Introduction
MicroRNAs (miRNAs/miRs) are a class of non-coding
RNAs that are 18 to 25 nucleotides in length (1,2).
These molecules can regulate the expression of hundreds of target
genes, and serve important regulatory roles in fundamental
biological processes, including cell proliferation, differentiation
and apoptosis via negative regulation of gene expression (3-5).
Consistent with their roles in these processes, a number of studies
have identified widespread alteration of miRNA expression in cancer
(6-8). miRNAs may function as tumor
suppressors or oncogenes, and dysregulation of miRNA expression is
associated with tumorigenesis, cancer progression and
metastasis.
In endometrial cancer, changes in miRNAs may
influence cancer proliferation and differentiation (9-12).
Several studies have investigated aberrant miR-34 family expression
and its roles in cancer (13-17).
The three members of the human miR-34 family (miR-34a, miR-34b and
miR-34c) induce cell cycle arrest and apoptosis, and are
downregulated in cancer (14,15,18).
Therefore, these molecules are tumor suppressor miRNAs.
Downregulation of miR-34 family expression may occur for several
reasons, including genetic mutation and aberrant DNA methylation of
the promoter region (19-22). Our previous studies have
demonstrated widespread aberrant DNA methylation in both
endometrial cancer tissue and cells (23-26).
Therefore, identification of epigenetically silenced miRNA and
exploration of dysregulation of miRNA and its functions in
endometrial cancer may be beneficial for diagnosis or therapy.
Indeed, miR-34 replacement therapy is currently undergoing clinical
trials for primary liver cancer (27). However, little is known regarding
the association between endometrial cancer and miR-34 family
dysregulation.
The present study investigated epigenetically
silenced tumor suppressor miRNAs in endometrial cancer cells, and
identified miR-34b as one such miRNA. Subsequently, miR-34b
function and effects on chemosensitivity of these cells were
explored. Downregulation of MET expression by miR-34b
overexpression enhanced the chemosensitivity of endometrial cancer
cells to paclitaxel. The present findings may contribute to
development of epigenetic medicine or individualized therapy for
endometrial cancer.
Materials and methods
Cell lines and culture
Six human endometrial cancer cell lines were used in
the present study: SNG-II and HHUA cells were established at Keio
University (Tokyo, Japan), Ishikawa cells were established at
National Kasumigaura Hospital (Ibaraki, Japan), HEC108 and HEC1B
cells were purchased from the Health Science Research Resources
Bank, and the KLE cell line was purchased from American Type
Culture Collection. KLE was maintained in DMEM/F12 (Sigma-Aldrich;
Merck KGaA) supplemented with 10% FBS. (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/strep-tomycin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a 5% CO2 humidified
incubator. All other cells were maintained in Ham's F12 medium
(Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS and 1%
penicillin/streptomycin at 37°C in a 5% CO2 humidified
incubator.
Demethylation treatment
Endometrial cancer cells were plated on a 10-cm dish
at a density of 106 cells/dish and cultured at 37°C with
5% CO2 for 72 h. Subsequently, 5-Aza-2′-deoxycytidine
(5-aza; Sigma-Aldrich; Merck KGaA), a demethylating agent, was
added at a final concentration of 1 µM in culture medium.
After 48 h of incubation at 37°C with 5% CO2, 1
µM 5-aza was added again and RNA was extracted 72 h after
the second addition of 5-aza.
DNA, RNA and protein extraction
An AllPrep DNA/RNA/miRNA Universal kit (Qiagen GmbH)
was used to extract DNA, RNA and miRNA from all samples according
to the manufacturer's protocol. For protein extraction, cells were
lysed using a Mammalian Cell Extraction kit (BioVision, Inc.)
according to the manufacturer's protocol.
miRNA microarrays and data analysis
Total RNA (100 ng) was labeled with cyanine 3-pCp
(Cy3) fluorescent dye using a miRNA Labeling Reagent and
Hybridization kit (Agilent Technologies, Inc.). Cy3-labeled RNA
from each sample was hybridized to an Agilent Human miRNA Version 2
Microarray (Agilent Technologies, Inc.). The hybridized array was
washed and scanned according to the manufacturer's protocols. Data
were extracted from scanned images using Feature Extraction
v.10.1.1.1 (Agilent Technologies, Inc.) and the results were
analyzed using GeneSpring GX 10 software (Agilent Technologies,
Inc.). Raw data were normalized and 798 miRNAs were used in further
analysis. Differentially expressed miRNAs in each cell line with or
without 5-aza treatment were identified using fold-change analysis.
A Venn diagram was used to show common altered miRNAs in the four
analyzed cell lines.
Reverse transcription and TaqMan
quantitative PCR (qPCR) validation
cDNA was reverse transcribed at 42°C for 15 min from
each total RNA sample using a TaqMan Advanced miRNA cDNA synthesis
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Prefabricated TaqMan MicroRNA assays (containing miRNA-specific
forward and reverse PCR primers and a miRNA-specific Taqman MGB
probe; Applied Biosystems; Thermo Fisher Scientific, Inc.) were
used to determine the expression levels of miR-34b (AB Assay ID,
002102, miRBase accession no. MIMAT0004676). The PCR conditions
were as follows: Initial denaturation at 95°C for 5 min, followed
by 14 cycles of 95°C for 3 sec and 60°C for 30 sec, and final
extension at 99°C for 10 min. Reverse transcription (RT)-qPCR was
performed using TaqMan Fast Advanced Master Mix in a QuantStudio 5
(Thermo Fisher Scientific, Inc.). miR-34b expression was calculated
using the ΔΔCq method (28) using
miR-423-5p (AB Assay ID, 002340; miRBase accession no.
MIMAT0004748) as an internal control.
miRNA transfection
Cells were plated on a 6-cm dish at a density of
2×105 cells/dish at 24 h before transfection. miR-34b
(5′-CAAUCACUAACUCCACUGCCAU-3′; 5 nM; Assay ID; PM12727; Applied
Biosystems; Thermo Fisher Scientific, Inc.) or Pre-miR miRNA
Precursor negative control miRNA #1 (cat. no. AM17110; 5 nM;
Applied Biosystems; Thermo Fisher Scientific, Inc.) was then
transfected using siPORT NeoFX transfection agent (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The medium was changed
every 2 days after transfection. After 48 h of miRNA transfection,
miR-34b and target gene expression levels were analyzed by RT-qPCR.
After 192 h of miRNA transfection, MET and MYC protein levels were
analyzed by western blotting.
Expression analysis of target genes
cDNA for GAPDH, MET and MYC
expression analysis was synthesized at 42°C for 50 min using a
SuperScript First-Strand Synthesis System for RT-PCR kit
(Invitrogen; Thermo Fisher Scientific, Inc.) from 1 µg total
RNA. PowerUp SYBR-Green Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for RT-qPCR, with 10 µl
PCR reaction mixture containing 1X qPCR mix, 0.25 µM primers
and 0.5 µl synthesized 1st strand cDNA as template. The
primers used for RT-qPCR were as follows: GAPDH forward,
5′-GAA GGT GAA GGT CGG AGT C-3′ and reverse, 5′-GAA GAT GGT GAT GGG
ATT TC-3′; MET forward, 5′-TGA AAT TCA TCC AAC CAA ATC TT-3′
and reverse, 5′-AAT AGA AAA CTG ACA ATG TTG AGA GG-3′; and
MYC forward, 5′-CTG GGA AGG GAG ATC CGG AGC-3′ and reverse,
5′-GGG GCA TCG TCG CGG GAG GCT G-3′. The PCR conditions were as
follows: 50°C for 2 min, 95°C for 15 sec, followed by 40 cycles of
95°C for 15 sec and 60°C for 60 sec. Quantification was performed
using the QuantStudio 5 (Thermo Fisher Scientific, Inc.).
Expression levels of MET and MYC were calculated
using the ΔΔCq method (28) with
GAPDH as an internal control. The present study used
TargetScan 6.2 (http://www.targetscan.org) for target prediction of
miR-34b.
Western blot analysis
Total protein concentration was deter-mined using
the UV absorption method (GeneQuant 100; GE Healthcare). A total of
50 µg protein was electrophoresed on a 10% polyacrylamide
gel and proteins were transferred to nitrocellulose membranes
(Bio-Rad Laboratories, Inc.). The membranes were soaked in PBS
containing 1% BSA (Sigma-Aldrich; Merck KGaA) and 0.1% Tween-20,
and incubated at room temperature for 1 h for blocking.
Subsequently, they were incubated with anti-β-actin antibody
(AC-74; cat. no. A2228; dilution, 1:5,000; Sigma-Aldrich; Merck
KGaA), anti-β-tubulin antibody (TU-20; can. no. CBL412; dilution,
1:100; Chemicon International; Thermo Fisher Scientific, Inc.),
anti-MET antibody (H-10; cat. no. sc-514149; dilution, 1:200; Santa
Cruz Biotechnology, Inc.) and anti-MYC antibody (c-19; cat. no.
sc-788; dilution, 1:200; Santa Cruz Biotechnology, Inc.) at 4°C
overnight, followed by three washes with PBS containing 0.1% Tween
(PBS-T). The samples were incubated with biotinylated anti-mouse
IgG antibody (cat. no. BA-2000; dilution, 1:200; Vector
Laboratories, Inc.) at room temperature for 1 h. The membranes were
rinsed with PBS-T three times and incubated with ATP binding
cassette transporters (ABC) complex at room temperature for 1 h.
Membranes were rinsed with PBS-T twice and PBS once, and signals
were visualized with 3,3′-diaminobenzidine (DAB; Sigma-Aldrich;
Merck KGaA).
