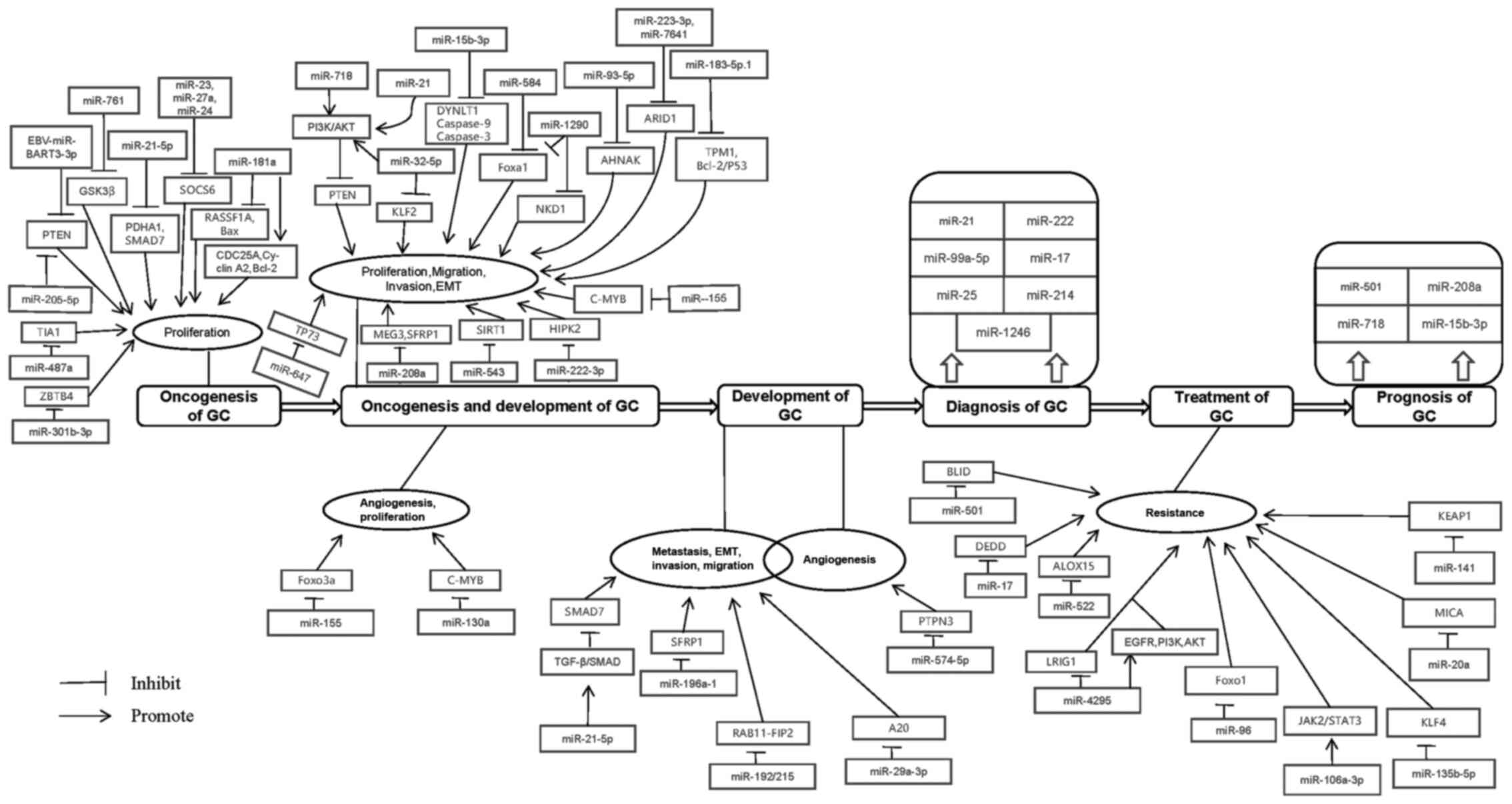
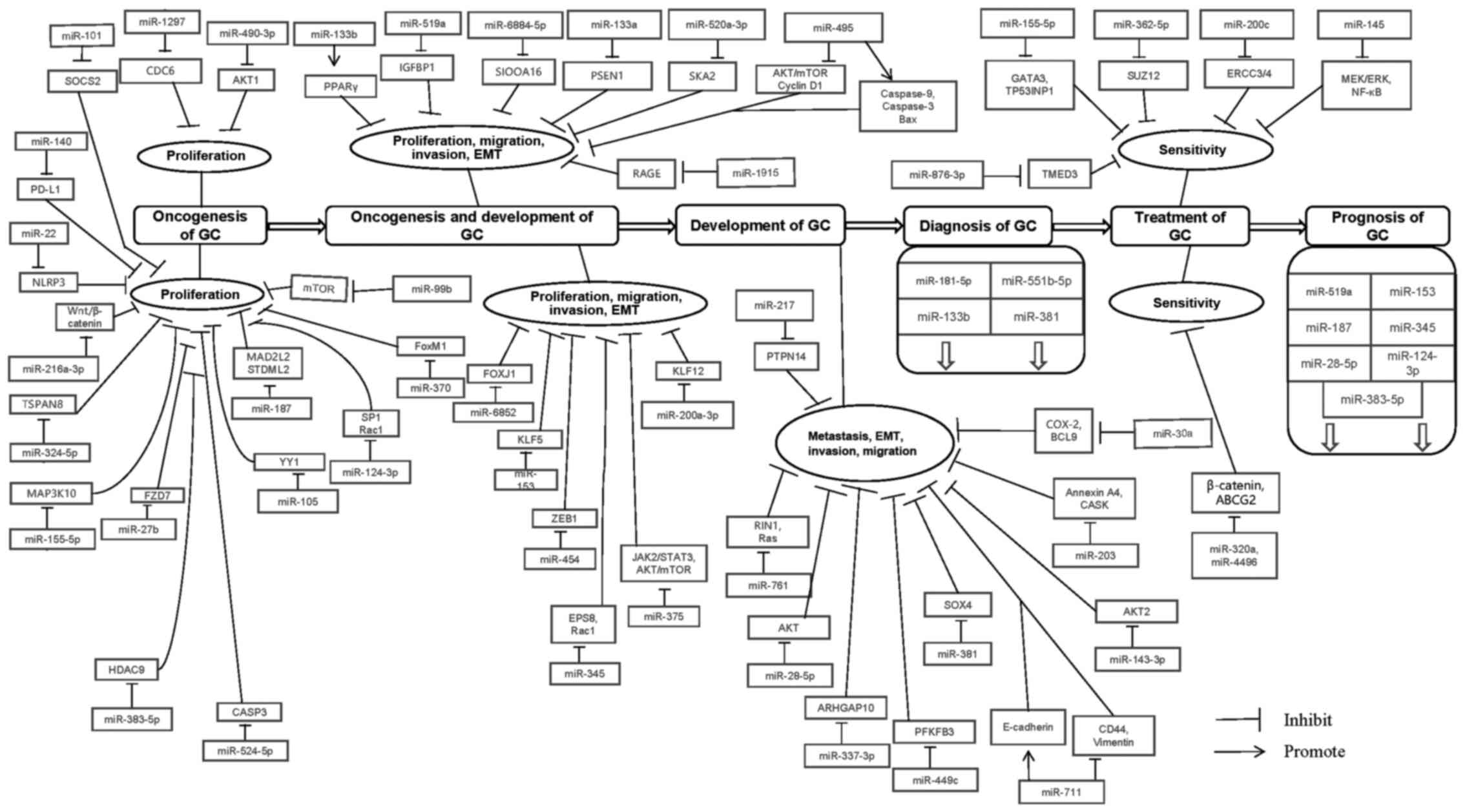
According to the newest data of WHO, cancer ranks
second among global causes of death, cumulative amount of cancer
deaths were 9.6 million, or one in six deaths, in 2018. The most
ordinary cancers are lung, breast, colorectal, prostate, skin and
stomach cancer (Table I;
https://www.who.int/). The most ordinary deadly
cancer are lung cancer, colorectal cancer, stomach cancer, liver
cancer and breast cancer (Table
II). From the above data, we can see that gastric cancer (GC)
is in the top five positions in terms of mortality. GC has a
severely bad impact on survival, constituting a significant
issue.
The cause of GC is not fully understood. At present,
relatively clear factors related to GC can be divided into
exogenous and endogenous factors. Exogenous factors include
lifestyle habits (e.g., low intake of fruits and vegetables, high
intake of salts, nitrates, and pickled foods, alcoholism, smoking
and obesity) (1-3), environmental factors, biological
infections [e.g., Helicobacter pylori (H. pylori) (4) and Epstein-Barr virus infections
(5,6), trauma and chronic irritation (e.g.,
chronic gastric ulcer)]. Hereditary factors (7,8)
are endogenous (Table III).
MicroRNAs (miRNAs) are non-coding RNAs of ~22 nt
nucleotides. The function of miRNAs is that they can avoid the
translation of specific mRNAs and regulate several homeostatic and
pathological processes within cells by acting as
post-transcriptional regulators binding to the 3′-untranslated
region (3′-UTR) of specific target mRNAs, specifically in the MRE
(miRNA recognition element) sequence (9-11).
