Introduction
Mucoepidermoid carcinoma (MEC) is the most common
malignant tumor of the salivary gland, and is well known for having
a considerable cellular heterogeneity, including epidermoid,
intermediate and mucin-producing cells (1,2).
Patients with high-grade and advanced stages of the disease have
been reported to be exhibit poor survival rates (3). Although radiotherapy and chemotherapy
with surgery are the main treatment modalities for MEC (4,5),
various side-effects associated with these treatments have been
reported (6). To overcome these
limitations, it is important to continue to identify novel
tumor‑specific molecular target and explore new, efficient and less
toxic drug candidates for the treatment of MEC.
B‑cell lymphoma 2 (Bcl‑2) family member is highly
conserved across species and is known to be a promising target for
chemotherapy (7). Myeloid cell
leukemia-1 (MCL-1), an anti‑apoptotic member of the Bcl‑2 family,
has been shown to play an anti-apoptotic role in cell survival
(8,9). MCL-1 is also overexpressed and is
associated with poor outcomes of various malignant tumors,
including hepatocellular carcinoma (10), breast cancer (11) and esophageal squamous cell
carcinoma (12). It sequesters
pro‑apoptotic members of the Bcl‑2 family, such as Bax, Bak, Bim
and t‑Bid through its direct binding to them, followed by blocking
their oligomerization for the formation of protein-permeable pores
on the mitochondrial outer membrane (13,14).
Finally, a decrease in MCL-1 expression allows for the release of
cytochrome c into the cytoplasm, leading to the activation
of the caspase cascade and ultimately, to the induction of
apoptosis (15,16). It is therefore important to
consider MCL-1 as a chemotherapeutic target for a variety of cancer
types.
Oridonin is a diterpenoid extracted from the
medicinal herb, Rabdosia rubescens, which has attracted much
research interest as it exhibits anticancer effects in various
cancer cells (17,18). The anticancer mechanisms of
oridonin include the Fas/FasL-mediated extrinsic apoptotic pathway,
phosphoinositide 3-kinase (PI3K)/Akt or mitogen-activated protein
kinase (MAPK) signaling pathway-related intrinsic apoptotic pathway
(19-22). In addition, recent studies have
demonstrated that oridonin exerts mitochondria-mediated apoptotic
effects on a variety of cancer cells through the Bcl‑2 family
(23,24). However, the precise effects of
oridonin on MEC cells and the underlying mechanism have not been
studied yet.
In the present study, the anticancer effects of
oridonin and the apparent underlying mechanisms were investigated
in MC-3 and YD-15 human MEC cell lines.
Materials and methods
Cell culture and chemical treatment
MC-3 and YD-15 cell lines were obtained from the
Fourth Military Medical University and Yonsei University,
respectively. Both cell lines were maintained in either DMEM/F12 or
RPMI-1640 medium (Welgene, Inc.) supplemented with 10% fetal bovine
serum (Welgene, Inc.) and 100 U/ml each penicillin (Welgene, Inc.)
in a humidified atmosphere containing 5% CO2 at 37°C.
During the culture process, the cells were routinely investigated
under a microscope (CKX53, Olympus Corp.) for fungal and mycoplasma
contamination and the mycoplasma removal agent (MP Biomedicals,
LLC) was used to treat the mycoplasma-free cultures if deemed
necessary. All experiments were performed with cells cultured at
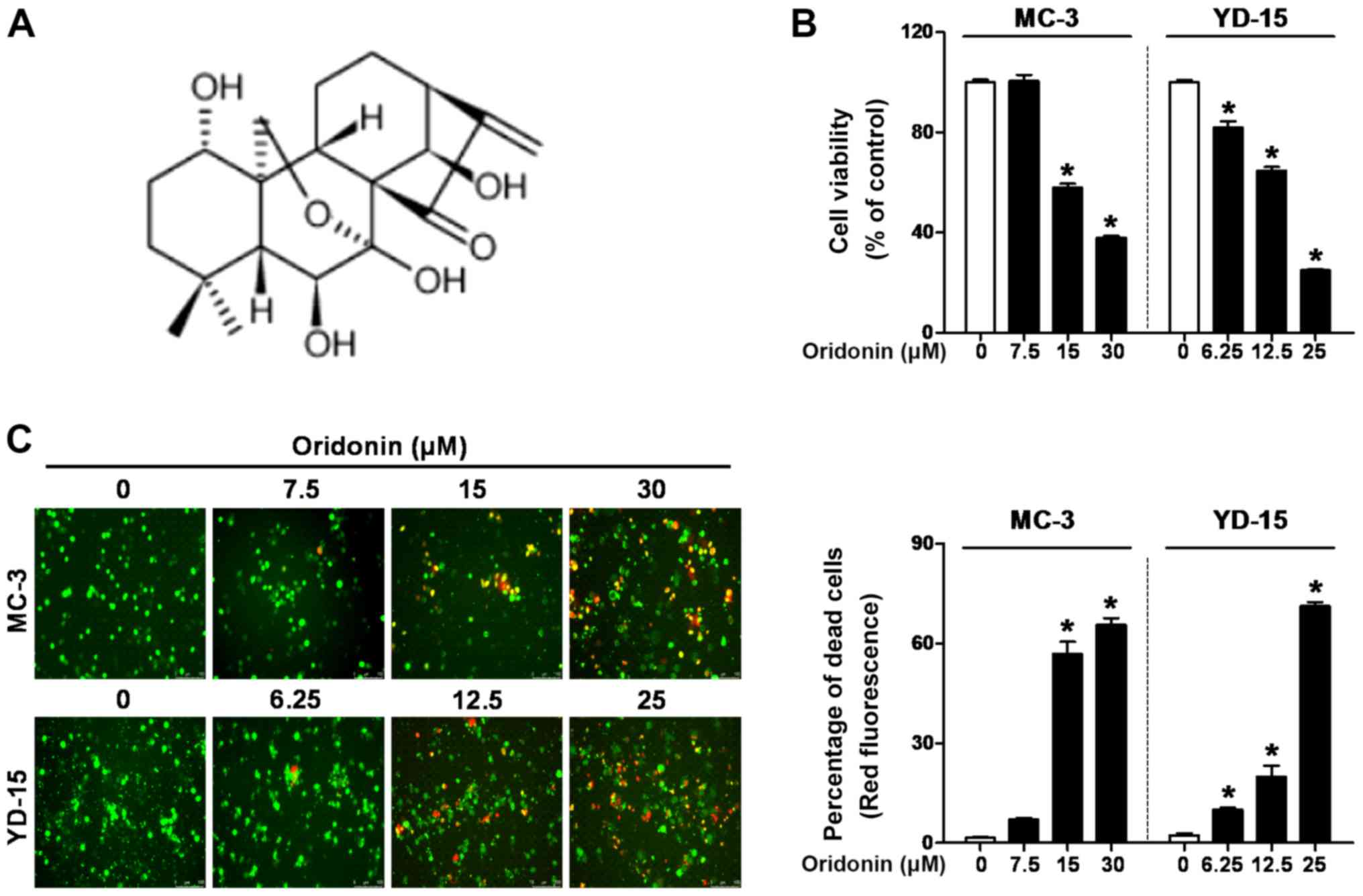
50‑60% confluency. Oridonin (chemical structure shown in Fig. 1A) was purchased from Abcam and
cycloheximide (CHX) were obtained from Sigma-Aldrich; Merck KGaA.
