Prostate cancer (PCa) is one of the major causes of
male cancer-associated death worldwide (1). Over the last few decades, screening
programs have increased early diagnosis and identified treatments
with the potential to cure the disease (2-5).
However, the high rates of recurrence and metastasis remain major
challenges in treating PCa (6-12).
During a long period of the disease, PCa can become sensitive to
androgen treatment (13,14). Testosterone controls cell
proliferation, tumor growth and, potentially, dissemination
(15-17), which is an advantage in treatments
that involve androgen deprivation (AD), when curative surgery
cannot be performed (18,19). Pharmacological castration using
gonadotropin-releasing hormone (GnRH) analogs to block the
hypothalamus-hypophysis-testicular axis provides the first-line
treatment for disseminated PCa (20-22).
However, during AD therapy, PCa cells frequently become
androgen-resistant, resulting in a castration-resistant form of the
disease with a poor prognosis (23-25).
The genetic and molecular mechanisms underlying androgen resistance
remain poorly understood (26-28).
Research suggests that, in certain cases, the androgen receptor
(AR) is involved in this resistance (29-31).
On the other hand, recurrence and metastasis progression are
complex processes that involve several mechanisms and genomic
modifications of malignant cells (32,33).
It is well-known that epithelial-mesenchymal transition (EMT) is
the main pathway via which malignant epithelial cells from
carcinomas alter their gene expression profile to display a
mesenchymal phenotype, acquiring, among other features, one of the
hallmarks of cancer cells: Invasive behavior (34-38).
However, increasing evidence indicates that tumors contain a
phenotypically heterogeneous cell population, and that the
cooperative action of these different types of malignant cells is
potentially required to accomplish a successful metastatic process
(39-42). In the past few years, a small
subpopulation of malignant cells with stem-like properties has been
identified in numerous types of cancer, including PCa (43,44).
These cells have been termed tumor-initiating cells (TICs) or
cancer stem cells (CSCs), and are hypothesized to be responsible
for recurrence and metastasis (45-48).
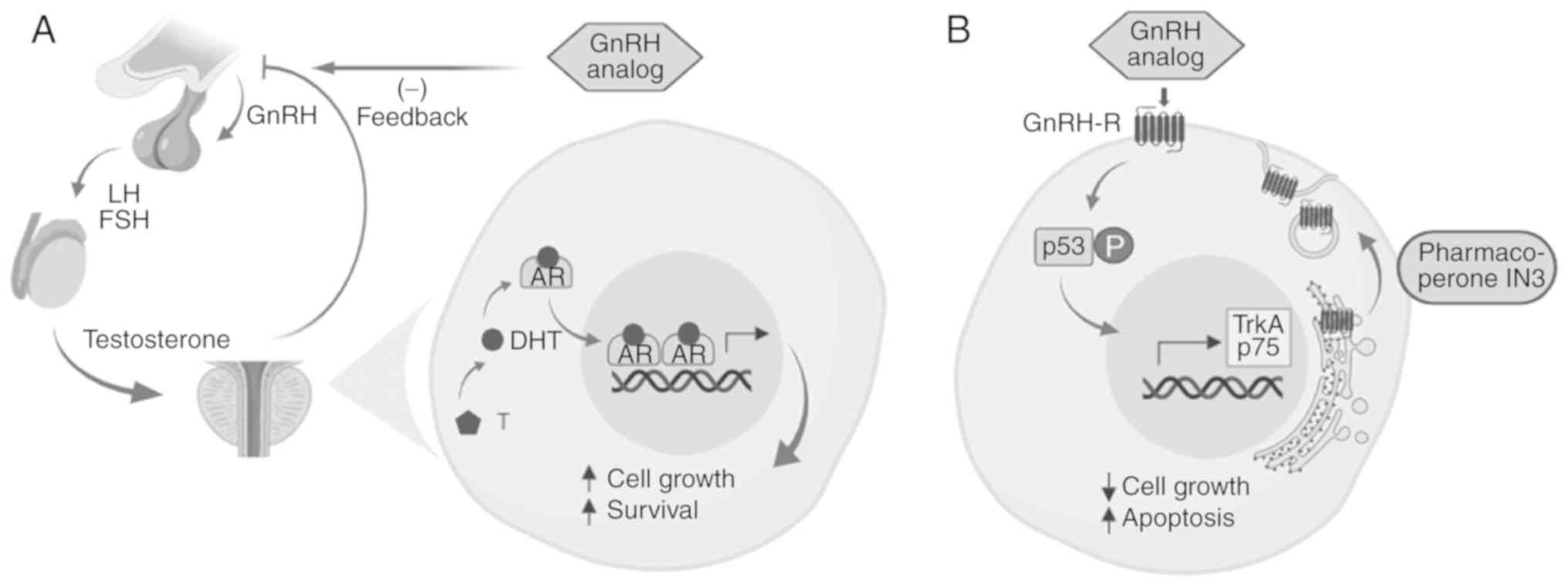
As aforementioned, GnRH analog therapy is the gold
standard to treat disseminated PCa (20,49).
This treatment induces AD by blocking the
hypothalamus-hypophysis-testicular axis, resulting in
pharmacological castration. This type of therapy is very efficient
at delaying tumor growth until PCa becomes castration-resistant
(20). Gene amplification,
mutations and other alterations in the AR gene have been identified
(29,50-52).
In addition, overexpression or constitutive activation of other
proliferation signaling pathways that overcome androgen control
have been reported (53,54). In addition, alterations in androgen
metabolism within the prostate gland have been associated with
androgen sensitivity (55,56). It is postulated that
castration-resistant PCa arises from a combination of these
different mechanisms. Our previous research, as well as other
studies, have reported the presence of GnRH receptor (GnRH-R) in
PCa cells (57-59). Furthermore, it has been observed
that GnRH analogs induce proliferation arrest and apoptosis in PCa
cells in a primary culture system (57,58).
GnRH-R expression increases from benign prostatic hyperplasia to
medium histological grade (Gleason score 6-7), and subsequently
decreases in samples from patients with higher Gleason scores
(60). Local cellular effects of
GnRH analogs may be of clinical relevance, as these effects remain
despite cell androgen insensitivity (58,60).
Concentrations >20 ng/ml are required to obtain significant
in vitro apoptotic effects (61); however, the plasma concentrations
in patients receiving AD treatment are below this level (62). This problem may be solved via
intraprostatic administration of GnRH analogs. Unfortunately,
patients who are castration-resistant often have a higher Gleason
score and, as aforementioned, GnRH-R expression decreases with
higher Gleason scores. There is evidence that GnRH-R in PCa,
specifically in the gonadotropic cells, is retained primarily in
the endoplasmic reticulum, where it can be moved to the plasma
membrane using peptide-mimetic compounds called pharmacoperones
(pharmacological chaperones) (60). Using this strategy, it is possible
to increase GnRH-R expression in cultured PCa cells and sensitize
them to the apoptotic effects of GnRH analogs (Fig. 1).
