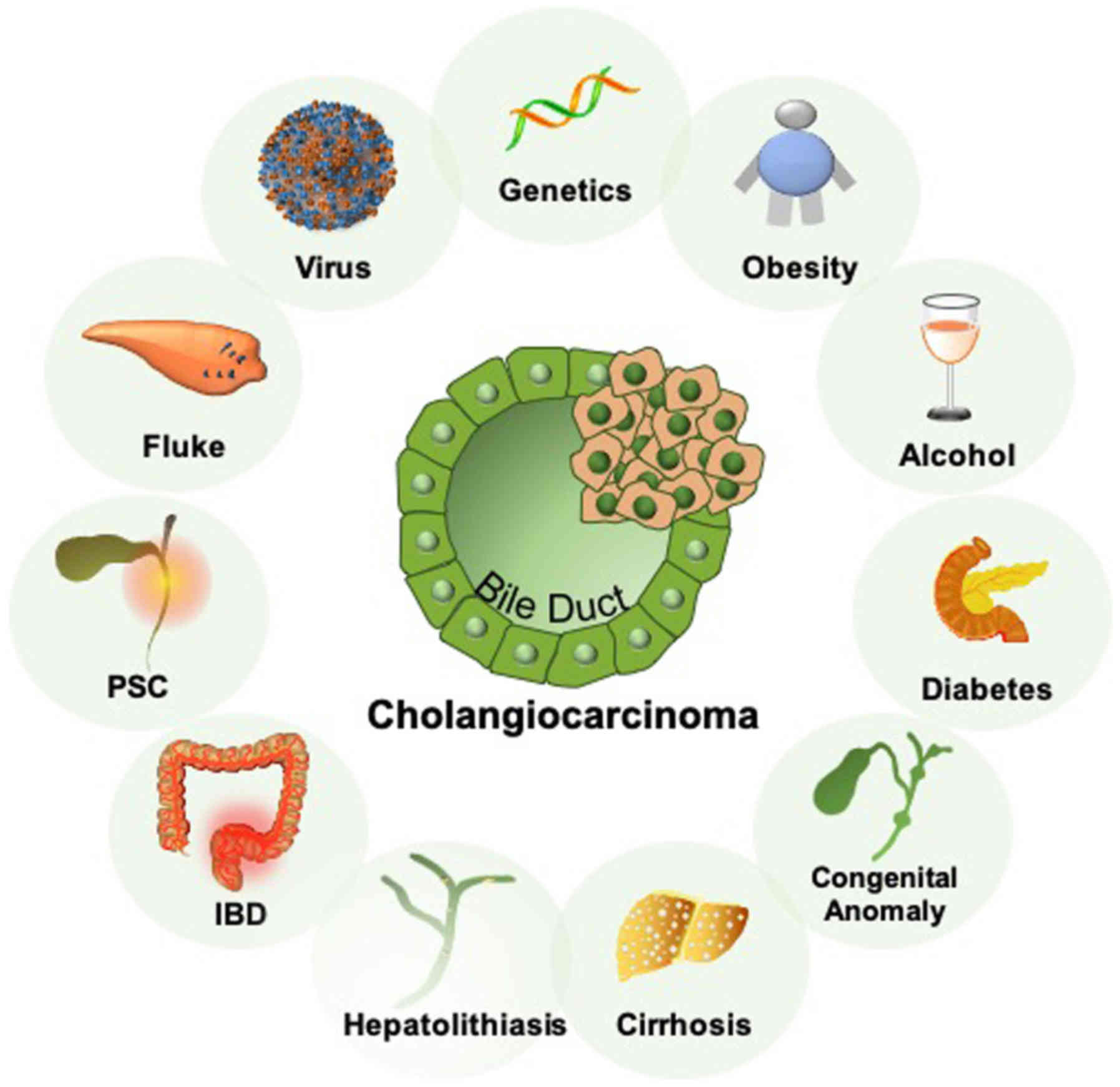
Cholangiocarcinoma (CCA) is a diverse and collective
malignancy that is derived from the biliary epithelium (1). Broadly recognized risk factors for
CCA include liver fluke infection, primary sclerosing cholangitis,
cirrhosis, viral hepatitis, congenital anomalies of the biliary
tree and hepatolithiasis (1). In
addition, inflammatory bowel disease, obesity and genetic
predisposition have been reported to be associated with a higher
risk of developing CCA (Fig. 1)
(1). Although the incidence of CCA
in the United States remains relatively low, at 1.26 cases per
100,000 people as of 2015, its prevalence has been steadily
increasing over the past 50 years (2). According to analysis by race, CCA has
the highest overall incidence in Asian Americans at 1.87 cases per
100,000 people, followed by Caucasian Americans at 1.23 cases per
100,000 people and lastly African Americans, which is 1.17 per
100,000 people (2). However, the
mortality rate of CCA in African Americans has increased
dramatically over the past decade, increasing 45% compared to ~20%
in Asian Americans and Caucasian Americans (3).
The current 5-year survival rate in patients with
early stage CCA who undergo curative-intent surgery is 30%
(4). Patients with advanced
disease at diagnosis have limited treatment options and poor
prognosis. In a previous surveillance program of 825 patients with
CCA, regardless of treatment modality or tumor pathology, the
median overall survival (OS) was found to be 7 months, whilst the
5-year survival rate was revealed to be 5.7% (5). Due to aggressive tumor growth and the
presence of metastases at diagnosis, ~86% of patients with CCA
patients are ineligible for either curative-intent surgery or
palliative resection (5). Patients
ineligible for curative surgery are provided a combinatorial
chemotherapy of gemcitabine and cisplatin either as adjuvant to
surgery or as the first line of care. However, combination
chemotherapy confers minimal survival benefit, where it modestly
prolongs the median OS to 11.7 months and progression free survival
to 8 months compared with a historical OS of 2.5-4.5 months with
supportive care (6,7). This highlights the urgent need for
the development of novel effective treatment options for patients
who are ineligible for surgery.
Newly developed targeted therapies have demonstrated
promising clinical efficacy against chemotherapy-refractory CCA. In
a phase II study of patients with fibroblast growth factor receptor
(FGFR)-altered advanced biliary tract cancer (BTC), a selective
pan-FGFR kinase inhibitor exerted impressive anti-tumor activity
with a disease-control rate (DCR) of 82% (8). Additionally, in another phase I study
involving patients with isocitrate dehydrogenase 1 (IDH)-positive
CCA, which represents ~25% of all cases of CCA, the IDH1 inhibitor
ivosidenib exhibited a well-tolerated safety profile with an
overall response rate (ORR) of 5% and an OS of 13.8 months
(9). However, these aforementioned
promising targeted therapies were only viable for a relatively
small percentage of patients with CCA with the specific IDH1 and
FGFR mutations aforementioned (9).
Within the last decade, immunotherapy has become a
major pillar of cancer therapy. Immune checkpoint inhibitors (ICIs)
act by targeting dysregulated immune checkpoints, including
programmed death protein 1 (PD-1) and programmed death ligand
(PD-L1), which affect anti-tumor immunity in several types of
cancers (10). Accumulating
evidence have demonstrated encouraging results in applications with
ICIs alone for treating hepatocellular carcinoma (HCC), with
response rates reaching ~15-20% (11,12).
However, ICIs have demonstrated minimal efficacy for the treatment
of CCA (13,14). In a recent study of advanced BTCs
that were treated with a combination of an ICI and microwave
ablation, the ORR was found to be 12.5% (15). Novel therapeutic strategies to
combat CCA progression are therefore urgently sought. One emerging
target in this research field is cancer stem cells (CSCs), also
referred to as tumor-initiating or -propagating cells.
There is increasing consensus supporting the
existence of a distinct cellular hierarchy within a tumor, where
CSCs are unique originators of all tumor cells and are responsible
for tumor growth (16-18). CSCs have been implicated in CCA
along with a variety of other solid tumors, including breast,
brain, colorectal (CRC), pancreatic, liver, melanoma, ovarian and
prostate cancer (Tables I and
II). It is hypothesized that CSCs
survive following the initial stages of cancer therapy and thereby
facilitate relapse and metastasis, where they are responsible for
acquired resistance to conventional cancer treatment regimens,
including radiation therapy and the more recently discovered
immunotherapy (18-20). CSCs may be able to evade the immune
system by altering their immunogenicity, enabling the avoidance of
rejection mediated by the immune system in vivo (21). CSCs are defined by their enriched
capacity for self-renewal and differentiation into explicit
malignant progenies. Tumors with CSC-enriched phenotypes are
considerably more plastic than originally anticipated, which are in
turn heavily influenced by the tumor microenvironment, rendering
the design of therapeutic methodology against them difficult
(22). In addition, although
previous reports suggested a frequency of <1 CSC per 1,000
cancer cells, the proportion of CSCs with tumorigenic capacity
could be much higher (23,24). CSCs can be uniquely characterized
by their cell-surface markers, where several markers have been used
to identify CSCs in various types of cancers such as CCA (Tables I and II).
