
blebbing, particularly in the LoVo DX cells treated with 18
µM spermine. Intense mitochondrial changes in the cells
treated with 21 µM spermine and ZmPAO were also observed.
The morphological and ultrastructural changes observed are the
typical features of apoptosis. The advantage of the apoptotic
process over necrosis is that it does not induce the inflammatory
response and immunoreaction after cell death. Apoptotic cells are
removed through phagocytosis by neighboring cells and macrophages
without releasing their contents that can cause damage to the
surrounding tissues. Indeed, the majority of cytotoxic drugs in
current clinical applications have been shown to induce the
apoptosis of cancer cells. Therefore, the induction of apoptosis by
spermine metabolites may be considered as one of the future
therapeutic strategies for cancer therapy.
It is known that the mitochondria play a pivotal
role in the intrinsic apoptotic pathway and a reduction in
Δψm is an early irreversible step (65). The observations reported in this
study indicated that spermine metabolite-induced apoptosis was
preceded by the Δψm collapse in the LoVo cells (Fig. 10). H2O2
generated by the oxidation of spermine is able to cross the cell
membrane and the inner membrane of mitochondria and directly
interacts with endogenous molecules and structures, inducing an
intense oxidative stress under appropriate conditions (40,61).
It is considered that oxidative stress is involved in the induction
of mitochondrial permeability transition by opening the transition
pore, leading to a loss of Δψm, mitochondrial swelling
and to the rupture of the outer membrane. It has been previously
demonstrated that treatment with 12 µM exogenous
H2O2 induced an increase in the mRNA levels
of the BAX pro-apoptotic gene in LoVo DX cells, while the
pro-survival Bcl-2 gene did not reveal any variation in expression
following treatment (66). In
order to further obtain information on the key molecular changes
following exposure to polyamine metabolites, a quantitative
proteomic approach with Stable-Isotope Labeling by Amino acids in
cell culture (SILAC) on tumor cells is promising and ongoing
(unpublished data).
The higher sensitivity to cytotoxic spermine
metabolites of LoVo DX cells as compared with their drug-sensitive
cells has previously been attributed to a higher basal production
of ROS, although the ROS levels were not related to the
glutathione-dependent H2O2 metabolism because
both cells have the same glutathione levels (38,39).
When LoVo DX cells are treated with polyamine metabolites, they may
be no longer able to eliminate additional ROS by the cellular
antioxidant system, leading to an earlier and greater loss of
mitochondrial functionality than LoVo WT cells. Higher levels of
ROS in LoVo DX cells than LoVo WT may be due to mitochondrial
electron transport chain activity as LoVo DX cells exhibit P-gp
overexpression that requires a higher ATP production. It is known
that there are differences in mitochondrial electron transport
chain activity between sensitive and resistant cell lines (39). A high activity of respiratory
complex protein subunits can induce a continuous and physiological
production of superoxide radical, H2O2 and
hydroxyl radical.
Whether the concentration of polyamine metabolites
is sufficient to induce cytotoxicity is unclear. The results
reported in this study demonstrated that at least 12 µM of
spermine in the presence of ZmPAO significantly reduced the
viability of both the LoVo WT and LoVo DX cells. However, although
polyamine concentrations are upregulated in tumors, free polyamines
which can be oxidized by ZmPAO are present only in small amounts as
polyamines preferentially bind to macromolecules, such as DNA, RNA,
and proteins (12). Moreover, slow
and low ROS production may be completely removed by enzymes of the
antioxidant system, such as catalase, glutathione peroxidase, and
superoxide dismutase (65). A
recent study demonstrated that recombinant ZmPAO overexpression in
human breast cancer cells did not inhibit cell growth under normal
growth conditions (64).
Nevertheless, the overexpression sensitized the cells to etoposide
as described above. The antitumor potential of BSAO has also been
demonstrated in vivo in a mouse melanoma model (43,44).
In the development of novel treatment strategies against tumors,
their side-effects against normal cells should be considered.
