Introduction
Osteoradionecrosis (ORN) is the most dangerous
adverse clinical complication in patients with head and neck cancer
following treatment with radiotherapy (RT), with an incidence of
5-15% (1-4). Most patients with head and neck
cancer receive radiation therapy (5). Marx (6) reported that ORN occurs following bone
tissue hypoxia, hypocellularity and hypovascularity in the early
stage; however, the underlying mechanisms remain unclear. In the
last decades, studies demonstrated that radiation-induced fibrosis
promotes the development of ORN (7,8).
Fibrosis is a common feature of end-stage ORN (9) and is rarely reversible. However, Xu
et al (10) reported that
reducing local blood flow and subsequent hypovascularity could lead
to an imbalance in bone remodeling, suggesting that microvascular
damage has a major impact on ORN early stage. However, whether IR
directly causes bone cell death, triggers other factors that
suppress bone cell function or results in a combination of both
effects, remain unknown.
RT can induce the generation of high levels of
reactive oxygen species (ROS), leading to microvascular structure
necrosis, local ischemia and subsequent tissue loss (11). ROS overproduction also inhibits the
survival of tissue-borne multipotent stromal cells in numerous
tissues (12). In addition, Mazur
et al (13) reported that
irradiation (IR) can induce bone marrow cell apoptosis. Although
the underlying mechanisms of radiation injury caused by ROS have
been extensively studied, a valid medical therapy designed to
prevent the deleterious side effects of radiation in patients is
not currently available (14).
α2-macroglobulin (α2M) is an acute-phase protein
that exerts radioprotective effects (15,16).
Pretreatment of rats by total-body irradiation with α2M can
significantly reduce radiation-induced DNA damage and completely
restore liver function and body weight (17,18).
In addition, a previous study reported that the rat acute-phase α2M
protein serves a central role in amifostine-mediated
radioprotection, increasing the protective effect by 45-fold
(19). As demonstrated in a study
from our laboratory, α2M maintains the osteogenic potential of
human bone marrow mesenchymal stem cells (hBMMSCs) following IR
(20). In particular, hBMMSCs are
among the main cells damaged during bone tissue radiation (21,22).
Radiation can alter hBMMSC proliferation, induce genomic DNA damage
and micronucleus formation and inhibit the osteogenic
differentiation of hBMMSCs (23).
The radioprotective effect of α2M in the late stage
of ORN, with a notable effect on the fibrosis in bone marrow, has
been demonstrated in our previous study (24). However, this treatment could not
reverse the development of ORN disease process. The present study
aimed therefore to examine whether α2M could exert a
radioprotective effect on early-stage ORN in vivo and in
vitro, and to explore its underlying mechanisms.
Materials and methods
Establishment of the ORN animal
model
A total of 72 Sprague-Dawley male rats (12-week old,
~350 g) that were purchased from the Laboratory Animal Center of
Sun Yat-sen University, were randomly assigned into four groups as
follows: control group, α2M group, RT group and α2M + RT group
(n=18 animals per group). Following anesthesia with pentobarbital
(1%; 50 mg/kg, intraperitoneal injection), a subperiosteal
injection of 0.5 ml of 2 mg/ml α2M (cat. no. 441251-10MG; EMD
Millipore) was prophylactically administered to mice from the α2M
and α2M + RT groups 30 min prior to IR, whereas the control and RT
groups were injected with the same volume of physiological saline.
Following preoperative anesthesia induction, a single dose of 30 Gy
(voltage, 160 kV; current, 25 mA, 2.75 Gy/min, 10.91 min; Rad
Source Technologies, Inc.) was delivered to the left mandible of
mice from the RT and α2M + RT groups. Improved 3D-printed mandible
fixtures and a lead sheet were used to precisely deliver radiation
to the mandible and protect the surrounding tissues by shielding
them from IR. The dosage was based on an extensive review of the
literature (25,26) and previous work from our laboratory
(20). All rats were fed a liquid
diet during the first week after IR. The flow diagram for the
experiment is shown in Fig. SI.
Six rats in each group were sacrificed with pentobarbital (1%, 120
mg/kg, intraperitoneal injection) on day 7, 14 and 28 following IR.
Death verification included cardiac arrest, respiratory arrest and
disappearance of various reflexes. Unilateral mandible, tongue and
buccal mucosal tissue were excised and examined immediately. In
addition, humane endpoints included the following: 20-25% body
weight loss, complete loss of appetite for 24 h or loss of appetite
(<50% of normal) for 3 days; weakness (inability to eat or
drink); dying (mental depression accompanied by hypothermia without
anesthesia or sedation); rapid and abdominal breathing with
pronounced effort; and severe infection. Six rats in RT group and
three rats in α2M + RT group reached the humane and experimental
endpoints exactly on day 7 after irradiation. After euthanasia by
injection of pentobarbital (1%, 120 mg/kg), the observation time
was 5-10 min to make sure animal died under fearless and painless
condition. If no observation of cardiac and respiratory arrest over
10 min, an overdose of the drug would be given.
Histological staining and quantitative
bone histomorphometry
Mandible bone, tongue and buccal mucosa tissues were
collected from rats and immediately immersed in 4% formaldehyde at
room temperature for 24 h. Tongue and buccal mucosa tissues were
embedded into paraffin wax through dehydration and wax soaking. In
addition, all bone samples were subjected to decalcification.
Briefly, mandibular bone tissues were transferred from 4%
paraformaldehyde into the decalcifying solution (cat. no. G1105;
Servicebio) and sealed at 25-30°C, and the solution was replaced
every three days until the bone softened. All tissues were then
sliced into 0.5 mm sections. Sections were then subjected to
hematoxylin and eosin (H&E) and Masson's trichrome staining.
The samples were observed under a scanning electron microscope
(NanoZoomer S360; Japan SLC, Inc.). The region of interest (ROI)
was drawn from the first molar to the last molar, and both buccal
and lingual cortices were visualized down to the nadir of the
incisor root. With ×200 magnification, nine high-power-field (HPF)
images were randomly selected from different sections of each
specimen, and empty lacunae were counted by three independent
reviewers, as previously described (27). The number of fat vacuoles was
manually counted in the different images and reported as the mean
number per HPF image in the marrow cavity (28).
Tartrate-resistant acid phosphatase
(TRAP) staining and terminal uridine deoxynucleotidyl nick end
labeling (TUNEL) staining
Osteoclasts in the four groups (control group, α2M
group, RT group and α2M + RT group) were stained with a TRAP kit
(cat. no. G1050; Servicebio) according to the manufacturers'
instructions. TRAP-positive multinucleated cells containing three
or more nuclei were counted as osteoclasts under a microscope
(Leica DMi1; Leica Microsystems GmbH). TRAP-positive osteoclasts
were quantified with Image J software (version 1.8.0; National
Institutes of Health), in terms of osteoclast number related to
total bone surface (N. Oc/BS) (29).
Apoptotic cells in the four groups (control group,
α2M group, RT group and α2M + RT group) were quantified with a
TUNEL apoptosis detection kit (cat. no. 11684817910; Roche
Diagnostics) according to the manufacturers' instructions. Five
images were randomly selected from each specimen, and the numbers
of TUNEL-positive cells were counted in HPFs (magnification, ×400).
The apoptosis index (AI) was determined as follows: AI = (number of
TUNEL-positive cells/total number of counted cells) x 100%.
Image-Pro Plus software (version 6.0, Media Cybernetics, Inc.) was
used for automatic counting as previously described (30).