Each chemical was dissolved in dimethyl sulfoxide (DMSO), aliquoted
and stored at ‑20°C. Stock solutions were diluted with culture
medium to the indicated concentrations (final DMSO concentration,
0.1%).
Trypan blue exclusion assay
The effects of oridonin on cell viability were
investigated using Trypan blue exclusion assay. Cells were
incubated with the vehicle control (0.1% DMSO) or oridonin for 48 h
at 37°C, stained with 0.4% trypan blue solution (Gibco; Thermo
Fisher Scientific, Inc.) for 2 min at room temperature, and viable
cells were counted using a hemocytometer (Marienfeld).
Live/dead assay
The Live/Dead and Viability/Cytotoxicity assay kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to determine
the cytotoxicity of oridonin in the MEC cell lines. Calcein-AM is
retained in living cells, producing intense green fluorescence
through intracellular esterase activity. Ethidium homodimer-1
enters dead cells with damaged membranes and binds to nucleic
acids, producing bright red fluorescence. Briefly, cells were
stained with 2 µM calcein‑AM and 4 µM ethidium
homodimer-1 and incubated for 30 min at room temperature. Cells
were analyzed under a fluorescence microscope (Leica DM5000B; Leica
Microsystems GmbH) with a suitable excitation and emission
filter.
Western blot analysis
Whole‑cell lysates were extracted with RIPA lysis
buffer (EMD Millipore) containing phosphatase inhibitors (Thermo
Fisher Scientific) and protease inhibitor cocktails (Roche
Diagnostics GmbH). Protein concentrations were measured using a DC
protein assay kit (Bio‑Rad Laboratories, Inc.). Proteins at the
same concentration (20 µg) were separated by
SDS-polyacrylamide gel electrophoresis in 12% acrylamide gel
(Bio‑Rad Laboratories, Inc.) and electrotransferred to a
polyvinylidene fluoride membrane (Pall Corporation). The membranes
were blocked with 5% skim milk dissolved in Tris‑buffered
saline‑Tween‑20 buffer (T‑BST) for 1 h at room temperature. The
membranes were then washed with T‑BST and incubated with primary
antibodies overnight at 4°C. Subsequently, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies at room temperature for 2 h. Rabbit anti-human
polyclonal antibodies against cleaved caspase-3 (cat. no. 9664;
1:1,000), cleaved poly (ADP-ribose) polymerase (PARP; cat. no.
9541; 1:1,000), Bcl‑xL (cat. no. 2764; 1:1,000), Bak (cat. no.
3814; 1:1,000), Bax (cat. no. 2772; 1:1,000), Bim (cat. no. 2819;
1:1,000) and MCL-1 (cat. no. 4572; 1:1,000) were obtained from Cell
Signaling Technology, Inc. Mouse anti-human monoclonal antibodies
against active‑Bak (cat. no. AM04; 1:1,000) was purchased from EMD
Millipore. Mouse anti-human monoclonal antibodies against
active‑Bax (cat. no. 556467; 1:1,000) was obtained from BD
Biosciences. Goat anti‑human polyclonal antibodies against t‑Bid
(cat. no. 34325; 1:1,000) and mouse anti-human monoclonal
antibodies against β-actin (cat. no. sc47778; 1:3,000) were
purchased from Santa Cruz Biotechnology, Inc. Antibody‑bound
proteins were detected using enhanced chemiluminescence Western
blotting Luminol reagent (Santa Cruz Biotechnology, Inc.) and
visualized using a LAS-500 imaging system (GE Healthcare Life
Sciences). The densitometric analysis of the western blots was
performed using ImageJ software (version 1.51k, NIH).
Flow cytometric analysis
Flow cytometry was performed to analyze the cell
cycle and apoptosis of the MEC cell lines. Cells were harvested
after being treated with various concentrations of oridonin (0-30
µM) for 48 h, washed twice with PBS, and fixed with 70%
ethanol at ‑20°C for overnight. Cells were then re‑suspended in PBS
containing 20 µg/ml RNase A and propidium iodide (P4170,
Sigma-Aldrich; Merck KGaA) for 15 min at 37°C. DNA contents were
detected using a fluorescence-activated cell sorter (FACS) Calibur
(BD Biosciences) and relative DNA content was calculated with Cell
Quest software (BD Biosciences).
4'‑6‑Diamidino‑2‑phenylindole (DAPI)
staining
DAPI solution (Sigma-Aldrich; Merck KGaA) was used
to investigate the nuclear morphological changes of apoptotic
cells. Cells seeded on 60 mm2 plates were treated with
various concentrations of oridonin (0-30 µM) for 48 h.
Following treatment, the cells were harvested, washed twice with
PBS, and fixed with 100% methanol at room temperature for 10 min.
The cells were washed again with PBS, plated on coated glass
slides, and stained with 2 µg/ml of DAPI solution for 1 min
at room temperature. The morphological changes of the cells were
observed under a fluorescence microscope (Leica DM5000B, Leica
Microsystems GmbH).
Annexin V/propidium iodide (PI)
staining
Apoptosis was measured using a FITC Annexin V
apoptosis detection kit (BD Pharmingen). Harvested cells were
washed twice with PBS and stained with Annexin V-FITC and PI dye at
room temperature for 15 min. The stained cells were then analyzed
using a FACSCalibur flow cytometer and calculated using Cell Quest
software (BD Biosciences).
