Introduction
Despite significant advances in anticancer therapy,
the recurrence, metastasis and therapeutic resistance of cancer
remain serious problems leading to poor clinical outcomes (1,2). In
particular, the drastic changes in cellular properties upon
epithelial mesenchymal transition (EMT) are being actively studied
to understand the molecular events underlying recurrence,
metastasis and therapy resistance, and a number of effectors, such
as zinc finger E-box binding homeobox 1 and snail family
transcriptional repressor 1, mediating these molecular events have
been identified and characterized (3,4).
Plasminogen activator inhibitor 1 (PAI-1), encoded
by Serpin family E member 1 (SERPINE1), serves key roles in
the regulation of extracellular matrix (ECM) remodeling by directly
inhibiting plasminogen activators (PAs), such as urokinase
plasminogen activator and tissue plasminogen activator (5). Considering the function of PAI-1 as
the main inhibitor of PAs in ECM remodeling, which can promote the
migration and invasion of cancer cells, SERPINE1 expression
in cancer has been hypothesized to inhibit these pro-metastatic
effects (6). However, recent
evidence has suggested that SERPINE1 expression is
associated with poor prognosis in lung cancer (7) and a high risk of metastasis in a
PA-independent manner (8-11), which has been summarized in a
review article (12).
While SERPINE1 expression is induced by TGFβ
stimuli, which controls PA activity (13), SERPINE1 induction in a
p53-dependent manner after TGFβ promotes replicative senescence
(14). A recent study also
demonstrated that TGFβ induced the formation of a complex of p53
and SMAD2/3 that enhances SERPINE1 expression, leading to
cytostatic activity (15). In
addition, 'non-SMAD pathways' such as p53, c-SRC, EGFR and MAPK are
involved in TGFβ-dependent SERPINE1 expression in the
context of vascular disorders (16) and pulmonary fibrosis (17). These reports suggest that other
signaling pathways may contribute to TGFβ-stimulated
SERPINE1 expression in a context-dependent manner.
The Hippo pathway, in which loss-of-function
mutations activate yes-associated protein (YAP) and transcriptional
co-activator with PDZ-binding motif (TAZ) and lead to robust cell
proliferation and survival, has been strongly implicated in several
cancer-associated processes, such as tumorigenesis (18), EMT (19,20),
metastasis (21) and therapy
resistance (22). Thus, the Hippo
pathway has been extensively studied in an effort to identify
molecular targets to inhibit YAP/TAZ-dependent gene responses.
Several small molecules have been reported to inhibit YAP/TAZ
activity, including Y-27632 as an inhibitor of Rho-associated
protein kinase (ROCK) (23,24).
In addition, YAP or TAZ can interact with the SMAD2/3-4 complex and
facilitate SMAD nuclear translocation (25,26)
to control SMAD-dependent gene responses. Crosstalk between YAP/TAZ
and the canonical TGFβ pathway has also been observed, as the
absence of YAP attenuates TGFβ-induced profibrotic gene responses
(27).
Using the lung cancer cell lines that demonstrate
clear cellular properties of EMT, the present study aimed to
examine the molecular mechanism of SERPINE1 induction in
cancer cells.
Materials and methods
Establishment of cell lines and cell
culture
The mesenchymal-like lung cancer cells
(Transdifferentiated cell; TD) were established by chronic exposure
of TGFβ to a A549 lung cancer cell line (purchased from Korean Cell
Line Bank; cat. no. 10185) as described previously (28). To maintain TD cells, cells were
treated with Hyclone DMEM/High glucose (Cytiva; cat. no.
SH30243.01), 10% FBS (cat. no. EF:35-015-CV; Corning, Inc.) and
Gentamicin (0.1%; cat. no. 15780-060; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2, along with a low
dose of TGFβ (2 ng/ml; cat. no. cyt-716; Prospec-Tany TechnoGene)
every 3 days at 37°C.
For generation of TD cell lines harboring
SERPINE1 knock-down via short hairpin (sh)RNA (cat. no.
SHCLNG-NM_000602; Sigma-Aldrich; Merck KGaA), pLKO.1-shControl
(cat. no. SHC016; Sigma-Aldrich; Merck KGaA) and shSERPINE1
vector were introduced using a lentivirus. The infected cells were
selected with puromycin. In order to examine TGFβ response, a 16 h
starvation with TGFβ prior to the experiment in TD cells was
performed. All genetic perturbations (e.g. knockdown or transient
transfection) were performed without TGFβ treatment.
For cell density dependent experiment, A549 or TD
cells were grown at the indicated cell density (magnification,
×100; scale bar, 80 µm). A cell number of 100% confluence
equalled ~3.0×106 cells. Indicative cell density was
obtained by a serial dilution with normal culture media.
Reagents and antibodies
TGFβ recombinant protein (cat. no. CYT-716) was
purchased from ProSpec-Tany TechnoGene Ltd., and Y27632 (cat. no.
1293823) was obtained from BioGems Ltd. Cells were treated with 10
µM Y27632 for 24 h at 37°C. Small interfering (si)RNA
targeting SERPINE1, SMAD4, YAP1 and TAZ were obtained from
Bioneer Corporation. Antibodies against E-cadherin (cat. no. 4065)
was purchased from Cell Signaling Technology, Inc. SERPINE1
(cat. no. ab125687) and β-actin (cat. no. sc-47778) were obtained
from Abcam and Santa Cruz Biotechnology, Inc., respectively.
Total RNA extraction via reverse
transcription-quantitative PCR (RT-qPCR)
Total cellular RNA was extracted using
TRIzol® (cat. no. 17061; Invitrogen; Thermo Fisher
Scientific, Inc.), followed by RT-PCR to generate the first strand
cDNA using RT Master mix (cat. no. RR036A; Takara Bio, Inc.). The
RT conditions were as follows: 37°C for 15 min, 85°C for 5 sec and
held at 4°C. The synthesized cDNA was subjected to qPCR using TB
green premix Ex Taq (Takara Bio, Inc.; cat. no. RR420),
PrimeScript™ 1st strand cDNA Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.) and SYBR-Green-based Real-time PCR (Takara
Bio, Inc.) using Roche Light Cycler 480 II (Roche Diagnostics,
Inc.) following the manufacturer's instructions. The data analysis
was performed as described previously, using the 2−ΔΔCq
method (29). Primer sequences are
presented in Table I.
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
GCATCCTGCACCACCAACTG |
| R:
GCCTGCTTCACCACCTTC TT |
| CDH1 | F:
TGCCCAGAAAATGAAAAACG |
| R:
GTGTATGTGGCAATGCGTTC |
| CDH2 | F:
GACAATGCCCCTCAAGTGTT |
| R:
CCATTAAGCCGAGTGATGGT |
| CTGF | F:
CCAATGACAACGCCTCCTG |
| R:
TGGTGCAGCCAGAAAGCTC |
|
SERPINE1 | F:
TTGAATCCCATAGCTGCTTGAAT |
| R:
ACCGCAACGTGGTTTTCTCA |
| YAP | F:
GTGAGCCTGTTTGGATGATG |
| R:
CACTGGACAAAGGAAGCTGA |
Immunoblotting
Cell were lysed with RIPA buffer with 10 µM
sodium vanadate and 1 mM protease inhibitor (Roche Diagnostics,
Inc.) followed by immunoblotting as described previously (30). Protein determination prior to
protein loading was performed with a BCA assay. The samples were
loaded to 7.5 or 10% SDS-PAGE gels and were blotted to a PVDF
membrane (Immobilon®-P; cat. no. IPVH00010; Merck KGaA).
The blotted membrane was incubated with skim milk [1 mg in
TBS-Tween (25%); 20 ml; cat. no. 232100; BD Difco; Becton-Dickinson
and Company] for 1 h at room temperature. Primary antibodies (all
1:1,000) for PAI-1 (cat. no. ab125687; Abcam), E-cadherin (cat. no.
3195S; Cell Signaling Technology, Inc.), phosphorylated (p)-YAP
(cat. no. 4911S; Cell Signaling Technology, Inc.), YAP (cat. no.
SC-101193; Santa Cruz Biotechnology, Inc.) and β-actin (cat. no.
