Introduction
Cholangiocarcinoma (CCA) is currently one of the
most common malignant tumors in the biliary tract (1,2). CCA
is prone to invasion and metastasis into blood vessels and distant
lymph nodes, thereby complicating clinical surgical treatment
(1,3). Tumor metastasis is the main cause of
cancer-related death (4). Once a
tumor metastasizes, the prognosis becomes extremely poor, despite
the prominent advances that have been made in cancer treatment over
the past few decades (4,5). Tumor invasion and metastasis are
complex biological processes involving multiple factors, during
which tumor cells detach from the primary site and interact with
the extracellular matrix, forming a migration channel for tumor
cells (4,6,7).
Tumor cells then enter the peripheral blood vessels through the
vascular basement membrane, leading to dissemination of the tumor
cells in distant tissues, where they finally colonize and form
metastatic foci (8,9). However, the underlying molecular
mechanisms have not been fully elucidated.
S100 calcium-binding protein A8 (S100A8), also known
as myeloid-related protein 8, is a calcium-binding protein
recognized as an endogenous ligand of Toll-like receptor 4 (TLR4).
S100A8 has been reported to be highly expressed in bone
marrow-derived cells, such as neutrophils (10). In addition, S100A8 protein can be
secreted in the form of heterodimers by necrotic cells and
pathogen-associated molecular pattern-activated immune cells
(10–12). Of note, S100A8 has been found to
positively regulate the progression of malignant tumors and the
dissemination of tumor cells (13,14).
Consistent with these findings, S100A8 protein is frequently
overexpressed in a variety of tumors, such as lung cancer, colon
cancer, gastric cancer, pancreatic cancer, and primary liver cancer
(4,10,15).
However, whether S100A8 is expressed in CCA remains unclear.
Vascular endothelial growth factor (VEGF) is a
protein that specifically affects endothelial cells (16,17).
The binding of VEGF to its receptor VEGFR, a tyrosine protein
kinase, serves a crucial role in promoting endothelial cell
proliferation, migration, budding, vascular growth and permeability
enhancement (17–19). Blood vessels in tumors are known to
be critical for malignant tumor growth, invasion and metastasis
(20–22), and in most cancers, microvessel
formation is a prognostic indicator of tumor metastasis, recurrence
and survival (20,23). Tumor remodeling and angiogenesis
are complex processes that require activation of multiple vascular
components: Endothelial cell division, vascular basement membrane
degradation, peripheral extracellular matrix degradation and
endothelial cell migration (7,24).
Previous studies have confirmed that VEGF is secreted by tumor
cells and has a major regulatory role in angiogenesis in various
types of cancer, by promoting the expansion of endothelial cells
(16,17); however, the exact role of VEGF in
CCA is poorly understood.
The present study demonstrated that S100A8 protein
expression was high in CCA tissues and significantly associated
with malignant pathology, differentiation, lymph node metastasis
and poor prognosis in patients with CCA. Further investigations
revealed that S100A8 activated the TLR4/NF-κB pathway to increase
VEGF secretion and to thereby promote CCA invasion and metastasis.
Collectively, the findings indicated that S100A8 may be a vital
molecule in promoting CCA invasion and metastasis.
Materials and methods
CCA tissue specimens
Pathological specimens were obtained from 134
patients with hilar cholangiocarcinoma who underwent surgical
treatment in the Department of Hepatobiliary Surgery at the
Southwest Hospital Affiliated with the Third Military Medical
University (Chongqing, China) from January 2010 to December 2015.
All patients were re-examined using upper abdominal color Doppler
ultrasound and computed tomography angiography (CTA) every three
months after discharge. Overall survival was calculated from the
date of surgery until the date of last contact. All neutrophil
counts were derived from routine blood tests. The present study was
approved by the Ethics Committee of the Third Military Medical
University and followed the guidelines of the Declaration of
Helsinki.
Animals
A total of 20 male BALB/c-nu mice (age, 4–6 weeks;
weight, 18–22 g) were purchased from the Institute of Laboratory
Animal Sciences of the Chinese Academy of Sciences. The mice were
randomly divided into four groups and were housed under specific
pathogen-free conditions (temperature, 24–26°C; humidity, 40–60%;
ventilation, 15 times/h; 12-h light/dark cycle; free access to food
and water). All animals received humane care according to the
criteria outlined in the 'Guide for the Care and Use of Laboratory
Animals' (National Institutes of Health), and the animal experiment
protocols were approved by the Institutional Animal Care and Use
Committee of the Third Military Medical University. All mice were
sacrificed via intraperitoneal injection of excessive sodium
pentobarbital.
Cell culture
The human CCA cell lines RBE and HCCC-9810 and the
human umbilical vein endothelial cell (HUVEC) line were obtained
from the Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences. The cells were cultured in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc.) medium containing 10% fetal bovine
serum (FBS; Zeta Life, Inc.) and maintained in a humidified
incubator containing 5% CO2 at 37°C.
Reagents
The TLR4 inhibitor TAK242 (cat. no. HY-11109) and
the NF-κB inhibitor ammonium pyrrolidinedithiocarbamate (PDTC; cat.
no. HY-18738) were purchased from MedChemExpress. The TAK-242
(25) and PDTC (26) doses used in the present study were
20 and 40 μmol/l, respectively. The neutralizing anti-human
VEGF-A antibody was purchased from Abcam (3 μg/ml; cat. no.
ab42609).
Lentivirus and stable cell lines
For S100A8 overexpression, full-length human S100A8
cDNA was amplified via polymerase chain reaction (PCR) and cloned
into a lentivirus vector to establish an RBE cell line that stably
expressed S100A8. Two clones were tested, and clone A was selected
for subsequent experiments. For S100A8 knockdown, lentivirus
encoding a specific shRNA against S100A8 was used to establish a
stable HCCC-9810 cell line with S100A8 knockdown. Two clones were
tested, and clone A was selected for subsequent experiments (target
sequence: ACT CTA TCA TCG ACG TCT ACT CGA GTA GAC GTC GAT GAT AGA
GT). S100A8 overexpression (HBLV-h-S100A8-3-flag-GFP-PURO) and
interference lentiviruses (HBLV-h-S100A8-shRNA1-GFP-PURO) were
purchased from Shanghai Genechem Co., Ltd., HCCC-9810 and RBE cells
were seeded onto 6-well plates, and lentiviral infection was
carried out at 50–70% confluency. For infection, the cells were
provided with 1 ml of fresh culture medium, and 30 μl of
lentivirus (1×108 transforming units/ml) was added to
each well. After 48–72 h, the infection rate was observed using a
fluorescence microscope, and S100A8 expression levels were detected
by western blotting.
Immunoblotting
Immunoblotting analyses were performed as described
previously (27). Antibodies
against S100A8 (cat. no. ab180735; Abcam), VEGF (cat. no. ab52917;
Abcam), NF-κB p65 (cat. no. 8242; Cell Signaling Technology, Inc.)
and phosphorylated (p-) NF-κB p65 Ser536 (cat. no. 3033; Cell
Signaling Technology, Inc.) were used at 1:1,000 dilution.
Membranes were washed with TBST for 30 min and incubated with an
HRP-conjugated anti-rabbit secondary antibody (1:4,000; cat. no.
ab6721; Abcam) for 2 h at room temperature.
Immunohistochemistry (IHC)
The expression of S100A8 in paraffin-embedded
clinical samples was investigated using IHC, according to a
previously described protocol (28). Antibodies against S100A8 (1:200;
cat. no. ab180735; Abcam) and VEGF (1:200; cat. no. ab52917; Abcam)
were used. IHC staining of S100A8 and VEGF revealed both membrane
and cytoplasmic localization. Hematoxylin and eosin (H&E)
staining of liver and spleen tumor tissue sections from mice was
performed, as previously described (29). The immunostained tissue slides were
scored according to stain intensity (0, no staining; 1, light
staining; 2, moderate staining; and 3, intense staining) multiplied
by a distribution score (1, staining of 0–33%; 2, staining of
33–66%; and 3, >66% staining). The final score was grouped as
low expression [negative, (0) or low (1–2)
scores], medium expression (moderate score, 3–4) or high expression
(high score, 6–9) for further non-parametric tests. Each
immunostained slide was scored by two pathologists in a
double-blind manner using a light microscope.
Tumor cell migration assay
Tumor cell migration assays were performed using
Transwell inserts (Corning, Inc.) with 8 μm pores. Tumor
cells (2×105) in 0.2 ml of serum-free medium were seeded
in the upper chamber of the insert, and 0.8 ml of medium containing
10% FBS was added to the lower chamber. Cells were cultured for 12
h in a humid environment at 37°C with 5% CO2. Cells
remaining in the upper part of the transwell were removed with a
cotton swab. Migrated cells were then stained with 0.5% crystal
violet, and the number of cells per field was determined using a
light microscope (Olympus Corporation; magnification, ×200).