Therefore, it was previously shown that normal human epidermal
melanocytes displayed a higher resistance than M14 cells against
BSAO/spermine-induced cytotoxicity (42). A further research is directed at
examining the differences between primary cells (neurons) and
neuroblastoma cancer cells in their sensitivity to treatment with
polyamine metabolites (unpublished data).
It would of interest to determine the intracellular
polyamine levels following treatment with ZmPAO in vivo.
Thus far, for the in vitro assay performed in this study, we
used exogenous spermine to observe cytotoxicity, although in
vivo, this enzymatic system is able to deaminate endogenous
polyamines. Since polyamine levels are high even in the tumor
microenvironment, extracellular polyamines to induce cytotoxicity
could also be used. However, ZmPAO-overexpressing MCF7 cells have
shown only a small, not statistically significant, decrease in the
levels of spermine and spermidine when compared with control cells
(64). However, the depletion of
polyamines in the tumor microenvironment may be able to affect
polyamine uptake and its distribution.
In conclusion, this study demonstrated that
treatment with ZmPAO and spermine induced cell death through an
apoptotic mechanism in human LoVo cancer cells. Microscopy analysis
also revealed that the treatment caused characteristic
morphological and ultrastructural changes associated with
apoptosis. Furthermore, the cytotoxicity was significantly greater
in the LoVo DX cells than the LoVo WT cells. Therefore, all
findings obtained in this study suggest that polyamine oxidation by
ZmPAO, specifically in tumor microenvironment, may be taken into
account for a future anticancer therapy, rendering this approach
attractive in combating cancer and mainly in treating MDR cancer
patients.
Funding
This study was funded by the generous support of
‘La Sapienza’ University of Rome and Italian MIUR (Ministero
dell’Istruzione, dell’Università e della Ricerca), AIRC IG 17575,
IG 20801; Istituto Pasteur - Fondazione Cenci-Bolognetti,
AFM-Telethon grant #21025. E.A. thanks Wakunaga Pharmaceutical Co.
Ltd. (Japan) for the scholarship given to SO for supporting his PhD
research work.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article or are available from the
corresponding author on reasonable request.
Authors’ contributions
EA and DAS conceived this study and coordinated the
collaboration among the authors. SO, RM, SM and AC performed all
the experiments on the LoVo and AGS cells. GC, RA and EG optimized
the protocols for the analyses and PAO/BSAO purification. All
authors wrote the manuscript and all authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Abbreviations:
|
AAP
|
4-aminoantipyrine
|
|
ALDH
|
aldehyde dehydrogenase
|
|
BSAO
|
bovine serum amine oxidase
|
|
DCHBS
|
3,5-dichloro-2-hydroxybenzene-sulfonic acid
|
|
MDR
|
multidrug resistance
|
|
Δψm
|
mitochondrial membrane potential
|
|
P-gp
|
P-glycoprotein
|
|
PI
|
propidium iodide
|
|
SEM
|
scanning electron microscopy
|
|
TEM
|
transmission electron microscopy
|
|
ZmPAO
|
maize polyamine oxidase
|
|
JC-1
|
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine
iodide
|
|
FAD
|
flavin-adenin-dinucleotide
|
|
GABA
|
γ-aminobutyric acid
|
|
ADR2
|
adriamycin
|
|
MDL 72527
|
N1,N4-bis(2,3-butadienyl)-1,4-butanediamine dihydrochloride
|
|
ED50
|
effective dose 50
|
|
TPQ
|
2,4,5-trihydroxyphenylalanine
quinone
|
|
FBS
|
fetal bovine serum
|
|
HRP
|
horseradish peroxidase
|
|
MEM
|
minimum essential medium
|
|
BSA
|
bovine serum albumin
|
|
DOX
|
doxorubicin
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
FITC
|
fluoresceine isothiocyanate
conjugated
|
|
IU
|
international units
|
|
MFC
|
means fluorescence channel
|
|
PBS
|
phosphate-buffered saline
|
|
RNAse A
|
ribonuclease A
|
|
ROS
|
reactive oxygen species
|
|
SD
|
standard deviation
|
|
SDS/PAGE
|
sodium dodecyl
sulphate/polyacrylamide gel electrophoresis
|
|
Spm
|
spermine
|
|
WT
|
wild-type
|
|
PEG
|
poly(ethylene glycol)
|
Acknowledgments
The authors would like to thank the ‘International
Polyamine Foundation - ONLUS’ for the availability to look up the
poly-amines documentation.
