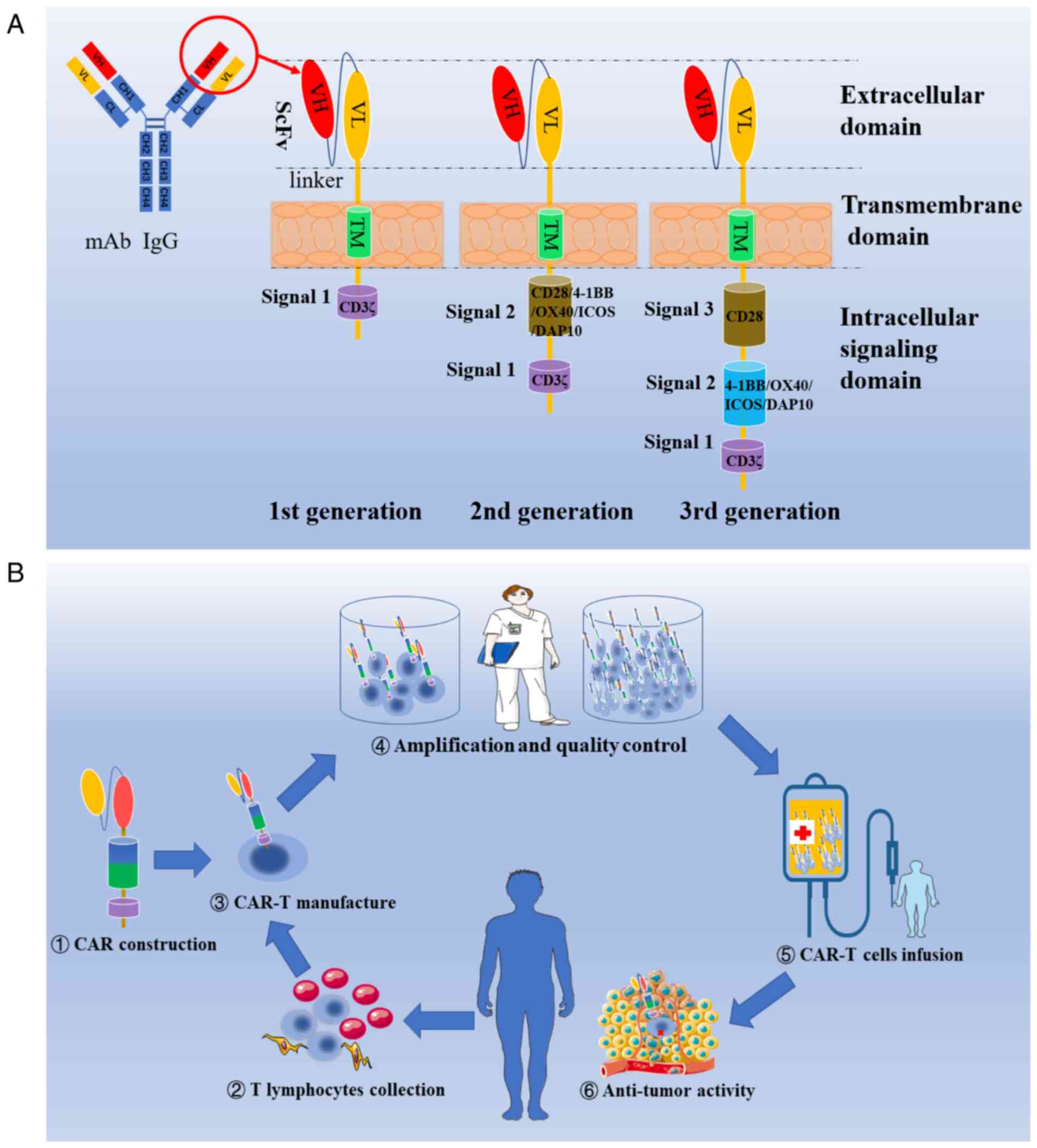
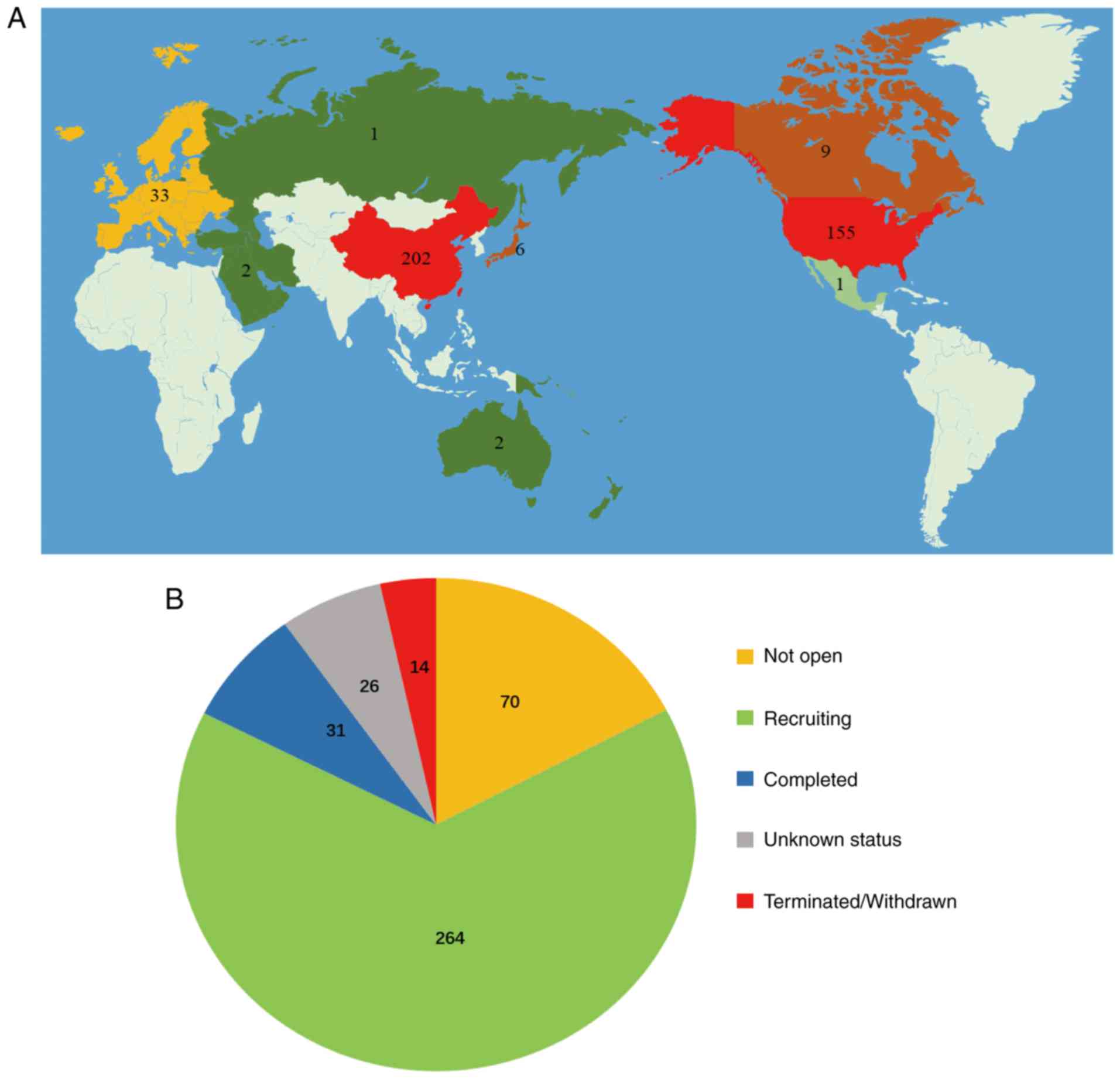
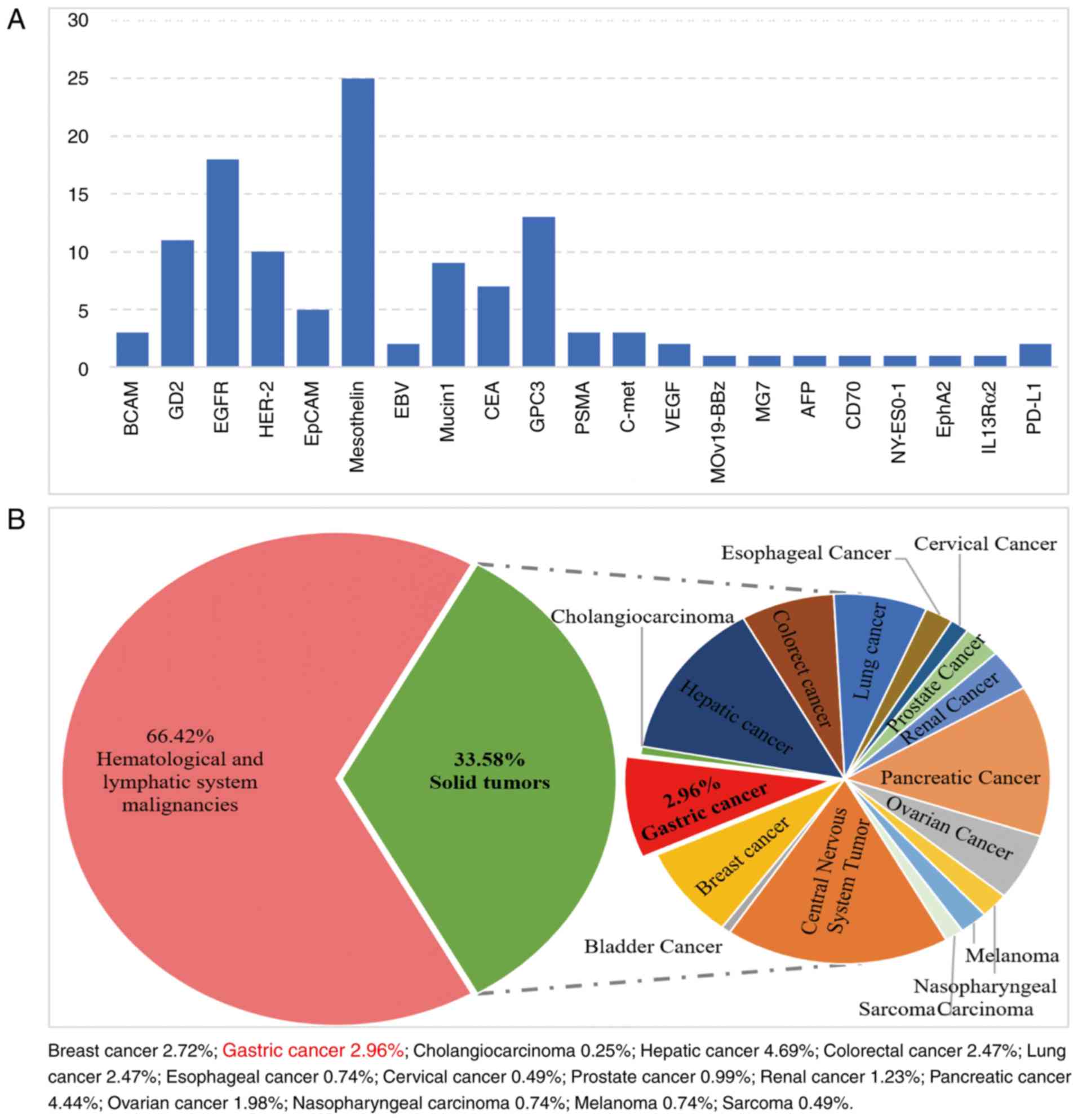
|
1
|
Khalil DN, Budhu S, Gasmi B, Zappasodi R,
Hirschhorn-Cymerman D, Plitt T, De Henau O, Zamarin D, Holmgaard
RB, Murphy JT, et al: The new era of cancer immunotherapy:
Manipulating T-cell activity to overcome malignancy. Adv Cancer
Res. 128:1–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosenberg SA, Yang JC, Sherry RM, Kammula
US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF,
Wunderlich JR, et al: Durable complete responses in heavily
pretreated patients with metastatic melanoma using T-cell transfer
immunotherapy. Clin Cancer Res. 17:4550–4557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schuster M, Nechansky A and Kircheis R:
Cancer immunotherapy. Biotechnol J. 1:138–147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fesnak AD, June CH and Levine BL:
Engineered T cells: The promise and challenges of cancer
immunotherapy. Nat Rev Cancer. 16:566–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow VA, Shadman M and Gopal AK:
Translating anti-CD19 CAR T-cell therapy into clinical practice for
relapsed/refractory diffuse large B-cell lymphoma. Blood.
