Introduction
Lung cancer is the leading cause of cancer-related
death in the world (1). Non-small
cell lung cancer (NSCLC) accounts for >85% of all lung cancer
cases (2). Patients are usually
diagnosed with NSCLC at an advanced or metastatic stage with poor
prognosis (3). However, the
molecular mechanisms driving NSCLC development and progression
remain poorly understood.
Fucosylation is one of the most common types of
mammalian glycosylation, in which fucose is conjugated to protein-
or lipid-bound oligosaccharides (4). Cell surface fucosylation serves a
crucial role in fine-tuning biological recognition processes that
mediate cell adhesion and migration (4). Aberrant fucosylation has been
observed in multiple types of cancer (5-7) and
is associated with tumor development and metastatic capability
(8-11). Therefore, fucosylation has gained
attention as a promising target for the development of novel cancer
therapeutics.
The fucosylation pathway consists of GDP-fucose
synthesis in the cytosol, GDP-fucose transport into the Golgi, and
fucose transfer to acceptor substrates inside the Golgi (12). GDP-fucose is synthesized by the
de novo or salvage enzymatic pathways involving GDP-mannose
4,6-dehydratase (GMDS), GDP-L-fucose synthase (TSTA3), L-fucose
kinase (FUK) and fucose-1-phosphate guanylyltransferase (FPGT)
(4,13). GDP-fucose is transported into the
Golgi lumen by GDP-fucose transporter 1 (SLC35C1) (13). A fucose residue from GDP-fucose is
transferred to the sugar moieties of glycoconjugates or the
serine/threonine residues on substrate proteins by
fucosyltransferases (FUTs) (14,15).
FUTs catalyze α-1,2 (by FUT1 and 2), α-1,3 (by FUT3-7 and 9-11),
α-1,4 (by FUT3 and 5) and α-1,6 (by FUT8) glycosidic bond formation
or protein O-fucosylation (by POFUT1 and 2) (4,16,17).
Certain FUT isotypes are aberrantly expressed in several types of
cancer, including NSCLC (18-26),
and this aberrant expression is associated with poor prognosis in
patients with cancer (21,26,27).
However, the molecular mechanism by which the fucosylation pathway
genes promote tumor progression has not been
well-characterized.
The present study aimed to analyze microarray data
obtained from NSCLC samples. The findings of this study may help
characterize the oncogenic role of fucosylation in NSCLC biology
and highlight its potential for therapeutic targeting.
Materials and methods
Data collection
Microarray data of samples from patients with lung
adenocarcinoma were obtained from the Gene Expression Omnibus (GEO)
database under the accession number GSE31210 (28) as the discovery dataset. The
GSE31210 data were obtained from 20 normal and 226 NSCLC samples
[68 epidermal growth factor receptor (EGFR)/KRAS/echinoderm
microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma
kinase (ALK) fusion-negative (triple-negative), 127 EGFR
mutation-positive, 20 KRAS mutation-positive and 11 EML4-ALK
fusion-positive samples]. The replication datasets were GSE30219
(29), GSE29013 (30), GSE37745 (31) and GSE50081 (32). RNA expression values of TCGA LUAD
dataset were obtained from the RNA-Seq V2 RSEM data of cBioPortal
(http://www.cbioportal.org). Survival
data of patients from TCGA LUAD dataset were collected using
TCGA-assembler 2.0.5 with R software (http://www.r-projects.org) (33). Baseline characteristics of the
survival datasets were summarized in Table SI.
Computational analysis
The microarray datasets were subjected to stringent
quality control tests to filter out low-quality microarray samples
(34). The criteria used were as
previously described (35,36). Of the 246 samples, 235 passed the
quality control test (Table SII).
These samples were normalized by the MAS5.0 algorithm in Expression
Console (Build 1.3.1.187, Affymetrix; Thermo Fisher Scientific,
Inc.), and 18 normal, 66 triple-negative, 121 EGFR
mutation-positive, 19 KRAS mutation-positive and 11 EML4-ALK
fusion-positive samples were used for further analysis. From the
microarray data, probes corresponding to the fucosylation pathway
genes (FUT1, FUT2, FUT3, FUT4, FUT5, FUT6, FUT7, FUT8, FUT9, FUT10,
FUT11, POFUT1, POFUT2, GMDS, TSTA3, FUK, FPGT and SLC35C1) were
extracted. When multiple probes were matched with one gene, the
probe with the highest expression was selected as the
representative value of gene expression. The fucosylation pathway
gene (FUT1, FUT2, FUT3, FUT4, FUT5, FUT6, FUT7, FUT8, FUT9, FUT10,
FUT11, POFUT1, POFUT2, GMDS, TSTA3, FUK, FPGT and SLC35C1)
expression values from tumor samples were then divided by those
from normal samples to calculate relative expression levels.
Correlation analysis was performed for the gene expression levels
between the TGFβ receptor complex pathway and the fucosylation
pathway to examine the association between the pathways. The 'TGFβ
receptor complex pathway gene set’ was created by collecting genes
listed in the gene signatures, such as 'TGFβ receptor signaling
activates SMADs’, 'TGFβ receptor signaling in EMT’ and
'transcriptional activity of SMAD2/SMAD3:SMAD4 heterotrimer’ in
Reactome pathway database version 65 (https://reactome.org) (37). Fucosylation pathway genes and TGFβ
receptor complex pathway genes were presented as scatterplots, and
correlations were expressed using a linear regression model.
Survival analysis
Survival analysis was performed using microarray
datasets. For each fucosylation pathway gene, the patient samples
were divided into four groups according to their gene expression
pattern. Kaplan-Meier survival curves, log-rank test, univariate
and multivariate Cox proportional hazards regression models were
used to determine the association between gene expression and
patient survival in the fourth quartile of expression of
fucosylation pathway genes in terms of relapse-free survival rate
(RFS) or overall survival rate (OS). A multivariate Cox
proportional hazard regression model was used to calculate hazard
ratios (HRs) and 95% confidence intervals (CIs) of the expression
on RFS or OS of NSCLC adjusting for age, sex, smoking status and
pathological stage. Among the 514 TCGA samples, 396 patients with
stage I and II were analyzed, as microarray datasets only consisted
of patients with tumor stage I or II (Table SI).
Cell culture and reagents
NCI-H3122 cells were kindly provided by Professor
Pasi A. Janne (Dana Faber Cancer Institute, Boston, MA, USA).
Calu-1 cells were supplied by the American Type Culture Collection.
Cells with passage number <20 were used in the experiments. All
cell culture reagents were obtained from Gibco; Thermo Fisher
Scientific, Inc. or HyClone; GE Healthcare Life Sciences. The FUT
inhibitor 2F-peracetyl-fucose (2F-PAF) was purchased from Merck
KGaA. 2F-PAF was dissolved in DMSO and stored at -20˚C. Prior to
treatment with TGFβ1 (R&D Systems, Inc.), cells were cultured
in RPMI-1640 medium (HyClone Laboratories, Inc.) containing 0.2%
FBS (HyClone Laboratories, Inc.) and/or 2F-PAF at the indicated
concentrations (25-200 µM) for 24 h. All other cell culture
reagents were supplied by Sigma-Aldrich.
Cell viability assay
NCI-H3122 and Calu-1 cells (3x104) were treated with
2F-PAF (0-200 µM) for 72 h in 12-well culture plates prior
to MTT assay using thiazolyl blue tetrazolium bromide
(Sigma-Aldrich; Merck KGaA). The purple formazan was dissolved in
DMSO and quantified by measuring the absorbance at 570 nm on a
BioTek Synergy MX microplate spectrophotometer (BioTek Instruments,
Inc.).
