Introduction
Clinically non-functioning pituitary adenoma (NFPA)
is one of the most common subtypes of pituitary adenoma. Being
unassociated with specific serum hormone changes, NFPA is usually
detected based on symptoms, such as headaches and visual
disturbance or incidental imaging examinations (1,2).
Surgical resection is the primary treatment choice for NFPA;
however, patients are often faced with tumor residue as the tumor
may have surrounded the internal carotid artery or invaded the
cavernous sinus (3,4). Approximately 12-58% of patients may
experience relapse within 5 years (5). Radiotherapy is often recommended to
patients with tumor residue; however, its long-term complications,
such as visual defects and hypopituitarism, pose concerns (6,7).
Thus, surgery remains the optimal treatment option for patients
with tumor regrowth. However, in contrast to clinically functioning
pituitary adenoma (FPA), for which serum hormone monitoring is used
for detection, NFPA lacks an effective evaluation method, resulting
in the failure of early intervention. Thus, research concerning the
molecular mechanisms of tumor regrowth and the development of
methods for predicting prognosis is of utmost significance.
Protein-coding genes (PCGs) are the most commonly
used class of molecular marker. Numerous studies have reported that
the altered expression of PCGs results in aggressive tumor behavior
and may affect the prognosis of patients with NFPA (1,8,9).
Long non-coding RNAs (lncRNAs) are a subgroup of non-protein-coding
RNAs >200 nucleotides in length. lncRNAs play pivotal roles in
the regulation of gene expression and have been used as prognostic
markers in multiple tumors types (8,10).
The expression of lncRNAs has been found to be related to the
growth and invasive behavior of pituitary adenoma (11,12).
However, there has been little research on the role of lncRNAs or
its combination with PCGs as potentially novel approaches for
prognostic evaluation in pituitary adenoma.
The present study aimed to identify PCGs and lncRNAs
that are asscoiated with the regrowth of NFPA, and to establish a
model which may be used to predict tumor regrowth based on the PCG
and lncRNA expression profiles of 66 NFPA samples through
microarray analyses. The findings of the present study may provide
an effective method for the prediction of the prognosis and
post-operative intervention of patients with NFPA.
Materials and methods
Patients and samples
NFPA specimens from 66 patients who had undergone
surgical treatment at Beijing Tiantan Hospital from October, 2007
to July, 2014 were selected, which included 34 females and 32 males
with a median age of 55 years (range, 25-66 years). The median
follow-up time was 78 months (range, 36-121 months). The definition
of tumor regrowth was a maximum increase in tumor diameter of >2
mm on an enhanced MRI from the time of surgery to the follow-up
endpoint with or without headaches, visual disturbances or other
mass effect symptoms. The clinical characteristics of the patients
are presented in Table I and
detailed clinical information is presented in Table SI. The present study was approved
by the Medical Ethics Committee of Beijing Tiantan Hospital and
written informed consent for the use of the resected samples for
research purposes was obtained from all patients.
 | Table IClinical characteristics of the 66
patients with NFPA. |
Table I
Clinical characteristics of the 66
patients with NFPA.
| Feature | Training set | Testing set | Entire set |
|---|
| Sex | | | |
| Female | 15 | 19 | 34 |
| Male | 18 | 14 | 32 |
| Age (years) | | | |
| ≤52 | 18 | 15 | 33 |
| >52 | 15 | 18 | 33 |
| Knosp
classification | | | |
| I | 5 | 9 | 14 |
| II | 5 | 4 | 9 |
| III | 5 | 5 | 10 |
| IV | 18 | 15 | 33 |
| Regrowth | | | |
| Yes | 23 | 23 | 46 |
| No | 10 | 10 | 20 |
| Invasion | | | |
| Yes | 21 | 18 | 36 |
| No | 12 | 15 | 30 |
Total RNA extraction and RNA
microarray
Total cellular RNA was extracted from the tumor
samples using the mirVana™ miRNA Isolation kit without phenol (cat.
no. AM1561, Ambion; Thermo Fisher Scientific, Inc.), which were
used to produce labeled fluorescent cRNA targets for the SBC human
ceRNA array v1.0 (4x180 K). The prepared cRNA targets were
hybridized with the slides and scanned on an Agilent Microarray
Scanner (Agilent Technologies, Inc.). Following data collection
with Feature Extraction software v10.7 (Agilent Technologies,
Inc.), the data were normalized by quantile normalization using a
package named limma from R program (bioinf.wehi.edu.au/limma).
Selection of PCGs and lncRNAs subsets
related to tumor regrowth
All patients We were randomly divided into the
training (n=33) and testing sets (n=33) using an algorithm called
sample from R program (www.r-project.org/). In the training set, the
evaluation of the association between the expression of candidate
PCGs and lncRNAs, and the PFS of each patient was performed using
univariate Cox regression analysis.
Selection of candidate PCGs and lncRNAs
as a predictive signature
A model for selecting PCGs and lncRNAs as a
predictive signature of prognosis was developed using the following
formula (13,14):
Risk score(RS)=∑Ni=1(Explg×Coef),
where 'N' in the formula represents the number of prognostic PCGs
and lncRNAs, 'Explg' represents the expression of PCGs and lncRNAs,
and 'Coef' represents the regression coefficient of PCGs and
lncRNAs.
Random survival forests-variable hunting (RSFVH)
analysis was used to screen 10 PCGs and lncRNAs, as previously
described (13). Considering that
a prediction model with a smaller number of PCGs and lncRNAs would
be more practical than one with a larger number, all of the
screened PCGs and lncRNAs were included. A time-dependent ROC curve
was used to evaluate the specificity and sensitivity of the
regrowth prediction of the RS of the 210-1=1023 signatures in the
training set. The patients with NFPA in each set were separated
into the high- and low-risk groups with the median RS in the
training set used as the cut-off value.
