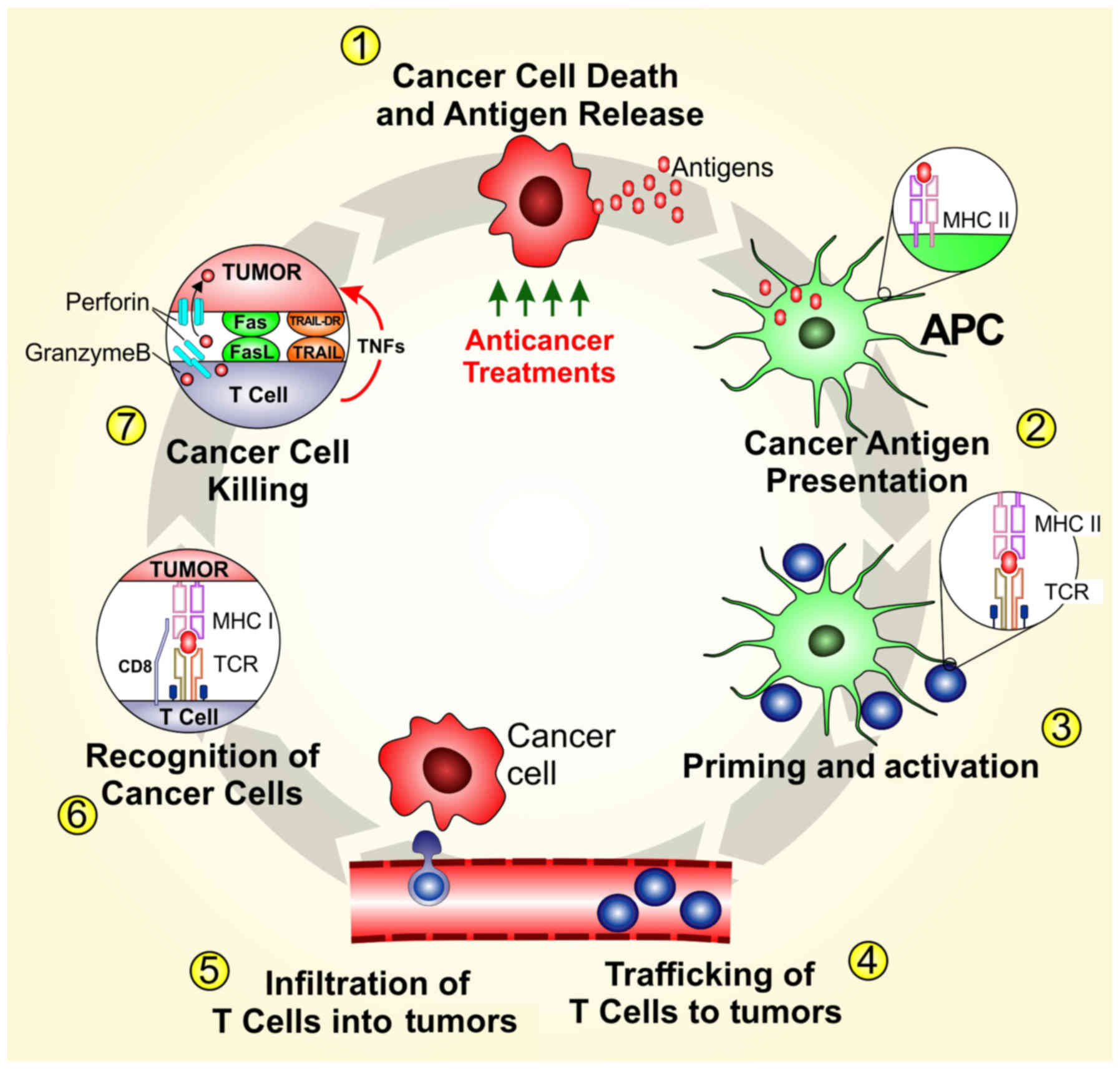
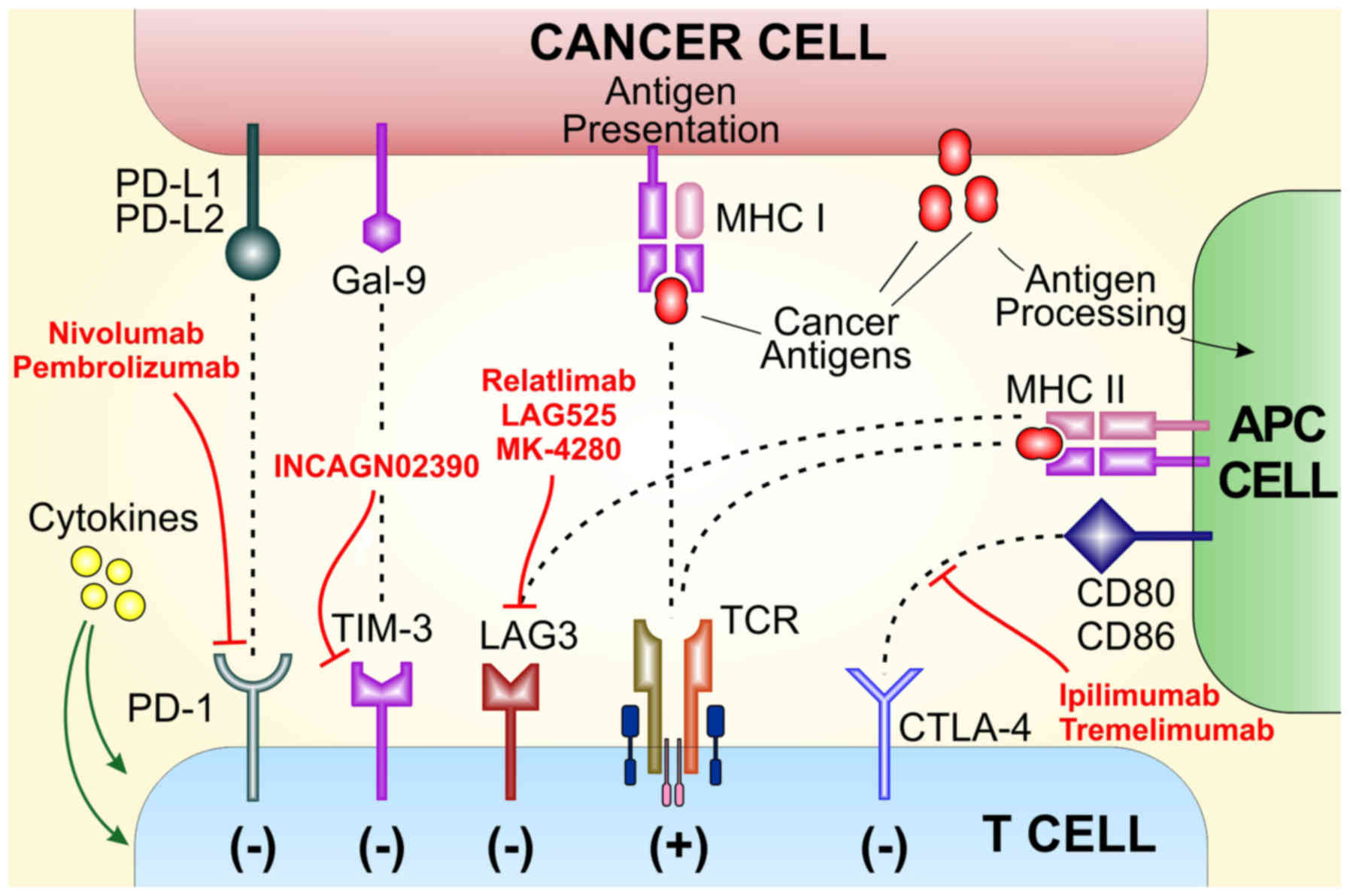
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leonardi GC, Falzone L, Salemi R, Zanghì
A, Spandidos DA, McCubrey JA, Candido S and Libra M: Cutaneous
melanoma: From pathogenesis to therapy (Review). Int J Oncol.
52:1071–1080. 2018.PubMed/NCBI
|
|
3
|
Candido S, Rapisarda V, Marconi A,
Malaponte G, Bevelacqua V, Gangemi P, Scalisi A, McCubrey JA,
Maestro R, Spandidos DA, et al: Analysis of the B-RafV600E mutation
in cutaneous melanoma patients with occupational sun exposure.