Luciferase assay
NCI-H3122 cells and Calu-1 cells (3x104)
were transfected with pGL2-3TP-luciferase (Addgene, Inc.) and
pCMV-β-galactosidase gene constructs (Takara Biotechnology Co.,
Ltd.) (38) using FuGENE 6 (Roche
Diagnostics) according to the manufacturer's instructions. After 24
h of transfection, cells were treated with 2F-PAF for 24 h and
further incubated with TGFβ for 24 h. Cells were harvested and
assayed for luciferase or β-galactosidase activity using a
Luciferase Assay System kit (Promega Corporation). Luciferase
activity was normalized to β-galactosidase activity as previously
described (38).
Western blot analysis. NCI-H3122 or Calu-1
cells (2x105) were seeded on 60 mm dishes. After 24 h, cells were
treated with the conditions indicated in figure legends (1 or 5
ng/ml TGFβ and 25-200 µM 2F-PAF). The crude extracts were
prepared by incubation with RIPA buffer containing protease and
phosphatase inhibitor cocktails (Merck KGaA). The protein
concentration was determined by bicinchoninic acid assay. The
samples were resolved using 6 or 10% SDS-PAGE and then transferred
to nitrocellulose membranes. The membranes were blocked with 5%
skim milk in TBS + 0.1% Tween-20 for 1 h at room temperature and
probed with the indicated antibodies. The signals were determined
using a SuperSignal West chemiluminescent substrate (Thermo Fisher
Scientific, Inc.). ImageJ software (ImageJ bundled with 64-bit Java
1.8.0_112; National Institutes of Health) was used to quantify band
intensity. The data were representative of at least three
independent experiments. Antibody information and experimental
conditions are presented in Table
SIII.
Confocal microscopy
NCI-H3122 cells (2x104) were seeded on
glass coverslips in 12-well plates. At 24 h, cells were treated
with the conditions indicated in figure legends (1 ng/ml TGFβ and
50-200 µM 2F-PAF). Cells were fixed with 4% formaldehyde in
PBS for 5 min, permeabilized with 0.3% Triton X-100 for 7 min, and
blocked with 3% normal goat serum in PBS for 1 h at room
temperature. Subsequently, cells were probed with an anti-Smad3
antibody (1:200; cat. no. 9523; Cell signaling Technology, Inc.)
overnight at 4˚C and stained with FITC-conjugated anti-rabbit IgG
antibody (1:1,000; cat. no. A21441; Invitrogen; Thermo Fisher
Scientific, Inc.) and DAPI (Roche Diagnostics GmbH). The stained
cells were imaged with a FluoView 1000 confocal microscope (x40
magnification; Olympus Corporation).
Wound-healing assay
Wound-healing assays were used to assess cell
migration as previously described (39). NCI-H3122 and Calu-1 cells were
cultured with 2F-PAF in 6-well plates for 24 h. A scratch was made
on the cell monolayer using a sterile 10 µl pipette tip, and
then cells were treated with TGFβ, 2F-PAF or TGFβ + 2F-PAF in
RPMI-1640 medium containing 0.2% FBS for 24 h. Migrated cells
within the scratch area were counted in five random fields using a
Nikon Eclipse TS100 phase-contrast microscope (Nikon Instruments,
Inc.).
Transwell migration assay
Transwell migration assays were performed in 24-well
chambers with 8 µm pore size Transwell inserts (Corning,
Inc.) as previously described (40,41).
The inserts were coated with 0.1 mg/ml collagen for 1 h. NCI-H3122
cells were trypsinized, and single-cell suspensions were placed
into the upper chamber (1×105 cells/well) in 100
µl serum-free medium. TGFβ (5 ng/ml) in serum-free medium
(800 µl) was placed in the lower chamber as a
chemoattractant. After 48-h incubation, the cells from the upper
surface of the chamber were removed using a cotton swab. The
migrated cells were fixed with 4% formaldehyde in PBS for 7 min and
stained with 0.5% crystal violet for 20 min at room temperature,
imaged and counted using a phase-contrast microscope (×100). The
number of migrated cells was counted in five random fields.
In vivo metastasis assay
Calu-1-Luc cells were established by infection with
RediFect Red-FLuc-Puromycin Lentiviral Particles (PerkinElmer,
Inc.) for 24 h at 37˚C and puromycin selection for 2 weeks with a
final concentration of 2 µg/ml. Calu-1-Luc cells were
treated with 20 µg/ml 2F-PAF for 96 h. BALB/c-nude mice
(male, 8 weeks old, n=5 per group) were housed in a specific
pathogen-free environment at 22±2˚C and 55±5% relative humidity
with light. BALB/c-nude mice injected intravenously with
1x106 2F-PAF-treated Calu-1-Luc cells and analyzed 2
weeks later. For in vivo bioluminescence imaging (BLI), mice
were injected intraperitoneally with D-Luciferin (150 mg/kg, 200
µl; PerkinElmer, Inc.) under gas anesthesia [1% (w/v)
isoflurane in 2 l oxygen] and imaged 10 min later using the IVIS
spectrum system (PerkinElmer, Inc.). BLI intensity was measured
using region of interest analysis. All experiments were conducted
under protocols approved by the Institutional Animal Care and Use
Committee of the Asan Institute for Life Sciences at the Asan
Medical Center (approval no. 2019-14-201).
Statistical analysis
GraphPad Prism 7.04 (GraphPad Software, Inc.) was
used for statistical analysis. Data are expressed as the mean ±
SEM. Comparison of mean values among experimental groups was
performed using one-way ANOVA followed by a Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of fucosylation pathway genes
is altered in NSCLC
To determine which fucosylation pathway genes were
differentially expressed between normal lung and NSCLC tissue
samples, 235 samples (18 normal and 217 NSCLC tissues) from the
public microarray dataset GSE31210 were analyzed. A heatmap
demonstrated distinct expression patterns of fucosylation pathway
genes (Fig. 1A). The expression
levels of FUT2, FUT3, FUT6, FUT8, GMDS, TSTA3, FUK and FPGT were
increased in NSCLC, whereas FUT1 expression decreased in NSCLC
(Fig. 1B). This result was
confirmed in the replication datasets GSE30219 (Fig. 1C and D) and GSE19188 (Fig. S3A and B). The expression levels of
FUT1, FUT2, FUT3, FUT6, FUT8, GMDS and TSTA3 were commonly altered
in NSCLC in the three datasets (Figs.
1 and S3).
