Breast cancer (BC) is one of the most common
malignancies affecting women, with approximately 1.5 million new
cases diagnosed per year, and accounts for 30% of all cancer types
in women. Despite advances in surgery, chemotherapy, radiotherapy,
endocrine therapy, molecular-targeted therapy and immunotherapy, BC
remains the cause of a vast number of deaths and is the second
leading cause of cancer-related mortality among women worldwide
(1-3).
BC is a heterogeneous disease, involving the
disruption of multiple oncogenic biological pathways and/or genetic
alterations. BC can be classified into different subtypes according
to gene expression profiling and/or molecular and receptor status.
Research has indicated that BC can be categorized into 5 major
subtypes as follows: Luminal A (LA), luminal B (LB), human
epidermal growth factor receptor 2 (HER2)-enriched, basal-like and
normal breast-like cancers (Table
I). The LA subtype expresses estrogen receptor (ER) and
progesterone receptor (PR), but is negative for HER2. LA is the
most common subtype, accounting for approximately 50-60% of all BC
cases. In the LB subtype, ER and PR expression is positive, and
HER2 expression can be either positive or negative. HER2-enriched
BC expresses HER2, whereas it does not express ER and PR, and this
subtype accounts for 5-20% of all BC cases (4-8).
The normal-like BC (ER- or PR-positive, HER2-negative) accounts for
approximately 5-10% of all BC cases (9). Basal-like subtype BC (ER-negative,
PR-negative, HER2-negative), also known as triple-negative breast
cancer (TNBC), is the most aggressive subtype of BC and accounts
for 15-20% of all BC cases. TNBC lacks the expression of all
hormone receptors, rendering it insensitive to the currently
available targeted and hormone therapies. Patients with TNBC thus
undergo earlier relapse and have higher mortality rates than
patients with other BC subtypes (10-12). No clearly defined TNBC-specific
therapeutic targets or markers have yet been identified, and
therefore, effective treatment methods have become major clinical
challenges for the treatment of patients with TNBC.
MicroRNAs (miRNAs or miRs) are a class of small
endogenous single-stranded RNA molecules that are 19-25 nucleotides
in length. miRNAs interact with the 3′untranslated region (UTR) of
the target messenger RNAs (mRNAs) to negatively regulate the gene
expression of specific mRNA targets. While the majority of miRNAs
are located in endonuclear noncoding regions, some studies have
reported miRNAs in the exons of genes (13-15). Each miRNA can regulate hundreds of
mRNAs and >60% of human mRNAs contain at least one miRNA binding
site (16).
Through the regulation of gene expression, miRNAs
are involved in various cellular processes, including
proliferation, differentiation, apoptosis, migration, metabolism
and the stress response (17-19). Over the past decade, a number of
studies have demonstrated that miRNA expression is dysregulated in
a number of types of cancer, including BC. The dysregulation of
miRNAs influences various processes that contribute to tumor
development, such as inflammation, the stress response, the cell
cycle, proliferation, differentiation, invasion, apoptosis and the
tumor microenvironment, promoting tumor development, morphogenesis,
development and metastasis (20-23). In cancer, miRNAs can act as tumor
suppressors or oncogenes and play important roles in resistance to
treatment (24-26).
Studies have demonstrated that the tumor cell
response to treatment can be consolidated using basic molecular
features explored by molecular technology (27,28). Two-thirds of BCs have similar
characteristics, depending on the interaction of estrogen with
nuclear ERα protein (29,30). In addition, the disorders of a
number of oncogenes or tumor suppressors are related to BC
(31-33).
A better understanding of the specific functions of
the molecules involved in BC progression and the regulatory
mechanisms is critical in order to identify effective treatment
strategies for BC. In addition, the identification of specific
biomarkers will help improve early diagnosis and establish
individualized treatments, as well predict recurrence and the
clinical efficacy of treatment in patients with BC to improve
patient prognosis.
Early diagnosis of BC is usually made by screening
or symptoms that prompt a diagnostic test (such as the detection of
a palpable mass). The prognosis of BC depends on the tumor
characteristics, patient factors and response to treatment. Despite
significant advances in the early detection, diagnosis and
treatment of BC, the overall survival rates for patients with BC
remain low due to acquired drug resistance, heterogeneity, relapse
and metastases (34-36). Metastasis and relapse following
treatment are the major factors that contribute to morbidity and
mortality in patients with BC, and approximately 30% of patients
still have a poor prognosis (37).
The early diagnosis and detection of BC can reduce
the rates of mortality. Currently, serum-based tumor markers are
the most effective screening method for the diagnosis of BC and
relapse detection in patients with BC. However, these biomarkers
are associated with a low specificity, low sensitivity, high
false-positive rates and complications, which limit their use in
diagnosis, and in monitoring disease progression and recurrence
(38). For example,
carcinoembryonic antigen and cancer antigen 15-3 have yielded
false-positive results and low sensitivity, limiting their clinical
application in detecting early-stage BC (39). Additionally, while some
circulating tumor biomarkers, such as tissue peptide-specific
antigens, have been used in clinical diagnosis, their diagnostic
specificity and sensitivity are low (40). Currently available serum markers
are unable to accurately diagnose early-stage BC due to a lack of
sensitivity and specificity (41). In addition, the hormone receptors,
ER, PR and HER2, have been established as markers for routine
analysis; however, their application is limited to specific BC
subtypes (42). For example, TNBC
lacks the expression of all 3 hormone receptors; thus, hormone
receptor-based biomarkers cannot be used to detect TNBC. Therefore,
the identification of highly specific and sensitive biomarkers is
essential for the early diagnosis and treatment of BC.
As key regulators in tumor progression, miRNAs have
been shown to act as potential biomarkers for clinical diagnosis
and as novel anticancer drugs (43-45). Over the past few decades, numerous
studies have focused on assessing the clinical utility of miRNAs as
potential biomarkers in BC. Several tumor-associated circulating
miRNAs in BC have been identified as promising biomarkers, such as
several circulating miRNAs that are significantly elevated in early
BC patients (46,47). Circulating miRNAs, including serum
and plasma miRNAs, are not only easy to access and measure, but
have also been shown to effectively distinguish cancer patients
from healthy individuals (48,49).
Plasma miR-21 can be used as a biomarker for
detecting primary and relapsed BC. In a previous study, a
significantly increased plasma miR-21 level was detected in
patients with primary (P<0.001) and recurrent (P<0.001) BC
compared with the levels in healthy subjects; miR-21 plays an
important role in the tumorigenesis, drug resistance and BC
recurrence (50). Another study
demonstrated that the expression of miR-34a was decreased in the
serum of patients with BC, and miRNA-34a can be used as a potential
non-invasive molecular marker for the early diagnosis of BC
(51). miR-891a-5p negatively
regulates the expression of ADAM10 by directly binding to its
3′UTR, resulting in the inhibition of proliferation and migration
of BC cells. miR-891a-5p is an important prognostic indicator for
hormone receptor-positive BC (52). miR-15b-5p promotes BC cell
proliferation, migration, and invasion by directly targeting HPSE2.
miR-15b-5p can be used as a prognostic tool and therapeutic target
for patients with BC (53)
(Table II).
Studies have demonstrated that miRNAs are very
stable in formalin-fixed paraffin-embedded tissue, suggesting their
presence in body fluids (54).
Due to the very high stability of miRNAs in tissues and body
fluids, such as serum, plasma, saliva, sweat and urine, miRNAs have
been considered promising markers for early detection, diagnosis,
and prognosis and targets for cancer treatment (55).
