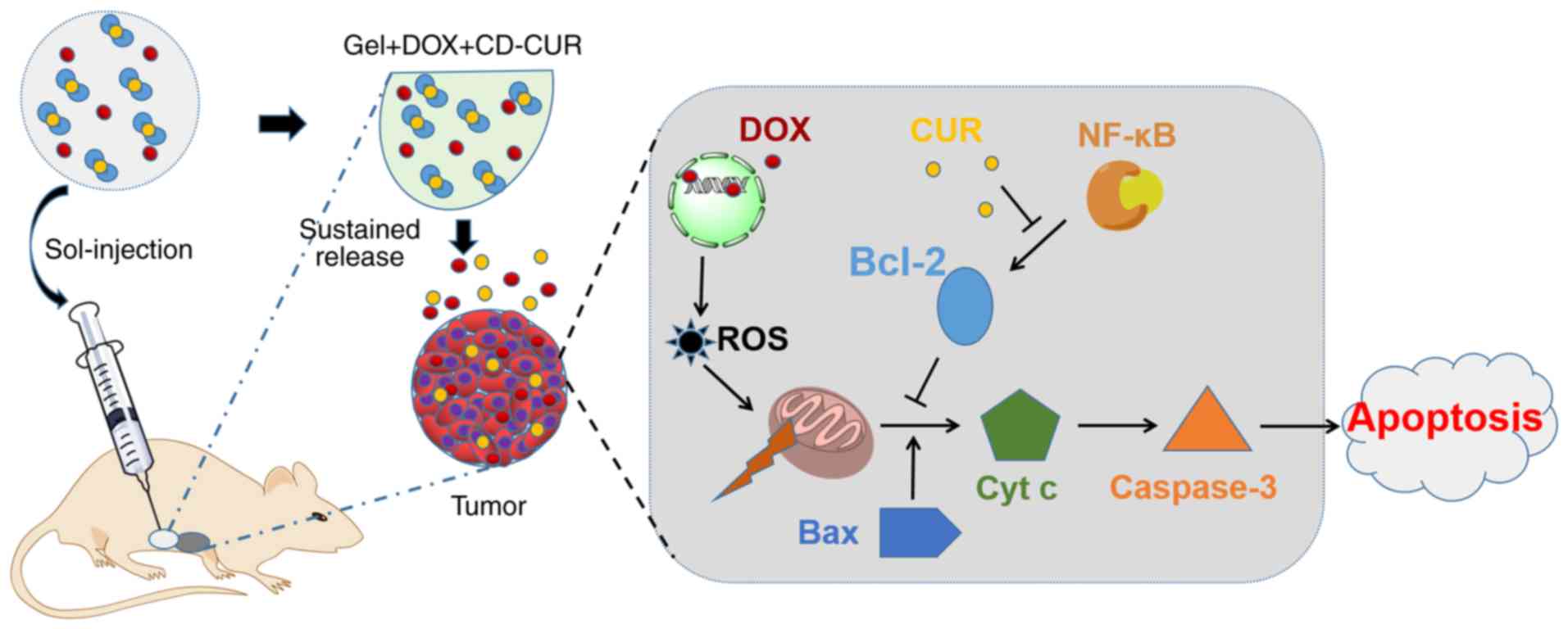
Introduction
The clinical treatment of osteosarcoma currently
includes surgical excision and chemotherapy (1). Combination of high-dose methotrexate,
doxorubicin (DOX) and cisplatin is the standard chemotherapy
strategy for osteosarcoma (2). DOX
is an effective chemotherapeutic agent that is widely used in the
treatment of tumors. However, due to the activation of NF-κB and
the anti-apoptosis gene Bcl-2, its therapeutic effect can be
affected by the development of chemoresistance (3,4).
Combination therapy, such as co-delivering chemotherapy drugs and a
chemosensitizer to tumor sites, provides a promising approach to
tackle this challenge (5). It has
been demonstrated that curcumin (CUR) is one of the optimal
chemosensitizers, being able to enhance the antitumor effect of
numerous traditional therapeutic drugs, including DOX (6,7).
CUR is a polyphenolic compound derived from the
rhizome of Curcuma longa and has been used as traditional
Chinese medicine for a long time (8). Modern medicine has demonstrated that
CUR possesses extensive pharmacological activities, including
antioxidant, anti-inflammatory, antimicrobial, antitoxic and
antitumor activities (9,10). The antitumor activity of CUR is
mainly achieved by blocking the activation of NF-κB and regulating
the mitogen activated protein kinase and PI3K/protein kinase B
signaling pathways (11,12). It has been demonstrated that CUR
exerts antitumor activities by improving cytotoxicity and inducing
apoptosis in various tumor cells, including K562 (13), MCF-7/Adr (14) and SKOV-3TR (15) cells. Despite its extensive
biological activities, the poor solubility and instability of
natural CUR in physiological circumstances has limited its clinical
application (16). To improve the
bioavailability of CUR, numerous encapsulation-based formulations
have been generated, including nanoparticles (17), micelles (18), conjugates (19) and cyclodextrins (20). β-cyclodextrin (β-CD) comprises a
hydrophobic inner cavity and hydrophilic hydroxyl moieties
surrounding the outer surface (21). It has been selected as a receptacle
for CUR to form an inclusion complex (22). The β-CD-CUR inclusion complex
(CD-CUR) circumvents the defects associated with the inherent
physicochemical properties of natural CUR and effectively improves
the solubility and stability of CU (20,23).
Poly(D,L-lactide-co-glycolide)-poly(ethylene-glycol)-poly
(D,L-lactide-co-glycolide) (PLGA-PEG-PLGA) thermosensitive hydrogel
has been widely used as a drug carrier due to its good
injectability, biodegradability and excellent biocompatibility
(24,25). This drug-vehicle continuously
delivers loaded drugs to the target and reduces the whole-body
exposure to drugs compared with systemic administration (26). In the present study, the CD-CUR
inclusion complex was prepared and the PLGA-PEG-PLGA hydrogel was
synthesized. Subsequently, a dual-drug delivery system
(gel+DOX+CD-CUR) was generated by physically mixing hydrogels with
DOX and CD-CUR. The release kinetics of CUR and DOX from
drug-loaded hydrogels was studied in vitro. MTT and
live/dead cell dual staining assays were performed to analyze the
antitumor efficiencies of different strategies. Furthermore, the
underlying mechanisms of the antitumor effect were analyzed by
western blotting and caspase-3 activity detection. Finally, the
in vivo antitumor effect of different strategies was
evaluated in tumor-bearing mice (Fig.
1).
Materials and methods
Materials and cell culture
PEG (Mn=1500), tin (II) 2-ethyl-hexanoate
[Sn(Oct)2], CU (≥95%), β-CD, D,L-lactide and glycolide
were purchased from Purac® (Corbion). DOX was purchased
from Zhejiang Hisun Chemical Co., Ltd. The following primary
antibodies were used: NF-κB (cat. no. 8242), IκB (cat. no. 4814),
phosphorylated-IκB (cat. no. 2859), PARP (cat. no. 9532) and
cleaved-PARP (cat. no. 9548; all from Cell Signaling Technology,
Inc.), Bcl-2 (cat. no. sc-509), Bax (cat. no. sc-20067), caspase 3
(cat. no. sc-7272) and GAPDH (cat. no. sc-47724; Santa Cruz
Biotechnology, Inc.), all dilutions were 1:1,000. Secondary
antibodies used were mouse anti-rabbit IgG-horseradish peroxidase
(HRP; cat. no. sc-2357) and m-IgGκBP-HRP (cat. no. sc-516102; both
Santa Cruz Biotechnology, Inc.), all secondary antibodies were
diluted 1:5,000. The osteosarcoma K-7 and Saos-2 cell lines were
obtained from the American Type Culture Collection and cultured in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and antibiotics (100 U/ml
penicillin and streptomycin; Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2.
PLGA-PEG-PLGA polymer polymerization and
characterization
PEG, as an initiator, was polymerized with
D,L-lactide (D,L-LA) and glycolide (GA) via a ring-opening
copolymerization method (25). The
molar ratio of D,L-LA/GA was set at 5:1. The molecular weight (MW)
and molar ratio of LA/GA were crucial to the gelling performance of
the synthesized hydrogels. When the MW of PEG was fixed, the rising
LA/GA molar ratio increased the hydrophobicity of this polymer
leading to a lower sol-gel transition temperature and a higher
stability of the hydrogel, as previously described (25). The crude polymers were prepared by
precipitating the mixture solution against ethyl alcohol after 24 h
of polymerization in nitrogen. Polymers were further purified by
dialysis for 3 days and subsequently collected by lyophilization.
The MW and chemical structure of PLGA-PEG-PLGA were determined by
proton nuclear magnetic resonance (1H NMR).
Preparation of the CD-CUR inclusion
complex
A methanol reflux method, previously described by
Tang et al (27), was used
to prepare the CD-CUR inclusion complex with slight modifications
to the inclusion complex collection step. A total of 35.6 mg CUR
was dissolved in 500 μl methanol and subsequently added
drop-wise to 5 ml β-CD deionized aqueous solution with intense
agitation. The set molar ratio of CUR/β-CD was 1.1:2. The reflux
condenser was used to continuously stir the mixture at 70°C for 5
h. Subsequently, methanol was evaporated by stirring without
reflux. The mixture was stirred for another 2 h at room temperature
and purified with a 0.45-μm filter. Finally, the inclusion
complex was collected via lyophilization rather than dried in a
vacuum oven, which was the collection method in the previous study
(27). The light-orange powder of
CD-CUR was collected for further analysis.
