|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodgers L, Peer CJ and Figg WD: Diagnosis,
staging and risk stratification in prostate cancer: Utilizing
diagnostic tools to avoid unnecessary therapies and side effects.
Cancer Biol Ther. 18:470–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah RB and Zhou M: Recent advances in
prostate cancer pathology: Gleason grading and beyond. Pathol Int.
66:260–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heijnsdijk EAM, Bangma CH, Borràs JM, de
Carvalho TM, Castells X, Eklund M, Espinàs JA, Graefen M, Grönberg
H, Lansdorp-Vogelaar I, et al: Summary statement on screening for
prostate cancer in Europe. Int J Cancer. 142:741–746. 2018.
View Article : Google Scholar
|
|
5
|
Litwin MS and Tan HJ: The diagnosis and
treatment of prostate cancer: A review. J Am Med Assoc.
317:2532–2542. 2017. View Article : Google Scholar
|
|
6
|
Ost P, Bossi A, Decaestecker K, De
Meerleer G, Giannarini G, Karnes RJ, Roach M III and Briganti A:
Metastasis-directed therapy of regional and distant recurrences
after curative treatment of prostate cancer: A systematic review of
the literature. Eur Urol. 67:852–863. 2015. View Article : Google Scholar
|
|
7
|
Fakhrejahani F, Madan RA and Dahut WL:
Management options for biochemically recurrent prostate cancer.
Curr Treat Options Oncol. 18:262017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artibani W, Porcaro AB, De Marco V,
Cerruto MA and Siracusano S: Management of biochemical recurrence
after primary curative treatment for prostate cancer: A review.
Urol Int. 100:251–262. 2018. View Article : Google Scholar
|
|
9
|
Sartor O and de Bono JS: Metastatic
prostate cancer. N Engl J Med. 378:645–657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song C, Kang T, Yoo S, Jeong IG, Ro JY,
Hong JH, Kim CS and Ahn H: Tumor volume, surgical margin, and the
risk of biochemical recurrence in men with organ-confined prostate
cancer. Urol Oncol. 31:168–174. 2013. View Article : Google Scholar
|
|
11
|
Suzman DL, Boikos SA and Carducci MA:
Bone-targeting agents in prostate cancer. Cancer Metastasis Rev.
33:619–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong L, Zieren RC, Xue W, de Reijke TM and
Pienta KJ: Metastatic prostate cancer remains incurable, why? Asian
J Urol. 6:26–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pelekanou V and Castanas E: Androgen
control in prostate cancer. J Cell Biochem. 2234:2224–2234. 2016.
View Article : Google Scholar
|
|
14
|
Tan MH, Li J, Xu HE, Melcher K and Yong
EL: Androgen receptor: Structure, role in prostate cancer and drug
discovery. Acta Pharmacol Sin. 36:3–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita K and Nonomura N: Role of androgen
receptor in prostate cancer: A review. World J Mens Health.
36:288–295. 2018.
|
|
16
|
Rodriguez KM, Pastuszak AW and Khera M:
The role of testosterone therapy in the setting of prostate cancer.
Curr Urol Rep. 19:672018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Obinata D, Takayama K, Takahashi S and
Inoue S: Crosstalk of the androgen receptor with transcriptional
collaborators: Potential therapeutic targets for
castration-resistant prostate cancer. Cancers (Basel). 9:pii: E22.
2017. View Article : Google Scholar
|
|
18
|
Grossmann M, Cheung AS and Zajac JD:
Androgens and prostate cancer; pathogenesis and deprivation
therapy. Best Pract Res Clin Endocrinol Metab. 27:603–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hahn AW, Hale P, Rathi N and Agarwal N:
Novel androgen axis systemic therapies for metastatic
hormone-sensitive prostate cancer. Curr Opin Urol. 27:559–565.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shore ND, Abrahamsson P, Anderson J,
Crawford ED and Lange P: New considerations for ADT in advanced
prostate cancer and the emerging role of GnRH antagonists. Prostate
Cancer Prostatic Dis. 16:7–15. 2013. View Article : Google Scholar
|
|
21
|
Lama G, Papi M, Angelucci C, Maulucci G,
Sica G and De Spirito M: Leuprorelin acetate long-lasting effects
on GnRH receptors of prostate cancer cells: An atomic force
microscopy study of agonist/receptor interaction. PLoS One.
8:e525302013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas BC and Neal DE: Androgen
deprivation treatment in prostate cancer. BMJ. 346:1–5. 2013.
View Article : Google Scholar
|
|
23
|
Katsogiannou M, Ziouziou H, Karaki S,
Andrieu C, Henry de Villeneuve M and Rocchi P: The hallmarks of
castration-resistant prostate cancers. Cancer Treat Rev.