Statistical analysis
Kaplan-Meier survival analyses were performed to
assess the regrowth distributions in the training and testing sets,
and the statistical significance was assessed using the two-sided
log-rank test. Chi-square tests were performed to analyze the
associations among the clinical features. Multivariable Cox
regression analysis was performed to evaluate the independence of
the risk score from clinical features. P<0.05 was considered to
indicate a statistically significant in the present study. All the
analyses in the present study were performed in the R program
(www.r-project.org) with the timeROC (cran.r-project.org/web/packages/timeROC/index.html),
survivalROC (cran.r-project.org/web/packages/survivalROC/index.html)
and randomForestSRC packages (cran.r-project.org/package=randomForestSRC).
Functional analysis of PCGs and
lncRNAs
The co-expression association between the candidate
PCGs and lncRNAs was evaluated with Pearson's correlation
coefficients. The expression data used for Pearson's correlation
were derived from the microarray analysis. Kyoto Encyclopedia of
Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses
of the PCGs and lncRNAs were performed to confirm the biological
functions of lncRNAs. The co-expression correlations between the
candidate PCGs and all other PCGs were assessed using Pearson's
correlation analysis, and genes with P-values <0.05 and absolute
values from the Pearson's correlation coefficient >0.6 were
selected for KEGG and GO enrichment analyses. The above-mentioned
analyses were performed with the clusterProfiler package (15,16).
Results
Construction of a PCG-lncRNA signature
for the prediction of NFPA regrowth
Expression profiles of 66 pituitary tumor tissues
were obtained using a ceRNA microarray. The expression levels of
18,829 PCGs and 19,741 lncRNAs were then determined. The 66 NFPAs
were randomly divided into a training set (n=33) and a testing set
(n=33). The training set was used for screening the candidate PCGs
and lncRNAs associated with tumor regrowth, and the testing set was
used for validating the classification power. In the selection
step, all analyses were based on the training set.
With regrowth as the dependent variable, univariate
Cox regression analysis was performed. In total, 1,245 PCGs and
1,214 lncRNAs (Table SII) were
identified that were signifi-cantly associated with tumor regrowth
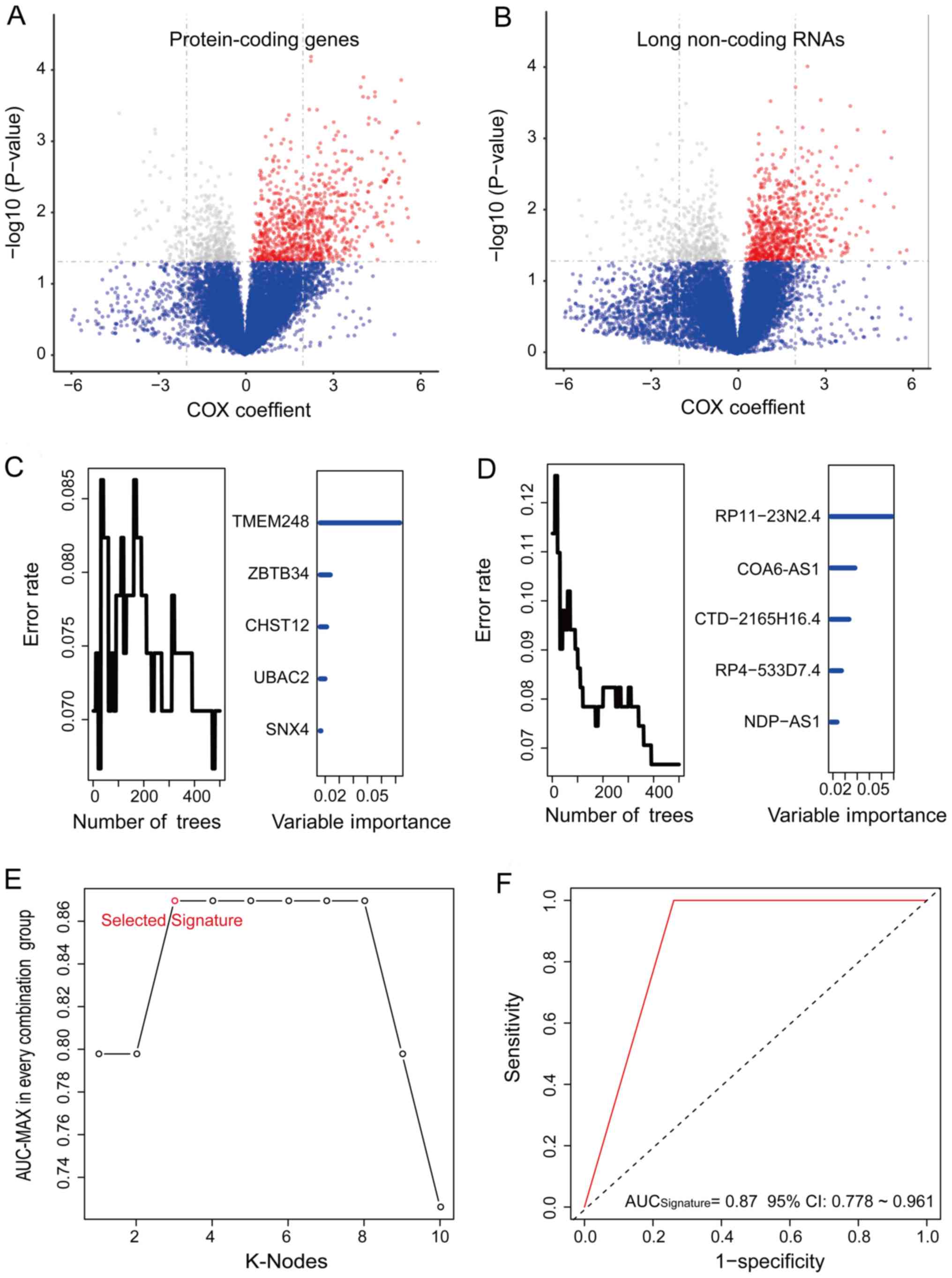
(P<0.05; Fig. 1A and B).