Characterization of the CD-CUR inclusion
complex 1H NMR spectra
The powder of CD-CUR was dissolved in
DMSO-d6 solution and analyzed using a Bruker
DMX300 NMR spectrometer (Bruker Corporation). The spectra of β-CD
and natural CUR were recorded at the same time.
Fourier transform infrared (FTIR)
spectrum
The FTIR spectra of CD-CUR, β-CD and natural CUR
were detected by a Nicolet 6700 FTIR spectrophotometer (Thermo
Fisher Scientific, Inc.). Briefly, 2 mg of each sample was placed
on the KBr discs container, and the absorbance was recorded from
4,000 to 400 cm−1 at a 4 cm−1 resolution with
30 scans.
Differential scanning calorimetry
(DSC)
DSC analysis of CD-CUR, β-CD and natural CUR was
carried out with a TA Instruments Q200 Differential Scanning
Calorimeter (TA Instruments, Inc.) in a 60 ml/min nitrogen
atmosphere. Each sample was placed on completely sealed aluminum
pans and heated from 25 to 300°C at a rate of 10°C/min.
Thermo-gravimetric analysis (TGA)
TGA analysis of CD-CUR, β-CD and natural CUR was
performed via TA Instruments Q50 Thermo-gravimetric analysis (TA
Instruments, Inc.). Samples were heated from 25 to 800°C at a rate
of 10°C/min.
Scanning electron microscopy (SEM)
The powders of CD-CUR, β-CD and natural CUR were
mounted on metal stubs and coated with gold film. The sample was
directly observed without fixation. The surface morphology of the
sample was observed using a S-3400 Scanning Electron Microscope
(Hitachi High-Technologies Corporation) at 20 kV.
CUR entrapment efficiency
The entrapment efficiency of CUR into β-CD was
estimated using the UV-265 UV spectrophotometer (Shimadzu
Corporation). Briefly, 1 mg CD-CUR was dissolved in 50 ml DMSO and
agitated in the dark for 24 h at 37°C. Through this process, the
captured CUR was dissociated from β-CD and extracted into DMSO.
β-CD was removed from the solution via centrifugation at 32,000 × g
for 10 min at 4°C. The supernatant containing CUR was collected,
and the content of CUR was analyzed by the UV spectrophotometer at
425 nm. Meanwhile, the equivalent natural CUR was prepared under
the same conditions to obtain a standard plot. The entrapment
efficiency was calculated using the following equation: Entrapment
efficiency (%)=(wt of CUR in CD-CUR)/(wt of total CUR) ×100.
Solubility and stability of the CD-CUR
inclusion complex
The solubility of CD-CUR and natural CUR in aqueous
solution was determined using the method of saturation solubility.
Excessive sample was dissolved in 20 ml water and transferred into
a centrifuge and centrifuged at 1.6 × g at 25°C for 5 min. After 24
h, the solution was filtered through a 0.45-μm filter
membrane. The clear filtrate was collected and measured by the UV
spectrophotometer at 425 nm.
CD-CUR and natural CUR at an equivalent dose of CUR
were prepared in PBS (pH 7.4) and subsequently transferred to a
shaker at 1.6 × g at 37°C for 24 h. At predetermined time points,
the absorbance of the sample solution was measured at 425 nm by a
UV spectrophotometer. The stability of these two samples was
calculated using the following equation: Stability of CUR (%) =
Ct/C0 ×100, where Ct and
C0 represented the concentration of CUR at testing time
(t h) and 0 h, respectively.
In vitro cytotoxicity of the CD-CUR
inclusion complex
The cytotoxicity effect of CD-CUR against
osteosarcoma K-7 and Saos-2 cells was evaluated by MTT assays in
vitro. Briefly, a series of concentrations (5, 10, 20 and 40
μg/ml) of CD-CUR and natural CUR were prepared in PBS (pH
7.4). Additionally, natural CUR was dissolved in DMSO (with a final
concentration of DMSO <0.5% v/v) as a positive control. K-7 or
Saos-2 cells at a density of 8,000 cells/well were seeded into
96-well plates and incubated at 37°C for 24 h. Subsequently, the
culture medium was removed, 200 μl fresh medium containing
different concentrations of formulations was added and the cells
were treated for 24 h. The cell growth ability was assessed by MTT
assay, and purple formazan was dissolved using DMSO. The absorbance
was measured using a microplate reader (Bio-Tek 680; Agilent
Technologies, Inc.) at 490 nm. Cell viability was calculated using
the following equation: Cell viability
(%)=(Asample/Acontrol) ×100, where
Asample and Acontrol represent the absorbance
of the different testing wells and control group, respectively.
Preparation of single- or dual-drug
delivery systems
The PLGA-PEG-PLGA polymer was dissolved in PBS (pH
7.4) at 4°C. The concentration of the polymer solution was 20% wt.
DOX and CD-CUR were co-loaded in the polymer solution to form a
homogeneous dual-drug-loaded hydrogel (gel+DOX+CD-CUR) under
continuous stirring. The samples of DOX, natural CUR and CD-CUR
were respectively mixed with the polymer solution to generate a
single-drug delivery system (gel+DOX, gel+CUR and gel+CD-CUR).
Phase transition and rheological
properties of single- and dual-drug delivery systems
The thermosensitive hydrogel PLGA-PEG-PLGA has the
ability of undergoing thermal-stimulated phase transition, which is
in the form of an aqueous solution (sol) at room temperature and
can transform to gel at body temperature. The phase transition
temperature of the drug-loaded hydrogel was investigated using a
vial inversion method at a rate of 2°C/10 min, and the intrinsic
gel-forming property of the PLGA-PEG-PLGA solution (20% wt) was
examined simultaneously. The mechanical properties of single- and
dual-drug-loaded hydrogels were investigated by an MCR 301
rheometer (Anton Paar GmbH). The sample was placed on the platform
with a 0.5-mm gap. The heating rate and frequency were set at 0.5
mm and 1.0 Hz, respectively.
In vitro drug release kinetics
The release kinetics of CUR from single- (gel+CD-CUR
and gel+CUR) and dual-drug-loaded hydrogels (gel+DOX+CD-CUR) were
determined in vitro. Single-drug-loaded hydrogels were
incubated with 3 ml PBS (pH 7.4) or PBS (pH 7.4) containing
Tween-80 (1% wt), while the dual-drug-loaded hydrogel was only
incubated with PBS (pH 7.4). The samples were transferred to a
shaker at 1.6 × g for 5 min at 37°C. At predetermined time
intervals, 2 ml released medium was removed and an equal volume of
fresh medium was re-added into the vials. The DOX release kinetics
of gel+DOX and gel+DOX+CD-CUR were determined in unique PBS (pH
7.4) medium.
The amount of released CUR was measured by the
method described by Gerola et al (28). The released medium was mixed with
an equal volume of tetrahydrofuran (50% v/v) and the absorbance was
measured by a UV spectrophotometer at 425 nm. The amount of
released DOX was determined by fluorescence measurements at an
excitation wavelength of 488 nm. Each experiment was performed in
triplicate.
In vitro cell viability of the single- or
dual-drug delivery systems
The cell viability of single and dual-drug-loaded
hydrogels were evaluated in K-7 and Saos-2 cells, respectively.
Briefly, K-7 or Saos-2 cells were seeded in 24-well plates at a
density of 5×104 cells/well in 1 ml DMEM culture medium.
After 24 h, the medium was replaced by fresh medium containing
different strategies (free DOX, DOX+CD-CUR, gel+DOX, gel+CD-CUR and
gel+DOX+CD-CUR) and cultured for another 48 h, with DMEM and free
gel as controls. MTT assays were used to evaluate the cell
viability. The purple formazan was dissolved by DMSO and the
absorbance of the solution was determined by using microplate
reader (Bio-Tek ELx800) at 490 nm. Cell viability was calculated
according to the aforementioned equation for cell viability.
Live/dead cell staining assays
The cell viability of K-7 cells incubated with
different formulations was investigated by a Live/Dead Cell Double
Staining kit (Shanghai Yeasen Biotechnology Co., Ltd.). Briefly,
The K-7 cells were seeded in 24-well plates at a density of
5×104 cells/well in 1 ml culture medium. After 24 h, the
medium was removed and the cells were incubated with different
strategies (DMEM, free gel, gel+DOX, gel+CD-CUR and gel+DOX+CD-CUR)
for another 24 h at 37°C. The concentrations of DOX and CUR used
were 1 and 20 μg/ml, respectively. Cells were stained by
staining assays [10 μl Calcein-AM and 5 μl propidium
iodide (PI) added in 1 ml 10X buffer] for 15 min at 37°C according
to the manufacturer's protocol. Living cells stained with
Calcein-AM appeared green, while dead cells stained with PI
appeared red. The stained cells were visualized using an Olympus
fluorescence microscope (Olympus Corporation) and captured via
ImageJ software version 1.46 (National Institutes of Health).