Oncol Rep. 31:1079–1082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawakami Y and Rosenberg SA: T-cell
recognition of self peptides as tumor rejection antigens. Immunol
Res. 15:179–190. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Faramarzi S and Ghafouri-Fard S: Melanoma:
A prototype of cancer-testis antigen-expressing malignancies.
Immunotherapy. 9:1103–1113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee N, Zakka LR, Mihm MC Jr and Schatton
T: Tumour-infiltrating lymphocytes in melanoma prognosis and cancer
immunotherapy. Pathology. 48:177–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kirkwood JM, Ibrahim JG, Sosman JA, Sondak
VK, Agarwala SS, Ernstoff MS and Rao U: High-dose interferon
alfa-2b significantly prolongs relapse-free and overall survival
compared with the GM2-KLH/QS-21 vaccine in patients with resected
stage IIB-III melanoma: Results of intergroup trial
E1694/S9512/C509801. J Clin Oncol. 19:2370–2380. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wheatley K, Ives N, Hancock B, Gore M,
Eggermont A and Suciu S: Does adjuvant interferon-alpha for
high-risk melanoma provide a worthwhile benefit? A meta-analysis of
the randomised trials. Cancer Treat Rev. 29:241–252. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atkins MB, Kunkel L, Sznol M and Rosenberg
SA: High-dose recombinant interleukin-2 therapy in patients with
metastatic melanoma: Long-term survival update. Cancer J Sci Am.
6(Suppl 1): S11–S14. 2000.PubMed/NCBI
|
|
10
|
Rosenberg SA: IL-2: The first effective
immunotherapy for human cancer. J Immunol. 192:5451–5458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klebanoff CA, Acquavella N, Yu Z and
Restifo NP: Therapeutic cancer vaccines: Are we there yet? Immunol
Rev. 239:27–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schwartzentruber DJ, Lawson DH, Richards
JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K,
Pockaj B, et al: gp100 peptide vaccine and interleukin-2 in
patients with advanced melanoma. N Engl J Med. 364:2119–2127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rohaan MW, van den Berg JH, Kvistborg P
and Haanen JBAG: Adoptive transfer of tumor-infiltrating
lymphocytes in melanoma: A viable treatment option. J Immunother
Cancer. 6:1022018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ascierto PA, McArthur GA, Dréno B,
Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, Demidov L,
Stroyakovskiy D, Thomas L, et al: Cobimetinib combined with
vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM):
Updated efficacy results from a randomised, double-blind, phase 3
trial. Lancet Oncol. 17:1248–1260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hodi FS, Kluger H, Sznol M, Carvajal R,
Lawrence D, Atkins M, Powderly J, Sharfman W, Puzanov I, et al:
Durable, long-term survival in previously treated patients with
advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy
in a phase I trial. Cancer Res. 76:CT0012016.
|
|
16
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christofi T, Baritaki S, Falzone L, Libra
M and Zaravinos A: Current perspectives in cancer immunotherapy.
Cancers (Basel). 11:14722019. View Article : Google Scholar
|
|
18
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schadendorf D, Hodi FS, Robert C, Weber
JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM and Wolchok JD:
Pooled analysis of long-term survival data from phase II and phase
III trials of ipilimumab in unresectable or metastatic melanoma. J
Clin Oncol. 33:1889–1894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weide B, Martens A, Wistuba-Hamprecht K,
Zelba H, Maier L, Lipp HP, Klumpp BD, Soffel D, Eigentler TK and
Garbe C: Combined treatment with ipilimumab and intratumoral
interleukin-2 in pretreated patients with stage IV melanoma-safety
and efficacy in a phase II study. Cancer Immunol Immunother.