Colony formation assay and cell cycle
analysis
Cells were plated at a density of 1×104
cells/10-cm dish. At 7 days after miRNA transfection, colonies were
stained with 0.1% crystal violet in 50% methanol at room
temperature for 20 min and imaged with an inverted phase contrast
microscope in a colony formation assay. The number of colonies was
quantified using ImageJ software (Ver. 1.4.6; National Institutes
of Health). For cell cycle analysis, 1×107 cells were
fixed in 70% cold ethanol at 4°C for 30 min, RNase-treated, stained
with propidium iodide (Sigma-Aldrich; Merck KGaA) at room
temperature for 15 min in the dark, and analyzed within 1 h. The
cell cycle profile was determined using a Gallios flow cytometer
(Beckman Coulter, Inc.). Cell cycle analysis was performed with
Kaluza software (version 1.1; Beckman Coulter, Inc.).
Transwell migration and invasion
assays
Migration and invasion assays were performed 7 days
after transfection of miRNAs. Cell culture insert 24-well plates
with an 8-µm pore size and BD BioCoat Matrigel invasion
chambers (BD Biosciences) were used for migration and invasion
assays. In the respective assays, 1×105 and
2×105 cells/well were plated in the upper Transwell
insert in 0.5 ml serum-free F12 medium (Sigma-Aldrich; Merck KGaA).
The lower compartment contained 0.75 ml F12 medium with 10% FCS
(Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated at
37°C in a 5% CO2 incubator for 30 h. Cells in the upper
Transwell chamber were carefully removed with a cotton swab. Cells
that migrated or invaded to the lower surface of the membrane were
fixed and stained using a Diff-Quik kit (Sysmex Corporation)
according to the manufacturer's protocol. Migrating and invading
cells were counted under a light microscope (magnification, ×200;
CX21; Olympus Corporation) in five randomly selected microscope
fields.
In vitro chemosensitivity analysis
After 48 h of miRNA transfection, endometrial cancer
cells were plated on 96-well plates at 5.0×104
cells/well and cultured for 48 h. These cells were treated with
various concentrations of paclitaxel (1.0×10−5,
0.5×10−5 and 1.0×10−6 M), cisplatin
(1.0×10−4, 0.5×10−4 and 1.0×10−5
M) or doxorubicin (1.0×10−5, 0.5×10−5 and
1.0×10−6 M) with or without miR-34b transfection. Viable
cells were quantified 48 h after administration of anticancer drugs
using a Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc.)
according to the manufacturer's protocol. Cytotoxicity was measured
by determining the IC50 of each anticancer drug in terms
of cell growth compared with the control.
In vivo experiments
The care and use of animals in the present study
were approved by the Animal Research Center at Keio University
(Tokyo, Japan). A total of 15 BALB/c nude mice housed at 20-25°C
with 30-60% humidity under a 12:12 h light/dark cycle with ad
libitum access to food and water were used for the xenograft
experiment. HEC1B cells (1×107) in 100 µl PBS
were subcutaneously injected at two sites in the flank of 15 female
6-week-old nude mice (weight, 18-22 g) obtained from CLEA Japan,
Inc. After 14 days, mice with tumor formation were randomly divided
into five groups [negative control miRNA (nega-miR), miR-34b,
paclitaxel, nega-miR and paclitaxel, and miR-34b and paclitaxel].
Paclitaxel was administered intraperitoneally at 20 mg/kg and a
mixture of miRNA and atelocollagen (Koken Co., Ltd.) was injected
at the tumor site on alternate days. Tumor diameters were measured
every week and the tumor volume (mm3) was calculated as
follows: V=length × width2 ×1/2. Five tumors were used
in each group. The mice were euthanized at 28 days post-injection
using CO2 inhalation (10-30%/min).
Immunohistochemical staining
Each extracted xenograft tumor was fixed in 4%
paraformaldehyde at room temperature for 30 min and embedded in
paraffin blocks. Tissue blocks were cut into 3-µm slices,
deparaffinized in xylene, rehydrated with graded alcohols (100, 90,
70 and 50%). Subsequently, the sections were autoclaved at 121°C
for 10 min with sodium citrate buffer (0.01 M, pH 7.0) for antigen
retrieval. Following a blocking step at room temperature for 30 min
using a Vectastain Elite ABC kit (Vector Laboratories, Inc.), the
sections were immunostained with anti-MET (EP1454Y; cat. no.
ab51067; dilution, 1:100; Abcam), anti-MYC (Y69; cat. no. ab32072;
dilution, 1:20; Abcam), anti-procaspase-3 (E61; cat. no. ab32150;
dilution, 1:50; Abcam), anti-cleaved caspase-3 (cat. no. ab2302;
dilution, 1:25; Abcam), anti-cleaved poly(ADP-ribose) polymerase 1
(PARP; E51; cat. no. ab32064; dilution, 1:100; Abcam) and anti-Ki67
(MIB-1; cat. no. M724001-2; dilution, 1:100; Dako; Agilent
Technologies, Inc.) at 4°C overnight. Indirect immunohistochemical
staining was performed by the avidin-biotin-peroxidase complex
method using a Vectastain Elite ABC kit (Vector Laboratories, Inc.)
according to the manufacturer's protocol, and visualized with DAB
(Sigma-Aldrich; Merck KGaA). Sections were counterstained with
hematoxylin at room temperature for 10 sec, dehydrated in a graded
series of ethanol, dried and a cover slip was applied. For
measurement of the proliferative index, staining data were captured
under a light microscope (magnification, ×200; CX21; Olympus
Corporation) randomly in five areas per slide. Ki67-positive cells
and cancer cells were counted and the Ki67 index was calculated as
Ki67-positive cells/cancer cells.
Statistical analysis
Each experiment was performed at least three times
and data are presented as the mean ± SD. Statcel3 statistical
software (OMS Ltd.) was used for analysis. Comparisons between two
groups were performed by Mann-Whitney U test. Comparisons among
three or more groups were conducted using one-way ANOVA with a
Tukey post hoc test or Kruskal-Wallis and a Steel-Dwass post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miRNAs upregulated by demethylation
treatment
To identify tumor suppressor miRNAs that are
epigenetically silenced by DNA methylation and to investigate the
function of these miRNAs in endometrial cancer, differentially
expressed miRNAs were analyzed using a microarray before and after
demethylation treatment in three well-differentiated endometrial
cancer cell lines (SNG-II, HHUA and Ishikawa) and one
poorly-differentiated endometrial cancer cell line (HEC-108). Among
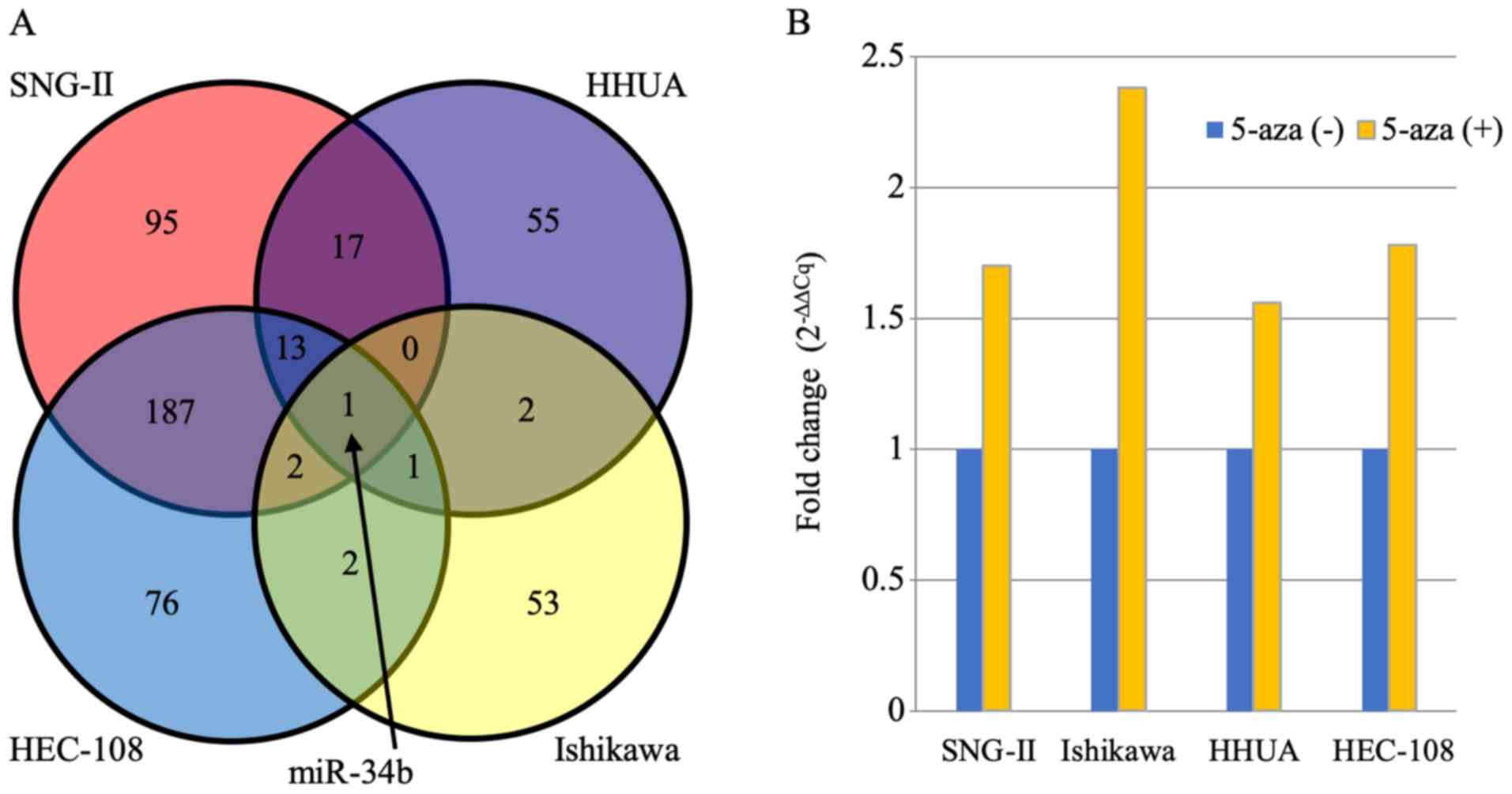
821 miRNAs, only miR-34b was upregulated (fold change >1.5) in
all four cell lines (Fig. 1A).
miR-34b upregulation after demethylation treatment was confirmed by
Taqman qPCR. In all four cell lines, miR-34b expression was
increased 1.56-2.38 fold compared with the level before treatment
(Fig. 1B). This result suggests
that miR-34b is commonly suppressed in endometrial cancer cells and
may serve an important role in endometrial cancer progression or
chemosensitivity.