CD133, also known as prominin-1, is a pentaspan
trans-membrane glycoprotein that appears to be an epithelial marker
in tissues in addition to being a CSC marker (24). Although the precise function of
CD133 remains unclear, considerable evidence exists for the
increased capabilities for tumor initiation in CD133+
cell cultures and tumor xenografts (24-28).
The presence of CD133 along with other suspected CSC markers has
also been associated with poorer overall survival in patients with
CCA (29). In a previous study of
29 patients with intrahepatic CCA who had undergone major
hepatectomies, only 8% of CD133+ patients remained alive
5 years following surgery, compared with 57% among
CD133- patients (P=0.02) (29). Consistent with this notion,
CD133+ liver cancer cell lines appear to exhibit
significantly higher resistance to autophagy as a result of IFN-γ
treatment compared with the corresponding CD133- cell
lines in vitro (28). This
finding has been corroborated in vivo, where
CD133- tumors shrank in cirrhosis-associated HCC cells
in mice treated with IFN-γ. By contrast, tumors of the
CD133+ phenotype were resistant and instead became
further enriched with CD133+ expression (28).
CD44 is a cell-surface glycoprotein that is involved
in cell-cell adhesion and migration (30,31).
Functionally, it is involved in leukocyte homing and activation,
wound healing and cell migration (30,31).
CD44s, the conventional isoform of CD44, is expressed in normal
epithelial cells and serves as an adhesion molecule in the
extracellular matrix (30,31). In some carcinomas of epithelial
origin, variant isoforms of CD44 have been implicated in tumor
metastasis and invasion (32).
Previous studies have suggested CD44 and its variant isoforms to be
responsible for cellular stemness characteristics, associated with
resistance to reactive oxygen species (ROS) in CSCs and implicated
in the progression of malignancies in gastrointestinal system
(30,31). However, it should be noted that the
clinical relevance of different CD44 isoforms are highly dependent
of the type of cancer. For example, CD44v6 appeared to be unrelated
to the CCA progression, whilst CD44v9 appears to be clinically
relevant to the disease (32-34).
In a previous immunohistochemistry analysis of CCA tumors, CD44v9
was found to associate with the expression of inflammatory markers
cyclooxygenase-2 (COX2) and S100 Calcium Binding Protein P, where
CD44v9 expression was found to be higher in CCA associated with
liver fluke infection (32).
There have been disparities in the findings
regarding the use of CD24 as a CSC marker. The
CD44high/CD24low cell phenotype has been
repeatedly utilized as a signature of CSCs in breast tumors, where
they were demonstrated to be chemoresistant following chemotherapy
(35). Similar findings were also
documented in a previous in vitro study of CCA (36). In CCA cell lines, a shift from
CD44high/CD24high to
CD44high/CD24low was observed in cells
resistant to epidermal growth factor receptor inhibition (36). By contrast, pharmacological
depletion of ROS scavengers resulted in increased sensitivity to
radiotherapy and depleted clonogenicity in the
CD24+CD90+-enriched cell population,
suggesting that the CD24+CD90+ combination
may be responsible for mediating resistance to radiation in CSCs
(37). In patients with CCA who
received chemotherapy and radiation, CD24 expression was previously
found to be associated with a lower median survival time (38). To verify these findings, further
research on the individual role of CD24 in CSCs and cancer
progression is required.
ALDH belong to a family of intracellular enzymes
that are involved in cellular detoxification, differentiation, and
drug resistance (46,47). Although ALDH1 has been most
commonly applied as a CSC marker in breast cancer, it has also been
previously implicated in CCA and HCC (46,47),
where the expression level of ALDH1 was found to be correlated with
poor prognosis in patients with CCA (46,47).
In addition, ALDH1 expression has been demonstrated to potentiate
mesenchymal properties in the CCA cell line TFK-1 (46). However, conflicting evidence exists
with regards to the role of ALDH1 in CRC compared with that in CCA.
In CRC, it was hypothesized that the expression of extracellular,
rather than intracellular CSC markers, may serve as superior
indicators of tumor stemness (23,24).
SOX2, NANOG and OCT4 are all transcription factors
essential for the maintenance of stemness in embryonic stem cells
and have been previously used as markers for CSCs (48). They directly communicate with each
other during embryonic development, where they suppress
differentiation into progenitor cells (48). NANOG, OCT4, and SOX2 expression
have all been previously revealed to be associated with poor
prognosis in rectal cancer, glioma and CCA (49). In rectal cancer, expression of ≥2
in comparison to ≤1 of these markers was found to associate
significantly with poorer OS. In particular, OCT4 was also
demonstrated to be independently associated with poor tumor
differentiation, higher N stage and larger tumor size in rectal
cancer (48). Likewise in CCA,
co-expression of OCT4 and Nanog was found to be associated with the
most inferior of the clinical outcomes (49). Elevated SOX2 expression has also
been previously associated with poorly differentiated tumors,
metastasis and insignificantly, vascular invasion and tumor stage
in CCA (50). Patients with CCA in
which SOX2 was overexpressed, exhibited significantly lower OS with
no difference in DFS (50).
Overall, based on the data available on CSC markers,
it is important to characterize CSCs in tumors by using multiple
markers instead of reliance on any single marker.
In addition to the expression of cell surface
molecules that are typically associated with stem cells, CSCs
exhibit classical features of stem cells such as the ability to
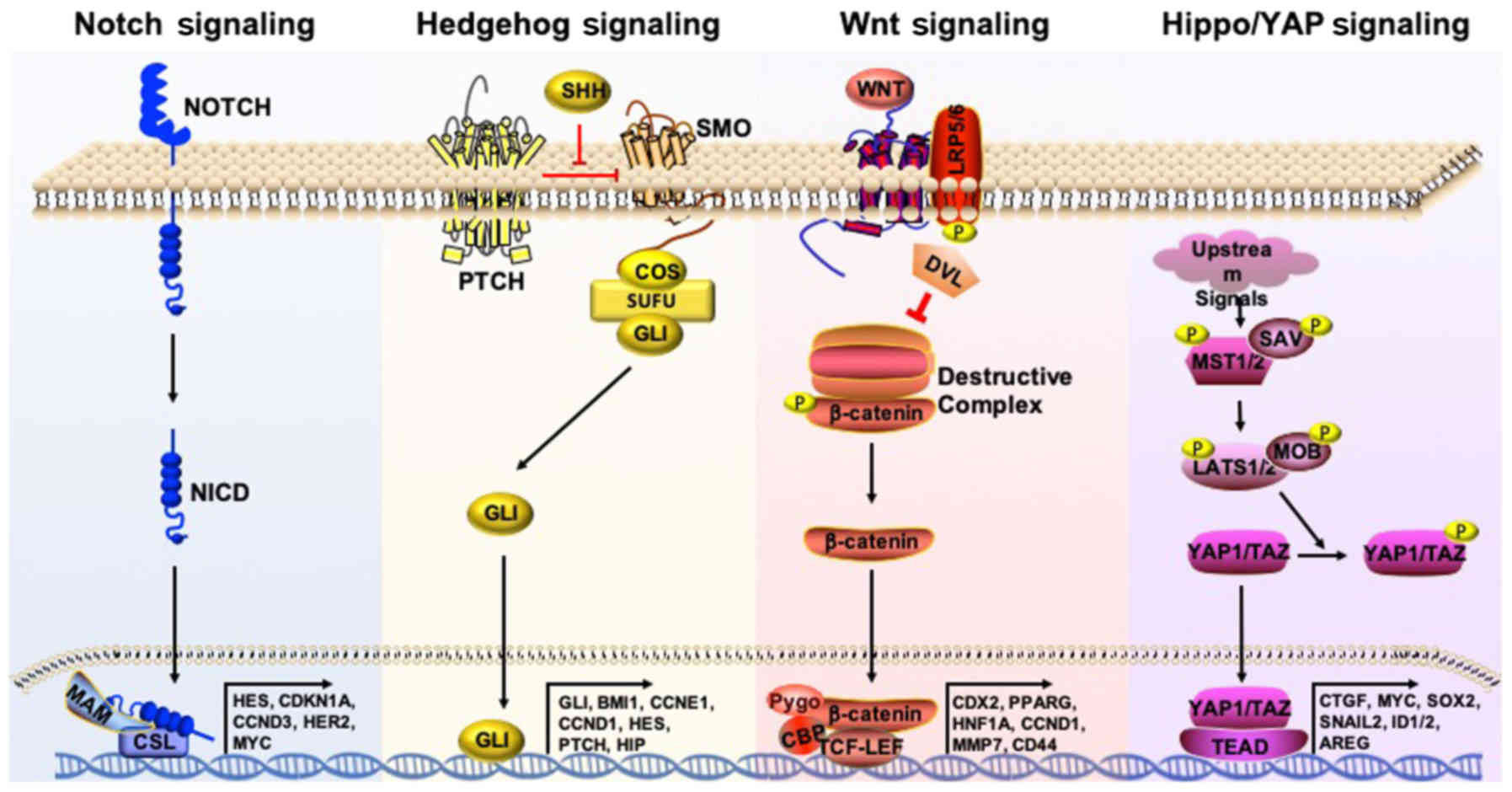
differentiate into explicit progenies (51). Signal transduction pathways that
are highly active in embryonic and adult stem cells, including
Notch, Hedgehog and Wnt, are also highly active in CSCs (Fig. 2) (19). In this section, the various
signaling pathways involved in CSC maintenance and research that
are focused on targeting these pathways will be outlined.
