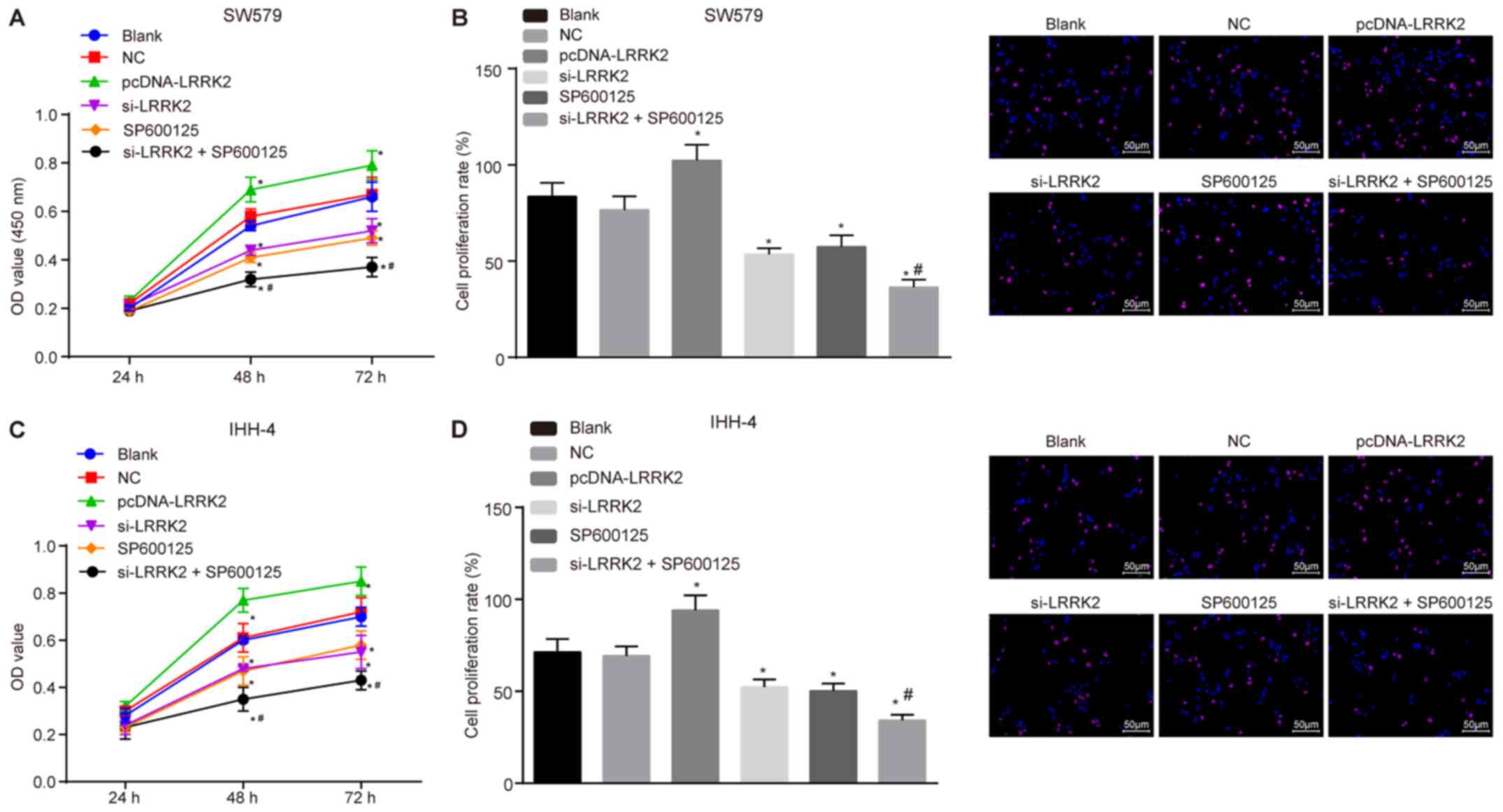
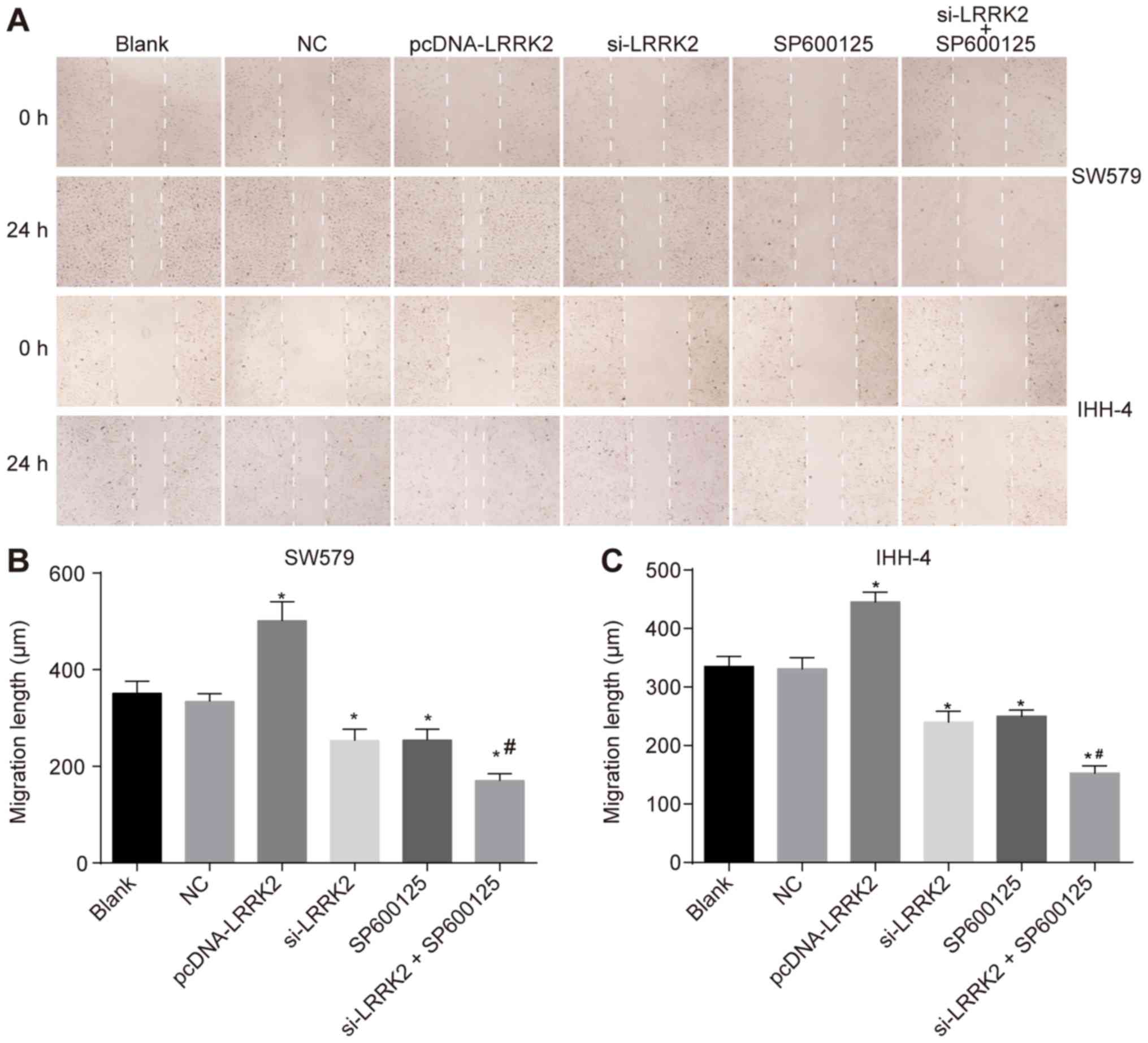
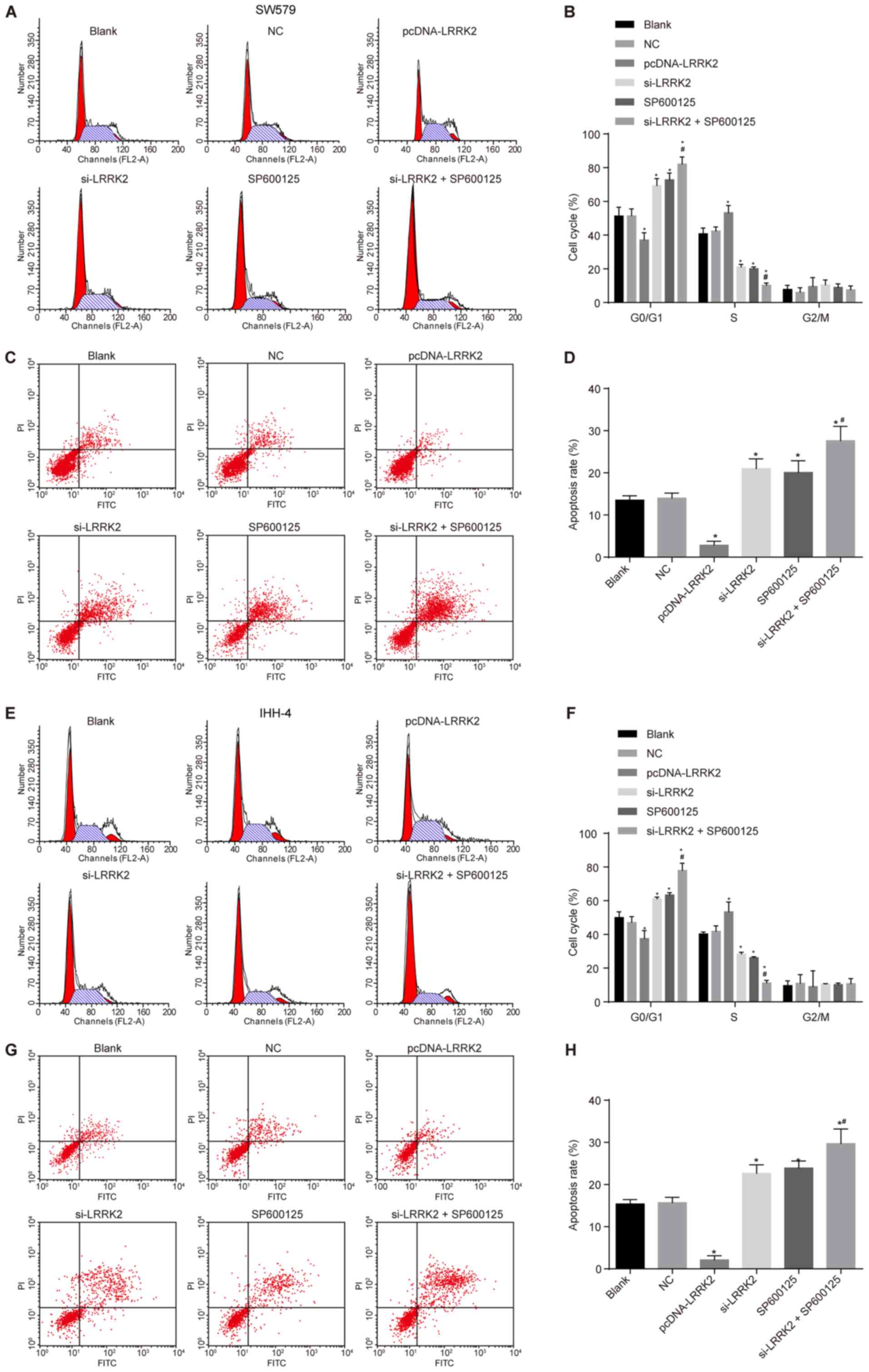
