Introduction
Melanin, which is found in various animal tissues,
such as the skin, eyes and hair, is a substance that determines
skin or hair colour and protects the skin from ultraviolet rays by
contributing to the reduction of cell damage by eliminating
reactive oxygen species (ROS) generated within the skin (1). Melanogenesis occurs in specialized
organelles, such as the melanosome of melanocytes, and can be
prompted a variety of paracrine cytokines, including α-melanocyte
stimulating hormone (α-MSH) (2,3). All
these factors activate the expression and activation of
pigment-related proteins, such as microphthalmia-associated
transcription factor (MITF), tyrosinase (TYR), tyrosine-related
protein-1 (TRP-1) and tyrosine-related protein-2 (TRP-2) (4). All signal transduction pathways
include MITF, the master regulator of melanogenesis, which
upregulates the expression of the melanogenesis-related enzymes,
TYR, TRP-1 and TRP-2 (5,6). Skin structure consists of the
epidermis, dermis and subcutaneous fat. In the epidermis,
keratinocytes are present in the spinous layer, and melanocytes are
present in the basal layer. Each melanocyte is surrounded by
approximately 10-40 keratinocytes (7). Melanogenesis refers to a series of
processes through which melanin is synthesized. When thymidine
dinucleotides (pTT), indicative of DNA damage in keratinocytes
found in the spinous layer caused by UV rays, are induced, p53, a
tumor suppressor protein, is activated and melanogenesis is
increased (8). Within
keratinocytes, activated p53 stimulates the promoter of
proopiomelanocortin (POMC), a precursor of α-MSH. α-MSH binds to
the melanocortin 1 receptor (MC1R) within melanocytes to activate
adenylate cyclase through a corresponding G-protein, ultimately
resulting in increases in the level of intracellular cAMP and the
activation of protein kinase A (PKA). PKA activates cAMP response
element binding protein (CREB) by phosphorylation, and activated
CREB induces the transcription of MITF (9,10).
Tyrosinase transcribed by MITF is a membrane protein that fuses
with melanosomes derived from lysosomes to oxidize L-tyrosine and
L-DOPA, and synthesize melanin through a series of processes
(9). Tyrosinase, a di-copper
metalloprotein (11), is an
essential element in melanogenesis that acts as a rate limiting
enzyme. The glycosylation of tyrosinase affects the activity of
tyrosinase (12). Melanosomes
undergo a 4-stage maturation process and migrate to dendrites
through microtubules, after which they are relayed to keratinocytes
by various mechanisms such as exocytosis, cytophagocytosis and the
fusion of plasma membrane vesicles to ultimately help prevent DNA
damage from UV radiation (13).
Malignant melanoma is a type of cancer that develops in
melanocytes, and UV exposure is the major cause of this malignancy
among individuals possessing low levels of skin pigment (14).
β-catenin is encoded by the CTNNB1 gene (15), and phosphorylation facilitated by
glycogen synthase kinase 3 (GSK3) and casein kinase (CK-1) that
form a complex with adenomatous polyposis coli (APC) and axin
protein (16). The GSK3β-induced
phosphorylation of the Ser-33 and 37 sites of β-catenin (16,17)
is essential for recognition by the β-TrCP E3 ubiquitin ligase
(18). β-catenin conjugated to
ubiquitin delivered by the E3 ubiquitin ligase is degraded by the
proteasome (17). The presence of
the cAMP and Wnt/β-catenin pathways in the upstream pathway of MITF
was confirmed through previous studies. The MITF promoter closest
to the common downstream exon is known as the M promoter and
appears to be selectively expressed in melanocytes (19). Wnt/β-catenin signal transduction is
important for the differentiation of melanocytes from neural ridges
(20). The binding of the WNT
protein to the Frizzled receptor causes the interaction of
β-catenin with the LEF/TCF transcription factor, leading to the
MITF-M promoter (21). In
addition, transcription factors involved in the regulation of the
MITF-M promoter include paired box gene 3 (PAX3), cAMP-reactive
element binding protein (CREB), SRY (sex determination region
Y)-box 10 (SOX10), lymphoid enhancer-binding factor (LEF, also
known as TCF), one cut domain 2 (ONECUT-2) and MITF itself
(22,23). In this study, the prevention of
melanin production by thymoquinone (TQ) was principally
investigated by WNT/β-catenin signaling.
Currently, a number of reagents are known to
supresses melanogenesis by inhibiting mRNA transcription,
interfering with glycosylation, inhibiting catalytic ability and
promoting the degradation of tyrosinase (24). Among these, albutin, which is well
known as a whitening agent, is an inhibitor that acts competitively
with the substrate L-tyrosine without affecting the transcription
of tyrosinase (25). Kojic acid
chelates copper, which is required in the active sites of
tyrosinase (26). Phenylthiourea
(PTU) induces the degradation of tyrosinase at the
post-translational level and also inhibits its catalytic activity
(27,28). It has been suggested, however, that
the use of these reagents as whitening or therapeutic agents for
pigmented lesions caused by the excessive secretion of melanin
pigment may be harmful to the skin. Consequently, the need to
develop ingredients containing natural extracts is increasing.
TQ is a phytochemical that is a component found in
the seeds of Nigella sativa, an annual plant that grows on
the Mediterranean coast. Seeds from Nigella sativa
containing TQ, a natural therapeutic agent, are processed in oil
and are sold as a health supplement. TQ has been shown to induce
the apoptosis of human colorectal cancer cells through a
p53-dependent mechanism (29), and
has also been shown to exert anti-inflammatory effects on
pancreatic cancer cells (30), and
to exert liver protective effects on isolated rat liver cells
(30). Recently, TQ has been
extensively studied in the context of both basic and clinical
research as a therapeutic agent for cancer treatment (31,32).
Various biological effects of TQ, including its anticancer,
anti-inflammatory (33) and
antioxidant effects, have been examined. However, to date, at least
to the best of our knowledge, studies on the molecular mechanisms
through which TQ inhibits melanogenesis are limited.
Therefore, the present study treated B16F10 mouse
melanoma cells with TQ and investigated the inhibition of
melanogenesis and the molecular mechanisms underlying this
inhibition. Treatment of the B16F10 cells with TQ resulted in a
dose-dependent decrease in the expression of MITF and tyrosinase
that was accompanied by decreased tyrosinase activity. These
treatments also simultaneously led to a decrease in the protein
expression and transcription of β-catenin, a Wnt signaling pathway
protein. Pre-treatment with MG132, a proteasome inhibitor, to
examine the inhibition of melanogenesis through the β-catenin
pathway by TQ treatment, revealed an increase in the expression of
β-catenin that was initially reduced by TQ, and the expression and
activity of MITF and tyrosinase was also increased. Pre-treatment
with LiCl, which is known to inactivate GSK3β by inducing the
phosphorylation of the Ser-9 site, resulted in an increased
phospho-GSK3β expression accompanied by β-catenin that was
initially reduced by TQ, and the recovery of the expression and
activity of tyrosinase was also confirmed. Transfection of S37A
cDNA into B16F10 cells that overexpress β-catenin resulted in the
recovery of β-catenin that was initially reduced by TQ, and this
treatment also recovered the expression and activity of tyrosinase.
Additionally, zebrafish were used in an animal experiment to
investigate the inhibition of melanogenesis by TQ in
vivo.
In this study, we aimed to determine whether TQ may
be used as a therapeutic potential compound due to its
anti-proliferative and anti-melanogenic effects by the inhibition
of the β-catenin pathway.
Materials and methods
Cells and cell culture
B16F10 mouse melanoma cells used in this study were
obtained from the Korean Cell Line Bank. The cells were added to
Dulbecco's modified Eagle's medium (DMEM, Gibco-BRL; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS, WELGENE),
50 µg/ml of streptomycin (Sigma-Aldrich) and 50 U/ml of
penicillin (Sigma-Aldrich) and incubated in 5% CO2, 37°C
incubator. The cells were subcultured in a culture dish at a
density of 2×105 cell/dish. The medium was exchanged
every 2-3 days, and when the cell density in the culture dish
reached 60%, the cells were treated with the reagents (TQ;
Sigma-Aldrich; 274666, PTU; Sigma-Aldrich; P7629, MG132;
Sigma-Aldrich; C221, LiCl; Calbiochem; 274666, NaCl; SAMCHUN;
S0484, KCl; Duchefa biochemie; 7447-40-7) used in the present
study. B16F10 melanoma cells were pre-incubated with 0-5 µM
MG132, 10-50 mM LiCl, 50 mM NaCl and KCl (negative control of LiCl)
for 3 h, followed by co-incubation with TQ for 24 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a
tetrazole (MTT) assay
MTT assay was performed to confirm cell viability.
B16F10 cells were dispensed to a 96-well plate (0.5×105
cells/well) and cultured at 37°C for 24 h and were subsequently
treated with 0-20 µM TQ (Sigma-Aldrich). Subsequently, 10
µl/well of MTT reagent I (10 mg/ml) were added followed by
culture for 4 h at 37°C. Subsequently, solubilization solution MTT
reagent II [10% sodium dodecyl sulfate (SDS) with 0,01 N HCl] was
added followed by culture for 12 h at 37°C. An ELISA reader (iMark
microplate absorbance reader, #1681130; Bio-Rad) was used to
measure the absorbance (550-600 nm) values.