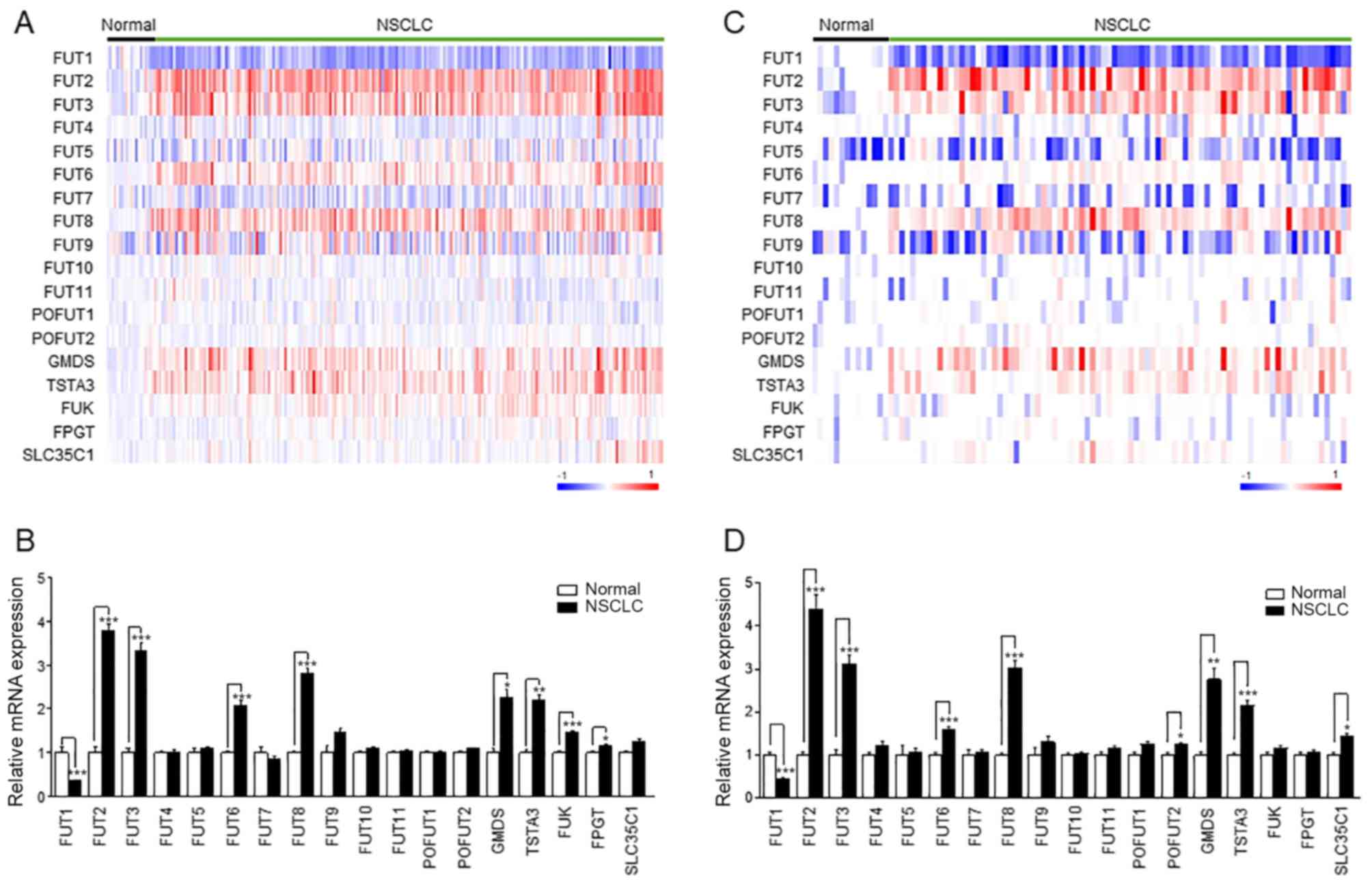 | Figure 1Expression of fucosylation pathway
genes in NSCLC. (A and C) The heatmap of the
log2-transformed expression level of fucosylation
pathway genes in each microarray sample. The heatmap was generated
using GenePattern HeatMapImage. (B and D) The expression levels of
fucosylation pathway genes in patients with NSCLC were normalized
to those in the control groups. Left panel, GSE31210; right panel,
GSE30219. Data are expressed as the mean ± SEM and compared using
an unpaired Student's t-test. *P<0.05,
**P<0.01, ***P<0.005. NSCLC, non-small
cell lung cancer; FUT, fucosyltransferase; TSTA3, GDP-L-fucose
synthase; GMDS, GDP-mannose 4,6-dehydratase; FUK, L-fucose kinase;
FPGT, fucose-1-phosphate guanylyltransferase; SLC35C1, GDP-fucose
transporter 1; POFUT, protein O-fucosyltransferase. |
The altered expression of these genes compared with
normal lung tissue was observed in NSCLC independently of the
triple-negative, EGFR mutation-positive, KRAS mutation-positive,
and EML4-ALK fusion-positive status. The analysis results
demonstrated that the gene expression levels of FUT1, FUT2, FUT3,
FUT6, FUT8, GMDS, TSTA3 and FUK were commonly altered in all four
NSCLC subtypes compared with those in normal tissues (Fig. S4 and Table SIV). However, the change was most
prominent in the EML4-ALK fusion-positive subtype of NSCLC.
Altered expression of fucosylation
pathway genes is associated with poor prognosis in patients with
NSCLC
Kaplan-Meier survival analysis with log-rank test
demonstrated that the altered expression of 7 out of 18
fucosylation pathway genes was associated with poor OS and RFS
(Figs. S5 and S6). Patients with NSCLC with high
expression of FUT2, FUT3, FUT6, FUT8 and TSTA3 and low expression
of FUT1 and FUT5 exhibited significantly worse RFS, even after
adjusting for epidemiological and clinicopathological factors
(Table I). To validate the results
of the survival analysis, OS analysis was performed using two
different datasets (Table SV).
These results demonstrated that patients with altered expression of
FUT1 (downregulated) and FUT8 (upregulated) exhibited significantly
worse survival rates. This result of the replication dataset was
consistent with the result of the discovery dataset (Tables I and SV).
 | Table IHRs for relapse-free survival based
on the expression of fucosylation pathway genes of 217 patients
with non-small cell lung cancer. |
Table I
HRs for relapse-free survival based
on the expression of fucosylation pathway genes of 217 patients
with non-small cell lung cancer.
