Introduction
Nonmelanoma skin cancer (NMSC) is a common type of
malignant neoplasm in Caucasian populations (1). NMSC accounts for more than one-third
of all cancers in the US with an estimated incidence of >600,000
cases per year (2). Cutaneous
squamous cell carcinoma (CSCC) is the second most frequent type of
NMSC, accounting for ~20% of all NMSC cases (3). The long-term prognosis of patients
with NMSC remains unsatisfactory despite promising progress in the
diagnosis and treatment for CSCC over the past few decades. Thus,
developing effective treatment methods for CSCC should be a
priority for researchers. A number of genes including p16 and p53
have been confirmed to be involved in the pathogenesis of CSCC
(4, 5). In addition, non-coding RNAs (ncRNAs)
such as microRNA (miRNA/miR)-186 and long non-coding RNA (lncRNA)
LINC00520 have also been verified to be involved in the pathology
of CSCC (6,7). Despite these observations, there is
still a need to explore the specific pathogenesis behind CSCC.
Therefore, further research investigating the occurrence and
development of CSCC in the aspect of epigenetics is pivotal to
provide further guidance for clinicians.
In terms of structure, ncRNAs can be divided into
circular (circ)RNAs and linear ncRNAs. The majority of research has
focused on linear ncRNAs, especially miRNAs (8,9) and
lncRNAs (10-12), whereas studies regarding circRNAs
are rare, mainly since circRNAs were once considered by-products of
incorrect slicing compared with the numerous classical linear
ncRNAs (13). Andreeva and Cooper
(13) reported in 2015 that
circRNAs widely existed in animal and plant cell tissues and
possessed a number of specific biological characteristics. This
report has ensured that circRNAs gained attention from numerous
researchers (14). Recent studies
have indicated that circRNAs exert crucial effects in the
pathological process of a number of diseases, such as a variety of
tumor types, cardiovascular and digestive system diseases (15-18).
For instance, Su et al (19) have revealed that the circRNA cTFRC
regulates the expression of target genes through competitive
binding with miR-107, which eventually participates in the
pathogenesis of bladder cancer (19).
The results of circRNA expression microarray
revealed that hsa_circ_0070933 and hsa_circ_0070934
(circ-0070934), two circRNAs associated with the La
ribonucleoprotein 1B (LARP1B) gene, exhibited high
expression levels in CSCC (20).
Circ-0070934 is located at chr4:128995614-129012667, and its
associated-gene symbol is LARP1B (http://www.circbase.org). The present study aimed to
explore whether circ-0070934 may enhance the invasive and
proliferative capacities of tumor cells and participate in the
pathogenesis of CSCC.
In the present study, a series of experiments were
performed to confirm whether circ-0070934 may function as a
competing endogenous RNA (ceRNA) to modulate homeobox B7
(HOXB7) gene expression by collating miR-1236-3p in CSCC.
Overall, the present study aimed to explore whether
circ-0070934 may have a crucial role in CSCC pathogenesis
and to provide a novel molecular target for the therapy of
CSCC.
Materials and methods
Cell culture and transfection
Human embryonic cell line 293T, human keratinocyte
cell line HaCaT and CSCC cell lines A431, HSC-5, SCC13 and SCL-1
were purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and were authenticated by STR
profiling. All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA) in a humidified
environment with 5% CO2 at 37°C. Cells with high
viability were seeded in 6-well plates when they reached the
logarithmic growth phase and were subjected to transfections with 5
µl circ-0070934 small interfering (si)RNAs,
circ-0070934 short hairpin (sh)RNA vectors,
circ-0070934 overexpression plasmids, miR-1236-3p inhibitors
and miR-1236-3p mimics (synthesized by Shanghai GenePhama Co.,
Ltd.) and corresponding negative controls (NCs) using 5 µl
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) for
6 h at 37°C according to the manufacturer's instructions.
The cells were collected 24 h post-transfection for further in
vitro experiments. For in vivo studies, lentiviral
particles carrying scrambled or circ-0070934 shRNA vectors
(pLVX-shRNA 2-GFP-Puro; TSINGKE Biological Technology Co., Ltd.)
were generated in 293T cells. A431 cells were then infected with
the recombinant lentivirus, followed by selection with 2
µg/ml puromycin. Detailed sequences are presented in
Table SI.
RNA separation and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was utilized to extract total RNA from
CSCC cells. The purity of the RNA was measured using a UV
spectrophotometer. Subsequently, RNA was placed in a refrigerator
at -80°C for later use. cDNAs were synthesized using the
PrimeScript™ Reverse Transcription reagent kit (Takara Bio, Inc.)
at 37°C for 15 min and 85°C for 5 sec. The expression
levels of circRNAs, mRNAs and miRNAs were then measured using an
ABI 7900HT PCR instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.) by initial denaturation at 94°C for 5 min,
followed by 35 cycles of denaturation at 94°C for 30 sec,
annealing at 55°C for 30 sec and extension at 72°C
for 90 sec. The 2−ΔΔCq method (21) was used to calculate the relative
gene expression normalized by GAPDH and U6. The primers used in the
present study are listed in Table
SI.
RNase R digestion
Total RNAs (5 µg) from A431 and SCL-1 cells
were incubated at 37°C for 15 min, and RNase R (Epicentre;
Illumina, Inc.) was used to remove linear RNAs at the ratio of 6
units: 1 µg. After RNase R treatment, the expression of
circ-0070934 was detected using RT-qPCR.
Sanger sequencing
The amplified PCR product was inserted into the T
vector (TSINGKE Biological Technology) for Sanger sequencing. After
determination of the full length sequence, different primers were
constructed by Invitrogen (Shanghai, China). Sanger sequencing was
performed by Realgene (Nanjing, China).
Western blotting detection
Cells in each group were collected and mixed into 1
ml prepared lysis buffer (Beyotime Institute of Biotechnology) in
each culture dish, followed by 5 min of lysis on ice. Lysate
solutions were collected using RIPA lysis buffer (Beyotime
Institute of Biotechnology) to extract total proteins. Protein
concentrations were measured by bicinchoninic acid (BCA) assay
(Beyotime Institute of Biotechnology). Protein samples (80
µg/lane) were subjected to 10% SDS-PAGE and transferred onto
a PVDF membrane, and the membranes were blocked for 1 h in 5%
skimmed milk at room temperature and incubated with anti-HOXB7
(1:1,000; cat. no. 12613-1-AP; ProteinTech Group, Inc.) and
anti-GAPDH (1:1,000; cat. no. AG019; Beyotime Institute of
Biotechnology) antibodies at 4°C overnight. The washing
reagent was TBS containing 0.1% Tween-20 (Beyotime Institute of
Biotechnology). The next day, the membranes were incubated with
anti-rabbit horseradish peroxidase (HRP)-conjugated (cat. no.
A0208) or anti-mouse HRP-conjugated (cat. no. A0216) secondary
antibodies (1:1,000; Beyotime Institute of Biotechnology) at
37°C for 2 h. Protein bands were detected on X-ray film
using an enhanced chemiluminescence detection system (Amersham;
Cytiva).
Bioinformatics prediction
The potential targets of circ-0070934 were
predicted through bioinformatics analyses using RegRNA (http://regrna2.mbc.nctu.edu.tw/) and
circinteractome (https://circinteractome.nia.nih.gov/). The potential
target genes of miR-1236-3p were searched and intersected using
TargetScan (http://www.targetscan.org/vert_72/) and miRDB
(http://mirdb.org/).
Dual-luciferase reporter assay
The 3′UTR sequences of circ-0070934 and
homeobox B7 (HOXB7) were downloaded from the NCBI website
(https://www.ncbi.nlm.nih.gov/), and
circ-0070934 wild-type (WT) 3′UTR and HOXB7 WT 3′UTR
sequences, as well as circ-0070934 mutant (MUT) 3′UTR and
HOXB7 MUT 3′UTR sequences were constructed. Subsequently,
5×103 cells A431 and SCL-1 cells were seeded onto
96-well plates and co-transfected with 80 ng WT or MUT plasmids and
50 pmol/l miR-1236-3p mimics or NC using Lipofectamine®
3000 (Thermo Fisher Scientific, Inc.) for 6 h at 37°C
according to the manufacturer's instructions. At 48 h
post-transfection, fluorescence intensity was detected using the
dual-luciferase reporter gene detection system and normalized to
that of Renilla luciferase (Promega Corporation).
