Introduction
Global cancer statistics are discouraging to
patients: Cancers are among the top lethal diseases (1). Skin cancers are most commonly found
in individuals with fair skin (2);
in the USA, approximately 9,000 patients die from melanoma each
year (3); the long-term survival
rates for patients with advanced metastatic melanoma range from 10
to 15% (4). Although the early
diagnosis and surgical removal of melanoma are efficient in
approximately 80% of cases (5),
melanoma cells are very likely to spread to other organs and
establish metastasis, which is responsible for the highest rates of
mortality among patients with skin cancer (6).
Considering the high metastatic capacity of
melanomas, some anti-metastatic drugs have been tested. For
example, selumetinib (7) or the
combination of celecoxib and dacarbazine (8) have been shown to be effective in
reducing melanoma metastasis to the liver and lungs, respectively,
in mouse models. However, the majority of clinically-available
conventional chemotherapeutic drugs are currently of limited value
for the treatment of metastatic melanoma (9). Over the past years, the treatment of
melanoma has undergone revolutionary breakthroughs alongside the
evolution of targeted therapies (10,11).
However, despite the increased response achieved by the combination
of chemotherapeutic agents (e.g., dacarbazine and temozolomide), or
those added to cytokines [such as interferon and interleukin
(IL)-2], up until the last decade, no significant impact on the
survival of patients with metastatic melanoma had been achieved
(12).
Cancer cells are well known to possess high
proliferation rates, sustaining growth signaling and evading cell
growth control, in addition to circumventing cell death (13). This is the reason why the majority
of chemotherapeutic agents currently used are directly or
indirectly cytotoxic to cancer cells. Although apparently
contradictory, cytotoxicity is in fact not desirable, since normal
tissues also present a proliferation of the cell population for
maintaining tissue homeostasis. Therefore, cytotoxic drugs are
often not selective to the tumor. On the other hand, modern
targeted therapies have been changing the way of treating melanoma.
However, they are extremely dependent on the tumor genetic
signature, which is highly variable among patients (14). Additionally, the astronomic cost of
modern cancer treatments has been recently brought to the attention
of the scientific community, costs that place such treatments out
of reach for the majority of individuals, even with public health
funds (15). In the USA, a
3.5-fold increase in the annual treatment of patients with melanoma
by 2030 has been foreseen (3).
Such forecasts call for advancements in treatment strategies;
however, state-of-the-art treatments are produced on a small scale,
rendering them inaccessible to the majority of patients (11).
In light of the aforementioned challenges, new
strategies for the treatment of diseases are being developed
worldwide, such as those based on traditional and complementary
medicine, including high dilutions as homeopathy. Individuals
choosing such treatments are often motivated by increased benefits
and lower costs (16). The
scientific understanding on the field has grown considerably. A
number of recent studies have described the antitumor activity of
highly diluted medicines for different cancer types in vitro
(17-22). In addition, the integration of high
dilutions into public health care systems is increasing. In 2006,
Brazil recognized homeopathy as part of the National Policy of
Integrative and Complementary Practices and since then, the demand
for this type of therapy has been increasing annually in the
country (23).
Finding cost-effective treatments for selected
diseases has been a priority in our research group. Over the past
years, we have investigated, in vitro (24-31)
and in vivo (32-36), the effects of highly diluted
natural complexes (HDNCs). Lately, we have focused on two specific
HDNCs, namely M1 and M8. Anti-melanoma effects have been described
for both HDNCs in melanoma-bearing mice. Lung colonization was
shown to be attenuated in mice injected with B16-F10 melanoma
cells, followed by M8 (36) or M1
(35) treatments. Decreased
proliferation, combined with the induction of cell death were found
in the tumor microenvironment of these M1-treated mice. In
addition, tumor growth was impaired in solid B16-F10-derived
subcutaneous tumors by a decrease in cell proliferation and
angiogenesis, as well as by an enhancement of cell death (35). From these studies, we have been
trying to elucidate the cellular mechanisms behind such
effects.
Metastasis is a multi-step process driven by
selective interactions that occur during circulation (37). Altered profiles of adhesion
molecules, leading to changes in migration patterns (38), coupled with the high production of
proteolytic enzymes, favors the degradation of the extracellular
matrix (ECM)-conferred barrier, rendering the invasion of adjacent
tissues possible (39). Finally,
metastatic colonization and solid tumor growth rely on individual
cell competence to generate a colony.
In spite of phenotypic modified characteristics,
malignant cancer cells do not act alone on disease development.
Tumors are complex structures composed of multiple cell types that
participate in heterotypic interactions, forming tumor
microenvironments (40).
Intercellular communication is driven by complex and dynamic
networks of adhesion molecules, cytokines, chemokines, growth
factors, inflammatory enzymes and ECM remodeling (41). In this context, fibroblasts are
stimulated by tumor cells to differentiate into cancer-associated
fibroblasts (CAFs), being able to influence virtually all processes
that lead to melanoma progression (40,42).
Not only direct cell contact, but also extracellular release
molecules, as well as paracrine factors are important for tumor
progression (43).
For a number of years, scientists and physicians
have considered that the anti-tumor effects of high dilutions were
due solely to immune system activation. However, we have found that
treatment with M1 in vivo did not affect immune cell numbers
in metastatic melanoma-bearing mouse lungs (35). Additionally, the effects of M1 seem
to occur irrespective of lymphocyte-mediated immunity (unpublished
data). Therefore, our current hypothesis is that M1 and M8 may act
directly on cancer cells, without cytotoxicity, suppressing the
metastatic phenotype. Bearing in mind the relatively low cost and
high effectiveness of both HDNCs, the aim of this study was to
elucidate the mechanisms that led to previous in vivo
observations. For this purpose, we used standard in vitro
assays to determine the mechanisms through which M1 or M8 directly
affect melanoma cell malignancy parameters, alone or in an
artificial tumor microenvironment (co‑cultured with fibroblasts),
acting as molecular and functional modulators for melanoma
cells.
Materials and methods
HDNCs, cell culture and in vitro
treatment
HDNCs were produced as recommended by the Brazilian
Homeopathic Pharmacopoeia. The components followed by their
dilution and final concentration in M8 were as follows: Aconitum
napellus (20 dH, 0.1×10−19), Arsenicum album
(18 dH, 0.1×10−17), Asafoetida (20 dH,
0.1×10−19), Calcarea carbonica (16 dH,
0.1×10−15), Conium maculatum (17 dH,
0.1×10−16), Ipecacuanha (13 dH,
0.1×10−12), phosphorus (20 dH, 0.1×10−19),
Rhus toxicodendron (17 dH, 0.1×10−16), Silicea
(20 dH, 0.1×10−19), sulphur (24 dH,
0.1×10−23) and Thuja occidentalis (19 dH,
0.1×10–18). M1 has the same basic composition as M8,
with the addition of Chelidonium majus (20 dH,
0.1×10−19), cinnamon (20 dH, 0.1×10−19),
Echinacea purpurea (20 dH, 0.1×10−19) and
Gelsemium sempervirens (20 dH, 0.1×10−19), as
previously described (29). They
were prepared trough serial decimal dilutions from mother
tinctures, which were obtained at Schraibmann Laboratory Ltd.
(Carapicuíba, Brazil). M1, as well as M8 preparations were achieved
by mixing and diluting those mother tinctures and pre-diluted
solutions to the desired final concentration of each component in
the HDNC. In the end, M1 and M8 were used separately and were not
mixed together. Between each dilution steps, the solutions were
vigorously shaken 100 times against a soft pad. Final products are
colorless and odorless and contain 0.1% ethanol. Traces of ethanol
present in highly diluted medicines may exert direct effects on
cell behavior in vitro (44,45).
For this reason, the last 3 M1 and M8 dilutions were performed in
distilled water, and water was used as a control. HDNCs and vehicle
control (water) were 0.22‑μm filtered, sterilized and stored
at room temperature, protected from light until use.
Murine B16-F10 melanoma (BCRJ, 0046) and Balb/3T3
fibroblasts (ATCC, CCL-163) were maintained in complete medium,
Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum
(FBS), 1 U/ml penicillin, 1 μg/ml streptomycin (all from
Gibco/Thermo Fisher Scientific, Waltham, MA, USA), at 37°C and 5%
CO2, for no more than 5 passages. For subculturing, the
cells were washed with PBS and incubated with trypsin for 3 min at
37°C, followed by complete medium inactivation. For co-culture
assays, the cells were simultaneously plated at a 1:1 ratio.
Following treatment, the mRNA of both cells and their supernatant
was collected for analysis. Both cell lineages were shown to be
mycoplasma-free after staining with DAPI and imaging under a
confocal laser scanning microscope [Nikon A1RMP (Tokyo, Japan) +
100X objective] (46).
At 6 h after cell seeding, M1, M8, or distilled
water were added to each respective group as 20% (v/v) of cell
culture media final volume on the well. Both M1 and M8 were used as
prepared, with no further dilutions. A 1% (v/v) booster dose was
provided every 24 h. For proliferation and viability assays, the
cells were treated for up to 96 h. For other functional and
molecular assays, the cells were pre-treated for 96 h. The
solutions were vigorously shaken 20 times against an open hand
palm, immediately prior to treatment.
Migration and invasion assays
Melanoma cell migration and invasion were determined
according to previously described protocols (47). For scratch assay, confluent treated
cells were scratched using 10 μl tip, and imaged under an
inverted AxioObserver Z1 microscope (Carl Zeiss, Jena, Germany),
over a heating plate at 37°C for up to 24 h to follow scratch
closure. The distance between the scratched edges was measured
using ImageJ 1.52n software.