41:588–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan X, Cai C, Chen S, Chen S, Yu Z and
Balk SP: Androgen receptor functions in castration-resistant
prostate cancer and mechanisms of resistance to new agents
targeting the androgen axis. Oncogene. 33:2815–2825. 2014.
View Article : Google Scholar
|
|
25
|
Fujimoto N: Role of the androgen-androgen
receptor axis in the treatment resistance of advanced prostate
cancer: From androgen-dependent to castration resistant and
further. J UOEH. 38:129–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chandrasekar T, Yang JC, Gao AC and Evans
CP: Mechanisms of resistance in castration-resistant prostate
cancer (CRPC). Transl Androl Urol. 4:365–380. 2015.
|
|
27
|
Tilki D, Schaeffer EM and Evans CP:
Understanding mechanisms of resistance in metastatic
castration-resistant prostate cancer: The role of the androgen
receptor. Eur Urol Focus. 2:499–505. 2019. View Article : Google Scholar
|
|
28
|
Huang Y, Jiang X, Liang X and Jiang G:
Molecular and cellular mechanisms of castration resistant prostate
cancer. Oncol Lett. 15:6063–6076. 2018.PubMed/NCBI
|
|
29
|
Ho Y and Dehm SM: Androgen receptor
rearrangement and splicing variants in resistance to endocrine
therapies in prostate cancer. Endocrinology. 158:1533–1542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Recouvreux MV, Wu JB, Gao AC, Zonis S,
Chesnokova V, Bhowmick N, Chung LW and Melmed S: Androgen receptor
regulation of local growth hormone in prostate cancer cells.
Endocrinology. 158:2255–2568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stelloo S, Nevedomskaya E, van der Poel
HG, de Jong J, van Leenders GJ, Jenster G, Wessels LF, Bergman AM
and Zwart W: Androgen receptor profiling predicts prostate cancer
outcome. EMBO Mol Med. 7:1450–1464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Höti N, Shah P, Hu Y, Yang S and Zhang H:
Proteomics analyses of prostate cancer cells reveal cellular
pathways associated with androgen resistance. Proteomics. 17:2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Den Eeden SK, Lu R, Zhang N,
Quesenberry CP Jr, Shan J, Han JS, Tsiatis AC, Leimpeter AD,
Lawrence HJ, Febbo PG and Presti JC: A Biopsy-based 17-gene genomic
prostate score as a predictor of metastases and prostate cancer
death in surgically treated men with clinically localized disease.
Eur Urol. 73:129–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hasegawa S, Nagano H, Konno M, Eguchi H,
Tomokuni A, Tomimaru Y, Asaoka T, Wada H, Hama N, Kawamoto K, et
al: A crucial epithelial to mesenchymal transition regulator,
Sox4/Ezh2 axis is closely related to the clinical outcome in
pancreatic cancer patients. Int J Oncol. 48:145–152. 2016.
View Article : Google Scholar
|
|
37
|
Frisch SM, Schaller M and Cieply B:
Mechanisms that link the oncogenic epithelial-mesenchymal
transition to suppression of anoikis. J Cell Sci. 126:21–29. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mateo F, Meca-Cortés O, Celià-Terrassa T,
Fernández Y, Abasolo I, Sánchez-Cid L, Bermudo R, Sagasta A,
Rodríguez-Carunchio L, Pons M, et al: SPARC mediates metastatic
cooperation between CSC and non-CSC prostate cancer cell
subpopulations. Mol Cancer. 13:2372014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin KC, Torga G, Sun Y, Axelrod R, Pienta
KJ, Sturm JC and Austin RH: The role of heterogeneous environment
and docetaxel gradient in the emergence of polyploid, mesenchymal
and resistant prostate cancer cells. Clin Exp Metastasis.
36:97–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bakker B, Taudt A, Belderbos ME, Porubsky
D, Spierings DC, de Jong TV, Halsema N, Kazemier HG,
Hoekstra-Wakker K, Bradley A, et al: Single-cell sequencing reveals
karyotype heterogeneity in murine and human malignancies. Genome
Biol. 17:1152016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chapman MP, Risom T, Aswani AJ, Langer EM,
Sears RC and Tomlin CJ: Modeling differentiation-state transitions
linked to therapeutic escape in triple-negative breast cancer. PLoS
Comput Biol. 15:e10068402019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eun K, Ham SW and Kim H: Cancer stem cell
heterogeneity: Origin and new perspectives on CSC targeting. BMB
Rep. 50:117–125. 2017. View Article : Google Scholar :
|
|
44
|
Adamowicz J, Pakravan K, Bakhshinejad B,
Drewa T and Babashah S: Prostate cancer stem cells: From theory to
practice. Scand J Urol. 51:95–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shahriari K, Shen F, Worrede-Mahdi A, Liu
Q, Gong Y, Garcia FU and Fatatis A: Cooperation among heterogeneous
prostate cancer cells in the bone metastatic niche. Oncogene.