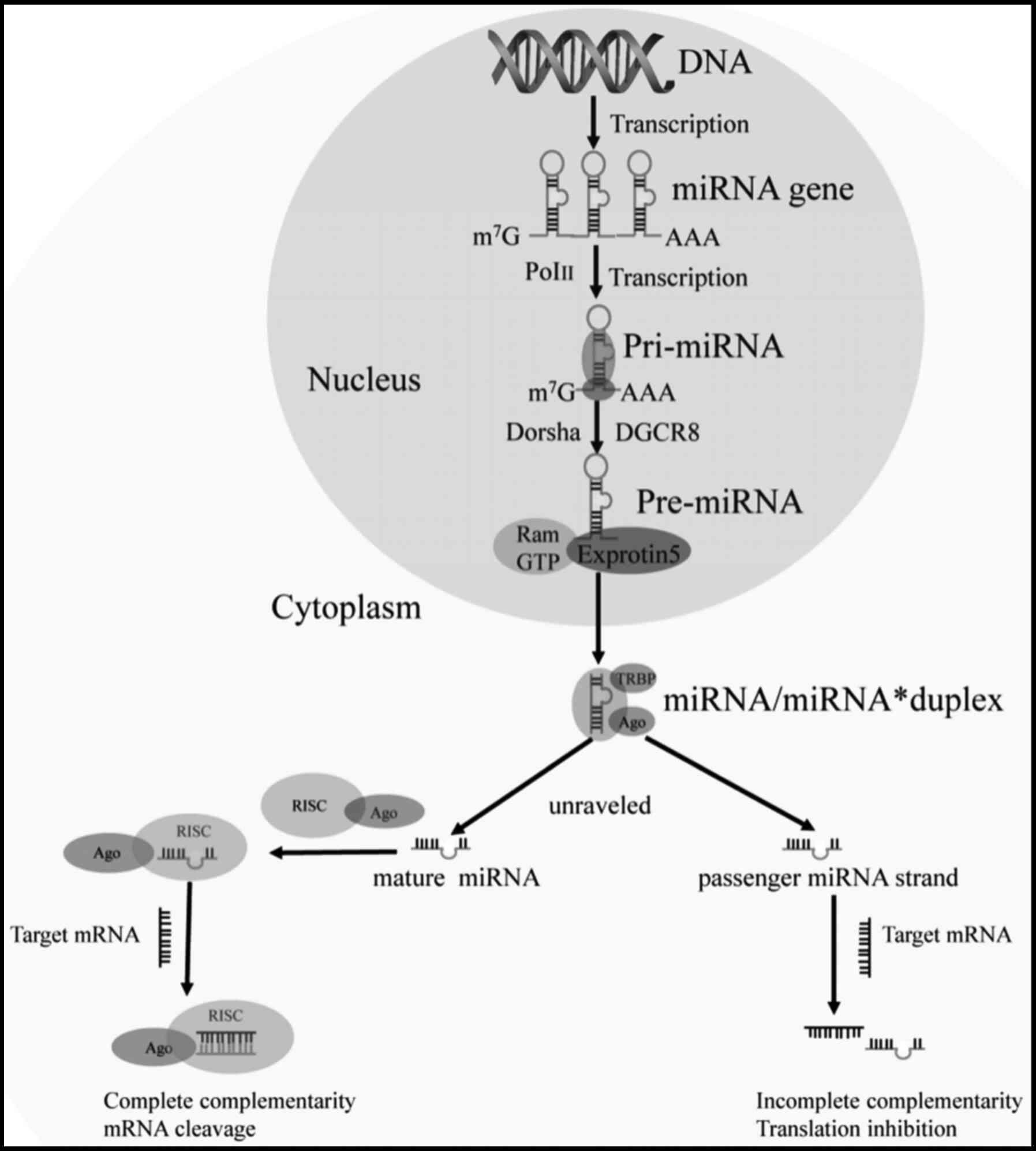
In animals, miRNAs are coded as monocistronic (as a
single gene), polycistronic (as a cluster), or introns (56). To date, >900 human miRNAs have
been identified and are transcribed as a single unit or in
polycistronic clusters or synergistically transcribed with host
protein-encoding genes (57,58). Mature miRNAs are derived from long
primary transcripts (59). miRNAs
are transcribed by RNA polymerase II (Pol II) to produce primary
miRNAs (pri-miRNA). After pri-miRNAs are transcribed in the
nucleus, they are cleaved by nuclear III Drosha to produce a
stem-loop intermediate known as precursor miRNAs (60,61). This process requires a series of
microprocessors, including RNase III Drosha, DiGeorge Critical
Region 8, DDX5 and DDX17 (62).
The processed pre-miRNAs interact with the receptor of exportin 5
(Exp5) and are exported into the cytoplasm, where they are
subsequently truncated by RNase III Dicer in the cytoplasm to
generate a miRNA duplex intermediate containing 20-25 nucleotides
(63-66). The duplex is then unraveled and
the mature single-stand miRNA is incorporated into the RNA-induced
silencing complex (RISC) to form a miRNA-induced silencing complex
(miRISC) with argonaute (Ago) family proteins (67). The miRISC complex pairs by
complementary target recognition to the 3′UTR of target mRNAs,
thereby silencing the expression of the target mRNAs through mRNA
cleavage or translation inhibition (68-72) (Fig.
1). Specific sequences in mature miRNAs known as 'seed
sequences' are necessary for target site recognition. The binding
of a fully complementary mature miRNA to a target site results in
the cleavage of the target mRNA, while binding with incomplete
complementarity results in translational inhibition. The main
function of miRNAs is to downregulate the expression of target
genes through mechanisms, such as RNA degradation, the induction of
capping, induction of adenylation, change in cap protein binding,
decreased ribosome occupancy and mRNA chelation (73). Generally, the seed sequence of a
miRNA is base-paired to the 3′UTR of the mRNA. The seed sequence
usually consists of 2-8 nucleotides, beginning at the second
nucleotide of the 5′end of the miRNA and ending at the eighth
nucleotide (74,75). The seed sequence plays an
important role in identifying the target mRNA and provides an
important basis for miRNA target prediction. A single miRNA usually
targets a number of mRNAs, and a single transcript may be targeted
by multiple miRNAs due to shared seed sequences (76).
miRNAs play a key role in human cancer. The
underlying mechanisms of abnormal miRNA expression in cancer
include chromosomal aberrations, defects in transcriptional
control, dysregulated epigenetic regulation and irregularities in
miRNA biogenesis. miRNAs can function as tumor suppressors by
negatively regulating molecules that are involved in the formation
of malignant tumors (77-80). miRNA dysregulation can disrupt
intracellular RNA networks in cancer cells, and a number of
researchers have focused on exploring the mechanisms of action of
miRNA-dependent molecular networks in cancer.
miRNA dysregulation is directly related to the
emergence of various aspects of tumorigenesis. miRNAs are involved
in tumorigenesis, tumor development, proliferation, metastasis,
epithelial-mesenchymal transition (EMT), stemness maintenance and
therapeutic resistance by downregulating target oncogenes or tumor
suppressor genes (81).
Therefore, these dysregulated miRNAs may serve as biomarkers for
cancer diagnosis and prognosis, and may be used as potential
targets for cancer treatment.
Researchers have performed RNA sequencing on BC
clinical specimens to identify potential tumor suppressor miRNAs in
BC. In a previous study, 64 miRNAs were identified as candidate
tumor suppressor miRNAs in BC cells. The expression levels of
miR-99a-5p/-3p, miR-101-5p/-3p, miR-126-5p/-3p, miR-143-5p/-3p and
miR-144-5p/-3p were downregulated in BC (82). That study demonstrated that the
low expression of miR-101-5p predicts a poor prognosis of patients
with BC (82). As previously
demonstrated, miR-302b was significantly downregulated in BC
tissues and cell lines compared with the controls. Patients with BC
with a lower miR-302b expression were found to have shorter
survival times than patients with a higher miR-302b expression.
miR-302b overexpression inhibited BC cell proliferation, migration
and invasion, while miR-302b silencing exerted the opposite effects
(83). miR-302b expression was
shown to be an independent prognostic factor for BC.
A previous study demonstrated that the expression
level of miR-9 in BC tissues was significantly decreased compared
with the controls; however, the expression level of miR-9 in serum
samples was not significantly altered (84). In another study, a decreased
miR-296 expression was associated with malignant phenotypes and a
poorer prognosis of patients with BC. The upregulation of miR-296
inhibited the proliferation, invasion and migration ability of BC
cells in vivo (85).
Another study also demonstrated that miR-539 expression was
downregulated in BC tissues and cell lines. The decreased
expression of miR-539 was closely related to lymph node metastasis
in patients with BC. The overexpression of miR-539 inhibited the
proliferation and promoted the apoptosis of BC cells (86). miR-124 was significantly reduced
in metastatic bone tissues from BC. The downregulation of miR-124
was found to be associated with aggressive clinical characteristics
and a shorter bone metastasis-free survival and overall survival
(87). miRNA-221-5p has been
shown to be upregulated in BC tissues, and its increased expression
is associated with lymph node metastasis, distant metastasis and a
poor prognosis of BC (88).
miR-34a has been found to be significantly downregulated in BC
tissues, and miR-34a can be used as a biomarker for the diagnosis
of BC in healthy women (89). A
previous study found that miRNA-663b was upregulated in BC with
tamoxifen (TAM) resistance. The downregulation of miRNA-663b
inhibited cell proliferative ability and promoted cell apoptosis,
resulting in an enhanced TAM sensitivity (90) (Table III).
Treatment of BC is mainly determined based on tumor
subtype, disease stage, the mutation status of the BC gene and
several genomic markers, and the health status and age of the
patient. Conventional treatments for BC include radiotherapy,
chemotherapy, molecular-targeted therapies, endocrine treatments
and surgical resection (91,92). Endocrine therapies (such as TAM
and aromatase inhibitors) are used for the treatment of hormone
receptor-positive BC tumors, while the monoclonal antibody,
trastuzumab, is widely used for the treatment of HER2-positive
tumors. TNBC does not respond to endocrine therapy as this subtype
lacks hormone receptors (7). TNBC
is the most aggressive and metastatic subtype of BC with poor
treatment outcomes (93), and
chemotherapy is currently the only treatment option for TNBC
(94).
The treatment of BC remains a clinical challenge.
Chemotherapy, hormone therapy and targeted therapy are
traditionally used in the treatment of BC. However, BC is a
heterogeneous disease, and patients often develop drug resistance.