SC-47778; Santa Cruz Biotechnology, Inc.) were incubated for
overnight at 4°C. After washing three time with TBS-Tween solution,
the mouse or rabbit secondary antibodies [Peroxidase AffiniPure
Goat Anti-Mouse IgG (H+L); cat. no. 115-035-003; Jackson
ImmuniResearch Laboratories, Inc.; and Peroxidase AffiniPure Goat
Anti-Rabbit IgG (H+L); cat. no. 111-035-003; Jackson ImmuniResearch
Laboratories, Inc.] were incubated for 1 h at room temerature. The
visualization was peformed with ECL blotting kit (cat. no. 16026;
West-Queen; iNtRON Biotechnology).
Transfection and dual-luciferase
assay
Transfections were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's
instructions. siRNA for SERPINE1 (Thermo Fisher Scientific, Inc.;
cat. no. AM16708) and control siRNA (Thermo Fisher Scientific,
Inc.; cat. no. AM4611) transfection was performed using DharmaFECT
(cat. no. T-2001-03; GE Healthcare Dharmacon, Inc.) according to
the manufacturer's instruction.
shSERPINE1 #2: 5′-CCGGTTTAGTGTTAATGACTCTT
TCCTCGAGGAAAGAGTCATTAACACTAAATTTTTG-3′; shSERPINE1 #4:
5′-CCGGAGACCAACAAGTTCAACTAT
ACTCGAGTATAGTTGAACTTGTTGGTCTTTTTTG-3′.
For the luciferase assay for SMAD4 activity, 8X
GTIIC luciferase reporter vector (kindly gifted by Professor Mo
Jung-Soon at Ajou University), as well as pRL Renilla
luciferase control reporter vector (cat. no. E223A; Promega
Corporation), were transfected into cells. A reporter assay was
conducted according to the Dual-Luciferase Reporter assay system
(Promega Corporation) using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.; cat. no. 52887). After 24 h transfection,
reporter activity was performed with the reporter assay kit
(Promega Corporation) as described previously (31). The luciferase activity was
normalized to Renilla luciferase activity. For YAP reporter
activity, 8X GTIIC (for recognition of TEAD binding domain; cat.
no. 34615; Addgene) was transfected to cells after incubation with
Lipofectamine® 2000 (cat. no. 52887; Invitrogen; Thermo
Fisher Scientific, Inc.) for 20 min according to the manufacturer's
instructions.
Migration and Transwell invasion
assay
For wound healing assay, after 24 h of incubation
under serum starvation (0.1% FBS) conditions, the TD cell layer was
scratched using a sterile micropipette tip. Cell migration was
monitored with the light microscope (magnification, ×40; Olympus
Corporation) and live images were captured using a JuLi stage
real-time imaging system (NanoEntek, Inc.) over 48 h.
For Transwell invasion assay, Transwells were
embedded with Matrigel. In brief, Transwells (6.5 mm) with
8-µm pore polycarbonate membrane insert (cat. no. 3422;
Corning, Inc.) were embedded with Matrigel for 2 h at 37°C (BD
Bioscience) and the bottom of the Matrigel-embedded insert was
coated with 0.2% gelatin. Cells were cultured under normal culture
medium Hyclone DMEM/High glucose (Cytiva, cat. no. SH30243.01), 10%
FBS (cat. no. EF:35-015-CV; Corning, Inc.) and Gentamicin (0.1%;
cat. no. 15780-060; Gibco; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2. At 24 h after 2×105 cells were
loaded in the Transwell assay, the Transwell membrane was fixed
with 4% formaldehyde for 10 min at room temperature and stained
with 0.1% violet at room temperature (25°C). Images of the
Transwell membrane were captured using light microscopy
(magnification, ×40) The area of invaded cells was measured using
ImageJ software (National Institutes of Health; version 1.52v).
Tumor spheroid invasion assay
The 3D tumor spheroid invasion assay was performed
as previously described (32). In
brief, spontaneously formed spheroids derived from TD cells were
implanted into self-assembling collagen I gels (Vitrogen; Cohesion
Technologies) supplemented with minimal essential media and 2% FBS
and were cultured for 7 days under standard cell culture
conditions.
Zymography
Conditioned media (Hyclone DMEM/High glucose; cat.
no. SH30243.01) with 10% FBS (cat. no. 35-15-CV; Corning, Inc.) and
0.1% gentamycin (REF:15780-060; Gibco; Thermo Fisher Scientific,
Inc.) from cells maintained at 37°C, 5% CO2 were
concentrated using centricon (EMD Millipore; 30 kDa cut).
Zymography analysis using Coomassie blue R250 staining (25°C, 15
min) after loading to 8% gelatin B gel, was performed as described
previously (33).
Cancer cell line transcriptome data
analysis
As described previously (34) RNA sequencing (RNA-seq) data of 932
cancer cell lines were obtained from the NCI's Genomic Data Commons
(https://gdc.cancer.gov/) in the BAM file format.
Gene-level read count and Transcripts per Million (TPM) were
quantified using RSEM v.1.3.1. (https://deweylab.github.io/RSEM/) with Gencode v19
annotation(https://www.gencodegenes.org/human/release_19). A
total of 18,965 genes annotated as 'protein-coding gene' were used
for subsequent analysis. The EMT score for each cell line was
defined as the Kolmogorov-Smirnov (K-S) statistic, which measures
the differences in the distribution of gene expression levels (TPM)
of EMT genes compared with those of the rest of the genes. EMT
genes were obtained from the MSigDB: 'hallmark epithelial
mesenchymal transition' (https://www.gsea-msigdb.org/gsea/msigdb). The K-S
statistic of each cell line was calculated using ks.test function
in R software(version 3.6.3). To compare the expression patterns of
mesenchymal-like and epithelial-like cell lines, the
EMT+ and EMT− cell groups were selected as
the top 10% and bottom 10% cell lines, respectively, based on the
EMT score. Gene-level read counts of A549 and TD cells were
downloaded from the Gene Expression Omnibus (GSE135402). The
differences in gene expression between TD cells and A549 cells or
between EMT+ and EMT− cell groups were
calculated using the DESeq2 package (35) (version 1.28.1) in R (version
3.6.3).
The Cancer Genome Atlas (TCGA) data
processing and analysis
RNA-seq and clinical data of patients with lung
adenocarcinoma (LUAD) in the TCGA study were obtained from the
Broad GDAC firehose (https://gdac.broadinsti-tute.org/; data version
2016_01_28). A total of 494 patients with ≥1 month of follow-up
clinical record information were used for survival analysis (women,
263; men, 231; median age, 66). Patients were divided into two
groups according to the median value (TPM) of SERPINE1 gene
expression. Upper (n=247) and lower (n=247) groups were defined as
'high' and 'low' groups. In similar, CTGF high (n-247) and low
(n=247) groups were defined. Overall survival and recurrence-free
survival analysis were performed to test the difference in the
survival rate between the groups using the survival package
(https://cran.r-project.org/package=survival; version
3.2-7) in R (version 3.6.3). The hazard ratio and P-value were
computed using Cox proportional hazards regression analysis and the
log-rank test, respectively.
Pathway enrichment analysis
RNAseq data of A549 and TD (GSE135402) was used.
Among the 3,675 differentially expressing genes (DEG) identified
between A549 and TD, 111 SMAD4 downstream genes were identified
using web-based tool 'EnrichR ' (https://maayanlab.cloud/Enrichr/). Pathway enrichment
analysis was performed based on the geneset of public annotation in
Gene Ontology (GO) via EnrichR.
Statistical analysis
Data are presented as the mean ± SD Statistical
significance between two groups was determined using t-test
analysis (unpaired). Statistical significance among the three
groups and between groups was determined using One-way ANOVA
following the Tukey's multiple comparison test (GraphPad Prism 7.0;
GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference. Number of experimental
repeats was >3.
Results
High SERPINE1 expression in mesenchymal
lung cancer cells
Our previous studies established isogenic
mesenchymal lung cancer cells [A549 transdifferentiated cells
(A549TD), hereafter TD] derived from A549 cells subjected to
long-term exposure to TGFβ (similar to a previously described
method) (36), with typical EMT
features such as chemoresis-tance and prometastatic activity
(28,37-39).
To identify key genes responsible for the mesenchymal cell state,
the current study examined genome-wide mRNA expression profiles
from A549 and TD cells, along with a panel of human cancer cell
lines (CCLE) (40). The most
upregulated gene in cells with enriched mesenchymal properties was
SERPINE1, followed by other known EMT markers such as
collagen type Iα1 chain (COL1A1), Coiled-Coil Domain
Containing 80, MMP2 and COL5A1 (Fig. 1A). As predicted, SERPINE1
expression was significantly increased in TD cells, in parallel
with lower levels of cadherin 1 (CDH1; encoding E-cadherin)
and higher CDH2 (encoding N-cadherin) compared with parental
A549 cells (Fig. 1B). The protein
level of PAI-1 (encoded by SERPINE1) was higher in TD cells
compared with A549 cells (Fig.
