1. Introduction
At present, cancer remains a serious threat to human
life and poses a significant burden to healthcare systems, and is
gradually becoming the leading cause of disease-related mortality.
A previous study assessing the global burden of 29 types of cancer
in 195 countries from 1990-2017 demonstrated that, when combining
all types of cancer in most countries, the average annual age
standardized incidence rates notably increased with time (1); 24.5 million cancer new cases and 9.6
million cancer-related deaths were reported globally in 2017, and
the most common types of cancer were non-melanoma skin cancer, lung
cancer, breast cancer, colon cancer, prostate cancer, stomach
cancer, liver cancer, cervical cancer, non-Hodgkin's lymphoma and
bladder cancer (1). The
development of drug resistance in cancer treatment is a major
obstacle. While the initial treatment is successful, acquired or
secondary resistance occurs during repetitive cycles of therapy,
subsequently resulting in tumor progression in several patients.
Additionally, tumor cells which develop resistance to a single
chemotherapeutic drug will often exhibit resistance to other
chemotherapeutic drugs, despite the different characteristics and
targets of the second-line therapeutic (2). This phenomenon is termed multi-drug
resistance (MDR), and is a significant contributor to the decreased
success rates of combination therapeutic regimens.
Drug resistance results in a reduction of the
therapeutic efficacy of the administered drugs, weakening the
inhibitory effects of chemotherapy on the growth and survival of
cancer cells, resulting in the failure of chemotherapy and
potentially increasing the risk of cancer-related mortality. The
emergence of MDR in advanced- and terminal-stage cancer is
typically associated with a poor prognosis and reduced survival
rates of patients (3). Thus, it is
necessary to elucidate the underlying molecular mechanisms of
resistance and to develop novel therapeutic approaches to counter
these. MDR in cancer cells during chemotherapy may be associated
with a diverse range of molecular mechanisms, including enhanced
drug efflux (4), decreased drug
uptake by influx transporters such as solute carriers (5), alterations in drug metabolism
(6), the inactivation of death
signaling pathways (7), the
mutation of drug targets (8),
genetic factors and changes in the tumor microenvironment (9,10).
Cholesterol is a significant component of plasma
membranes and a precursor of steroid hormones and bile acids.
Cholesterol is considered to play a vital role in human life
(11). Cholesterol has been shown
to accumulate in malignant tissues, and this is now considered a
common characteristic of cancer cells (12). Recently, mechanistic investigations
have demonstrated that cholesterol pathways are involved in the
development of MDR in cancer cells (13,14).
Furthermore, epidemiological studies have suggested that
combination chemotherapy (including targeted therapy) and
cholesterol-lowing drugs improve survival times and lower cancer
mortality rates (15,16). The present review focuses on the
roles of cholesterol as signaling molecules in the regulation of
cancer drug resistance and the therapeutic potential of lowering
cholesterol levels in cancer treatment.
2. Cholesterol metabolism
Cholesterol synthesis
Cholesterol plays a crucial role in life, performing
numerous roles. including serving as a structural component of
membranes to function as a precursor for several compounds, such as
vitamin D, bile acids or steroid hormones. Cholesterol synthesis
occurs through the mevalonate pathway, in which the progress of
synthesis is rate-limited by various enzymes and transcription
factors (17).
The first step in cholesterol synthesis is the
formation of mevalonate from acetate (18). Acetyl-CoA is produced from the
glycolysis of glucose in the mitochondria, and the remaining steps
of cholesterol synthesis occur in the endoplasmic reticulum (ER)
and cytoplasm (19). Acetyl-CoA
condenses with acetoacetyl-CoA to form
hydroxyl-methyl-glutaryl-coenzyme A (HMG-CoA) which is catalyzed by
HMG-CoA reductase (HMGCR/HMGR). HMGCR is not only the rate-limiting
enzyme for the reduction of HMG-CoA to mevalonate, but is also a
committal step for the entire process. Mevalonate acts as a
precursor of numerous products, such as geranyl-pyro-phosphate and
farnesylpyrophospate, both of which can induce the isoprenylation
of the intracellular G proteins Ras and Rho, which in-turn
modulates cell proliferation and apoptosis (20). The following step is the conversion
of mevalonate into squalene; mevalonate kinase forms
meva-onate-5-phosphate from mevalonate (21). Following a series of successive
condensation reactions of activated isoprenes, squalene is formed
in the presence of squalene synthase. To form cholesterol, squalene
has to initially undergo reactions in which squalene monooxygenase
(SQLE) and lanosterol synthase catalyze the transformed squalene
into cholesterol following several successive reactions (22).
In order to maintain the balance of cholesterol
homeostasis in the plasma and intracellular membrane, with the
involvement of enzyme, acyl-CoA acyl-transferase (ACAT) in the ER,
free cholesterol is converted into cholesterol esters, which are
stored as cytosolic lipid droplets when their levels increase above
the threshold, and is used as cholesterol ester hydrolase when
required (23). The disposal of
cholesterol through 7α-hydroxylated bile acids exerts a significant
effect on cholesterol homeostasis. 7α-hydroxylation is catalyzed by
the enzyme, cytochrome P450 7A1 (CYP7A1), which also promotes the
excretion of fecal sterols (24).
Statins are competitive inhibitors of HMGCR, the
rate-limiting enzyme of the mevalonate pathway required for the
biosynthesis of cholesterol (25).
Recently, several preclinical studies discovered that the
cholesterol levels in drug-resistant cell lines were considerably
higher than those in drug-sensitive cell lines (26-28).
Additionally, an additive effect of statins in terms of the
inhibition of cell proliferation was observed; the related statins
are presented in Table I. Multiple
mechanisms connected with cholesterol have been identified, which
lead to drug resistance in different types of cancer. Serum
cholesterol levels, as well as intracellular cholesterol levels
appear to be crucial in cancer drug resistance.
 | Table ICombination of chemotherapeutic drugs
and statins in different cancer entities. |
Table I
Combination of chemotherapeutic drugs
and statins in different cancer entities.
| First author,
year | Chemotherapeutic
drug | Statin | In
vitro | In vivo | (Refs.) |
|---|
Yun et al
2019
Greife et al 2015 | Doxorubicin | Simvastatin | Human epidermoid
carcinoma cell line, A431; Human bladder cancer cell line,
BFTC-905-DOXO-II | BLAB-C nu/nu mice
with A431 | (29,30) |
| Kong et al
2018 | Enzalutamide | Simvastatin | Human prostate
cancer cell lines, MR49F/C4-2R/22RV1 | Nude mice with
22RV1 | (31) |
| Kim et al
2019 | Paclitaxel | Simvastatin | Human cell line,
A549T | BLAB/C nude mice
with A549/T | (32) |
| Gupta et al
2018 | Paclitaxel | Lovastatin | | Athymic nude mice
with human pancreatic cancer cell line, SU.86.86 | (33) |
| Glodkowska-Mrowka
et al 2014 | Imatinib | Lovastatin | Human chronic
myeloid leukemia cell line, K562 | | (34) |
| Chen et al
2015 | Cetuximab | Nystatin | Human epidermoid
carcinoma cell line, A431; human pulmonary adenocarcinoma cell
line, A549; human colon carcinoma cell line, HCT-116 | BLAB/C nude mice
with A431/A549 | (35) |
| Chen et al
2016 | Docetaxel | Atorvastatin | Human prostate
carcinoma cell lines, PC-3/LNCaP | | (36) |
| Chen et al
2018 | Gefitinib | Lovastatin | Human non-small
cell lung cancer cell lines, H1975/PC-9-GR | BLAB/C nude mice
with H1975 | (28) |
Cholesterol influx
To sustain whole-body cholesterol levels within a
physiological range, lipoproteins mediate the management and
delivery of dietary cholesterol to cells. In addition to de
novo synthesis, cells capture cholesterol through the uptake of
circulating plasma lipoproteins via low-density lipoprotein
receptor (LDL-R) and scavenger receptor class B type I (SR-BI).
LDL-R, which is present on the plasma membrane of
the majority of cells, is the major endocytic route for uptake of
exogenous cholesterol. LDL or other apolipoprotein E/apolipoprotein
B-containing lipoproteins bind to the LDL-R, and the complex is
endocytosed by clathrincoated vesicles. LDL becomes disassociated
in the early endosome, and the LDL-R is recycled back to plasma
membrane. As the early endosome progresses into a late
endosome/lysosome, the non-recycled cholesterol is delivered to
other organs, such as the ER, mitochondria and plasma membrane
(37,38). SR-BI has been shown to be of utmost
importance in mediating the uptake of cholesterol from high-density
lipoprotein (HDL). HDL binds to an HDL-receptor with a high
affinity (39). SR-BI is expressed
primarily in the liver and non-placental steroidogenic tissues and
mediates selective cholesterol uptake by a mechanism distinct from
the classical LDL receptor pathway (40). Although SR-BI binds to a variety of
ligands, including HDL, LDL, very low-density lipoproteins and
modified lipoproteins, the most important property of SR-BI is
considered its ability to function as an HDL receptor.
Niemann pick C1-like 1 (NPC1L1), a protein which is
localized at the brush border membrane of enterocytes, is
responsible for absorption of free cholesterol into cells. In
humans, it is also expressed in the liver, where it transports
newly secreted biliary cholesterol back into the hepatocytes, and
prevents the loss of endogenous cholesterol (41).
Cholesterol efflux
To prevent cholesterol retention, excess cellular
cholesterol from cells is removed. As cells cannot degrade
cholesterol, reverse cholesterol transport (RCT), a mechanism
through which redundant cholesterol is transported from peripheral
tissues to the liver for reuse or excretion, is necessary for
cholesterol homeostasis (42). It
has been shown that ABC transporters, one of the largest families
of integral plasma membrane proteins, are involved in the
cholesterol transport across the cell membrane. The dysfunction of
ATP-binding cassette (ABC) transporters may contribute to various
cholesterol-related diseases. For example, mutations in ABC,
subfamily A, member 1 (ABCA1) result in familial HDL deficiency
(43).
ABCA1 promotes cholesterol efflux to extracellular
acceptors, such as apolipoprotein A1, resulting in the formation of
HDL particles; the first step in RCT (44). Synergistic mediation of ABCA1 and
ABC, subfamily G, member 1 (ABCG1) for cholesterol efflux to HDL
has been shown (45).
Additionally, studies have demonstrated that the combined actions
of ABCA1 and ABCG1 contributes to the maturation of HDL through the
addition of cellular lipids to the nascent particles in macrophages
(46). Two other ABC transporters,
ABCG5 and ABCG8, which are both expressed on the brush border
membrane of enterocytes and the canalicular membrane of
hepatocytes, transport cholesterol from enterocytes into the gut
lumen for fecal disposal (47).
Of note, SR-BI can mediate the bi-directional
exchange of free cholesterol between cells and HDL (48). For example, J774 macrophages stably
overexpressing SR-BI have been shown to export more cholesterol to
HDL than the controls, indicating the potential role of SR-BI in
cholesterol efflux (49).