| Gene | Expression
quartile | Patients (n) | Death (n) | Crude HR (95%
CI) | Adjusted HRa (95% CI) |
|---|
| FUT2 | Q1 (0-25%) | 54 | 11 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 15 | 1.299
(0.601-2.809) | 1.458
(0.673-3.161) |
| Q3 (50-75%) | 54 | 12 | 1.023
(0.459-2.277) | 1.255
(0.559-2.817) |
| Q4 (75-100%) | 54 | 22 | 2.275
(1.125-4.601) | 2.527
(1.228-5.203) |
| FUT3 | Q1 (0-25%) | 54 | 13 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 17 | 1.364
(0.663-2.809) | 2.207
(1.042-4.679) |
| Q3 (50-75%) | 54 | 10 | 0.736
(0.323-1.679) | 1.027
(0.440-2.395) |
| Q4 (75-100%) | 54 | 20 | 1.737
(0.864-3.493) | 2.190
(1.073-4.470) |
| FUT4 | Q1 (0-25%) | 54 | 13 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 14 | 1.144
(0.537-2.434) | 0.868
(0.400-1.880) |
| Q3 (50-75%) | 54 | 11 | 0.843
(0.378-1.883) | 0.869
(0.386-1.954) |
| Q4 (75-100%) | 54 | 22 | 2.020
(1.016-4.018) | 1.605
(0.791-3.258) |
| FUT6 | Q1 (0-25%) | 54 | 14 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 11 | 0.614
(0.276-1.367) | 0.754
(0.336-1.690) |
| Q3 (50-75%) | 54 | 12 | 0.803
(0.376-1.715) | 0.834
(0.385-1.805) |
| Q4 (75-100%) | 54 | 23 | 1.765
(0.920-3.385) | 1.962
(1.018-3.780) |
| FUT8 | Q1 (0-25%) | 54 | 8 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 13 | 1.869
(0.774-4.510) | 1.666
(0.685-4.052) |
| Q3 (50-75%) | 54 | 18 | 2.511
(1.092-5.777) | 2.489
(1.078-5.748) |
| Q4 (75-100%) | 54 | 21 | 3.096
(1.371-6.990) | 2.530
(1.112-5.760) |
| FUT9 | Q1 (0-25%) | 54 | 13 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 9 | 0.702
(0.300-1.641) | 0.637
(0.271-1.497) |
| Q3 (50-75%) | 54 | 17 | 1.535
(0.745-3.162) | 1.496
(0.721-3.106) |
| Q4 (75-100%) | 54 | 21 | 1.854
(0.928-3.706) | 1.424
(0.698-2.906) |
| FUT10 | Q1 (0-25%) | 54 | 16 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 15 | 0.958
(0.473-1.938) | 0.988
(0.479-2.040) |
| Q3 (50-75%) | 54 | 13 | 0.840
(0.404-1.745) | 0.947
(0.448-2.000) |
| Q4 (75-100%) | 54 | 16 | 1.031
(0.516-2.063) | 1.188
(0.578-2.444) |
| FUT11 | Q1 (0-25%) | 54 | 11 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 11 | 1.070
(0.464-2.468) | 1.058
(0.457-2.453) |
| Q3 (50-75%) | 54 | 16 | 1.629
(0.756-3.511) | 1.394
(0.639-3.039) |
| Q4 (75-100%) | 54 | 22 | 2.411
(1.168-4.977) | 1.834
(0.868-3.876) |
| POFUT1 | Q1 (0-25%) | 54 | 11 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 13 | 1.266
(0.567-2.827) | 1.030
(0.455-2.331) |
| Q3 (50-75%) | 54 | 16 | 1.551
(0.720-3.342) | 1.159
(0.527-2.548) |
| Q4 (75-100%) | 54 | 20 | 2.273
(1.088-4.747) | 1.638
(0.760-3.530) |
| GMDS | Q1 (0-25%) | 54 | 7 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 19 | 3.180
(1.337-7.567) | 2.620
(1.092-6.291) |
| Q3 (50-75%) | 54 | 18 | 2.916
(1.218-6.983) | 2.656
(1.103-6.396) |
| Q4 (75-100%) | 54 | 16 | 2.649
(1.089-6.442) | 2.160
(0.881-5.296) |
| TSTA3 | Q1 (0-25%) | 54 | 9 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 16 | 2.069
(0.922-4.645) | 1.847
(0.817-4.175) |
| Q3 (50-75%) | 54 | 15 | 1.689
(0.731-3.902) | 1.475
(0.632-3.442) |
| Q4 (75-100%) | 54 | 20 | 2.563
(1.167-5.629) | 2.464
(1.118-5.431) |
| FUK | Q1 (0-25%) | 54 | 18 | 1 (Reference) | 1 (Reference) |
| Q2 (25-50%) | 55 | 12 | 0.620
(0.299-1.288) | 0.669
(0.318-1.410) |
| Q3 (50-75%) | 54 | 10 | 0.497
(0.229-1.077) | 0.633
(0.281-1.426) |
| Q4 (75-100%) | 54 | 20 | 1.062
(0.562-2.008) | 1.052
(0.547-2.024) |
| FUT1 | Q1 (75-100%) | 54 | 26 | 1 (Reference) | 1 (Reference) |
| Q2 (50-75%) | 55 | 12 | 3.839
(1.406-10.483) | 3.030
(1.094-8.397) |
| Q3 (25-50%) | 54 | 17 | 2.636
(0.929-7.485) | 1.838
(0.626-5.398) |
| Q4 (0-25%) | 54 | 5 | 6.768
(2.604-17.591) | 4.469
(1.657-12.051) |
| FUT5 | Q1 (75-100%) | 54 | 26 | 1 (Reference) | 1 (Reference) |
| Q2 (50-75%) | 55 | 11 | 1.104
(0.487-2.502) | 1.064
(0.467-2.425) |
| Q3 (25-50%) | 54 | 12 | 1.036
(0.449-2.390) | 1.099
(0.469-2.577) |
| Q4 (0-25%) | 54 | 11 | 2.882
(1.423-5.836) | 2.619
(1.286-5.332) |
| FUT7 | Q1 (75-100%) | 54 | 16 | 1 (Reference) | 1 (Reference) |
| Q2 (50-75%) | 55 | 9 | 1.616
(0.822-3.177) | 1.593
(0.809-3.137) |
| Q3 (25-50%) | 54 | 21 | 0.623
(0.270-1.439) | 0.657
(0.281-1.534) |
| Q4 (0-25%) | 54 | 14 | 1.133
(0.552-2.321) | 1.130
(0.538-2.371) |
| POFUT2 | Q1 (75-100%) | 54 | 19 | 1 (Reference) | 1 (Reference) |
| Q2 (50-75%) | 55 | 17 | 1.168
(0.523-2.607) | 1.275
(0.569-2.857) |
| Q3 (25-50%) | 54 | 13 | 1.510
(0.707-3.223) | 1.634
(0.759-3.520) |
| Q4 (0-25%) | 54 | 11 | 1.874
(0.891-3.939) | 1.570
(0.741-3.327) |
| FPGT | Q1 (75-100%) | 54 | 17 | 1 (Reference) | 1 (Reference) |
| Q2 (50-75%) | 55 | 19 | 0.615
(0.266-1.422) | 0.646
(0.278-1.503) |
| Q3 (25-50%) | 54 | 10 | 1.424
(0.714-2.842) | 1.291
(0.645-2.587) |
| Q4 (0-25%) | 54 | 14 | 1.309
(0.651-2.633) | 0.958
(0.466-1.971) |
| SLC35C1 | Q1 (75-100%) | 54 | 14 | 1 (Reference) | 1 (Reference) |
| Q2 (50-75%) | 55 | 17 | 1.825
(0.862-3.866) | 1.759
(0.829-3.733) |
| Q3 (25-50%) | 54 | 18 | 1.613
(0.755-3.443) | 1.802
(0.835-3.892) |
| Q4 (0-25%) | 54 | 11 | 1.294
(0.587-2.852) | 1.528
(0.688-3.391) |
Inhibition of FUTs attenuates
TGFβ-induced cell migration and tumor metastasis
Fucosylation of the TGFβ receptor has previously
been demonstrated to enhance TGFβ signaling (42,43).
To determine the association between TGFβ signaling and
fucosylation in patients with lung cancer, the correlation of gene
expression between the fucosylation pathway and the TGFβ receptor
complex pathway was first analyzed. The TGFβ receptor complex
pathway gene set was constructed using the Reactome database, and
the expression levels of these genes were extracted from GSE31210.
The hierarchical clustering results demonstrated that the
expression of TGFβ receptor complex pathway genes such as cbl
proto-oncogene, cadherin1, desmoplakin, E2F transcription factor 4
(E2F4), histone deacetylase 1, matrix metallopepti-dase 9,
partitioning defective 6 homolog α, poly (ADP-ribose) polymerase 1,
protein phosphatase 1A, protein phosphatase 1 catalytic subunit α,
ras homolog family member A, SKI-like proto oncogene, SMAD3,
SRY-box transcription factor 9, Sp1 transcription factor, zinc
finger E-box-binding homeobox 1, TGFβR2, tight junction protein-1,
ubiquitin-conjugating enzyme E2, WW domain containing transcription
regulator 1, zinc finger E-box binding homeobox 2 and zinc finger
FYVE-type containing 9 exhibited strong correlations with the
expression of FUT1, FUT2, FUT3, FUT4, FUT6, FUT7, FUT8, FUT11,
POFUT2, GMDS, FPGT, FUK and TSTA3 in patients with NSCLC (Fig. S7). The expression of E2F4
exhibited a fair correlation with the expression of FUT4 and GMDS
(R=0.53 and R=0.57, respectively) 30191186. In the linear
regression model, E2F4-FUT4 (R2=0.28) and E2F4-GDMS (R2=0.32) had
explanatory power (Fig. S8).