Tyrosinase activity assay
Tyrosinase activity assay was performed to measure
intracellular tyrosinase activity. The protease inhibitor,
phenylmethylsulfonyl fluoride (PMSF, Sigma-Aldrich) was mixed with
pH 6.8 phosphate buffer containing 1% Triton X-100 to bring the
concentration to 1 mM, and proteins were then extracted using lysis
buffer. The extracted proteins were quantified by BSA assay and
reacted with 0.5 mg/ml L-DOPA (Sigma-Aldrich) at 37°C for 1 h.
Tyrosinase activity was determined by measuring the ability of
tyrosinase to degrade L-DOPA, a substrate, resulting in a purple
substance. Subsequently, an ELISA reader (iMark microplate
absorbance reader, #1681130, Bio-Rad) was used to measure the
absorbance value at 450 nm. The image was scanned using a cannon
MP276 scanner.
Measurement of the expression of
β-catenin and tyrosinase
B16F10 cells were seeded in a 96-well plate and
transfected with S37A or control vector using TurboFect™
transfection reagent. After 24 h, TQ (0-20 µM) was added to
the cells for 24 h. The stimulated cells were washed with PBS and
fixed with 3.5% paraformaldehyde for 30 min at 4°C. FBS (1% in PBS)
was used as a blocking reagent. The cells were incubated with
anti-tyrosinase (sc-15341; Santa Cruz Biotechnology, Inc.) or
anti-β-catenin (sc-7963; Santa Cruz Biotechnology, Inc.) polyclonal
antibodies for 1 h at room temperature. Thecells were then
subsequently washed with PBS and incubated with FITC-conjugated
anti-mouse IgG (F0257; Sigma-Aldrich) or FITC-conjugated
anti-rabbit IgG (F0382; Sigma-Aldrich) for 1 h at room temperature.
The expression levels of tyrosinase or β-catenin were measured
using a fluorometer (Flx800, BioTek Instruments, Inc.) at a 485 nm
excitation wavelength and 535 nm emission wavelength.
Melanin content assay
Melanin content assay was performed to measure the
intracellular melanin content. B16F10 cells (0.5x105)
were solubilized in 1 ml of 1 N NaOH/10% DMSO for 2 h at 80°C. The
solubilized solution was centrifuged at 12,000 x g for 10 min at
room temperature and the supernatants were transferred to fresh
tubes. Subsequently, an ELISA reader (iMark microplate absorbance
reader, #1681130, Bio-Rad) was used to measure the absorbance value
at 470 nm.
Western blot analysis
Following the addition of protease inhibitor [10
µg/ml leupeptin, 10 µg/ml pepstatin A, 10
µg/ml aprotinin, 1 mM 4-(2-aminoethyl) benzensulfonyl
fluoride] and phosphatase inhibitor (1 mM NaF, 1 mM
Na3VO4) to buffer containing 50 mM Tris-HCl,
pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, proteins were
extracted using lysis buffer. These proteins were electrophoresed
using a 9% SDS-polyacrylamide gel and transferred onto
nitrocellulose (NC) film. After attaching the primary antibodies
β-catenin (1:1,000; sc-7963; Santa Cruz Biotechnology, Inc.), MITF
(1:1,000; ab140606; Abcam), tyrosinase (1:1,000; sc-15341; Santa
Cruz Biotechnology, Inc.;), p-GSK3β (Cell Signaling Technology
Inc.; #9339; 1:1000), GSK3β (1:1,000; sc-7291; Santa Cruz
Biotechnology Inc.), proliferating cell nuclear antigen (PCNA;
1:1,000; sc-25280; Santa Cruz Biotechnology Inc.) and GAPDH
(1:1,000; sc-166545; Santa Cruz Biotechnology Inc.) and the
secondary antibodies (goat-anti-rabbit IgG, 1:2,000, ADI-SAB-300-J,
Enzo Life Sciences; goat-anti-mouse IgG, 1:2,000, ADI-SAB-100-J,
Enzo Life Sciences; rabbit-anti-goat IgG, 1:2,000, AP106P, EMD
Millipore) for 2 h at room temperature. ECL (Daeillab Service Co.,
Korea) system was used for protein analysis. The quantification of
protein expression was performed by densitometric analysis (ImageJ
software, National Institutes of Health; NIH)
Reverse transcription-polymerase chain
reaction (RT-PCR) and semi-quantitative PCR
Following treatment of the cultured B16F10 cells
with the reagent, TRIzol reagent (Thermo Fisher Scientific, Inc.)
was then used for RNA extraction. RT-PCR was performed on
β-catenin, MITF, tyrosinase and GAPDH with RNA extracted using
SuPrimeScript RT-PCR premix (GeNet Bio; #SR-8000). The primers used
were as follows: β-catenin (50°C, 25 cycle) sense
5′-GTGCAATTCCTGAGCTGACA-3′ and antisense,
5′-CTTAAAGATGGCCAGCAAGC-3′; MITF (52°C, 23 cycle) sense,
5′-GCTGGAAATGCTAGAATACA-3′ and antisense,
5′-TGTTCATACCTGGGCACTCA-3′; tyrosinase (55°C, 23 cycle) sense,
5′-ATGGGTGTTGACCCATTGTT-3′ and antisense,
5′-GCACCATCTGGACCTCAGTT-3′; and GAPDH (55°C, 23 cycle) sense,
5′-TGTTCCTACCCCCAATGTGT-3′ and antisense,
5′-CCCTGTTGCTGTAGCCGTAT-3′. The DNA were separated on a 1% agarose
gel and stained with ethidium bromide staining solution (#161-0433;
Bio-Rad). The band was quantified using ImageJ software (NIH).
cDNA and siRNA transfection
TurboFect™ transfection reagent (Thermo Fisher
Scientific, Inc.) was used to transfect the cells with scrambled
siRNA, pCMV6, S37A cDNA, β-catenin siRNA and MITF siRNA. The siRNA
(Shanghai Solarbio Bioscience & Technology Co., Ltd.) sequences
used in this study were as follows: Scrambled siRNA,
5′-UUCUCCGAACGUGUCACGUTT-3′; β-catenin siRNA,
5′-GGGUUCAGAUGAUAUAAAUd(TT)-3′; and MITF siRNA,
5′-AGCAGUACCUUUCUACCACd(TT)-3′. The transfected cells were then
incubated for 6 h at 4°C and subsequently treated with TQ (20
µM) or the vehicle (DMSO) for 48 h.
Immunofluorescence assay
Immunofluorescence assay was performed to confirm
the differences in the intracellular expression of β-catenin and
tyrosinase proteins in B16F10 melanoma cells. Following fixation
with 3.5% paraformaldehyde for 20 min at room temperature, 0.1%
Triton X-100 was used for 15 min at room temperature to increase
the cell membrane permeability. Subsequently, β-catenin (1:100,
sc-7963; Santa Cruz Biotechnology, Inc.) and tyrosinase (1:100,
sc-15341; Santa Cruz Biotechnology, Inc.) primary antibodies were
reacted for 2 h each at room temperature, while secondary
antibodies with tetramethylrhodamine (TRITC; 1:100, T5393;
Sigma-Aldrich) and fluorescein isothiocyanate (FITC; 1:100, F0382;
Sigma-Aldrich) attached were reacted for 2 h at room temperature.
The nuclei were stained using 4′6′-diamidi-no-2-phenylindole
dihydrochloride (DAPI) for 20 min at room temperature.
Subsequently, a BX51 fluorescence microscope (Olympus) was used to
observe the cells.
Reagent treatment and heart-beating rate
measurement of zebrafish
Zebrafish (3 to 12 months old) were used to obtain
embryonic eggs. Males and females were separated in the chamber at
a 2:1 ratio and kept in the dark for 12 h. Subsequently, most of
the water in the chamber was removed and the fish were mated for 1
h. Using zebrafish embryo medium containing 55 mM NaCl, 0.174 mM
KCl, 0.333 mM CaCl2·2H20, 0.332 mM
MgS04, and sodium bicarbonate in a 12-well plate, the
zebrafish embryo eggs were treated with the reagent using TQ (0, 1,
2.5 and 5 µM) or PTU (200 µM, Sigma-Aldrich) at 10 h
following fertilization and the medium was exchanged every 24 h.
Dorsal and lateral images of zebrafish were acquired, and the
concentration and distribution of melanin was quantified using an
image J software (NIH). The zebrafish were anesthetized and
euthanised by tricaine methane sulfonate (200-300 mg/l) by
prolonged immersion (34-36). The euthanasia of zebrafish was
confirmed by cardiac arrest. At 72 h following treatment with the
reagents, a stereo microscope (cat. no. SMZ745T; Nikon) was used to
measure the heartbeats of zebrafish juveniles for 3 min. The
results were used to derive the mean beats per min.
Statistical analysis
The present study used the mean values from multiple
rounds of experiments. One-way ANOVA statistical analyses at 95%
confidence (with a post hoc Tukey's test) was performed. At a
confidence interval (CI) of 5%, a P-value <0.05 was considered
to indicate a statistically significant difference.
