Introduction
Renal cell carcinoma (RCC) is a genitourinary cancer
with a high mortality rate (1,2).
Major subtypes of RCC include clear cell RCC (ccRCC), papillary RCC
and chromophobe RCC, of which ccRCC is the most common and accounts
for most cancer-related deaths (3).
The standard of care for localized RCC is surgical
excision; however, it has been reported that overall distant
recurrence rates at 5 years after surgical resection are 27.6 and
64% for localized and locally advanced (nodal) disease,
respectively (4). Approximately
one-third of patients already have metastatic disease at diagnosis
(5). For the treatment of
metastatic or recurrent RCC, cytokines such as interferon (IFN)-α
and high-dose IL-2 are the standard of care before the introduction
of sunitinib (6). Tyrosine kinase
inhibitors, including sunitinib (7), sorafenib (8), pazopanib (9) and axitinib (10), which inhibit hypoxia-inducible
factor and vascular endothelial growth factor (VEGF) signaling, are
the mainstay of treatment for advanced RCC in the front-line
setting in addition to other targeting therapies, such as the
anti-VEGF monoclonal antibody bevacizumab (11), and the mTOR inhibitors everolimus
(12) and temsirolimus (13). Recently, multi-kinase inhibitors,
including cabozantinib (14) and
lenvatinib (15), have also been
approved for the treatment of metastatic RCC (mRCC).
At present, immune checkpoint inhibitors are the
standard first- and second-line treatments for RCC. The
anti-programmed cell death protein 1 (PD-1) antibody nivolumab has
been approved for the treatment of patients whose previous therapy
has failed (16); treatment with
nivolumab together with the anti-cytotoxic T-lymphocyte-associated
protein 4 antibody ipilimumab has been approved as first-line
treatment (17). Furthermore,
pembrolizumab (anti-PD-1) plus axitinib has been shown to be
superior to sunitinib for the first-line treatment of mRCC
regardless of risk groups with an acceptable safety profile
(18). These results resulted in a
change to the National Comprehensive Cancer Network and European
Urological Association guidelines (19,20).
However, not all patients benefit, and response rates of 25% with
nivolumab alone (16) and 42% with
the combination therapy (17) have
been documented. In addition, many immune-related adverse events
have been reported, and no predictive biomarker is available to
select which patients will benefit from which treatment. Therefore,
although these checkpoint inhibitors are promising, there is still
an urgent need to improve the treatment of RCC.
Combinations of molecular targeted agents with
immune checkpoint inhibitors are now beginning to be extensively
studied (21). Because molecular
targeted agents not only impact directly on cancer cells but also
affect immune cells and modulate the tumor microenvironment
(22,23), a better understanding of the
immunological properties of these drugs will contribute to the
rational design of combination therapies. This study performed
extensive immune monitoring of patients' peripheral blood
mononuclear cells (PBMCs) to investigate the immunological effects
of the molecular targeted agents sunitinib, everolimus and
temsirolimus.
Materials and methods
Patients
This clinical study analyzed the immunological
impact of molecular targeted agents in patients with RCC and was
conducted at The University of Tokyo Hospital. The research
protocol was approved by the Ethical Committee of The University of
Tokyo (approval no. 3652) and written informed consent was obtained
from each patient before they entered the study. All procedures in
the present study were performed according to the ethical standards
of the institutions and were in conformity with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards. Blood was collected before treatment started and after 4
weeks of treatment. Between June 2012 and November 2015, 31
patients (seven favorable, 20 intermediate and four poor risk
patients, according to the Memorial Sloan Kettering Cancer Center
risk criteria) were enrolled to the present study (24); 11, 12 and 8 patients received
sunitinib, everolimus and temsirolimus, respectively, according to
the then current guidelines for RCC treatment (Table I).
 | Table IDemographic and clinical
characteristics of the patients. |
Table I
Demographic and clinical
characteristics of the patients.
| Variables | Sunitinib
(n=11) | Everolimus
(n=12) | Temsirolimus
(n=8) |
|---|
| Age (years) | 65 (20-83) | 69 (53-84) | 70 (62-85) |
| Sex | | | |
| Male | 10 (91%) | 5 (42%) | 5 (63%) |
| Female | 1 (9%) | 7 (58%) | 3 (38%) |
| Histology | | | |
| Clear cell renal
cell carcinoma | 8 (73%) | 10 (83%) | 3 (38%) |
| Papillary cell
renal cell carcinoma | 2 (18%) | 1 (8%) | 2 (25%) |
| Chromophobe renal
cell carcinoma | 0 (0%) | 0 (0%) | 1 (13%) |
| Other types | 1 (9%) | 1 (8%) | 2 (25%) |
| Karnofsky
performance status | | | |
| ≥80 | 8 (73%) | 10 (83%) | 5 (63%) |
| <80 | 3 (27%) | 2 (17%) | 3 (38%) |
| MSKCC risk
criteria | | | |
| Favorable | 2 (18%) | 4 (33%) | 1 (13%) |
| Intermediate | 9 (82%) | 7 (58%) | 4 (50%) |
| Poor | 0 (0%) | 1 (8%) | 3 (38%) |
| Prior systemic
treatments | | | |
| 0 | 9 (82%) | 0 (0%) | 5 (63%) |
| 1 | 2 (18%) | 1 (8%) | 1 (13%) |
| ≥2 | 0 (0%) | 11 (92%) | 2 (25%) |
| Median duration of
treatment (days) | 91 | 161 | 43 |
| Best overall
response | | | |
| CR | 0 (0%) | 0 (0%) | 1 (13%) |
| PR | 4 (36%) | 1 (8%) | 1 (13%) |
| SD | 4 (36%) | 6 (50%) | 3 (38%) |
| PD | 3 (27%) | 5 (42%) | 3 (38%) |
PBMC isolation and flow cytometry
Peripheral blood samples were collected twice, just
before treatment and after 4 weeks of treatment. PBMCs were
isolated by density gradient centrifugation at 1,100 × g for 20 min
at room temperature using Lymphoprep™ (cat. no. 1114547; Alere
Technologies AS), and cryopreserved in Bambanker™ freezing medium
(cat. no. CS-02-001; Nippon Genetics Co., Ltd.). Cryopreserved
PBMCs were thawed in RPMI-1640 (cat. no. 189-02025; Wako Pure
Chemical Industries, Ltd.) supplemented with 50 IU/ml
Benzonase® Nuclease (cat. no. E1014; Sigma-Aldrich;
Merck KGaA). Cells (2×105) were blocked with 10
µl Clear Back (cat. no. MTG-001; MBL International Co.) for
5 min at room temperature and then stained with 100 µl
phosphate-buffered saline containing 1% FBS (cat. no. 17012;
Sigma-Aldrich; Merck KGaA) and 0.1% sodium azide (cat. no.
195-11092; Wako Pure Chemical Industries, Ltd.) with antibodies
(1:100 dilution) against human leukocyte antigen (HLA)-DR (cat. no.
347367; BD Biosciences), tumor necrosis factor (TNF)-α (cat. no.
557996; BD Biosciences), CD8 (cat. no. 6603861; Beckman Coulter,
Inc.), CD28 (cat. no. 6607111; Beckman Coulter, Inc.), 7-AAD
Viability Dye (cat. no. A07704; Beckman Coulter, Inc.), CD3 (cat.
no. A07746; Beckman Coulter, Inc.), CD19 (cat. no. A07769; Beckman
Coulter, Inc.), CD56 (cat. no. A07788; Beckman Coulter, Inc.),
NKG2D (cat. no. A08934; Beckman Coulter, Inc.), CD8 (cat. no.
B08467; Beckman Coulter, Inc.), CD45RA (cat. no. IM2711U; Beckman
Coulter, Inc.), CD3 (cat. no. 300328; BioLegend, Inc.), CD4 (cat.
no. 300512; BioLegend, Inc.), CD4 (cat. no. 300514; BioLegend,
Inc.), CD4 (cat. no. 300521; BioLegend, Inc.), CD4 (cat. no.
300538; BioLegend, Inc.), CD8 (cat. no. 300926; BioLegend, Inc.),
CD11b (cat. no. 301325; BioLegend, Inc.), CD14 (cat. no. 301828;
BioLegend, Inc.), CD16 (cat. no. 302006; BioLegend, Inc.), CD20
(cat. no. 302304; BioLegend, Inc.), CD25 (cat. no. 302606;
BioLegend, Inc.), CD28 (cat. no. 302906; BioLegend, Inc.), CD33
(cat. no. 303408; BioLegend, Inc.), CD45 (cat. no. 304012;
BioLegend, Inc.), CD45RA (cat. no. 304112; BioLegend, Inc.), CD95
(cat. no. 305624; BioLegend, Inc.), CD56 (cat. no. 318304;
BioLegend, Inc.), forkhead box P3 (FOXP3; cat. no. 320212;
BioLegend, Inc.), CD57 (cat. no. 322312; BioLegend, Inc.), CD15
(cat. no. 323020; BioLegend, Inc.), PD-1 (cat. no. 329919;
BioLegend, Inc.), DNAX accessory molecule 1 (DNAM1; cat. no.
338306; BioLegend, Inc.), Ki67 (cat. no. 350514; BioLegend, Inc.),
killer cell lectin-like receptor subfamily G member 1 (KLRG1; cat.
no. 368605; BioLegend, Inc.), IL-2 (cat. no. 500306; BioLegend,
Inc.), IFN-γ (cat. no. 502522; BioLegend, Inc.), CD19 (cat. no.