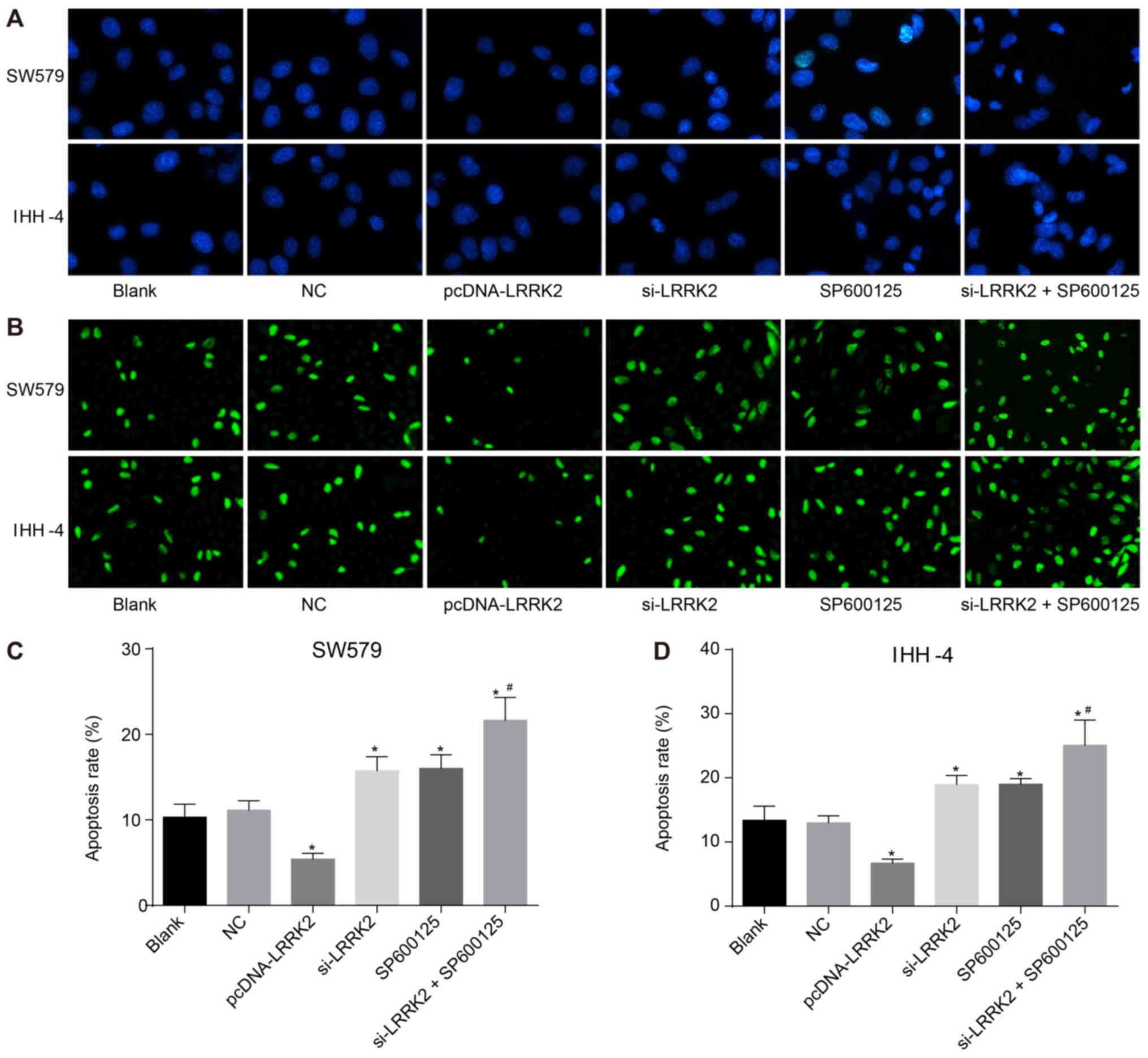
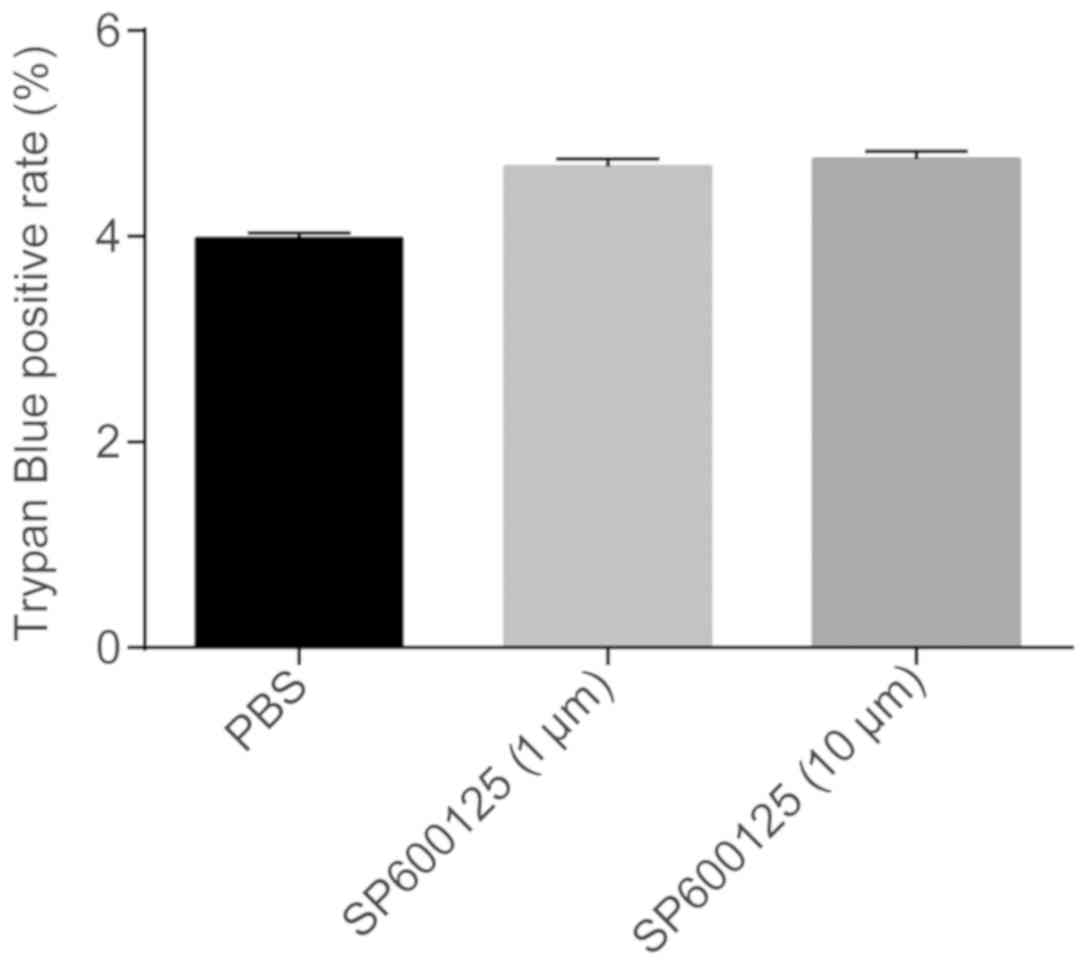
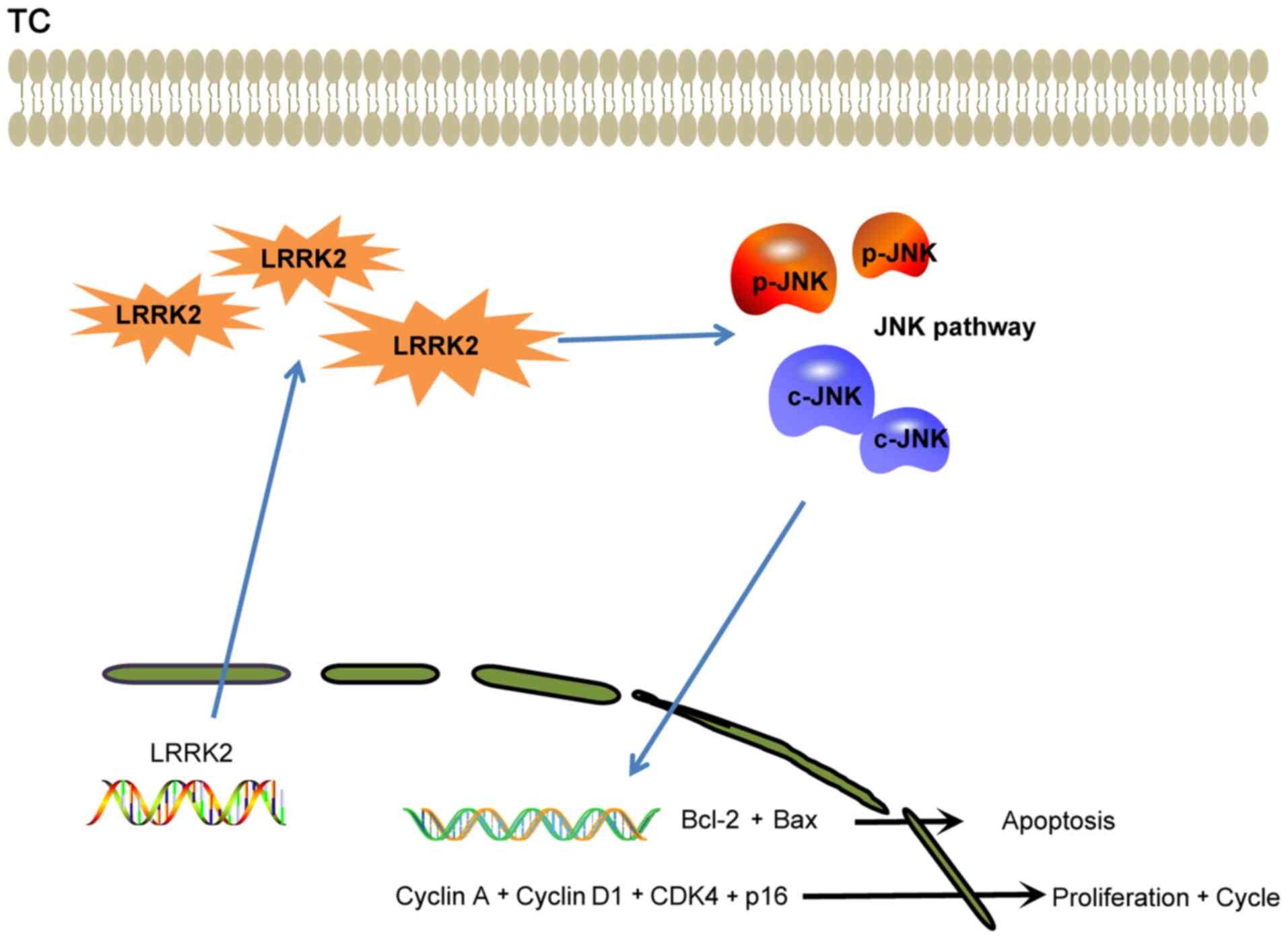
|
1
|
Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z,
Wang F, Duan Z, Xin S and Zhang J: Hashimoto's thyroiditis as a
risk factor of papillary thyroid cancer may improve cancer
prognosis. Otolaryngol Head Neck Surg. 148:396–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nikiforov YE and Nikiforova MN: Molecular
genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol.