Tumor cell/HUVEC co-culture migration
assay
HUVEC migration assays were performed using
Transwell inserts (Corning, Inc.) with 8 μm pores. HUVECs
(1×105) in 0.2 ml of serum-free medium were seeded in
the upper chamber, and 0.8 ml of tumor cell culture supernatant was
added to the lower chamber. For the tumor cell supernatant, tumor
cells (5×105) were cultured for 24 h in 1.0 ml of medium
containing 10% FBS in a humid environment at 37°C and with 5%
CO2. HUVECs were allowed to migrate for 5 h. Then, cells
remaining in the upper part of the Transwell were removed with a
cotton swab. Migrated cells on the lower part of the Transwell were
stained with 0.5% crystal violet, and the number of cells per field
was determined using a light microscope (Olympus Corporation;
magnification, ×200). In some experiments (Fig. S2), tumor cells
(2×105/ml) in 0.2 ml of serum-free medium were added to
the upper chamber and HUVECs were placed into the lower chamber,
and migration of lentivirus-transduced tumor cells or control cells
was observed.
HUVEC tube formation assay
For HUVEC tube formation assays, 0.3 ml of Matrigel
(BD Biosciences) was pipetted into a 24-well plate and then allowed
to solidify for 1 h at 37°C. HUVECs (1×105) in 0.2 ml of
conditioned medium were seeded into each well and incubated for 10
h. The average number of HUVEC tubule structures per field was
determined using a light microscope (Olympus Corporation;
magnification, ×100).
ELISA
VEGF levels in tumor cells and tissues were detected
using a human VEGF ELISA kit (cat. no. DY293B; R&D Systems,
Inc.), according to the manufacturer's instructions. In addition,
the S100A8 levels in the CCA tissue samples were determined using
an ELISA kit (cat. no. DY4570-05; R&D Systems, Inc.), according
to the manufacturer's instructions.
In vivo experiments
After anesthetizing with 1% sodium pentobarbital (60
mg/kg; Sigma-Aldrich; Merck KGaA) by intraperitoneal injection, a
median abdominal incision of 1 cm was made under the xiphoid of
nude mice. For experimental liver metastasis, RBE and HCCC-9810
cells were trypsinized and resuspended in PBS, and 5×105
cells or the indicated number of cells (in 0.1 ml of PBS) were
injected into the spleen of the mice. After 45 days, a 7.0 T small
animal MRI (Bruker Biospec) was used to scan the mice and to
observe metastasis. Mice were sacrificed at 45 days after injection
(preliminary experiments were performed to determine the optimal
time after injection to evaluate metastasis; data not shown),
livers were isolated and fixed in 10% neutral-buffered formalin for
24 h at room temperature, and then dehydrated and embedded in
paraffin. Metastatic foci on the surface of the livers were counted
under a dissecting microscope, and the nodule size was
measured.
Distant initial dissemination of tumor cells can be
observed with the first few hours after the tumor cells enter the
blood circulation, which is a key step to the metastatic cascade
(30). For experimental initial
liver colonization, 5×105 RBE and HCCC-9810 cells were
preloaded with Vybrant DiO Cell-labelling Solution (cat. no.
V22886; Invitrogen; Thermo Fisher Scientific, Inc.) for 15 min at
37°C following the manufacturer's instructions. Cells were then
injected into the spleen of the mice, as aforementioned. After 6 h,
livers were isolated, embedded in OCT compound (Tissue-Tek; Sakura
Finetek USA, Inc.) and frozen in liquid nitrogen. Liver sections (8
μm thickness) were analyzed using an Olympus fluorescence
microscope (VS120; Olympus Corporation), and the number of RBE and
HCCC-9810 cells (green signal) was determined.
To assess tumor volumes, the longest (length) and
shortest (width) diameters of the metastatic tumor foci in the
liver were measured, and the volumes were calculated using the
following formula: Length × width2 ×0.52. The maximum
volume of liver tumors was 91 mm3, and the maximum
diameter was 7 mm.
Statistical analysis
Data were analyzed with Prism 6.0 (GraphPad
Software, Inc.) or SPSS 19.0 software (IBM Corp.). For categorical
data, chi-square analysis or Fisher's exact test was used.
Kaplan-Meier and Cox proportional hazards regression analysis were
applied to assess overall survival. Experiments were repeated at
least three times independently. Comparisons among multiple groups
were made using ANOVA followed by least significant difference post
hoc test. Normally distributed data are presented as the mean ±
standard error and were analyzed using Student's t-test.
Non-normally distributed data were analyzed using Mann-Whitney U
test. Spearman rank correlation coefficient was used to analyze the
association between the protein expression levels of S100A8 and
VEGF. P<0.05 was considered to indicate a statistically
significant difference.
Results
S100A8 is highly expressed in CCA, and
its expression is closely associated with CCA severity
To investigate the role of S100A8 in CCA,
pathological CCA tumor tissue and adjacent normal tissue specimens
were collected from 41 patients with CCA. S100A8 expression was
found to be higher in CCA tissues compared with adjacent normal
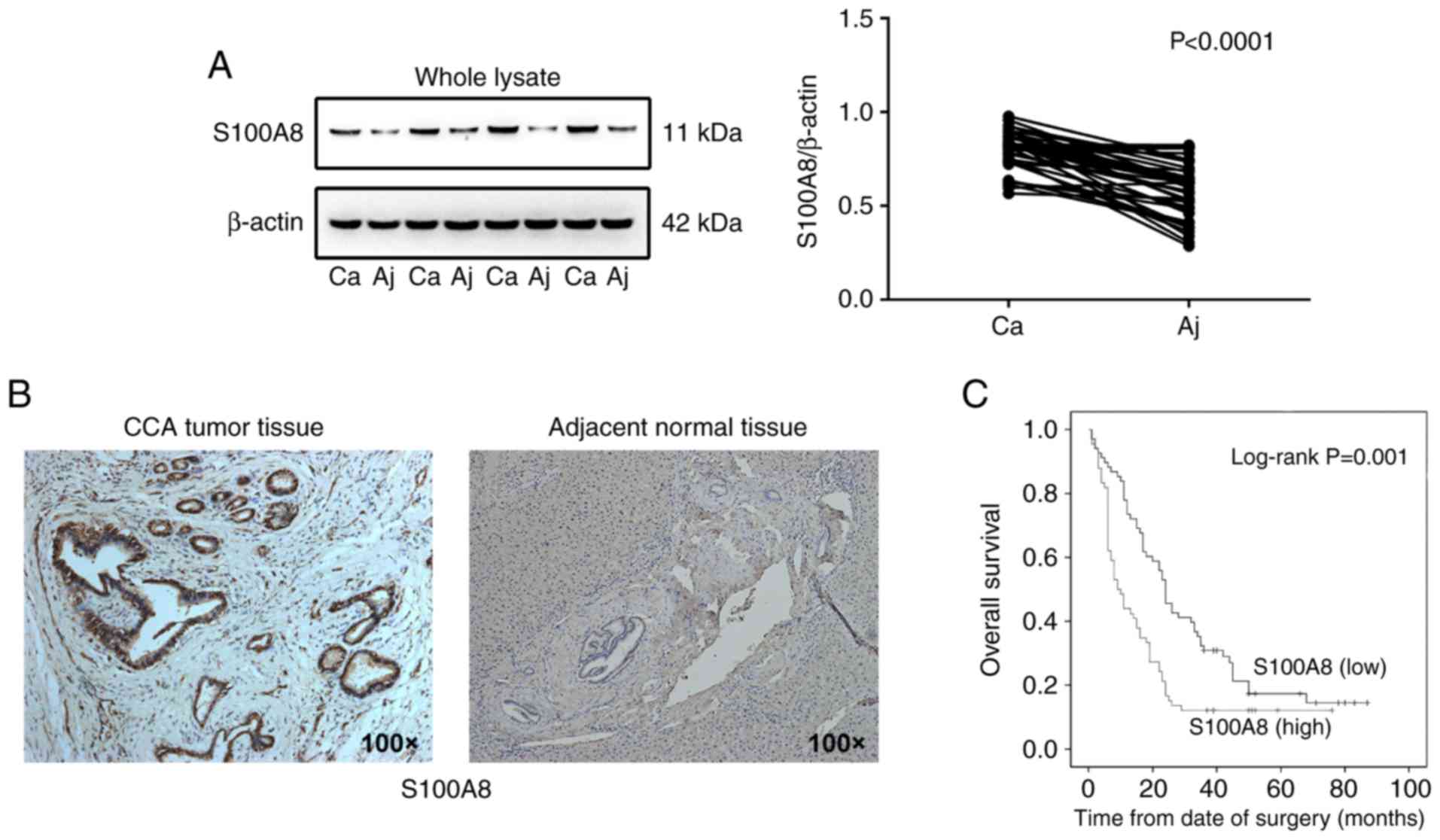
tissues, as detected by western blot analysis (Fig. 1A). In addition, increased S100A8
expression was observed in CCA primary tumor tissues compared with
the adjacent normal tissues by immunohistochemical staining
(Fig. 1B and Fig. S1A). To further evaluate the
association between S100A8 protein expression and CCA malignancy,
the S100A8 protein levels were detected in 134 pathological
specimens from patients with CCA via ELISA. S100A8 expression
levels were significantly associated with tumor differentiation
status and lymph node metastasis, but not with age or sex (Table I). In accordance with this finding,
S100A8 expression levels were also significantly associated with
poor prognosis in patients with CCA following surgical resection,
as evaluated by multivariate Cox proportional hazards regression
analysis (Table II). In addition,
Kaplan-Meier survival analysis revealed that high S100A8 expression
was correlated with a significant reduction in overall survival of
patients with CCA (Fig. 1C).