11-0199-42; eBioscience; Thermo Fisher Scientific, Inc.), CCR7
(cat. no. FAB197P; R&D Systems, Inc.), lymphocyte activation
gene 3 protein (LAG-3; cat. no. FAB2319P; R&D Systems, Inc.)
and T cell immunoglobulin and mucin protein 3 (TIM-3; cat. no.
FAB2365G; R&D Systems, Inc.). Mouse IgG1, κ (cat. no. 400114;
BioLegend, Inc.), Mouse IgG1, κ (cat. no. 400122; BioLegend, Inc.),
Mouse IgG1 (cat. no. 400134; BioLegend, Inc.), Mouse IgG1, κ (cat.
no. 400134; BioLegend, Inc.), Mouse IgG1, κ (cat. no. 400158;
BioLegend, Inc.), Mouse IgG2a, κ (cat. no. 400212; BioLegend,
Inc.), Mouse IgG2a, κ (cat. no. 400222; BioLegend, Inc.), Mouse
IgM, κ (cat. no. 401609; BioLegend, Inc.), Rat IgG2A (cat. no.
IC006G; R&D Systems, Inc.), Mouse IgG2a (cat. no. A12689;
Beckman Coulter, Inc.), and Goat IgG (cat. no. IC108P; R&D
Systems, Inc.) were used as isotype controls. Dead cells were
excluded by staining with Zombie Yellow™ Fixable Viability kit
(cat. no. 423104; BioLegend, Inc.) or Fixable Viability Dye eFluor™
780 (cat. no. 65-0865-18; eBioscience; Thermo Fisher Scientific,
Inc.).
For intracellular cytokine staining, cells were
stimulated with 10 ng/ml PMA (cat. no. P1585; Sigma-Aldrich; Merck
KGaA) and 1 µg/ml ionomycin (cat. no. I0634; Sigma-Aldrich;
Merck KGaA) in the presence of 10 µg/ml brefeldin A (cat.
no. B7651; Sigma-Aldrich; Merck KGaA) at 37°C for 4 h.
Cytokine-producing cells were then evaluated by intracellular
cytokine staining, which was conducted according to the
manufacturer's instructions using IntraPrep Permeabilizaton Reagent
(cat. no. A07803; Beckman Coulter, Inc.). Cells were cultured at
37°C for 30 min with 80 µM
2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose
(2-NBDG; cat. no. 23002-v; Peptide Institute, Inc.) in glucose-free
RPMI-1640 containing 10% FBS in order to analyze the uptake of the
glucose analog. To analyze mitochondria, cells were cultured with
MitoTracker Green (MTG; cat. no. M7514; Invitrogen; Thermo Fisher
Scientific, Inc.) or tetramethylrhodamine, ethyl ester (TMRE; cat.
no. 87917; Abcam) for 30 min at 37°C. Stained cells were analyzed
on a Gallios flow cytometer (Beckman Coulter, Inc.) and data
processed using Kaluza software (version 2.1; Beckman Coulter,
Inc.) and FlowJo (version 7.6.5; FlowJo, LLC).
Statistical analysis
Comparison of results was performed with the
Wilcoxon signed-rank test using GraphPad Prism 5 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of molecular targeted agents on
the composition of the PBMC population
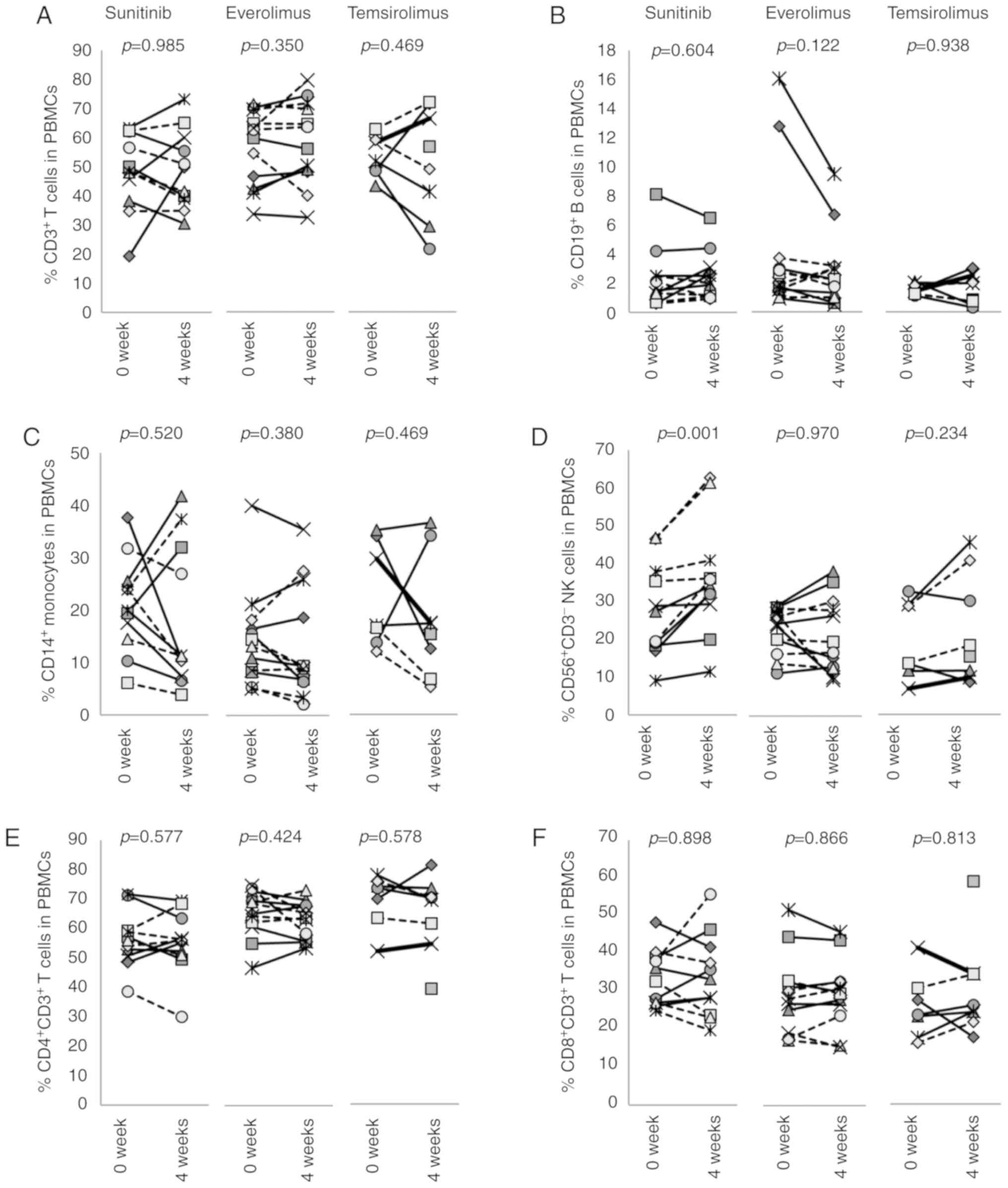
The effects of sunitinib, everolimus or temsirolimus
on the composition of PBMCs were examined by flow cytometry using
samples from patients with RCC before and 4 weeks after treatment
initiation. The frequencies of CD3+ T cells,
CD19+ B cells, CD14+ monocytes and
CD56+CD3− natural killer (NK) cells were
assessed in each patient (Figs.
1A-D and S1). The frequencies
of T cells, B cell and monocytes were either increased or decreased
by sunitinib; the individual differences and variations were such
that no significant differences emerged when all patients were
grouped together. Conversely, CD56+CD3− NK
cells were consistently increased from 27.8±11.9 to 36.0±14.5%
(P=0.001) following treatment with sunitinib (Figs. 1D and S2). In patients who received everolimus
or temsirolimus, no significant differences in the frequencies of
CD3+ T cells, CD19+ B cells, CD14+
monocytes or CD56+CD3− NK cells were seen
after 4 weeks of treatment in all patients as a group. The
frequencies of CD4+ and CD8+ T cells were not
changed by any of these molecular targeted agents (Fig. 1E and F).