66:441–449. 2017. View Article : Google Scholar
|
|
23
|
Brohl AS, Khushalani NI, Eroglu Z,
Markowitz J, Thapa R, Chen YA, Kudchadkar R and Weber JS: A phase
IB study of ipilimumab with peginterferon alfa-2b in patients with
unre-sectable melanoma. J Immunother Cancer. 4:852016. View Article : Google Scholar
|
|
24
|
Ribas A, Kefford R, Marshall MA, Punt CJ,
Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, et
al: Phase III randomized clinical trial comparing tremelimumab with
standard-of-care chemotherapy in patients with advanced melanoma. J
Clin Oncol. 31:616–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eggermont AM, Chiarion-Sileni V, Grob JJ,
Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA,
Richards JM, et al: Prolonged survival in stage III melanoma with
ipilimumab adjuvant therapy. N Engl J Med. 375:1845–1855. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al
KEYNOTE-006 investigators: Pembrolizumab versus ipilimumab in
advanced melanoma. N Engl J Med. 372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ribas A, Hamid O, Daud A, Hodi FS, Wolchok
JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al:
Association of pembrolizumab with tumor response and survival among
patients with advanced melanoma. JAMA. 315:1600–1609. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schachter J, Ribas A, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab for advanced melanoma: Final
overall survival results of a multicentre, randomised, open-label
phase 3 study (KEYNOTE-006). Lancet. 390:1853–1862. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, et al:
Five-year survival outcomes for patients with advanced melanoma
treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 30:582–588.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robert C, Ribas A, Schachter J, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006):
Post-hoc 5-year results from an open-label, multicentre,
randomised, controlled, phase 3 study. Lancet Oncol. 20:1239–1251.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar
|
|
32
|
Ascierto PA, Long GV, Robert C, Brady B,
Dutriaux C, Di Giacomo AM, Mortier L, Hassel JC, Rutkowski P,
McNeil C, et al: Survival outcomes in patients with previously
untreated BRAF wild-type advanced melanoma treated with nivolumab
therapy: Three-year follow-up of a randomized phase 3 trial. JAMA
Oncol. 5:187–194. 2019. View Article : Google Scholar :
|
|
33
|
Weiss SA, Wolchok JD and Sznol M:
Immunotherapy of melanoma: Facts and hopes. Clin Cancer Res.
25:5191–5201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weber J, Mandala M, Del Vecchio M, Gogas
HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al CheckMate 238 Collaborators: Adjuvant
nivolumab versus ipilimumab in resected stage III or IV melanoma. N
Engl J Med. 377:1824–1835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eggermont AMM, Blank CU, Mandala M, Long
GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A,
Carlino MS, et al: Adjuvant pembrolizumab versus placebo in
resected stage III melanoma. N Engl J Med. 378:1789–1801. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J,
Dummer R, et al: Five-year survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 381:1535–1546. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kirchberger MC, Hauschild A, Schuler G and
Heinzerling L: Combined low-dose ipilimumab and pembrolizumab after
sequential ipilimumab and pembrolizumab failure in advanced
melanoma. Eur J Cancer. 65:182–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Long GV, Atkinson V, Cebon JS, Jameson MB,
Fitzharris BM, McNeil CM, Hill AG, Ribas A, Atkins MB, Thompson JA,
et al: Standard-dose pembrolizumab in combination with reduced-dose
ipilimumab for patients with advanced melanoma (KEYNOTE-029): An
open-label, phase 1b trial. Lancet Oncol. 18:1202–1210. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zimmer L, Apuri S, Eroglu Z, Kottschade
LA, Forschner A, Gutzmer R, Schlaak M, Heinzerling L, Krackhardt
AM, Loquai C, et al: Ipilimumab alone or in combination with
nivolumab after progression on anti-PD-1 therapy in advanced
melanoma. Eur J Cancer. 75:47–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Long GV, Robert C, Blank C, Ribas A,
Mortier L, Schachter J, Middleton MR, et al: Outcomes in patients
treated with ipilimumab after pembrolizumab in KEYNOTE-006. Eur J
Cancer. 72:S128–S129. 2017. View Article : Google Scholar
|
|
42
|
Ascierto PA, Butterfield LH, Demaria S,
Ferris RL, Freeman GJ, Lo RS, Mantovani A, Nathan P, Hamid O,
Politi K, et al: The great debate at 'Immunotherapy Bridge 2018',
Naples, November 29th, 2018. J Immunother Cancer. 7:2212019.