Transwell invasion assay
Transwell assays were used to determine the invasive
abilities of CSCC cells. The upper chamber was pre-coated with
Matrigel (BD Biosciences) at 37°C for 30 min, and
4×105 transfected A431 and SCL-1 cells were placed in
100 µl DMEM without serum, whereas 500 µl DMEM
containing 10% FBS was placed in the bottom chamber. After 24-h
incubation at 37°C, the cells on the upper surface of the
membrane were wiped using cotton swabs, and the culture medium was
removed. Formaldehyde was used at room temperature for 10 min to
fix the cells, which were subsequently stained using 0.5% crystal
violet at room temperature for 30 min. The number of invaded cells
was counted in 10 randomly selected fields of view under a light
microscope (×200 magnification).
Cell proliferation assays
After transfection, A431 and SCL-1 cells were
cultured in 96-well plates (100 µl/well) at 1×106 cells/ml.
After 24 h, 10 µl Cell Counting Kit-8 (CCK-8; Beyotime
Institute of Biotechnology) solution was added and incubated with
5% CO2 at 37°C for 1 h. Finally, the culture
medium was removed, and the absorbance at was measured at 450 nm
using the TECAN infinite M200 multimode micro-plate reader (Tecan
Group, Ltd.).
Cells seeded in 96-well plates with 5×103
cells/well were labeled with 50 µM medium containing
5-ethynyl-2′-deoxy-uridine (EdU; Guangzhou Ribobio Co., Ltd.) for 2
h, fixed with 4% paraformaldehyde and 0.5% Triton X-100 and
incubated with anti-EdU working solution according to the
manufacturer's instructions. Cell nuclei were dyed with DAPI
(Beyotime Institute of Biotechnology). A total of five randomly
selected fields of view in each well were captured using
fluorescence microscopy (×200 magnification) to calculate
EdU-positive cells. The experiments were performed in
triplicate.
Flow cytometry assay
At 24 h post-transfection, 1×106 A431 and
SCL-1 cells were cultured in 6-well plates. After 48 h, Annexin
V-FITC/Propidium Iodide kit (BD Biosciences) was used to stain the
treated cells according to the manufacturer's instructions. Next,
the samples were detected with a BeamCyte flow cytometer (Changzhou
Beam Diagnostics Automation Co. Ltd.) and analyzed early and late
apoptosis with CytoSYS 1.0 software (Changzhou Beam Diagnostics
Automation Co. Ltd.). All assays were repeated three times
independently.
Determination of localization by
subcellular fractionation
The PARIS kit (Thermo Fisher Scientific, Inc.) was
used to separate cytoplasmic and nuclear RNAs according to the
manufacturer's protocol. Total RNA was separated from each fraction
and determined using RT-qPCR, with U6 as a nuclear control marker
and GAPDH as a cytoplasmic control marker.
RNA binding protein immunoprecipitation
(RIP) assay
Magna RIP kit (EMD Millipore) was utilized to
perform the RIP assay as previously described (22). The immunoprecipitation mixture was
applied for RNA extraction and detection.
Mouse xenograft model
A total of 6 female BALB/c nude mice (age, 6 weeks;
weight, 18-22 g) were purchased from the Model Animal Research
Center of Nanjing University. Mice were housed in a sterile room
under a 12-h light/dark cycle at ~23°C and 50% humidity,
with ad libitum access to food and water. A total of
2×106 A431 cells transfected with
hsa_circ_0070934 shRNA or NC were injected subcutaneously
into the BALB/c nude mice. Tumor volumes were calculated every 4
days using the following formula: Tumor volume = (length ×
width2) / 2. At 4 weeks post-injection, the mice were
anesthetized by intraperitoneal injection of sodium pentobarbital
(40 mg/kg) and sacrificed by 10% formalin perfusion fixation of
central nervous system; death was confirmed by complete stopping of
the heartbeat and breathing, as well as disappearance of the foot
withdrawal reflex. The tumor tissues were isolated and weighed.
Then, the tumor tissues were analyzed using a TUNEL Apoptosis
Detection kit (cat. no. C1086; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. The study was
approved by the Ethics Committee of The Affiliated Huaian No. 1
People's Hospital of Nanjing Medical University, and the
experiments were performed following the National Institutes of
Health guidelines on animal welfare.
Statistical analysis
SPSS 22.0 (IBM Corp.) and GraphPad Prism 6.0
(GraphPad Software, Inc.) were used for statistical processing. For
normally distributed data with equal variance, the difference was
evaluated by two-tailed Student's t-test (two group comparisons) or
one-way ANOVA followed by the Bonferroni post hoc test (multigroup
comparisons) as appropriate. For non-normally distributed data or
data with unequal variances, the difference was evaluated by a
nonparametric Mann-Whitney U test (two group comparisons) or the
Kruskal-Wallis test followed by the Bonferroni post hoc test
(multigroup comparisons). Data are presented as the mean ± SD.
P<0.05 was considered to indicate a statistically signifi-cant
difference.
Results
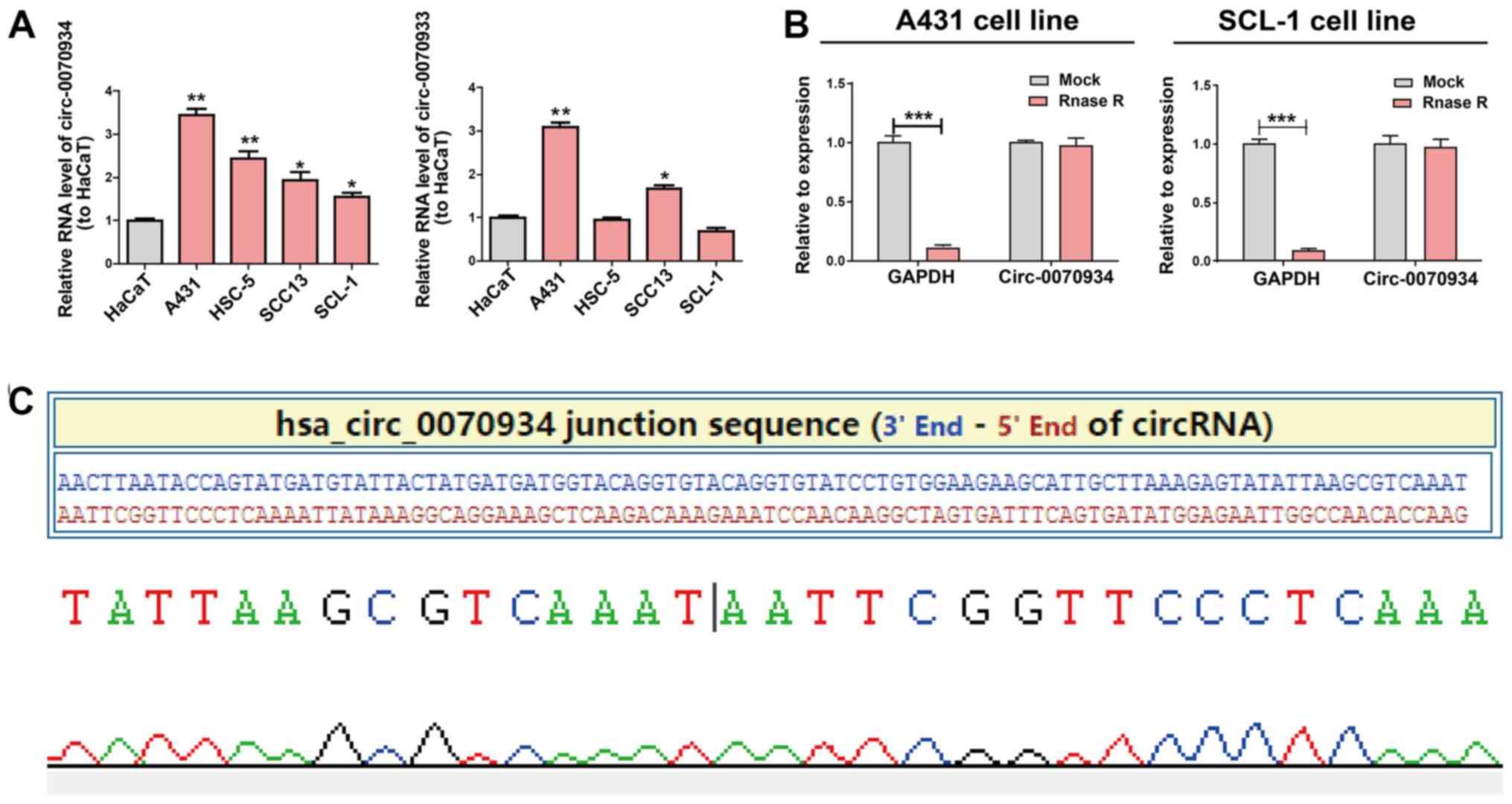
Expression of circ-0070934 in CSCC
Based on the results of circRNA expression
microarray (20),
hsa_circ_0070933 and hsa_circ_0070934 were selected
as the candidate circRNAs of interest. The expression patterns of
these two circRNAs in CSCC cell lines and a human keratinocyte cell
line (HaCaT) were examined using RT-qPCR. circ-0070934, but
no circ-0070933, exhibited a stable high level in CSCC cell
lines compared with those in HaCaT cells. (Fig. 1A). Thus, circ-0070934 was
selected for subsequent experiments. RNase R was added to the total
RNA samples to further determine the circular nature of
circ-0070934, as RNase R dissolved linear RNAs that
contained a free 3′ terminus, but did not affect circRNAs. The
results revealed that circ-0070934 was indeed a circRNA with
resistance to RNase R digestion (Fig.