Effects of molecular targeted agents on T
cell phenotypes
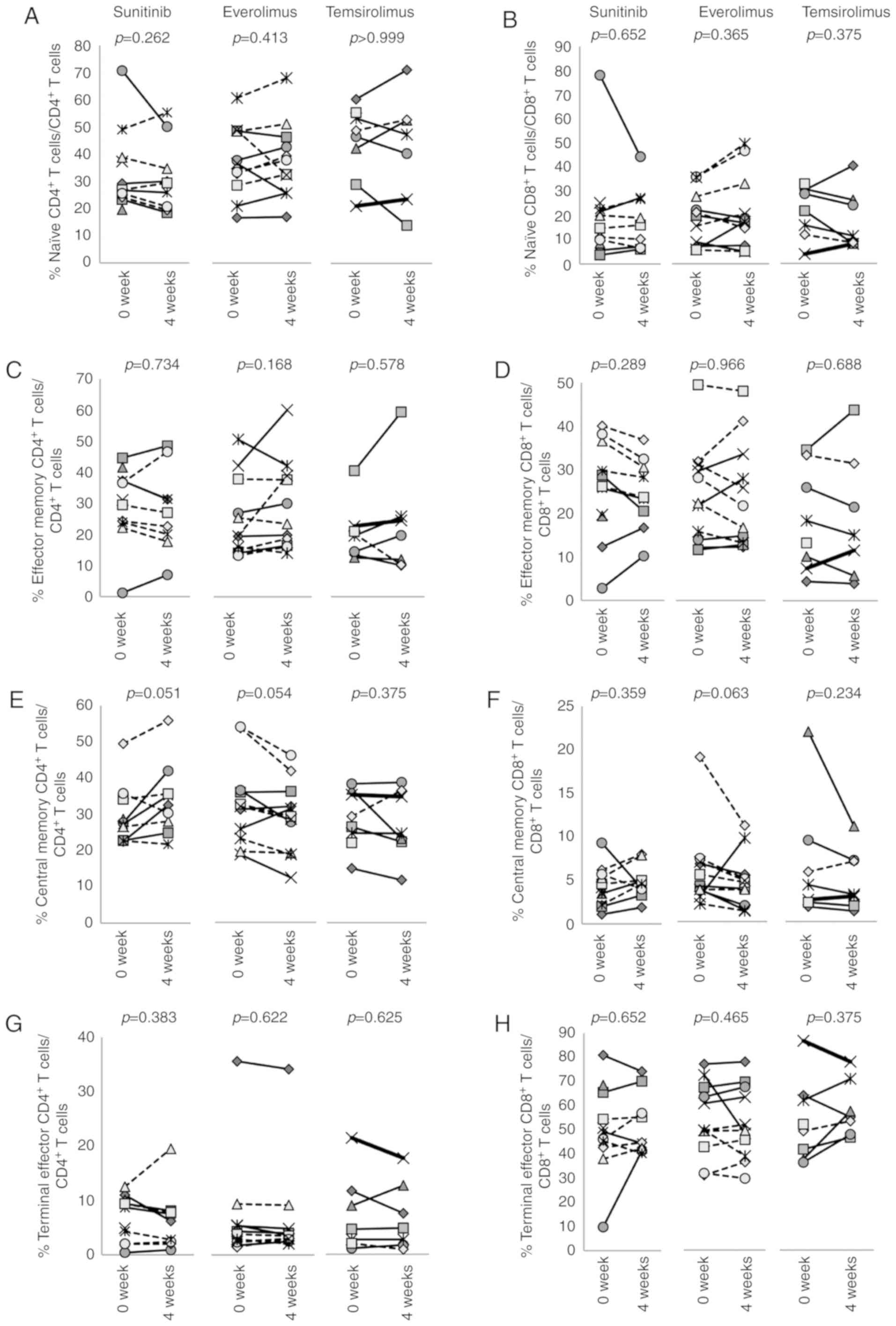
To determine the phenotypes of T cells, PBMCs were
stained for CD3, CD4, CD8, CCR7 and CD45RA (Figs. 2 and S3). Naïve, effector memory, central
memory and terminal effector memory T cells were defined as
CCR7+CD45RA+,
CCR7+CD45RA−,
CCR7−CD45RA− and
CCR7−CD45RA+, respectively. The results
revealed that none of the three drugs affected these memory
markers.
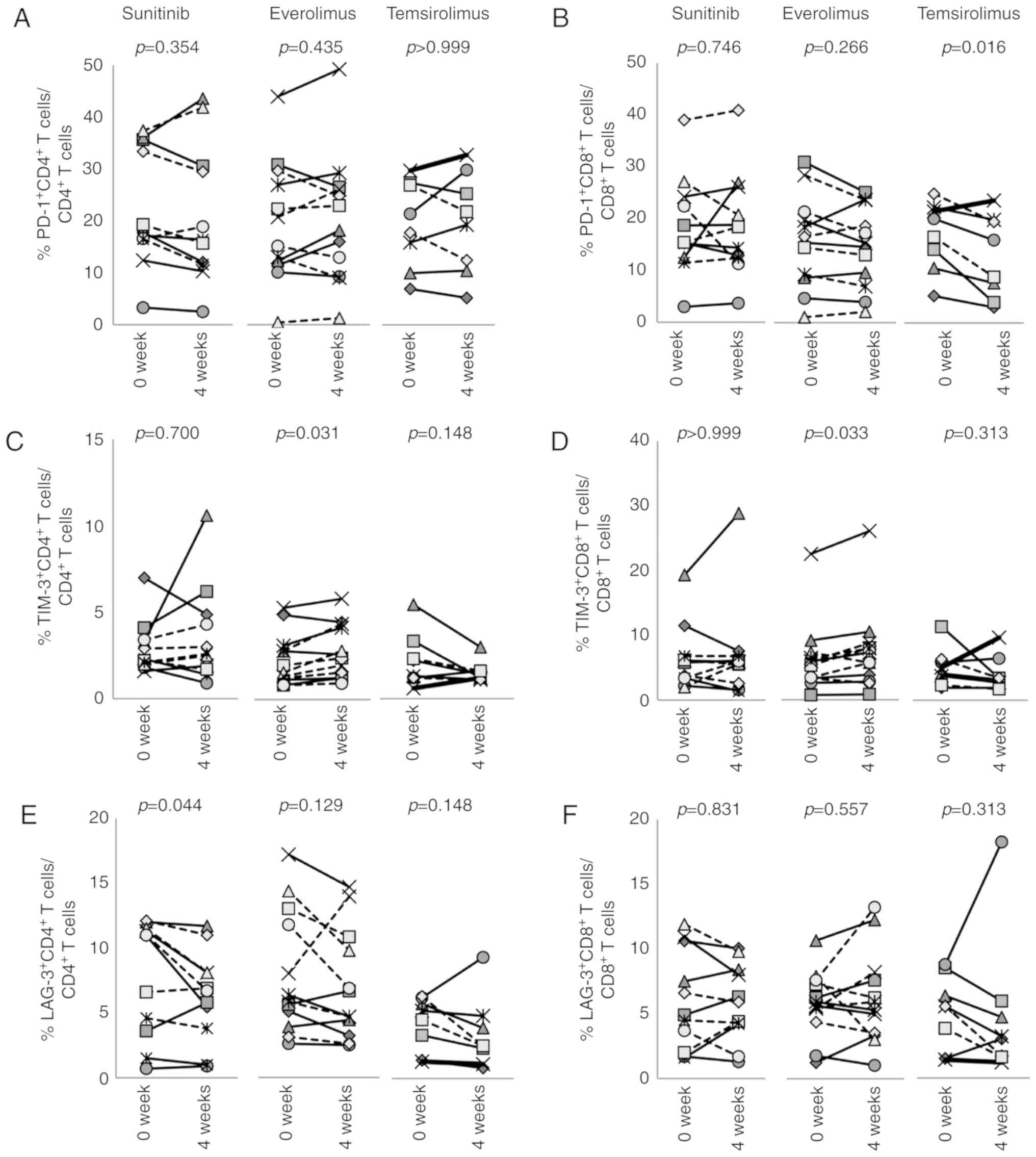
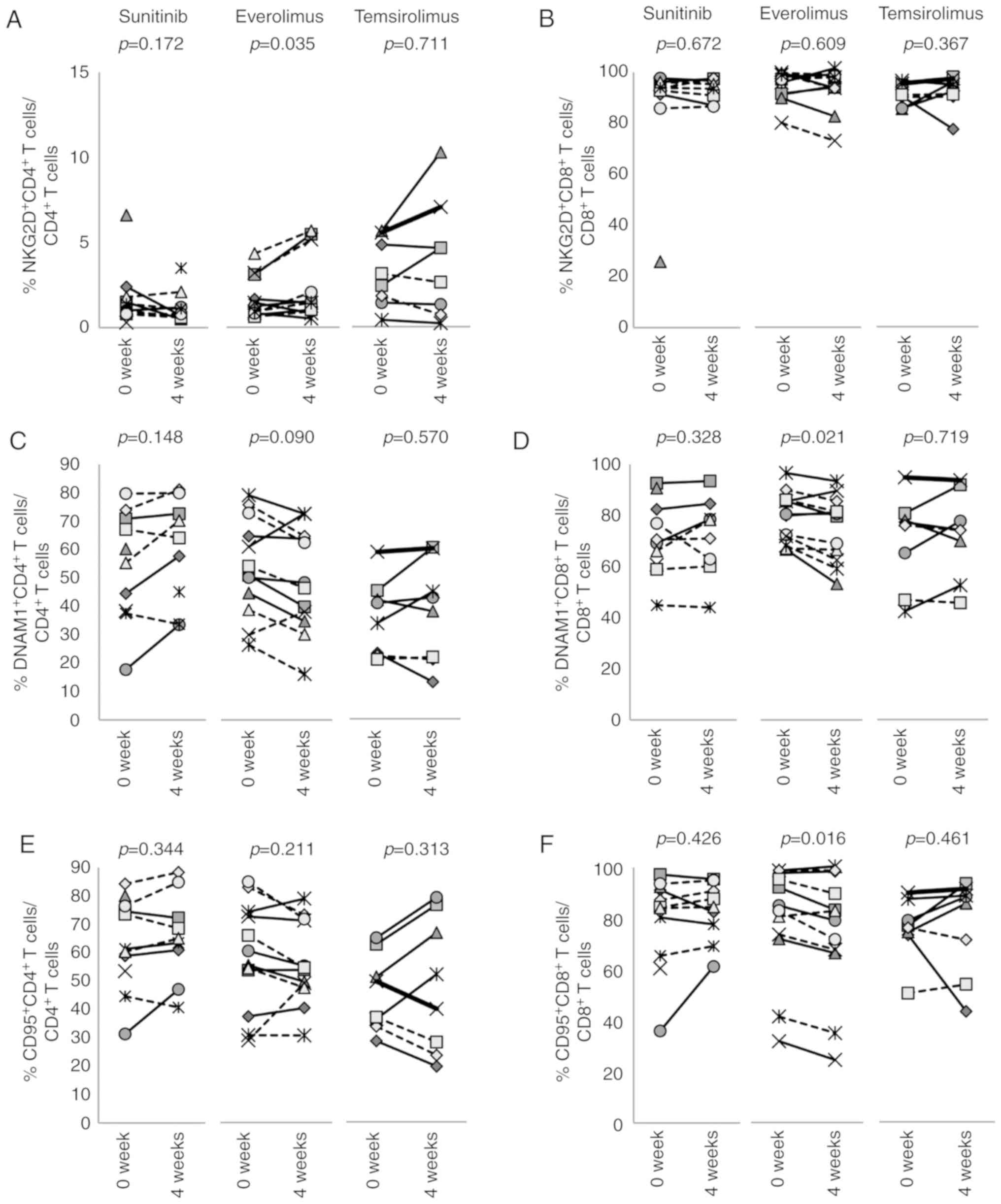
This study also examined the expression of
inhibitory receptors and activation markers on CD4+ and
CD8+ T cells (Figs. 3,
4 and S4). Sunitinib reduced the
percentage of LAG-3+CD4+ T cells (Fig. 3E, P=0.044), whereas the expression
of other molecules, including PD-1 and TIM-3 was not changed
(Fig. 3A and C). Conversely, 4
weeks of treatment with everolimus increased the expression of
TIM-3 on CD4+ (P=0.031) and CD8+ T cells
(P=0.033; Fig. 3C and D). In
addition, everolimus increased NKG2D+CD4+ T
cells (P= 0.035) and decreased DNAM1+CD8+ (P=
0.021) and CD95+CD8+ T cells (P= 0.016;
Fig. 4A, D and F). Temsirolimus
treatment had no effect on the expression of these molecules on
CD8+ T cells, with the exception that
PD-1+CD8+ T cells decreased from 16.8±6.2% to
12.7±7.4% (P=0.016; Fig. 3B).