36:2846–2856. 2017. View Article : Google Scholar :
|
|
46
|
Chang L, Graham P, Hao J, Ni J, Deng J,
Bucci J, Malouf D, Gillatt D and Li Y: Cancer stem cells and
signaling pathways in radioresistance. Oncotarget. 7:11002–11017.
2016.
|
|
47
|
Geng SQ, Alexandrou AT and Li JJ: Breast
cancer stem cells: Multiple capacities in tumor metastasis. Cancer
Lett. 349:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leão R, Domingos C, Figueiredo A, Hamilton
R, Tabori U and Castelo-Branco P: Cancer stem cells in prostate
cancer: Implications for targeted therapy. Urol Int. 99:125–136.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rosario DJ, Davey P, Green J, Greene D,
Turner B, Payne H and Kirby M: The role of gonadotrophin-releasing
hormone antagonists in the treatment of patients with advanced
hormone-dependent prostate cancer in the UK. World J Urol.
34:1601–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Poelaert F, Kumps C, Lumen N, Verschuere
S, Libbrecht L, Praet M, Rottey S, Claeys T, Ost P, Decaestecker K,
et al: Androgen receptor gene copy number and protein expression in
treatment-naïve prostate cancer. Urol Int. 99:222–228. 2017.
View Article : Google Scholar
|
|
51
|
Prekovic S, van Royen ME, Voet AR, Geverts
B, Houtman R, Melchers D, Zhang KY, Van den Broeck T, Smeets E,
Spans L, et al: The effect of F877L and T878A mutations on androgen
receptor response to enzalutamide. Mol Cancer Ther. 15:1702–1712.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sutinen P, Malinen M, Heikkinen S and
Palvimo JJ: SUMOylation modulates the transcriptional activity of
androgen receptor in a target gene and pathway selective manner.
Nucleic Acids Res. 42:8310–8319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rowlands MA, Holly JM, Hamdy F, Phillips
J, Goodwin L, Marsden G, Gunnell D, Donovan J, Neal DE and Martin
RM: Serum insulin-like growth factors and mortality in localised
and advanced clinically detected prostate cancer. Cancer Causes
Control. 23:347–354. 2012. View Article : Google Scholar
|
|
54
|
Lescarbeau RM, Seib FP, Prewitz M, Werner
C and Kaplan DL: In vitro model of metastasis to bone marrow
mediates prostate cancer castration resistant growth through
paracrine and extracellular matrix factors. PLoS One. 7:e403722012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Penning TM and Tamae D: Current advances
in intratumoral androgen metabolism in castration-resistant
prostate cancer. Curr Opin Endocrinol Diabetes Obes. 23:264–270.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Price DK, Chau CH, Till C, Goodman PJ,
Leach RJ, Johnson-Pais TL, Hsing AW, Hoque A, Parnes HL, Schenk JM,
et al: Association of androgen metabolism gene polymorphisms with
prostate cancer risk and androgen concentrations: Results from the
prostate cancer prevention trial. Cancer. 122:2332–2340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
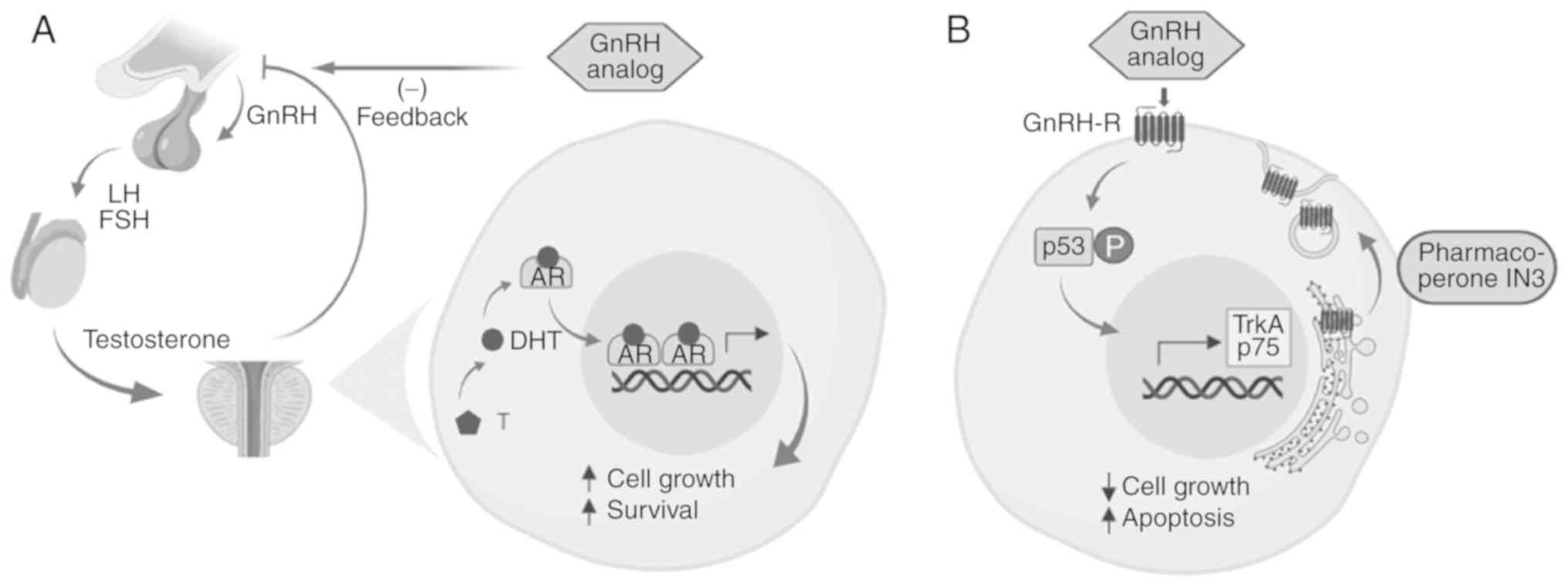
Clementi M, Sánchez C, Benitez DA,
Contreras HR, Huidobro C, Cabezas J, Acevedo C and Castellón EA:
