Introduction
Thyroid cancer is among the most common types of
endocrine cancer worldwide, and its incidence is increasing
(1). It has been reported that
thyroid cancer has the most rapidly increasing rate of incidence of
all cancer types among women, and the second among men (2). Thyroid cancer can be characterized
into papillary (PTC), follicular (FTC), poorly differentiated
(PDTC) and anaplastic thyroid cancer (ATC) by histopathological
characteristics. PTC is the most common type of thyroid cancer and
accounts for >90% of all thyroid cancer cases (3). Although multiple studies have
investigated the molecular mechanisms of PTC, it remains to be
fully characterized. Recent studies suggest that, as an endocrine
cancer, the pathogenesis of PTC may be associated with cell
metabolism, and that targeting of cancer cell metabolism may be a
novel approach for the prevention or treatment of PTC (4).
Metformin, a first-line antidiabetic agent, is known
to activate AMP-activated protein kinase (AMPK) and has been
demonstrated to be a potential anticancer agent (5,6).
Epidemiologists have reported that diabetic patients who receive
metformin demonstrate a lower risk and incidence of multiple types
of cancer (7,8). Furthermore, a number of
pharmacological studies have revealed the antiproliferative and
antimetastatic effects of metformin in multiple types of cancer,
including thyroid cancer (9-11).
Although the use of metformin decreases the incidence of cancer and
induces cell death in cancer cells, its mechanism of action has not
been fully investigated in thyroid cancer.
Endoplasmic reticulum (ER) stress-associated
apoptosis is a major pathway in the regulation of cell apoptosis.
The ER is an essential organelle for protein synthesis, folding and
trafficking. A number of cellular stress conditions, such as
hypoxia and metabolic stress, lead to the accumulation of nascent
or unfolded proteins in the ER lumen. ER stress-induced unfolded or
misfolded proteins regulate ER chaperone proteins to inhibit
protein aggregation and translation and induce degradation by the
proteasome; this process is termed the unfolded protein response
(UPR) (12). If ER stress is
persistent and unresolved, UPR triggers several signaling pathways
leading to apoptosis. Recent studies have suggested that ER stress
induces apoptosis via increasing the expression of DNA
damage-inducible transcript 3 (DDIT3, also known as CHOP), JNK and
caspase-12 (13). Granato et
al (14) revealed that
metformin enhances the cytotoxic effect of bortezomib against PEL
cells by altering UPR activation and upregulating the expression of
CHOP.
The present study investigated the effect of
metformin on the proliferation and apoptosis of thyroid cancer
TPC-1 cells, and explored the underlying molecular mechanism in
vitro and in vivo. The results demonstrated that
metformin inhibited cell proliferation and induced apoptosis via an
ER stress-associated mechanism. The present findings suggested that
metformin treatment may have therapeutic potential for patients
with papillary thyroid cancer.
Materials and methods
Cell culture
The human thyroid cancer cell line, TPC-1, was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences. The cells were cultured in RPMI-1640 (HyClone; GE
Healthcare Life Sciences), supplemented with 10% fetal bovine serum
(Gemini Bio Products) and antibiotics (100 U/ml penicillin and 100
µg/ml streptomycin), at 37°C in a humidified
atmosphere with 5% CO2.
Cell viability assay
Metformin, thapsigargin and 4-phen-ylbutyrate
(4-PBA) were purchased from Sigma-Aldrich (Merck KGaA) and
dissolved in dimethyl sulfoxide (DMSO). Cell viability was detected
by Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc.) assay. TPC-1 cells were seeded into 96-well plates at a
density of 5×103 cells/well. The medium was removed and
replaced with medium containing metformin at 1.25, 2.5, 5, 10 or 20
mmol/l for 24 or 48 h. Cells treated with 20 mmol/l metformin for
24 h were pretreated with 1 µmol/l thapsigargin and/or 1
mmol/l 4-phenylbutyrate for 1 h. Then, the cells were incubated
with 10 µl CCK-8 solution for 1 h. The OD values were
measured by absorbance using a microplate reader (Thermo Fisher
Scientific, Inc.) at a wavelength of 450 nm, and cell viability was
quantified as a percentage of the control using the OD values.
Apoptosis analysis
For cell apoptosis analysis, TPC-1 cells were
analyzed using an Annexin V-FITC Apoptosis Detection kit (BD
Biosciences), according to the manufacturer's instructions.
Briefly, cells were collected after treatment with various
concentrations of metformin for 24 h. The cell suspension was
transferred to a 5 ml tube prior to the addition of 5 µl
Annexin V and 5 µl propidium iodide (PI). After incubation
in the dark at room temperature for 15 min, 400 µl binding
buffer was added. Apoptosis was detected with a Epics XL-MCL ADC
flow cytometer (Beckman Coulter, Inc.), according to the
manufacturer's instructions. Data were analyzed using the EXP032ADC
operation system.
Western blot analysis
TPC-1 cells were harvested and total protein was
extracted on ice using RIPA lysis buffer (Beyotime Institute of
Biotechnology) with a protease inhibitor cocktail (104 mM AEBSF, 80
µM aprotinin, 5 mM bestatin, 1.5 mM E-64, 2 mM leupeptin,
and 1.5 mM pepstatin A; MedChemExpress LLC) for 20 min. The protein
concentration was determined using a bicinchoninic acid kit
(Beyotime Institute of Biotechnology). Equal amounts of protein ~30
µg were separated by 12% SDS-PAGE and transferred into PVDF
membranes (Roche Diagnostics). The membranes were immunoblotted at
4°C overnight, with the following primary antibodies: heat
shock protein family A member 5 (HSPA5, also known as Bip; 1:800
dilution; cat. no. 11587-1-AP), CHOP (1:300 dilution; cat. no.
15204-1-AP), caspase-12 (1:500 dilution; cat. no. 55238-1-AP) and
β-actin (1:5,000 dilution; cat. no. 60008-1-Ig), all from
ProteinTech Group, Inc. This was followed by six 5-min washes with
PBS. Then the membranes were incubated with a horseradish
peroxidase-conjugated goat anti-rabbit IgG antibody (1:5,000
dilution; cat. no. JH-0011; DingGuo BioTech Co., Ltd.) for 1 h at
room temperature, and washed six more times with PBS. The proteins
were visualized using an enhanced chemiluminescence western
blotting detection kit (Thermo Fisher Scientific, Inc.), according
to the manufacturer's instruction. β-actin served as internal
control.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from TPC-1 cells using TRIzol
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. cDNA was synthesized using a Reverse script RT
reagent kit (Takara Biotechnology Co., Ltd.). SYBR-Green (Takara
Biotechnology Co., Ltd.) was used for the qPCR to quantify the
expression of Bip, caspase-12 and CHOP on the real-time PCR
detection system Mx-3005P (Agilent Technologies, Inc.), according
to the manufacturer's instructions. β-actin was used as a
housekeeping control gene. The primer sequences used were as
follows: Bip, forward, 5′-GAACGTCTG ATTGGCGATGC-3′
and reverse, 5′-ACCACCTTGAACGG CAAGAA-3′; caspase-12,
forward, 5′-GCTCAGGAAATGGA AACAGC-3′ and reverse,
5′-AGTGCTTGGTCCCACAGATT-3′; CHOP, forward,
5′-TGGAAGCCTGGTATGAGGAC-3′ and reverse,
5′-AAGCAGGGTCAAGAGTGGTG-3′; and β-actin, forward,
5′-CCTGGCACCCAGCACAAT-3′ and reverse,
5′-GGGCCGGACTCGTCATAC-3′. The thermocycling
conditions were: 95°C for 60 sec, and 40 cycles of
95°C for 15 sec, 60°C for 15 sec, and 72°C for
45 sec. Relative fold changes in mRNA expression were calculated
using the formula 2−ΔΔCq (15).