Western blotting
K-7 cells were treated with different strategies,
including DMEM, free gel, gel+DOX, gel+CD-CUR and gel+DOX+CD-CUR
for 2 h at 37°C. The concentrations of DOX and CUR used were 1 and
20 μg/ml, respectively. Subsequently, cells were harvested,
and total protein was extracted using RIPA lysis buffer (Beyotime
Institute of Biotechnology) and quantified using the bicinchoninic
acid assay. A total of 40 μg protein/lane was separated via
10% SDS-PAGE and then transferred onto PDVF membranes. The
membranes were blocked with Tris-buffered saline with 0.1% Tween-20
containing 5% skimmed milk at room temperature for 1 h. The
membranes were probed using primary antibodies overnight at 4°C and
then incubated with secondary antibodies for 2 h at room
temperature. GAPDH was used as the internal control. Proteins were
visualized using Luminata Western HRP substrate (EMD Millipore) and
the Gene Genius Bio-imaging system, bands were imaged using the
ChemiDOX XRS (both Bio-Rad Laboratories, Inc.).
Quantitative analysis of caspase-3
activity
Caspase-3 activity was determined by the Caspase-3
Fluorimetric assay kit (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol. Briefly, the cells were lysed with lysis
buffer and incubated at 4°C for 10 min. The lysate was centrifuged
at 16,000 × g for 5 min at room temperature. The supernatant was
removed and incubated with an equal volume of assay buffer
containing substrate (Ac-DEVD-AMC) at 37°C for 2 h. The absorbance
of samples was measured at 405 nm using a BioTek microplate reader
(Agilent Technologies, Inc.). The caspase-3 activity of each
formulation was compared with that of the control group. The
experiments were performed in triplicate.
In vivo antitumor activity
The present study was conducted according to the
Animal Research Reporting In vivo Experiments guidelines
(29). A total of 14 female BALB/c
mice, weighing 18-20 g and aged 5 weeks, were purchased from Model
Animal Research Center of Nanjing University, and housed under a
12-h light/12-h dark cycle and sterile conditions (temperature,
26-28°C; humidity, 40-60%) with ad libitum access to water
and food. Mice were sacrificed by cervical dislocation and
comprehensive judgment was made to confirm mouse death by observing
respiration, heartbeat and nerve reflex. K-7 tumor-bearing mice
were prepared through subcutaneous injection of K-7 cells
(2×106/mouse in PBS) into the right flank. When the
average tumor volume reached ~100 mm3, tumor-bearing
mice were randomly divided into seven groups (n=5 for each group).
The different treatment strategies, including normal saline (NS),
free gel, free DOX, gel+DOX, gel+CD-CUR, DOX+CD-CUR and
gel+DOX+CD-CUR, were peritumorally administrated to tumor-bearing
mice. The concentrations of DOX and CUR were set at 2.5 and 50
mg/kg, respectively. The tumor volume and body weight were
monitored every 2 days after administration. The tumor volume was
calculated using the following equation: V (mm3)=L ×
S2/2, where L and S (mm) were the longest and shortest
tumor diameters, respectively.
Histological analysis
The mice were sacrificed on the 14th day after
treatment. The tumor tissues and major organs (heart, liver, lung,
kidney and spleen) were separated and collected. The tissue slices
were stained at room temperature with hematoxylin (2 min) and eosin
(1 min). (H&E), and the histological changes of tissues were
observed via an Eclipse Ti light microscope (magnification, ×40;
Nikon Corporation).
Statistical analysis
All experiments were performed in triplicate, and
data are presented as the mean ± SD. For comparisons among the
different groups, a one-way ANOVA was used, followed by Tukey's
post hoc test for multiple comparisons using SPSS v22.0 (IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
Synthesis of the PLGA-PEG-PLGA
polymers
The PLGA-PEG-PLGA polymers were synthesized via
ring-opening copolymerization. D,L-LA and GA were copolymerized
onto the PEG initiator with the catalysis of Sn(Oct)2.
As presented in Figs. S1 and
S2, the characteristic peaks on
the 1H NMR spectrum were at 5.28, 4.37, 4.8, 3.6 and 1.54 ppm,
belonging to the CH of D,L-LA, the CH3 of D,L-LA, the CH2 of GA,
the CH2 of PEG and the CH2 of ester bond, respectively. The MW of
the synthesized polymer was calculated based on the results of the
1H NMR analysis. Since the Mn of PEG was constant in the
PLGA-PEG-PLGA molecule, the proton number of PEG was decided. As
presented in Fig. S2, there was
most likely 50 of LA and 10 of GA (molar ratio of D,L-LA/GA was
4.8:1) in one molecule. The MW of the PLGA-PEG-PLGA polymer was
5,500, which was consistent with the current design. The hydrogel
PLGA-PEG-PLGA flowed freely at room temperature and formed a stable
gel rapidly with rising temperatures. This phenomenon revealed that
PLGA-PEG-PLGA had good thermosensitivity.
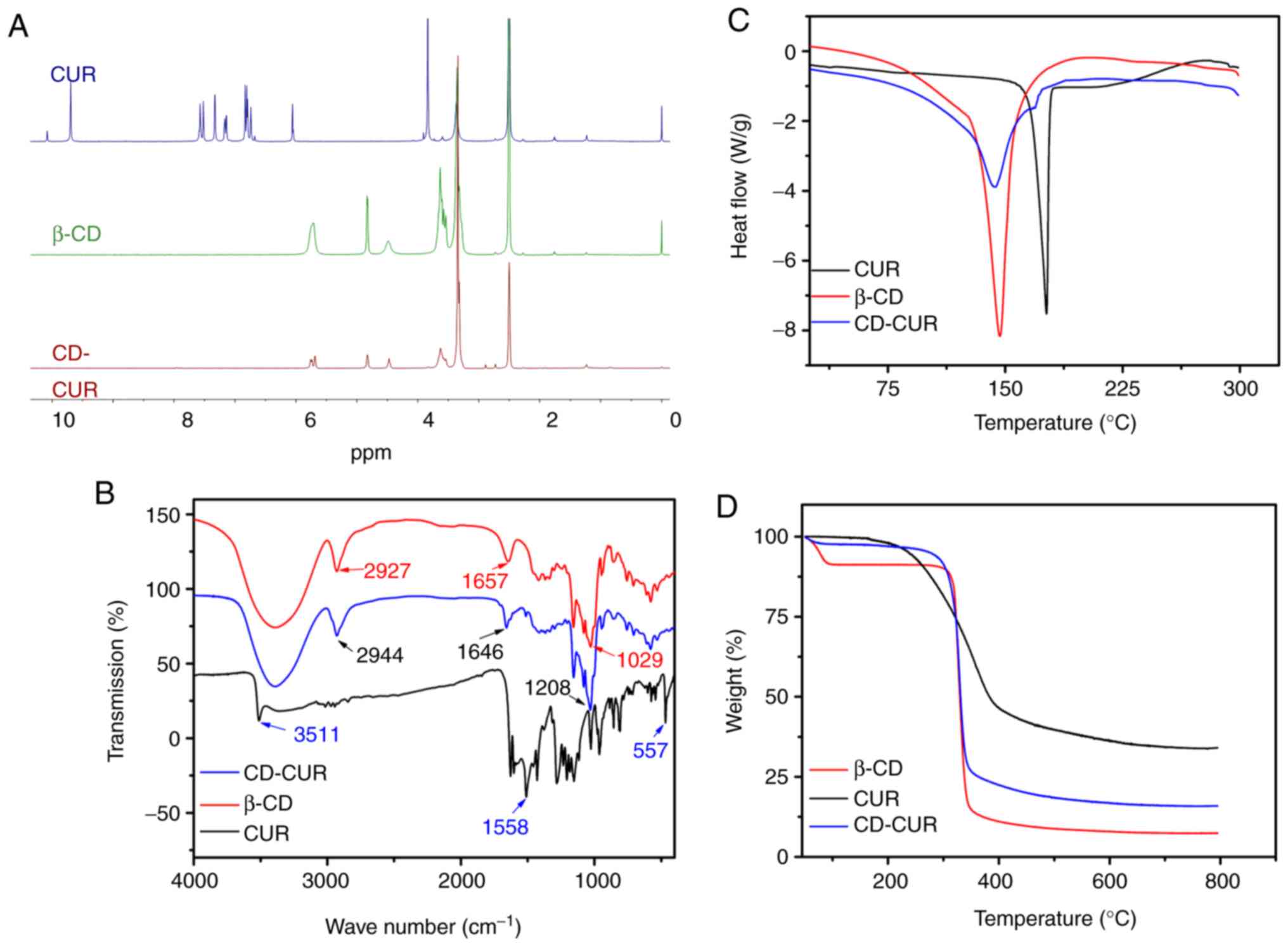
Characterization of the CD-CUR inclusion
complex
The CD-CUR inclusion complex was prepared using a
methanol reflux method. The obtained CD-CUR was characterized by
1H NMR, FTIR, DSC, TGA and SEM (Fig. S3). The formation of CD-CUR was
confirmed by 1H NMR (Fig.
2A). The spectrum of CD-CUR revealed that all peaks belonged to
β-CD, while the typical peaks between 8 and 6 ppm belonging to
natural CUR were almost absent. The present results confirmed that
CUR was successfully entrapped in the inner cavity of β-CD
(21).
