Introduction
ETS variant 1 (ETV1; previously also known as ER81)
is a transcription factor endowed with an ETS domain that binds to
DNA sequences with a GGA(A/T) core (1-3).
Nucleotides flanking this core or neighboring DNA-binding sites for
interaction partners determine which ETS protein can bind to a
specific gene regulatory element (4,5).
However, ETV1 has two close homologs, ETV4 and ETV5, and they can
bind potentially to the same sites throughout the genome. However,
tissue-specific expression and possibly different interactomes
endow each of these three transcription factors with a specific
role during development (6).
Indeed, compared to ETV4 and ETV5 ablation,
ETV1 knockout leads to different phenotypic alterations in
mice, such as deficient connection between muscle sensory and
spinal motor neurons, abnormal numbers of spindles in limb muscles,
the lack of Pacinian corpuscle limb mechanoreceptors, cardiac
conduction defects, and the abnormal development of the ventricular
conduction system (7-10). Furthermore, the activity of ETV1 is
heavily regulated by post-translational modification, which
includes phosphorylation, acetylation and ubiquitylation (11-18);
however, the mechanisms thorough which these post-translational
modifications affect the developmental functions of ETV1 remain
elusive.
ETV1 has been implicated in tumorigenesis, most
prominently in prostate cancer (6). Chromosomal translocations occur in
~5-10% of all human prostate tumors that lead to the overexpression
of ETV1 (19). Furthermore, ETV1
overexpression is associated with an increased Gleason score and
disease recurrence, suggesting that ETV1 promotes the development
of aggressive prostate cancer in particular (20-22).
Transgenic mice that prostate-specifically overexpress ETV1
develop prostatic intraepithelial neoplasia and the simultaneous
homozygous loss of the tumor suppressor, Pten, leads to the
development of invasive adenocarcinomas (21-23).
Likewise, ETV1 transgenic mice develop prostate carcinoma
when Jumonji C domain-containing (JMJD)2A is concurrently
over-expressed in a heterozygous Pten knockout background
(24). JMJD2A is a member of the
JMJD protein family that encompasses numerous, diverse histone
demethylases (25-27). The JMJD2A enzyme particularly
demethylates tri-methylated lysine 9 and 36 on histone H3, and can
thereby induce changes in gene transcription (28-30).
It also binds directly to ETV1 and serves the function of an ETV1
coactivator (24,31).
Notably, JMJD2A is robustly expressed in several
established colorectal cancer cell lines (32). In addition, the downregulation of
JMJD2A in HCT116, DLD-1 and HT-29 human colorectal cancer cells has
been shown to reduce their proliferative potential and increase
apoptosis (33), while a small
molecule inhibitor of JMJD2A catalytic activity has been shown to
suppress the growth of HCT116 colorectal cancer cells (34). These data suggest that JMJD2A
promotes colorectal cancer growth. Furthermore, ETV1 is expressed
in human colorectal cancer cell lines and tumors (35,36).
Hence, the present study examined the possible association between
ETV1 and JMJD2A in colon cancer. Another histone demethylase,
JMJD1A (37,38), was also included in the present
study, since this enzyme is reportedly expressed in HCT116 cells
and is overexpressed in human colorectal tumors (39-42).
Materials and methods
Cell culture and transfection
HCT116 cells (CCL-247; American Type Culture
Collection), 293T cells (CRL-3216; American Type Culture
Collection) and LNCaP cells (CRL-1740; American Type Culture
Collection) were cultured at 37°C in a 5% CO2 atmosphere
in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum
(43). The calcium phosphate
coprecipitation method was employed to transfect 293T cells
cultured in poly-L-lysine coated 6-cm plates (44), while transfection of HCT116 cells
grown in 12-wells was accomplished with the help of 2 µg
polyethylenimine (45).
Retrovirus
In order to downregulate a gene, respective shRNA
was cloned into the pSIREN-RetroQ retroviral vector (Clontech
Laboratories, Inc.). The sequences targeted by ETV1 shRNAs were as
previously published (46), while
JMJD1A shRNA#1 targeted the sequence 5′-GCAGGUGUCAAUAGUGAUA-3′ and
shRNA#2 the sequence 5′-GUAGACCUAGUUAAUUGUA-3′. The sequence of the
control shRNA was 5′-CAACAAGAU GAAGAGCACCAA-3′, which has at least
5 mismatches to known human genes. The production of retrovirus in
293T cells has been previously described (47). HCT116 cells were then infected two
or three times, temporally spaced by 12 h, with the indicated
retrovirus, grown for 24 h in fresh media and then selected with 1
µg/ml puromycin for 2-3 days. Changes in protein expression
were determined by western blot analysis (48); for this, cells were lysed in
Laemmli sample buffer, resulting protein extracts run on SDS
polyacrylamide gels and proteins transferred to PVDF membranes. For
overexpression experiments, HA-tagged FOXQ1 and TBX6 were cloned
into the pQCXIH retroviral vector (Clontech). HCT116 cells were
infected twice within 12 h, grown for 24 h with fresh media,
selected with 200 µg/ml hygromycin B for 3 days and then
expanded under continuous hygromycin B selection. To detect
expression of proteins in retro-virally transduced cells, rabbit
polyclonal antibodies directed against ETV1 [#959; as previously
described (46); 1:1,000
dilution], actin (A2066; Sigma-Aldrich; Merck KGaA; 1:5,000
dilution) or JMJD1A (NB100-77282; Novus Biologicals; 1:1,500
dilution) and goat polyclonal antibodies directed against FOXQ1
(NB100-1283; Novus Biologicals; 1:1,000 dilution) or TBX6 (AF4744;
R&D Systems; 1:1,000 dilution) were used. Secondary goat
anti-rabbit (170-6515; Biorad) or mouse anti-goat (sc-2768; Santa
Cruz Biotechnology, Inc.) antibodies coupled to horse-radish
peroxidase were used at a 1:3,000 dilution. All antibody
incubations were performed in TRIS-buffered saline/0.05% Tween-20
with 4% non-fat dry milk. Detection was done with enhanced
chemiluminescence using an ECL kit (GERPN2106; Sigma-Aldrich; Merck
KGaA).
Cell growth and clonogenic assays
A total of 2,000 (for Fig. 1) or 2,400 (for Fig. 9) cells were seeded in 96-wells
(49) and growth was determined
using the PrestoBlue Cell Viability kit (A13262, Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
recommendations. For clonogenic assays, 3,000 (for Fig. 1) or 2,400 (for Fig. 9) cells were spread into 6-wells
(50) and the formation of
colonies was assayed after 8 days by staining with 0.4% crystal
violet (C0775; Sigma-Aldrich; Merck KGaA) in 10% methanol/10%
glacial acetic acid for 10 min at room temperature.
Analysis of mRNA expression levels in
public databases
All respective bioinformatics analyses were
performed using the Oncomine tool (51). Data for mRNA expression levels were
downloaded through the website www.oncomine.org and imported into GraphPad Prism 6
for Mac OS X for analysis.
Co-immunoprecipitation and GST pulldown
experiments
Human 293T cells were transfected with the following
amounts of plasmids: 3 µg Flag-tagged JMJD expression vector
or empty vector pEV3S (52), 1
µg 6Myc-tagged ETV1 expression vector or empty vector
pCS3+-6Myc (kindly provided by Professor Tony Hunter,
Salk Institute), and 5 µg pBluescript KS+
(Stratagene; Agilent Technologies, Inc.). At 2 days following
transfection, cells were lysed in 650 µl of 50 mM Tris-HCl
(pH 7.4), 150 mM NaCl, 0.5% Igepal CA-630, 50 mM NaF, 0.1 mM
Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 2
µg/ml aprotinin, 10 µg/ml leupeptin, 1 µg/ml
pepstatin A, 0.1 mM dithiothreitol and immunoprecipitations were
performed with 1 µg of anti-Flag M2 (F1804; Sigma-Aldrich;
Merck KGaA) mouse monoclonal antibody (53); the nature of the beads used,
incubation times and washing procedures, including centrifugation
steps, for the immunoprecipitations were as previously described
(54). Following western blot
analysis, as described above, with mouse monoclonal anti-Myc 9E10
antibody (M4439; Sigma-Aldrich; Merck KGaA; 1:3,000 dilution) and
secondary goat anti-mouse antibodies coupled to horseradish
peroxidase (170-6516, Bio-Rad Laboratories, Inc.; 1:3,000
dilution), the blots were developed utilizing chemiluminescence
(55). For endogenous
coimmunoprecipitation, ETV1 was immunoprecipitated with 0.5
µg of rabbit poly-clonal antibody H-70 (sc-28681; Santa Cruz
Biotechnology, Inc.) and JMJD1A was detected with rabbit polyclonal
anti-body NB100-77282 (Novus Biologicals; 1:1,500 dilution).
Glutathione S-transferase (GST) pulldown experiments were
performed essentially as described (56) utilizing as a binding and washing
buffer phosphate-buffered saline supplemented with 0.05% Tween-20,
1 mM dithiothreitol, 0.2 mM phenyl-methylsulfonyl fluoride, 2
µg/ml aprotinin, 10 µg/ml leupeptin and 1
µg/ml pepstatin A. The GST-ETV1 fusion protein or the GST
moiety was expressed in Escherichia coli and puri-fied with
the help of glutathione agarose, while Flag- and His-tagged JMJD1A
was produced in baculovirus-infected Sf9 cells and purified with
the help of Ni2+-NTA agarose (24).
Luciferase assays
The following amounts of plasmids were used to
transfect the HCT116 cells: 0.25 µg matrix
metallo-proteinase (MMP)1(-525/+15) luciferase reporter
plasmid (12), 0.75 µg
pBluescript KS+ (Stratagene; Agilent Technologies,
Inc.), 20 ng ETV1 expression vector or empty vector pEV3S (52), and 50 ng Flag-tagged JMJD
expression vector or empty vector pEV3S (52). For experiments comparing pGL2-Basic
to the FOXQ1(-2000/+124) luciferase reporter, 100 ng pEV3S
or ETV1 expression vector, as well as 75 ng pEV3S or JMJD1A
expression vector were employed. Cells were washed once with
phosphate-buffered saline 8 h after transfection and lysed 40 h
later as previously described (57). Subsequently, light emission
following the addition of D-luciferin was measured using a
luminometer (Berthold Lumat LB9507), as previously described
(58). Please note that, similar
to recent publications (59-62),
no internal control plasmid was used to normalize for transfection
efficiency, since the authors have observed that such internal
control plasmids are often themselves affected upon transcription
factor overexpression, furthering the notion that the use of
internal control plasmids can lead to artefacts (63-66).
RNA analyses
RNA was isolated from the HCT116 cells using TRIzol
reagent (67) and reverse
transcribed into cDNA using the GoScript Reverse Transcription kit
(A5000; Promega Corporation) utilizing random p(dN)6
primers according to the manufacturer's recommendations. The
resultant cDNA was then amplified by polymerase chain reaction, as
previously described (68). The
temperature program was as follows: 97°C for 1 min; 9 cycles of
97°C for 25 sec, 65°C (−1°C per cycle) for 25 sec, 72°C for 25 sec;
16-29 cycles of 97°C for 25 sec, 56°C for 25 sec, 72°C for 25 sec;
72°C for 4 min followed by cooling down to 4°C (69). The PCR products were visualized on
ethidium bromide-stained agarose gels (70). Alternatively, qPCR was performed
with the AccuPower 2X Greenstar qPCR kit (Bioneer K-6251) with
initial denaturation at 95°C for 6 min followed by 32 cycles of
95°C for 15 sec, 56°C for 15 sec and 72°C for 35 sec. Relative
quantification was performed with the ΔΔCq method (71). The primer sequences are listed in
Table SI.
RNA-sequencing was performed in the Targeted DNA
Methylation and Mitochondrial Heteroplasmy Core of the Nathan Shock
Center of Excellence in the Biology of Aging in Oklahoma City.