1B). The circ-0070934 sequence was amplified using its
primer and was identified to be identical to the sequence in
circbase through Sanger sequencing (Fig. 1C).
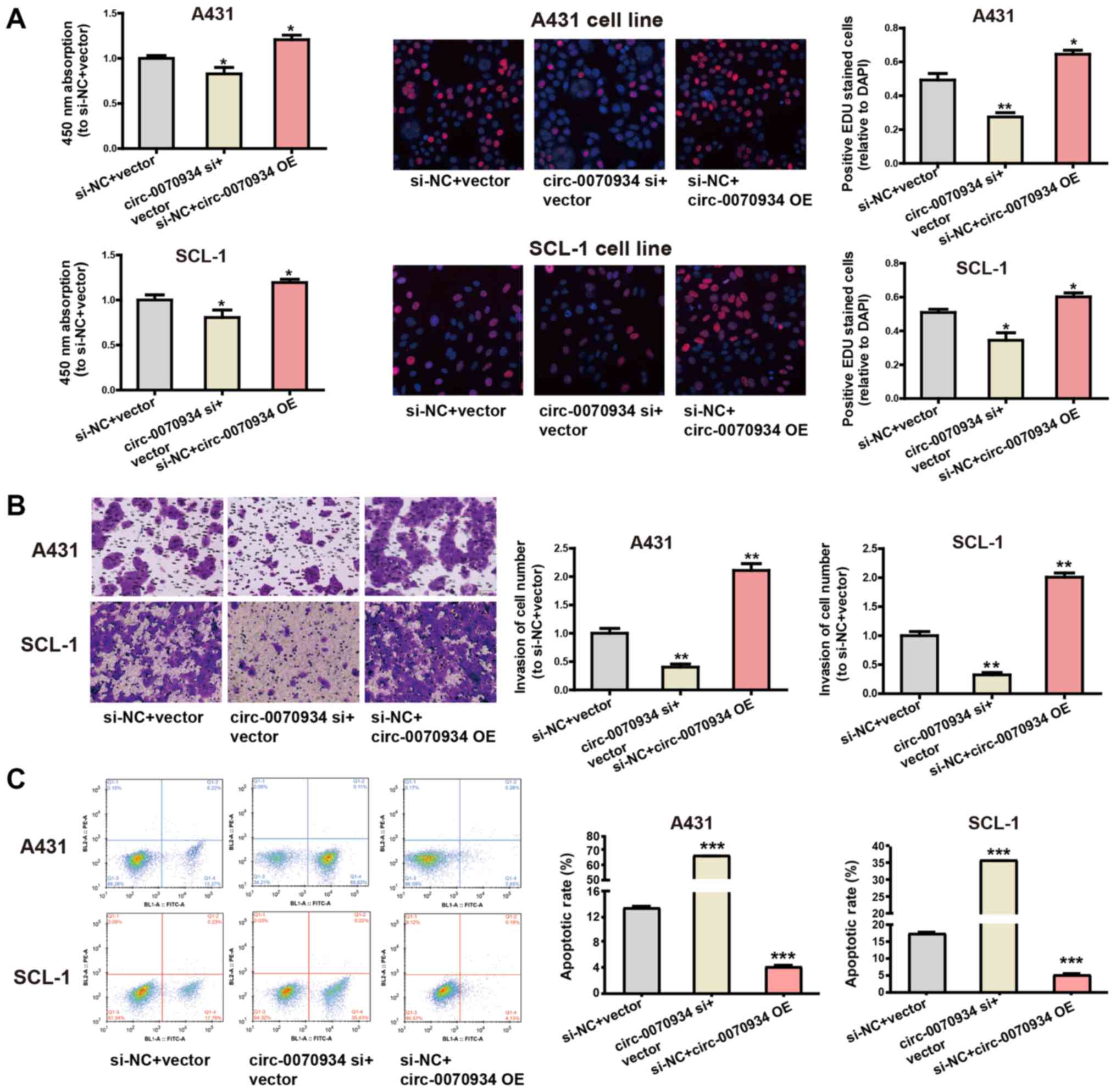
Overexpression of circ-0070934 stimulates
the invasion and proliferation of CSCC cells and inhibits
apoptosis
The expression of circ-0070934 was silenced
using targeted siRNAs, and circ-0070934 overexpression
plasmids were used for ectopic expression. After the
circ-0070934 overexpression plasmids or siRNAs were
transfected into CSCC cells, the transfection efficiency was
determined by RT-qPCR (Fig. S1A).
According to CCK-8 and EdU assays, knockdown of circ-0070934
decreased the rate of cell proliferation, whereas
circ-0070934 overexpression enhanced the proliferative
capacity of CSCC cells compared with that of the control cells
(Fig. 2A). In addition, Transwell
assay results demonstrated that knockdown of circ-0070934
inhibited the invasive capability of CSCC cells, whereas
circ-0070934 overexpression promoted the invasive capability
of CSCC cells compared with that of the control cells (Fig. 2B). Flow cytometric analysis of
apoptosis revealed that knockdown of circ-0070934 promoted
CSCC cell apop-tosis, which was suppressed by circ-0070934
overexpression compared with that in the control cells (Fig. 2C). Overall, these results indicated
that elevated circ-0070934 enhanced the proliferative and
invasive capabilities of CSCC cells, as well as inhibited
apoptosis.
