Introduction
Rhabdomyosarcoma (RMS) is one of the three most
frequent extracranial solid tumors in childhood, directly after
nephroblastoma and malignant bone tumors. In the group of soft
tissue and other extraosseous sarcomas it represents the most
frequent type (1). The outcome is
mainly influenced by tumor localization, histological subtype and
molecular characterization, IRS stage, and age at presentation
(2). Embryonal and alveolar RMS
are the most common histological subtypes, while botryoid and
spindle cell variants are less common. The embryonal RMS subtype
has a favorable 5-year survival rate compared to the alveolar
subtype. The alveolar RMS subtype is in 80% characterized by
chromosomal translocations t(2;13) or t(1;13) resulting in FOXO1
fusion genes (60% PAX3, 20% PAX7); FOXO1 fusion genes in embryonal
RMS are rare. According to the Children Oncology Group (COG), RMS
with FOXO1 fusion genes correlate with a worse prognosis (3). The spindle cell/sclerosing subtype is
a markedly aggressive RMS accounting for 5-10% of all RMS cases
with a wide age distribution (4).
Using the information about the pre-treatment staging (TNM, based
on anatomic site tumor-node-metastasis), the extent of the disease,
after surgical resection (clinical group), primary tumor site, and
histology/fusion status, a risk stratification system has been
established with an accurate prediction of patient outcomes
dividing the patients in low-, intermediate-, and high-risk groups
(5). Multimodality treatment
includes chemotherapy and surgery with or without radiotherapy
(6). The backbone of most
chemotherapeutical treatment protocols is a combination of a
three-drug regimen with vincristine (VCR), dactinomycin (DAC), and
cyclophosphamide (VAC); eventually low-risk patients are treated
only with a two-drug regimen of VCR and DAC (6). Even in high-risk and metastatic RMS
patients, VAC remains the chemotherapy with the highest effects,
and treatment regimens with additional or substitute components
could not rule out the benefits of VAC therapy (7). Even in cases with relapse, VCR is
also part of common therapeutic regimens (8). VCR as well as other similar vinca
alkaloid agents may cause acute and long-term damage to peripheral
nerves. As a consequence, severe neuropathy leads to dose reduction
or even cessation of therapy; in a great number of patients
significant long-term impairments persist after completion of
treatment (9).
Since 1970, the overall survival rate of RMS has
increased from 25 to 70% in 1990. However, compared to a high
failure-free survival (FFS) of 88% in the low-risk group, and
55-76% in the intermediate group, the outcome of the high-risk
group remains poor with only 10-30% FFS and has not improved during
the last 30 years (10). This is
mainly due to the relatively low incidence of the disease and even
lower rates of high-risk cases. The result is a limited number and
timing of new clinical trials that tests new potential treatments.
Preclinical efforts to identify potential new treatments with in
vitro cell-line research are an important cornerstone of
initiating further clinical trials with promising new drugs. Recent
attempts include investigations of natural or synthetic
chemopreventive small molecules and drugs (11).
One of the most extensively studied phytochemicals
of complementary oncology is curcumin (CUR), a yellow-orange dye
derived from the rhizome of the plant Curcuma longa. In
tumors it induces apoptosis, inhibits cell proliferation, and
efficiently affects several pathways associated with cancer stem
cell self-renewal (12). Moreover,
it facilitates absorption of radiation between 350-500 nm and
causes oxygen-dependent phototoxicity (13). Since more than three decades, basic
research has revealed effects on tumors; it has been analyzed in
multiple Phase-II and -III studies in adult patients with varying
malignant tumors as a supplement treatment in high-risk cases
(14). CUR should be considered
for pediatric oncological therapy due to its tolerability and
minimal side effects (15).
Furthermore, there is some evidence that curcumin significantly
attenuates the neurotoxic side effects of VCR (16,17).
The low bioavailability of native CUR was recently overcome by
micellar galenics (18). In an
in vivo model of pediatric hepatocellular carcinoma in mice
we could demonstrate both, the antitumor effects on orthotopic
tumors in a combination therapy of CUR and cisplatin and relevant
CUR concentrations in blood and organs after oral administration of
micellar CUR (19). Furthermore,
additional phototherapy (PDT) amplified the inhibitory effect of
CUR on hepatoma cells in vitro up to 20-fold (13). Few studies have elucidated the
effects of CUR on sarcoma, especially RMS cells (20,21).
The present study explored the effects of CUR alone
or in combination with the cytotoxic drugs VCR, or DAC or with PDT
on cell viability, proliferation and migration in RMS cells in
vitro.
Materials and methods
Drugs and chemicals
The native CUR powder of the plant Curcuma
longa Linn. used in all formulations contained 82% CUR, 16%
demethoxycurcumin (DMC), and 2% bis-deme-thoxycurcumin (BDMC) and
was purchased by Aquanova. The stock solution consisted of 15 mg
CUR dissolved in 1 ml DMSO. The final maximal concentration of CUR
was 30 µM, and the final concentrations of DMSO used in
experiments were <0.0007% (v/v). Control groups were treated
with 0.01% DMSO. The cytotoxic drug VCR was purchased from Teva,
and the stock solution contained 1 mg VCR sulfate per ml. The
cytotoxic drug dactinomycin (DAC; Cosmegen®-Lyevac) was
purchased from MSD Sharp & Dome GmbH, and the stock solution
contained 500 mg DAC with 20 mg mannitol. Stock solutions of VCR,
and DAC were further diluted in DMEM.
Cell lines and culture conditions
The embryonal RMS (ERMS) cell line RD was purchased
from ATCC. It was initially obtained from a seven-year old girl
with relapsing pelvic RMS. The cells have 51-hyperdiploid
chromosomes and show amplification of MYC oncogene, Q61H mutation
of NRAS, and homozygous mutations of TP53. RD cells are one of the
most commonly used cells lines in RMS research (22,23).
The alveolar RMS (ARMS) cell line RH30 expressing the Pax3/FOXO1
fusion protein secondary to the t(2;13)(q35;q14) translocation was
obtained from DSMZ. It was initially isolated from a bone
metastasis of a 17-year old boy with untreated RMS (22).
Normal human skeletal muscle cells (SKMC) from adult
donors were purchased from PromoCell GmbH. All cell lines were
cultured in Dulbecco's modified Eagle's medium (DMEM) with
GlutaMAX, 4.5 g/l D-glucose (Gibco, Life Technologies; Thermo
Fisher Scientific, Inc.) supplemented with 10% FCS (Biochrom GmbH)
and 1% penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. No additional mitogen was added,
except FCS, neither in the cell culture nor in the experiments. All
cell cultures were mycoplasma species-negative. For subculturing,
cells were detached from the culture surface using 0.05%
Trypsin-EDTA (Gibco Life Technologies; Thermo Fisher Scientific,
Inc.).
Viability assays
Cell viability was assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide (MTT)
assay (AppliChem GmbH) as previously described (24). All assays were performed at least
three times independently in quadruplicates. Percentages of
viability were calculated through normalization between background
of cultures without cells and untreated cultures as control
experiments. Dose-dependent viability curves were computed by
sigmoidal curves with variable slopes to determine IC50
values.
Tumor cells (1×104 cells/100 µl)
were cultured in 96-well plates. After 24 h of incubation the
supernatant was exchanged, and therapeutic agents were added in
increasing concentrations: native CUR (5-30 µM), VCR (0.3-80
nM) or DAC (0.1-13 nM); cells were incubated for 48 or 72 h. In
another series of experiments, a combination treatment with CUR and
VCR, or CUR and DAC was added for 48 or 72 h. Furthermore, the
effects of PDT were analyzed: cells were treated with CUR for 48 h
in concentrations of approximately 10-fold lower than the
IC50. Cells were washed and 1 h after CUR treatment PDT
was performed (lambda 488 nm, 5 sec; 300 W xenon short-arc lamp;
Karl Storz SE & Co. KG).