Expression levels of miR-34b target genes
in endometrial cancer cells
Identification of target genes of miR-34b is
important for understanding the functions and molecular mechanisms
of miR-34b. Binding of miRNAs to the 3′-untranslated region (UTR)
is the basis of most target site prediction algorithms. The present
study used TargetScan 6.2 for target prediction of miR-34b, and
identified MET and MYC as the predicted target genes.
Although no statistically significant difference was demonstrated,
after increased miR-34b expression following miR-34b transfection
was demonstrated (Fig. 2A), target
gene expression was analyzed. The expression levels of MET
and MYC were downregulated in HEC-108, HEC-1B and KLE cells
after miR-34b transfection at the RNA and protein levels with no
statistically significant differences observed (Fig. 2B-F). This demonstrated that
MET and MYC may be targets of miR-34b.
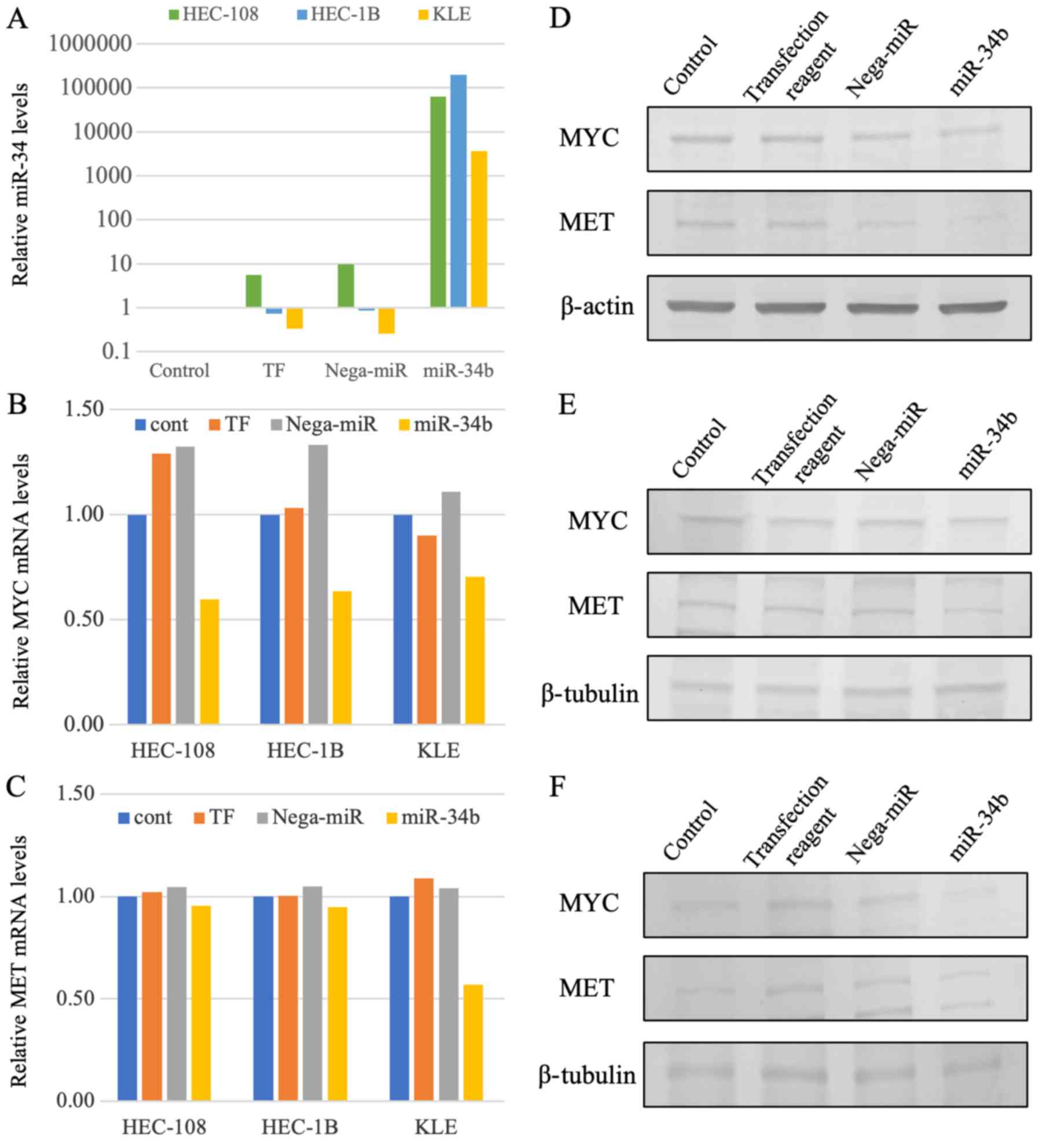 | Figure 2miR-34b and target gene expression.
(A) miR-34b expression assessed by RT-qPCR. After 48 h of miR-34b
transfection, miR-34b expression was markedly upregulated compared
with that in HEC-108, HEC-1B and KLE cell lines. (B) MYC
gene expression assessed by RT-qPCR. After 48 h of miR-34b
transfection, MYC expression was reduced by 54.9, 52.1 and
36.5% compared with that in HEC-108, HEC-1B and KLE cells
transfected with nega-miR, respectively. (C) MET gene
expression assessed by RT-qPCR. After 48 h of miR-34b transfection,
MET expression was reduced by 8.7, 9.4 and 45.1% compared
with that in HEC-108, HEC-1B and KLE cells transfected with
nega-miR, respectively. (D-F) MET and MYC protein expression was
assessed by western blotting. MET and MYC protein expression was
reduced after 192 h of miR-34b transfection in (D) HEC-108, (B)
HEC-1B and (F) KLE cells. β-actin or β-tubulin was used as the
internal control. cont, Control; miR-34b, microRNA-34b; nega-miR,
negative control microRNA; RT-qPCR, reverse
transcription-quantitative PCR; TF, transfection reagent. |
Cell growth following miR-34b
treatment
Further analysis was performed to investigate the
effect of miR-34b via its target genes. To examine the effect of
miR-34b via MYC in cell proliferation and the cell cycle in
endometrial cancer cells, a colony formation assay and flow
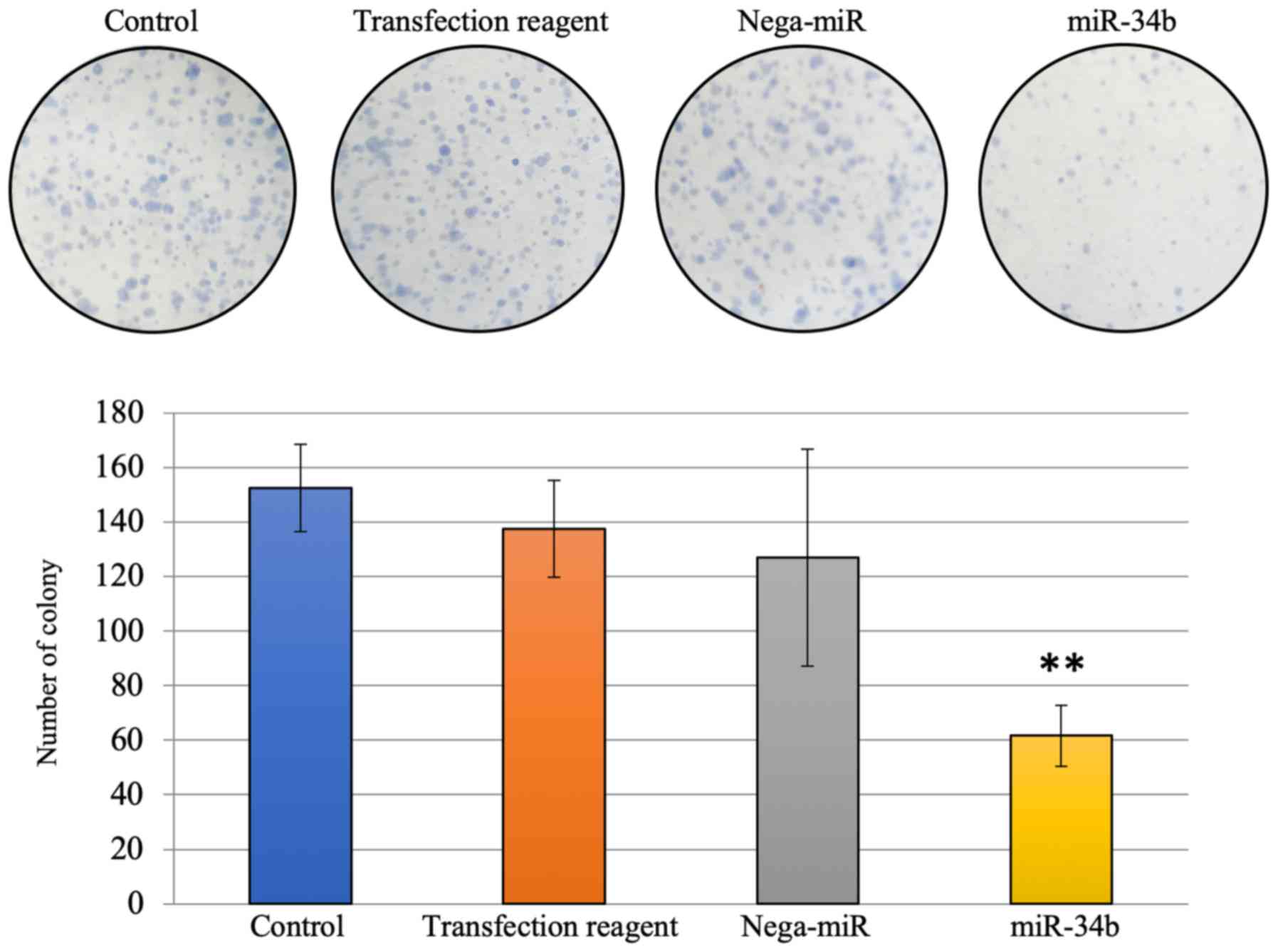
cytometry analysis were performed. The number of colonies was
significantly reduced 7 days after miR-34b treatment compared with
the nega-miR treatment group (Fig.