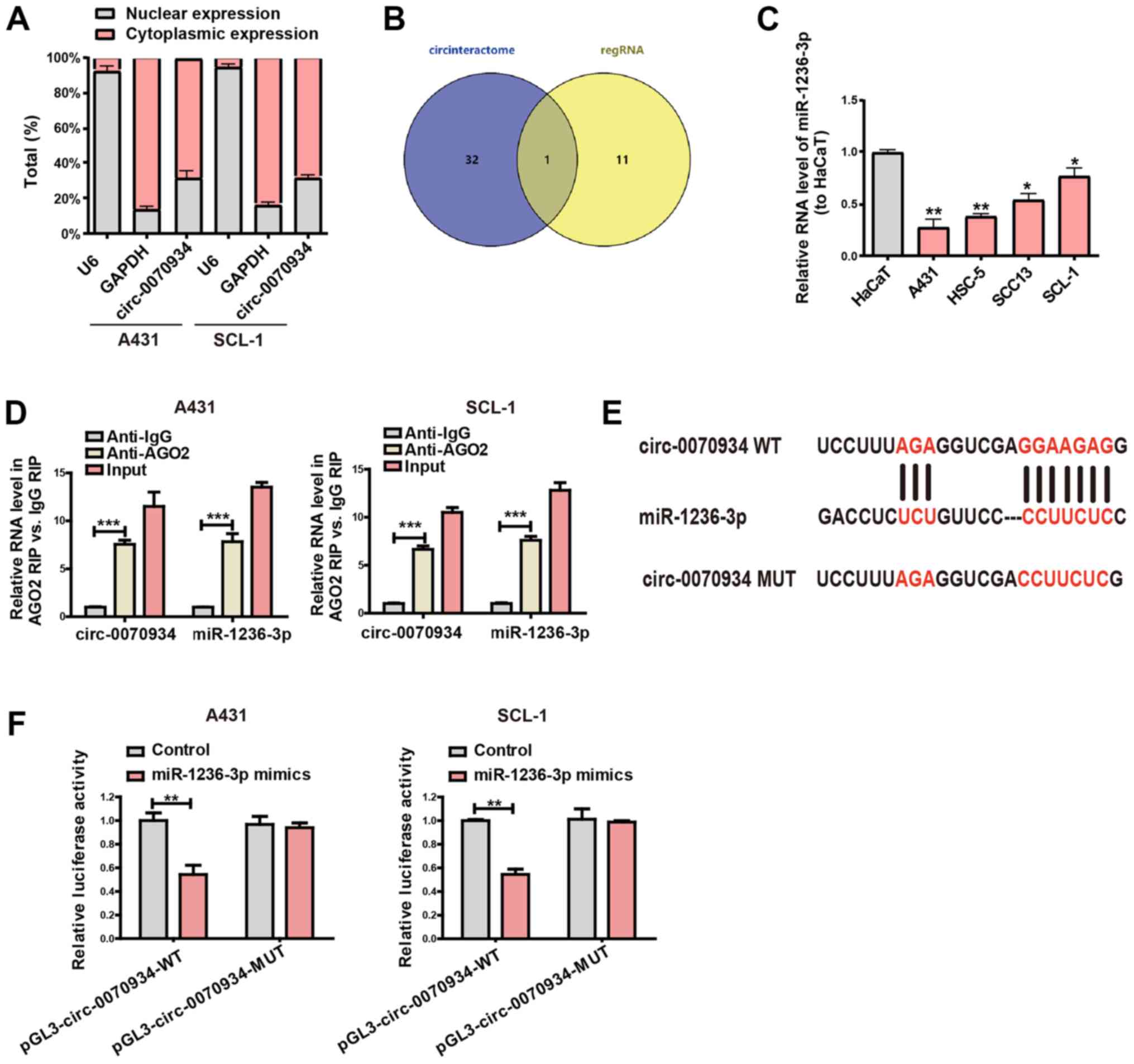
Circ-0070934 serves as a sponge for
miR-1236-3p
To further analyze the exact mechanisms of action
through which circ-0070934 acts in CSCC, the subcellular
localization of circ-0070934 was determined using separation
experiments in the cytoplasm and the nucleus. The results
demonstrated that circ-0070934 was primarily located within
the cytoplasm of CSCC cells (Fig.
3A). This observation suggested that circ-0070934 may
function through post-translational modifications. Considering that
circRNAs can serve as miRNA sponge (23), the potential targets of
circ-0070934 were predicted through bioinformatics analyses
(RegRNA, circinteractome), and miR-1236-3p was indicated to be the
potential complementary miRNA that can bind to circ-0070934
(Fig. 3B). To determine the
association between circ-0070934 and the predicted miRNA,
miR-1236-3p expression levels in CSCC cell lines and HaCaT cells
were measured; the results of RT-qPCR demonstrated that compared
with that in HaCaT, miR-1236-3p expression in the CSCC cell lines
was downregulated (Fig. 3C). In
addition, the results of the RIP binding assay demonstrated that
the levels of circ-0070934 and miR-1236-3p were higher in
the anti-Ago2 group than that in the anti-normal IgG group, which
indicated that circ-0070934 and miR-1236-3p were in the same
RNA-induced silencing complex (Fig.
3D). Subsequently, plasmids containing the MUT or WT
circ-0070934 sequences were constructed (Fig. 3E). Dual-luciferase reporter gene
assays demonstrated that cells co-transfected with
circ-0070934 WT and miR-1236-3p mimics exhibited reduced
luciferase activity compared with that in the control groups
(Fig. 3F). However, the relative
luciferase activity was not notably different between the cells
co-transfected with circ-0070934 MUT and miR-1236-3p mimics
and those transfected with the NC (Fig. 3F). These results indicated that
circ-0070934 may serve as a collator for miR-1236-3p.
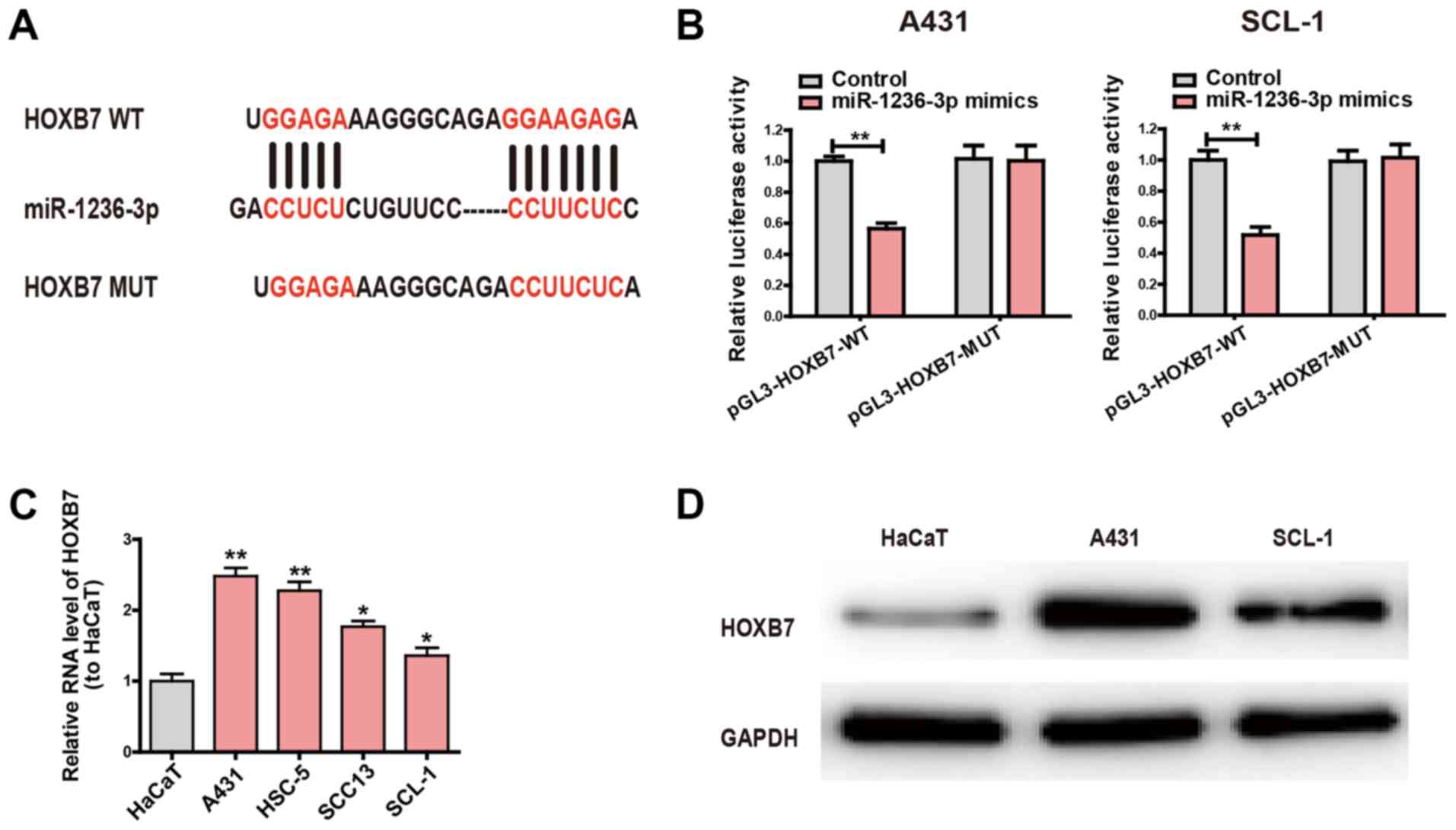
Circ-0070934 regulates the miR-1236-3p
target gene HOXB7
The potential target genes of miR-1236-3p were
searched and intersected by bioinformatics analysis (TargetScan,
miRDB). It was identified that the binding site between HOXB7 and
miR-1236-3p was highly consistent with that of circ-0070934
and miR-1236-3p. In order to further examine the interactions
between miR-1236-3p and HOXB7, pGL3-HOXB7-WT and pGL3-HOXB7-MUT
plasmids were constructed (Fig.