Chemical adsorptions of the samples were
characterized by FTIR spectroscopy (Fig. 2B). The specific IR absorption bands
of natural CUR were at 3,511 (phenolic O-H), 1,558 (C=C of benzene)
and 557 cm−1 (C-O-C glucose unit). The typical
absorption bands of β-CD were at 2,927 (O-H), 1,657 (C-H) and 1,029
cm−1 (C-O). The IR absorption band at 3,511
cm−1 belonging to natural CUR was absent on the spectrum
of CD-CUR. The typical IR absorption bands of β-CD were at 2,927,
1,657 and 1,208 cm−1 corresponding to O-H, C-H, C-O
units, which was similar to those reported for β-CD (20,22).
The thermal properties of CD-CUR, β-CD and natural
CUR were characterized by DSC and TGA (Fig. 2C and D, respectively). The DSC
thermogram of natural CUR exhibited a single endothermic peak at
180°C, since natural CUR existed in the crystalline state. However,
in the thermogram of CD-CUR, the aforementioned typical peak
belonging to natural CUR was almost absent. An endothermic peak of
CD-CUR was observed at 145°C, which was slightly lower than that at
147°C of β-CD. The TGA curves of CD-CUR, β-CD and natural CUR in
Fig. 2D revealed that the weight
loss rate of β-CD was nearly 100% at 600°C, while the degradation
rate of natural CUR was 69% at this temperature. The thermal
stability of CD-CUR was improved to an 86% weight loss rate at
600°C.
CUR entrapment efficiency
The content of CUR in 1 mg CD-CUR inclusion complex
was determined by UV spectroscopy. The entrapment efficiency of CUR
was 92.0% (data not shown). Gerola et al (28) have described that the CD-CUR
inclusion complex exhibited the highest binding constant when the
molar ratio of β-CD to CUR was 2:1 according to the steric factors
of forming inclusion, since the aromatic ring of CUR was suitable
to enter into the inner cavity of β-CD.
In vitro solubility and stability of the
CD-CUR inclusion complex
Excessive CD-CUR and natural CUR dissolved in
aqueous solution at 25°C. CD-CUR was completely dissolved to form a
well-dispersed solution. By contrast, natural CUR hardly dissolved
in aqueous solution and most of it aggregated at the bottom of
vials. CD-CUR achieved a solubility of 1.43 mg/ml in water,
equivalent to 636 times that of natural CUR (2.21 μg/ml;
data not shown).
The in vitro stabilities of CD-CUR and
natural CUR in PBS were investigated. As presented in Fig. S4, natural CUR was unstable and was
rapidly degraded in neutral PBS solution, while CD-CUR had a good
stability and remained at 86.7% of total CUR mass under the same
conditions after 24 h. The poor solubility and rapid degradation of
natural CUR in neutral solution has limited its clinical
application (16,30). Preparation of the CD-CUR inclusion
complex could easily circumvent these obstacles (31). Hydrophobic CUR was entrapped in the
inner cavity of β-CD, and the outer surface of β-CD was covered by
hydrophilic moieties, allowing the inclusion complex to have good
solubility. Additionally, β-CD guarded the entrapped CUR by
reducing the hydrolysis and biotransformation of CUR. The
improvement of the solubility and stability of CUR has been
analyzed in the aforementioned experiments. Therefore, it was
hypothesized that, compared with natural CUR, CD-CUR possesses
advanced physiochemical properties that may improve its antitumor
efficiency.
In vitro cytotoxicity of CD-CUR
The cytotoxicity efficiencies of different
strategies (CD-CUR in PBS and natural CUR in PBS or DMSO) were
investigated via MTT assays in the osteosarcoma K-7 and Saos-2 cell
lines. As shown in Fig. S5,
natural CUR in PBS had a weak cytotoxicity effect on both cell
lines, even at a dose of 40 μg/ml. By contrast, CD-CUR in
PBS exhibited significant cytotoxicity effects compared with
natural CUR in PBS on both cell lines, due to the improved
solubility and stability of CD-CUR compared with natural CUR in
PBS. However, the best a cytotoxicity effect was observed from the
strategy of natural CUR in DMSO, which was attributed to it being
completely dissolved in organic solvent (DMSO), and to the free
uptake of CUR by cells without the restriction of β-CD. Although
the cytotoxicity of low-concentration DMSO (<0.5%) was not
apparent on the cytotoxicity of both cell lines compared with the
control group, it should be taken into consideration when applied
to humans (32). Therefore, it is
a feasible method to improve the solubility of CUR by the formation
of inclusion compounds, particularly β-CD.
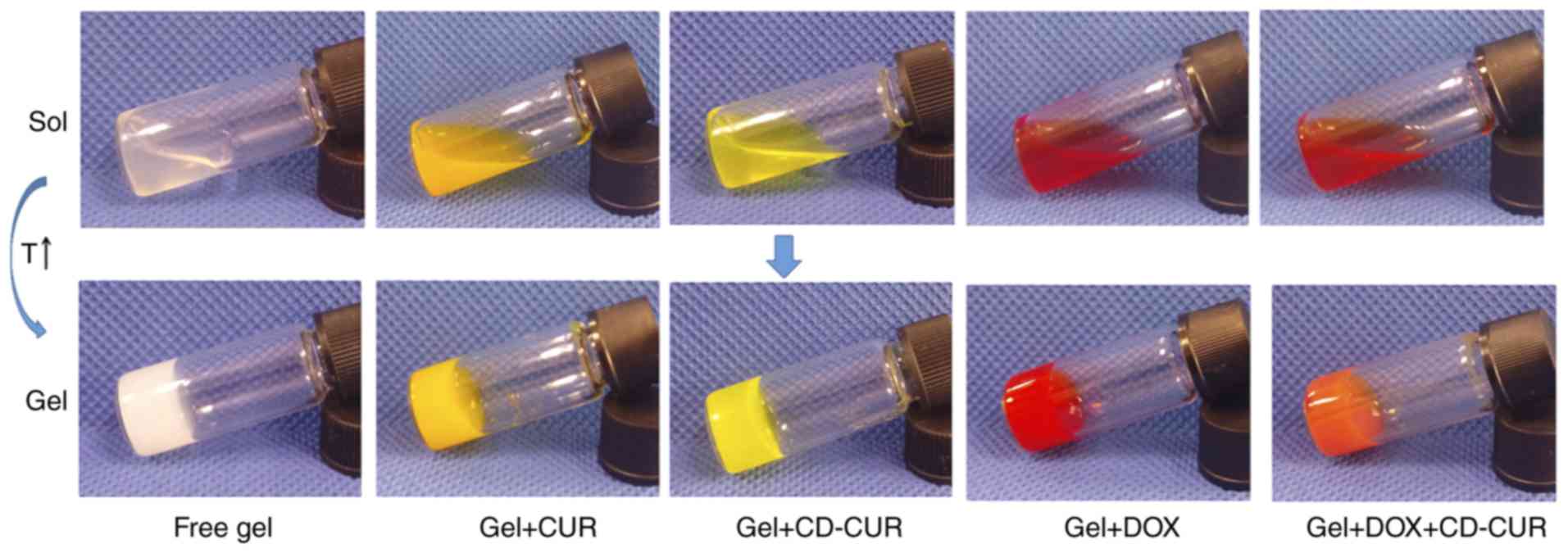
Phase transition and rheological
properties of single- or dual-drug-loaded hydrogel solution
Phase transition temperature was detected by the
vial inversion method (Fig. 3).
The sol-gel transition temperature (Tgel) was affected
by polymer MW, hydrophobic block length, polymer concentration, and
interactions between loaded drugs and polymer blocks (25,26).
As shown in Fig. S6 and Table I, the phase transition diagram of
DOX (1 mg/ml) loaded into the PLGA-PEG-PLGA hydrogel was similar to
that of drug-free hydrogel, which is consistent with the study by
Yu et al (33). β-CD-loaded
hydrogel (gel+β-CD) had a lower Tgel due to the
interaction between β-CD and hydrophilic PEG blocks. Cesteros et
al (34) reported that
acylated PEG could cross-link with β-CD to form a new hydrogel
network. The phase diagram of gel+CD-CUR was similar to
gel+DOX+CD-CUR, but slightly different from gel+β-CD. As the
amphiphilic PLGA-PEG-PLGA copolymer was self-assembled into
micelles in PBS solution and the core of the micelle was composed
of hydrophobic PLGA blocks, natural CUR was able to be encapsulated
in hydrogels through hydrophobic forces to form a homogeneous
solution. Gel+CUR exhibited the lowest Tgel compared
with other formulations, partly due to the increase of hydrophobic
interactions between the hydrogel and CUR.
 | Table IPhase transition temperatures and
rheological properties of different strategies. |
Table I
Phase transition temperatures and
rheological properties of different strategies.
| Groups | Tgel
(°C)c | Storage modulus
(Pa)d |
|---|
| Free gela | 21.6±1.2 | 812 |
| Gel+CDb | 19±2.0 | 881 |
| Gel+CUR | 17.6±1.2 | 1,206 |
| Gel+DOX | 21±2.0 | 1,009 |
| Gel+CD-CUR | 19.6±1.2 | 1,077 |
| Gel+DOX+CD-CUR | 18.3±1.2 | 1,392 |
Additionally, dynamic rheological properties of
various formulations were investigated in vitro. A rapid
increase of the storage modulus (G′) indicates the formation of a
hydrogel network (35). As shown
in Fig. S6C, gel+CUR exhibited a
sharp rise in G′ compared with other formulations. The formation
rate of hydrogel networks and the mechanical strength of drug-free
hydrogel was the lowest. The gel+β-CD, gel+CD-CUR and
gel+DOX+CD-CUR obtained a modest gel formation rate and G′. The
present results are consistent with the phase transition diagram
detected by the vial inversion method.