EMT is a process in which an epithelial genetic
program switches to a mesenchymal program; as a result, an
epithelial cell loses its polarity, proliferation, and
differentiation control and positioning, changing to a mesenchymal
phenotype (63-65). This is a physiologically normal
process occurring primarily during embryonic development (66). During carcinogenesis, similar
genetic changes occur in carcinomas that transform a malignant
epithelial cell into a highly proliferative and invasive
mesenchymal-like cell (65,67).
Epithelial malignant cells progressively lose adhesion molecules,
such as E-cadherin, syndecans and tight junction molecules, whereas
gene-regulating factors, including Snail family transcriptional
repressor SNAI1, SNAI2, zinc finger E-box-binding homeobox 1/2 and
TWIST increase their expression, together with mesenchymal markers
such as vimentin, N-cadherin and metalloproteinases, resulting in
an invasive cell phenotype (35,38,68-70).
It is becoming apparent that tumors present a
significant degree of cell heterogeneity (80,81).
Tumor heterogeneity may be understood at the phenotypic and genetic
level (82). Tumor cell phenotypic
heterogeneity will specifically be discussed. Cellular and
molecular mechanisms responsible for this heterogeneous cell
population remain poorly understood. There remains controversy
regarding the origin of CSCs, and several hypotheses have been
suggested (76,83-85).
However, regardless of the origin of CSCs, the relevant point,
particularly for clinical application, is that such a population is
present in the majority of cancers studied. The multifocal origin
of cancer cells within the organ and the distinct differentiation
fate during EMT process may explain, in part, this phenomenon
(75,86). As with the process of
microevolution, cancer cells adapt to different microenvironments,
first within the tumor niche and subsequently in potential
metastatic niches (87,88). Within the tumor, it is possible to
find a hypoxic microenvironment, for instance in the center of a
solid tumor, whereas in the periphery, where neoangiogenesis is
occurring, a more oxygenated milieu is more prevalent (89,90);
cancer cells adapt differently to these distinct microenvironments.
Therefore, it is possible that during EMT progression, certain
cells express a stem gene program, forming a stable CSC population
within a tumor (91-93).
Metastasis is an inefficient process; it is
estimated that <2% of total cancer cells entering the blood
stream from a solid tumor will be able to colonize a premetastatic
niche (94). Furthermore,
<0.02% will be able to survive in that niche and support
sustained growth to give rise to clinically evident metastatic foci
(94). Evidence suggests that this
is not a stochastic process, indicating that not all malignant
cells are able to sustain metastasis (94). Furthermore, very few cells have the
ability to colonize, survive and grow in a tissue or organ
different to the one from which it originated (95). The majority of researchers
investigating CSCs have concluded that these metastatic cells
express stemness genes and exhibit little invasive capacity
(96). Previous results from our
laboratory in CSCs from PCa are consistent with this hypothesis
(78). Instead, PCa CSCs, as with
other CSCs, have a low proliferation rate, high resistance to drugs
and apoptosis (particularly anoikis), sphere-growing ability and a
high clonogenic capacity (97-99).
Determining how these CSCs, with little invasive activity, can
leave the tumor and colonize premetastatic niches will be
subsequently addressed.
Metastasis is a complex process. Premetastatic
niches are developed in advance by several signals originating from
the initiating tumor determining the tissue tropism of the future
metastatic foci (100,101). It is proposed that, once in the
blood stream, CSCs are guided by homing signals from these
premet-astatic niches (102,103). Once colonizing a metastatic site
has begun, CSCs can be induced by niche milieu factors to survive
and proliferate, or to become quiescent (104,105). In the event of quiescence, future
microenvironmental changes can subsequently induce cell
proliferation and tumor growth, resulting in relapse, even if
curative surgery was performed to remove the primary tumor
(104). In human PCa, bone is one
of the main sites of distant metastasis (106). Stromal-cell-derived-factor 1,
acting through C-X-C chemokine receptor 4 on malignant cells, is
hypothesized to promote cell survival in the niche (106). Secretion of several interleukins,
tumor necrosis factor-α and other factors by cancer cells
stimulates secretion of the receptor activator of NF-κB ligand
(RANKL), which in turn stimulates osteoclast differentiation
(107). Increased osteoclast
activity releases bone matrix and growth factors that promote CSC
survival and growth for metastatic progression (106-108). Exosomes secreted by CSCs and bulk
cancer cell cultures derived from PCa contain various microRNAs
(miRNAs/miRs). Comparing those miRNAs using next-generation
sequencing followed by bioinformatics analysis, specific miRNAs,
such as miR-100-5p, miR-21-5p and miR-139-5p were found to be
overexpressed and, analyzed in an in vitro system, they
increased the expression of metalloproteinases-2, -9 and -13, and
RANKL, as well as fibroblast migration, supporting the idea that
the different PCa cells contribute cooperatively to prepare the
premetastatic niche (100).