Following deep exploration of miRNAs, it was identified that miRNAs
can regulate gene expression in many normal and pathological
cellular processes and become particularly attractive targets in
the study of malignancies, including the study of GC. Several lines
of study have verified that miRNA expression correlates with a
variety of cancers, and abnormal expression occurs in many cancer
types (12-14). Some miRNAs also have functions
similar to tumor suppressor genes (such miRNAs are named tsmiRs)
and oncogenes (such miRNAs are named oncomiRs) (15). The target gene of miRNA determines
the role of specific miRNA. Some miRNAs could suppress tumor by
targeting oncogenes and becoming tsmiRs. However, some miRNAs
target tumor suppressor genes and become potential oncomiRs. The
latest researches have verified that miRNAs are closely correlated
with a series of oncogenesis and development processes as well as
the clinical application of GC (12-14). In summary, miRNAs are involved in
the entire process of GC (Figs. 1
and 2).
Cancer cells can uncontrollably grow and spread
affect the body in an adverse manner. The most basic biological
characteristics of tumor cells are imbalance in proliferation,
abnormal differentiation, and ability to invade and metastasize.
miRNAs are involved in oncogenesis of GC (Table IV).
EBVaGC is an Epstein-Barr virus-associated GC,
accounting for approximately 10% of all GC cases, and has
distinctive pathological and molecular characteristics. Tumor
protein p53 is encoded by the TP53 gene. p53 is one of the most
important tumor suppressors and is activated by DNA damage and
other stresses (20,21). EBV-miR-BART3-3p (BART3-3p) has a
high expression state in EBVaGC and directly targets the CDS region
of TP53 and inhibits PTEN to promote the proliferation and inhibit
the senescence of GC cells (22).
PTEN expression can affect cell apoptosis, proliferation, migration
and intracellular mitochondria and inhibit AKT phosphorylation and
suppress tumor growth (23,24). miR-205-5p may directly reduce PTEN
expression and activity, then promote the growth of GC (25). miR-301b-3p attenuates zinc finger
and BTB domain containing 4 (ZBTB4) expression and acceler- ates
the growth of GC cells. The knockdown of miR-301b-3p markedly
induces cell cycle arrest at G1 phase and apoptosis, finally
inhibiting proliferation in MGC-803 cells (26). TIA-1 is a protein that inhibits
proliferation and promotes apoptosis of GC cells. It was identified
that TIA-1 presented a low level in GC tissues. miR-487a can
promote the progression of GC by attenuating TIA-1 (27). RASSF1A can promote apoptosis via
suppressing G1/S transition and cell proliferation in GC. miR-181a
could promote gastric carcinogenesis, but RASSF1A knockdown could
attenuate the effects of miR-181a downregulation. miR-181a promotes
gastric carcinogenesis via downregulating RASSF1A. In addition,
miR-181a can upregulate CDC25A, cyclin A2 and Bcl-2, and
downregulate Bax protein expression in GC cell lines (28).
MAP3K10 is a target gene of miR-155-5p in GC cell
lines. miR-155-5p mimics markedly reduce the expression level of
MAP3K10 proteins in AGS-1 cells. miR-155-5p could down- regulate
MAP3K10 and then suppress GC cell proliferation and promote
apoptosis (34). miR-1297 could
reduce cell division control protein 6 (CDC6) expression to inhibit
proliferation and promote apoptosis in GC cells (35). If there is miR-187 overexpression
in MGC-803 cells, the cell proliferation will be suppressed and the
cell cycle will be altered via capturing cells in the G0/G1 phase.
MAD2 mitotic arrest deficient-like 2 (MAD2L2) and stomatin
(EPB72)-like 2 (STOML2) are targets of miR-187. The expression of
MAD2L2 and STOML2 is downregulated by overexpression of miR-187 in
GC (36). Ying Yang 1 (YY1) is an
important oncogene (37). YY1 was
confirmed that it could destabilize p53 and HIF-1 to promote cell
proliferation (38,39). Overexpression of miR-105 could
reduce cell viability and proliferation in GC. YY1 is a direct
target of miR-105, by downregulating which miR-105 has an
anti-proliferative effect (40).
HDAC9 knockout or miR-383-5p mimics both cause growth suppression
and increase apoptosis in AGS and SGC-7901 cells. Furthermore,
miR-383-5p could suppress HDAC9 to inhibit the cell proliferation
of GC (41).
The protein kinase B (AKT1) signaling pathway
promotes cell proliferation, growth and glucose metabolism, but
inhibit apoptosis (42-44). The downregulation of miR-490-3p
causes the enhancement of cell proliferation and suppression of
apoptosis. In addition, overexpression of AKT1 partially reversed
the effects of miR-490-3p in GC. It indicated that miR-490-3p
reduced AKT1 to effect proliferation and apoptosis in GC cells
(45). In vitro,
miR-124-3p reduces cell viability and plate colony formation. In
vivo, miR-124-3p suppresses tumor growth as well. miR-124-3p
has a negative effect on tumor growth by negatively regulating SP1
and Rac1 in GC (46). The
overexpression of miR-524-5p markedly reduces cell proliferation
capacity and induces cell cycle arrest at G0/G1 phase in GC cells.
Dual-luciferase, RT-qPCR and western blot analysis confirmed CASP3
as a target gene of miR-524-5p. Furthermore, recovery of CASP3
expression attenuated the negative effect of miR-524-5p on cell
growth. In conclusion, miR-524-5p suppresses cell proliferation in
GC via negatively adjusting CASP3 (47).
Using immune checkpoint blockades is a promising
therapeutic strategy in the treatment of various human
malignancies. miR-140 is significantly reduced in Hp-positive GC.
PD-L1 is a direct target of miR-140 in GC. In addition, PD-L1 is
significantly increased in Hp-positive GC. miR-140 significantly
restrained GC cell proliferation through repressing PD-L1 (48). FZD7 is an important co-receptor in
the WNT signaling pathway and significantly induced by H.
pylori infection in a dose- and time-dependent manner. miR-27b
overexpression significantly inhibited H. pylori
infection-induced cell proliferation and WNT signaling pathway
activation in GC cells through negatively regulating Frizzled7
(49). Downregulation of SOCS2
can inhibit cell growth and cell-cycle progression in GC but SOCS2
is overexpressed in H. pylori-positive tissues. miR-101
restricts cell growth and tumorigenesis of H. pylori-related
GC via repressing SOCS2 (50).
FoxM1 is a critical positive regulator of cell proliferation and
directly downregulated by miR-370 in GC. H. pylori and CagA
restrain miR-370 expression, which promotes FoxM1 expression and
cell proliferation (51). miR-99b
is increased in H. pylori+ cancer samples and promotes H.
pylori-induced autophagy to play a tumor-suppressive function
through the inhibition of mTOR expression (52).
miR-718 could activate PI3K/Akt signaling, which
directly downregulates PTEN and promotes the proliferation and
invasion in GC (53). KLF2 binds
to the PTEN promoter to induce its expression. miRNA-32-5p
downregulates the expression of KLF2 to reduce PTEN and activate
the PI3K/AKT signaling to enhance the development of GC (54). miR-21 markedly reduces PTEN level,
which in turn increases Akt phosphorylation at Thr308 and Ser473.
Conclusively, miR-21 enhances cell proliferation and migration via
targeting PTEN/Akt signaling pathway in human GC cells (55). miR-15b-3p promotes the enhancement
of migration, invasion, proliferation and inhibition of apoptosis
by restraining DYNLT1, and cleaved caspase-9 expression in GC. In
addition, caspase-9 induced Caspase-3 expression and then promoted
apoptosis together (56).
miR-1290 increased the proliferation and
invasiveness of GC cells via directly targeting and suppressing
NKD1 (57). As a transcriptional
regulator, NKD1 is a negative antagonist of the Wnt signaling
pathway, which not only upregulates the expression of the
downstream tumor suppressor gene, but also regulates the biological
behavior of a variety of tumors in combination with the upstream
related proteins (58-60). CagA of H. pylori can
promote the expression of miR-584 and miR-1290 in an NF-κB and
Erk1/2-dependent manner, respectively. miR-584 and miR-1290 can
immediately inhibit Foxa1 to promote EMT. It indicates that miR-584
and miR-1290 induced by CagA can enhance EMT via repressing Foxa1
(61). miR-93-5p causes the
occurrence of epithelial-mesenchymal transition and enhances
proliferation by repressing AHNAK through Wnt signaling pathway in
GC (62). miR-223-3p accelerates
the cell cycle transition from the G1 to the S phase, which
enhances DNA synthesis in the cell and cell proliferation. Arid1a
is an essential constituent subunit of SWI/SNF, which is encoded by
Arid1a (63). It plays a
necessary role in the assembly of SWI/SNF complexes (64,65). Arid1a is a tumor depressor and
target of miR-223-3p and miR-7641 in GC. Thus, miR-223-3p (66) and miR-7641 (67) enhance cell proliferation and
invasion via downregulating Arid1a in GC. In addition, miR-647
could enhance proliferation, migration and invasion through
repressing TP73 in GC (68).
Furthermore, miR-223-3p/ARID1A axis is involved in CagA-induced
cell proliferation and migration. miR-223-3p is significantly
higher in H. pylori-positive GC tissues than that in H.
pylori-negative tissues. NF-κB/miR-223-3p/ARID1A axis may link
the process of H. pylori-induced chronic inflammation to GC
(69).