Gonadotropin releasing hormone analogs induce apoptosis by
extrinsic pathway involving p53 phosphorylation in primary cell
cultures of human prostatic adenocarcinomas. Prostate.
69:1025–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sánchez C, Clementi M, Benitez D,
Contreras H, Huidobro C and Castellón E: Effect of GnRH analogs on
the expression of TrkA and p75 neurotrophin receptors in primary
cell cultures from human prostate adenocarcinoma. Prostate.
65:195–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Angelucci C, Lama G, Iacopino F, Ferracuti
S, Bono AV, Millar RP and Sica G: GnRH receptor expression in human
prostate cancer cells is affected by hormones and growth factors.
Endocrine. 36:87–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sánchez CA, Mercado AJ, Contreras HR,
Cabezas JC, Huidobro CC and Castellón EA: Pharmacoperone IN3
enhances the apoptotic effect of leuprolide in prostate cancer
cells by increasing the gonadotropin-releasing hormone receptor in
the cell membrane. Anticancer Drugs. 23:959–969. 2012.PubMed/NCBI
|
|
61
|
Castellón E, Clementi M, Hitschfeld C,
Sánchez C, Benítez D, Sáenz L, Contreras H and Huidobro C: Effect
of leuprolide and cetrorelix on cell growth, apoptosis, and GnRH
receptor expression in primary cell cultures from human prostate
carcinoma. Cancer Invest. 24:261–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Saltzstein D, Shore ND, Moul JW, Chu F,
Concepcion R, de la Motte S, McLane JA, Atkinson S, Yang A and
Crawford ED: Pharmacokinetic and pharmacodynamic comparison of
subcutaneous versus intramuscular leuprolide acetate formulations
in male subjects. Ther Adv Urol. 10:43–50. 2017. View Article : Google Scholar
|
|
63
|
Nieto MA and Cano A: The
epithelial-mesenchymal transition under control: Global programs to
regulate epithelial plasticity. Semin Cancer Biol. 22:361–368.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
García de Herreros A and Baulida J:
Cooperation, amplification, and feed-back in epithelial-mesenchymal
transition. Biochim Biophys Acta. 1825:223–228. 2012.PubMed/NCBI
|
|
65
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21(Suppl 7): vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial- mesen-chymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Osorio LA, Farfán NM, Castellón EA and
Contreras HR: SNAIL transcription factor increases the motility and
invasive capacity of prostate cancer cells. Mol Med Rep.
13:778–786. 2016. View Article : Google Scholar :
|
|
70
|
Orellana-Serradell O, Herrera D, Castellón
EA and Contreras HR: The transcription factor ZEB1 promotes an
aggressive phenotype in prostate cancer cell lines. Asian J Androl.
20:294–299. 2018. View Article : Google Scholar :
|
|
71
|
Contreras HR, Ledezma RA, Vergara J,
Cifuentes F, Barra C, Cabello P, Gallegos I, Morales B, Huidobro C
and Castellón EA: The expression of syndecan-1 and -2 is associated
with Gleason score and epithelial-mesenchymal transition markers,
E-cadherin and beta-catenin, in prostate cancer. Urol Oncol.