132:777–781. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grupp SA, Michael K, David B, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015. View Article : Google Scholar :
|
|
8
|
Wang Z, Wu Z, Liu Y and Han W: New
development in CAR-T cell therapy. J Hematol Oncol. 10:532017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37513025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
FDA approves second CAR T-cell therapy.
Cancer Discov. 8:5–6. 2018. View Article : Google Scholar
|
|
12
|
June CH, O'Connor RS, Kawalekar OU,
Ghassemi S and Milone MC: CAR T cell immunotherapy for human
cancer. Science. 359:1361–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han Y, Liu C, Li G, Li J, Lv X, Shi H, Liu
J, Liu S, Yan P, Wang S, et al: Antitumor effects and persistence
of a novel HER2 CAR T cells directed to gastric cancer in
preclinical models. Am J Cancer Res. 8:106–119. 2018.PubMed/NCBI
|
|
14
|
Han H, Wang S, Hu Y, Li Z, Yang W, Lv Y,
Wang L, Zhang L and Ji J: Monoclonal antibody 3H11 chimeric antigen
receptors enhance T cell effector function and exhibit efficacy
against gastric cancer. Oncol Lett. 15:6887–6894. 2018.PubMed/NCBI
|
|
15
|
Kim M, Pyo S, Kang CH, Lee CO, Lee HK,
Choi SU and Park CH: Folate receptor 1 (FOLR1) targeted chimeric
antigen receptor (CAR) T cells for the treatment of gastric cancer.
PLoS One. 13:e01983472018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo
Y, Zhao X, Wang Y, Wang Z, Han W and Chen L: Effective and
persistent antitumor activity of HER2-directed CAR-T cells against
gastric cancer cells in vitro and xenotransplanted tumors in vivo.
Protein Cell. 9:867–878. 2018. View Article : Google Scholar :
|
|
17
|
Luo F, Qian J, Yang J, Deng Y, Zheng X,
Liu J and Chu Y: Bifunctional αHER2/CD3 RNA-engineered CART-like
human T cells specifically eliminate HER2(+) gastric cancer. Cell
Res. 26:850–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gross G, Waks T and Eshhar Z: Expression
of immunoglobulin-T-cell receptor chimeric molecules as functional
receptors with antibody-type specificity. Proc Natl Acad Sci USA.
86:10024–10028. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eshhar Z, Waks T, Gross G and Schindler
DG: Specific activation and targeting of cytotoxic lymphocytes
through chimeric single chains consisting of antibody-binding
domains and the gamma or zeta subunits of the immunoglobulin and
T-cell receptors. Proc Natl Acad Sci USA. 90:720–724. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jaspers JE and Brentjens RJ: Development
of CAR T cells designed to improve antitumor efficacy and safety.
Pharmacol Ther. 178:83–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brocker T and Karjalainen K: Signals
through T cell receptor-zeta chain alone are insufficient to prime
resting T lymphocytes. J Exp Med. 181:1653–1659. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Finney HM, Lawson AD, Bebbington CR and
Weir AN: Chimeric receptors providing both primary and
costimulatory signaling in T cells from a single gene product. J
Immunol. 161:2791–2797. 1998.PubMed/NCBI
|
|
23
|
Savoldo B, Ramos CA, Liu E, Mims MP,
Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al:
CD28 costimulation improves expansion and persistence of chimeric
antigen receptor-modified T cells in lymphoma patients. J Clin
Invest. 121:1822–1826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brentjens RJ, Rivière I, Park JH, Davila
ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda
O, et al: Safety and persistence of adoptively transferred
autologous CD19-targeted T cells in patients with relapsed or
chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brentjens RJ, Davila ML, Riviere I, Park
J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska
M, et al: CD19-targeted T cells rapidly induce molecular remissions
in adults with chemotherapy-refractory acute lymphoblastic
leukemia. Sci Transl Med. 5:177ra382013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalos M, Levine BL, Porter DL, Katz S,
Grupp SA, Bagg A and June CH: T cells with chimeric antigen
receptors have potent antitumor effects and can establish memory in
patients with advanced leukemia. Sci Transl Med. 3:95ra732011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Finney HM, Akbar AN and Lawson AD:
Activation of resting human primary T cells with chimeric
receptors: Costimulation from CD28, inducible costimulator, CD134,
and CD137 in series with signals from the TCR zeta chain. J
Immunol. 172:104–113. 2004. View Article : Google Scholar
|
|
28
|
Knochelmann HM, Smith AS, Dwyer CJ, Wyatt
MM, Mehrotra S and Paulos CM: CAR T cells in solid tumors:
Blueprints for building effective therapies. Front Immunol.