Subsequently, 10 candidates, including 5 PCGs and 5 lncRNAs related
to tumor regrowth were identified among the 2,459 members of the
PCG and lncRNA set according to the permutation importance values
of the random forest supervised classification (RSFC) analysis
(Fig. 1C and D).
To obtain an optimized prediction signature,
time-dependent ROC analysis was performed and the specificity and
sensitivity of the regrowth prediction of the RS was evaluated for
each patient according to the RS model of 210-1=1023 in the
training set (Table SIII). The
PCG and lncRNA combination with the max AUC was composed of CHST12,
COA6-AS1 and RP11-23N2.4 (Fig. 1C and
D; Table II). The RS of the
signature was obtained as follows: RS = (4.95 x CHST12) + (3.41 x
COA 6-AS1) + (1.90 x RP11-23N2.4). The AUC for the PCG and lncRNA
signature was 0.869 in the training set, which indicated a strong
performance for regrowth prediction (Fig. 1E and F).
 | Table IIIdentification of PCGs and lncRNAs in
the predicting signature and the univariable Cox association with
regrowth. |
Table II
Identification of PCGs and lncRNAs in
the predicting signature and the univariable Cox association with
regrowth.
| Gene symbol | Coefficienta | P-valuea | Expression level
association with poor prognosis | Chromosome location
(GRCh38/hg38) |
|---|
| CHST12 | 4.95 | 0.003 | High | chr7:
2403588-2448484: + |
| COA6-AS1 | 3.41 | 0.001 | High |
chr1:234372807-234373593: − |
| RP11-23N2.4 | 1.90 | 0.007 | High |
chr15:52584907-52587652: + |
Predictive ability of the molecular
signature for tumor regrowth
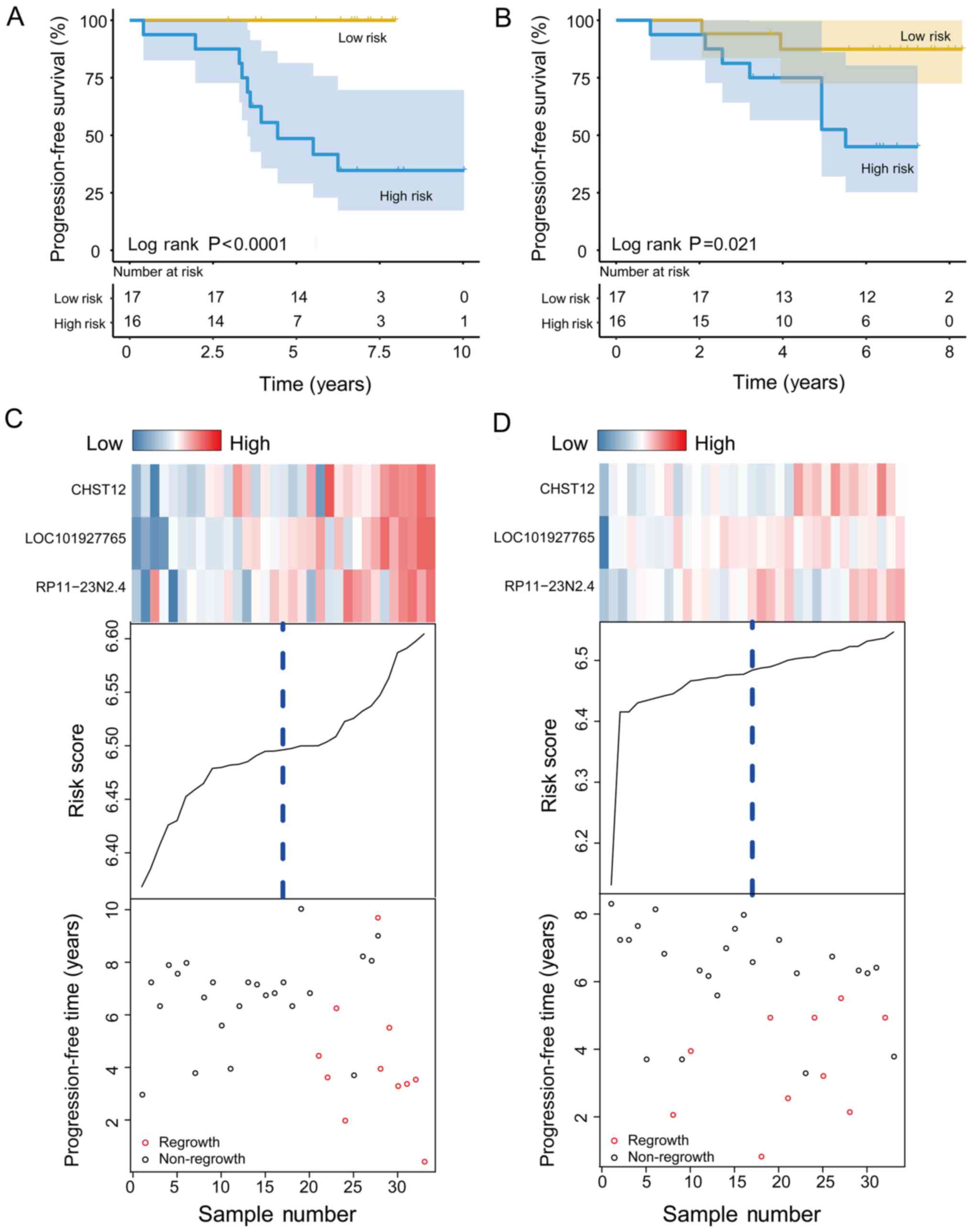
In the training set, patients were separated into
the high-risk (n=16) and low-risk (n=17) group using the median RS
of the signature as the cut-off. The results revealed that patients
in the high-risk group had a shorter PFS than patients in the
low-risk group (median PFS, 4.44 years vs. <7.97 years;
P<0.001; Fig. 2A). The regrowth
rate in the high-risk group was >50%, whereas that of the
low-risk group was <1%. In the testing set, patients were
similarly separated into the high-risk (n=16) and low-risk (n=17)
groups. To confirm the predictive power of the CHST12, COA6-AS1 and
RP11-23N2.4 signature, the results of Kaplan-Meier analysis for the
patients in the testing set were plotted, as shown in Fig. 2B (median PFS, 5.51 years vs.