Reverse transcription and polymerase
chain reaction (RT‑PCR) and semi‑quantitative PCR
Total RNA was isolated using the Easy‑BLUE total RNA
extraction kit (iNtRON Biotechnology) and cDNA was synthesized
using a cDNA synthesis kit (Enzo Life Sciences, Inc.). The
resulting target cDNA was subjected to PCR using HiPi PCR
PreMix (ELPISBIOTECH, Inc.) and amplified using the following
primers: MCL-1 sense, 5′-GAG GAG GAG GAC GAG TTG TA-3′ and
antisense 5′-CCT TAC GAG AAC GTC TGT TGT GAT AC-3′; and GAPDH
sense, 5′-CGG AGT CAA CGG ATT TGG TCG TAT-3′ and antisense, 5′-AGC
CTT CTC CAT GGT GGT GAA GAC‑3′. The amplification of MCL‑1 and
GAPDH was performed for 28 cycles (30 sec at 95°C, 35 sec at 60°C
and 45 sec at 72°C). The amplified PCR products were detected using
2% agarose gel electrophoresis and visualized by ethidium bromide
staining. The densitometric analysis of the amplified PCR products
was performed using ImageJ software (version 1.51k, NIH).
Quantitative (real‑time) PCR (qPCR)
The resulting target cDNA was subjected to PCR using
AMPIGENE qPCR Green Mix Hi‑Rox (Enzo Life Sciences, Inc). qPCR was
performed by a StepOne Plus Real-Time PCR System (Applied
Biosystems) and the resulting target cDNA was amplified using the
following primers: MCL-1 sense, 5′-GTA TCA CAG ACG TTC TCG TAA
GG-3′ and antisense, 5′-CCA CCT TCT AGG TCC TCT ACA T-3′; and GAPDH
sense, 5′-GTG GTC TCC TCT GAC TTC AAC-3′ and antisense, 5′-CCT GTT
GCT GTA GCC AAA TTC‑3′. The amplification of MCL‑1 and GAPDH was
performed for 40 cycles (2 min at 95°C 10 sec at 95°C and 30 sec at
60°C). Each PCR product was run in triplicate. The relative MCL-1
mRNA expression was calculated using the 2−ΔΔCq method
(25).
Cycloheximide (CHX) chase assay
Cycloheximide (Sigma-Aldrich; Merck KGaA) chase
assay was carried out to examine whether oridonin affected the
half-life of MCL-1 protein. In brief, the cells were treated with
0.05 µg/ml of CHX 1 h prior to oridonin treatment, and were
subsequently treated with or without oridonin for the indicated
periods of time (0‑12 h). Western blot analysis was then
performed.
Mitochondrial membrane potential (ΔΨm)
assay
Changes in ΔΨm were determined using a MitoScreen
kit (BD Pharmingen). Harvested cells were washed twice with PBS and
incubated with JC‑1 solution at 37°C for 30 min. The cells washed
twice using 1X Assay Buffer and JC‑1 fluorescence was analyzed by
flow cytometry (FACSCalibur, BD Biosciences).
Construction of MCL‑1 overexpression
vector and transient transfection
The open reading frame of the human MCL-1
(NM_021960) gene was amplified from cDNA using the following
primers: MCL-1 sense, 5′-GAA TTC ATG TTT GGC CTC AAA AGA-3′, with
an included EcoRI site; and MCL-1 antisense, 5′-GAA TTC CTA
TCT TAT TAG ATA TGC-3′, with an included EcoRI site and
cloned into a pGEM®-T Easy Vector System (Promega
Corporation). The genes were cloned into the multiple cloning site
of pcDNA3.1(+) vector (Invitrogen; Thermo Fisher Scientific, Inc.).
The MC‑3 and YD-15 cells were transfected with the empty pcDNA3.1
or the pcDNA3.1‑ MCL‑1 vector (0.5 µg) construct using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using SPSS 22
software (SPSS, Inc.). A one-way analysis of variance (ANOVA) was
applied with Tukey's post hoc test and all data are presented as
the means ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Oridonin inhibits the viability and
induces the death of human MEC cell lines
To investigate the growth-inhibitory effects of
oridonin, the MC-3 and YD-15 cells were incubated with various
concentrations of oridonin (0‑30 µM) for 48 h. The results
revealed that statistical significance was observed at the 15 and
30 µM concentrations of oridonin for the MC‑3 cells and at
the 6.25, 12.5 and 25 µM concentrations for the YD‑15 cells
(Fig. 1B). To determine the
cytotoxic effects of oridonin, a Live/Dead assay was performed
under the same conditions as a Trypan blue exclusion assay in the
MEC cell lines. As illustrated in Fig.
1C, treatment with oridonin led to an increase in the ratio of
dead cells (red fluorescence) in a concentration-dependent manner.
These results indicate that oridonin inhibits the viability and
induces the death of MEC cell lines.