Immunohistochemistry of bone and soft
tissues
The 0.5-mm thick embedded sections of four groups
(control group, α2M group, RT group and α2M + RT group) were
dewaxed by microwave-heating at 60°C for 2 h and hydrated using
graded ethanol (100, 95, 90 and 80%) at room temperature for 5 min
each time. Antigen retrieval was completed at 98°C for 20 min
followed by quenching for 20 min. Sections were blocked with 1%
bovine serum albumin (cat. no. A8010; Beijing Solarbio Science
& Technology Co., Ltd.) dissolved in double distilled
H2O for 30 min at room temperature. The bone and tongue
sections were incubated with primary antibodies against 8-OHdG
(1:100; cat. no. ab26842; Abcam) whereas the buccal mucosa and
tongue tissues were incubated with primary antibody against SOD2
(1:100; cat. no. 24127-1-AP; ProteinTech Group, Inc.) in a wet box
at 4°C overnight. After washing twice in PBS for 5 min, sections
were incubated with goat anti-rabbit IgG (1:100; cat. no. GAR007;
Multi Sciences Biotech, Co., Ltd.) or goat anti-mouse IgG (1:100;
cat. no. GAM007; Multi Sciences Biotech, Co., Ltd.) at room
temperature for 30 min. Following incubation with DAB reagent (cat.
no. DA1010; Beijing Solarbio Science & Technology Co., Ltd.)
for 10 min at room temperature and counterstain in haematoxylin,
sections were mounted with neutral balsam (cat. no. G8590; Beijing
Solarbio Science & Technology Co., Ltd.). Images were captured
using a light microscopy (Leica Microsystems GmbH). The relative
optical density (ROD) was calculated using Image-Pro Plus software
as the ratio of the integrated optical density (IOD) to the
measured area, as previously described (31).
Cell proliferation and apoptotic
sensitivity of irradiated hBMMSCs
The primary hBMMSCs obtained from ScienCell Research
Laboratories, Inc. (cat. no. 7500) were cultured in Mesenchymal
Stem Cell Medium (cat. no. 7501; ScienCell Research Laboratories,
Inc.) supplemented with 1% mesen-chymal stem cell growth supplement
(ScienCell Research Laboratories, Inc.), 1% penicillin/streptomycin
solution (ScienCell Research Laboratories, Inc.) and 5% fetal
bovine serum (ScienCell Research Laboratories, Inc.) and placed in
a humidified 5% CO2 incubator at 37°C. Cells were seeded
in 96-well plates at a density of 5×103 cells/well (6
duplicate wells per group) and were divided into four groups:
Control group, no treatment; α2M group, 0.5 mg/ml α2M pretreatment
for 24 h with no irradiation; RT group, no α2M pretreatment +
irradiation with 8 Gy; and α2M + RT group, 0.5 mg/ml α2M
pretreatment for 24 h + one dose of 8 Gy dose irradiation (20). After cell treatment for 24, 48 and
72 h, 10 µl of the Cell Counting Kit-8 solution (CCK-8; cat.
no. C0038; Beyotime Institute of Biotechnology) was added to each
well. After incubation for 2 h at 37°C (5% CO2),
absorbance value was measured at a wavelength of 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Cell apoptosis was assessed with an Annexin
V-FITC/PI apoptosis detection kit (cat. no. KGA108-1; Nanjing
KeyGen Biotech Co., Ltd.) according to the manufacturers'
instructions. Briefly, hBMMSCs were seeded at the density of
2×105 cells/well in 6-well plates and incubated for 24
h, followed by 0.5 mg/ml α2M treatment 30 min before irradiation.
Treated cells were cultured for 24 h and harvested. Cells were
washed with cold PBS and resuspended in 100 µl of Annexin
V-FITC binding buffer. Next, 5 µl of Annexin V-FITC and 10
µl of PI Staining Solution were added to the samples and
incubated for 15 min in the dark at room temperature. Subsequently,
400 µl of 1X binding buffer was added, and cells were
analyzed using flow cytometry (Beckman Cytomic FC500; Beckman
Coulter). Data were analyzed using FlowJoTM software (v 10.6.2; BD
Biosciences)..
ROS detection in irradiated hBMMSCs
The hBMMSCs were cultured in 6-well plates at a
density of 2×105 cells/well in cell culture medium for
24 h before ROS detection. Cultured cells were divided into four
groups: control group; α2M group; RT group; and α2M + RT group).
ROS detection was performed with a ROS assay kit (cat. no. S0033;
Beyotime Institute of Biotechnology) according to the
manufacturers' instructions. Culture medium was removed and cells
were incubated with 10 µmol/l DCFH-DA solution diluted in
serum-free medium in the dark at 37°C for 20 min. Cells were washed
three times with serum-free medium to remove excess dye. ROS
intensity was observed through inverted fluorescence microscope
(Olympus IX73; Olympus Soft Imaging Solutions GmbH).
ROS level was also detected by flow cytometry. At 24
h after IR, hBMMSCs were harvested and washed three times with PBS.
DCFH-DA (cat. no. S0033; Beyotime Institute of Biotechnology) was
added to each group at a final concentration of 10 µmol/l
and incubated for 20 min at 37°C. Finally, the samples were washed
and assessed using flow cytometer (BD FACSCanto II; BD Biosciences)
and data were analyzed using FlowJo™ software (version 10.6.2; BD
Biosciences).
Western blotting analysis of Akt, HO-1
and SOD2 expression
The expression of Akt, heme oxygenase-1 (HO-1), and
SOD2 proteins was detected by western blotting. Briefly, cells were
lysed using RIPA lysis buffer (cat. no. P0013B; Beyotime Institute
of Biotechnology) for 15 min on ice and centrifuged at 12,000 rpm
for 15 min at 4°C. Protein concentration was measured using a BCA
assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.).
Proteins (50 µg) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes.
Membranes were blocked with 5% skimmed milk at room
temperature for 1 h and were incubated with primary antibodies
presented in Table SII at 4°C
overnight. Membranes were washed twice with TBST (cat. no. T1085;
Beijing Solarbio Science & Technology Co., Ltd.) for 5 min and
were incubated with secondary antibodies presented in Table SII at room temperature for 2 h.
Bands were detected with Immobilon western electrochemiluminescence
HRO substrate (cat. no. P36599, Millipore) using a
chemiluminescence equipment (Bio-Rad Laboratories, Inc.). The data
were analyzed via densitometry using ImageJ software (version
1.8.0; National Institutes of Health).
Statistical analysis
Data were analyzed using one-way ANOVA followed by
Bonferroni post hoc test for multiple comparisons. If the collected
data were not normally distributed, a Kruskal-Wallis ANOVA with
ranks was used. The results were analyzed using SPSS 20.0 software
(IBM Corp.), and all data with a normal distribution are presented
as the means ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
α2M alleviates alopecia and mucositis at
an early stage
Within 7 days and during the recovery phase, all IR
rats exhibited signs and symptoms of IR side effects, including
alopecia (Fig. 1A and B) and
inflammation of the buccal mucosa and tongue (Fig. 1C and D). In addition, as presented
in Fig. 1E, α2M-treated rats
presented a tendency to gain weight earlier and recovered faster
compared with rats in the RT group on days 7 and 14 after IR
(P<0.05). As presented in Fig.
1F, the food intake of all IR rats began to decrease suddenly
after IR and reached the lowest level on day 7 after IR, before
returning to normal until the end of the study period.