Results
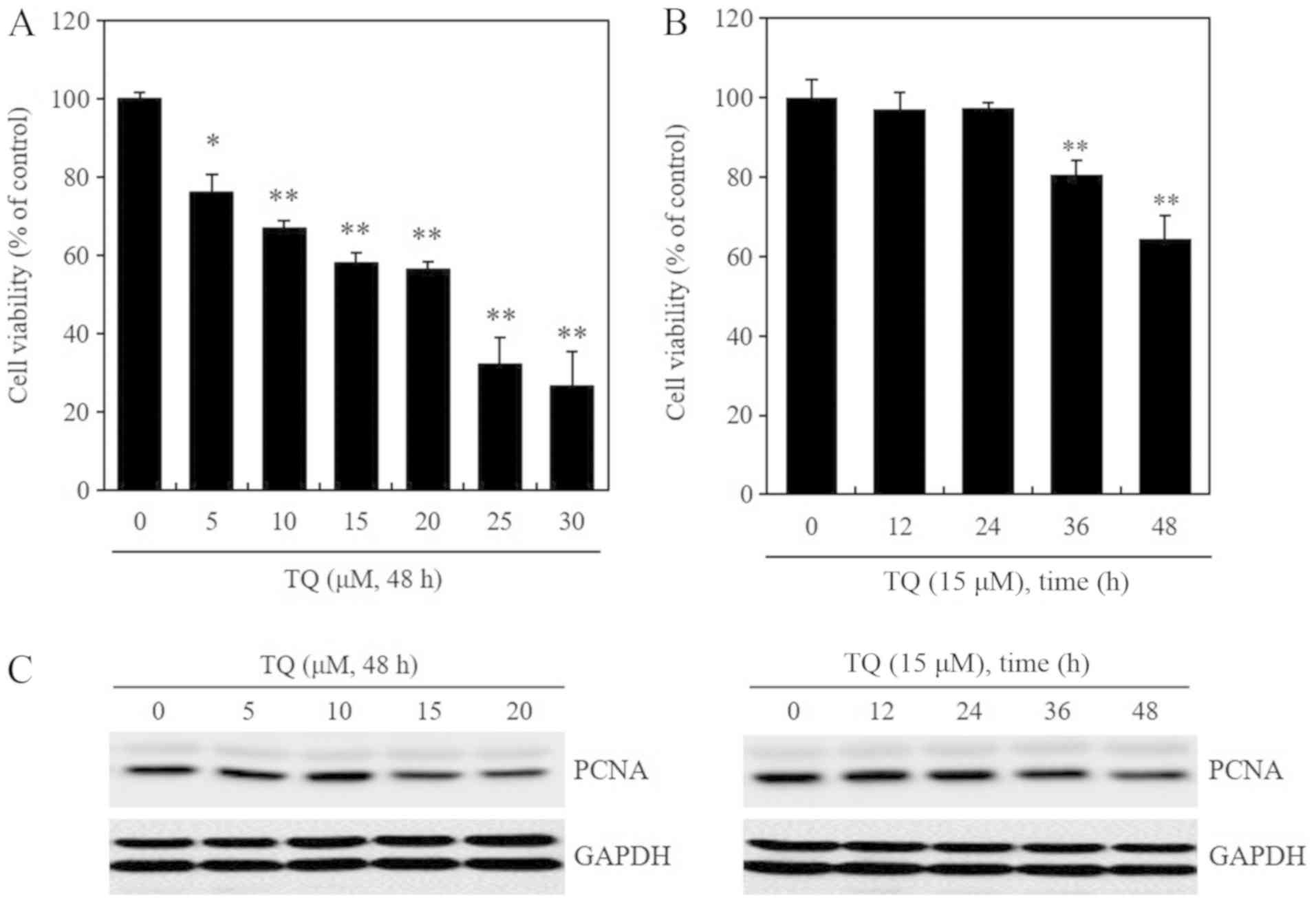
TQ treatment reduces the viability and
proliferation of B16F10 mouse melanoma cells
In the experiments in this study, the negative
control represents the control that corresponds to the vehicle
(DMSO) or mock (empty vector, scrambled siRNA). An MTT assay was
performed to confirm whether treatment of the B16F10 mouse melanoma
cells with TQ affected their viability. Following treatment with 5,
10, 15 and 20 µM of TQ for 48 h, a dose-dependent decrease
in cell viability was observed (Fig.
1A). Additionally, following treatment with 15 µM of TQ
for 12, 24, 36 and 48 h, a time-dependent decrease in cell
viability was observed (Fig. 1B).
The expression of PCNA, a marker of cell proliferation, was
decreased following treatment with TQ at 15 and 20 µM for 48
h (left panel) and 15 µM for 24-48 h (right panel) (Fig. 1C). GAPDH protein expression is
represented by 2 sets of bands. In our laboratory and in other
previous studies, GAPDH bands are represented by 2 sets of bands in
western blot analysis (37-39).
Therefore, this phenomenon, in which several bands of antibody are
present, can be considered as the general reaction of the
antibodies used. Unfortunately, the equipment with which to perform
mass spectrometry was not available and thus this was not done
(Fig. 1C). Taken together, these
results confirmed that TQ treatment reduced the viability and
proliferation of B16F10 mouse melanoma cells. At the concentration
of 25 and 30 µM, TQ decreased cell viability decreased to
more than the IC50 value (Fig.
1A), indicating a marked decrease in viability; in addition, at
these concentrations, the majority of the had died. Thus, TQ was
used at concentrations between 10-20 µM in the subsequent
experiments. Although these concentrations were low, they
effectively reduced the levels of the melanogenesis-related
proteins MITF, tyrosinase and β-catenin (Fig. 2).
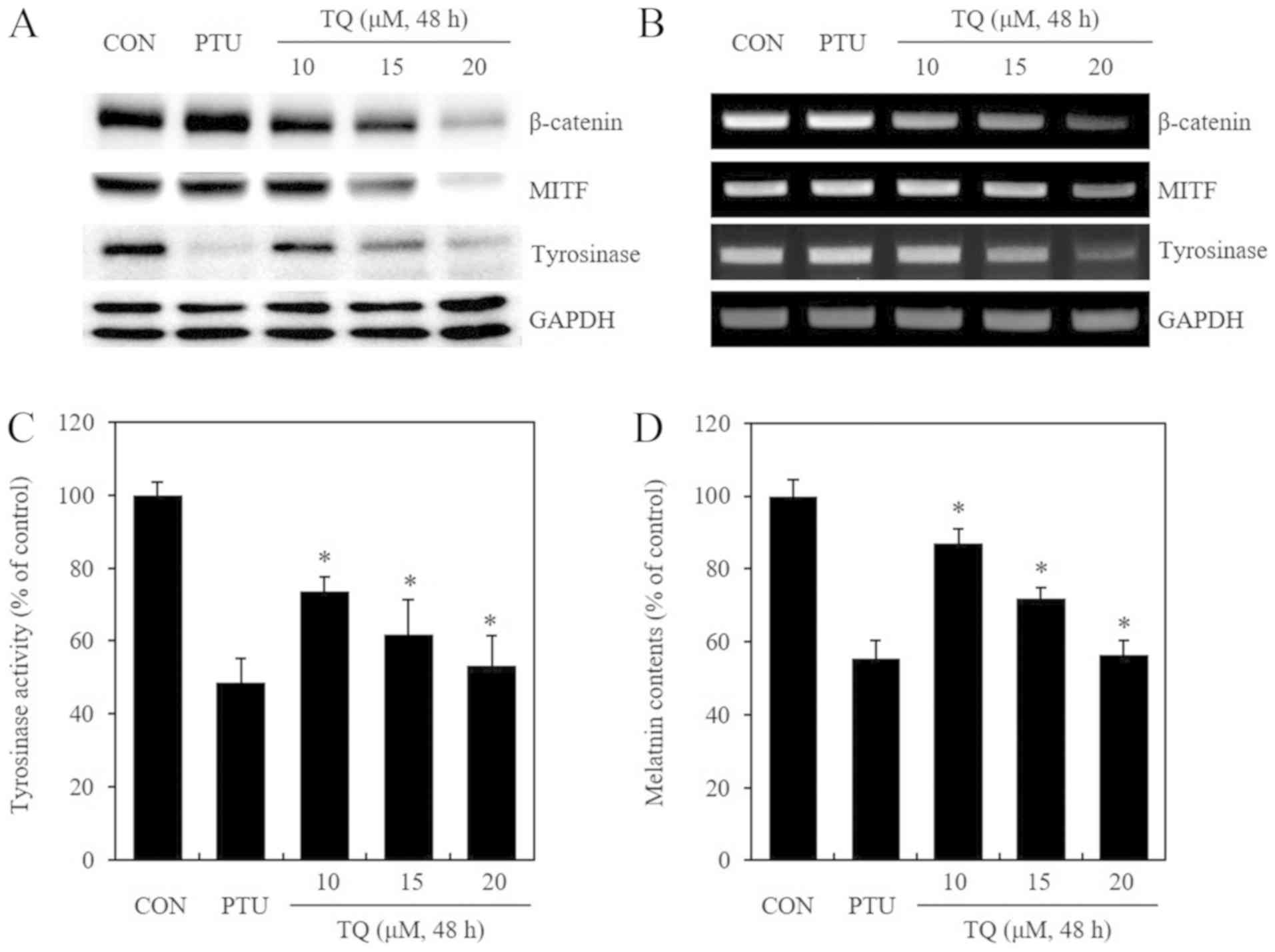
TQ treatment reduces the protein
expression and mRNA transcription of β-catenin, MITF and
tyrosinase, and this treatment also inhibits tyrosinase activity in
B16F10 mouse melanoma cells
This study then examined whether TQ exerts an effect
on MITF and tyrosinase, which are proteins associated with
melanogenesis, as well as whether these proteins are regulated
through the β-catenin pathway. PTU, a known inhibitor of
melanogenesis, was used as a positive control. Following treatment
with TQ for 48 h in a dose-dependent manner, western blot analysis
was performed to confirm the expression of β-catenin, MITF and
tyrosinase proteins (Fig. 2A).