References
|
1
|
Pegg AE: Polyamine metabolism and its
importance in neoplastic growth and a target for chemotherapy.
Cancer Res. 48:759–774. 1988.PubMed/NCBI
|
|
2
|
Bachrach U, Wang YC and Tabib A:
Polyamines: New cues in cellular signal transduction. News Physiol
Sci. 16:106–109. 2001.PubMed/NCBI
|
|
3
|
Mattoo AK, Minocha SC, Minocha R and Handa
AK: Polyamines and cellular metabolism in plants: Transgenic
approaches reveal different responses to diamine putrescine versus
higher poly-amines spermidine and spermine. Amino Acids.
38:405–413. 2010. View Article : Google Scholar
|
|
4
|
Migliaccio N, Martucci NM, Ruggiero I,
Sanges C, Ohkubo S, Lamberti A, Agostinelli E and Arcari P: Ser/Thr
kinases and polyamines in the regulation of non-canonical functions
of elongation factor 1A. Amino Acids. 48:2339–2352. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schuber F: Influence of polyamines on
membrane functions. Biochem J. 260:1–10. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ficker E, Taglialatela M, Wible BA, Henley
CM and Brown AM: Spermine and spermidine as gating molecules for
inward rectifier K+ channels. Science. 266:1068–1072.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neel BG and Tonks NK: Protein tyrosine
phosphatases in signal transduction. Curr Opin Cell Biol.
9:193–204. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grancara S, Dalla Via L, García-Argáez AN,
Ohkubo S, Pacella E, Manente S, Bragadin M, Toninello A and
Agostinelli E: Spermine cycling in mitochondria is mediated by
adenine nucleotide translocase activity: Mechanism and
pathophysiological implications. Amino Acids. 48:2327–2337. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madeo F, Eisenberg T, Pietrocola F and
Kroemer G: Spermidine in health and disease. Science.
359:eaan27882018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee CY, Su GC, Huang WY, Ko MY, Yeh HY,
Chang GD, Lin SJ and Chi P: Promotion of homology-directed DNA
repair by polyamines. Nat Commun. 10:652019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cervelli M, Angelucci E, Germani F,
Amendola R and Mariottini P: Inflammation, carcinogenesis and
neurodegeneration studies in transgenic animal models for polyamine
research. Amino Acids. 46:521–530. 2014. View Article : Google Scholar
|
|
12
|
Park MH and Igarashi K: Polyamines and
their metabolites as diagnostic markers of human diseases. Biomol
Ther (Seoul). 21:1–9. 2013. View Article : Google Scholar
|
|
13
|
Wallace HM and Fraser AV: Inhibitors of
polyamine metabolism: Review article. Amino Acids. 26:353–365.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCann PP and Pegg AE: Ornithine
decarboxylase as an enzyme target for therapy. Pharmacol Ther.
54:195–215. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakanishi S and Cleveland JL: Targeting
the polyamine-hypusine circuit for the prevention and treatment of
cancer. Amino Acids. 48:2353–2362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tabib A and Bachrach U: Role of polyamines
in mediating malignant transformation and oncogene expression. Int
J Biochem Cell Biol. 31:1289–1295. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel AR and Wang JY: Polyamines modulate
transcription but not posttranscription of c-myc and c-jun in IEC-6
cells. Am J Physiol. 273:C1020–C1029. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao L and Wang JY: Posttranscriptional
regulation of gene expression in epithelial cells by polyamines.