Briefly, Illumina Truseq Stranded HT library generation was
performed according to the manufacturer's instructions. For
strand-specificity, the incorporation of dUTP instead of dTTP in
the second strand cDNA synthesis did not allow amplification past
this dUTP with the polymerase. Following cDNA synthesis, each
product underwent end repair process, the addition of a single
A-base and ligation of adpaters. The cDNA products were further
purified and enriched using PCR to make the final library for
sequencing. Library sizing was performed by TapeStation (Agilent
Technologies, Inc.) and the RNA-seq libraries were sequenced using
an Illumina NextSeq (2×75 bp) instrument. Following sequencing,
reads were trimmed and aligned against the GRCh38 build of the
human genome, and differential expression statistics and
correlation analyses were performed with the help of the Strand NGS
software package (Avadis). Normalization was performed with the
DESeq algorithm and a z-test was used to determine differential
expression. P-values were adjusted according to the Benjamini and
Hochberg method and deemed significant for a value <0.05
(72). No biological/technical
replicates were used. Ingenuity Pathway Analysis (Qiagen GmbH) was
employed to identify pathways affected upon ETV1 downregulation.
Sequencing data were deposited in Gene Expression Omnibus (GEO)
under accession number GSE158294.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation assays were performed
on formaldehyde-treated HCT116 cells essentially as previously
described (73). ETV1 and JMJD1A
were precipitated with the rabbit polyclonal antibodies, ab81086
(Abcam) and NB100-77282 (Novus Biologicals), respectively, while
control rabbit IgG was purchased from Santa Cruz Biotechnology,
Inc. (sc-2027). Approximately 2 µg of the antibodies were
employed for the immunoprecipitations that were performed at 4°C
over-night (74). Genomic DNA was
amplified in two steps with nested primers (75). The first PCR encompassed the
following temperature steps: 97°C for 1 min; 9 cycles of 97°C for
20 sec, 65°C (-1°C per cycle) for 20 sec, 72°C for 40 sec; 20
cycles of 97°C for 20 sec, 56°C for 20 sec, 72°C for 40 sec; 72°C
for 4 min followed by cooling down to 4°C. The second PCR was
performed in the same manner except that 31 instead of 20 cycles
were used. The primer sequences are presented in Table SII.
Statistical analyses
Means with standard deviations are presented in all
applicable figures. Statistical tests that were used are indicated
in the figure legends and included an unpaired, two-tailed
Student's t-test, as well as one-way or two-way ANOVA with post hoc
tests (Dunnett's, Sidak's or Tukey's) for multiple comparisons. All
calculations were performed with GraphPad Prism 6 for Mac OS X. A
P-value <0.05 was considered to reflect a statistical
significance difference.
Results
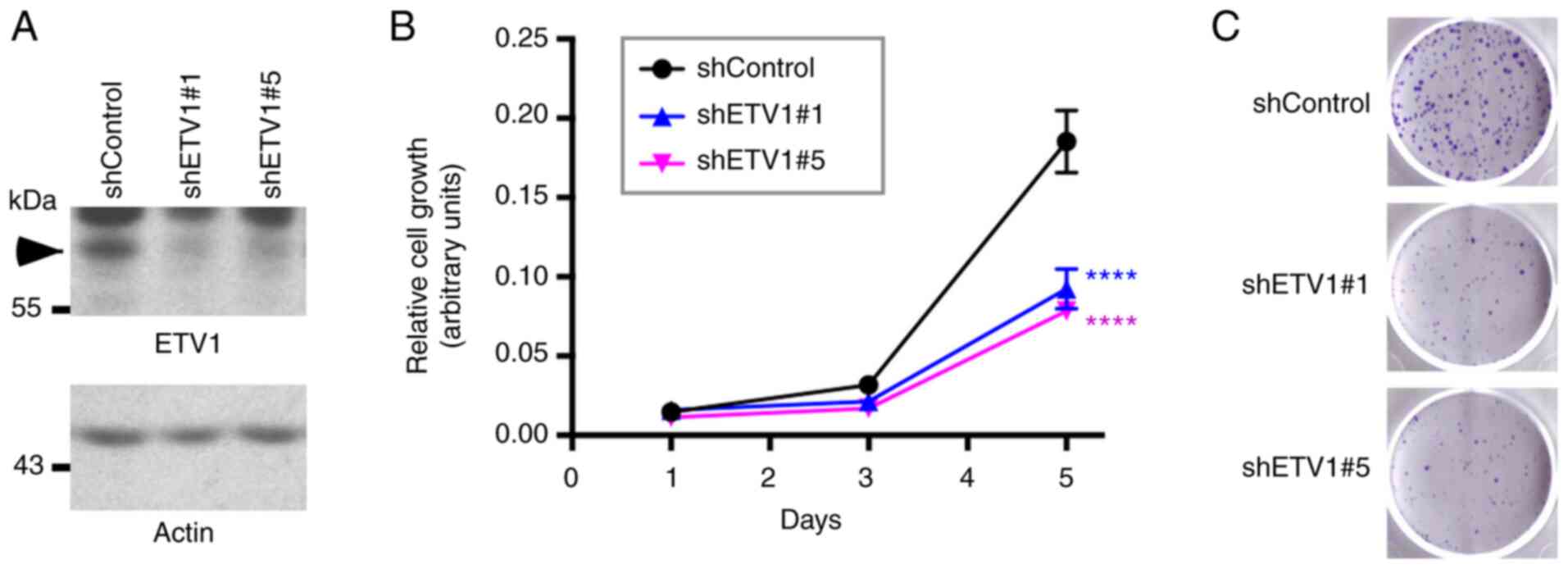
Effect of ETV1 on HCT116 cells
Previously, it was reported that ETV1 downregulation
reduced the viability of HT-29 colorectal cancer cells (76). The present study wished to assess
whether ETV1 is also a promoter of HCT116 colorectal cancer cell
growth; this particular cell line was selected for use in the
present study, as ETV1, JMJD1A and JMJD2A were all robustly
expressed in HCT116 cells (see below). Indeed, the downregulation
of ETV1 with two different shRNAs significantly decreased the
growth of HCT116 cells (Fig. 1A and
B). In addition, the cell clonogenic activity was markedly
reduced in the presence of ETV1 shRNA (Fig. 1C). Hence, similar to JMJD2A
(33) or JMJD1A (39,41,42),
ETV1 is a promoter of HCT116 colorectal cancer cell growth; this
may therefore serve as a model system to study the potential
cooperation of ETV1 with JMJD histone demethylases.
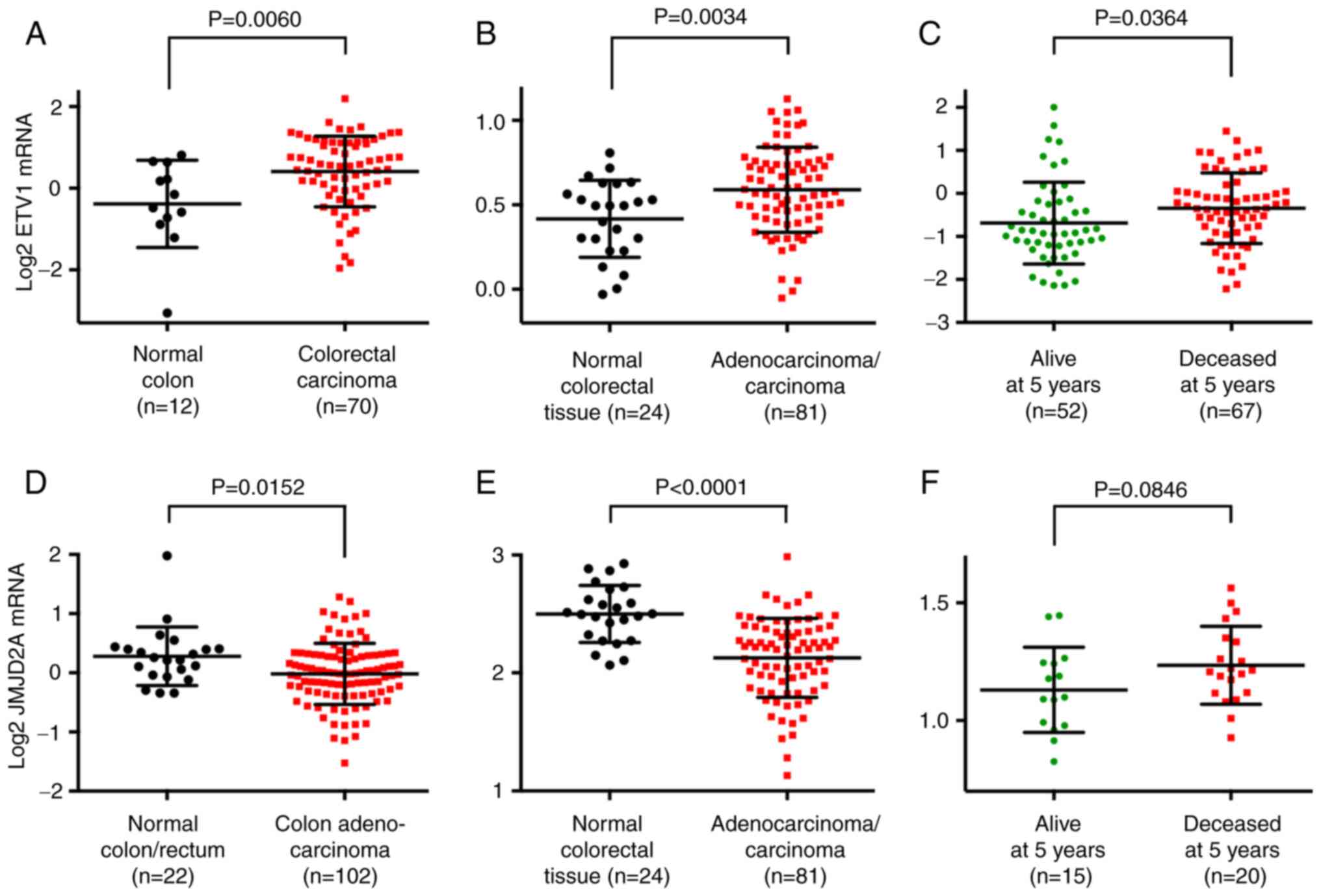
ETV1 and JMJD2A mRNA levels in colorectal
cancer
In order to substantiate that ETV1 is
overexpressed in colorectal tumors, bioinformatics analyses were
performed through the Oncomine website (www.oncomine.org). Utilizing published microarray data
(77,78), it was found that ETV1 mRNA
expression was significantly upregulated in colorectal tumors
compared with normal tissue (Fig. 2A
and B). Moreover, a high ETV1 expression was
significantly associated with a reduced survival at 5 years
[Fig. 2C; data from a previous
study (79)], 3 years and 1 year
(Fig. S1A) following diagnosis
and was also positively associated with the stage of the disease
(Fig. S1B), the latter being
consistent with the reported presence of ETV1 in lymph node
metastases (80). These data
strongly support the notion that ETV1 promotes colorectal tumor
formation and may be a predictor of an unfavorable outcome.
Since ETV1 and JMJD2A synergize in prostate cancer
(24), the present study also
examined the mRNA levels of the JMJD2A histone demethylase.
Surprisingly, it was discovered that the JMJD2A mRNA levels
were downregulated in colorectal cancer [Fig. 2D and E; data from previous studies
(78,81)]. Notably, high JMJD2A mRNA
levels were associated with a reduced survival (Figs. 2F and S1C) and were associated with an
increased disease stage (Fig.
S1D). These data suggest that JMJD2A is not required for the
initiation of disease, but rather for the progression to the more
aggressive and lethal end stages of colorectal cancer.