<8.30 years; P=0.021). The regrowth rate of the patients in the
low-risk group was approximately 12.6%, whereas that of patients in
the high-risk group was 55.0%.
The regrowth status of patients with NFPA from the
training and testing sets is illustrated in the dot plots (Fig. 2C and D). Moreover, the expression
patterns of the 3 PCG and lncRNAs in the 2 groups are presented.
Consistent with the pattern observed in the training set, patients
with low-risk scores tended to have lower expression levels of the
1 PCG and 2 lncRNAs than the patients in the high-risk group.
Associations between patient clinical
characteristics and the molecular signature
To better understand the associations between
clinical characteristics and the CHST12, COA6-AS1 and RP11-23N2.4
signature, the associations of the signature with the clinical
characteristics of the whole patient set (n=66) were investigated.
Unlike age, sex, and invasion status based on Knosp classification
(17,18) exhibited no association with the
PCG-lncRNA signature (Table
III).
 | Table IIIAssociation of the signature with the
clinical characteristics of the patients with NFPA. |
Table III
Association of the signature with the
clinical characteristics of the patients with NFPA.
| Variable | Training set
| P-valuea | Testing set
| P-valuea | Entire set
| P-valuea |
|---|
| Low risk | High risk | Low risk | High risk | Low risk | High risk |
|---|
| Sex | | | > 0.99 | | | 0.62 | | | 0.63 |
| Female | 8 | 7 | | 11 | 8 | | 19 | 15 | |
| Male | 9 | 9 | | 6 | 8 | | 15 | 17 | |
| Age (years) | | | 0.59 | | | 0.02 | | | 0.03 |
| ≤52 | 8 | 10 | | 4 | 11 | | 12 | 21 | |
| >52 | 9 | 6 | | 13 | 5 | | 22 | 11 | |
| Invasion | | | > 0.99 | | | 0.39 | | | 0.48 |
| Yes | 11 | 10 | | 11 | 7 | | 22 | 17 | |
| No | 6 | 6 | | 6 | 9 | | 12 | 15 | |
To explore the independency of our signature from
the clinical characteristics, multivariable Cox regression analysis
was performed of the entire patient set using the PCG-lncRNA
signature-based RS. It was found that the CHST12, COA6-AS1 and
RP11-23N2.4 signature was indeed independent of these clinical
characteristics (high-risk vs. low-risk, HR=1.47; 95% CI,
1.22-1.79; P<0.001, n=66; Table
IV).
 | Table IVUnivariate and multivariate Cox
regression analysis of the signature of the patients with NFPA in
the training, testing set and entire set. |
Table IV
Univariate and multivariate Cox
regression analysis of the signature of the patients with NFPA in
the training, testing set and entire set.
A, Univariable
analysis
|
|---|
| Variable | HR | Training set (n=33)
95% CI
| P-value | HR | Testing set (n=33)
95% CI
| P-value | HR | Entire set (n=66)
95% CI
| P-value |
|---|
| Lower | Upper | Lower | Upper | Lower | Upper |
|---|
| Sex | | | | | | | | | | | | |
| Male vs.
female | 1.05 | 0.29 | 3.74 | 0.94 | 0.53 | 0.14 | 2.03 | 0.35 | 0.75 | 0.31 | 1.81 | 0.52 |
| Age (years) | | | | | | | | | | | | |
| >52 vs.
≤52 | 0.23 | 0.05 | 1.09 | 0.06 | 0.35 | 0.09 | 1.36 | 0.13 | 0.29 | 0.10 | 0.79 | 0.02 |
| Knosp
classification | | | | | | | | | | | | |
| III IV vs. I
II | 1.36 | 0.72 | 2.54 | 0.34 | 1.11 | 0.68 | 1.83 | 0.67 | 1.21 | 0.82 | 1.78 | 0.33 |
| Signature | | | | | | | | | | | | |
| High-risk vs.
low-risk | 1.88 | 1.37 | 2.57 | <0.001 | 1.24 | 0.92 | 1.66 | 0.04 | 1.47 | 1.23 | 1.76 | <0.001 |
|
| B, Multivariable
analysis |
|
| Variable | HR | Training set (n=33)
95% CI
| P-value | HR | Testing set (n=33)
95% CI
| P-value | HR | Entire set (n=66)
95% CI
| P-value |
| Lower | Upper | Lower | Upper | Lower | Upper |
|
| Sex | | | | | | | | | | | | |
| Male vs.
female | 0.54 | 0.09 | 3.40 | 0.52 | 0.49 | 0.12 | 2.03 | 0.33 | 0.50 | 0.17 | 1.45 | 0.20 |
| Age (years) | | | | | | | | | | | | |
| >52 vs.