SFRP1 and MEG3 are targets of miR-208a, which
enhances cell proliferation and invasion by passively regulating
MEG3 and SFRP1 in GC (70).
miR-183-5p.1 plays a positive role in the promotion of cell
proliferation, migration and invasion through repressing TPM1 and
the Bcl-2/P53 signaling pathways in GC (71). c-MYB may regulate proliferation,
growth, differentiation and survival of many cell types (72). The expression changes of miR-155
and c-MYB are opposite in GC. In addition, c-MYB was a direct
target of miR-155. miR-155 was able to inhibit c-MYB, which
promotes the metastasis, growth and tube formation of vascular
cells and leads to the occurrence and development of tumors
(73). FOXO3a inhibited
angiogenesis via suppressing the growth of vascular smooth muscle
(74). miR-155 is an angiogenesis
driver. It can accelerate the generation of new vessels through
inhibiting Forkhead box O3 (FOXO3a) protein (75). Similar to miR-155, miR-130a also
activates angiogenesis of GC cells through repressing c-MYB in
vascular endothelial cells (76).
H. pylori increased the expression of miR-543 in GC and
increased miR-543 induced by CagA is a strong promoter of cell
proliferation, migration, and invasion via SIRT1. miR-543
significantly restrained SIRT1 in GC (77). miR-222-3p aberrantly upregulates
in GC. miR-222-3p is significantly upregulated in the H.
pylori (+) group and homeodomain-interacting protein kinase 2
(HIPK2) is a novel target of miR-222-3p in GC. HIPK2 levels were
decreased in H. pylori (+) GC patients. miR-222-3p
overexpression promoted the proliferation and invasion via
repressing HIPK2 in GC infected by H. pylori (78).
miR-133b may become a tumor suppressor because of
repressing the proliferation and invasion of GC cells through
affecting ATP citrate lyase (ACLY) and peroxisome
proliferator-activated receptor-γ (PPARγ) in GC, which inhibits
ACLY and increases the levels of nuclear PPARγ (79). IGFBP1 is a target of miR-519a and
they have an inverse relationship in GC cells. Therefore, miR-519a
could suppress the proliferation, migration and invasion of GC
cells via attenuating IGFBP1 (80). Similarly, S100A16 is a target of
miR-6884-5p and they have an opposite relationship in GC tissues
and cell lines. miR-6884-5p inhibited the proliferation, invasion
and EMT via attenuating S100A16 expression (81). Presenilin 1 (PSEN1) was a direct
target of miR-133a, the suppression of which would abolish the
promoting functions of miR-133a suppression on cell growth and
metastasis. miR-133a represses GC cell growth, migration, and
epithelial-mesenchymal transition by attenuating presenilin 1
(82). In addition, miR-6852
could attenuate forkhead box J1 (FOXJ1) to suppress cell
proliferation and invasion in GC (83). Upregulation of miR-153 in the GC
SNU-5 cells repress cell proliferation, migration and invasion. The
expression of Kruppel-like factor 5 (KLF5) is negatively regulated
by miR-153 in SNU-5 cells (84).
miR-200a-3p overexpression increases the G1/S cell
ratio and attenuates cell proliferation and colony formation and
directly interacts with the 3′-UTR of KLF12. However, there is a
negative correlation between miR-200a-3p and KLF12. These indicated
that miR-200a-3p repressed cell proliferation via downregulating
KLF12 (85). miR-520a-3p could
markedly inhibit the cell proliferation, invasion and migration of
SGC-7901 and MGC-803 cell lines. Spindle and kinetochore-associated
2 (SKA2) is a target gene of miR-520a-3p. miR-520a-3p plays a tumor
suppressor effect via downregulating SKA2 in GC cell lines
(86). miR-454 could target zinc
finger E-box-binding homeobox 1 (ZEB1) and repress its expression.
Therefore, miR-454 attenuates the proliferation, migration and
invasion by repressing ZEB1 in GC (87). EPS8 is downregulated by miR-345
and Rac1 signaling is its downstream. miR-345 could attenuate
migration and the stem-like cell phenotype via inactivation of Rac1
by downregulating EPS8 in GC (88). Overexpression of miR-495
suppressed cell proliferation and migration of GC, which could
promote caspase-3/-9 and Bax protein expression and suppress cyclin
D1 protein expression and the PI3K/Akt/mTOR pathway (89). In addition, overexpression of
miR-375 attenuated the AKT/mammalian target of rapamycin signaling
pathway to suppress the proliferation and migration of GC cells
(90). In addition, miR-375
repressed H. pylori-induced gastric carcinogenesis by
attenuating JAK2-STAT3 signaling (91). miR-1915 was under-expressed but
RAGE was overexpressed in H. pylori-infected GC tissues and
cells. Overexpression of miR-1915 restrains the proliferation,
invasion, and migration of H. pylori-infected GC by
repressing RAGE (92).
miR-574-5p bound to the 3′-UTR of protein tyro- sine
phosphatase non-receptor type 3 (PTPN3) mRNA, which represses PTPN3
to enhance phosphorylation of p44/42 MAPKs and promote angiogenesis
in GC (93). Peritoneal
mesothelial cells (PMCs) promoted peritoneal metastasis of GC cells
via mesothelial-to-mesenchymal transition (MMT), which provides a
convenient environment for metastatic GC cells. miR-21-5p could
repress SMAD7 expression and induce MMT of PMCs and promote tumor
peritoneal metastasis through activation of TGF-β/SMAD pathway
(94). SFRP1 is one of the
antagonists of the Wnt/β-catenin signaling pathway, the 3-UTR of
which could bind miR-196a-1. Therefore, miR-196a-1 plays a positive
effect in promoting GC cell invasion and metastasis by repressing
SFRP1 (95). RAB11-FIP2 is a
target of miR-192/215 that affects the establishment of cell
polarity and tight junction formation in GC cells via attenuating
RAB11-FIP2 (96). Similarly,
H. pylori infection can upregulate the expression of
miR-29a-3p in GC, while A20 expression is decreased in H.
pylori-positive gastric mucosa tissues. miR-29a-3p can enhance
the migration of gastric epithelial cells. A20 is a direct target
of miR-29a-3p and the miR-29a-3p mimic significantly blocked A20
expression. The miR-29a-3p is a tumor promotive miRNA and enhances
migration through directly repressing A20 gene in H.
pylori-infected GC (97).
miR-761 suppressed the expression of Ras and Rab
interactor 1 (RIN1) to play a repressive role in the metastasis of
GC (98). miR-28-5p, a tumor
suppressor, represses the phosphorylation of RAC serine/threonine
protein kinase (AKT) that affects invasion and metastasis to
inhibit GC cell migration and invasion (99). ARHGAP10 has a passive effect in
regulating the small G protein Rho- and Cdc42-mediated downstream
signal transduction (100).
miR-337-3p bound to the 3′-untranslated region of ARHGAP10 and
repressed the mRNA and protein levels of ARHGAP10 to attenuate
gastric tumor metastasis (101).
It has been reported that PFKFB3 was a versatile protein in human
cancers (102,103). It is a cancer-promoting protein
and a target of miR-449c. Overexpression of PFKFB3 abrogates the
inhibitory effect of miR-449c on the migration and invasion of GC
cells (104).
miR-711 could repress vimentin protein expression
but upregulate E-cadherin protein expression, which causes the
downregulation of CD44 to inhibit EMT of GC cells (105). In GC tissues and cell lines, the
expression changes between miR-203 and Annexin A4 are opposite and
the invasion and EMT are suppressed by overexpression of miR-203
via repressing Annexin A4 (106). In addition miR-203 is also a
tumor suppressor in H. pylori-related GC via repressing CASK
expression (107). PTPN14 is a
target of miR-217. miR-217 abrogated PTPN14 expression by directly
targeting its 3′-UTR and epithelial-to-mesenchymal transition,
metastasis and invasion through repressing PTPN14 in GC (108). SRY-Box 4 (SOX4) plays a
stimulatory effect on epithelial-mesenchymal transition in GC.
miR-381 could abrogate migration and invasion in human gastric
carcinoma via downregulating it (109). A study identified that
miR-143-3p was significantly increased in H. pylori-positive
GC tissues but decreased in GC tissues and cells. AKT2 is a certain
direct target of miR-143-3p and they have an opposite relationship.
Knockdown of miR-143-3p can promote AKT2 expression. miR-143-3p
acts as a novel tumor suppressive miRNA via repressing migration
and invasion by directly targeting AKT2 gene (110). A study identified that miR-30a
acts as a tumor suppressor to inhibit migration of H.
pylori-infected GC by double-restraining COX-2 and BCL9
(111).
Some miRNAs could become potential diagnostic
candidates during the early stage of GC (Table VII).
Kaplan-Meier survival analysis is a common method
used to determine whether a certain miRNA can become a diagnostic
marker. miR-181b-5p is significantly decreased in GC-associated
malignant ascites (116).