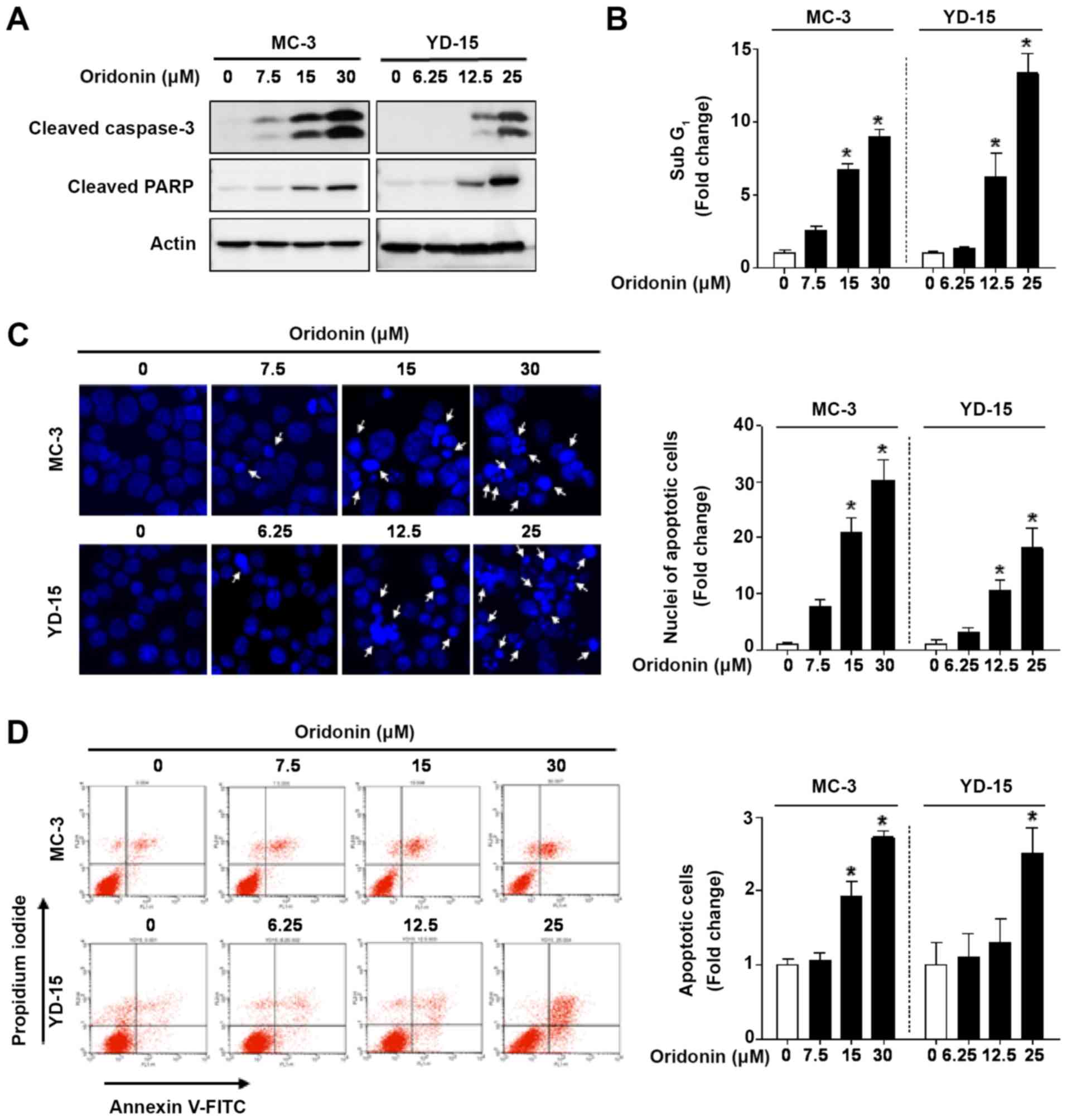
Oridonin induces the apoptosis of MEC
cell lines
To determine the type of cell death induced by
oridonin, the levels of PARP and caspase-3, as markers of
apoptosis, were detected by western blot analysis. In response to
oridonin treatment, the cleavage of caspase-3 and PARP was markedly
increased compared with the vehicle control (Fig. 2A). To examine the effects of
oridonin on the sub G1 cell population, flow cytometry
was performed. The cell population in the sub G1 phase
increased from 8.96- to 13.31-fold in the MC-3 and YD‑15 cell lines
treated with oridonin (Fig. 2B).
When visualizing cell apoptosis by DAPI staining, oridonin was
found to significantly increase the number of apoptotic nuclei with
condensation or fragmentation (white arrows) in the MEC cell lines
(Fig. 2C). To further verify the
apoptotic effects of oridonin, Annexin V/PI double staining was
performed. The results revealed that the number of Annexin-positive
cells was significantly increased in a concentration‑dependent
manner (Fig. 2D). Taken together,
these results suggest that oridonin enhances the apoptotic death of
human MEC cell lines.
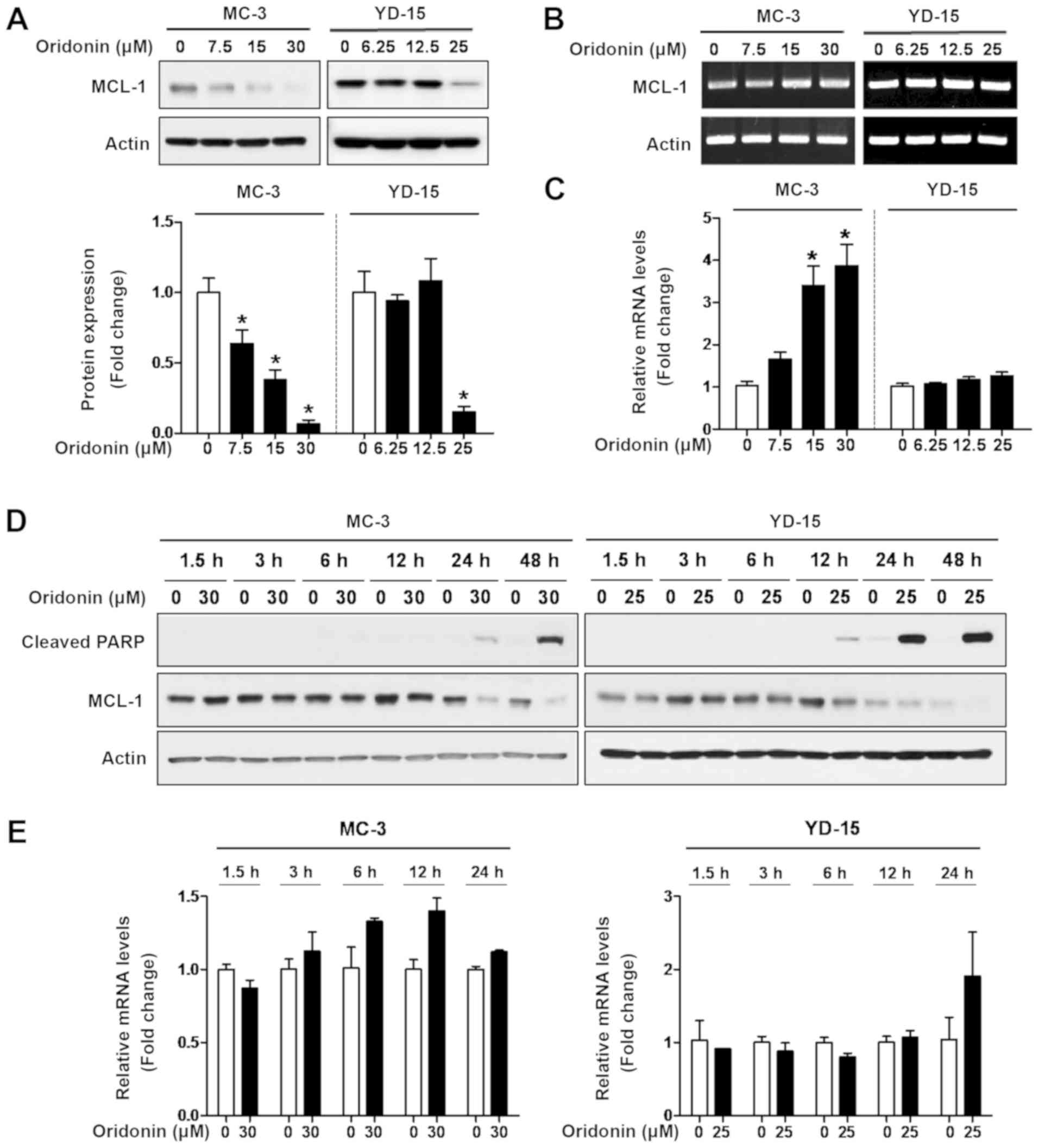
Oridonin downregulates MCL‑1 protein
expression through post‑translational modification
To elucidate the fundamental mechanisms of
oridonin-induced apoptosis, the expression of Bcl‑2 family was
identified as a key mediator of apoptosis. The results of western
blot analysis revealed that treatment with oridonin significantly
decreased the expression level of MCL‑1 protein and increased PARP
cleavage in a concentration- and time-dependent manner (Fig. 3A and D); however, oridonin did not
affect the expression of other anti-apoptotic proteins (e.g.,
Bcl‑xL) in both cell lines (Fig.