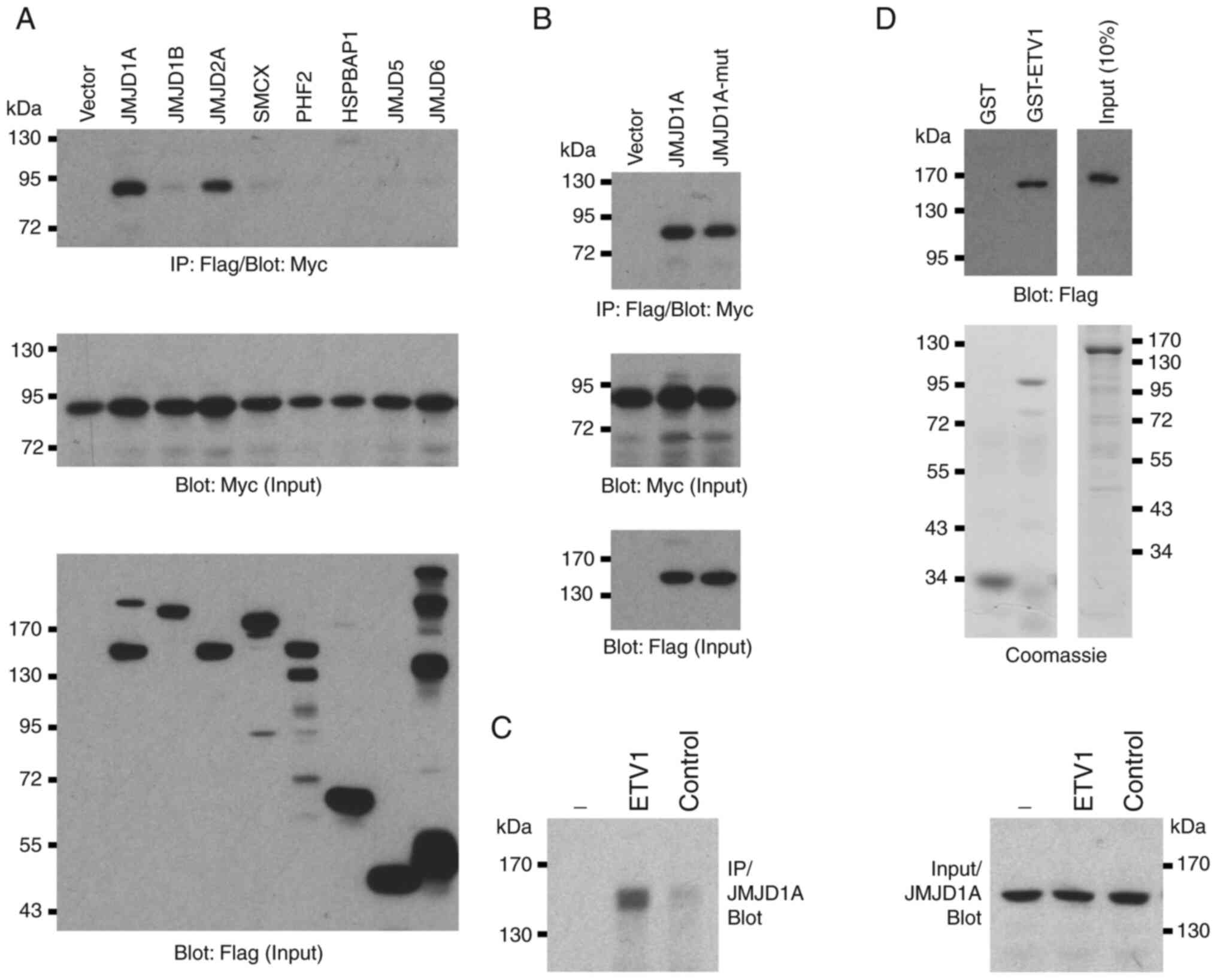
Physical interaction between ETV1 and
JMJD1A
It has been previously reported that JMJD1A is
overexpressed in colorectal tumors (39,40).
Since it has been found that ETV1 is upregulated in
colorectal cancer and as ETV1 has been shown to physically bind to
JMJD2A (24), the present study
wished to determine whether JMJD1A may also form complexes with
ETV1. Hence, the present study investigated the potential
interaction between these two proteins in co-immunoprecipitation
assays. Indeed, overexpressed ETV1 robustly co-immunoprecipitated
with JMJD1A to a similar degree as with JMJD2A (Fig. 3A), but not with several other JMJD
proteins (JMJD1B, SMCX, PHF2, HSPBAP1, JMJD5 and JMJD6). This
complex formation of JMJD1A with ETV1 was independent of catalytic
activity, as the H1120A/D1122G catalytic mutant of JMJD1A was
indistinguishable from the wild-type in its ability to
co-immunoprecipitate with ETV1 (Fig.
3B). Furthermore, endogenous JMJD1A co-immunopre-cipitated with
endogenous ETV1 in HCT116 cells (Fig.
3C). In addition, recombinant JMJD1A purified from baculovirus
bound to bacterially expressed, purified GST-ETV1, but not to GST
(Fig. 3D). Taken together, these
data demonstrate that ETV1 and JMJD1A can directly bind to each
other in vitro and in vivo.
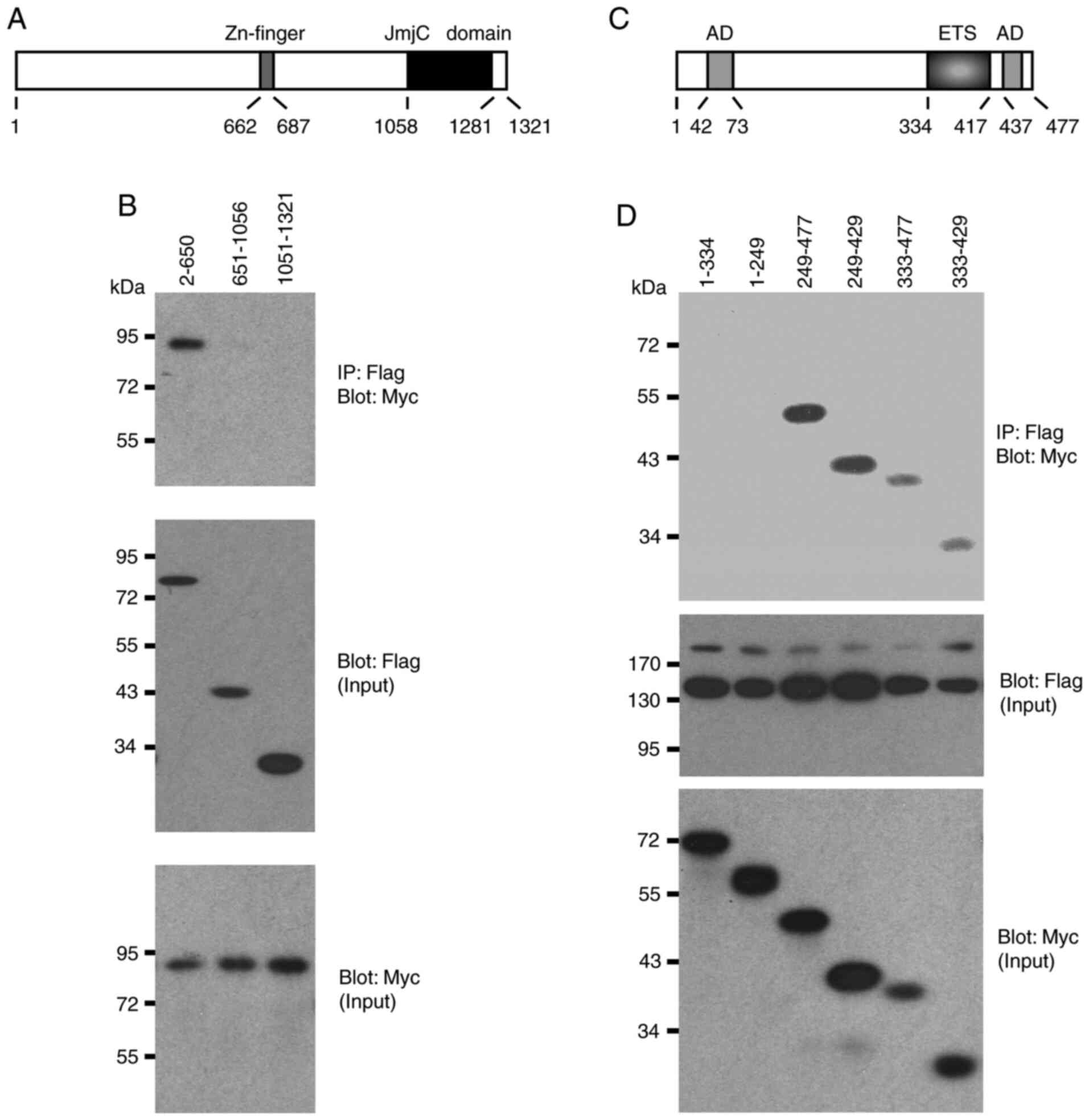
Domain mapping experiments
Two structural domains were identified in JMJD1A, a
central Zn-finger and the C-terminal Jumonji C domain that is its
catalytic center (Fig. 4A). The
present study then examined whether any of these domains are
involved in the interaction with ETV1 by utilizing truncation
mutants of JMJD1A. ETV1 co-immunoprecipitated with the N-terminal
650 JMJD1A amino acids, whereas the 651-1056 and 1051-1321
truncations, which encompass the Zn-finger or Jumonji C domain, did
not form complexes with ETV1 (Fig.
4B). Likewise, the Jumonji C domain of JMJD2A was reportedly
not required for binding to ETV1 (24), which may be the reason why ETV1 did
not promiscuously interact with all of the Jumonji C
domain-containing proteins tested in Fig. 3A.
Conversely, the present study assessed which domains
in ETV1 mediate its interaction with JMJD1A. Neither amino acids
1-334 nor 1-249, which encompass a strong N-terminal
transactivation domain of ETV1 (3), co-immunoprecipitated with JMJD1A
(Fig. 4C and D). In contrast, all
other truncations tested formed complexes with JMJD1A. The smallest
fragment that still interacted with JMJD1A encompassed ETV1 amino
acids 333-429, suggesting that the ETS DNA-binding domain of ETV1
mediates the interaction with JMJD1A. Similarly, the ETS domain was
reportedly crucial for binding of ETV1 to JMJD2A (24), suggesting that the DNA-binding ETS
domain of ETV1 is responsible for recruitment of either JMJD1A or
JMJD2A. This also suggests that the highly homologous ETV4 and ETV5
proteins are likely to bind to JMJD1A and JMJD2A through their ETS
domains.