The Hippo/YAP1 is a signaling pathway that is highly
conserved among the majority of mammalian species. It has recently
garnered significant attention due to its reported role in
regulating CSCs (52). Core
kinases involved in this pathway include macrophage stimulating
(MST) 1/2 and large tumor suppressor kinase (LATS) 1/2, which are
under regulation by scaffolding proteins Salvador family WW domain
containing protein 1 and phocein (MOB), respectively (Fig. 2). Activation of MST and LATS leads
to the phosphorylation and subsequent inactivation of YAP1. This
inhibits YAP1 from entering the nucleus, where it would normally
bind to the transcriptional enhancer factor (TEAD) and SMAD
families of transcription factors, leading to the transcription of
a number of oncogenic genes, including SOX9, amphiregulin, MYC and
Gli1 (53-55).
YAP1 overexpression is associated with
tumorigenesis, epithelial-to-mesenchymal transition (EMT), in
addition to tumor angiogenesis and invasion, physiological
processes that have been previously demonstrated to lead to
unfavorable prognoses in malignancies, including CCA, HCC, lung,
brain, ovarian, breast, bladder and colon cancer (56-60).
YAP1 expression was found to be an independent prognostic factor in
both human and animal studies of CCA (57,59).
Previous studies have demonstrated that liver-specific MOB1a/1b
double knockout (61) and MST1/2
conditional knockout mice (62)
exhibited phenotypically mixed HCC/CCA inducible tumors. These
findings suggest that the Hippo/YAP1 signaling pathway is critical
for liver tumorigenesis. Intriguingly, the introduction of
activated YAP1 and myristoylated AKT in the biliary tract, coupled
with biliary ligation, triggered CCA formation in an IL-6-dependent
manner within 6-8 weeks in >70% of the mice tested (63). This was performed through the
ectopic expression of the constitutively active AKT and YAP1 using
the Sleeping Beauty transposon transfection system. This study not
only highlighted the potential role of inflammatory cytokines on
CCA oncogenesis but also suggests YAP1 to be an important driver of
CCA tumorigenesis.
A number of studies have indicated that the
Hippo/YAP1 pathway serves an integral role in the maintenance of
CSCs. YAP1 overexpression directly upregulates SOX9 in esophageal
cancer, which in turn endows cancer cells with stem-like properties
(64,65). In addition, YAP1 can induce the
expression of the embryonic stem cell transcription factor SOX2 and
co-operate with the pro-inflammatory COX2 pathway to expand the
population of CSCs in urothelial cancer (66). Immunohistochemistry analysis of
COX2 and YAP1 expression in bladder cancer samples previously
demonstrated that these two proteins can mediate resistance to
treatment, whilst the in vitro inhibition of these proteins
significantly reduced tumor growth (66). Additionally, the expression of YAP1
was found to be upregulated in CSCs in non-small cell lung cancer
(NSCLC) cell lines, which was believed to contribute to their
capacity for self-renewal forming angiogenic tubules (67). This previous study also showed that
knocking down YAP1 expression can significantly reduce the spheroid
forming and proliferative capacity of NSCLC (67). Interestingly, the effects of YAP1
were revealed to be mediated through induction of SOX2, which then
directly interacts with OCT4, in a manner that was independent of
TEAD2 (67). It was reported
previously that long noncoding RNAs are highly expressed in CSCs of
HCC, which are required for the self-maintenance of liver CSCs
(68). Among those, lncBRM
initiates YAP1 signaling activation to drive the self-renewal of
liver CSCs (68). In conclusion,
these findings suggest that YAP1 likely serve a pivotal role in the
maintenance of stemness in CSCs.
Until recently, targeting the Hippo/YAP1 signaling
pathway has proved challenging due to its complexity and
substantial crosstalk with other pathways. Verteporfin, a
photodynamic drug that was traditionally used for treating macular
degeneration, has recently emerged as a YAP/tafazzin (TAZ)
inhibitor, where it has demonstrated promising preclinical results
in cancers such as CCA (57). The
combination of verteporfin and rapamycin was previously found to
inhibit intrahepatic CCA cell proliferation and tumor growth
(57), where verteporfin activated
mTOR whilst inhibiting STAT3 phosphorylation in CCA (57). However, to the best of our
knowledge, no clinical studies on the effects of verteporfin on CCA
or CSCs in other cancers have been performed.
In a recent study, LEE011 was found to inhibit
cyclin-dependent kinase 6 (CDK6), whilst CA3 inhibited YAP1, using
both in vivo and in vitro models of esophageal cancer
(69). YAP1 and CDK6 expression
were also revealed to associate positively with each other and with
resistance to radiation (69).
Combined treatment using both CA3 and LEE011 reduced tumor volume
to a greater degree compared with either treatment alone. These
findings suggest that YAP1 may synergize with CDK6 to induce cell
proliferation and resistance to chemotherapy in cancer (69). However, to date, no clinical
studies have been conducted using the novel Hippo/YAP1 inhibitors.
Further research devoted to the effects of Hippo/YAP1 on CSC
formation may prove to be beneficial for the treatment of
refractory cancers such as CCA.
Hedgehog (Hh) protein activation by Patched triggers
a signaling cascade that regulates proliferation, metastasis and
invasion in cancer cells (70). In
addition, it has also been implicated in the stemness maintenance
of CSCs (19). A previous study
has found that hypoxia induces Hh activation in CCA, which was
demonstrated to be positively associated with the induction of EMT,
and upregulation of the stemness markers, including NANOG, Oct4,
SOX2, CD133 in vitro (27).
Application of the Hh inhibitor Saridegib, in
combination with gemcitabine, did not exert notable therapeutic
effects on metastatic pancreatic cancer in phase II trials
(71). Additionally, whilst there
were promising preclinical and phase I data for this trial, control
patients exhibited longer OS compared with those treated with the
Hh inhibitor (71,72). Glasdegib and Vismodegib are
currently the two FDA-approved Hh signaling inhibitors that have
been extensively studied in relation to malignancies such as acute
myeloid leukemia (73). However,
since research on their effects in other cancers have been mostly
discontinued, they have not been studied clinically in CCA.
The Notch signaling pathway has been extensively
studied in cancer, where it was suggested to be important for the
regulation of cell survival and apoptosis (74). Notch is cleaved sequentially by
tumor necrosis factor-α converting enzyme (TACE) and γ-secretase,
resulting in the release of the intracellular domain of Notch
(NICD). NICD then trans-locates into the nucleus where it mediates
the transcription of target genes, including hairy and enhancer of
split-1, NF-κB, cyclin D1 and c-myc (75). The Notch1 receptor and the Notch
ligand Jagged 1 was found to be overexpressed in four human CCA
cell lines (76). In addition,
overexpression of NICD in mouse livers has been previously found to
induce cystic CCA tumor development (77). These observations suggest that
Notch can serve as a potential target for controlling CSCs
(78).