For the analysis of potential CUR resistance, cells
were incubated with increasing CUR concentrations as a long-term
treatment model [start concentration 0.5 µg/ml (1.357
µM); increase steps 0.5 µg/ml (1.357 µM); and
end concentration 8 µg/ml (21.72 µM)]. After the end
concentration was reached, cells were cultured without CUR for 72
h. Subsequently, after another 72-h CUR incubation, an MTT-assay
was performed comparing initial untreated cells with cells after
long-term treatment.
Evaluation of drug interaction
To analyze the inhibitory effect of drug
combinations, the coefficient of drug interaction (CDI) was
calculated. This was performed according to the Bliss Independence
model, which is considered one of the most popular models to assess
the combined effects of drugs (25). CDI was calculated as following: CDI
= (A + B - A x B)/AB. AB is the ratio of the absorbance in the
combination drugs to control; A or B is the ratio of the absorbance
of the single agent group to the control group. CDI values <1,
=1, or >1 indicated that the drugs were synergistic, additive,
or antagonistic, respectively. Inhibition rates obtained from the
MTT assays were used for these calculations.
Clonogenic assays
RMS cells were plated in 6-well-plates with 750
cells/well and treated with increasing CUR concentrations. After 72
h cells were washed with PBS and fresh medium (without treatment)
was added. After 14 days, subsequent grown cell colonies were
washed with PBS and fixed twice in methanol for 5 min, at room
temperature (RT). Visualization of fixed cell colonies was achieved
by incubation with 1% (w/v) crystal violet for 30 min for RD cells
and 120 min for RH30 cells, at RT. Visible colonies consisting of
>50 cells were counted. Dividing the number of colonies by the
number of plated cells and multiplying by 100 yielded the colony
formation rate according to Franken et al (26). Images were captured using
phase-contrast microscope Zeiss Axiovert 135 microscope (original
magnification, ×5; Carl Zeiss Microscopy GmbH).
Cell migration
A wound healing assay was performed to assess the
migratory inhibition effect of CUR and of CUR in combination with
VCR. Each test was performed at least in triplicates. Cells were
grown in the presence of the complete growth media (FCS >5%) to
90% confluence and subdivided into a 6-well cell culture plate
(1×106/well). A scratch across each well was produced
using a 10-100 µl pipette tip. Cellular debris was gently
washed with PBS. The growth media was exchanged, CUR, and/or VCR
were added, and migration potential was estimated for RD cells
after 0, 24 and 30 h, and for RH30 cells after 0, 24, 30 and 48 h.
The cell migration area was quantified by analyzing images, and
images were acquired at the defined time-points using a
phase-contrast microscope Zeiss Axiovert 135 microscope (×5,
magnification), and AxioVision software LE64, 4.9.1 (Carl Zeiss
Microscopy GmbH). The percentage of cell migration was
calculated.
Flow cytometry
Apoptosis was analyzed using flow cytometry with
Annexin V staining with allophycocyanin (APC) conjugation (BD
Biosciences) after single treatment with the following
concentrations below the respective IC50: CUR 10.86
µM, or 13.57 µM for RD cells, and 13.57 µM, or
16.29 µM for RH30 cells; VCR 0.91 nM, DAC 1.20 nM) and
combination treatment (CUR and VCR, CUR and DAC). After 48 h, cells
were detached from the culture surface using 0.05% Trypsin-EDTA
(Gibco Life Technologies; Thermo Fisher Scientific, Inc.) and
washed with Annexin binding buffer (1:10). The staining with
APC-conjugated Annexin V was performed according to a standard BD
Bioscience protocol. The staining with propidium iodide was
interfered by the fluorescence of intracellular CUR in the PE
channel and thus could not be performed (Fig. S1). Samples were analyzed on a
FACSCanto II flow cytometer and evaluated with BD FACS Diva
software Version 8.0 (BD Biosciences).
Statistical analyses
Data analysis was carried out using GraphPad Prism
8.4.0.00 (GraphPad Software, Inc.). In MTT, the IC50 was
calculated from the sigmoid dose-response curves with variable
slopes. In fluorescence measurements, LOG half maximal effective
concentration (EC50)-values were calculated on the basis
of sigmoidal dose-response curves with variable slopes. The
obtained curves on RMS cells for each treatment were compared to
their IC50 or LOGEC50 values, and the slope
and the P-value were determined with 95% confidence intervals
(CI).
Comparison of two regression curves was performed by
an F-test and a significant difference was obtained at P-values
<0.05. All numeric data are expressed as arithmetic means and
standard error of means (SEM).
All data were tested for significance with either a
one-way ANOVA (post hoc Dunnett's multiple comparison test) or
two-way ANOVA (post hoc Bonferroni's multiple comparison test).
Results
Effects of curcumin on cell viability and
evaluation of drug interaction
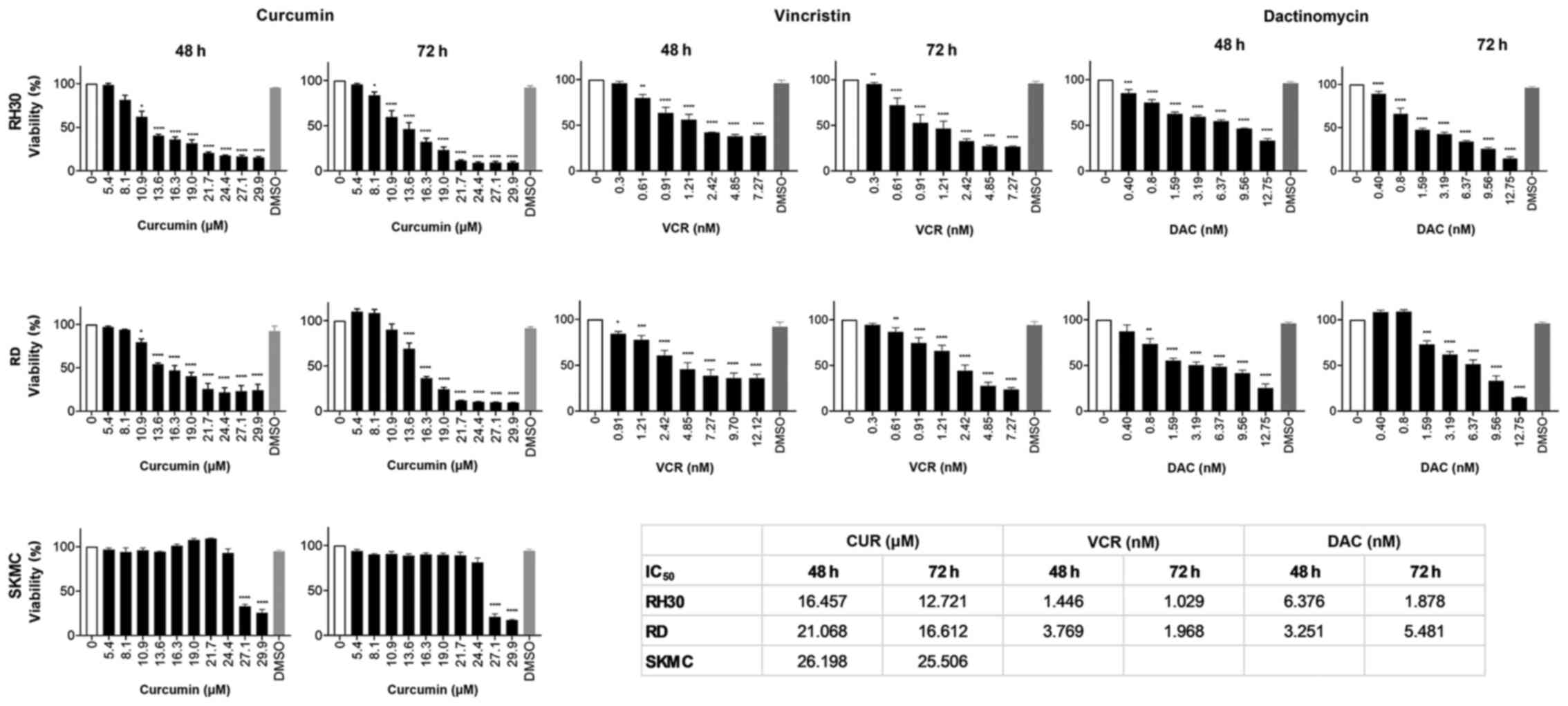
As revealed in Fig.