Tumor xenograft mouse models
Ten male immunodeficient BALB/c nude mice (age, 4-6
weeks; weight, 16±2 g) were purchased from Vital River Laboratories
Co., Ltd. The animals were housed under pathogen-free conditions at
the Institute of Medicine Zhengzhou University at 25±2°C and
70±5% humidity, under a 12-h light/dark cycle and access to food
and water ad libitum. TPC-1 cells in the log-phase were
suspended in serum-free culture medium with 50% Matrigel (BD
Biosciences) at a density of 1×107 TPC-1 cells in 200
µl. Tumor xenografts were established by subcutaneous
inoculation into the right flank of nude mice. The mice were
randomly divided into two groups of 5 mice: the control group,
administered with PBS containing 10% DMSO; and the metformin group,
administered with 350 mg/kg intragastric metformin infusion, daily
(16-18). The volume of the tumors was
measured using calipers every week. Tumor volume was calculated
according to the following formula: Tumor volume (mm3) =
(length × width2)/2. The maximum tumor diameter in the
present study was 15.67 mm, which was acceptable under the ethical
guidelines. After 6 weeks, the animals were sacrificed by cervical
dislocation following anesthesia by 1% pentobarbital (50 mg/kg)
injection (19), and the tumor
tissues were removed and measured. Xenograft tumors were harvested,
fixed in 10% formalin for 24 h at room temperature, embedded in
paraffin, and cut into 4 µm sections for immunohistochemical
analysis.
Immunohistochemistry
The slides were immersed in heated antigen retrieval
solution (10 mmol/l citrate buffer, pH 6.0), and subsequently
treated with 3% hydrogen peroxide for 10 min. After washing with
PBS, the slides were incubated with primary antibody (Bip, cat. no.
11587-1-AP, 1:50 dilution; CHOP, cat. no. 15204-1-AP, 1:50
dilution; both from ProteinTech Group, Inc.) at 4°C
overnight, and then with secondary antibody (cat. no. PV9000; 1:500
dilution; OriGene Technologies, Inc.) for 20 min at room
temperature. The reaction was developed using a
3,3'-diaminobenzidine kit (1:50 dilution in buffer; OriGene
Technologies, Inc.). Finally, the slides were counterstained in
hematoxylin prior to dehydration and mounting. Staining results
were observed under a light microscope (CX31; Olympus Corporation;
original magnification, ×200). Sections were scored
semi-quantitatively for the extent of immunoreaction as follows: 0,
0% immunoreactive cells; 1, <5% cells; 2, 5-50% immunoreactive
cells; and 3, >50% immunoreactive cells. In addition, the
intensity of staining was scored semi-quantitatively as: 0,
negative; 1, weak; 2, intermediate; and 3, strong. The final
immunoreaction score was calculated as the sum of both parameters
(extent and intensity) (20).
Statistical analysis
All data are presented as the mean ± standard
deviation from at least three independent experiments. Differences
between groups were assessed using Student's t-test followed by
Shapiro-Wilk W test, or one-way ANOVA followed by Bonferroni test.
P<0.05 was considered to indicate a statistically significant
difference, and analyses were performed using SPSS 17.0 software
(SPSS Inc.).
Results
Metformin inhibits cell viability in
TPC-1 cells
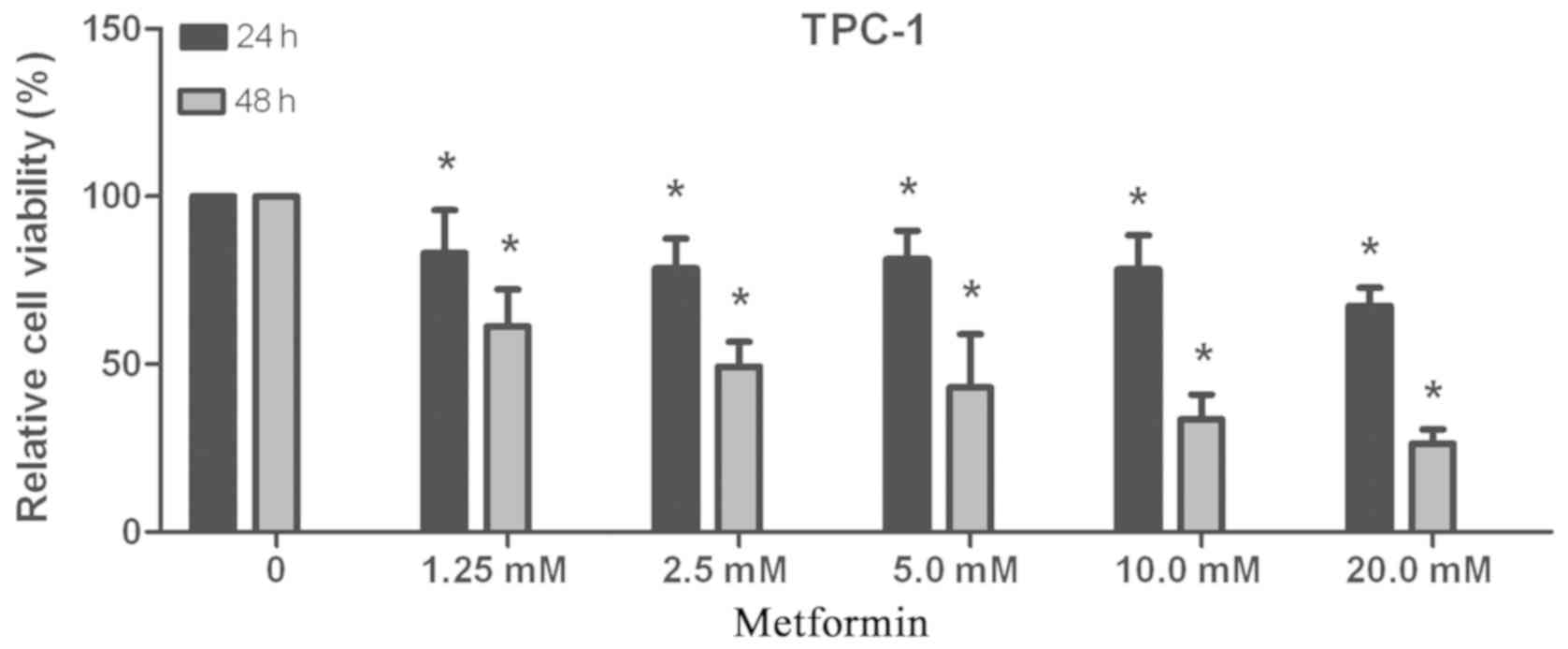
TPC-1 cells were seeded into 96-well plates.