To confirm the association between TGFβ signaling
and fucosylation, the FUT inhibitor 2F-PAF was used. Since altered
expression of fucosylation pathway genes is most prominent in the
EML4-ALK fusion-positive subtype of NSCLC, NCI-H3122, which is an
NSCLC cell line that expresses the EML4-ALK fusion, was selected
for the experiments. 2F-PAF suppressed TGFβ-induced Smad activation
in a dose-dependent manner (Fig.
2A). In addition, 2F-PAF attenuated the TGFβ-mediated
phosphorylation of Smad2/3 (Fig.
2B) and translocation of Smad3 into the nucleus (Fig. 2C). Additionally, 2F-PAF inhibited
TGFβ signaling in NCI-H3122 cells without exhibiting any
cytotoxicity effects (Fig. S9A).
The effects of 2F-PAF on TGFβ-mediated phenotypes in NCI-H3122
cells were further examined; 2F-PAF suppressed TGFβ-induced
downregulation of E-cadherin and upregulation of vimentin,
N-cadherin and fibronectin (Fig.
2D), suggesting that 2F-PAF inhibited TGFβ-mediated
epithelial-mesenchymal transition (EMT). In addition, 2F-PAF
suppressed TGFβ-induced cell migration and invasion in a
wound-healing (Figs. 2E and
S10A-C) and Transwell invasion
(Figs. 2F and S11) assays, respectively. These results
demonstrated that 2F-PAF inhibited TGFβ-induced EMT, migration and
invasion of NSCLC cells.
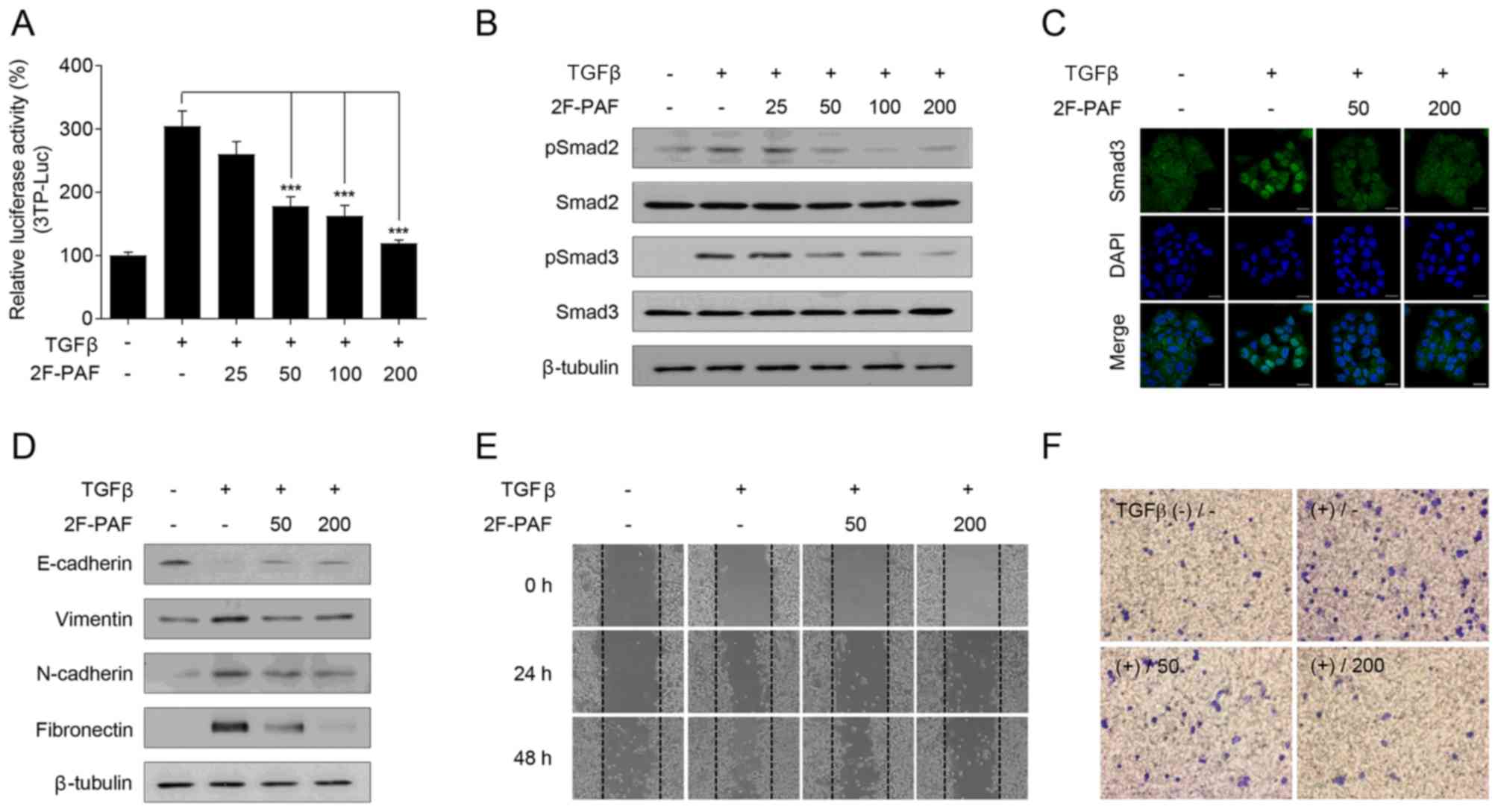 | Figure 2Inhibition of FUTs suppresses
TGFβ-induced cell migration and invasion in NCI-H3122 cells. (A)
NCI-H3122 cells were transfected with a 3TP-Luc reporter construct
for 24 h and incubated with TGFβ (1 ng/ml) and 2F-PAF at the
indicated concentrations (0, 25, 50, 100, and 200 µM) for 24
h. The luciferase activity was expressed as a relative value
compared with that of the untreated cells. Data are expressed as
the mean ± SEM (n=3). ***P<0.005. (B and C) Cells
were treated with TGFβ (1 ng/ml) and 2F-PAF at the indicated
concentrations for 1 h prior to (B) western blot analysis and (C)
confocal microscopy. The localization of Smad3 was assessed using
an anti-Smad3 antibody and a FITC-conjugated IgG antibody. DAPI was
used to visualize the nucleus. Scale bar, 20 µm. (D) Cells
were treated with TGFβ (5 ng/ml) and/or 2F-PAF for 48 h prior to
western blot analysis. (E) In a wound-healing assay, cells were
treated with TGFβ (5 ng/ml) and/or 2F-PAF at 50 and 200 µM
for the indicated times after the wound was created. (F) The
invasive cells in the Transwell assay were fixed with 4%
formaldehyde, stained with 0.5% crystal violet, imaged and counted
using a phase-contrast microscope. FUT, fucosyltransferase; TGFβ,
transforming growth factor β; 2F-PAF, 2F-peracetyl-fucose; p,
phosphorylated; Luc, luciferase. |
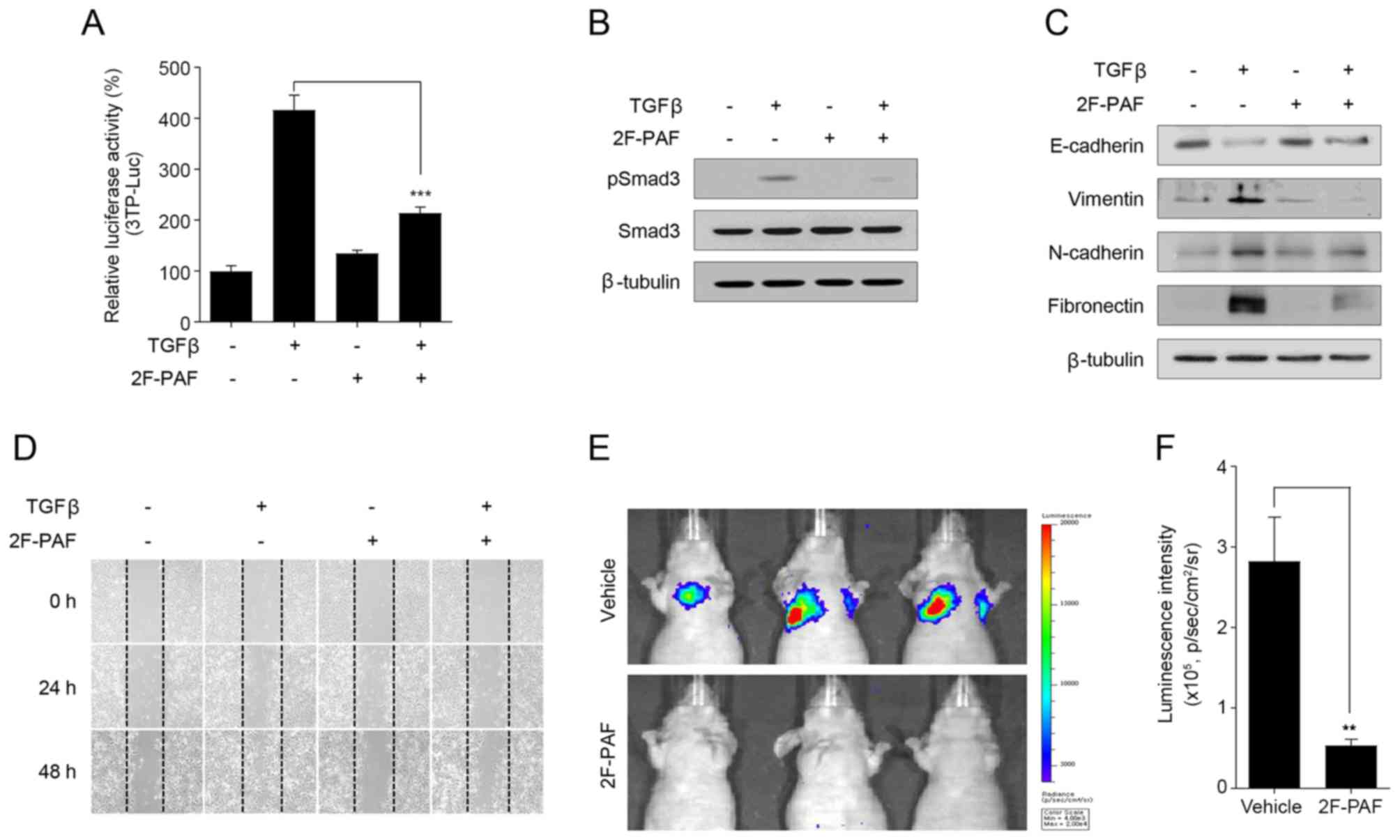
Inhibition of FUTs attenuates NSCLC
metastasis
In addition to in vitro experiments, further
experiments were performed to examine whether 2F-PAF may attenuate
tumor metastasis in vivo. The anti-metastatic activity of
2F-PAF was examined using highly metastatic Calu-1 NSCLC cells.
2F-PAF inhibited TGFβ signaling in Calu-1 cells (Fig. 3A and B) without exhibiting any
cytotoxicity effects 27683099 (Fig.
S9B). The expression levels of EMT marker proteins in Calu-1