Because S100A8 is known to be highly expressed by neutrophils, the
relationship between S100A8 expression and neutrophil numbers was
investigated in the 134 patients with CCA in the present study, but
no significant correlation of S100A8 expression with the number of
neutrophils in the peripheral blood was observed (Fig. S1B). The present results indicated
that S100A8 expression was significantly associated with the
severity of CCA.
 | Table IAssociation between S100A8 expression
levels and clini-copathological features in 134 patients with
cholangiocarcinoma. |
Table I
Association between S100A8 expression
levels and clini-copathological features in 134 patients with
cholangiocarcinoma.
| Clinicopathological
feature | S100A8 expression
| P-value |
|---|
| Low 68 (%) | High 66 (%) |
|---|
| Sex | | | 0.468 |
| Male | 43 (63) | 46 (70) | |
| Female | 25 (37) | 20 (30) | |
| Age (years) | | | 0.729 |
| <55 | 30 (44) | 32 (48) | |
| ≥55 | 38 (56) | 34 (52) | |
|
Differentiation | | | 0.037 |
| Poor | 19 (28) | 24 (36) | |
| Moderate | 43 (63) | 42 (64) | |
| High | 6 (9) | 0 (0) | |
| Lymph node
metastasis | | | 0.01 |
| Yes | 30 (44) | 44 (67) | |
| No | 38 (56) | 22 (33) | |
 | Table IIMultivariate Cox proportional hazards
regression analysis for S100A8 expression levels and overall
survival in patients with cholangiocarcinoma. |
Table II
Multivariate Cox proportional hazards
regression analysis for S100A8 expression levels and overall
survival in patients with cholangiocarcinoma.
| Factor | Hazard ratio (95%
CI) | P-value |
|---|
| Expression of
S100A8 (low vs. high) | 1.654
(1.130–2.421) | 0.01 |
| Sex | 0.850
(0.568–1.272) | 0.429 |
| Age | 1.404
(0.960–2.055) | 0.08 |
|
Differentiation | 0.610
(0.420–0.884) | 0.009 |
| Lymph node
metastasis | 0.312
(0.206–0.473) | <0.001 |
S100A8 facilitates CCA cell invasion and
migration in vitro
Next, the present study hypothesized that S100A8 may
be involved in promoting CCA metastasis. To investigate this, the
RBE CCA cell line, which has low intrinsic S100A8 protein
expression, was transduced with lentivirus to overexpress S100A8.
The transduction efficiency was confirmed by immunoblotting; the
results revealed that S100A8 protein expression was significantly
upregulated in the RBE-overexpressing cells compared with the
negative control cells (Fig. 2A;
clone A was selected for further experiments). In vitro
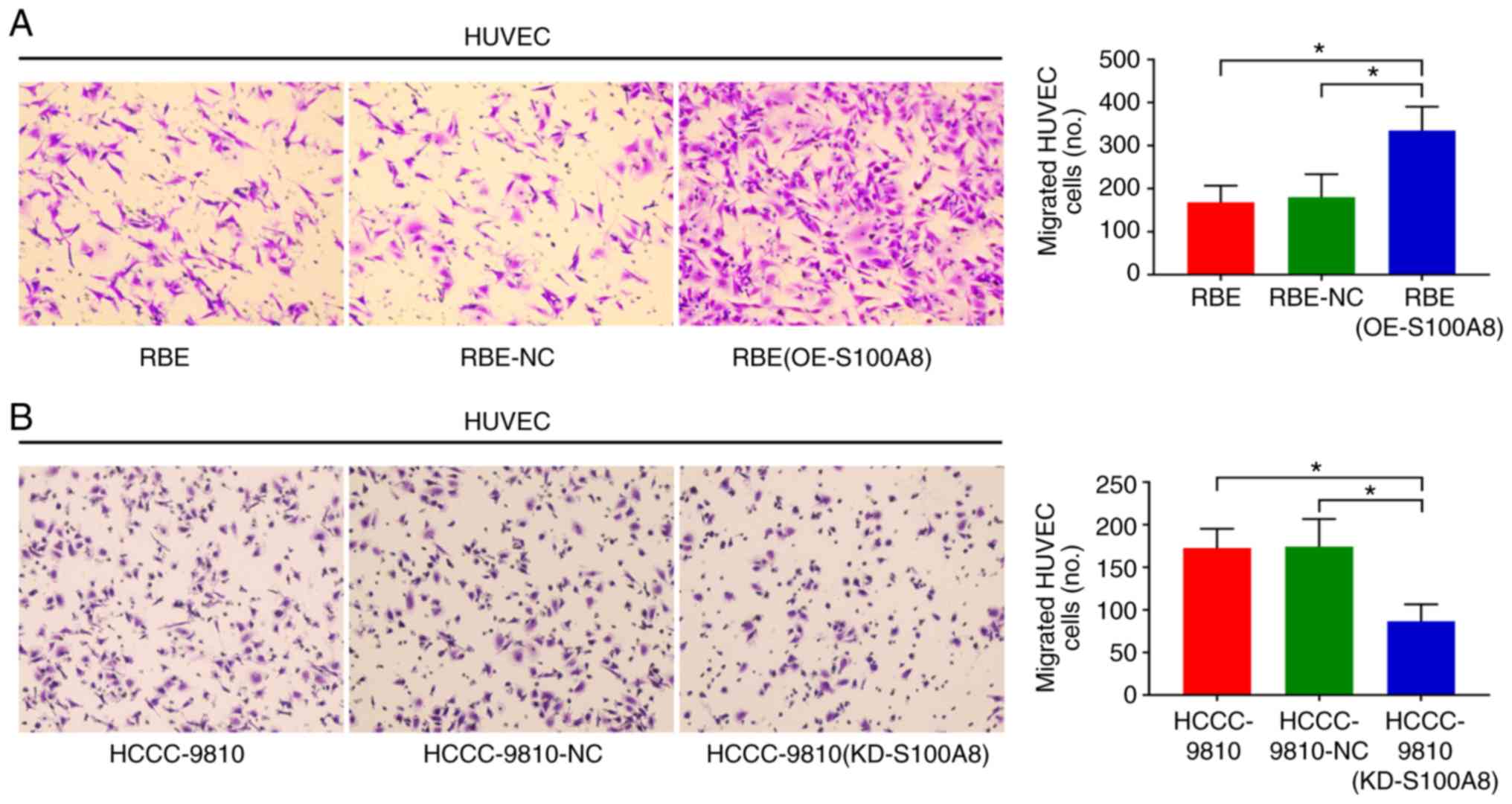
Transwell assays revealed that S100A8-overexpressing RBE cells
displayed a significant increase in migration ability compared with
control cells (Fig. 2C). Transwell
invasion assays (with Matrigel-coated inserts) gave similar results
for cell invasion (data not shown).