PTU, which is known to promote the intracellular degradation of
tyrosinase proteins, only reduced the expression of tyrosinase
protein. Conversely, TQ treatment resulted in a dose-dependent
decrease in the expression of not only tyrosinase, but also of
β-catenin and MITF proteins. Following treatment with PTU and TQ
under the same conditions described above, differences in the
levels of β-catenin, MITF and tyrosinase mRNA transcription were
confirmed by semi-quantitative RT-PCR (Fig. 2B). PTU did not affect the level of
β-catenin, MITF and tyrosinase mRNA transcription, while TQ
treatment resulted in a dose-dependent decrease in the
transcription of these genes. When tyrosinase activity was analyzed
following treatment with PTU and TQ under the same conditions
described above, TQ treatment led to a dose-dependent inhibition of
tyrosinase activity (Fig. 2C).
Herein, the decrease in the intracellular melanin content was
examined by analyzing pigment deposits in cell pellets and the
melanin content (Fig. 2D). On the
whole, the results suggested that TQ inhibited both cell
proliferation and melanogenesis. As shown in Fig. 1, the inhibitory effect of TQ on
cell proliferation seemed to inhibit melanin production.
Furthermore, as shown in Fig. 2D,
the amount of melanin was quantified by the same number of cells
and the amount of melanin was measured. This result confirmed that
TQ decreased the synthesis of melanin in a concentration-dependent
manner. In other words, TQ inhibited cell proliferation and also
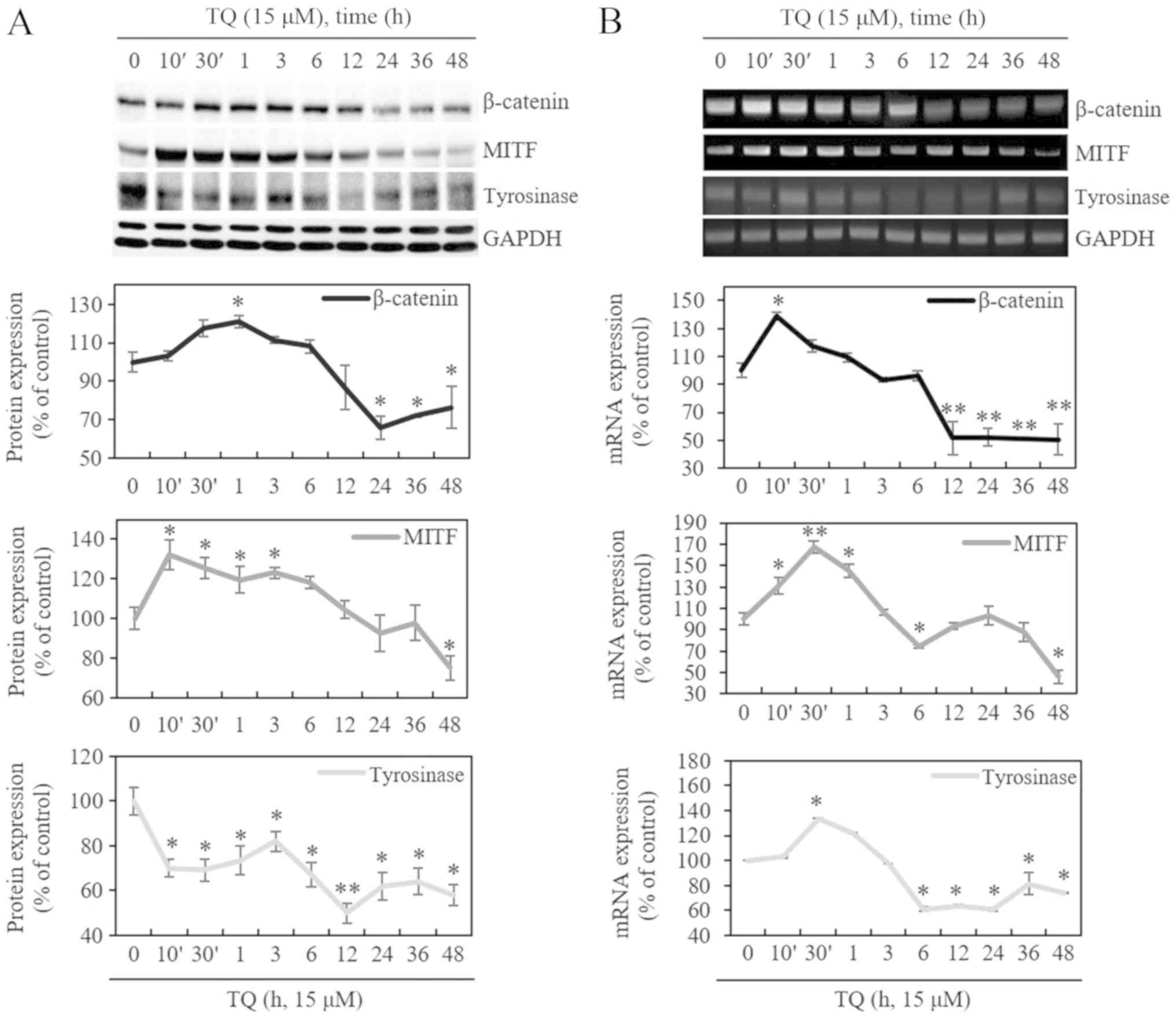
inhibited the synthesis of melanin. Following treatment with 15
µM of TQ in a time-dependent manner, the protein levels of
β-catenin and MITF increased at 10 min to 6 h and decreased after 6
h. The mRNA levels were increased at 10 min to 1 h and decreased
after 3 h. On the other hand, the mRNA levels of tyrosinase
increased at 30 min and decreased after 3 h, whereas they decreased
immediately after 10 min at the protein level, and increased at 30
min to 3 h. It was found that the mRNA and protein levels of
β-catenin and tyrosinase were increased at up to approximately 6 h
by TQ and were then downregulated after 6 h. The expression of MITF
protein was increased at up to approximately 3 h by TQ and was then
downregulated after 6 h. However, the mRNA expression of MITF was
increased at up to approximately 6 h by TQ and was then
downregulated after 6 h. (Fig. 3A and
B). In the subsequent experiments, the cells were treated with
TQ for 48 h.
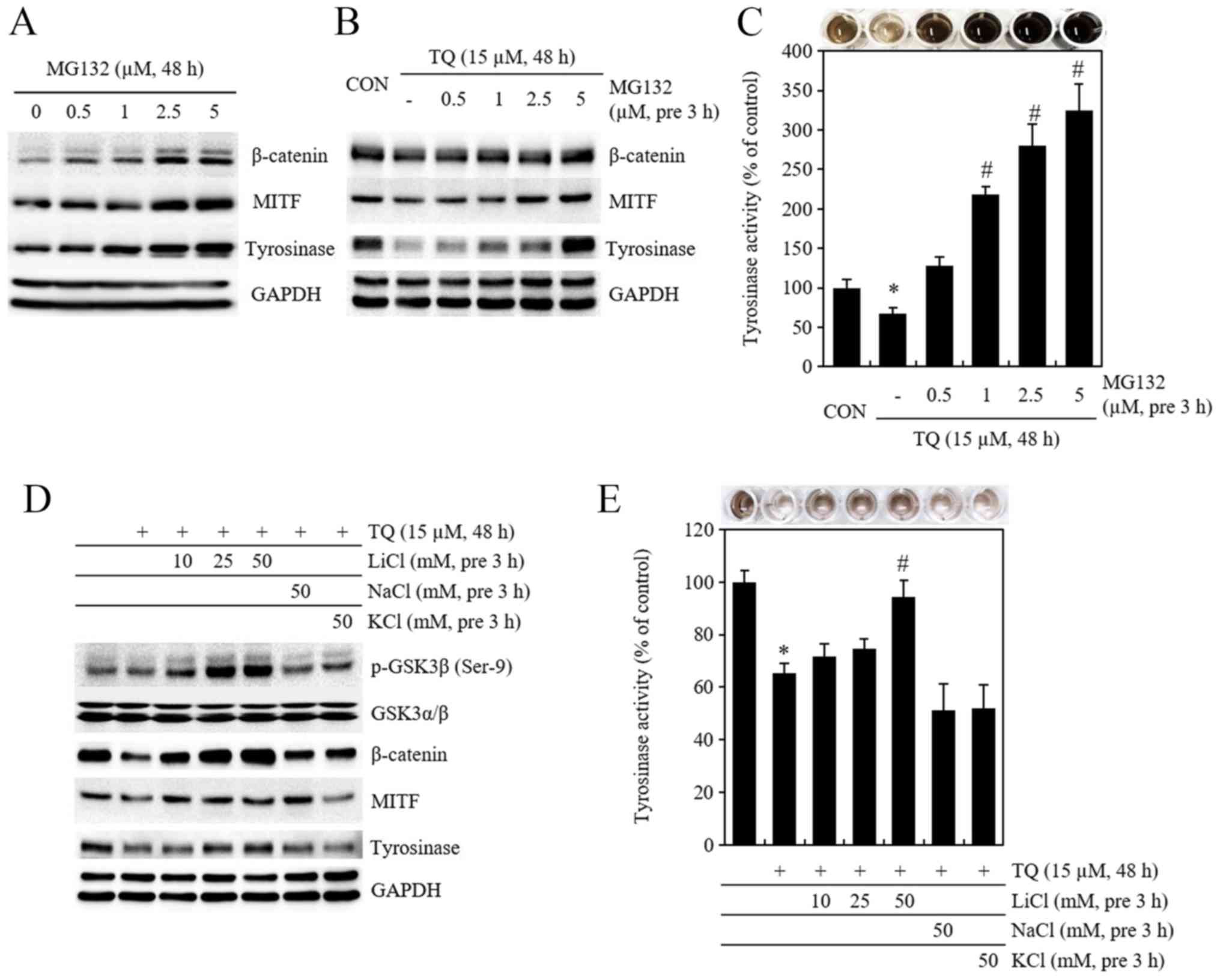
Pre-treatment with MG132 and LiCl results
in the recovery of β-catenin and tyrosinase protein expression and
activity initially reduced by TQ
To examine whether melanogenesis inhibited by TQ is
regulated by β-catenin, 0,5, 1, 2.5 and 5 µM of MG132, a
proteasome inhibitor, was used for treatment (Fig. 4A) and for pre-treatment at 3 h
prior to TQ treatment. Subsequently, the expression of β-catenin,
MITF and tyrosinase proteins (Fig.