Transcriptional regulation of cholesterol
synthesis-related genes
In mammary tissues, the homeostatic control of
cholesterol is reflected in the balance of biosynthesis, and the
influx and efflux of cholesterol. The balance is primarily
regulated by the cooperation of two transcription factor families:
The sterol regulatory element-binding proteins (SREBPs) and the
liver X receptors (LXRs) (50,51).
SREBP family members are synthesized as membrane
proteins in the ER, and SREBP2 primarily regulates the
transcription of genes involved in cholesterol biosynthesis, and
its activity is controlled via a negative feedback loop (52). Under sterol-rich conditions, SREBP
is sequestered by the SREBP-cleavage-activating protein (SCAP) in
the ER, in which SCAP binds to the insulin-induced gene (INSIG) and
this interaction traps SREBP in the ER membrane. However, when
cholesterol levels decrease below homeostatic levels, SCAP
dissociates from INSIG and escorts SREBP to the Golgi where the
complex is cleaved, subsequently leading to the translocation of
the mature transcription factor to the nucleus and transcription of
SREBP-targeted genes, such as HMGCR and LDL-R (Fig. 1) (53,54).
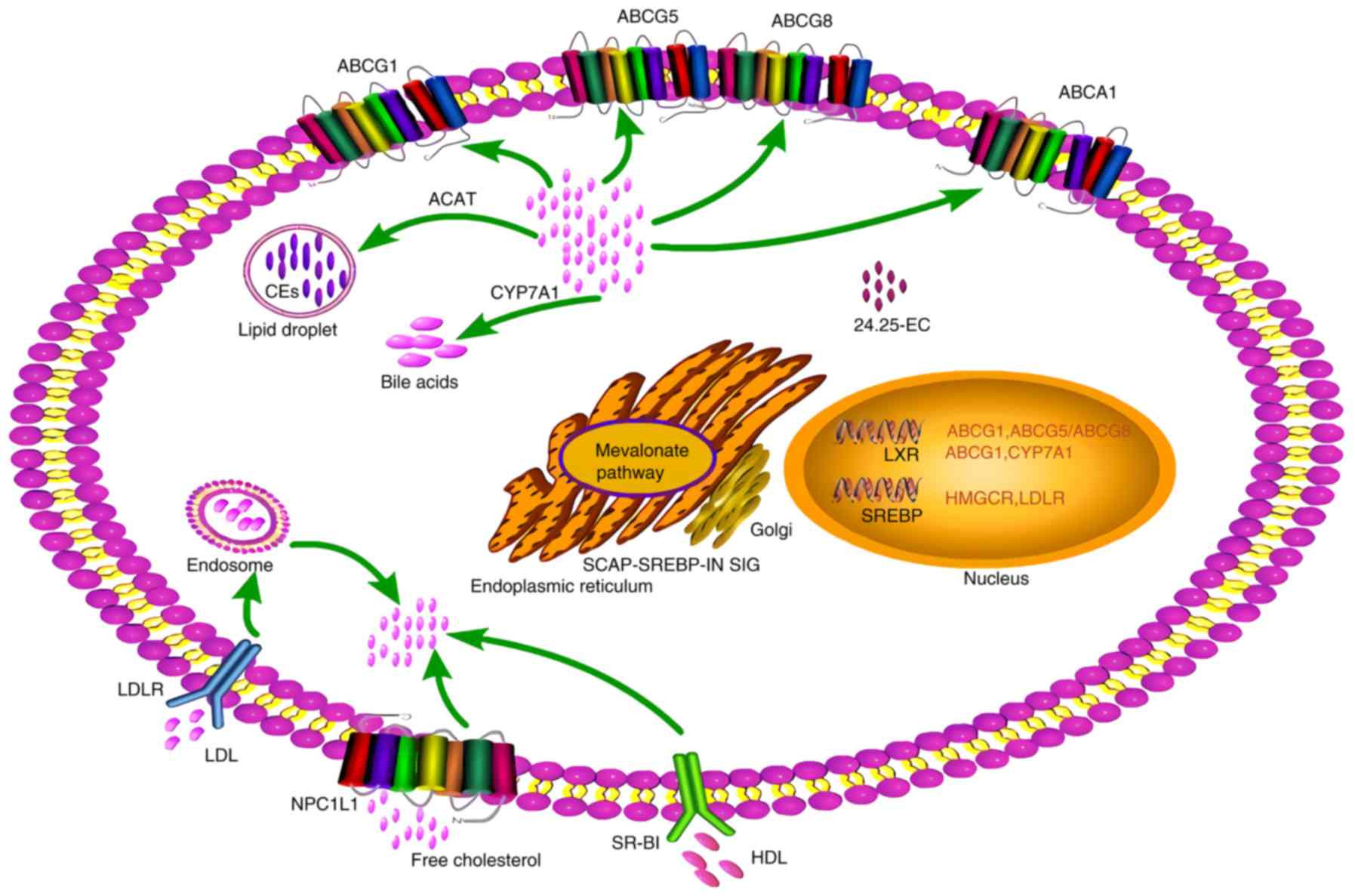 | Figure 1Regulation of cholesterol metabolism
through modulation of intracellular sterol levels. Under conditions
of high sterol levels, endogenous ligands activate LXRs, thus
upregulating the expression of transporters such as ABCG1, ABCG5/G8
and ABCA1 to mediate cholesterol efflux. Additionally, LXRs target
CYP7A1 to transform cholesterol into bile acids. Excess sterols are
converted into esters and stored within lipid droplets. Conversely,
under conditions of low sterol levels, the SCAP-SREBP complex
migrates to the Golgi and is cleaved, releasing SREBP, resulting in
the migration of the mature transcription factor to the nucleus and
the transcription of SREBP-targeted genes, such as members of the
HMGCR activating mevalonate pathway and LDL-R enhancing exogenous
cholesterol capture. ABC, ATP binding cassette; LXR, liver X
receptor; SREBP, sterol regulatory element binding protein; SCAP,
SREBP-cleavage-activating protein; HMGCR,
hydroxyl-methyl-glutaryl-coenzyme A reductase; LDL-R, low density
lipoprotein-receptor. |
In addition to SREBPs, LXRs, which are members of
the nuclear receptor superfamily, also participate in cholesterol
metabolism. LXRs are activated by endogenous ligands, including
oxysterols, desmosterol, and 24S,25-epoxycholesterol. Once
activated, LXRs upregulate the transcriptional levels of genes
involved in cholesterol transport, including ABCA1, ABCG1, ABCG5
and ABCG8 (Fig. 1) (50). When intracellular cholesterol
levels exceed physiological limits, LXRs facilitate the
transcription of the aforementioned target genes and thus RCT,
thereby functioning to maintain homeostatic levels (55). Furthermore, CYP7A1, which catalyzes
the formation of bile acids, is a target gene of LXRs (Fig. 1). LXR activation can downregulate
NPC1L1 expression in human enterocytes, accompanied by the
reduction of cholesterol absorption (56).
3. Role of cholesterol in drug resistance in
cancer
The association between cholesterol homeostasis and
drug resistance has been the subject of numerous studies. Recently,
using data obtained from The Cancer Genome Atlas, it was
demonstrated that there was an association between cholesterol
synthesis and a decreased patient survival, as well as progression
in patients with cancer (57,58).
Considering the role of cholesterol in cancer development and the
adverse outcomes of MDR in cancer patients, it is necessary to
examine the role of cholesterol in cancer drug resistance (14).
Preclinical studies have also demonstrated the role
of cholesterol in drug resistance in several types of cancer,
including prostate, pancreatic, bladder and breast cancer, amongst
others (30,31,59-61).
In breast cancer, aromatase inhibitor-resistant
cells exhibit activated endogenous cholesterol biosynthesis,
resulting in the constitutive activation of estrogen receptor-α;
estrogen receptor-α binding can be reduced using statins, which
in-turn reduces cell invasion. Furthermore, patients with high
levels of cholesterol biosynthesis are less likely to benefit from
treatment with aromatase inhibitors (62). Another example in breast cancer
includes the association between tamoxifen resistance and
cholesterol. As previously demonstrated, in tamoxifen-resistant
cells, the expression of peroxisome proliferator-activated
receptor-γ, which regulates several lipid droplet proteins, is
altered, and the expression of ABCA1, which functions a cholesterol
efflux pump, is downregulated. Notably, a substantial increase was
also observed in neutral lipids (cholesterol esters and
triglycerides), as well as an accumulation of free cholesterol in
the resistant cells (60). Similar
results have been reported in non-small cell lung cancer cells,
where cholesterol levels in gefitinib-resistant cells were notably
higher than in the gefitinib-sensitive cells (28). Notably, it has been reported that
radioresistance in pancreatic cancer cells is associated with the
expression of ACAT-2, fatty acid synthesis (FASN) and SQLE at the
mRNA level, all of which are involved in cholesterol homeostasis,
suggesting that cholesterol may also be associated with
radioresistance (63). The
expression of HMGCR, a crucial enzyme in the mevalonate pathway of
cholesterol synthesis, is increased in enzalutamide-resistant
prostate cancer cell lines, and HMGCR knockdown or HMGCR inhibition
has been shown to result in the re-sensitization of resistant cells
to enzalutamide, whereas HMGCR overexpression confers resistance to
the drug (31). The induction of
the mevalonate pathway has also been reported as a mechanism of
doxorubicin resistance in bladder cancer, and co-treatment with
simvastatin restores the sensitivity of resistant cells to
doxorubicin (30). Doxorubicin
downregulates HMGCR protein levels, resulting in decreased levels
of cholesterol, which is associated with the inactivation of the
epidermal growth factor receptor (EGFR)-src pathway (29). Furthermore, HMGCR inhibition or
knockdown enhances doxorubicin toxicity (29), and these results are in accordance
with those of aforementioned studies (30,31).
In addition to cholesterol, the increased accumulation of
cholesterol esters has also been observed in drug-resistant
pancreatic ductal adenocarcinoma and chronic myelogenous leukemia,
and cholesterol esterification inhibition enhances the sensitivity
to gemcitabine and imatinib (59,64).
Elevated cholesterol levels in the mitochondria have
been shown to confer resistance to apoptotic signals, thus
contributing to chemotherapeutic resistance in several types of
cancer (Fig. 2) (26,65,66).
The mitochondrial cholesterol content in cases of hepatoma has been
shown to be notably increased compared with that of normal tissues
(67). Furthermore, the
mitochondria of hepatocellular carcinoma cells, which are resistant
to mitochondrial membrane permeabilization and other various
stimuli, exhibit increased cholesterol levels. The sensitivity of
cells to chemotherapy increases upon cholesterol depletion through
the inhibition of HMGCR or SQLE. In a previous study, when
steroidogenic acute regulatory protein, a mitochondrial
cholesterol-transporting polypeptide that is upregulated in
hepatocellular carcinoma cells, was knocked down by small
interfering RNA, the cells exhibited increased sensitivity to
chemotherapy (26). Another
previous study using animal experiments also confirmed that
cholesterol overload in the liver contributed to mitochondrial
changes, which ultimately conferred resistance to cell death
(65).