28:534–540. 2010. View Article : Google Scholar
|
|
72
|
Poblete CE, Fulla J, Gallardo M, Muñoz V,
Castellón EA, Gallegos I and Contreras HR: Increased SNAIL
expression and low syndecan levels are associated with high Gleason
grade in prostate cancer. Int J Oncol. 44:647–654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Farfán N, Ocarez N, Castellón EA, Mejía N,
de Herreros AG and Contreras HR: The transcriptional factor ZEB1
represses Syndecan 1 expression in prostate cancer. Sci Rep.
8:114672018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Montanari M, Rossetti S, Cavaliere C,
D'Aniello C, Malzone MG, Vanacore D, Di Franco R, La Mantia E,
Iovane G, Piscitelli R, et al: Epithelial-mesenchymal transition in
prostate cancer: An overview. Oncotarget. 8:35376–35389. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10699–10710. 2015. View Article : Google Scholar
|
|
76
|
Peitzsch C, Tyutyunnykova A, Pantel K and
Dubrovska A: Cancer stem cells: The root of tumor recurrence and
metastases. Semin Cancer Biol. 44:10–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ajani JA, Song S, Hochster HS and
Steinberg IB: Cancer stem cells: The promise and the potential.
Semin Oncol. 42(Suppl 1): S3–S17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Castellón EA, Valenzuela R, Lillo J,
Castillo V, Contreras HR, Gallegos I, Mercado A and Huidobro C:
Molecular signature of cancer stem cells isolated from prostate
carcinoma and expression of stem markers in different Gleason
grades and metastasis. Biol Res. 45:297–305. 2012. View Article : Google Scholar
|
|
79
|
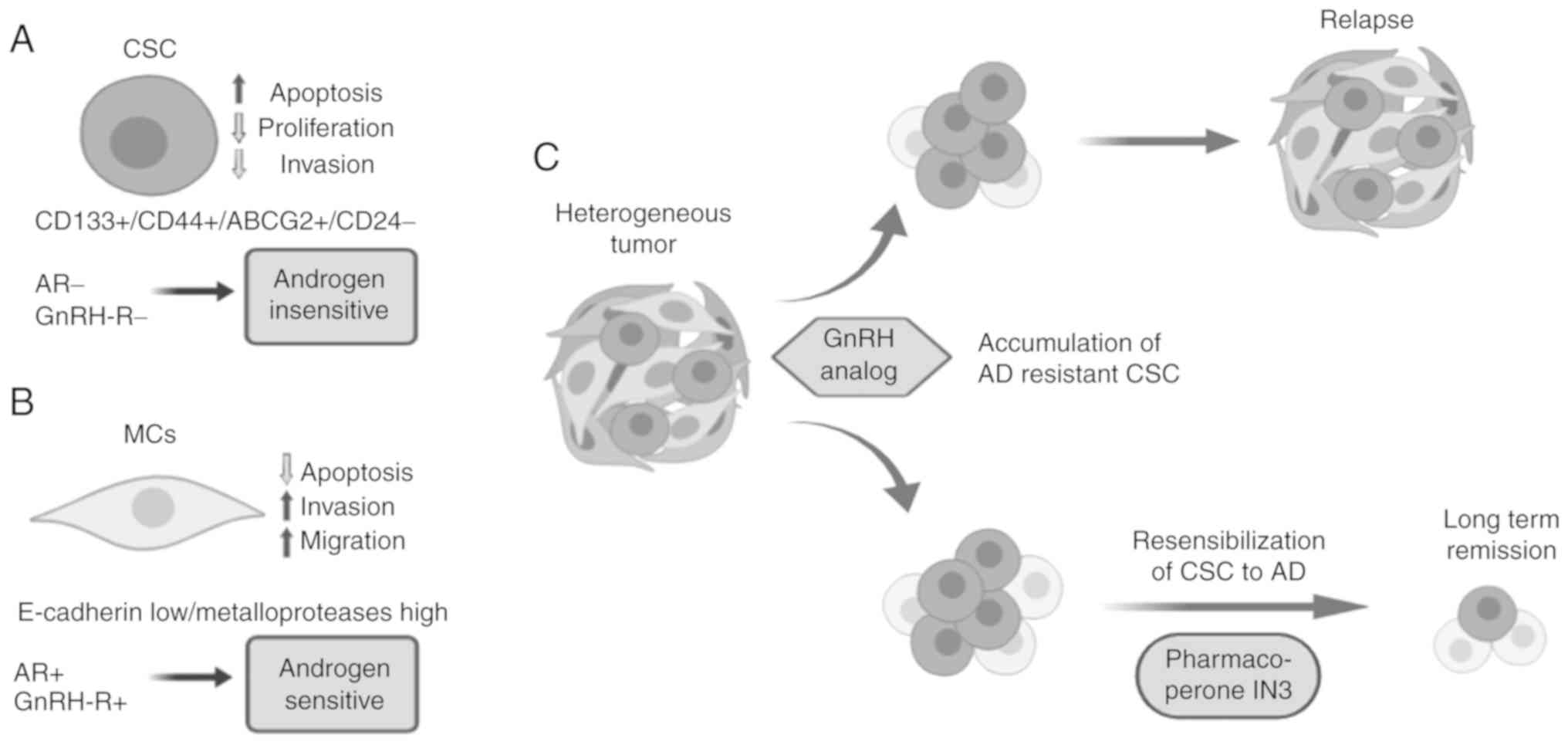
Castillo V, Valenzuela R, Huidobro C,
Contreras HR and Castellon EA: Functional characteristics of cancer
stem cells and their role in drug resistance of prostate cancer.