cells were also analyzed. Similar to NCI-H3122 cells (Fig. 2D), TGFβ decreased the expression
level of E-cadherin and increased those of vimentin, N-cadherin,
and fibronectin, and these effects were reversed by 2F-PAF
(Fig. 3C). In addition,
wound-healing assay demonstrated that 2F-PAF decreased TGFβ-induced
Calu-1 cell migration (Fig. 3D and
Fig. S10D-F), which was similar
to the results observed in NCI-H3122 cells (Fig. 2E).
At 200 µM, 2F-PAF completely suppressed
TGFβ-induced reporter gene activity and Smad phosphorylation
(Figs. 2A and B, and 3A and B), but partially rescued
TGFβ-induced EMT markers (Figs. 2D
and 3C) in the two NSCLC cell
lines. At 800 µM 2F-PAF fully rescued TGFβ-induced changes
in EMT markers (data not shown). However, to minimize the adverse
drug response, 200 µM 2F-PAF in the in vivo studies.
Bioluminescence imaging analysis of a mouse NSCLC metastasis model
revealed that 2F-PAF inhibited the colonizing ability of Calu-1-Luc
(Fig. 3E and F), a
luciferase-expressing Calu-1 cell line that has the ability to
colonize to the lung following intravenous injection (44). These results indicated that
inhibition of FUTs attenuated the in vivo metastatic
capacity of Calu-1 cells.
Discussion
Fucosylation of cell surface receptors serves a
crucial role in fine-tuning cellular responses to extracellular
stimuli (13). Previous studies
have reported that cellular fucosylation patterns are altered
during cancer development and progression (21,45).
The results of the present study demonstrated that altered
expression of fucosylation pathway genes is associated with poor
prognosis in patients with NSCLC. In addition, inhibition of FUTs
suppressed TGFβ signaling and tumor metastasis.
TGFβ serves a crucial role in cancer metastasis by
affecting various cellular processes, including cell migration
(46,47). The results of the present study
demonstrated that FUTs were aberrantly expressed in NSCLC and that
2F-PAF inhibited TGFβ signaling and cell migration. These results
suggested that the altered expression of FUTs may stimulate cancer
metastasis by potentiating TGFβ signaling in NSCLC. In addition,
these results indicated that FUT inhibitors, including 2F-PAF, may
be promising agents against metastasis of NSCLC. A previous study
reported the feasibility of FUT inhibitors as anti-metastatic
agents in prostate cancer (45).
However, to corroborate the clinical significance of the present
study, further studies will be required to measure the expression
levels of FUTs and fucosylation on their substrates from a mouse
model and patient samples. In addition, it is important to
investigate how long the effect of 2F-PAF lasts in the experimental
conditions and the possible secondary adverse drug effects.
The effect of each fucosylation pathway gene on TGFβ
signaling was assessed in the present study using siRNAs against
FUT2, FUT3, FUT6, FUT8 and TSTA3; however, siRNA-mediated silencing
of any of these gene alone did not recapitulate the effects of
2F-PAF on inhibiting TGFβ signaling in NCI-H3122 cells (data not
shown). These results suggested that simultaneous inhibition of all
FUTs, and thus multiple types of fucosylation, may be effective in
inhibiting TGFβ signaling and metastasis.