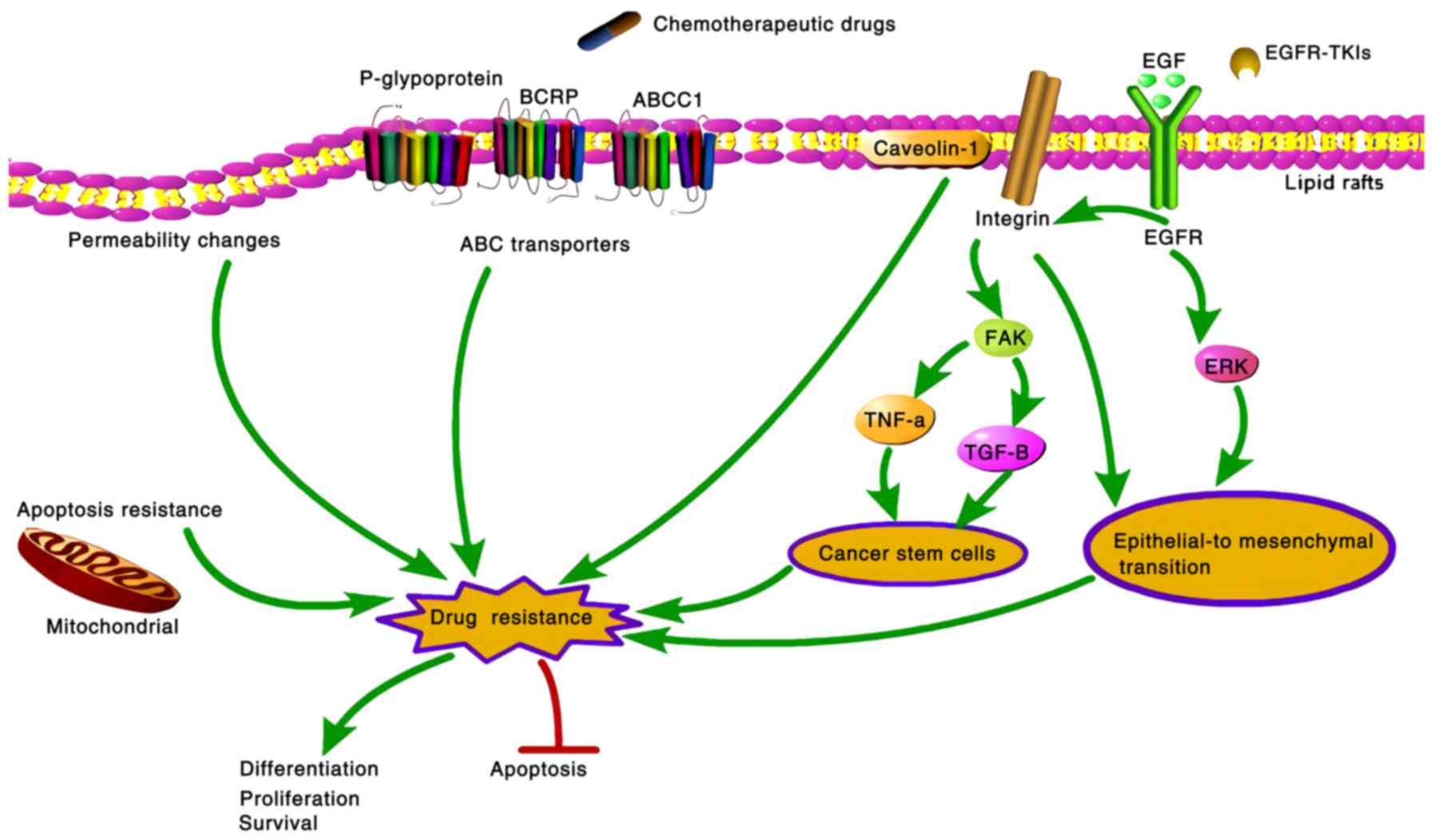 | Figure 2Mechanisms through which cholesterol
regulates drug resistance in cancer cells. Cholesterol regulates
drug resistance in cancer cells via different mechanisms. Elevated
mitochondrial cholesterol levels can induce resistance to apoptotic
signals. Moreover, cholesterol levels in lipid rafts regulate
related signal transduction pathways, such as the EGFR signaling
pathway and caveolin-1, leading to epithelial-mesenchymal
transition or acquisition of cancer-stem cell like properties. As
the cholesterol composition of the cellular membrane is altered,
the function of ABC transporters (p-glycoprotein, BCRP and ABCC1)
is changed accordingly. In addition, the change in cholesterol
content in cell membranes may affect the permeability to
therapeutic drugs and the uptake of agents. Eventually, drug
resistance in cancer cells influences survival, proliferation,
differentiation and apoptosis. ABC, ATP binding cassette; EGFR,
epidermal growth factor receptor. |
However, another previous in vitro study
demonstrated an opposite effect of cholesterol on drug resistance
(68). Combined treatment with
cholesterol and temozolomide reversed temozolomide resistance,
whereas clinical anti-hypercholesterolemia agents (lovastatin or
simvastatin) suppressed temozolomide-induced cell death. These
conflicting results suggest drug resistance mediated by elevated
cholesterol levels may be restricted to certain cancer types.
4. Mechanisms through which cholesterol
regulates drug resistance
Lipid rafts
In addition to serving as a precursor for steroid
hormones and a key component of the plasma membrane, cholesterol
also plays an essential role in intracellular signal transduction
(69). The regulation of signal
transduction path-ways in cancer resistance by cholesterol, to a
certain degree, is partly due to membrane microdomains termed lipid
rafts, which are characterized by the enrichment of cholesterol and
sphingolipids, resulting in a lipid phase that is more ordered than
the surrounding membrane (70). A
wide number of transduction signals related to cell survival and
proliferation, have been reported to be connected with lipid rafts,
such as receptor tyrosine kinases, platelet-derived growth factor
receptor and EGFR. Of note, cancer cells possess higher numbers of
lipid rafts than their normal counterparts, and the cholesterol
levels in lipid rafts of drug-resistant cancer cells are higher
than drug-sensitive cancer cells (28,71).
Thus far, there are numerous studies available on cancer resistance
is mediated through lipid rafts, within which signaling molecules
associated with cell proliferation, apoptosis, invasion and
migration, are impeded by cholesterol changes, as described
below.
In non-small cell lung cancer, the sensitivity of
gefitinib, an EGFR-tyrosine kinase inhibitor, has been shown to be
affected by cholesterol levels in lipid rafts. Following the
depletion of cholesterol in lipid rafts, gefitinib-resistant cell
lines exhibit a high affinity for gefitinib and EGFR, leading to an
enhanced sensitivity to gefitinib, as well as to the decreased
phosphorylation of AKT, MEK1/2 and ERK1/2 (28). The attenuation of EGFR signaling is
attributed to multiple mechanisms, one of which is endocytosis, a
process through which receptors are removed from the cell surface
and delivered to sites of inactivation, such as through ER-based
phosphatases (72).
Clathrin-independent endocytosis is activated by high levels of
EGF, but is also dependent on the cholesterol levels in the
membrane (73). Thus, sensitivity
to gefitinib may be mediated by cholesterol via endocytosis.
Similarly, a study in multiple human carcinoma cell lines
highlighted the presence of a mechanism whereby the uptake of an
EGFR-targeting monoclonal antibody was improved through cholesterol
sequestration, which also enhanced drug internalization by
regulating EGFR trafficking/turnover and facilitating a switch from
lipid rafts to clathrin-mediated endocytosis (35). ERK1/2 pathway activation mediated
by EGFR, and adipocyte plasma membrane-associated protein (APMAP)
accumulation are involved in the cholesterol-mediated induction of
epithelial-mesenchymal transition (EMT) (Fig. 2). Mechanistically, APMAP increases
the interaction between EGFR substrate15-related protein,
inhibiting the endocytosis of EGFR by cholesterol, thus promoting
EMT (74). Another example of the
role of cholesterol in lipid rafts influencing EMT-associated drug
resistance concerns cell adhesion proteins. Lipid raft disruption
by simvastatin suppresses integrin-β3 and focal adhesion formation,
thus inhibiting the FAK signaling pathway and re-sensitizing
drug-resistant cancer cells to paclitaxel, and repolarizes
tumor-associated macrophages, increasing TNF-α and attenuating
TGF-β, which ultimately results in the suppression of EMT (Fig. 2) (32). Of note, integrin signaling is also
required for the maintenance of properties of cancer stem cells
(CSCs) (Fig. 2) (75). CSCs are a subset of cells within
the tumor which possess self-renewal, differentiation and
tumorigenic capacity, often underlying the failure of cancer
therapy and eventually resulting in tumor recurrence and eventually
metastasis, due to their considerable chemoresistant properties
(76). Taken together. these data
indicate that cholesterol-rich lipid rafts may play a critical role
in cancer drug resistance.
Caveolae, a specific subclass of lipid rafts, can be
readily identified by the presence of the cholesterol binding
protein, caveolin-1 (77). In
pancreatic cancer cells, high caveolin-1 levels promote resistance
to chemotherapeutic agents, such as gemcitabine and 5-fluorouracil
(78). Caveolin-1 expression is
upregulated in resistant colorectal cancer cells (79). In aggressive and metastatic
prostate cancer, a high caveolin-1 expression increases acetyl-CoA
carboxylase-1 and FASN expression in an androgen
receptor-independent manner, suggesting that caveolin-1 promotes
hormone resistance through the regulation of lipid synthesis
(80). Cholesterol depletion using
methyl-β-cyclodextrin potentiates the tamoxifen-induced anticancer
effects, and this sensitization is associated with the
downregulation of caveolin-1 (Fig.
2). Thus, cholesterol and caveolin-1 may interact and influence
each other (81).
ABC transporters
The importance of ABC transporters in the
development of drug resistance in several types of cancer has been
the subject of numerous studies spanning several decades. In view
of their localization in the membrane, it has been hypothesized
that the membrane environment of the transporters is crucial for
their function. Several studies have demonstrated that when the
cholesterol composition of the cellular membrane is altered, the
function of these ABC transporters is altered accordingly.
In human peripheral blood mononuclear cells,
elevated cellular cholesterol levels significantly increase
p-glycoprotein activity (82). In
addition, p-glycoprotein that has been reconstituted in
cholesterol-containing liposomes, exhibit increased p-glycoprotein
ATPase activity (83). In
agreement with these findings, the removal of cholesterol, which
modulates the membrane lipid composition, alters the localization
of p-glycoprotein and results in the loss of p-glycoprotein
function (Fig. 2) (84). Furthermore, in human CEM acute
lymphoblastic leukemia cells, which exhibit varying degrees of
chemoresistance, the amount of cholesterol ester increases linearly
with the level of resistance to vinblastine, whereas the amounts of
total and free cholesterol increase in a non-linear manner.