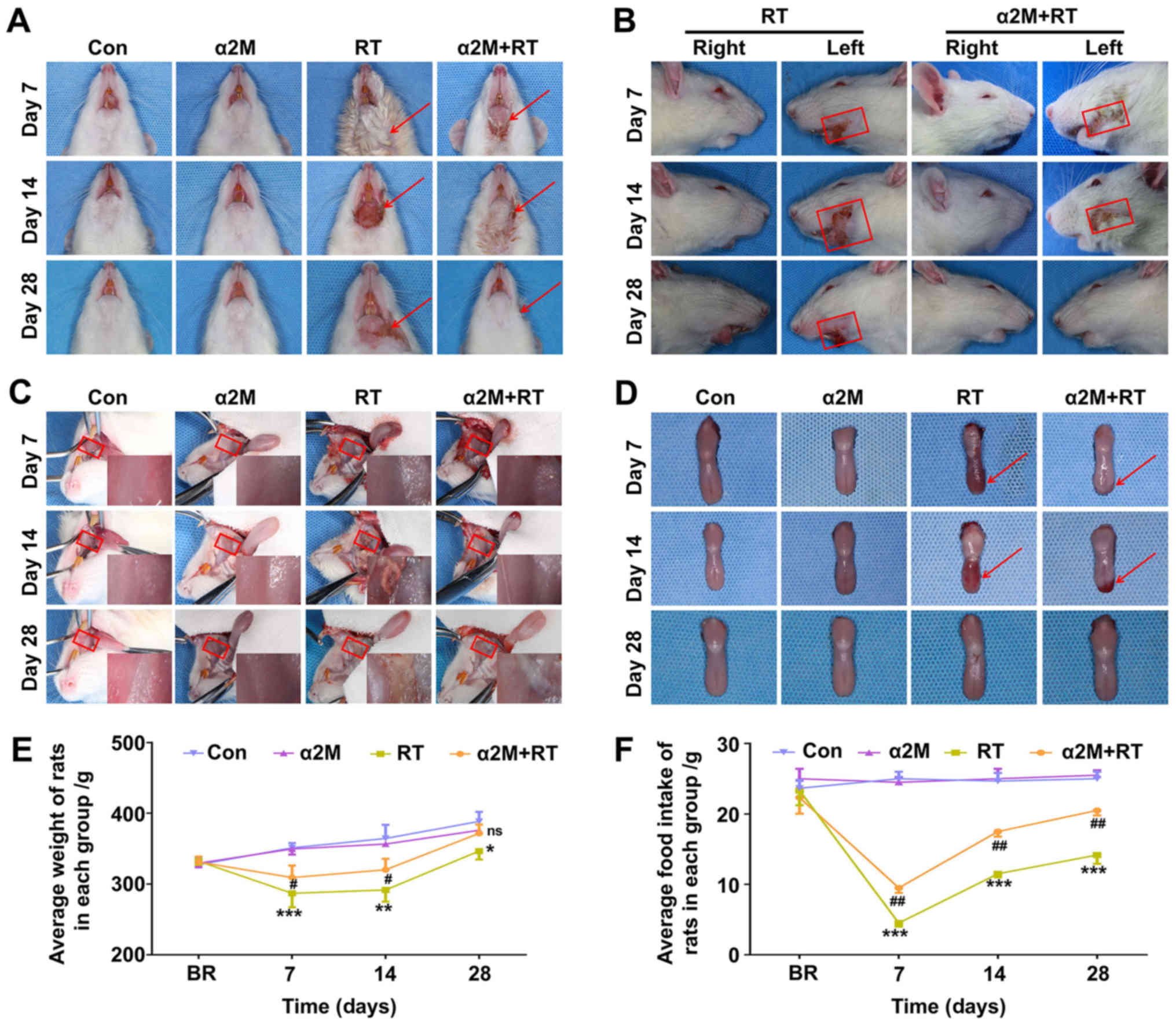 | Figure 1Radiation-induced injury and clinical
symptoms and quantitation of body weight and food intake in rats.
(A) RT group showed serious symptoms, including drooling, alopecia,
ulceration, exudation and superficial incrustation in the
irradiated area at the early stage of osteoradionecrosis. (B) α2M +
RT group exhibited mild damage and low levels of alopecia. Red
arrows in Fig. 1A and the
rectangles in Fig. 1B indicate
areas affected by alopecia. (C and D) Signs of irradiation are red
and swollen tongue with erosive lesions and atrophied mucosa in the
RT group, whereas conditions of the mucosa and tongue were
substantially better in the rats in the α2M + RT group compared
with rats in the RT group. Red arrows in Fig. 1D indicate the ulcerated areas. (E
and F) Weight and food intake of rats measured weekly.
*P<0.05, **P<0.01 and
***P<0.001 vs. control group. #P<0.05
and ##P<0.01 vs. RT group. n=6 per group at D7, D14
or D28. Con, control; D, day; ns, not significant; RT,
radiotherapy; α2M, α2-macroglobulin. |
α2M protect against IR-induced
pathophysiological changes induced in bone and soft tissues
Compared with the control group, the cell loss and
vascular obstruction in bone marrow was observed in the
pathological sections from the RT group. However, compared with the
RT group, the α2M + RT group presented a decreased cell loss and
vascular obstruction following IR (Fig. 2A). The number of empty bone lacunae
in the RT group was significantly increased compared with the
control group; however, the α2M + RT group showed lower bone cell
death within 28 days compared with the RT group (Fig. 2B). Results from Masson's trichrome
staining demonstrated no fibrosis in the bone marrow cavity in the
IR groups until day 28 (Fig. 2C).
In particular, a 1.5-fold increase in fat vacuoles was observed in
the marrow cavity of the RT group compared with the control group
(Fig. 2D). Mucosal inflammation
and ulcers were observed in the IR rats at the early stage but were
significantly decreased in the α2M + RT group compared with the RT
group in the buccal mucosa (Fig.
2E) and lingual mucosa (Fig.
2F).