The results of the present study demonstrated that
FUT1 was downregulated and associated with poor prognosis in
patients with NSCLC. By contrast, the expression levels of FUT1
were elevated in samples from patient with prostate cancer
(48). These studies provide
insight into the cancer type-specific roles of FUT isotypes.
However, further studies are needed to investigate how the
expression levels of fucosylation pathway genes vary across a wide
range of cancer types.
The results of the present study indicated that the
change in the expression levels of fucosylation pathway genes is
most prominent in the EML4-ALK fusion-positive subtype of NSCLC.
These findings suggested that fucosylation may exert differing
roles in different NSCLC subtypes. However, due to the small sample
size in our dataset, no statistically significant differences were
observed in patient prognosis across the different molecular
subtypes of NSCLC.
One limitation of the present study was that
patients were recruited from different datasets, and could
therefore possess different clinicopathological characteristics
that affect lung adenocarcinoma survival. In the discovery set
(GSE31210), the expression levels of FUT1, FUT2, FUT3, FUT6, FUT8
and TSTA3 correlated with poor RFS. However, in the replication
dataset, only FUT1 and FUT8 were associated with poor OS. The
different results between the discovery and replication datasets
result from individual heterogeneity, including the distribution of
sex and smoking status in the datasets. In addition, OS analysis
was performed in the replication datasets as there are not enough
publicly accessible RFS datasets. Despite these limitations, the
strength of the present study is that the target genes extracted
from large amounts of disease tran-scriptome data were verified
through survival analysis, in vitro and in vivo
experiments.
In conclusion, the present study applied a
data-driven approach to increase the understanding of the role of
fucosylation pathway genes in NSCLC and to assess the clinical
relevance of fucosylation pathway genes. In addition, the results
of the present study demonstrated that inhibition of FUTs
attenuated TGFβ signaling and tumor metastasis. These results
suggested that targeting fucosylation may represent a promising
strategy for the development of novel NSCLC therapeutics.
Supplementary Data
Funding
This study was supported by a grant of the Korea
Health Technology R&D Project, Ministry of Health &
Welfare, Republic of Korea (grant no. A110057).
Availability of data and materials
All data generated or analyzed during this study are
including in this published article.
Authors' contributions
All authors contributed to the acquisition,
analysis, or interpretation of data for this work. TMK, DWK, SYK,
IS, HGK, JYC and JHJ made substantial contributions to the
conception or design of the work. SP, JML, JNC, JYC and JHJ
contributed to drafting the manuscript. SHL, TMK, DWK, DJB, SMB,
SYK, IS and HGK revised the manuscript. All authors approved the
final version to be published and agreed to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
All animal experiments were conducted under
protocols approved by the Institutional Animal Care and Use
Committee (IACUC) of the Asan Institute for Life Sciences at the
Asan Medical Center (2019-14-201).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chansky K, Sculier JP, Crowley JJ, et al:
The International Association for the Study of Lung Cancer Staging
Project: prognostic factors and pathologic TNM stage in surgically
managed non-small cell lung cancer. J Thorac Oncol. 4:792–801.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma B, Simala-Grant JL and Taylor DE:
Fucosylation in prokaryotes and eukaryotes. Glycobiology.
16:158R–184R. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adamczyk B, Tharmalingam T and Rudd PM:
Glycans as cancer biomarkers. Biochim Biophys Acta. 1820:1347–1353.
2012. View Article : Google Scholar
|
|
6
|
Miyoshi E, Moriwaki K, Terao N, Tan CC,
Terao M, Nakagawa T, Matsumoto H, Shinzaki S and Kamada Y:
Fucosylation is a promising target for cancer diagnosis and
therapy. Biomolecules. 2:34–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tuccillo FM, de Laurentiis A, Palmieri C,
Fiume G, Bonelli P, Borrelli A, Tassone P, Scala I, Buonaguro FM,
Quinto I, et al: Aberrant glycosylation as biomarker for cancer:
Focus on CD43. BioMed Res Int. 2014:7428312014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyoshi E, Moriwaki K and Nakagawa T:
Biological function of fucosylation in cancer biology. J Biochem.
143:725–729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HJ, Kim SC, Ju W, Kim YH, Yin SY and
Kim HJ: Aberrant sialylation and fucosylation of intracellular
proteins in cervical tissue are critical markers of cervical
carcinogenesis. Oncol Rep. 31:1417–1422. 2014. View Article : Google Scholar
|
|
10
|
Zhu J, Wang Y, Yu Y, et al: Aberrant
fucosylation of glycosphin-golipids in human hepatocellular
carcinoma tissues. Liver Int. 34:147–160. 2014. View Article : Google Scholar
|
|
11
|
Munkley J, Mills IG and Elliott DJ: The
role of glycans in the development and progression of prostate
cancer. Nat Rev Urol. 13:324–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson
SR, Vogt PK and Wong CH: Glycoproteomic probes for fluorescent
imaging of fucosylated glycans in vivo. Proc Natl Acad Sci USA.
103:12371–12376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Becker DJ and Lowe JB: Fucose:
Biosynthesis and biological function in mammals. Glycobiology.
13:41R–53R. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oriol R, Mollicone R, Cailleau A,
Balanzino L and Breton C: Divergent evolution of fucosyltransferase
genes from vertebrates, invertebrates, and bacteria. Glycobiology.
9:323–334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu Z, Lin YN and Lin CH: Development of
fucosyltransferase and fucosidase inhibitors. Chem Soc Rev.
42:4459–4475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Merino P, Tejero T, Delso I,
Hurtado-Guerrero R, Gómez-SanJuan A and Sádaba D: Recent progress
on fucosyltransferase inhibitors. Mini Rev Med Chem. 12:1455–1464.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mollicone R, Moore SE, Bovin N,
Garcia-Rosasco M, Candelier JJ, Martinez-Duncker I and Oriol R:
Activity, splice variants, conserved peptide motifs, and phylogeny
of two new alpha1,3-fucosyltransferase families (FUT10 and FUT11).
J Biol Chem. 284:4723–4738. 2009. View Article : Google Scholar
|
|
18
|
Guo Q, Guo B, Wang Y, Wu J, Jiang W, Zhao
S, Qiao S and Wu Y: Functional analysis of
α1,3/4-fucosyltransferase VI in human hepatocellular carcinoma
cells. Biochem Biophys Res Commun. 417:311–317. 2012. View Article : Google Scholar
|
|
19
|
Barthel SR, Wiese GK, Cho J, Opperman MJ,
Hays DL, Siddiqui J, Pienta KJ, Furie B and Dimitroff CJ: Alpha 1,3
fucosyltransferases are master regulators of prostate cancer cell
trafficking. Proc Natl Acad Sci USA. 106:19491–19496. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muinelo-Romay L, Vázquez-Martín C,
Villar-Portela S, Cuevas E, Gil-Martín E and Fernández-Briera A:
Expression and enzyme activity of alpha(1,6)fucosyltransferase in
human colorectal cancer. Int J Cancer. 123:641–646. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CY, Jan YH, Juan YH, Yang CJ, Huang
MS, Yu CJ, Yang PC, Hsiao M, Hsu TL and Wong CH: Fucosyltransferase
8 as a functional regulator of nonsmall cell lung cancer. Proc Natl
Acad Sci USA. 110:630–635. 2013. View Article : Google Scholar
|
|
22
|
Carvalho AS, Harduin-Lepers A, Magalhães
A, Machado E, Mendes N, Costa LT, Matthiesen R, Almeida R, Costa J
and Reis CA: Differential expression of
alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyltransferases
regulates the levels of sialyl Lewis a and sialyl Lewis x in
gastrointestinal carcinoma cells. Int J Biochem Cell Biol.