γ-secretase inhibitors are among the largest class
of Notch inhibitors, with >12 different clinical trials having
previously applied this class of drug (19). However, none have progressed to
phase III trials due to inefficacy or intolerance and therefore
development of drugs of this class has been discontinued.
Antibodies specifically targeting Notch 1-3 have also been
developed, but like γ-secretase inhibitors, research on this class
of drug has been repeatedly discontinued (19). By contrast, the anti-delta like
canonical notch ligand 4 antibody, Demcizumab, has progressed to
randomized phase II trials in lung and pancreatic cancer after
exhibiting a manageable safety profile and an ORR of 51% in phase I
NSCLC trials (19). No data on its
use in CCA are currently available.
The Wnt/β-catenin signaling pathway is one of widely
studied pathways in cancer research. Notably, CRC is initiated by
mutations in genes such as adenomatous polyposis coli (APC), which
activates the Wnt/β-catenin pathway (79). In the canonical Wnt/β-catenin
pathway, Wnt ligands bind to Frizzled and low-density lipoprotein
receptor-related protein receptor complexes, initiating the
recruitment of scaffold proteins and disruption of the β-catenin
destruction complex. Mutations in this complex, including that of
APC, can lead to β-catenin accumulation in the cytosol. The
accumulated β-catenin then translocates into the nucleus where it
associates with the TCF family of transcription factors and a
number of co-activators, including TAZ, to initiate the
transcription of target genes. It has been previously reported that
the canonical Wnt/β-catenin signaling pathway is activated in human
CCA, where the inhibition of Wnt/β-catenin signaling reduced
proliferation whilst inducing apoptosis in vivo (80). However, it remains unclear how the
Wnt signaling pathway serves a role on the stemness of CSCs. The
canonical Wnt/β-catenin pathway has been previously reported to be
directly regulated by TAZ, an effector of the Hippo/YAP1 pathway
(81).
LY2090314 is a glycogen synthase kinase 3 inhibitor,
which induces the accumulation of β-catenin (Fig. 2). It has been shown that LY2090314
treatment in conjunction with nab-paclitaxel in a preclinical model
of pancreatic cancer prolonged mice survival (82). However, regimens consisting of
LY2090314 in combination with pemetrexed and carboplatin,
demonstrated suboptimal safety profiles and minimal clinical
efficacy in patients with advanced pancreatic cancer in a previous
phase I clinical trial (83).
BBI503 is a novel stemness kinase inhibitor that inhibits Nanog and
serine/threonine kinase 17a, which induces β-catenin accumulation
by stabilizing the β-catenin destruction complex (Fig. 2). Phase I data on BBI503 indicated
prolonged OS and disease control in patients with CRC tumors with
positive Nanog expression (84,85).
Phase II trials in both patients with HCC and CCA in addition to
those with other types of solid tumors are under way (84,85).
STATs are a family of cytoplasmic transcription
factors that serve key roles in maintaining cancer stemness. They
exert significant influence on cellular survival, proliferation,
differentiation and apoptosis by mediating responses to cytokine,
hormone and growth factor signaling. Genetic variations in the
JAK/STAT signaling pathway appear to be associated with CRC
(86). Notably, abnormalities in
STAT3 have been revealed to be involved in the oncogenesis of a
number of cancers, where it was demonstrated that STAT3 and both
JAK1 and JAK2 are involved in CRC cell growth, survival, invasion
and migration through the regulation of target gene expression,
including Bcl-2, E-cadherin, vascular endothelial growth factor and
matrix metalloproteinases (86).
Napabucasin (BBI608), a drug that targets the
transcription factor STAT3 to reduce stemness characteristics, has
reached phase III trials (87). In
phase II trials in CRC, napabucasin, in combination with standard
chemotherapy, exhibited an impressive DCR of 93% and an ORR of 33%
(88). Napabucasin has also been
studied preclinically in CCA, where treatment with this drug
resulted in general cytotoxicity and inhibited cancer stemness
(89). In addition, napabucasin
has been shown to inhibit colony formation and significantly
downregulate the expression of several stemness-related genes,
including CSC markers ALDH1 and CD133 in CCA cells (89).
To the best of our knowledge, no single agent is
currently available that can effectively target CSCs. One of the
reasons is the substantial crosstalk among the pathways
aforementioned. For example, phosphorylated YAP in the cytoplasm
can associate with β-catenin to promote its degradation (90). Furthermore, nuclear β-catenin can
associate with TAZ and SMAD to induce transcription of Wnt target
genes. Recently, it was discovered that the loss of MST1/2 in
hepatocytes, a core kinase of the Hippo pathway, led to the
activation of Notch signaling (91). This resulted in severe liver
enlargement and HCC formation due to a positive feedback loop with
YAP/TAZ (91). Knockdown of
β-catenin expression in the livers of MST1/2-null mice was found to
increase tumorigenesis, revealing an inhibitory role of Wnt in
relation to YAP (91).
Additionally, Hh can interact with both the Wnt and Notch pathways,
where increased Hh signaling can upregulate the expression of the
Notch ligand and Jagged 2 in neuronal stem cells, thereby
maintaining stemness (92). It is
possible that this phenomena is conserved in cancer. Secreted
frizzled protein 1 is an integral protein upstream in the Wnt
signaling cascade that was previously identified as a target of Hh
signaling through the actions of Gli1/2 (93). All aforementioned crosstalk between
the signaling pathways increase the complexity and challenge of
targeting CSCs using a single agent.
Given the role of CSCs in cancer relapse and
metastasis, there is an urgent need for the development of novel
therapies that target CSCs to effectively eradicate cancer
(Fig. 3). Even with the knowledge
of the pathways involved in the development and maintenance of
CSCs, designing pharmacological agents for targeting these pathways
has proven to be exceedingly difficult. Although there are only a
small number of clinical drugs currently available that are
hypothesized to target CSCs exclusively, a number of clinical
trials targeting CSCs in different types of cancer, including CCA,
are currently ongoing (Table
III).
CSCs have the ability to hide from the immune system
and resist therapies designed to kill cancer cells. YAP1 is a
downstream effector of the Hippo/YAP1 pathway, where the
dysregulation of this pathway leads to oncogenesis and the
enhancement of cell stemness. A number of drugs have attempted to
targeted cancer stemness with modest effects in clinical trials.
The Hippo/YAP1 pathway has become an attractive target for novel
CSC inhibition. Although novel therapeutic agents targeting CSCs
have demonstrated promise in preclinical research, none have
demonstrated satisfactory outcomes in clinical settings. The
complexity of crosstalk between signaling pathways therefore
warrants further research. Given the resistance of CCA to
non-surgical treatment, patients with CCA may benefit substantially
from research on this topic.
The present study is supported by
Physician-Scientist Early Investigator Program at CCR of NIH/NCI to
CX.
The datasets generated during the current study are
not publicly available because they consist of publicly accessible
information but are available on reasonable request.
NAM, JF, and CX collected information and wrote the
manuscript. SZG collected information and edited the manuscript.
All authors read and approved the final version of this
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Shaib Y and El-Serag HB: The epidemiology
of cholangiocarcinoma. Semin Liver Dis. 24:115–125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel N and Benipal B: Incidence of
Cholangiocarcinoma in the USA from 2001 to 2015: A US Cancer
Statistics Analysis of 50 States. Cureus. 11:e39622019.PubMed/NCBI
|
|
3
|
Yao KJ, Jabbour S, Parekh N, Lin Y and
Moss RA: Increasing mortality in the United States from
cholangiocarcinoma: An analysis of the National Center for Health
Statistics Database. BMC Gastroenterol. 16:1172016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mavros MN, Economopoulos KP, Alexiou VG
and Pawlik TM: Treatment and Prognosis for Patients With
Intrahepatic Cholangiocarcinoma: Systematic Review and
Meta-analysis. JAMA Surg. 149:565–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arrington AK, Nelson RA, Falor A, Luu C,
Wiatrek RL, Fakih M, Singh G and Kim J: Impact of medical and
surgical intervention on survival in patients with
cholangiocarcinoma. World J Gastrointest Surg. 5:178–186. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: ABC-02 Trial Investigators: Cisplatin plus gemcitabine
versus gemcitabine for biliary tract cancer. N Engl J Med.