Transwell migration was determined using 8 μm
PVP-free carbonate Transwell plates (Millipore, Tullagreen,
Ireland). Chemoattractants were added to lower filters surface (0.1
μg/μl fibronectin) and lower chamber (DMEM + 20%
FBS). Pre-treated cells were mechanically detached, suspended in
DMEM and added to the upper Transwell chamber (2×105
cells/chamber). The plates were incubated for 28 h under culture
conditions at 37°C. The cells were fixed in 2% paraformaldehyde and
then stained with 0.25 mg/ml crystal violet (CV) (both from EMS,
Hatfield, PA, USA) for 10 min. The upper surface cells were then
removed, and the migrating cells were imaged under an optic
microscope Eclipse E200 (Nikon). CV was eluted in 33% acetic acid,
followed by absorbance reading at 570 nm using an Epoch™ Microplate
Spectrophotometer (BioTek Instruments, Winooski, VT, USA).
BioCoat Matrigel Invasion Chambers 8 μm
PET-membrane (Corning Inc., Corning, NY, USA) were used for the
invasion assay. The complete medium was added to the lower chamber
as a chemoattractant. Pre-treated cells were mechanically detached,
suspended in DMEM, added to the upper chamber (8×104
cells/chamber), and incubated for 72 h under culture conditions at
37°C. The cells were fixed with 2% paraformalde-hyde (EMS), the
upper surface cells were wiped off, and the invaded cells were
stained/mounted in Dapi-Fluoromount-G™ (EMS) and imaged using an
Axio Imager Z2 microscope (Carl Zeiss).
Reactive oxygen species (ROS)
detection
The H2DCFDA probe (Sigma-Aldrich, St.
Louis, MO, USA) was used to determine the intracellular ROS levels
(48). Pre-treated cells were
incubated with 2.5 μM H2DCFDA in PBS for 30 min
at 37°C, and the fluorescence intensity detected using a
FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA) flow
cytometer. H2O2 was used as an internal
positive control.
Hyaluronic acid detection
Hyaluronic acid (HA) was quantified using HA‑binding
probes (49). Culture supernatants
were incubated overnight at 56°C with 6 mg/ml maxatase, then boiled
and incubated overnight at 4°C in ELISA plates pre-adsorbed with
probes. Biotinilated probes and europium-conjugated streptavidin
were used to detect HA. DELFIA Enhancement Solution (PerkinElmer,
Waltham, MA, USA) was added and fluorescence detected using
Victor-2 fluorimeter (PerkinElmer‑Wallac®; PerkinElmer).
Data were normalized by the cellular protein concentration
following the manufacturer's instructions for the BCA Protein Assay
kit (Thermo Fisher Scientific).
Cells immunolabeling
The cells were fixed in 2% paraformaldehyde (EMS),
blocked with 0.1 M glycine (VETEC/Sigma-Aldrich, Rio de Janeiro,
Brazil) and permeabilized with 0.01% saponin (Sigma-Aldrich). The
cells were incubated with primary antibodies in 1% BSA
(Sigma-Aldrich) for 1 h and with appropriate secondary antibody for
45 min, both at room temperature. Information regarding the source
and target species, as well as the dilutions used is presented in
Table I. The cell fluorescence
intensity and/or positive cell percentage were obtained using a
FACSCalibur (BD Biosciences) flow cytometer.
 | Table IList of antibodies used in flow
cytometry and western blot analysis. |
Table I
List of antibodies used in flow
cytometry and western blot analysis.
| Antibody | Company (cat.
no.) | Dilution |
|---|
| Monoclonal rat
anti-mouse CD44 IgG2b | BD Biosciences, San
Jose, CA, USA (550538) | 0.625
μg/ml |
| Polyclonal goat
anti-human N-cadherin IgG | Santa Cruz
Biotechnology, Santa Cruz, CA, USA (sc-31030) | 40
μg/ml |
| Monoclonal rabbit
anti-human β1-integrin IgG | Millipore,
Billerica, MA, USA (04-1109) | 1:500 |
| Polyclonal rabbit
anti-mouse heparanase IgG | Santa Cruz
Biotechnology, Santa Cruz, CA, USA (sc-25826) | 2 μg/ml |
| Polyclonal goat
FITC-conjugated anti-rat IgG | Jackson
Laboratories, Bar Harbor, ME, USA (112-095-003) | 1:200 |
| Polyclonal rabbit
Alexa Fluor 488-conjugated anti-goat IgG | Invitrogen/Thermo
Fisher Scientific, Waltham, MA, USA (A11078) | 6.7
μg/ml |
| Polyclonal donkey
FITC-conjugated anti-rabbit IgG | Jackson
Laboratories, Bar Harbor, ME, USA (711-095-152) | 5 μg/ml |
RT-qPCR
Total RNA was extracted using the E.Z.N.A. RNA
Extraction kit (Omega Bio-tek, Norcross, GA, USA) cDNA was reverse
transcribed from 500 ng total RNA using the High Capacity cDNA
Reverse Transcription kit (Applied Biosystems/Thermo Fisher
Scientific, Foster City, CA, USA) as per the manufacturer's
instructions. qPCR was performed using the Power SYBR-Green kit
(Applied Biosystems) using 2 μl of cDNA diluted 1:10 in
water. The primer concentration was 800 nM for all targets. The
primer sequences are listed in Table
II. The reaction was performed on a StepOne Plus thermal cycler
(Applied Biosystems/Thermo Fisher Scientific), using the following
cycle conditions: 95°C for 10 min, followed by 40 cycles of 95°C
for 15 sec and 60°C for 1 min. Finally, a melting step was
performed, ramping from 60 to 95°C, to ensure that no secondary PCR
product was interfering with the analysis. The Cp was obtained
using StepOne Software version 2.3. Expression data were analyzed
by geometric averaging of multiple internal control genes
(GAPDH, HPRT and ACTB) expression values,
calculated using geNorm application, as previously described by
Vandesompele et al. GeNorm calculates the gene-stability for
all control genes in a given set of samples, allowing normalization
by multiple housekeeping genes instead of one (50). After the normalization of the
target gene by multiple housekeeping ones, treated samples were
compared to the respective control of each individual
experiment.
 | Table IIPrimers sequences used for
RT-qPCR. |
Table II
Primers sequences used for
RT-qPCR.
| Gene | Forward
sequence | Reverse
sequence |
|---|
| N-cadherin |
5′-TTGCTTCAGGCGTCTGTGGAG-3′ |
5′-ACATCCTTCGGTAAGACTGCG-3′ |
|
Hyaluronidase-1 |
5′-AAAGTTTGGAGAATGAAGCCCT-3′ |
5′-GGTTGGATACCACGGAACCT-3′ |
| HSPG2 |
5′-CCGTCCTGGTCTCGATTACA-3′ |
5′-AGGAAGTCTCGATGCGGATG-3′ |
| Sulfatase-2 |
5′-CCTTCGCCGTGTATCTCAAC-3′ |
5′-CACGTATGAGCCGTTGTACTC-3′ |
| PDGF-A |
5′-AGGAGGAGACAGATGTGAGGT-3′ |
5′-TTCAGGAATGTCACACGCCA-3′ |
| PDGF-B |
5′-GGAGTCGGCATGAATCGCT-3′ |
5′-GAATGGGATCCCCCTCGG-3′ |
| PDGF-C |
5′-GGAACAGAACGGAGTGCAAGA-3′ |
5′-GGCTGTGGATGCTCCCATTA-3′ |
| GAPDH |
5′-ATCTTCTTGTGCAGTGCCAG-3′ |
5′-GGCAACAATCTCCACTTTGCC-3′ |
| HPRT |
5′-TCCCTGGTTAAGCAGTACAGCCCC-3′ |
5′-AGTCTGGCCTGTATCCAACACTTCG-3′ |
| ACTB |
5′-AAGATCAAGATCATTGCTCCTG-3′ |
5′-CGTACTCCTGCTTGCTGATC-3′ |
Gelatin zymography
Zymography was performed as previously described
(51). Sample media were replaced
for DMEM after 96 h of treatment. Supernatants were collected 24 h
later, mixed with non-reducing sample buffer and
electrophoretically separated on an 8% polyacrylamide gel
containing 1 mg/ml gelatin (Fluka, Steinheim, Germany). The gels
were incubated in matrix metalloproteinase (MMP) optimum incubation
buffer for 72 h at 37°C, stained with Coomassie Brilliant Blue
R-250 (VETEC/Sigma-Aldrich) 0.5% (w/v) in 30% methanol and 10%
glacial acetic acid for 30 min at room temperature, and imaged in
Eu-88 A3 Transparency Unit (Epson, Tokyo, Japan). Degradation bands
related to MMP-2 activity were quantified using ImageJ 1.52n
software. Data were normalized by the adherent cell number/well,
which was obtained by CV staining.
Clonogenic assay
Pre-treated B16-F10 cells were mechanically
detached, suspended in complete medium containing 10% firming
buffer, and added to the AlgiMatrix 3D Culture System (both from
Gibco/Thermo Fisher Scientific) (2.5×103 cells/well).
The plates were incubated for 6 days under culture conditions at
37°C. The gels were fixed in 1% paraformaldehyde (EMS), stained
with 0.25 mg/ml CV for 20 min at room temperature, washed with
water, transferred to checkered Petri dishes, and photographed
using a smartphone, keeping always the same distance between the
camera and the alginate gel for all samples. The colony number and
size were quantified using ImageJ 1.52n software.
Proliferation, metabolic activity and
cell death assays
For proliferation assessment, the cells were fixed
in 2% paraformaldehyde (EMS), stained with CV for 20 min at room
temperature, and the staining was eluted in 33% acetic acid (Merck,
Darmstadt, Germany) (52). For
metabolic activity determination, the cells were incubated with 5
mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT; Sigma-Aldrich) for 3 h at 37°C. Formazan crystals were
dissolved in DMSO (Sigma-Aldrich), as previously described
(53). CV and MTT absorbances were
read at 570 or 550 nm, respectively, using an Epoch™ Microplate
Spectrophotometer (BioTek Instruments).