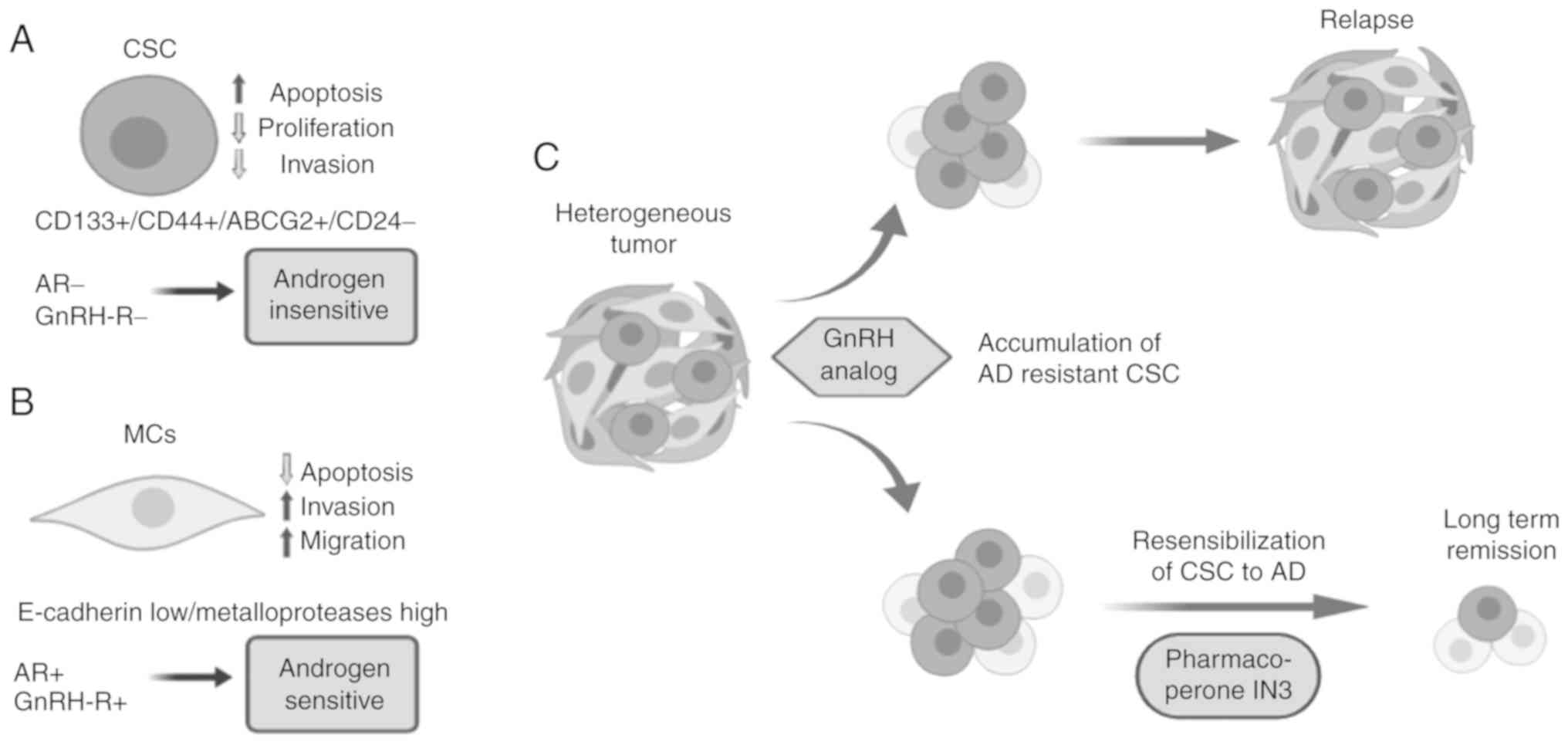
Considering that CSCs appear to be the only cells
within a tumor with the ability to form metastasis, it is
reasonable to propose that any increase in circulating CSCs will
raise the risk of metastasis or recurrence (88,109-111). Our previous study investigated
the expression of stem signatures in PCa samples of different
histological grades, using a tissue microarray and quantitative
immunohistochemistry (78). It was
observed that the number of cells expressing stem markers increases
with Gleason grade, reaching maximal levels at medium Gleason, and
decreasing thereafter in high-Gleason grade, lymph node and bone
metastatic samples (78).
Considering that malignant cells begin to enter the blood stream
shortly after the tumor becomes locally invasive (low-to-medium
histological grade), it is possible that a patient with a localized
tumor with a medium Gleason score will contain the maximal number
of CSCs potentially leaving the tumor and spreading throughout
blood stream. At this stage, the indicated therapy is surgical
removal of prostate gland (112).
However, if CSCs already released from the tumor have seeded the
metastatic niches, recurrence risk would be high. This is an
important point to consider, particularly in patients with
localized tumors of low Gleason grade where the therapeutic
recommendation is active surveillance (5,113).
Therefore, identifying and quantifying CSCs in PCa biopsies may be
a valuable prognostic factor for relapse.
Reanalyzing the problem of how CSCs with little
invasive activity can leave the tumor and colonize premetastatic
niches, it is reasonable to suggest that some type of collaboration
with highly invasive mesenchymal-like cells occurs (45,96).
Previously, Celià-Terrassa et al (114) provided evidence regarding this
potential cooperative action. Using commercial cell lines derived
from PCa (PC3) and bladder cancer (TSU-Pr1), these were enriched
with metastatic TICs, a cell population with a strong epithelial
profile. In turn, they deprived TICs, a cell population with a
mesenchymal profile. Overexpression of mesenchymal genes in the
former cell population (epithelial phenotype) decreased its TIC
ability, whereas knockdown of these genes in the latter cell
population (mesenchymal phenotype) enhanced its TIC capacity
(114). Using immunocompromised
nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice,
it was observed that, injected in combination, mesen-chymal-like
cells increased the metastatic potential of epithelial TIC-enriched
cell populations, suggesting a cooperative action between both cell
types (114). Subsequently, the
same research group described that secreted protein acidic and rich
in cysteine (SPARC) mediates the metastatic cooperation between CSC
and non-CSC cell subpopulations (39).
Recently, it was reported that SPARC induced EMT,
increasing the invasive capacities of PCa cells (115). Collectively, these findings
support the hypothesis that within a tumor, MCs become the
predominant population via EMT, increasing the invasive capacity of
the tumor. However, it has been proposed that a small cell
population that expresses a stem-like program (CSCs) remains in the
tumor and can escape passively with the bulk of MCs. Once in the
metastatic niche, it is hypothesized that CSCs proliferate and
produce progenitor cells that may further differentiate to an
epithelial-like phenotype. This may explain certain findings
revealing that in metastatic PCa samples, an increase in epithelial
markers and a decrease in mesenchymal markers is observed, which
has been called mesenchymal-epithelial transition (116,117). It is postulated that the
metastatic foci will generate the full heterogeneity of the
original tumor, in which epithelial-like cells will undergo EMT
again, whilst a small number of CSCs are retained in the tumor. On
the other hand, tumor cell plasticity influences the phenotypic
heterogeneity of tumor cells, with the varied cell abilities
enabling cooperation to promote cancer progression and metastasis.
Differential cell distribution within the tumor, and spatial and
temporal patterns during EMT-stemness processes may influence cell
frequencies and the results of the proposed cell cooperation
(118). This may contribute to
why different patients with PCa at the same stage may have
different outcomes.
Personalized medicine should take into consideration
this evidence to develop novel and innovative therapeutic
strategies. In this context, resensibilization of PCa cells
(including CSCs) to GnRH analogs using pharmacoperones or
lentiviral transduction may provide an effective treatment against
metastatic castration-resistant PCa. It is necessary to validate
this hypothesis using CSCs and MCs derived from the tumors of
various patients. Metastasis is, by definition, a process that
occurs in a living organism. Therefore, there are no in
vitro models for investigating this complex pathological
process. In previous years, several in vivo models have been
developed (119-124). The majority of these use
immunocompromised mice, and several mouse strains have been
obtained, a number of them via transgenic manipulation (124-126). One of the most used models, at
present, is the NOD/SCID mouse (127).