4A), which were then used to transfect CSCC cells. Compared
with the control group, cells co-transfected with pGL3-HOXB7-WT and
miR-1236-3p mimics exhibited significantly decreased luciferase
activity; however, luciferase activity did not change in cells
co-transfected with pGL3-HOXB7-MUT and miR-1236-3p mimics (Fig. 4B). Subsequently, the mRNA levels of
HOXB7 in the CSCC cell lines was examined. Compared with HaCaT, the
CSCC cell lines exhibited higher HOXB7 expression levels (Fig. 4C). Similarly, the expression levels
of the HOXB7 protein in A431 and SCL-1 cells were also increased
compared with those in HaCaT cells (Fig. 4D). These results suggested that
HOXB7 was the target gene of miR-1236-3p.
Following from these observations, the present study
explored whether circ-0070934 was able to bind miR-1236-3p
to mediate the expression of HOXB7. In cells transfected with the
circ-0070934 siRNA, the protein and mRNA expression levels
of HOXB7 were decreased compared with those in cells transfected
with the NC (Fig. 5A and B).
However, co-transfection with the miR-1236-3p inhibitor prevented
the circ-0070934 siRNA-induced downregulation of HOXB7
(Fig. 5A and B). In cells treated
with the miR-1236-3p mimics, the mRNA and protein expression levels
of HOXB7 were reduced, but this reduction was reversed by
co-transfection with circ-0070934 overex-pression plasmids
(Fig. 5C and D). Overall, these
results demonstrated that circ-0070934 upregulated HOXB7
expression by suppressing miR-1236-3p.
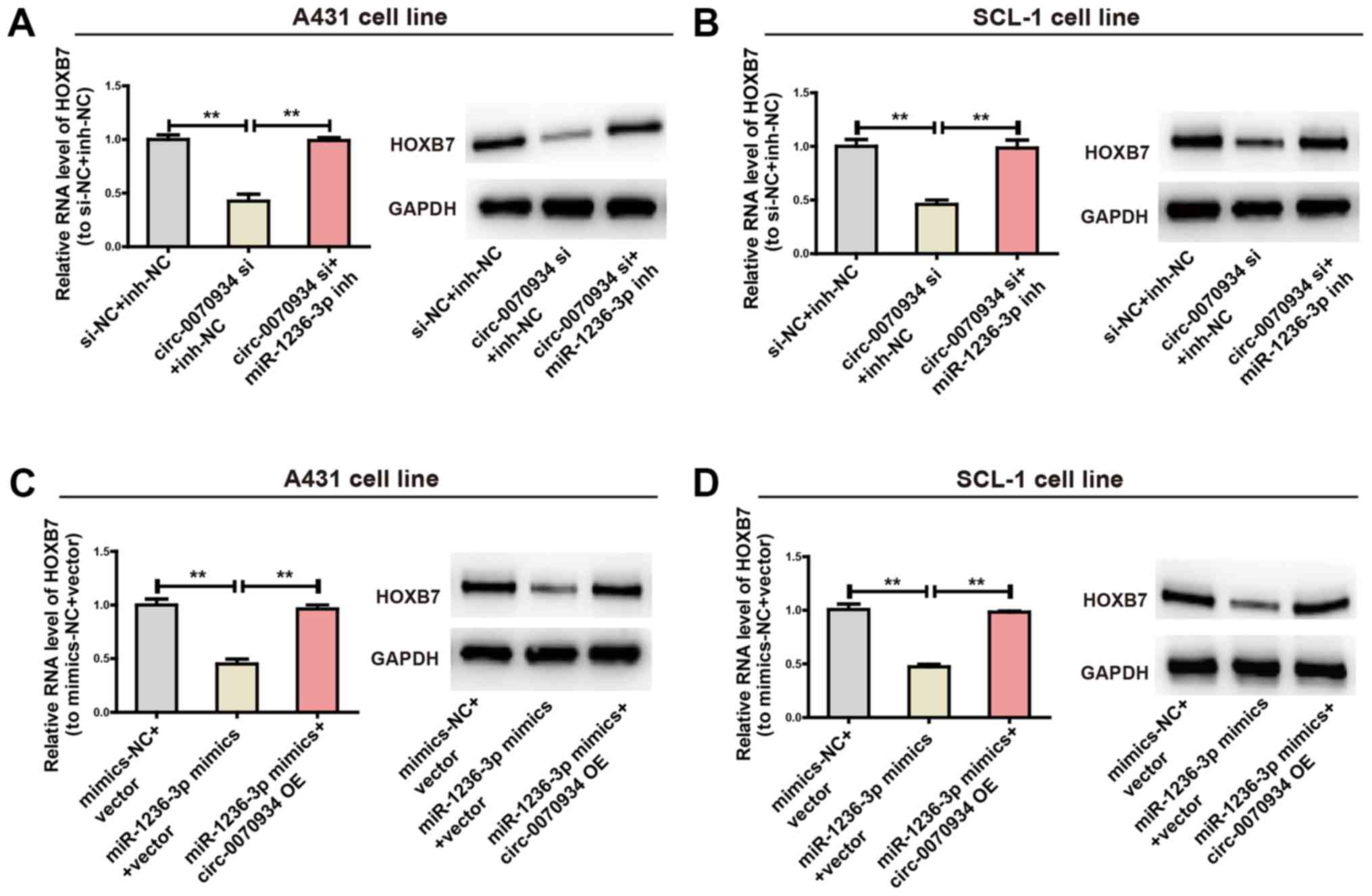 | Figure 5The circ-0070934/miR-1236-3p
regulatory axis is crucial for the expression of HOXB7. (A and B)
After CSCC cells were transfected with circ-0070934 siRNAs
with or without miR-1236-3p inhibitors, the mRNA and protein
expression levels of HOXB7 mRNA and protein were determined, with
GAPDH used as a control. (C and D) After CSCC cells were
transfected with miR-1236-3p mimics with or without
circ-0070934 overexpression plasmids, the mRNA and protein
expression levels of HOXB7 were detected. All experiments were
conducted in triplicate. Data are reported as the mean ± SD.
**P<0.01. CSCC, cutaneous squamous cell carcinoma;
circ, circular RNA; miR, microRNA; HOX7B, homeobox 7B; siRNA, small
interfering RNA; NC, negative control; inh, inhibitor; OE,
overexpression plasmid. |
The circ-0070934/miR-1236-3p regulatory
axis is pivotal for CSCC cell function
The role of miR-1236-3p in CSCC cell function was
analyzed. The effects of transfections with the miRNA mimics or
inhibitors on miR-1236-3p expression levels in CSCC cells was
determined by RT-qPCR (Fig. S1B).