7:569–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikiforov YE: Is ionizing radiation
responsible for the increasing incidence of thyroid cancer? Cancer.
116:1626–1628. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leux C, Truong T, Petit C,
Baron-Dubourdieu D and Guénel P: Family history of malignant and
benign thyroid diseases and risk of thyroid cancer: A
population-based case-control study in New Caledonia. Cancer Causes
Control. 23:745–755. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brindel P, Doyon F, Bourgain C, Rachédi F,
Boissin JL, Sebbag J, Shan L, Bost-Bezeaud F, Petitdidier P,
Paoaafaite J, et al: Family history of thyroid cancer and the risk
of differentiated thyroid cancer in French polynesia. Thyroid.
20:393–400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nexø MA, Watt T, Cleal B, Hegedüs L,
Bonnema SJ, Rasmussen AK, Feldt-Rasmussen U and Bjorner JB:
Exploring the experiences of people with hypo- and hyperthyroidism.
Qual Health Res. 25:945–953. 2015. View Article : Google Scholar
|
|
11
|
Rowe CW, Bendinelli C and McGrath S:
Charting a course through the CEAs: Diagnosis and management of
medullary thyroid cancer. Clin Endocrinol (Oxf). 85:340–343. 2016.
View Article : Google Scholar
|
|
12
|
Lubitz CC and Sosa JA: The changing
landscape of papillary thyroid cancer: Epidemiology, management,
and the implications for patients. Cancer. 122:3754–3759. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Humadi H, Zarros A, Al-Saigh R and
Liapi C: Genetic basis and gene therapy trials for thyroid cancer.
Cancer Genomics Proteomics. 7:31–49. 2010.PubMed/NCBI
|
|
14
|
Nikonova EV, Xiong Y, Tanis KQ, Dawson VL,
Vogel RL, Finney EM, Stone DJ, Reynolds IJ, Kern JT and Dawson TM:
Transcriptional responses to loss or gain of function of the
leucine-rich repeat kinase 2 (LRRK2) gene uncover biological
processes modulated by LRRK2 activity. Hum Mol Genet. 21. pp.
163–174. 2012, View Article : Google Scholar
|
|
15
|
Kubo M, Kamiya Y, Nagashima R, Maekawa T,
Eshima K, Azuma S, Ohta E and Obata F: LRRK2 is expressed in B-2
but not in B-1 B cells, and downregulated by cellular activation. J
Neuroimmunol. 229:123–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maekawa T, Mori S, Sasaki Y, Miyajima T,
Azuma S, Ohta E and Obata F: The I2020T Leucine-rich repeat kinase
2 transgenic mouse exhibits impaired locomotive ability accompanied
by dopaminergic neuron abnormalities. Mol Neurodegener. 7:152012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piccoli G, Condliffe SB, Bauer M, Giesert
F, Boldt K, De Astis S, Meixner A, Sarioglu H, Vogt-Weisenhorn DM,
Wurst W, et al: LRRK2 controls synaptic vesicle storage and
mobilization within the recycling pool. J Neurosci. 31:2225–2237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zechel S, Meinhardt A, Unsicker K and von
Bohlen Und Halbach O: Expression of leucine-rich-repeat-kinase 2
(LRRK2) during embryonic development. Int J Dev Neurosci.