The NOD/SCID mouse has been widely used to
investigate the metastasis of several types of human cancer
(128). A critical issue is the
type of injection used to introduce human cancer cells. Numerous
researchers use subcutaneous, intravenous or intracardiac
administration, with varying results (114,129). Additionally, orthotopic models
have been developed (injection in the same mouse organ or tissue
from which human cells were derived). This model mimics the
metastatic process more precisely (129). Reports of orthotopic models for
human PCa have been published (130-132). A modification of the orthotopic
model for PCa using a cell injection in one of the anterior lobes
of the NOD/SCID mouse prostate has been developed by our laboratory
(133,134). This orthotopic injection results
in consistent and reproducible metastatic progression. First, a
fraction of tumor cells injected in the mouse prostate survives and
generates a tumor derived from surviving injected cells (transduced
with luciferase and red fluorescent protein genes). The
fluorescence allows the tracking of metastatic progression in
vivo using in vivo imaging equipment. In a chronological
sequence, metastatic foci begin to appear in the liver, lungs and
the kidneys. Injection of cells into the anterior lobe, instead of
the ventral prostate, has the advantage that it is possible to
surgically remove the prostate tumor to evaluate the progression of
metastasis, with or without the primary prostate tumor. In this
orthotopic model, the utility of prostatectomy during metastasis
progression has been demonstrated (134), as has the effect of knocking down
the stemness gene Sox2 on metastasis (unpublished data). In current
studies, progression towards a castration-resistant PCa mouse model
using surgical castration as an AD strategy is being
established.
In conclusion, it is proposed that there is
cooperation between CSCs and MCs during metastatic progression.
Further development of preclinical orthotopic models of PCa may
provide additional evidence supporting this hypothesis. In
addition, the role of stem genes, as well as AR, GnRH-R and
differentiation genes, in metastasis progression and hormone
resistance may have critical relevance. Further investigation of
these aspects will contribute to the understanding of the cellular
and molecular mechanisms of metastasis, recurrence and hormone
resistance in PCa, which remain major challenges for the treatment
of this disease. It is predicted that evidence obtained using
preclinical models, will be beneficial for clinical purposes in the
near future, identifying novel prognostic factors and therapeutic
targets.
The present study was funded by Fondecyt (grant nos.
1140417 and 1151214), ENLACE-VID (grant nos. ENL-22/19 and
ENL-23/19) and URedes URC (grant no. 007/17).
All data generated or analyzed during this study are
included in this published article.
HRC and FLM contributed to reviewing and discussing
the literature, and selecting relevant studies. EAC analyzed the
subject and wrote the review.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors thank the professional contribution of
Mrs Graciela Caroca (Laboratory of Cellular and Molecular Oncology,
Department of Basic and Clinical Oncology, Faculty of Medicine,
University of Chile) for assistance in the laboratory.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodgers L, Peer CJ and Figg WD: Diagnosis,
staging and risk stratification in prostate cancer: Utilizing
diagnostic tools to avoid unnecessary therapies and side effects.
Cancer Biol Ther. 18:470–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah RB and Zhou M: Recent advances in
prostate cancer pathology: Gleason grading and beyond. Pathol Int.
66:260–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heijnsdijk EAM, Bangma CH, Borràs JM, de
Carvalho TM, Castells X, Eklund M, Espinàs JA, Graefen M, Grönberg
H, Lansdorp-Vogelaar I, et al: Summary statement on screening for
prostate cancer in Europe. Int J Cancer. 142:741–746. 2018.
View Article : Google Scholar
|
|
5
|
Litwin MS and Tan HJ: The diagnosis and
treatment of prostate cancer: A review. J Am Med Assoc.
317:2532–2542. 2017. View Article : Google Scholar
|
|
6
|
Ost P, Bossi A, Decaestecker K, De
Meerleer G, Giannarini G, Karnes RJ, Roach M III and Briganti A:
Metastasis-directed therapy of regional and distant recurrences
after curative treatment of prostate cancer: A systematic review of
the literature. Eur Urol. 67:852–863. 2015. View Article : Google Scholar
|
|
7
|
Fakhrejahani F, Madan RA and Dahut WL:
Management options for biochemically recurrent prostate cancer.
Curr Treat Options Oncol. 18:262017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artibani W, Porcaro AB, De Marco V,
Cerruto MA and Siracusano S: Management of biochemical recurrence
after primary curative treatment for prostate cancer: A review.
Urol Int. 100:251–262. 2018. View Article : Google Scholar
|
|
9
|
Sartor O and de Bono JS: Metastatic
prostate cancer. N Engl J Med. 378:645–657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song C, Kang T, Yoo S, Jeong IG, Ro JY,
Hong JH, Kim CS and Ahn H: Tumor volume, surgical margin, and the
risk of biochemical recurrence in men with organ-confined prostate
cancer. Urol Oncol. 31:168–174. 2013. View Article : Google Scholar
|
|
11
|
Suzman DL, Boikos SA and Carducci MA:
Bone-targeting agents in prostate cancer. Cancer Metastasis Rev.
33:619–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong L, Zieren RC, Xue W, de Reijke TM and
Pienta KJ: Metastatic prostate cancer remains incurable, why? Asian
J Urol. 6:26–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pelekanou V and Castanas E: Androgen
control in prostate cancer. J Cell Biochem. 2234:2224–2234. 2016.
View Article : Google Scholar
|
|
14
|
Tan MH, Li J, Xu HE, Melcher K and Yong
EL: Androgen receptor: Structure, role in prostate cancer and drug
discovery. Acta Pharmacol Sin. 36:3–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita K and Nonomura N: Role of androgen
receptor in prostate cancer: A review. World J Mens Health.
36:288–295. 2018.
|
|
16
|
Rodriguez KM, Pastuszak AW and Khera M:
The role of testosterone therapy in the setting of prostate cancer.
Curr Urol Rep. 19:672018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Obinata D, Takayama K, Takahashi S and
Inoue S: Crosstalk of the androgen receptor with transcriptional
collaborators: Potential therapeutic targets for
castration-resistant prostate cancer. Cancers (Basel). 9:pii: E22.