362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JO, Oh DY, Hsu C, Chen JS, Chen LT,
Orlando M, Kim JS and Lim HY: Gemcitabine Plus Cisplatin for
Advanced Biliary Tract Cancer: A Systematic Review. Cancer Res
Treat. 47:343–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Javle M, Lowery M, Shroff RT, Weiss KH,
Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S,
Macarulla T, et al: Phase II Study of BGJ398 in Patients With
FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 36:276–282.
2018. View Article : Google Scholar :
|
|
9
|
Lowery MA, Burris HA III, Janku F, Shroff
RT, Cleary JM, Azad NS, Goyal L, Maher EA, Gore L, Hollebecque A,
et al: Safety and activity of ivosidenib in patients with
IDH1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet
Gastroenterol Hepatol. 4:711–720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akinleye A and Rasool Z: Immune checkpoint
inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol.
12:922019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH III, et
al: Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: KEYNOTE-224 investigators: Pembrolizumab in patients with
advanced hepatocellular carcinoma previously treated with sorafenib
(KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet
Oncol. 19:940–952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gou M, Zhang Y, Si H and Dai G: Efficacy
and safety of nivolumab for metastatic biliary tract cancer.
OncoTargets Ther. 12:861–867. 2019. View Article : Google Scholar
|
|
14
|
Ueno M, Chung HC, Nagrial A, Marabelle A,
Kelley RK, Xu L, Mahoney J, Pruitt SK and Oh D: Pembrolizumab for
advanced biliary adenocarcinoma: Results from the multicohort,
phase 2 KEYNOTE-158 study. Ann Oncol. 29(Suppl 8): pp.
viii205–viii270. 2018, View Article : Google Scholar
|
|
15
|
Xie C, Duffy AG, Mabry-Hrones D, Wood B,
Levy E, Krishnasamy V, Khan J, Wei JS, Agdashian D, Tyagi M, et al:
Tremelimumab in Combination With Microwave Ablation in Patients
With Refractory Biliary Tract Cancer. Hepatology. 69:2048–2060.
2019. View Article : Google Scholar :
|
|
16
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashita T and Wang XW: Cancer stem cells
in the development of liver cancer. J Clin Invest. 123:1911–1918.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clara JA, Monge C, Yang Y and Takebe N:
Targeting signalling pathways and the immune microenvironment of
cancer stem cells - a clinical update. Nat Rev Clin Oncol.
17:204–232. 2020. View Article : Google Scholar
|
|
20
|
Lytle NK, Barber AG and Reya T: Stem cell
fate in cancer growth, progression and therapy resistance. Nat Rev
Cancer. 18:669–680. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Silver DJ, Sinyuk M, Vogelbaum MA,
Ahluwalia MS and Lathia JD: The intersection of cancer, cancer stem
cells, and the immune system: Therapeutic opportunities.
Neuro-oncol. 18:153–159. 2016. View Article : Google Scholar :
|
|
22
|
Lu W and Kang Y: Epithelial-Mesenchymal
Plasticity in Cancer Progression and Metastasis. Dev Cell.
49:361–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lugli A, Iezzi G, Hostettler I, Muraro MG,
Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L and Zlobec
I: Prognostic impact of the expression of putative cancer stem cell
markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer.
Br J Cancer. 103:382–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cardinale V, Renzi A, Carpino G, Torrice
A, Bragazzi MC, Giuliante F, DeRose AM, Fraveto A, Onori P,
Napoletano C, et al: Profiles of cancer stem cell subpopulations in
cholangiocarcinomas. Am J Pathol. 185:1724–1739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh SK, Clarke ID, Hide T and Dirks PB:
Cancer stem cells in nervous system tumors. Oncogene. 23:7267–7273.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glumac PM and LeBeau AM: The role of CD133
in cancer: A concise review. Clin Transl Med. 7:182018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhuria V, Xing J, Scholta T, Bui KC,
Nguyen MLT, Malek NP, Bozko P and Plentz RR: Hypoxia induced Sonic
Hedgehog signaling regulates cancer stemness,
epithelial-to-mesenchymal transition and invasion in
cholangiocarcinoma. Exp Cell Res. 385:1116712019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Chen JN, Zeng TT, He F, Chen SP, Ma
S, Bi J, Zhu XF and Guan XY: CD133+ liver cancer stem cells resist
interferon-gamma-induced autophagy. BMC Cancer. 16:152016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimada M, Sugimoto K, Iwahashi S,
Utsunomiya T, Morine Y, Imura S and Ikemoto T: CD133 expression is
a potential prognostic indicator in intrahepatic
cholangiocarcinoma. J Gastroenterol. 45:896–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mima K, Okabe H, Ishimoto T, Hayashi H,
Nakagawa S, Kuroki H, Watanabe M, Beppu T, Tamada M, Nagano O, et
al: CD44s regulates the TGF-β-mediated mesenchymal phenotype and is
associated with poor prognosis in patients with hepatocellular
carcinoma. Cancer Res. 72:3414–3423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc(-) and thereby promotes tumor growth.
Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suwannakul N, Ma N, Thanan R, Pinlaor S,
Ungarreevittaya P, Midorikawa K, Hiraku Y, Oikawa S, Kawanishi S
and Murata M: Overexpression of CD44 Variant 9: A Novel Cancer Stem
Cell Marker in Human Cholangiocarcinoma in Relation to
Inflammation. Mediators Inflamm. 2018:48672342018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morrin M and Delaney PV: CD44v6 is not
relevant in colorectal tumour progression. Int J Colorectal Dis.
17:30–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coppola D, Hyacinthe M, Fu L, Cantor AB,
Karl R, Marcet J, Cooper DL, Nicosia SV and Cooper HS: CD44V6
expression in human colorectal carcinoma. Hum Pathol. 29:627–635.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vaquero J, Lobe C, Tahraoui S, Clapéron A,
Mergey M, Merabtene F, Wendum D, Coulouarn C, Housset C,
Desbois-Mouthon C, et al: The IGF2/IR/IGF1R Pathway in Tumor Cells
and Myofibroblasts Mediates Resistance to EGFR Inhibition in
Cholangiocarcinoma. Clin Cancer Res. 24:4282–4296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Agrawal S, Kuvshinoff BW, Khoury T, Yu J,
Javle MM, LeVea C, Groth J, Coignet LJ and Gibbs JF: CD24
expression is an independent prognostic marker in
cholangiocarcinoma. J Gastrointest Surg. 11:445–451. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou FQ, Qi YM, Xu H, Wang QY, Gao XS and
Guo HG: Expression of EpCAM and Wnt/β-catenin in human colon
cancer. Genet Mol Res. 14:4485–4494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamashita T, Budhu A, Forgues M and Wang
XW: Activation of hepatic stem cell marker EpCAM by
Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res.
67:10831–10839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sulpice L, Rayar M, Turlin B, Boucher E,
Bellaud P, Desille M, Meunier B, Clément B, Boudjema K and
Coulouarn C: Epithelial cell adhesion molecule is a prognosis
marker for intrahepatic cholangiocarcinoma. J Surg Res.
192:117–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vasanthakumar S, Sasikala P, Padma M,
Balachandar V, Venkatesh B and Ganesan S: EpCAM as a novel
therapeutic target for hepatocellular carcinoma. J Oncological Sci.
3:71–76. 2017. View Article : Google Scholar
|
|
43
|
Breuhahn K, Baeuerle PA, Peters M, Prang
N, Töx U, Köhne-Volland R, Dries V, Schirmacher P and Leo E:
Expression of epithelial cellular adhesion molecule (Ep-CAM) in
chronic (necro-)inflammatory liver diseases and hepatocellular
carcinoma. Hepatol Res. 34:50–56. 2006. View Article : Google Scholar
|
|
44
|
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu
SJ, Shi RY, Hu B, Zhou J and Fan J: Circulating stem cell-like
epithelial cell adhesion molecule-positive tumor cells indicate
poor prognosis of hepatocellular carcinoma after curative
resection. Hepatology. 57:1458–1468. 2013. View Article : Google Scholar
|
|
45
|
Wang M, Xiao J, Shen M, Yahong Y, Tian R,
Zhu F, Jiang J, Du Z, Hu J, Liu W, et al: Isolation and
characterization of tumorigenic extrahepatic cholangiocarcinoma
cells with stem cell-like properties. Int J Cancer. 128:72–81.