28:391–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Looyenga BD, Furge KA, Dykema KJ, Koeman
J, Swiatek PJ, Giordano TJ, West AB, Resau JH, Teh BT and MacKeigan
JP: Chromosomal amplification of leucine-rich repeat kinase-2
(LRRK2) is required for oncogenic MET signaling in papillary renal
and thyroid carcinomas. Proc Natl Acad Sci USA. 108:1439–1444.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang LL, Huang H, Zhang CR, Xia J, Liu SS
and Wang XW: Cloning and functional characterization of c-Jun
NH2-terminal kinase from the Mediterranean species of the Whitefly
Bemisia tabaci complex. Int J Mol Sci. 14:13433–13446. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu B, Yang H, Sun M, Chen H, Jiang L,
Zheng X, Ding G, Liu Y, Sheng Y, Cui D, et al:
2,3′,4,4′,5-pentachlorobiphenyl induces inflammatory responses in
the thyroid through JNK and Aryl hydrocarbon receptor-mediated
pathway. Toxicol Sci. 149:300–311. 2016. View Article : Google Scholar
|
|
22
|
Shang J, Ding Q, Yuan S, Liu JX, Li F and
Zhang H: Network analyses of integrated differentially expressed
genes in papillary thyroid carcinoma to identify characteristic
genes. Genes (Basel). 10. pp. E452019, View Article : Google Scholar
|
|
23
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45(D1): D362–D368. 2017. View Article : Google Scholar
|
|
24
|
Piñero J, Bravo À, Queralt-Rosinach N,
Gutiérrez-Sacristán A, Deu-Pons J, Centeno E, García-García J, Sanz
F and Furlong LI; DisGeNET: A comprehensive platform integrating
information on human disease-associated genes and variants. Nucleic
Acids Res. 45(D1): D833–D839. 2017. View Article : Google Scholar
|
|
25
|
Piñero J, Queralt-Rosinach N, Bravo À,
Deu-Pons J, Bauer-Mehren A, Baron M, Sanz F and Furlong LI;
DisGeNET: A discovery platform for the dynamical exploration of
human diseases and their genes. Database (Oxford). 2015. pp.
bav0282015, View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-∆ ∆ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
27
|
Santos LS, Branco SC, Silva SN, Azevedo
AP, Gil OM, Manita I, Ferreira TC, Limbert E, Rueff J and Gaspar
JF: Polymorphisms in base excision repair genes and thyroid cancer
risk. Oncol Rep. 28:1859–1868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stjepanovic N and Capdevila J: Multikinase
inhibitors in the treatment of thyroid cancer: Specific role of
lenvatinib. Biologics. 8:129–139. 2014.PubMed/NCBI
|
|
29
|
Maekawa T, Kubo M, Yokoyama I, Ohta E and
Obata F: Age-dependent and cell-population-restricted LRRK2
expression in normal mouse spleen. Biochem Biophys Res Commun.
392:431–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sabapathy K: Role of the JNK pathway in
human diseases. Prog Mol Biol Transl Sci. 106:145–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dou J, Li X, Cai Y, Chen H, Zhu S, Wang Q,
Zou X, Mei Y, Yang Q, Li W, et al: Human cytomegalovirus induces
caspase-dependent apoptosis of megakaryocytic CHRF-288-11 cells by
activating the JNK pathway. Int J Hematol. 91:620–629. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Chao L, Li X, Ma G, Chen L, Zang Y
and Zhou G: Elevated expression of phosphorylated c-Jun
NH2-terminal kinase in basal-like and 'triple-negative' breast
cancers. Hum Pathol. 41:401–406. 2010. View Article : Google Scholar
|
|
33
|
Trancikova A, Mamais A, Webber PJ, Stafa
K, Tsika E, Glauser L, West AB, Bandopadhyay R and Moore DJ:
Phosphorylation of 4E-BP1 in the mammalian brain is not altered by
LRRK2 expression or pathogenic mutations. PLoS One. 7:e477842012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kwon SH, Kim JA, Hong SI, Jung YH, Kim HC,
Lee SY and Jang CG: Loganin protects against hydrogen
peroxide-induced apoptosis by inhibiting phosphorylation of JNK,
p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 58:533–541.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim MK, Choi HS, Cho SG, Shin YC and Ko
SG: Rubus coreanus Miquel extract causes apoptosis of
doxorubicin-resistant NCI/ADR-RES ovarian cancer cells via JNK
phosphorylation. Mol Med Rep. 13:4065–4072. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Tian Z, Yang Q, Li H, Guan H, Shi
B, Hou P and Ji M: Sulforaphane inhibits thyroid cancer cell growth
and invasiveness through the reactive oxygen species-dependent
pathway. Oncotarget. 6:25917–25931. 2015.PubMed/NCBI
|
|
37
|
Guan H, Liang W, Liu J, Wei G, Li H, Xiu
L, Xiao H and Li Y: Transmembrane protease serine 4 promotes
thyroid cancer proliferation via CREB phosphorylation. Thyroid.