1, CUR decreased cell viability in all assessed cell lines in a
concentration-dependent manner. The IC50 values were
lower after 72 h of incubation than after 48 h. Longer incubation
of CUR for more than 72 h did not result in a further loss of
viability in any of the cell lines (data not shown). The viability
of SKMC was not impaired by CUR, only high concentrations of >25
µM led to a decrease. The decrease of cell viability by VCR
and DAC was also concentration-dependent with IC50 1-14
nM for VCR, and IC50 1-6 nM for DAC. As VCR and DAC are
routinely used in chemotherapeutic regimens for decades, these
substances were not assessed for SKMC cells.
A further series of experiments evaluated the impact
of combined incubation of CUR either with VCR or DAC and revealed
increased effects indicating that CUR may have some chemo-sparing
effects (Fig. 2). The calculated
coefficients of drug interaction revealed synergistic effects of
CUR and VCR in RH30 and RD cells. The combination of CUR with DAC
had an antagonistic effect in RD cells and a synergistic effect in
RH30 cells (Fig. 2).
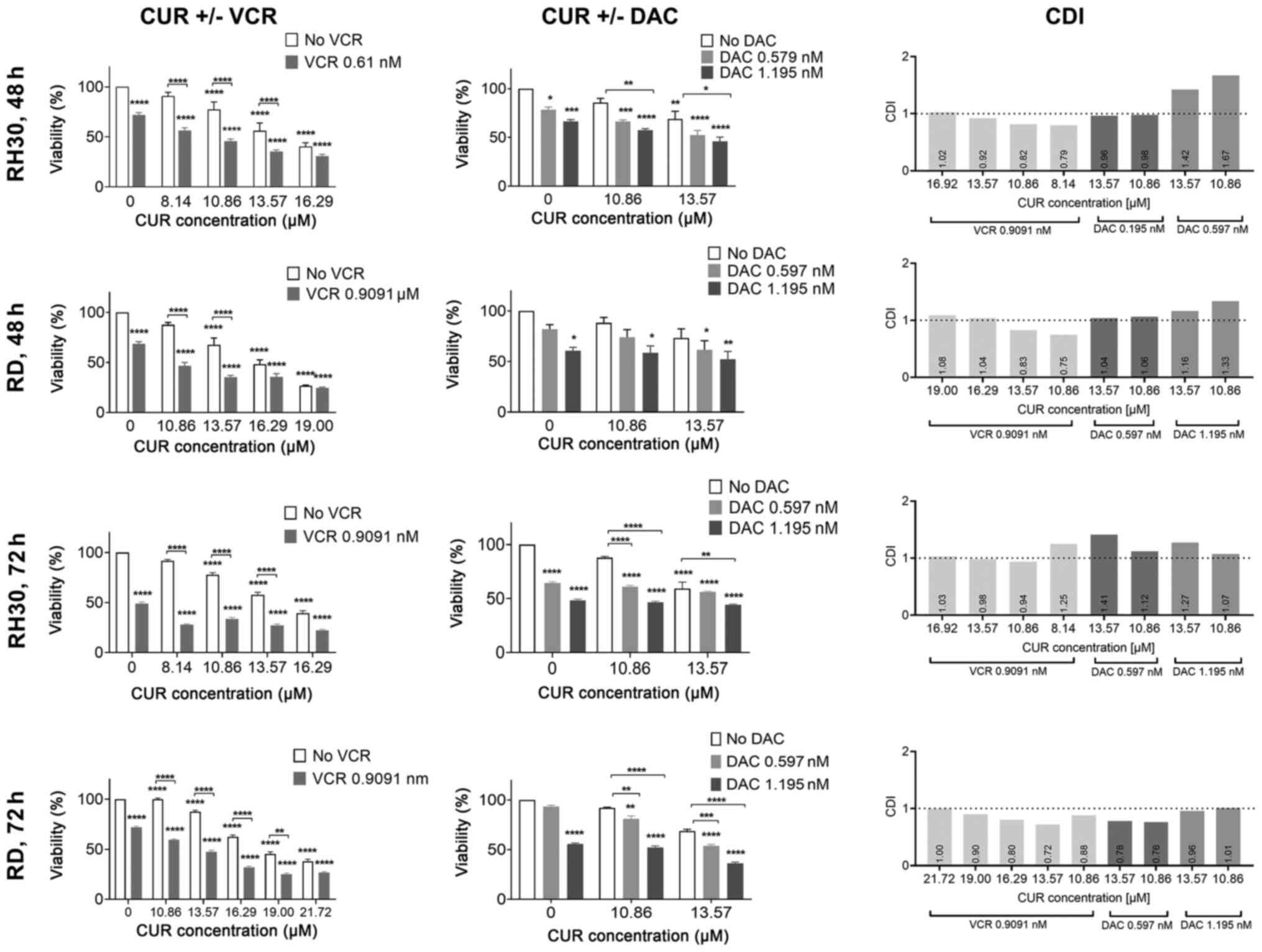 | Figure 2Effect of combination therapies of
CUR and VCR, or CUR and DAC on cell viability. Left rows, effect of
combination therapy with CUR and VCR. Middle rows, effect of
combination therapy with CUR and DAC. Arithmetic means ± SEM (n=6)
of the relative number of viable RMS cells following a 48 or 72 h
incubation in the presence of 13.57 µM CUR along with VCR
(RH30 and RD, 0.61 or 0.91 nM), or DAC (RH30 and RD, 0.597 or 1.195
nM). Two-way ANOVA with Bonferroni's multiple comparison test was
performed. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 indicates
statistical significance compared to the control (simple asterisks)
or compared to each other (asterisks with a square bracket). Right
rows, CDI according to Bliss Indepencence. Values below 1 (broken
line) indicate synergism, values above 1 indicate antagonism, and 1
indicates additive effects. CUR, curcumin; VCR, vincristine; DAC,
dactinomycin; RMS, rhabdomyosarcoma; CDI, coefficients of drug
interaction. |
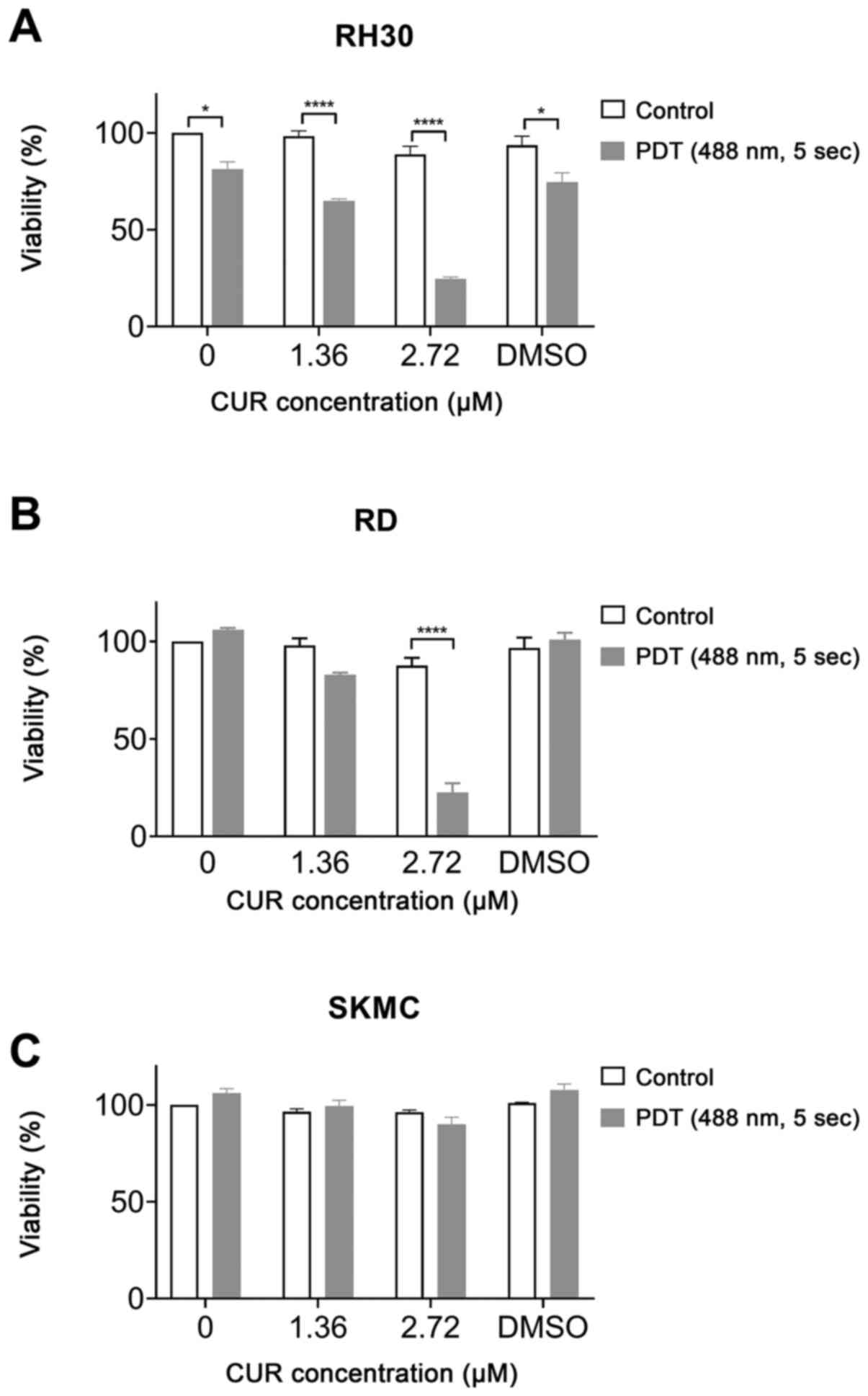
Effects of CUR and PDT on RMS cell
viability
The combination of CUR and PDT had higher potency
than CUR alone. IC50 values were decreased compared to
treatment without PDT (RH30 cells, 1.67 µM; RD cells, 1.93
µM; Fig. 3). In SKMC cells
the combination of CUR with PDT did not significantly decrease cell
viability (Fig. 3C).