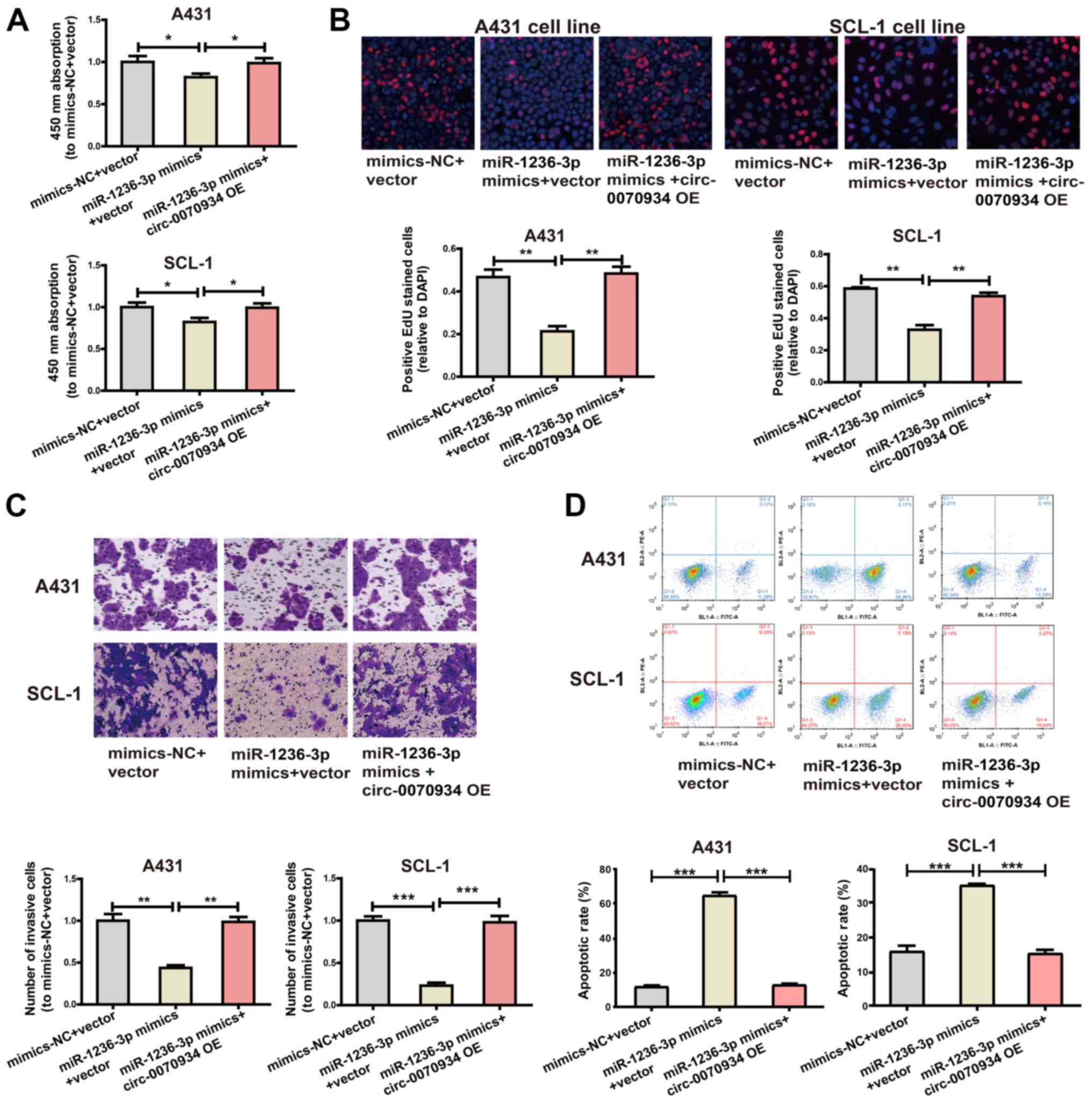
Cell proliferative and invasive capabilities were impeded by
miR-1236-3p mimics, whereas the overexpression of
circ-0070934 exerted the opposite effect (Fig. 6A-C). In addition, the miR-1236-3p
mimics promoted CSCC cell apop-tosis, whereas overexpression of
circ-0070934 suppressed apoptosis (Fig. 6D). These results further identified
the regulatory role of the circ-0070934/miR-1236-3p/HOXB7
axis on CSCC cell function.
Knockdown of circ-0070934 in tumors
inhibits CSCC growth
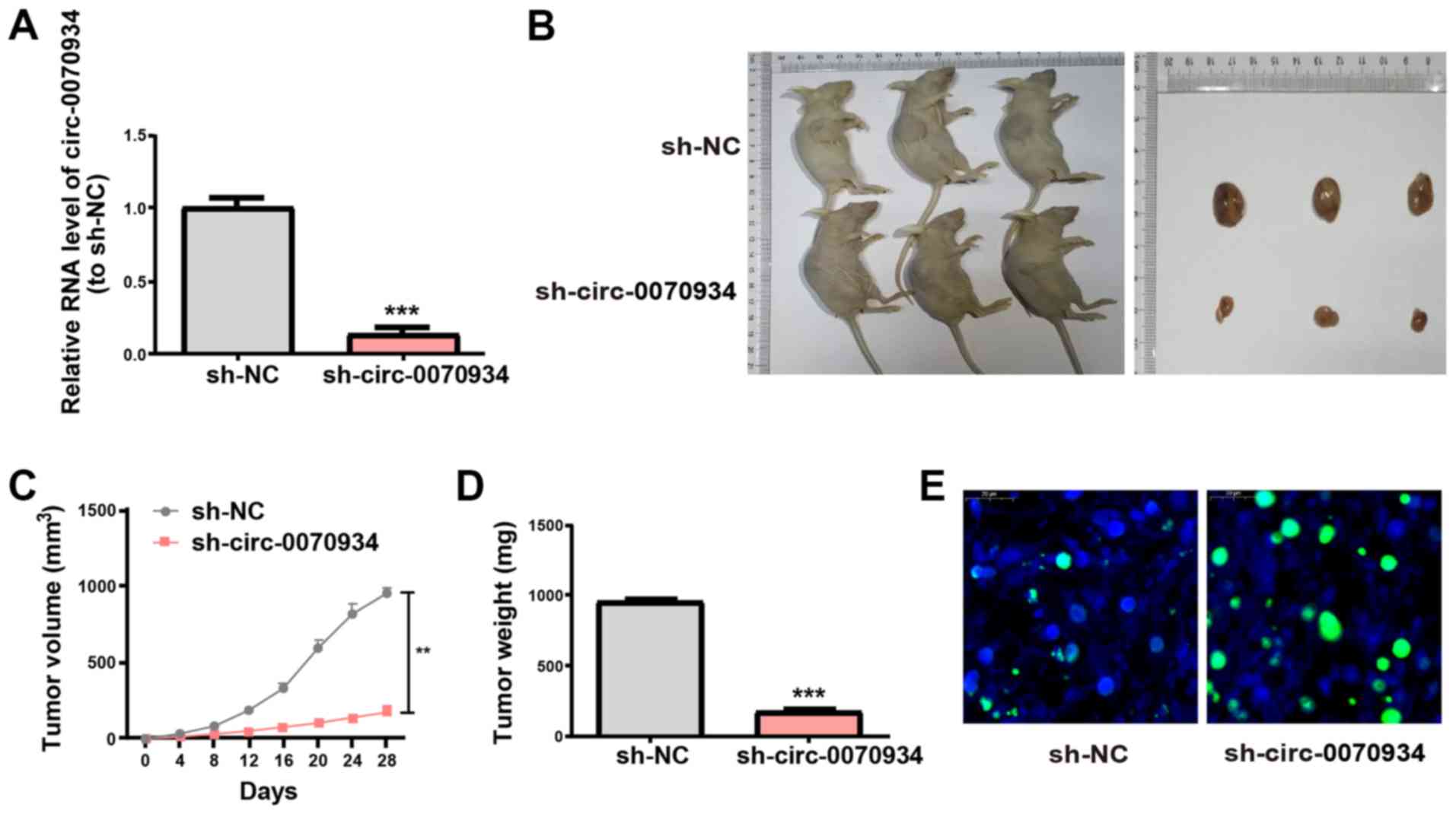
To investigate the role of circ-0070934 in
CSCC tumor growth in vivo, A431 cells transfected with
scrambled or circ-0070934 shRNAs were subcutaneously
injected into nude mice. After the scrambled or circ-0070934
shRNAs were transfected into A431 cells, the transfection
efficiency was detected (Fig. 7A).
The results of the xenograft assay demonstrated that knockdown of
circ-0070934 decreased the tumor volume and weight after
four weeks after tumor inoculation (Fig. 7B-D). In addition, TUNEL assays
demonstrated that xenograft tumors from the circ-0070934
shRNA group appeared to exhibit higher levels of apoptosis compared
with those from the sh-NC group (Fig.
7E). These results were consistent with the in vitro
results of the present study.