Following treatment with metformin at different concentrations
(1.25, 2.5, 5, 10 or 20 mmol/l) for 24 or 48 h, cell viability was
detected by CCK-8 assay. As presented in Fig. 1, the inhibitory effect of metformin
on proliferation increased with the increase in concentration and
duration of treatment. The IC50 values of metformin for
24 and 48 h were 324.865 and 2.684 mmol/l, respectively. These data
indicated that metformin inhibited cell viability in a
concentration- and time-dependent manner.
Metformin induces cell apoptosis in TPC-1
cells
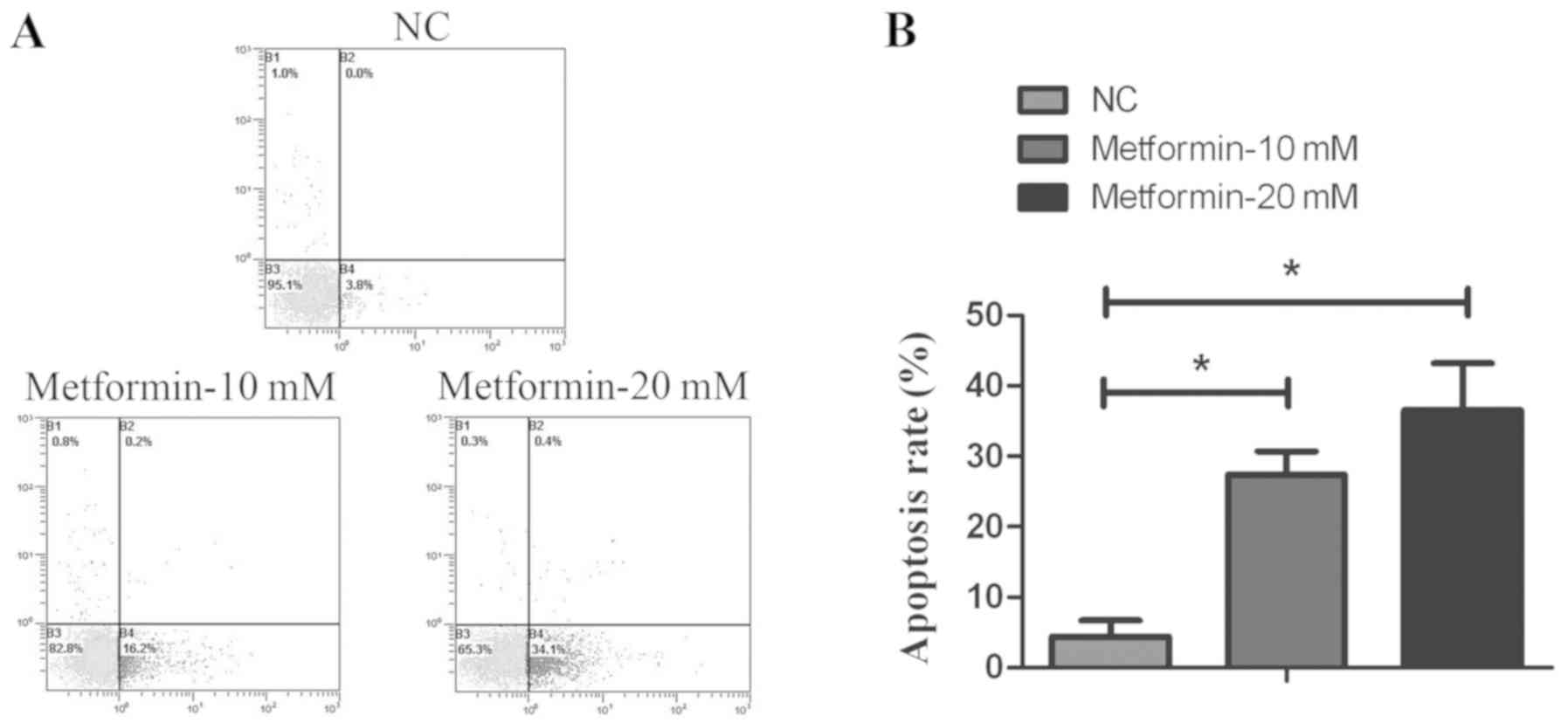
To investigate whether the inhibition of cell
proliferation was due to an increased rate of apoptosis, the
proportion of apoptotic cells was detected by flow cytometry using
Annexin V-FITC/PI staining, following treatment with different
concentrations of metformin. As presented in Fig. 2, the apoptosis rate following
metformin treatment was significantly increased compared with the
control group. These findings indicated that metformin induced
apoptosis of thyroid cancer TPC-1 cells.
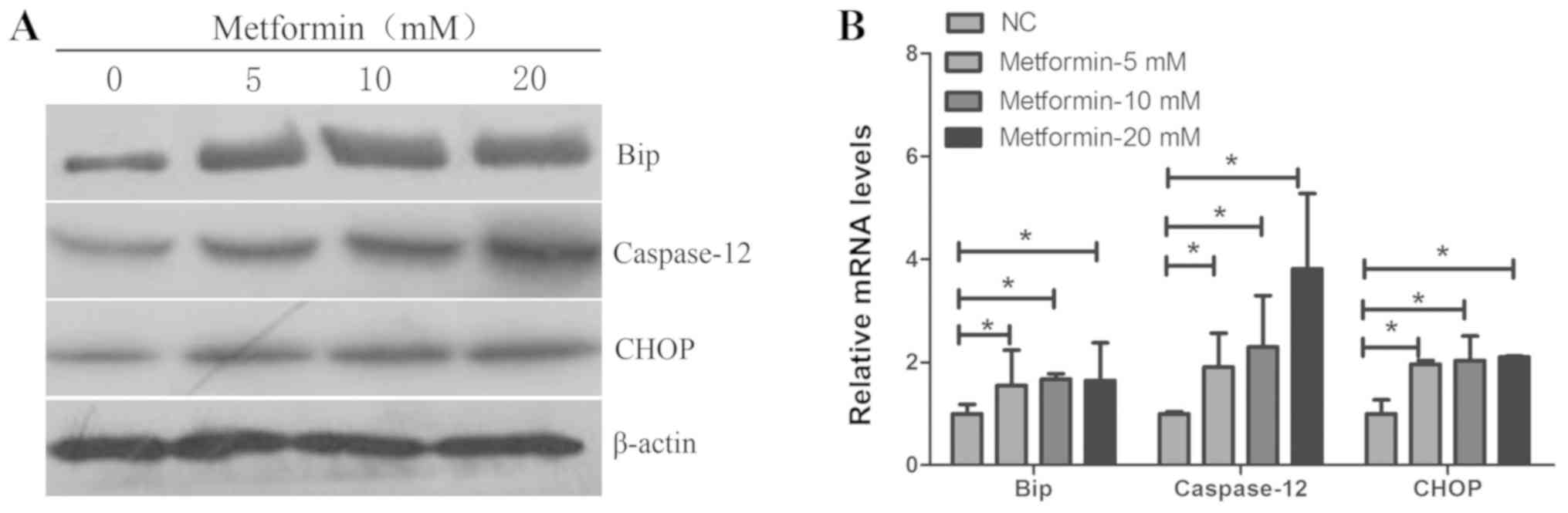
Metformin induces ER stress and ER
stress-associated apoptosis in TPC-1 cells
The ER stress-associated apoptotic pathway serves an
important role in apoptosis induced by anticancer agents (22). Following treatment with metformin
at different concentrations for 24 h, the mRNA and protein
expression levels of the ER molecular chaperone Bip, and the
ER-associated apoptosis genes CHOP and caspase-12, were detected in
TPC-1 cells by RT-qPCR and western blotting. As presented in
Fig. 3, the mRNA and protein
expression levels of Bip, CHOP and caspase-12 were significantly
increased with increased concentrations of metformin for 24 h.