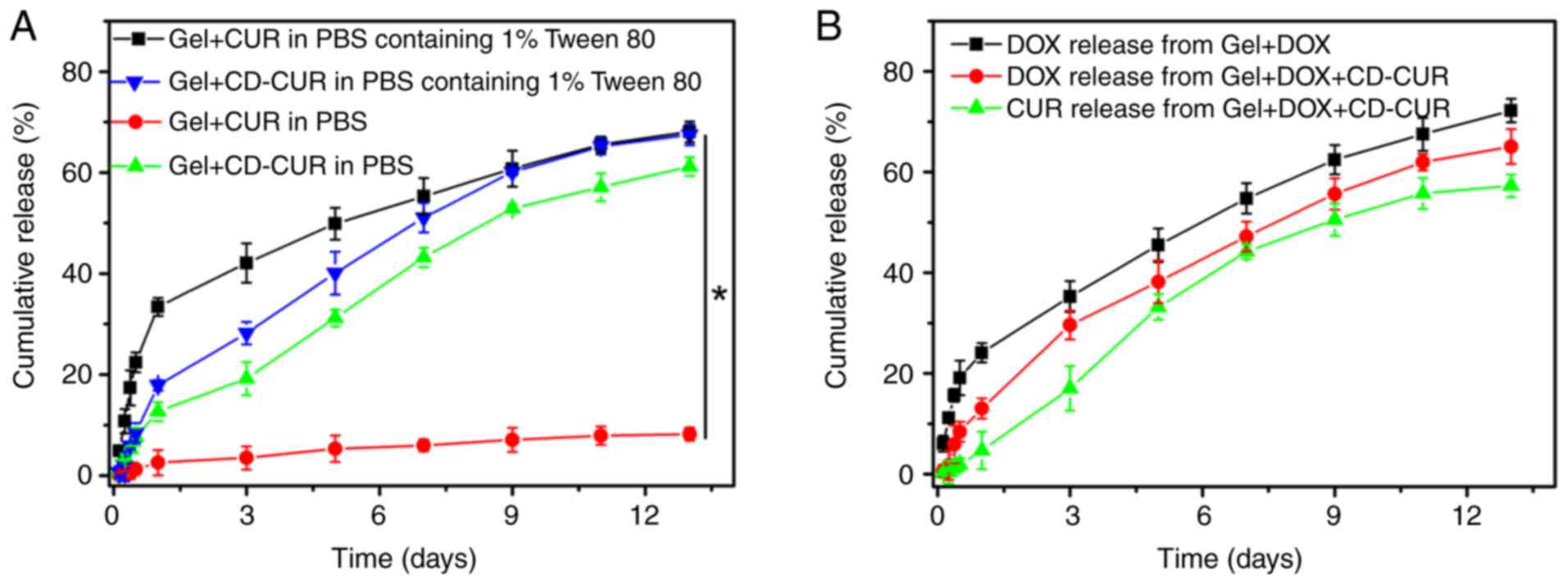
In vitro drug release kinetics
The release kinetics of CUR from gel+CUR and
gel+CD-CUR were investigated in PBS with or without Tween 80 (0.5%
wt; Fig. 4A). As a surfactant,
Tween 80 was capable of increasing the solubility of hydrophobic
CUR in aqueous solution without affecting the polymer networks.
Although a small amount of CUR was released from gel+CUR in PBS
over 13 days, <75% of CUR was released from gel+CUR in PBS
containing Tween 80 over the same time. The present result
indicates that when the thermosensitive hydrogel PLGA-PEG-PLGA was
used as a hydrophobic drug-vehicle, the drug release rate was
partially dependent on the release medium in vitro (25). Notably, when the extremely
hydrophobic natural CUR was loaded into hydrogel, the hydrophobic
forces between CUR and the cores of the micelles strongly affected
the drug release kinetic. By contrast, ~60% of CUR was released
from gel+CD-CUR in the PBS release medium over 13 days. Due to the
good solubility of the CD-CUR inclusion complex in neutral medium,
CUR was released from gel+CD-CUR in a sustained manner. It was
noticeable that Tween 80 had a low impact on the release rate of
CUR from gel+CD-CUR. While the release of CUR in PBS with Tween 80
on day 1 was ~35% from gel+CUR, only 18% of CUR was released from
gel+CD-CUR. This may be due to the formation of the CD-CUR
inclusion complex. Xu et al (36) reported that the poly(2-hydroxyethyl
methacrylate) (pHEMA) hydrogel containing β-CD has a lower burst
release of puerarin than pHEMA hydrogel in tears, due to the
formation of an inclusion complex between β-CD and puerarin. The
complex of drug and β-CD decreases the average mobility of the drug
and regulates the drug release from hydrogels (37).
The release kinetics of CUR and DOX from
dual-drug-loaded hydrogel (gel+DOX+CD-CUR) were investigated in
PBS. As shown in Fig. 4B, the
release behavior of both drugs from the dual-drug delivery system
was similar to that of the single-drug delivery system.
In vitro antitumor efficiencies of the
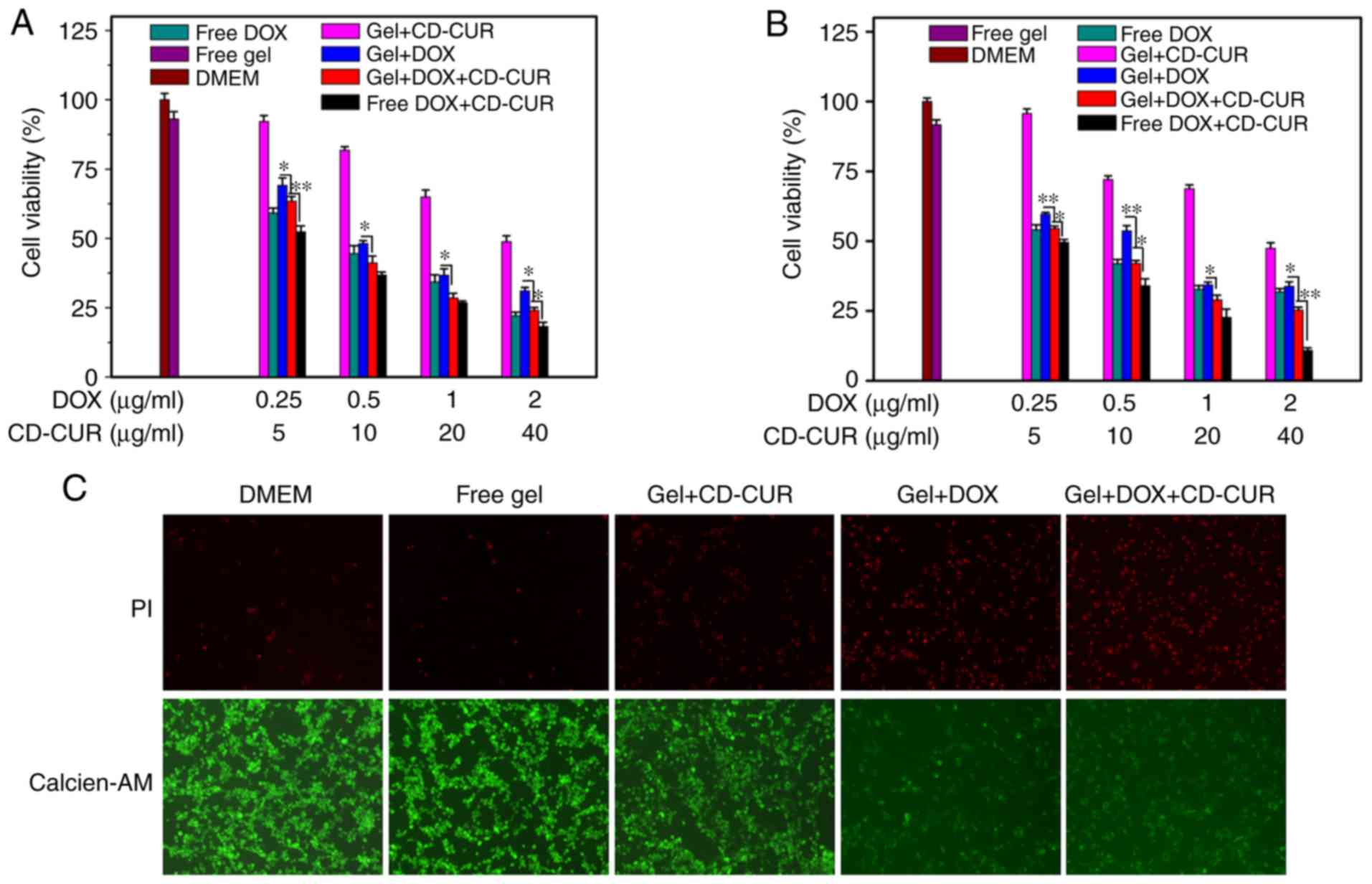
dual-drug delivery system
The antitumor efficiencies of different formulations
against K-7 and Saos-2 cells were examined via MTT assays in
vitro. As displayed in Fig. 5A and
B, both strategies of free DOX and gel+DOX exhibited slightly
dose-dependent cytotoxicity effects on both osteosarcoma cell
lines. Combination therapy of DOX and CD-CUR, both when loaded in
hydrogel or not, had a large cytotoxicity effect on both cell
lines. The IC50 values for both cell lines of
gel+DOX+CD-CUR (0.34 μg/ml vs. K-7 and 0.40 μg/ml vs.