9:17402018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramos CA and Gianpietro D: Chimeric
antigen receptor (CAR)-engineered lymphocytes for cancer therapy.
Expert Opin Biol Ther. 11:855–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jackson HJ, Rafiq S and Brentjens RJ:
Driving CAR T-cells forward. Nat Rev Clin Oncol. 13:370–383. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Till BG, Jensen MC, Wang J, Qian X, Gopal
AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE, et al:
CD20-specific adoptive immunotherapy for lymphoma using a chimeric
antigen receptor with both CD28 and 4-1BB domains: Pilot clinical
trial results. Blood. 119:3940–3950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jensen MC and Riddell SR: Designing
chimeric antigen receptors to effectively and safely target tumors.
Curr Opin Immunol. 33:9–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McAdam AJ, Greenwald RJ, Levin MA,
Chernova T, Malenkovich N, Ling V, Freeman GJ and Sharpe AH: ICOS
is critical for CD40-mediated antibody class switching. Nature.
409:102–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lipowska-Bhalla G, Gilham DE, Hawkins RE
and Rothwell DG: Targeted immunotherapy of cancer with CAR T cells:
Achievements and challenges. Cancer Immunol Immunother. 61:953–962.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haynes NM, Trapani JA, Teng MW, Jackson
JT, Cerruti L, Jane SM, Kershaw MH, Smyth MJ and Darcy PK:
Single-chain antigen recognition receptors that costimulate potent
rejection of established experimental tumors. Blood. 100:3155–3163.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu J, Song Y, Bakker AB, Bauer S, Spies T,
Lanier LL and Phillips JH: An activating immunoreceptor complex
formed by NKG2D and DAP10. Science. 285:730–732. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pulè MA, Straathof KC, Dotti G, Heslop HE,
Rooney CM and Brenner MK: A chimeric T cell antigen receptor that
augments cytokine release and supports clonal expansion of primary
human T cells. Mol Ther. 12:933–941. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Milone MC, Fish JD and Carmine C: Chimeric
receptors containing CD137 signal transduction domains mediate
enhanced survival of T cells and increased antileukemic efficacy in
vivo. Mol Ther. 17:1453–1464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hua Z, Snyder KM, Suhoski MM, Maus MV,
Kapoor V, June CH and Mackall CL: 4-1BB is superior to CD28
costimulation for generating CD8+ cytotoxic lymphocytes for
adoptive immunotherapy. J Immunol. 179:4910–4918. 2007. View Article : Google Scholar
|
|
41
|
Friedmann-Morvinski D, Bendavid A, Waks T,
Schindler D and Eshhar Z: Redirected primary T cells harboring a
chimeric receptor require costimulation for their antigen-specific
activation. Blood. 105:30872005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Porter DL, Hwang WT, Frey NV, Lacey SF,
Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al:
Chimeric antigen receptor T cells persist and induce sustained
remissions in relapsed refractory chronic lymphocytic leukemia. Sci
Transl Med. 7:303ra1392015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawalekar OU, O'Connor RS, Fraietta JA,
Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J,
Keith B, et al: Distinct signaling of coreceptors regulates
specific metabolism pathways and impacts memory development in CAR
T cells. Immunity. 44:380–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maude SL, Noelle F, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheadle EJ, Rothwell DG, Bridgeman JS,
Sheard VE, Hawkins RE and Gilham DE: Ligation of the CD2
co-stimulatory receptor enhances IL-2 production from
first-generation chimeric antigen receptor T cells. Gene Ther.