These data suggested that metformin may induce ER stress-mediated
apoptosis.
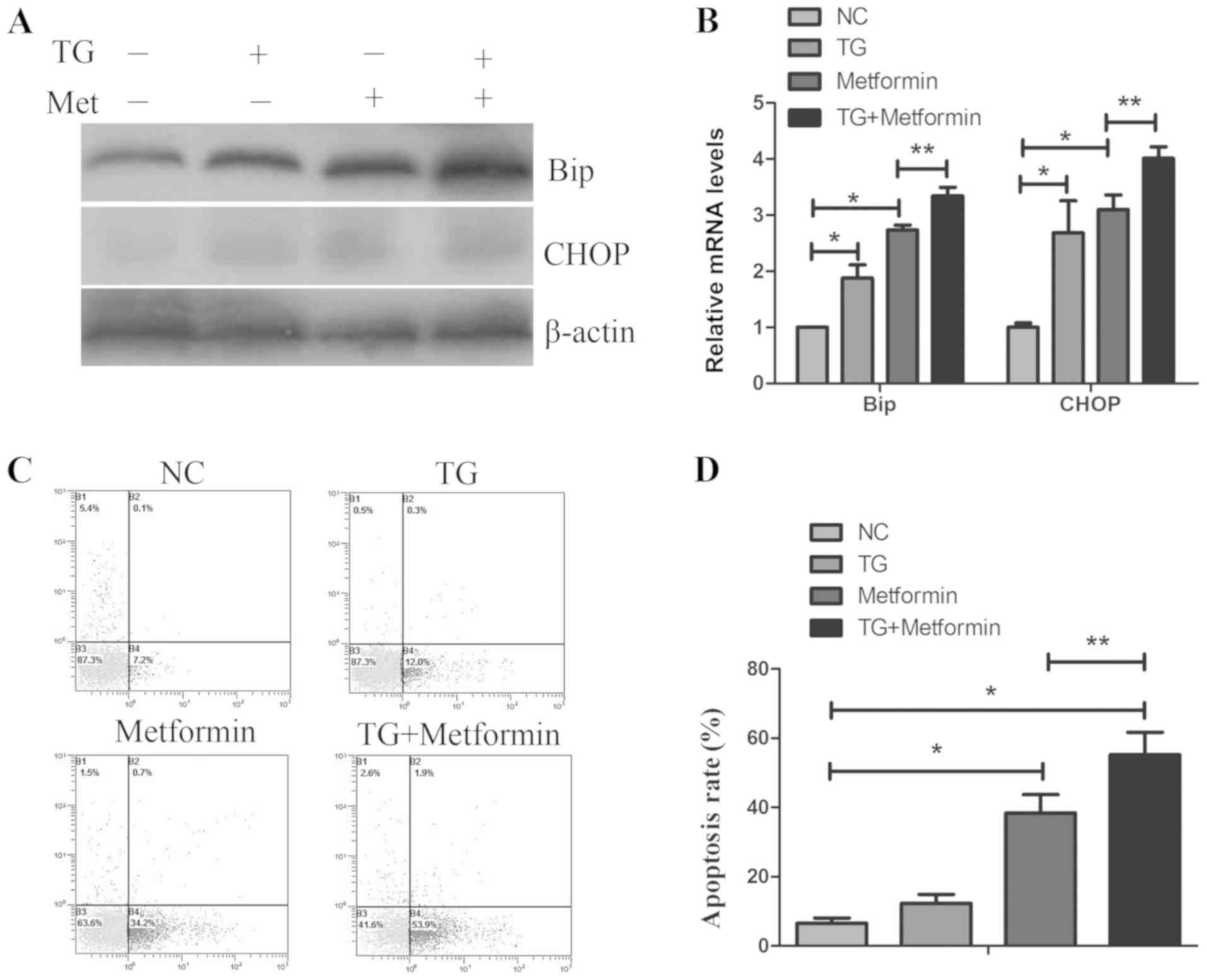
Activation of ER stress by thapsigargin
enhances metformin- mediated apoptosis in TPC-1 cells
To detect whether ER stress serves an important role
in apoptosis mediated by metformin, TPC-1 cells were pretreated
with the ER stress activator thapsigargin (1µmol/l) for 1 h,
followed by treatment with metformin for 24 h. As presented in
Fig. 4C and D, treatment with
thapsigargin enhanced the metformin-mediated apoptosis.
Furthermore, treatment with thapsigargin and metformin further
increased the expression levels of Bip and CHOP compared with
either treatment alone (Fig. 4A and
B). These data indicated that activation of ER stress enhanced
the anticancer effect of metformin in TPC-1 cells.