Methods Mol Biol. 720:67–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Šebela M, Radová A, Angelini R,
Tavladoraki P, Frébort I and Peč P: FAD-containing polyamine
oxidases: A timely challenge for researchers in biochemistry and
physiology of plants. Plant Sci. 160:197–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki Y and Yanagisawa H: Purification
and properties of maize polyamine oxidase: A flavoprotein. Plant
Cell Physiol. 21:1085–1094. 1980.
|
|
21
|
Cona A, Moreno S, Cenci F, Federico R and
Angelini R: Cellular re-distribution of flavin-containing polyamine
oxidase in differentiating root and mesocotyl of Zea mays L.
seedlings. Planta. 221:265–276. 2005. View Article : Google Scholar
|
|
22
|
Cona A, Cenci F, Cervelli M, Federico R,
Mariottini P, Moreno S and Angelini R: Polyamine oxidase, a
hydrogen peroxide-producing enzyme, is up-regulated by light and
down-regulated by auxin in the outer tissues of the maize
mesocotyl. Plant Physiol. 131:803–813. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pennell RI and Lamb C: Programmed cell
death in plants. Plant Cell. 9:1157–1168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kärkönen A and Kuchitsu K: Reactive oxygen
species in cell wall metabolism and development in plants.
Phytochemistry. 112:22–32. 2015. View Article : Google Scholar
|
|
25
|
Houen G, Struve C, Søndergaard R, Friis T,
Anthoni U, Nielsen PH, Christophersen C, Petersen BO and Duus JØ:
Substrate specificity of the bovine serum amine oxidase and in situ
characterisation of aminoaldehydes by NMR spectroscopy. Bioorg Med
Chem. 13:3783–3796. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Matteis G, Agostinelli E, Mondovì B and
Morpurgo L: The metal function in the reactions of bovine serum
amine oxidase with substrates and hydrazine inhibitors. J Biol
Inorg Chem. 4:348–353. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Agostinelli E and Seiler N:
Non-irradiation-derived reactive oxygen species (ROS) and cancer:
Therapeutic implications. Amino Acids. 31:341–355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sharmin S, Sakata K, Kashiwagi K, Ueda S,
Iwasaki S, Shirahata A and Igarashi K: Polyamine cytotoxicity in
the presence of bovine serum amine oxidase. Biochem Biophys Res
Commun. 282:228–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Binda C, Coda A, Angelini R, Federico R,
Ascenzi P and Mattevi A: A 30-angstrom-long U-shaped catalytic
tunnel in the crystal structure of polyamine oxidase. Structure.
7:265–276. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tavladoraki P, Cona A and Angelini R:
Copper-containing amine oxidases and FAD-dependent polyamine
oxidases are key players in plant tissue differentiation and organ
development. Front Plant Sci. 7:824–834. 2016.Review. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cona A, Federico R, Gramiccia M, Orsini S
and Gradoni L: The amino aldehydes produced by spermine and
spermidine oxidation with maize polyamine oxidase have
anti-leishmanial effect. Biotechnol Appl Biochem. 14:54–59.
1991.PubMed/NCBI
|
|
32
|
Cona A, Rea G, Angelini R, Federico R and
Tavladoraki P: Functions of amine oxidases in plant development and
defence. Trends Plant Sci. 11:80–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bouchereau A, Aziz A, Larher F and
Martin-Tanguy J: Polyamines and environmental challenges: Recent
development. Plant Sci. 140:103–125. 1999. View Article : Google Scholar
|
|
34
|
Massa S, Spanò D, Pintus F, Medda R and
Floris G: Oxidation of di- and polyamines: In vitro effect of amino
aldehydes on the vitality of Leishmania promastigotes. Med Chem
Res. 19:77–83. 2010. View Article : Google Scholar
|
|
35
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cole SP: Targeting multidrug resistance
protein 1 (MRP1, ABCC1): Past, present, and future. Annu Rev
Pharmacol Toxicol. 54:95–117. 2014. View Article : Google Scholar
|
|
37
|
Meschini S, Calcabrini A, Monti E, Del
Bufalo D, Stringaro A, Dolfini E and Arancia G: Intracellular
P-glycoprotein expression is associated with the intrinsic
multidrug resistance phenotype in human colon adenocarcinoma cells.