S1). To determine whether MCL-1 is regulated at the
transcriptional level, RT-PCR and qPCR were performed. The effect
of oridonin on MCL‑1 mRNA levels was variable in both cell lines
(Fig. 3B, C and E). To further
determine the effect of oridonin on MCL-1 protein turnover, chasing
analysis was performed using cycloheximide (CHX), an inhibitor of
protein synthesis, followed by western blot analysis. As a result,
the MCL-1 protein levels were markedly decreased by simultaneous
treatment with oridonin and CHX compared to CHX treatment only
(Fig. S2, top panel). The
half-life of MCL-1 protein in the cells treated with both oridonin
and CHX was approximately 11.5 and 10.8 h compared with 23.3 and
44.0 h in the CHX-treated group in the MC-3 and YD-15 cells,
respectively (Fig. S2 bottom
panel). These results suggest that the mode of action of oridonin
is through the inhibition of the MCL-1 protein level.
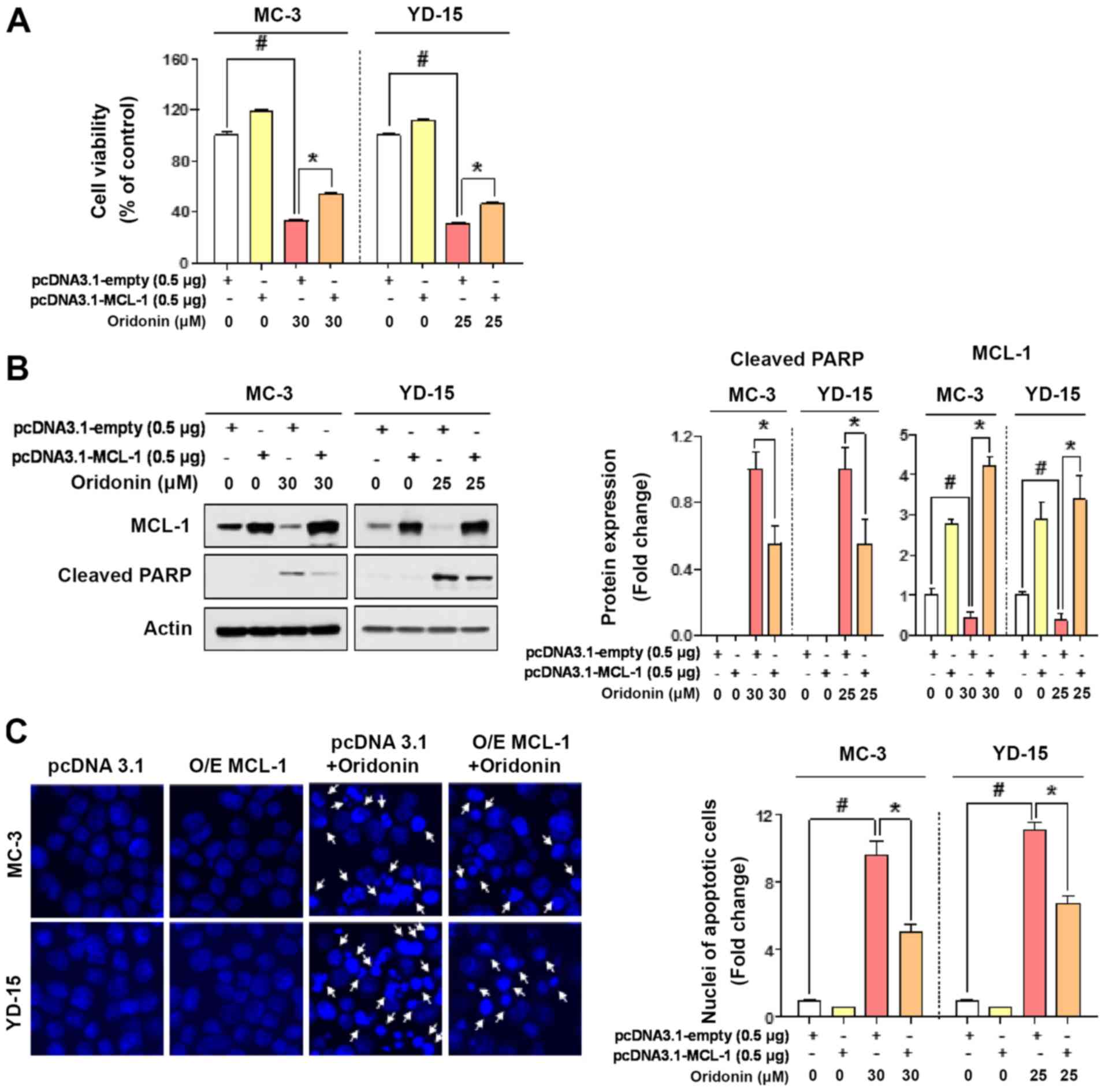
Depletion of MCL‑1 protein is associated
with the oridonin‑induced apoptosis of MEC cell lines
To verify whether the effects of oridonin-mediated
apoptosis are dependent on MCL-1, the two cell lines were
transfected with an empty vector or a MCL-1 expression vector.
MCL-1 overexpression significantly restored cell viability and PARP
cleavage, which were previously affected by oridonin in both cell
lines (Fig. 4A and B). This
observation was further confirmed by the result that the ratio of
apoptotic nuclei in MCL-1 overexpressing cells was reduced compared
with the control (Fig. 4C). These
results suggest that the principal mechanism of oridonin-mediated
apoptosis may involve the targeting of MCL-1 protein in human MEC
cell lines.