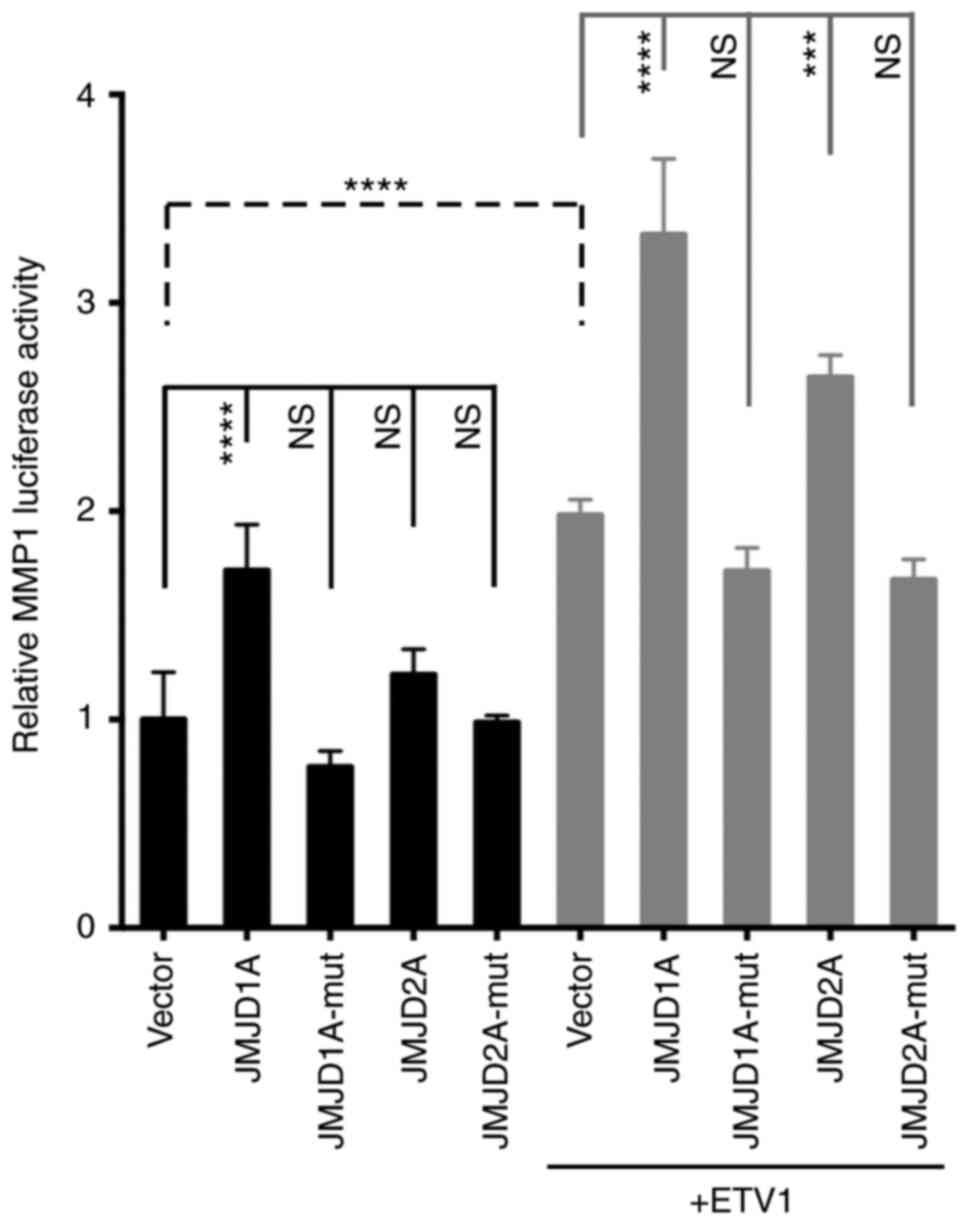
Cooperation between ETV1 and JMJD histone
demethylases
To assess whether ETV1 and JMJD1A (or JMJD2A)
function-ally cooperate in gene transcription in colon cancer
cells, the MMP1 gene promoter that is a bona fide
target of ETV1 (12) was examined.
When an MMP1 luciferase reporter gene was transfected into
HCT116 cells, expectedly, a significant stimulation of luciferase
activity was observed upon ETV1 co-transfection (Fig. 5). Notably, JMJD1A, but not JMJD2A
co-transfection alone also significantly increased MMP1
luciferase activity; this different behavior may be attributable to
the fact that endogenous levels of ETV1 were only sufficient in the
case of JMJD1A to detect transactivation. It should be noted that
JMJD1A and JMJD2A were over-expressed at comparable levels
(Fig. S2A). When JMJD1A or JMJD2A
was co-expressed with ETV1, a cooperative transcriptional
activation was observed (Fig. 5).
As previously reported in prostate cells (24), the JMJD2A-H188A catalytic mutant
did not cooperate with ETV1, and neither did the
JMJD1A-H1120A/D1122G catalytic mutant (Fig. 5), indicating that the catalytic
activity of JMJD1A is required for the coactivation of ETV1.
Similarly, JMJD1A was capable of cooperating with ETV1 in LNCaP
prostate cancer cells, although in this case, JMJD2A was much more
potent (Fig. S2B). Taken
together, these data reveal the novel finding (to the best of our
knowledge) that enzymatically active JMJD1A can serve as a
co-activator of ETV1.
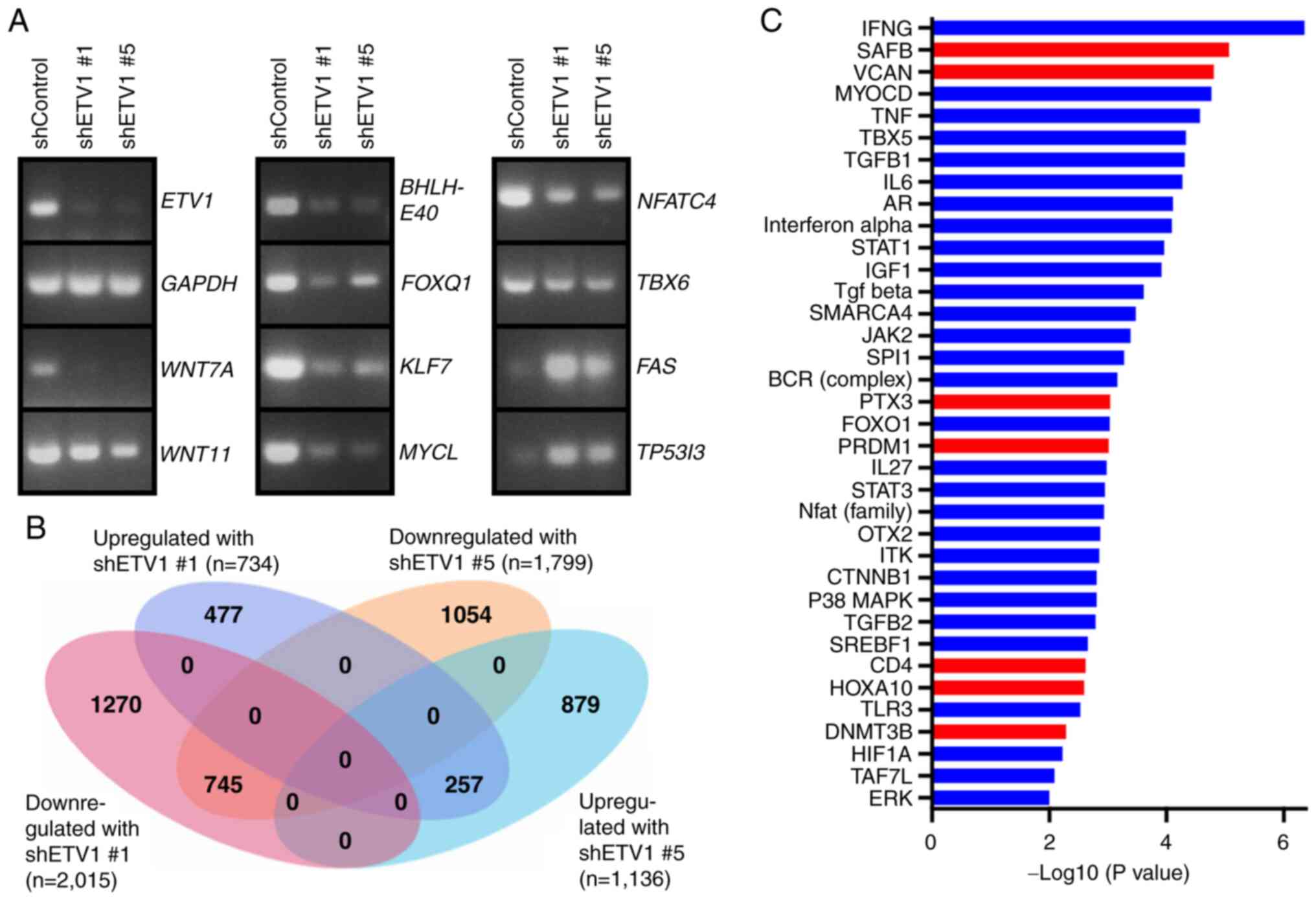
Regulation of the transcriptome by
ETV1
To gain deeper insight into the mechanisms through
which ETV1 may affect colon cancer cells, ETV1 expression was
downregulated with shRNA in HCT116 cells and RNA-sequencing was
performed (Fig. 6A and B). A total
of 745 genes were downregulated upon the expression of either of
the two ETV1 shRNAs used, and 257 genes were upregulated,
indicating that ETV1 regulates approximately a thousand genes in
HCT116 cells. Amongst the downregulated genes, RT-qPCR confirmed
the reduced expression of signaling molecules (WNT7A and WNT11) and
transcription factors (BHLHE40, FOXQ1, KLF7, MYCL, NFATC4 and
TBX6), whereas two examples of validated upregulated genes were
FAS and TP53I3 (Fig.
6A). WNT7A is a secreted morphogen that is overexpressed in
colorectal cancer and may activate cancer-associated fibroblasts,
which enhances tumor aggressiveness (82,83).
Likewise, WNT11 is overexpressed in colon cancer and may promote
colorectal cancer cell growth and invasion (84,85).
Hence, the upregulation of WNT7A and WNT11 may be
involved in the mechanisms through which ETV1 overexpression
contributes to colon tumorigenesis.
This may also pertain to the upregulation of
FOXQ1, as the encoded transcription factor is overexpressed
in colorectal cancer and promotes tumor initiation and metastasis
(86-89). By contrast, the authors were unable
to find substantial evidence in the literature that BHLHE40, KLF7,
MYCL, NFATC4 or TBX6 promote colon cancer. However, the
downregulation of the FAS cell surface death receptor has been
reported in colon tumors and seems to be associated with a reduced
survival, likely due to the fact that FAS downregulation
facilitates escape from immune surveillance and thus, also
resistance to immune checkpoint inhibitors (90,91).
Accordingly, the suppression of FAS expression by ETV1 could
contribute to colon cancer aggressiveness. By contrast, the
suppression of the expression of the oxidoreduc-tase TP53I3 by ETV1
cannot account for the pro-growth role of ETV1 in HCT116 cells,
since TP53I3 is also a pro-growth factor (92). This indicates that results from
RNA-sequencing, although they can provide some insight into how
ETV1 facilitates its oncogenic effects, should be treated with
caution and identifying seminal downstream effectors of ETV1
requires experimental validation (see below).
The present study also applied Ingenuity Pathway
Analysis to better comprehend ETV1 function. This revealed multiple
upstream regulatory pathways that were affected by ETV1
downregulation (Fig. 6C). Amongst
the downregulated path-ways, there were interleukin (IL)-6, signal
transducer and activator of transcription (STAT)3 and β-catenin
(CTNNB1). Given that IL6/STAT3 as well as β-catenin are potent
oncogenic agents in colorectal cancer (93,94),
this outlines how ETV1 overexpression may facilitate tumorigenesis
by stimulating the IL6/STAT3 and β-catenin pathways.