≤52 | 0.29 | 0.05 | 1.82 | 0.19 | 0.54 | 0.13 | 2.24 | 0.39 | 0.47 | 0.18 | 1.23 | 0.12 |
| Knosp
classification | | | | | | | | | | | | |
| III IV vs. I
II | 1.39 | 0.66 | 2.92 | 0.38 | 1.14 | 0.66 | 1.96 | 0.64 | 1.20 | 0.82 | 1.75 | 0.35 |
| Signature | | | | | | | | | | | | |
| High-risk vs.
low-risk | 1.95 | 1.34 | 2.86 | <0.001 | 1.23 | 0.89 | 1.70 | 0.05 | 1.47 | 1.22 | 1.79 | <0.001 |
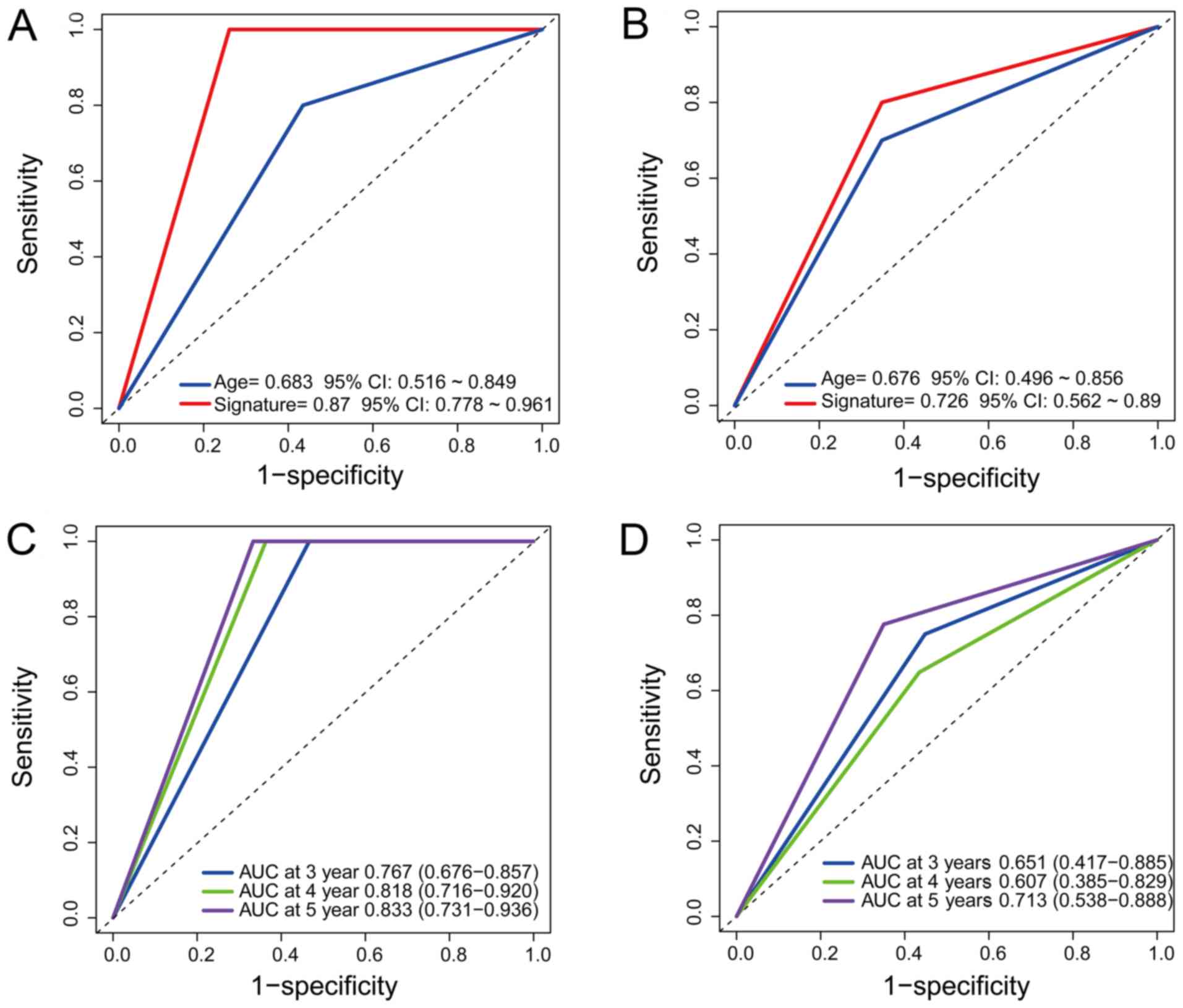
Comparison of the predictive power
between the molecular signature and age
To compare the specificity and sensitivity of
regrowth prediction between age and our signature, ROC analysis was
performed. The predictive power of our signature was significantly
better than that of age in the training/testing set
(AUC=0.870/0.683 vs. AUC=0.726/0.676; Fig. 3A and B), which demonstrated that
our signature is an effective for prediction.
To further understand the predictive ability of the
signature regarding 3- to 5-year PFS, timeROC analysis was used
within the 2 sets. The AUC of our signature was 0.767/0.818/0.833
at 3/4/5 years in the training set and 0.651/0.607/0.713 at 3/4/5
years in the testing set, respectively (Fig. 3C and D). The results indicated that
our predictive signature had a better performance for 5-year PFS
than for 3- or 4-year PFS.