Discussion
Previous studies have demonstrated that genes have
various methods of transcriptional regulation. For example, miRNA,
as an important regulatory factor, is a short-chain RNA with a
length of ~22 nt that can serve a negative regulatory role on
target gene expression by blocking the degradation and translation
of the target gene (24). Numerous
ncRNAs also share binding sites with miRNAs, appearing to collate
miRNAs within cells, thus preventing miRNAs from inhibiting their
target genes and subsequently allowing for the upregulation of the
expression levels of the target gene; this mechanism of action is
termed the ceRNA mechanism (25).
Numerous recent studies have reported that circRNAs can regulate
the invasion and proliferation of tumor cells in a similar manner
to that of ceRNAs (26-29); however, limited attention has been
paid to the mechanism of circRNA action in CSCC as ceRNAs. Thus,
the present study aimed to investigate whether circ-0070934
may be used as a ceRNA to affect CSCC cell proliferation and
invasion.
The results of the present study demonstrated that
circ-0070934 expression levels in various CSCC cell lines
were higher compared with those in the normal human kerati-nocyte
cell line HaCaT. This result is consistent with a previous study,
which has demonstrated that circ-0070934 expression is
notably increased in CSCC samples compared with that in the
corresponding control samples (20); however, the effect of
circ-0070934 on CSCC cell function has yet to be confirmed.
Additionally, knockdown of circ-0070934 expression inhibited
the invasion and proliferation of CSCC cells and stimulated
apoptosis compared with that in the negative control group. This
suggested that circ-0070934 may be a pivotal factor,
positively modulating CSCC cell proliferation and serving as an
oncogene. Therefore, investigating the mechanism of action behind
the effects of circ-0070934 on CSCC cell proliferation may
be valuable for evaluating the occurrence, development and
metastasis of CSCC.
Through separation of RNAs in the nucleus and the
cytoplasm, the present study verified that the subcellular
localization of circ-0070934 was primarily in the cytoplasm,
indicating that circ-0070934 may act as a ceRNA. RIP assays
demonstrated that circ-0070934 and miR-1236-3p were in the
same RNA-induced silencing complex. And subsequent dual-luciferase
reporter assays demonstrated that circ-0070934 was a
molecular collator that upregulated HOXB7 expression through
competitively binding miR-1236-3p. Previous studies have
demonstrated that miR-1236-3p exerts an inhibitory effect on
numerous tumor types such as lung adenocarcinoma, gastric and
bladder cancer (30-32). Wang et al (33) have demonstrated that miR-1236-3p
targets ZEB1 in high-grade serous ovarian carcinoma, reducing the
migratory and invasive abilities of the cells (33). The results of the present study
demonstrated that miR-1236-3p was downregulated in CSCC cell lines,
and that the miR-1236-3p mimics inhibited CSCC cell invasion and
proliferation as well as promoted apoptosis. In addition, HOXB7 was
expressed at a higher level in CSCC cell lines compared with that
in the normal control. Transfection with circ-0070934 siRNAs
reduced the expression of HOXB7, which was consistent with the
inhibitory effect of the miR-1236-3p mimics on HOXB7 expression
levels. Of note, the invasive and proliferative capabilities of
CSCC cells were inhibited by the miR-1236-3p mimics compared with
those in the NC group, and this inhibition was reversed by
overexpressing circ-0070934. In addition, the miR-1236-3p
mimics increased the apoptotic rates of CSCC cells, whereas
co-transfection with circ-0070934 overexpression plasmids
rescued apoptosis. These results suggested that the
circ-0070934/miR-1236-3p/HOXB7 regulatory axis may be
involved in the occurrence and development of CSCC by modulating
the invasive and proliferative abilities of CSCC cells and
regulating their apoptosis.
HOX genes encode the transcription factor family
that is crucial for growth regulation and differentiation in the
process of embryonic development and maintaining adult tissue
homeostasis (34) These genes
serve roles in tumor cell migration, proliferation, invasion and
apoptosis, and are often abnormally regulated in cancer (35,36).
A total of 39 HOX genes are classified into chromosomal clusters
(A, B, C and D) in humans, with each ~100 kb long, and they are
located on chromosomes 7, 17, 2 and 12, respectively (37). The transcription factor HOXB7, a
member of class I HOX genes, exerts a pivotal effect on
tumorigenesis in several types of cancer, including gastric
(38), pancreatic (39), lung (40), oral squamous (41) and breast (42) cancers. Tu et al (43) have reported that HOXB7 upregulation
is associated with poor prognosis in patients with gastric cancer.
Gao and Chen (44) have
demonstrated that specific HOXB7 knockdown impedes CSCC cell
migration and invasion and triggers apoptosis through the
Wnt/β-catenin signaling pathway. The results of the present study
demonstrated that overexpression of circ-0070934 led to an
increase in the expression levels of HOXB7, a target of
miR-1236-3p, which may result in abnormal proliferation, invasion
and apoptosis of CSCC cells. However, the present study had a
number of limitations. Firstly, tissue samples from patients with
CSCC are required to further explore the clinical value of
circ-0070934. Secondly, in situ hybridization
fluorescence would be valuable to verify the association between
circ-0070934 and miR-1236-3p in future studies.
Additionally, whether there are other target genes or miRNAs which
can interact with circ-0070934 needs to be explored.
In conclusion, the present study revealed the
significance of circ-0070934 modulation of HOXB7 expression
levels by acting as a ceRNA and collating miR-1236-3p in the
pathogenesis of CSCC. Thus, further studies investigating circRNAs
may be clinically valuable for diagnosing and treating CSCC and
other diseases.
Supplementary Data
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
DWZ and CRZ designed the experiments. DWZ and HYW
performed the experiments. CRZ and DDW wrote the manuscript. All
authors discussed the results and revised the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Huaian No. 1 People's Hospital of Nanjing Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Apalla Z, Lallas A, Sotiriou E, Lazaridou
E and Ioannides D: Epidemiological trends in skin cancer. Dermatol
Pract Concept. 7:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leiter U, Eigentler T and Garbe C:
Epidemiology of skin cancer. Adv Exp Med Biol. 810:120–140.
2014.PubMed/NCBI
|
|
3
|
Newlands C, Currie R, Memon A, Whitaker S
and Woolford T: Non-melanoma skin cancer: United Kingdom National
Multidisciplinary Guidelines. J Laryngol Otol. 130(Suppl 2): pp.
S125–S132. 2016, View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Satgunaseelan L, Chia N, Suh H, Virk S,
Ashford B, Lum T, Ranson M, Clark J and Gupta R: p16 expression in
cutaneous squamous cell carcinoma of the head and neck is not
associated with integration of high risk HPV DNA or prognosis.
Pathology. 49:494–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen H, Takahara M, Xie L, Takeuchi S, Tu
Y, Nakahara T, Uchi H, Moroi Y and Furue M: Levels of the
EMT-related protein Snail/Slug are not correlated with p53/p63 in
cutaneous squamous cell carcinoma. J Cutan Pathol. 40:651–656.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu X, Liu Y, Ai P, He S, Liu L, Chen C,
Tan Y and Wang T: MicroRNA-186 promotes cell proliferation and
inhibits cell apoptosis in cutaneous squamous cell carcinoma by
targeting RETREG1. Exp Ther Med. 17:1930–1938. 2019.PubMed/NCBI
|
|
7
|
Mei XL and Zhong S: Long noncoding RNA
LINC00520 prevents the progression of cutaneous squamous cell
carcinoma through the inactivation of the PI3K/Akt signaling
pathway by downregulating EGFR. Chin Med J (Engl). 132:454–465.
2019. View Article : Google Scholar
|
|
8
|
Hausser J and Zavolan M: Identification
and consequences of miRNA-target interactions - beyond repression
of gene expression. Nat Rev Genet. 15:599–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA Deregulation in Cancer Cells and the Tumor
Microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hubé F, Ulveling D, Sureau A, Forveille S
and Francastel C: Short intron-derived ncRNAs. Nucleic Acids Res.