Cell death was determined using an Annexin V-FITC/
7-AAD Apoptosis Detection kit (BD Biosciences) according to the
manufacturer's instructions. The percentage of cells positive for
each marker was obtained using a FACSCalibur (BD Biosciences) flow
cytometer.
Statistical analysis
Images and flow cytometry data were analyzed using
ImageJ 1.52n (54) or Flowing 2
(55) software, respectively. The
treated samples were compared to the vehicle control of each
independent experiment using appropriate statistical tests (Paired
t-test) for each experiment. For multiple comparisons, the
Kruskal-Wallis test followed by Dunn's multiple comparisons test
were used. P-values <0.05 were considered to indicate
statistically significant differences.
Results
Dissemination of melanoma cells and
modulation of tumor progression signature Migratory and invasive
capacities are reduced by HDNCs
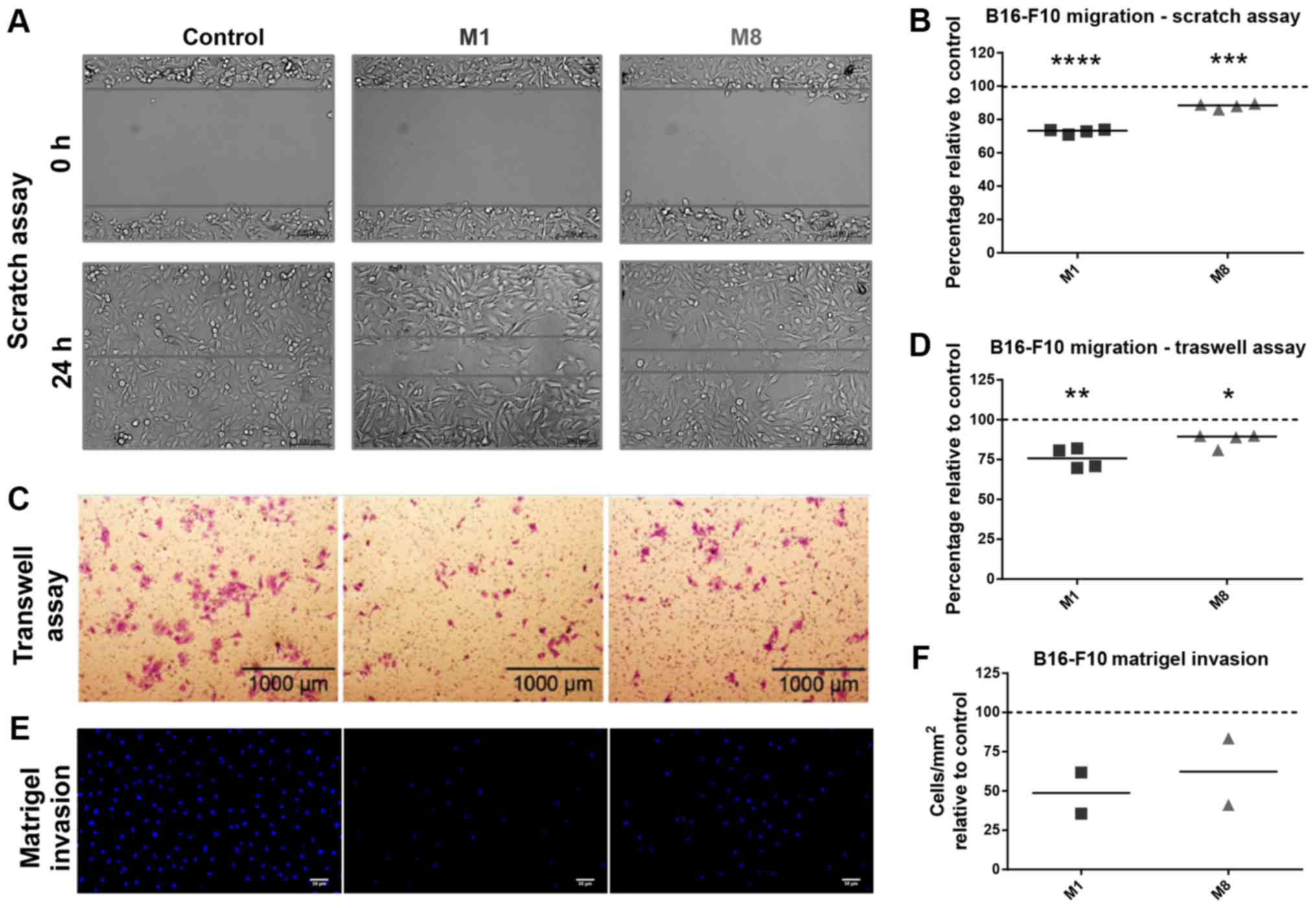
First, we sought to determine whether the HDNCs can
restrict the migration of melanoma cells. Scratch wound (Fig. 1A) and Transwell (Fig. 1C) assays revealed that both the
HDNCs were capable of reducing the melanoma cell migratory
capacity. M1 was more effective than M8 in reducing the cell
migratory capacity by 27.2% (P<0.0001) and 24.15% (P=0.0049), as
shown by scratch wound and Transwell assays, respectively, against
approximately 11.93% (P=0.0006) and 12.54% (P=0.0102) of M8
(Fig. 1B andD).
Next, the invasive capacity of the melanoma cells
through Matrigel was evaluated. We found that the cells pre-treated
with M1 and M8 exhibited a decreased ability to invade the ECM
barrier (Fig. 1E and F) by 51 and
38%, respectively.
Cell migration-related molecules are
modulated by HDNCs
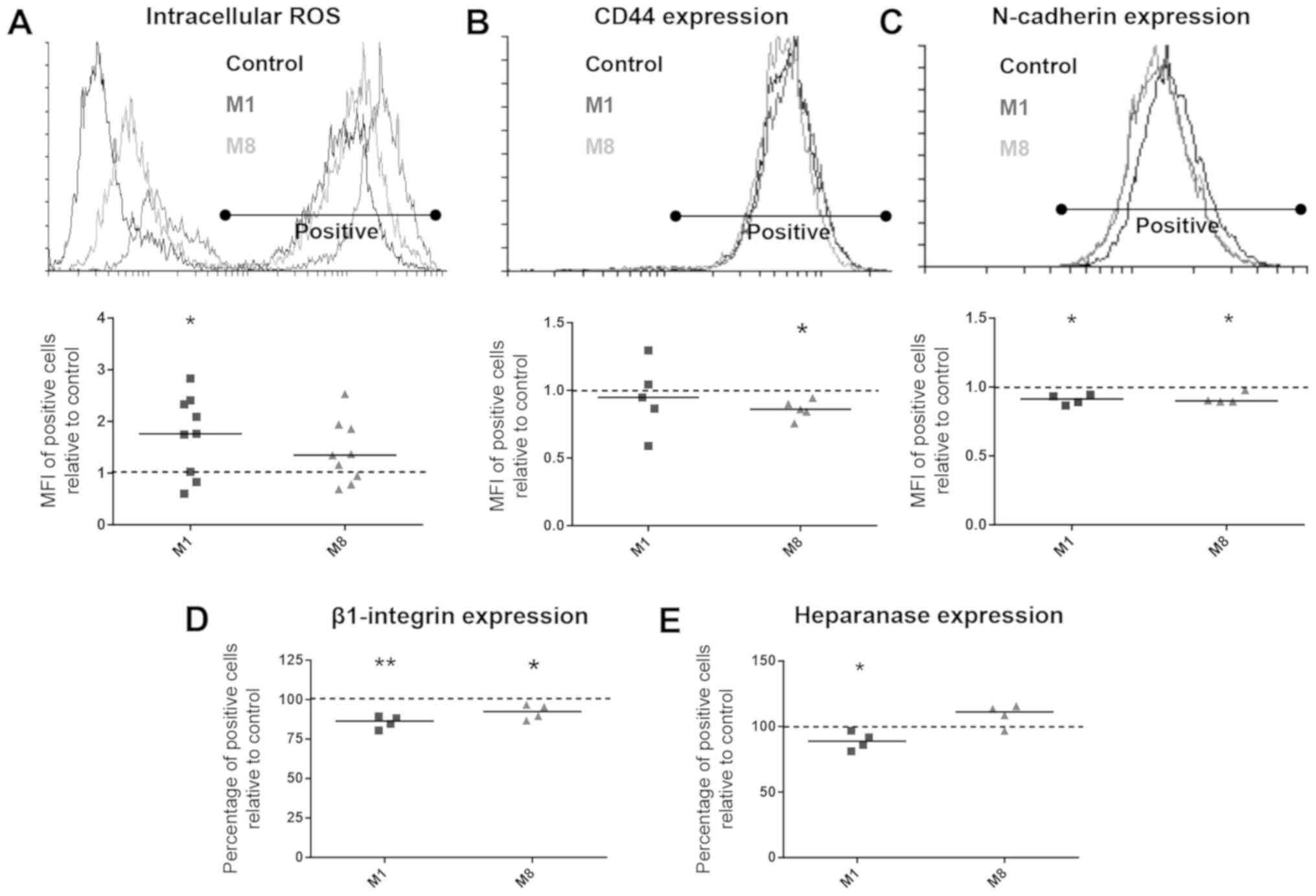
Flow cytometry detection revealed that M1 was able
to enhance the intracellular ROS levels by 73.63% (P=0.0209). No
significant difference was found over M8 treatment (Fig. 2A). On the other hand, only the
M8-treated cells presented an CD44 expression reduced by 13.84%
(P=0.0115) (Fig. 2B).