Int J Oncol. 45:985–994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
McGranahan N and Swanton C: Clonal
heterogeneity and tumor evolution: Past, present, and the future.
Cell. 168:613–628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bosman FT: Tumor heterogeneity: Will it
change what pathologists do. Pathobiology. 85:18–22. 2018.
View Article : Google Scholar
|
|
82
|
Jolly MK and Celià-Terrassa T: Dynamics of
phenotypic heterogeneity during EMT and stemness in cancer
progression. J Clin Med. 8:pii: E1542. 2019. View Article : Google Scholar
|
|
83
|
Bu Y and Cao D: The origin of cancer stem
cells. Front Biosci (Schol Ed). 4:819–830. 2012.
|
|
84
|
Parsons BL: Multiclonal tumor origin:
Evidence and implications. Mutat Res. 777:1–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Vicente-Dueñas C, Hauer J, Cobaleda C,
Borkhardt A and Sánchez-García I: Epigenetic priming in cancer
initiation. Trends Cancer. 4:408–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ye X and Weinberg RA:
Epithelial-mesenchymal plasticity: A central regulator of cancer
progression. Trends Cell Biol. 25:675–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Graham TA and Sottoriva A: Measuring
cancer evolution from the genome. J Pathol. 241:183–191. 2017.
View Article : Google Scholar
|
|
88
|
Francart M, Lambert J, Vanwynsberghe AM,
Thompson EW, Bourcy M, Polette M and Gilles C:
Epithelial-mesenchymal plasticity and circulating tumor cells:
Travel companions to metastases. Dev Dyn. 247:432–450. 2018.
View Article : Google Scholar
|
|
89
|
Carnero A and Lleonart M: The hypoxic
microenvironment: A determinant of cancer stem cell evolution.
Bioessays. 38(Suppl 1): S65–S74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yeo CD, Kang N, Choi SY, Kim BN, Park CK,
Kim JW, Kim YK and Kim SJ: The role of hypoxia on the acquisition
of epithelial-mesenchymal transition and cancer stemness: A
possible link to epigenetic regulation. Korean J Intern Med.
32:589–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Rhim AD: Epithelial to mesenchymal
transition and the generation of stem-like cells in pancreatic
cancer. Pancreatology. 13:114–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lan L, Luo Y, Cui D, Shi BY, Deng W, Huo
LL, Chen HL, Zhang GY and Deng LL: Epithelial-mesenchymal
transition triggers cancer stem cell generation in human thyroid
cancer cells. Int J Oncol. 43:113–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li N, Babaei-Jadidi R, Lorenzi F,
Spencer-Dene B, Clarke P, Domingo E, Tulchinsky E, Vries RGJ, Kerr
D, Pan Y, et al: An FBXW7-ZEB2 axis links EMT and tumour
microenvironment to promote colorectal cancer stem cells and
chemoresistance. Oncogenesis. 8:132019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Croker AK and Allan AL: Cancer stem cells:
Implications for the progression and treatment of metastatic
disease. J Cell Mol Med. 12:374–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Massagué J and Obenauf AC: Metastatic
colonization by circulating tumour cells. Nature. 529:298–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hayashida T, Jinno H, Kitagawa Y and
Kitajima M: Cooperation of cancer stem cell properties and
epithelial-mesenchymal transition in the establishment of breast
cancer metastasis. J Oncol. 2011:5914272011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hsu CL, Chung FH, Chen CH, Hsu TT, Liu SM,
Chung DS, Hsu YF, Chen CL, Ma N and Lee HC: Genotypes of cancer
stem cells characterized by epithelial-to-mesenchymal transition
and proliferation related functions. Sci Rep. 6:325232016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yun EJ, Lo UG and Hsieh JT: The evolving
landscape of prostate cancer stem cell: Therapeutic implications
and future challenges. Asian J Urol. 3:203–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lin CJ, Lo UG and Hsieh JT: The regulatory
pathways leading to stem-like cells underlie prostate cancer