45:4768–4781. 2017.PubMed/NCBI
|
|
11
|
Leisegang MS, Fork C, Josipovic I, Richter
FM, Preussner J, Hu J, Miller MJ, Epah J, Hofmann P, Günther S, et
al: Long Noncoding RNA MANTIS Facilitates Endothelial Angiogenic
Function. Circulation. 136:65–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang
Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, et al: Long noncoding RNA
MALAT1 suppresses breast cancer metastasis. Nat Genet.
50:1705–1715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu LP, He YJ, Hou JC, Chen X, Zhou SY,
Yang SJ, Li J, Zhang HD, Hu JH, Zhong SL, et al: The role of
circRNAs in cancers. Biosci Rep. 37:BSR201707502017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andreeva K and Cooper NG: MicroRNAs in the
Neural Retina. Int J Genomics. 2014:1658972014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patop IL and Kadener S: circRNAs in
Cancer. Curr Opin Genet Dev. 48:121–127. 2018. View Article : Google Scholar :
|
|
16
|
Greene J, Baird AM, Brady L, Lim M, Gray
SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, Function
and Role in Human Diseases. Front Mol Biosci. 4:382017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan X, Weng X, Zhao Y, Chen W, Gan T and
Xu D: Circular RNAs in Cardiovascular Disease: An Overview. BioMed
Res Int. 2017:51357812017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheng JQ, Liu L, Wang MR and Li PY:
Circular RNAs in digestive system cancer: Potential biomarkers and
therapeutic targets. Am J Cancer Res. 8:1142–1156. 2018.PubMed/NCBI
|
|
19
|
Su H, Tao T, Yang Z, Kang X, Zhang X, Kang
D, Wu S and Li C: Circular RNA cTFRC acts as the sponge of
MicroRNA-107 to promote bladder carcinoma progression. Mol Cancer.
18:272019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sand M, Bechara FG, Gambichler T, Sand D,
Bromba M, Hahn SA, Stockfleth E and Hessam S: Circular RNA
expression in cutaneous squamous cell carcinoma. J Dermatol Sci.
83:210–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bi W, Huang J, Nie C, Liu B, He G, Han J,
Pang R, Ding Z, Xu J and Zhang J: CircRNA circRNA_102171 promotes
papillary thyroid cancer progression through modulating
CTNNBIP1-dependent activation of β-catenin pathway. J Exp Clin
Cancer Res. 37:2752018. View Article : Google Scholar
|
|
23
|
Su Y, Xu C, Liu Y, Hu Y and Wu H: Circular
RNA hsa_circ_0001649 inhibits hepatocellular carcinoma progression
via multiple miRNAs sponge. Aging (Albany NY). 11:3362–3375.
2019.
|
|
24
|
Towler BP, Jones CI and Newbury SF:
Mechanisms of regulation of mature miRNAs. Biochem Soc Trans.
43:1208–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Conte F, Fiscon G, Chiara M, Colombo T,
Farina L and Paci P: Role of the long non-coding RNA PVT1 in the
dysregulation of the ceRNA-ceRNA network in human breast cancer.
PLoS One. 12:pp. e01716612017, View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen G, Shi Y, Zhang Y and Sun J:
CircRNA_100782 regulates pancreatic carcinoma proliferation through
the IL6-STAT3 pathway. OncoTargets Ther. 10:5783–5794. 2017.
View Article : Google Scholar
|
|
27
|
Chen L, Zhang S, Wu J, Cui J, Zhong L,
Zeng L and Ge S: circRNA_100290 plays a role in oral cancer by
functioning as a sponge of the miR-29 family. Oncogene.
36:4551–4561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He JH, Li YG, Han ZP, Zhou JB, Chen WM, Lv
YB, He ML, Zuo JD and Zheng L: The CircRNA-ACAP2/Hsa-miR-21-5p/
Tiam1 Regulatory Feedback Circuit Affects the Proliferation,
Migration, and Invasion of Colon Cancer SW480 Cells. Cell Physiol
Biochem. 49:1539–1550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma HB, Yao YN, Yu JJ, Chen XX and Li HF:
Extensive profiling of circular RNAs and the potential regulatory
role of circRNA-000284 in cell proliferation and invasion of
cervical cancer via sponging miR-506. Am J Transl Res. 10:592–604.
2018.PubMed/NCBI
|
|
30
|
Bian T, Jiang D, Liu J, Yuan X, Feng J, Li
Q, Zhang Q, Li X, Liu Y and Zhang J: miR-1236-3p suppresses the
migration and invasion by targeting KLF8 in lung adenocarcinoma
A549 cells. Biochem Biophys Res Commun. 492:461–467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An JX, Ma MH, Zhang CD, Shao S, Zhou NM
and Dai DQ: miR-1236-3p inhibits invasion and metastasis in gastric
cancer by targeting MTA2. Cancer Cell Int. 18:662018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Manthei U, Nickells MW, Barnes SH, Ballard
LL, Cui WY and Atkinson JP: Identification of a C3b/iC3 binding
protein of rabbit platelets and leukocytes. A CR1-like candidate
for the immune adherence receptor. J Immunol. 140:1228–1235.
1988.PubMed/NCBI
|
|
33
|
Wang Y, Yan S, Liu X, Zhang W, Li Y, Dong
R, Zhang Q, Yang Q, Yuan C, Shen K, et al: miR-1236-3p represses
the cell migration and invasion abilities by targeting ZEB1 in
high-grade serous ovarian carcinoma. Oncol Rep. 31:1905–1910. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miksiunas R, Mobasheri A and Bironaite D:
Homeobox Genes and Homeodomain Proteins: New Insights into Cardiac
Development, Degeneration and Regeneration. Adv Exp Med Biol.
1212:155–178. 2020. View Article : Google Scholar
|
|
35
|
Carrera M, Bitu CC, de Oliveira CE,
Cervigne NK, Graner E, Manninen A, Salo T and Coletta RD: HOXA10
controls proliferation, migration and invasion in oral squamous
cell carcinoma. Int J Clin Exp Pathol. 8:3613–3623. 2015.PubMed/NCBI
|
|
36
|
Hur H, Lee JY, Yun HJ, Park BW and Kim MH:
Analysis of HOX gene expression patterns in human breast cancer.
Mol Biotechnol. 56:64–71. 2014. View Article : Google Scholar
|
|
37
|
Holland PW: Evolution of homeobox genes.
Wiley Interdiscip Rev Dev Biol. 2:31–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Joo MK, Park JJ, Yoo HS, Lee BJ, Chun HJ,
Lee SW and Bak YT: The roles of HOXB7 in promoting migration,
invasion, and anti-apoptosis in gastric cancer. J Gastroenterol
Hepatol. 31:1717–1726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsuboi M, Taniuchi K, Shimizu T, Saito M
and Saibara T: The transcription factor HOXB7 regulates ERK kinase
activity and thereby stimulates the motility and invasiveness of
pancreatic cancer cells. J Biol Chem. 292:17681–17702. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Monterisi S, Lo Riso P, Russo K, Bertalot
G, Vecchi M, Testa G, Di Fiore PP and Bianchi F: HOXB7
overexpression in lung cancer is a hallmark of acquired stem-like
phenotype. Oncogene. 37:3575–3588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang K, Jin J, Ma T and Zhai H:
MiR-376c-3p regulates the proliferation, invasion, migration, cell
cycle and apoptosis of human oral squamous cancer cells by
suppressing HOXB7. Biomed Pharmacother. 91:517–525. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heinonen H, Lepikhova T, Sahu B, Pehkonen
H, Pihlajamaa P, Louhimo R, Gao P, Wei GH, Hautaniemi S, Jänne OA,
et al: Identification of several potential chromatin binding sites
of HOXB7 and its downstream target genes in breast cancer. Int J
Cancer. 137:2374–2383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tu W, Zhu X, Han Y, Wen Y, Qiu G and Zhou
C: Overexpression of HOXB7 is associated with a poor prognosis in
patients with gastric cancer. Oncol Lett. 10:2967–2973. 2015.
View Article : Google Scholar
|
|
44
|
Gao D and Chen HQ: Specific knockdown of
HOXB7 inhibits cutaneous squamous cell carcinoma cell migration and
invasion while inducing apoptosis via the Wnt/beta-catenin
signaling pathway. Am J Physiol Cell Physiol. 315:C675–C86. 2018.
View Article : Google Scholar
|