1C). Consistently, four genes (COL1A1, CDC80,
MMP2 and COL5A1) were found to be closely associated
with SERPINE1 expression in 494 patients with lung
adenocarcinoma (TCGA) (Fig.
1D).
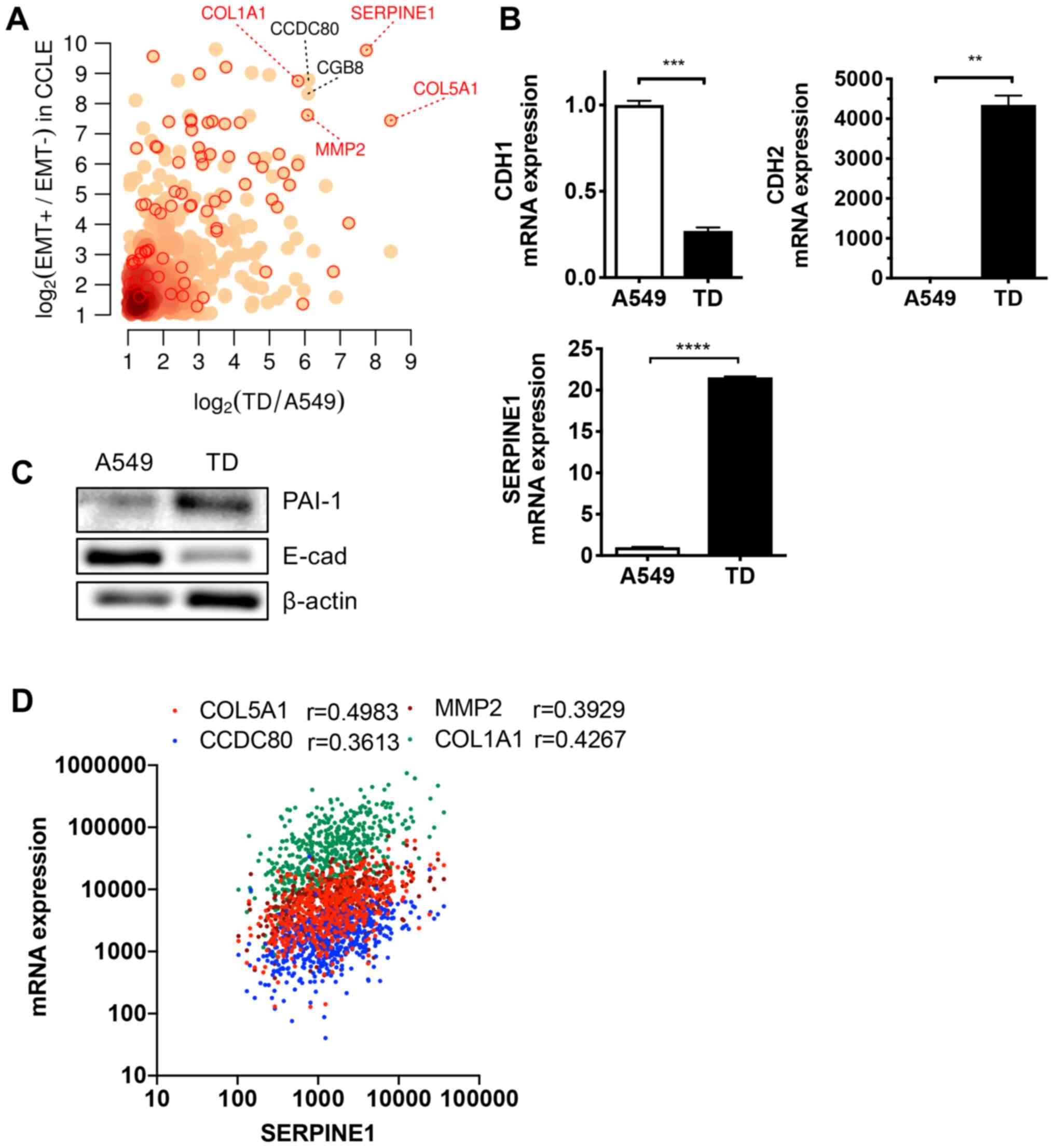 | Figure 1High SERPINE1 expression in
mesenchymal lung cancer cells. (A) Differentially upregulated genes
in both the EMT+ vs. EMT− cell groups of the
CCLE dataset and in TD vs. A549 cell groups. Red circles indicate
known EMT genes annotated by MSigDB (http://software.broadinstitute.org/gsea/msigdb). (B)
Relative mRNA expression levels of CDH1, CDH2 and
SERPINE1 in A549 and TD cells. (C) Immunoblotting for PAI-1
and E-cad in A549 and TD cells. β-actin was used as an equal
protein loading control. (D) Correlation of SERPINE1 mRNA
expression with indicated genes in patients with lung
adenocarcinoma. **P<0.01, ***P<0.001,
****P<0.0001. E-cad, E-cadherin; SERPINE1,
serpin family E member 1; COL1A1, collagen type Iα1 chain; EMT,
epithelial mesenchymal transition; CCLE, Cancer Cell Line
Encyclopedia; CDH, cadherin; PAI-1, Plasminogen activator
inhibitor 1. |
SERPINE1 contributes to cancer cell
invasive properties
Despite the well-known physiological role of
SERPINE1 in inhibiting PAs, SERPINE1 induction
promotes invasion and migration in diverse cancer types in a
PA-independent manner (11). To
examine whether the elevated expression of SERPINE1 in TD
cells (Fig. 1B and C) accounted
for the high invasiveness of TD cells (28,37),
SERPINE1 expression was knockdown using siRNA in TD cells
(Fig. 2A), and a cell invasion
assay was performed. Consistent with previous reports (9,41,42),
the highly invasive properties of TD cells (28,37)
were significantly attenuated by knockdown of SERPINE1,
while knockdown in A549 cells had no significant effect on cell
invasion (Fig. 2B). Stable
knockdown of SERPINE1 with shRNA was achieved; the clone#2
demonstrated the most significant knockdown efficiency (Fig. S1A) and was selected for further
study. Consistent with the results of siRNA, stable knockdown of
SERPINE1 using shRNA had an inhibitory effect on cell
invasion (Fig. 2C). The attenuated
invasiveness by loss of SERPINE1 expression was further
validated via tumor spheroid invasion assay (Fig. 2D) (32).