Effects of molecular targeted agents on
inhibitory cells
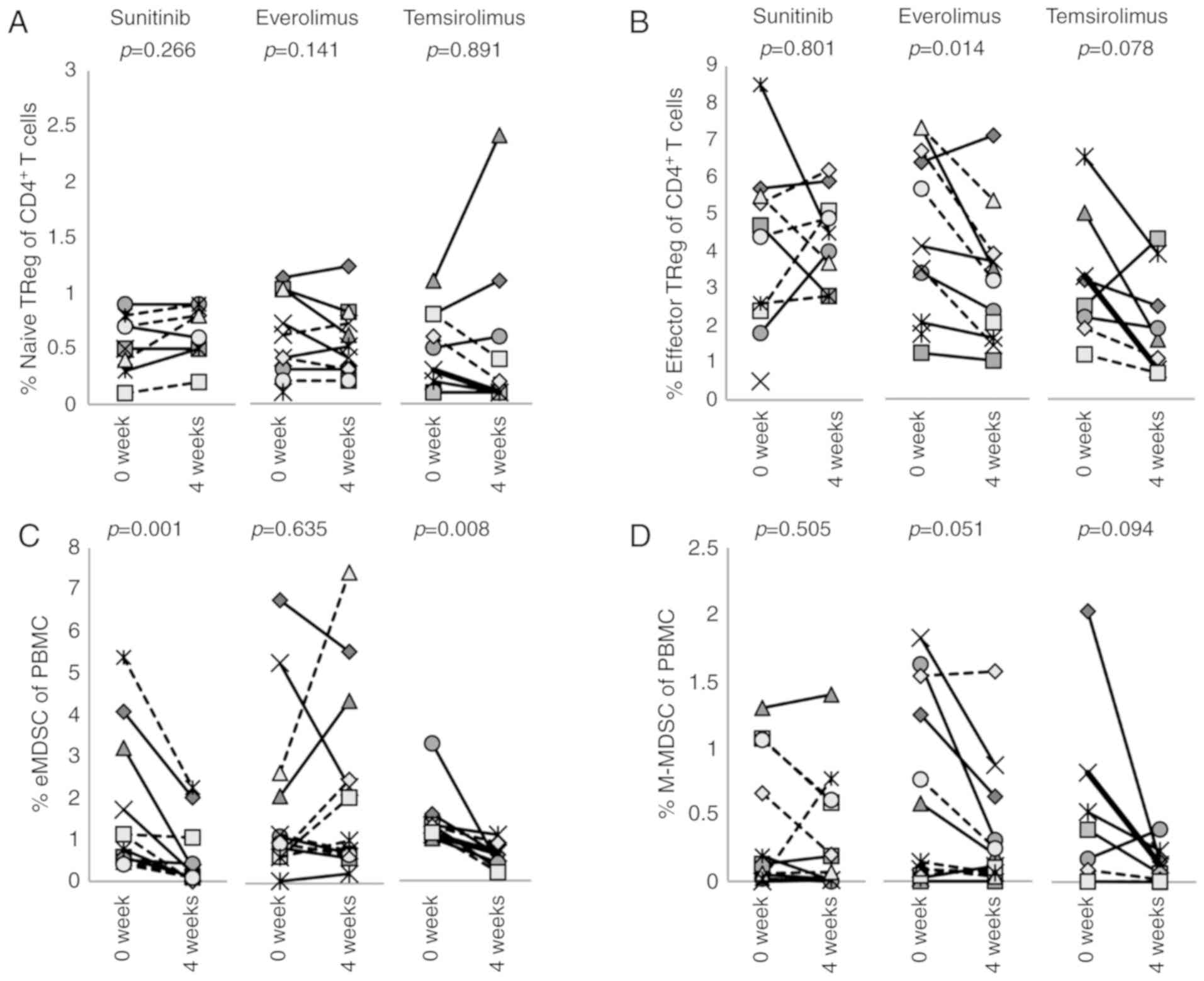
The frequencies of regulatory T (TReg)
cells and myeloid-derived suppressor cells (MDSCs) in PBMCs were
enumerated (Fig. 5).
TReg cells were subdivided into two populations:
CD45RA+FOXP3low naïve TReg cells
(Fig. 5A) and
CD45RA−FOXPhigh effector TReg
cells (Figs. 5B and S5) (25). There were more effector
TReg cells than naïve TReg cells in these
patients' PBMCs, and their frequencies were not affected by
sunitinib. However, effector TReg cells were
significantly decreased from 4.36±2.1 to 3.1±1.7% by everolimus
(P=0.014) and although not statistically significant, temsirolimus
also decreased the percentage of effector TReg cells
from 3.2±1.6 to 2.1±1.3% (P=0.078).
MDSCs can also be subdivided into polymorphonuclear
(PMN)-MDSCs, monocytic MDSCs (M-MDSCs) or early-stage MDSCs
(eMDSCs) (26) defined as
CD14−CD11b+CD15+ (or
CD66b+),
Lin(CD3/19/20/56)−CD11b+CD14+HLA-
DRlow/−CD15−, and
Lin−CD14−CD15−HLA-DR−CD33+,
respectively (Fig. S6). Since the
present study utilized cryo-preserved PBMCs that were isolated by
density-gradient centrifugation with Lymphoprep™, most PMN-MDSCs
were lost to the study. Therefore, this study primarily evaluated
eMDSCs (Fig. 5C) and M-MDSCs
(Fig. 5D). Sunitinib reduced the
percentage of eMDSCs (P= 0.001), but not M-MDSCs (Figs. 5C and D, and S7). Similarly, temsirolimus reduced the
percentage of eMDSCs from 1.5±0.69 to 0.58±0.28% (P=0.008). In
contrast, everolimus did not affect either eMDSCs or M-MDSCs.