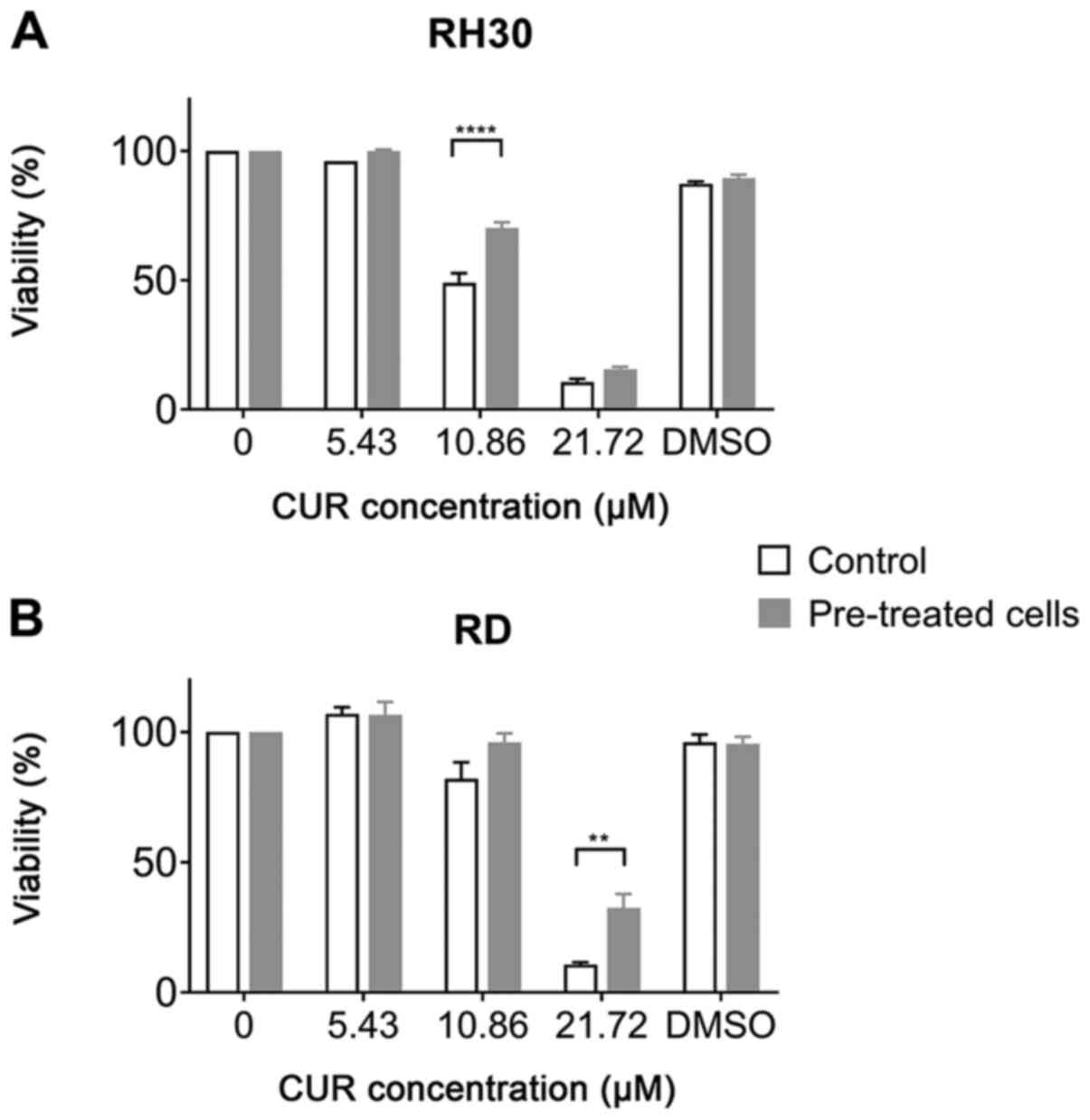
Effects of pretreatment and resistance to
CUR
Another series of experiments explored whether a
long-term pretreatment of the cells with increasing CUR
concentrations resulted in any resistance to CUR. While the
viability of pretreated cells was less decreased with CUR in high
doses of 21.72 µM in RD, and 10.86 µM in RH30 cells,
with a significant difference obtained with one-way ANOVA test, in
a comparison of the IC50, no significance was obtained
concerning the IC50 of RD cells untreated,
IC50 11.85 µM; RD cells pretreated,
IC50 14.11 µM; RH30 cells untreated,
IC50 10.76 µM; and RH30 cells pretreated,
IC50 11.19 µM. (Fig.
4).