4B) and tyrosinase activity were measured by tyrosinase
activity assay (Fig. 4C). The
expression of β-catenin, MITF and tyrosinase proteins, which was
initially reduced by TQ, was recovered by pre-treatment with MG132,
and tyrosinase activity also increased. The cells were also
pre-treated with LiCl, a GSK3β inhibitor, at 10, 25, and 50 mM
concentrations at 3 h prior to TQ treatment, and the expression of
β-catenin, MITF, and tyrosinase proteins and tyrosinase activity
were then measured (Fig. 4D and
E). LiCl pre-treatment led to an increase in Ser-9
phosphorylated GSK3β protein, and as a result, the expression of
β-catenin, MITF and tyrosinase proteins and tyrosinase activity,
which were initially reduced by TQ, increased (Fig. 4D and E).
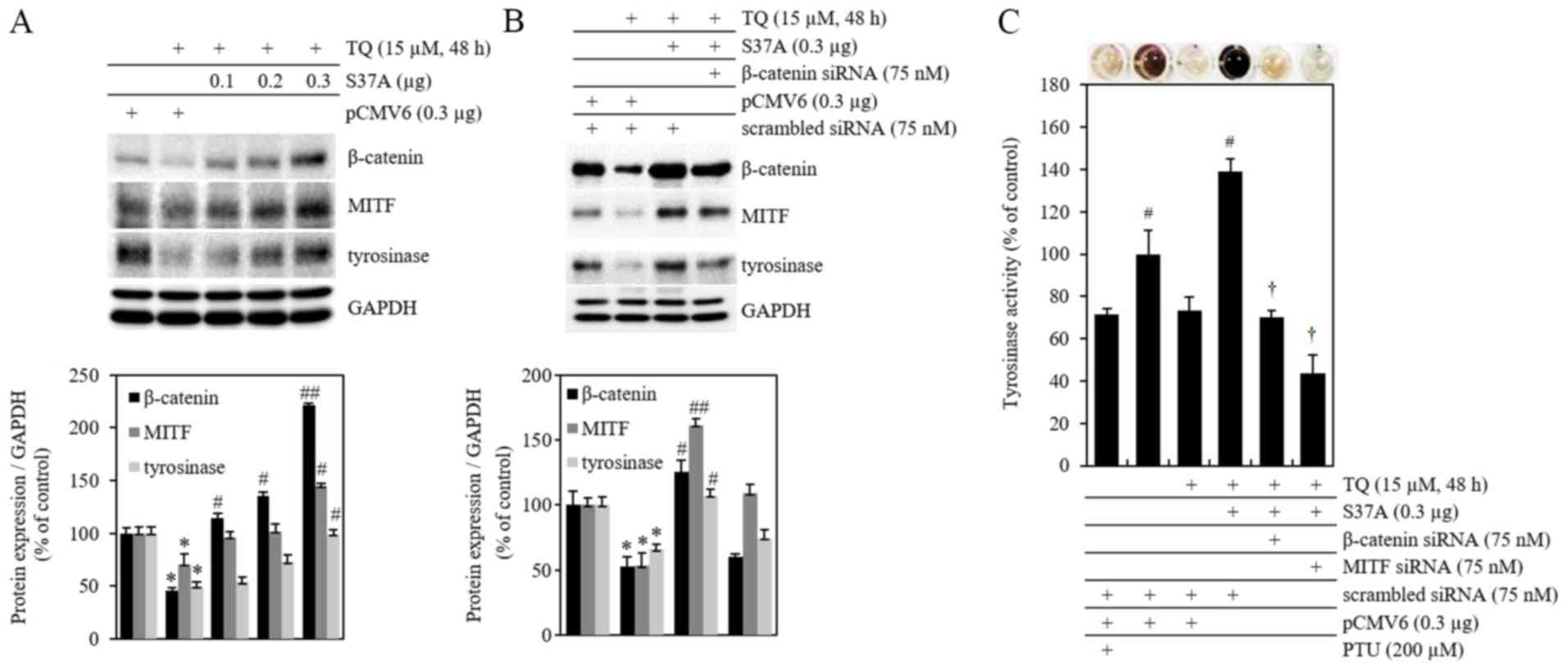
Overexpression of β-catenin increases
MITF and tyrosinase protein expression, and activity initially
reduced by TQ treatment
The expression of β-catenin, MITF and tyrosinase
proteins was confirmed following treatment of the cells with TQ and
transfection with 0.1, 0.2 and 0.3 µg of cDNA β-catenin
overexpression vector with an Ala mutation at the β-catenin Ser-37
site (S37A). The results indicated that intracellular accumulation
occurred due to the blockage of the ubiquitin-mediated proteasome
degradation of β-catenin in B16F10 cells, while the expression of
MITF and tyrosinase proteins increased (Fig. 5A). pCMV6 is an empty vector that
was used as a negative control. To confirm whether the inhibition
of melanogenesis by TQ is regulated by the β-catenin signaling
pathway, the cells were treated with TQ following simultaneous
transfection with S37A β-catenin and β-catenin siRNA. Subsequently,
the expression of β-catenin, MITF and tyrosinase proteins (Fig. 5B) and the levels of tyrosinase
activity were measured by tyrosinase activity assay (Fig. 5C). The expression of MITF that was
reduced by TQ was increased by S37A mutant β-catenin and thus
tyrosinase expression increased. This suggested that the
accumulation of S37A mutant β-catenin, which is limited by
proteasomal degradation, induced an increase in MITF and tyrosinase
expression (Fig. 5B). The
expression of β-catenin, MITF and tyrosinase proteins, and the
levels of tyrosinase activity, that had increased subsequent to TQ
treatment following transfection with S37A β-catenin, were reduced
again by simultaneous transfection with β-catenin siRNA.
Additionally, simultaneous transfection with MITF siRNA and S37A
cDNA also reduced tyrosinase activity. Scrambled siRNA was used as
a negative control (Fig. 5C).
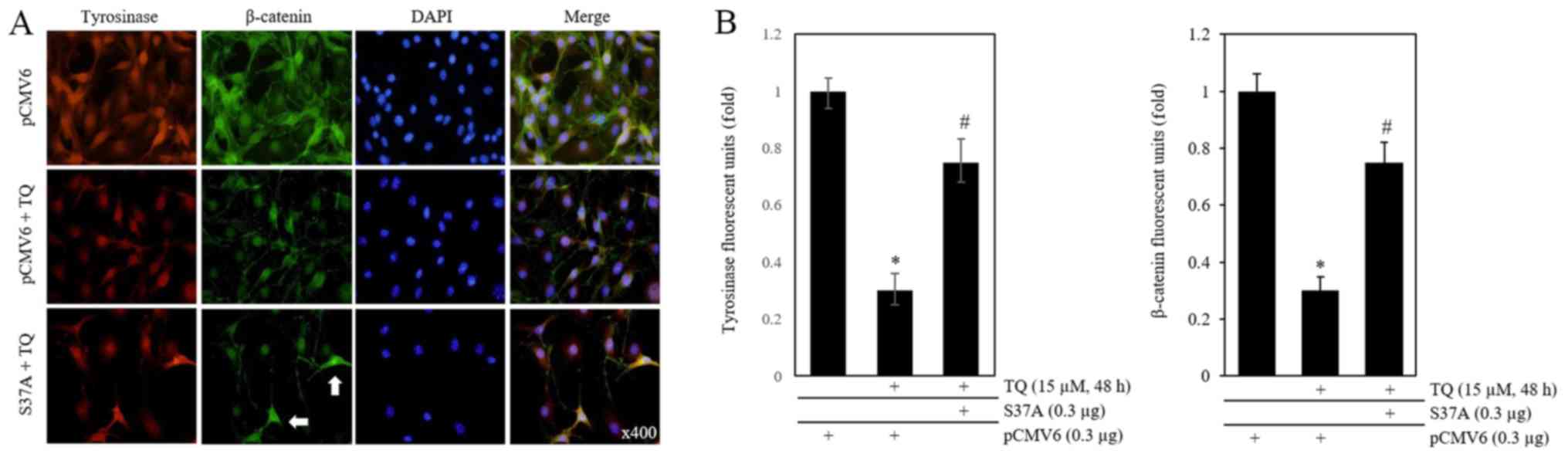
An immunofluorescence assay was then performed
subsequent to TQ treatment at 6 h following transfection with 0.3
µg of S37A β-catenin cDNA (Fig.