Effects of molecular targeted agents on
senescent T cells
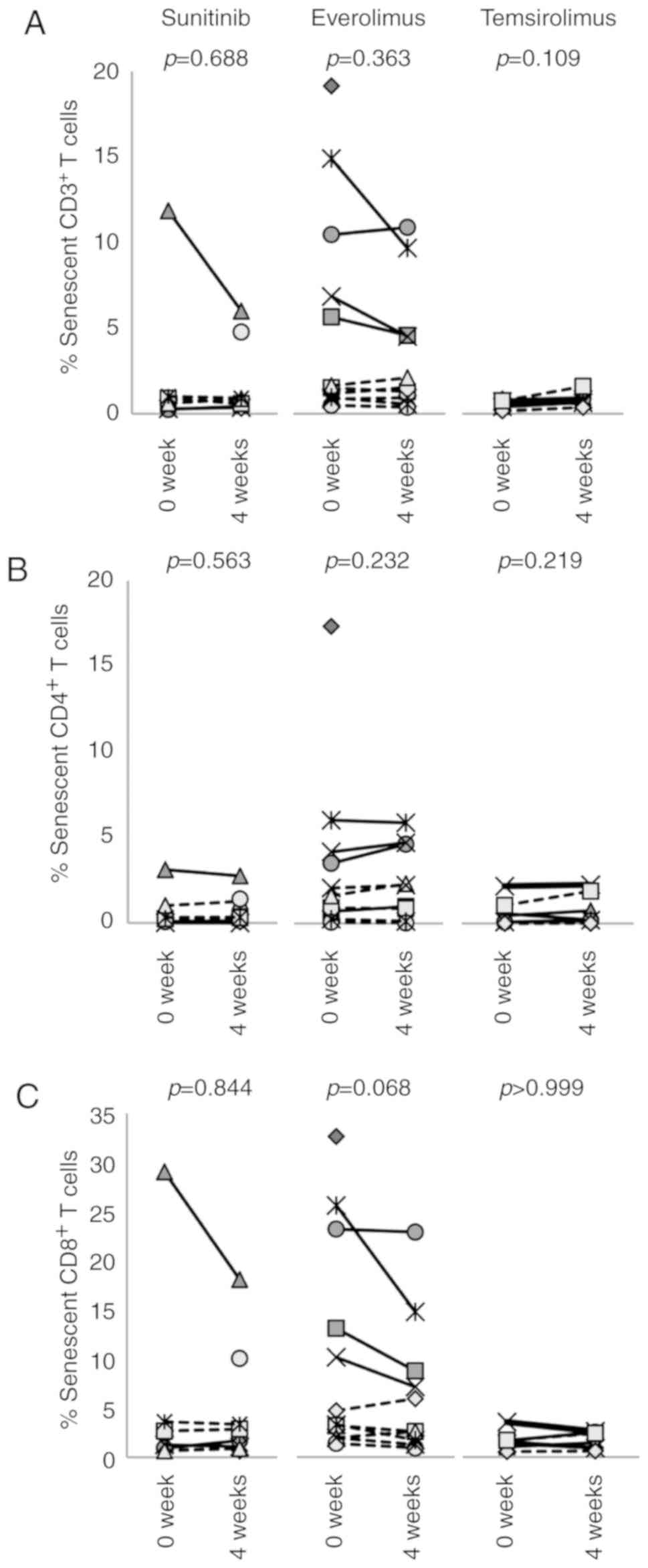
Aging is associated with a decline of the immune
system known as immunosenescence, which could influence the
efficacy and safety profile of immunotherapy. It is known that
immunosenescence is accompanied by an increase in CD28−,
KLRG1+, CD152+, CD45RO+ and
CD57+ cells;
CD28−CD57+KLRG1+CD3+ T
cells are defined as immunose-nescent T cells (Fig. S8). This study revealed that 4
weeks of treatment with any of the three agents analyzed had no
impact on the percentage of senescent T cells (Fig. 6).
Effects of molecular targeted agents on
T-cell metabolism
T-cell phenotypes and functions are closely
associated with cellular metabolism. Therefore, this study examined
the effect of molecular targeted agents on this variable (Fig. 7). Glucose uptake was evaluated by
2-NBDG incorporation, and mitochondrial mass and membrane potential
were determined by MTG and TMRE, respectively (Figs. S9 and S10). Sunitinib and temsirolimus did not
affect 2-NBDG uptake by CD4+ or CD8+ T cells
(Fig. 7A and B), whereas the
effect of everolimus differed substantially between individuals.
Thus, the mean fluorescent intensity (MFI) of 2-NBDG in
CD4+ or CD8+ T cells was either increased or
decreased by the treatment. Sunitinib and temsirolimus did not
affect the TMRE or MTG of T cells in any patient; however,
everolimus decreased the MFI of MTG from 117,194±10,626 to
106,488±11,724 (P=0.016) in CD4+ T cells (Fig. 7C) and from 82,767±16,244 to
64,020±11,349 (P=0.016) in CD8+ T cells (Fig. 7D), with no effect on TMRE (Fig. 7E and F).
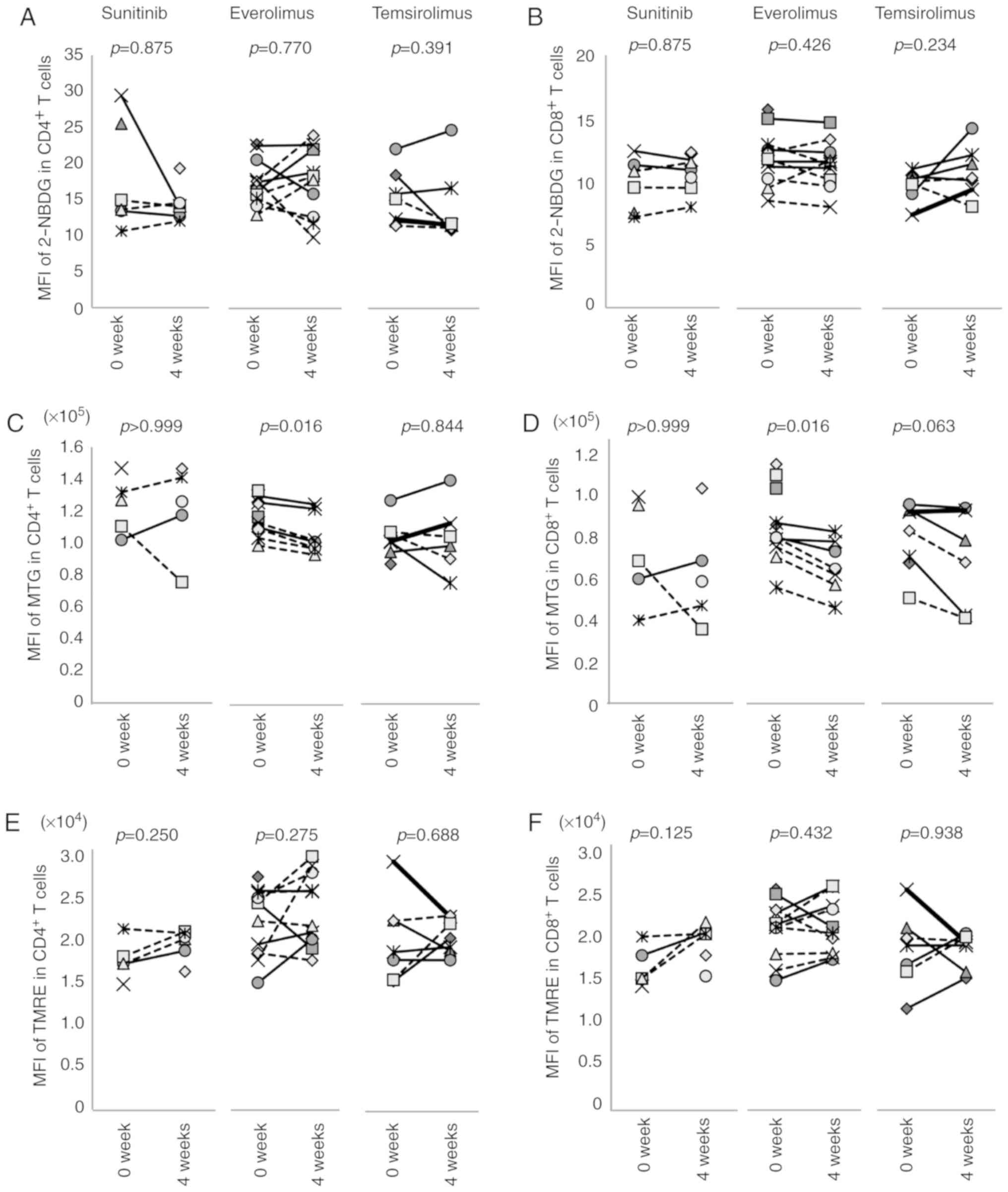 | Figure 7Effects of treatment with molecular
targeted agents on T-cell metabolism. The effects of sunitinib,
everolimus or temsirolimus on the uptake of (A and B) 2-NBDG, (C
and D) MTG staining and (E and F) TMRE staining of (A, C and E)
CD4+ and (B, D and F) CD8+ T cells are shown.