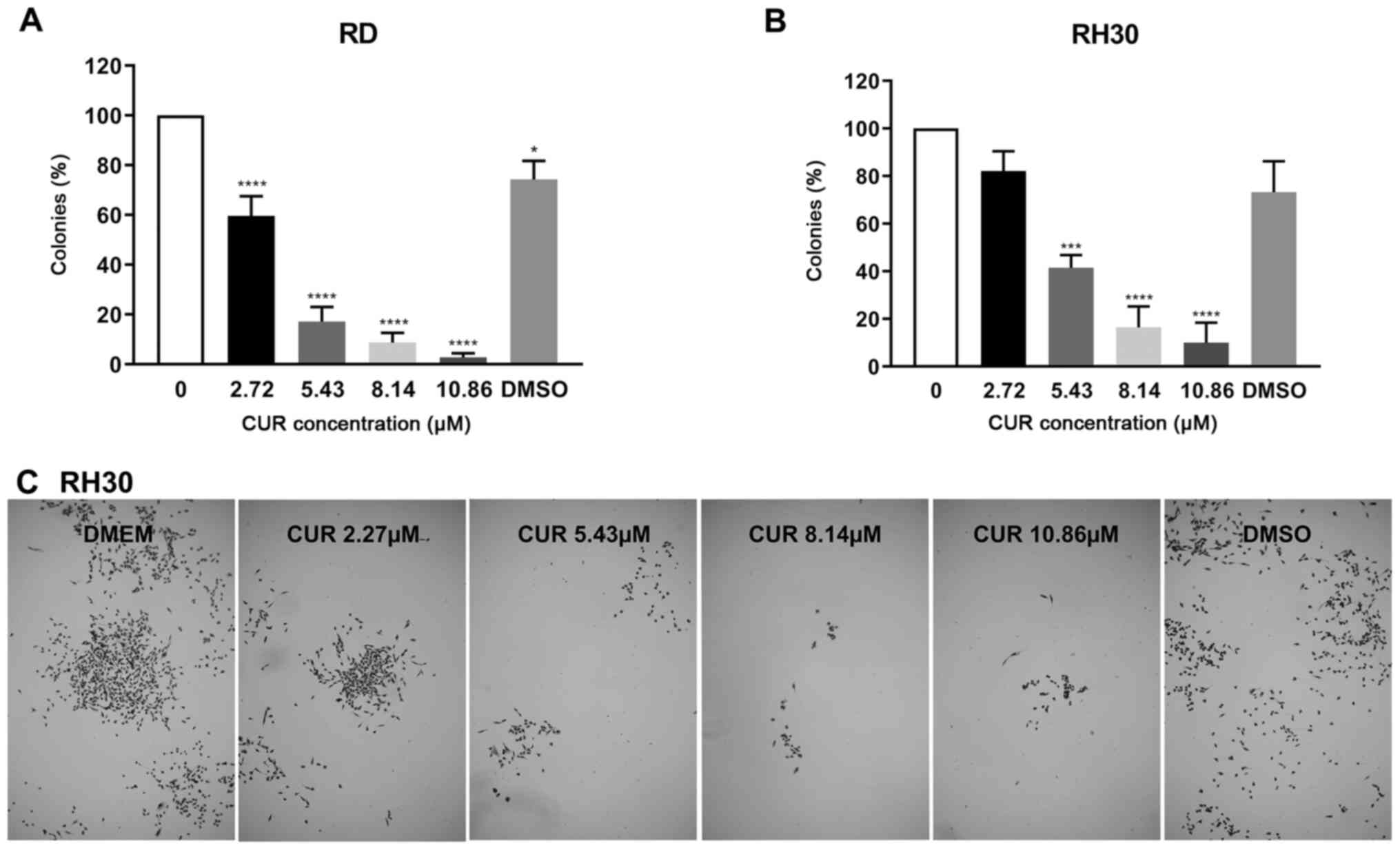
Effects of CUR on colony formation of RMS
cells
Since the colony forming potential of a single tumor
cell is a marker of its malignant potential, the effect of CUR on
colony formation was analyzed. Even concentrations that were
markedly lower than the respective IC50 led to a
significant decrease of colonies: in RD cells 2.72 µM and in
RH30 5.43 µM (Fig. 5).
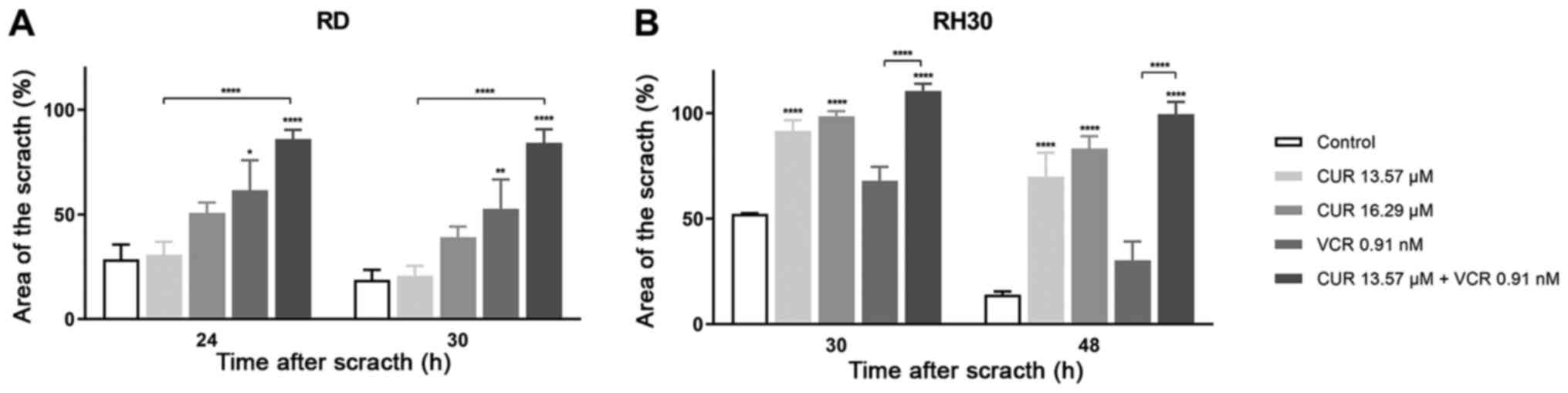
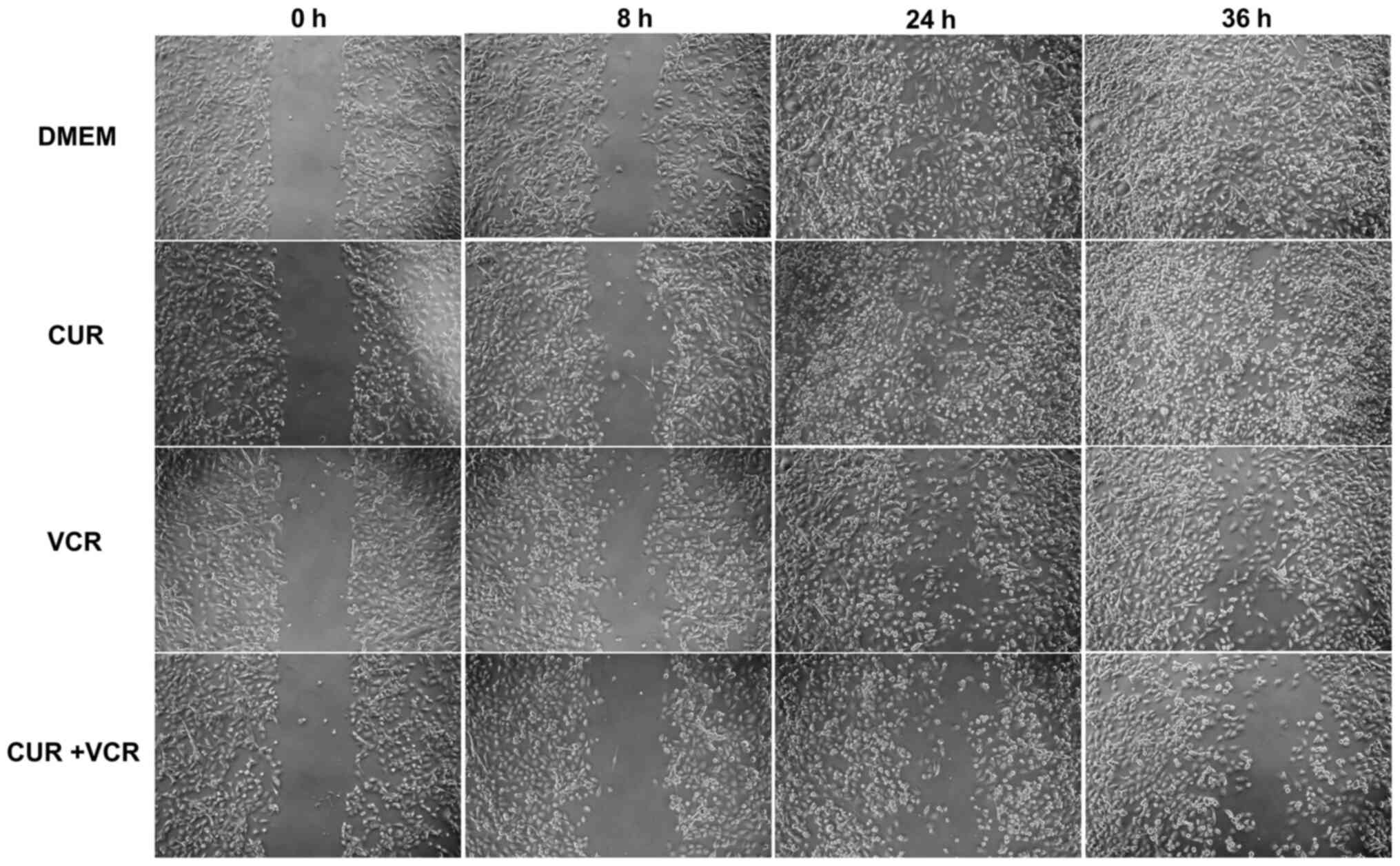
Effects of CUR and drug combinations on
RMS cell migration
One aspect of the increased effects of CUR with VCR
was revealed in the migration assays. The migration abilities of
the RMS cells RH30 and RD were inhibited more effectively by CUR
and VCR in combination with concentrations below the
IC50 compared to VCR or CUR alone (Figs. 6-8). Notably, CUR alone did not
significantly decrease the migration at the IC50 value
in RD cells, while it did so in the RH30 cells. In contrast, VCR
decreased the migration as a single drug in RD cells, but not in
RH30 cells. The cells in the cell migration assay were supplemented
with >5% FCS; lower concentrations led to a significant increase
of apoptotic cells and an insufficient healing of the scratch
during 48 h. Furthermore, the long doubling times for the cells (RD
cells 23 h, RH30 cells 37 h) (27)
also minimize a falsification of the migration assay by the use of
FCS >5%.