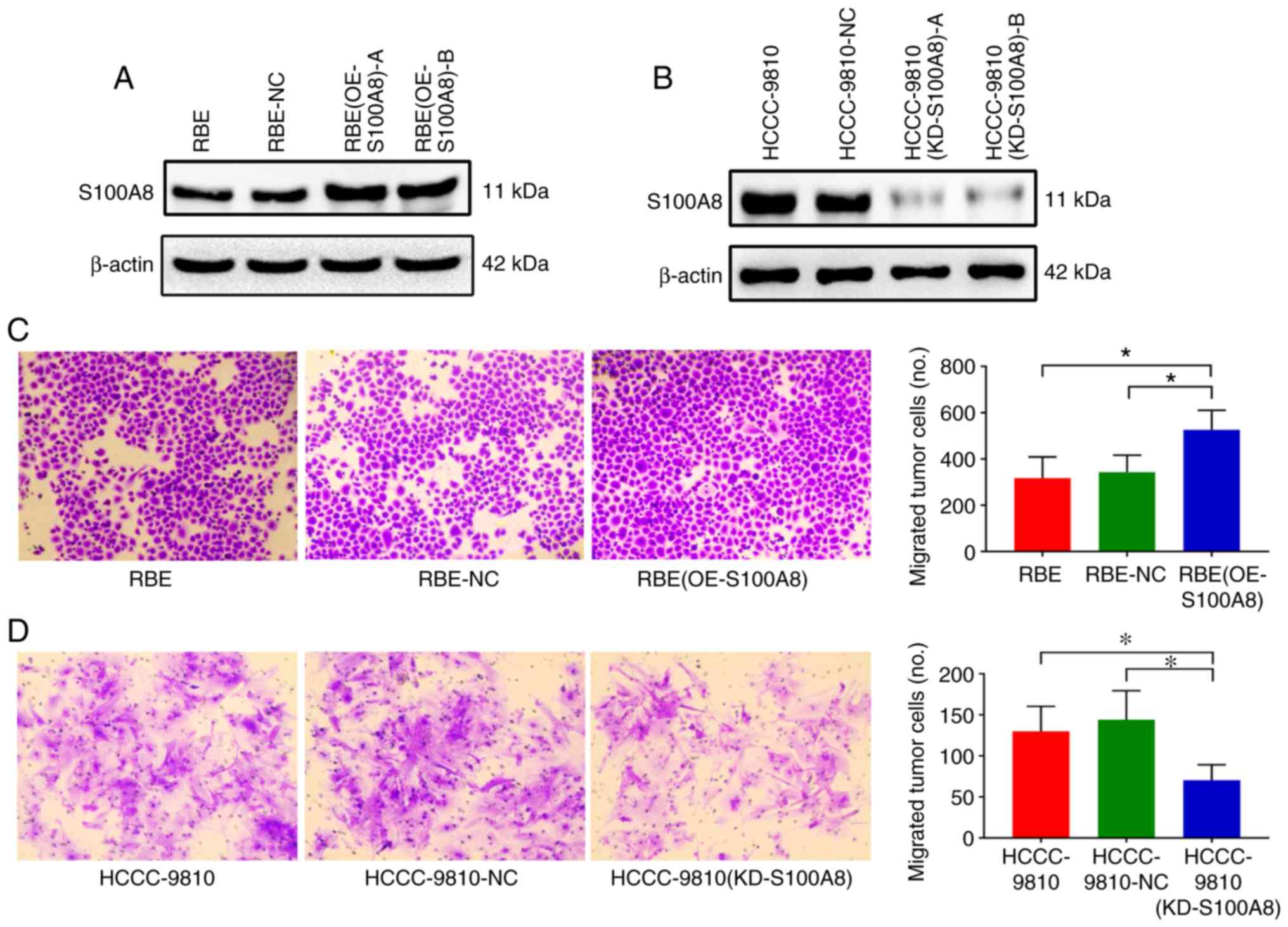 | Figure 2S100A8 facilitates CCA cell migration
in vitro. (A) Overexpression of S100A8 in the RBE CCA cell
line following lentivirus transduction was confirmed by western
blotting. Two separate overexpressing clones, (OE-S100A8)-A and
(OE-S100A8)-B, were tested. (B) Knockdown of S100A8 in the
HCCC-9810 CCA cell line following lentivirus transduction was
confirmed by western blotting. Two separate knockdown clones,
(KD-S100A8)-A and (KD-S100A8)-B, were tested. (C) Transwell
migration assays of control parental, lentiviral
control-transduced, and S100A8-overexpressing RBE cells or (D)
S100A8-knockdown HCCC-9810 cells. *P<0.05 with
comparisons indicated by brackets. S100A8, S100 calcium-binding
protein A8; CCA, cholangiocarcinoma; OE, overexpression; KD,
knockdown; NC, negative control. |
To determine whether silencing of S100A8 expression
could attenuate the migration of CCA cells, another CCA cell line,
HCCC-9810, which has high intrinsic S100A8 expression, was used.
Knockdown of S100A8 in HCCC-9810 cells was established via a
shRNA-encoding lentivirus, and successful transduction was
confirmed by western blotting (Fig.
2B; clone A was selected for further experiments). In
vitro Transwell assays revealed that S100A8-silenced HCCC-9810
cells had significantly reduced migration ability compared with
control cells (Fig. 2D). These
results indicated that S100A8 may participate in promoting CCA
migration.
S100A8 enhances the tumor-induced
migration of vascular endothelial cells in vitro
Because angiogenesis is a key process contributing
in malignant tumor growth, invasion and metastasis, the role of
S100A8 in promoting angiogenesis was investigated next. The ability
of RBE cells overexpressing S100A8 to promote migration of HUVECs
was analyzed using a Transwell assay. Conditioned media from
S100A8-overexpressing and control RBE cells was placed into the
lower chamber of the Transwell system, and HUVECs were placed into
the upper chamber. The number of migratory HUVECs was found to be
significantly increased towards the conditioned media of the
S100A8-overexpressing RBE cells compared with the control group
(Fig. 3A). However, no migration
of lentivirus-transduced RBE tumor cells or control cells was
observed when HUVECs were placed into the lower chamber and
lentivirus-transduced RBE cells and control cells were placed into
the upper chamber (Fig. S2). In
addition, these observations were further confirmed using the
second CCA cell line, HCCC-9810; S100A8 knockdown reduced the
ability of HCCC-9810 to induce HUVEC migration (Fig. 3B). These findings indicated that
S100A8 had a pro-angiogenesis function in CCA.
S100A8 promotes CCA dissemination and
metastasis in vivo
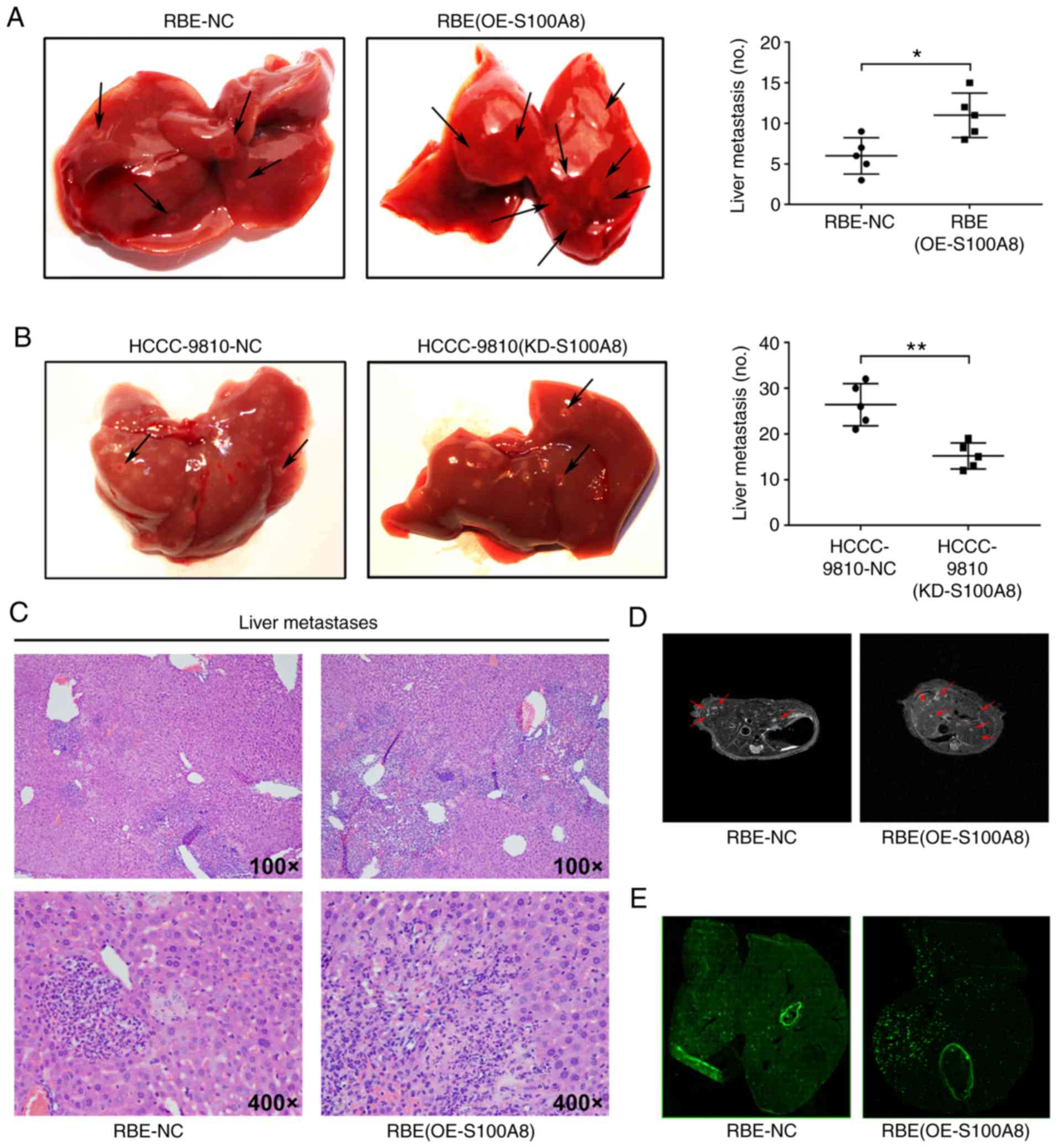
To investigate whether S100A8 increases CCA
metastasis in vivo, a liver experimental metastasis model
was established in mice. Control RBE and S100A8-overexpressing RBE
cells were injected into the spleens of wild-type mice, and then,
liver metastasis was monitored. Both S100A8-overexpressing RBE
cells and control RBE cells formed metastatic foci in the liver,
but S100A8-overexpressing RBE cells resulted in significantly
increased numbers of metastatic foci (Fig. 4A, C and D). By contrast, S100A8
knockdown reduced the metastatic ability of HCCC-9810 CCA cells
in vivo (Fig. 4B). To
investigate whether S100A8 protein overexpression or knockdown
affects metastatic growth, the volume of the liver metastatic foci
was measured, and no significant difference was found between the
S100A8-overexpressing, the S100A8-silenced and the control CCA
cells (data not shown).