Moreover, membrane cholesterol controls both ATPase activity and
the drug efflux activity of p-glycoprotein. CEM cells that express
increasing levels of elevated chemoresistance have been shown to
increase the amount of p-glycoprotein to a peak of 40% of total
membrane proteins and this remains unvaried (85). In terms of increasing the quantity
of cholesterol in the membrane and its association with MDR, it has
been strongly suggested that cholesterol may directly underlie the
acquisition of a typical MDR phenotype. The mechanisms through
which the presence of cholesterol enhances the activity of
p-glyco-protein have also been the subject of numerous studies.
Both the ability of drug binding to p-glycoprotein and drug
trans-port are affected by cholesterol by altering the partitioning
of hydrophobic drug substrates into the membrane and altering the
local lipid environments of p-glycoprotein (86). A recent study found that
p-glycoprotein substrates may preferentially accumulate in
cholesterol-rich regions of the membrane; thus, the transport
activity of p-glycoprotein is increased in the presence of
cholesterol (87). In agreement
with this finding, in colon cancer cells, following the reduction
of cholesterol synthesis and incorporation in detergent resistant
membranes, the amount of p-glycoprotein, as well as transport
activity, are decreased. The inhibition of cholesterol synthesis by
simvastatin has been found to facilitate the degradation of
β-catenin and decrease p-glycoprotein expression, subsequently
contributing to the sensitivity to drugs in canine mammary CSCs
(88). Of note, β-catenin is
usually activated in CSCs and leads to the upregulation of
p-glycoprotein (89).
Several studies have established an association
between the levels of cholesterol and the activity of BCRP. A
previous study found that the ATPase activity of human BCRP
transfected sf9 cell membranes differed from that of
BCRP-overexpressing human cell membranes, and that the lipid
compositions of the two cell lines differed from each other
(90). Of note, in both cell
lines, cholesterol loading prominently improved drug transport into
inside-out membrane vesicles, indicating the vital role of membrane
cholesterol in the function of BCRP. A similar study later
suggested that the cholesterol enrichment of cell membrane vesicles
increases BCRP-driven substrate uptake, substrate-stimulated ATPase
activity, as well as the formation of a catalytic cycle
intermediate, also highlighting the importance of membrane
cholesterol in BCRP transport activity (Fig. 2) (91). Analogous results have been found in
human erythrocyte membranes in which lucifer yellow (a fluorescent
BCRP substrate) uptake decreased when membrane cholesterol content
was increased following treatment with cholesterol hemisuccinate
(92). Taken together, it is
reasonable to ascribe chemoresistance to cholesterol-induced BCRP
expression.
ABC subfamily C, member 1 (ABCC1) is another ABC
transporter whose functionality appears to be regulated by
cholesterol. It was suggested that ABCC1 functionality was
associated with its localization in cholesterol-rich membrane
microdomains, and depletion of membrane cholesterol below 40%
caused a partial shift of ABCC1 to the high-density fraction, and
decreased functionality (Fig. 2)
(93). In this regard, there is
some resemblance between BCRP and ABCC1, as their localization in
the cell membrane and functionality is regulated by
cholesterol.
Drug uptake is modulated by
cholesterol
There have been some studies which have demonstrated
the role of cholesterol in modulating the uptake of
chemotherapeutic drugs. Compared with the parental cell line,
vincristine-resistant cells exhibit lower rates of drug delivery in
murine leukemic lymphoblasts. Furthermore, the cholesterol content
in resistant cells is directly proportional to the relative
resistance to vincristine; cholesterol depletion results in an
increase in the rate of drug uptake, which is reversed by
cholesterol reloading (94).
Similar results have been found in breast cancer cells, where
decreased cholesterol levels have been shown to result in the
increased uptake of doxorubicin (27). A possible explanation for these
findings is that the change in the cholesterol content in cancer
cell membranes significantly affects their permeability to
therapeutic drugs (Fig. 2)
(95). The cholesterol-modulated
uptake of chemotherapeutic agents is considered one of the
mechanisms underlying the initiation of drug resistance.
5. Clinical relevance of cholesterol in
cancer
Cholesterol levels in patients with
drug-resistant cancer
The accumulation of cholesterol is a well-known
feature observed in several types of cancer (96). The mechanisms through which
cholesterol influences carcinogenesis have been extensively
investigated for decades. Clinical studies have also suggested an
association between cancer drug resistance and cholesterol levels
in certain types of cancer. In lung cancer, compared with patients
who exhibit the delayed acquisition of resistance, patients who
acquire chemoresistance exhibit elevated serum levels of
cholesterol at a relatively rapid rate (14). In breast cancer, cholesterol
biosynthesis-related genes are progressively upregulated as the
cancer progresses, and patients with high levels of cholesterol
biosynthesis are unlikely to benefit from aromatase inhibitor
treatment (62). Similarly, the
increased expression of enzymes in the cholesterol biosynthesis
pathway has been shown to be significantly associated with a poor
response to endocrine therapy (97), and a high expression of cholesterol
biosynthesis genes is an independent prognostic factor of shorter
recurrence-free and overall survival (98). For patients with ovarian cancer,
elevated cholesterol levels in ascites have been shown to be
associated with chemoresistance, as well as shorter recurrence-free
survival times (99). However,
additional studies are required to fully determine the effects of
cholesterol on cancer development and to elucidate the mechanisms
through it modulates cancer drug resistance.
Prognostic significance of cholesterol in
cancer
The prognostic significance of serum total
cholesterol (TC), triglycerides (TG), HDL-C and LDL-C in cancer has
been extensively studied. Recently, a systematic review and
meta-analysis covering 26 studies, including 24,655 individuals,
found that only the TC and HDL-C levels were significantly
associated with cancer mortality (100). Similarly, the existing literature
are more supportive of the role of TC and HDL-C as prognostic
predictors than TG and LDL-C. Herein, the prognostic value of TC
and HDL-C in different types of cancer are discussed (101-103).
A retrospective study comprising 184 patients with
gastric cancer undergoing gastrectomy found that the patients in
the low-HDL-C group had a significantly higher rate of gastric
cancer mortality, as well as increased lymphatic and vascular
invasion compared with the normal-HDL-C group (104). Moreover, a multivariate analysis
of the factors influencing gastric cancer mortality rates revealed
that the HDL-C value was an independent prognostic factor. In soft
tissue sarcoma, both univariate and multivariate analysis revealed
that decreased HDL-C levels were significantly associated with the
decreased overall survival and decreased disease-free survival of
patients with extensive and radical surgical resection, suggesting
the potential prognostic utility of plasma HDL-C levels as an
independent factor (105).
Similarly, in breast cancer, the prognostic significance of HDL-C
has been shown. A case-controlled study, including 1,081 patients
demonstrated that HDL-C levels in the patient group were notably
lower than those in the control group (106). For patients with triple-negative
breast cancer, a higher proportion of HDL-C to TC represents a
lower overall risk of mortality. However, no associations have been
observed between HDL-C and prognostic outcome amongst all the
breast cancer cases (including luminal A, luminal B, HER2-enriched
and triple-negative breast cancer), suggesting that the impact of
pre-diagnostic HDL-C on breast cancer recurrence and survival may
be limited by cancer subtype (102). In agreement with these studies, a
recent meta-analysis demonstrated that disease-free survival and
overall survival were increased in patients with high HDL-C levels
compared with patients with low HDL-C levels. Across the included
types of cancer (breast cancer, lung cancer, hepatocellular
carcinoma and renal cell carcinoma), the most prominent prognostic
effect of HDL-C was observed in lung cancer (100). The HDL-C level has emerged as a
valuable prognostic factor in different types of cancer, and the
clinical value of HDL-C levels requires further verification.
The prognostic role of TC also has been established
by numerous independent studies. The negative association of serum
cholesterol levels with cancer mortality was previously
investigated in a prospective study as early as 1980 (107). Similar results were later found
in the multiple risk factor intervention trial (108). In a study on gastric cancer, the
serum cholesterol levels were significantly lower in the cancer
patients compared with the healthy subjects. Additionally,
decreased levels of cholesterol were accompanied by tumor
progression, suggesting that serum cholesterol levels may be used
as a marker of cancer progression (109). In clear cell renal cell
carcinoma, lower pre-operative serum TC levels were associated with
a lower recurrence-free survival and cancer-specific survival
rates. Furthermore, multivariate analysis indicated that serum TC
levels were an independent predictor of clear cell renal cell
carcinoma (110). A study on 198
patients with non-small cell lung cancer revealed the prognostic
role of pre-operative TC levels in both univariate and multivariate
analysis. Lower serum TC levels were shown to be associated with
shorter overall survival times (111). Conversely, a positive association
between high levels of TC and overall mortality has been observed
in breast cancer patients (103).
Given the contrasting results based on the type of cancer studied,
the prognostic role of TC requires further investigations; however,
it appears to be cancer type-specific.
6. Strategies targeting cholesterol
metabolism to overcome cancer resistance
The characteristics of cholesterol, as well as its
involvement in several mechanisms associated with drug resistance
in various types of cancer are discussed below. Targeting
cholesterol homeostasis pathways is a potential target for
modulating drug resistance. Examples of drugs targeting cholesterol
homeostasis pathways are summarized in Table II.
 | Table IIAgents targeting cholesterol in
different types of cancer. |
Table II
Agents targeting cholesterol in
different types of cancer.
| First author,
year | Cholesterol-related
targets | Agent | Cancer | (Refs.) |
|---|
| Glodkowska-Mrowka
et al 2014 | Synthesis | HMGCR | Statins | Chronic myeloid
leukemia | (34) |
| Gelsomino et
al 2013 | | | DHA | Colon cancer | (88) |
| Kim et al
2018 | | FDPS | Alendronate,
zoledronate | Glioblastoma | (114) |
| Ginestier et
al 2012 | | GGTI | GGTI-288 | Breast cancer | (115) |
| Yang et al
2016 | Transport | LXRs | T0901317 | Prostate
cancer | (116) |
| El Roz A et
al 2012 | | |
22(R)-hydroxycholesterol | Breast cancer | (117) |
| El Roz A et
al 2013 | | | t9, t11-CLA,
Lycopene | Breast cancer | (118) |
| Kuzu et al
2014 | | NPC1 | Leelamine | Colon cancer,
melanoma | (121) |
| Rios-Marco et
al 2013 | | | Alkylphospholipids,
itraconazole | Glioblastoma | (123,124) |
Liu et al
2014
Kuzu et al 2017 | | | Perphenazine | Melanoma | (125) |
| Solomon et
al 2009 | Absorption | | Ezetimibe | Prostate
cancer | (131) |
| Xu et al
2016 | | | SC09 | Multiple
myeloma | (132) |
| Li et al
2018 | Esterification | ACAT-1 | Avasimibe | Chronic myeloid
leukemia | (59) |
Targeting cholesterol synthesis
Multiple enzymes and proteins are involved in
cholesterol homeostasis as mentioned above. Therapeutic drugs
targeting cholesterol synthesis have been investigated, and statins
are the most extensively studied class. Statins are potent
competitive inhibitors of HMGCR, with exhibit synergistic activity
with numerous chemotherapeutic agents, preventing the development
of MDR in cancer cells. For example, lovastatin synergistically
potentiates the anti-leukemic activity of imatinib with chronic
myeloid leukemia cells from patients with different stages of the
disease, including those resistant to imatinib (34). The effect was ascribed to increased
intracellular concentration of imatinib through lovastatin,
inhibiting efflux of the drug by ABCB1 and ABCG2. Several
retrospective studies have demonstrated that statin use is
associated with the prognosis and survival of various types of
cancer, and the benefit of drugs appears to be statin-type and
follow-up time dependent (15,16,112,113). A recent example of a cohort study
of patients with endometrial cancer demonstrated that, compared
with patients who had never used statins, the continuous use of
statins (pre-diagnosis and post-diagnosis) and those who had only
used statins at post-diagnosis, exhibited reduced mortality rates
and an improved survival (15).