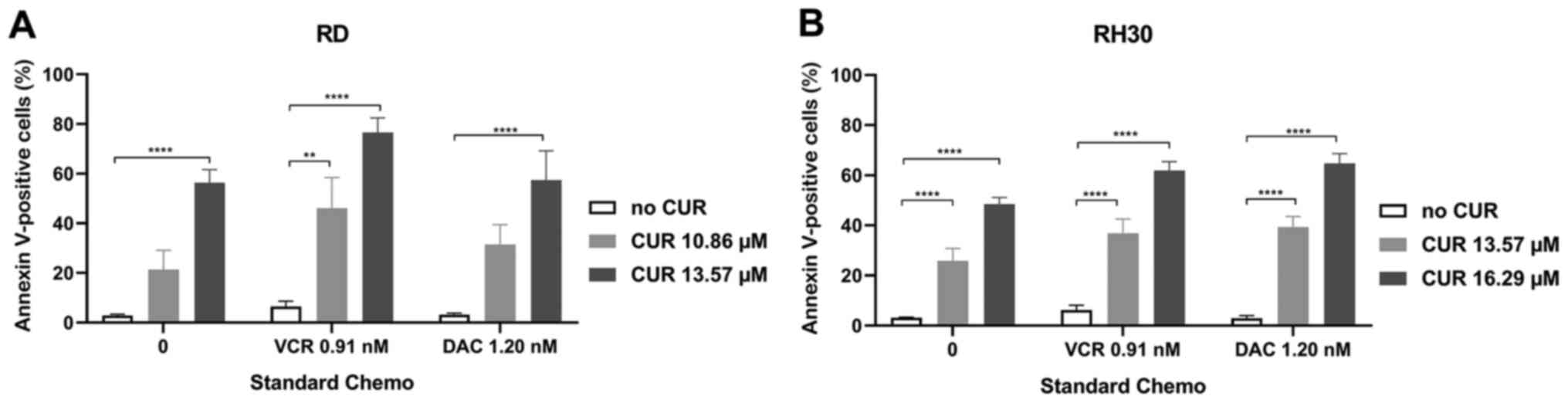
Effects of CUR and drug combinations on
apoptosis in RMS cells
Another important aspect of the increased effects in
the combination therapies was revealed by apoptosis assays
(Fig. 9). With VCR or DAC alone,
nearly no apoptosis was detectable. With CUR alone, apoptosis was
induced only by high doses near the respective IC50 (RD,
13.57 µM; RH30, 16.3 µM). This effect was markedly
enhanced in the combination treatment of CUR along with VCR, or
DAC.
Discussion
Soft tissue sarcomas represent less than 6% of
childhood malignancies including the subgroup of RMS accounting for
4.5%. Similar to other childhood malignancies, the overall survival
of RMS patients has improved from less than 20% to more than 80%
since 1950, but the outcome of patients with advanced tumor stages
remains poor (1). Children with
high-risk RMS tumors represent approximately 15% of patients with
initially diagnosed RMS (5). These
relatively low numbers hamper randomization and thus are a limiting
effect of further optimization studies. In an attempt to increase
survival even in these high-risk tumors new therapeutic strategies
are required. Well-tolerated phytotherapeutics with additional
chemotherapeutic-sparing effects are favorable. CUR has been in the
focus of medical research for more than three decades and meets
these criteria perfectly. In addition to varying anti-inflammatory
and neuroprotective properties, its demonstrated antineoplastic
potential has been summarized in several reviews (28-30).
However, little research has been conducted on the effect of CUR on
pediatric solid tumors.
The present study evaluated the anticancer activity
of CUR on RMS cells. Its effect on cell viability, proliferation,
colony forming, and apoptosis was analyzed and was compared to the
effects of the standard chemotherapeutic agents, VCR and DAC.
In the recent study, it was revealed, for the first
time to the best of our knowledge, that treatment of the RMS cell
lines RH30 and RD with CUR significantly decreased the viability,
clonogenic ability, and capacity to migrate of RMS cells. In a
prior study, high concentrations of CUR (20 µM) only
slightly reduced the viability of one liposarcoma cell line and one
synovial sarcoma cell line, while in several other sarcoma cell
lines CUR treatment had no effect on cell viability (21). The present study revealed that low
concentrations of CUR (12-15 µM) resulted in a 50% viability
reduction in pediatric RD and RH30 cells. In SKMC, CUR had lower
potency (IC50 >25 µM). Another study used cell
proliferation assays and obtained even lower IC50 values
in RMS cell lines (RH30 5.1 µM and RH1 2.7 µM)
(31).
RD cells (embryonal RMS cells), one of the most
commonly used cell lines in RMS research, have been demonstrated to
undergo growth inhibition when treated with VCR and VAC, but not
DAC, and doxorubicin in in vivo mouse models (32). The cell line RH30 represents the
alveolar RMS subtype with FOXO1 fusion. In RH30 xenografts, VCR led
to tumor regression while DAC or doxorubicin induced no growth
inhibition (32). In the present
in vitro results, RD and RH30 cell lines were inhibited only
by high concentrations of DAC, but CUR resulted in a viability
decrease in all RMS cell lines. The highest reported peak serum
concentration of oral administered CUR (micellar galenics) in a
human trial was ~3.2 µM (18). In our previous study involving mice
we measured peak serum concentrations ~2-3 µM but also
analyzed the concentrations of curcumin in inner organs and liver
tumors and the highest concentration in the lungs was ~0.008
µM/kG (19). Compared to
the required IC50 in cell culture, the achievable serum
concentrations are markedly low. On the other hand, these
comparatively low serum concentrations, in a murine tumor model for
hepatocellular carcinoma, were able to induce a significant
inhibitory effect on tumor growth (19). To the best of our knowledge, no
investigations concerning CUR concentrations in muscle cells or
even muscle tumors exist. In a recent study we analyzed the effects
of CUR on normal SKMC and observed the same phenomena of increased
viability in concentrations of ~20 µM, indicating a good
conservation of the surrounding muscular tissue in vivo.
This was described before in other studies with fibroblasts; CUR
below a concentration of 25 µM led to an increase in
viability, while concentrations higher than 25 µM had an
inhibitory effect (21,33).