Although substantial progress has been made in the treatment of BC,
novel therapeutic targets are still required to overcome the
current obstacles to BC treatment. Studies have demonstrated that
the abnormal expression of specific miRNAs has been associated with
resistance to chemotherapy, radiation therapy and hormone therapy.
The dysregulation of miRNAs can affect target protein expression in
cells, the ability for anticancer drugs to reach their targets
within cells and apoptotic pathways (95).
In recent years, the inhibition of cellular and
molecular mechanisms that interfere with the development of BC is
one of the critical diagnostic and therapeutic strategies (96,97). The upregulation of nicotinamide
phosphoribosyltransferase (NAMPT) in patients with BC has been
associated with the increased adverse effects of doxorubicin. Thus,
inhibiting NAMPT is a strategy for the treatment of BC (98). NAMP is a rate-limiting enzyme of
rescues the biosynthetic pathway of nicotinamide adenine
dinucleotide (NAD) (99,100). NAD disorders may be related to
the progression of BC. NAMPT is a target of miR-154, and the
expression level of miR-154 has been found to be inversely related
to the mRNA and protein levels of NAMPT in BC cell lines. miR-154
inhibits the NAD rescue pathway, leading to a significant decrease
in cell viability and an increase in cell mortality. In BC cells
co-treated with doxorubicin and miR-154 mimics, cell viability was
markedly reduced compared with cells treated with doxorubicin
alone. Therefore, targeting the inhibitory effect of miR-154 on NAD
may be an effective strategy to improve the therapeutic effect on
BC (101).
TAM is an endocrine therapy that is commonly used in
the treatment of patients with BC expressing ER. The downregulation
of miRNA-663b has been shown to inhibit the proliferative ability
and promote the apoptosis of BC cells, resulting in an enhanced TAM
sensitivity. miRNA-663b may therefore be a critical therapeutic
target in BC (90). Sevoflurane
significantly suppresses BC cell proliferation by arresting the
cell cycle at the G1 phase. A previous study demonstrated that
sevoflurane inhibits BC cell proliferation by upregulating the
expression of miR-203 (102).
Due to the low immune response and low toxicity of
miRNAs, miRNAs have become a promising therapeutic strategy for
cancer treatment. At present, the main challenge of miRNA-based
cancer therapy is to achieve the specificity of miRNA therapy and
the effective and safe delivery of miRNAs to cancer cells. In
addition to free miRNAs in patient serum or plasma, miRNAs have
also been identified in exosomes (103). Exosomes or microvesicles are
small endosomally-derived vesicles that are secreted by a variety
of cell types and tissues. Engineered exosomes have become a new
drug delivery vehicle for cancer treatment, and exosomes carrying
miRNAs can be transferred among different cell lines through direct
uptake.
Researchers have indicated that MDA-MB-231 BC
cell-derived exosomes (231-Exo) can be specifically internalized by
non-small cell lung cancer cells through specific interactions
between overexpressed integrin β4 (on exosomes) and surfactant
protein C (SPC) on cancer cells. miRNA-231-Exo (miRNA-126 loaded in
231-Exo) has been shown to significantly inhibit the proliferation
and migration of A549 lung cancer cells by interrupting the
phosphatase and tensin homolog (PTEN)/PI3K/AKT signaling pathway.
In addition, miRNA-231-Exo also inhibits the formation of lung
metastases (104). A previous
study demonstrated that treatment with exosomes derived from
MDA-MB-231 cells enhanced the viability, migration and chemotherapy
resistance of non-malignant HMLE cells (105). Some researchers used
three-layered polyplex with folic acid as a targeting group to
deliver miR-210 systemically to BC cells, thereby inhibiting the
growth of BC (106). miRNAs and
exosomes also have been shown to function as novel diagnostic and
therapeutic biomarkers for monitoring patients with BC (107).
miRNAs can positively or negatively regulate
signaling pathways, promoting or preventing signal transmission to
downstream effectors. Multiple studies have demonstrated that
miRNAs play key functions in tumorigenesis by regulating tumor
suppressors or oncogenes (108,109). Over the past decade, research on
the pathogenesis of BC has led to the discovery of a number of
signaling pathways and corresponding therapeutic targets involved
in BC, such as transforming growth factor β (110,111), phosphoinositide-3-kinase (PI3K),
v-akt murine thymoma viral oncogene homolog (AKT), mechanistic
target of rapamycin (mTOR) (112-114), Ras/mitogen-activated protein
kinase (MAPK) (115,116), nuclear factor (NF)-κB (117-120), Notch (121-123), Wnt/β (124,125), HER2 (126), vascular endothelial growth
factor (VEGF) (127,128), epidermal growth factor receptor
(EGFR) (129,130), cyclin-dependent kinase 4/6
(CDK4/6) (131), poly(adenosine
diphosphate-ribose) polymerase (PARP) (132,133) and programmed death-1 (PD-1)
(134). Below, several key
signaling pathways in BC and the involvements of miRNAs in BC are
reviewed.
TGF-β is a polypeptide growth factor. The TGF-β
signaling pathway is involved in hindering the growth and
proliferation of early-stage cancer cells, and increasing the
metastasis and invasion of late-stage tumor cells. TGF-β plays a
key role in EMT in cells and cancer metastasis, and promotes EMT in
BC (135). EMT is the conversion
of epithelial phenotype to a highly active fibroblast or
mesenchymal phenotype (136).
EMT is generally considered to be one of the most important steps
which triggers migration, thus also promoting tumor invasion and
metastasis (137-139).
The TGF-β signaling pathway is controlled by
multiple molecules. miR-200b-200a-429 or miR-200c-141 in Madin
Darby canine kidney epithelial cells lead to the suppression of
TGF-β-stimulated EMT (140).
TMEPAI can transform TGF-β from a tumor suppressor to a tumor
promoter and can induce the tumorigenic function of EMT (141). The overexpression of miR-133b
has been shown to significantly reduce the expression of TGFβR1, an
essential receptor of TGF-β/SMAD signaling, and to inhibit
TGF-β-induced EMT and BC cell invasion in vitro (142). GATA3 has been shown to play a
role in inhibiting EMT in BC by activating miR-455-3p expression.
The enforced expression of miR-455-3p alone partially prevents EMT
induced by TGF-β in cells and tumor xenografts by directly
inhibiting key components of TGF-β signaling (143). Zinc finger E-box binding
homeobox 1 (ZEB1) is an important member of the zinc finger
homeodomain transcription factor family and was originally
identified as a binding protein of the lens-specific δ1-crystalline
enhancer. ZEB1 is also a key transcription factor in the EMT
process and plays a vital role in the progression of BC.
The PI3K/Akt/mTOR pathway is involved in tumor
growth, proliferation, survival, motility, metabolism and in the
regulation of the immune response. The activation of this pathway
is one of the main mechanisms underlying the resistance of cancer
cells to antitumor therapy (144,145). Lysosomal-associated protein
transmembrane 4 beta (LAPTM4B) is a proto-oncogene and a positive
regulator of cancer progression. A previous study demonstrated that
miR-132-3p inhibited the migration and invasion of BC cells through
LAPTM4B by mediating EMT signals and partially reversed the
carcinogenesis induced by LAPTM4B by inhibiting the PI3K/AKT/mTOR
signaling pathway (146). miR-21
targets PTEN by inhibiting the PI3K/AKT/mTOR pathway to coordinate
the functions of autophagy and apoptosis. The silencing of miR-21
has been shown to enhance the sensitivity of ER(+) BC cells to TAM
and fulvestrant by increasing autophagic cell death (147). miR-122 can inhibit cancer by
targeting insulin-like growth factor 1 receptor (IGF1R) and
regulating the PI3K/Akt/mTOR/p70S6K pathway. miR-122 may thus be a
treatment or diagnosis or prognosis target for the treatment of BC
(148).
Ras is a signal transduction effector that acts as a
second-messenger to initiate intracellular signaling pathways. In
BC, Ras may be overactivated by growth factor receptors, such as
HER2 and IGF-1, and they may subsequently activate downstream
pathways, such as the MAPK and PI3K/AKT pathways through Raf, MEK
and extracellular signal-regulated kinase (ERK)1/2, ultimately
leading to the survival and proliferation of BC cells (149). The MAPK pathway superfamily
involves several members constituting seven groups: ERK1/2, Jun
N-terminal kinase (JNK) 1/2/3, ERK3/4, p38 α/β/γ/δ, ERK5, ERK7/8
and Nemo-like kinases (NLKs). The MAPK pathways do not individually
regulate cell functions, but interact with each other, as well as
with other signaling pathways such as the TGFβ/Smads,
PI3K/Akt/mTor, Wnt/β-catenin and Rho/actin pathways (150,151). A major targeted therapeutic
strategy for BC is to block its downstream effectors with specific
small molecule inhibitors targeting the Ras/MAPK pathway (152). miR-200c has been shown to
suppress the expression of KRas, and thus, miR-200c hinders the
proliferation and survival of breast tumor cells via negative
control of Akt and ERK (153).