Following treatment with the HDNCs, the percentage
of melanoma cells expressing N-cadherin and β1-integrin was
quantified by flow cytometry. Both M1 and M8 were able to
downregulate the intensity of N-cadherin surface detection by 9.09%
(P=0.0155) and 8.23% (P=0.0264), respectively (Fig. 2C), and the percentage of cells
expressing β1-integrin by 14.32% (P=0.0057) and 7.89% (P=0.0455),
respectively (Fig. 2D).
ECM-related molecules were differentially
modulated by M1 and M8
The gelatinolytic activity of MMP-2 was examined
trough gelatin zymography. Band densitometry revealed no
significant changes after the melanoma cells treatment with the
HDNCs (data not shown).
We also observed that neither HA secretion (data not
shown) nor hyaluronidase-1 mRNA expression were altered in
HDNC-treated melanoma cells (Table
III). However, the melanoma cells treated with M1 expressed
30.1% (P=0.0249) more HSPG2 mRNA (Table III), although by contrast,
exhibited heparanase protein levels decreased by 11.06% (P=0.0481)
(Fig. 2E). On the other hand, the
cells treated with M8 exhibited a 36.9% (P=0.0189) enhancement of
sulfatase-2 mRNA expression (Table
III).
 | Table IIIGene expression of adhesion- and
extracellular matrix remodeling-related proteins in B16-F10
melanoma cells following treatment with M1 or M8. |
Table III
Gene expression of adhesion- and
extracellular matrix remodeling-related proteins in B16-F10
melanoma cells following treatment with M1 or M8.
| Gene | M1 | M8 |
|---|
|
Hyaluronidase-1 | No change | No change |
| HSPG2 | + (30.1%) | No change |
| Sulfatase-2 | No change | + (36.9%) |
MMP-2 activity is modulated in the
artificial tumor microenvironment by HDNCs
Balb/3T3 fibroblasts were used as CAF model and
co-cultured with B16-F10 melanoma cells. We observed that neither
M1 nor M8 affected N-cadherin mRNA expression (Table IV). We also investigated HA
synthesis/secretion in a co-culture context. HA synthesis was not
affected by any of the HDNCs (data not shown). Using RT-qPCR, we
accessed the mRNA expression of commonly produced molecules within
the tumor microenvironment. M8 treatment did not induce changes in
the expression of these genes. However, M1 reduced platelet-derived
growth factor (PDGF)-B expression by 32.21% (P=0.0036) (Table IV).
 | Table IVGene expression of adhesion- and
soluble signaling protein-related molecules in B16-F10-Balb/3T3
co-cultured following treatment with M1 or M8. |
Table IV
Gene expression of adhesion- and
soluble signaling protein-related molecules in B16-F10-Balb/3T3
co-cultured following treatment with M1 or M8.
| Gene | M1 | M8 |
|---|
| N-cadherin | No change | No change |
| PDGF-A | No change | No change |
| PDGF-B | − (32.2%) | No change |
| PDGF-C | No change | No change |
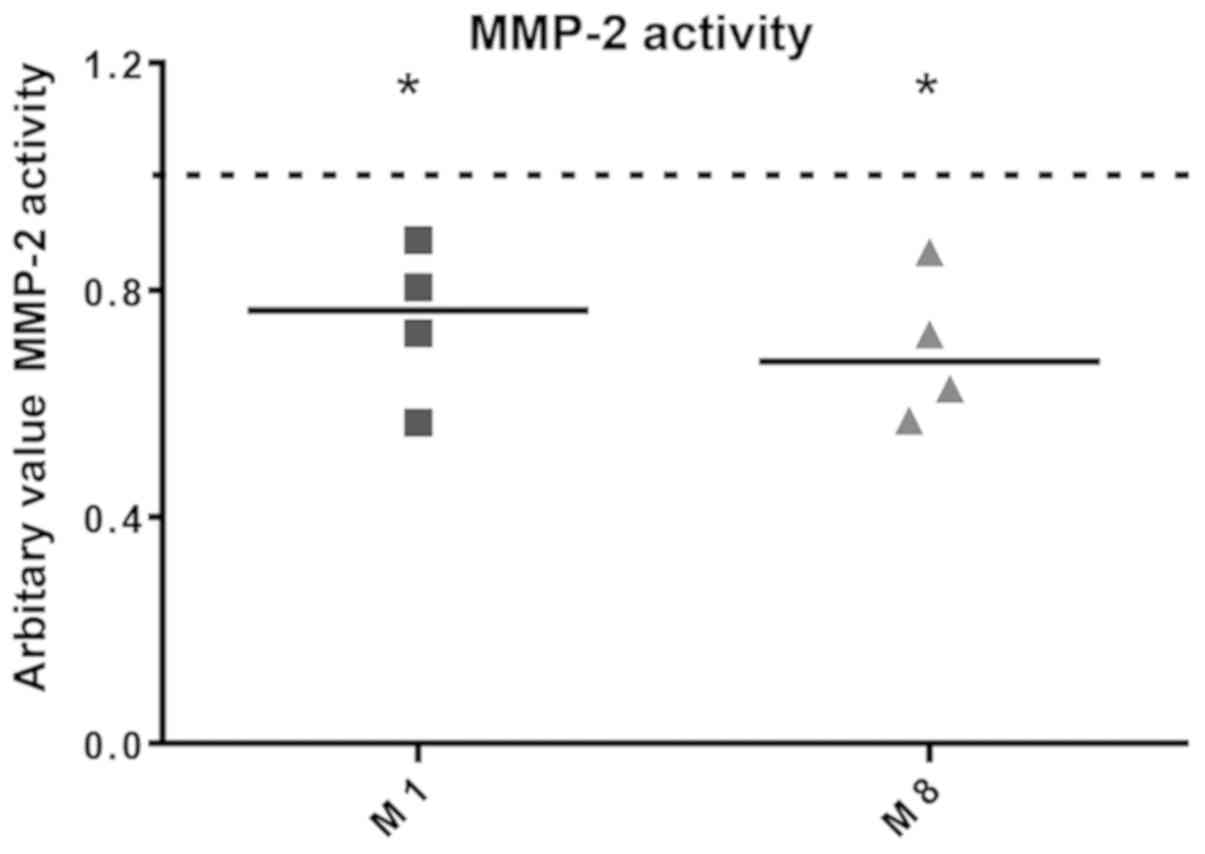
Co-culture supernatants were used to determine MMP-2
gelatinolytic activity by gelatin zymography. We observed that both
M1 and M8 were able to significantly reduce the secreted MMP-2
activity by 25.48% (P=0.0340) and 30.42% (P=0.0186), respectively
(Fig. 3).
Clonogenic capacity of melanoma
cells
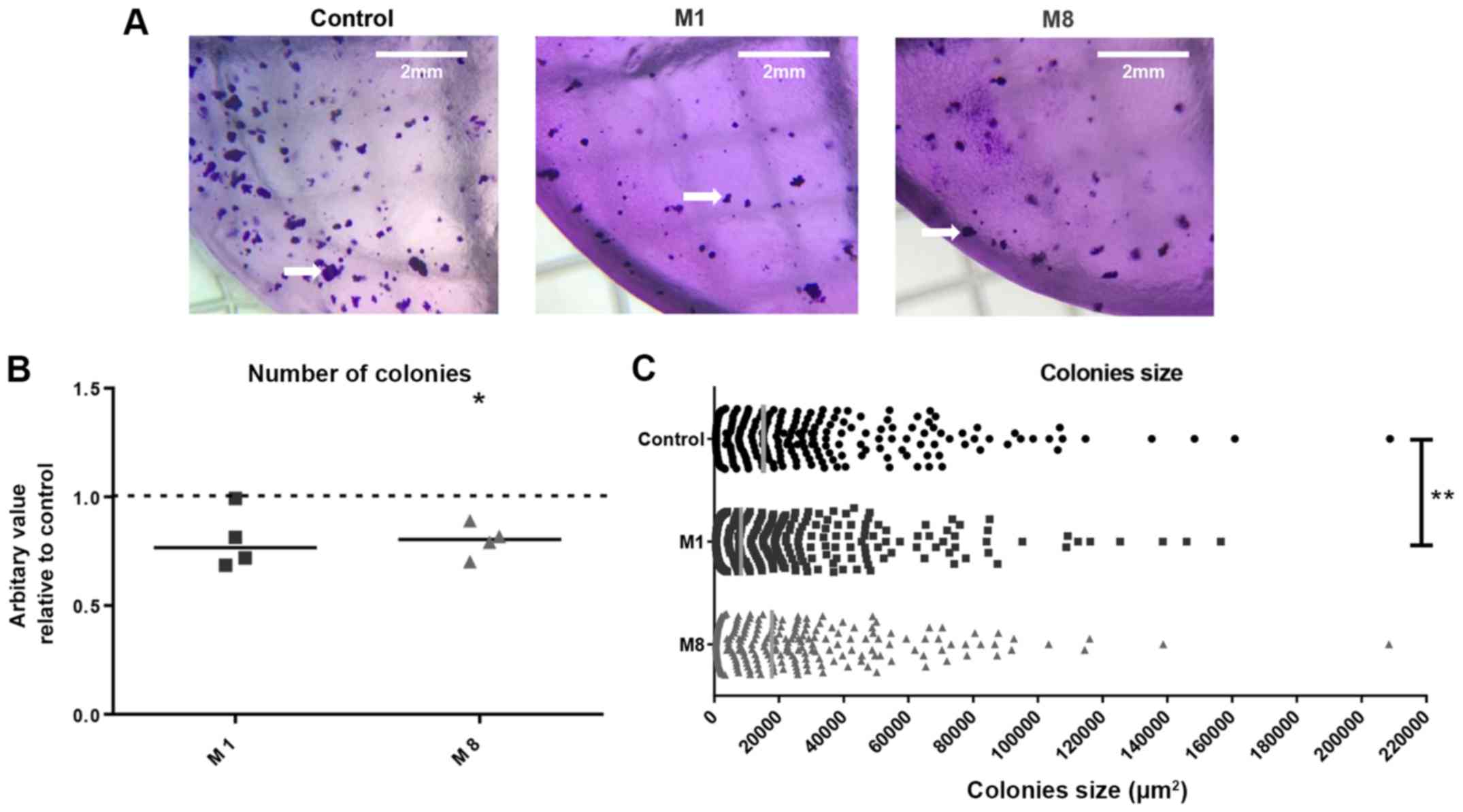
Alginate gel matrix was used to determine the colony
formation capacity of the melanoma cells following pre-treatment
with the HDNCs (Fig. 4A). M8
reduced the number of colonies derived from the pre-treated
melanoma cells by 19.96% (P=0.0144), while M1 did not significantly
affect the number of colonies (Fig.