2011. View Article : Google Scholar
|
|
46
|
Shuang ZY, Wu WC, Xu J, Lin G, Liu YC, Lao
XM, Zheng L and Li S: Transforming growth factor-β1-induced
epithelial-mesenchymal transition generates ALDH-positive cells
with stem cell properties in cholangiocarcinoma. Cancer Lett.
354:320–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lingala S, Cui YY, Chen X, Ruebner BH,
Qian XF, Zern MA and Wu J: Immunohistochemical staining of cancer
stem cell markers in hepatocellular carcinoma. Exp Mol Pathol.
89:27–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
You L, Guo X and Huang Y: Correlation of
Cancer Stem-Cell Markers OCT4, SOX2, and NANOG with
Clinicopathological Features and Prognosis in Operative Patients
with Rectal Cancer. Yonsei Med J. 59:35–42. 2018. View Article : Google Scholar
|
|
49
|
Zhang MX, Gan W, Jing CY, Zheng SS, Yi Y,
Zhang J, Xu X, Lin JJ, Zhang BH and Qiu SJ: High expression of Oct4
and Nanog predict poor prognosis in intrahepatic cholangiocarcinoma
patients after curative resection. J Cancer. 10:1313–1324. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gu MJ and Jang BI: Clinicopathologic
significance of Sox2, CD44 and CD44v6 expression in intrahepatic
cholangiocarcinoma. Pathol Oncol Res. 20:655–660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Elaimy AL and Mercurio AM: Convergence of
VEGF and YAP/TAZ signaling: Implications for angiogenesis and
cancer biology. Sci Signal. 11:pp. eaau11652018, View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guo L and Teng L: YAP/TAZ for cancer
therapy: Opportunities and challenges (Review). Int J Oncol.
46:1444–1452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sugihara T, Isomoto H, Gores G and Smoot
R: YAP and the Hippo pathway in cholangiocarcinoma. J
Gastroenterol. 54:485–491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim MK, Jang JW and Bae SC: DNA binding
partners of YAP/TAZ. BMB Rep. 51:126–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim HM, Jung WH and Koo JS: Expression of
Yes-associated protein (YAP) in metastatic breast cancer. Int J
Clin Exp Pathol. 8:11248–11257. 2015.PubMed/NCBI
|
|
57
|
Sugiura K, Mishima T, Takano S, Yoshitomi
H, Furukawa K, Takayashiki T, Kuboki S, Takada M, Miyazaki M and
Ohtsuka M: The Expression of Yes-Associated Protein (YAP) Maintains
Putative Cancer Stemness and Is Associated with Poor Prognosis in
Intrahepatic Cholangiocarcinoma. Am J Pathol. 189:1863–1877. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT,
Zender L, Lowe SW, Poon RT and Luk JM: Yes-associated protein is an
independent prognostic marker in hepatocellular carcinoma. Cancer.
115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee K, Lee KB, Jung HY, Yi NJ, Lee KW, Suh
KS and Jang JJ: The correlation between poor prognosis and
increased yes-associated protein 1 expression in keratin 19
expressing hepatocellular carcinomas and cholangiocarcinomas. BMC
Cancer. 17:4412017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu JY, Li YH, Lin HX, Liao YJ, Mai SJ,
Liu ZW, Zhang ZL, Jiang LJ, Zhang JX, Kung HF, et al:
Overexpression of YAP 1 contributes to progressive features and
poor prognosis of human urothelial carcinoma of the bladder. BMC
Cancer. 13:3492013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nishio M, Sugimachi K, Goto H, Wang J,
Morikawa T, Miyachi Y, Takano Y, Hikasa H, Itoh T, Suzuki SO, et
al: Dysregulated YAP1/TAZ and TGF-β signaling mediate
hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc Natl Acad Sci
USA. 113:E71–E80. 2016. View Article : Google Scholar
|
|
62
|
Song H, Mak KK, Topol L, Yun K, Hu J,
Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al: Mammalian
Mst1 and Mst2 kinases play essential roles in organ size control
and tumor suppression. Proc Natl Acad Sci USA. 107:1431–1436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yamada D, Rizvi S, Razumilava N, Bronk SF,
Davila JI, Champion MD, Borad MJ, Bezerra JA, Chen X and Gores GJ:
IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an
interleukin-6-sensitive mechanism. Hepatology. 61:1627–1642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song S, Xie M, Scott AW, Jin J, Ma L, Dong
X, Skinner HD, Johnson RL, Ding S and Ajani JA: A Novel YAP1
Inhibitor Targets CSC-Enriched Radiation-Resistant Cells and Exerts
Strong Antitumor Activity in Esophageal Adenocarcinoma. Mol Cancer
Ther. 17:443–454. 2018. View Article : Google Scholar
|
|
65
|
Song S, Ajani JA, Honjo S, Maru DM, Chen
Q, Scott AW, Heallen TR, Xiao L, Hofstetter WL, Weston B, et al:
Hippo coactivator YAP1 upregulates SOX9 and endows esophageal
cancer cells with stem-like properties. Cancer Res. 74:4170–4182.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ooki A, Del Carmen Rodriguez Pena M,
Marchionni L, Dinalankara W, Begum A, Hahn NM, VandenBussche CJ,
Rasheed ZA, Mao S, Netto GJ, et al: YAP1 and COX2 Coordinately
Regulate Urothelial Cancer Stem-like Cells. Cancer Res. 78:168–181.
2018. View Article : Google Scholar :
|
|
67
|
Bora-Singhal N, Nguyen J, Schaal C,
Perumal D, Singh S, Coppola D and Chellappan S: YAP1 Regulates OCT4
Activity and SOX2 Expression to Facilitate Self-Renewal and
Vascular Mimicry of Stem-Like Cells. Stem Cells. 33:1705–1718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhu P, Wang Y, Wu J, Huang G, Liu B, Ye B,
Du Y, Gao G, Tian Y, He L, et al: LncBRM initiates YAP1 signalling
activation to drive self-renewal of liver cancer stem cells. Nat
Commun. 7:136082016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu Li F, Liu Y, Singh B, Zhao PK, Jin W,
Han J, Scott G, Dong AW, Huo XL, et al: YAP1-Mediated CDK6
Activation Confers Radiation Resistance in Esophageal Cancer -
Rationale for the Combination of YAP1 and CDK4/6 Inhibitors in
Esophageal Cancer. Clin Cancer Res. 25:2264–2277. 2019. View Article : Google Scholar
|
|
70
|
Syed IS, Pedram A and Farhat WA: Role of
Sonic Hedgehog (Shh) Signaling in Bladder Cancer Stemness and
Tumorigenesis. Curr Urol Rep. 17:112016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
U.S. National Library of Medicine: A Study
Evaluating IPI-926 in Combination With Gemcitabine in Patients With
Metastatic Pancreatic Cancer. http://ClinicalTrials.govurisimpleClinicalTrials.gov
Identifier: NCT01130142. https://clinicaltrials.gov/ct2/show/NCT01130142.
Accessed May 25, 2010.
|
|
72
|
Ko AH, LoConte N, Tempero MA, Walker EJ,
Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci
DV, et al: A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog
Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma.
Pancreas. 45:370–375. 2016. View Article : Google Scholar
|
|
73
|
Xie H, Paradise BD, Ma WW and
Fernandez-Zapico ME: Recent Advances in the Clinical Targeting of
Hedgehog/GLI Signaling in Cancer. Cells. 8:E3942019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ranganathan P, Weaver KL and Capobianco
AJ: Notch signalling in solid tumours: A little bit of everything
but not all the time. Nat Rev Cancer. 11:338–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang Z, Li Y, Banerjee S and Sarkar FH:
Emerging role of Notch in stem cells and cancer. Cancer Lett.
279:8–12. 2009. View Article : Google Scholar :
|
|
76
|
Cigliano A, Wang J, Chen X and Calvisi DF:
Role of the Notch signaling in cholangiocarcinoma. Expert Opin Ther
Targets. 21:471–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Fan B, Malato Y, Calvisi DF, Naqvi S,
Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X,
et al: Cholangiocarcinomas can originate from hepatocytes in mice.