2017. View Article : Google Scholar
|
|
18
|
Grossmann M, Cheung AS and Zajac JD:
Androgens and prostate cancer; pathogenesis and deprivation
therapy. Best Pract Res Clin Endocrinol Metab. 27:603–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hahn AW, Hale P, Rathi N and Agarwal N:
Novel androgen axis systemic therapies for metastatic
hormone-sensitive prostate cancer. Curr Opin Urol. 27:559–565.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shore ND, Abrahamsson P, Anderson J,
Crawford ED and Lange P: New considerations for ADT in advanced
prostate cancer and the emerging role of GnRH antagonists. Prostate
Cancer Prostatic Dis. 16:7–15. 2013. View Article : Google Scholar
|
|
21
|
Lama G, Papi M, Angelucci C, Maulucci G,
Sica G and De Spirito M: Leuprorelin acetate long-lasting effects
on GnRH receptors of prostate cancer cells: An atomic force
microscopy study of agonist/receptor interaction. PLoS One.
8:e525302013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas BC and Neal DE: Androgen
deprivation treatment in prostate cancer. BMJ. 346:1–5. 2013.
View Article : Google Scholar
|
|
23
|
Katsogiannou M, Ziouziou H, Karaki S,
Andrieu C, Henry de Villeneuve M and Rocchi P: The hallmarks of
castration-resistant prostate cancers. Cancer Treat Rev.
41:588–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan X, Cai C, Chen S, Chen S, Yu Z and
Balk SP: Androgen receptor functions in castration-resistant
prostate cancer and mechanisms of resistance to new agents
targeting the androgen axis. Oncogene. 33:2815–2825. 2014.
View Article : Google Scholar
|
|
25
|
Fujimoto N: Role of the androgen-androgen
receptor axis in the treatment resistance of advanced prostate
cancer: From androgen-dependent to castration resistant and
further. J UOEH. 38:129–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chandrasekar T, Yang JC, Gao AC and Evans
CP: Mechanisms of resistance in castration-resistant prostate
cancer (CRPC). Transl Androl Urol. 4:365–380. 2015.
|
|
27
|
Tilki D, Schaeffer EM and Evans CP:
Understanding mechanisms of resistance in metastatic
castration-resistant prostate cancer: The role of the androgen
receptor. Eur Urol Focus. 2:499–505. 2019. View Article : Google Scholar
|
|
28
|
Huang Y, Jiang X, Liang X and Jiang G:
Molecular and cellular mechanisms of castration resistant prostate
cancer. Oncol Lett. 15:6063–6076. 2018.PubMed/NCBI
|
|
29
|
Ho Y and Dehm SM: Androgen receptor
rearrangement and splicing variants in resistance to endocrine
therapies in prostate cancer. Endocrinology. 158:1533–1542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Recouvreux MV, Wu JB, Gao AC, Zonis S,
Chesnokova V, Bhowmick N, Chung LW and Melmed S: Androgen receptor
regulation of local growth hormone in prostate cancer cells.
Endocrinology. 158:2255–2568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stelloo S, Nevedomskaya E, van der Poel
HG, de Jong J, van Leenders GJ, Jenster G, Wessels LF, Bergman AM
and Zwart W: Androgen receptor profiling predicts prostate cancer
outcome. EMBO Mol Med. 7:1450–1464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Höti N, Shah P, Hu Y, Yang S and Zhang H:
Proteomics analyses of prostate cancer cells reveal cellular
pathways associated with androgen resistance. Proteomics. 17:2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Den Eeden SK, Lu R, Zhang N,
Quesenberry CP Jr, Shan J, Han JS, Tsiatis AC, Leimpeter AD,
Lawrence HJ, Febbo PG and Presti JC: A Biopsy-based 17-gene genomic
prostate score as a predictor of metastases and prostate cancer
death in surgically treated men with clinically localized disease.
Eur Urol. 73:129–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hasegawa S, Nagano H, Konno M, Eguchi H,
Tomokuni A, Tomimaru Y, Asaoka T, Wada H, Hama N, Kawamoto K, et
al: A crucial epithelial to mesenchymal transition regulator,
Sox4/Ezh2 axis is closely related to the clinical outcome in
pancreatic cancer patients. Int J Oncol. 48:145–152. 2016.
View Article : Google Scholar
|
|
37
|
Frisch SM, Schaller M and Cieply B:
Mechanisms that link the oncogenic epithelial-mesenchymal
transition to suppression of anoikis. J Cell Sci. 126:21–29. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mateo F, Meca-Cortés O, Celià-Terrassa T,
Fernández Y, Abasolo I, Sánchez-Cid L, Bermudo R, Sagasta A,
Rodríguez-Carunchio L, Pons M, et al: SPARC mediates metastatic
cooperation between CSC and non-CSC prostate cancer cell
subpopulations. Mol Cancer. 13:2372014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin KC, Torga G, Sun Y, Axelrod R, Pienta
KJ, Sturm JC and Austin RH: The role of heterogeneous environment
and docetaxel gradient in the emergence of polyploid, mesenchymal
and resistant prostate cancer cells. Clin Exp Metastasis.
36:97–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bakker B, Taudt A, Belderbos ME, Porubsky
D, Spierings DC, de Jong TV, Halsema N, Kazemier HG,
Hoekstra-Wakker K, Bradley A, et al: Single-cell sequencing reveals
karyotype heterogeneity in murine and human malignancies. Genome
Biol. 17:1152016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chapman MP, Risom T, Aswani AJ, Langer EM,
Sears RC and Tomlin CJ: Modeling differentiation-state transitions
linked to therapeutic escape in triple-negative breast cancer. PLoS
Comput Biol. 15:e10068402019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eun K, Ham SW and Kim H: Cancer stem cell
heterogeneity: Origin and new perspectives on CSC targeting. BMB
Rep. 50:117–125. 2017. View Article : Google Scholar :
|
|
44
|
Adamowicz J, Pakravan K, Bakhshinejad B,
Drewa T and Babashah S: Prostate cancer stem cells: From theory to
practice. Scand J Urol. 51:95–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shahriari K, Shen F, Worrede-Mahdi A, Liu
Q, Gong Y, Garcia FU and Fatatis A: Cooperation among heterogeneous
prostate cancer cells in the bone metastatic niche. Oncogene.
36:2846–2856. 2017. View Article : Google Scholar :
|
|
46
|
Chang L, Graham P, Hao J, Ni J, Deng J,
Bucci J, Malouf D, Gillatt D and Li Y: Cancer stem cells and
signaling pathways in radioresistance. Oncotarget. 7:11002–11017.
2016.
|
|
47
|
Geng SQ, Alexandrou AT and Li JJ: Breast
cancer stem cells: Multiple capacities in tumor metastasis. Cancer
Lett. 349:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leão R, Domingos C, Figueiredo A, Hamilton
R, Tabori U and Castelo-Branco P: Cancer stem cells in prostate
cancer: Implications for targeted therapy. Urol Int. 99:125–136.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rosario DJ, Davey P, Green J, Greene D,
Turner B, Payne H and Kirby M: The role of gonadotrophin-releasing
hormone antagonists in the treatment of patients with advanced
hormone-dependent prostate cancer in the UK. World J Urol.