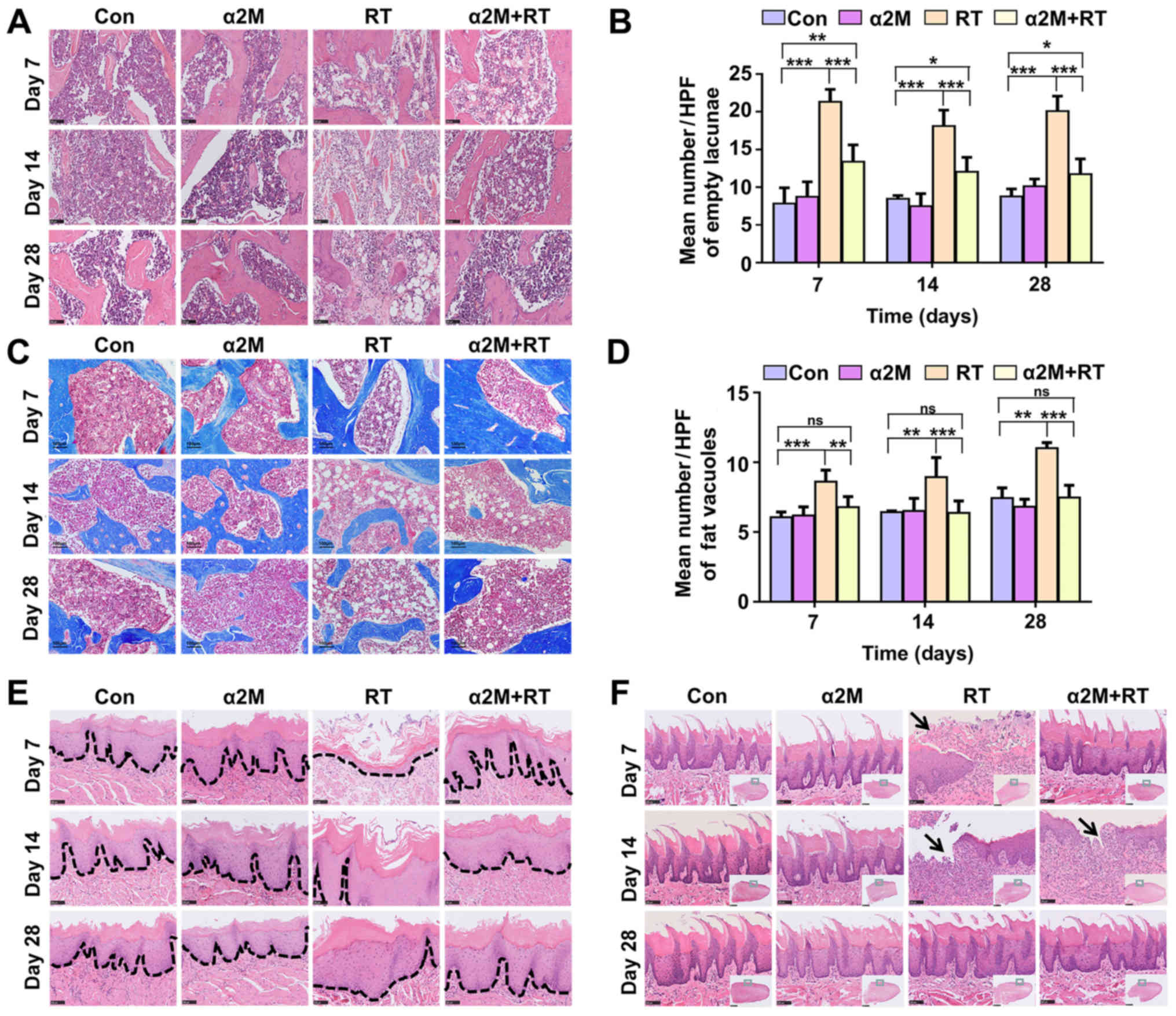 | Figure 2Pathophysiological changes associated
with osteoradionecrosis in the jaws of mice in the four groups. (A)
Haematoxylin and eosin staining. RT group showed significant
increase in cell loss and vessel injury at each time point compared
with α2M + RT group (scale bar, 100 µm). (B) Empty lacunae
were observed in HPFs in all groups. (C) Masson's trichrome
staining. α2M+RT group exhibited fewer fat vacuoles compared with
RT group, but more than in the control group (scale bar, 100
µm). (D) Fat vacuoles were evaluated in HPFs in all groups.
Pathological changes were more serious in the RT group than in the
α2M+RT group at each time point. (E and F) RT group showed serious
injuries with more regions of ulceration and erosion in the mucosa
(E, scale bar, 2.5 mm) and tongue (F, scale bar, 100 µm).
Dotted lines indicate the basement membrane and black arrows
indicate the ulcerated area. (B) ***P<0.001 vs.
control group; ***P<0.001 vs. RT group; (D)
***P<0.001 vs. control group; **P<0.01
vs. RT group. Con, control; D, day; HFP, high-power-field; ns, not
significant; RT, radiotherapy; α2M, α2-macroglobulin. |
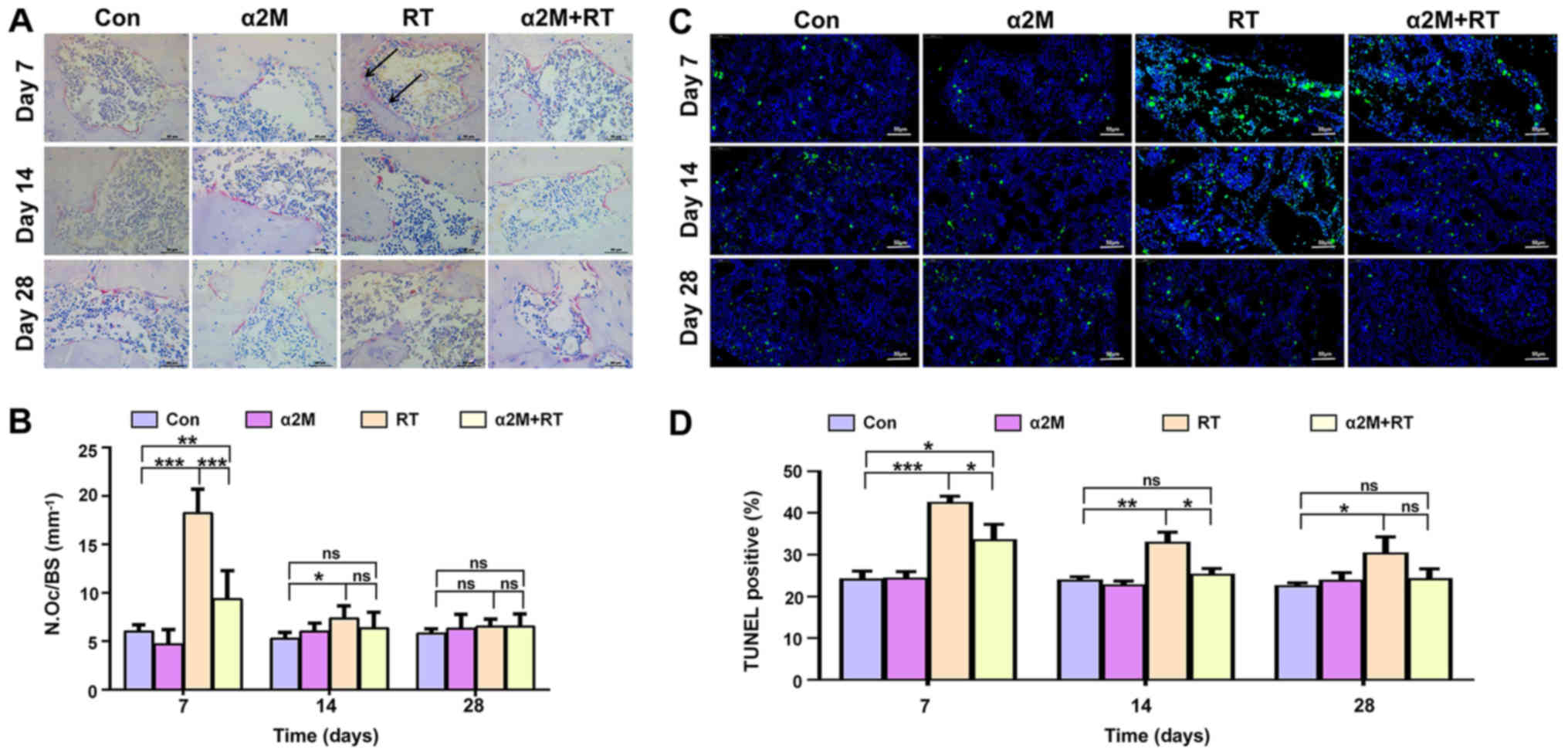
α2M inhibits osteoclasts activity and
apoptosis of cells in the bone tissue
As presented in Fig. 3A
and B, a significant increase in the number of TRAP-positive
multinucleated cells was observed in the RT group compared with the
control group on days 7 and 14 following IR (P<0.05). In
addition, the results from the TUNEL assay demonstrated a
significant increase in the percentage of TUNEL-positive cells in
the RT group compared with the control group on days 7 and 14 after
IR (Fig. 3C and D; P<0.05).
SOD2 and 8-OHdG levels in bone and soft
tissues
Pretreatment with α2M significantly inhibited the
IR-induced increase in 8-OHdG level (P<0.05; Fig. 4A-D). Furthermore, following
pretreatment with α2M, SOD2 expression was significantly increased
in both the mucosa and tongue, as presented in Fig. 4E-H (P<0.05).