Compared with normal tissues, miR-551b-5p emerged a low expression
state in GC tissues. Furthermore, it is verified that the
downregulation of miR-551b-5p indicates the appearance of GC by
serum miRNA microarray analysis (117). As with the miRNAs mentioned
above, miR-133b (114) and
miRNA-381 (118) were markedly
decreased in GC patient groups compared with normal cases. In
addition, these miRNAs are associated with lymph node metastasis
and the development of GC. Thus, miR-133b and miR-381 are potential
candidates for the diagnosis in GC patients in the early stage.
miR-501 directly regulates BLID at the
post-transcriptional level in multiple GC cell lines. Furthermore,
in doxorubicin-resistant GC SGC7901/ADR cells, endogenous miR-501
was higher but BLID was lower than parental SGC7901 cells. miR-501
could therefore become a tumor suppressor because it inhibited GC
cell apoptosis and enhanced cell proliferation, migration, and
invasion to induce resistance to doxorubicin. The suppression of
BLID by miR-501 subsequently inactivates caspase-9 and caspase-3
and phosphorylation of Akt (119). In a similar manner to miR-501
and BLID, miR-17 presents high expression but DEDD presents low
expression in GC. DEDD is a target gene of miR-17. In a study, it
has been verified that miR-17 could induce resistance to cisplatin
or 5-Fu and suppress cell apoptosis via repressing DEDD in GC cells
(120). Cell death occurs in
various ways, including ferroptosis which is induced by lipid-ROS
in an iron-dependent manner. However, miR-522 inhibited ferroptosis
by targeting ALOX15 and repressing lipid-ROS accumulation in cancer
cells. Cancer-associated fibroblasts (CAFs) secretes various
bioactive substances to promote tumor progression and drug
resistance including exosomes. In addition, CAFs secrete miR-522 in
the tumor microenvironment and consequently promote acquired
chemo-resistance by suppressing ferroptosis in GC (121). miR-21 is a typical oncomiRNA,
the overexpression of which is ordinary in GC cells. Phosphatase
and tensin homolog (PTEN) is a typical tumor suppressor that
passively regulates the Akt/PKB signaling pathway by repressing
phosphoinositide 3-kinase (PI3K), a target of miR-21 in cancer
cells (122,123). miR-21 enhances the
chemo-resistance of DDP and curcumin, as well as tumor growth,
migration and invasion via abrogating autophagy through the
miR-21/PTEN/PI3K/Akt/mTOR pathway in GC cells (124,125). Overexpression of miR-4295 is
relevant to the higher expression of EGFR, PI3K, Akt, p-PI3K and
p-Akt and the lower expression of LRIG1 in GC cells. In addition,
miR-4295 directly targets LRIG1. In a recent study, it was
confirmed that miR-4295 could activate the EGFR/PI3K/Akt signaling
pathway via negatively regulating LRIG1 expression, which then
promoted cell proliferation and abrogated apoptosis-induced DDP in
GC cells (126).
miR-96 repressed the post-transcriptional expression
of FOXO1. Subsequently, the downexpression of FOXO1 led to
downregulation of transcriptional activity of the cyclin-dependent
kinase inhibitor 1A (CDKN1A, also known as p21) promoter region.
Consequently, the expression of p21 would be downregulated in a
tumor protein p53-independent manner. It was recently identified
that the induction of miR-96 could cause chemoresistance and
promote proliferation via repressing FOXO1 and p21 in SGC7901 cells
(127). Apatinib is a common
chemotherapy drug used in the treatment of GC. miR-106a-3p actived
Janus-Activated Kinase 2 (JAK2)/Signal Transducer and Activator of
Transcription 3 (STAT3) by increasing the level of SOCS family,
which induced resistance of Apatinib (128). H. pylori infection could
induce the secretion of many substances in GC, including
miR-135b-5p. miR-135b-5p abrogates KLF4 expression by directly
targeting its 3′-UTR to induce cisplatin resistance in H.
pylori GC cells (129). NK
cells could directly kill tumor cells, and NKG2D plays an essential
role in mediating the anti-tumor immunity of NK cells. NKG2D
specifically binds to its ligand MICA (MHC class I chain-related
protein A), transmits an activation signal through an adaptor
protein (DAP10 or DAP12), and mobilizes particles containing
perforin and granzyme B to connect with tumor cells to cause the
tumor cells to lyse to exert antitumor effects. MICA suppressed the
immune-evasion of GC cells. However, miR-20a could repress MICA
expression by directly targeting MICA (130). Upregulation of miR-20a could
promote the harmful effect of Docetaxel on abrogation of MICA to
repress the curative effect of Docetaxel (131). To some extent, H. pylori
infection is good for the treatment of GC. H. pylori
infection significantly downregulates miR-141. Knockdown of miR-141
expression significantly improves cisplatin sensitivity via
suppressing KEAP1. KEAP1 is a direct target of miR-141 (132).
miR-155-5p has a large number of targets, which
includes GATA binding protein 3 (GATA3) and tumor protein p53
inducible nuclear protein 1 (TP53INP1). miR-155-5p could interact
with their 3′-untranslated regions. Exosome miR-155-5p can induce
EMT and the transition of chemo-resistant phenotypes from
paclitaxel-resistant GC cells to the sensitive cells, the mechanism
of which may is that miR-155-5p suppresses the expression of GATA3
and TP53INP1 (133). Suppressor
of zeste 12 protein (SUZ12) is one of the targets of miR-362-5p. It
is relevant to the stimulatory effect of miR-362-5p on cisplatin
sensitivity of GC cells. In a recent study, it was verified that
miR-362-5p could promote cisplatin sensitivity via repressing SUZ12
(134). Numerous miRNAs are
relevant to reversal of drug resistance in human GC cells. miR-200c
is a miRNAs whose overexpression may reverse drug resistance in the
SGC7901/DDP GC cell line via repressing ERCC3 and ERCC4 through the
NER-ERCC3/4 pathway (135).
Lidocaine is an anti-cancer chemotherapy drug which abrogates the
viability, proliferation, migration, and invasion of GC cells.
miR-145 plays a positive role in promoting the effect of lidocaine
on GC cells. It was confirmed that lidocaine could have a stronger
inhibitory action via up-regulating miR-145 expression which
further inactivates the MEK/ERK and NF-κB signaling path- ways
(136). TMED3 is one of the
direct targets of miR-876-3p. miR-876-3p inhibitor may
significantly increase mRNA and protein expression of TMED3, which
represses cisplatin sensitivity and restricts stem cell-like
features of GC (137). miR-320a
and miR-4496 attenuate H. pylori cytotoxin-associated gene A
(CagA)-induced chemoresistance by repressing β-catenin and
ATP-binding cassette, subfamily G, member 2 (ABCG2) (138).
Some miRNAs are abnormally expressed in patients
after treatment and these abnormal expressions can become
indicators of poor prognosis. miR-501 presents a high expression
state in patients with poor prognosis, thereby making it an
indicator thereof (119).
Kaplan-Meier analysis is also used to discern indicators of poor
prognosis. Kaplan-Meier analysis confirmed that overexpression of
miR-208a was obviously associated with shorter overall survival
(OS) time. Furthermore, univariate and multivariate Cox analysis
revealed that lymph node metastasis, TNM stage and higher miR-208a
were independent risks factors of OS time (70). In conclusion, miR-208a is another
indicator of poor prognosis of GC. Similarly, miR-718 (53) and exo-miR-15b-3p (56) are upregulated in patients with
poor prognosis and indicators of worse overall survival and poor
prognosis.
There is no doubt that there are some miRNAs
abnormally expressed in patients after treatment and these abnormal
expressions can become indicators of good prognosis as well.
Increased expression of miR-519a is relevant to the good overall
survival of GC patients and is identified as an independent
prognostic biomarker for the patients (80). miR-153 (84) and miR-187 (36) are reduced in GC patients with
aggressiveness and poor prognosis. Furthermore, downregulation of
the two miRNAs is correlated to cell differentiation, TNM staging
and poor prognosis in GC patients. These miRNAs could also be
indicators of good prognosis in GC patients after treatment. As
with miR-153 and miR-187, GC patients with a stronger expression of
miR-345 have a better prognosis (88). Compared with GC patients with
lower miR-28-5p expression, patients with higher expression have a
longer overall survival time and good prognosis, as indicated via
the Kaplan Meier survival curve analysis (99). Kaplan-Meier analysis also revealed
that the downregulation of miR-124-3p (46) and miR-383-5p (41) correlates with advanced clinical
stage, larger tumor size, lymph node metastases and shorter OS
time. Furthermore, multivariate Cox proportional hazards regression
analyses revealed that miR-124-3p and miR-383-5p were independent
prognostic factors for predicting the prognosis of GC patients
after treatment.
The relationship between miRNAs and GC is very
close, miRNAs can be divided into oncomiRNA and tsmiRNA. miRNAs
correlate with the entire clinical course of GC, but currently
there are not many miRNAs that can be truly used in clinical
practice. Since the specific mechanism of most miRNAs is not yet
clear, the current review uses the clinical process of GC as a clue
to comprehensively elaborate the latest miRNAs with specific
targets or pathways and GC research results, which are of great
significance for the prevention, diagnosis, treatment and prognosis
of GC. As miRNAs are relevant to various tumors, this review can
also provide ideas for other tumor research.
This work was funded by the Natural Science
Foundation of Hunan Province (grant no. 2019JJ50500), the National
Natural Science Foundation of China (grant no. 81372134), the
Scientific Research Fund Project of Hunan Provincial Health
Commission (grant no. 20201921), the Hunan Provincial Key
Laboratory of Tumor Microenvironment Responsive Drug Research
[Hunan Provincial Science and Technology Department Document (grant
no. 2019-56)], the Hunan Province Cooperative Innovation Center for
Molecular Target New Drug Study (grant no. 2014-405), and the
Undergraduate Research-based Learning and Innovative Experimental
Program of University of South China (grant nos. 2017XJYZ040,
2018XJXZ348, CX2019010).
Not applicable.
JO and XY contributed to writing the original draft.
ZX, XL, GT and RG contributed to revising the manuscript. All
authors read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors have no competing interests to
disclose.
Not applicable.
This work was funded by the Natural Science Foundation of Hunan
Province (grant no. 2019JJ50500), the National Natural Science
Foundation of China (grant no. 81372134), the Scientific Research
Fund Project of Hunan Provincial Health Commission (grant no.