Saos-2 cells) were lower than those of the gel+DOX system (0.59
μg/ml vs. K-7 and 0.47 μg/ml vs. Saos-2 cells)
(Table SI). Although the
solubility and stability of CUR were improved by the CD-CUR
inclusion complex, gel+CD-CUR exhibited moderate cytotoxicity
against both cell lines even at the concentration of 40
μg/ml (Fig. 5A and B).
Nevertheless, the combination of DOX and CD-CUR had significant
cytotoxicity effects compared with gel+DOX alone.
Following K-7 cell incubation with different
strategies for 24 h, cell viability was analyzed with the live/dead
cell staining kit (Fig. 5C). The
cells in the control groups (DMEM and free gel) maintained high
viability. The dual-drug delivery system (gel+DOX+CD-CUR) exhibited
more dead cells than any other single-drug therapies. The present
results are consistent with the aforementioned investigation of
antitumor activity determined via MTT assays.
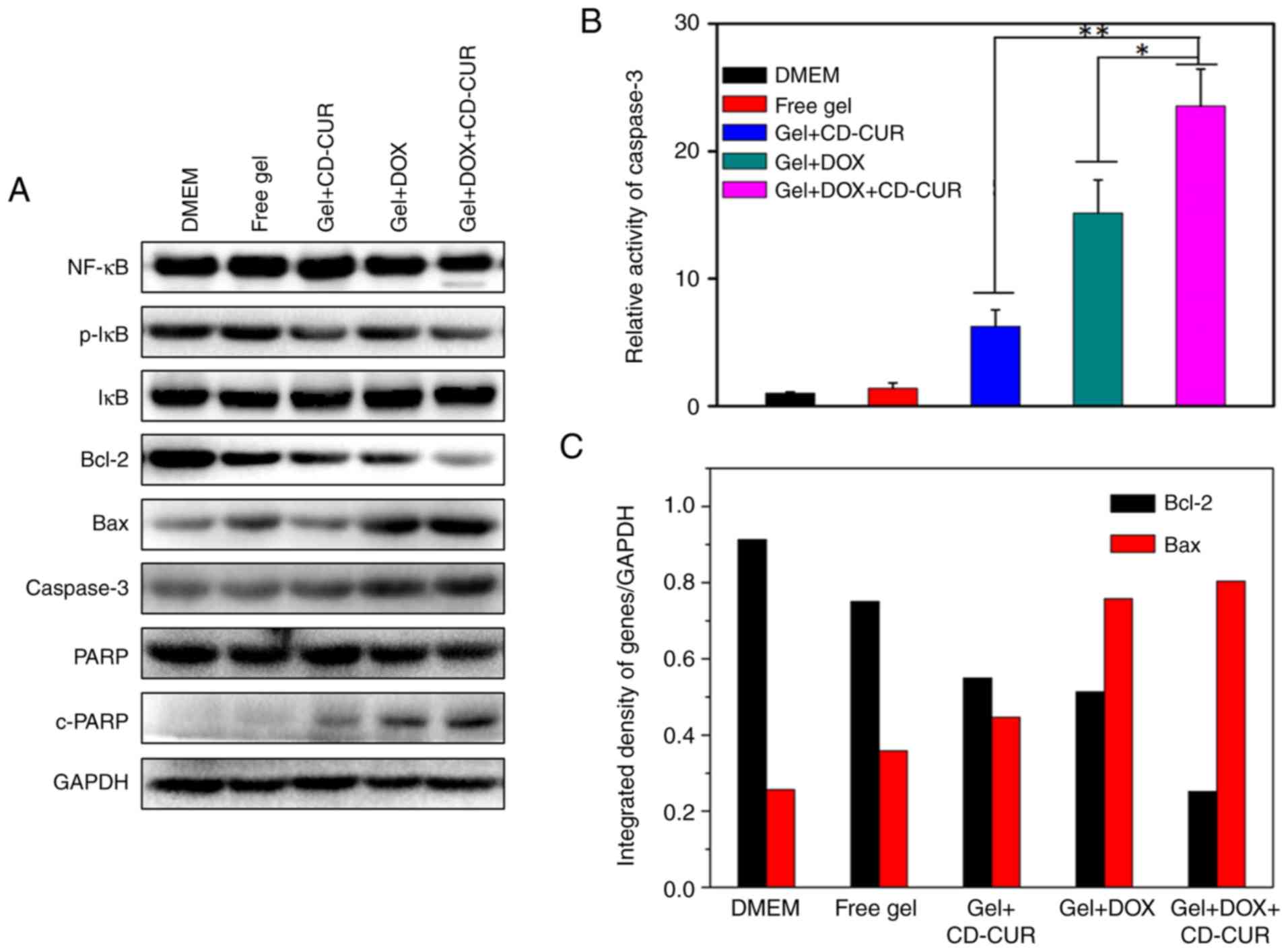
Antitumor mechanisms of CUR and DOX
combination
To explore the pro-apoptotic effects of different
strategies, the expression levels of the anti-apoptotic protein
Bcl-2 and the pro-apoptotic protein Bax were detected by western
blotting. As displayed in Fig. 6A and
C, all treatment strategies significantly decreased the
expression levels of Bcl-2 compared with the controls and
simultaneously increased the expression levels of Bax. Notably, the
combination therapy of DOX and CD-CUR exhibited the strongest
downregulation effect on Bcl-2 expression. The endogenous activity
of CUR inhibits the activation of NF-κB (38) and downregulates the expression of
Bcl-2 (13). Although the
expression levels of NF-κB were not affected by CUR, the protein
levels of phosphorylated-IκB, which is as an indicator of NF-κB
activation (39), were decreased
for gel+CD-CUR and gel+DOX+CD-CUR (Fig. 6A). Caspase-3 is a key molecule in
the mitochondrial apoptotic pathway (39). The expression levels of caspase-3
in gel+DOX and gel+DOX+CD-CUR were higher than those in the control
groups. In caspase-3 activity assays, the dual-drug delivery system
(gel+DOX+CD-CUR) displayed the highest caspase-3 activity (Fig. 6B). It was hypothesized that the
DOX-induced apoptosis was mainly associated with the upregulation
of caspase-3. Poly (ADP-ribose) polymerase (PARP) and cleaved-PARP
are indicators of apoptosis in tumor cells (40). The Gel+DOX+CD-CUR group exhibited
the highest cleaved-PARP expression, suggesting that this group has
the strongest apoptosis-inducing efficiency.
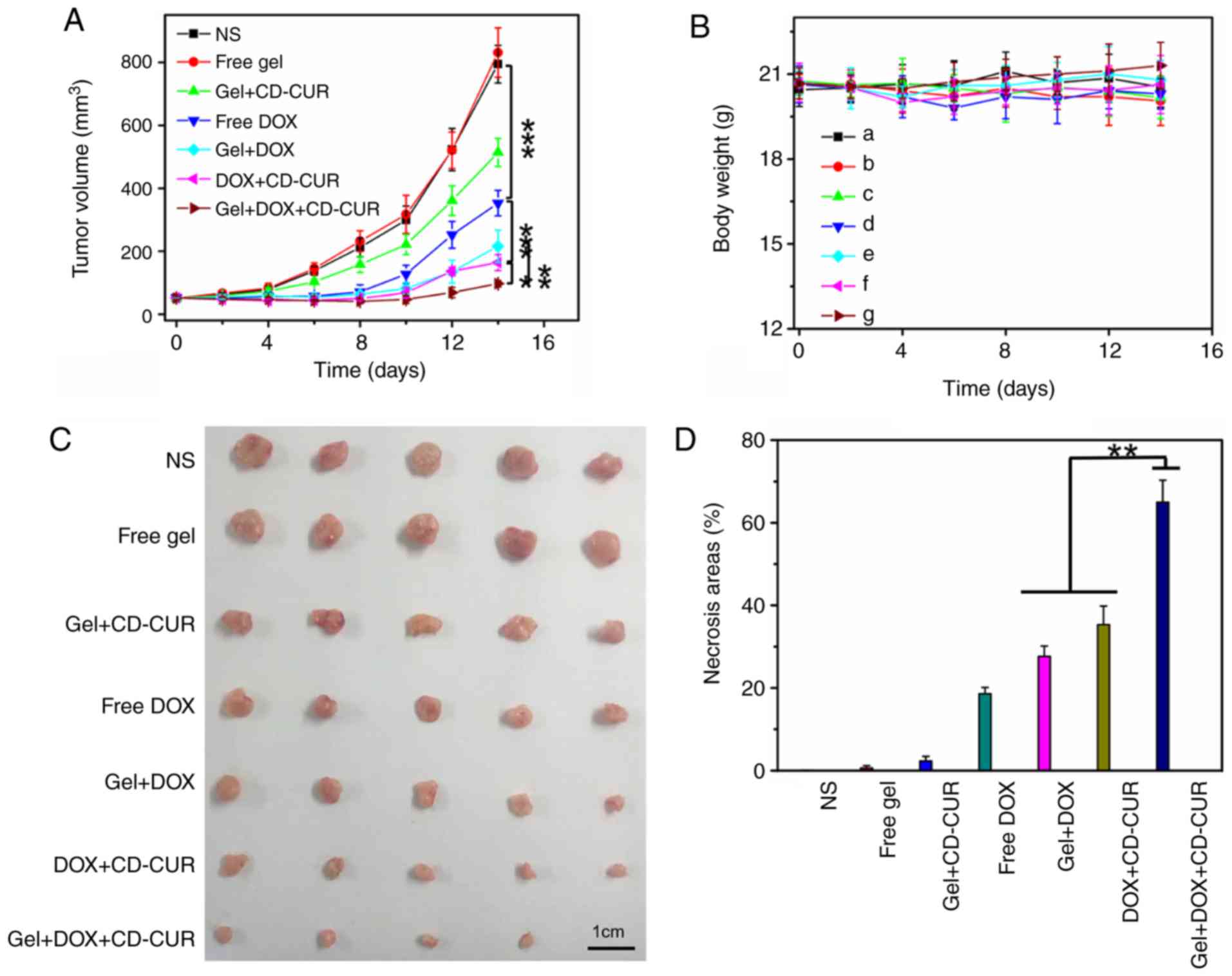
In vivo antitumor efficiencies of
different strategies
The in vivo antitumor efficiencies of
different strategies were evaluated using K-7 tumor-bearing mice.