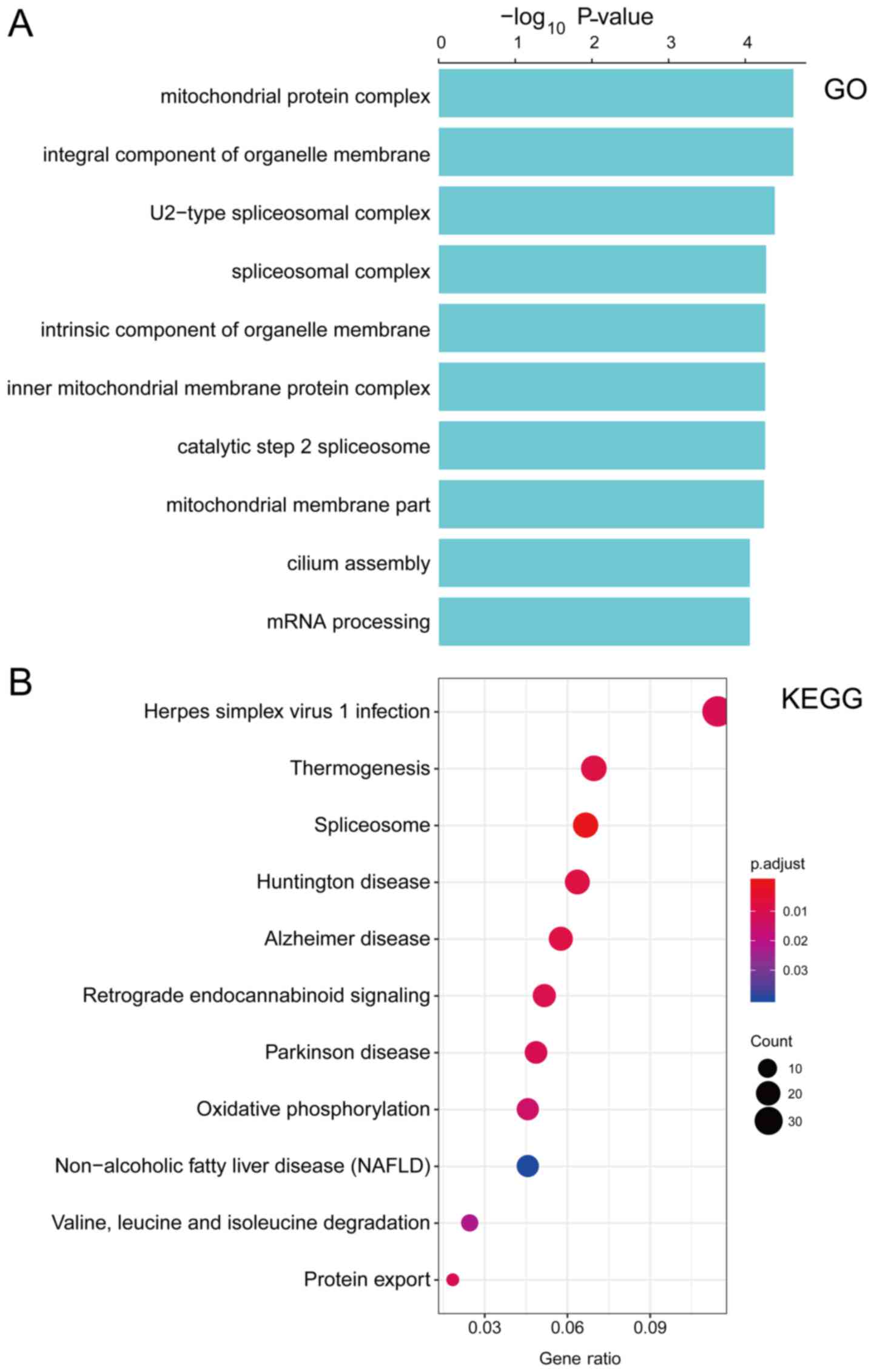
Functional characterization of the PCG
and lncRNAs composing the prognostic signature
To explore the biological functions of the PCG and
lncRNAs in our signature, the co-expression network of the one PCG
and two lncRNAs with other PCGs were assessed using Pearson's
correlation analysis. The expression levels of 1,076 PCGs highly
correlated with those of one or more PCG and lncRNAs (Pearson's
correlation coefficient >0.60, P<0.05, Table SIV). Subsequently, performed KEGG
pathway and GO function enrichment analyses were performed for the
co-expressed PCGs. GO function annotation analysis revealed that
1,074 PCGs were enriched in 98 GO/KEGG terms (Table SV), which indicated that the PCG
and lncRNAs may be associated with important biological processes,
including those involving the spliceosome and mitochondrial protein
complex, etc., (Fig. 4).
Discussion
Unlike tumor regrowth in functioning pituitary
adenoma, which can be monitored by monitoring the levels of
specific serum hormones, tumor regrowth in NFPA is difficult to
monitor. By the time patients are re-examined due to optic nerve
compression symptoms, the tumor may have acquired a large volume,
which presents a number of obstacles to total resection and
post-operative recovery. Therefore, the present study aimed to
develop novel predictive signatures that can identify early
regrowth and serve as predictive markers of prognosis. The present
study aimed to categorize patients into a high- and low-risk group;
thus the most effective and timely treatment can be applied to
patients with NFPA.
A previous study focused on factors associated with
tumor regrowth in NFPA with the ultimate goal of ameliorating the
prognosis of patients at the post-operative stage (18). Age is recognized as an important
independent factor influencing the prognosis of patients with NFPA,
with a younger age being associated with a greater chance of tumor
regrowth (19,20). However, the prognostic value of age
is not as effective as the PCG-lncRNA signature identified in the
present study. Ki-67 is another commonly used pathological
prognostic evaluation index (21);
however, the use of a single indicator for prognostic assessment
has limitations in accurately evaluating patient prognosis. A
previous study attempted to establish a statistical model that
considers both combines clinical features (age and tumor volume)
and molecular markers (p16, WIF1 and TGF-β) to evaluate regrowth
probability among patients with NFPA post-operatively (1). In the present study, the inclusion of
clinical characteristics did not improve predictive efficacy.