4B). Additionally, M1 reduced the size of individual colonies
by approximately 30% (P=0.0029), while no significant changes were
observed with M8 (Fig. 4C).
Anchorage-dependent growth and
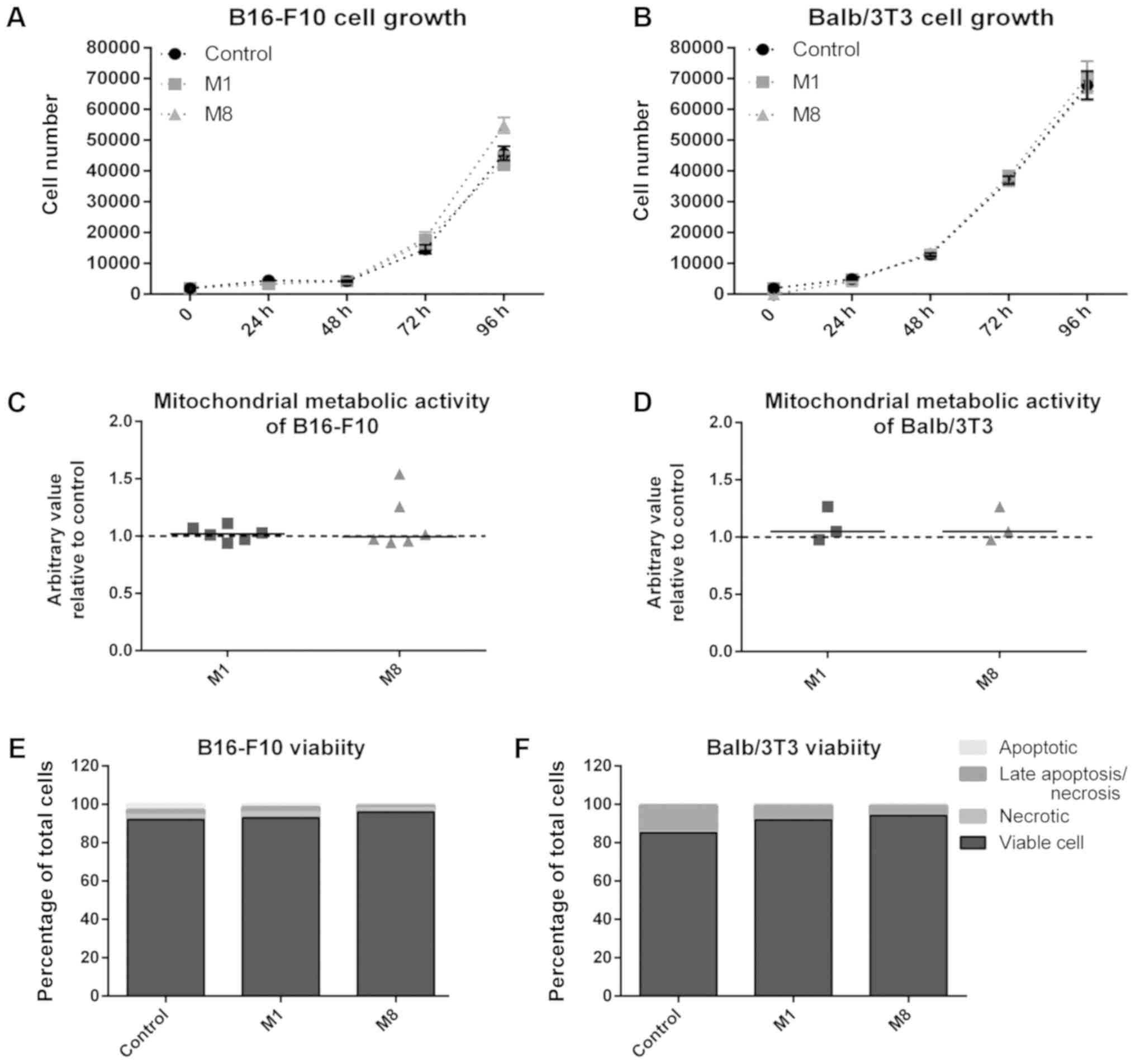
cytotoxicity
Anchorage-dependent growth and cell viability were
assayed in both melanoma cells and fibroblasts (non-tumor cell
model). When cultured in vitro, the B16-F10 melanoma and
Balb/3T3 fibroblasts are adherent cells. CV assay was used to
determine the effects of the HDNCs on cell proliferation. A
time-course growth curve for up to 96 h was performed and CV
staining revealed no differences in the number of adherent cells
for M1 and M8 treatments, for either the melanoma cells (Fig. 5A) or the fibroblasts (Fig. 5B).
Next, regular cell features were used to determine
the loss of cell viability, as well as the extent of cell damage.
MTT standard assay to access cytotoxicity by measuring
mitochondrial enzymes activity was used. No marked changes in
metabolic activity of the melanoma cells (Fig. 5C) and fibroblasts (Fig. 5D) treated with the HDNCs were
observed. In addition, to confirm that no cell damage had occurred,
phosphatidylserine externalization and membrane permeabilization
were determined by Annexin V and 7-AAD labeling, respectively. This
assay provides a clear picture of cytotoxicity, as it can
distinguish viable cells from early apoptotic and necrotic cells,
as well as late apoptotic/necrotic (on the samples, you have the
percentage of each population). We found no statistically
significant differences for both cell types and experimental groups
(Fig. 5E and F). Therefore, no
cytotoxicity was found.
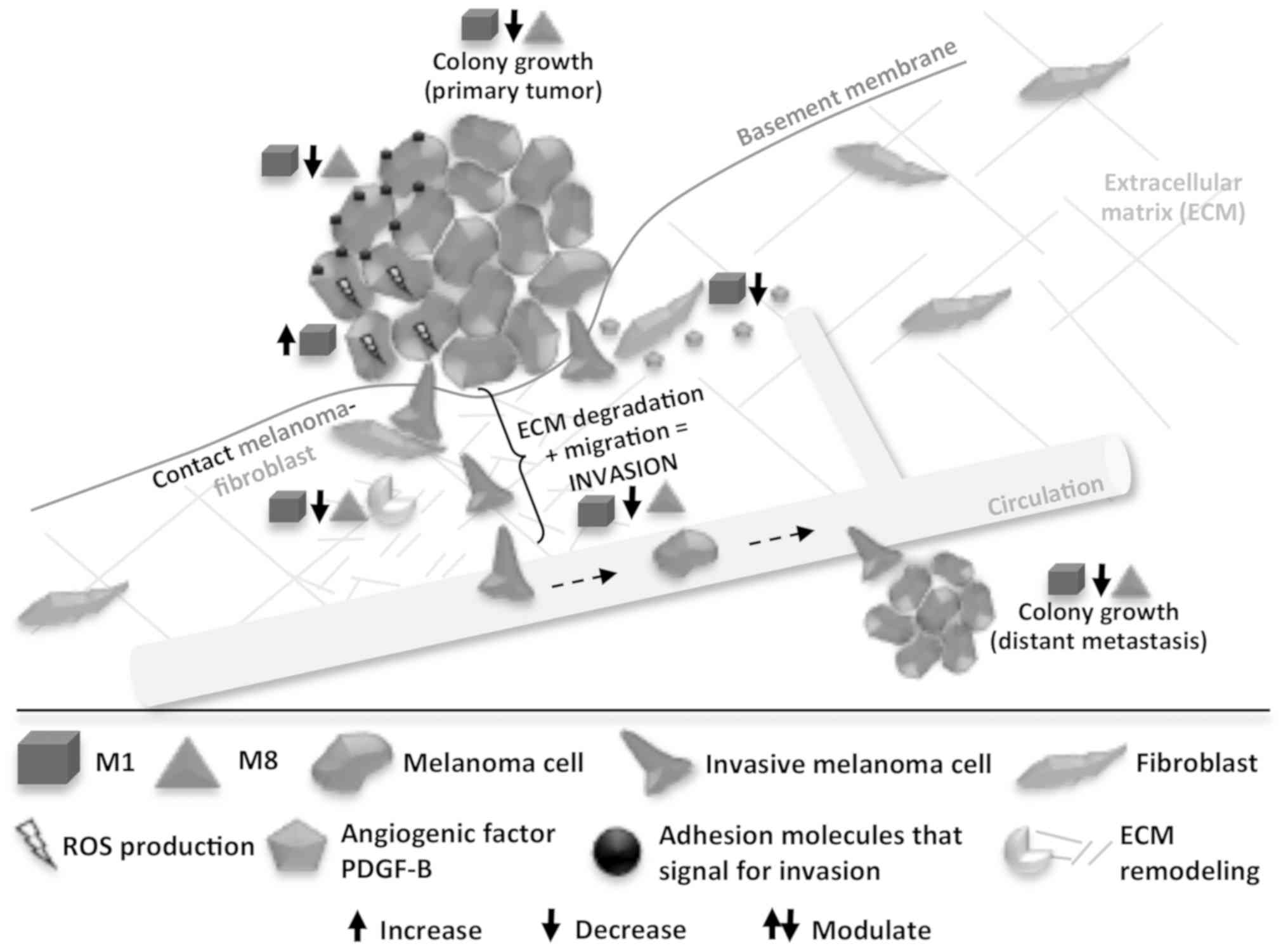
In summary, in this study, we observed that M1 and
M8 exerted direct effects on melanoma cells in vitro, when
isolated and co-cultured with fibroblasts, in a non-cytotoxic
manner. Through different mechanisms, both HDNCs modulated key
molecules (ROS, PDGF-B, surface adhesion molecules, and MMP-2) for
the melanoma cell metastatic phenotype, leading to a reduction in
the capacity of the cells to generate new colonies and invade other
tissues (Fig. 6).