As shown in Fig. 7A, all treatment
strategies resulted in anti-proliferative effects compared with the
control groups (NS and free gel). The tumor growth curve of free
gel was not significantly different from that of the NS group,
suggesting that hydrogel as a drug-carrier has a small effect on
tumor growth. While the free DOX group exhibited notable antitumor
effects, the tumor volume of the gel+DOX group was smaller than
that of the free DOX group; the former depended on the sustained
release of DOX from hydrogel to maintain a relatively high DOX
concentration in tumor sites for a long time. Due to the
downregulation of Bcl-2 by CUR, the combination therapy of
gel+DOX+CD-CUR served a more powerful role in killing tumor cells
than free DOX (Fig. 7D).
Therefore, although the antitumor effect of gel+CD-CUR was weak,
the combination therapy based on gel+DOX+CD-CUR exhibited a
stronger antitumor effect than monotherapy. This localized
dual-drug delivery system could deliver DOX and CD-CUR to the tumor
site simultaneously. Furthermore, hydrogel served as a drug depot
to maintain effective drug concentration for long periods of time;
therefore, this promising strategy greatly inhibited tumor growth
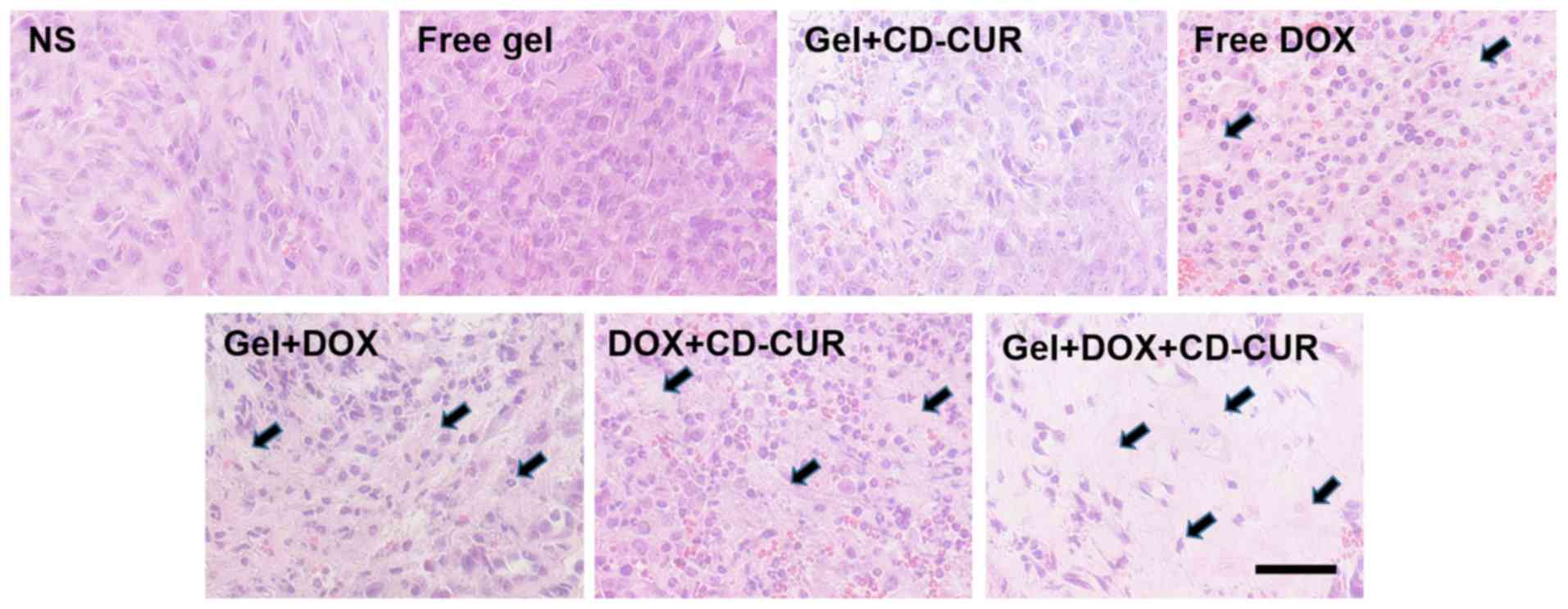
(Fig. 7C). To further investigate
the antitumor efficiencies of different strategies, the tumor
tissues were sliced and stained with H&E for histological
analysis. According to Fig. 8,
both combination therapy strategies (DOX+CD-CUR and gel+DOX+CD-CUR)
produced larger necrotic areas than single-drug therapies. The
present results were consistent with the outcome of the in
vivo antitumor evaluation.
Systemic security
Body weight was an important index to evaluate the
systemic toxicity of different strategies. As shown in Fig. 7B, none of the treatment strategies
resulted in significant weight loss throughout the treatment period
compared with control groups, although the free DOX group exhibited
slight weight loss at the end of the treatment. The present result
suggests that the localized treatment strategies had high systemic
safety. The histological analysis of major organs was carried out
to further explore the safety of the system. As shown in Fig. S7, no marked histological changes
were observed in all treated groups compared with in the control
groups.
Conclusion
In the present study, the thermosensitive hydrogel
PLGA-PEG-PLGA copolymer and the CD-CUR inclusion complex were
successfully prepared. A 20% wt PLGA-PEG-PLGA hydrogel, with a
suitable sol-gel transition temperature, was adopted to deliver
drugs. The solubility and stability of CD-CUR were significantly
improved compared with natural CUR. Single- or dual-drug delivery
systems were prepared by mixing drugs with the polymer solution.
Although natural CUR could be readily dissolved into polymer
solution without aggregation, the release rate of CUR from the
PLGA-PEG-PLGA hydrogel was extremely slow in PBS without Tween 80.
By contrast, gel+CD-CUR could release CUR in PBS with or without
Tween 80 in a sustained manner.
CUR can potentiate the cytotoxicity of most
first-line chemotherapy drugs and combination therapy is an
important strategy in the treatment of osteosarcoma (6). In the present study, co-loading DOX
and CD-CUR into hydrogel to form a dual-drug delivery system
(gel+DOX+CD-CUR) was able to improve the cytotoxicity efficiency
and promote the pro-apoptotic effect of DOX compared with
single-drug treatment. Gel+DOX+CD-CUR markedly downregu-lated Bcl-2
expression and increased the protein levels of caspase-3. In
vivo, the gel+DOX+CD-CUR group exhibited the strongest
antitumor effect compared with other groups. Additionally, the good
systemic safety of this dual-drug delivery system has been
demonstrated. In summary, combination therapy based on DOX and
CD-CUR co-loaded hydrogel may be a promising strategy for the
localized treatment of osteosarcoma.