It has been demonstrated that the increased expression of miR-543
inhibits the progression of BC cells by targeting ERK2 and
inhibiting the MAPK/ERK pathway (154). An increase in miR-148a/152 has
also been shown to prevent the development of BC via the
downregulation of the expression levels of IGF1R and IRS-1, and the
inactivation of the Akt and MAPK/ERK pathways (155).
The NF-κB family consists of 5 members: NF-κB1
(p105/p50), NF-κB2 (p100/p52), RelA (p65), RelB and c-Rel. The
NF-κB family consists of 5 members, including NF-κB1 (p105/p50),
NF-κB2 (p100/p52) and RelA (p65), which are influenced by miRNAs
and have major functions in the tumorigenesis of BC (156). A previous study demonstrated
that miR-132/-212 were overexpressed in doxorubicin (DOX)-resistant
BC, and the silencing of miR-132/-212 expression induced DOX
accumulation, while the overexpression of miR-132/-212 led to BC
resistance protein (BCRP)-based DOX efflux. The upregulation of
miR-132/-212 suppressed the expression of PTEN, a target gene of
miR-132/-212, which activated AKT phosphorylation and the NF-κB
members, including NF-κB1 (p105/p50), NF-κB2 (p100/p52) and RelA
(p65). The miR-132/-212-PTEN-AKT/NF-κB-BCRP pathway plays an
important role in the development of BC drug resistance and
provides a potential method to reverse drug resistance (157). The upregulation of miRNA-181b
has been shown to suppress BC cell survival and migration via the
NF-κB signaling pathway (158).
The therapeutic administration of glucocorticoids is frequently
used as an add-on chemotherapy for palliative purposes during the
of treatment BC. In a previous study, glucocorticoid receptor
agonists induced miR-708 and the downstream suppression of NF-κB
signaling, which may be applicable as a novel therapeutic
intervention in the treatment of BC (159).
The Notch signaling pathway regulates a number of
biological processes, such as cell proliferation, differentiation
and apoptosis. The interplay between the Notch pathway and miRNAs
is associated with the progression of BC. The long non-coding RNA
(lncRNA) small nucleolar RNA host gene 3 (SNHG3) has been found to
promote BC cell proliferation and invasion by regulating the
miR-101/zinc-finger enhancer binding axis in BC. lncRNA SNHG3
promotes BC cell proliferation and metastasis by activating the
Notch signaling pathway (160).
The small nucleolar RNA host gene 7 has been shown to promote BC
tumorigenesis and progression by sponging miR-34a through the
initiation of EMT and the Notch-1 pathway (161). Rhamnetin has been shown to
significantly promote the expression of p53 protein and miR-34a and
suppresses the expression of Notch1 protein in MCF-7 cells.
Rhamnetin induces the apoptosis of human BC cells via the
miR-34a/Notch-1 signaling pathway (162). In a previous study, miR-34a
inhibited BC stemness and enhanced chemosensitivity to paclitaxel
partially by downregulating the Notch1 pathway. Thus, miR-34a is a
potential target for the prevention and therapy for BC (163). The overexpression of miR-1179
has also been shown to significantly inhibit the proliferation,
migration and invasion of BC cells. miR-1179, a tumor suppressor,
may act as a novel potential prognostic biomarker or molecular
therapeutic target for BC (164).
The Wnt/β-catenin pathway is constitutively active
in BC and promotes metastasis in BC. miR-454-3p is overexpressed in
metastatic BC. The inhibitory effect of miR-454-3p on RPRD1A
activates Wnt/β-catenin signaling, thereby promoting metastasis.
miR-454-3p and RPRD1A may be potential diagnostic and therapeutic
targets for BC metastasis (165). In a previous study, miRNA-216a
overexpression was shown to lead to a decrease in the proliferation
and migration of MCF-7 cells, and to inhibit Wnt and β-catenin
expression in MCF-7 cells. The anticancer effects of miRNA-216a
were reversed by anti-miRNA-216a by promoting the Wnt/β-catenin
signaling pathway. The inactivation of the Wnt pathway enhanced the
anticancer effects of miRNA-216a on MCF-7 cells. miRNA-216a
suppressed the growth of human BC cells by targeting the
Wnt/β-catenin signaling pathway (166). miR-221/222 activates the
Wnt/β-catenin signaling to promote aggressiveness and TNBC
properties of BC (167). The
constitutive activation of the Wnt/β-catenin pathway is inversely
associated with the prognosis of patients with BC. The expression
of miR-1229 is significantly upregulated in BC and is associated
with a poor survival. The overexpression of miR-1229 activates the
Wnt/β-catenin signaling pathway in BC (168). miR-224 downregulates the
Wnt/β-catenin signaling possibly by binding to Frizzled 5 and
inhibited proliferation and migration of BC cells (169).
BC is the most frequently diagnosed type of cancer
among women worldwide and one of the leading causes of
cancer-related mortality in women. miRNAs play an important role in
the tumorigenesis and development of BC. In the present review, the
recent findings of miRNAs involved in the occurrence and
development of BC and the current research on miRNAs as potential
biomarkers and therapeutic targets for BC were summarized. In
addition, several signaling pathways that function in the
development of BC and the roles of miRNAs in these pathways were
reviewed.
Multiple studies have demonstrated that miRNAs are
involved in cell proliferation, apoptosis and metastasis in BC.
Some miRNAs function as oncogenes and activate cancer-related
signaling pathways, while others act as tumor suppressors,
negatively regulating many biomolecules that are critical for the
formation of malignant tumors. Several miRNAs are frequently
dysregulated in BC and represent not only potential diagnostic
markers, but also precise targets for therapies. Increasing
research has been exploring miRNA as therapeutic agents in
chemotherapy or combined with anti-cancer therapies. The currently
available serum markers exhibit a low diagnostic specificity and
sensitivity, thus limiting their applicability for the early
diagnosis of BC. The exact molecular mechanisms responsible for BC
progression remain unknown. Therefore, future research on BC is
required to focus on elucidating the molecular mechanisms of BC in
order to identify effective molecular biomarkers and develop novel
treatment strategies.
Not applicable.
HMU, WZ and YQ were involved in the writing of the
article and critically revised the manuscript. TT and HW were
involved in data collection for the purposes of the review. ZC and
GX conceived the review and revised the manuscript. All authors
have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by Hunan Natural Science
Foundation (C2019143).
|
1
|
Ding L, Li J, Wu C, Yan F, Li X and Zhang
S: A self-assembled RNA-triple helix hydrogel drug delivery system
targeting triple-negative breast cancer. J Mater Chem B.
8:3527–3533. 2020. View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Song Y, Wang Z, Zhang Z, Lu M and
Wang Y: Long Non-coding RNA LINC01787 drives breast cancer
progression via disrupting miR-125b generation. Front Oncol.
9:11402019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ding L, Gu H, Xiong X, Ao H, Cao J, Lin W,
Yu M, Lin J and Cui Q: MicroRNAs involved in carcinogenesis,
prognosis, therapeutic resistance and applications in human
triple-negative breast cancer. Cells. 8:14922019. View Article : Google Scholar
|
|
5
|
Denkiewicz M, Saha I, Rakshit S, Sarkar JP
and Plewczynski D: Identification of breast cancer subtype specific
MicroRNAs using survival analysis to find their role in
transcriptomic regulation. Front Genet. 10:10472019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao Y, Humphries B, Yang C and Wang Z:
MiR-205 Dysregulations in breast cancer: The complexity and
opportunities. Noncoding RNA. 5:532019.
|
|
7
|
Ediriweera MK and Cho SK: Targeting miRNAs
by histone deacetylase inhibitors (HDACi): Rationalizing
epigenetics-based therapies for breast cancer. Pharmacol Ther.
206:1074372020. View Article : Google Scholar
|
|
8
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J
and Shi B: Breast cancer intrinsic subtype classification, clinical
use and future trends. Am J Cancer Res. 5:2929–2943.
2015.PubMed/NCBI
|
|
10
|
Kapadia CH, Ioele SA and Day ES:
Layer-by-layer assembled PLGA nanoparticles carrying miR-34a cargo
inhibit the proliferation and cell cycle progression of
triple-negative breast cancer cells. J Biomed Mater Res A.
108:601–613. 2020. View Article : Google Scholar :
|
|
11
|
Tian Y, Xia S, Ma M and Zuo Y: LINC00096
promotes the proliferation and invasion by sponging miR-383-5p and
regulating RBM3 expression in triple-negative breast cancer. Onco
Targets Ther. 12:10569–10578. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng S, Li M, Miao K and Xu H: lncRNA
GAS5-promoted apoptosis in triple-negative breast cancer by
targeting miR-378a-5p/SUFU signaling. J Cell Biochem.
121:2225–2235. 2020. View Article : Google Scholar
|
|
13
|
Umeh-Garcia M, Simion C, Ho PY, Batra N,
Berg AL, Carraway KL, Yu A and Sweeney C: A novel bioengineered
miR-127 prodrug suppresses the growth and metastatic potential of
triple-negative breast cancer cells. Cancer Res. 80:418–429. 2020.