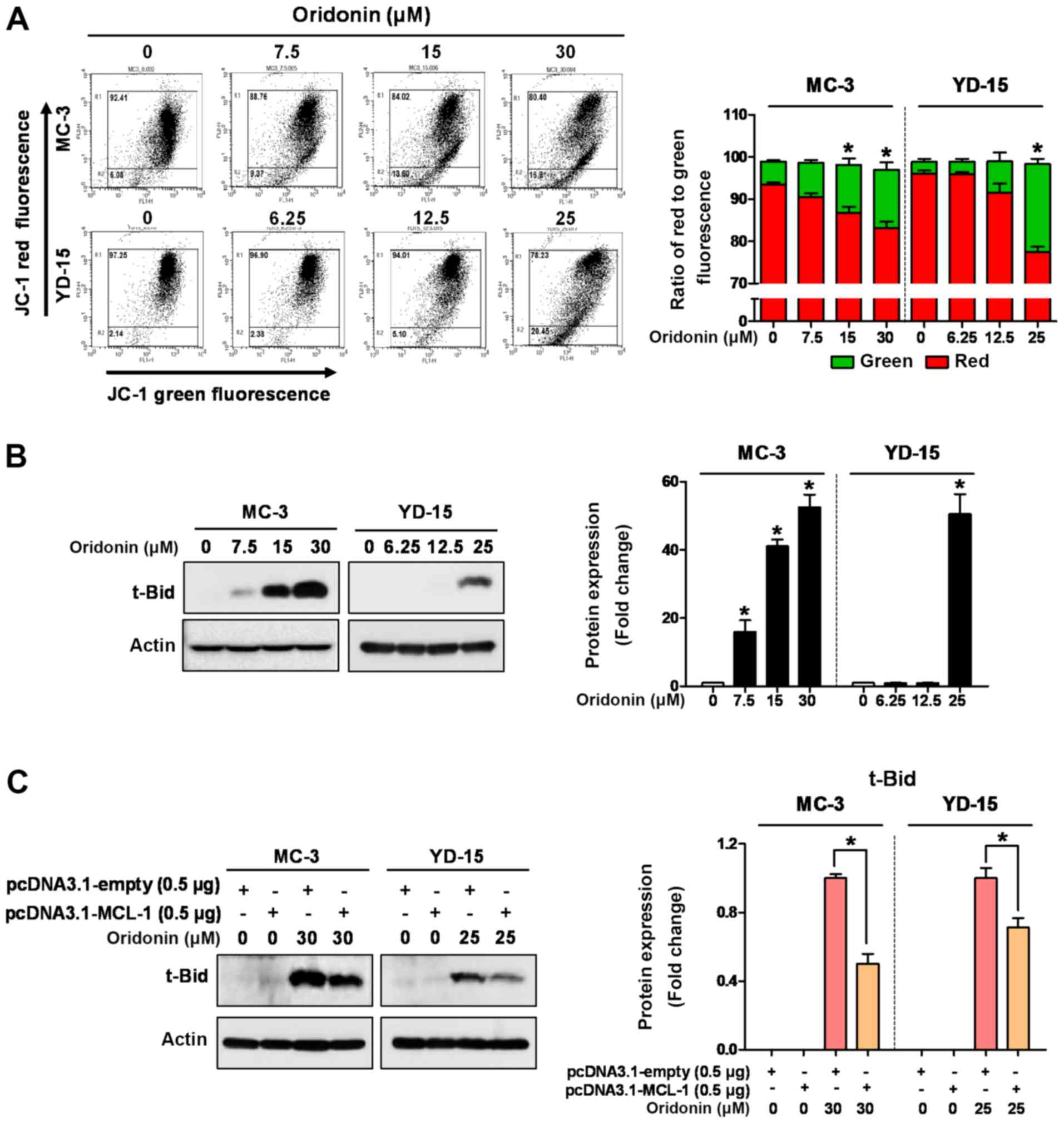
Oridonin induces mitochondrial apoptosis
by regulating the MCL‑1/t‑Bid signaling axis
The majority of apoptotic stimuli require
mitochondrial outer membrane permeabilization (MOMP) by
pro‑apoptotic proteins, such as Bax, Bak, Bim and t‑Bid (26). In the present study, JC‑1 staining
was performed to determine whether oridonin induces apoptosis
through mitochondrial dysfunction. Oridonin exerted a significant
decrease in red fluorescence compared to the vehicle control,
confirming that the loss of ΔΨm was induced in both cell lines
(Fig. 5A). Subsequently, to
further examine the mitochondrial‑dependent pathway involved in
oridonin-mediated apoptosis, the expression levels of pro-apoptotic
proteins were analyzed. As shown in Fig. 5B, the expression of t‑Bid was
markedly increased by oridonin in a concentration-dependent manner,
whereas the levels of other pro‑apoptotic proteins, such as Bak,
Bax and Bim were not commonly affected in both cell lines (Fig. S1). To ascertain the involvement of
MCL‑1 in t‑Bid expression during oridonin-induced apoptosis, an
MCL-1 expression vector was used. As was expected, the ectopic
expression of MCL‑1 protein significantly decreased the expression
of t‑Bid in the oridonin-treated cells (Fig. 5C). These results suggest that t‑Bid
may be an essential downstream molecule of MCL‑1 during the
oridonin-induced apoptosis of MEC cell lines.
Discussion
MCL‑1, a pro‑survival member of the Bcl‑2 family,
has been shown to be highly expressed in human malignancies, which
has led to increased attention in oral cancer, including other
solid tumors (27,28). Notably, human oral cancer cells
have been shown to exhibit an elevated expression of MCL-1 protein
compared to their normal counterparts, human oral keratinocytes,
while Bcl‑2 exhibits a negligible expression in most cells
(28,29). Furthermore, it has been reported
that MCL‑1 overexpression is resistant to Bcl‑2 family inhibitors,
such as paclitaxel, vincristine and gemcitabine, which are
well-known anticancer therapeutic agents (30,31).
Thus, the downregulation of MCL-1 may be a key step in promoting
the apoptosis of cancer cells and the use of natural compounds to
modulate the expression or function of MCL-1 protein has recently
been viewed as a promising alternative for the development of
preventive and therapeutic regimens (32,33).