19:1114–1120. 2012. View Article : Google Scholar
|
|
46
|
Han S, Latchoumanin O, Wu G, Zhou G,
Hebbard L, George J and Qiao L: Recent clinical trials utilizing
chimeric antigen receptor T cells therapies against solid tumors.
Cancer Lett. 390:188–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kochenderfer JN, Wilson WH, Janik JE,
Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M,
Nathan DA, Lanier BJ, et al: Eradication of B-lineage cells and
regression of lymphoma in a patient treated with autologous T cells
genetically engineered to recognize CD19. Blood. 116:4099–4102.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Y, Zhang WY, Han QW, Liu Y, Dai HR,
Guo YL, Bo J, Fan H, Zhang Y, Zhang YJ, et al: Effective response
and delayed toxicities of refractory advanced diffuse large B-cell
lymphoma treated by CD20-directed chimeric antigen
receptor-modified T cells. Clin Immunol. 155:160–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Till BG, Jensen MC, Wang J, Chen EY, Wood
BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al:
Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle
cell lymphoma using genetically modified autologous CD20-specific T
cells. Blood. 112:2261–2271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang WY, Wang Y, Guo YL, Dai HR, Yang QM,
Zhang YJ, Zhang Y, Chen MX, Wang CM, Feng KC, et al: Treatment of
CD20-directed Chimeric Antigen Receptor-modified T cells in
patients with relapsed or refractory B-cell non-Hodgkin lymphoma:
An early phase IIa trial report. Signal Transduct Target Ther.
1:160022016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kochenderfer JN, Dudley ME, Feldman SA,
Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes
MS, Sherry RM, et al: B-cell depletion and remissions of malignancy
along with cytokine-associated toxicity in a clinical trial of
anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood.
119:2709–2720. 2012. View Article : Google Scholar :
|
|
52
|
Locke FL, Neelapu SS, Bartlett NL, Siddiqi
T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, et
al: Phase 1 results of ZUMA-1: A multicenter study of KTE-C19
anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol
Ther. 25:285–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kochenderfer JN, Somerville RPT, Lu T, Shi
V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, et al:
Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T
cells are associated with high serum interleukin-15 levels. J Clin
Oncol. 35:1803–1813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schuster SJ, Svoboda J, Chong EA, Nasta
SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V,
Landsburg D, et al: Chimeric antigen receptor T cells in refractory
B-cell lymphomas. N Engl J Med. 377:2545–2554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kershaw MH, Westwood JA, Parker LL, Wang
G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S,
Rogers-Freezer L, et al: A phase I study on adoptive immuno-therapy
using gene-modified T cells for ovarian cancer. Clin Cancer Res.
12:6106–6115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lamers CH, Sleijfer S, Vulto AG, Kruit WH,
Kliffen M, Debets R, Gratama JW, Stoter G and Oosterwijk E:
Treatment of metastatic renal cell carcinoma with autologous
T-lymphocytes genetically retargeted against carbonic anhydrase IX:
First clinical experience. J Clin Oncol. 24:e20–e22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo Y, Wang Y and Han W: Chimeric antigen
receptor-modified T cells for solid tumors: Challenges and
prospects. J Immunol Res. 2016:38508392016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sadelain M, Brentjens R and Rivière I: The
promise and potential pitfalls of chimeric antigen receptors. Curr
Opin Immunol. 21:215–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fousek K and Ahmed N: The evolution of
T-cell therapies for solid malignancies. Clin Cancer Res.