View Article : Google Scholar
|
|
43
|
Tawbi HA, Forsyth PA, Algazi A, Hamid O,
Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, et
al: Combined nivolumab and ipilimumab in melanoma metastatic to the
brain. N Engl J Med. 379:722–730. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Long GV, Atkinson V, Lo S, Sandhu S,
Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer
RA, et al: Combination nivolumab and ipilimumab or nivolumab alone
in melanoma brain metastases: A multicentre randomised phase 2
study. Lancet Oncol. 19:672–681. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Conry RM, Westbrook B, McKee S and Norwood
TG: Talimogene laherparepvec: First in class oncolytic virotherapy.
Hum Vaccin Immunother. 14:839–846. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Andtbacka RH, Kaufman HL, Collichio F,
Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I,
Agarwala SS, et al: Talimogene laherparepvec improves durable
response rate in patients with advanced melanoma. J Clin Oncol.
33:2780–2788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chesney J, Puzanov I, Collichio F, Singh
P, Milhem MM, Glaspy J, Hamid O, Ross M, Friedlander P, Garbe C, et
al: Randomized, open-label phase II study evaluating the efficacy
and safety of talimogene laherparepvec in combination with
ipilimumab versus ipilimumab alone in patients with advanced,
unresectable melanoma. J Clin Oncol. 36:1658–1667. 2018. View Article : Google Scholar :
|
|
48
|
Ribas A, Dummer R, Puzanov I, Vander Walde
A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J,
Fernandez E, et al: Oncolytic virotherapy promotes intratumoral T
cell infiltration and improves anti-PD-1 immunotherapy. Cell.
174:1031–1032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Durham NM, Nirschl CJ, Jackson CM, Elias
J, Kochel CM, Anders RA and Drake CG: Lymphocyte activation gene 3
(LAG-3) modulates the ability of CD4 T-cells to be suppressed in
vivo. PLoS One. 9:e1090802014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Andrews LP, Marciscano AE, Drake CG and
Vignali DA: LAG3 (CD223) as a cancer immunotherapy target. Immunol
Rev. 276:80–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Woo SR, Turnis ME, Goldberg MV, Bankoti J,
Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et
al: Immune inhibitory molecules LAG-3 and PD-1 synergistically
regulate T-cell function to promote tumoral immune escape. Cancer
Res. 72:917–927. 2012. View Article : Google Scholar
|
|
52
|
Ascierto PA, Bono P, Bhatia S, Melero I,
Nyakas MS, Svane I, Larkin J, Gomez-Roca C, Schadendorf D, Dummer
R, et al: LBA18Efficacy of BMS-986016, a monoclonal antibody that
targets lymphocyte activation gene-3 (LAG-3), in combination with
nivolumab in pts with melanoma who progressed during prior
anti-PD-1/PD-L1 therapy (mel prior IO) in all-comer and
biomarker-enriched populations. Ann Oncol. 28(Suppl 5): v605–v649.
2017. View Article : Google Scholar
|
|
53
|
Das M, Zhu C and Kuchroo VK: Tim-3 and its
role in regulating anti-tumor immunity. Immunol Rev. 276:97–111.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ricciuti B, Leonardi GC, Puccetti P,
Fallarino F, Bianconi V, Sahebkar A, Baglivo S, Chiari R and Pirro
M: Targeting indoleamine-2,3-dioxygenase in cancer: Scientific
rationale and clinical evidence. Pharmacol Ther. 196:105–116. 2019.
View Article : Google Scholar
|
|
55
|
Long GV, Dummer R, Hamid O, Gajewski TF,
Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM, et al:
Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in
patients with unresectable or metastatic melanoma
(ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study.
Lancet Oncol. 20:1083–1097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nicholas C and Lesinski GB:
Immunomodulatory cytokines as therapeutic agents for melanoma.