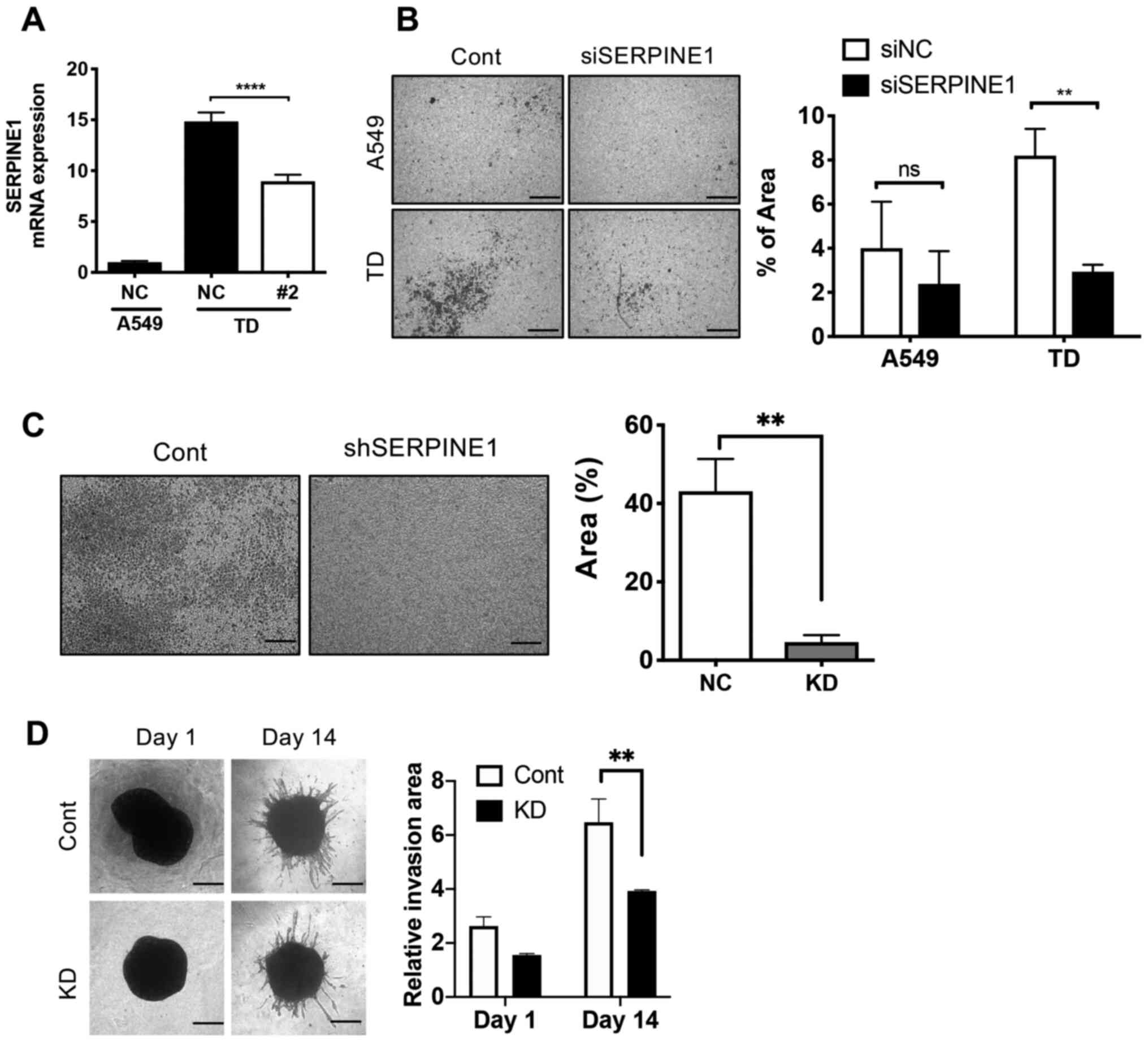 | Figure 2SERPINE1 contributes to cancer
cell invasive properties. (A) mRNA expression of SERPINE1 in
A549 and TD cells after knockdown with SERPINE1 shRNA.
Representative microscopic images (left) of invaded cell numbers in
a two-chamber invasion model after knockdown either with (B)
SERPINE1 siRNA or (C) shRNA, relative invasion area was
graphically presented (right). Magnification, ×40; scale bar, 200
µm. (D) Microscope images of tumor spheroid of control TD
cells (left), and graphical presentation of invasive area (right).
Magnification, ×100; scale bar, 80 µm.
**P<0.01, ****P<0.0001. si, small
interfering RNA; SERPINE1, serpin family E member 1; NC,
negative control; Cont, control; shRNA, short hairpin RNA; KD,
knockdown. |
However, inconsistent with the findings of a
previous report (43),
SERPINE1 knockdown in TD cells with shRNA (Fig. S1A) only had marginal effects on
cellular migration (Fig. S1B).
Moreover, transient knockdown of SERPINE1 with siRNA (Fig. S1C) had a negligible effect on the
high activity of MMP9 in TD cells (Fig. S1D).
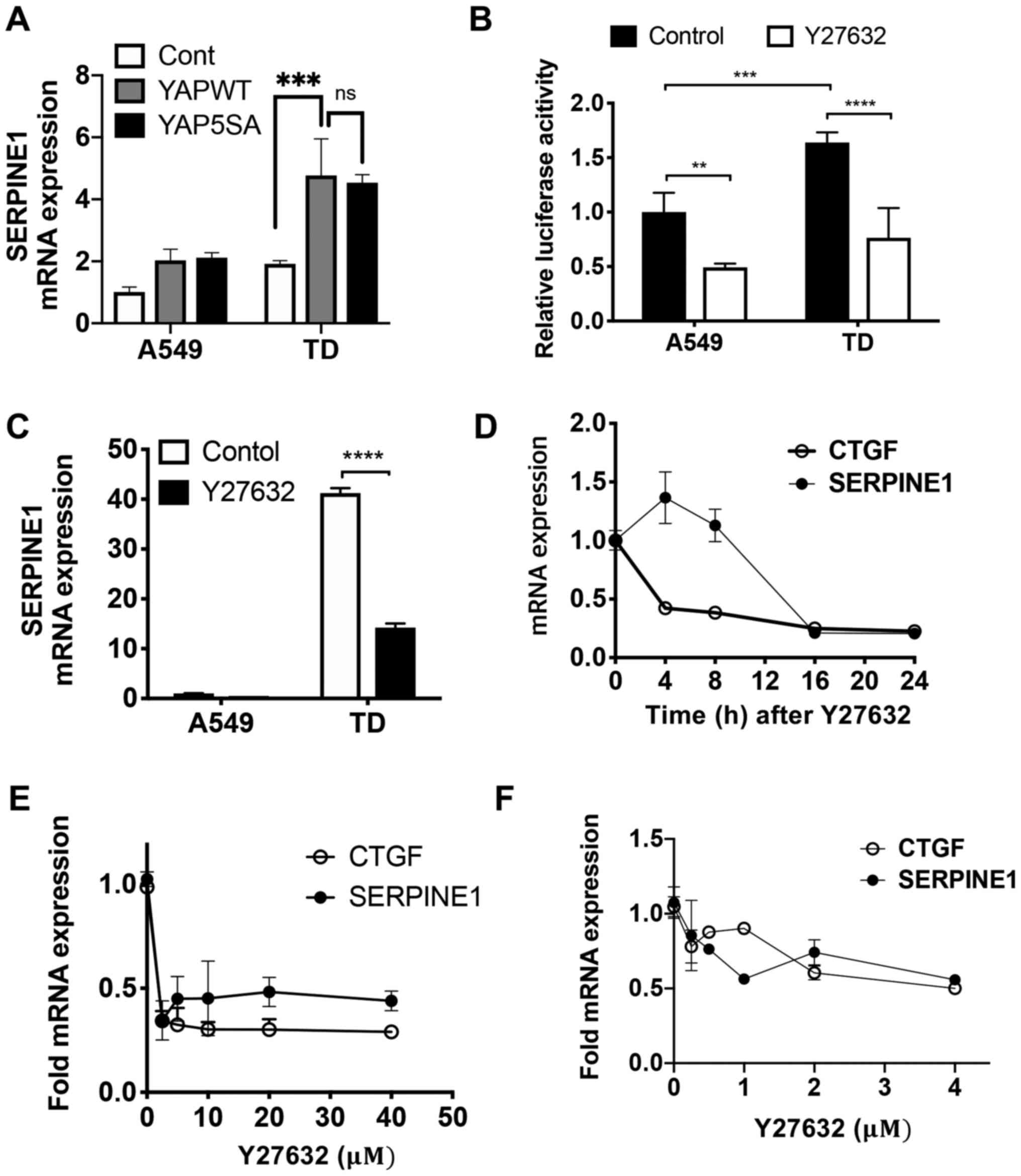
YAP-dependent SERPINE1 expression
Previous work has revealed that SERPINE1 is
induced by ectopic expression of TAZ, along with connective tissue
growth factor (CTGF), a well-established YAP/TAZ downstream target
gene (44), which is frequently
used as a marker to determine endogenous YAP-TEAD activity in a
number of study (44,45). The present study identified that
ectopic expression of either wild-type YAP or a constitutively
active mutant lacking inhibitory phosphorylation of YAP was
sufficient to induce SERPINE1 expression in both A549 and TD
cells (Fig. 3A). It is important
to note that despite a comparable level of YAP expression in A549
and TD (data not shown), the nuclear level of YAP appeared to be
significantly higher in TD cells compared with A549 cells,
regardless of cell density (Fig.
S2A). This observation was consistent with the higher mRNA
expression level of SERPINE1 in TD cells compared with A549
cells (Fig. 3A).
To confirm the involvement of the Hippo-YAP pathway
in SERPINE1 expression, Hippo signaling was re-activated by
Y27632, a ROCK inhibitor (46)
that markedly decreases YAP reporter activity (e.g. 8X GTIIC
reporter activity) (Fig. 3B).