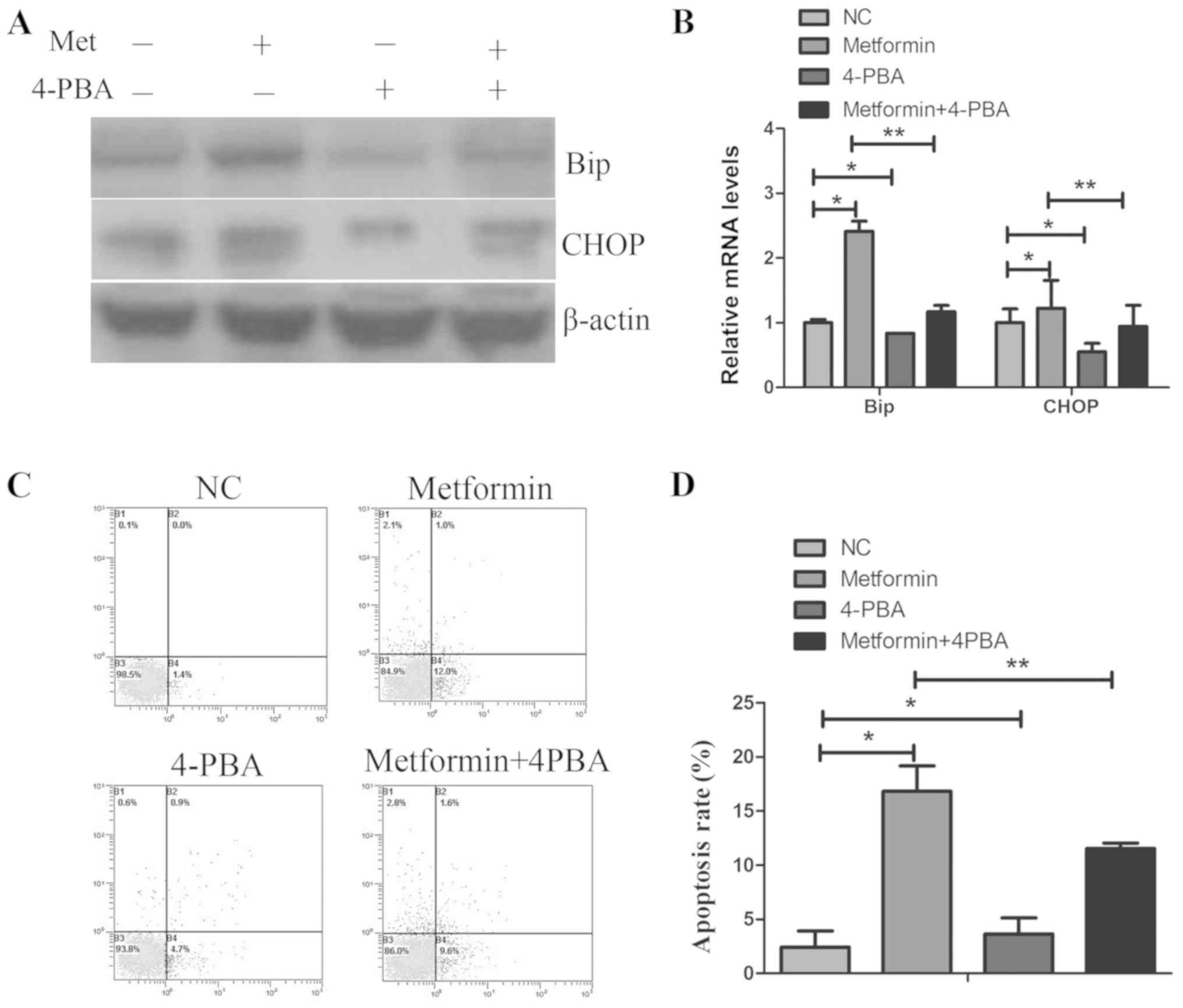
Inhibition of ER stress by 4-PBA reverses
metformin-induced apoptosis in TPC-1 cells
To further confirm whether ER stress has an
important role in apoptosis induced by metformin, TPC-1 cells were
pretreated with the ER stress inhibitor 4-PBA (1 mmol/l) for 1 h,
followed by treatment with metformin for 24 h. As presented in
Fig. 5C and D, treatment with
4-PBA decreased the metformin-mediated growth apoptosis.
Furthermore, 4-PBA and metformin co-treatment decreased the Bip and
CHOP mRNA and protein expression levels, compared with metformin
treatment alone. These results further confirmed that the ER stress
process was required for metformin-induced apoptosis in TPC-1
cells.
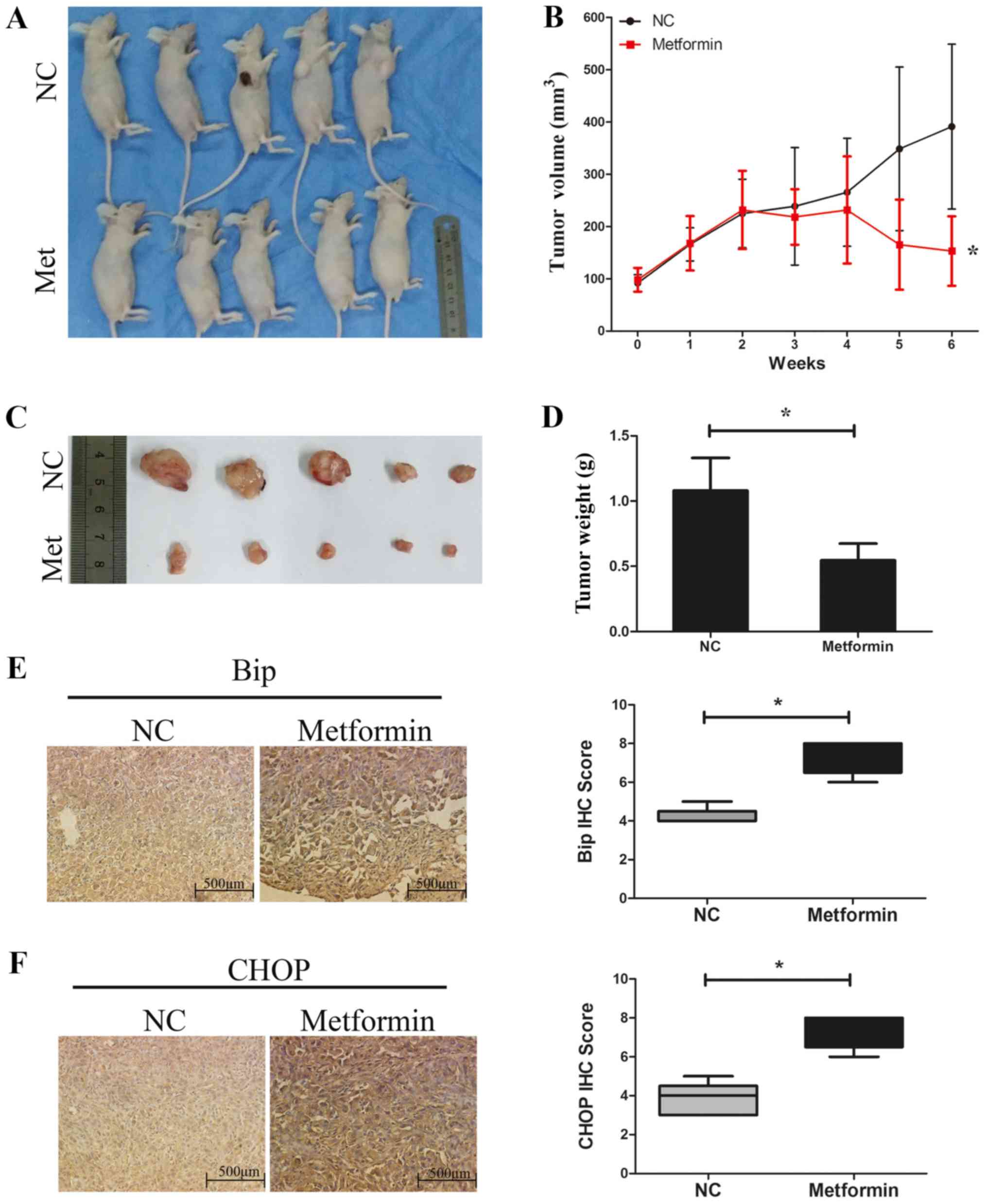
Metformin inhibits TPC-1 tumor cell
growth and increases the expression of Bip and CHOP in vivo
Next, the effects of metformin on TPC-1 cells were
investigated in vivo using a xenograft mouse model (Fig. 6A). The results demonstrated that
the tumor volume (Fig. 6B and C)
and the tumor weight (Fig. 6D) in
the control group at the end of the treatments were 391.0±157.6
mm3 and 1.08±0.25 g, respectively, whereas those in the
metformin group were 153.1±66.5 mm3 and 0.55±0.13 g,
respectively. These data indicated that treatment with metformin
significantly decreased the tumor growth rate and tumor weight
compared with the control group (Fig.