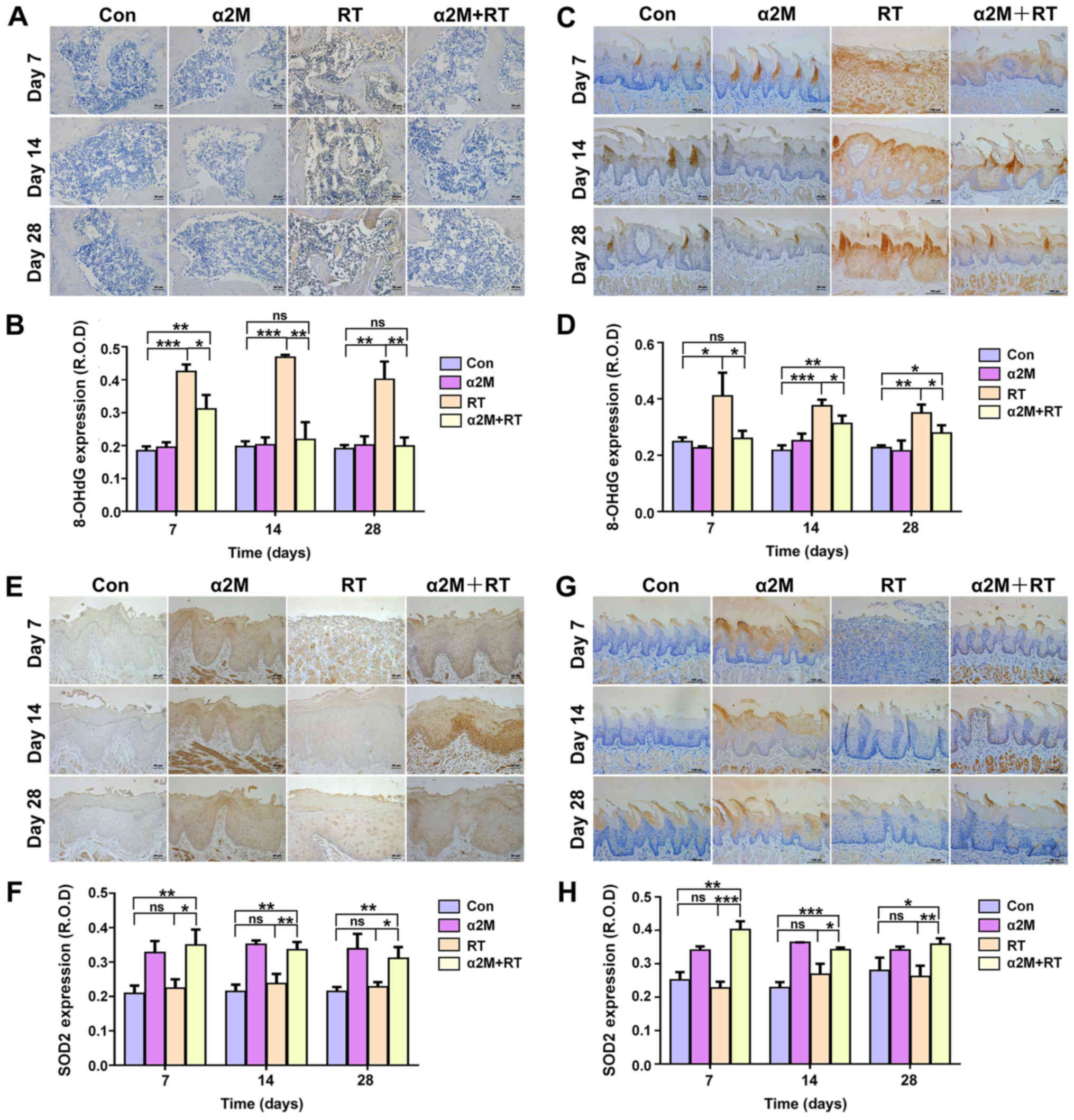 | Figure 4α2M increased SOD2 expression and
decreased 8-OHdG level in bone and soft tissues after irradiation.
(A and B) Level of 8-OHdG in bone from the α2M + RT group was lower
than in the RT group. Scale bar, 100 µm. (C and D) Levels of
8-OHdG in the tongue tissue after IR was lower in the α2M + RT
group than in the RT group. Scale bar, 100 µm. (E and F)
SOD2 was expressed at higher level in the mucosa of the α2M + RT
group than in the RT group. Scale bar, 100 µm. (G and H)
SOD2 was expressed at a higher level in the tongue tissue after IR
in the α2M+RT group than the RT group. Scale bar, 100 µm.
*P<0.05, **P<0.01 and
***P<0.001 vs. control group or RT group. Con,
control; D, day; HFP, high-power-field; ns, not significant; RT,
radiotherapy; α2M, α2-macroglobulin; 8-OHdG,
8-hydroxy-2′-deoxyguanosine; ROD, relative optical density; SOD2,
superoxide dismutase 2. |
α2M reduced the apoptosis rate, improves
the antioxidant capacity and increases Akt phosphorylation in
IR-treated hBMMSCs
Four groups of cells were incubated in 6-well plates
for 24 h after IR (Fig. 5A).
IR-treated hBMMSCs had a lower proliferation rates than cells in
the control group at 48 and 72 h (P<0.001). However, α2M
pretreatment resulted in a higher proliferation rate compared with
the RT group (P<0.01; Fig. 5B).
In addition, significantly increased apoptosis rate was observed in
the RT group compared with the control group (P<0.01), and
pretreatment with α2M prior to IR decreased the apoptosis rate
(P<0.05; Fig. 5C and D).
Significant decrease in ROS levels were detected following IR in
the α2M-treated group compared the RT group (P<0.05; Fig. 5E-H). Furthermore, the protective
effects of α2M on the Akt signaling pathway were observed in the
IR-treated hBMMSCs using Western blotting. Increased expression of
SOD2 and HO-1 was observed in groups pretreated with α2M (Fig. 5I and J).