Supplementary Data
Funding
The present study was supported by the National
Natural Science Foundation of China (project no. 51390484), the
Jilin Province Science and Technology Development Program (program
no. 20130521011JH) and the Doctoral Fund Project of Hunan
Provincial People's Hospital (program no. BSJJ201812).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY, YL and JL conceived and designed the
experiments. ZY and YL performed the experiments. JL analyzed the
data. ZY and YL wrote the manuscript. ZY, YL and JL modified the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Medical
Ethics Committee of Hunan Normal University and were in accordance
with the Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Schwartz CL, Gorlick R, Teot L, Krailo M,
Chen Z, Goorin A, Grier HE, Bernstein ML and Meyers P: Multiple
drug resistance in osteogenic sarcoma: INT0133 from the Children's
Oncology Group. J Clin Oncol. 25:2057–2062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nedelcu T, Kubista B, Koller A, Sulzbacher
I, Mosberger I, Arrich F, Trieb K, Kotz R and Toma CD: Livin and
Bcl-2 expression in high-grade osteosarcoma. J Cancer Res Clin
Oncol. 134:237–244. 2008. View Article : Google Scholar
|
|
3
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–133.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sen GS, Mohanty S, Hossain DM,
Bhattacharyya S, Banerjee S, Chakraborty J, Saha S, Ray P,
Bhattacharjee P, Mandal D, et al: Curcumin enhances the efficacy of
chemotherapy by tailoring p65NFκB-p300 cross-talk in favor of
p53-p300 in breast cancer. J Biol Chem. 286:42232–42247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yardley DA: Drug resistance and the role
of combination chemotherapy in improving patient outcomes. Int J
Breast Cancer. 2013:1374142013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwon Y: Curcumin as a cancer chemotherapy
sensitizing agent. J Korean Soc Appl Biol Chem. 57:273–280. 2014.
View Article : Google Scholar
|
|
7
|
Wang J, Ma W and Tu P: Synergistically
Improved anti-tumor efficacy by co-delivery doxorubicin and
curcumin polymeric micelles. Macromo Biosci. 15:1252–1261. 2015.
View Article : Google Scholar
|
|
8
|
Tsai YM, Jan WC, Chien CF, Lee WC, Lin LC
and Tsai TH: Optimised nano-formulation on the bioavailability of
hydrophobic polyphenol, curcumin, in freely-moving rats. Food chem.
127:918–925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strimpakos AS: Preventive and therapeutic
properties in laboratory studies and clinical trials. Antioxid
Redox Signal. 10:511–545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
11
|
Meiyanto E, Putri DD, Susidarti RA,
Murwanti R, Sardjiman, Fitriasari A, Husnaa U, Purnomo H and
Kawaichi M: Curcumin and its analogues (PGV-0 and PGV-1) enhance
sensitivity of resistant MCF-7 cells to doxorubicin through
inhibition of HER2 and NF-kB activation. Asian Pac J Cancer Prev.
15:179–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chuah AM, Jacob B, Jie Z, Ramesh S, Mandal
S, Puthan JK, Deshpande P, Vaidyanathan VV, Gelling RW, Patel G, et
al: Enhanced bioavailability and bioefficacy of an amorphous solid
dispersion of curcumin. Food Chem. 156:227–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Misra R and Sahoo SK: Coformulation of
doxorubicin and curcumin in poly(D, L-lactide-co-glycolide)
nanoparticles suppress the development of multi drug resistance in
K562 cells. Mol Pharm. 8:52–866. 2011. View Article : Google Scholar
|
|
14
|
Nabekura T, Kamiyama S and Kitagawa S:
Effects of dietary chemopreventive phytochemicals on P-glycoprotein
function. Biochem Biophys Res Commun. 327:866–870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lv L, Qiu K, Yu X, Chen C, Qin F, Shi Y,
Ou J, Zhang T, Zhu H, Wu J, et al: Amphiphilic copolymeric micelles
for doxorubicin and curcumin co-delivery to reverse multidrug
resistance in breast cancer. J Biomed Nanotechnol. 12:973–985.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aggarwal BB, Kumar A and Bharti AC:
Anticancer potential of curcumin: Preclinical and clinical studies.
Anticancer Res. 23:363–398. 2003.PubMed/NCBI
|
|
17
|
Liu J, Chen S, Lv L, Song L, Guo S and
Huang S: Recent progress in studying curcumin and its
nano-preparations for cancer therapy. Curr Pharm Des. 19:1974–1993.
2013.
|
|
18
|
Zhang W, Cui T, Liu L, Wu Q, Sun L, Li L,
Wang N and Gong C: Improving anti-tumor activity of curcumin by
polymeric micelles in thermosensitive hydrogel system in colorectal
peritoneal carcinomatosis model. J Biomed Nanotechnol.
11:1173–1182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pillarisetti S, Maya S, Sathianarayanan S
and Jayakumar R: Tunable pH and redox-responsive drug release from
curcumin conjugated γ-polyglutamic acid nanoparticles in cancer
microenvironment. Colloids Surf B Biointerfaces. 159:809–819. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yallapu MM, Jaggi M and Chauhan SC:
Beta-cyclodextrin-curcumin self-assembly enhances curcumin delivery
in prostate cancer cells. Colloids Surf B Biointerfaces.
79:113–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horvath G, Premkumar T, Boztas A, Lee E,
Jon S and Geckeler KE: Supramolecular nanoencapsulation as a tool:
Solubilization of the anticancer drug trans-dichloro(dipyridine)
platinum(II) by complexation with beta-cyclodextrin. Mol Pharm.
5:358–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rahman S, Cao S, Steadman KJ, Wei M and
Parekh HS: Natural and β-cyclodextrin-enclosed curcumin: Entrapment
within liposomes and their in vitro cytotoxicity in lung and colon
cancer. Drug Deliv. 19:346–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rachmawati H, Edityaningrum CA and
Mauludin R: Molecular inclusion complex of curcumin-β-cyclodextrin
nanoparticle to enhance curcumin skin permeability from hydrophilic
matrix gel. AAPS PharmSciTech. 14:1303–1312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alexander A, Ajazuddin, Khan J and Saraf S
and Saraf S: Poly(ethylene glycol)-poly(lactic-co-glycolic acid)
based thermosensitive injectable hydrogels for biomedical
applications. J Control Release. 172:715–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu L and Ding J: Injectable hydrogels as
unique biomedical materials. Chem Soc Rev. 37:1473–1481. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He C, Tang Z, Tian H and Chen X:
Co-delivery of chemotherapeutics and proteins for synergistic
therapy. Adv Drug Deliv Rev. 98:64–76. 2016. View Article : Google Scholar
|
|
27
|
Tang B, Ma L, Wang HY and Zhang GY: Study
on the supramolecular interaction of curcumin and beta-cyclodextrin
by spectrophotometry and its analytical application. J Agric Food
Chem. 50:1355–1361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gerola AP, Silva DC, Jesus S, Carvalha RA,
Rubria AF, Muniz EC, Borges O and Valente AJM: Synthesis and
controlled curcumin supramolecular complex release from
pH-sensitive modified gumarabic-based hydrogels. RSC Adv.
5:94519–94533. 2015. View Article : Google Scholar
|
|
29
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG; National Centre for the Replacement, Refinement
and Reduction of Amimals in Research: Animal research: Reporting in
vivo experiments-the ARRIVE guidelines. J Cereb Blood Flow Metab.
31:991–993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohanty C and Sahoo SK: The in vitro
stability and in vivo pharmacokinetics of curcumin prepared as an
aqueous nanoparticulate formulation. Biomaterials. 31:6597–6611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marcolino VA, Zanin GM, Durrant LR,
Benassi Mde T and Matioli G: Interaction of curcumin and bixin with
β-cyclodextrin: Complexation methods, stability, and applications
in food. J Agric Food Chem. 59:3348–3357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiong X, Luo S, Wu B and Wang J:
Comparative developmental toxicity and stress protein responses of
dimethyl sulfoxide to rare minnow and zebrafish embryos/larvae.
ZEBRAFISH. 14:60–68. 2017. View Article : Google Scholar
|
|
33
|
Yu L, Ci T, Zhou S, Zeng W and Ding J: The
thermogelling PLGA-PEG-PLGA block copolymer as a sustained release
matrix of doxorubicin. Biomater Sci. 1:411–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cesteros LC, Gonzalez-Teresa R and Katime
I: Hydrogels of β-cyclodextrin crosslinked by acylated
poly(ethylene glycol): Synthesis and properties. Eur Polym J.
45:674–679. 2009. View Article : Google Scholar
|
|
35
|
Yoon SJ, Hyun H, Lee DW and Yang DH:
Visible light-cured glycol chitosan hydrogel containing a
beta-cyclodextrin-curcumin inclusion complex improves wound healing
in vivo. Molecules. 22:pp. E15132017, View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu J, Li X and Sun F:
Cyclodextrin-containing hydrogels for contact lenses as a platform
for drug incorporation and release. Acta Biomater. 6:486–493. 2010.
View Article : Google Scholar
|
|
37
|
Quaglia F, Varricchio G, Miro A, La
Rotonda MI, Larobina D and Mensitieri G: Modulation of drug release
from hydrogels by using cyclodextrins: The case of
nicardipine/beta-cyclodextrin system in crosslinked
polyethylenglycol. J Control Release. 71:329–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fryer RA, Galustian C and Dalgleish AG:
Recent advances and developments in treatment strategies against
pancreatic cancer. Curr Clin Pharmacol. 4:102–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao M, Fan S, Goldberg ID, Laterra J,
Kitsis RN and Rosen EM: Hepatocyte growth factor/scatter factor
blocks the mitochondrial pathway of apoptosis signaling in breast
cancer cells. J Biol Chem. 276:47257–47265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sa G and Das T: Anti cancer effects of
curcumin: Cycle of life and death. Cell Div. 3:142008. View Article : Google Scholar : PubMed/NCBI
|