More comprehensive studies are required to confirm the benefits of
statins in cancer.
Omega 3 polyunsaturated fatty acids docosahexaenoic
acid (DHA) is another therapeutic targeting the ubiquitination of
HMG-CoA reductase, thus reducing cholesterol biosynthesis. It has
been shown that DHA restores the antitumor effects of different
chemotherapeutic drugs in MDR cells (88).
Farnesyl diphosphate synthesis (FDPS) is a key
enzyme involved in cholesterol biosynthesis. Alendronate and
zoledronate, which both inhibit FDPS, significantly reduce the
formation and the embryonic stem cell signature of glioblastoma
spheres (114). The acquisition
of stem-cell-like characteristics (stemness) has been hypothesized
to be closely associated with the chemoresistance of glioblastoma.
Protein geranylgeranylation, a branch of the cholesterol synthesis
pathway, has been found to be critical for breast CSC maintenance.
An inhibitor of the geranylgeranyl transferase I (GGTI) enzyme,
GGTI-288, reduces the CSC subpopulation in breast cancer both in
vitro and in primary breast cancer xenografts (115). Taken together, these preclinical
studies suggest that targeting cholesterol synthesis pathways may
be beneficial for modulating drug resistance.
Targeting cholesterol transport
Preclinical studies have demonstrated the potential
of disrupting cholesterol trans-port in cancer chemotherapy. LXR,
which plays key roles in the regulation of the expression of ABC
transporters, is implicated in cholesterol efflux as mentioned
above. LXR agonists, including T0901317, 22(R)-hydroxycholesterol
and conjugated linoleic acids (CLA) isomers (t9 and t11-CLA),
inhibit the proliferation of multiple types of cancer cells by
increasing the expression of LXR target proteins (such as ABCA1 and
ABCG1) involved in cholesterol efflux, thus reducing the
intracellular and membrane-associated cholesterol levels (116-118). Lycopene increases the protein and
mRNA expression levels of LXR and ABCA1 in androgen-independent
prostate cancer cells. Moreover, the combination of lycopene and
T0901317 has been shown to exert synergistic effects on cell
proliferation (116). Notably,
etoposide and teniposide, which are DNA topoisomerase II inhibitors
that are frequently used in the treatment of various types of
cancer, have been reported to increase the expression of ABCA1 and
ABCG1 in macrophages in vitro and to enhance RCT from
macrophages to the feces in vivo, highlighting the potential
role of ABCA1 and ABCG1 in anti-tumorigenesis (119).
NPC1 is a transmembrane efflux pump involved in
cholesterol trafficking. Previous studies have indicated that NPC1
is associated with resistance against imatinib in acute
lymphoblastic leukemia cells (120). It was demonstrated that leelamine
mediated cancer cell death through inhibiting autophagic flux and
inducing cholesterol accumulation in lysosomal/endosomal cell
compartments (121). A subsequent
study found that the active derivatives of leelamine hindered
xenografted melanoma tumor development by binding to NPC1 (122). Alkylphospholipids, itraconazole
and perphenazine are examples of inhibitors targeting intracellular
cholesterol transport, which in preclinical studies suppresses
tumor cell growth by disrupting autophagic flux and inducing
autophagy (123-125). The potential of these agents in
suppressing melanoma cells or modulating cancer resistance remains
to be determined, as well as the clinical value of targeting
cholesterol transport.
Other cholesterol-directed treatment
approaches
The role of dietary cholesterol in cancer
development and prognosis is contested. A recent study found that a
high-cholesterol-diet attenuated the anticancer activity of
doxorubicin in epidermoid carcinoma xenografts (29). Several case-control studies have
suggested a positive association between the risk of several types
of cancer and dietary cholesterol uptake (126-129). However, several other studies
have not found any evidence of an association between dietary
cholesterol intake, and the risk of lung cancer and ovarian cancer
(127,130). Furthermore, dietary surveys may
be unreliable. Based on the above, the effect of increased dietary
cholesterol on the development of cancer requires further study.
The inhibition of intestinal cholesterol absorption reduces the
levels of cholesterol in cancer cells. For example, ezetimibe, an
FDA-approved drug for blocking cholesterol uptake, reduces the
growth of human prostate cancer xenograft tumors by inhibiting
cholesterol absorption (131).
Similarly, in a separate study, the tumor-promoting effects of a
Western diet on human xenografts were reduced when ezetimibe was
used to inhibit the intestinal uptake of cholesterol (126). SC09, an inhibitor of cholesterol
absorption, has been shown to induce multiple myeloma cell death
in vitro and attenuate multiple myeloma tumor growth in
vivo (132). While targeting
dietary cholesterol uptake reduces tumor development in preclinical
studies, the clinical efficacy of this approach requires further
validation.
Targeting cholesterol esterification
inhibition is another method with which to reduce cancer
resistance
For example, avasimibe, a potent inhibitor of
ACAT-1, combined with imatinib, has been shown to result in a
significant synergistic inhibition of cell proliferation in chronic
myelogenous leukemia (64). The
synergistic effects were confirmed in a xenograft mouse model. Of
note, similar results have been observed in pancreatic ductal
adenocarcinoma when avasimibe was used in combination with
gemcitabine (59).
7. Concluding remarks and future
directions
The potential causative factors and therapeutic
strategies of cancer and cancer drug resistance are both
substantial health-care concerns. To date, numerous studies have
demonstrated the significant role of cholesterol in cancer
development (57,58). The effect of cholesterol on the
acquisition of drug resistance in cancer is increasingly being
studied. Increased cholesterol levels, as well as altered protein
expression levels are involved in cholesterol metabolism and have
been observed in various types of chemoresistant cancer cells.
These proteins include FASN, SQLE, ACAT and HMGCR, amongst others.
The mechanisms through which increased cellular cholesterol levels
enhance drug resistance are summarized as follows: i) Lipid
rafts/caveolae disruption induces altered signal transduction in
several cellular behaviors including apoptosis, invasion and
proliferation; ii) The improved functionality of ABC transporters
accelerates the efflux of chemotherapeutic drugs from cancer cells;
and iii) Decreased membrane permeability to therapeutic agents
directly results in reduced drug uptake (Fig. 2). Furthermore, epidemiological
studies (106-108) have provided additional support
for the use of cholesterol as a prognostic factor for certain
cancer types, indicating the importance of regulating cholesterol
homeostasis.
Lipids are a varied class of molecules, which
include cholesterol. Over the past few years, the importance of
lipids in various aspects of cancer biology has been elucidated,
and lipid signal mediators involved in the development of cancer
drug resistance have garnered increasing attention. In addition to
cholesterol, other types of lipids, including fatty acids and
sphingolipids have also been shown to participate in drug
resistance. FASN is the major lipogenic enzyme catalyzing the
synthesis of fatty acids. The inhibition of FASN-driven lipid rafts
negatively affects EGFR-her2/neu crosstalk, thus reducing
trastuzumab resistance (133). In
a spontaneous pancreatic cancer mouse model, a significant increase
in FASN expression was associated with increased disease
progression (134). In accordance
with this, a high FASN expression has been shown to be associated
with the poor survival of patients, and with less sensitivity to
gemcitabine in cell lines via the induction of ER stress that
results in apoptosis. Another example of the lipid-mediated
regulation of drug resistance is sphin-gomyelinase, an essential
component of the cell membrane. Decitabine treatment may increase
sphingomyelinase activity, thus leading to decreased sphingomyelin
levels, which affects lipid composition and membrane fluidity
(135). Such alterations in
doxorubicin-resistant cells eventually facilitate drug transport
and enhance drug efficacy. The function of membrane fluidity based
on lipid profile, which ultimately affects anticancer drug
transport and drug resistance, has also been studied (136). However, the influence of lipid
profiles on cancer drug resistance requires further study.
In conclusion, although not conclusive, dysregulated
cholesterol metabolism appears to be an essential contributing
factor in the acquisition of drug resistance in several types of
cancer. Therefore, preclinical models are required to confirm the
regulation of cholesterol metabolism in cancer resistance, and
preclinical results translating into useful practices in the clinic
may provide an additional therapeutic approach to slow the
progression of tumors.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AY contributed the general conception of the study
and wrote the initial draft of the manuscript. ZJ was involved in
the conception and design of the study, and revised the manuscript.
CQ, MW and XD assisted in the literature search and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Global Burden of Disease Cancer
Collaboration; Fitzmaurice C, Abate D, Abbasi N, Abbastabar H,
Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I,
et al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2017:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 5:1749–1768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fojo A, Hamilton TC, Young RC and Ozols
RF: Multidrug resistance in ovarian cancer. Cancer. 60:2075–2080.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang F, Gao B, Li R, Li W, Chen W, Yu Z
and Zhang J: Expression levels of resistant genes affect cervical
cancer prognosis. Mol Med Rep. 15:2802–2806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chun SY, Kwon YS, Nam KS and Kim S:
Lapatinib enhances the cytotoxic effects of doxorubicin in MCF-7
tumorspheres by inhibiting the drug efflux function of ABC
transporters. Biomed Pharmacother. 72:37–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
International Transporter Consortium;
Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X,
Dahlin A, Evers R, Fischer V, et al: Membrane transporters in drug
development. Nat Rev Drug Discov. 9:215–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bedi A, Barber JP, Bedi GC, el-Deiry WS,
Sidransky D, Vala MS, Akhtar AJ, Hilton J and Jones RJ:
BCR-ABL-mediated inhibition of apoptosis with delay of G2/M
transition after DNA damage: A mechanism of resistance to multiple
anticancer agents. Blood. 86:1148–1158. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Camidge DR, Pao W and Sequist LV: Acquired
resistance to TKIs in solid tumours: Learning from lung cancer. Nat
Rev Clin Oncol. 11:473–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maier S, Dahlstroem C, Haefliger C, Plum A
and Piepenbrock C: Identifying DNA methylation biomarkers of cancer
drug response. Am J Pharmacogenomics. 5:223–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taylor ST, Hickman JA and Dive C:
Epigenetic determinants of resistance to etoposide regulation of
Bcl-X(L) and Bax by tumor microenvironmental factors. J Natl Cancer
Inst. 92:18–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maxfield FR and Tabas I: Role of
cholesterol and lipid organization in disease. Nature. 438:612–621.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gabitova L, Gorin A and Astsaturov I:
Molecular pathways: Sterols and receptor signaling in cancer. Clin
Cancer Res. 20:28–34. 2014. View Article : Google Scholar
|
|
13
|
Zhang P, Wang D, Zhao Y, Ren S, Gao K, Ye
Z, Wang S, Pan CW, Zhu Y, Yan Y, et al: Intrinsic BET inhibitor
resistance in SPOP-mutated prostate cancer is mediated by BET
protein stabi-lization and AKT-mTORC1 activation. Nat Med.