Int J Cancer. 87:615–628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arancia G, Calcabrini A, Marra M, Crateri
P, Artico M, Martone A, Martelli F and Agostinelli E: Mitochondrial
alterations induced by serum amine oxidase and spermine on human
multidrug resistant tumor cells. Amino Acids. 26:273–282. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Calcabrini A, Arancia G, Marra M, Crateri
P, Befani O, Martone A and Agostinelli E: Enzymatic oxidation
products of spermine induce greater cytotoxic effects on human
multidrug-resistant colon carcinoma cells (LoVo) than on their
wild-type counterparts. Int J Cancer. 99:43–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agostinelli E, Belli F, Molinari A,
Condello M, Palmigiani P, Vedova LD, Marra M, Seiler N and Arancia
G: Toxicity of enzymatic oxidation products of spermine to human
melanoma cells (M14): Sensitization by heat and MDL 72527. Biochim
Biophys Acta. 1763:1040–1050. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Agostinelli E, Condello M, Molinari A,
Tempera G, Viceconte N and Arancia G: Cytotoxicity of spermine
oxidation products to multidrug resistant melanoma M14 ADR2 cells:
Sensitization by the MDL 72527 lysosomotropic compound. Int J
Oncol. 35:485–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Agostinelli E, Condello M, Tempera G,
Macone A, Bozzuto G, Ohkubo S, Calcabrini A, Arancia G and Molinari
A: The combined treatment with chloroquine and the enzymatic
oxidation products of spermine overcomes multidrug resistance of
melanoma M14 ADR2 cells: A new therapeutic approach. Int J Oncol.
45:1109–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Averill-Bates DA, Chérif A, Agostinelli E,
Tanel A and Fortier G: Anti-tumoral effect of native and
immobilized bovine serum amine oxidase in a mouse melanoma model.
Biochem Pharmacol. 69:1693–1704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Averill-Bates DA, Ke Q, Tanel A, Roy J,
Fortier G and Agostinelli E: Mechanism of cell death induced by
spermine and amine oxidase in mouse melanoma cells. Int J Oncol.
32:79–88. 2008.
|
|
45
|
Polticelli F, Basran J, Faso C, Cona A,
Minervini G, Angelini R, Federico R, Scrutton NS and Tavladoraki P:
Lys300 plays a major role in the catalytic mechanism of maize
polyamine oxidase. Biochemistry. 44:16108–16120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Averill-Bates DA, Agostinelli E,
Przybytkowski E, Mateescu MA and Mondovì B: Cytotoxicity and
kinetic analysis of purified bovine serum amine oxidase in the
presence of spermine in Chinese hamster ovary cells. Arch Biochem
Biophys. 300:75–79. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Agostinelli E, De Matteis G, Mondovì B and
Morpurgo L: Reconstitution of Cu2+-depleted bovine serum
amine oxidase with Co2+. Biochem J. 330:383–387. 1998.
View Article : Google Scholar
|
|
48
|
Cervelli M, Cona A, Angelini R, Polticelli
F, Federico R and Mariottini P: A barley polyamine oxidase isoform
with distinct structural features and subcellular localization. Eur
J Biochem. 268:3816–3830. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Federico R, Alisi C and Forlani F:
Properties of the polyamine oxidase from the cell wall of maize
seedlings. Phytochemistry. 28:45–46. 1989. View Article : Google Scholar
|
|
50
|
Turini P, Sabatini S, Befani O, Chimenti
F, Casanova C, Riccio PL and Mondovì B: Purification of bovine
plasma amine oxidase. Anal Biochem. 125:294–298. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Janes SM, Mu D, Wemmer D, Smith AJ, Kaur
S, Maltby D, Burlingame AL and Klinman JP: A new redox cofactor in
eukaryotic enzymes: 6-hydroxydopa at the active site of bovine
serum amine oxidase. Science. 248:981–987. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Angelini R, Cona A and Tavladoraki P:
Determination of copper amine oxidase activity in plant tissues.