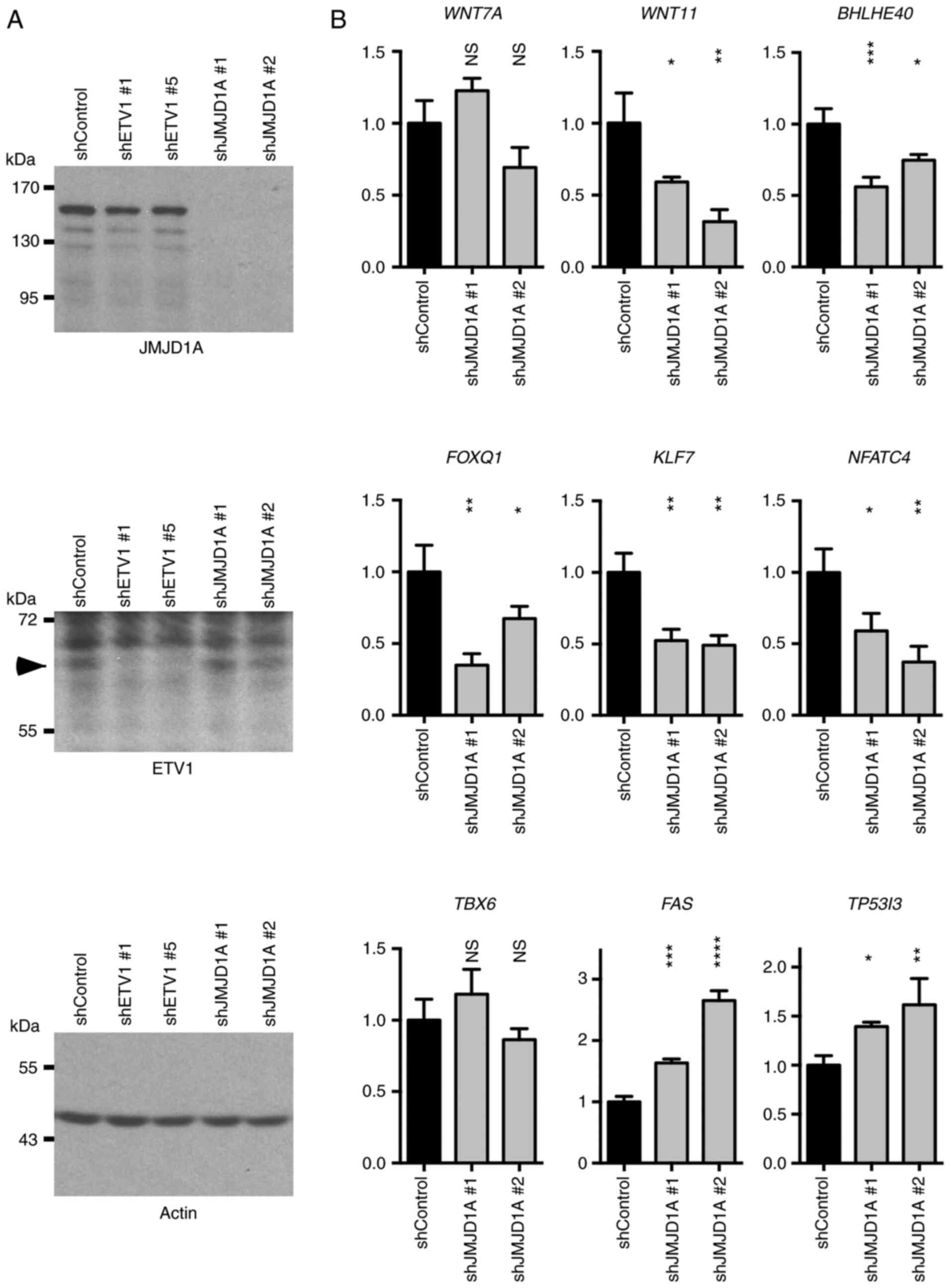
Subsequently, the authors wished to determine
whether JMJD1A may also regulate the newly identified ETV1 target
genes. To this end, two different JMJD1A shRNAs were transfected
into HCT116 cells. This robustly ablated JMJD1A expression, but did
not alter the ETV1 levels, and neither did ETV1 downregulation
affect JMJD1A levels (Fig. 7A).
The mRNA levels of WNT7A, WNT11, BHLHE40,
FOXQ1, KLF7, NFATC4, TBX6, FAS
and TP53I3 were then measured (Fig. 7B). As observed with ETV1
downregulation, JMJD1A shRNA led to a decrease in the WNT11,
BHLHE40, FOXQ1, KLF7 and NFATC4 mRNA
levels, while the FAS and TP53I3 levels were
increased. However, in contrast to ETV1, WNT7A and
TBX6 mRNA levels were not significantly affected by JMJD1A
shRNAs. Collectively, these results suggest that JMJD1A cooperates
with ETV1 in the regulation of several, but not all of ETV1 target
genes.
FOXQ1 as a downstream effector of ETV1
and JMJD1A
Out of the validated ETV1-regulated genes,
FOXQ1 was selected for further analysis. The reasons were
that the pro-oncogenic association of FOXQ1 with colorectal
cancer has been established (87,89)
and it could be corroborated that FOXQ1 was upregulated in
colorectal tumors (Fig. S3A-C).
In addition, FOXQ1 mRNA levels were regulated by JMJD1A
(Fig. 7B), but not by JMJD2A
(Fig. S4), suggesting that ETV1
and JMJD1A cooperate in the transcriptional control of
FOXQ1.
Thus, the human FOXQ1 promoter from -2,000
to +124 was cloned and it was demonstrated that it was
dose-dependently inducible by ETV1 (Fig. 8A). Accordingly, multiple potential
ETV1 binding sites were identified within these 2.1 kbp of genomic
DNA (Fig. S5). Subsequently,
JMJD1A was expressed and it was observed that JMJD1A, but not the
H1120A/D1122G catalytic mutant, was capable of inducing the
FOXQ1 gene promoter only in the presence of ectopic ETV1
(Fig. 8B); as a control, the
parental luciferase construct pGL2-Basic was not affected by ETV1
or JMJD1A. In addition, as determined by chromatin
immunoprecipitation experiments, both ETV1 and JMJD1A bound to the
FOXQ1 promoter within region A (Fig. 8C), but not within region C. Of
note, JMJD1A, but not ETV1, also bound to region B (Fig. 8C), suggesting that a transcription
factor(s) other than ETV1 likewise recruits JMJD1A to the
FOXQ1 gene promoter. Taken together, these data strongly
support the notion that FOXQ1 is a valid target of the
ETV1/JMJD1A complex.
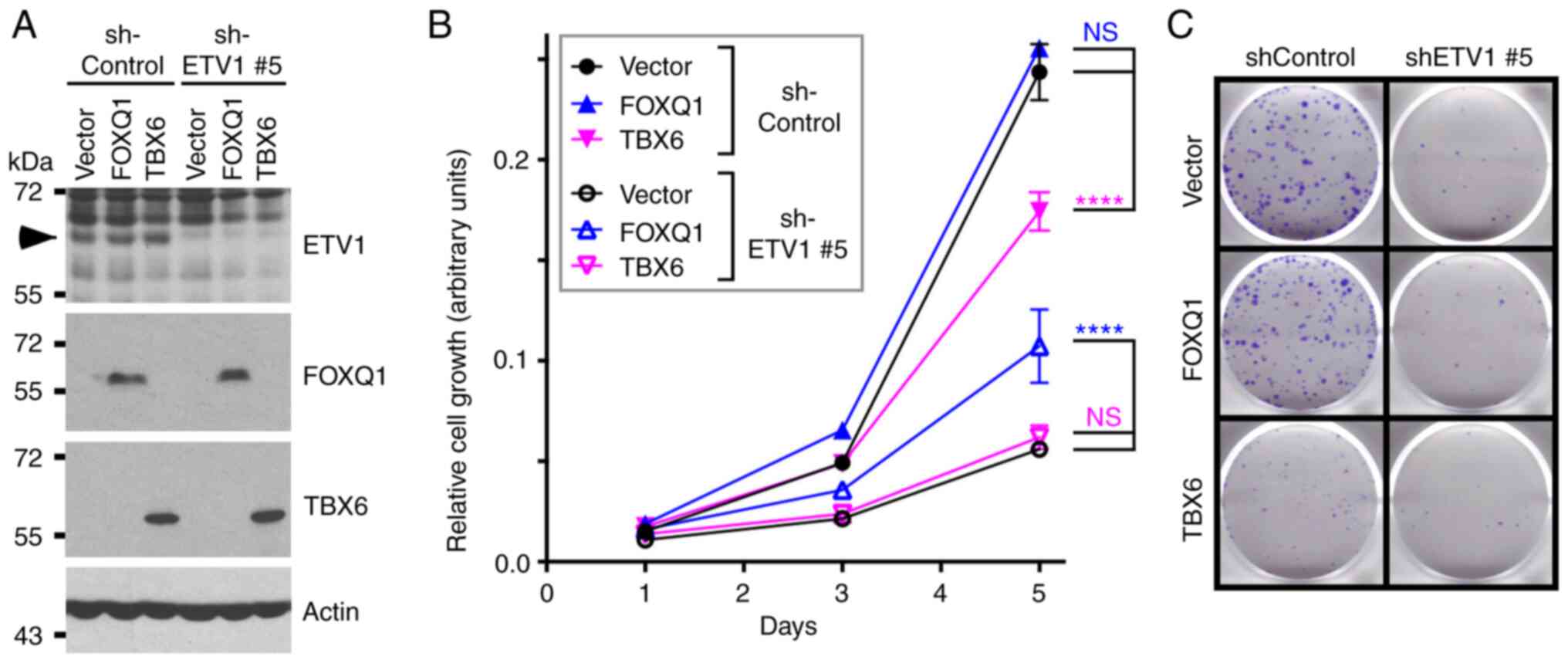
Finally, the present study wished to determine
whether FOXQ1 is seminal for ETV1 function in HCT116 cells. To this
end, ETV1 was downregulated and FOXQ1 was overexpressed
simultaneously. As also shown above (Fig. 1), ETV1 downregulation suppressed
HCT116 cell growth and clonogenic activity (Fig. 9). The overexpression of FOXQ1 had
no effect in the presence of control shRNA, suggesting that
endogenous levels of FOXQ1 were already sufficient for a maximal
growth-stimulatory effect of this transcription factor in the
presence of normal ETV1 levels. However, FOXQ1 overexpression
partially reversed the growth-suppressive effect of ETV1 shRNA on
HCT116 cells, although not the clonogenic activity. These data
demonstrate that FOXQ1 is indeed one of the crucial target
genes of ETV1.
The present study also attempted to rescue ETV1
ablation by TBX6 overexpression, since our interest was piqued by
the fact that we did not find any literature convincingly linking
the TBX6 transcription factor to cancer at all. This was despite
conflicting bioinformatics results demonstrating either the up- or
downregulation of TBX6 mRNA in colorectal cancer (Fig. S3D-F) and JMJD1A as well as JMJD2A
not regulating TBX6 gene transcription (Figs. 7B and S4). Notably, TBX6 overexpression
significantly impaired HCT116 cell growth and clonogenic activity
in the presence of control shRNA, but had no effect in the presence
of ETV1 shRNA (Fig. 9). This
indicates that TBX6 transcriptional upregulation does not
contribute to the pro-growth activity of ETV1, but may instead
suppress it. In addition, to the best of our knowledge, these data
suggest for the first time that TBX6 may perform a
tumor-suppressive activity in colorectal cancer cells.
Discussion
The present study provided evidence that the ETS
transcription factor, ETV1, may contribute to the development of
colorectal cancer. This is primarily based on ETV1 being
overexpressed in respective tumors, its expression levels being
associated with the aggressiveness of the disease, and being
required for the efficient growth and clonogenic activity of HCT116
colorectal cancer cells. Moreover, it was discovered that ETV1
binds directly to the JMJD1A histone demethylase that appears to
coregulate many ETV1 target genes. Given that JMJD1A itself is
overexpressed in colorectal cancer and has been implicated as a
respective tumor promoter (39-42,95),
this interaction may have lethal consequences. Furthermore, the
relevance of the ETV1-JMJD1A complex may extend to other
malignancies, including prostate cancer, where ETV1 is known to be
a driver of tumorigenesis (21-23,96)
and some in vitro evidence points to a similar role for
JMJD1A (97-100). Consistently, JMJD1A is
upregulated in prostate tumors and even more so at metastatic sites
(Fig. S6).
The depletion of ETV1 in HCT116 cells resulted in
the inhibition of multiple regulatory pathways (Fig. 6C), including IL6/STAT3 and
β-catenin signaling that facilitate colon cancer (93,94).
Accordingly, the stimulation of these pathways upon ETV1
overexpression could contribute to tumorigenesis. Seemingly
inconsistent with the oncogenic role of ETV1 in colon cancer,
interferon-γ (IFNG) signaling (Fig.