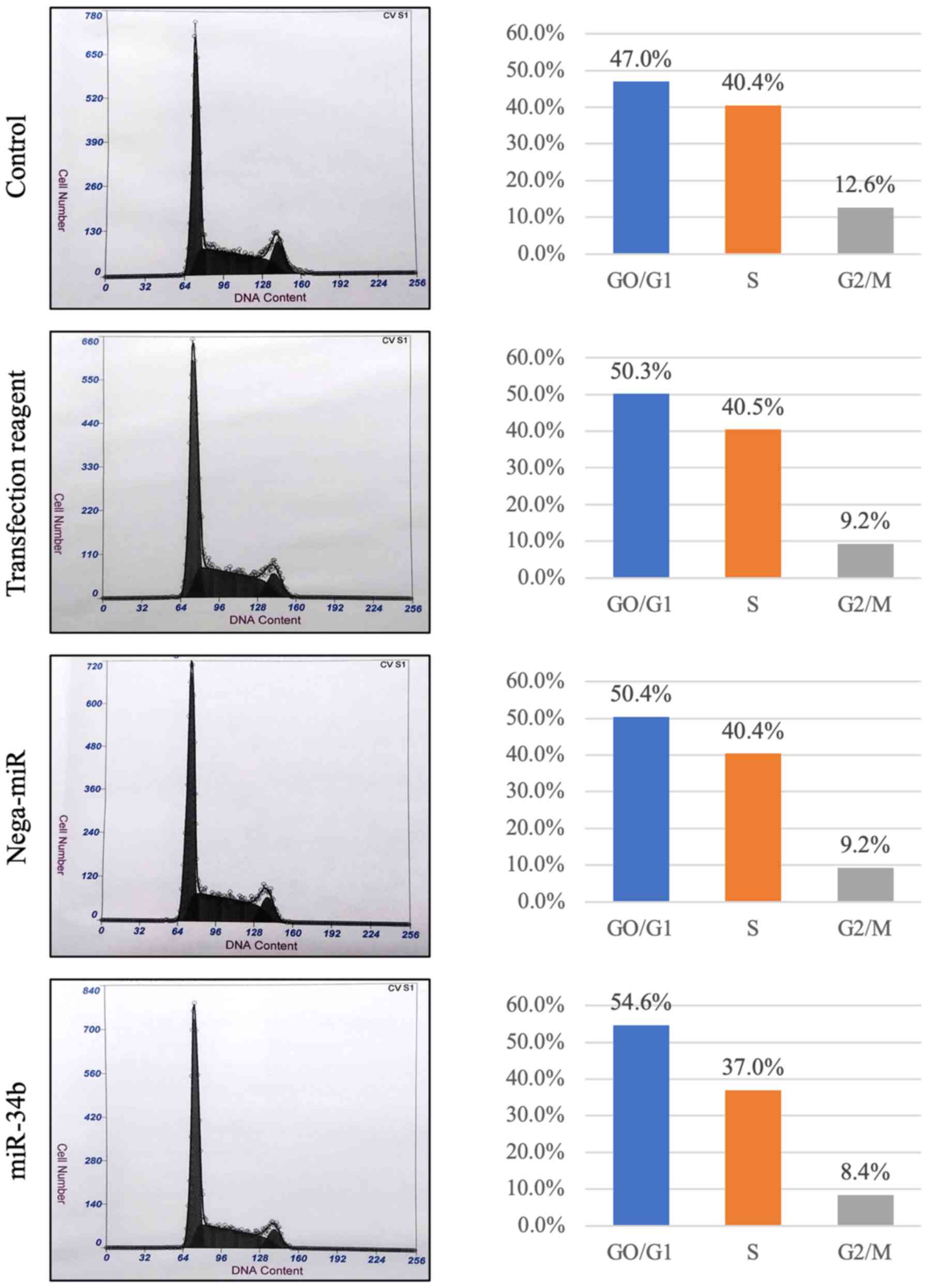
3). In flow cytometry, The proportion of
G0/G1 phase cells was increased in
miR-34b-treated cells compared with the others (Fig. 4). Subsequently, it was investigated
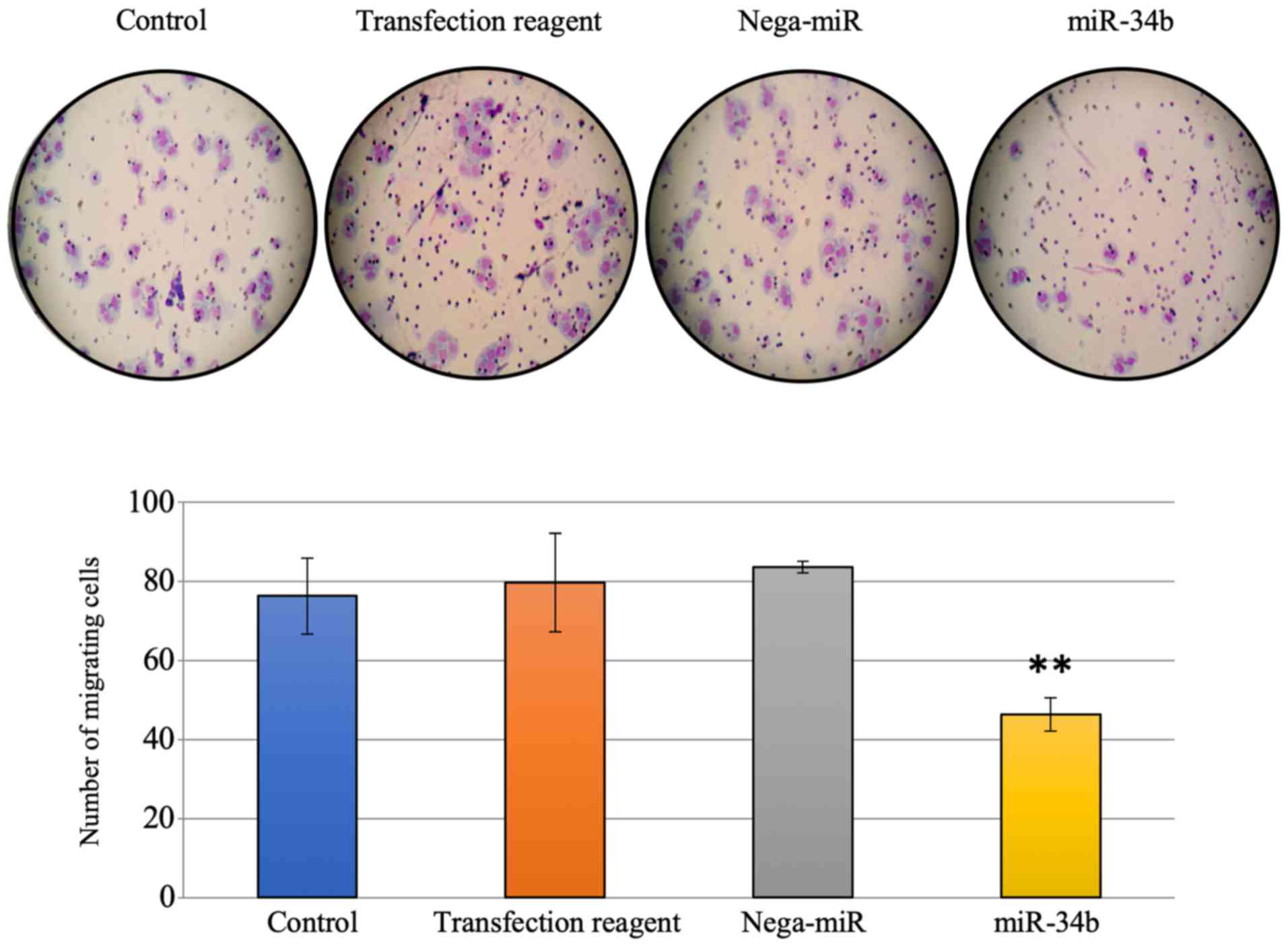
whether miR-34b affects cell invasion and motility, since
MET is associated with cell migration and invasion in lung
cancer cells (1). In Transwell
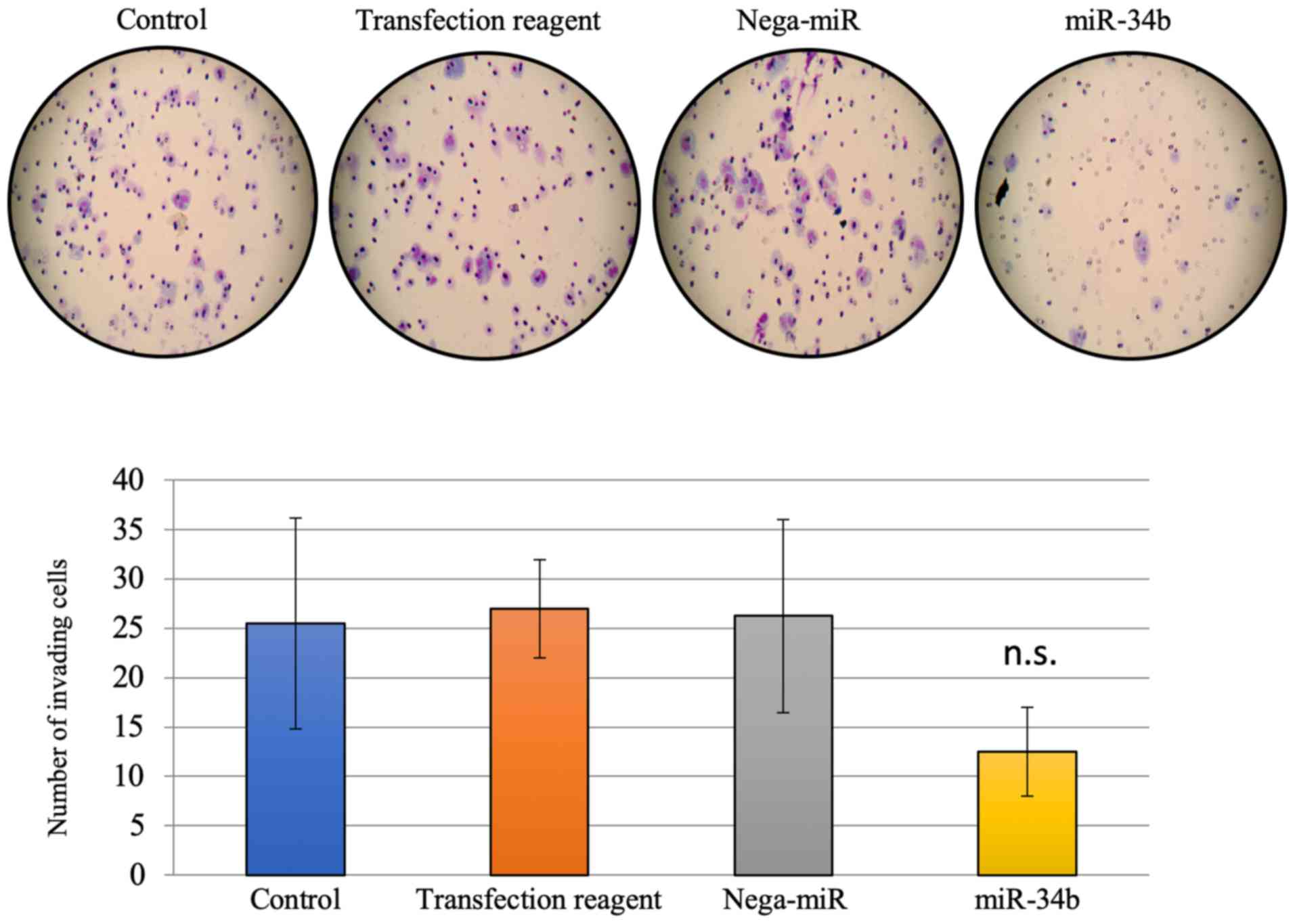
assays, significantly reduced migration, but not invasion, was
observed after miR-34b treatment compared with treatment with
nega-miR in HEC-108 cells (Figs. 5
and 6).