20201921), the Hunan Provincial Key Laboratory of Tumor
Microenvironment Responsive Drug Research [Hunan Provincial Science
and Technology Department Document (grant no. 2019-56)], the Hunan
Province Cooperative Innovation Center for Molecular Target New
Drug Study (grant no. 2014-405), and the Undergraduate
Research-based Learning and Innovative Experimental Program of
University of South China (grant nos. 2017XJYZ040, 2018XJXZ348,
CX2019010).
|
1
|
Lunet N, Valbuena C, Vieira AL, Lopes C,
Lopes C, David L, Carneiro F and Barros H: Fruit and vegetable
consumption and gastric cancer by location and histological type:
Case-control and meta-analysis. Eur J Cancer Prev. 16:312–327.
2007.
|
|
2
|
Ladeiras-Lopes R, Pereira AK, Nogueira A,
Pinheiro-Torres T, Pinto I, Santos-Pereira R and Lunet N: Smoking
and gastric cancer: Systematic review and meta-analysis of cohort
studies. Cancer Causes Control. 19:689–701. 2008.
|
|
3
|
Yang P, Zhou Y, Chen B, Wan HW, Jia GQ,
Bai HL and Wu XT: Overweight, obesity and gastric cancer risk:
Results from a meta-analysis of cohort studies. Eur J Cancer.
45:2867–2873. 2009.
|
|
4
|
Bornschein J, Selgrad M, Warnecke M,
Kuester D, Wex T and Malfertheiner P: H. pylori infection is
a key risk factor for proximal gastric cancer. Dig Dis Sci.
55:3124–3131. 2010.
|
|
5
|
Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT,
Chang MC, Wang HP and Lin JT: Epstein-Barr virus-associated gastric
carcinomas: Relation to H. pylori infection and genetic
alterations. Gastroenterology. 118:1031–1038. 2000.
|
|
6
|
Wang HH, Wu MS, Shun CT, Wang HP, Lin CC
and Lin JT: Lymphoepithelioma-like carcinoma of the stomach: A
subset of gastric carcinoma with distinct clinicopathological
features and high prevalence of Epstein-Barr virus infection.
Hepatogastroenterology. 46:1214–1219. 1999.
|
|
7
|
Oliveira C, Pinheiro H, Figueiredo J,
Seruca R and Carneiro F: Familial gastric cancer genetic
susceptibility, pathology, and implications for management. Lancet
Oncol. 16:e60–e70. 2015.
|
|
8
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016.
|
|
9
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. dv Exp Med Biol. 774:1–20. 2013.
|
|
10
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531.
2004.
|
|
11
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.
|
|
12
|
Shi Y, Wang Z, Zhu X, Chen L, Ma Y, Wang
J, Yang X and Liu Z: Exosomal miR-1246 in serum as a potential
biomarker for early diagnosis of gastric cancer. Int J Clin Oncol.
25:89–99. 2020.
|
|
13
|
Ishikawa D, Yoshikawa K, Takasu C,
Kashihara H, Nishi M, Tokunaga T, Higashijima J and Shimada M:
Expression level of MicroRNA-449a predicts the prognosis of
patients with gastric cancer. Anticancer Res. 40:239–244. 2020.
|
|
14
|
Chen P, Guo H, Wu X, Li J, Duan X, Ba Q
and Wang H: Epigenetic silencing of microRNA-204 by Helicobacter
pylori augments the NF-κB signaling pathway in gastric cancer
development and progression. Carcinogenesis. 41:430–441. 2020.
|
|
15
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010.
|
|
16
|
Sun X, Hou H, Li K and Zheng M:
microRNA-761 regulates glycogen synthase kinase 3β expression and
promotes the proliferation and cell cycle of human gastric cancer
cells. Oncol Lett. 16:3459–3464. 2018.
|
|
17
|
Liu Z, Yu M, Fei B, Fang X, Ma T and Wang
D: miR-21-5p targets PDHA1 to regulate glycolysis and cancer
progression in gastric cancer. Oncol Rep. 40:2955–2963. 2018.
|
|
18
|
Jiang Y, Zhang M, Guo T, Yang C, Zhang C
and Hao J: MicroRNA-21-5p promotes proliferation of gastric cancer
cells through targeting SMAD7. Onco Targets Ther. 11:4901–4911.
2018.
|
|
19
|
Hua K, Chen YT, Chen CF, Tang YS, Huang
TT, Lin YC, Yeh TS, Huang KH, Lee HC, Hsu MT, et al:
MicroRNA-23a/27a/24-2 cluster promotes gastric cancer cell
proliferation synergistically. Oncol Lett. 16:2319–2325. 2018.
|
|
20
|
Sherr CJ: Principles of tumor suppression.
Cell. 116:235–246. 2004.
|
|
21
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000.
|
|
22
|
Wang J, Zheng X, Qin Z, Wei L, Lu Y, Peng
Q, Gao Y, Zhang X, Zhang X, Li Z, et al: Epstein-Barr virus
miR-BART3-3p promotes tumorigenesis by regulating the senescence
pathway in gastric cancer. J Biol Chem. 294:4854–4866. 2019.
|
|
23
|
Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S and
Navab R: MicroRNA-21 (miR-21) regulates cellular proliferation,
invasion, migration, and apoptosis by targeting PTEN, RECK and
Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS
One. 9:e1036982014.
|
|
24
|
Chen X, Wang W, Zhang J, Li S, Zhao Y, Tan
L and Luo A: Involvement of caspase-3/PTEN signaling pathway in
isoflurane-induced decrease of self-renewal capacity of hippocampal
neural precursor cells. Brain Res. 1625:275–286. 2015.
|
|
25
|
Yao L, Shi W and Gu J: Micro-RNA 205-5p is
involved in the progression of gastric cancer and targets
phosphatase and tensin homolog (PTEN) in SGC-7901 human gastric
cancer cells. Med Sci Monit. 25:6367–6377. 2019.
|
|
26
|
Fan H, Jin X, Liao C, Qiao L and Zhao W:
MicroRNA-301b-3p accelerates the growth of gastric cancer cells by
targeting zinc finger and BTB domain containing 4. Pathol Res
Pract. 215:1526672019.
|
|
27
|
Yang X, Wang M, Lin B, Yao D, Li J, Tang
X, Li S, Liu Y, Xie R and Yu S: miR-487a promotes progression of
gastric cancer by targeting TIA1. Biochimie. 154:119–126. 2018.
|
|
28
|
Yu J, Qi J, Sun X, Wang W, Wei G, Wu Y,
Gao Q and Zheng J: MicroRNA-181a promotes cell proliferation and
inhibits apoptosis in gastric cancer by targeting RASSF1A. Oncol
Rep. 40:1959–1970. 2018.
|
|
29
|
Li S, Liang X, Ma L, Shen L, Li T, Zheng
L, Sun A, Shang W, Chen C, Zhao W and Jia J: miR-22 sustains NLRP3
expression and attenuates H. pylori-induced gastric
carcinogenesis. Oncogene. 37:884–896. 2018.
|
|
30
|
Takaishi S, Okumura T and Wang TC: Gastric
cancer stem cells. J Clin Oncol. 26:2876–2882. 2008.
|
|
31
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005.
|
|
32
|
Song H, Shi L, Xu Y, Xu T, Fan R, Cao M,
Xu W and Song J: BRD4 promotes the stemness of gastric cancer cells
via attenuating miR-216a-3p-mediated inhibition of Wnt/β-catenin
signaling. Eur J Pharmacol. 852:189–197. 2019.
|
|
33
|
Lin H, Zhou AJ, Zhang JY, Liu SF and Gu
JX: miR-324-5p reduces viability and induces apoptosis in gastric
cancer cells through modulating TSPAN8. J Pharm Pharmacol.
70:1513–1520. 2018.
|
|
34
|
Li S, Zhang T, Zhou X, Du Z, Chen F, Luo J
and Liu Q: The tumor suppressor role of miR-155-5p in gastric
cancer. Oncol Lett. 16:2709–2714. 2018.
|
|
35
|
Zhang X, Zhang M, Guo Q, Hu X, Zhao Z, Ni
L, Liu L, Wang X, Wang Z, Tong D, et al: MicroRNA-1297 inhibits
proliferation and promotes apoptosis in gastric cancer cells by
downregulating CDC6 expression. Anticancer Drugs. 30:803–811.
2019.
|
|
36
|
Chen W, Cui Y, Wang J, Yuan Y, Sun X,
Zhang L, Shen S and Cheng J: Effects of downregulated expression of
microRNA-187 in gastric cancer. Exp Ther Med. 16:1061–1070.
2018.
|
|
37
|
Shi Y, Lee JS and Galvin KM: Everything
you have ever wanted to know about Yin Yang 1. Biochim Biophys
Acta. 1332:F49–F66. 1997.
|
|
38
|
Sui G, Affar el B and Shi Y, Brignone C,
Wall NR, Yin P, Donohoe M, Luke MP, Calvo D, Grossman SR and Shi Y:
Yin Yang 1 is a negative regulator of p53. Cell. 117:859–872.
2004.
|
|
39
|
Wu S, Kasim V, Kano MR, Tanaka S, Ohba S,
Miura Y, Miyata K, Liu X, Matsuhashi A, Chung UI, et al:
Transcription factor YY1 contributes to tumor growth by stabilizing
hypoxia factor HIF-1α in a p53-independent manner. Cancer Res.