6C), whose tumor suppressive role has long been recognized
(101), was inhibited upon ETV1
downregulation. However, IFNG can also contribute to the
progression of cancer, in particular by promoting the immunoevasion
of tumor cells, which may occur through the upregulation of e.g.,
PD-L1 or the increased secretion of immunosuppressive molecules
that is associated with a reduced survival (102). Another pathway diminished by ETV1
downregulation was TGF-β signaling (Fig. 6C). TGF-β normally suppresses the
growth of epithelial cells, but it can also promote
epithe-lial-to-mesenchymal transition and thereby metastasis
(103). Hence, it is plausible
that the stimulation of TGF-β signaling by ETV1 may particularly be
relevant during the progression from localized to disseminated
tumors. Conversely, from the RNA-sequencing data in the present
study, it was predicted that ETV1 causes the inhibition of scaffold
attachment factor B (SAFB) signaling (Fig. 6C). SAFB is downregulated in
colorectal tumors, being associated with a reduced survival, and
its overexpression leads to the inhibition of metastasis (104), outlining another mechanism
through which ETV1 could potentially drive colon cancer. However,
whether the aforementioned pathways are indeed crucial for the role
of ETV1 in colorectal cancer needs to be validated in the
future.
FOXQ1 is overexpressed in colorectal tumors
(86-88,105) and this transcription factor can
promote in vitro migration, invasion, angiogenesis and
proliferation, as well as the xenograft tumor growth of colorectal
cancer cells (87-89). The present study discovered
FOXQ1 was a target gene of ETV1 and JMJD1A. Notably, FOXQ1
overexpression partially reversed the growth suppressive effect of
ETV1 shRNA on HCT116 cells, strongly arguing that FOXQ1 is a
seminal downstream effector of ETV1 in colorectal and possible
other cancers.
However, albeit FOXQ1 is in general considered as
an oncoprotein, a recent study demonstrated that FOXQ1 can also act
as a tumor suppressor in melanomas (106). It is possible that the same holds
true for prostate cancer, as it was found that FOXQ1 was
downregulated in prostate tumors, and this down-regulation was even
accentuated upon metastasis (Fig.
S7). Accordingly, FOXQ1 may not always be a crucial downstream
effector of ETV1, and it remains to be determined whether and why
ETV1 would potentially be ineffectual to induce FOXQ1
transcription in prostate tumors.
FOXQ1 overexpression only partially reversed ETV1
ablation, indicating that other crucial ETV1 downstream effectors
await discovery. For instance, one of these could be the
transcription factor, MYCL, as MYCL transcription was
reduced upon ETV1 shRNA expression. The MYCL gene has been
found amplified and/or overexpressed in small cell lung cancer,
ovarian carcinomas and prostate cancer (107-109). In mice in which both the
TP53 and RB1 tumor suppressor genes were deleted, the
overexpression of MYCL promoted, while the knockout of
MYCL suppressed small cell lung cancer formation, indicating
an oncogenic activity for MYCL (110,111). This was further substantiated by
the fact that transgenic mice over-expressing MYCL in T
cells developed lymphoid tumors (112). Consistent with being another
downstream effector of ETV1, MYCL was found overexpressed in
both colon and prostate cancer (Fig.
S8).
It was also unraveled that TBX6
transcription was stimulated by ETV1, but not by JMJD1A. The T-box
transcription factor TBX6 is essential for embryonic development
(113), yet its role in cancer
has, to our knowledge, not yet been investigated. TBX6
overexpression reduced HCT116 cell growth and clonogenicity,
suggesting that TBX6 exerts a tumor suppressive function, an
important finding in its own right. Accordingly, TBX6
upregulation may dampen the oncogenic activity of ETV1. Since it is
conceivable that TBX6 gene activity is modulated by
transcription factors other than ETV1, TBX6 downregulation
may occur in a number of instances in colorectal cancer and promote
tumorigenesis. Similar to numerous cancer-critical transcription
factors, TBX6 has been shown to be associated with congenital
developmental diseases, including Müllerian aplasia, scoliosis,
anomalies of the kidney and urinary tract, and cervical vertebral
malformations (114-117). One possible mechanism through
which TBX6 could affect development is through TBX6-mediated
repression of the stem cell factor SOX2 (118,119). The same mechanism may apply for
TBX6-mediated tumor suppression; consistently, the elevated
expression of SOX2 is associated with increased metastasis,
recurrence and lethality in colorectal cancer patients (120-122).
In conclusion, the data of the present study
revealed plausible mechanisms through which an ETV1-JMJD1A protein
complex could facilitate tumorigenesis in the colon, including by
upregulating the FOXQ1 gene. This knowledge suggests that
interfering with ETV1, JMJD1A and/or FOXQ1 function may be a valid
strategy with which to combat metastatic colorectal cancer.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was in part funded by grants R01
CA154745, R03 CA223615 and R01 CA233613 from the National Cancer
Institute of the USA (to RJ) and a team science seed grant from the
Stephenson Cancer Center (to RJ and WMF). In addition, RJ was
supported in part by the Oklahoma Tobacco Settlement Endowment
Trust through an award made to the University of
Oklahoma/Stephenson Cancer Center. Additional assistance was
provided by the Stephenson Cancer Center Molecular Biology Core
that was supported by NIH grants P30 CA225520 and P20 GM103639. The
funding sources had no involvement in the study design, data
collection, analysis, interpretation and the writing of this
manuscript. The content of this manuscript is solely the
responsibility of the authors and does not necessarily represent
the official views of the granting agencies.
Availability of data and materials
Original data and material will be made available
upon reasonable request. RNA-sequencing data have been deposited in
Gene Expression Omnibus (GSE158294).
Authors' contributions
SO, HS and RJ designed and performed the
experiments. SO, HS, WMF, SS and RJ analyzed and interpreted the
data. RJ supervised the study and wrote the manuscript with input
from all other authors. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown TA and McKnight SL: Specificities of
protein-protein and protein-DNA interaction of GABP alpha and two
newly defined ets-related proteins. Genes Dev. 6:2502–2512. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monte D, Coutte L, Baert JL, Angeli I,
Stehelin D and de Launoit Y: Molecular characterization of the
ets-related human transcription factor ER81. Oncogene. 11:771–779.
1995.PubMed/NCBI
|
|
3
|
Janknecht R: Analysis of the
ERK-stimulated ETS transcription factor ER81. Mol Cell Biol.
16:1550–1556. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei GH, Badis G, Berger MF, Kivioja T,
Palin K, Enge M, Bonke M, Jolma A, Varjosalo M, Gehrke AR, et al:
Genome-wide analysis of ETS-family DNA-binding in vitro and in
vivo. EMBO J. 29:2147–2160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hollenhorst PC, McIntosh LP and Graves BJ:
Genomic and biochemical insights into the specificity of ETS
transcription factors. Annu Rev Biochem. 80:437–471. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oh S, Shin S and Janknecht R: ETV1, 4 and
5: An oncogenic subfamily of ETS transcription factors. Biochim
Biophys Acta. 1826:1–12. 2012.PubMed/NCBI
|
|
7
|
Arber S, Ladle DR, Lin JH, Frank E and
Jessell TM: ETS gene Er81 controls the formation of functional
connections between group Ia sensory afferents and motor neurons.
Cell. 101:485–498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kucera J, Cooney W, Que A, Szeder V,
Stancz-Szeder H and Walro J: Formation of supernumerary muscle
spindles at the expense of Golgi tendon organs in ER81-deficient
mice. Dev Dyn. 223:389–401. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sedy J, Tseng S, Walro JM, Grim M and
Kucera J: ETS transcription factor ER81 is required for the
pacinian corpuscle development. Dev Dyn. 235:1081–1089. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shekhar A, Lin X, Liu FY, Zhang J, Mo H,
Bastarache L, Denny JC, Cox NJ, Delmar M, Roden DM, et al:
Transcription factor ETV1 is essential for rapid conduction in the
heart. J Clin Invest. 126:4444–4459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Papoutsopoulou S and Janknecht R:
Phosphorylation of ETS transcription factor ER81 in a complex with
its coactivators CREB-binding protein and p300. Mol Cell Biol.
20:7300–7310. 2000.PubMed/NCBI
|
|
12
|
Bosc DG, Goueli BS and Janknecht R:
HER2/Neu-mediated activation of the ETS transcription factor ER81
and its target gene MMP-1. Oncogene. 20:6215–6224. 2001.PubMed/NCBI
|
|
13
|
Wu J and Janknecht R: Regulation of the
ETS transcription factor ER81 by the 90-kDa ribosomal S6 kinase 1
and protein kinase A. J Biol Chem. 277:42669–42679. 2002.PubMed/NCBI
|
|
14
|
Xie L, Gazin C, Park SM, Zhu LJ, Debily
MA, Kittler EL, Zapp ML, Lapointe D, Gobeil S, Virbasius CM and
Green MR: A synthetic interaction screen identifies factors
selectively required for proliferation and TERT transcription in
p53-deficient human cancer cells. PLoS Genet. 8:e10031512012.
|
|
15
|
Janknecht R: Regulation of the ER81
transcription factor and its coactivators by mitogen- and
stress-activated protein kinase 1 (MSK1). Oncogene. 22:746–755.
2003.PubMed/NCBI
|
|
16
|
Goel A and Janknecht R:
Acetylation-mediated transcriptional activation of the ETS protein
ER81 by p300, P/CAF, and HER2/Neu. Mol Cell Biol. 23:6243–6254.
2003.PubMed/NCBI
|
|
17
|
Baert JL, Monte D, Verreman K, Degerny C,
Coutte L and de Launoit Y: The E3 ubiquitin ligase complex
component COP1 regulates PEA3 group member stability and
transcriptional activity. Oncogene. 29:1810–1820. 2010.PubMed/NCBI
|
|
18
|
Vitari AC, Leong KG, Newton K, Yee C,
O'Rourke K, Liu J, Phu L, Vij R, Ferrando R, Couto SS, et al: COP1
is a tumour suppressor that causes degradation of ETS transcription
factors. Nature. 474:403–406. 2011.PubMed/NCBI
|
|
19
|
Tomlins SA, Rhodes DR, Perner S,
Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J,
Kuefer R, et al: Recurrent fusion of TMPRSS2 and ETS transcription
factor genes in prostate cancer. Science. 310:644–648.
2005.PubMed/NCBI
|
|
20
|
Attard G, Clark J, Ambroisine L, Mills IG,
Fisher G, Flohr P, Reid A, Edwards S, Kovacs G, Berney D, et al:
Heterogeneity and clinical significance of ETV1 translocations in
human prostate cancer. Br J Cancer. 99:314–320. 2008.PubMed/NCBI
|
|
21
|
Shin S, Kim TD, Jin F, van Deursen JM,
Dehm SM, Tindall DJ, Grande JP, Munz JM, Vasmatzis G and Janknecht
R: Induction of prostatic intraepithelial neoplasia and modulation
of androgen receptor by ETS variant 1/ETS-related protein 81.
Cancer Res. 69:8102–8110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baena E, Shao Z, Linn DE, Glass K, Hamblen
MJ, Fujiwara Y, Kim J, Nguyen M, Zhang X, Godinho FJ, et al: ETV1
directs androgen metabolism and confers aggressive prostate cancer
in targeted mice and patients. Genes Dev. 27:683–698. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomlins SA, Laxman B, Dhanasekaran SM,
Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, et
al: Distinct classes of chromosomal rearrangements create oncogenic
ETS gene fusions in prostate cancer. Nature. 448:595–599. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim TD, Jin F, Shin S, Oh S, Lightfoot SA,
Grande JP, Johnson AJ, van Deursen JM, Wren JD and Janknecht R:
Histone demethylase JMJD2A drives prostate tumorigenesis through
transcription factor ETV1. J Clin Invest. 126:706–720. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kooistra SM and Helin K: Molecular
mechanisms and potential functions of histone demethylases. Nat Rev
Mol Cell Biol. 13:297–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berry WL and Janknecht R: KDM4/JMJD2
histone demethylases: Epigenetic regulators in cancer cells. Cancer
Res. 73:2936–2942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh S, Shin S and Janknecht R: The small
members of the JMJD protein family: Enzymatic jewels or jinxes?