Immunotherapy. 3:673–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Charych DH, Hoch U, Langowski JL, Lee SR,
Addepalli MK, Kirk PB, Sheng D, Liu X, Sims PW, VanderVeen LA, et
al: NKTR-214, an engineered cytokine with biased IL2 receptor
binding, increased tumor exposure, and marked efficacy in mouse
tumor models. Clin Cancer Res. 22:680–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Diab A, Haymaker C, Bernatchez C,
Andtbacka RHI, Shaheen M, Johnson D, Markowitz J, Puzanov I, Murthy
R, Johnson DH, et al: Intratumoral (it) injection of the TLR9
agonist tilsotolimod (imo-2125) in combination with ipilimumab
(ipi) triggers durable responses in pd-1 inhibitor refractory
metastatic melanoma (rmm): results from a multicenter, phase 1/2
study. Ann Oncol. 29(Suppl 8): viii442–viii466. 2018. View Article : Google Scholar
|
|
59
|
Milhem MM, Long GV, Hoimes CJ, Amin A, Lao
CD, Conry RM, Hunt J, Daniels GA, Almubarak M, Shaheen MF, et al:
Phase 1b/2, open label, multicenter, study of the combination of
SD-101 and pembrolizumab in patients with advanced melanoma who are
naïve to anti-PD-1 therapy. J Clin Oncol. 37(Suppl 15): 9534. 2019.
View Article : Google Scholar
|
|
60
|
Wiehagen KR, Girgis NM, Yamada DH, Smith
AA, Chan SR, Grewal IS, Quigley M and Verona RI: Combination of
CD40 agonism and CSF-1R blockade reconditions tumor-associated
macrophages and drives potent antitumor immunity. Cancer Immunol
Res. 5:1109–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cannarile MA, Weisser M, Jacob W, Jegg AM,
Ries CH and Rüttinger D: Colony-stimulating factor 1 receptor
(CSF1R) inhibitors in cancer therapy. J Immunother Cancer.
5:532017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sarnaik AA, Yu B, Yu D, Morelli D, Hall M,
Bogle D, Yan L, Targan S, Solomon J, Nichol G, et al: Extended dose
ipilimumab with a peptide vaccine: Immune correlates associated
with clinical benefit in patients with resected high-risk stage
IIIc/IV melanoma. Clin Cancer Res. 17:896–906. 2011. View Article : Google Scholar :
|
|
64
|
Ribas A, Comin-Anduix B, Chmielowski B,
Jalil J, de la Rocha P, McCannel TA, Ochoa MT, Seja E, Villanueva
A, Oseguera DK, et al: Dendritic cell vaccination combined with
CTLA4 blockade in patients with metastatic melanoma. Clin Cancer
Res. 15:6267–6276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wilgenhof S, Van Nuffel AM, Benteyn D,
Corthals J, Aerts C, Heirman C, Van Riet I, Bonehill A, Thielemans
K and Neyns B: A phase IB study on intravenous synthetic mRNA
electroporated dendritic cell immunotherapy in pretreated advanced
melanoma patients. Ann Oncol. 24:2686–2693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wilgenhof S, Corthals J, Heirman C, van
Baren N, Lucas S, Kvistborg P, Thielemans K and Neyns B: Phase II
study of autologous monocyte-derived mRNA electroporated dendritic
cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated
advanced melanoma. J Clin Oncol. 34:1330–1338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chick RC, Faries MB, Hale DF, Kemp Bohan
PM, Hickerson A, Vreelandc TJ, Myers JW, Cindass JL, Brown TA, et
al: Multi-institutional, prospective, randomized, double-blind,
placebo-controlled phase IIb trial of the tumor lysate,
particle-loaded, dendritic cell (TLPLDC) vaccine to prevent
recurrence in high-risk melanoma patients: A subgroup analysis. J
Clin Oncol. 38(Suppl 5): 63. 2020. View Article : Google Scholar
|
|
68
|
Kvistborg P, Philips D, Kelderman S,
Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der
Burg S, Kapiteijn E, Michielin O, et al: Anti-CTLA-4 therapy
broadens the melanoma-reactive CD8+ T cell response. Sci
Transl Med. 6:254ra1282014. View Article : Google Scholar
|
|
69
|
Daud AI, Loo K, Pauli ML,
Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A,
Nardo L, Alvarado MD, et al: Tumor immune profiling predicts
response to anti-PD-1 therapy in human melanoma. J Clin Invest.