Moreover, in the present study CUR enhanced the
sensitivity of RMS cells to the cytotoxic drugs VCR and DAC. This
enhanced sensitivity affected cell viability, capacity to migrate,
and apoptosis. Notably, the colony forming potential was
significantly decreased even with low concentrations of CUR. As the
IC50 for cell viability describes only one main aspect
of the effect of a drug, even drug concentrations below the
IC50 value may have an impact on cells. It was
demonstrated in the present study that CUR decreased the ability of
colony formation of RD cells in concentrations 10-fold lower than
the IC50 value and of RH30 cells in concentrations
3-fold lower than the IC50 value. This fact may be
indicative of the ability of CUR to inhibit the self-renewal
properties of RMS cells. Similar findings of low-dose CUR effects
on the colony-forming potential of glioblastoma stem cells have
been reported (34). Moreover, in
combination with VCR, CUR in doses beyond the IC50
values inhibited migration of RD cells, and CUR in doses of the
IC50 inhibited migration of RH30 cells, which may be
interpreted as an effect on metastatic potential, since most
patients with relapsing RMS suffer from local recurrence due to
invasive disease.
One the major causes of failure in treating children
with RMS is the development of resistance to chemotherapy.
Especially in advanced and relapsed cases several of these tumors
are only initially chemosensitive, but may acquire multidrug
resistance during chemotherapy (35). While the enhanced expression of
multidrug resistance-related proteins including MDR1, MRP, or LPR
may lead to an enhanced efflux of chemotherapeutic agents out of
the tumor cells (36), the
blocking of apoptosis signaling is another important characteristic
feature of RMS that has been associated with poor treatment
response (37). Herein it was
revealed that the single treatment of VCR or DAC did not induce
apoptosis in RMS cells, but high doses of CUR did. Furthermore, the
combination of CUR with VCR, or DAC resulted in a further increase
of apoptosis. Apoptosis induction as one effect of the
antineoplastic potential of CUR has been described in different
solid tumors, as well as in RMS cell lines, RH30 and RH1 (31,38-41).
While Beevers et al described the pro-apoptotic effects of
CUR at a concentration of 20 µM (31), we revealed similar and
dose-dependent effects in concentrations of 13.57 µM in RH30
cells and 10.86 µM in RD cells. A limitation of our
apoptosis assay is the limited usability of the necrosis marker
propidium iodide due to the overlapping emissions of CUR and the PI
dye in the PE channel. In the present study, it was also
demonstrated that PDT (480 nm) of RMS cells shortly after CUR
treatment amplified the cytotoxicity of CUR in both assessed RMS
tumor cell lines but not in normal SKMC. Transferring these
findings in an in vivo model, after tumor resection, the PDT
of the tumor bed with blue light shortly after CUR administration
may result in a further destruction of remaining invisible
micrometastases without any harm to surrounding tissues. An
orthotopic in vivo model to explore the impact of CUR and
PDT on surrounding normal tissue is currently under
development.
Although the present study did not focus on the
pathways involved in the inhibitory effects of CUR on RMS cells, it
did indicate that the additive use of CUR should be a therapeutic
option in the treatment of RMS. A further limitation of the present
study was the fact, that we used only two RMS cell lines and not
more fusion-negative and fusion-positive cell lines. On the other
hand, there are hardly any studies on solid tumors in children
treated with CUR, thus the present results have an important impact
as a basis for further investigations. Furthermore, we hope to be
able to add in vivo assessment to our current knowledge in a
future model with orthotopic RMS tumors in mice.
In summary, it was demonstrated that CUR effectively
decreased the viability of RMS cells in a dose-dependent manner. In
combination with VCR or DAC, CUR inhibited cell viability in an
additive and in some combinations even in a synergistic way. In
combination with PDT, markedly low doses far beyond the
IC50 values of CUR decreased RMS cell viability.
Additionally, it was revealed that low doses of CUR inhibited
important abilities of RMS cells including colony formation
capacity. Moreover, pretreatment of RMS cells with CUR did not lead
to signs of CUR resistance. The present findings indicated that CUR
may be an effective and safe additional chemotherapeutic drug for
the treatment of RMS in children.
Supplementary Data
Funding
The research was partly supported by the
Interdisciplinary Centre for Clinical Research (IZKF), Tuebingen
(Junior Research Group, grant no. PK 2016-1-03) and by the Open
Access Publishing Fund of Tuebingen University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VE, ES, and JF conceived the study. CS, VE, ES, and
NB acquired and analyzed the data. All authors drafted the work and
revised it, and provided their final approval of the version to be
published. All authors agree to be accountable for all aspects of
the work.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no financial or
non-financial competing interests.
Acknowledgments
The authors acknowledge the technical support by
Mrs. Bettina Kirchner, Mrs. Julia Wenz and Mrs. Melanie Hauth,
Department of Pediatric Surgery and Pediatric Urology and the IT
support by Mr. Olaf Kupka, IT Service Center, University Hospital
Tuebingen (Tuebingen, Germany). The authors acknowledge Mrs.
Vanessa Di Cecco, McMaster University (Hamilton, Canada) for
English language editing.
Abbreviations:
|
CUR
|
curcumin
|
|
DAC
|
dactinomycin
|
|
PDT
|
phototherapy
|
|
RMS
|
rhabdomyosarcoma
|
|
VCR
|
vincristine
|
References
|
1
|
Kaatsch P: Epidemiology of childhood
cancer. Cancer Treat Rev. 36:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma X, Huang D, Zhao W, Sun L, Xiong H,
Zhang Y, Jin M, Zhang D, Huang C, Wang H, et al: Clinical
characteristics and prognosis of childhood rhabdomyosarcoma: A
ten-year retro-spective multicenter study. Int J Clin Exp Med.
8:17196–17205. 2015.
|
|
3
|
Hibbitts E, Chi YY, Hawkins DS, Barr FG,
Bradley JA, Dasgupta R, Meyer WH, Rodeberg DA, Rudzinski ER, Spunt
SL, et al: Refinement of risk stratification for childhood
rhabdomyosarcoma using FOXO1 fusion status in addition to
established clinical outcome predictors: A report from the
Children's Oncology Group. Cancer Med. 8:6437–6448. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gui H, Lhospital E, Staddon AP, Nagda SN,
Zager EL, Zhang PJL and Brooks JS: Combined sclerosing and spindle
cell rhabdomyosarcoma in previous craniotomy site: A case report
and a review of the literature. Int J Surg Pathol. 27:328–335.
2019. View Article : Google Scholar
|
|
5
|
Dasgupta R, Fuchs J and Rodeberg D:
Rhabdomyosarcoma. Semin Pediatr Surg. 25:276–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raney RB, Walterhouse DO, Meza JL,
Andrassy RJ, Breneman JC, Crist WM, Maurer HM, Meyer WH, Parham DM
and Anderson JR: Results of the Intergroup Rhabdomyosarcoma Study
Group D9602 protocol, using vincristine and dactinomycin with or
without cyclophosphamide and radiation therapy, for newly diagnosed
patients with low-risk embryonal rhabdomyo-sarcoma: A report from
the Soft Tissue Sarcoma Committee of the Children's Oncology Group.
J Clin Oncol. 29:1312–1318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malempati S and Hawkins DS:
Rhabdomyosarcoma: Review of the Children's Oncology Group (COG)
Soft-Tissue Sarcoma Committee experience and rationale for current
COG studies. Pediatr Blood Cancer. 59:5–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mascarenhas L, Lyden ER, Breitfeld PP,
Walterhouse DO, Donaldson SS, Rodeberg DA, Parham DM, Anderson JR,
Meyer WH and Hawkins DS: Risk-based treatment for patients with
first relapse or progression of rhabdomyosarcoma: A report from the
Children's Oncology Group. Cancer. 125:2602–2609. 2019.PubMed/NCBI
|
|
9
|
Cliff J, Jorgensen AL, Lord R, Azam F,
Cossar L, Carr DF and Pirmohamed M: The molecular genetics of
chemo-therapy-induced peripheral neuropathy: A systematic review
and meta-analysis. Crit Rev Oncol Hematol. 120:127–140. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egas-Bejar D and Huh WW: Rhabdomyosarcoma
in adolescent and young adult patients: Current perspectives.