Biochim Biophys Acta Rev Cancer. 1871:406–418. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Whetstine JR, Nottke A, Lan F, Huarte M,
Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M and Shi
Y: Reversal of histone lysine trimethylation by the JMJD2 family of
histone demethylases. Cell. 125:467–481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klose RJ, Yamane K, Bae Y, Zhang D,
Erdjument-Bromage H, Tempst P, Wong J and Zhang Y: The
transcriptional repressor JHDM3A demethylates trimethyl histone H3
lysine 9 and lysine 36. Nature. 442:312–316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shin S and Janknecht R: Diversity within
the JMJD2 histone demethylase family. Biochem Biophys Res Commun.
353:973–977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim TD, Oh S, Lightfoot SA, Shin S, Wren
JD and Janknecht R: Upregulation of PSMD10 caused by the JMJD2A
histone demethylase. Int J Clin Exp Med. 9:10123–10134.
2016.PubMed/NCBI
|
|
32
|
Kim TD, Fuchs JR, Schwartz E, Abdelhamid
D, Etter J, Berry WL, Li C, Ihnat MA, Li PK and Janknecht R:
Pro-growth role of the JMJD2C histone demethylase in HCT-116 colon
cancer cells and identification of curcuminoids as JMJD2
inhibitors. Am J Transl Res. 6:236–247. 2014.PubMed/NCBI
|
|
33
|
Kim TD, Shin S, Berry WL, Oh S and
Janknecht R: The JMJD2A demethylase regulates apoptosis and
proliferation in colon cancer cells. J Cell Biochem. 113:1368–1376.
2012. View Article : Google Scholar
|
|
34
|
Lee HJ, Kim BK, Yoon KB, Kim YC and Han
SY: Novel inhibitors of lysine (K)-specific demethylase 4A with
anticancer activity. Invest New Drugs. 35:733–741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crawford HC, Fingleton B, Gustavson MD,
Kurpios N, Wagenaar RA, Hassell JA and Matrisian LM: The PEA3
subfamily of Ets transcription factors synergizes with
beta-catenin-LEF-1 to activate matrilysin transcription in
intestinal tumors. Mol Cell Biol. 21:1370–1383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horiuchi S, Yamamoto H, Min Y, Adachi Y,
Itoh F and Imai K: Association of ets-related transcriptional
factor E1AF expression with tumour progression and overexpression
of MMP-1 and matrilysin in human colorectal cancer. J Pathol.
200:568–576. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoo J, Jeon YH, Cho HY, Lee SW, Kim GW,
Lee DH and Kwon SH: Advances in histone demethylase KDM3A as a
cancer therapeutic target. Cancers (Basel). 12:10982020. View Article : Google Scholar
|
|
38
|
Sui Y, Gu R and Janknecht R: Crucial
functions of the JMJD1/KDM3 epigenetic regulators in cancer. Mol
Cancer Res. Jun 30–2020.Epub ahead of print. View Article : Google Scholar
|
|
39
|
Uemura M, Yamamoto H, Takemasa I, Mimori
K, Hemmi H, Mizushima T, Ikeda M, Sekimoto M, Matsuura N, Doki Y
and Mori M: Jumonji domain containing 1A is a novel prognostic
marker for colorectal cancer: In vivo identification from hypoxic
tumor cells. Clin Cancer Res. 16:4636–4646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Yu B, Deng P, Cheng Y, Yu Y, Kevork
K, Ramadoss S, Ding X, Li X and Wang CY: KDM3 epigenetically
controls tumorigenic potentials of human colorectal cancer stem
cells through Wnt/β-catenin signalling. Nat Commun. 8:151462017.
View Article : Google Scholar
|
|
41
|
Peng K, Su G, Ji J, Yang X, Miao M, Mo P,
Li M, Xu J, Li W and Yu C: Histone demethylase JMJD1A promotes
colorectal cancer growth and metastasis by enhancing Wnt/β-catenin
signaling. J Biol Chem. 293:10606–10619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Oh S, Song H, Shin S, Zhang B,
Freeman WM and Janknecht R: A potential common role of the Jumonji
C domain-containing 1A histone demethylase and chromatin remodeler
ATRX in promoting colon cancer. Oncol Lett. 16:6652–6662.
2018.PubMed/NCBI
|
|
43
|
Dowdy SC, Mariani A and Janknecht R:
HER2/Neu- and TAK1-mediated up-regulation of the transforming
growth factor beta inhibitor Smad7 via the ETS protein ER81. J Biol
Chem. 278:44377–44384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mooney SM, Goel A, D'Assoro AB, Salisbury
JL and Janknecht R: Pleiotropic effects of p300-mediated
acetylation on p68 and p72 RNA helicase. J Biol Chem.
285:30443–30452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim TD, Shin S and Janknecht R: ETS
transcription factor ERG cooperates with histone demethylase KDM4A.
Oncol Rep. 35:3679–3688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oh S, Shin S, Lightfoot SA and Janknecht
R: 14-3-3 proteins modulate the ETS transcription factor ETV1 in
prostate cancer. Cancer Res. 73:5110–5119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Berry WL, Shin S, Lightfoot SA and
Janknecht R: Oncogenic features of the JMJD2A histone demethylase
in breast cancer. Int J Oncol. 41:1701–1706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim J, Shin S, Subramaniam M, Bruinsma E,
Kim TD, Hawse JR, Spelsberg TC and Janknecht R: Histone demethylase
JARID1B/KDM5B is a corepressor of TIEG1/KLF10. Biochem Biophys Res
Commun. 401:412–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim TD, Oh S, Shin S and Janknecht R:
Regulation of tumor suppressor p53 and HCT116 cell physiology by
histone demethylase JMJD2D/KDM4D. PLoS One. 7:e346182012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Berry WL, Kim TD and Janknecht R:
Stimulation of β-catenin and colon cancer cell growth by the KDM4B
histone demethylase. Int J Oncol. 44:1341–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Matthias P, Muller MM, Schreiber E,
Rusconi S and Schaffner W: Eukaryotic expression vectors for the
analysis of mutant proteins. Nucleic Acids Res. 17:64181989.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li X, Moon G, Shin S, Zhang B and
Janknecht R: Cooperation between ETS variant 2 and Jumonji
domain-containing 2 histone demethylases. Mol Med Rep.
17:5518–5527. 2018.PubMed/NCBI
|
|
54
|
Rossow KL and Janknecht R: The Ewing's
sarcoma gene product functions as a transcriptional activator.
Cancer Res. 61:2690–2695. 2001.PubMed/NCBI
|
|
55
|
Mooney SM, Grande JP, Salisbury JL and
Janknecht R: Sumoylation of p68 and p72 RNA helicases affects
protein stability and transactivation potential. Biochemistry.
49:1–10. 2010. View Article : Google Scholar
|
|
56
|
Knebel J, De Haro L and Janknecht R:
Repression of transcription by TSGA/Jmjd1a, a novel interaction
partner of the ETS protein ER71. J Cell Biochem. 99:319–329. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Goel A and Janknecht R: Concerted
activation of ETS protein ER81 by p160 coactivators, the
acetyltransferase p300 and the receptor tyrosine kinase HER2/Neu. J
Biol Chem. 279:14909–14916. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Janknecht R and Hunter T: Activation of
the Sap-1a transcription factor by the c-Jun N-terminal kinase
(JNK) mitogen-activated protein kinase. J Biol Chem. 272:4219–4224.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang YL, Wang PW, Wang FS, Lin HY and
Huang YH: miR-29a modulates GSK3β/SIRT1-linked mitochondrial
proteostatic stress to ameliorate mouse non-alcoholic
steatohepatitis. Int J Mol Sci. 21:E68842020. View Article : Google Scholar
|
|
60
|
Lin X, Feng D, Li P and Lv Y: LncRNA
LINC00857 regulates the progression and glycolysis in ovarian
cancer by modulating the Hippo signaling pathway. Cancer Med. Sep
12–2020.Epub ahead of print. View Article : Google Scholar
|
|
61
|
Chen X, Liu X, Gao Y, Lin J, Liu X, Pang
X, Lin J and Chen L: Application of firefly luciferase (Luc) as a
reporter gene for the chemoautotrophic and acidophilic
Acidithiobacillus spp. Curr Microbiol. 77:3724–3730. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ou Y, Liao C, Li H and Yu G: LncRNA
SOX2OT/Smad3 feed-back loop promotes myocardial fibrosis in heart
failure. IUBMB Life. Sep 21–2020.Epub ahead of print. View Article : Google Scholar
|
|
63
|
Sims RJ III, Liss AS and Gottlieb PD:
Normalization of luciferase reporter assays under conditions that
alter internal controls. Biotechniques. 34:938–940. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shifera AS and Hardin JA: Factors
modulating expression of Renilla luciferase from control plasmids
used in luciferase reporter gene assays. Anal Biochem. 396:167–172.
2010. View Article : Google Scholar
|
|
65
|
Wu GQ, Wang X, Zhou HY, Chai KQ, Xue Q,
Zheng AH, Zhu XM, Xiao JY, Ying XH, Wang FW, et al: Evidence for
transcriptional interference in a dual-luciferase reporter system.
Sci Rep. 5:176752015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Repele A and Manu: Robust normalization of
luciferase reporter data. Methods Protoc. 2:622019. View Article : Google Scholar :
|
|
67
|
Shin S, Oh S, An S and Janknecht R: ETS
variant 1 regulates matrix metalloproteinase-7 transcription in
LNCaP prostate cancer cells. Oncol Rep. 29:306–314. 2013.
View Article : Google Scholar
|
|
68
|
Shin S, Bosc DG, Ingle JN, Spelsberg TC
and Janknecht R: Rcl is a novel ETV1/ER81 target gene upregulated
in breast tumors. J Cell Biochem. 105:866–874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Oh S, Shin S, Song H, Grande JP and
Janknecht R: Relationship between ETS transcription factor ETV1 and
TGF-β-regulated SMAD proteins in prostate cancer. Sci Rep.
9:81862019. View Article : Google Scholar
|
|
70
|
Oh S and Janknecht R: Histone demethylase
JMJD5 is essential for embryonic development. Biochem Biophys Res
Commun. 420:61–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
72
|
Sui Y, Li X, Oh S, Zhang B, Freeman WM,
Shin S and Janknecht R: Opposite roles of the JMJD1A interaction
partners MDFI and MDFIC in colorectal cancer. Sci Rep. 10:87102020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Goueli BS and Janknecht R: Upregulation of
the catalytic telomerase subunit by the transcription factor ER81
and oncogenic HER2/Neu, Ras, or Raf. Mol Cell Biol. 24:25–35. 2004.
View Article : Google Scholar :
|
|
74
|
Goueli BS and Janknecht R: Regulation of
telomerase reverse transcriptase gene activity by upstream
stimulatory factor. Oncogene. 22:8042–8047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
DiTacchio L, Bowles J, Shin S, Lim DS,
Koopman P and Janknecht R: Transcription factors ER71/ETV2 and SOX9
participate in a positive feedback loop in fetal and adult mouse
testis. J Biol Chem. 287:23657–23666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Orlando G, Law PJ, Cornish AJ, Dobbins SE,
Chubb D, Broderick P, Litchfield K, Hariri F, Pastinen T, Osborne
CS, et al: Promoter capture Hi-C-based identification of recurrent
noncoding mutations in colorectal cancer. Nat Genet. 50:1375–1380.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hong Y, Downey T, Eu KW, Koh PK and Cheah
PY: A 'metastasis-prone' signature for early-stage mismatch-repair
proficient sporadic colorectal cancer patients and its implications
for possible therapeutics. Clin Exp Metastasis. 27:83–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Skrzypczak M, Goryca K, Rubel T, Paziewska
A, Mikula M, Jarosz D, Pachlewski J, Oledzki J and Ostrowski J:
Modeling oncogenic signaling in colon tumors by multidirectional
analyses of microarray data directed for maximization of analytical
reliability. PLoS One. 5:e130912010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Smith JJ, Deane NG, Wu F, Merchant NB,
Zhang B, Jiang A, Lu P, Johnson JC, Schmidt C, Bailey CE, et al:
Experimentally derived metastasis gene expression profile predicts
recurrence and death in patients with colon cancer.