25:85–94. 2015. View Article : Google Scholar :
|
|
38
|
Lu Y, Wei C and Xi Z: Curcumin suppresses
proliferation and invasion in non-small cell lung cancer by
modulation of MTA1-mediated Wnt/β-catenin pathway. In Vitro Cell
Dev Biol Anim. 50:840–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sui C, Zhuang C, Sun D, Yang L, Zhang L
and Song L: Notch1 regulates the JNK signaling pathway and
increases apoptosis in hepatocellular carcinoma. Oncotarget.
8:45837–45847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Husdal A, Bukholm G and Bukholm IR: The
prognostic value and overexpression of cyclin A is correlated with
gene amplification of both cyclin A and cyclin E in breast cancer
patient. Cell Oncol. 28:107–116. 2006.PubMed/NCBI
|
|
41
|
Romagosa C, Simonetti S, López-Vicente L,
Mazo A, Lleonart ME, Castellvi J and Ramon y Cajal S: p16(Ink4a)
over-expression in cancer: A tumor suppressor gene associated with
senescence and high-grade tumors. Oncogene. 30:2087–2097. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Shen L, Xu H, Pang Y, Xu Y, Ling M,
Zhou J, Wang X and Liu Q: Up-regulation of cyclin D1 by JNK1/c-Jun
is involved in tumorigenesis of human embryo lung fibroblast cells
induced by a low concentration of arsenite. Toxicol Lett.
206:113–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Poomsawat S, Buajeeb W, Khovidhunkit SO
and Punyasingh J: Alteration in the expression of cdk4 and cdk6
proteins in oral cancer and premalignant lesions. J Oral Pathol
Med. 39:793–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schapansky J, Nardozzi JD and LaVoie MJ:
The complex relationships between microglia, alpha-synuclein, and
LRRK2 in Parkinson's disease. Neuroscience. 302:74–88. 2015.
View Article : Google Scholar
|
|
45
|
Berwick DC and Harvey K: LRRK2 signaling
pathways: The key to unlocking neurodegeneration? Trends Cell Biol.
21:257–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen CY, Weng YH, Chien KY, Lin KJ, Yeh
TH, Cheng YP, Lu CS and Wang HL: (G2019S) LRRK2 activates MKK4-JNK
pathway and causes degeneration of SN dopaminergic neurons in a
transgenic mouse model of PD. Cell Death Differ. 19:1623–1633.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luerman GC, Nguyen C, Samaroo H, Loos P,
Xi H, Hurtado-Lorenzo A, Needle E, Stephen Noell G, Galatsis P,
Dunlop J, et al: Phosphoproteomic evaluation of pharmacological
inhibition of leucine-rich repeat kinase 2 reveals significant
off-target effects of LRRK-2-IN-1. J Neurochem. 128:561–576. 2014.
View Article : Google Scholar
|
|
48
|
Moehle MS, Webber PJ, Tse T, Sukar N,
Standaert DG, DeSilva TM, Cowell RM and West AB: LRRK2 inhibition
attenuates microglial inflammatory responses. J Neurosci.
32:1602–1611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Prabhudesai S, Bensabeur FZ, Abdullah R,
Basak I, Baez S, Alves G, Holtzman NG, Larsen JP and Møller SG:
LRRK2 knockdown in zebrafish causes developmental defects, neuronal
loss, and synuclein aggregation. J Neurosci Res. 94:717–735. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu P, Xia X, Yang Z, Tian Y, Di J and Guo
M: Silencing of TCTN1 inhibits proliferation, induces cell cycle
arrest and apoptosis in human thyroid cancer. Exp Ther Med.
14:3720–3726. 2017. View Article : Google Scholar : PubMed/NCBI
|