73:1787–1799. 2013.
|
|
40
|
Zhou GQ, Han F, Shi ZL, Yu L, Li XF, Yu C,
Shen CL, Wan DW, Zhu XG, Li R and He SB: DNMT3A-mediated
down-regulation of microRNA-105 promotes gastric cancer cell
proliferation. Eur Rev Med Pharmacol Sci. 21:3377–3383. 2017.
|
|
41
|
Xu G, Li N, Zhang Y, Zhang J, Xu R and Wu
Y: MicroRNA-383-5p inhibits the progression of gastric carcinoma
via targeting HDAC9 expression. Braz J Med Biol Res.
52:e83412019.
|
|
42
|
Ooms LM, Binge LC, Davies EM, Rahman P,
Conway JR, Gurung R, Ferguson DT, Papa A, Fedele CG, Vieusseux JL,
et al: The inositol polyphosphate 5-phosphatase PIPP regulates
AKT1-dependent breast cancer growth and metastasis. Cancer Cell.
28:155–169. 2015.
|
|
43
|
Sahlberg SH, Spiegelberg D, Glimelius B,
Stenerlöw B and Nestor M: Evaluation of cancer stem cell markers
CD133, CD44, CD24: Association with AKT isoforms and radiation
resistance in colon cancer cells. PLoS One. 9:e946212014.
|
|
44
|
Etemadmoghadam D and Bowtell D: AKT1 gene
amplification as a biomarker of treatment response in ovarian
cancer: Mounting evidence of a therapeutic target. Gynecol Oncol.
135:409–410. 2014.
|
|
45
|
Yu H, Sun J, Jiang S and Xu Y:
MicroRNA-490-3p regulates cell proliferation and apoptosis in
gastric cancer via direct targeting of AKT1. Exp Ther Med.
17:1330–1336. 2019.
|
|
46
|
Liu F, Hu H, Zhao J, Zhang Z, Ai X, Tang L
and Xie L: miR-124-3p acts as a potential marker and suppresses
tumor growth in gastric cancer. Biomed Rep. 9:147–155. 2018.
|
|
47
|
Zhu CY, Meng FQ and Liu J: MicroRNA-524-5p
suppresses cell proliferation and promotes cell apoptosis in
gastric cancer by regulating CASP3. Eur Rev Med Pharmacol Sci.
23:7968–7977. 2019.
|
|
48
|
Zhao M, Liu Q, Liu W, Zhou H, Zang X and
Lu J: MicroRNA-140 suppresses Helicobacter pylori-positive
gastric cancer growth by enhancing the antitumor immune response.
Mol Med Rep. 20:2484–2492. 2019.
|
|
49
|
Geng Y, Lu X, Wu X, Xue L, Wang X and Xu
J: MicroRNA-27b suppresses Helicobacter pylori-induced
gastric tumorigenesis through negatively regulating Frizzled7.
Oncol Rep. 35:2441–2450. 2016.
|
|
50
|
Zhou X, Xia Y, Li L and Zhang G: miR-101
inhibits cell growth and tumorigenesis of Helicobacter
pylori related gastric cancer by repression of SOCS2. Cancer
Biol Ther. 16:160–169. 2015.
|
|
51
|
Feng Y, Wang L, Zeng J, Shen L, Liang X,
Yu H, Liu S, Liu Z, Sun Y, Li W, et al: FoxM1 is overexpressed in
Helicobacter pylori-induced gastric carcinogenesis and is
negatively regulated by miR-370. Mol Cancer Res. 11:834–844.
2013.
|
|
52
|
Yang L, Li C and Jia Y: MicroRNA-99b
promotes Helicobacter pylori-induced autophagyand suppresses
carcinogenesis by targeting mTOR. Oncol Lett. 16:5355–5360.
2018.
|
|
53
|
Liu S, Tian Y, Zhu C, Yang X and Sun Q:
High miR-718 suppresses phosphatase and tensin homolog (PTEN)
expression and correlates to unfavorable prognosis in gastric
cancer. Med Sci Monit. 24:5840–5850. 2018.
|
|
54
|
Wang Q, He Y, Kan W, Li F, Ji X, Wu X,
Wang X, Zhang Y and Chen J: microRNA-32-5p targets KLF2 to promote
gastric cancer by activating PI3K/AKT signaling pathway. Am J
Transl Res. 11:4895–4908. 2019.
|
|
55
|
Zhou H, Liu H, Jiang M, Zhang S, Chen J
and Fan X: Targeting MicroRNA-21 suppresses gastric cancer cell
proliferation and migration via PTEN/Akt signaling axis. Cell
Transplant. 28:306–317. 2019.
|
|
56
|
Wei S, Peng L, Yang J, Sang H, Jin D, Li
X, Chen M, Zhang W, Dang Y and Zhang G: Exosomal transfer of
miR-15b-3p enhances tumorigenesis and malignant transformation
through the DYNLT1/Caspase-3/Caspase-9 signaling pathway in gastric
cancer. J Exp Clin Cancer Res. 39:322020.
|
|
57
|
Huang J, Shen M, Yan M, Cui Y, Gao Z and
Meng X: Exosome-mediated transfer of miR-1290 promotes cell
proliferation and invasion in gastric cancer via NKD1. Acta Biochim
Biophys Sin (Shanghai). 51:900–907. 2019.
|
|
58
|
Angonin D and Van Raay TJ: Nkd1 functions
as a passive antagonist of Wnt signaling. PLoS One.
8:e746662013.
|
|
59
|
Yan D, Wallingford JB, Sun TQ, Nelson AM,
Sakanaka C, Reinhard C, Harland RM, Fantl WJ and Williams LT: Cell
autonomous regulation of multiple dishevelled-dependent pathways by
mammalian Nkd. Proc Natl Acad Sci USA. 98:3802–3807. 2001.
|
|
60
|
Wharton KA Jr, Zimmermann G, Rousset R and
Scott MP: Vertebrate proteins related to drosophila naked cuticle
bind dishevelled and antagonize Wnt signaling. Dev Biol.
234:93–106. 2001.
|
|
61
|
Zhu Y, Jiang Q, Lou X, Ji X, Wen Z, Wu J,
Tao H, Jiang T, He W, Wang C, et al: MicroRNAs up-regulated by CagA
of Helicobacter pylori induce intestinal metaplasia of
gastric epithelial cells. PLoS One. 7:e351472012.
|
|
62
|
Shen E, Wang X, Liu X, Lv M, Zhang L, Zhu
G and Sun Z: MicroRNA-93-5p promotes epithelial-mesenchymal
transition in gastric cancer by repressing tumor suppressor AHNAK
expression. Cancer Cell Int. 20:762020.
|
|
63
|
Nagl NG Jr, Patsialou A, Haines DS, Dallas
PB, Beck GR Jr and Moran E: The p270 (ARID1A/SMARCF1) subunit of
mammalian SWI/SNF-related complexes is essential for normal cell
cycle arrest. Cancer Res. 65:9236–9244. 2005.
|
|
64
|
Shigetomi H, Oonogi A, Tsunemi T, Tanase
Y, Yamada Y, Kajihara H, Yoshizawa Y, Furukawa N, Haruta S, Yoshida
S, et al: The role of components of the chromatin modification
machinery in carcinogenesis of clear cell carcinoma of the ovary
(Review). Oncol Lett. 2:591–597. 2011.
|
|
65
|
Cheng S, Wang L, Deng CH, Du SC and Han
ZG: ARID1A represses hepatocellular carcinoma cell proliferation
and migration through lncRNA MVIH. Biochem Biophys Res Commun.
491:178–182. 2017.
|
|
66
|
Zhu Y, Li K, Yan L, He Y, Wang L and Sheng
L: miR-223-3p promotes cell proliferation and invasion by targeting
Arid1a in gastric cancer. Acta Biochim Biophys Sin (Shanghai).
52:150–159. 2020.
|
|
67
|
Yang Y, Yin ZX, Wang ZY, Tian SB, Wang HC,
Zhang FX, Li LP, Zheng C and Kong S: miR-7641 depletion suppresses
proliferation of gastric cancer cells by targeting ARID1A.
Anticancer Drugs. 31:368–376. 2020.
|
|
68
|
Zhang X, Zhang M, Wang G, Tian Y and He X:
Tumor promoter role of miR-647 in gastric cancer via repression of
TP73. Mol Med Rep. 18:3744–3750. 2018.
|
|
69
|
Yang F, Xu Y, Liu C, Ma C, Zou S, Xu X,
Jia J and Liu Z: NF-κB/miR-223-3p/ARID1A axis is involved in
Helicobacter pylori CagA-induced gastric carcinogenesis and
progression. Cell Death Dis. 9:122018.
|
|
70
|
Cui HB, Ge HE, Wang YS and Bai XY:
miR-208a enhances cell proliferation and invasion of gastric cancer
by targeting SFRP1 and negatively regulating MEG3. Int J Biochem
Cell Biol. 102:31–39. 2018.
|
|
71
|
Lin J, Shen J, Yue H and Cao Z:
miRNA-183-5p.1 promotes the migration and invasion of gastric
cancer AGS cells by targeting TPM1. Oncol Rep. 42:2371–2381.
2019.
|
|
72
|
Pelengaris S and Khan M: The many faces of
c-MYC. Arch Biochem Biophys. 416:129–136. 2003.
|
|
73
|
Deng T, Zhang H, Yang H, Wang H, Bai M,
Sun W, Wang X, Si Y, Ning T, Zhang L, et al: Exosome miR-155
derived from gastric carcinoma promotes angiogenesis by targeting
the c-MYB/VEGF axis of endothelial cells. Mol Ther Nucleic Acids.