Protein expression levels of YAP, TAZ and p-YAP at serine 127 were
not different between A549 and TD cells (Fig. S2B). Treatment with Y27632 was
sufficient to attenuate SERPINE1 expression (Fig. 3C) in a time- (Fig. 3D) and dose- (Fig. 3E and F) dependent manner, along
with a reduction in CTGF. As Y27632 treatment had no effect on
either CDH1 or CDH2, the repression of
SERPINE1 by Y27632 may not be mediated by a loss of
mesenchymal properties in the TD cells (Fig. S2C).
Involvement of the TGFβ-SMAD4 axis in
YAP-dependent SERPINE1 expression
It has been well established that TGFβ serves as a
key stimuli for SERPINE1 expression in diverse cell line
models (13). In addition,
crosstalk with 'non-SMAD pathways' is involved in TGFβ-dependent
SERPINE1 expression (15,16,47).
Although TGFβ treatment alone upregulated SERPINE1
expression in both cells, TGFβ-mediated SERPINE1 expression
was induced to a greater degree in TD cells compared with A549 in a
dose- (Fig. 4A) and time-dependent
manner (Fig. 4B). Increased
expression of PAI-1 protein by TGFβ also occurred, while knockdown
of SERPINE1 using siRNA markedly lowered PAI-1 protein
expression (Fig. 4C).
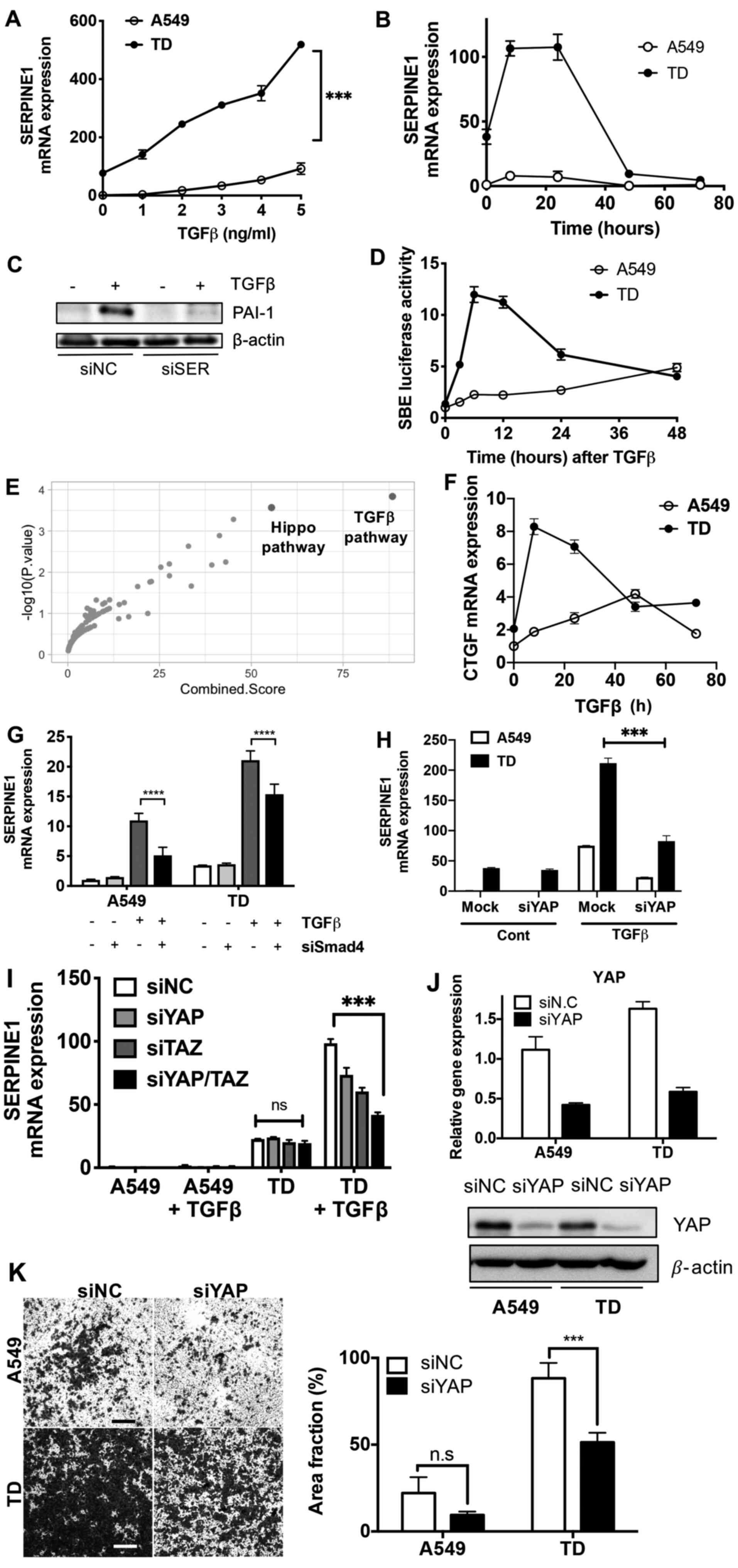 | Figure 4Involvement of the TGFβ-SMAD4 axis in
YAP-dependent SERPINE1 expression. (A) SERPINE1 mRNA
expression in A549 and TD cells at 24 h after TGFβ treatment with
the indicated concentrations. (B) Relative mRNA expression of
SERPINE1 in A549 and TD cells at indicative time-point after
TGFβ (2 ng/ml) treatment. (C) Immunoblotting analysis for PAI-1
protein expression at 24 h after TGFβ (2 ng/ml) treatment with siNC
or siSER. (D) SBE luciferase activity in A549 and TD cell lines at
indicated time-points after TGFβ (2 ng/ml) treatment. (E) Enriched
pathway in the 111 genes regulated by SMAD4 in DEG of A549 and TD
cells. Highlighted red dots indicate the TGFβ and Hippo pathways.
(F) mRNA expression of CTGF in A549 and TD cells at indicated
time-point after TGFβ treatment (2 ng/ml). mRNA expression of
SERPINE1 in A549 and TD cells at 24 h after TGFβ (2 ng/ml)
treatment after knockdown of (G) SMAD4, (H) YAP and (I) YAP/TAZ
with siRNA transfection. (J) mRNA expression (top) and protein
(bottom) of YAP after knockdown of YAP, β-actin was used for
protein loading control. (K) Representative images (magnification,
×40) of invaded cells from a two-chamber invasion model after
knockdown with YAP siRNA (left), graphical representation of area
fraction of invaded area (right). ***P<0.001,
****P<0.0001. siSER, small interfering RNA serpin
family E member 1; NC, negative control; SERPINE1, serpin
family E member 1; YAP, yes-associated protein; TAZ,
transcriptional co-activator with PDZ-binding motif; CTGF,
connective tissue growth factor; PAI-1, plasminogen activator
inhibitor 1; SBE, Smad-binding element; ns, not significant. |
Smad-binding element reporter activity, which is
used to determine SMAD2/3 dependent gene responses (48), was markedly enhanced in TD cells
(Fig. 4D). These data suggested
that SERPINE1 induction may be facilitated by the
enhancement of TGFβ-dependent gene responses. Given that
SERPINE1 expression was regulated by both YAP and TGFβ, it
was conclude that the YAP pathway and SMAD4-dependent TGFβ
signaling interact upon TGFβ stimulation. To evaluate the
hypothesis of a crosstalk between TGFβ signaling and YAP, pathway
enrichment analysis of 111 genes, which are regulated by the SMAD4
transcription factor in the DEGs in A549 and TD cells, was
performed. The second most enriched pathway was the Hippo pathway
(P=1.4×10−4) after TGFβ signaling (P=0.2×104)
(Fig. 4E). As predicted,
CTGF expression, indicating a YAP-dependent gene response,
was rapidly enhanced upon TGFβ treatment in TD cells (Fig. 4F), similar to SRE reporter activity
(Fig. 4D). Of interest, knockdown
of SMAD4 in TD cells failed to affect basal levels of
SERPINE1 expression, whereas TGFβ-mediated SERPINE1
expression was attenuated by SMAD4 knockdown (Fig. 4G). Moreover, TGFβ-mediated
SERPINE1 expression was significantly decreased by knockdown
of YAP (Fig. 4H). This effect was
more pronounced when YAP and TAZ were simultaneously depleted
(Fig. 4I), suggesting that YAP and
TAZ may be required for TGFβ-SMAD4-dependent SERPINE1
expression in mesenchymal lung cancer cells. The knockdown
efficiencies of siRNAs of SMAD4, YAP and TAZ with or without
treatment of TGFβ were validated (Fig. S3A-C). It is also important to note
that knockdown of YAP (Fig. 4J)
significantly attenuated the invasiveness in TD cells, which was
similar to the effect identified after SERPINE1 expression
knockdown (Fig. 4K).