MFI before treatment (0 week) and after 4 weeks of treatment in
each individual case is indicated. 2-NBDG,
2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]-D-glucose;
MFI, mean fluorescence intensity; MTG, MitoTracker Green; TMRE,
tetramethylrhodamine, ethyl ester. |
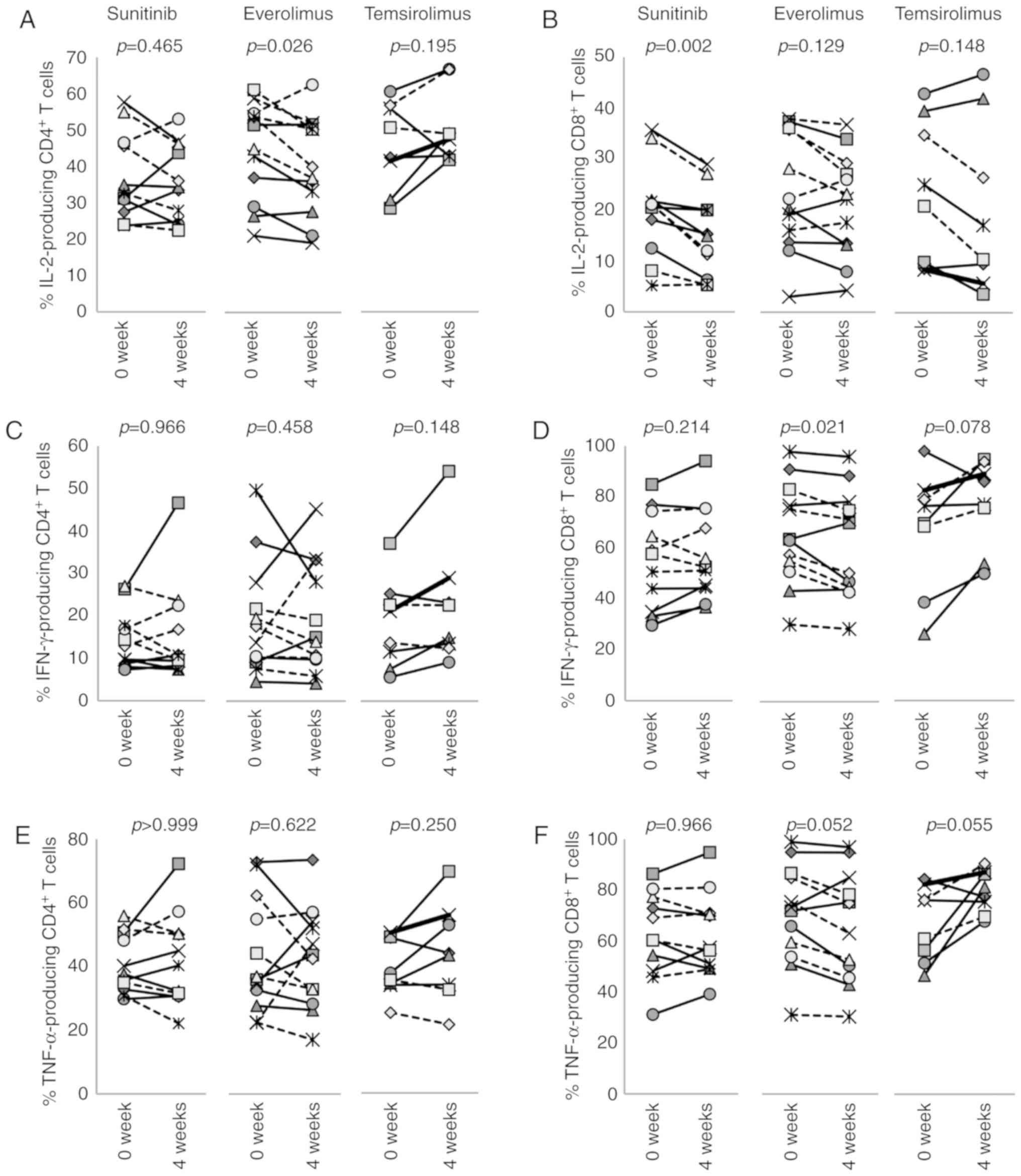
Effects of molecular targeted agents on
T-cell functionality
T-cell functionality was evaluated by intracellular
cytokine staining for the production of IFN-γ, TNF-α and IL-2
following stimulation with PMA and ionomycin (Figs. S11-S13). The results revealed that
the percentages of CD4+ T cells producing these
cytokines were not changed following treatment of patients with
sunitinib or temsirolimus (Fig. 8A, C
and E). In everolimus-treated patients, the percentage of
IL-2-producing CD4+ T cells was slightly decreased from
43.6±12.6 to 39.2±12.7 after 4 weeks (P=0.026), whereas the
frequencies of IFN-γ- or TNF-α-producing CD4+ T cells
were not changed (Fig. 8C and
E).
All three drugs had a greater impact on
CD8+ T cells than CD4+ T cells. The frequency
of IL-2-producing CD8+ T cells was decreased by
sunitinib from a pretreatment level of 20.0±8.9 to 15.1±7.8% after
4 weeks (P=0.002) (Fig. 8B).
However, IFN-γ or TNF-α single-producer CD8+ T cells
(Fig. 8D and F), and IFN-γ and
TNF-α double-producers (Fig. 9D and
F) were not affected. In everolimus-treated patients, the
percentages of IFN-γ-producing CD8+ T cells were
significantly reduced from 63.6±18.5 to 59.4±19.6% (P=0.021), and
the percentages of IL-2- and TNF-α-producing CD8+ T
cells were decreased from 23.1±10.9 to 20.9±9.6% (P=0.129) and from
67.8±18.1 to 63.2±19.8% (P=0.052), respectively (Fig. 8B, D and F). While frequencies of
IFN-γ and TNF-α double-producers were not changed (Fig. 9D and F), the percentage of
dysfunctional CD8+ T cells that could not produce any of
these three cytokines was increased from 22.0±15.2 to 26.4±16.6%
(P=0.012) (Fig. 9J), suggesting
that everolimus treatment has some immunosuppressive activity on
CD8+ T cells. Conversely, temsirolimus treatment
increased the percentage of cytokine-producing CD8+ T
cells. Although not statistically significant, differences between
pretreatment and 4 week samples for single cytokine producers were
altered, and the percentage of IFN-γ and TNF-α double-producers was
increased from 45.7±19.6 to 59.2±16.1% by temsirolimus treatment
(P=0.023) (Fig. 9D and F).
Reciprocally, albeit not significantly, the percentage of
dysfunctional CD8+ T cells that could not produce any of
these three cytokines was decreased from 20.7±10.9 to 12.6±0.11%
(P=0.109) (Fig. 9J). These results
suggested that temsirolimus may have a positive impact on cytokine
production of CD8+ T cells.
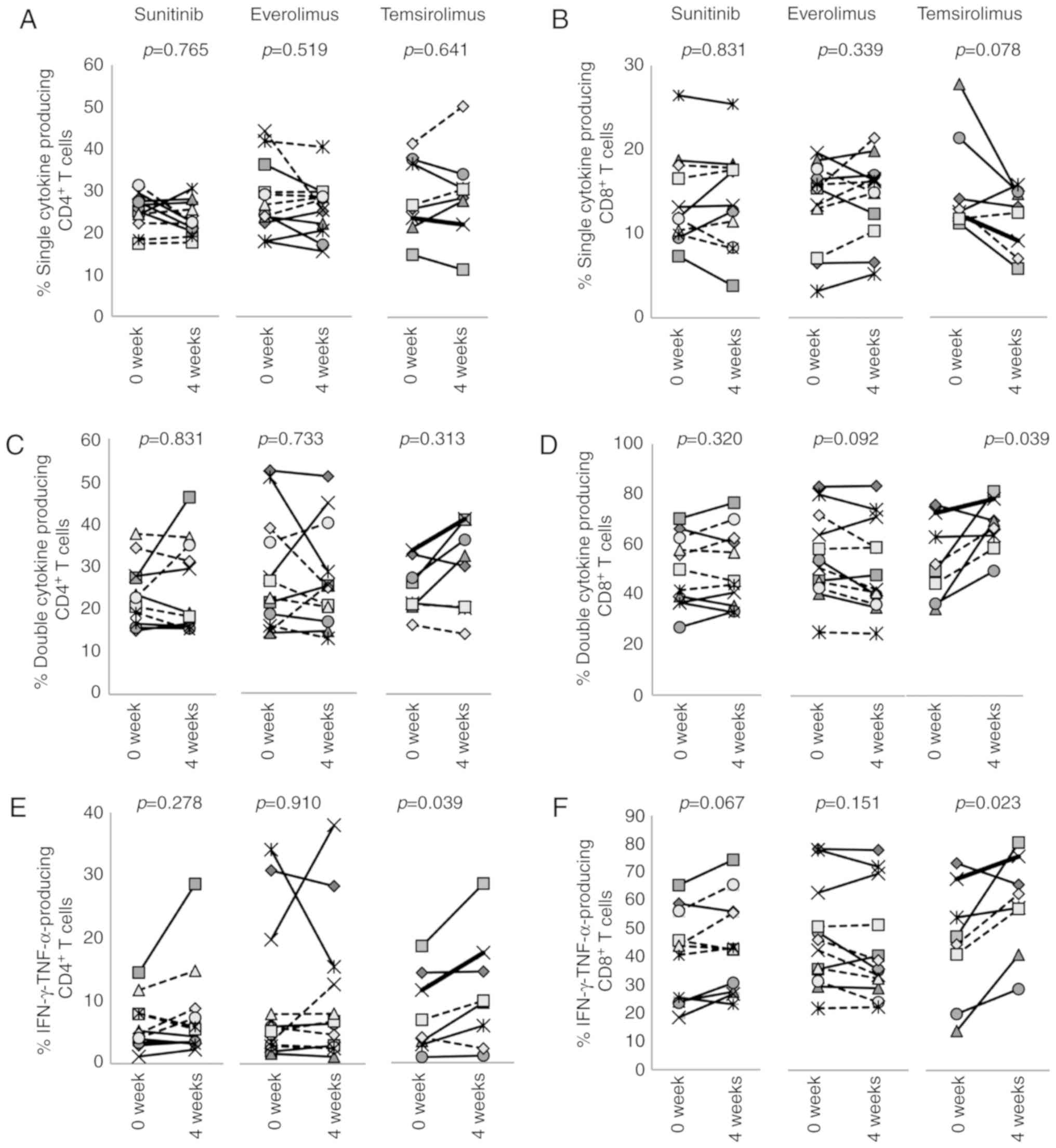 | Figure 9Effects of treatment with molecular
targeted agents on polyfunctional T cells. The effects of
sunitinib, everolimus or temsirolimus on polyfunc-tionality of (A,
C, E, G and I) CD4+ and (B, D, F, H and J)
CD8+ T cells were examined by intracellular cytokine
staining. Production of IL-2, IFN-γ and TNF-α were simultaneously
evaluated. T cells positive for one or more of these three
cytokines were recognized as (A and B) single cytokine producers or
(C and D) double cytokine producers. (E and F) T cells positive for
IFN-γ and TNF-α but negative for IL-2 were present. (G and H)
Triple cytokine producers and (I and J) dysfunctional T cells that
could not produce any of these three cytokines were also detected.