Adolesc Health Med Ther. 5:115–125. 2014.PubMed/NCBI
|
|
11
|
Carvalho LF, Silva AMF and Carvalho AA:
The use of anti-oxidant agents for chemotherapy-induced peripheral
neuropathy treatment in animal models. Clin Exp Pharmacol Physiol.
44:971–979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Norris L, Karmokar A, Howells L, Steward
WP, Gescher A and Brown K: The role of cancer stem cells in the
anti-carcinogenicity of curcumin. Mol Nutr Food Res. 57:1630–1637.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ellerkamp V, Bortel N, Schmid E, Kirchner
B, Armeanu-Ebinger S and Fuchs J: Photodynamic Therapy Potentiates
the Effects of Curcumin on Pediatric Epithelial Liver Tumor Cells.
Anticancer Res. 36:3363–3372. 2016.PubMed/NCBI
|
|
14
|
Allegra A, Innao V, Russo S, Gerace D,
Alonci A and Musolino C: Anticancer activity of curcumin and its
analogues: preclinical and clinical studies. Cancer Invest.
35:1–22. 2017. View Article : Google Scholar
|
|
15
|
Suskind DL, Wahbeh G, Burpee T, Cohen M,
Christie D and Weber W: Tolerability of curcumin in pediatric
inflam-matory bowel disease: a forced-dose titration study. J
Pediatr Gastroenterol Nutr. 56:277–279. 2013. View Article : Google Scholar :
|
|
16
|
Babu A, Prasanth KG and Balaji B: Effect
of curcumin in mice model of vincristine-induced neuropathy. Pharm
Biol. 53:838–848. 2015. View Article : Google Scholar
|
|
17
|
Greeshma N, Prasanth KG and Balaji B:
Tetrahydrocurcumin exerts protective effect on vincristine induced
neuropathy: Behavioral, biochemical, neurophysiological and
histological evidence. Chem Biol Interact. 238:118–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schiborr C, Kocher A, Behnam D, Jandasek
J, Toelstede S and Frank J: The oral bioavailability of curcumin
from micronized powder and liquid micelles is significantly
increased in healthy humans and differs between sexes. Mol Nutr
Food Res. 58:516–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bortel N, Armeanu-Ebinger S, Schmid E,
Kirchner B, Frank J, Kocher A, Schiborr C, Warmann S, Fuchs J and
Ellerkamp V: Effects of curcumin in pediatric epithelial liver
tumors: Inhibition of tumor growth and alpha-fetoprotein in vitro
and in vivo involving the NFkappaB- and the beta-catenin pathways.
Oncotarget. 6:40680–40691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei CC, Ball S, Lin L, Liu A, Fuchs JR, Li
PK, Li C and Lin J: Two small molecule compounds, LLL12 and FLLL32,
exhibit potent inhibitory activity on STAT3 in human
rhabdomyo-sarcoma cells. Int J Oncol. 38:279–285. 2011.
|
|
21
|
Harati K, Behr B, Daigeler A, Hirsch T,
Jacobsen F, Renner M, Harati A, Wallner C, Lehnhardt M and
Becerikli M: Curcumin and viscum album extract decrease
proliferation and cell viability of soft-tissue sarcoma cells: an
in vitro analysis of eight cell lines using real-time monitoring
and colorimetric assays. Nutr Cancer. 69:340–351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hinson AR, Jones R, Crose LE, Belyea BC,
Barr FG and Linardic CM: Human rhabdomyosarcoma cell lines for
rhabdomyosarcoma research: Utility and pitfalls. Front Oncol.
3:1832013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McAllister RM, Melnyk J, Finkelstein JZ,
Adams EC Jr and Gardner MB: Cultivation in vitro of cells derived
from a human rhabdomyosarcoma. Cancer. 24:520–526. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ellerkamp V, Lieber J, Nagel C, Wenz J,
Warmann SW, Fuchs J and Armeanu-Ebinger S: Pharmacological
inhibition of beta-catenin in hepatoblastoma cells. Pediatr Surg
Int. 29:141–149. 2013. View Article : Google Scholar
|
|
25
|
Foucquier J and Guedj M: Analysis of drug
combinations: Current methodological landscape. Pharmacol Res
Perspect. 3:e001492015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Franken NAP, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
27
|
Kang MH, Smith MA, Morton CL, Keshelava N,
Houghton PJ and Reynolds CP: National Cancer Institute pediatric
preclinical testing program: Model description for in vitro
cytotoxicity testing. Pediatr Blood Cancer. 56:239–249. 2011.
View Article : Google Scholar
|
|
28
|
Banik U, Parasuraman S, Adhikary AK and
Othman NH: Curcumin: the spicy modulator of breast carcinogenesis.
J Exp Clin Cancer Res. 36:982017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kunnumakkara AB, Bordoloi D, Harsha C,
Banik K, Gupta SC and Aggarwal BB: Curcumin mediates anticancer
effects by modulating multiple cell signaling pathways. Clin Sci
(Lond). 131:1781–1799. 2017. View Article : Google Scholar
|
|
30
|
Panda AK, Chakraborty D, Sarkar I, Khan T
and Sa G: New insights into therapeutic activity and anticancer
properties of curcumin. J Exp Pharmacol. 9:31–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beevers CS, Li F, Liu L and Huang S:
Curcumin inhibits the mammalian target of rapamycin-mediated
signaling pathways in cancer cells. Int J Cancer. 119:757–764.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Houghton JA, Houghton PJ and Green AA:
Chemotherapy of childhood rhabdomyosarcomas growing as xenografts
in immune-deprived mice. Cancer Res. 42:535–539. 1982.PubMed/NCBI
|
|
33
|
Kang JY, Huang H and Zhu FQ: Effect of
curcumin on growth and function of fibroblast in human hyperplastic
scar. Zhongguo Zhong Xi Yi Jie He Za Zhi. 29:1100–1103. 2009.In
Chinese.
|
|
34
|
Gersey ZC, Rodriguez GA, Barbarite E,
Sanchez A, Walters WM, Ohaeto KC, Komotar RJ and Graham RM:
Curcumin decreases malignant characteristics of glioblastoma stem
cells via induction of reactive oxygen species. BMC Cancer.
17:992017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seitz G, Warmann SW, Vokuhl CO, Heitmann
H, Treuner C, Leuschner I and Fuchs J: Effects of standard
chemotherapy on tumor growth and regulation of multidrug resistance
genes and proteins in childhood rhabdomyosarcoma. Pediatr Surg Int.
23:431–439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fruci D, Cho WC, Nobili V, Locatelli F and
Alisi A: Drug trans-porters and multiple drug resistance in
pediatric solid tumors. Curr Drug Metab. 17:308–316. 2016.
View Article : Google Scholar
|
|
37
|
Fulda S: Targeting apoptosis resistance in
rhabdomyosarcoma. Curr Cancer Drug Targets. 8:536–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dhivya R, Ranjani J, Bowen PK, Rajendhran
J, Mayandi J and Annaraj J: Biocompatible curcumin loaded
PMMA-PEG/ZnO nanocomposite induce apoptosis and cytotoxicity in
human gastric cancer cells. Mater Sci Eng C. 80:59–68. 2017.
View Article : Google Scholar
|
|
39
|
Liu L, Sun L, Wu Q, Guo W, Li L, Chen Y,
Li Y, Gong C, Qian Z and Wei Y: Curcumin loaded polymeric micelles
inhibit breast tumor growth and spontaneous pulmonary metastasis.
Int J Pharm. 443:175–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Picone P, Nuzzo D, Caruana L, Messina E,
Scafidi V and Di Carlo M: Curcumin induces apoptosis in human
neuroblastoma cells via inhibition of AKT and Foxo3a nuclear
translocation. Free Radic Res. 48:1397–1408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shafiee M, Mohamadzade E, ShahidSales S,
Khakpouri S, Maftouh M, Parizadeh SA, Hasanian SM and Avan A:
Current status and perspectives regarding the therapeutic potential
of targeting EGFR pathway by curcumin in lung cancer. Curr Pharm
Des. 23:2002–2008. 2017. View Article : Google Scholar : PubMed/NCBI
|