Regulation of SEPINE1 expression in the
patients with lung adenocarcinoma
To verify the results from the lung cancer cell line
model, a transcriptome dataset from 494 patients with lung
adenocarcinoma (TCGA; PanCancer Atlas) was obtained from cBioPoral
(https//www.cbioportal.org/).
SERPINE1 expression was closely correlated with typical EMT
marker genes such as zinc finger E-box binding homeobox 1,
CDH2 and snail family transcriptional repressor 2 (Fig. 5A). Additionally, the YAP-dependent
nature of SERPINE1 expression (Fig. 3) was also supported by the close
correlation of SERPINE1 to CTGF expression (Pearson
r=0.5183; Fig. 5B). The TGFβ
dependency of SERPINE1 expression (Fig. 4) was supported by a close
correlation of SERPINE1 expression to COL1A1, inhibin
subunit βA (INHBA) and fibronectin 1 (FN1; Pearson
r=0.4267, 0.6200 and 0.4244, respectively), which are typical SMAD4
targets (Fig. 5C). Of note, the
CTGF-high patient group demonstrated upregulated expression levels
of SMAD4 downstream targets (COL1A1, INHBA and
FN1), as well as high SERPINE1 expression (Fig. 5D), suggesting a crosstalk between
TGFβ downstream targets, including SERPINE1, and YAP/TAZ
gene responses.
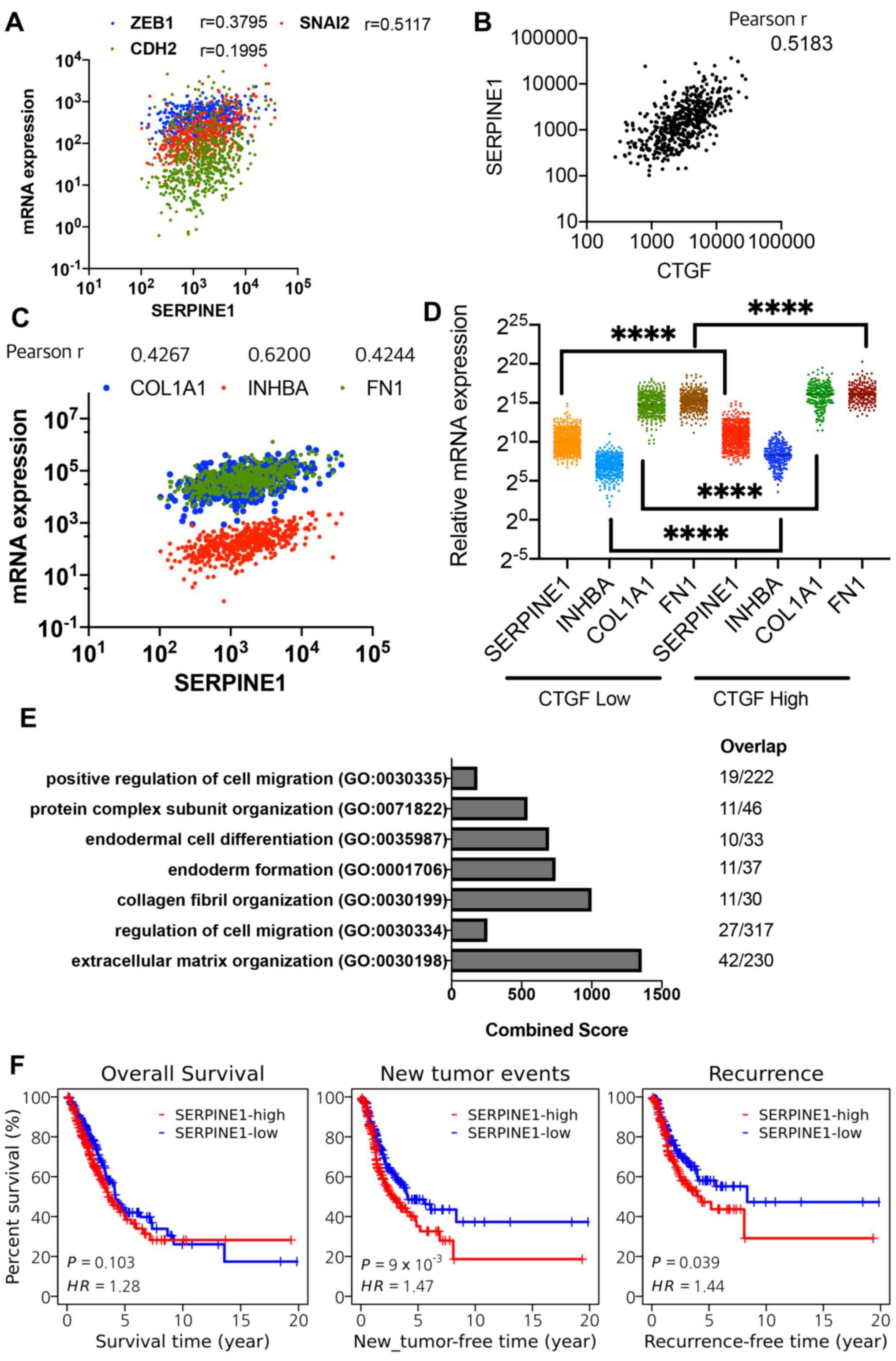 | Figure 5Regulation of SEPINE1
expression in the patients with LUAD. Correlation of mRNA
expression of SERPINE1 with (A) epithelial mesenchymal
transition marker genes, (B) CTGF and (C) SMAD4 downstream genes in
patients with LUAD. (D) Comparison between the high- and
low-expression group for CTGF of average expression levels from 566
patients with lung cancer from TCGA cohort, in terms of relative
mRNA expression of indicated genes in patients with LUAD. (E) GO
analysis with genes highly correlated with SERPINE1 in
patients with LUAD. Combined score of each GO is presented as a bar
graph (left) and number of genes overlapped in each GO is
illustrated (right). (F) Comparison between the high- and
low-expression groups for SERPINE1 in terms of overall
survival, new tumor event-free survival and locoregional
recurrence-free survival in patients with LUAD.
****P<0.0001. GO, Gene Ontology; SERPINE1,
serpin family E member 1; HR, hazard ratio; CTGF, connective tissue
growth factor; ZEB1, zinc finger E-box binding homeobox 1; SNAI2,
snail family transcriptional repressor 2; COL1A1, collagen type Iα1
chain; INHBA, inhibin subunit βA; FN1, fibronectin 1; LUAD, lung
adenocarcinoma. |
From the mRNA expression database involving 566
patients with lung adenocarcinoma, a total of 237 genes were found
to be highly correlated with SERPINE1 expression (Pearson
R>0.4), which were then subjected to GO analysis. As predicted,
gene signatures of 'ECM matrix organization' (GO: 0030198) and
'cell migration' (GO: 0030334 and 0030335) were highly associated
with a SERPINE1 gene signature in the patients (Fig. 5E; Table SI). Consistent with previous
studies (10,43,49),
high SERPINE1 expression was associated with a poor
prognosis for relapse or recurrence-free survival but not overall
survival (Fig. 5F), indicating
that SERPINE1 expression may have particular relevance to
metastasis or recurrence.
Discussion
TGFβ has been extensively characterized as a key
stimulus for SERPINE1 expression and underlies the diverse
roles of SERPINE1 in senescence, fibrosis, vascular
disorders and cancer (13,16,50-53).
Considering the significant roles of the PA system in controlling
ECM remodeling, which itself promotes the invasive and metastatic
potential of cancer (5,54), induction of SERPINE1 in
cancer cells is unexpectedly associated with poor prognosis due to
its ability to promote metastasis or therapy resistance independent
of PA activity(6,10,41,55,56).
While the mesenchymal features acquired via EMT (4) have been extensively characterized in
both cancer metastasis and therapy resistance, the signaling
components responsible for these cancer-promoting mesenchymal
features may be impor-tant targets for future anti-cancer
strategies.