34:1601–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Poelaert F, Kumps C, Lumen N, Verschuere
S, Libbrecht L, Praet M, Rottey S, Claeys T, Ost P, Decaestecker K,
et al: Androgen receptor gene copy number and protein expression in
treatment-naïve prostate cancer. Urol Int. 99:222–228. 2017.
View Article : Google Scholar
|
|
51
|
Prekovic S, van Royen ME, Voet AR, Geverts
B, Houtman R, Melchers D, Zhang KY, Van den Broeck T, Smeets E,
Spans L, et al: The effect of F877L and T878A mutations on androgen
receptor response to enzalutamide. Mol Cancer Ther. 15:1702–1712.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sutinen P, Malinen M, Heikkinen S and
Palvimo JJ: SUMOylation modulates the transcriptional activity of
androgen receptor in a target gene and pathway selective manner.
Nucleic Acids Res. 42:8310–8319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rowlands MA, Holly JM, Hamdy F, Phillips
J, Goodwin L, Marsden G, Gunnell D, Donovan J, Neal DE and Martin
RM: Serum insulin-like growth factors and mortality in localised
and advanced clinically detected prostate cancer. Cancer Causes
Control. 23:347–354. 2012. View Article : Google Scholar
|
|
54
|
Lescarbeau RM, Seib FP, Prewitz M, Werner
C and Kaplan DL: In vitro model of metastasis to bone marrow
mediates prostate cancer castration resistant growth through
paracrine and extracellular matrix factors. PLoS One. 7:e403722012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Penning TM and Tamae D: Current advances
in intratumoral androgen metabolism in castration-resistant
prostate cancer. Curr Opin Endocrinol Diabetes Obes. 23:264–270.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Price DK, Chau CH, Till C, Goodman PJ,
Leach RJ, Johnson-Pais TL, Hsing AW, Hoque A, Parnes HL, Schenk JM,
et al: Association of androgen metabolism gene polymorphisms with
prostate cancer risk and androgen concentrations: Results from the
prostate cancer prevention trial. Cancer. 122:2332–2340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Clementi M, Sánchez C, Benitez DA,
Contreras HR, Huidobro C, Cabezas J, Acevedo C and Castellón EA:
Gonadotropin releasing hormone analogs induce apoptosis by
extrinsic pathway involving p53 phosphorylation in primary cell
cultures of human prostatic adenocarcinomas. Prostate.
69:1025–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sánchez C, Clementi M, Benitez D,
Contreras H, Huidobro C and Castellón E: Effect of GnRH analogs on
the expression of TrkA and p75 neurotrophin receptors in primary
cell cultures from human prostate adenocarcinoma. Prostate.
65:195–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Angelucci C, Lama G, Iacopino F, Ferracuti
S, Bono AV, Millar RP and Sica G: GnRH receptor expression in human
prostate cancer cells is affected by hormones and growth factors.
Endocrine. 36:87–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sánchez CA, Mercado AJ, Contreras HR,
Cabezas JC, Huidobro CC and Castellón EA: Pharmacoperone IN3
enhances the apoptotic effect of leuprolide in prostate cancer
cells by increasing the gonadotropin-releasing hormone receptor in
the cell membrane. Anticancer Drugs. 23:959–969. 2012.PubMed/NCBI
|
|
61
|
Castellón E, Clementi M, Hitschfeld C,
Sánchez C, Benítez D, Sáenz L, Contreras H and Huidobro C: Effect
of leuprolide and cetrorelix on cell growth, apoptosis, and GnRH
receptor expression in primary cell cultures from human prostate
carcinoma. Cancer Invest. 24:261–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Saltzstein D, Shore ND, Moul JW, Chu F,
Concepcion R, de la Motte S, McLane JA, Atkinson S, Yang A and
Crawford ED: Pharmacokinetic and pharmacodynamic comparison of
subcutaneous versus intramuscular leuprolide acetate formulations
in male subjects. Ther Adv Urol. 10:43–50. 2017. View Article : Google Scholar
|
|
63
|
Nieto MA and Cano A: The
epithelial-mesenchymal transition under control: Global programs to
regulate epithelial plasticity. Semin Cancer Biol. 22:361–368.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
García de Herreros A and Baulida J:
Cooperation, amplification, and feed-back in epithelial-mesenchymal
transition. Biochim Biophys Acta. 1825:223–228. 2012.PubMed/NCBI
|
|
65
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21(Suppl 7): vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial- mesen-chymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Osorio LA, Farfán NM, Castellón EA and
Contreras HR: SNAIL transcription factor increases the motility and
invasive capacity of prostate cancer cells. Mol Med Rep.
13:778–786. 2016. View Article : Google Scholar :
|
|
70
|
Orellana-Serradell O, Herrera D, Castellón
EA and Contreras HR: The transcription factor ZEB1 promotes an
aggressive phenotype in prostate cancer cell lines. Asian J Androl.
20:294–299. 2018. View Article : Google Scholar :
|
|
71
|
Contreras HR, Ledezma RA, Vergara J,
Cifuentes F, Barra C, Cabello P, Gallegos I, Morales B, Huidobro C
and Castellón EA: The expression of syndecan-1 and -2 is associated
with Gleason score and epithelial-mesenchymal transition markers,
E-cadherin and beta-catenin, in prostate cancer. Urol Oncol.
28:534–540. 2010. View Article : Google Scholar
|
|
72
|
Poblete CE, Fulla J, Gallardo M, Muñoz V,
Castellón EA, Gallegos I and Contreras HR: Increased SNAIL
expression and low syndecan levels are associated with high Gleason
grade in prostate cancer. Int J Oncol. 44:647–654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Farfán N, Ocarez N, Castellón EA, Mejía N,
de Herreros AG and Contreras HR: The transcriptional factor ZEB1
represses Syndecan 1 expression in prostate cancer. Sci Rep.
8:114672018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Montanari M, Rossetti S, Cavaliere C,
D'Aniello C, Malzone MG, Vanacore D, Di Franco R, La Mantia E,
Iovane G, Piscitelli R, et al: Epithelial-mesenchymal transition in
prostate cancer: An overview. Oncotarget. 8:35376–35389. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10699–10710. 2015. View Article : Google Scholar
|
|
76
|
Peitzsch C, Tyutyunnykova A, Pantel K and
Dubrovska A: Cancer stem cells: The root of tumor recurrence and
metastases. Semin Cancer Biol. 44:10–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ajani JA, Song S, Hochster HS and
Steinberg IB: Cancer stem cells: The promise and the potential.