6B-D). The protein expression levels of Bip and CHOP in the
tumor xenograft tissues were detected by immunohistochemistry. As
presented in Fig. 6E and F, the
expression levels of Bip and CHOP were significantly increased in
the tumor tissues from the metformin group compared with the
control group, which is consistent with the aforementioned in
vitro results of the present study.
Discussion
Metformin, commonly used for the treatment of
diabetes mellitus through increasing insulin sensitivity and
improving glycemic control, has been demonstrated to inhibit cancer
cell proliferation and induce cancer cell death (23). Previous studies have reported that
metformin inhibits the carcinogenesis of endocrine tumors through
activating AMPK, leading to the downregulation of mTOR activation,
which serves an important role in cell metabolism (24-26).
Evidence also suggests that metformin reduces chemoresistance in
ovarian cancer (27), bladder
cancer (28), breast cancer
(6) and esophageal squamous
carcinoma (29). The present
results suggested that cellular proliferation was suppressed
following treatment with metformin in TPC-1 cells. Flow cytometry
analysis revealed that metformin significantly increased the rate
of apoptosis in TPC-1 cells, in a concentration and time-dependent
manner. Mechanistically, metformin may induce TPC-1-cell apoptosis
through its regulation of ER-stress associated pathways.
In the present study, the metformin dosing range
used in vitro was 1.25-20 mmol/l, while the serum levels of
metformin in patients with type 2 diabetes (average 1,500 mg oral
daily dose) have been reported at 2-6 µmol/l, with peak
levels of 38 µmol/l and steady state ranges of 15.5
µmol/l (21). A recent
study has indeed noted that the dosing of metformin in vitro
is a contentious issue, with concerns that supra-physiological
doses result in off-target effects and are not reflective of in
vivo events (21). However, it
can be speculated that the purpose of using metformin for diabetes
treatment is the regulation of insulin sensitivity and improvement
of glycemic control, while the use of metformin for cancer
treatment would aim at inducing apoptosis. In the present study,
the anticancer effect of metformin was explored in thyroid cancer
cells, instead of a diabetic model. Our previous study also
indicated that metformin at 20 mM exhibits anticancer effects in
gallbladder cancer (18). Similar
in vitro concentrations have been used in other studies
(16,30,31).
Deregulation of apoptosis can lead to the
development or progression of tumorigenesis. To investigate the
mechanism of metformin-induced cell death, the ER stress
pathway-mediated apoptosis was examined. Increasing evidence
suggests that ER stress-associated apoptosis is involved in the
apoptosis induced by anticancer agents (32). The ER triggers adaptive protective
processes with the accumulation of unfolded or misfolded proteins
(33). This homeostatic mechanism
is mediated by UPR via three ER transmembrane receptors: Activating
transcription factor 6 (ATF6), protein kinase dsRNA-like ER kinase
(PERK) and inositol-requiring enzyme 1α (IRE1α) (34). Increasing evidence suggests that ER
stress serves a protective role against tumor invasion, metastasis
and chemoresistance (35,36). However, when ER stress is severe or
prolonged, the ATF6, PERK and IRE1α signaling pathways are
activated, resulting in increased expression of CHOP, caspase-12
and JNK (37), and subsequently
leading to apoptosis.
Recent studies have suggested that ER stress serves
a role in chemoresistance in colorectal cancer (38). A previous study has reported that
ER stress induces apoptosis via the JNK/p38 pathway following
treatment with protodioscin (39).
However, the role of metformin in thyroid cancer remained unclear.
The present study demonstrated that the mRNA and protein expression
levels of Bip increased with metformin treatment in TPC-1 cells.
Bip is an abundant and key ER chaperone. It has been proposed that
under conditions of ER stress, the expression of Bip is increased
and the three ER stress sensors IRE1α, PERK and ATF6, are activated
to alter transcriptional and translational programs, indicating
that the expression and activation of Bip has a key role in ER
stress (40). The present study
also observed that the expression of the ER stress-associated
apoptosis genes CHOP and caspase-12 increased when TPC-1 cells were
treated with metformin. CHOP, a key transcription factor, is the
most well-characterized proapoptotic pathway in the ER (41). Investigating the relationship
between ER stress and apoptosis, it was demonstrated that
activation of ER stress by thapsigargin enhanced the sensitivity of
TPC-1 cells to metformin, while inhibition of ER stress by 4-PBA
reversed metformin-induced apoptosis in TPC-1 cells. Furthermore, a
xenograft mouse model was used to investigate the role of metformin
in vivo. Treatment with metformin significantly decreased
tumor growth compared with the control group. Furthermore, the
protein expression levels of Bip and CHOP were increased in tumor
tissues treated with metformin compared with the control group,
which was consistent with the in vitro results. These data
indicated that metformin may suppress cell proliferation via
induction of ER stress-associated apoptosis. Cho et al
(42) revealed that treatment with
100 mg/ml metformin decreases tumor growth by 47-60% compared with
the control group in a thyroid cancer BPH10-3SC xenograft mouse
model. Thakur et al (43)
reported that treatment with metformin leads to a significant
reduction in thyroid cancer metastatic growth in the FTC133 model,
but not in the BCPAP model. A recent study also reported that
metformin is a potential anticancer agent in multiple types of
cancer (44). Based on these
studies, metformin may possess anticancer effect in other thyroid
cancer cell models. In the present study, treatment with metformin
in the TPC-1 xenograft model decreased tumor growth by ~60%
compared with the control group. However, there are several
limitations to the present results: the use of only one thyroid
cancer cell line; the lack of a positive control (for example
sorafenib, a known anticancer drug used to treat thyroid cancer);
and the lack of other mechanistic controls to compare the effects
of metformin treatment. Therefore, further studies are required in
order to fully investigate the anticancer effect of metformin in
ER-induced apoptosis in thyroid cancer.
Recently, Yang et al (45) reported that metformin induced ER
stress-dependent apoptosis, suggesting a promising therapeutic
strategy for prostate cancer. In the present study, the results
demonstrated that metformin could also inhibit proliferation and
induce apoptosis in thyroid cancer TPC-1 cells, by targeting the ER
stress-associated apoptotic pathway. These findings indicated that
metformin may have therapeutic application in the treatment of
thyroid cancer. Multiple studies have recently focused on the
relationship between apoptosis and autophagy. Autophagy and
apoptosis are both important biological processes and their
relationship is complex. Wang et al (46) revealed that autophagy activated by
metformin reversed hyperglycemia-induced cardiomyocyte apoptosis in
H9c2 cells. Similar results were observed in the Xiao et al
and Li et al studies (47,48),
indicating that autophagy may have a protective role in
metformin-induced apoptosis. The role and mechanism of autophagy in
metformin-induced apoptosis in thyroid cancer remains unclear, and
it will be investigated further in our future study.