23:1055–1062. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Si R, Tang H, He Z, Zhu H, Wang L,
Fan Y, Xia S, He Z and Wang Q: Cholesterol reduces the sensitivity
to platinum-based chemotherapy via upregulating ABCG2 in lung
adenocarcinoma. Biochem Biophyes Res Commun. 457:614–620. 2015.
View Article : Google Scholar
|
|
15
|
Sperling CD, Verdoodt F, Hansen MK,
Dehlendorff C, Friis S and Kjaer SK: Statin use and mortality among
endometrial cancer patients: A danish nationwide cohort study. Int
J Cancer. 143:2668–2676. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murtola TJ, Peltomaa AI, Talala K,
Määttänen L, Taari K, Tammela TL and Auvinen A: Statin use and
prostate cancer survival in the finnish randomized study of
screening for prostate cancer. Eur Urol Focus. 3:212–220. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rezen T, Rozman D, Pascussi JM and
Monostory K: Interplay between cholesterol and drug metabolism.
Biochim Biophys Acta. 1814:146–160. 2011. View Article : Google Scholar
|
|
18
|
Cerqueira NM, Oliveira EF, Gesto DS,
Santos-Martins D, Moreira C, Moorthy HN, Ramos MJ and Fernandes PA:
Cholesterol biosynthesis: A mechanistic overview. Biochemistry.
55:5483–5506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bloch K: Summing up. Ann Rev Biochem.
56:1–19. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williamson IP and Kekwick RG: The
formation of 5-phospho-mevalonate by mevalonate kinase in hevea
brasiliensis latex. Biochem J. 96:862–871. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ačimovič J and Rozman D: Steroidal
triterpenes of cholesterol synthesis. Molecules. 18:4002–4017.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brown MS and Goldstein JL: Multivalent
feedback regulation of HMG CoA reductase, a control mechanism
coordinating isoprenoid synthesis and cell growth. J Lipid Res.
21:505–517. 1980.PubMed/NCBI
|
|
24
|
Gilardi F, Mitro N, Godio C, Scotti E,
Caruso D, Crestani M and De Fabiani E: The pharmacological
exploitation of cholesterol 7alpha-hydroxylase, the key enzyme in
bile acid synthesis: From binding resins to chromatin remodelling
to reduce plasma cholesterol. Pharmacol Ther. 116:449–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakakura Y, Shimano H, Sone H, Takahashi
A, Inoue N, Toyoshima H, Suzuki S and Yamada N: Sterol regulatory
element-binding proteins induce an entire pathway of cholesterol
synthesis. Biochem Biophys Res Commun. 286:176–183. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montero J, Morales A, Llacuna L, Lluis JM,
Terrones O, Basañez G, Antonsson B, Prieto J, García-Ruiz C, Colell
A, et al: Mitochondrial cholesterol contributes to chemotherapy
resistance in hepatocellular carcinoma. Cancer Res. 68:5246–5256.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weber P, Wagner M and Schneckenburger H:
Cholesterol dependent uptake and interaction of doxorubicin in
MCF-7 breast cancer cells. Int J Mol Sci. 14:8358–8366. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen QF, Pan ZZ, Zhao M, Wang Q, Qiao C,
Miao L and Ding X: High cholesterol in lipid rafts reduces the
sensitivity to EGFR-TKI therapy in non-small cell lung cancer. J
Cell Physiol. 233:6722–6732. 2018. View Article : Google Scholar
|
|
29
|
Yun UJ, Lee JH, Shim J, Yoon K, Goh SH, Yi
EH, Ye SK, Lee JS, Lee H, Park J, et al: Anti-Cancer effect of
doxorubicin is mediated by downregulation of HMG-Co A reductase via
inhibition of EGFR/Src pathway. Lab Invest. 99:1157–1172. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greife A, Tukova J, Steinhoff C, Scott SD,
Schulz WA and Hatina J: Establishment and characterization of a
bladder cancer cell line with enhanced doxorubicin resistance by
mevalonate pathway activation. Tumor Biol. 36:3293–3300. 2015.
View Article : Google Scholar
|
|
31
|
Kong YF, Cheng LJ, Mao FY, Zhang ZZ, Zhang
YQ, Farah E, Bosler J, Bai YF, Ahmad N, Kuang S, et al: Inhibition
of cholesterol biosynthesis overcomes enzalutamide resistance in
castration-resistant prostate cancer (CRPC). J Biol Chem.
293:14328–14341. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim YN, Jin H, He Y, Zhao P, Hu Y, Tao J,
Chen J and Huang Y: Targeting lipid metabolism to overcome
EMT-associated drug resistance via integrin β3/FAK pathway and
tumor-associated macrophage repolarization using
legumain-activatable delivery. Theranostics. 9:265–278. 2019.
View Article : Google Scholar
|
|
33
|
Gupta VK, Sharma NS, Kesh K, Dauer P,
Nomura A, Giri B, Dudeja V and Banerjee S, Bhattacharya S, Saluja A
and Banerjee S: Metastasis and chemoresistance in CD133 expressing
pancreatic cancer cells are dependent on their lipid raft
integrity. Cancer Lett. 439:101–112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Glod kowska-M rowka E, M rowka P, Basa k
GW, Niesiobedzka-Krezel J, Seferynska I, Wlodarski PK, Jakobisiak M
and Stoklosa T: Statins inhibit ABCB1 and ABCG2 drug trans-porter
activity in chronic myeloid leukemia cells and potentiate
antileukemic effects of imatinib. Exp Hematol. 42:439–447. 2014.
View Article : Google Scholar
|
|
35
|
Chen Y, Liu G, Guo L, Wang H, Fu Y and Luo
Y: Enhancement of tumor uptake and therapeutic efficacy of
EGFR-targeted antibody cetuximab and antibody-drug conjugates by
cholesterol sequestration. Int J Cancer. 136:182–194. 2015.
View Article : Google Scholar
|
|
36
|
Chen X, Liu Y, Wu J, Huang HR, Du ZY,
Zhang K, Zhou DY, Hung K, Goodin S and Zheng X: Mechanistic study
of inhibitory effects of atorvastatin and docetaxel in combination
on prostate cancer. Cancer Genomics Proteomics. 13:151–160.
2016.PubMed/NCBI
|
|
37
|
Brown MS and Goldstein JL:
Receptor-Mediated control of cholesterol metabolism. Science.
191:150–154. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brown MS and Goldstein JL: A
receptor-mediated pathway for cholesterol homeostasis. Science.
232:34–47. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Acton S, Rigotti A, Landschulz KT, Xu S,
Hobbs HH and Krieger M: Identification of scavenger receptor SR-BI
as a high density lipoprotein receptor. Science. 271:518–520. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landschulz KT, Pathak RK, Rigotti A,
Krieger M and Hobbs HH: Regulation of scavenger receptor, class B,
type I, a high density lipoprotein receptor, in liver and
steroidogenic tissues of the rat. J Clin Invest. 98:984–995. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Betters JL and Yu L: NPC1L1 and
cholesterol transport. FEBS Lett. 584:2740–2747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rader DJ, Alexander ET, Weibel GL,
Billheimer J and Rothblat GH: The role of reverse cholesterol
transport in animals and humans and relationship to
atherosclerosis. J Lipid Res. 50(Suppl): S189–S194. 2009.
View Article : Google Scholar :
|
|
43
|
Maranghi M, Truglio G, Gallo A, Grieco E,
Verrienti A, Montali A, Gallo P, Alesini F, Arca M and Lucarelli M:
A novel splicing mutation in the ABCA1 gene, causing tangier
disease and familial HDL deficiency in a large family. Biochem
Biophys Res Commun. 508:487–493. 2019. View Article : Google Scholar
|
|
44
|
Vedhachalam C, Duong PT, Nickel M, Nguyen
D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S and Phillips
MC: Mechanism of ATP-binding cassette transporter A1-mediated
cellular lipid efflux to apolipoprotein A-I and formation of high
density lipoprotein particles. J Biol Chem. 282:25123–25130. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gelissen IC, Harris M, Rye KA, Quinn C,
Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L and
Jessup W: ABCA1 and ABCG1 synergize to mediate cholesterol export
to apoA-I. Arterioscler Thromb Vasc Biol. 26:534–540. 2006.
View Article : Google Scholar
|
|
46
|
Jessup W, Gelissen IC, Gaus K and
Kritharides L: Roles of ATP binding cassette transporters A1 and
G1, scavenger receptor BI and membrane lipid domains in cholesterol
export from macro-phages. Curr Opin Lipidol. 17:247–257. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang J, Mitsche MA, Lutjohann D, Cohen JC,
Xie XS and Hobbs HH: Relative roles of ABCG5/ABCG8 in liver and
intestine. J Lipid Res. 56:319–330. 2015. View Article : Google Scholar :
|
|
48
|
Connelly MA and Williams DL: Scavenger
receptor BI: A scavenger receptor with a mission to transport high
density lipo-protein lipids. Curr Opin Lipidol. 15:287–295. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang ZH, Gu D, Lange Y and Mazzone T:
Expression of scavenger receptor BI facilitates sterol movement
between the plasma membrane and the endoplasmic reticulum in
macrophages. Biochemistry. 42:3949–3955. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Calkin AC and Tontonoz P: Transcriptional
integration of metabolism by the nuclear sterol-activated receptors
LXR and FXR. Nat Rev Mol Cell Biol. 13:213–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Goldstein JL, DeBose-Boyd RA and Brown MS:
Protein sensors for membrane sterols. Cell. 124:35–46. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Brown MS and Goldstein JL: The SREBP
pathway: Regulation of cholesterol metabolism by proteolysis of a
membrane-bound transcription factor. Cell. 89:331–340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shimano H: SREBPs: Physiology and
pathophysiology of the SREBP family. FEBS J. 276:616–621. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Radhakrishnan A, Goldstein JL, McDonald JG
and Brown MS: Switch-Like control of SREBP-2 transport triggered by
small changes in ER cholesterol: A delicate balance. Cell Metab.