Percentages of CD4+ and CD8+
cytokine-producing T cells before treatment (0 week) and after 4
weeks of treatment in each individual case are indicated. IFN-γ,
interferon-γ; TNF-α, tumor necrosis factor-α. |
Discussion
This study performed flow cytometric
immunophenotyping and assessed the functionality of PBMCs from
patients with RCC receiving the molecular targeted agents
sunitinib, everolimus or temsirolimus, in order to assess the
immunological impact of these drugs. It was revealed that these
molecular targeted agents had different effects on the distribution
and functionality of numerous immune cells in the peripheral
blood.
The therapeutic landscape of advanced RCC has
totally changed since nivolumab was approved by the United States
Food and Drug Administration in 2015 as second-line therapy for
mRCC (16), and the combination of
nivolumab plus ipili-mumab was approved as first-line therapy for
intermediate and poor-risk patients (17). In addition, pembrolizumab plus
axitinib has been revealed to be superior to sunitinib in
first-line management of mRCC, regardless of the risk groups, with
an acceptable safety profile (18). The Javelin Renal-101 trial revealed
the PD-L1 blocker avelumab plus axitinib to be more efficacious
than sunitinib (27). Furthermore,
the IMmotion151 trial demonstrated that atezolizumab plus
bevacizumab prolonged progression-free survival compared with
sunitinib with a favourable safety profile (28). Ongoing phase III trials aim to
investigate the combination of nivolumab plus cabozantinib compared
to sunitinib (Checkmate-9ER, NCT01984242), and pembrolizumab plus
lenvatinib compared to lenvatinib plus everolimus or sunitinib
(Clear, NCT02811861). Thus, a large number of trials are testing
the combination of immune checkpoint inhibitors and other molecular
targeted agents.
For establishing effective combination immunotherapy
for mRCC, it is important to understand the immunological
properties of molecular targeted therapy. The mode of action of
molecular targeted agents is to inhibit oncogenic activation of
several processes required for the growth, survival and
proliferation of cancer cells. They are used in the clinical
setting in cancer therapy, and in the case of mTOR inhibitors also
for immunosuppression. However, the effects of molecular targeted
agents on the immune system may be more complex than previously
thought. A variety of immune cell populations infiltrate into the
tumor and contribute to the complexity of the tumor
microenvironment, which can either promote or limit tumor
progression and result in anti- or pro-tumor immunity (29,30).
Therefore, understanding the effects of molecular targeted agents
on overall immune responsiveness in vivo is important.
Because current checkpoint blockade therapies depend on the
invigoration of pre-existing anti-tumor T cells (31), this study focused particularly on
the distribution of T-cell subsets and their functions in patients
with RCC, particularly during early treatment, to determine the
effects of several molecular targeted agents.
As summarized in Table
II, sunitinib treatment decreased the percentage of eMDSCs and
increased NK cells; suni-tinib did not affect the phenotypes and
effector function of CD4+ and CD8+ T cells.
Everolimus decreased effector TReg cells. However, it
also decreased IL-2-producing CD4+ T cells and increased
the percentage of dysfunctional CD8+ T cells. In
contrast, temsirolimus decreased the percentage of
PD-1+CD8+ T cells and eMDSCs; the percentage
of IFN-γ and TNF-α double-producers was increased and the
percentage of dysfunctional CD8+ T cells was decreased.
Although everolimus and temsirolimus are both mTOR inhibitors, they
behaved as a potential immunosuppressor and immunostimulator,
respectively. The underlying molecular mechanisms responsible for
these different immunological effects are not clear, and several
other differences between these two drugs were observed. In
addition, everolimus increased the expression of TIM-3 on
CD4+ and CD8+ T cells, and also affected
2-NBDG uptake and the MFI of MTG. These results suggested that
everolimus has more direct effects on T cells than temsirolimus and
that it may predominantly suppress effector functions.
 | Table IIImmunological effects of treatment of
patients with renal cell carcinoma with molecular targeted drugs on
peripheral blood mononuclear cells. |
Table II
Immunological effects of treatment of
patients with renal cell carcinoma with molecular targeted drugs on
peripheral blood mononuclear cells.
| Treatment | NK | TIM-3+
CD4+ | LAG-3+
CD4+ | PD-1+
CD8+ | TIM-3+
CD8+ |
eTReg | eMDSC | M-MDSC |
2-NBDG+T | TMRE+
T | MTG+
T | IL-2+
CD4+ T | IL-2+
CD8+ T | IFN-γ+
CD8+ T | TNF-α+
CD8+ T | IFN-γ+
TNF-α+ CD8+ T | Dysfunctional
CD8+ T |
|---|
| Sunitinib | ↑ | → | ↓ | → | → | → | ↓ | → | → | → | → | → | ↓ | → | → | → | → |
| Everolimus | → | ↑ | → | → | ↑ | ↓ | → | → | → | → | ↓ | ↓ | → | ↓ | → | → | ↑ |
| Temsirolimus | → | → | → | ↓ | → | | ↓ | → | ⇅ | → | → | → | ↘ | → | ↘ | ↑ | ↘ |
It has been reported that sunitinib and sorafenib
may inhibit T-cell activation, proliferation and cytokine
production (32,33). On the other hand, it has been
suggested that sunitinib reduces TReg cells (34) and MDSCs (35); and may therefore potentiate
antitumor immune responses (36).
This study observed decreasing percentages of eMDSCs in response to
sunitinib treatment; however, the overall T-cell response was not
affected by 4 weeks of sunitinib treatment.
The expansion of TReg cells has been
reported in patients with mRCC treated with everolimus (37,38),
which induced an overall increased level of immunosuppression via
an increase in TReg cells and MDSCs (38,39).
In the present study, no increase was observed in TReg
cells, but an increase in dysfunctional CD8+ T cells was
detected in everolimus-treated patients. This may be at least
partly because the mTOR pathway is directly involved in the control
of T-cell proliferation and functions, and immune signals are
integrated with cellular metabolism by mTOR signaling (40).
mTOR signaling is also associated with
CD8+ T cell differentiation programs (41). Activation of mTOR facilitates the
differentiation of naïve T cells to activated and effector T cells,
whereas its inhibition in effector T cells leads to memory T cell
formation (42,43). Therefore, based on T-cell
differentiation status, inhibition of mTOR should result in reduced
CD8+ effector T-cell numbers and functions, and an
increase of memory T cells. Because the differentiation status of
peripheral blood T cells varies in different individuals, this may
explain the patient-to-patient variations observed in the effects
of molecular targeted agents on T-cell immunity.
Immunosenescence, or age-associated changes to
adaptive and innate immunity, has been associated with increased
susceptibility to infection and cancer (44). Since the response to vaccination is
reduced in the elderly, the efficiency of checkpoint blockade may
also be associated with immunosenescence. None of the molecular
targeted agents examined in the present study affected the
frequency of CD28−CD57+KLRG1+
immunosenescent T cells in PBMC. However, temsirolimus decreased
PD-1+CD8+ T cells and enhanced cytokine
production. This is consistent with a previous study. which
reported that the mTOR inhibitor RAD001 decreased the percentage of
PD-1+CD4+ and CD8+ T cells, and
improved the response to influenza vaccination (45). The low-grade inflammation observed
in elderly people and continuous antigenic stimulation by chronic
infection or cancer results in a slight activation of mTOR that can
be ameliorated by mTOR inhibitors. Unlike everolimus, overall
responses in temsirolimus-treated patients were more
immunostimulatory, which may contribute to enhanced immune function
and improve the quality of T-cell responses. Therefore,
temsirolimus might be a good candidate for combination with other
immunomodulators. Notably, a combination of temsirolimus with a
CCR4 antagonist targeting this receptor that is highly expressed on
TReg cells, together with cancer vaccination, has been
reported to be more effective in amplifying functional
tumor-specific CD8+ T cells than monotherapy alone
(46).
There are several limitations to the present study.
Firstly, the sample size was small. Secondly, sunitinib is approved
as a first-line option, whereas the mTOR inhibitors everolimus and
temsirolimus are approved in the second-line setting and only in
the first-line setting for patients with high-risk status (12,13).
Therefore, baseline immunological conditions might have differed in
the patients receiving these different agents. Nonetheless, the
data obtained in the present study derive from real-world patients
with RCC. They include comprehensive immunomonitoring that covers
phenotype, function and metabolism, and this should be valuable for
further comparisons. Thirdly, only the immune monitoring of
patients' PBMCs was conducted; the changes in PBMC do not
necessarily reflect changes in tumor tissue. The phenotypes and
functions of tumor-infiltrating lymphocytes and PBMCs have been
reported to differ in patients with RCC (47). However, tumor biopsies are
generally costly, invasive, cause treatment delays and increase the
risk of adverse events. The analysis of readily accessible
peripheral blood is preferred for developing biomarkers with
clinical utility. In fact, numerous studies provide compelling
evidence that subtypes and status of PBMCs are associated with
responses to immunotherapy (48).