Semin Oncol. 42(Suppl 1): S3–S17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Castellón EA, Valenzuela R, Lillo J,
Castillo V, Contreras HR, Gallegos I, Mercado A and Huidobro C:
Molecular signature of cancer stem cells isolated from prostate
carcinoma and expression of stem markers in different Gleason
grades and metastasis. Biol Res. 45:297–305. 2012. View Article : Google Scholar
|
|
79
|
Castillo V, Valenzuela R, Huidobro C,
Contreras HR and Castellon EA: Functional characteristics of cancer
stem cells and their role in drug resistance of prostate cancer.
Int J Oncol. 45:985–994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
McGranahan N and Swanton C: Clonal
heterogeneity and tumor evolution: Past, present, and the future.
Cell. 168:613–628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bosman FT: Tumor heterogeneity: Will it
change what pathologists do. Pathobiology. 85:18–22. 2018.
View Article : Google Scholar
|
|
82
|
Jolly MK and Celià-Terrassa T: Dynamics of
phenotypic heterogeneity during EMT and stemness in cancer
progression. J Clin Med. 8:pii: E1542. 2019. View Article : Google Scholar
|
|
83
|
Bu Y and Cao D: The origin of cancer stem
cells. Front Biosci (Schol Ed). 4:819–830. 2012.
|
|
84
|
Parsons BL: Multiclonal tumor origin:
Evidence and implications. Mutat Res. 777:1–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Vicente-Dueñas C, Hauer J, Cobaleda C,
Borkhardt A and Sánchez-García I: Epigenetic priming in cancer
initiation. Trends Cancer. 4:408–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ye X and Weinberg RA:
Epithelial-mesenchymal plasticity: A central regulator of cancer
progression. Trends Cell Biol. 25:675–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Graham TA and Sottoriva A: Measuring
cancer evolution from the genome. J Pathol. 241:183–191. 2017.
View Article : Google Scholar
|
|
88
|
Francart M, Lambert J, Vanwynsberghe AM,
Thompson EW, Bourcy M, Polette M and Gilles C:
Epithelial-mesenchymal plasticity and circulating tumor cells:
Travel companions to metastases. Dev Dyn. 247:432–450. 2018.
View Article : Google Scholar
|
|
89
|
Carnero A and Lleonart M: The hypoxic
microenvironment: A determinant of cancer stem cell evolution.
Bioessays. 38(Suppl 1): S65–S74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yeo CD, Kang N, Choi SY, Kim BN, Park CK,
Kim JW, Kim YK and Kim SJ: The role of hypoxia on the acquisition
of epithelial-mesenchymal transition and cancer stemness: A
possible link to epigenetic regulation. Korean J Intern Med.
32:589–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Rhim AD: Epithelial to mesenchymal
transition and the generation of stem-like cells in pancreatic
cancer. Pancreatology. 13:114–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lan L, Luo Y, Cui D, Shi BY, Deng W, Huo
LL, Chen HL, Zhang GY and Deng LL: Epithelial-mesenchymal
transition triggers cancer stem cell generation in human thyroid
cancer cells. Int J Oncol. 43:113–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li N, Babaei-Jadidi R, Lorenzi F,
Spencer-Dene B, Clarke P, Domingo E, Tulchinsky E, Vries RGJ, Kerr
D, Pan Y, et al: An FBXW7-ZEB2 axis links EMT and tumour
microenvironment to promote colorectal cancer stem cells and
chemoresistance. Oncogenesis. 8:132019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Croker AK and Allan AL: Cancer stem cells:
Implications for the progression and treatment of metastatic
disease. J Cell Mol Med. 12:374–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Massagué J and Obenauf AC: Metastatic
colonization by circulating tumour cells. Nature. 529:298–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hayashida T, Jinno H, Kitagawa Y and
Kitajima M: Cooperation of cancer stem cell properties and
epithelial-mesenchymal transition in the establishment of breast
cancer metastasis. J Oncol. 2011:5914272011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hsu CL, Chung FH, Chen CH, Hsu TT, Liu SM,
Chung DS, Hsu YF, Chen CL, Ma N and Lee HC: Genotypes of cancer
stem cells characterized by epithelial-to-mesenchymal transition
and proliferation related functions. Sci Rep. 6:325232016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yun EJ, Lo UG and Hsieh JT: The evolving
landscape of prostate cancer stem cell: Therapeutic implications
and future challenges. Asian J Urol. 3:203–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lin CJ, Lo UG and Hsieh JT: The regulatory
pathways leading to stem-like cells underlie prostate cancer
progression. Asian J Androl. 21:233–240. 2019. View Article : Google Scholar :
|
|
100
|
Sánchez CA, Andahur EI, Valenzuela R,
Castellón EA, Fullá JA, Ramos CG and Triviño JC: Exosomes from bulk
and stem cells from human prostate cancer have a differential
microRNA content that contributes cooperatively over local and
pre-metastatic niche. Oncotarget. 7:3993–4008. 2016. View Article : Google Scholar :
|
|
101
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organo-tropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited-The role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Miftakhova R, Hedblom A, Semenas J,
Robinson B, Simoulis A, Malm J, Rizvanov A, Heery DM, Mongan NP,
Maitland NJ, et al: Cyclin A1 and P450 aromatase promote metastatic
homing and growth of stem-like prostate cancer cells in the bone
marrow. Cancer Res. 76:2453–2464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shiozawa Y, Berry JE, Eber MR, Jung Y,
Yumoto K, Cackowski FC, Yoon HJ, Parsana P, Mehra R, Wang J, et al:
The marrow niche controls the cancer stem cell phenotype of
disseminated prostate cancer. Oncotarget. 7:41217–41232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sharma S, Xing F, Liu Y, Wu K, Said N,
Pochampally R, Shiozawa Y, Lin HK, Balaji KC and Watabe K: Secreted
protein acidic and rich in cysteine (SPARC) mediates metastatic
dormancy of prostate cancer in the bone. J Biol Chem.
291:19351–19363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jin J, Dayyani F and Gallick G: Steps in
prostate cancer progression that lead to bone metastasis. Int J
Cancer. 128:2545–2561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Peyruchaud O, Leblanc R and David M:
Pleiotropic activity of lysophosphatidic acid in bone metastasis.
Biochim Biophys Acta. 1831:99–104. 2013. View Article : Google Scholar
|
|
108
|
Roodman GD: Genes associate with abnormal
bone cell activity in bone metastasis. Cancer Metastasis Rev.