Effect of miR-34b on chemosensitivity in
vitro
To investigate the effect of miR-34b on
chemosensitivity in endometrial cancer cells, a MTT assay with
miR-34b treatment was performed in HEC-108, HEC-1B and KLE cells.
These cell lines were used since our previous studies demonstrated
that they exhibit chemoresistance under normal conditions (24-26).
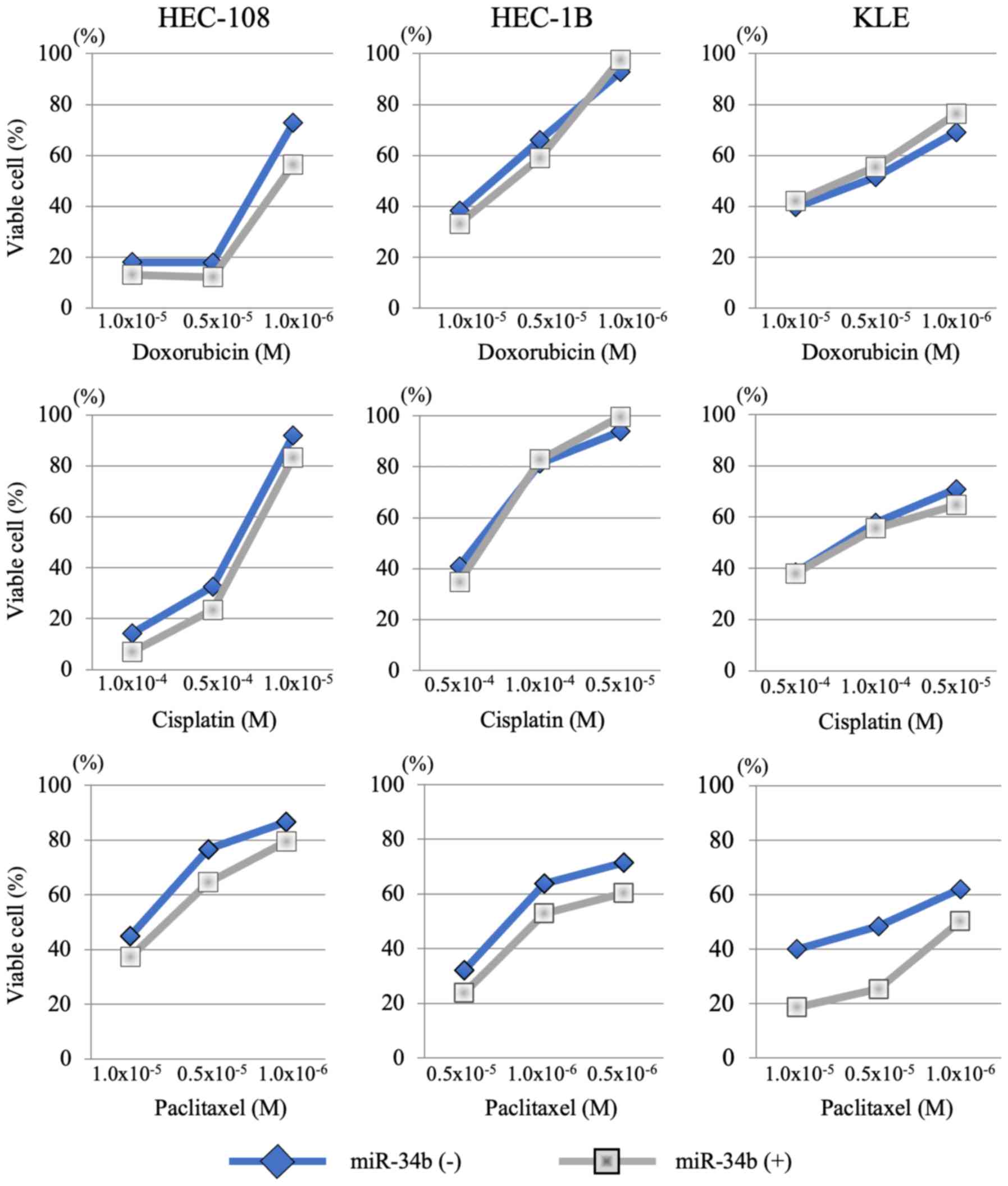
There was little change in chemosensitivity to cisplatin and
doxorubicin in HEC-108, HEC-1B and KLE cell lines with and without
miR-34b overexpression at the concentrations examined (Fig. 7). However, paclitaxel sensitivity
was enhanced by miR-34b transfection in all three cell lines
(Fig. 7). Treatment with miR-34b
changed the IC50 for paclitaxel from 0.9×10−5
to 0.8×10−5 in HEC-108 cells, from 0.3×10−5
to 1.0×10−6 in HEC-1B cells and from 0.4×10−5
to 1.0×10−6 in KLE cells (Table I).
 | Table IIC50 (M) of anticancer
drugs for inhibition of growth of endometrial cancer cells with or
without miR-34b treatment. |
Table I
IC50 (M) of anticancer
drugs for inhibition of growth of endometrial cancer cells with or
without miR-34b treatment.