Tumor metastasis is primarily dependent on
hematogenous dissemination in the blood circulation (30). To further investigate whether
S100A8-overexpressing RBE cells increase metastatic dissemination
in the liver, equal quantities of fluorescently-labelled RBE and
S100A8-overexpressing RBE cells were injected into the spleen of
mice. Compared with control cells, S100A8-overexpressing RBE cells
resulted in a significantly increased liver metastatic tumor burden
(Fig. 4E). In addition,
fluorescence microscopy analysis revealed that the tumor cells were
primarily distributed around the vasculature (Fig. 4E), indicating that these tumor
cells may have entered surrounding tissues through the blood
vessels to form metastases. These data suggested that S100A8 may
enhance the dissemination and metastasis ability of CCA cells in
vivo.
S100A8 upregulates VEGF expression in CCA
cells
Given that VEGF is a key factor in tumor invasion
and metastasis (7,22), the present study next evaluated the
VEGF expression levels in the patient tissues. The results
demonstrated that VEGF was more highly expressed in CCA tissues
compared with adjacent normal tissues from patients with CCA
(Fig. 5A and B, and Fig. S3). These results were similar to
the S100A8 expression results. Consistently, VEGF expression was
also significantly associated with tumor differentiation status,
lymph node metastasis and poor prognosis in patients with CCA
following surgical resection (Tables
III and IV). In addition,
Kaplan-Meier survival analysis revealed that high VEGF expression
was associated with a significant decrease in the overall survival
of patients with CCA (Fig.
5C).
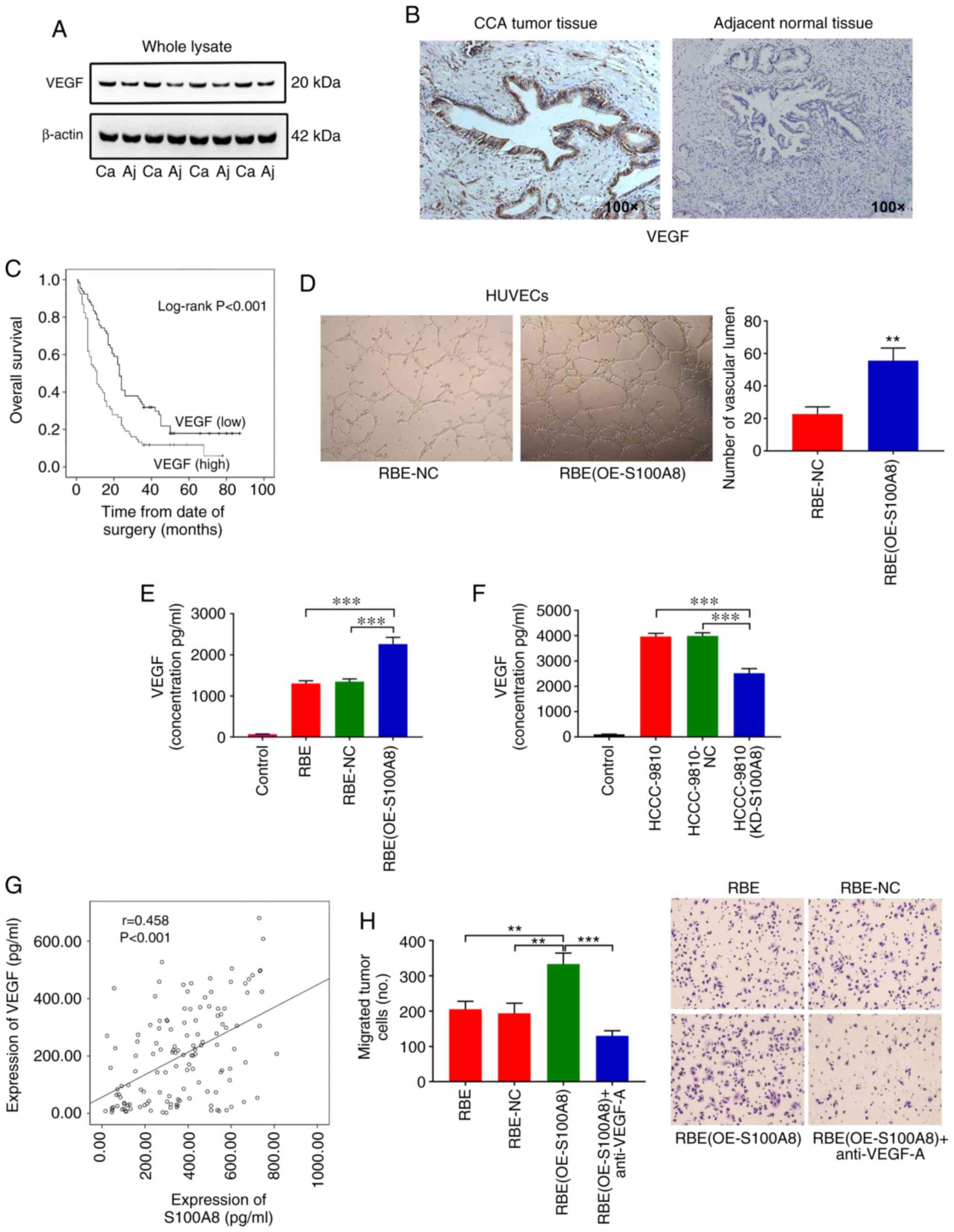 | Figure 5S100A8 upregulates VEGF expression in
CCA cells. (A) VEGF protein expression levels were determined by
western blotting in 41 tumor tissues and adjacent normal tissues
from patients with CCA. Representative blots from 4 paired samples
are shown. (B) Representative images from immunohistochemical
staining of VEGF in CCA tumor (high expression) and adjacent normal
tissues (low expression). (C) Kaplan-Meier plot for overall
survival of 134 CCA patients with low or high VEGF expression
(based the mean expression level). (D) Representative images and
quantification of vascular tube-like formation in HUVECs treated
with supernatants from RBE cells with or without S100A8
overexpression (n=3). (E) RBE cells with or without S100A8
overexpression were cultured in 1.0 ml of medium containing 10% FBS
for 24 h. The total VEGF levels were then determined by ELISA. (F)
HCCC-9810 cells with or without S100A8 knockdown were cultured in
1.0 ml of medium containing 10% FBS for 24 h. The total VEGF levels
were then determined by ELISA. (G) Correlation between S100A8 and
VEGF protein levels in 134 patients with CCA, as determined by
ELISA. (H) Migration of RBE cells (with or without S100A8
overexpression) following treatment with a neutralizing VEGF
antibody. **P<0.01 and ***P<0.001, with
comparisons indicated by brackets. S100A8, S100 calcium-binding
protein A8; VEGF, vascular endothelial growth factor; CCA,
cholangiocarcinoma; HUVEC, human umbilical vein endothelial cell;
Ca, carcinoma; Aj, adjacent normal; OE, overexpression; KD,
knockdown; NC, negative control. |
 | Table IIIAssociation between VEGF expression
levels and clinicopathological features in 134 patients with
cholangiocarcinoma. |
Table III
Association between VEGF expression
levels and clinicopathological features in 134 patients with
cholangiocarcinoma.