Discussion
The fact that metastatic melanoma is highly
refractory to existing therapies has driven several research groups
to develop and test potential anti-melanoma products. The majority
of anticancer chemotherapeutics used clinically are cytotoxic,
which causes severe side-effects for patients with limited
effective results against metastatic melanoma. Even though novel
leading therapies present better results against this type of
cancer, they are closely dependent on the tumor's individual
genetic signature and are prohibitively expensive. Therefore, the
use of HDNCs may be a cost-effective method for the treatment of
melanoma, as lung colonization (35,36)
and solid tumor growth (35) have
been shown to be impaired in metastatic melanoma-bearing mice
treated with M1 and M8. In the present study, we assessed whether
these HDNCs can directly act on the malignant parameters of
melanoma cells, alone or in an artificial tumor microenvironment
(co-cultured with fibroblasts), leading to the attenuation of cell
metastasis-related features.
The cell invasive ability is directly related to
metastatic competence (38).
Cancer cells often exhibit an altered expression of cell-cell and
cell-ECM adhesion molecules, such as β1-integrins (56), N-cadherin (57) and CD44 (58), leading to changes in migration
patterns and promoting the invasion of adjacent tissues (38). In this study, we found that HDNC
treatment decreased the migratory and invasive capacities of the
melanoma cells (Fig. 1) by the
differential modulation of related molecules (Figs. 2 and 3). Although statistically limited, the
reduction of the cell invasive capacity following HDNC treatment
was very expressive. The results corroborated the reduction in
migratory capacity revealed by two different approaches. By
comparing the results from the migration and invasion assays, it is
possible to note that approximately half of the reduction on
invasive capacity promoted by M1 was due to migratory attenuation.
For M8, such a contribution accounted for approximately 1/3 of the
effect. Although controversial, ROS are also implicated in cell
migration (59). M1 treatment, but
not M8, enhanced the intracellular ROS levels. On the other hand,
M8 reduced CD44 expression, the main receptor for HA that triggers
intracellular signal transductions, stimulating migration and
invasion processes of melanoma cells (60). Both HDNCs reduced B16-F10 surface
β1-integrin and N-cadherin, key adhesion molecules that can
increase cell migratory capacity.
In addition, cancer cell-ECM interaction,
degradation and remodeling (61)
are critical steps to invasion (61). In this study, we detected that M1
and M8 differentially modulated ECM-related molecules. Tumor cells
often produce MMPs, but upon stimulation, stromal cells are also
able to do so (62), and thus the
co-culture model is more appropriate for detecting MMP activity.
MMP-2 activity was decreased by both HDNCs in the artificial tumor
microenvironment, but not when the B16-F10 cells were cultured
alone. MMP-2 is the main gelatinolytic enzyme in melanoma, mainly
produced at the tumor edges, its invasive front (63). Therefore, the notable finding of
ECM degradation reduction by HDNCs may contribute to the impairment
of the capacity of tumor cells to escape and spread, thus reducing
metastasis.
ECM components and their modulators were also
investigated. Hyaluronidase-induced HA fragments can trigger MMP-2
production via TLR4 signaling, stimulating tumor progression
(64). In this study, neither HA
synthesis nor hyaluronidase-1 expression levels were altered in
melanoma cells alone or in the co-culture model. However, HSPG2, an
important basement membrane constituent, was upregulated by
melanoma cells following M1 treatment. By contrast, the expression
of heparanase - the enzyme responsible for heparan sulfate (HS)
chain degradation, was downregulated. However, M8 caused no changes
in HSPG2 or heparanase expression, but instead upregulated
sulfatase-2 mRNA expression. These results are quite noteworthy as
proteoglycan HS chain cleavage by heparanase releases growth
factors trapped in the ECM, enhancing its bioavailability (65). In addition, heparanase inhibition
on B16 cells was shown to be responsible for the inhibition of
invasion in the reconstituted basement membrane (66). Moreover, sulfatases remove 6-O
sulfates from HS chains, which are important for binding to growth
factors and their receptors, sustaining its signaling (65). Thus, these results suggest that M1
and M8 interfere via distinct mechanisms with the enzymatic
processing of HS chains from proteoglycans, but at the same time
can reduce the activity of pro-tumor growth factors.
Growth factors are commonly released in large
amounts in the melanoma microenvironment due to cancer
cell-fibroblast communications. Along with cytokines, they can
stimulate angiogenesis, recruit stromal cells, and promote
inflammation and the invasive capacity (67). Melanoma-produced PDGFs A and C are
the main signaling molecules involved in HA synthesis stimulation
by CAFs (68). PDGFs A and C
expression levels were not affected by HDNCs, which is likely the
reason for unaffected HA synthesis. Notably, M1 downregulated
PDGF-B expression, a stimulatory signal for connective tissue
production around melanoma tumors (69) and for pericyte recruitment
(70), which contributes to the
formation of new blood vessels. Other important molecules for
melanoma migration, invasion and the tumor microenvironment [such
as p-AKT, β-catenin, melanocyte inducing transcription factor
(MTIF), melanin, α-smooth muscle actin (α-SMA), transforming growth
factor (TGF)β1 and vascular endothelial growth factor (VEGF)] were
addressed, but no changes were found (data not shown).
The extraordinary capacity of cancer cells to
overcome growth arrest in growth-limiting conditions allows them to
proliferate in new environments (38). In this study, no effects on cell
proliferation or cytotoxicity were observed in the
anchorage-dependent growth model (2D), although the
anchorage-independent growth of pre-treated melanoma cells was
decreased (Fig. 4). M8 reduced the
number of melanoma-derived colonies, while M1 restrained colony
size. The lack of clonogenic capacity by cancer cells is a
determinant of reproductive death (71), as it represents the final result of
the metastatic process.
Despite scientific evidence of the effectiveness
high dilutions for cancer treatment, as well as for other diseases,
we often face prejudiced claims that there is no active molecule in
such solutions. Fortunately, science regarding the use of
homeopathy has evolved. It is currently known that molecules can be
kept at the air-liquid interface in between dilutions and be
carried to the next ones. In addition, the dilution process
involving vigorous shaking may release silicon and silica from the
glass vial where it is being produced, which can maintain
information from original material under the form of nanoparticles.
Therefore, we can now understand high dilutions as a nanomedicine
system (72,73). Initial studies on cancer were
clinical trials in which the objective was to treat pain or the
side-effects caused by chemotherapy (74-77).
Recently, a number of other studies have been carried out, with an
aim to identify the direct effects of these medicines on cancer
cells in vitro. Notably, the majority of the existing
literature investigated different highly diluted compounds
searching for cytotoxic effects (19,21,28,78-83)
or alterations in cell death-related gene expression (20,84,85).
In addition, in vitro studies have primarily investigated
medications made of single substances. Therefore, we strongly
believe that our findings are valuable, not only as they may help
to increase the survival of patients with metastasis, but may also
lead to important advancements in the field of high dilutions
research. Using standard in vitro assays, we were able to
elucidate the molecular and functional alterations caused by M1 and
M8 directly on isolated cancer cells, and under the tumor
microenvironment simulation. The main results are summarized in
Fig. 6. The data obtained herein
are strongly connected, as we were able to see that the sum of
discrete changes on many molecules expression related to cell
communication, adhesion, migration, and invasion led to the final
effect of reducing melanoma cells invasive and clonogenic
capacities in vitro. Likewise, these cell function changes
are in strong agreement with previous in vivo results
showing M1 and M8 as potent agents against tumor growth and
metastasis. However, future studies are warranted in order to
validate M1 and M8 as anti-melanoma candidates. Strategies to block
specific molecule function should provide more detailed information
regarding the contribution of each molecule whose expression has
been altered herein for the final effect of M1 or M8. In addition,
approaches to determine the active agents must be explored.
In conclusion, previous in vivo studies using
murine melanoma models have demonstrated the success of HDNCs in
preventing solid tumors to grow and lung nodules to develop as fast
as in non-treated animals (35,36).
In this study, we were able to elucidate the in vitro
cellular mechanisms likely responsible for these in vivo
effects. These are important findings as those HDNCs are
non-cytotoxic and yet still present prominent effects on reducing
main malignancy parameters (migratory, invasive, and clonogenic
capacities) of cancer cells in vitro. Unraveling the
mechanisms behind such low cost and non-cytotoxic HDNCs could
support future studies regarding their possible therapeutic use as
one integrative and complementary therapy for patients with
metastatic melanoma.