| Treatment | HEC-108 cells | HEC-1B cells | KLE cells |
|---|
| Doxorubicin, M |
0.3×10−5 |
0.2×10−5 |
0.7×10−5 |
0.6×10−5 |
0.5×10−5 |
0.6×10−5 |
| Cisplatin, M |
0.4×10−4 |
0.3×10−4 |
0.4×10−5 |
0.4×10−5 |
0.3×10−4 |
0.3×10−4 |
| Paclitaxel, M |
0.9×10−5 |
0.8×10−5 |
0.3×10−5 |
1.0×10−6 |
0.4×10−5 |
1.0×10−6 |
| miR-34b | (−) | (+) | (−) | (+) | (−) | (+) |
Effect of miR-34b on chemosensitivity in
vivo
To investigate whether miR-34b has an effect on
chemosensitivity in endometrial cancer in vivo, as well as
in vitro, a xenograft tumor model was established. After 4
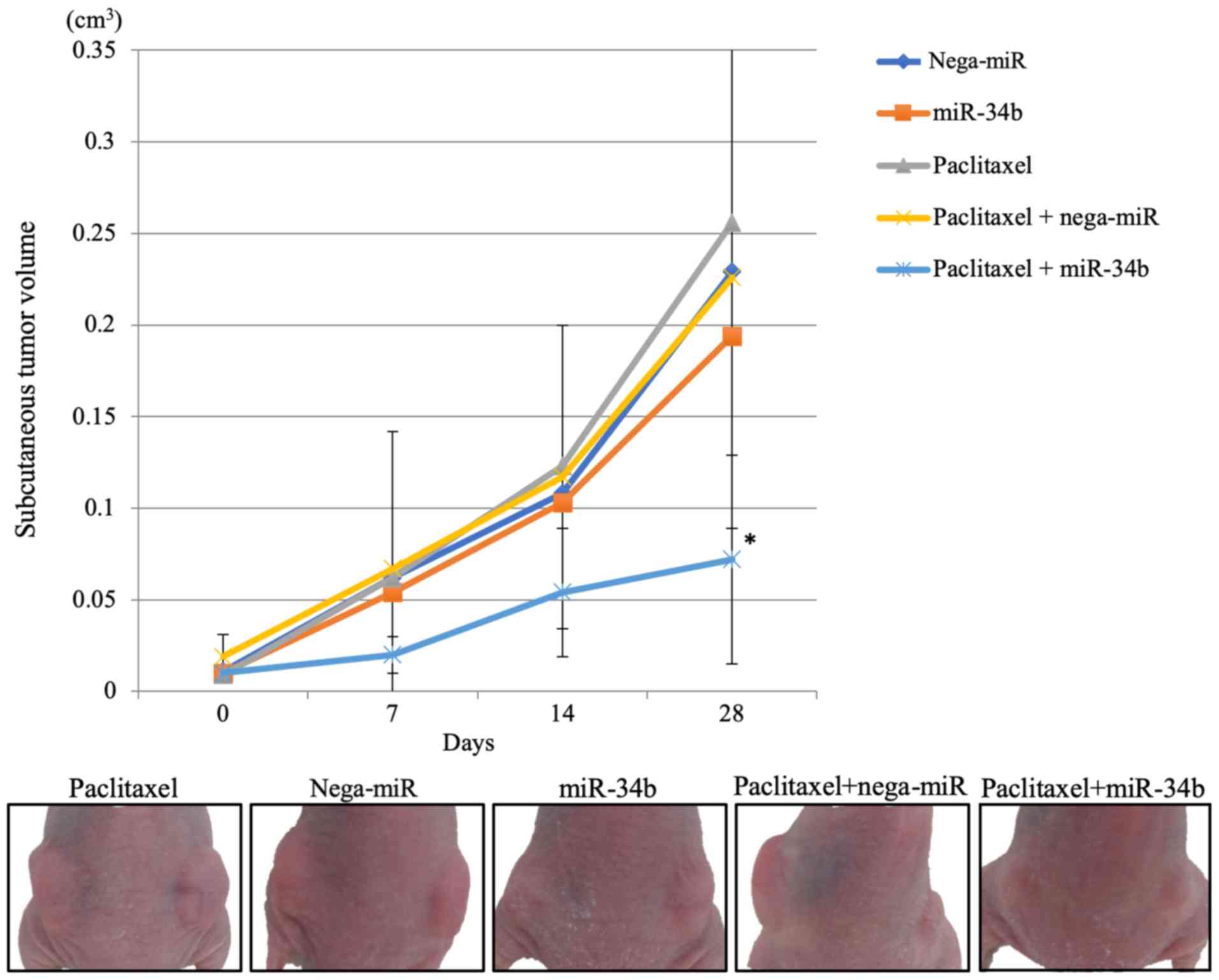
weeks of treatment, tumor growth was significantly suppressed in
the paclitaxel + miR-34b group compared with in the paclitaxel +
nega-miR group (Fig. 8). After
measuring the tumor diameter, all xenograft tumors were removed and
paraffin-embedded sections were prepared. MET and MYC protein
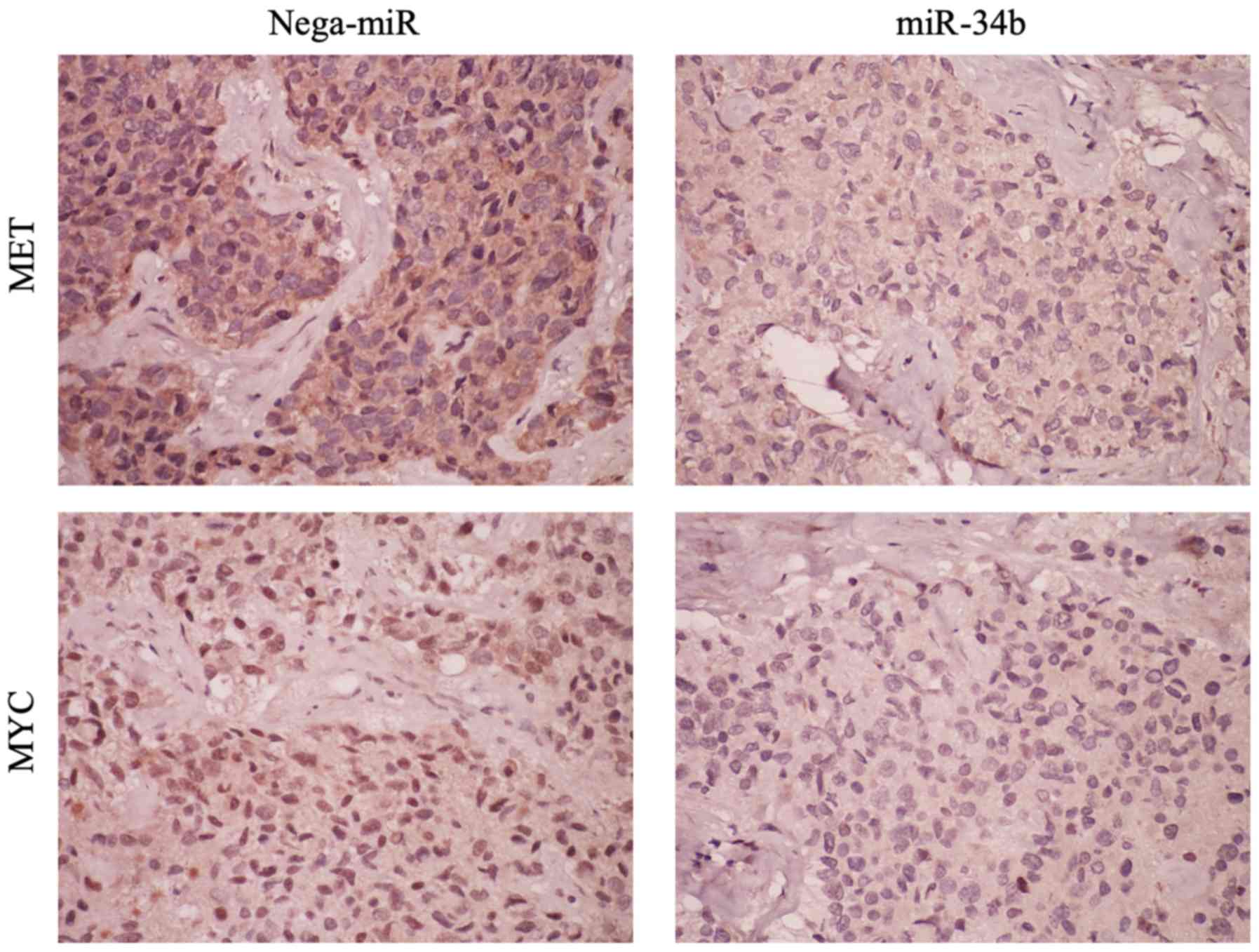
levels were examined by immunohistochemistry. Following miR-34b
treatment, both MET and MYC expression were
suppressed, indicating that miR-34b has an effect on these genes
in vivo as well as in vitro (Fig. 9). Subsequently, it was examined
whether miR-34b affects cell growth and apoptosis in vivo
using immunohistochemical staining of Ki67, caspase-3 and PARP.
Tumor growth was suppressed in the paclitaxel + miR-34b treatment
group compared with the paclitaxel + nega-miR treatment group;
however, no significant difference was observed for Ki67 staining
(Fig. 10A). Pro caspase-3,
cleaved caspase-3 and PARP staining was positive after treatment
with paclitaxel + miR-34b, but not after treatment with paclitaxel
+ nega-miR (Fig. 10B). Caspase-3
serves a central role in the execution of cancer cell apoptosis and
is responsible for cleavage of PARP during cell death (29). Cleavage of caspase-3 and PARP to
its active form is considered to be an important event in cancer
cell apoptosis. Therefore, these results demonstrated that
paclitaxel + miR-34b combination treatment induces apoptosis.
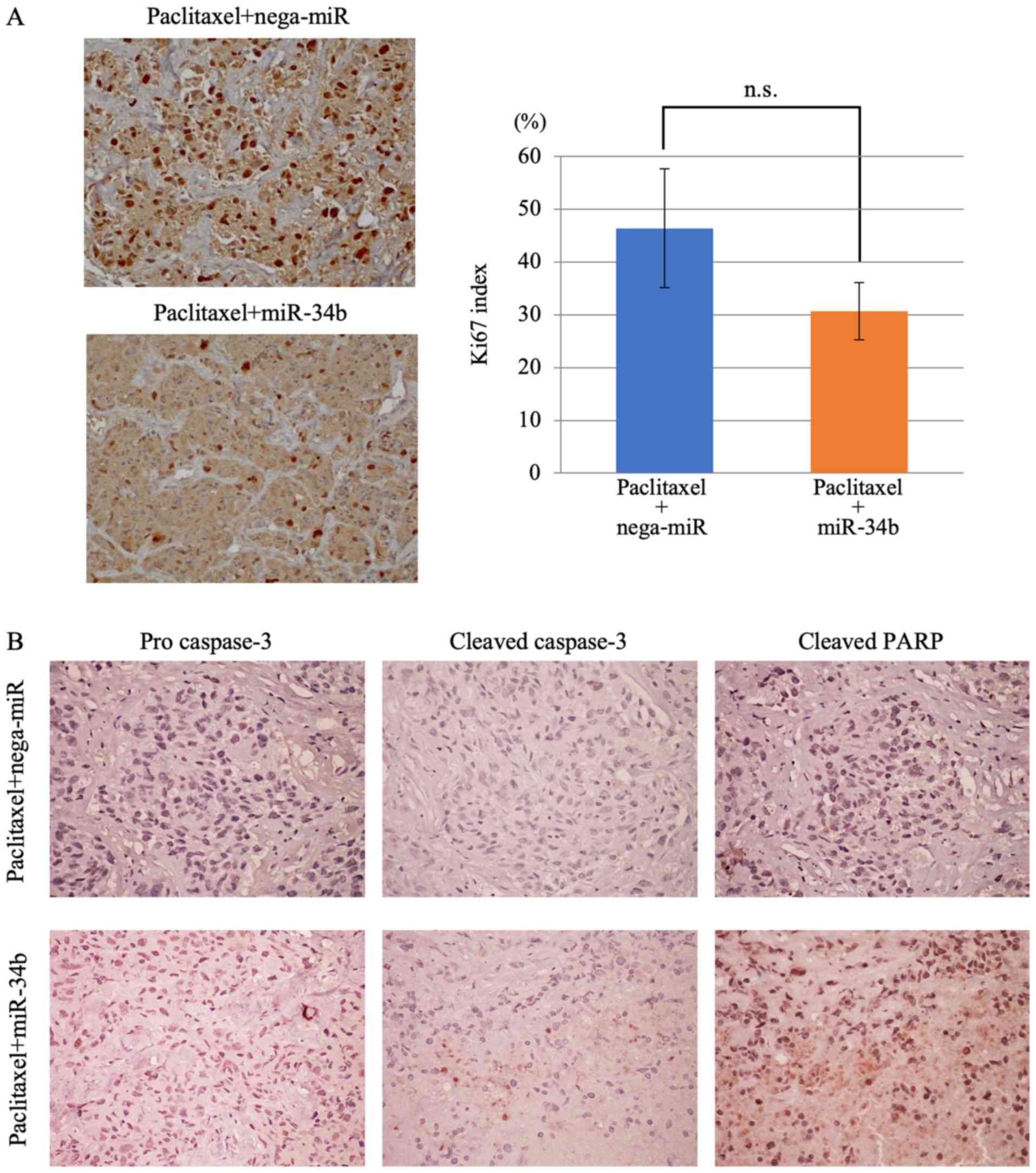 | Figure 10Decreased proliferative index and
increased apoptosis following paclitaxel + miR-34b combination
treatment. (A) Ki67 staining of xenograft tumors. Proliferative
indexes in the paclitaxel + nega-miR and paclitaxel + miR-34b
groups and representative images from each group are shown.