19:1449–1459. 2020.
|
|
74
|
Maiese K, Hou J, Chong ZZ and Shang YC: A
fork in the path: Developing therapeutic inroads with FoxO
proteins. Oxid Med Cell Longev. 2:119–129. 2009.
|
|
75
|
Zhou Z, Zhang H, Deng T, Ning T, Liu R,
Liu D, Bai M, Ying G and Ba Y: Exosomes carrying MicroRNA-155
target forkhead box O3 of endothelial cells and promote
angiogenesis in gastric cancer. Mol Ther Oncolytics. 15:223–233.
2019.
|
|
76
|
Yang H, Zhang H, Ge S, Ning T, Bai M, Li
J, Li S, Sun W, Deng T, Zhang L, et al: Exosome-derived miR-130a
activates angiogenesis in gastric cancer by targeting C-MYB in
vascular endothelial cells. Mol Ther. 26:2466–2475. 2018.
|
|
77
|
Shi Y, Yang Z, Zhang T, Shen L, Li Y and
Ding S: SIRT1-targeted miR-543 autophagy inhibition and
epithelial-mesenchymal transition promotion in Helicobacter
pylori CagA-associated gastric cancer. Cell Death Dis.
10:6252019.
|
|
78
|
Tan X, Tang H, Bi J, Li N and Jia Y:
MicroRNA-222-3p associated with Helicobacter pylori targets
HIPK2 to promote cell proliferation, invasion, and inhibits
apoptosis in gastric cancer. J Cell Biochem. 119:5153–5162.
2018.
|
|
79
|
Cheng Y, Jia B, Wang Y and Wan S: miR-133b
acts as a tumor suppressor and negatively regulates ATP citrate
lyase via PPARγ in gastric cancer. Oncol Rep. 38:3220–3226.
2017.
|
|
80
|
Cai H, Lin H, Cao W, Sun J, Huang Y and
Fang Y: Downregulation of miR-519a predicts poor prognosis and
contributes to tumor progression in gastric cancer. Oncol Res
Treat. 43:19–26. 2020.
|
|
81
|
Lv H, Hou H, Lei H, Nie C, Chen B, Bie L,
Han L and Chen X: MicroRNA-6884-5p regulates the proliferation,
invasion and EMT of gastric cancer cells by directly targeting
S100A16. Oncol Res. 28:225–236. 2020.
|
|
82
|
Chen XB, Li W and Chu AX: MicroRNA-133a
inhibits gastric cancer cells growth, migration, and
epithelial-mesenchymal transition process by targeting presenilin
1. J Cell Biochem. 120:470–480. 2019.
|
|
83
|
Yu H, Zhang J, Wen Q, Dai Y, Zhang W, Li F
and Li J: MicroRNA-6852 suppresses cell proliferation and invasion
via targeting forkhead box J1 in gastric cancer. Exp Ther Med.
16:3249–3255. 2018.
|
|
84
|
Ouyang Y, Yuan W and Qiu S: MicroRNA-153
functions as a tumor suppressor in gastric cancer via targeting
Kruppel-like factor 5. Exp Ther Med. 16:473–482. 2018.
|
|
85
|
Jia C, Zhang Y, Xie Y, Ren Y, Zhang H,
Zhou Y, Gao N, Ding S and Han S: miR-200a-3p plays tumor suppressor
roles in gastric cancer cells by targeting KLF12. Artif Cells
Nanomed Biotechnol. 47:3697–3703. 2019.
|
|
86
|
Su H, Ren F, Jiang H, Chen Y and Fan X:
Upregulation of microRNA-520a-3p inhibits the proliferation,
migration and invasion via spindle and kinetochore associated 2 in
gastric cancer. Oncol Lett. 18:3323–3330. 2019.
|
|
87
|
Song Z, Li W, Wang L, Jia N and Chen B:
MicroRNA-454 inhibits tumor cell proliferation, migration and
invasion by downregulating zinc finger E-box-binding homeobox 1 in
gastric cancer. Mol Med Rep. 16:9067–9073. 2017.
|
|
88
|
Zhang J, Wang C, Yan S, Yang Y, Zhang X
and Guo W: miR-345 inhibits migration and stem-like cell phenotype
in gastric cancer via inactivation of Rac1 by targeting EPS8. Acta
Biochim Biophys Sin (Shanghai). 52:259–267. 2020.
|
|
89
|
Wang J, Feng W, Dong Y, Mao X, Guo F and
Luo F: MicroRNA-495 regulates human gastric cancer cell apoptosis
and migration through Akt and mTOR signaling. Oncol Rep.
40:3654–3662. 2018.
|
|
90
|
Yuan KT, Li BX, Yuan YJ, Tan M, Tan JF,
Dai WG, Feng WD and Zuo JD: Deregulation of MicroRNA-375 inhibits
proliferation and migration in gastric cancer in association with
autophagy-mediated AKT/mTOR signaling pathways. Technol Cancer Res
Treat. 17:15330338188064992018.
|
|
91
|
Miao L, Liu K, Xie M, Xing Y and Xi T:
miR-375 inhibits Helicobacter pylori-induced gastric
carcinogenesis by blocking JAK2-STAT3 signaling. Cancer Immunol
Immunother. 63:699–711. 2014.
|
|
92
|
Xu XC, Zhang WB, Li CX, Gao H, Pei Q, Cao
BW and He TH: Up-regulation of miR-1915 inhibits proliferation,
invasion, and migration of Helicobacter pylori-infected
gastric cancer cells via targeting RAGE. Yonsei Med J. 60:38–47.
2019.
|
|
93
|
Zhang S, Zhang R, Xu R, Shang J, He H and
Yang Q: MicroRNA-574-5p in gastric cancer cells promotes
angiogenesis by targeting protein tyrosine phosphatase non-receptor
type 3 (PTPN3). Gene. 733:1443832020.
|
|
94
|
Li Q, Li B, Li Q, Wei S, He Z, Huang X,
Wang L, Xia Y, Xu Z, Li Z, et al: Exosomal miR-21-5p derived from
gastric cancer promotes peritoneal metastasis via
mesothelial-to-mesenchymal transition. Cell Death Dis.
9:8542018.
|
|
95
|
Feng C, She J, Chen X, Zhang Q, Zhang X,
Wang Y, Ye J, Shi J, Tao J, Feng M, et al: Exosomal miR-196a-1
promotes gastric cancer cell invasion and metastasis by targeting
SFRP1. Nanomedicine (Lond). 14:2579–2593. 2019.
|
|
96
|
Zhang X, Peng Y, Huang Y, Deng S, Feng X,
Hou G, Lin H, Wang J, Yan R, Zhao Y, et al: Inhibition of the
miR-192/215-Rab11-FIP2 axis suppresses human gastric cancer
progression. Cell Death Dis. 9:7782018.
|
|
97
|
Sun F, Ni Y, Zhu H, Fang J, Wang H, Xia J,
Ding F, Shen H and Shao S: microRNA-29a-3p, Up-regulated in human
gastric cells and tissues with H. Pylori infection, promotes
the migration of GES-1 cells via A20-mediated EMT pathway. Cell
Physiol Biochem. 51:1250–1263. 2018.
|
|
98
|
Zhang Q, Sui Y and Sui X: MicroRNA-761
inhibits the metastasis of gastric cancer by negatively regulating
Ras and Rab interactor 1. Oncol Lett. 18:3097–3103. 2019.
|
|
99
|
Xiao F, Cheng Z, Wang P, Gong B, Huang H,
Xing Y and Liu F: MicroRNA-28-5p inhibits the migration and
invasion of gastric cancer cells by suppressing AKT
phosphorylation. Oncol Lett. 15:9777–9785. 2018.
|
|
100
|
Bassères DS, Tizzei EV, Duarte AA, Costa
FF and Saad ST: ARHGAP10, a novel human gene coding for a
potentially cytoskeletal Rho-GTPase activating protein. Biochem
Biophys Res Commun. 294:579–585. 2002.
|
|
101
|
Wang Z, Yao L, Li Y, Hao B, Wang M, Wang
J, Gu W, Zhan H, Liu G and Wu Q: miR-337-3p inhibits gastric tumor
metastasis by targeting ARHGAP10. Mol Med Rep. 21:705–719.
2020.
|
|
102
|
Clem BF, O'Neal J, Tapolsky G, Clem AL,
Imbert-Fernandez Y, Kerr DA II, Klarer AC, Redman R, Miller DM,
Trent JO, et al: Targeting 6-phosphofructo-2-kinase (PFKFB3) as a
therapeutic strategy against cancer. Mol Cancer Ther. 12:1461–1470.
2013.
|
|
103
|
Imbert-Fernandez Y, Clem A, Clem B,
Tapolsky G, Telang S and Chesney J: Suppression of
6-phosphofructo-2-kinase (PFKFB3) for the treatment of breast
cancer. Cancer Res. 76(14 Suppl): S562016.
|
|
104
|
Chen X, Wang A and Yue X: miR-449c
inhibits migration and invasion of gastric cancer cells by
targeting PFKFB3. Oncol Lett. 16:417–424. 2018.
|
|
105
|
Xiao WS, Li DF, Tang YP, Chen YZ, Deng WB,
Chen J, Zhou WW and Liao AJ: Inhibition of epithelial-mesenchymal
transition in gastric cancer cells by miR-711-mediated down-
regulation of CD44 expression. Oncol Rep. 40:2844–2853. 2018.
|
|
106
|
Li J, Zhang B, Cui J, Liang Z and Liu K:
miR-203 inhibits the invasion and EMT of gastric cancer cells by
directly targeting annexin A4. Oncol Res. 27:789–799. 2019.
|
|
107
|
Zhou X, Xu G, Yin C, Jin W and Zhang G:
Down-regulation of miR-203 induced by Helicobacter pylori
infection promotes the proliferation and invasion of gastric cancer
by targeting CASK. Oncotarget. 5:11631–11640. 2014.
|
|
108
|
Chen G, Yang Z, Feng M and Wang Z:
microRNA-217 suppressed epithelial-to-mesenchymal transition
through targeting PTPN14 in gastric cancer. Biosci Rep.