Funding
This research was supported by the National Natural
Science Foundation of China (grant no. 81702863), the High School
Key Science and Technology Project of Henan Province (grant no.
19B320039), the Outstanding Young Talent Research Fund of Zhengzhou
University (grant no. 1421412090) and the Medical Science and
Technology Project of Henan Province (grant no. SBGJ2018021).
Availability of data and materials
All data generated or analyzed during the study are
included in this published article.
Authors' contributions
JY, LQ, KC, SS and RL performed the experiments, WZ
and CZ designed the study, JY and LQ prepared and wrote the study.
All authors read and approved the final manuscript and agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal protocol was approved by the
Institutional Animal Care and Use Committee of the First Affiliated
Hospital of Zhengzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Xu Y, Zheng X, Qiu Y, Jia W, Wang J and
Yin S: Distinct Metabolomic Profiles of Papillary Thyroid Carcinoma
and Benign Thyroid Adenoma. J Proteome Res. 14:3315–3321. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Z, Liu Y, Ma M and Chang L: Knockdown
of TRIM44 inhibits the proliferation and invasion in papillary
thyroid cancer cells through suppressing the Wnt/β-catenin
signaling pathway. Biomed Pharmacother. 96:98–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han G, Gong H, Wang Y, Guo S and Liu K:
AMPK/mTOR-mediated inhibition of survivin partly contributes to
metformin-induced apoptosis in human gastric cancer cell. Cancer
Biol Ther. 16:77–87. 2015. View Article : Google Scholar :
|
|
5
|
Feng T, Li L, Ling S, Fan N, Fang M, Zhang
H, Fang X, Lan W, Hou Z, Meng Q, et al: Metformin enhances
radiation response of ECa109 cells through activation of ATM and
AMPK. Biomed Pharmacother. 69:260–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu C, Zhang W, Zheng G, Zhang Z, Yin J and
He Z: Metformin reverses multidrug resistance and
epithelial-mesenchymal transition (EMT) via activating
AMP-activated protein kinase (AMPK) in human breast cancer cells.
Mol Cell Biochem. 386:63–71. 2014. View Article : Google Scholar
|
|
7
|
Chung HH, Moon JS, Yoon JS, Lee HW and Won
KC: The relationship between metformin and cancer in patients with
type 2 diabetes. Diabetes Metab J. 37:125–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hall C, Stone RL, Gehlot A, Zorn KK and
Burnett AF: Use of metformin in obese women with type I endometrial
cancer is associated with a reduced incidence of cancer recurrence.
Int J Gynecol Cancer. 26:313–317. 2016. View Article : Google Scholar
|
|
9
|
Zhang X, Zhang X, Huang T, Geng J, Liu M
and Zheng J: Combination of metformin and valproic acid
synergistically induces cell cycle arrest and apoptosis in clear
cell renal cell carcinoma. Int J Clin Exp Pathol. 8:2823–2828.
2015.PubMed/NCBI
|
|
10
|
Chen X, Hu C, Zhang W, Shen Y, Wang J, Hu
F and Yu P: Metformin inhibits the proliferation, metastasis, and
cancer stem-like sphere formation in osteosarcoma MG63 cells in
vitro. Tumour Biol. 36:9873–9883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bikas A, Jensen K, Patel A, Costello J Jr,
McDaniel D, Klubo-Gwiezdzinska J, Larin O, Hoperia V, Burman KD,
Boyle L, et al: Glucose-deprivation increases thyroid cancer cells
sensitivity to metformin. Endocr Relat Cancer. 22:919–932. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung TW, Lee MW, Lee YJ and Kim SM:
Metformin prevents endoplasmic reticulum stress-induced apoptosis
through AMPK-PI3K-c-Jun NH2 pathway. Biochem Biophys Res Commun.
417:147–152. 2012. View Article : Google Scholar
|
|
13
|
Sun X, Liao W, Wang J, Wang P, Gao H, Wang
M, Xu C, Zhong Y and Ding Y: CSTMP induces apoptosis and
mitochondrial dysfunction in human myeloma RPMI8226 cells via
CHOP-dependent endoplasmic reticulum stress. Biomed Pharmacother.
83:776–784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Granato M, Gilardini Montani MS, Romeo MA,
Santarelli R, Gonnella R, D'Orazi G, Faggioni A and Cirone M:
Metformin triggers apoptosis in PEL cells and alters
bortezomib-induced Unfolded Protein Response increasing its
cytotoxicity and inhibiting KSHV lytic cycle activation. Cell
Signal. 40:239–247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Brodowska K, Theodoropoulou S, Meyer Zu
Hörste M, Paschalis EI, Takeuchi K, Scott G, Ramsey DJ, Kiernan E,
Hoang M, Cichy J, et al: Effects of metformin on retinoblastoma
growth in vitro and in vivo. Int J Oncol. 45:2311–2324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rico M, Baglioni M, Bondarenko M, Laluce
NC, Rozados V, André N, Carré M, Scharovsky OG and Menacho Márquez
M: Metformin and propranolol combination prevents cancer
progression and metastasis in different breast cancer models.
Oncotarget. 8:2874–2889. 2017. View Article : Google Scholar :
|
|
18
|
Ye J, Chen K, Qi L, Li R, Tang H, Zhou C
and Zhai W: Metformin suppresses hypoxia induced migration via the
HIF 1α/VEGF pathway in gallbladder cancer in vitro and in vivo.
Oncol Rep. 40:3501–3510. 2018.PubMed/NCBI
|
|
19
|
He W, He S, Wang Z, Shen H, Fang W, Zhang
Y, Qian W, Lin M, Yuan J, Wang J, et al: Astrocyte elevated
gene-1(AEG-1) induces epithelial-mesenchymal transition in lung
cancer through activating Wnt/β-catenin signaling. BMC Cancer.
15:1072015. View Article : Google Scholar
|
|
20
|
Suwei D, Liang Z, Zhimin L, Ruilei L,
Yingying Z, Zhen L, Chunlei G, Zhangchao L, Yuanbo X, Jinyan Y, et
al: NLK functions to maintain proliferation and stemness of NSCLC
and is a target of metformin. J Hematol Oncol. 8:1202015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies G, Lobanova L, Dawicki W, Groot G,
Gordon JR, Bowen M, Harkness T and Arnason T: Metformin inhibits
the development, and promotes the resensitization, of
treatment-resistant breast cancer. PLoS One. 12:e01871912017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Y, Li D, Zeng L, Wang C, Zhang L, Wang
Y, Yu Y, Liu S and Li Z: Proteasome inhibitor lactacystin enhances
cisplatin cyto-toxicity by increasing endoplasmic reticulum
stress-associated apoptosis in HeLa cells. Mol Med Rep. 11:189–195.
2015. View Article : Google Scholar
|
|
23
|
Wang J, Gao Q, Wang D, Wang Z and Hu C:
Metformin inhibits growth of lung adenocarcinoma cells by inducing
apoptosis via the mitochondria-mediated pathway. Oncol Lett.