126:3447–3452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Foley KC, Nishimura MI and Moore TV:
Combination immunotherapies implementing adoptive T-cell transfer
for advanced-stage melanoma. Melanoma Res. 28:171–184. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rosenberg SA, Packard BS, Aebersold PM,
Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp
CA, et al: Use of tumor-infiltrating lymphocytes and interleukin-2
in the immunotherapy of patients with metastatic melanoma. A
preliminary report N Engl J Med. 319:1676–1680. 1988.
|
|
72
|
Rosenberg SA, Yang JC, Sherry RM, Kammula
US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF,
Wunderlich JR, et al: Durable complete responses in heavily
pretreated patients with metastatic melanoma using T-cell transfer
immunotherapy. Clin Cancer Res. 17:4550–4557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Feins S, Kong W, Williams EF, Milone MC
and Fraietta JA: An introduction to chimeric antigen receptor (CAR)
T-cell immunotherapy for human cancer. Am J Hematol. 94:S3–S9.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Robbins PF, Kassim SH, Tran TL, Crystal
JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR,
Sherry RM, et al: A pilot trial using lymphocytes genetically
engineered with an NY-ESO-1-reactive T-cell receptor: Long-term
follow-up and correlates with response. Clin Cancer Res.
21:1019–1027. 2015. View Article : Google Scholar
|
|
75
|
Falzone L, Salomone S and Libra M:
Evolution of cancer pharmacological treatments at the turn of the
third millennium. Front Pharmacol. 9:13002018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pelster MS and Amaria RN: Combined
targeted therapy and immunotherapy in melanoma: A review of the
impact on the tumor microenvironment and outcomes of early clinical
trials. Ther Adv Med Oncol. 11:17588359198308262019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ribas A, Hodi FS, Callahan M, Konto C and
Wolchok J: Hepatotoxicity with combination of vemurafenib and
ipilimumab. N Engl J Med. 368:1365–1366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Minor DR, Puzanov I, Callahan MK, Hug BA
and Hoos A: Severe gastrointestinal toxicity with administration of
trametinib in combination with dabrafenib and ipilimumab. Pigment
Cell Melanoma Res. 28:611–612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ribas A, Butler M, Lutzky J, Lawrence DP,
Robert C, Miller W, Linette WMGP, Ascierto PA, Kuzel T, Algazi AP,
et al: Phase I study combining anti-PD-L1 (MEDI4736) with BRAF
(dabrafenib) and/or MEK (trametinib) inhibitors in advanced
melanoma. J Clin Oncol. 33(Suppl 15): 30032015. View Article : Google Scholar
|
|
80
|
Shui L, Yang X, Li J, Yi C, Sun Q and Zhu
H: Gut microbiome as a potential factor for modulating resistance
to cancer immuno-therapy. Front Immunol. 10:29892020. View Article : Google Scholar
|
|
81
|
Longo V, Brunetti O, Azzariti A, Galetta
D, Nardulli P, Leonetti F and Silvestris N: Strategies to improve
cancer immune checkpoint inhibitors efficacy, other than abscopal
effect: A systematic review. Cancers (Basel). 11:5392019.
View Article : Google Scholar
|
|
82
|
Velez MA, Burns TF and Stabile LP: The
estrogen pathway as a modulator of response to immunotherapy.
Immunotherapy. 11:1161–1176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Matsushita M and Kawaguchi M:
Immunomodulatory Effects of Drugs for Effective Cancer
Immunotherapy. J Oncol. 2018:86534892018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Vivarelli S, Falzone L, Basile MS,
Nicolosi D, Genovese C, Libra M and Salmeri M: Benefits of using
probiotics as adjuvants in anticancer therapy (Review). World A Sci
J. 1:125–135. 2019.
|
|
85
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar
|
|
87
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar
|
|
88
|
Banna GL, Torino F, Marletta F, Santagati
M, Salemi R, Cannarozzo E, Falzone L, Ferraù F and Libra M:
Lactobacillus rhamnosus GG: An overview to explore the rationale of
its use in cancer. Front Pharmacol. 8:6032017. View Article : Google Scholar :
|
|
89
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Elkrief A, Derosa L, Kroemer G, Zitvogel L
and Routy B: The negative impact of antibiotics on outcomes in
cancer patients treated with immunotherapy: A new independent
prognostic factor? Ann Oncol. 30:1572–1579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Eskiocak B, McMillan EA, Mendiratta S,
Kollipara RK, Zhang H, Humphries CG, Wang C, Garcia-Rodriguez J,
Ding M, Zaman A, et al: Biomarker accessible and chemically
addressable mechanistic subtypes of BRAF melanoma. Cancer Discov.