40:BSR201931762020.
|
|
109
|
Zhang M, Huang S and Long D: miR-381
inhibits migration and invasion in human gastric carcinoma through
downregulatedting SOX4. Oncol Lett. 14:3760–3766. 2017.
|
|
110
|
Wang F, Liu J, Zou Y, Jiao Y, Huang Y, Fan
L, Li X, Yu H, He C, Wei W, et al: MicroRNA-143-3p, up-regulated in
H. pylori-positive gastric cancer, suppresses tumor growth,
migration and invasion by directly targeting AKT2. Oncotarget.
8:28711–28724. 2017.
|
|
111
|
Liu X, Ji Q, Zhang C, Liu X, Liu Y, Liu N,
Sui H, Zhou L, Wang S and Li Q: miR-30a acts as a tumor suppressor
by double-targeting COX-2 and BCL9 in H. pylori gastric
cancer models. Sci Rep. 7:71132017.
|
|
112
|
Emami SS, Nekouian R, Akbari A, Faraji A,
Abbasi V and Agah S: Evaluation of circulating miR-21 and miR-222
as diagnostic biomarkers for gastric cancer. J Cancer Res Ther.
15:115–119. 2019.
|
|
113
|
Saito R, Maruyama S, Kawaguchi Y, Akaike
H, Shimizu H, Furuya S, Kawaida H and Ichikawa D: miR-99a-5p as
possible diagnostic and prognostic marker in patients with gastric
cancer. J Surg Res. 250:193–199. 2020.
|
|
114
|
ZiaSarabi P, Sorayayi S, Hesari A and
Ghasemi F: Circulating microRNA-133, microRNA-17 and microRNA-25 in
serum and its potential diagnostic value in gastric cancer. J Cell
Biochem. 120:12376–12381. 2019.
|
|
115
|
Ji B, Huang Y, Gu T, Zhang L, Li G and
Zhang C: Potential diagnostic and prognostic value of plasma long
noncoding RNA LINC00086 and miR-214 expression in gastric cancer.
Cancer Biomark. 24:249–255. 2019.
|
|
116
|
Yun J, Han SB, Kim HJ, Go SI, Lee WS, Bae
WK, Cho SH, Song EK, Lee OJ, Kim HK, et al: Exosomal miR-181b-5p
down- regulation in ascites serves as a potential diagnostic
biomarker for gastric cancer-associated malignant ascites. J
Gastric Cancer. 19:301–314. 2019.
|
|
117
|
Jiang X, Jiang M, Xu M, Xu J and Li Y:
Identification of diagnostic utility and molecular mechanisms of
circulating miR-551b-5p in gastric cancer. Pathol Res Pract.
215:900–904. 2019.
|
|
118
|
Li Y, Sun H, Guan J, Ji T and Wang X:
Serum microRNA-381: A potential marker for early diagnosis of
gastric cancer. Yonsei Med J. 60:720–726. 2019.
|
|
119
|
Xu YC, Liu X, Li M, Li Y, Li CY, Lu Y,
Sanches J, Wang L, Du Y, Mao LM, et al: A novel mechanism of
doxorubicin resistance and tumorigenesis mediated by
MicroRNA-501-5p-suppressed BLID. Mol Ther Nucleic Acids.
12:578–590. 2018.
|
|
120
|
Wu DM, Hong XW, Wang LL, Cui XF, Lu J,
Chen GQ and Zheng YL: MicroRNA-17 inhibition overcomes
chemoresistance and suppresses epithelial-mesenchymal transition
through a DEDD-dependent mechanism in gastric cancer. Int J Biochem
Cell Biol. 102:59–70. 2018.
|
|
121
|
Zhang H, Deng T, Liu R, Ning T, Yang H,
Liu D, Zhang Q, Lin D, Ge S, Bai M, et al: CAF secreted miR-522
suppresses ferroptosis and promotes acquired chemo-resistance in
gastric cancer. Mol Cancer. 19:432020.
|
|
122
|
Wang Z, Cai Q, Jiang Z, Liu B, Zhu Z and
Li C: Prognostic role of microRNA-21 in gastric cancer: A
meta-analysis. Med Sci Monit. 20:1668–1674. 2014.
|
|
123
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026.
2012.
|
|
124
|
Roy S, Yu Y, Padhye SB, Sarkar FH and
Majumdar AP: Difluorinated-curcumin (CDF) restores PTEN expression
in colon cancer cells by down-regulating miR-21. PLoS One.
8:e685432013.
|
|
125
|
Qiang Z, Meng L, Yi C, Yu L, Chen W and
Sha W: Curcumin regulates the miR-21/PTEN/Akt pathway and acts in
synergy with PD98059 to induce apoptosis of human gastric cancer
MGC-803 cells. J Int Med Res. 47:1288–1297. 2019.
|
|
126
|
Yan R, Li K, Yuan DW, Wang HN, Zhang Y,
Dang CX and Zhu K: Downregulation of microRNA-4295 enhances
cisplatin-induced gastric cancer cell apoptosis through the
EGFR/PI3K/Akt signaling pathway by targeting LRIG1. Int J Oncol.
53:2566–2578. 2018.
|
|
127
|
Lang C, Xu M, Zhao Z, Chen J and Zhang L:
MicroRNA-96 expression induced by low-dose cisplatin or doxorubicin
regulates chemosensitivity, cell death and proliferation in gastric
cancer SGC7901 cells by targeting FOXO1. Oncol Lett. 16:4020–4026.
2018.
|
|
128
|
Guo W, Li W, Yuan L, Mei X and Hu W:
MicroRNA-106a-3p induces apatinib resistance and activates
janus-activated kinase 2 (JAK2)/signal transducer and activator of
transcription 3 (STAT3) by targeting the SOCS system in gastric
cancer. Med Sci Monit. 25:10122–10128. 2019.
|
|
129
|
Shao L, Chen Z, Soutto M, Zhu S, Lu H,
Romero-Gallo J, Peek R, Zhang S and El-Rifai W: Helicobacter
pylori-induced miR-135b-5p promotes cisplatin resistance in
gastric cancer. FASEB J. 33:264–274. 2019.
|
|
130
|
Tang S, Fu H, Xu Q and Zhou Y: miR-20a
regulates sensitivity of colorectal cancer cells to NK cells by
targeting MICA. Biosci Rep. 39:BSR201806952019.
|
|
131
|
Shekari N, Javadian M, Ghaffari S,
Baradaran B, Darabi M and Kazemi T: DHA abolishes the detrimental
effect of docetaxel on downregulation of the MICA via decreasing
the expression level of MicroRNA-20a in gastric cancer. J
Gastrointest Cancer. 51:545–551. 2020.
|
|
132
|
Zhou X, Su J, Zhu L and Zhang G:
Helicobacter pylori modulates cisplatin sensitivity in
gastric cancer by down-regulating miR-141 expression. Helicobacter.
19:174–181. 2014.
|
|
133
|
Wang M, Qiu R, Yu S, Xu X, Li G, Gu R, Tan
C, Zhu W and Shen B: Paclitaxel-resistant gastric cancer MGC-803
cells promote epithelial-to-mesenchymal transition and
chemoresistance in paclitaxel-sensitive cells via exosomal delivery
of miR155-5p. Int J Oncol. 54:326–338. 2019.
|
|
134
|
Wei X, Gao M, Ahmed Y, Gao M, Liu W, Zhang
Y, Xie X, Zhao Q, Wang H and Gu K: MicroRNA-362-5p enhances the
cisplatin sensitivity of gastric cancer cells by targeting
suppressor of zeste 12 protein. Oncol Lett. 18:1607–1616. 2019.
|
|
135
|
Li M, Gao M, Xie X, Zhang Y, Ning J, Liu P
and Gu K: MicroRNA-200c reverses drug resistance of human gastric
cancer cells by targeting regulation of the NER-ERCC3/4 pathway.
Oncol Lett. 18:145–152. 2019.
|
|
136
|
Sui H, Lou A, Li Z and Yang J: Lidocaine
inhibits growth, migration and invasion of gastric carcinoma cells
by up-regulation of miR-145. BMC Cancer. 19:2332019.
|
|
137
|
Peng C, Huang K, Liu G, Li Y and Yu C:
miR-876-3p regulates cisplatin resistance and stem cell-like
properties of gastric cancer cells by targeting TMED3. J
Gastroenterol Hepatol. 34:1711–1719. 2019.
|
|
138
|
Kang DW, Yang ES, Noh YN, Hwang WC, Jo SY,
Suh YA, Park WS, Choi KY and Min DS: MicroRNA-320a and
microRNA-4496 attenuate Helicobacter pylori
cytotoxin-associated gene A (CagA)-induced cancer-initiating
potential and chemoresistance by targeting β-catenin and
ATP-binding cassette, subfamily G, member 2. J Pathol. 241:614–625.
2017.
|