Furthermore, unlike the above-mentioned study, a time component was
added to the prognosis assessment and independently assessed the
prognosis of patients at different time points.
In recent years, long non-coding RNAs have been
reported in a number of types of tumor and represent a promising
novel group of molecular markers for tumor biological behavior,
disease diagnosis and prognosis evaluation (22-24).
The expression of lncRNA H19 has been found to be decreased in
pituitary adenomas, and its overexpression can markedly inhibit the
growth of pituitary tumor cells; furthermore, it has been
identified as a potential drug resistance marker (11). Xing et al (25), identified differentially expressed
mRNAs and lncRNAs in clinically NFPA and normal pituitary, and
constructed a mRNA-lncRNA co-expression network. However, their
research failed to illustrate the regulatory mechanisms of key
genes or lncRNAs or their effects on patient prognosis. Guo et
al (26) successfully
constructed a circRNA signature using the random survival forest
algorithm, Kaplan-Meier analysis and ROC analysis. They randomly
divided patients into a training and testing set and obtained an
accuracy of 0.87 and 0.67 in each group. Compared with the single
signature, the present study included PCGs and lncRNAs into the
model and achieved a better accuracy in this model. In addition,
the present study introduced a concept of time into our predictive
signature, which could be more instructive in clinical practice.
The present study found 2 lncRNAs that could be used as prognostic
signatures. However, the function and regulatory mechanisms of
these two lncRNA have not yet been reported. A PCG marker, CHST12,
was also identified, which plays an important role in articular
cartilage; however, there is limited research available on its role
in tumors (27,28). The biological roles of the one PCG
and two lncRNAs composing the signature in the present study are
not yet fully illustrated and warrant further investigation in
future studies.
There are a few limitations to the present study
which need to be acknowledged. First, there is limited research
available on the functions and molecular mechanisms of these PCGs
and lncRNAs in tumors. The biological roles of CHST12, COA6-AS1 and
RP11-23N2.4 have not yet been fully illustrated and thus require
further investigation in the future. Second, there are limited
sequencing data available regarding NFPA; thus, the verification of
the present results with an independent validating set was not
possible. In addition, further studies are required using larger
cohort sizes and for the validation of the present results in the
future. It should be noted that further research based on these
data is still ongoing and the authors would like to share their
data privately to researchers who are interested in pituitary
adenoma. Finally, the application of our signature in clinical
practice should be tested prospectively. Despite the
above-mentioned limitations, the consistent and significant
correlation of our CHST12, COA6-AS1 and RP11-23N2.4 signature with
tumor regrowth indicates that this signature is a potentially
potent prognostic predictive model for NFPA.
In conclusion, to the best of our knowledge, this is
the first study to integrate PCGs and lncRNAs to predict tumor
regrowth in patients with NFPA. The findings of the present study
may provide a new aspect of prognostic evaluation and may help
patients to benefit from early intervention.
Supplementary Data
Acknowledgments
The authors would like to thank Dr Yazhuo Miao and
Mr. Qiuyue Fang from Capital Medical University for the selfless
assistance in collecting clinical data.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81771489, 81672495 and
81601205), the National High Technology Research and Development
Program of China (863 Program, grant no. 2014AA020610), the Beijing
Municipal Science and Technology Commission (grant no.
Z171100000117002), the Beijing Talents Fund (grant no.
2015000021223ZK24) and the China National Key Research and
Development Program (grant no. 2017YFC0908300).
Availability of data and materials
Further research regarding the topic of the present
study is ongoing and all data in this study are available from the
corresponding author on reasonable request.
Authors' contributions
SC was involved in data collection and analysis, and
in the writing of the manuscript. JG and ZL were involved in data
collection. CL and YZ were involved in the conception and design of
the research, funding management and in the final editing of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Beijing Tiantan Hospital and written informed consent
for the use of the resected samples for research purposes was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Cheng S, Wu J, Li C, Li Y, Liu C, Li G, Li
W, Hu S, Ying X and Zhang Y: Predicting the regrowth of clinically
non-functioning pituitary adenoma with a statistical model. J
Transl Med. 17:1642019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ebersold MJ, Quast LM, Laws ER Jr,
Scheithauer B and Randall RV: Long-term results in transsphenoidal
removal of nonfunctioning pituitary adenomas. J Neurosurg.
64:713–719. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meij BP, Lopes MB, Ellegala DB, Alden TD
and Laws ER Jr: The long-term significance of microscopic dural
invasion in 354 patients with pituitary adenomas treated with
transsphenoidal surgery. J Neurosurg. 96:195–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shomali ME and Katznelson L: Medical
therapy of gonadotropin-producing and nonfunctioning pituitary
adenomas. Pituitary. 5:89–98. 2002. View Article : Google Scholar
|
|
5
|
Brochier S, Galland F, Kujas M, Parker F,
Gaillard S, Raftopoulos C, Young J, Alexopoulou O, Maiter D and
Chanson P: Factors predicting relapse of nonfunctioning pituitary
macroadenomas after neurosurgery: A study of 142 patients. Eur J
Endocrinol. 163:193–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brada M and Jankowska P: Radiotherapy for
pituitary adenomas. Endocrinol Metab Clin North Am. 37:pp. 263–275.
pp. xi2008, https://doi.org/10.1016/j.ecl.2007.10.005.