Original magnification, ×200. In each group, staining images were
captured in five random areas, and positively stained cells were
counted in all areas. Each bar in the graph represents the mean ±
SD. The significance of differences between groups (paclitaxel +
nega-miR vs. paclitaxel + miR-34b) was assessed using a
Mann-Whitney U test. (B) Detection of apoptosis-related proteins in
xenograft tumors. Few tumor cells in the paclitaxel + nega-miR
group exhibited positive staining for pro caspase-3, cleaved
caspase-3 and cleaved PARP, whereas tumor cells in the paclitaxel +
miR-34b group exhibited weak positive procaspase-3 staining, and
positive staining for cleaved caspase-3 and cleaved PARP,
indicating that apoptosis was increased. Original magnification,
×200. miR-34b, microRNA-34b; nega-miR, negative control microRNA;
n.s., not significant; PARP, poly(ADP-ribose) polymerase 1. |
Discussion
Aberrant DNA methylation of promoter regions is
widely observed in human cancer and results in suppression of the
expression of genes and miRNA (7,8). In
our previous studies, it was demonstrated that genes silenced by
DNA methylation serve important roles in the progression and
chemosensitivity of endometrial cancer (23-26).
MiR-34b, one of the tumor suppressor miRNA is downregulated in
cancer by aberrant DNA methylation (19-22).
MET and MYC, the predicted target genes of miR-34b,
serve important roles in cancer progression and metastasis,
including in migration, invasion, cell proliferation and cell cycle
progression (18,30-34).
The results of the present study demonstrated that
miR-34b expression was downregulated in four endometrial cancer
cells following DNA methylation. Restoration of miR-34b reduced
cell growth, invasion and migration, and increased cell cycle
arrest. Several reports have demonstrated that the 3′-UTR of
MYC contains a binding site for miR-34b, and transient
miR-34b expression decreases the expression of endogenous
MYC (31,32,35).
Increased MYC expression induces growth and proliferation
and strongly sensitizes cells toward proapoptotic stimuli,
including DNA damaging agents (35,36).
Consequently, downregulation of MYC is required to ensure
cell cycle arrest and survival of cells in response to DNA damage
(35,36). Similarly, the ability to
downregulate MYC in the presence of strong mitogenic signals
is required for oncogene-induced senescence, and may therefore
constitute a tumor-suppressive mechanism (37). G1 arrest and reduced
cell growth in endometrial cancer cells were observed in the
present study, which may have been induced by downregulation of
MYC expression due to miR-34b transfection.
Furthermore, reduced cell invasion of endometrial
cancer cells was observed. Previous reports have revealed that
MET oncogene expression is suppressed by miR-34b
overexpression in lung, colon and ovarian cancer cells (19,30,38).
MET small interfering RNA reduces adhesion, invasion, metastasis
and tumor burden in a cancer xenograft model, and miR-34b
downregulation is associated with metastasis in human cancers
(30,39). The present results suggest that the
miR-34b-MET signaling pathway may serve an important role in
invasion and migration of endometrial cancer cells, as has been
demonstrated in other types of cancer.
MET is associated with chemosensitivity of
cancer cells, with recent studies revealing that inhibition of
MET expression increases paclitaxel sensitivity and
chemotherapy-induced apoptosis (33,34).
An effect of a MET inhibitor and paclitaxel combination treatment
has been identified in ovarian cancer cells and glioma cells
(33,39). This combination treatment results
in inhibition of cell growth and widespread death of cancer cells.
It was concluded that reduced expression of MET could block
progression to S phase and DNA synthesis, and could abrogate
G2/M phase arrest in cancer cells by chemotherapy.
MET gene downregulation via miR-34b overexpression may have
caused a similar chemosensitivity change to paclitaxel in
endometrial cancer cells in the present study. Therefore, the
present results are consistent with previous reports (33,39).
Cells are most sensitive to chemotherapy during the G2/M
phase, and less sensitive in G1 and S phases (40). In a previous study, it has been
demonstrated that after exposure to chemotherapy, G2/M
phase arrest is the main protective effect that allows cells to
repair DNA damage, maintain genetic stability of their daughter
cells, and avoid mutations in cellular DNA (40). Drugs or agents that abrogate
G2/M arrest after chemotherapy will increase
chemosensitivity, as illustrated by abrogation of the
G2/M checkpoint and chemosensitization of ovarian cancer
cells following treatment with a MET inhibitor (33). The present results demonstrated
that miR-34b overexpression induces G1 phase arrest and
decreases cell numbers in S phase in endometrial cancer cells, and
this redistribution of the cell cycle may establish a
chemosensitive state. This may explain why only chemosensitivity to
paclitaxel was increased, but not that to cisplatin and
doxorubicin, since paclitaxel affects cells in the G2/M
phase (41).
Furthermore, it was revealed that miR-34b
overexpression enhanced paclitaxel sensitivity in endometrial
cancer cells in vivo, as well as in vitro. Following
paclitaxel + miR-34b combination treatment, induction of apoptosis
was observed compared with paclitaxel + nega-miR treatment.
Therefore, MET downregulation via overexpression of miR-34b
may increase the sensitivity to treatment with paclitaxel as a
single agent.
Suppression of miR-34b and increased MET
expression were identified in endometrial cancer tissues compared
with normal endometrium tissues (data not shown), although the
sample size was small. Therefore, aberrant miR-34b suppression may
occur in endometrial cancer cells and tissues. Numerous targeted
inhibitors of MET have been developed, and clinical trials
of the efficiency of these inhibitors for solid tumor treatment are
being conducted (42-44). The MET gene is a key target
for cancer therapy, and the present data support these studies.
In summary, the present study identified miR-34b as
an upregulated miRNA from among 821 candidate miRNAs after
demethylation treatment in four endometrial cancer cell lines.
Inhibition of cell growth, invasion, migration and cell cycle
arrest were observed following miR-34b overexpression, and
increased sensitivity to paclitaxel was reported in vitro
and in vivo. The present study only included preliminary
data for miR-34b and MET expression in endometrial cancer
tissues, and a large scale analysis is required. However, the
present results may contribute to the development of endometrial
cancer treatment with a MET inhibitor, miR-34 mimic or a
demethylation agent in combination with an anticancer drug.
Funding
The present study was supported by grants from JSPS
KAKENHI Grants-in-Aid for Scientific Research (C) (grant nos.
15K10727 and 16K11154; to MY and KB).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KB designed the experiments, and analyzed and
interpreted the data. MY performed the experiments and analyzed the
data. DA contributed to statistical analysis and interpretation of
the data. All authors drafted, reviewed and edited the manuscript,
and all authors agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Animal protocols were in compliance with the Guide
for the Care and Use of Laboratory Animals at Keio University of
School of Medicine. All experiments were approved by the Ethics
Committee of Keio University School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
miRNA
|
microRNA
|
|
nega-miR
|
negative control miRNA
|
|
5-aza
|
5-Aza-2′-deoxycytidine
|
|
DAB
|
3,3′-diaminobenzidine
|
Acknowledgments
The authors would like to thank Professor Shiro
Nozawa and Dr Isamu Ishiwata (Keio University, Tokyo, Japan) for
providing SNG-II and HHUA cells, and Dr Masato Nishida (National
Kasumigaura Hospital, Ibaraki, Japan) for providing Ishikawa
cells.
References
|
1
|
Lee JM, Yoo JK, Yoo H, Jung HY, Lee DR,
Jeong HC, Oh SH, Chung HM and Kim JK: The novel miR-7515 decreases
the proliferation and migration of human lung cancer cells by
targeting c-Met. Mol Cancer Res. 11:43–53. 2013. View Article : Google Scholar
|
|
2
|
Kozloski GA, Jiang X, Bhatt S, Ruiz J,
Vega F, Shaknovich R, Melnick A and Lossos IS: miR-181a negatively
regulates NF-κB signaling and affects activated B-cell-like diffuse
large B-cell lymphoma pathogenesis. Blood. 127:2856–2866. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ivey KN and Srivastava D: MicroRNAs as
regulators of differ-entiation and cell fate decisions. Cell Stem
Cell. 7:36–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bueno MJ and Malumbres M: MicroRNAs and
the cell cycle. Biochim Biophys Acta. 1812:592–601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rachagani S, Macha MA, Menning MS, Dey P,
Pai P, Smith LM, Mo YY and Batra SK: Changes in microRNA (miRNA)
expression during pancreatic cancer development and progression in
a genetically engineered KrasG12D;Pdx1-Cre mouse (KC) model.