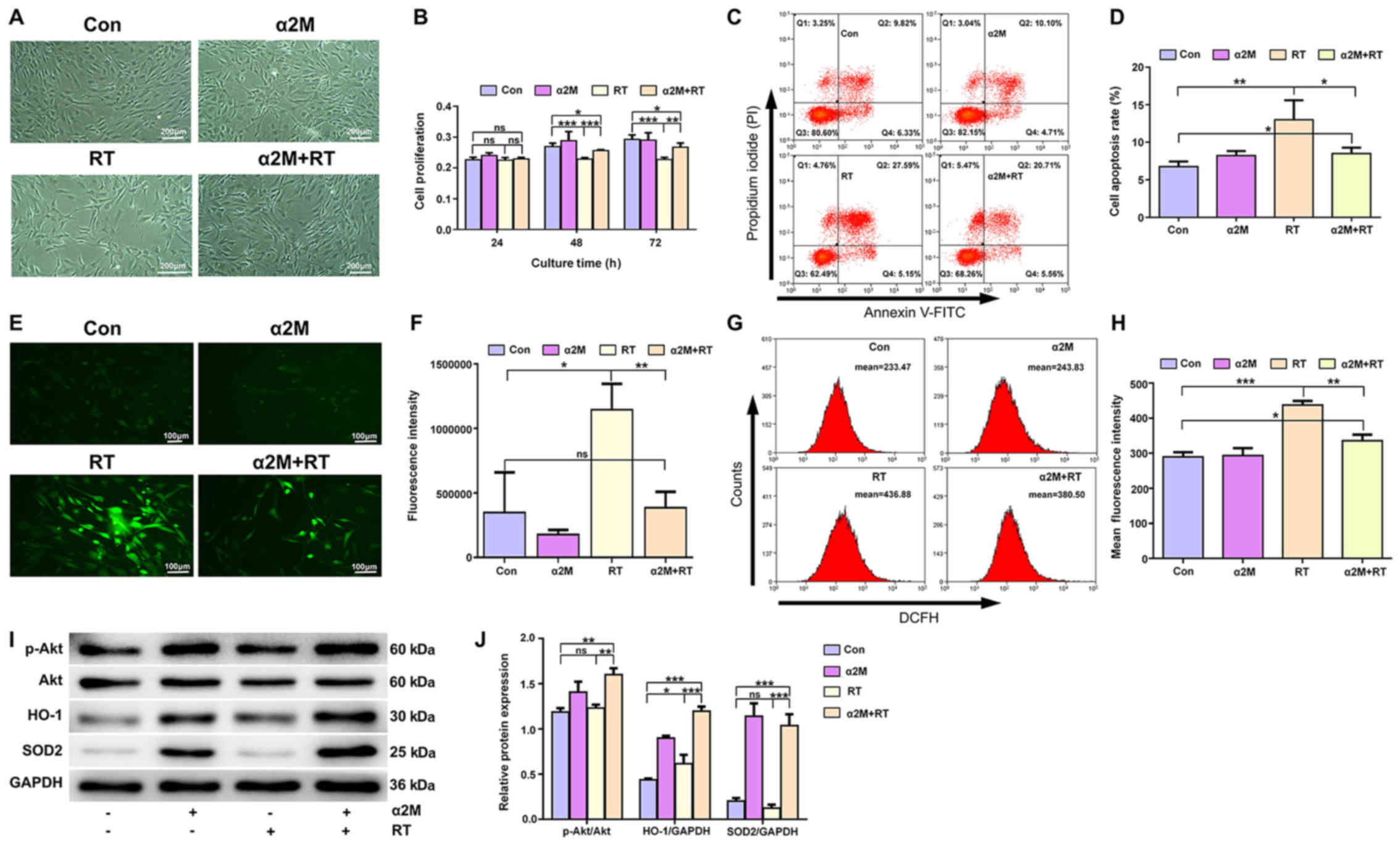 | Figure 5α2M reduced the apoptosis rate,
improved the antioxidant capacity and upregulated Akt
phosphorylation in the IR-treated hBMMSCs. (A) Different groups of
hBMMSCs were evaluated 24 h after IR. Scale bar, 200 µm. (B)
CCK-8 assay was used to detect the effects of IR on hBMMSC
proliferation 24, 48 and 72 h after IR. (C and D) α2M decreased the
apoptosis rate in hBMMSCs 24 h after IR. Apoptotic rate was
calculated by adding the Q2 and Q4 percentages (Q2, Annexin V and
PI-positive cells; Q4, Annexin V-positive and PI-negative cells).
(E and F) α2M decreased ROS level in the α2M + RT group detected by
fluorescent microscopy (E, scale bar, 100 µm) followed by
statistical analysis. (G and H) α2M decreased ROS level in the α2M
+ RT group detected by flow cytometry followed by a statistical
analysis. (I and J) Western blotting used to detect the effect of
α2M on p-Akt, Akt, HO-1 and SOD2 expression in hBMMSCs 24 h after
IR. *P<0.05, **P<0.01 and
***P<0.001 vs. control group or RT group. DCFH,
dichloro-dihydro-fluorescein; HO-1, heme oxygenase-1; p,
phosphorylated; PI, propidium iodide; IR, irradiation; Con,
control; D, day; HFP, high-power-field; ns, not significant; RT,
radiotherapy; α2M, α2-macroglobulin; 8-OHdG,
8-hydroxy-2′-deoxyguanosine; ROD, relative optical density; SOD2,
superoxide dismutase 2. |
Discussion
Numerous studies have reported some changes in the
rat mandible 3 months following IR (32,33).
Other studies demonstrated changes in the soft tissues within two
weeks after IR (34-36). However, the sequential changes
observed in bone and soft tissues at the early stage after IR
remain unclear. Furthermore, ORN is a disease that causes both bone
and soft tissue injuries. To the best of our knowledge, the present
study was the first to use a rat ORN model to investigate the early
stage of ORN in bone and soft tissues.
As previously reported, IR with 30 Gy represents an
ideal dose to induce a significant change in bone injury in rats
(25,26). However, based on a previous study
that applied graded doses of 2, 4, 8 and 12 Gy, the 8 Gy IR dose
should be used to induce hBMMSC injury in vitro (20).
In the present study, cells in the marrow cavity
were largely lost, vascular injury was significantly expanded and
more fat vacuoles were present in the RT group compared with the
control group. Conversely, the α2M + RT group presented resistance
to IR-induced damage. As previously described by Xu et al
(10), irreversible damage to the
blood vessels caused by radiation serves a crucial role in the
development of ORN. Bléry et al (25) reported an increase in the number of
fat vacuoles in mandibular bone marrow following IR. In the present
study, serious soft tissue injury was observed in the RT group,
which was inhibited by α2M treatment, specifically by reducing
ulceration and erosion. These findings were consistent with those
from previous studies (18,19),
and suggested that the potent effect of α2M may be due to its
ability to decrease the IR-induced toxicity in the bone and the
soft tissue.
Osteocytes serve an essential role in bone
reconstruction and metabolism (37). The number of osteocytes undergoing
cell death is commonly calculated by counting empty lacunae during
histological observations (38).
IR induces bone loss and increases osteoclast numbers and activity
(39,40). In the present study, the number of
empty lacunae increased after IR, and IR increased the activity of
osteoclasts within the first week, which was consistent with
previous in vivo studies describing significant increase in
the number and activity of osteoclasts within the first three days
after irradiation (41,42). However, after the first week, the
number of osteoclasts decreased in the RT group, which was
consistent with a previous in vivo study reporting that the
cell number was decreased after 10 days (43). Because bone remodeling involves a
balance between osteoclast resorption and osteoblasts activation,
the findings from the present study suggested that the IR-induced
disruption of this balance may contribute to the development of
ORN. However, α2M treatment had a significant inhibitory effect on
the number of TRAP-positive osteoclasts.
ROS levels are indicators of the degree of oxidative
stress (44). The biomarker 8-OHdG
was first reported by Kasai et al (45) in 1984 and is now commonly used as a
sensitive indicator of oxidative stress-induced DNA damage
(46). Furthermore, SOD2, which is
a well-known enzyme located in the mitochondria, serves
anti-apoptotic role by directly decreasing cellular ROS levels
(47). α2M was previously reported
to inhibit ROS production by increasing SOD2 activity and to reduce
oxidative stress-induced DNA damage, confirming its protective
effect on radiation-induced injury in liver tissue (48). The present study aimed therefore to
determine whether the radioprotective effect of α2M could be
associated with an antioxidant activity. The results reported
increased ROS and 8-OHdG levels following IR, which was reversed
after treatment with α2M. Furthermore, results from western
blotting demonstrated an increased expression of SOD2 and HO-1
following α2M treatment, suggesting that α2M may possess some
antioxidative effects. In addition, α2M stimulated Akt activation,
which is known to be activated by oxidative stress (49,50).
The present study presented some promising results,
which suggested the potential use of α2M prophylactically in
clinical trials. The radioprotective effect of α2M has been
demonstrated by pathological alteration of rat jaw bones in the
early stage of irradiation, and not only physical signs of hard and
soft tissues alteration were attenuated, but the pathological
changes in irradiation jaw bone were also alleviated.
This study presented some limitations. Firstly, it
was not clarified how α2M could regulate SOD2 expression, and
further investigation is required. Regarding the radiation-induced
impairment of oral epithelial cell proliferation and cell
production, further investigation about the underlying mechanism of
α2M on irradiated soft tissue is required. Secondly, no in
vitro experiments on the effect of α2M on the apoptosis rate,
antioxidant capacity and Akt pathway in oral epithelial cells was
performed. Further in vivo experiments will explore the
underlying mechanism of α2M in order to determine whether SOD2
expression was affected by gene knockout or Akt over-expression. In
addition, the duration and time points of α2M management in
patients must be further evaluated in order to determine its
effectiveness.