21:3384–3392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lamers CH, Langeveld SC, Grootvan Ruijven
CM, Debets R, Sleijfer S and Gratama JW: Gene-modified T cells for
adoptive immunotherapy of renal cell cancer maintain
transgene-specific immune functions in vivo. Cancer Immunol
Immunother. 56:1875–1883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pule MA, Savoldo B, Myers GD, Rossig C,
Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al:
Virus-specific T cells engineered to coexpress tumor-specific
receptors: Persistence and antitumor activity in individuals with
neuroblastoma. Nat Med. 14:1264–1270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Brown CE, Badie B, Barish ME, Weng L,
Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, et
al: Bioactivity and safety of IL13Rα2-redirected chimeric antigen
receptor CD8+ T cells in patients with recurrent glioblastoma. Clin
Cancer Res. 21:4062–4072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Whilding LM and Maher J: ErbB-targeted CAR
T-cell immunotherapy of cancer. Immunotherapy. 7:229–241. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fanotto V, Ongaro E, Rihawi K, Avallone A,
Silvestris N, Fornaro L, Vasile E, Antonuzzo L, Leone F, Rosati G,
et al: HER-2 inhibition in gastric and colorectal cancers: Tangible
achievements, novel acquisitions and future perspectives.
Oncotarget. 7:69060–69074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang J, Zhang Y, Chuai S, Wang Z, Zheng
D, Xu F, Zhang Y, Li C, Liang Y and Chen Z: Trastuzumab (herceptin)
targets gastric cancer stem cells characterized by CD90 phenotype.
Oncogene. 31:671–682. 2012. View Article : Google Scholar
|
|
67
|
Lo PK and Chen H: Cancer stem cells and
cells of origin in MMTV-Her2/neu-induced mammary tumorigenesis.
Oncogene. 32:1338–1340. 2013. View Article : Google Scholar
|
|
68
|
Shah D, Wyatt D, Baker AT, Simms P,
Peiffer DS, Fernandez M, Rakha E, Green A, Filipovic A, Miele L and
Osipo C: Inhibition of HER2 increases JAGGED1-dependent breast
cancer stem cells: Role for membrane JAGGED1. Clin Cancer Res.
24:4566–4578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nagorsen D, Kufer P, Baeuerle PA and
Bargou R: Blinatumomab: A historical perspective. Pharmacol Ther.
136:334–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun M, Shi H, Liu C, Liu J, Liu X and Sun
Y: Construction and evaluation of a novel humanized HER2-specific
chimeric receptor. Breast Cancer Res. 16:1–10. 2014. View Article : Google Scholar
|
|
72
|
Schönfeld K, Sahm C, Zhang C, Naundorf S,
Brendel C, Odendahl M, Nowakowska P, Bönig H, Köhl U, Kloess S, et
al: Selective inhibition of tumor growth by clonal NK cells
expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol
Ther. 23:330–338. 2015. View Article : Google Scholar :
|
|
73
|
Rainusso N, Brawley VS, Ghazi A, Hicks MJ,
Gottschalk S, Rosen JM and Ahmed N: Immunotherapy targeting HER2
with genetically modified T cells eliminates tumor-initiating cells
in osteosarcoma. Adv Exp Med Biol. 19:212–217. 2012.
|
|
74
|
Hartmann J, Schüßler-Lenz M, Bondanza A
and Buchholz CJ: Clinical development of CAR T cells-challenges and
opportunities in translating innovative treatment concepts. EMBO
Mol Med. 9:1183–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Frey NV and Porter DL: Cytokine release
syndrome with novel therapeutics for acute lymphoblastic leukemia.
Hematology Am Soc Hematol Educ Program. 2016:567–572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ali SA, Shi V, Maric I, Wang M, Stroncek
DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG,
et al: T cells expressing an anti-B-cell maturation antigen
chimeric antigen receptor cause remissions of multiple myeloma.
Blood. 128:1688–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Morgan RA, Yang JC, Kitano M, Dudley ME,
Laurencot CM and Rosenberg SA: Case report of a serious adverse
event following the administration of T cells transduced with a
chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kim MG, Kim D, Suh SK, Park Z, Choi MJ and
Oh YK: Current status and regulatory perspective of chimeric
antigen receptor-modified T cell therapeutics. Arch Pharm Res.
39:437–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Teachey DT, Lacey SF, Shaw PA, Melenhorst
JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein
J, et al: Identification of predictive biomarkers for cytokine
release syndrome after chimeric antigen receptor T-cell therapy for
acute lymphoblastic leukemia. Cancer Discov. 6:664–679. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Brudno JN and Kochenderfer JN: Toxicities
of chimeric antigen receptor T cells: Recognition and management.