progression. Asian J Androl. 21:233–240. 2019. View Article : Google Scholar :
|
|
100
|
Sánchez CA, Andahur EI, Valenzuela R,
Castellón EA, Fullá JA, Ramos CG and Triviño JC: Exosomes from bulk
and stem cells from human prostate cancer have a differential
microRNA content that contributes cooperatively over local and
pre-metastatic niche. Oncotarget. 7:3993–4008. 2016. View Article : Google Scholar :
|
|
101
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organo-tropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited-The role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Miftakhova R, Hedblom A, Semenas J,
Robinson B, Simoulis A, Malm J, Rizvanov A, Heery DM, Mongan NP,
Maitland NJ, et al: Cyclin A1 and P450 aromatase promote metastatic
homing and growth of stem-like prostate cancer cells in the bone
marrow. Cancer Res. 76:2453–2464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shiozawa Y, Berry JE, Eber MR, Jung Y,
Yumoto K, Cackowski FC, Yoon HJ, Parsana P, Mehra R, Wang J, et al:
The marrow niche controls the cancer stem cell phenotype of
disseminated prostate cancer. Oncotarget. 7:41217–41232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sharma S, Xing F, Liu Y, Wu K, Said N,
Pochampally R, Shiozawa Y, Lin HK, Balaji KC and Watabe K: Secreted
protein acidic and rich in cysteine (SPARC) mediates metastatic
dormancy of prostate cancer in the bone. J Biol Chem.
291:19351–19363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Jin J, Dayyani F and Gallick G: Steps in
prostate cancer progression that lead to bone metastasis. Int J
Cancer. 128:2545–2561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Peyruchaud O, Leblanc R and David M:
Pleiotropic activity of lysophosphatidic acid in bone metastasis.
Biochim Biophys Acta. 1831:99–104. 2013. View Article : Google Scholar
|
|
108
|
Roodman GD: Genes associate with abnormal
bone cell activity in bone metastasis. Cancer Metastasis Rev.
31:569–578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang T and Armstrong AJ: Clinical utility
of circulating tumor cells in advanced prostate cancer. Curr Oncol
Rep. 18:32016. View Article : Google Scholar
|
|
110
|
Barriere G, Fici P, Gallerani G, Fabbri F,
Zoli W and Rigaud M: Circulating tumor cells and epithelial,
mesenchymal and stemness markers: Characterization of cell
subpopulations. Ann Transl Med. 2:1092014.PubMed/NCBI
|
|
111
|
Vogelzang NJ, Fizazi K, Burke JM, De Wit
R, Bellmunt J, Hutson TE, Crane E, Berry WR, Doner K, Hainsworth
JD, et al: Circulating tumor cells in a phase 3 study of docetaxel
and prednisone with or without lenalidomide in metastatic
Castration-resistant prostate cancer. Eur Urol. 71:168–171. 2017.
View Article : Google Scholar
|
|
112
|
Srivatsa N, Nagaraja H, Shweta S and
Raghunath S: Radical prostatectomy for locally advanced prostate
cancers-review of literature. Indian J Surg Oncol. 8:175–180. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wilt T, Brawe M, Jones K, Barry MJ,
Aronson WJ, Fox S, Gingrich JR, Wei JT, Gilhooly P, Grob BM, et al:
Radical prostatectomy versus observation for localized prostate
cancer. N Engl J Med. 367:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Celià-Terrassa T, Meca-Cortés Ó, Mateo F,
Martínez de Paz A, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz
A, Guerra-Rebollo M, et al: Epithelial-mesenchymal transition can
suppress major attributes of human epithelial tumor-initiating
cells. J Clin Invest. 122:1846–1868. 2012. View Article : Google Scholar
|
|
115
|
López-Moncada F, Torres MJ, Castellón EA
and Contreras HR: Secreted protein acidic and rich in cysteine
(SPARC) induces epithelial-mesenchymal transition, enhancing
migration and invasion, and is associated with high Gleason score
in prostate cancer. Asian J Androl. 21:557–564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gunasinghe NP, Wells A, Thompson EW and
Hugo HJ: Mesenchymal-epithelial transition (MET) as a mechanism for
metastatic colonisation in breast cancer. Cancer Metastasis Rev.