J Clin Invest. 122:2911–2915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo
J, Huang H, Du Q, Geller DA and Cheng B: Notch and Wnt/β-catenin
signaling pathway play important roles in activating liver cancer
stem cells. Oncotarget. 7:5754–5768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Schatoff EM, Leach BI and Dow LE: Wnt
Signaling and Colorectal Cancer. Curr Colorectal Cancer Rep.
13:101–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Boulter L, Guest RV, Kendall TJ, Wilson
DH, Wojtacha D, Robson AJ, Ridgway RA, Samuel K, Van Rooijen N,
Barry ST, et al: WNT signaling drives cholangiocarcinoma growth and
can be pharmacologically inhibited. J Clin Invest. 125:1269–1285.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Park HW, Kim YC, Yu B, Moroishi T, Mo JS,
Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al: Alternative
Wnt Signaling Activates YAP/TAZ. Cell. 162:780–794. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Santoro R, Zanotto M, Simionato F,
Zecchetto C, Merz V, Cavallini C, Piro G, Sabbadini F, Boschi F,
Scarpa A and Melisi D: Modulating TAK1 expression inhibits YAP and
TAZ oncogenic functions in pancreatic cancer. Mol Cancer Ther.
19:247–257. 2020. View Article : Google Scholar
|
|
83
|
Gray JE, Infante JR, Brail LH, Simon GR,
Cooksey JF, Jones SF, Farrington DL, Yeo A, Jackson KA, Chow KH, et
al: A first-in-human phase I dose-escalation, pharmacokinetic, and
pharmacodynamic evaluation of intravenous LY2090314, a glycogen
synthase kinase 3 inhibitor, administered in combination with
pemetrexed and carboplatin. Invest New Drugs. 33:1187–1196. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
U.S. National Library of Medicine: A Study
of BBI503 in Adult Patients With Advanced Hepatobiliary Cancer.
http://ClinicalTrials.govurisimpleClinicalTrials.gov
Identifier: NCT02232633. https://ClinicalTrials.gov/show/NCT02232633.
Accessed September 5, 2014.
|
|
85
|
Jonker DJ, Laurie SA, Cote GM, Flaherty K,
Fuchs CS, Chugh R, Smith DC, Edenfield WJ, Conkling PR, Mier JW, et
al: Phase 1 extension study of BBI503, a first-in-class cancer
stemness kinase inhibitor, in patients with advanced colorectal
cancer. J Clin Oncol. 33(Suppl 15): pp. 36152015, View Article : Google Scholar
|
|
86
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, Zhang YJ, Lu R, Chen YX and Fang JY: Inhibition of JAK1,
2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces
tumor cell invasion in colorectal cancer cells. Neoplasia.
10:287–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
U.S. National Library of Medicine: A Study
of Napabucasin (BBI-608) in Combination With FOLFIRI in Adult
Patients With Previously Treated Metastatic Colorectal Cancer.
ClinicalTrials. gov Identifier: NCT02753127. https://clinicaltrials.gov/ct2/show/NCT02753127.
Accessed April 27, 2016.
|
|
88
|
Bendell JC, Hubbard JM, O'Neil BH, Jonker
DJ, Starodub A, Peyton JD, Pitot HC, Halfdanarson TR, Nadeau BR,
Zubkus JD, et al: Phase 1b/II study of cancer stemness inhibitor
napabucasin (BBI-608) in combination with FOLFIRI +/- bevacizumab
(bev) in metastatic colorectal cancer (mCRC) patients (pts). J Clin
Oncol. 35(Suppl 15): pp. 35292017, View Article : Google Scholar
|
|
89
|
Beyreis M, Gaisberger M, Jakab M,
Neureiter D, Helm K, Ritter M, Kiesslich T and Mayr C: The Cancer
Stem Cell Inhibitor Napabucasin (BBI608) Shows General Cytotoxicity
in Biliary Tract Cancer Cells and Reduces Cancer Stem Cell
Characteristics. Cancers (Basel). 11. pp. E2762019, View Article : Google Scholar
|
|
90
|
Piersma B, Bank RA and Boersema M:
Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front Med
(Lausanne). 2:pp. 592015
|
|
91
|
Kim W, Khan SK and Yang Y: Interacting
network of Hippo, Wnt/β-catenin and Notch signaling represses liver
tumor formation. BMB Rep. 50:1–2. 2017. View Article : Google Scholar :
|
|
92
|
Rabadán MA, Cayuso J, Le Dréau G, Cruz C,
Barzi M, Pons S, Briscoe J and Martí E: Jagged2 controls the
generation of motor neuron and oligodendrocyte progenitors in the
ventral spinal cord. Cell Death Differ. 19:209–219. 2012.
View Article : Google Scholar :
|
|
93
|
He J, Sheng T, Stelter AA, Li C, Zhang X,
Sinha M, Luxon BA and Xie J: Suppressing Wnt signaling by the
hedgehog pathway through sFRP-1. J Biol Chem. 281:35598–35602.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Beier D, Hau P, Proescholdt M, Lohmeier A,
Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U and Beier
CP: CD133(+) and CD133(-) glioblastoma-derived cancer stem cells
show differential growth characteristics and molecular profiles.
Cancer Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Croker AK, Goodale D, Chu J, Postenka C,
Hedley BD, Hess DA and Allan AL: High aldehyde dehydrogenase and
expression of cancer stem cell markers selects for breast cancer
cells with enhanced malignant and metastatic ability. J Cell Mol
Med. 13:2236–2252. 2009. View Article : Google Scholar
|
|
96
|
Shmelkov SV, Butler JM, Hooper AT, Hormigo
A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et
al: CD133 expression is not restricted to stem cells, and both
CD133+ and CD133- metastatic colon cancer cells initiate tumors. J
Clin Invest. 118:2111–2120. 2008.PubMed/NCBI
|
|
97
|
Schmelzer E, Zhang L, Bruce A, Wauthier E,
Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, et al:
Human hepatic stem cells from fetal and postnatal donors. J Exp
Med. 204:1973–1987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sagrinati C, Netti GS, Mazzinghi B,
Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M,
Squecco R, et al: Isolation and characterization of multipotent
progenitor cells from the Bowman's capsule of adult human kidneys.
J Am Soc Nephrol. 17:2443–2456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ma S, Chan KW, Lee TK, Tang KH, Wo JY,
Zheng BJ and Guan XY: Aldehyde dehydrogenase discriminates the
CD133 liver cancer stem cell populations. Mol Cancer Res.
6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar
|
|
101
|
Jaksch M, Múnera J, Bajpai R, Terskikh A
and Oshima RG: Cell cycle-dependent variation of a CD133 epitope in
human embryonic stem cell, colon cancer, and melanoma cell lines.
Cancer Res. 68:7882–7886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kryczek I, Liu S, Roh M, Vatan L, Szeliga
W, Wei S, Banerjee M, Mao Y, Kotarski J, Wicha MS, et al:
Expression of aldehyde dehydrogenase and CD133 defines ovarian
cancer stem cells. Int J Cancer. 130:29–39. 2012. View Article : Google Scholar
|
|
103
|
Silva IA, Bai S, McLean K, Yang K,
Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds
RK, et al: Aldehyde dehydrogenase in combination with CD133 defines
angiogenic ovarian cancer stem cells that portend poor patient
survival. Cancer Res. 71:3991–4001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lardon J, Corbeil D, Huttner WB, Ling Z
and Bouwens L: Stem cell marker prominin-1/AC133 is expressed in
duct cells of the adult human pancreas. Pancreas. 36:pp. e1–e6.
2008, View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar
|
|
108
|
Zhou J, Wang H, Cannon V, Wolcott KM, Song
H and Yates C: Side population rather than CD133(+) cells
distinguishes enriched tumorigenicity in hTERT-immortalized primary
prostate cancer cells. Mol Cancer. 10:1122011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Avril T, Etcheverry A, Pineau R, Obacz J,
Jegou G, Jouan F, Le Reste PJ, Hatami M, Colen RR, Carlson BL, et
al: CD90 expression controls migration and predicts dasatinib
response in glioblastoma. Clin Cancer Res. 23:7360–7374. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yamashita T, Honda M, Nakamoto Y, Baba M,
Nio K, Hara Y, Zeng SS, Hayashi T, Kondo M, Takatori H, et al:
Discrete nature of EpCAM+ and CD90+ cancer stem cells in human
hepato-cellular carcinoma. Hepatology. 57:1484–1497. 2013.