Our previous study observed that SERPINE1
expression was correlated with chemoresistance score, a common
feature of mesenchymal cancer cells (39). Consistent with this finding, the
present study identified that SERPINE1 expression was
significantly elevated in TD cells, and was closely correlated with
other mesenchymal marker genes in patients with lung cancer. This
close correlation with EMT features was recently reported in
gastric cancer types via transcriptomic analysis of a large dataset
(49). Despite the strong
correlation of SERPINE1 to chemoresistance score,
SERPINE1 knockdown failed to sensitize TD cells to
conventional chemotherapeutics (data not shown). Instead, knockdown
of SERPINE1 in TD cells impaired invasive properties in a
manner independent of MMP activity. These results were in
accordance with data indicating that SERPINE1 expression
served as a more favorable prognostic marker for recurrence-free
survival in lung cancer compared with overall survival (Fig. 5F).
The present results suggested that TGFβ-dependent
SERPINE1 expression was distinct between A549 and TD cells.
A significant induction of SERPINE1 expression upon TGFβ
treatment was found in TD cells, indicating that a factor(s)
activated in TD cells contributed to TGFβ-mediated SERPINE1
expression, a finding that was similar to previous studies
revealing crosstalk among other signaling components underlying the
induction of SERPINE1 expression upon TGFβ stimulation
(15,16). The current study demonstrated that
YAP, uncontrolled activation of which promotes both development and
malignancy in diverse cancer types (57), itself induced SERPINE1
expression and also contributed to SERPINE1 expression upon
TGFβ treatment. Moreover, a functional inter-action between YAP and
TGFβ was identified in the regulation of CTGF, which functions as
an important growth modulator of malignant mesothelioma (58). The significant reduction of
SERPINE1 expression upon TGFβ by knockdown of YAP suggested
that high YAP activity may prime TGFβ-dependent expression. Thus,
the significant induction of CTGF by TGFβ in TD cells indicated
that the functional interaction between YAP and TGFβ was
substantially enhanced in TD cells by an unknown mechanism, which
represents as an interesting research question for subsequent
studies. It was also observed that immunoblotting for PAI-1 with
two commercially available antibodies was technically challenging,
unlike that found in previous studies (59,60),
for unknown reasons. With multiple attempts, high PAI-1 protein
expression in TD cells was barely detected.
In conclusion, the present study demonstrated that
SERPINE1 induction in the mesenchymal lung cancer cells
resulted from the synergic effect of YAP and TGFβ, which promoted
invasive features. As a cancer prone effect of SERPINE1
(e.g. invasion) occurs in a PA activity-independent manner, instead
of direct inhibitor for SERPINE1, a drug(s) to reverse
transcriptome signature responsible for SERPINE1-dependent
invasiveness based on drug-transcripome data analysis (e.g.
Connectivity MAP) could be a feasible approach to target the cancer
prone effect of SERPINE1 (39, 61).
Supplementary Data
Funding
This work was supported by the National Research
Foundation of Korea (grant no. NRF-2020R1A2C2005914) and by the
Global Core Research Center (grant no. 2011-0030001). This work was
also supported by Creative-Pioneering Researchers Program through
Seoul National University.
Availability of data and materials
RNA-seq of A549 and TD cell lines can be obtained
from Gene Expression Omnibus (GEO) under the accession number
GSE135402.
Authors' contributions
HJC conceived the overall study design and led the
experiments. HJK and EJK mainly conducted the experiments and data
analysis, as well as provided critical discussion of the results.
OSK, HL and WK analyzed clinicogenomics and RNAseq data. JYC and
YJK performed the most of genetic perturbation study. All authors
contributed to manuscript writing and revising, and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing of interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Park
Hyun-Woo for providing the YAP expression vectors and GTIIC
reporter construct.
References
|
1
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marcucci F, Stassi G and De Maria R:
Epithelial-mesenchymal transition: A new target in anticancer drug
discovery. Nat Rev Drug Discov. 15:311–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andreasen PA, Egelund R and Petersen HH:
The plasminogen activation system in tumor growth, invasion, and
metastasis. Cell Mol Life Sci. 57:25–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pavón MA, Arroyo-Solera I, Céspedes MV,
Casanova I, León X and Mangues R: uPA/uPAR and SERPINE1 in head and
neck cancer: Role in tumor resistance, metastasis, prognosis and
therapy. Oncotarget. 7:57351–57366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pedersen H, Brünner N, Francis D,
Osterlind K, Rønne E, Hansen HH, Danø K and Grøndahl-Hansen J:
Prognostic impact of urokinase, urokinase receptor, and type 1
plasminogen activator inhibitor in squamous and large cell lung
cancer tissue. Cancer Res. 54:4671–4675. 1994.PubMed/NCBI
|
|
8
|
Lin X, Lin BW, Chen XL, Zhang BL, Xiao XJ,
Shi JS, Lin JD and Chen X: PAI-1/PIAS3/Stat3/miR-34a forms a
positive feedback loop to promote EMT-mediated metastasis through
Stat3 signaling in Non-small cell lung cancer. Biochem Biophys Res
Commun. 493:1464–1470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirahata M, Osaki M, Kanda Y, Sugimoto Y,
Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Kawai A, Ito H, et
al: PAI-1, a target gene of miR-143, regulates invasion and
metastasis by upregulating MMP-13 expression of human osteosarcoma.
Cancer Med. 5:892–902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seker F, Cingoz A, Sur-Erdem İ, Erguder N,
Erkent A, Uyulur F, Esai Selvan M, Gümüş ZH, Gönen M, Bayraktar H,
et al: Identification of SERPINE1 as a regulator of glioblastoma
cell dispersal with transcriptome profiling. Cancers (Basel).
11:112019. View Article : Google Scholar
|
|
11
|
Pavón MA, Arroyo-Solera I, Téllez-Gabriel
M, León X, Virós D, López M, Gallardo A, Céspedes MV, Casanova I,
López-Pousa A, et al: Enhanced cell migration and apoptosis
resistance may underlie the association between high SERPINE1
expression and poor outcome in head and neck carcinoma patients.
Oncotarget. 6:29016–29033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kubala MH and DeClerck YA: The plasminogen
activator inhibitor-1 paradox in cancer: A mechanistic
understanding. Cancer Metastasis Rev. 38:483–492. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerwin BI, Keski-Oja J, Seddon M, Lechner
JF and Harris CC: TGF-beta 1 modulation of urokinase and PAI-1
expression in human bronchial epithelial cells. Am J Physiol.
259:L262–L269. 1990.PubMed/NCBI
|
|
14
|
Kortlever RM, Higgins PJ and Bernards R:
Plasminogen activator inhibitor-1 is a critical downstream target
of p53 in the induction of replicative senescence. Nat Cell Biol.
8:877–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawarada Y, Inoue Y, Kawasaki F, Fukuura
K, Sato K, Tanaka T, Itoh Y and Hayashi H: TGF-β induces p53/Smads
complex formation in the PAI-1 promoter to activate transcription.
Sci Rep. 6:354832016. View Article : Google Scholar
|
|
16
|
Samarakoon R and Higgins PJ: Integration
of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen
activator inhibitor type-1 gene expression in vascular smooth
muscle cells. Thromb Haemost. 100:976–983. 2008. View Article : Google Scholar
|
|
17
|
Higgins SP, Tang Y, Higgins CE, Mian B,
Zhang W, Czekay RP, Samarakoon R, Conti DJ and Higgins PJ:
TGF-β1/p53 signaling in renal fibrogenesis. Cell Signal. 43:1–10.
2018. View Article : Google Scholar
|
|
18
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F,
Pei XH, Zhao S, Xiong Y and Guan KL: TAZ promotes cell
proliferation and epithelial-mesenchymal transition and is
inhibited by the hippo pathway. Mol Cell Biol. 28:2426–2436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Liu J, Ying X, Lin PC and Zhou BP:
Twist-mediated epithelial-mesenchymal transition promotes breast
tumor cell invasion via inhibition of Hippo pathway. Sci Rep.
6:246062016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:E2441–E2450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sebio A and Lenz HJ: Molecular pathways:
Hippo signaling, a critical tumor suppressor. Clin Cancer Res.