7:832–851. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Salemi R, Falzone L, Madonna G, Polesel J,
Cinà D, Mallardo D, Ascierto PA, Libra M and Candido S: MMP-9 as a
candidate marker of response to BRAF inhibitors in melanoma
patients with BRAFV600E mutation detected in circulating-free DNA.
Front Pharmacol. 9:8562018. View Article : Google Scholar :
|
|
94
|
Guarneri C, Bevelacqua V, Polesel J,
Falzone L, Cannavò PS, Spandidos DA, Malaponte G and Libra M: NFκB
inhibition is associated with OPN/MMP 9 downregulation in cutaneous
melanoma. Oncol Rep. 37:737–746. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Luke JJ, Flaherty KT, Ribas A and Long GV:
Targeted agents and immunotherapies: Optimizing outcomes in
melanoma. Nat Rev Clin Oncol. 14:463–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gajewski TF, Louahed J and Brichard VG:
Gene signature in melanoma associated with clinical activity: A
potential clue to unlock cancer immunotherapy. Cancer J.
16:399–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Harlin H, Meng Y, Peterson AC, Zha Y,
Tretiakova M, Slingluff C, McKee M and Gajewski TF: Chemokine
expression in melanoma metastases associated with CD8+
T-cell recruitment. Cancer Res. 69:3077–3085. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ji RR, Chasalow SD, Wang L, Hamid O,
Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M,
Siemers NO, et al: An immune-active tumor microenvironment favors
clinical response to ipilimumab. Cancer Immunol Immunother.
61:1019–1031. 2012. View Article : Google Scholar
|
|
100
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumour
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C,
Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of
PTEN promotes resistance to T cell-mediated immunotherapy. Cancer
Discov. 6:202–216. 2016. View Article : Google Scholar :
|
|
102
|
Zaretsky JM, Garcia-Diaz A, Shin DS,
Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY,
Abril-Rodriguez G, Sandoval S, Barthly L, et al: Mutations
associated with acquired resistance to PD 1 blockade in melanoma. N
Engl J Med. 375:819–829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Shin DS, Zaretsky JM, Escuin-Ordinas H,
Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W,
Sandoval S, Torrejon DY, et al: Primary resistance to PD 1 blockade
mediated by JAK1/2 mutations. Cancer Discov. 7:188–201. 2017.
View Article : Google Scholar
|
|
104
|
Liu D, Schilling B, Liu D, Sucker A,
Livingstone E, Jerby-Arnon L, Zimmer L, Gutzmer R, Satzger I,
Loquai C, et al: Integrative molecular and clinical modeling of
clinical outcomes to PD1 blockade in patients with metastatic
melanoma. Nat Med. 25:1916–1927. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tuaeva NO, Falzone L, Porozov YB, Nosyrev
AE, Trukhan VM, Kovatsi L, Spandidos DA, Drakoulis N, Kalogeraki A,
Mamoulakis C, et al: Translational application of circulating DNA
in oncology: review of the last decades achievements. Cells.
8:12512019. View Article : Google Scholar :
|
|
106
|
Lee EY and Kulkarni RP: Circulating
biomarkers predictive of tumor response to cancer immunotherapy.
Expert Rev Mol Diagn. 19:895–904. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Napoli S, Scuderi C, Gattuso G, Bella VD,
Candido S, Basile MS, Libra M and Falzone L: Functional roles of
matrix metallopro-teinases and their inhibitors in melanoma. Cells.
9:E11512020. View Article : Google Scholar
|
|
108
|
Falzone L, Salemi R, Travali S, Scalisi A,
McCubrey JA, Candido S and Libra M: MMP-9 overexpression is
associated with intragenic hypermethylation of MMP9 gene in
melanoma. Aging (Albany NY). 8:933–944. 2016. View Article : Google Scholar
|
|
109
|
Moogk D, da Silva IP, Ma MW, Friedman EB,
de Miera EV, Darvishian F, Scanlon P, Perez-Garcia A, Pavlick AC,
Bhardwaj N, et al: Melanoma expression of matrix
metallopro-teinase-23 is associated with blunted tumor immunity and
poor responses to immunotherapy. J Transl Med. 12:3422014.
View Article : Google Scholar
|