Previous studies by the authors have found that natural products,
such as mithramycin A, fisetin and fucoidan inhibit the expression
of MCL-1 protein, leading to the apoptosis of human oral cancer
cells (29,34,35).
The authors have also previously reported that oridonin induces
apoptosis by increasing the expression of γH2AX in response to DNA
damage (18). Despite several
studies being published the apoptotic effects of oridonin, its mode
of action in downregulating MCL-1 has not yet been fully elucidated
(36,37). In the present study, for the first
time, to the best of our knowledge, it was found that oridonin
induced apoptosis through the downregulation of MCL-1 in human MEC
cell lines, independent of transcriptional regulation (Figs. 2 and 3). It was also demonstrated that the
overexpression of MCL-1 effectively abrogated oridonin-induced
apoptosis (Fig. 4). In present
study, these results support the notion that MCL-1 is an important
survival factor and that its downregulation is a potential
therapeutic strategy for MEC.
In response to apoptotic stimuli, the BH3‑only
proteins are upregulated to allow the activation of Bax and Bak for
oligomerization in the outer-mitochondrial membrane known as MOMP,
leading to the release of cytochrome c and other
apoptosis-inducing factors (38).
This indicates that the impairment of MOMP by the Bcl‑2 family is
the cornerstone of intrinsic apoptotic pathways. In the present
study, it was found that oridonin treatment significantly induced
the loss of MOMP through the upregulation of t‑Bid expression in a
concentration-dependent manner, accompanied by marked apoptotic
cell death. Liu et al obtained similar results,
demonstrating that the gradual increase in the MOMP destruction
rate by oridonin in the HPB‑ALL cell line occurred through the
upregulation of t‑Bid (39). Thus,
these findings emphasize that oridonin-induced apoptosis is closely
related to or is dependent on the loss of MOMP through the
MCL‑1/t‑Bid signaling axis in human MEC cell lines.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that the
downregulation of MCL-1 by oridonin promotes apoptosis through the
loss of MOMP and that t‑Bid is a critical downstream target of
MCL‑1 in the oridonin-induced apoptosis of human MEC cell lines.
Taken together, MCL-1 may prove to be a valuable molecular target
for oridonin-mediated anticancer activity. Oridonin is thus
recommended as a naturally derived chemotherapeutic drug candidate
for the treatment of MEC.
Supplementary Data
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT, and Future Planning
(grant nos. 2017R1D1A1B03029317, 2018R1D1A1B07043080 and
2019R1A2C10858 96).
Availability of data and materials
All data generated or analyzed during this study are
included in the current published article.
Authors' contributions
JMH designed the study, analyzed and interpreted the
results, and drafted the manuscript. KOH contributed to the
manuscript preparation, the study design and statistical analysis.
IHY managed the cellular samples and was involved in data
acquisition. CHA contributed to the quality control of the data. BJ
and WL performed the statistical analysis and wrote the methods
section. JAS performed the statistical analysis and was involved in
data interpretation. KAK and YCJ contributed to writing parts of
the Material and methods section and conducted data analysis. KAK
contributed to the interpretation of the revised data (Figs. 4 and 5) and wrote the revised manuscript. SDC
and SDH interpreted the results and supervised the study and
contributed to manuscript reviewing and editing, and critically
revised the manuscript. All authors were involved in the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
MEC
|
mucoepidermoid carcinoma
|
|
t‑Bid
|
truncated Bid
|
|
MCL‑1
|
myeloid cell leukemia‑1
|
|
PARP
|
poly (ADP-ribose) polymerase
|
|
MOMP
|
mitochondrial outer membrane
permeabilization
|
References
|
1
|
Venkata V and Irulandy P: The frequency
and distribution pattern of minor salivary gland tumors in a
government dental teaching hospital, Chennai, India. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 111:e32–e39. 2011. View Article : Google Scholar
|
|
2
|
Schwarz S, Stiegler C, Müller M, Ettl T,
Brockhoff G, Zenk J and Agaimy A: Salivary gland mucoepidermoid
carcinoma is a clinically, morphologically and genetically
heterogeneous entity: A clinicopathological study of 40 cases with
emphasis on grading, histological variants and presence of the
t(11;19) translocation. Histopathology. 58:557–570. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Birkeland AC, Foltin SK, Michmerhuizen NL,
Hoesli RC, Rosko AJ, Byrd S, Yanik M, Nor JE, Bradford CR, Prince
ME, et al: Correlation of Crtc1/3-Maml2 fusion status, grade and
survival in mucoepidermoid carcinoma. Oral Oncol. 68:5–8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rapidis AD, Givalos N, Gakiopoulou H,
Stavrianos SD, Faratzis G, Lagogiannis GA, Katsilieris I and
Patsouris E: Mucoepidermoid carcinoma of the salivary glands.
Review of the literature and clinicopathological analysis of 18
patients. Oral Oncol. 43:130–136. 2007. View Article : Google Scholar
|
|
5
|
Green B, Rahimi S and Brennan PA: Salivary
gland malignancies-an update on current management for oral
healthcare practitioners. Oral Dis. 22:735–739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan HK and Ismail S: Side effects of
chemotherapy among cancer patients in a Malaysian General Hospital:
Experiences, perceptions and informational needs from clinical
pharmacists. Asian Pac J Cancer Prev. 15:5305–5309. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas LW, Lam C and Edwards SW: Mcl‑1;
the molecular regulation of protein function. FEBS Lett.
584:2981–2989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Senichkin VV, Streletskaia AY, Zhivotovsky
B and Kopeina GS: Molecular comprehension of Mcl-1: From gene
structure to cancer therapy. Trends Cell Biol. 29:549–562. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Opferman JT, Letai A, Beard C, Sorcinelli
MD, Ong CC and Korsmeyer SJ: Development and maintenance of B and T
lymphocytes requires antiapoptotic MCL‑1. Nature. 426:671–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sieghart W, Losert D, Strommer S, Cejka D,
Schmid K, Rasoul‑Rockenschaub S, Bodingbauer M, Crevenna R, Monia
BP, Peck‑Radosavljevic M and Wacheck V: Mcl-1 overexpression in
hepatocellular carcinoma: A potential target for antisense therapy.
J Hepatol. 44:151–157. 2006. View Article : Google Scholar
|
|
11
|
Campbell KJ, Dhayade S, Ferrari N, Sims
AH, Johnson E, Mason SM, Dickson A, Ryan KM, Kalna G, Edwards J, et
al: MCL-1 is a prognostic indicator and drug target in breast
cancer. Cell Death Dis. 9:192018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin J, Fu D, Dai Y, Lin J and Xu T: Mcl-1
inhibitor suppresses tumor growth of esophageal squamous cell
carcinoma in a mouse model. Oncotarget. 8:114457–114462. 2017.