View Article : Google Scholar
|
|
7
|
Pollock BE, Cochran J, Natt N, Brown PD,
Erickson D, Link MJ, Garces YI, Foote RL, Stafford SL and Schomberg
PJ: Gamma knife radiosurgery for patients with nonfunctioning
pituitary adenomas: Results from a 15-year experience. Int J Radiat
Oncol Biol Phys. 70:1325–1329. 2008. View Article : Google Scholar
|
|
8
|
Aydin B and Arga KY: Co-expression network
analysis elucidated a core module in association with prognosis of
non-functioning non-invasive human pituitary adenoma. Front
Endocrinol (Lausanne). 10:3612019. View Article : Google Scholar
|
|
9
|
Zhenye L, Chuzhong L, Youtu W, Xiaolei L,
Lei C, Lichuan H, Hongyun W, Yonggang W, Fei W and Yazhuo Z: The
expression of TGF-β1, Smad3, phospho-Smad3 and Smad7 is correlated
with the development and invasion of nonfunctioning pituitary
adenomas. J Transl Med. 12:712014. View Article : Google Scholar
|
|
10
|
Dykes IM and Emanueli C: Transcriptional
and post-transcriptional gene regulation by long non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Liu YT, Tang H, Xie WQ, Yao H, Gu
WT, Zheng YZ, Shang HB, Wang Y, Wei YX, et al: Exosome-transmitted
lncRNA H19 inhibits the growth of pituitary adenoma. J Clin
Endocrinol Metab. 104:6345–6356. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu H, Guo J, Shen Y, Dong W, Gao H, Miao
Y, Li C and Zhang Y: Functions and mechanisms of tumor necrosis
factor-α and noncoding RNAs in bone-invasive pituitary adenomas.
Clin Cancer Res. 24:5757–5766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo JC, Li CQ, Wang QY, Zhao JM, Ding JY,
Li EM and Xu LY: Protein-coding genes combined with long non-coding
RNAs predict prognosis in esophageal squamous cell carcinoma
patients as a novel clinical multi-dimensional signature. Mol
Biosyst. 12:3467–3477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu
K, Fan Q, Li J, Ning T, Tian F, et al: Exosome circRNA secreted
from adipocytes promotes the growth of hepatocellular carcinoma by
targeting deubiquitination-related USP7. Oncogene. 38:2844–2859.
2019. View Article : Google Scholar :
|
|
17
|
Knosp E, Steiner E, Kitz K and Matula C:
Pituitary adenomas with invasion of the cavernous sinus space: A
magnetic resonance imaging classification compared with surgical
findings. Neurosurgery. 33:610–617; discussion 617-618.
1993.PubMed/NCBI
|
|
18
|
Lee EH, Kim KH, Kwon JH, Kim HD and Kim
YZ: Results of immunohistochemical staining of cell-cycle
regulators: The prediction of recurrence of functioning pituitary
adenoma. World Neurosurg. 81:563–575. 2014. View Article : Google Scholar
|
|
19
|
Tampourlou M, Ntali G, Ahmed S, Arlt W,
Ayuk J, Byrne JV, Chavda S, Cudlip S, Gittoes N, Grossman A, et al:
Outcome of nonfunctioning pituitary adenomas that regrow after
primary treatment: A study from two large UK centers. J Clin
Endocrinol Metab. 102:1889–1897. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie Y, Burcu M, Linn DE, Qiu Y and Baer
MR: Pim-1 kinase protects P-glycoprotein from degradation and
enables its glycosylation and cell surface expression. Mol
Pharmacol. 78:310–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hasanov R, Aydoğan BI, Kiremitçi S, Erden
E and Güllü S: The prognostic roles of the Ki-67 proliferation
index, P53 expression, mitotic index, and radiological tumor
invasion in pituitary adenomas. Endocr Pathol. 30:49–55. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Dai M, Zhu H, Li J, Huang Z, Liu
X, Huang Y, Chen J and Dai S: Evaluation on the diagnostic and
prognostic values of long non-coding RNA BLACAT1 in common types of
human cancer. Mol Cancer. 16:1602017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu ZR, Yan L, Liu YT, Cao L, Guo YH, Zhang
Y, Yao H, Cai L, Shang HB, Rui WW, et al: Inhibition of mTORC1 by
lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary
tumours. Nat Commun. 9:46242018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamoah K, Johnson MH, Choeurng V, Faisal
FA, Yousefi K, Haddad Z, Ross AE, Alshalafa M, Den R, Lal P, et al:
Novel biomarker signature that may predict aggressive disease in
African American men with prostate cancer. J Clin Oncol.
33:2789–2796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xing W, Qi Z, Huang C, Zhang N, Zhang W,
Li Y, Qiu M, Fang Q and Hui G: Genome-wide identification of
lncRNAs and mRNAs differentially expressed in non-functioning
pituitary adenoma and construction of an lncRNA-mRNA co-expression
network. Biol Open. 8:82019. View Article : Google Scholar
|
|
26
|
Guo J, Wang Z, Miao Y, Shen Y, Li M, Gong
L, Wang H, He Y, Gao H, Liu Q, et al: A two-circRNA signature
predicts tumour recurrence in clinical non-functioning pituitary
adenoma. Oncol Rep. 41:113–124. 2019.
|
|
27
|
Guo Y, Zhou Y, Yan S, Qu C, Wang L, Guo X
and Han J: Decreased expression of CHST-12, CHST-13, and UST in the
proximal interphalangeal joint cartilage of school-age children
with Kashin-Beck disease: An endemic osteoarthritis in China caused
by selenium deficiency. Biol Trace Elem Res. 191:276–285. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han J, Li D, Qu C, Wang D, Wang L, Guo X
and Lammi MJ: Altered expression of chondroitin sulfate structure
modifying sulfotransferases in the articular cartilage from adult
osteoarthritis and Kashin-Beck disease. Osteoarthritis Cartilage.
25:1372–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|