The association between the early stage development
of ORN and oxidative damage remains unclear. Our previous study
discussed the radioprotective effect of α2M on an animal model of
jaw osteonecrosis established by fraction dose irradiation
(24). In the present study, a
modified rat model of osteoradionecrosis with shorter experimental
period, which in the early stage of osteoradionecrosis of the jaw
(ORNJ), was successfully established by a single 30 Gy dose of
radiation. The present study demonstrated that ORN was associated
with oxidative damage, and that α2M may upregulate the activity of
the antioxidant enzymes SOD2 and HO-1, leading to decreased levels
of ROS and 8-OHdG. Furthermore, the expression of p-Akt in hBMMSCs
was elevated following α2M administration, which indicated that Akt
phosphorylation may serve a role in α2M effect against
radiation-induced mandibular bone damage. The present study not
only focused on the physical signs and pathological changes of
irradiated bone tissues, but also on radiation-induced responses in
soft tissues, at the early stage of ORN rat model. Based on these
results, this study demonstrated that α2M may protect rats from
IR-induced injury by improving the endogenous antioxidant system
and inhibiting the oxidative damage.
In summary, the present study was the first to
demonstrate that α2M may exert a radioprotective effect in
early-stage ORN. The results from this study suggested that α2M may
represent a novel radioprotective and therapeutic agent for ORN in
patients with cancer. In addition, oxidative damage observed during
early-stage ORN provides a new target for preclinical studies.
Supplementary Data
Funding
This study was supported by the Natural Science
Foundation of Guangdong Province (grant no. 2015A030313064), the
Science and Technology Planning Project of Guangdong Province
(grant no. 2017A010105027) and the Science and Technology Planning
Project of Guangdong Province (grant no. 2014A020212167).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL and PY conceived, planned and carried out the
in vivo and in vitro experiments, respectively. JL
drafted the manuscript. XK, XC and SF contributed to the study
concepts and design, and critically revised the manuscript for
important intellectual content. PY, WZ, YG, LQ and ZL participated
in the experiments, data acquisition, quality control of the data
and manuscript review. YS and XX performed the statistical analysis
and scientific discussion. JM revised the manuscript. All the
authors agree to be accountable for all aspects of the work. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Sun Yat-sen
University (approval no. IACUC-DB-16-1107) and conducted in
accordance with the guidelines published in the Guide for the Care
and Use of Laboratory Animals (8th Edition).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
8-OHdG
|
8-hydroxy-2′-deoxyguanosine
|
|
α2M
|
α2-macroglobulin
|
|
hBMMSCs
|
human bone marrow mesenchymal stem
cells
|
|
HO-1
|
heme oxygenase-1
|
|
ORN
|
osteoradionecrosis
|
|
ROS
|
reactive oxygen species
|
|
RT
|
radiotherapy
|
|
SOD2
|
superoxide dismutase 2
|
References
|
1
|
Sciubba JJ and Goldenberg D: Oral
complications of radiotherapy. Lancet Oncol. 7:175–183. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chrcanovic BR, Reher P, Sousa AA and
Harris M: Osteoradionecrosis of the jaws - a current overview -
part 1: Physiopathology and risk and predisposing factors. Oral
Maxillofac Surg. 14:3–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGowan K, Ivanovski S and Acton C:
Osteonecrosis of the jaws: A 14-year retrospective survey of
hospital admissions. Aust Dent J. 63:202–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chronopoulos A, Zarra T, Ehrenfeld M and
Otto S: Osteoradionecrosis of the jaws: Definition, epidemiology,
staging and clinical and radiological findings. A concise review
Int Dent J. 68:22–30. 2018. View Article : Google Scholar
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marx RE: Osteoradionecrosis: A new concept
of its pathophysiology. J Oral Maxillofac Surg. 41:283–288. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Madrid C, Abarca M and Bouferrache K:
Osteoradionecrosis: An update. Oral Oncol. 46:471–474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poort LJ, Ludlage JH, Lie N, Böckmann RA,
Odekerken JC, Hoebers FJ and Kessler PA: The histological and
histomorphometric changes in the mandible after radiotherapy: An
animal model. J Craniomaxillofac Surg. 45:716–721. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marx RE and Johnson RP: Studies in the
radiobiology of osteoradionecrosis and their clinical significance.
Oral Surg Oral Med Oral Pathol. 64:379–390. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu J, Zheng Z, Fang D, Gao R, Liu Y, Fan
ZP, Zhang CM and Wang SL: Early-stage pathogenic sequence of jaw
osteoradione-crosis in vivo. J Dent Res. 91:702–708. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J, Zheng Z, Fang D, Gao R, Liu Y, Fan
Z, Zhang C, Shi S and Wang S: Mesenchymal stromal cell-based
treatment of jaw osteoradionecrosis in Swine. Cell Transplant.
21:1679–1686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazur L, Augustynek A, Halicka HD and
Deptała A: Induction of apoptosis in bone marrow cells after
treatment of mice with WR-2721 and gamma-rays: Relationship to the
cell cycle. Cell Biol Toxicol. 19:13–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao X, Wu X, Frassica D, Yu B, Pang L,
Xian L, Wan M, Lei W, Armour M, Tryggestad E, et al: Irradiation
induces bone injury by damaging bone marrow microenvironment for
stem cells. Proc Natl Acad Sci USA. 108:1609–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rehman AA, Zaman M, Zia MK, Ahsan H, Khan
RH and Khan FH: Conformational behavior of alpha-2-macroglobulin:
Aggregation and inhibition induced by TFE. Int J Biol Macromol.
104:539–546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Kong X, Zhang Z, Chen W, Chen J,
Li H, Cao W, Ge Y and Fang S: Alpha-2-macroglobulin as a
radioprotective agent: A review. Chin J Cancer Res. 26:611–621.
2014.PubMed/NCBI
|
|
17
|
Uskoković A, Dinić S, Mihailović M,
Grigorov I, Ivanović-Matić S, Bogojević D, Grdović N, Arambasić J,
Vidaković M, Martinović V, et al: STAT3/NFkappaB interplay in the
regulation of alpha2-macroglobulin gene expression during rat liver
development and the acute phase response. IUBMB Life. 59:170–178.
2007. View Article : Google Scholar
|
|
18
|
Bogojević D, Poznanović G, Grdović N,
Grigorov I, Vidaković M, Dinić S and Mihailović M: Administration
of rat acute-phase protein α(2)-macroglobulin before total-body
irradiation initiates cytoprotective mechanisms in the liver.
Radiat Environ Biophys. 50:167–179. 2011. View Article : Google Scholar
|
|
19
|
Mirjana M, Goran P, Nevena G, Melita V,
Svetlana D, Ilijana G and Desanka B: The rat acute-phase protein
α2-macroglobulin plays a central role in amifostine-mediated
radioprotection. J Radiol Prot. 30:567–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Cao W, Kong X, Li J, Chen X, Ge Y,
Zhong W and Fang S: Protective effects of α2 macroglobulin on human
bone marrow mesenchymal stem cells in radiation injury. Mol Med
Rep. 18:4219–4228. 2018.PubMed/NCBI
|
|
21
|
Moroni L and Fornasari PM: Human
mesenchymal stem cells: A bank perspective on the isolation,
characterization and potential of alternative sources for the
regeneration of musculoskeletal tissues. J Cell Physiol.