Gastroenterology. 138:958–968. 2010. View Article : Google Scholar
|
|
80
|
Xie N, Yao Y, Wan L, Zhu T, Liu L and Yuan
J: Next-generation sequencing reveals lymph node metastasis
associated genetic markers in colorectal cancer. Exp Ther Med.
14:338–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang Y, Wei J, Zhang S, Li G, Zhang T, Yu
X, Chen H and Liu M: Overexpression of Wnt7α protein predicts poor
survival in patients with colorectal carcinoma. Tumour Biol.
36:8781–8787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Avgustinova A, Iravani M, Robertson D,
Fearns A, Gao Q, Klingbeil P, Hanby AM, Speirs V, Sahai E, Calvo F
and Isacke CM: Tumour cell-derived Wnt7a recruits and activates
fibroblasts to promote tumour aggressiveness. Nat Commun.
7:103052016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nishioka M, Ueno K, Hazama S, Okada T,
Sakai K, Suehiro Y, Okayama N, Hirata H, Oka M, Imai K, et al:
Possible involvement of Wnt11 in colorectal cancer progression. Mol
Carcinog. 52:207–217. 2013. View Article : Google Scholar
|
|
85
|
He D, Yue Z, Liu L, Fang X, Chen L and Han
H: Long noncoding RNA ABHD11-AS1 promote cells proliferation and
invasion of colorectal cancer via regulating the miR-1254-WNT11
pathway. J Cell Physiol. 234:12070–12079. 2019. View Article : Google Scholar
|
|
86
|
Kaneda H, Arao T, Tanaka K, Tamura D,
Aomatsu K, Kudo K, Sakai K, De Velasco MA, Matsumoto K, Fujita Y,
et al: FOXQ1 is overexpressed in colorectal cancer and enhances
tumorigenicity and tumor growth. Cancer Res. 70:2053–2063. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Abba M, Patil N, Rasheed K, Nelson LD,
Mudduluru G, Leupold JH and Allgayer H: Unraveling the role of
FOXQ1 in colorectal cancer metastasis. Mol Cancer Res.
11:1017–1028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Peng X, Luo Z, Kang Q, Deng D, Wang Q,
Peng H, Wang S and Wei Z: FOXQ1 mediates the crosstalk between
TGF-β and Wnt signaling pathways in the progression of colorectal
cancer. Cancer Biol Ther. 16:1099–1109. 2015. View Article : Google Scholar :
|
|
89
|
Liu JY, Wu XY, Wu GN, Liu FK and Yao XQ:
FOXQ1 promotes cancer metastasis by PI3K/AKT signaling regulation
in colorectal carcinoma. Am J Transl Res. 9:2207–2218.
2017.PubMed/NCBI
|
|
90
|
Möller P, Koretz K, Leithäuser F,
Brüderlein S, Henne C, Quentmeier A and Krammer PH: Expression of
APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in
normal and neoplastic colon epithelium. Int J Cancer. 57:371–377.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xiao W, Ibrahim ML, Redd PS, Klement JD,
Lu C, Yang D, Savage NM and Liu K: Loss of Fas expression and
function Is coupled with colon cancer resistance to immune
checkpoint inhibitor immunotherapy. Mol Cancer Res. 17:420–430.
2019. View Article : Google Scholar :
|
|
92
|
Park SJ, Kim HB, Kim J, Park S, Kim SW and
Lee JH: The oncogenic effects of p53-inducible gene 3 (PIG3) in
colon cancer cells. Korean J Physiol Pharmacol. 21:267–273. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer. Int J Oncol. 44:1032–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Krieg AJ, Rankin EB, Chan D, Razorenova O,
Fernandez S and Giaccia AJ: Regulation of the histone demethylase
JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene
expression and tumor growth. Mol Cell Biol. 30:344–353. 2010.
View Article : Google Scholar
|
|
96
|
Zong Y, Xin L, Goldstein AS, Lawson DA,
Teitell MA and Witte ON: ETS family transcription factors
collaborate with alternative signaling pathways to induce carcinoma
from adult murine prostate cells. Proc Natl Acad Sci USA.
106:12465–12470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fan L, Peng G, Sahgal N, Fazli L, Gleave
M, Zhang Y, Hussain A and Qi J: Regulation of c-Myc expression by
the histone demethylase JMJD1A is essential for prostate cancer
cell growth and survival. Oncogene. 35:2441–2452. 2016. View Article : Google Scholar :
|
|
98
|
Wilson S, Fan L, Sahgal N, Qi J and Filipp
FV: The histone demethylase KDM3A regulates the transcriptional
program of the androgen receptor in prostate cancer cells.
Oncotarget. 8:30328–30343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Fan L, Zhang F, Xu S, Cui X, Hussain A,
Fazli L, Gleave M, Dong X and Qi J: Histone demethylase JMJD1A
promotes alter-native splicing of AR variant 7 (AR-V7) in prostate
cancer cells. Proc Natl Acad Sci USA. 115:E4584–E4593. 2018.
View Article : Google Scholar
|
|
100
|
Tang DE, Dai Y, Fan LL, Geng XY, Fu DX,
Jiang HW and Xu SH: Histone demethylase JMJD1A promotes tumor
progression via activating Snail in prostate cancer. Mol Cancer
Res. 18:698–708. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ikeda H, Old LJ and Schreiber RD: The
roles of IFN gamma in protection against tumor development and
cancer immunoediting. Cytokine Growth Factor Rev. 13:95–109. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mojic M, Takeda K and Hayakawa Y: The dark
side of IFN-ү: Its role in promoting cancer immunoevasion. Int J
Mol Sci. 19:892017. View Article : Google Scholar
|
|
103
|
Jung B, Staudacher JJ and Beauchamp D:
Transforming growth factor β superfamily signaling in development
of colorectal cancer. Gastroenterology. 152:36–52. 2017. View Article : Google Scholar
|
|
104
|
Jiao HL, Ye YP, Yang RW, Sun HY, Wang SY,
Wang YX, Xiao ZY, He LQ, Cai JJ, Wei WT, et al: Downregulation of
SAFB sustains the NF-κB pathway by targeting TAK1 during the
progression of colorectal cancer. Clin Cancer Res. 23:7108–7118.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Weng W, Okugawa Y, Toden S, Toiyama Y,
Kusunoki M and Goel A: FOXM1 and FOXQ1 are promising prognostic
biomarkers and novel targets of tumor-suppressive miR-342 in human
colorectal cancer. Clin Cancer Res. 22:4947–4957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bagati A, Bianchi-Smiraglia A, Moparthy S,
Kolesnikova K, Fink EE, Lipchick BC, Kolesnikova M, Jowdy P,
Polechetti A, Mahpour A, et al: Melanoma suppressor functions of
the carcinoma oncogene FOXQ1. Cell Rep. 20:2820–2832. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nau MM, Brooks BJ, Battey J, Sausville E,
Gazdar AF, Kirsch IR, McBride OW, Bertness V, Hollis GF and Minna
JD: L-myc, a new myc-related gene amplified and expressed in human
small cell lung cancer. Nature. 318:69–73. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wu R, Lin L, Beer DG, Ellenson LH, Lamb
BJ, Rouillard JM, Kuick R, Hanash S, Schwartz DR, Fearon ER and Cho
KR: Amplification and overexpression of the L-MYC proto-onco-gene
in ovarian carcinomas. Am J Pathol. 162:1603–1610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Boutros PC, Fraser M, Harding NJ, de Borja
R, Trudel D, Lalonde E, Meng A, Hennings-Yeomans PH, McPherson A,
Sabelnykova VY, et al: Spatial genomic heterogeneity within
localized, multifocal prostate cancer. Nat Genet. 47:736–745. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Huijbers IJ, Bin Ali R, Pritchard C,
Cozijnsen M, Kwon MC, Proost N, Song JY, de Vries H, Badhai J,
Sutherland K, et al: Rapid target gene validation in complex cancer
mouse models using re-derived embryonic stem cells. EMBO Mol Med.
6:212–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kim DW, Wu N, Kim YC, Cheng PF, Basom R,
Kim D, Dunn CT, Lee AY, Kim K, Lee CS, et al: Genetic requirement
for Mycl and efficacy of RNA Pol I inhibition in mouse models of
small cell lung cancer. Genes Dev. 30:1289–1299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Möröy T, Fisher P, Guidos C, Ma A,
Zimmerman K, Tesfaye A, DePinho R, Weissman I and Alt FW: IgH
enhancer deregulated expression of L-myc: Abnormal T lymphocyte
development and T cell lymphomagenesis. EMBO J. 9:3659–3666. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chapman DL and Papaioannou VE: Three
neural tubes in mouse embryos with mutations in the T-box gene
Tbx6. Nature. 391:695–697. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sandbacka M, Laivuori H, Freitas E,
Halttunen M, Jokimaa V, Morin-Papunen L, Rosenberg C and Aittomaki
K: TBX6, LHX1 and copy number variations in the complex genetics of
Mullerian aplasia. Orphanet J Rare Dis. 8:1252013. View Article : Google Scholar
|
|
115
|
Wu N, Ming X, Xiao J, Wu Z, Chen X,
Shinawi M, Shen Y, Yu G, Liu J, Xie H, et al: TBX6 null variants
and a common hypomorphic allele in congenital scoliosis. N Engl J
Med. 372:341–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Verbitsky M, Westland R, Perez A, Kiryluk
K, Liu Q, Krithivasan P, Mitrotti A, Fasel DA, Batourina E, Sampson
MG, et al: The copy number variation landscape of congenital
anomalies of the kidney and urinary tract. Nat Genet. 51:117–127.
2019. View Article : Google Scholar :
|
|
117
|
Ren X, Yang N, Wu N, Xu X, Chen W, Zhang
L, Li Y, Du RQ, Dong S, Zhao S, et al: Increased TBX6 gene dosages
induce congenital cervical vertebral malformations in humans and
mice. J Med Genet. 57:371–379. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Takemoto T, Uchikawa M, Yoshida M, Bell
DM, Lovell-Badge R, Papaioannou VE and Kondoh H: Tbx6-dependent
Sox2 regulation determines neural or mesodermal fate in axial stem
cells. Nature. 470:394–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sadahiro T, Isomi M, Muraoka N, Kojima H,
Haginiwa S, Kurotsu S, Tamura F, Tani H, Tohyama S, Fujita J, et
al: Tbx6 induces nascent mesoderm from pluripotent stem cells and
temporally controls cardiac versus somite lineage diversification.
Cell Stem Cell. 23:382–395.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Saigusa S, Tanaka K, Toiyama Y, Yokoe T,
Okugawa Y, Ioue Y, Miki C and Kusunoki M: Correlation of CD133,
OCT4, and SOX2 in rectal cancer and their association with distant
recurrence after chemoradiotherapy. Ann Surg Oncol. 16:3488–3498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ong CW, Kim LG, Kong HH, Low LY, Iacopetta
B, Soong R and Salto-Tellez M: CD133 expression predicts for
non-response to chemotherapy in colorectal cancer. Mod Pathol.
23:450–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Neumann J, Bahr F, Horst D, Kriegl L,
Engel J, Luque RM, Gerhard M, Kirchner T and Jung A: SOX2
expression correlates with lymph-node metastases and distant spread
in right-sided colon cancer. BMC Cancer. 11:5182011. View Article : Google Scholar : PubMed/NCBI
|