21:5002–5007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Varelas X, Sakuma R, Samavarchi-Tehrani P,
Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW and Wrana JL:
TAZ controls Smad nucleocytoplasmic shuttling and regulates human
embryonic stem-cell self-renewal. Nat Cell Biol. 10:837–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grannas K, Arngården L, Lönn P,
Mazurkiewicz M, Blokzijl A, Zieba A and Söderberg O: Crosstalk
between Hippo and TGFβ: subcellular localization of YAP/TAZ/Smad
complexes. J Mol Biol. 427:3407–3415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szeto SG, Narimatsu M, Lu M, He X, Sidiqi
AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, et al:
YAP/TAZ Are mechanoregulators of TGF-β-Smad signaling and renal
fibrogenesis. J Am Soc Nephrol. 27:3117–3128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bae GY, Hong SK, Park JR, Kwon OS, Kim KT,
Koo J, Oh E and Cha HJ: Chronic TGFβ stimulation promotes the
metastatic potential of lung cancer cells by Snail protein
stabilization through integrin β3-Akt-GSK3β signaling. Oncotarget.
7:25366–25376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Kwon OS, Lee H, Kong HJ, Kwon EJ, Park JE,
Lee W, Kang S, Kim M, Kim W and Cha HJ: Connectivity map-based drug
repositioning of bortezomib to reverse the metastatic effect of
GALNT14 in lung cancer. Oncogene. 39:4567–4580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dupont S: Luciferase reporter assays to
determine YAP/TAZ activity in mammalian cells. Methods Mol Biol.
1893:121–135. 2019. View Article : Google Scholar
|
|
32
|
Vinci M, Box C and Eccles SA:
Three-dimensional (3D) tumor spheroid invasion assay. J Vis Exp.
May 1–2015.Epub ahead of print. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bae GY, Choi SJ, Lee JS, Jo J, Lee J, Kim
J and Cha HJ: Loss of E-cadherin activates EGFR-MEK/ERK signaling,
which promotes invasion via the ZEB1/MMP2 axis in non-small cell
lung cancer. Oncotarget. 4:2512–2522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kwon OS, Kwon EJ, Kong HJ, Choi JY, Kim
YJ, Lee EW, Kim W, Lee H and Cha HJ: Systematic identification of a
nuclear receptor-enriched predictive signature for erastin-induced
ferroptosis. Redox Biol. 37:1017192020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Katsuno Y, Meyer DS, Zhang Z, Shokat KM,
Akhurst RJ, Miyazono K and Derynck R: Chronic TGF-β exposure drives
stabilized EMT, tumor stemness, and cancer drug resistance with
vulnerability to bitopic mTOR inhibition. Sci Signal. 12:122019.
View Article : Google Scholar
|
|
37
|
Hong SK, Park JR, Kwon OS, Kim KT, Bae GY
and Cha HJ: Induction of integrin β3 by sustained ERK activity
promotes the invasiveness of TGFβ-induced mesenchymal tumor cells.
Cancer Lett. 376:339–346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwon OS, Hong SK, Kwon SJ, Go YH, Oh E and
Cha HJ: BCL2 induced by LAMTOR3/MAPK is a druggable target of
chemo-radioresistance in mesenchymal lung cancer. Cancer Lett.
403:48–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hong SK, Lee H, Kwon OS, Song NY, Lee HJ,
Kang S, Kim JH, Kim M, Kim W and Cha HJ: Large-scale
pharmacogenomics based drug discovery for ITGB3 dependent
chemoresistance in mesenchymal lung cancer. Mol Cancer. 17:1752018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghandi M, Huang FW, Jané-Valbuena J,
Kryukov GV, Lo CC, McDonald ER III, Barretina J, Gelfand ET,
Bielski CM, Li H, et al: Next-generation characterization of the
Cancer Cell Line Encyclopedia. Nature. 569:503–508. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Klein RM, Bernstein D, Higgins SP, Higgins
CE and Higgins PJ: SERPINE1 expression discriminates site-specific
metastasis in human melanoma. Exp Dermatol. 21:551–554. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arroyo-Solera I, Pavón MA, León X, López
M, Gallardo A, Céspedes MV, Casanova I, Pallarès V, López-Pousa A,
Mangues MA, et al: Effect of serpinE1 overexpression on the primary
tumor and lymph node, and lung metastases in head and neck squamous
cell carcinoma. Head Neck. 41:429–439. 2019.
|
|
43
|
Yang JD, Ma L and Zhu Z: SERPINE1 as a
cancer-promoting gene in gastric adenocarcinoma: Facilitates tumour
cell proliferation, migration, and invasion by regulating EMT. J
Chemother. 31:408–418. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu
J, Lin JD, Wang CY, Chinnaiyan AM, et al: TEAD mediates
YAP-dependent gene induction and growth control. Genes Dev.
22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moroishi T, Park HW, Qin B, Chen Q, Meng
Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, et al: A
YAP/TAZ-induced feedback mechanism regulates Hippo pathway
homeostasis. Genes Dev. 29:1271–1284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wada K, Itoga K, Okano T, Yonemura S and
Sasaki H: Hippo pathway regulation by cell morphology and stress
fibers. Development. 138:3907–3914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kutz SM, Hordines J, McKeown-Longo PJ and
Higgins PJ: TGF-beta1-induced PAI-1 gene expression requires MEK
activity and cell-to-substrate adhesion. J Cell Sci. 114:3905–3914.
2001.PubMed/NCBI
|
|
48
|
Dennler S, Itoh S, Vivien D, ten Dijke P,
Huet S and Gauthier JM: Direct binding of Smad3 and Smad4 to
critical TGF beta-inducible elements in the promoter of human
plasminogen activator inhibitor-type 1 gene. EMBO J. 17:3091–3100.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu B, Bai Z, Yin J and Zhang Z: Global
transcriptomic analysis identifies SERPINE1 as a prognostic
biomarker associated with epithelial-to-mesenchymal transition in
gastric cancer. PeerJ. 7:e70912019. View Article : Google Scholar :
|
|
50
|
Hirashima Y, Kobayashi H, Suzuki M, Tanaka
Y, Kanayama N and Terao T: Transforming growth factor-beta1
produced by ovarian cancer cell line HRA stimulates attachment and
invasion through an up-regulation of plasminogen activator
inhibitor type-1 in human peritoneal mesothelial cells. J Biol
Chem. 278:26793–26802. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Allan EH, Zeheb R, Gelehrter TD, Heaton
JH, Fukumoto S, Yee JA and Martin TJ: Transforming growth factor
beta inhibits plasminogen activator (PA) activity and stimulates
production of urokinase-type PA, PA inhibitor-1 mRNA, and protein
in rat osteoblast-like cells. J Cell Physiol. 149:34–43. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Higgins CE, Tang J, Mian BM, Higgins SP,
Gifford CC, Conti DJ, Meldrum KK, Samarakoon R and Higgins PJ:
TGF-β1-p53 cooperativity regulates a profibrotic genomic program in
the kidney: Molecular mechanisms and clinical implications. FASEB
J. 33:10596–10606. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Samarakoon R, Higgins SP, Higgins CE and
Higgins PJ: The TGF-β1/p53/PAI-1 signaling axis in vascular
senescence: Role of caveolin-1. Biomolecules. 9:92019. View Article : Google Scholar
|
|
54
|
Andreasen PA, Kjøller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: A review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ghosh AK, Rai R, Park KE, Eren M, Miyata
T, Wilsbacher LD and Vaughan DE: A small molecule inhibitor of
PAI-1 protects against doxorubicin-induced cellular senescence.
Oncotarget. 7:72443–72457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kang J, Kim W, Kwon T, Youn H, Kim JS and
Youn B: Plasminogen activator inhibitor-1 enhances radioresistance
and aggressiveness of non-small cell lung cancer cells. Oncotarget.
7:23961–23974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fujii M, Toyoda T, Nakanishi H, Yatabe Y,
Sato A, Matsudaira Y, Ito H, Murakami H, Kondo Y, Kondo E, et al:
TGF-β synergizes with defects in the Hippo pathway to stimulate
human malignant mesothelioma growth. J Exp Med. 209:479–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Uchiyama T, Okajima F, Mogi C, Tobo A,
Tomono S and Sato K: Alamandine reduces leptin expression through
the c-Src/p38 MAP kinase pathway in adipose tissue. PLoS One.
12:e01787692017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li C, Zhu HY, Bai WD, Su LL, Liu JQ, Cai
WX, Zhao B, Gao JX, Han SC, Li J, et al: MiR-10a and miR-181c
regulate collagen type I generation in hypertrophic scars by
targeting PAI-1 and uPA. FEBS Lett. 589:380–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kwon OS, Kim W, Cha HJ and Lee H: In
silico drug repositioning: From large-scale transcriptome data to
therapeutics. Arch Pharm Res. 42:879–889. 2019. View Article : Google Scholar : PubMed/NCBI
|