| Clinicopathological
feature | VEGF expression
| P-value |
|---|
| Low 68 (%) | High 66 (%) |
|---|
| Sex | | | 0.361 |
| Male | 41 (62) | 48 (71) | |
| Female | 25 (38) | 20 (29) | |
| Age (years) | | | 0.392 |
| <55 | 28 (42) | 34 (50) | |
| ≥55 | 38 (58) | 34 (50) | |
|
Differentiation | | | 0.008 |
| Poor | 16 (24) | 27 (40) | |
| Moderate | 44 (67) | 41 (60) | |
| High | 6 (9) | 0 (0) | |
| Lymph node
metastasis | | | 0.015 |
| Yes | 29 (44) | 45 (66) | |
| No | 37 (56) | 23 (34) | |
 | Table IVMultivariate Cox proportional hazards
regression analysis for VEGF expression levels and overall survival
in patients with cholangiocarcinoma. |
Table IV
Multivariate Cox proportional hazards
regression analysis for VEGF expression levels and overall survival
in patients with cholangiocarcinoma.
| Factor | Hazard ratio (95%
CI) | P-value |
|---|
| Expression of VEGF
(low vs. high) | 1.781
(1.213–2.614) | 0.003 |
| Sex | 0.842
(0.563–1.259) | 0.401 |
| Age | 1.498
(1.020–2.201) | 0.039 |
|
Differentiation | 0.619
(0.424–0.903) | 0.013 |
| Lymph node
metastasis | 0.307
(0.202–0.466) | <0.001 |
Treatment of HUVECs with supernatants from
S100A8-overexpressing RBE cells distinctly promoted the formation
of vascular tube-like structures (Fig.
5D). In addition, a previous study has reported that S100A8 can
promote the secretion of vasoactive factors, including VEGF
(7). These findings suggested that
VEGF may be responsible for the role of S100A8 in facilitating CCA
dissemination and metastasis. Notably, overexpression or knockdown
of S100A8 significantly increased or reduced the secretion of VEGF
from CCA cells, respectively (Fig. 5E
and F). In addition, S100A8 expression levels were
significantly correlated with VEGF expression levels in the tissues
of the 134 patients with CCA (Fig.
5G). Multivariate Cox proportional hazards regression analyses
revealed that combined high S100A8 and high VEGF expression levels
were significantly associated with poor prognosis in patients with
CCA following surgical resection (Table V). Finally, inhibition of VEGF via
a neutralizing antibody abrogated the S100A8-enhanced migration of
RBE cells (Fig. 5H). Therefore,
these results suggested that S100A8 may play a crucial role in CCA
progression through upregulation of VEGF.
 | Table VMultivariate Cox proportional hazards
regression analysis for combined S100A8/VEGF expression levels and
overall survival in patients with cholangiocarcinoma. |
Table V
Multivariate Cox proportional hazards
regression analysis for combined S100A8/VEGF expression levels and
overall survival in patients with cholangiocarcinoma.
| Factor | Hazard ratio (95%
CI) | P-value |
|---|
| Expression of
high | 1.819
(1.210–2.734) | 0.004 |
| S100A8/high
VEGF | | |
| Sex | 0.896
(0.599–1.340) | 0.593 |
| Age | 1.380
(0.944–2.018) | 0.096 |
|
Differentiation | 0.652
(0.447–0.950) | 0.026 |
| Lymph node
metastasis | 0.316
(0.208–0.480) | <0.001 |
S100A8 increases VEGF expression by
activating the TLR4/NF-κB pathway
Finally, the present study sought to determine the
underlying mechanism by which S100A8 upregulates VEGF expression.
TLR4 has been reported to be a receptor of S100A8, and NF-κB, which
is downstream of TLR4, is responsible for VEGF production (31,32).
Therefore, it was speculated that S100A8 might promote VEGF
expression through the TLR4/NF-κB pathway. As anticipated,
overexpression of S100A8 in RBE cells activated the NF-κB pathway
(Fig. 6A). Notably, TLR4 and NF-κB
inhibition completely abrogated the S100A8 overexpression-induced
upregulation of VEGF expression in RBE cells (Fig. 6E), and significantly inhibited RBE
cell migration (Fig. 6C). In
addition, results from these assays in HCCC-9810 cells revealed
that the NF-κB pathway was crucial to the migration of the CCA
cells and the secretion of VEGF (Fig.
6B, D and F). Taken together, the present findings demonstrated
that the TLR4/NF-κB pathway mediated the role of S100A8 in
promoting VEGF expression in CCA cells.
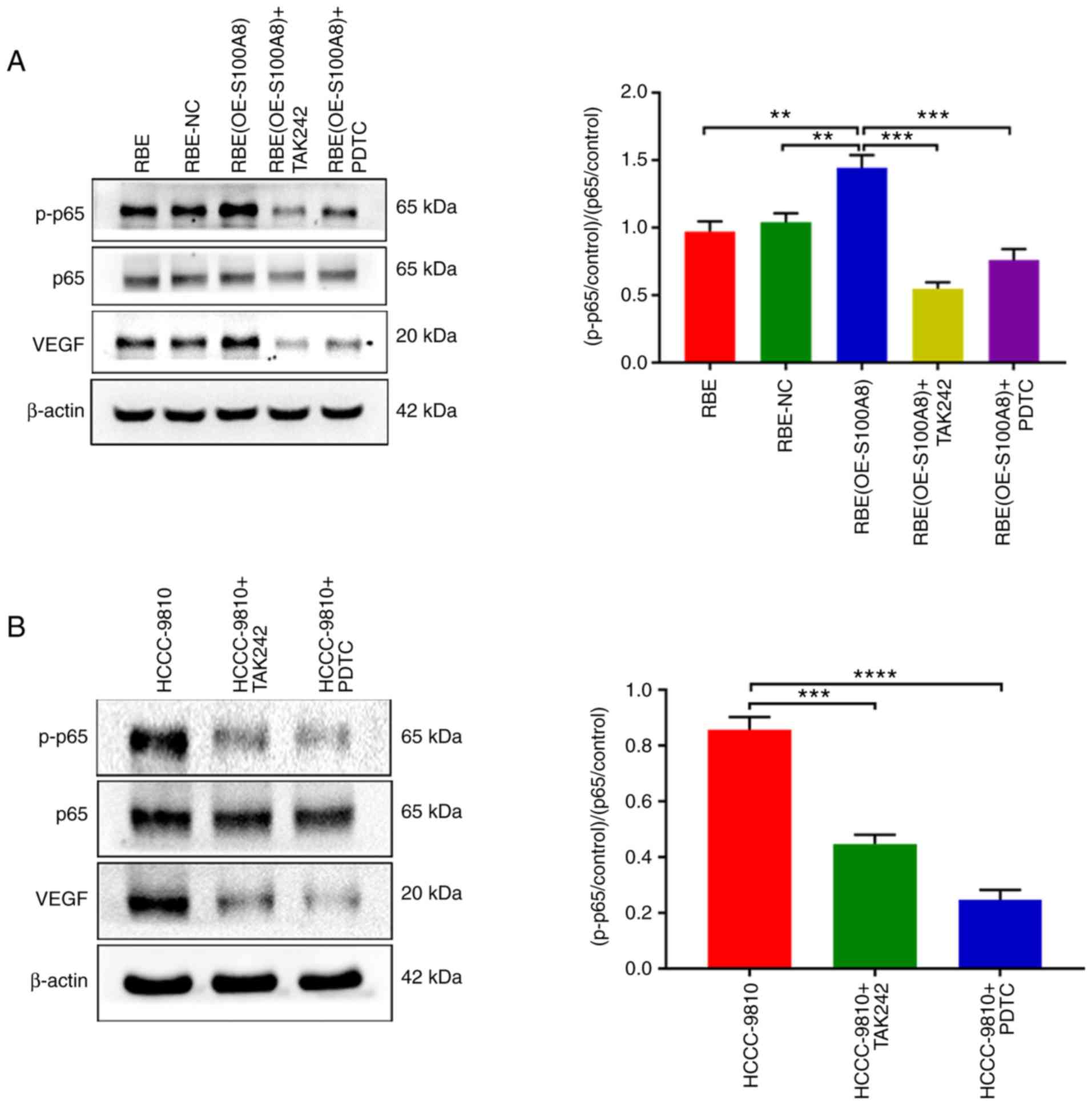 | Figure 6S100A8 increases VEGF expression by
activating the TLR4/NF-κB pathway. (A) p-p65, total p65 and VEGF
protein expression levels were determined by western blotting in
RBE parental, NC and S100A8-overexpressing cells, with or without
treatment with the TAK242 and PDTC inhibitors. (B) p-p65, total p65
and VEGF protein expression levels were determined by western
blotting in HCCC-9810 cells with or without treatment with the
TAK242 and PDTC inhibitors. (C) RBE parental, NC and
S100A8-overexpressing cells, with or without treatment with the
TAK242 and PDTC inhibitors, were allowed to migrate in Transwell
chambers for 12 h, then the numbers of migrated cells were
determined by microcopy (magnification, ×200). (D) HCCC-9810 cells
with or without treatment with the TAK242 and PDTC inhibitors were
allowed to migrate in Transwell chambers for 12 h, then the numbers
of migrated cells were determined by microcopy (magnification,
×200). (E) RBE parental, NC and S100A8-overexpressing cells, with
or without treatment with the TAK242 and PDTC inhibitors, were
seeded separately into 24-well dishes in 1 ml of medium containing
10% FBS and cultured for 24 h. The total VEGF levels were then
determined by ELISA. (F) HCCC-9810 cells with or without treatment
with the TAK242 and PDTC inhibitors were seeded separately into
24-well dishes in 1 ml of medium containing 10% FBS and cultured
for 24 h. The total VEGF levels were then determined by ELISA.