Methods Mol Biol. 1694:129–139. 2018. View Article : Google Scholar
|
|
53
|
Dolfini E, Dasdia T, Arancia G, Molinari
A, Calcabrini A, Scheper RJ, Flens MJ, Gariboldi MB and Monti E:
Characterization of a clonal human colon adenocarcinoma line
intrinsically resistant to doxorubicin. Br J Cancer. 76:67–76.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Grandi M, Geroni C and Giuliani FC:
Isolation and characterization of a human colon adenocarcinoma cell
line resistant to doxorubicin. Br J Cancer. 54:515–518. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
van Engeland M, Nieland LJ, Ramaekers FC,
Schutte B and Reutelingsperger CP: Annexin V-affinity assay: A
review on an apoptosis detection system based on phosphatidylserine
exposure. Cytometry. 31:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Federico R, Cona A, Angelini R, Schininà
ME and Giartosio A: Characterization of maize polyamine oxidase.
Phytochemistry. 29:2411–2414. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Damaghi M, Wojtkowiak JW and Gillies RJ:
pH sensing and regulation in cancer. Front Physiol. 4:370–379.
2013. View Article : Google Scholar
|
|
59
|
De Biase D, Agostinelli E, De Matteis G,
Mondovì B and Morpurgo L: Half-of-the-sites reactivity of bovine
serum amine oxidase. Reactivity and chemical identity of the second
site. Eur J Biochem. 237:93–99. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Agostinelli E, Arancia G, Vedova LD, Belli
F, Marra M, Salvi M and Toninello A: The biological functions of
polyamine oxidation products by amine oxidases: Perspectives of
clinical applications. Amino Acids. 27:347–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Agostinelli E, Belli F, Dalla Vedova L,
Marra M, Crateri P and Arancia G: Hyperthermia enhances
cytotoxicity of amine oxidase and spermine on drug-resistant LoVo
colon adenocar-cinoma cells. Int J Oncol. 28:1543–1553.
2006.PubMed/NCBI
|
|
62
|
Agostinelli E, Vedova LD, Belli F,
Condello M, Arancia G and Seiler N: Sensitization of human colon
adenocarcinoma cells (LoVo) to reactive oxygen species by a
lysosomotropic compound. Int J Oncol. 29:947–955. 2006.PubMed/NCBI
|
|
63
|
Iyamu E, Perdew H and Woods G: Growth
inhibitory and differentiation effects of chloroquine and its
analogue on human leukemic cells potentiate fetal hemoglobin
production by targeting the polyamine pathway. Biochem Pharmacol.
77:1021–1028. 2009. View Article : Google Scholar
|
|
64
|
Marcocci L, Casadei M, Faso C, Antoccia A,
Stano P, Leone S, Mondovì B, Federico R and Tavladoraki P:
Inducible expression of maize polyamine oxidase in the nucleus of
MCF-7 human breast cancer cells confers sensitivity to etoposide.
Amino Acids. 34:403–412. 2008. View Article : Google Scholar
|
|
65
|
Grancara S, Zonta F, Ohkubo S, Brunati AM,
Agostinelli E and Toninello A: Pathophysiological implications of
mitochondrial oxidative stress mediated by mitochondriotropic
agents and poly-amines: The role of tyrosine phosphorylation. Amino
Acids. 47:869–883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Amendola R, Cervelli M, Fratini E,
Sallustio DE, Tempera G, Ueshima T, Mariottini P and Agostinelli E:
Reactive oxygen species spermine metabolites generated from amine
oxidases and radiation represent a therapeutic gain in cancer
treatments. Int J Oncol. 43:813–820. 2013. View Article : Google Scholar : PubMed/NCBI
|