31:569–578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang T and Armstrong AJ: Clinical utility
of circulating tumor cells in advanced prostate cancer. Curr Oncol
Rep. 18:32016. View Article : Google Scholar
|
|
110
|
Barriere G, Fici P, Gallerani G, Fabbri F,
Zoli W and Rigaud M: Circulating tumor cells and epithelial,
mesenchymal and stemness markers: Characterization of cell
subpopulations. Ann Transl Med. 2:1092014.PubMed/NCBI
|
|
111
|
Vogelzang NJ, Fizazi K, Burke JM, De Wit
R, Bellmunt J, Hutson TE, Crane E, Berry WR, Doner K, Hainsworth
JD, et al: Circulating tumor cells in a phase 3 study of docetaxel
and prednisone with or without lenalidomide in metastatic
Castration-resistant prostate cancer. Eur Urol. 71:168–171. 2017.
View Article : Google Scholar
|
|
112
|
Srivatsa N, Nagaraja H, Shweta S and
Raghunath S: Radical prostatectomy for locally advanced prostate
cancers-review of literature. Indian J Surg Oncol. 8:175–180. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wilt T, Brawe M, Jones K, Barry MJ,
Aronson WJ, Fox S, Gingrich JR, Wei JT, Gilhooly P, Grob BM, et al:
Radical prostatectomy versus observation for localized prostate
cancer. N Engl J Med. 367:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Celià-Terrassa T, Meca-Cortés Ó, Mateo F,
Martínez de Paz A, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz
A, Guerra-Rebollo M, et al: Epithelial-mesenchymal transition can
suppress major attributes of human epithelial tumor-initiating
cells. J Clin Invest. 122:1846–1868. 2012. View Article : Google Scholar
|
|
115
|
López-Moncada F, Torres MJ, Castellón EA
and Contreras HR: Secreted protein acidic and rich in cysteine
(SPARC) induces epithelial-mesenchymal transition, enhancing
migration and invasion, and is associated with high Gleason score
in prostate cancer. Asian J Androl. 21:557–564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gunasinghe NP, Wells A, Thompson EW and
Hugo HJ: Mesenchymal-epithelial transition (MET) as a mechanism for
metastatic colonisation in breast cancer. Cancer Metastasis Rev.
31:469–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bullock MD, Sayan AE, Packham GK and
Mirnezami AH: MicroRNAs: Critical regulators of epithelial to
mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in
cancer progression. Biol Cell. 104:3–12. 2012. View Article : Google Scholar
|
|
118
|
Bocci F, Gearhart-Serna L, Boareto M,
Ribeiro M, Ben-Jacob E, Devi GR, Levine H, Onuchic JN and Jolly MK:
Toward understanding cancer stem cell heterogeneity in the tumor
microenvironment. Proc Natl Acad Sci USA. 116:148–157. 2019.
View Article : Google Scholar
|
|
119
|
Harris JE, Shin J, Lee B, Pelosky K,
Hooker CM, Harbom K, Hulbert A, Zahnow C, Yang SC, Baylin S, et al:
A murine xenograft model of spontaneous metastases of human lung
adenocarcinoma. J Surg Res. 171:e75–e79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Rea D, Del Vecchio V, Palma G, Barbieri A,
Falco M, Luciano A, De Biase D, Perdonà S, Facchini G and Arra C:
Mouse models in prostate cancer translational research: From
Xenograft to PDX. Biomed Res Int. 2016:112016. View Article : Google Scholar
|
|
121
|
Daphu I, Sundstrøm T, Horn S, Huszthy PC,
Niclou SP, Sakariassen PØ, Immervoll H, Miletic H, Bjerkvig R and
Thorsen F: In vivo animal models for studying brain metastasis:
Value and limitations. Clin Exp Metastasis. 30:695–610. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Romano G, Chagani S and Kwong LN: The path
to metastatic mouse models of colorectal cancer. Oncogene.
37:2481–2489. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kahn J, Tofilon PJ and Camphausen K:
Preclinical models in radiation oncology. Radiat Oncol. 7:2232012.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Loi M, Di Paolo D, Becherini P, Zorzoli A,
Perri P, Carosio R, Cilli M, Ribatti D, Brignole C, Pagnan G, et
al: The use of orthotopic models to validate antivascular therapies
for cancer. Int J Dev Biol. 55:547–555. 2011. View Article : Google Scholar
|
|
125
|
Grabowska MM, Degraff DJ, Yu X, Jin RJ,
Chen Z, Borowsky AD and Matusik RJ: Mouse models of prostate
cancer: Picking the best model for the question. Cancer Metastasis
Rev. 33:377–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Usary J, Zhao W, Darr D, Roberts PJ, Liu
M, Balletta L, Karginova O, Jordan J, Combest A, Bridges A, et al:
Predicting drug responsiveness in human cancers using genetically
engineered mice. Clin Cancer Res. 19:4889–4899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bastide C, Bagnis C, Mannoni P, Hassoun J
and Bladou F: A Nod Scid mouse model to study human prostate
cancer. Prostate Cancer Prostatic Dis. 5:311–315. 2002. View Article : Google Scholar
|
|
128
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Dai J, Hensel J, Wang N, Kruithof-de Julio
M and Shiozawa Y: Mouse models for studying prostate cancer bone
metastasis. Bonekey Rep. 5:7772016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tumati V, Mathur S, Song K, Hsieh JT, Zhao
D, Takahashi M, Dobin T, Gandee L, Solberg TD, Habib AA and Saha D:
Development of a locally advanced orthotopic prostate tumor model
in rats for assessment of combined modality therapy. Int J Oncol.
42:1613–1619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lee ST, Wong PF, He H, Hooper JD and
Mustafa MR: Alpha-tomatine attenuation of in vivo growth of
subcutaneous and orthotopic xenograft tumors of human prostate
carcinoma PC-3 cells is accompanied by inactivation of nuclear
factor-Kappa B signaling. PLoS One. 8:e577082013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang Y, Xue H, Cutz JC, Bayani J, Mawji
NR, Chen WG, Goetz LJ, Hayward SW, Sadar MD, Gilks CB, et al: An
orthotopic metastatic prostate cancer model in SCID mice via
grafting of a transplantable human prostate tumor line. Lab Invest.
85:1392–1404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cifuentes FF, Valenzuela RH, Contreras HR
and Castellón EA: Development of an orthotopic model of human
metastatic prostate cancer in the NOD-SCIDγ mouse (Mus musculus)
anterior prostate. Oncol Lett. 10:2142–2148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cifuentes FF, Valenzuela RH, Contreras HR
and Castellón EA: Surgical cytoreduction of the primary tumor
reduces metastatic progression in a mouse model of prostate cancer.
Oncol Rep. 34:2837–2844. 2015. View Article : Google Scholar : PubMed/NCBI
|