*P<0.05, **P<0.01 and
***P<0.001, with comparisons indicated by brackets.
S100A8, S100 calcium-binding protein A8; VEGF, vascular endothelial
growth factor; TLR, Toll-like receptor; p-, phosphorylated; NC,
negative control; OE, overexpression; KD, knockdown. |
Discussion
Patients with CCA have a poor prognosis and high
mortality due to the increased invasiveness and early metastasis of
CCA (33–35). Although many studies have focused
on CCA, the molecular mechanisms underlying CCA invasion and
metastasis have not been fully elucidated. The present study
demonstrated for the first time that S100A8 promoted CCA migration
and metastasis via the TLR4/NF-κB/VEGF signaling axis.
Previous studies have shown that S100A8 protein is
localized in the cytoplasm of multiple cell types and that it has
multiple biological functions, including regulating cell cycle
progression, affecting cell differentiation and inducing cell
apoptosis (36–38). S100A8 can also function as a
cytokine that is primarily secreted by neutrophils to activate
monocytes and macrophages (39,40).
Notably, other studies have observed increased expression of S100A8
protein in multiple tumor types (37,41),
however, the expression and role of S100A8 protein in malignant
bile duct tumors have not been previously elucidated. The present
study reported that S100A8 was more highly expressed in CCA tissues
compared with adjacent normal tissues, suggesting that S100A8 may
have a unique role in CCA. Additionally, S100A8 expression was
significantly associated with CCA severity and poor clinical
prognosis. Furthermore, in vitro and in vivo
experiments revealed that S100A8 had a crucial role in CCA tumor
migration and metastasis.
New blood vessels in tumor tissues are required for
tumor growth and metastasis (42–44),
and VEGF is well-established as a factor required to promote
angiogenesis, which markedly enhances tumor invasion and metastasis
(23). In the present study,
through analysis of pathological specimens, it was demonstrated
that VEGF expression levels were increased in CCA tumor tissues
compared with adjacent normal tissues, and that they were
significantly associated with the poor prognosis of CCA, which was
similar to the S100A8 findings. Furthermore, in vitro
overexpression or knockdown of S1000A8 in CCA cells resulted in
increased or decreased, respectively, expression of VEGF. According
to a previous study, the migration of endothelial cells induced by
tumor cells greatly increases the microvessel density in tumors,
thus serving a pivotal role in tumor invasion and metastasis
(7,45). In line with this notion, the
present study confirmed that S100A8-overexpressing CCA cells
increased the migration of endothelial cells, therefore potentially
leading to an enhancement in tumor dissemination and metastasis
ability. However, other studies have reported that a low S100A8
concentration promotes endothelial cell migration and expansion and
enhances vascular permeability and a high S100A8 concentrations
leads to necrosis of endothelial cells (46–48).
Thus, the biological functions of S100A8 may be different depending
on the cell or tumor type.
Multiple studies have reported that the NF-κB
pathway has an important role in the progression multiple types of
cancer, by promoting cell proliferation and inhibiting cell
apoptosis (49,50). In addition, the NF-κB pathway is
responsible for generation of many of the cytokines associated with
tumor angiogenesis, such as VEGF (51,52).
The current study provided experimental evidence that S100A8
promoted CCA cell migration, at least in part, through the
TLR4/NF-κB/VEGF signaling pathway. However, the possibility that
there could be other mechanisms that also mediate the role of
S100A8 in CCA migration and metastasis cannot be excluded, and
further research will be required to fully elucidate this.
In conclusion, the present study highlighted the
importance of S100A8 in upregulation of VEGF expression through
activation of the TLR4/NF-κB pathway, thereby providing a favorable
microenvironment for tumor invasion and metastasis. Therefore,
S100A8 may serve as a potential target for tumor metastasis.
Additionally, the present findings suggested that S100A8 may be a
candidate prognostic marker for CCA.
Supplementary Data
Funding
This work was supported by grants from the National
Natural Science Fund of China (nos. 81725019, 81502755 and
81500087) and the Scientific Research Project of The Chinese
People's Liberation Army (grant no. AWS16J014).
Availability of data and materials
The datasets used and analyzed in the current study
are available from the corresponding author upon reasonable
request.
Authors' contributions
JW and YH designed and supervised all experiments.
MC and PZ prepared the materials and contributed to data
acquisition. YH, YX, CD, JL, YZ, JB and JZ assisted with the animal
and molecular biology experiments. JC and PJ analyzed the data. SP
and MH drafted the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Experiments were approved by the Ethics Committee of
the Southwest Hospital of the Third Military Medical University of
China. Written, informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We thank the Institute of Hepatobiliary Surgery of
Southwest Hospital for providing human cholangiocarcinoma tissue.
We also thank the Statistical Teaching and Research Department of
the Third Military Medical University for technical assistance.
References
|
1
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tyson GL and El-Serag HB: Risk factors for
cholangiocarcinoma. Hepatology. 54:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al: Guidelines for the diagnosis and
treatment of cholangiocarcinoma: An update. Gut. 61:1657–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim SY, Yuzhalin AE, Gordon-Weeks AN and
Muschel RJ: Tumor-infiltrating monocytes/macrophages promote tumor
invasion and migration by upregulating S100A8 and S100A9 expression
in cancer cells. Oncogene. 35:5735–5745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu LX, Yan L, Yang W, Wu FQ, Ling Y, Chen
SZ, Tang L, Tan YX, Cao D, Wu MC, et al: Platelets promote tumour
metastasis via interaction between TLR4 and tumour cell-released
high-mobility group box1 protein. Nat Commun. 5:52562014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brantley-Sieders DM, Fang WB, Hwang Y,
Hicks D and Chen J: Ephrin-A1 facilitates mammary tumor metastasis
through an angiogenesis-dependent mechanism mediated by EphA
receptor and vascular endothelial growth factor in mice. Cancer
Res. 66:10315–10324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalinsky K, Mayer JA, Xu X, Pham T, Wong
KL, Villarin E, Pircher TJ, Brown M, Maurer MA and Bischoff FZ:
Correlation of hormone receptor status between circulating tumor
cells, primary tumor, and metastasis in breast cancer patients.
Clin Transl Oncol. 17:539–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srikrishna G: S100A8 and S100A9: New
insights into their roles in malignancy. J Innate Immun. 4:31–40.
2012. View Article : Google Scholar :
|
|
11
|
Leukert N, Vogl T, Strupat K, Reichelt R,
Sorg C and Roth J: Calcium-dependent tetramer formation of S100A8
and S100A9 is essential for biological activity. J Mol Biol.
359:961–972. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim SY, Raftery MJ and Geczy CL: Oxidative
modifications of DAMPs suppress inflammation: The case for S100A8
and S100A9. Antioxid Redox Signal. 15:2235–2248. 2011. View Article : Google Scholar
|
|
13
|
Porta C, Larghi P, Rimoldi M, Totaro MG,
Allavena P, Mantovani A and Sica A: Cellular and molecular pathways
linking inflammation and cancer. Immunobiology. 214:761–777. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hiratsuka S, Watanabe A, Sakurai Y,
Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S,
Aburatani H and Maru Y: The S100A8-serum amyloid A3-TLR4 paracrine
cascade establishes a pre-metastatic phase. Nat Cell Biol.