View Article : Google Scholar
|
|
111
|
Wang P, Gao Q, Suo Z, Munthe E, Solberg S,
Ma L, Wang M, Westerdaal NA, Kvalheim G and Gaudernack G:
Identification and characterization of cells with cancer stem cell
properties in human primary lung cancer cell lines. PLoS One. 8:pp.
e570202013, View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chen WC, Hsu HP, Li CY, Yang YJ, Hung YH,
Cho CY, Wang CY, Weng TY and Lai MD: Cancer stem cell marker CD90
inhibits ovarian cancer formation via β3 integrin. Int J Oncol.
49:1881–1889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jiang J, Zhang Y, Chuai S, Wang Z, Zheng
D, Xu F, Zhang Y, Li C, Liang Y and Chen Z: Trastuzumab (herceptin)
targets gastric cancer stem cells characterized by CD90 phenotype.
Oncogene. 31:671–682. 2012. View Article : Google Scholar
|
|
114
|
Flahaut M, Jauquier N, Chevalier N, Nardou
K, Balmas Bourloud K, Joseph JM, Barras D, Widmann C, Gross N,
Renella R, et al: Aldehyde dehydrogenase activity plays a Key role
in the aggressive phenotype of neuroblastoma. BMC Cancer.
16:7812016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ricardo S, Vieira AF, Gerhard R, Leitão D,
Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes
J: Breast cancer stem cell markers CD44, CD24 and ALDH1: expression
distribution within intrinsic molecular subtype. J Clin Pathol.
11:937–946. 2011. View Article : Google Scholar
|
|
116
|
Feng H and Liu Y, Bian X, Zhou F and Liu
Y: ALDH1A3 affects colon cancer in vitro proliferation and invasion
depending on CXCR4 status. Br J Cancer. 118:224–232. 2018.
View Article : Google Scholar :
|
|
117
|
Khorrami S, Zavaran Hosseini A, Mowla SJ
and Malekzadeh R: Verification of ALDH Activity as a Biomarker in
Colon Cancer Stem Cells-Derived HT-29 Cell Line. Iran J Cancer
Prev. 8:pp. e34462015, View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Moreb JS, Baker HV, Chang LJ, Amaya M,
Lopez MC, Ostmark B and Chou W: ALDH isozymes downregulation
affects cell growth, cell motility and gene expression in lung
cancer cells. Mol Cancer. 7:872008. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yan J, De Melo J, Cutz JC, Aziz T and Tang
D: Aldehyde dehydrogenase 3A1 associates with prostate
tumorigenesis. Br J Cancer. 110:2593–2603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Li W, Ma H, Zhang J, Zhu L, Wang C and
Yang Y: Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem
cell markers in tumorigenesis and metastasis. Sci Rep. 7:138562017.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Luo Y, Dallaglio K, Chen Y, Robinson WA,
Robinson SE, McCarter MD, Wang J, Gonzalez R, Thompson DC, Norris
DA, et al: ALDH1A isozymes are markers of human melanoma stem cells
and potential therapeutic targets. Stem Cells. 30:2100–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mueller MT, Hermann PC, Witthauer J,
Rubio-Viqueira B, Leicht SF, Huber S, Ellwart JW, Mustafa M,
Bartenstein P, D'Haese JG, et al: Combined targeted treatment to
eliminate tumorigenic cancer stem cells in human pancreatic cancer.
Gastroenterology. 137:1102–1113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Pietras A, Katz AM, Ekström EJ, Wee B,
Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT and
Holland EC: Osteopontin-CD44 signaling in the glioma perivascular
niche enhances cancer stem cell phenotypes and promotes aggressive
tumor growth. Cell Stem Cell. 14:357–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Fu J, Yang QY, Sai K, Chen FR, Pang JC, Ng
HK, Kwan AL and Chen ZP: TGM2 inhibition attenuates ID1 expression
in CD44-high glioma-initiating cells. Neuro-oncol. 15:1353–1365.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Todaro M, Gaggianesi M, Catalano V,
Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S,
Cocorullo G, et al: CD44v6 is a marker of constitutive and
reprogrammed cancer stem cells driving colon cancer metastasis.
Cell Stem Cell. 14:342–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Paradis V, Ferlicot S, Ghannam E, Zeimoura
L, Blanchet P, Eschwége P, Jardin A, Benoît G and Bedossa P: CD44
is an independent prognostic factor in conventional renal cell
carcinomas. J Urol. 161:1984–1987. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J and
Li J: Cancer stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer.
126:2067–2078. 2010.
|
|
128
|
He QZ, Luo XZ, Wang K, Zhou Q, Ao H, Yang
Y, Li SX, Li Y, Zhu HT and Duan T: Isolation and characterization
of cancer stem cells from high-grade serous ovarian carcinomas.
Cell Physiol Biochem. 33:173–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra
D, Zhou J, Claypool K, et al: Highly purified CD44+ prostate cancer
cells from xenograft human tumors are enriched in tumorigenic and
metastatic progenitor cells. Oncogene. 25:1696–1708. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y,
Uraoka N, Anami K, Sentani K, Oue N and Yasui W: Expression of
cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and
lymph node metastasis of gastric cancer. Pathol Int. 62:pp.
112–119. 2012, View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kimura Y, Goi T, Nakazawa T, Hirono Y,
Katayama K, Urano T and Yamaguchi A: CD44variant exon 9 plays an
important role in colon cancer initiating cells. Oncotarget.
4:785–791. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yae T, Tsuchihashi K, Ishimoto T, Motohara
T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H,
et al: Alternative splicing of CD44 mRNA by ESRP1 enhances lung
colonization of metastatic cancer cell. Nat Commun. 3:8832012.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wang T, Gantier MP, Xiang D, Bean AG,
Bruce M, Zhou SF, Khasraw M, Ward A, Wang L, Wei MQ, et al: EpCAM
aptamer-mediated survivin silencing sensitized cancer stem cells to
doxorubicin in a breast cancer model. Theranostics. 5:14562015.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Münz M, Kieu C, Mack B, Schmitt B, Zeidler
R and Gires O: The carcinoma-associated antigen EpCAM upregulates
c-myc and induces cell proliferation. Oncogene. 23:57482004.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yeung TM, Gandhi SC, Wilding JL, Muschel R
and Bodmer WF: Cancer stem cells from colorectal cancer-derived
cell lines. Proc Natl Acad Sci USA. 107:3722–3727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lee TK, Castilho A, Cheung VC, Tang KH, Ma
S and Ng IO: CD24(+) liver tumor-initiating cells drive
self-renewal and tumor initiation through STAT3-mediated NANOG
regulation. Cell Stem Cell. 9:50–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Gangemi RM, Griffero F, Marubbi D, Perera
M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A and Corte G:
SOX2 silencing in glioblastoma tumor-initiating cells causes stop
of proliferation and loss of tumorigenicity. Stem Cells. 27:40–48.
2009. View Article : Google Scholar
|
|
140
|
Chen Y, Shi L, Zhang L, Li R, Liang J, Yu
W, Sun L, Yang X, Wang Y, Zhang Y, et al: The molecular mechanism
governing the oncogenic potential of SOX2 in breast cancer. J Biol
Chem. 283:17969–17978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Chou YT, Lee CC, Hsiao SH, Lin SE, Lin SC,
Chung CH, Chung CH, Kao YR, Wang YH, Chen CT, et al: The emerging
role of SOX2 in cell proliferation and survival and its crosstalk
with oncogenic signaling in lung cancer. Stem Cells. 31:2607–2619.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Higgins DM, Wang R, Milligan B, Schroeder
M, Carlson B, Pokorny J, Cheshier SH, Meyer FB, Weissman IL,
Sarkaria JN, et al: Brain tumor stem cell multipotency correlates
with nanog expression and extent of passaging in human glioblastoma
xenografts. Oncotarget. 4:792–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Jeter CR, Badeaux M, Choy G, Chandra D,
Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ and Tang
DG: Functional evidence that the self-renewal gene NANOG regulates
human tumor development. Stem Cells. 27:993–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hoei-Hansen CE, Almstrup K, Nielsen JE,
Brask Sonne S, Graem N, Skakkebaek NE, Leffers H and Rajpert-De
Meyts E: Stem cell pluripotency factor NANOG is expressed in human
fetal gonocytes, testicular carcinoma in situ and germ cell
tumours. Histopathology. 47:48–56. 2005. View Article : Google Scholar : PubMed/NCBI
|