View Article : Google Scholar
|
|
14
|
Das PK, Siddika MA, Asha SY, Aktar S,
Rakib MA, Khanam JA, Pillai S and Islam F: MicroRNAs, a promising
target for breast cancer stem cells. Mol Diagn Ther. 24:69–83.
2020. View Article : Google Scholar
|
|
15
|
Ge JH, Zhu JW, Fu HY, Shi WB and Zhang CL:
An antisense oligonucleotide drug targeting miR-21 induces H1650
apoptosis and caspase activation. Technol Cancer Res Treat.
18:15330338198922632019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Ann Rev Pathol. 9:287–314. 2014. View Article : Google Scholar
|
|
18
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdolvahabi Z, Nourbakhsh M, Hosseinkhani
S, Hesari Z, Alipour M, Jafarzadeh M, Ghorbanhosseini SS, Seiri P,
Yousefi Z, Yarahmadi S and Golpour P: MicroRNA-590-3P suppresses
cell survival and triggers breast cancer cell apoptosis via
targeting sirtuin-1 and deacetylation of p53. J Cell Biochem.
120:9356–9368. 2019. View Article : Google Scholar
|
|
20
|
Shaffi SK, Galas D, Etheridge A and
Argyropoulos C: Role of MicroRNAs in renal parenchymal diseases-a
new dimension. Int J Mol Sci. 19:17972018. View Article : Google Scholar
|
|
21
|
Chen E, Xu X, Liu R and Liu T: Small but
heavy role: MicroRNAs in hepatocellular carcinoma progression.
Biomed Res Int. 2018:67846072018.PubMed/NCBI
|
|
22
|
Svoronos AA, Engelman DM and Slack FJ:
OncomiR or tumor suppressor? The duplicity of microRNAs in cancer.
Cancer Res. 76:3666–3670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu H, Wang Q, Zhong H, Li L, Zhang Q,
Huang Q and Yu Z: Differentially expressed microRNAs in exosomes of
patients with breast cancer revealed by next-generation sequencing.
Oncol Rep. 43:240–250. 2020.
|
|
24
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar :
|
|
25
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Visone R and Croce CM: MiRNAs and cancer.
Am J Pathol. 174:1131–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reis-Filho JS, Weigelt B, Fumagalli D and
Sotiriou C: Molecular profiling: Moving away from tumor philately.
Sci Transl Med. 2:47ps432010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sotiriou C and Pusztai L: Gene-expression
signatures in breast cancer. N Engl J Med. 360:790–800. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Yu H, Qiao Y, Yang J, Shu J, Zhang
J, Zhang Z, He J and Li Z: Salivary Glycopatterns as potential
biomarkers for screening of early-stage breast cancer.
EBioMedicine. 28:70–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong C, Tan W, Chen K, You N, Zhu S, Liang
G, Xie X, Li Q, Zeng Y, Ouyang N, et al: Prognostic value of a
BCSC-associated MicroRNA signature in hormone receptor-positive
HER2-negative breast cancer. EBioMedicine. 11:199–209. 2016.
View Article : Google Scholar :
|
|
31
|
Do SI, Kim HS, Kim K, Lee H, Do IG, Kim
DH, Chae SW and Sohn JH: Predictive and prognostic value of
sphingosine kinase 1 expression in patients with invasive ductal
carcinoma of the breast. Am J Transl Res. 9:5684–5695. 2017.
|
|
32
|
Phillips SL, Williams CB, Zambrano JN,
Williams CJ and Yeh ES: Connexin 43 in the development and
progression of breast cancer: What's the connection? (Review) Int J
Oncol. 51:1005–1013. 2017. View Article : Google Scholar
|
|
33
|
Katoh M: Canonical and non-canonical WNT
signaling in cancer stem cells and their niches: Cellular
heterogeneity, omics reprogramming, targeted therapy and tumor
plasticity (Review). Int J Oncol. 51:1357–1369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Javadian M, Shekari N, Soltani-Zangbar MS,
Mohammadi A, Mansoori B, Maralbashi S, Shanehbandi D, Baradaran B,
Darabi M and Kazemi T: Docosahexaenoic acid suppresses migration of
triple-negative breast cancer cell through targeting
metastasis-related genes and microRNA under normoxic and hypoxic
conditions. J Cell Biochem. 121:2416–2427. 2020. View Article : Google Scholar
|
|
35
|
Zhao CH, Qu L, Zhang H and Qu R:
Identification of breast cancer-related circRNAs by analysis of
microarray and RNA-sequencing data: An observational study.
Medicine. 98:e180422019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang W, Li GS, Li JD, Pan WY, Shi Q, Xiong
DD, Mo CH, Zeng JJ, Chen G, Feng ZB, et al: The role of upregulated
miR-375 expression in breast cancer: An in vitro and in silico
study. Pathol Res Pract. 216:1527542020. View Article : Google Scholar
|
|
37
|
Tungsukruthai S, Petpiroon N and
Chanvorachote P: Molecular mechanisms of breast cancer metastasis
and potential Anti-metastatic compounds. Anticancer Res.
38:2607–2618. 2018.PubMed/NCBI
|
|
38
|
Li X, Dai D, Chen B, Tang H, Xie X and Wei
W: Determination of the prognostic value of preoperative CA15-3 and
CEA in predicting the prognosis of young patients with breast
cancer. Oncol Lett. 16:4679–4688. 2018.PubMed/NCBI
|
|
39
|
Duffy MJ, Evoy D and McDermott EW: CA
15-3: Uses and limitation as a biomarker for breast cancer. Clin
Chim Acta. 411:1869–1874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie S, Ding X, Mo W and Chen J: Serum
tissue polypeptide-specific antigen is an independent predictor in
breast cancer. Acta Histochem. 116:372–376. 2014. View Article : Google Scholar
|
|
41
|
Duffy MJ: Serum tumor markers in breast
cancer: Are they of clinical value? Clin Chem. 52:345–351. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weigel MT and Dowsett M: Current and
emerging biomarkers in breast cancer: Prognosis and prediction.
Endocr Relat Cancer. 17:R245–R262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo S, Zhang J, Wang B, Zhang B, Wang X,
Huang L, Liu H and Jia B: A 5-serum miRNA panel for the early
detection of colorectal cancer. Onco Targets Ther. 11:2603–2614.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Adams BD, Arem H, Hubal MJ, Cartmel B, Li
F, Harrigan M, Sanft T, Cheng CJ, Pusztai L and Irwin ML: Exercise
and weight loss interventions and miRNA expression in women with
breast cancer. Breast Cancer Res Treat. 170:55–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paszek S, Gabło N, Barnaś E, Szybka M,
Morawiec J, Kołacińska A and Zawlik I: Dysregulation of microRNAs
in triple-negative breast cancer. Ginekol Pol. 88:530–536. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heneghan HM, Miller N, Lowery AJ, Sweeney
KJ, Newell J and Kerin MJ: Circulating microRNAs as novel minimally
invasive biomarkers for breast cancer. Ann Surg. 251:499–505. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao H, Shen J, Medico L, Wang D,
Ambrosone CB and Liu S: A pilot study of circulating miRNAs as
potential biomarkers of early stage breast cancer. PLoS One.
5:e137352010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang N, Wang L, Yang Y, Gong L, Xiao B and
Liu X: A serum exosomal microRNA panel as a potential biomarker
test for gastric cancer. Biochem Biophys Res Commun. 493:1322–1328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhou X, Wen W, Zhu J, Huang Z, Zhang L,
Zhang H, Qi LW, Shan X, Wang T, Cheng W, et al: A six-microRNA
signature in plasma was identified as a potential biomarker in
diagnosis of esophageal squamous cell carcinoma. Oncotarget.
8:34468–34480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Motamedi M, Hashemzadeh Chaleshtori M,
Ghasemi S and Mokarian F: Plasma level of miR-21 and miR-451 in
primary and recurrent breast cancer patients. Breast Cancer (Dove
Med Press). 11:293–301. 2019.
|
|
51
|
Raheem AR, Abdul-Rasheed OF and Al-Naqqash
MA: The diagnostic power of circulating micro ribonucleic acid 34a
in combination with cancer antigen 15-3 as a potential biomarker of
breast cancer. Saudi Med J. 40:1218–1226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang Z, Xu L, He L, Wang J, Shi X, Li Z,
Shi S, Hou K, Teng Y and Qu X: MiR-891a-5p as a prognostic marker
and therapeutic target for hormone receptor-positive breast cancer.
J Cancer. 11:3771–3782. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu B, Liu G, Jin Y, Yang T, Zhang D, Ding
L, Zhou F, Pan Y and Wei Y: miR-15b-5p promotes growth and
metastasis in breast cancer by targeting HPSE2. Front Oncol.
10:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xi Y, Nakajima G, Gavin E, Morris CG, Kudo
K, Hayashi K and Ju J: Systematic analysis of microRNA expression
of RNA extracted from fresh frozen and formalin-fixed
paraffin-embedded samples. RNA. 13:1668–1674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Treiber T, Treiber N and Meister G:
Regulation of microRNA biogenesis and its crosstalk with other
cellular pathways. Nat Rev Mol Cell Biol. 20:5–20. 2019. View Article : Google Scholar
|
|
57
|
Griffiths-Jones S, Saini HK, van Dongen S
and Enright AJ: miRBase: Tools for microRNA genomics. Nucleic Acids
Res. 36:D154–D158. 2008. View Article : Google Scholar :
|
|
58
|
Altuvia Y, Landgraf P, Lithwick G, Elefant
N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T and Margalit H:
Clustering and conservation patterns of human microRNAs. Nucleic
Acids Res. 33:2697–2706. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek
SH and Kim VN: MicroRNA genes are transcribed by RNA polymerase II.
EMBO J. 23:4051–4060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim VN: MicroRNA precursors in motion:
Exportin-5 mediates their nuclear export. Trends Cell Biol.
14:156–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gregory RI, Yan KP, Amuthan G, Chendrimada
T, Doratotaj B, Cooch N and Shiekhattar R: The Microprocessor
complex mediates the genesis of microRNAs. Nature. 432:235–240.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lund E, Güttinger S, Calado A, Dahlberg JE
and Kutay U: Nuclear export of microRNA precursors. Science.
303:95–98. 2004. View Article : Google Scholar
|
|
64
|
Grishok A, Pasquinelli AE, Conte D, Li N,
Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G and Mello CC: Genes
and mechanisms related to RNA interference regulate expression of
the small temporal RNAs that control C. elegans developmental
timing. Cell. 106:23–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentate ribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hutvágner G, McLachlan J, Pasquinelli AE,
Bálint E, Tuschl T and Zamore PD: A cellular function for the
RNA-interference enzyme Dicer in the maturation of the let-7 small
temporal RNA. Science. 293:834–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Parker R and Song H: The enzymes and
control of eukaryotic mRNA turnover. Nat Struct Mol Biol.
11:121–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mathe A, Scott RJ and Avery-Kiejda KA:
MiRNAs and other epigenetic changes as biomarkers in triple
negative breast cancer. Int J Mol Sci. 16:28347–28376. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Petersen CP, Bordeleau ME, Pelletier J and
Sharp PA: Short RNAs repress translation after initiation in
mammalian cells. Mol Cell. 21:533–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sharma S and Lu HC: microRNAs in
Neurodegeneration: Current findings and potential impacts. J
Alzheimers Dis Parkinsonism. 8:4202018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ma F, Liu X, Li D, Wang P, Li N, Lu L and
Cao X: MicroRNA-466l upregulates IL-10 expression in TLR-triggered
macrophages by antagonizing RNA-binding protein
tristetraprolin-mediated IL-10 mRNA degradation. J Immunol.
184:6053–6059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Abolghasemi M, Tehrani SS, Yousefi T,
Karimian A, Mahmoodpoor A, Ghamari A, Jadidi-Niaragh F, Yousefi M,
Kafil HS, Bastami M, et al: MicroRNAs in breast cancer: Roles,
functions, and mechanism of actions. J Cell Physiol. 235:5008–5029.
2020. View Article : Google Scholar
|
|
77
|
Tavakolian S, Goudarzi H, Eslami G and
Faghihloo E: Transcriptional regulation of epithelial to
mesenchymal transition related genes by lipopolysaccharide in human
cervical cancer cell line HeLa. Asian Pac J Cancer Prev.
20:2455–2461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: Bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pourbagheri-Sigaroodi A, Bashash D,
Safaroghli-Azar A, Farshi-Paraasghari M, Momeny M, Mansoor FN and
Ghaffari SH: Contributory role of microRNAs in anti-cancer effects
of small molecule inhibitor of telomerase (BIBR1532) on acute
promyelocytic leukemia cell line. Eur J Pharmacol. 846:49–62. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang W and Luo YP: MicroRNAs in breast
cancer: Oncogene and tumor suppressors with clinical potential. J
Zhejiang Univ Sci B. 16:18–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Toda H, Seki N, Kurozumi S, Shinden Y,
Yamada Y, Nohata N, Moriya S, Idichi T, Maemura K, Fujii T, et al:
RNA-sequence-based microRNA expression signature in breast cancer:
Tumor-suppressive miR-101-5p regulates molecular pathogenesis. Mol
Oncol. 14:426–446. 2020. View Article : Google Scholar
|
|
83
|
Ma J and Zhou Z: Downregulation of
miR-302b is associated with poor prognosis and tumor progression of
breast cancer. Breast Cancer. 27:291–298. 2020. View Article : Google Scholar
|
|
84
|
Tavakolian S, Goudarzi H, Torfi F and
Faghihloo E: Evaluation of microRNA-9 and -192 expression levels as
biomarkers in patients suffering from breast cancer. Biomed Rep.
12:30–34. 2020.
|
|
85
|
Sun WM, Tao W, Li JC, Zhu DM and Miao Y:
MicroRNA-296 functions as a tumor suppressor in breast cancer by
targeting FGFR1 and regulating the Wnt/β-catenin signaling pathway.
Eur Rev Med Pharmacol Sci. 23:10422–10432. 2019.PubMed/NCBI
|
|
86
|
Cai F, Chen L, Sun Y, He C, Fu D and Tang
J: MiR-539 inhibited the malignant behaviors of breast cancer cells
by targeting SP1. Biochem Cell Biol. 98:426–433. 2020. View Article : Google Scholar
|
|
87
|
Cai WL, Huang WD, Li B, Chen TR, Li ZX,
Zhao CL, Li HY, Wu YM, Yan WJ and Xiao JR: microRNA-124 inhibits
bone metastasis of breast cancer by repressing Interleukin-11. Mol
Cancer. 17:92018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Niu XY, Zhang ZQ and Ma PL: MiRNA-221-5p
promotes breast cancer progression by regulating E-cadherin
expression. Eur Rev Med Pharmacol Sci. 23:6983–6990.
2019.PubMed/NCBI
|
|
89
|
Orangi E and Motovali-Bashi M: Evaluation
of miRNA-9 and miRNA-34a as potential biomarkers for diagnosis of
breast cancer in Iranian women. Gene. 687:272–279. 2019. View Article : Google Scholar
|
|
90
|
Jiang H, Cheng L, Hu P and Liu R:
MicroRNA-663b mediates TAM resistance in breast cancer by
modulating TP73 expression. Mol Med Rep. 18:1120–1126.
2018.PubMed/NCBI
|
|
91
|
Aoki N, Amano S, Ando M, Fukuda A, Ami K,
Imai K, Ganno H, Sugita H, Amagasa H, Arai K, et al: A study of
therapy for locally advanced breast cancer with metastasis. Gan To
Kagaku Ryoho. 43:1432–1434. 2016.In Japanese.
|
|
92
|
Anderson GM: Breast-cancer recurrence
after stopping endocrine therapy. N Engl J Med. 378:8702018.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Neophytou C, Boutsikos P and Papageorgis
P: Molecular mechanisms and emerging therapeutic targets of
triple-negative breast cancer metastasis. Front Oncol. 8:312018.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Huang L, Tang X, Shi X and Su L:
miR-532-5p promotes breast cancer proliferation and migration by
targeting RERG. Exp Ther Med. 19:400–408. 2020.
|
|
95
|
Robertson NM and Yigit MV: The role of
microRNA in resistance to breast cancer therapy. Wiley Interdiscip
Rev RNA. 5:823–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Vranic S, Palazzo J, Sanati S, Florento E,
Contreras E, Xiu J, Swensen J and Gatalica Z: Potential novel
therapy targets in neuroendocrine carcinomas of the breast. Clin
Breast Cancer. 19:131–136. 2019. View Article : Google Scholar
|
|
97
|
Ang D, Ballard M, Beadling C, Warrick A,
Schilling A, O'Gara R, Pukay M, Neff TL, West RB, Corless CL and
Troxell ML: Novel mutations in neuroendocrine carcinoma of the
breast: Possible therapeutic targets. Appl Immunohistochem Mol
Morphol. 23:97–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Folgueira MA, Carraro DM, Brentani H,
Patrão DF, Barbosa EM, Netto MM, Caldeira JR, Katayama ML, Soares
FA, Oliveira CT, et al: Gene expression profile associated with
response to doxorubicin-based therapy in breast cancer. Clin Cancer
Res. 11:7434–7443. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shackelford RE, Mayhall K, Maxwell NM,
Kandil E and Coppola D: Nicotinamide phosphoribosyltransferase in
malignancy: A review. Genes Cancer. 4:447–456. 2013. View Article : Google Scholar
|
|
100
|
Grolla AA, Travelli C, Genazzani AA and
Sethi JK: Extracellular nicotinamide phosphoribosyltransferase, a
new cancer metabokine. Br J Pharmacol. 173:2182–2194. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bolandghamat Pour Z, Nourbakhsh M,
Mousavizadeh K, Madjd Z, Ghorbanhosseini SS, Abdolvahabi Z, Hesari
Z and Ezzati Mobasser S: Suppression of nicotinamide
phosphoribosyltransferase expression by miR-154 reduces the
viability of breast cancer cells and increases their susceptibility
to doxorubicin. BMC Cancer. 19:10272019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Liu J, Yang L, Guo X, Jin G, Wang Q, Lv D,
Liu J, Chen Q, Song Q and Li B: Sevoflurane suppresses
proliferation by upregulating microRNA-203 in breast cancer cells.