6A). The endogenous β-catenin and mutant form (S37A) of
β-catenin in one cell are indistinguishable. However, cells with
exogenous β-catenin are brighter than β-catenin in the surrounding
cells. S37A used in this study was cDNA without a tag (Fig. 6A). The expression of tyrosinase
(red) and β-catenin (green), that had been reduced by TQ, increased
when the cells were transfected with S37A cDNA. A fluorometer was
also used to quantify the expression of β-catenin and tyrosinase
(Fig. 6B). The results confirmed
that the inhibition of melanogenesis in B16F10 mouse melanoma cells
by TQ was due to the inhibition of the β-catenin signaling
pathway.
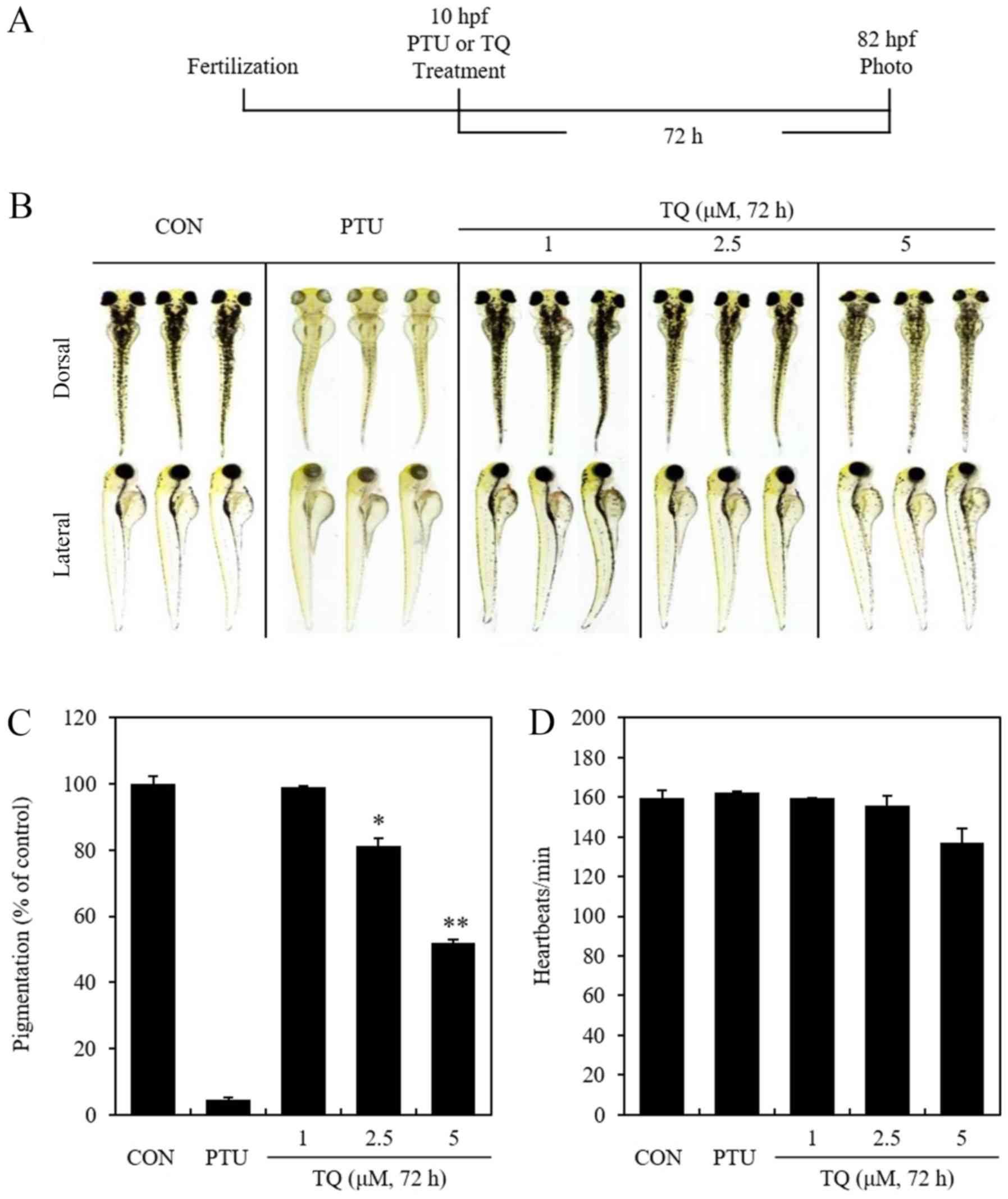
TQ inhibits melanogenesis in
zebrafish
To determine the toxic effects of TQ on zebrafish,
vital signs (movement, heartbeat and circulation) were measured as
previously described (40).
Zebrafish were also euthanized after anesthesia to determine the
amount of melanin synthesis and euthanasia was confirmed by
measuring vital signs (Fig. 7). At
72 h after the TQ or PTU treatment of zebrafish embryo eggs that
had been fertilized for 10 h, heartbeats per min were measured
(Fig. 7A). Additionally, dorsal
and lateral images of zebrafish were acquired, and the
concentration and distribution of melanin was quantified using
imageJ software. (Fig. 7B and C).
The results confirmed the dose-dependent inhibition of
melanogenesis in zebrafish induced by TQ treatment. When heartbeats
per min were measured in specimens treated with 200 µM PTU
and 1, 2.5, or 5 µM of TQ to investigate the toxicity of TQ,
the toxicity of TQ was confirmed at 5 µM, as indicated by a
slight decrease in the heartbeats of the specimens treated with 5
µM of TQ (Fig. 7D). Given
these observations, these results confirm that TQ treatment
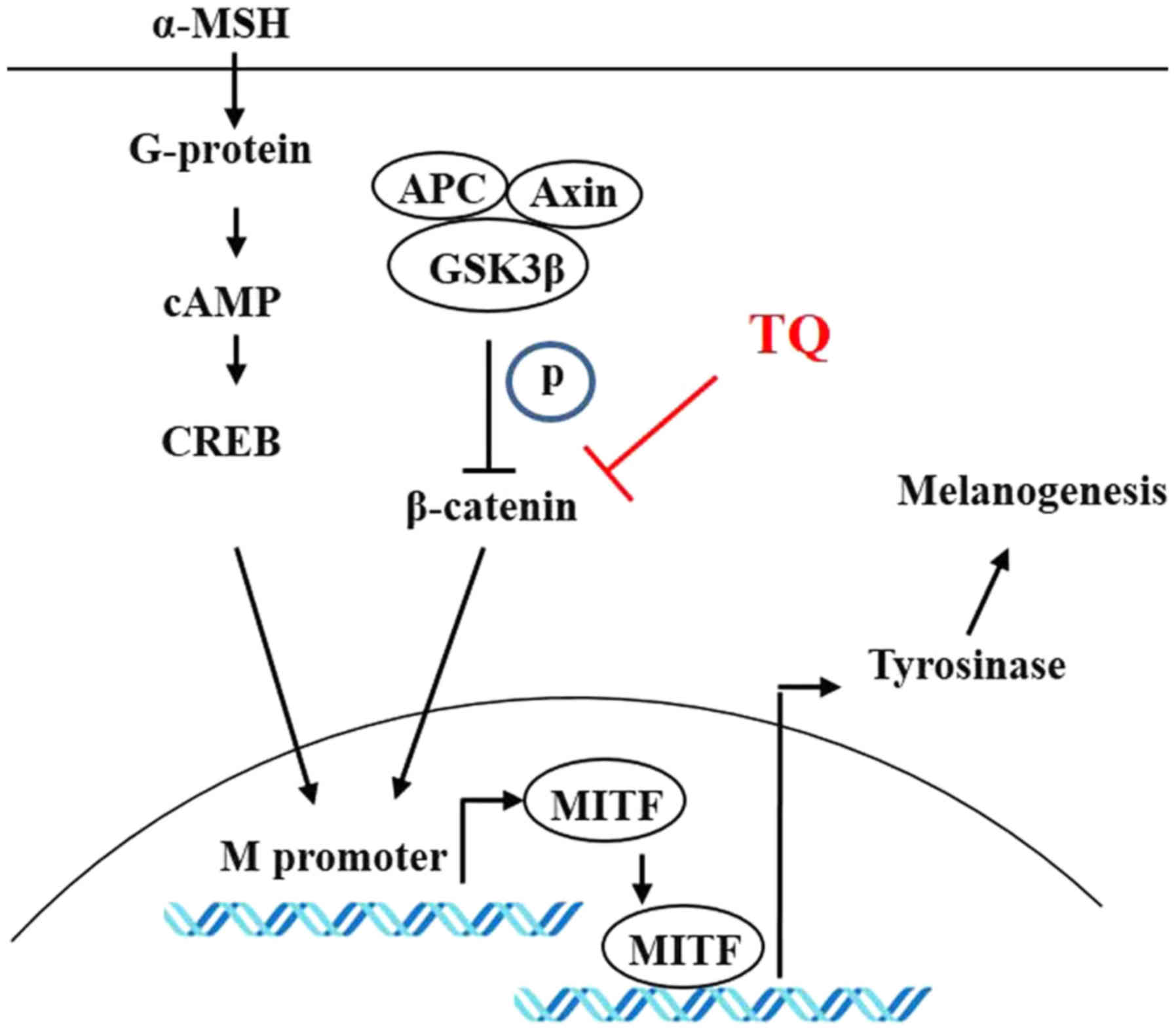
inhibits melanogenesis in zebrafish. Fig. 8 provides a schematic diagram
detailing the control of melanin production through the β-catenin
pathway regulated by TQ.