Oncotarget. 6:40295–40309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balacescu O, Sur D, Cainap C, Visan S,
Cruceriu D, Manzat-Saplacan R, Muresan MS, Balacescu L, Lisencu C
and Irimie A: The Impact of miRNA in colorectal cancer progression
and its liver metastases. Int J Mol Sci. 19:37112018. View Article : Google Scholar
|
|
8
|
Mavrogiannis AV, Kokkinopoulou I, Kontos
CK and Sideris DC: Effect of vinca alkaloids on the expression
levels of microRNAs targeting apoptosis-related genes in breast
cancer cell lines. Curr Pharm Biotechnol. 19:1076–1086. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao W, Zhang Y, Li S, Fan Q, Qiu M, Wang
Y, Li Y, Ji X, Yang Y, Sang Z, et al: miR1075p promotes tumor
proliferation and invasion by targeting estrogen receptoralpha in
endometrial carcinoma. Oncol Rep. 41:1575–1585. 2019.
|
|
10
|
Li L, Shou H, Wang Q and Liu S:
Investigation of the potential theranostic role of KDM5B/miR-29c
signaling axis in paclitaxel resistant endometrial carcinoma. Gene.
694:76–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shu S, Liu X, Xu M, Gao X, Chen S, Zhang L
and Li R: MicroRNA-320a acts as a tumor suppressor in endometrial
carcinoma by targeting IGF-1R. Int J Mol Med. 43:1505–1512.
2019.PubMed/NCBI
|
|
12
|
Tan A, Luo R and Ruan P: miR-495 promotes
apoptosis and inhibits proliferation in endometrial cells via
targeting PIK3R1. Pathol Res Pract. 215:594–599. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engkvist ME, Stratford EW, Lorenz S,
Meza-Zepeda LA, Myklebost O and Munthe E: Analysis of the miR-34
family functions in breast cancer reveals annotation error of
miR-34b. Sci Rep. 7:96552017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang LL, Sun BF, Huang LR, Yuan HB, Zhang
S, Chen J, Yu ZJ and Luo H: Potent inhibition of miR-34b on
migration and inva-sion in metastatic prostate cancer cells by
regulating the TGF-β pathway. Int J Mol Sci. 18:27622017.
View Article : Google Scholar
|
|
15
|
Wang Y, Wu Z and Hu L: The regulatory
effects of metformin on the [SNAIL/miR-34]:[ZEB/miR-200] system in
the epithelial-mesenchymal transition (EMT) for colorectal cancer
(CRC). Eur J Pharmacol. 834:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Metheetrairut C, Chotigavanich C,
Amornpichetkul K, Keskool P, Ongard S and Metheetrairut C:
Expression levels of miR-34-family microRNAs are associated with
TP53 mutation status in head and neck squamous cell carcinoma. Eur
Arch Otorhinolaryngol. 276:521–533. 2019. View Article : Google Scholar
|
|
17
|
Zhang L, Wang L, Dong D, Wang Z, Ji W, Yu
M, Zhang F, Niu R and Zhou Y: MiR-34b/c-5p and the neurokinin-1
receptor regulate breast cancer cell proliferation and apoptosis.
Cell Prolif. 52:e125272019. View Article : Google Scholar
|
|
18
|
Yang L, Song X, Zhu J, Li M, Ji Y, Wu F,
Chen Y, Cui X, Hu J, Wang L, et al: Tumor suppressor microRNA-34a
inhibits cell migration and invasion by targeting
MMP-2/MMP-9/FNDC3B in esophageal squamous cell carcinoma. Int J
Oncol. 51:378–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Corney DC, Hwang CI, Matoso A, Vogt M,
Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH,
Hermeking H and Nikitin AY: Frequent downregulation of miR-34
family in human ovarian cancers. Clin Cancer Res. 16:1119–1128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Javeri A, Ghaffarpour M, Taha MF and
Houshmand M: Downregulation of miR-34a in breast tumors is not
associated with either p53 mutations or promoter hypermethylation
while it correlates with metastasis. Med Oncol. 30:4132013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okada N, Lin CP, Ribeiro MC, Biton A, Lai
G, He X, Bu P, Vogel H, Jablons DM, Keller AC, et al: A positive
feedback between p53 and miR-34 miRNAs mediates tumor suppression.
Genes Deve. 28:438–450. 2014. View Article : Google Scholar
|
|
22
|
Xie K, Liu J, Chen J, Dong J, Ma H, Liu Y
and Hu Z: Methylation-associated silencing of microRNA-34b in
hepatocellular carcinoma cancer. Gene. 543:101–107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawaguchi M, Yanokura M, Banno K,
Kobayashi Y, Kuwabara Y, Kobayashi M, Nomura H, Hirasawa A, Susumu
N and Aoki D: Analysis of a correlation between the BRAF V600E
mutation and abnormal DNA mismatch repair in patients with sporadic
endometrial cancer. Int J Oncol. 34:1541–1547. 2009.PubMed/NCBI
|
|
24
|
Yanokura M, Banno K, Kawaguchi M, Hirao N,
Hirasawa A, Susumu N, Tsukazaki K and Aoki D: Relationship of
aberrant DNA hypermethylation of CHFR with sensitivity to taxanes
in endometrial cancer. Oncol Rep. 17:41–48. 2007.
|
|
25
|
Yanokura M, Banno K, Susumu N, Kawaguchi
M, Kuwabara Y, Tsukazaki K and Aoki D: Hypermethylation in the p16
promoter region in the carcinogenesis of endometrial cancer in
Japanese patients. Anticancer Res. 26:851–856. 2006.PubMed/NCBI
|
|
26
|
Banno K, Yanokura M, Susumu N, Kawaguchi
M, Hirao N, Hirasawa A, Tsukazaki K and Aoki D: Relationship of the
aberrant DNA hypermethylation of cancer-related genes with
carcinogenesis of endometrial cancer. Oncol Reps. 16:1189–1196.
2006.
|
|
27
|
Agostini M and Knight RA: miR-34: From
bench to bedside. Oncotarget. 5:872–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Mondal A and Bennett LL: Resveratrol
enhances the efficacy of sorafenib mediated apoptosis in human
breast cancer MCF7 cells through ROS, cell cycle inhibition,
caspase 3 and PARP cleavage. Biomed Pharmacother. 84:1906–1914.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Migliore C, Petrelli A, Ghiso E, Corso S,
Capparuccia L, Eramo A, Comoglio PM and Giordano S: MicroRNAs
impair MET-mediated invasive growth. Cancer Res. 68:10128–10136.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seviour EG, Sehgal V, Lu Y, Luo Z, Moss T,
Zhang F, Hill SM, Liu W, Maiti SN, Cooper L, et al: Functional
proteomics identifies miRNAs to target a p27/Myc/phospho-Rb
signature in breast and ovarian cancer. Oncogene. 35:691–701. 2016.
View Article : Google Scholar
|
|
32
|
Siemens H, Jackstadt R, Hunten S, Kaller
M, Menssen A, Gotz U and Hermeking H: miR-34 and SNAIL form a
double-negative feedback loop to regulate epithelial-mesenchymal
transitions. Cell Cycle. 10:4256–4271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J and Cheng JX: c-Met inhibition
enhances chemosensitivity of human ovarian cancer cells. Clin Exp
Pharmacol Physiol. 44:79–87. 2017. View Article : Google Scholar
|
|
34
|
Yang L, Song Z, Wang X, Yang W, Wang M and
Liu H: Huaier extract enhances the treatment efficacy of paclitaxel
in breast cancer cells via the NF-κB/IκBα pathway. Oncol Rep.
38:3455–3464. 2017.PubMed/NCBI
|
|
35
|
Kress TR, Cannell IG, Brenkman AB, Samans
B, Gaestel M, Roepman P, Burgering BM, Bushell M, Rosenwald A and
Eilers M: The MK5/PRAK kinase and Myc form a negative feedback loop
that is disrupted during colorectal tumorigenesis. Mol Cell.
41:445–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cannell IG, Kong YW, Johnston SJ, Chen ML,
Collins HM, Dobbyn HC, Elia A, Kress TR, Dickens M, Clemens MJ, et
al: p38 MAPK/MK2-mediated induction of miR-34c following DNA damage
prevents Myc-dependent DNA replication. Proc Natl Acad Sci USA.
107:5375–5380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hydbring P, Bahram F, Su Y, Tronnersjo S,
Hogstrand K, von der Lehr N, Sharifi HR, Lilischkis R, Hein N, Wu
S, et al: Phosphorylation by Cdk2 is required for Myc to repress
Ras-induced senescence in cotransformation. Proc Natl Acad Sci USA.
107:58–63. 2010. View Article : Google Scholar
|
|
38
|
Watanabe K, Emoto N, Hamano E, Sunohara M,
Kawakami M, Kage H, Kitano K, Nakajima J, Goto A, Fukayama M, et
al: Genome structure-based screening identified epigenetically
silenced microRNA associated with invasiveness in non-small-cell
lung cancer. Int J Cancer. 130:2580–2590. 2012. View Article : Google Scholar
|
|
39
|
Chu SH, Ma YB, Feng DF, Zhang H, Qiu JH
and Zhu ZA: c-Met antisense oligodeoxynucleotides increase
sensitivity of human glioma cells to paclitaxel. Oncol Rep.
24:189–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shah MA and Schwartz GK: Cell
cycle-mediated drug resistance: An emerging concept in cancer
therapy. Clin Cancer Res. 7:2168–2181. 2001.PubMed/NCBI
|
|
42
|
Choi J, Lee HE, Kim MA, Jang BG, Lee HS
and Kim WH: Analysis of MET mRNA expression in gastric cancers
using RNA in situ hybridization assay: Its clinical implication and
comparison with immunohistochemistry and silver in situ
hybridization. PLoS One. 9:e1116582014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu P, Du F, Yao M, Lv K and Liu Y:
MicroRNA-185 inhibits proliferation by targeting c-Met in human
breast cancer cells. Exp Ther Med. 8:1879–1883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rubin MA: Insights into the mechanism of
organ-specific cancer metastasis. Cancer Discov. 4:1262–1264. 2014.
View Article : Google Scholar : PubMed/NCBI
|