31:469–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bullock MD, Sayan AE, Packham GK and
Mirnezami AH: MicroRNAs: Critical regulators of epithelial to
mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in
cancer progression. Biol Cell. 104:3–12. 2012. View Article : Google Scholar
|
|
118
|
Bocci F, Gearhart-Serna L, Boareto M,
Ribeiro M, Ben-Jacob E, Devi GR, Levine H, Onuchic JN and Jolly MK:
Toward understanding cancer stem cell heterogeneity in the tumor
microenvironment. Proc Natl Acad Sci USA. 116:148–157. 2019.
View Article : Google Scholar
|
|
119
|
Harris JE, Shin J, Lee B, Pelosky K,
Hooker CM, Harbom K, Hulbert A, Zahnow C, Yang SC, Baylin S, et al:
A murine xenograft model of spontaneous metastases of human lung
adenocarcinoma. J Surg Res. 171:e75–e79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Rea D, Del Vecchio V, Palma G, Barbieri A,
Falco M, Luciano A, De Biase D, Perdonà S, Facchini G and Arra C:
Mouse models in prostate cancer translational research: From
Xenograft to PDX. Biomed Res Int. 2016:112016. View Article : Google Scholar
|
|
121
|
Daphu I, Sundstrøm T, Horn S, Huszthy PC,
Niclou SP, Sakariassen PØ, Immervoll H, Miletic H, Bjerkvig R and
Thorsen F: In vivo animal models for studying brain metastasis:
Value and limitations. Clin Exp Metastasis. 30:695–610. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Romano G, Chagani S and Kwong LN: The path
to metastatic mouse models of colorectal cancer. Oncogene.
37:2481–2489. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kahn J, Tofilon PJ and Camphausen K:
Preclinical models in radiation oncology. Radiat Oncol. 7:2232012.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Loi M, Di Paolo D, Becherini P, Zorzoli A,
Perri P, Carosio R, Cilli M, Ribatti D, Brignole C, Pagnan G, et
al: The use of orthotopic models to validate antivascular therapies
for cancer. Int J Dev Biol. 55:547–555. 2011. View Article : Google Scholar
|
|
125
|
Grabowska MM, Degraff DJ, Yu X, Jin RJ,
Chen Z, Borowsky AD and Matusik RJ: Mouse models of prostate
cancer: Picking the best model for the question. Cancer Metastasis
Rev. 33:377–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Usary J, Zhao W, Darr D, Roberts PJ, Liu
M, Balletta L, Karginova O, Jordan J, Combest A, Bridges A, et al:
Predicting drug responsiveness in human cancers using genetically
engineered mice. Clin Cancer Res. 19:4889–4899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bastide C, Bagnis C, Mannoni P, Hassoun J
and Bladou F: A Nod Scid mouse model to study human prostate
cancer. Prostate Cancer Prostatic Dis. 5:311–315. 2002. View Article : Google Scholar
|
|
128
|
Hidalgo M, Amant F, Biankin AV, Budinská
E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo
GM, et al: Patient derived xenograft models: An emerging platform
for translational cancer research. Cancer Discov. 4:998–1013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Dai J, Hensel J, Wang N, Kruithof-de Julio
M and Shiozawa Y: Mouse models for studying prostate cancer bone
metastasis. Bonekey Rep. 5:7772016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tumati V, Mathur S, Song K, Hsieh JT, Zhao
D, Takahashi M, Dobin T, Gandee L, Solberg TD, Habib AA and Saha D:
Development of a locally advanced orthotopic prostate tumor model
in rats for assessment of combined modality therapy. Int J Oncol.
42:1613–1619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Lee ST, Wong PF, He H, Hooper JD and
Mustafa MR: Alpha-tomatine attenuation of in vivo growth of
subcutaneous and orthotopic xenograft tumors of human prostate
carcinoma PC-3 cells is accompanied by inactivation of nuclear
factor-Kappa B signaling. PLoS One. 8:e577082013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang Y, Xue H, Cutz JC, Bayani J, Mawji
NR, Chen WG, Goetz LJ, Hayward SW, Sadar MD, Gilks CB, et al: An
orthotopic metastatic prostate cancer model in SCID mice via
grafting of a transplantable human prostate tumor line. Lab Invest.
85:1392–1404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cifuentes FF, Valenzuela RH, Contreras HR
and Castellón EA: Development of an orthotopic model of human
metastatic prostate cancer in the NOD-SCIDγ mouse (Mus musculus)
anterior prostate. Oncol Lett. 10:2142–2148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cifuentes FF, Valenzuela RH, Contreras HR
and Castellón EA: Surgical cytoreduction of the primary tumor
reduces metastatic progression in a mouse model of prostate cancer.
Oncol Rep. 34:2837–2844. 2015. View Article : Google Scholar : PubMed/NCBI
|