Discussion
Melanoma, which is the most aggressive and fatal
form of skin cancer, occurs in melanocytes, the pigment cells found
mainly in the skin. Incidences of malignant melanoma continue to
increase at a steady pace. This malignancy can be treated with
surgical excision at an early stage; however, once the disease
advances to metastatic stages, treatment becomes extremely
difficult. Recent discoveries involving the cell signaling and
molecular mechanisms underlying this disease have provided a basic
and deep understanding of melanoma that is being used to develop
targeted drugs and novel therapeutic methods (41).
TQ is an active ingredient isolated from the seeds
of Nigella sativa, and since it was first extracted in 1960,
its anticancer, anti-inflammatory and antioxidant activities have
been investigated using both in vitro and in vivo
models. The anti-inflammatory and antioxidant effects of TQ have
been reported in various disease models, including
encephalomyelitis, diabetes, asthma and cancer. The anticancer
effects of TQ are mediated through various actions, including
anti-proliferative actions, the induction of apoptosis, the
termination of the cell cycle, the production of ROS,
anti-metastatic actions and anti-angiogenic actions (42). The induction of ROS by TQ in breast
cancer cells induces anti-proliferative effects (43). In human non-small cell lung cancer
cells, TQ has been shown to inhibit proliferation and invasion
through the ERK pathway (44).
Hatiboglu et al (45)
suggested that the inhibition of proliferation by TQ in B16F10
cells occurs via the inhibition of p-STAT3. Previously, in a study
published by the authors, it was reported that TQ induces the
apoptosis of rabbit chondrocytes (46). In this study, it was also confirmed
that the proliferation of B16F10 cells was reduced by the
inhibition of PCNA by TQ, and this study also identified the
inhibition of melanogenesis and the mechanism of action of TQ in
B16F10 mouse melanoma cells. In another previous study, researchers
focused on effects, such as anticancer or anti-inflammatory effects
mediated by TQ (31-33,43-45).
However, this study focused on the identification of signal
pathways regulated by TQ in melanogenesis in B16F10 cells. It was
found that the β-catenin signaling pathway plays an important role
in melanogenesis in B16F10 cells. These results are a novel finding
which, to the best of our knowledge, has not yet been previously
demonstrated.
The findings of this study suggested that TQ
inhibited both cell proliferation and melanogenesis (Figs. 1 and 2). Therefore, when the cells were stained
with DAPI, the number of cells was lower in the cells treated with
TQ than in the control cells, since all of the experiments revealed
a quantitative result in proportion to the number of cells
(Fig. 6A). In this study,
experiments were conducted using B16f10 melanocytes, but not with
normal cells. It has previously been demonstrated that thymoquinone
inhibits cell proliferation and induces apoptosis even in normal
cells (46). That is, TQ may exert
an inhibitory effect on melanogenesis and may suppress the
proliferation of cancer cells; however, it may also have a
side-effect of inhibiting the proliferation of normal cells. The
inhibition of melanogenesis by TQ treatment was confirmed through a
decrease in the intracellular melanin content and tyrosinase
transcription, protein expression and activity. To examine whether
the inhibition of melanogenesis by TQ in B16F10 mouse melanoma
cells involved the β-catenin pathway, 3 independent experiments
were conducted.
First, pre-treatment with MG132 was applied to block
the degradation of β-catenin by the ubiquitin-mediated proteasome
pathway. The expression of β-catenin, that was previously decreased
by TQ treatment, increased inside the cells due to degradation
being blocked by MG132, and this also resulted in an increased MITF
and tyrosinase expression and tyrosinase activity. Proteasomal
degradation occurs inside the cells via the phosphorylation of the
Ser-33, 37 and 45 and Thr-41 sites of β-catenin by GSK3β, which is
an upstream pathway (16,17). The phosphorylation of the Tyr-216
site of GSK3β increases the enzymatic activity of GSK3, while the
phosphorylation of the Ser-9 site of GSK3β induces inactivation
(47). Lithium (Li+) is
known to cause the inactivation of this pathway by inducing the
phosphorylation of the Ser-9 site of GSK3β (48). Accordingly, pre-treatment with LiCl
was applied in this study, and this resulted in an increased
phospho-GSK3β expression that led to the blockage of the
proteasomal degradation pathway of β-catenin, and increased the
expression and activity of tyrosinase. Finally, the cells were
transfected with S37A β-catenin cDNA to induce the overexpression
of β-catenin. The amino acids 32-37 of β-catenin
(Asp-Ser-Gly-Ile-His-Ser) comprise a motif that is recognized by
the β-TrCP E3 ubiquitin ligase, and the phosphorylation of Ser-33
and 37 is essential for recognition by the E3 ligase (18). S37A β-catenin exhibits a blocked
phosphorylation of the Ala-33 site of GSK3β and the β-TrCP E3
ubiquitin ligase cannot recognize the ligase motif, ultimately
resulting in intracellular accumulation due to the degradation
pathway being blocked by the proteasome. Consequently, the recovery
of MITF and tyrosinase expression and tyrosinase activity that were
initially reduced by TQ was confirmed. When the cells were
simultaneously transfected with S37A β-catenin and β-catenin siRNA,
β-catenin expression again decreased, while the expression of MITF
and tyrosinase, and the activity of tyrosinase also decreased
(Fig. 5). These results indicated
that β-catenin is an important protein for the expression and
activity of tyrosinase.
The MITF promoter that is located most proximal to
the common downstream exons is known as the M promoter and appears
to be selectively expressed in melanocytes. The MITF-M promoter is
targeted by several transcription factors that are important in
neural-crest development and signaling (19). At the MITF-M promoter,
Wnt/β-catenin signaling is crucial for the differentiation of
melanocytes from the neural crest. In embryogenesis, the
Wnt/β-catenin signaling pathway plays a deterministic role in the
formation of body parts in the early embryo stage and is also
involved in gastrulation and blastopore lip formation (49). The overexpression of β-catenin or
the inhibition of translation by GSK3 antisense mRNA injection in
zebrafish can lead to the formation of an extra blastopore and body
axis (50). When the same
concentration of TQ used to treat the B16F10 cells was used to
treat zebrafish embryo eggs, this treatment caused deformations or
death during embryogenesis (data not shown). Accordingly, zebrafish
eggs were treated with TQ concentrations that were reduced
3-15-fold from the cell treatment concentration, and as a result, a
reduction in melanogenesis was confirmed (Fig. 7). Additional studies, however, are
warranted to determine whether the inhibition of melanogenesis in
zebrafish by TQ is mediated via the β-catenin pathway.
In conclusion, the findings of this study
demonstrated that TQ inhibited melanogenesis in B16F10 mouse
melanoma cells by inhibiting the β-catenin signaling pathway, and
it also impeded melanogenesis in zebrafish. The findings of the
present study may prove to be useful for the future development of
therapeutic agents targeting pigmented lesions and melanoma.
Funding
This study was supported by grants from the National
Research Foundation of Korea (NRF) and funded by the Korean
government (MSIP) (nos. 2017R1D1A3B03033401 and
2018R1D1A1B07051064).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HJ and SMY conceived and designed the study and the
experiments, performed research, and wrote the manuscript. SJK
conceived and designed the study and the experiments, conducted
research, and wrote the manuscript.
Ethics approval and consent to
participate
This research conformed to the guidelines of and was
approved by the Kongju National University Ethical Review
Board.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
TQ
|
thymoquinone
|
|
PTU
|
phenylthiourea
|
|
MITF
|
microphthalmia-associated
transcription factor
|
|
GSK3
|
glycogen synthase kinase 3
|
|
α-MSH
|
α-melanocyte stimulating hormone
|
|
PMSF
|
phenylmethylsulfonyl fluoride
|
References
|
1
|
Morison WL: What is the function of
melanin? Arch Dermatol. 121:1160–1163. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schallreuter KU, Kothari S, Chavan B and
Spencer JD: Regulation of melanogenesis - controversies and new
concepts. Exp Dermatol. 17:395–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu Y, Zhu WY, Tan C, Yu GH and Gu JX:
Melanocytes are potential immunocompetent cells: Evidence from
recognition of immunological characteristics of cultured human
melanocytes. Pigment Cell Res. 15:454–460. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plonka PM, Passeron T, Brenner M, Tobin
DJ, Shibahara S, Thomas A, Slominski A, Kadekaro AL, Hershkovitz D,
Peters E, et al: What are melanocytes really doing all day long?