42:80–89. 2010. View Article : Google Scholar
|
|
23
|
Li W, Zhang W, Luo J, Cao A, Zhang Y,
Huang D, Sheng W, Cai S and Li J: Alpha1,3 fucosyltransferase VII
plays a role in colorectal carcinoma metastases by promoting the
carbohy-dration of glycoprotein CD24. Oncol Rep. 23:1609–1617.
2010.PubMed/NCBI
|
|
24
|
Wang X, Chen J, Li QK, Peskoe SB, Zhang B,
Choi C, Platz EA and Zhang H: Overexpression of α (1,6)
fucosyltransferase associated with aggressive prostate cancer.
Glycobiology. 24:935–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang X, Liu S and Yan Q: Role of
fucosyltransferase IV in epithelial-mesenchymal transition in
breast cancer cells. Cell Death Dis. 4:e7352013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Honma R, Kinoshita I, Miyoshi E, Tomaru U,
Matsuno Y, Shimizu Y, Takeuchi S, Kobayashi Y, Kaga K, Taniguchi N,
et al: Expression of fucosyltransferase 8 is associated with an
unfavorable clinical outcome in non-small cell lung cancers.
Oncology. 88:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma L, Dong P, Liu L, Gao Q, Duan M, Zhang
S, Chen S, Xue R and Wang X: Overexpression of protein
O-fucosyltransferase 1 accelerates hepatocellular carcinoma
progression via the Notch signaling pathway. Biochem Biophys Res
Commun. 473:503–510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okayama H, Kohno T, Ishii Y, Shimada Y,
Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S,
et al: Identification of genes upregulated in ALK-positive and
EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res.
72:100–111. 2012. View Article : Google Scholar
|
|
29
|
Rousseaux S, Debernardi A, Jacquiau B,
Vitte AL, Vesin A, Nagy-Mignotte H, Moro-Sibilot D, Brichon PY,
Lantuejoul S, Hainaut P, et al: Ectopic activation of germline and
placental genes identifies aggressive metastasis-prone lung
cancers. Sci Transl Med. 5:186ra662013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie Y, Xiao G, Coombes KR, Behrens C,
Solis LM, Raso G, Girard L, Erickson HS, Roth J, Heymach JV, et al:
Robust gene expression signature from formalin-fixed
paraffin-embedded samples predicts prognosis of non-small-cell lung
cancer patients. Clin Cancer Res. 17:5705–5714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Botling J, Edlund K, Lohr M, Hellwig B,
Holmberg L, Lambe M, Berglund A, Ekman S, Bergqvist M, Pontén F, et
al: Biomarker discovery in non-small cell lung cancer: Integrating
gene expression profiling, meta-analysis, and tissue microarray
validation. Clin Cancer Res. 19:194–204. 2013. View Article : Google Scholar
|
|
32
|
Der SD, Sykes J, Pintilie M, Zhu CQ,
Strumpf D, Liu N, Jurisica I, Shepherd FA and Tsao MS: Validation
of a histology-independent prognostic gene signature for
early-stage, non-small-cell lung cancer including stage IA
patients. J Thorac Oncol. 9:59–64. 2014. View Article : Google Scholar
|
|
33
|
Zhu Y, Qiu P and Ji Y: TCGA-assembler:
Open-source software for retrieving and processing TCGA data. Nat
Methods. 11:599–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eijssen LM, Jaillard M, Adriaens ME, Gaj
S, de Groot PJ, Müller M and Evelo CT: User-friendly solutions for
microarray quality control and pre-processing on ArrayAnalysis.org.
Nucleic Acids Res. 41(W1): W71–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee S, Chun JN, Kim SH, So I and Jeon JH:
Icilin inhibits E2F1-mediated cell cycle regulatory programs in
prostate cancer. Biochem Biophys Res Commun. 441:1005–1010. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee S, Park YR, Kim SH, Park EJ, Kang MJ,
So I, Chun JN and Jeon JH: Geraniol suppresses prostate cancer
growth through down-regulation of E2F8. Cancer Med. 5:2899–2908.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fabregat A, Korninger F, Viteri G,
Sidiropoulos K, Marin-Garcia P, Ping P, Wu G, Stein L, D'Eustachio
P and Hermjakob H: Reactome graph database: Efficient access to
complex pathway data. PLOS Comput Biol. 14:e10059682018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park EJ, Chun JN, Kim SH, Kim CY, Lee HJ,
Kim HK, Park JK, Lee SW, So I and Jeon JH: Schisandrin B suppresses
TGFβ1 signaling by inhibiting Smad2/3 and MAPK pathways. Biochem
Pharmacol. 83:378–384. 2012. View Article : Google Scholar
|
|
39
|
Chun JN, Kim SY, Park EJ, Kwon EJ, Bae DJ,
Kim IS, Kim HK, Park JK, Lee SW, Park HH, et al: Schisandrin B
suppresses TGFβ1-induced stress fiber formation by inhibiting
myosin light chain phosphorylation. J Ethnopharmacol. 152:364–371.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim JS, Kim JG, Moon MY, Jeon CY, Won HY,
Kim HJ, Jeon YJ, Seo JY, Kim JI, Kim J, et al: Transforming growth
factor-beta1 regulates macrophage migration via RhoA. Blood.
108:1821–1829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu J, Hu G, Chen D, Gong AY, Soori GS,
Dobleman TJ and Chen XM: Suppression of SCARA5 by Snail1 is
essential for EMT-associated cell migration of A549 cells.
Oncogenesis. 2:e732013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hirakawa M, Takimoto R, Tamura F, Yoshida
M, Ono M, Murase K, Sato Y, Osuga T, Sato T, Iyama S, et al:
Fucosylated TGF-β receptors transduces a signal for
epithelial-mesenchymal transition in colorectal cancer cells. Br J
Cancer. 110:156–163. 2014. View Article : Google Scholar
|
|
43
|
Lin H, Wang D, Wu T, Dong C, Shen N, Sun
Y, Sun Y, Xie H, Wang N and Shan L: Blocking core fucosylation of
TGF-β1 receptors downregulates their functions and attenuates the
epithelial-mesenchymal transition of renal tubular cells. Am J
Physiol Renal Physiol. 300:F1017–F1025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Larsen JE, Nathan V, Osborne JK, Farrow
RK, Deb D, Sullivan JP, Dospoy PD, Augustyn A, Hight SK, Sato M, et
al: ZEB1 drives epithelial-to-mesenchymal transition in lung
cancer. J Clin Invest. 126:3219–3235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li J, Guillebon AD, Hsu JW, Barthel SR,
Dimitroff CJ, Lee YF and King MR: Human fucosyltransferase 6
enables prostate cancer metastasis to bone. Br J Cancer.
109:3014–3022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Padua D and Massagué J: Roles of TGFbeta
in metastasis. Cell Res. 19:89–102. 2009. View Article : Google Scholar
|
|
47
|
Drabsch Y and ten Dijke P: TGF-β
signalling and its role in cancer progression and metastasis.
Cancer Metastasis Rev. 31:553–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fukushima K, Satoh T, Baba S and Yamashita
K: alpha1,2-Fucosylated and beta-N-acetylgalactosaminylated
prostate-specific antigen as an efficient marker of prostatic
cancer. Glycobiology. 20:452–460. 2010. View Article : Google Scholar
|