View Article : Google Scholar
|
|
13
|
Gény C, Rivière G, Bignon J, Birlirakis N,
Guittet E, Awang K, Litaudon M, Roussi F and Dumontet V: Anacardic
acids from knema hookeriana as modulators of Bcl‑xL/Bak and
Mcl‑1/Bid interactions. J Nat Prod. 79:838–844. 2016. View Article : Google Scholar
|
|
14
|
Choi ES, Kim JS, Kwon KH, Kim HS, Cho NP
and Cho SD: Methanol extract of Sanguisorba officinalis L. with
cytotoxic activity against PC3 human prostate cancer cells. Mol Med
Rep. 6:670–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clohessy JG, Zhuang J, de Boer J,
Gil‑Gómez G and Brady HJ: Mcl‑1 interacts with truncated Bid and
inhibits its induction of cytochrome c release and its role in
receptor-mediated apoptosis. J Biol Chem. 281:5750–5759. 2006.
View Article : Google Scholar
|
|
16
|
Bolaños JP, Moro MA, Lizasoain I and
Almeida A: Mitochondria and reactive oxygen and nitrogen species in
neurological disorders and stroke: Therapeutic implications. Adv
Drug Deliv Rev. 61:1299–1315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen
M and Wang Y: Oridonin induces apoptosis, inhibits migration and
invasion on highly-metastatic human breast cancer cells. Am J Chin
Med. 41:177–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang IH, Shin JA, Lee KE, Kim J, Cho NP
and Cho SD: Oridonin induces apoptosis in human oral cancer cells
via phosphorylation of histone H2AX. Eur J Oral Sci. 125:438–443.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin S, Shen JN, Wang J, Huang G and Zhou
JG: Oridonin induced apoptosis through Akt and MAPKs signaling
pathways in human osteosarcoma cells. Cancer Biol Ther. 6:261–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera
S and Ikejima T: Oridonin induces G2/M arrest and apoptosis via
activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK
survival pathway in murine fibrosarcoma L929 cells. Arch Biochem
Biophys. 490:70–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu HZ, Yang YB, Xu XD, Shen HW, Shu YM,
Ren Z, Li XM, Shen HM and Zeng HT: Oridonin induces apoptosis via
PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta
Pharmacol Sin. 28:1819–1826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Chen X, Qu S, Yao B, Xu Y, Wu J, Jin
Y and Ma C: Oridonin induces G2/M cell cycle arrest and
apoptosis via the PI3K/Akt signaling pathway in hormone-independent
prostate cancer cells. Oncol Lett. 13:2838–2846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Zhu L, Feng X, Zhang H, Luo Q and
Chen F: Oridonin induces G2/M cell cycle arrest and apoptosis in
human oral squamous cell carcinoma. Eur J Pharmacol. 815:282–289.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Shi D, Zhang L, Yang F and Cheng G:
Oridonin enhances the radiosensitivity of lung cancer cells by
upregulating Bax and downregulating Bcl‑2. Exp Ther Med.
16:4859–4864. 2018.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real‑time quantitative PCR and
the 2(‑Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akgul C: Mcl-1 is a potential therapeutic
target in multiple types of cancer. Cell Mol Life Sci.
66:1326–1336. 2009. View Article : Google Scholar
|
|
28
|
Maji S, Samal SK, Pattanaik L, Panda S,
Quinn BA, Das SK, Sarkar D, Pellecchia M, Fisher PB and Dash R:
Mcl-1 is an important therapeutic target for oral squamous cell
carcinomas. Oncotarget. 6:16623–16637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin JA, Jung JY, Ryu MH, Safe S and Cho
SD: Mithramycin A inhibits myeloid cell leukemia-1 to induce
apoptosis in oral squamous cell carcinomas and tumor xenograft
through activation of Bax and oligomerization. Mol Pharmacol.
83:33–41. 2013. View Article : Google Scholar
|
|
30
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei SH, Dong K, Lin F, Wang X, Li B, Shen
JJ, Zhang Q, Wang R and Zhang HZ: Inducing apoptosis and enhancing
chemosensitivity to gemcitabine via RNA interference targeting
Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother
Pharmacol. 62:1055–1064. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muller F, Cerella C, Radogna F, Dicato M
and Diederich M: Effects of natural products on Mcl-1 expression
and function. Curr Med Chem. 22:3447–3461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Won DH, Chung SH, Shin JA, Hong KO, Yang
IH, Yun JW and Cho SD: Induction of sestrin 2 is associated with
fisetin‑mediated apoptosis in human head and neck cancer cell
lines. J Clin Biochem Nutr. 64:97–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee HE, Choi ES, Shin JA, Lee SO, Park KS,
Cho NP and Cho SD: Fucoidan induces caspase-dependent apoptosis in
MC3 human mucoepidermoid carcinoma cells. Exp Ther Med. 7:228–232.
2014. View Article : Google Scholar
|
|
36
|
Zhang HP, Li GQ, Guo WZ, Chen GH, Tang HW,
Yan B, Li J, Zhang JK, Wen PH, Wang ZH, et al: Oridonin
synergistically enhances JQ1-triggered apoptosis in hepatocellular
cancer cells through mitochondrial pathway. Oncotarget.
8:106833–106843. 2017. View Article : Google Scholar :
|
|
37
|
Ikezoe T, Yang Y, Bandobashi K, Saito T,
Takemoto S, Machida H, Togitani K, Koeffler HP and Taguchi H:
Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits
the proliferation of cells from lymphoid malignancies in
association with blockade of the NF‑kappa B signal pathways. Mol
Cancer Ther. 4:578–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roy MJ, Vom A, Czabotar PE and Lessene G:
Cell death and the mitochondria: Therapeutic targeting of the BCL‑2
family‑driven pathway. Br J Pharmacol. 171:1973–1987. 2014.
View Article : Google Scholar :
|
|
39
|
Liu JJ, Huang RW, Lin DJ, Wu XY, Peng J,
Pan XL, Lin Q, Hou M, Zhang MH and Chen F: Antiproliferation
effects of oridonin on HPB‑ALL cells and its mechanisms of action.
Am J Hematol. 81:86–94. 2006. View Article : Google Scholar : PubMed/NCBI
|