Exp Dermatol. 18:799–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park HY, Kosmadaki M, Yaar M and Gilchrest
BA: Cellular mechanisms regulating human melanogenesis. Cell Mol
Life Sci. 66:1493–1506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tachibana M: MITF: A stream flowing for
pigment cells. Pigment Cell Res. 13:230–240. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilchrest BA, Park HY, Eller MS and Yaar
M: Mechanisms of ultraviolet light-induced pigmentation. Photochem
Photobiol. 63:1–10. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Videira IF, Moura DF and Magina S:
Mechanisms regulating melanogenesis. An Bras Dermatol. 88:76–83.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matoba Y, Kumagai T, Yamamoto A, Yoshitsu
H and Sugiyama M: Crystallographic evidence that the dinuclear
copper center of tyrosinase is flexible during catalysis. J Biol
Chem. 281:8981–8990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Branza-Nichita N, Negroiu G, Petrescu AJ,
Garman EF, Platt FM, Wormald MR, Dwek RA and Petrescu SM: Mutations
at critical N-glycosylation sites reduce tyrosinase activity by
altering folding and quality control. J Biol Chem. 275:8169–8175.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wasmeier C, Hume AN, Bolasco G and Seabra
MC: Melanosomes at a glance. J Cell Sci. 121:3995–3999. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanavy HE and Gerstenblith MR: Ultraviolet
radiation and melanoma. Semin Cutan Med Surg. 30:222–228. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kraus C, Liehr T, Hülsken J, Behrens J,
Birchmeier W, Grzeschik KH and Ballhausen WG: Localization of the
human beta-catenin gene (CTNNB1) to 3p21: A region implicated in
tumor development. Genomics. 23:272–274. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orford K, Crockett C, Jensen JP, Weissman
AM and Byers SW: Serine phosphorylation-regulated ubiquitination
and degradation of beta-catenin. J Biol Chem. 272:24735–24738.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aberle H, Bauer A, Stappert J, Kispert A
and Kemler R: beta-catenin is a target for the ubiquitin-proteasome
pathway. EMBO J. 16:3797–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winston JT, Strack P, Beer-Romero P, Chu
CY, Elledge SJ and Harper JW: The SCFbeta-TRCP-ubiquitin ligase
complex associates specifically with phosphorylated destruction
motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha
ubiquitination in vitro. Genes Dev. 13:270–283. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levy C, Khaled M and Fisher DE: MITF:
Master regulator of melanocyte development and melanoma oncogene.
Trends Mol Med. 12:406–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bellei B, Pitisci A, Catricalà C, Larue L
and Picardo M: Wnt/β-catenin signaling is stimulated by
α-melanocyte-stimulating hormone in melanoma and melanocyte cells:
Implication in cell differentiation. Pigment Cell Melanoma Res.
24:309–325. 2011. View Article : Google Scholar
|
|
21
|
Yasumoto K, Takeda K, Saito H, Watanabe K,
Takahashi K and Shibahara S: Microphthalmia-associated
transcription factor interacts with LEF-1, a mediator of Wnt
signaling. EMBO J. 21:2703–2714. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mollaaghababa R and Pavan WJ: The
importance of having your SOX on: Role of SOX10 in the development
of neural crest-derived melanocytes and glia. Oncogene.
22:3024–3034. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Mello SA, Finlay GJ, Baguley BC and
Askarian-Amiri ME: Signaling pathways in melanogenesis. Int J Mol
Sci. 17:E11442016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ando H, Kondoh H, Ichihashi M and Hearing
VJ: Approaches to identify inhibitors of melanin biosynthesis via
the quality control of tyrosinase. J Invest Dermatol. 127:751–761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maeda K and Fukuda M: Arbutin: Mechanism
of its depigmenting action in human melanocyte culture. J Pharmacol
Exp Ther. 276:765–769. 1996.PubMed/NCBI
|
|
26
|
Battaini G, Monzani E, Casella L,
Santagostini L and Pagliarin R: Inhibition of the catecholase
activity of biomimetic dinuclear copper complexes by kojic acid. J
Biol Inorg Chem. 5:262–268. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DuBOIS KP and Erway WF: Studies on the
mechanism of action of thiourea and related compounds; inhibition
of oxidative enzymes and oxidations catalyzed by copper. J Biol
Chem. 165:711–721. 1946.PubMed/NCBI
|
|
28
|
Hall AM and Orlow SJ: Degradation of
tyrosinase induced by phenylthiourea occurs following Golgi
maturation. Pigment Cell Res. 18:122–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gali-Muhtasib H, Diab-Assaf M, Boltze C,
Al-Hmaira J, Hartig R, Roessner A and Schneider-Stock R:
Thymoquinone extracted from black seed triggers apoptotic cell
death in human colorectal cancer cells via a p53-dependent
mechanism. Int J Oncol. 25:857–866. 2004.PubMed/NCBI
|
|
30
|
Daba MH and Abdel-Rahman MS:
Hepatoprotective activity of thymoquinone in isolated rat
hepatocytes. Toxicol Lett. 95:23–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asaduzzaman Khan M, Tania M, Fu S and Fu
J: Thymoquinone, as an anticancer molecule: From basic research to
clinical investigation. Oncotarget. 8:51907–51919. 2017.PubMed/NCBI
|
|
32
|
Hossen MJ, Yang WS, Kim D, Aravinthan A,
Kim JH and Cho JY: Thymoquinone: An IRAK1 inhibitor with in vivo
and in vitro anti-inflammatory activities. Sci Rep. 7:429952017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mostofa AGM, Hossain MK, Basak D and Bin
Sayeed MS: Thymoquinone as a potential adjuvant therapy for cancer
treatment: Evidence from preclinical studies. Front Pharmacol.
8:2952017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matthews M and Varga ZM: Anesthesia and
euthanasia in zebrafish. ILAR J. 53:192–204. 2012. View Article : Google Scholar
|
|
35
|
Strykowski JL and Schech JM: Effectiveness
of recommended euthanasia methods in larval zebrafish (Danio
rerio). J Am Assoc Lab Anim Sci. 54:81–84. 2015.PubMed/NCBI
|
|
36
|
Wilk R, Ali N, England SJ and Lewis KE:
Using zebrafish to bring hands-on laboratory experiences to urban
classrooms. Zebrafish. 15:156–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Liu S, Dong W, Qu X, Huang C, Yan
T and Du J: Combination of hesperetin and platinum enhances
anticancer effect on lung adenocarcinoma. Biomed Pharmacother.
113:1087792019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Y, Wang C, Zhao K, Zhang G, Wang D
and Mei Y: TRMP, a p53-inducible long noncoding RNA, regulates G1/S
cell cycle progression by modulating IRES-dependent p27
translation. Cell Death Dis. 9:8862018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ouchida AT, Li Y, Geng J, Najafov A,
Ofengeim D, Sun X, Yu Q and Yuan J: Synergistic effect of a novel
autophagy inhibitor and quizartinib enhances cancer cell death.
Cell Death Dis. 9:1382018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stones DH, Fehr AGJ, Thompson L, et al:
Zebrafish (Danio rerio) as a vertebrate model host to study
colonization, pathogenesis, and transmission of foodborne
Escherichia coli O157. mSphere. 2:e003652017. View Article : Google Scholar :
|
|
41
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Woo CC, Kumar AP, Sethi G and Tan KH:
Thymoquinone: Potential cure for inflammatory disorders and cancer.
Biochem Pharmacol. 83:443–451. 2012. View Article : Google Scholar
|
|
43
|
Woo CC, Hsu A, Kumar AP, Sethi G and Tan
KH: Thymoquinone inhibits tumor growth and induces apoptosis in a
breast cancer xenograft mouse model: The role of p38 MAPK and ROS.
PLoS One. 8:e753562013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang J, Kuang XR, Lv PT and Yan XX:
Thymoquinone inhibits proliferation and invasion of human
nonsmall-cell lung cancer cells via ERK pathway. Tumour Biol.
36:259–269. 2015. View Article : Google Scholar
|
|
45
|
Hatiboglu MA, Kocyigit A, Guler EM, Akdur
K, Nalli A, Karatas E and Tuzgen S: Thymoquinone induces apoptosis
in B16-F10 melanoma cell through inhibition of p-STAT3 and inhibits
tumor growth in a murine intracerebral melanoma model. World
Neurosurg. 114:e182–e190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu SM and Kim SJ: Thymoquinone-induced
reactive oxygen species causes apoptosis of chondrocytes via
PI3K/Akt and p38kinase pathway. Exp Biol Med (Maywood).
238:811–820. 2013. View Article : Google Scholar
|
|
47
|
Jope RS, Yuskaitis CJ and Beurel E:
Glycogen synthase kinase-3 (GSK3): Inflammation, diseases, and
therapeutics. Neurochem Res. 32:577–595. 2007. View Article : Google Scholar :
|
|
48
|
De Sarno P, Li X and Jope RS: Regulation
of Akt and glycogen synthase kinase-3 beta phosphorylation by
sodium valproate and lithium. Neuropharmacology. 43:1158–1164.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Haegel H, Larue L, Ohsugi M, Fedorov L,
Herrenknecht K and Kemler R: Lack of beta-catenin affects mouse
development at gastrulation. Development. 121:3529–3537.
1995.PubMed/NCBI
|
|
50
|
Kelly GM, Erezyilmaz DF and Moon RT:
Induction of a secondary embryonic axis in zebrafish occurs
following the overexpression of beta-catenin. Mech Dev. 53:261–273.
1995. View Article : Google Scholar : PubMed/NCBI
|