10:1343–1349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reni M, Dugnani E, Cereda S, Belli C,
Balzano G, Nicoletti R, Liberati D, Pasquale V, Scavini M, Maggiora
P, et al: (Ir)relevance of metformin treatment in patients with
metastatic pancreatic cancer: An open-label, randomized phase II
trial. Clin Cancer Res. 22:1076–1085. 2016. View Article : Google Scholar
|
|
25
|
Coperchini F, Leporati P, Rotondi M and
Chiovato L: Expanding the therapeutic spectrum of metformin: From
diabetes to cancer. J Endocrinol Invest. 38:1047–1055. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai H, Zhang Y, Han TK, Everett RS and
Thakker DR: Cation-selective transporters are critical to the
AMPK-mediated antiproliferative effects of metformin in human
breast cancer cells. Int J Cancer. 138:2281–2292. 2016. View Article : Google Scholar
|
|
27
|
Mert I, Chhina J, Allo G, Dai J, Seward S,
Carey MS, Llaurado M, Giri S, Rattan R and Munkarah AR: Synergistic
effect of MEK inhibitor and metformin combination in low grade
serous ovarian cancer. Gynecol Oncol. 146:319–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su Q, Tao T, Tang L, Deng J, Darko KO,
Zhou S, Peng M, He S, Zeng Q, Chen AF, et al: Down-regulation of
PKM2 enhances anticancer efficiency of THP on bladder cancer. J
Cell Mol Med. 22:2774–2790. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mynhardt C, Damelin LH, Jivan R, Peres J,
Prince S, Veale RB and Mavri-Damelin D: Metformin-induced
alterations in nucleotide metabolism cause 5-fluorouracil
resistance but gemcitabine susceptibility in oesophageal squamous
cell carcinoma. J Cell Biochem. 119:1193–1203. 2018. View Article : Google Scholar
|
|
30
|
Tang JC, An R, Jiang YQ and Yang J:
Effects and mechanisms of metformin on the proliferation of
esophageal cancer cells in vitro and in vivo. Cancer Res Treat.
49:778–789. 2017. View Article : Google Scholar :
|
|
31
|
Zhang J, Li G, Chen Y, Fang L, Guan C, Bai
F, Ma M, Lyu J and Meng QH: Metformin inhibits tumorigenesis and
tumor growth of breast cancer cells by upregulating miR-200c but
downregu-lating AKT2 expression. J Cancer. 8:1849–1864. 2017.
View Article : Google Scholar :
|
|
32
|
Zhang J, Feng Z, Wang C, Zhou H, Liu W,
Kanchana K, Dai X, Zou P, Gu J, Cai L, et al: Curcumin derivative
WZ35 efficiently suppresses colon cancer progression through
inducing ROS production and ER stress-dependent apoptosis. Am J
Cancer Res. 7:275–288. 2017.PubMed/NCBI
|
|
33
|
Martins AS, Alves I, Helguero L, Domingues
MR and Neves BM: The Unfolded Protein Response in Homeostasis and
Modulation of Mammalian Immune Cells. Int Rev Immunol. 35:457–476.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hetz C, Chevet E and Harding HP: Targeting
the unfolded protein response in disease. Nat Rev Drug Discov.
12:703–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu SM, Lin WY, Shen CC, Pan HC, Keh-Bin W,
Chen YC, Jan YJ, Lai DW, Tang SC, Tien HR, et al: Melatonin set out
to ER stress signaling thwarts epithelial mesenchymal transition
and peritoneal dissemination via calpain-mediated C/EBPβ and NFκB
cleavage. J Pineal Res. 60:142–154. 2016. View Article : Google Scholar
|
|
36
|
Fan L, Song B, Sun G, Ma T, Zhong F and
Wei W: Endoplasmic reticulum stress-induced resistance to
doxorubicin is reversed by paeonol treatment in human
hepatocellular carcinoma cells. PLoS One. 8:e626272013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
KoraMagazi A, Wang D, Yousef B, Guerram M
and Yu F: Rhein triggers apoptosis via induction of endoplasmic
reticulum stress, caspase-4 and intracellular calcium in primary
human hepatic HL-7702 cells. Biochem Biophys Res Commun.
473:230–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen MC, Hsu HH, Chu YY, Cheng SF, Shen
CY, Lin YJ, Chen RJ, Viswanadha VP, Lin YM and Huang CY: Lupeol
alters ER stress-signaling pathway by downregulating ABCG2
expression to induce Oxaliplatin-resistant LoVo colorectal cancer
cell apoptosis. Environ Toxicol. 33:587–593. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin CL, Lee CH, Chen CM, Cheng CW, Chen
PN, Ying TH and Hsieh YH: Protodioscin induces apoptosis through
ROS-mediated endoplasmic reticulum stress via the JNK/p38
activation pathways in human cervical cancer cells. Cell Physiol
Biochem. 46:322–334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang G, Yang ZQ and Zhang K: Endoplasmic
reticulum stress response in cancer: Molecular mechanism and
therapeutic potential. Am J Transl Res. 2:65–74. 2010.PubMed/NCBI
|
|
41
|
Malhi H and Kaufman RJ: Endoplasmic
reticulum stress in liver disease. J Hepatol. 54:795–809. 2011.
View Article : Google Scholar
|
|
42
|
Cho SW, Yi KH, Han SK, Sun HJ, Kim YA, Oh
BC, Park YJ and Park DJ: Therapeutic potential of metformin in
papillary thyroid cancer in vitro and in vivo. Mol Cell Endocrinol.
393:24–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thakur S, Daley B, Gaskins K, Vasko VV,
Boufraqech M, Patel D, Sourbier C, Reece J, Cheng SY, Kebebew E, et
al: Metformin targets mitochondrial glycerophosphate dehydrogenase
to control rate of oxidative phosphorylation and growth of thyroid
cancer in vitro and in vivo. Clin Cancer Res. 24:4030–4043. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morales DR and Morris AD: Metformin in
cancer treatment and prevention. Annu Rev Med. 66:17–29. 2015.
View Article : Google Scholar
|
|
45
|
Yang J, Wei J, Wu Y, Wang Z, Guo Y, Lee P
and Li X: Metformin induces ER stress-dependent apoptosis through
miR-708-5p/NNAT pathway in prostate cancer. Oncogenesis.
4:e1582015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang GY, Bi YG, Liu XD, Zhao Y, Han JF,
Wei M and Zhang QY: Autophagy was involved in the protective effect
of metformin on hyperglycemia-induced cardiomyocyte apoptosis and
Connexin43 downregulation in H9c2 cells. Int J Med Sci. 14:698–704.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiao Z, Gaertner S, Morresi-Hauf A, Genzel
R, Duell T, Ullrich A and Knyazev PG: Metformin triggers autophagy
to attenuate drug-induced apoptosis in NSCLC cells, with minor
effects on tumors of diabetic patients. Neoplasia. 19:385–395.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li J, Gui Y, Ren J, Liu X, Feng Y, Zeng Z,
He W, Yang J and Dai C: Metformin protects against
cisplatin-induced tubular cell apoptosis and acute kidney injury
via AMPKα-regulated autophagy induction. Sci Rep. 6:239752016.
View Article : Google Scholar
|