228:680–687. 2013. View Article : Google Scholar
|
|
22
|
Baker N, Boyette LB and Tuan RS:
Characterization of bone marrow-derived mesenchymal stem cells in
aging. Bone. 70:37–47. 2015. View Article : Google Scholar
|
|
23
|
Wang Y, Zhu G, Wang J and Chen J:
Irradiation alters the differentiation potential of bone marrow
mesenchymal stem cells. Mol Med Rep. 13:213–223. 2016. View Article : Google Scholar :
|
|
24
|
Li J, Kong XB, Chen XY, Zhong WZ, Chen JY,
Liu Y, Yin P and Fang SL: Protective role of α2-macroglobulin
against jaw osteoradionecrosis in a preclinical rat model. J Oral
Pathol Med. 48:166–173. 2019. View Article : Google Scholar
|
|
25
|
Bléry P, Espitalier F, Hays A, Crauste E,
Demarquay C, Pilet P, Sourice S, Guicheux J, Malard O, Benderitter
M, et al: Development of mandibular osteoradionecrosis in rats:
Importance of dental extraction. J Craniomaxillofac Surg.
43:1829–1836. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cohen M, Nishimura I, Tamplen M, Hokugo A,
Beumer J, Steinberg ML, Suh JD, Abemayor E and Nabili V: Animal
model of radiogenic bone damage to study mandibular
osteoradionecrosis. Am J Otolaryngol. 32:291–300. 2011. View Article : Google Scholar
|
|
27
|
Tchanque-Fossuo CN, Monson LA, Farberg AS,
Donneys A, Zehtabzadeh AJ, Razdolsky ER and Buchman SR:
Dose-response effect of human equivalent radiation in the murine
mandible: Part I. A histomorphometric assessment. Plast Reconstr
Surg. 128:114–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spencer M, Yang L, Adu A, Finlin BS, Zhu
B, Shipp LR, Rasouli N, Peterson CA and Kern PA: Pioglitazone
treatment reduces adipose tissue inflammation through reduction of
mast cell and macrophage number and by improving vascularity. PLoS
One. 9:e1021902014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Liu S, Zhao Y, Liu D, Liu Y, Chen
C, Karray S, Shi S and Jin Y: Osteoblast-induced osteoclast
apoptosis by fas ligand/FAS pathway is required for maintenance of
bone mass. Cell Death Differ. 22:1654–1664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan H, Qi J, Fan BY, Zhang J, Su FF and
Wang HT: MicroRNA-24-3p attenuates myocardial ischemia/reperfusion
injury by suppressing RIPK1 expression in mice. Cell Physiol
Biochem. 51:46–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Jin X, Hu CF, Li R, Zhou Z and Shen
CX: Exosomes derived from mesenchymal stem cells rescue myocardial
ischaemia/reper-fusion injury by inducing cardiomyocyte autophagy
via AMPK and Akt pathways. Cell Physiol Biochem. 43:52–68. 2017.
View Article : Google Scholar
|
|
32
|
Niehoff P, Springer IN, Açil Y, Lange A,
Marget M, Roldán JC, Köppe K, Warnke PH, Kimmig B and Wiltfang J:
HDR brachy-therapy irradiation of the jaw - as a new experimental
model of radiogenic bone damage. J Craniomaxillofac Surg.
36:203–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monson LA, Jing XL, Donneys A, Farberg AS
and Buchman SR: Dose-response effect of human equivalent radiation
in the mandible. J Craniofac Surg. 24:1593–1598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang JW, Choi JW, Lee BH, Park JK, Shin
YS, Oh YT, Noh OK and Kim CH: Protective effects of Korean red
ginseng on radiation-induced oral mucositis in a preclinical rat
model. Nutr Cancer. 66:400–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han G, Bian L, Li F, Cotrim A, Wang D, Lu
J, Deng Y, Bird G, Sowers A, Mitchell JB, et al: Preventive and
therapeutic effects of Smad7 on radiation-induced oral mucositis.
Nat Med. 19:421–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maria OM, Shalaby M, Syme A, Eliopoulos N
and Muanza T: Adipose mesenchymal stromal cells minimize and repair
radiation-induced oral mucositis. Cytotherapy. 18:1129–1145. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sapir-Koren R and Livshits G: Osteocyte
control of bone remodeling: Is sclerostin a key molecular
coordinator of the balanced bone resorption-formation cycles?
Osteoporos Int. 25:2685–2700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zong C, Cai B, Wen X, Alam S, Chen Y, Guo
Y, Liu Y and Tian L: The role of myofibroblasts in the development
of osteoradionecrosis in a newly established rabbit model. J
Craniomaxillofac Surg. 44:725–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Willey JS, Lloyd SA, Nelson GA and Bateman
TA: Ionizing radiation and none loss: Space exploration and
clinical therapy applications. Clin Rev Bone Miner Metab. 9:54–62.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sibonga JD: Spaceflight-induced bone loss:
Is there an osteoporosis risk? Curr Osteoporos Rep. 11:92–98. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Willey JS, Lloyd SA, Robbins ME, Bourland
JD, Smith-Sielicki H, Bowman LC, Norrdin RW and Bateman TA: Early
increase in osteoclast number in mice after whole-body irradiation
with 2Gy X rays. Radiat Res. 170:388–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Green DE, Adler BJ, Chan ME and Rubin CT:
Devastation of adult stem cell pools by irradiation precedes
collapse of trabecular bone quality and quantity. J Bone Miner Res.
27:749–759. 2012. View Article : Google Scholar
|
|
43
|
Sawajiri M, Mizoe J and Tanimoto K:
Changes in osteoclasts after irradiation with carbon ion particles.
Radiat Environ Biophys. 42:219–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sakuma S, Abe M, Kohda T and Fujimoto Y:
Hydrogen peroxide generated by xanthine/xanthine oxidase system
represses the proliferation of colorectal cancer cell line Caco-2.
J Clin Biochem Nutr. 56:15–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kasai H, Hayami H, Yamaizumi Z, Saitô H
and Nishimura S: Detection and identification of mutagens and
carcinogens as their adducts with guanosine derivatives. Nucleic
Acids Res. 12:2127–2136. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Valavanidis A, Vlachogianni T and Fiotakis
C: 8-hydroxy-2′-de-oxyguanosine (8-OHdG): A critical biomarker of
oxidative stress and carcinogenesis. J Environ Sci Health Part C
Environ Carcinog Ecotoxicol Rev. 27:120–139. 2009. View Article : Google Scholar
|
|
47
|
Ruiz-Perera LM, Schneider L, Windmöller
BA, Müller J, Greiner JF, Kaltschmidt C and Kaltschmidt B: NF-κB
p65 directs sex-specific neuroprotection in human neurons. Sci Rep.
8:160122018. View Article : Google Scholar
|
|
48
|
Ivanov VE, Usacheva AM, Chernikov AV,
Bruskov VI and Gudkov SV: Formation of long-lived reactive species
of blood serum proteins induced by low-intensity irradiation of
helium-neon laser and their involvement in the generation of
reactive oxygen species. J Photochem Photobiol B. 176:36–43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bautista E, Vergara P and Segovia J:
Iron-induced oxidative stress activates AKT and ERK1/2 and
decreases Dyrk1B and PRMT1 in neuroblastoma SH-SY5Y cells. J Trace
Elem Med Biol. 34:62–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lim SW, Jin L, Luo K, Jin J, Shin YJ, Hong
SY and Yang CW: Klotho enhances FoxO3-mediated manganese superoxide
dismutase expression by negatively regulating PI3K/AKT pathway
during tacrolimus-induced oxidative stress. Cell Death Dis.
8:e29722017. View Article : Google Scholar : PubMed/NCBI
|