Mol Med Rep. 18:455–460. 2018.PubMed/NCBI
|
|
103
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Nie H, Xie X, Zhang D, Zhou Y, Li B, Li F,
Li F, Cheng Y, Mei H, Meng H and Jia L: Use of lung-specific
exosomes for miRNA-126 delivery in non-small cell lung cancer.
Nanoscale. 12:877–887. 2020. View Article : Google Scholar
|
|
105
|
Li XJ, Ren ZJ, Tang JH and Yu Q: Exosomal
MicroRNA MiR-1246 promotes cell proliferation, invasion and drug
resistance by targeting CCNG2 in breast cancer. Cell Physiol
Biochem. 44:1741–1748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li Y, Dai Y, Zhang X and Chen J:
Three-layered polyplex as a microRNA targeted delivery system for
breast cancer gene therapy. Nanotechnology. 28:2851012017.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Jafari SH, Saadatpour Z, Salmaninejad A,
Momeni F, Mokhtari M, Nahand JS, Rahmati M, Mirzaei H and Kianmehr
M: Breast cancer diagnosis: Imaging techniques and biochemical
markers. J Cell Physiol. 233:5200–5213. 2018. View Article : Google Scholar
|
|
108
|
Zarredar H, Ansarin K, Baradaran B,
Shekari N, Eyvazi S, Safari F and Farajnia S: Critical microRNAs in
lung cancer: Recent advances and potential applications. Anticancer
Agents Med Chem. 18:1991–2005. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hu J, Markowitz GJ and Wang X: Noncoding
RNAs regulating cancer signaling network. Adv Exp Med Biol.
927:297–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen W, Zhou S, Mao L, Zhang H, Sun D,
Zhang J, Li J and Tang JH: Crosstalk between TGF-beta signaling and
miRNAs in breast cancer metastasis. Tumour Biol. 37:10011–10019.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Smith B, Agarwal P and Bhowmick NA:
MicroRNA applications for prostate, ovarian and breast cancer in
the era of precision medicine. Endocr Relat Cancer. 24:R157–R172.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kia V, Sharif Beigli M, Hosseini V,
Koochaki A, Paryan M and Mohammadi-Yeganeh S: Is miR-144 an
effective inhibitor of PTEN mRNA: A controversy in breast cancer.
In vitro Cell Dev Biol Anim. 54:621–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yue D and Qin X: miR-182 regulates
trastuzumab resistance by targeting MET in breast cancer cells.
Cancer Gene Ther. 26:1–10. 2019. View Article : Google Scholar
|
|
114
|
Zhang Y, Zhao Z, Li S, Dong L, Li Y, Mao
Y, Liang Y, Tao Y and Ma J: Inhibition of miR-214 attenuates the
migration and invasion of triple-negative breast cancer cells. Mol
Med Rep. 19:4035–4042. 2019.PubMed/NCBI
|
|
115
|
Samadi P, Saki S, Dermani FK, Pourjafar M
and Saidijam M: Emerging ways to treat breast cancer: Will promises
be met? Cell Oncol (Dordr). 41:605–621. 2018. View Article : Google Scholar
|
|
116
|
Xu C, Sun X, Qin S, Wang H, Zheng Z, Xu S,
Luo G, Liu P, Liu J, Du N, et al: Let-7a regulates mammosphere
formation capacity through Ras/NF-κB and Ras/MAPK/ERK pathway in
breast cancer stem cells. Cell Cycle. 14:1686–1697. 2015.
View Article : Google Scholar :
|
|
117
|
Zhao Y, Yang F, Li W, Xu C, Li L, Chen L,
Liu Y and Sun P: miR-29a suppresses MCF-7 cell growth by
downregulating tumor necrosis factor receptor 1. Tumour Biol.
39:10104283176922642017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang R and Nakshatri H: Systemic actions
of breast cancer facilitate functional limitations. Cancers.
12:1942020. View Article : Google Scholar :
|
|
119
|
Ruan L and Qian X: MiR-16-5p inhibits
breast cancer by reducing AKT3 to restrain NF-κB pathway. Biosci
Rep. 39:BSR201916112019. View Article : Google Scholar
|
|
120
|
D'Souza LC, Mishra S, Chakraborty A,
Shekher A, Sharma A and Gupta SC: Oxidative stress and cancer
development: Are noncoding RNAs the missing links? Antioxid Redox
Signal. 33:1209–1229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Mohammady M, Ghetmiri SI, Baharizade M,
Morowvat MH and Torabi S: Expanding the Biotherapeutics realm via
miR-34a: 'Potent Clever Little' agent in breast cancer therapy.
Curr Pharm Biotechnol. 20:665–673. 2019. View Article : Google Scholar
|
|
122
|
Pan JY, Zhang F, Sun CC, Li SJ, Li G, Gong
FY, Bo T, He J, Hua RX, Hu WD, et al: miR-134: A human cancer
suppressor? Mol Ther Nucleic Acids. 6:140–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Majumder M, Dunn L, Liu L, Hasan A,
Vincent K, Brackstone M, Hess D and Lala PK: COX-2 induces
oncogenic micro RNA miR655 in human breast cancer. Sci Rep.
8:3272018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zou Y, Lin X, Bu J, Lin Z, Chen Y, Qiu Y,
Mo H, Tang Y, Fang W and Wu Z: Timeless-Stimulated
miR-5188-FOXO1/β-Catenin-c-Jun feedback loop promotes stemness via
Ubiquitination of β-catenin in breast cancer. Mol Ther. 28:313–327.
2020. View Article : Google Scholar
|
|
125
|
Han B, Peng X, Cheng D, Zhu Y, Du J, Li J
and Yu X: Delphinidin suppresses breast carcinogenesis through the
HOTAIR/microRNA-34a axis. Cancer Sci. 110:3089–3097. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ju J, Zhu AJ and Yuan P: Progress in
targeted therapy for breast cancer. Chronic Dis Transl Med.
4:164–175. 2018.PubMed/NCBI
|
|
127
|
Liu H, Li A, Sun Z, Zhang J and Xu H: Long
non-coding RNA NEAT1 promotes colorectal cancer progression by
regulating miR-205-5p/VEGFA axis. Hum Cell. 33:386–396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kaban K, Salva E and Akbuga J: Modulation
of the dual-faced effects of miR-141 with chitosan/miR-141
nanoplexes in breast cancer cells. J Gene Med. 21:e31162019.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Han M, Wang F, Gu Y, Pei X, Guo G, Yu C,
Li L, Zhu M, Xiong Y and Wang Y: MicroRNA-21 induces breast cancer
cell invasion and migration by suppressing smad7 via EGF and TGF-β
pathways. Oncol Rep. 35:73–80. 2016. View Article : Google Scholar
|
|
130
|
Qian B, Katsaros D, Lu L, Preti M, Durando
A, Arisio R, Mu L and Yu H: High miR-21 expression in breast cancer
associated with poor disease-free survival in early stage disease
and high TGF-beta1. Breast Cancer Res Treat. 117:131–140. 2009.
View Article : Google Scholar
|
|
131
|
Citron F, Segatto I, Vinciguerra GLR,
Musco L, Russo F, Mungo G, D'Andrea S, Mattevi MC, Perin T,
Schiappacassi M, et al: Downregulation of miR-223 expression is an
early event during mammary transformation and confers resistance to
CDK4/6 inhibitors in luminal breast cancer. Cancer Res.
80:1064–1077. 2020. View Article : Google Scholar
|
|
132
|
Lee A, Moon BI and Kim TH: BRCA1/BRCA2
pathogenic variant breast cancer: Treatment and prevention
strategies. Ann Lab Med. 40:114–121. 2020. View Article : Google Scholar
|
|
133
|
Vinayak S, Tolaney SM, Schwartzberg L,
Mita M, McCann G, Tan AR, Wahner-Hendrickson AE, Forero A, Anders
C, Wulf GM, et al: Open-label clinical trial of Niraparib combined
with pembrolizumab for treatment of advanced or metastatic
triple-negative breast cancer. JAMA Oncol. 5:1132–1140. 2019.
View Article : Google Scholar :
|
|
134
|
Wang X and Liu Y: PD-L1 expression in
tumor infiltrated lymphocytes predicts survival in triple-negative
breast cancer. Pathol Res Pract. 216:1528022020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yahya SM and Elsayed GH: A summary for
molecular regulations of miRNAs in breast cancer. Clin Biochem.
48:388–396. 2015. View Article : Google Scholar
|
|
136
|
Grelet S and Howe PH: hnRNP E1 at the
crossroads of translational regulation of epithelial-mesenchymal
transition. J Cancer Metastasis Treat. 5:162019.PubMed/NCBI
|
|
137
|
Campbell K and Casanova J: A common
framework for EMT and collective cell migration. Development.