8:512–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lo Sasso G, Murzilli S, Salvatore L,
D'Errico I, Petruzzelli M, Conca P, Jiang ZY, Calabresi L, Parini P
and Moschetta A: Intestinal specific LXR activation stimulates
reverse cholesterol transport and protects from atherosclerosis.
Cell Metab. 12:187–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Duval C, Touche V, Tailleux A, Fruchart
JC, Fievet C, Clavey V, Staels B and Lestavel S: Niemann-Pick C1
like 1 gene expression is downregulated by LXR activators in the
intestine. Biochem Biophys Res Commun. 340:1259–1263. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cancer Genome Atlas Research Network;
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kuzu OF, Noory MA and Robertson GP: The
role of cholesterol in cancer. Cancer Res. 76:2063–2070. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li JJ, Qu XC, Tian J, Zhang JT and Cheng
JX: Cholesterol esterification inhibition and gemcitabine
synergistically suppress pancreatic ductal adenocarcinoma
proliferation. PLoS One. 13:e01933182018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hultsch S, Kankainen M, Paavolainen L,
Kovanen RM, Ikonen E, Kangaspeska S, Pietiäinen V and Kallioniemi
O: Association of tamoxifen resistance and lipid reprogramming in
breast cancer. BMC Cancer. 18:8502018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
May GL, Wright LC, Dyne M, Mackinnon WB,
Fox RM and Mountford CE: Plasma membrane lipid composition of
vinblastine sensitive and resistant human leukaemic lymphoblasts.
Int J Cancer. 42:728–733. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nguyen VT, Barozzi I, Faronato M, Lombardo
Y, Steel JH, Patel N, Darbre P, Castellano L, Győrffy B, Woodley L,
et al: Differential epigenetic reprogramming in response to
specific endocrine therapies promotes cholesterol biosynthesis and
cellular invasion. Nat Commun. 6:100442015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Souchek JJ, Baine MJ, Lin C, Rachagani S,
Gupta S, Kaur S, Lester K, Zheng D, Chen S, Smith L, et al:
Unbiased analysis of pancreatic cancer radiation resistance reveals
cholesterol biosynthesis as a novel target for radiosensitisation.
Br J Cancer. 111:1139–1149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bandyopadhyay S, Li J, Traer E, Tyner JW,
Zhou A, Oh ST and Cheng JX: Cholesterol esterification inhibition
and imatinib treatment synergistically inhibit growth of BCR-ABL
mutation-independent resistant chronic myelogenous leukemia. PLoS
One. 12:e01795582017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dominguez-Perez M, Simoni-Nieves A,
Rosales P, Nuño-Lámbarri N, Rosas-Lemus M, Souza V, Miranda RU,
Bucio L, Carvajal SU, Marquardt JU, et al: Cholesterol burden in
the liver induces mitochondrial dynamic changes and resistance to
apoptosis. J Cell Physiol. 234:7213–7223. 2019. View Article : Google Scholar
|
|
66
|
Smith B and Land H: Anticancer activity of
the cholesterol exporter ABCA1 gene. Cell Rep. 2:580–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Crain RC, Clark RW and Harvey BE: Role of
lipid transfer proteins in the abnormal lipid content of morris
hepatoma mitochondria and microsomes. Cancer Res. 43:3197–3202.
1983.PubMed/NCBI
|
|
68
|
Yamamoto Y, Tomiyama A, Sasaki N,
Yamaguchi H, Shirakihara T, Nakashima T, Kumagai K, Takeuchi S,
Toyooka T, Otani N, et al: Intracellular cholesterol level
regulates sensitivity of glioblastoma cells against
temozolo-mide-induced cell death by modulation of caspase-8
activation via death receptor 5-accumulation and activation in the
plasma membrane lipid raft. Biochem Biophys Res Commun.
495:1292–1299. 2018. View Article : Google Scholar
|
|
69
|
Ikonen E: Cellular cholesterol trafficking
and compartmentalization. Nat Rev Mol Cell Biol. 9:125–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pike LJ: Lipid rafts: Heterogeneity on the
high seas. Biochem J. 378:281–292. 2004. View Article : Google Scholar
|
|
71
|
Li YC, Park MJ, Ye SK, Kim CW and Kim YN:
Elevated levels of cholesterol-rich lipid rafts in cancer cells are
correlated with apoptosis sensitivity induced by
cholesterol-depleting agents. Am J Pathol. 168:1107–1118. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Vieira AV, Lamaze C and Schmid SL: Control
of EGF receptor signaling by clathrin-mediated endocytosis.
Science. 274:2086–2089. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sigismund S, Argenzio E, Tosoni D,
Cavallaro E, Polo S and Di Fiore PP: Clathrin-Mediated
internalization is essential for sustained EGFR signaling but
dispensable for degradation. Dev Cell. 15:209–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jiang S, Wang X, Song D, Liu X, Gu Y, Xu
Z, Wang X, Zhang X, Ye Q, Tong Z, et al: Cholesterol induces
epithelial-to-mesenchymal transition of prostate cancer cells by
suppressing degradation of EGFR through APMAP. Cancer Res.
15:3063–3075. 2019. View Article : Google Scholar
|
|
75
|
Su YJ, Lin WH, Chang YW, Wei KC, Liang CL,
Chen SC and Lee JL: Polarized cell migration induces cancer
type-specific CD133/integrin/Src/Akt/GSK3 beta/beta-catenin
signaling required for maintenance of cancer stem cell properties.
Oncotarget. 6:38029–38045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Prieto-Vila M, Takahashi RU, Usuba W,
Kohama I and Ochiya T: Drug resistance driven by cancer stem cells
and their niche. Int J Mol Sci. 18:25742017. View Article : Google Scholar
|
|
77
|
Drab M, Verkade P, Elger M, Kasper M, Lohn
M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al:
Loss of caveolae, vascular dysfunction, and pulmonary defects in
caveolin-1 gene-disrupted mice. Science. 293:2449–2452. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chatterjee M, Ben-Josef E, Thomas DG,
Morgan MA, Zalupski MM, Khan G, Andrew Robinson C, Griffith KA,
Chen CS, Ludwig T, et al: Caveolin-1 is associated with tumor
progression and confers a multi-modality resistance phenotype in
pancreatic cancer. Sci Rep. 5:108672015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li Z, Wang N, Huang C, Bao Y, Jiang Y and
Zhu G: Downregulation of caveolin-1 increases the sensitivity of
drug-resistant colorectal cancer HCT116 cells to 5-fluorouracil.
Oncol Lett. 13:483–487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Karantanos T, Karanika S, Wang J, Yang G,
Dobashi M, Park S, Ren C, Li L, Basourakos SP, Hoang A, et al:
Caveolin-1 regulates hormone resistance through lipid synthesis,
creating novel therapeutic opportunities for castration-resistant
prostate cancer. Oncotarget. 7:46321–46334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mohammad N, Malvi P, Meena AS, Singh SV,
Chaube B, Vannuruswamy G, Kulkarni MJ and Bhat MK: Cholesterol
depletion by methyl-beta-cyclodextrin augments tamoxifen induced
cell death by enhancing its uptake in melanoma. Mol Cancer.
13:2042014. View Article : Google Scholar
|
|
82
|
Troost J, Albermann N, Haefeli WE and
Weiss J: Cholesterol modulates P-glycoprotein activity in human
peripheral blood mononuclear cells. Biochem Biophys Res Commun.
316:705–711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rothnie A, Theron D, Soceneantu L, Martin
C, Traikia M, Berridge G, Higgins CF, Devaux PF and Callaghan R:
The importance of cholesterol in maintenance of P-glycoprotein
activity and its membrane perturbing influence. Eur Biophys J.
30:430–442. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kamau SW, Krämer SD, Günthert M and
Wunderli-Allenspach H: Effect of the modulation of the membrane
lipid composition on the localization and function of
P-glycoprotein in MDR1-MDCK cells. In Vitro Cell Dev Anim.
41:207–216. 2005. View Article : Google Scholar
|
|
85
|
Gayet L, Dayan G, Barakat S, Labialle S,
Michaud M, Cogne S, Mazane A, Coleman AW, Rigal D and Baggetto LG:
Control of P-glycoprotein activity by membrane cholesterol amounts
and their relation to multidrug resistance in human CEM leukemia
cells. Biochemistry. 44:4499–4509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Eckford PD and Sharom FJ: Interaction of
the P-glycoprotein multidrug efflux pump with cholesterol: Effects
on ATPase activity, drug binding and transport. Biochemistry.
47:13686–13698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Subramanian N, Schumann-Gillett A, Mark AE
and O'Mara ML: Understanding the accumulation of P-glycoprotein
substrates within cells: The effect of cholesterol on membrane
partitioning. Biochim Biophys Acta. 1858:776–782. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Gelsomino G, Corsetto PA, Campia I,
Montorfano G, Kopecka J, Castella B, Gazzano E, Ghigo D, Rizzo AM
and Riganti C: Omega 3 fatty acids chemosensitize multidrug
resistant colon cancer cells by down-regulating cholesterol
synthesis and altering detergent resistant membranes composition.
Mol Cancer. 12:1372013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen W, Zhang YW, Li Y, Zhang JW, Zhang T,
Fu BS, Zhang Q and Jiang N: Constitutive expression of
wnt/betacatenin target genes promotes proliferation and invasion of
liver cancer stem cells. Mol Med Rep. 13:3466–3474. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pál A, Méhn D, Molnár E, Gedey S, Mészáros
P, Nagy T, Glavinas H, Janáky T, von Richter O, Báthori G, et al:
Cholesterol potentiates ABCG2 activity in a heterologous expression
system: Improved in vitro model to study function of human ABCG2. J
Pharmacol Exp Ther. 321:1085–1094. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Telbisz A, Müller M, Ozvegy-Laczka C,
Homolya L, Szente L, Váradi A and Sarkadi B: Membrane cholesterol
selectively modulates the activity of the human ABCG2 multidrug
transporter. Biochem Biophys Acta. 1768:2698–2713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Takano M, Higashi M, Ito H, Toyota S,
Hirabayashi Y and Yumoto R: Functional expression of breast cancer
resistance protein and cholesterol effect in human erythrocyte
membranes. Pharmazie. 73:700–705. 2018.PubMed/NCBI
|
|
93
|
Marbeuf-Gueye C, Stierle V, Sudwan P,
Salerno M and Garnier-Suillerot A: Perturbation of membrane
microdomains in GLC4 multidrug-resistant lung cancer
cells-modification of ABCC1 (MRP1) localization and functionality.
FEBS J. 274:1470–1480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Pallarés-Trujillo J, Domènech C,
Grau-Oliete MR and Rivera-Fillat MP: Role of cell cholesterol in
modulating vincristine uptake and resistance. Int J Cancer.