Funding
This study was financed in part by the Coordenação
de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) -
Finance Code 001.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JPG and FBP planned and conducted the experiments,
analyzed and interpreted the obtained data. JPG wrote the
manuscript. MLFDS and VSCG helped on the execution of most
experiments. GRR and AM under the supervision of HBN planned and
conducted HA measurements. TJ under the supervision of SMBW
planned, conducted, and analyzed RT-qPCR assays and results. EST
and CCDO supervised all steps of assays planning and results
interpretation, and also revised the manuscript. All authors have
read and approved the final manuscript
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Homeoterápica
manipulation Pharmacy for preparing and kindly providing the HDNCs,
as well as LabCet (ICC/Fiocruz) for purchasing the Balb/3T3 cells
from ATCC and kindly providing them so that we could perform the
experiments described herein. The authors would also like to thank
Dr Silvio M. Zanata [Universidade Federal do Paraná (UFPR)] for
providing fibronectin for the Transwell migration assay, and
CTAF-UFPR for the acquisition of the migration and invasion assay
images. The authors are also grateful to the UFPR Academic
Publishing Advisory Center [Centro de Assessoria de Publicação
Acadêmica (CAPA)] for assistance with English language editing.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lomas A, Leonardi-Bee J and Bath-Hextall
F: A systematic review of worldwide incidence of nonmelanoma skin
cancer. Br J Dermatol. 166:1069–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guy GP Jr, Thomas CC, Thompson T, Watson
M, Massetti GM and Richardson LC; Centers for Disease Control and
Prevention (CDC): Vital signs: Melanoma incidence and mortality
trends and projections - United States, 1982-2030. MMWR Morb Mortal
Wkly Rep. 64:591–596. 2015.PubMed/NCBI
|
|
4
|
American Cancer Society: Survival Rates
for Melanoma Skin Cancer, by Stage. American Cancer Society;
Atlanta, GA: 2017, https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html.
Accessed February 1, 2019.
|
|
5
|
Maverakis E, Cornelius LA, Bowen GM, Phan
T, Patel FB, Fitzmaurice S, He Y, Burrall B, Duong C, Kloxin AM, et
al: Metastatic melanoma - A review of current and future treatment
options. Acta Derm Venereol. 95:516–524. 2015. View Article : Google Scholar
|
|
6
|
American Cancer Society Skin Cancer:
American Cancer Society, Inc.; Atlanta, GA: 2016, http://www.cancer.org/cancer/cancercauses/sunanduvexposure/skin-cancer-facts
Accessed May 10 , 2018.
|
|
7
|
Ryu SH, Heo SH, Park EY, Choi KC, Ryu JW,
Lee SH and Lee SW: Selumetinib inhibits melanoma metastasis to
mouse liver via suppression of EMT-targeted genes. Anticancer Res.
37:607–614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadhu SS, Wang S, Averineni RK, Seefeldt
T, Yang Y and Guan X: In-vitro and in-vivo inhibition of melanoma
growth and metastasis by the drug combination of celecoxib and
dacarbazine. Melanoma Res. 26:572–579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ibrahim N and Haluska FG: Molecular
pathogenesis of cutaneous melanocytic neoplasms. Annu Rev Pathol.
4:551–579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luke JJ, Flaherty KT, Ribas A and Long GV:
Targeted agents and immunotherapies: Optimizing outcomes in
melanoma. Nat Rev Clin Oncol. 14:463–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo C and Shen J: Research progress in
advanced melanoma. Cancer Lett. 397:120–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eggermont AM and Robert C: New drugs in
melanoma: It's a whole new world. Eur J Cancer. 47:2150–2157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melis C, Rogiers A, Bechter O and van den
Oord JJ: Molecular genetic and immunotherapeutic targets in
metastatic melanoma. Virchows Arch. 471:281–293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Workman P, Draetta GF, Schellens JH and
Bernards R: How much longer will we put up with $100,000 cancer
drugs? Cell. 168:579–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
World Health Organization: WHO Traditional
Medicine Strategy: 2014-2023. WHO Press; Geneva: pp. 762013
|
|
17
|
Ghosh S, Sikdar S, Mukherjee A and
Khuda-Bukhsh AR: Evaluation of chemopreventive potentials of
ethanolic extract of Ruta graveolens against A375 skin melanoma
cells in vitro and induced skin cancer in mice in vivo. J Integr
Med. 13:34–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mondal J, Samadder A and Khuda-Bukhsh AR:
Psorinum 6× triggers apoptosis signals in human lung cancer cells.
J Integr Med. 14:143–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sikdar S, Kumar Saha S and Rahman
Khuda-Bukhsh A: Relative apoptosis-inducing potential of
homeopa-thic Condurango 6C and 30C in H460 lung cancer cells in
vitro: -Apoptosis-induction by homeopathic Condurango in H460
cells. J Pharmacopuncture. 17:59–69. 2014. View Article : Google Scholar
|
|
20
|
Saha SK, Roy S and Khuda-Bukhsh AR:
Ultra-highly diluted plant extracts of Hydrastis canadensis and
Marsdenia condurango induce epigenetic modifications and alter gene
expression profiles in HeLa cells in vitro. J Integr Med.
13:400–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arora S and Tandon S: DNA fragmentation
and cell cycle arrest: A hallmark of apoptosis induced by Ruta
graveolens in human colon cancer cells. Homeopathy. 104:36–47.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khuda-Bukhsh AR: Modulation of TERT and
Top II activities by the homeopathic nosode, Hep C 30 in
demonstrating its anticancer potential against Hep G2 liver cancer
cells: A commentary on one of our Published Research Anisur. BAOJ
Med Nurs. 4:22018.
|
|
23
|
da Saúde Ministério: Política Nacional de
Práticas Integrativas e Complementares no SUS. 2nd edition.
Biblioteca Virtual em Saúde do Ministério da Saúde; Brasil: pp.
982015, In Portuguese.
|
|
24
|
de Oliveira CC, de Oliveira SM, Godoy LM,
Gabardo J and Buchi DF: Canova, a Brazilian medical formulation,
alters oxidative metabolism of mice macrophages. J Infect.
52:420–432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abud AP, Cesar B, Cavazzani LF, de
Oliveira CC, Gabardo J and Buchi DF: Activation of bone marrow
cells treated with Canova in vitro. Cell Biol Int. 30:808–816.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lopes L, Godoy LM, de Oliveira CC, Gabardo
J, Schadeck RJ and de Freitas Buchi D: Phagocytosis,
endosomal/lysosomal system and other cellularaspects of macrophage
activation by Canova medication. Micron. 37:277–287. 2006.
View Article : Google Scholar
|
|
27
|
Cesar B, Abud AP, de Oliveira CC, Cardoso
F, Gremski W, Gabardo J and Buchi DF: Activation of mononuclear
bone marrow cells treated in vitro with a complex homeopathic
medication. Micron. 39:461–470. 2008. View Article : Google Scholar
|
|
28
|
Guimarães FS, Abud AP, Oliveira SM,
Oliveira CC, César B, Andrade LF, Donatti L, Gabardo J, Trindade ES
and Buchi DF: Stimulation of lymphocyte anti-melanoma activity by
co-cultured macrophages activated by complex homeopathic
medication. BMC Cancer. 9:2932009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Oliveira CC, Abud AP, de Oliveira SM,
Guimarães FS, de Andrade LF, Di Bernardi RP, Coletto EL, Kuczera D,
Da Lozzo EJ, Gonçalves JP, et al: Developments on drug discovery
and on new therapeutics: Highly diluted tinctures act as biological
response modifiers. BMC Complement Altern Med. 11:1012011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Oliveira SM, de Oliveira CC, Abud AP,
Guimarães FS, Di Bernardi RP, Coletto EL and Buchi DF: Mercurius
solubilis: Actions on macrophages. Homeopathy. 100:228–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonçalves JP, Dos Santos MLF, Rossi GR,
Costa Gagosian VS and de Oliveira CC: Differential effects of
Zincum metallicum on cell models. Homeopathy. 106:171–180. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sato DY, Wal R, de Oliveira CC, Cattaneo
RII, Malvezzi M, Gabardo J and Buchi DF: Histopathological and
immunopheno-typing studies on normal and sarcoma 180-bearing mice
treated with a complex homeopathic medication. Homeopathy.
94:26–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Oliveira CC, de Oliveira SM, Goes VM,
Probst CM, Krieger MA and Buchi DF: Gene expression profiling of
macrophages following mice treatment with an immunomodulator
medication. J Cell Biochem. 104:1364–1377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cesar B, Abud AP, de Oliveira CC, Cardoso
F, Bernardi RP, Guimarães FS, Gabardo J and de Freitas Buchi D:
Treatment with at homeopathic complex medication modulates
mononuclear bone marrow cell differentiation. Evid Based Complement
Alternat Med. 2011:2124592011. View Article : Google Scholar :
|
|
35
|
Ferrari de Andrade L, Mozeleski B, Leck
AR, Rossi G, da Costa CR, de Souza Fonseca, Guimarães F, Zotz R,
Fialho do Nascimento K, Camargo de Oliveira C, de Freitas Buchi D,
et al: Inhalation therapy with M1 inhibits experimental melanoma
development and metastases in mice. Homeopathy. 105:109–118. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guimarães FS, Andrade LF, Martins ST, Abud
AP, Sene RV, Wanderer C, Tiscornia I, Bollati-Fogolín M, Buchi DF
and Trindade ES: In vitro and in vivo anticancer properties of a
Calcarea carbonica derivative complex (M8) treatment in a murine
melanoma model. BMC Cancer. 10:1132010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lambert AW, Pattabiraman DR and Weinberg
RA: Emerging Biological Principles of Metastasis. Cell.