In conclusion, different immunological effects of
the molecular targeted agents sunitinib, everolimus and
temsirolimus were observed in patients with RCC. Everolimus tended
towards suppression of T-cell functions, whereas temsirolimus
increased T-cell functionality. Although it may increase the risk
of immune-related toxicity, it may be proposed that temsirolimus
combined with checkpoint blockade will result in enhanced activity
of cancer immunotherapy.
Supplementary Data
Funding
This work was conducted with the institutional
support of RIKEN (grant no. 177100000958).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YK, AH, KN, TKar and HM analyzed and interpreted
the flow cytometry data. DY, TKaw, YS, TT, YY, MN, MS, AM, TN and
HK recruited patients, collected samples and interpreted the data
regarding RCC. KK conceived and designed the study, analyzed and
interpreted the data, and wrote and revised the paper. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This clinical study on the immunological impact of
molecular targeted agents in patients with RCC was conducted at The
University of Tokyo Hospital. All procedures in this study were
performed following the ethical standards of the institutions, and
in conformity with the 1964 Helsinki declaration and its later
amendments or comparable ethical standards. The research protocol
was approved by the Ethical Committee of The University of Tokyo
(approval no. #3652). Written informed consent to participate in
the study was obtained from each patient before they entered the
study.
Patient consent for publication
Written informed consent was obtained from each
patient before they entered the study.
Competing interests
The authors declare that have no competing
interests.
Acknowledgments
The authors would like to thank Mr. Kosuke Odaira
and Ms. Nao Fujieda for their excellent technical assistance.
References
|
1
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-Part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lam JS, Shvarts O, Leppert JT, Pantuck AJ,
Figlin RA and Belldegrun AS: Postoperative surveillance protocol
for patients with localized and locally advanced renal cell
carcinoma based on a validated prognostic nomogram and risk group
stratification system. J Urol. 174:466–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta K, Miller JD, Li JZ, Russell MW and
Charbonneau C: Epidemiologic and socioeconomic burden of metastatic
renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev.
34:193–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McDermott DF, Regan MM, Clark JI, Flaherty
LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff
MS, et al: Randomized phase III trial of high-dose interleukin-2
versus subcutaneous interleukin-2 and interferon in patients with
metastatic renal cell carcinoma. J Clin Oncol. 23:133–141. 2005.
View Article : Google Scholar
|
|
7
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motzer RJ, McCann L and Deen K: Pazopanib
versus sunitinib in renal cancer. N Engl J Med. 369:19702013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME,
Rusakov IG, et al: Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): A randomised
phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: Bevacizumab plus interferon alfa-2a for
treatment of metastatic renal cell carcinoma: A randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Efficacy of everolimus in advanced renal cell
carcinoma: A double-blind, randomised, placebo-controlled phase III
trial. Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hudes G, Carducci M, Tomczak P, et al:
Temsirolimus, interferon alfa, or both for advanced renal-cell
carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choueiri TK, Escudier B, Powles T,
Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL,
Peltola K, et al: Cabozantinib versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1814–1823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motzer RJ, Hutson TE, Glen H, Michaelson
MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B,
et al: Lenvatinib, everolimus, and the combination in patients with
metastatic renal cell carcinoma: A randomised, phase 2, open-label,
multicentre trial. Lancet Oncol. 16:1473–1482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jonasch E: NCCN guidelines updates:
Management of metastatic kidney cancer. J Natl Compr Canc Netw.
17:587–589. 2019.PubMed/NCBI
|
|
20
|
Albiges L, Powles T, Staehler M, Bensalah
K, Giles RH, Hora M, Kuczyk MA, Lam TB, Ljungberg B, Marconi L, et
al: Updated european association of urology guidelines on renal
cell carcinoma: Immune checkpoint inhibition is the new backbone in
first-line treatment of metastatic clear-cell renal cell carcinoma.
Eur Urol. 76:151–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuca G, de Braud F and Di Nicola M:
Immunotherapy-based combinations: An update. Curr Opin Oncol.
30:345–351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Neill LA, Kishton RJ and Rathmell J: A
guide to immunome-tabolism for immunologists. Nat Rev Immunol.
16:553–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Donnell JS, Massi D, Teng MWL and
Mandala M: PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux.
Semin Cancer Biol. 48:91–103. 2018. View Article : Google Scholar
|
|
24
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune system.
Nat Rev Immunol. 10:490–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Motzer RJ, Penkov K, Haanen J, Rini B,
Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S,
Uemura M, et al: Avelumab plus axitinib versus sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 380:1103–1115. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rini BI, Powles T, Atkins MB, Escudier B,
McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL,
et al: Atezolizumab plus bevacizumab versus sunitinib in patients
with previously untreated metastatic renal cell carcinoma
(IMmotion151): A multicentre, open-label, phase 3, randomised
controlled trial. Lancet. 393:2404–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fridman WH, Pages F, Sautes-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kerkar SP and Restifo NP: Cellular
constituents of immune escape within the tumor microenvironment.
Cancer Res. 72:3125–3130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao W, Gu YH, Song R, Qu BQ and Xu Q:
Sorafenib inhibits activation of human peripheral blood T cells by
targeting LCK phosphorylation. Leukemia. 22:1226–1233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu Y, Zhao W, Meng F, Qu B, Zhu X, Sun Y,
Shu Y and Xu Q: Sunitinib impairs the proliferation and function of
human peripheral T cell and prevents T-cell-mediated immune
response in mice. Clin Immunol. 135:55–62. 2010. View Article : Google Scholar
|
|
34
|
Finke JH, Rini B, Ireland J, Rayman P,
Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R and
Bukowski R: Sunitinib reverses type-1 immune suppression and
decreases T-regulatory cells in renal cell carcinoma patients. Clin
Cancer Res. 14:6674–6682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ko JS, Rayman P, Ireland J, Swaidani S, Li
G, Bunting KD, Rini B, Finke JH and Cohen PA: Direct and
differential suppression of myeloid-derived suppressor cell subsets
by sunitinib is compartmentally constrained. Cancer Res.
70:3526–3536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adotevi O, Pere H, Ravel P, Haicheur N,
Badoual C, Merillon N, Medioni J, Peyrard S, Roncelin S, Verkarre
V, et al: A decrease of regulatory T cells correlates with overall
survival after sunitinib-based antiangiogenic therapy in metastatic
renal cancer patients. J Immunother. 33:991–998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huijts CM, Santegoets SJ, van den Eertwegh
AJ, Pijpers LS, Haanen JB, de Gruijl TD, Verheul HM and van der
Vliet HJ: Phase I-II study of everolimus and low-dose oral
cyclophos-phamide in patients with metastatic renal cell cancer.
BMC Cancer. 11:5052011. View Article : Google Scholar
|
|
38
|
Beziaud L, Mansi L, Ravel P, Marie-Joseph
EL, Laheurte C, Rangan L, Bonnefoy F, Pallandre JR, Boullerot L,
Gamonet C, et al: Rapalogs efficacy relies on the modulation of
antitumor T-cell immunity. Cancer Res. 76:4100–4112. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huijts CM, Santegoets SJ, de Jong TD,
Verheul HM, de Gruijl TD and van der Vliet HJ: Immunological
effects of everolimus in patients with metastatic renal cell
cancer. Int J Immunopathol Pharmacol. 30:341–352. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Powell JD, Pollizzi KN, Heikamp EB and
Horton MR: Regulation of immune responses by mTOR. Annu Rev
Immunol. 30:39–68. 2012. View Article : Google Scholar
|
|
41
|
Chi H: Regulation and function of mTOR
signalling in T cell fate decisions. Nat Rev Immunol. 12:325–338.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Araki K, Turner AP, Shaffer VO, Gangappa
S, Keller SA, Bachmann MF, Larsen CP and Ahmed R: mTOR regulates
memory CD8 T-cell differentiation. Nature. 460:108–112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rao RR, Li Q, Odunsi K and Shrikant PA:
The mTOR kinase determines effector versus memory CD8+ T cell fate
by regulating the expression of transcription factors T-bet and
Eomesodermin. Immunity. 32:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sadighi Akha AA: Aging and the immune
system: An overview. J Immunol Methods. 463:21–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mannick JB, Del Giudice G, Lattanzi M,
Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT,
Kovarik J, Carson S, et al: mTOR inhibition improves immune
function in the elderly. Sci Transl Med. 6:268ra1792014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Beziaud L, Boullerot L, Tran T, Mansi L,
Marie-Joseph EL, Ravel P, Johannes L, Bayry J, Tartour E and
Adotévi O: Rapalog combined with CCR4 antagonist improves
anticancer vaccines efficacy. Int J Cancer. 143:3008–3018. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kopecký O, Lukesová S, Vroblová V,
Vokurková D, Morávek P, Safránek H, Hlávková D and Soucek P:
Phenotype analysis of tumour-infiltrating lymphocytes and
lymphocytes in peripheral blood in patients with renal carcinoma.
Acta Medica (Hradec Kralove). 50:207–212. 2007. View Article : Google Scholar
|
|
48
|
Nixon AB, Schalper KA, Jacobs I, Potluri
S, Wang IM and Fleener C: Peripheral immune-based biomarkers in
cancer immunotherapy: Can we realize their predictive potential? J
Immunother Cancer. 7:3252019. View Article : Google Scholar : PubMed/NCBI
|