55:667–671. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rivel T, Ramseyer C and Yesylevskyy S: The
asymmetry of plasma membranes and their cholesterol content
influence the uptake of cisplatin. Sci Rep. 9:56272019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sharma B, Gupta V, Dahiya D, Kumar H,
Vaiphei K and Agnihotri N: Clinical relevance of cholesterol
homeostasis genes in colorectal cancer. Biochim Biophys Acta Mol
Cell Biol Lipids. 1864:1314–1327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Simigdala N, Gao Q, Pancholi S,
Roberg-Larsen H, Zvelebil M, Ribas R, Folkerd E, Thompson A, Bhamra
A, Dowsett M and Martin LA: Cholesterol biosynthesis pathway as a
novel mechanism of resistance to estrogen deprivation in estrogen
receptor-positive breast cancer. Breast Cancer Res. 18:582016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kimbung S, Lettiero B, Feldt M, Bosch A
and Borgquist S: High expression of cholesterol biosynthesis genes
is associated with resistance to statin treatment and inferior
survival in breast cancer. Oncotarget. 7:59640–59651. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kim S, Lee M, Dhanasekaran DN and Song YS:
Activation of LXRa/beta by cholesterol in malignant ascites
promotes chemo-resistance in ovarian cancer. BMC Cancer.
18:12322018. View Article : Google Scholar
|
|
100
|
Zhou P, Li B, Liu B, Chen T and Xiao J:
Prognostic role of serum total cholesterol and high-density
lipoprotein cholesterol in cancer survivors: A systematic review
and meta-analysis. Clin Chim Acta. 477:94–104. 2018. View Article : Google Scholar
|
|
101
|
Rodrigues Dos Santos C, Fonseca I, Dias S
and de Almeida JC: Plasma level of LDL-cholesterol at diagnosis is
a predictor factor of breast tumor progression. BMC Cancer.
14:1322014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lofterød T, Mortensen ES, Nalwoga H,
Wilsgaard T, Frydenberg H, Risberg T, Eggen AE, McTiernan A, Aziz
S, Wist EA, et al: Impact of pre-diagnostic triglycerides and
HDL-cholesterol on breast cancer recurrence and survival by breast
cancer subtypes. BMC Cancer. 18:6542018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wulaningsih W, Vahdaninia M, Rowley M,
Holmberg L, Garmo H, Malmstrom H, Lambe M, Hammar N, Walldius G,
Jungner I, et al: Prediagnostic serum glucose and lipids in
relation to survival in breast cancer patients: A competing risk
analysis. BMC Cancer. 15:9132015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tamura T, Inagawa S, Hisakura K, Enomoto T
and Ohkohchi N: Evaluation of serum high-density lipoprotein
cholesterol levels as a prognostic factor in gastric cancer
patients. J Gastroenterol Hepatol. 27:1635–1640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Que Y, Jiang F, Liu L, Li Y, Chen Y, Qiu
H, Zhou Z and Zhang X: Clinical significance of preoperative serum
high density lipoprotein cholesterol levels in soft tissue sarcoma.
Medicine (Baltimore). 94:e8442015. View Article : Google Scholar
|
|
106
|
Wei LJ, Zhang C, Zhang H, Wei X, Li SX,
Liu JT and Ren XB: A case-control study on the association between
serum lipid level and the risk of breast cancer. Zhonghua Yu Fang
Yi Xue Za Zhi. 50:1091–1095. 2016.In Chinese.
|
|
107
|
Cambien F, Ducimetiere P and Richard J:
Total serum cholesterol and cancer mortality in a middle-aged male
population. Am J Epidemiol. 112:388–394. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sherwin RW, Wentworth DN, Cutler JA,
Hulley SB, Kuller LH and Stamler J: Serum cholesterol levels and
cancer mortality in 361,662 men screened for the multiple risk
factor intervention trial. JAMA. 257:943–948. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kim TH, Ahn SJ, Jung WT, Lee OJ, Ha WS and
Jang JS: Clinical significance of the levels of serum cholesterol
in patients with gastric cancer. Cancer Res Treat. 35:335–340.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ohno Y, Nakashima J, Nakagami Y, Gondo T,
Ohori M, Hatano T and Tachibana M: Clinical implications of
preopera-tive serum total cholesterol in patients with clear cell
renal cell carcinoma. Urology. 83:154–158. 2014. View Article : Google Scholar
|
|
111
|
Sok M, Ravnik J and Ravnik M: Preoperative
total serum cholesterol as a prognostic factor for survival in
patients with resectable non-small-cell lung cancer. Wien Klin
Wochenschr. 121:314–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Larsen SB, Dehlendorff C, Skriver C,
Dalton SQ, Jespersen CG, Borre M, Brasso K, Nørgaard M, Johansen C,
Sørensen HT, et al: Postdiagnosis statin use and mortality in
danish patients with prostate cancer. J Clin Oncol. 35:3290–3297.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu B, Yi Z, Guan X, Zeng YX and Ma F: The
relationship between statins and breast cancer prognosis varies by
statin type and exposure time: A meta-analysis. Breast Cancer Res
Treat. 164:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim HY, Kim DK, Bae SH, Gwak H, Jeon JH,
Kim JK, Lee BI, You HJ, Shin DH, Kim YH, et al: Farnesyl
diphosphate synthase is important for the maintenance of
glioblastoma stemness. Exp Mol Med. 50:1–12. 2018.PubMed/NCBI
|
|
115
|
Ginestier C, Monville F, Wicinski J,
Cabaud O, Cervera N, Josselin E, Finetti P, Guille A, Larderet G,
Viens P, et al: Mevalonate metabolism regulates Basal breast cancer
stem cells and is a potential therapeutic target. Stem Cells.
30:1327–1337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yang CM, Lu YL, Chen HY and Hu ML:
Lycopene and the LXRalpha agonist T0901317 synergistically inhibit
the proliferation of androgen-independent prostate cancer cells via
the PPARγ-LXRα-ABCA1 pathway. J Nutr Biochem. 23:1155–1162. 2012.
View Article : Google Scholar
|
|
117
|
EL Roz A, Bard JM, Huvelin JM and Nazih H:
LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast
cancer cells: Relation to proliferation and apoptosis. Anticancer
Res. 32:3007–3013. 2012.PubMed/NCBI
|
|
118
|
El Roz A, Bard JM, Huvelin JM and Nazih H:
The anti-proliferative and pro-apoptotic effects of the trans9,
trans11 conjugated linoleic acid isomer on MCF-7 breast cancer
cells are associated with LXR activation. Prostaglandins Leukot
Essent Fatty Acids. 88:265–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang L, Jiang M, Shui Y, Chen Y, Wang Q,
Hu W, Ma X, Li X, Liu X, Cao X, et al: DNA topoisomerase II
inhibitors induce macro-phage ABCA1 expression and cholesterol
efflux-an LXR-dependent mechanism. Biochim Biophys Acta.
1831:1134–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Naren D, Wu J, Gong Y, Yan T, Wang K, Xu
W, Yang X, Shi F and Shi R: Niemann-Pick disease type C1(NPC1) is
involved in resistance against imatinib in the imatinib-resistant
Ph+ acute lymphoblastic leukemia cell line SUP-B15/RI. Leuk Res.
42:59–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kuzu OF, Gowda R, Sharma A and Robertson
GP: Leelamine mediates cancer cell death through inhibition of
intracellular cholesterol transport. Mol Cancer Ther. 13:1690–1703.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Gowda R, Inamdar GS, Kuzu O, Dinavahi SS,
Krzeminski J, Battu MB, Voleti SR, Amin S and Robertson GP:
Identifying the structure-activity relationship of leelamine
necessary for inhibiting intracellular cholesterol transport.
Oncotarget. 8:28260–28277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rios-Marco P, Martín-Fernández M,
Soria-Bretones I, Ríos A, Carrasco MP and Marco C:
Alkylphospholipids deregulate cholesterol metabolism and induce
cell-cycle arrest and autophagy in U-87 MG glioblastoma cells.
Biochim Biophys Acta. 1831:1322–1334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Liu R, Li J, Zhang T, Zou L, Chen Y, Wang
K, Lei Y, Yuan K, Li Y, Lan J, et al: Itraconazole suppresses the
growth of glioblastoma through induction of autophagy: Involvement
of abnormal cholesterol trafficking. Autophagy. 10:1241–1255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kuzu OF, Gowda R, Noory MA and Robertson
GP: Modulating cancer cell survival by targeting intracellular
cholesterol transport. Br J Cancer. 117:513–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Pelton K, Coticchia CM, Curatolo AS,
Schaffner CP, Zurakowski D, Solomon KR and Moses MA:
Hypercholesterolemia induces angiogenesis and accelerates growth of
breast tumors in vivo. Am J Pathol. 184:2099–2110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lin X, Liu L, Fu Y, Gao J, He Y, Wu Y and
Lian X: Dietary cholesterol intake and risk of lung cancer: A
meta-analysis. Nutrients. 10:1852018. View Article : Google Scholar :
|
|
128
|
Li C, Yang L, Zhang D and Jiang W:
Systematic review and meta-analysis suggest that dietary
cholesterol intake increases risk of breast cancer. Nutr Res.
36:627–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hu J, La Vecchia C, de Groh M, Negri E,
Morrison H and Mery L; Canadian Cancer Registries Epidemiology
Research Group: Dietary cholesterol intake and cancer. Ann Oncol.
23:491–500. 2012. View Article : Google Scholar
|
|
130
|
Genkinger JM, Hunter DJ, Spiegelman D,
Anderson KE, Beeson WL, Buring JE, Colditz GA, Fraser GE,
Freudenheim JL, Goldbohm RA, et al: A pooled analysis of 12 cohort
studies of dietary fat, cholesterol and egg intake and ovarian
cancer. Cancer Causes Control. 17:273–285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Solomon KR, Pelton K, Boucher K, Joo J,
Tully C, Zurakowski D, Schaffner CP, Kim J and Freeman MR:
Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol.
174:1017–1026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Xu X, Han K, Zhu J, Mao H, Lin X, Zhang Z,
Cao B, Zeng Y and Mao X: An inhibitor of cholesterol absorption
displays anti-myeloma activity by targeting the JAK2-STAT3
signaling pathway. Oncotarget. 7:75539–75550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Menendez JA, Vellon L and Lupu R:
Targeting fatty acid synthase-driven lipid rafts: A novel strategy
to overcome trastuzumab resistance in breast cancer cells. Med
Hypotheses. 64:997–1001. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tadros S, Shukla SK, King RJ, Gunda V,
Vernucci E, Abrego J, Chaika NV, Yu F, Lazenby AJ, Berim L, et al:
De novo lipid synthesis facilitates gemcitabine resistance through
endoplasmic reticulum stress in pancreatic cancer. Cancer Res.
77:5503–5517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Vijayaraghavalu S, Peetla C, Lu S and
Labhasetwar V: Epigenetic modulation of the biophysical properties
of drug-resistant cell lipids to restore drug transport and
endocytic functions. Mol Pharm. 9:2730–2742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Todor IN, Lukyanova NY and Chekhun VF: The
lipid content of cisplatin- and doxorubicin-resistant MCF-7 human
breast cancer cells. Exp Oncol. 34:97–100. 2012.PubMed/NCBI
|
