168:670–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kam Y, Rejniak KA and Anderson AR:
Cellular modeling of cancer invasion: Integration of in silico and
in vitro approaches. J Cell Physiol. 227:431–438. 2012. View Article : Google Scholar
|
|
40
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Balkwill FR, Capasso M and Hagemann T: The
tumor microenvironment at a glance. J Cell Sci. 125:5591–5596.
2012. View Article : Google Scholar
|
|
42
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bogenrieder T and Herlyn M: Axis of evil:
Molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lima LF, Rocha RM, Duarte AB, Brito IR,
Silva GM, Rodrigues GQ, Nunes-Pinheiro DC, Sales AD, Moura AA,
Wheeler MB, et al: Unexpected effect of the vehicle (grain ethanol)
of homeopathic FSH on the in vitro survival and development of
isolated ovine preantral follicles. Microsc Res Tech. 80:406–418.
2017. View Article : Google Scholar
|
|
45
|
Chirumbolo S and Bjørklund G: Homeopathic
potencies of Arnica montana L. change gene expression in a
Tamm-Horsfall protein-1 cell line in vitro model: The role of
ethanol as a possible confounder and statistical bias. J Integr
Med. 15:255–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Young L, Sung J, Stacey G and Masters JR:
Detection of Mycoplasma in cell cultures. Nat Protoc. 5:929–934.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Justus CR, Leffler N, Ruiz-Echevarria M
and Yang LV: In vitro cell migration and invasion assays. J Vis
Exp. 88:e510462014.
|
|
48
|
Eruslanov E and Kusmartsev S:
Identification of ROS using oxidized DCFDA and flow-cytometry.
Methods Mol Biol. 594:57–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Martins JR, Passerotti CC, Maciel RM,
Sampaio LO, Dietrich CP and Nader HB: Practical determination of
hyaluronan by a new noncompetitive fluorescence-based assay on
serum of normal and cirrhotic patients. Anal Biochem. 319:65–72.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol. Jun
18–2002.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Toth M and Fridman R: Assessment of
gelatinases (MMP-2 and MMP-9 by gelatin zymography. Methods Mol
Med. 57:163–174. 2001.PubMed/NCBI
|
|
52
|
Bonnekoh B, Wevers A, Jugert F, Merk H and
Mahrle G: Colorimetric growth assay for epidermal cell cultures by
their crystal violet binding capacity. Arch Dermatol Res.
281:487–490. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Terho P: Flowing Software. Turku Centre
for Biotechnology. 2013, http://flowingsoftware.btk.fi/index.php?page=1.
|
|
56
|
Kuphal S, Bauer R and Bosserhoff A-K:
Integrin signaling in malignant melanoma. Cancer Metastasis Rev.
24:195–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li G, Satyamoorthy K and Herlyn M:
N-cadherin-mediated intercellular interactions promote survival and
migration of melanoma cells. Cancer Res. 61:3819–3825.
2001.PubMed/NCBI
|
|
58
|
Dietrich A, Tanczos E, Vanscheidt W,
Schöpf E and Simon JC: High CD44 surface expression on primary
tumours of malignant melanoma correlates with increased metastatic
risk and reduced survival. Eur J Cancer. 33:926–930. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Herraiz C, Crosas-Molist E and Sanz-Moreno
V: Reactive oxygen species and tumor dissemination: Allies no
longer. Mol Cell Oncol. 3:e11273132016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sironen RK, Tammi M, Tammi R, Auvinen PK,
Anttila M and Kosma VM: Hyaluronan in human malignancies. Exp Cell
Res. 317:383–391. 2011. View Article : Google Scholar
|
|
61
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hofmann UB, Westphal JR, Zendman AJW,
Becker JC, Ruiter DJ and van Muijen GN: Expression and activation
of matrix metallo-proteinase-2 (MMP-2) and its co-localization with
membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with
melanoma progression. J Pathol. 191:245–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kurschat P, Wickenhauser C, Groth W, Krieg
T and Mauch C: Identification of activated matrix
metalloproteinase-2 (MMP-2) as the main gelatinolytic enzyme in
malignant melanoma by in situ zymography. J Pathol. 197:179–187.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Voelcker V, Gebhardt C, Averbeck M,
Saalbach A, Wolf V, Weih F, Sleeman J, Anderegg U and Simon J:
Hyaluronan fragments induce cytokine and metalloprotease
upregulation in human melanoma cells in part by signalling via
TLR4. Exp Dermatol. 17:100–107. 2008. View Article : Google Scholar
|
|
65
|
Sanderson RD, Yang Y, Kelly T, MacLeod V,
Dai Y and Theus A: Enzymatic remodeling of heparan sulfate
proteoglycans within the tumor microenvironment: Growth regulation
and the prospect of new cancer therapies. J Cell Biochem.
96:897–905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nakajima M, DeChavigny A, Johnson CE,
Hamada J, Stein CA and Nicolson GL: Suramin. A potent inhibitor of
melanoma heparanase and invasion. J Biol Chem. 266:9661–9666.
1991.PubMed/NCBI
|
|
67
|
Herlyn M and Shih IM: Interactions of
melanocytes and melanoma cells with the microenvironment. Pigment
Cell Res. 7:81–88. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Willenberg A, Saalbach A, Simon JC and
Anderegg U: Melanoma cells control HA synthesis in peritumoral
fibroblasts via PDGF-AA and PDGF-CC: Impact on melanoma cell
proliferation. J Invest Dermatol. 132:385–393. 2012. View Article : Google Scholar
|
|
69
|
Forsberg K, Valyi-Nagy I, Heldin CH,
Herlyn M and Westermark B: Platelet-derived growth factor (PDGF) in
oncogenesis: Development of a vascular connective tissue stroma in
xenotransplanted human melanoma producing PDGF-BB. Proc Natl Acad
Sci USA. 90:393–397. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Abramsson A, Lindblom P and Betsholtz C:
Endothelial and nonendothelial sources of PDGF-B regulate pericyte
recruitment and influence vascular pattern formation in tumors. J
Clin Invest. 112:1142–1151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
recommendations of the Nomenclature Committee on Cell Death 2009.
Cell Death Differ. 16:3–11. 2009. View Article : Google Scholar :
|
|
72
|
Chikramane PS, Kalita D, Suresh AK, Kane
SG and Bellare JR: Why extreme dilutions reach non-zero asymptotes:
A nanoparticulate hypothesis based on froth flotation. Langmuir.
28:15864–15875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Upadhyay RP and Nayak C: Homeopathy
emerging as nano-medicine. Int J High Dilution Res. 10:299–310.
2011.
|
|
74
|
Thompson EA and Reillly D: The homeopathic
approach to symptom control in the cancer patient: A prospective
observational study. Palliat Med. 16:227–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Burton MJ, Couch ME and Rosenfeld RM:
Extracts from the Cochrane Library: Homeopathic medicines for
adverse effects of cancer treatments. Otolaryngol Head Neck Surg.
141:162–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Orellana Alvarellos G, Ruiz de Viñaspre
Alvear P and Kaszkin-Bettag M: A series of case reports: Clinical
evaluation of a complex homeopathic injection therapy in the
management of pain in patients after breast cancer treatment.
Altern Ther Health Med. 16:54–59. 2010.PubMed/NCBI
|
|
77
|
Oberbaum M, Yaniv I, Ben-Gal Y, Stein J,
Ben-Zvi N, Freedman LS and Branski D: A randomized, controlled
clinical trial of the homeopathic medication TRAUMEEL S in the
treatment of chemotherapy-induced stomatitis in children undergoing
stem cell transplantation. Cancer. 92:684–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Samadder A, Das S, Das J, Paul A,
Boujedaini N and Khuda-Bukhsh AR: The potentized homeopathic drug,
Lycopodium clavatum (5C and 15C) has anti-cancer effect on hela
cells in vitro. J Acupunct Meridian Stud. 6:180–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Preethi K, Ellanghiyil S, Kuttan G and
Kuttan R: Induction of apoptosis of tumor cells by some potentiated
homeopathic drugs: Implications on mechanism of action. Integr
Cancer Ther. 11:172–182. 2012. View Article : Google Scholar
|
|
80
|
Arora S, Aggarwal A, Singla P, Jyoti S and
Tandon S: Anti-proliferative effects of homeopathic medicines on
human kidney, colon and breast cancer cells. Homeopathy.
102:274–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Benkendorff K, McIver CM and Abbott CA:
Bioactivity of the murex homeopathic remedy and of extracts from an
australian muricid mollusc against human cancer cells. Evid Based
Complement Alternat Med. 2011:8795852011. View Article : Google Scholar :
|
|
82
|
Frenkel M, Mishra BM, Sen S, Yang P,
Pawlus A, Vence L, Leblanc A, Cohen L and Banerji P and Banerji P:
Cytotoxic effects of ultra-diluted remedies on breast cancer cells.
Int J Oncol. 36:395–403. 2010.PubMed/NCBI
|
|
83
|
MacLaughlin BW, Gutsmuths B, Pretner E,
Jonas WB, Ives J, Kulawardane DV and Amri H: Effects of homeopathic
preparations on human prostate cancer growth in cellular and animal
models. Integr Cancer Ther. 5:362–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Saha SK, Roy S and Khuda-Bukhsh AR:
Evidence in support of gene regulatory hypothesis: Gene expression
profiling manifests homeopathy effect as more than placebo. Int J
Hight Dilution Res. 12:162–167. 2013.
|
|
85
|
Thangapazham RL, Gaddipati JP, Rajeshkumar
NV, Sharma A, Singh AK, Ives JA, Maheshwari RK and Jonas WB:
Homeopathic medicines do not alter growth and gene expression in
prostate and breast cancer cells in vitro. Integr Cancer Ther.
5:356–361. 2006. View Article : Google Scholar : PubMed/NCBI
|