Blood. 127:3321–3330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Brudno JN and Kochenderfer JN: Recent
advances in CAR T-cell toxicity: Mechanisms, manifestations and
management. Blood Rev. 34:45–55. 2019. View Article : Google Scholar :
|
|
84
|
Park JH, Rivière I, Gonen M, Wang X,
Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, et
al: Long-term follow-up of CD19 CAR therapy in acute lymphoblastic
leukemia. N Engl J Med. 378:449–459. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Thistlethwaite FC, Gilham DE, Guest RD,
Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW,
Sharma SK, et al: The clinical efficacy of first-generation
carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited
by poor persistence and transient pre-conditioning-dependent
respiratory toxicity. Cancer Immunol Immunothe. 66:1425–1436. 2017.
View Article : Google Scholar
|
|
86
|
Gross G and Eshhar Z: Therapeutic
potential of T cell chimeric antigen receptors (CARs) in cancer
treatment: Counteracting off-tumor toxicities for safe CAR T cell
therapy. Annu Rev Pharmacol Toxicol. 56:59–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kenderian SS, Ruella M, Shestova O,
Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter
DL, Carroll M, et al: CD33-specific chimeric antigen receptor T
cells exhibit potent preclinical activity against human acute
myeloid leukemia. Leukemia. 29:1637–1647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lamers CH, Sleijfer S, van Steenbergen S,
van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M,
Oosterwijk E, Debets R and Gratama JW: Treatment of metastatic
renal cell carcinoma with CAIX CAR-engineered T cells: Clinical
evaluation and management of on-target toxicity. Mol Ther.
21:904–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mei H, Jiang H, Wu Y, Guo T, Xia L, Jin R
and Hu Y: Neurological toxicities and coagulation disorders in the
cytokine release syndrome during CAR-T therapy. Br J Haematol.
181:689–692. 2018. View Article : Google Scholar
|
|
90
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra252014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Schultz DR and Arnold PI: Properties of
four acute phase proteins: C-reactive protein, serum amyloid A
protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis
Rheum. 20:129–147. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lee DW, Kochenderfer JN, Stetlerstevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar
|
|
93
|
Lee DW, Rebecca G, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Pardridge WM: CNS drug design based on
principles of blood-brain barrier transport. J Neurochem.
70:1781–1792. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Brudno JN, Maric I, Hartman SD, Rose JJ,
Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, et
al: T cells genetically modified to express an anti-B-cell
maturation antigen chimeric antigen receptor cause remissions of
poor-prognosis relapsed multiple myeloma. J Clin Oncol.
36:2267–2280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Maude SL, Laetsch TW, Buechner J, Rives S,
Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers
GD, et al: Tisagenlecleucel in children and young adults with
B-cell lymphoblastic leukemia. N Engl J Med. 378:439–448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Beatty GL and O'Hara M: Chimeric antigen
receptor-modified T cells for the treatment of solid tumors:
Defining the challenges and next steps. Pharmacol Ther. 166:30–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kang TH, Mao CP, Lee SY, Chen A, Lee JH,
Kim TW, Alvarez RD, Roden RB, Pardoll D, Hung CF and Wu TC:
Chemotherapy acts as an adjuvant to convert the tumor
microenvironment into a highly permissive state for
vaccination-induced antitumor immunity. Cancer Res. 73:2493–2504.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shahabi V, Postow MA, Tuck D and Wolchok
JD: Immune-priming of the tumor microenvironment by radiotherapy:
Rationale for combination with immunotherapy to improve anticancer
efficacy. Am J Clin Oncol. 38:90–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Nanki K, Toshimitsu K, Takano A, Fujii M,
Shimokawa M, Ohta Y, Matano M, Seino T, Nishikori S, Ishikawa K, et
al: Divergent routes toward Wnt and R-spondin niche independency
during human gastric carcinogenesis. Cell. 174:856–869.e17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient-derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lv J, Zhao R, Wu D, Zheng D, Wu Z, Shi J,
Wei X, Wu Q, Long Y, Lin S, et al: Mesothelin is a target of
chimeric antigen receptor T cells for treating gastric cancer. J
Hematol Oncol. 12:182019. View Article : Google Scholar : PubMed/NCBI
|