10:1349–1355. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kapanadze T, Gamrekelashvili J, Ma C, Chan
C, Zhao F, Hewitt S, Zender L, Kapoor V, Felsher DW, Manns MP, et
al: Regulation of accumulation and function of myeloid derived
suppressor cells in different murine models of hepatocellular
carcinoma. J Hepatol. 59:1007–1013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamerlik P, Lathia JD, Rasmussen R, Wu Q,
Bartkova J, Lee M, Moudry P, Bartek J Jr, Fischer W, Lukas J, et
al: Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma
stem-like cell viability and tumor growth. J Exp Med. 209:507–520.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chatterjee S, Heukamp LC, Siobal M,
Schöttle J, Wieczorek C, Peifer M, Frasca D, Koker M, König K,
Meder L, et al: Tumor VEGF:VEGFR2 autocrine feed-forward loop
triggers angiogenesis in lung cancer. J Clin Invest. 123:1732–1740.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alessi C, Scapulatempo Neto C, Viana CR
and Vazquez VL: PD-1/PD-L1 and VEGF-A/VEGF-C expression in lymph
node microenvironment and association with melanoma metastasis and
survival. Melanoma Res. 27:565–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X, Zhang Y, Hosaka K, Andersson P,
Wang J, Tholander F, Cao Z, Morikawa H, Tegnér J, Yang Y, et al:
VEGF-B promotes cancer metastasis through a VEGF-A-independent
mechanism and serves as a marker of poor prognosis for cancer
patients. Proc Natl Acad Sci USA. 112:E2900–E2909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gay LJ and Felding-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dehghani S, Nosrati R, Yousefi M, Nezami
A, Soltani F, Taghdisi SM, Abnous K, Alibolandi M and Ramezani M:
Aptamer-based biosensors and nanosensors for the detection of
vascular endothelial growth factor (VEGF): A review. Biosens
Bioelectron. 110:23–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang D, Song SJ, Wu ZZ, Wu W, Cui XY,
Chen JN, Zeng MS and Su SC: Epstein-barr virus-induced VEGF and
GM-CSF drive nasopharyngeal carcinoma metastasis via recruitment
and activation of macrophages. Cancer Res. 77:3591–3604. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Ji S, Zhang B, Liu J, Qin Y, Xu J
and Yu X: Role of angiogenesis in pancreatic cancer biology and
therapy. Biomed Pharmacother. 108:1135–1140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Dreden P, Epsilonlalamy I and
Gerotziafas GT: The role of tissue factor in cancer-related
hypercoagulability, tumor growth, angiogenesis and metastasis and
future therapeutic strategies. Crit Rev Oncog. 22:219–248. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Long T, Liu Z, Shang J, Zhou X, Yu S, Tian
H and Bao Y: Polygonatum sibiricum polysaccharides play anti-cancer
effect through TLR4-MAPK/NF-κB signaling pathways. Int J Biol
Macromol. 111:813–821. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu JW, Young E, Busby B, Covington J and
Johnson JW: Oral administration of pyrrolidine dithiocarbamate
(PDTC) inhibits VEGF expression, tumor angiogenesis, and growth of
breast cancer in female mice. Cancer Biol Ther. 8:514–521. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gan L, Pan S, Cui J, Bai J, Jiang P and He
Y: Functional analysis of the correlation between ABCB11 gene
mutation and primary intrahepatic stone. Mol Med Rep. 19:195–204.
2019.
|
|
28
|
Pan S, Li X, Jiang P, Jiang Y, Shuai L, He
Y and Li Z: Variations of ABCB4 and ABCB11 genes are associated
with primary intrahepatic stones. Mol Med Rep. 11:434–446. 2015.
View Article : Google Scholar
|
|
29
|
Zhang B, Wang D, Ji TF, Shi L and Yu JL:
Overexpression of lncRNA ANRIL up-regulates VEGF expression and
promotes angiogenesis of diabetes mellitus combined with cerebral
infarction by activating NF-κB signaling pathway in a rat model.
Oncotarget. 8:17347–17359. 2017.PubMed/NCBI
|
|
30
|
Labelle M and Hynes RO: The initial hours
of metastasis: The importance of cooperative host-tumor cell
interactions during hematogenous dissemination. Cancer Discov.
2:1091–1099. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin JM, Park JH, Kim HJ, Park IH and Lee
HM: Cigarette smoke extract increases vascular endothelial growth
factor production via TLR4/ROS/MAPKs/NF-kappaB pathway in nasal
fibroblast. Am J Rhinol Allergy. 31:78–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naruishi K and Nagata T: Biological
effects of interleukin-6 on Gingival Fibroblasts: Cytokine
regulation in periodontitis. J Cell Physiol. 233:6393–6400. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sonbare DJ: Influence of surgical margins
on outcome in patients with intrahepatic cholangiocarcinoma: A
multicenter study by the AFC-IHCC-2009 Study Group. Ann Surg.
259:e362014. View Article : Google Scholar
|
|
34
|
Lau SH and Lau WY: Current therapy of
hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 11:12–17.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin DL, Liang YJ, Zheng TS, Song RP, Wang
JB, Sun BS, Pan SH, Qu LD, Liu JR, Jiang HC and Liu LX: EF24
inhibits tumor growth and metastasis via suppressing NF-kappaB
dependent pathways in human cholangiocarcinoma. Sci Rep.
6:321672016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghavami S, Rashedi I, Dattilo BM, Eshraghi
M, Chazin WJ, Hashemi M, Wesselborg S, Kerkhoff C and Los M:
S100A8/A9 at low concentration promotes tumor cell growth via RAGE
ligation and MAP kinase-dependent pathway. J Leukoc Biol.
83:1484–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shabani F, Farasat A, Mahdavi M and Gheibi
N: Calprotectin (S100A8/S100A9): A key protein between inflammation
and cancer. Inflamm Res. 67:801–812. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miller P, Kidwell KM, Thomas D, Sabel M,
Rae JM, Hayes DF, Hudson BI, El-Ashry D and Lippman ME: Elevated
S100A8 protein expression in breast cancer cells and breast tumor
stroma is prognostic of poor disease outcome. Breast Cancer Res
Treat. 166:85–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Steinckwich N, Schenten V, Melchior C,
Bréchard S and Tschirhart EJ: An essential role of STIM1, Orai1,
and S100A8-A9 proteins for Ca2+ signaling and FcgammaR-mediated
phagosomal oxidative activity. J Immunol. 186:2182–2191. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ieguchi K, Omori T, Komatsu A, Tomita T,
Deguchi A and Maru Y: Ephrin-A1 expression induced by S100A8 is
mediated by the toll-like receptor 4. Biochem Biophys Res Commun.
440:623–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tanriover G, Eyinc MB, Aliyev E, Dilmac S
and Erin N: Presence of S100A8/Gr1-positive myeloid-derived
suppressor cells in primary tumors and visceral organs invaded by
breast carcinoma cells. Clin Breast Cancer. 18:e1067–e1076. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gacche RN and Meshram RJ: Angiogenic
factors as potential drug target: Efficacy and limitations of
anti-angiogenic therapy. Biochim Biophys Acta. 1846:161–179.
2014.PubMed/NCBI
|
|
44
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang W and Ferrara N: The Complex role of
neutrophils in tumor angiogenesis and metastasis. Cancer Immunol
Res. 4:83–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li C, Li S, Jia C, Yang L, Song Z and Wang
Y: Low concentration of S100A8/9 promotes angiogenesis-related
activity of vascular endothelial cells: Bridges among inflammation,
angiogenesis, and tumorigenesis? Mediators Inflamm.
2012:2485742012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Viemann D, Strey A, Janning A, Jurk K,
Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, et al:
Myeloid-related proteins 8 and 14 induce a specific inflammatory
response in human microvascular endothelial cells. Blood.
105:2955–2962. 2005. View Article : Google Scholar
|
|
48
|
Viemann D, Barczyk K, Vogl T, Fischer U,
Sunderkötter C, Schulze-Osthoff K and Roth J: MRP8/MRP14 impairs
endothelial integrity and induces a caspase-dependent and
-independent cell death program. Blood. 109:2453–2460. 2007.
View Article : Google Scholar
|
|
49
|
Li Y, Xie G, Li L, Jiang Z, Yue Z and Pan
Z: The effect of TLR4/MyD88/NF-κB signaling pathway on
proliferation and apoptosis in human nasopharyngeal carcinoma 5-8F
cells induced by LPS. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 29:1012–1015. 2015.In Chinese.
|
|
50
|
Jiang N, Xie F, Guo Q, Li MQ, Xiao J and
Sui L: Toll-like receptor 4 promotes proliferation and apoptosis
resistance in human papillomavirus-related cervical cancer cells
through the Toll-like receptor 4/nuclear factor-κB pathway. Tumour
Biol. 39:10104283177105862017. View Article : Google Scholar
|
|
51
|
O'Driscoll CM, Lima MP, Kaufmann WE and
Bressler JP: Methyl CpG binding protein 2 deficiency enhances
expression of inflammatory cytokines by sustaining NF-κB signaling
in myeloid derived cells. J Neuroimmunol. 283:23–29. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Low P, Clark AM, Chou TC, Chang TC,
Reynolds M and Ralph SJ: Immunomodulatory activity of Melaleuca
alternifolia concentrate (MAC): Inhibition of LPS-induced NF-κB
activation and cytokine production in myeloid cell lines. Int
Immunopharmacol. 26:257–264. 2015. View Article : Google Scholar : PubMed/NCBI
|