143:4291–4300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Schaeffer D, Somarelli JA, Hanna G, Palmer
GM and Garcia-Blanco MA: Cellular migration and invasion uncoupled:
Increased migration is not an inexorable consequence of
epithelial-to-mesenchymal transition. Mol Cell Biol. 34:3486–3499.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Son H and Moon A: Epithelial-mesenchymal
transition and cell invasion. Toxicol Res. 26:245–252. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wardhani BW, Puteri MU, Watanabe Y, Louisa
M, Setiabudy R and Kato M: TGF-β-induced TMEPAI attenuates the
response of triple-negative breast cancer cells to doxorubicin and
paclitaxel. J Exp Pharmacol. 12:17–26. 2020. View Article : Google Scholar :
|
|
142
|
Wang S, Huang M, Wang Z, Wang W, Zhang Z,
Qu S and Liu C: MicroRNA-133b targets TGFβ receptor I to inhibit
TGF-β-induced epithelial-to-mesenchymal transition and metastasis
by suppressing the TGF-β/SMAD pathway in breast cancer. Int J
Oncol. 55:1097–1109. 2019.PubMed/NCBI
|
|
143
|
Zeng Y, Gao T, Huang W, Yang Y, Qiu R, Hou
Y, Yu W, Leng S, Feng D, Liu W, et al: MicroRNA-455-3p mediates
GATA3 tumor suppression in mammary epithelial cells by inhibiting
TGF-β signaling. J Biol Chem. 294:15808–15825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Guerrero-Zotano A, Mayer IA and Arteaga
CL: PI3K/AKT/mTOR: Role in breast cancer progression, drug
resistance, and treatment. Cancer Metastasis Rev. 35:515–524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Fruman DA, Chiu H, Hopkins BD, Bagrodia S,
Cantley LC and Abraham RT: The PI3K pathway in human disease. Cell.
170:605–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Li S, Xu JJ and Zhang QY: MicroRNA-132-3p
inhibits tumor malignant progression by regulating
lysosomal-associated protein transmembrane 4 beta in breast cancer.
Cancer Sci. 110:3098–3109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yu X, Li R, Shi W, Jiang T, Wang Y, Li C
and Qu X: Silencing of MicroRNA-21 confers the sensitivity to
tamoxifen and fulvestrant by enhancing autophagic cell death
through inhibition of the PI3K-AKT-mTOR pathway in breast cancer
cells. Biomed Pharmacother. 77:37–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wang B, Wang H and Yang Z: MiR-122
inhibits cell proliferation and tumorigenesis of breast cancer by
targeting IGF1R. PLoS One. 7:e470532012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Thompson KN, Whipple RA, Yoon JR, Lipsky
M, Charpentier MS, Boggs AE, Chakrabarti KR, Bhandary L, Hessler
LK, Martin SS and Vitolo MI: The combinatorial activation of the
PI3K and Ras/MAPK pathways is sufficient for aggressive tumor
formation, while individual pathway activation supports cell
persistence. Oncotarget. 6:35231–35246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Carlomagno F and Chiariello M: Growth
factor transduction pathways: Paradigm of anti-neoplastic targeted
therapy. J Mol Med (Berl). 92:723–733. 2014. View Article : Google Scholar
|
|
151
|
Osaki LH and Gama P: MAPKs and signal
transduction in the control of gastrointestinal epithelial cell
proliferation and differentiation. Int J Mol Sci. 14:10143–10161.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Johnston SR, Semiglazov VF, Manikhas GM,
Spaeth D, Romieu G, Dodwell DJ, Wardley AM, Neven P, Bessems A,
Park YC, et al: A phase II, randomized, blinded study of the
farnesyltransferase inhibitor tipifarnib combined with letrozole in
the treatment of advanced breast cancer after antiestrogen therapy.
Breast Cancer Res Treat. 110:327–335. 2008. View Article : Google Scholar
|
|
153
|
Song C, Liu LZ, Pei XQ, Liu X, Yang L, Ye
F and Xie X, Chen J, Tang H and Xie X: miR-200c inhibits breast
cancer proliferation by targeting KRAS. Oncotarget. 6:34968–34978.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chen P, Xu W, Luo Y, Zhang Y, He Y, Yang S
and Yuan Z: MicroRNA 543 suppresses breast cancer cell
proliferation, blocks cell cycle and induces cell apoptosis via
direct targeting of ERK/MAPK. Onco Targets Ther. 10:1423–1431.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y,
Qi YT, Xu Q, Li W, Lu B, et al: A regulatory circuit of
miR-148a/152 and DNMT1 in modulating cell transformation and tumor
angiogenesis through IGF-IR and IRS1. J Mol Cell Biol. 5:3–13.
2013. View Article : Google Scholar :
|
|
156
|
Wu H, Wang G, Wang Z, An S, Ye P and Luo
S: A negative feedback loop between miR-200b and the nuclear
factor-κB pathway via IKBKB/IKK-β in breast cancer cells. FEBS J.
283:2259–2271. 2016. View Article : Google Scholar
|
|
157
|
Xie M, Fu Z, Cao J, Liu Y, Wu J, Li Q and
Chen Y: MicroRNA-132 and microRNA-212 mediate doxorubicin
resistance by down-regulating the PTEN-AKT/NF-κB signaling pathway
in breast cancer. Biomed Pharmacother. 102:286–294. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Wang L, Wang YX, Chen LP and Ji ML:
Upregulation of microRNA-181b inhibits CCL18-induced breast cancer
cell metastasis and invasion via the NF-κB signaling pathway. Oncol
Lett. 12:4411–4418. 2016. View Article : Google Scholar
|
|
159
|
Senthil Kumar KJ, Gokila Vani M, Hsieh HW,
Lin CC, Liao JW, Chueh PJ and Wang SY: MicroRNA-708 activation by
glucocorticoid receptor agonists regulate breast cancer
tumorigenesis and metastasis via downregulation of NF-κB signaling.
Carcinogenesis. 40:335–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Jiang H, Li X, Wang W and Dong H: Long
non-coding RNA SNHG3 promotes breast cancer cell proliferation and
metastasis by binding to microRNA-154-3p and activating the notch
signaling pathway. BMC Cancer. 20:8382020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Sun X, Huang T, Liu Z, Sun M and Luo S:
LncRNA SNHG7 contributes to tumorigenesis and progression in breast
cancer by interacting with miR-34a through EMT initiation and the
Notch-1 pathway. Eur J Pharmacol. 856:1724072019. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Lan L, Wang Y, Pan Z, Wang B, Yue Z, Jiang
Z, Li L, Wang C and Tang H: Rhamnetin induces apoptosis in human
breast cancer cells via the miR-34a/Notch-1 signaling pathway.
Oncol Lett. 17:676–682. 2019.PubMed/NCBI
|
|
163
|
Kang L, Mao J, Tao Y, Song B, Ma W, Lu Y,
Zhao L, Li J, Yang B and Li L: MicroRNA-34a suppresses the breast
cancer stem cell-like characteristics by downregulating Notch1
pathway. Cancer Sci. 106:700–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Li WJ, Xie XX, Bai J, Wang C, Zhao L and
Jiang DQ: Increased expression of miR-1179 inhibits breast cancer
cell metastasis by modulating Notch signaling pathway and
correlates with favorable prognosis. Eur Rev Med Pharmacol Sci.
22:8374–8382. 2018.PubMed/NCBI
|
|
165
|
Ren L, Chen H, Song J, Chen X, Lin C,
Zhang X, Hou N, Pan J, Zhou Z, Wang L, et al: MiR-454-3p-Mediated
Wnt/β-catenin signaling Antagonists suppression promotes breast
cancer metastasis. Theranostics. 9:449–465. 2019. View Article : Google Scholar :
|
|
166
|
Xie Q, Wang S, Zhao Y, Zhang Z, Qin C and
Yang X: MicroRNA-216a suppresses the proliferation and migration of
human breast cancer cells via the Wnt/β-catenin signaling pathway.
Oncol Rep. 41:2647–2656. 2019.PubMed/NCBI
|
|
167
|
Liu S, Wang Z, Liu Z, Shi S, Zhang Z,
Zhang J and Lin H: miR-221/222 activate the Wnt/β-catenin signaling
to promote triple-negative breast cancer. J Mol Cell Biol.
10:302–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Tan Z, Zheng H, Liu X, Zhang W, Zhu J, Wu
G, Cao L, Song J, Wu S, Song L and Li J: MicroRNA-1229
overexpression promotes cell proliferation and tumorigenicity and
activates Wnt/β-catenin signaling in breast cancer. Oncotarget.
7:24076–24087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liu F, Liu Y, Shen J, Zhang G and Han J:
MicroRNA-224 inhibits proliferation and migration of breast cancer
cells by down-regulating Fizzled 5 expression. Oncotarget.
7:49130–49142. 2016. View Article : Google Scholar : PubMed/NCBI
|